Introduction
Inflammation is a crucial physiological response of
the immune system to infection, and this complex biological symptom
is frequently caused by the assault of pathogens during destructive
injury of human tissues (1-4).
Inflammation is an acute phase that is the initial response, which
involves the increased motion of plasma and inflammatory cells from
the bronchial tubes to the tissue. Inflammation is described in the
pathogenesis of several diseases, including metabolic disease,
infection, pulmonary disease and neoplasm (5-7).
Then, the lipopolysaccharides (LPS) of gram-negative bacteria
activate macrophages, as indicated by the production of tumour
necrosis factor-α (TNF-α), interleukins (ILs) and leukotrienes
(8,9).
MAPKs and nuclear factor-κB (NF-κB) signalling
pathways are major adjusters in the expression of inflammatory
mediators involving cyclooxygenase-2 (COX-2) (10,11). The dimeric form of NF-κB is taken
into the cytoplasm by the physical combination in unstimulated
cells with an inhibitory protein, IκBα (7,12-14). With degradation of IκBα, NF-κB is
translocated to the nucleus, and specific NF-κB inhibitors check
COX-2 expression (15).
Activation of MAPKs leads to the production of inflammatory
mediators such as COX-2 in activated macrophage cells (16). In this study, as part of a
continuing search to establish the anti-inflammatory mechanism of
methanolic extracts of P. foetida L. (PFME), we demonstrated
that PFME is a strong inhibitor of COX-2 expression in
LPS-stimulated RAW264.7 macrophage cells. Moreover, the
anti-inflammatory mechanism by which PFME blocks LPS-induced
inflammation via NF-κB and MAPKs signalling pathways is novel.
Passiflora foetida L. (Passifloraceae)
is better known as 'Wild maracuja', 'Bush Passion fruit' or 'Buah
tikus' in Indonesia. The 'foetid' stinking passionflower is a
species of passionflower with a stinky smell that is indigenous to
tropical America in Mexico, the Southwestern United States
(Arizona, Southern Texas) the Caribbean and much of South America.
During the Age of Exploration, the species was introduced to
Europe, India and Southeast Asia for cultivation and gardening,
which was followed by naturalization in other tropical countries
(17,18). The plant is a climbing herb with
tendrils, and the flowers are white to pale cream and ~5-6 cm in
diameter with 2-4-fold pinnatifid bracts at the base. Leaves are
ovate to obovate, with 3 shallow lobes, 6-9 cm in length, with a
heart-shaped base and frequently a winding and ciliate and pointed
tip. Black seeds are embedded in the pulp. The woody and wiry stems
are sheer, covered by yellow tacky hairs, and the 2-3 cm diameter
fruit is yellowish-orange to red (when ripe). The plant is
generally scattered, growing in wayside thickets and riverbeds and
dried-up forest floors and is similarly found close to human
settlements (18). Extracts from
the leaves and fruit of P. foetida (PF) have been used to
treat biliousness and asthma (19), and leaf and root extracts are used
to treat hysteria, and in the case of headache, a paste of the leaf
is rubbed on the head. The herb is used in lotions or poultices for
skin diseases with erysipelas and inflammation in Brazil (20). Present study has focused on the
pain reliever, anti-diarrhoeal and cytotoxic activities of PF
(17), in addition to the
antiulcer effects, antioxidant activity (21), analgesic activities, biological
and pharmacological activities (17,22,23) and anti-inflammatory effects
(22,23). However, the anti-inflammatory
activity of PFME and the underlying mechanisms have not been
closely examined. In the present study, we investigate the
anti-inflammatory effects of PFME and the fundamental mechanisms in
RAW264.7 macrophage cells.
Materials and methods
Preparation of Passiflora foetida L. (PF)
extract
Plants were collected from the 'Pirangrung, Ujung
Kulon National Park' West Java of Indonesia in 2008. The Center for
Pharmaceutical and Medical Technology (PTFM, BPPT, Jawa Timur,
Indonesia) collected and identified the plants, with the
identifications verified by Herbarium Bogoriense (LIPI, Jakarta,
Indonesia). Voucher specimens are deposited in the herbarium of the
Korea Research Institute of Bioscience and Biotechnology (KRIBB)
and in the PTFM and Herbarium Bogoriense. Passiflora foetida
was treated with methanol and sonicated repeatedly for 3 days at
room temperature to produce the extract.
Cell culture and reagents
Murine macrophage RAW264.7 cells were purchased from
the American Tissue Culture Collection (ATCC, Manassas, VA, USA).
The RAW264.7 cells were grown in Dulbecco's modified Eagle's medium
(DMEM) supplemented with 1% (v/v) penicillin (100
U/ml)/streptomycin (100 μg/ml) and 10% fetal bovine serum
(FBS). The RAW264.7 cells were cultured three times a week, and the
RAW264.7 cells were used for experimentation at 70-80% confluence.
After pre-incubation of the cells for 4 h, 5, 10, 20 and 30
μg/ml of the extract was added. The cell lines were
incubated under a humidified condition of 5% CO2 at
37°C. Escherichia coli LPS and the Griess reagent were
purchased from Sigma (St. Louis, MO, USA). DMEM (Welgene,
Gyeongsan-si, Korea), FBS (HyClone, Logan, UT, USA), and the 1%
(v/v) penicillin (100 U/ml)/streptomycin (100 μg/ml)
(Invitrogen, Grand Island, NY, USA) were also purchased. In another
set of cultures, the cells were co-incubated with the p42/44
inhibitor, PD98059 (10 μM), the p38 inhibitor, SB203580 (10
μM), the c-Jun N-terminal kinase (JNK) inhibitor, SP600125
(10 μM), and the IκBα inhibitor (BAY11-7082) (all from Merck
Millipore, Darmstadt, Germany).
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyl
tetrazolium bromide (MTT) assay
Briefly, the RAW264.7 cells were seeded in a 96-well
plate (1×105 cells/ml) and treated with 5, 10, 20 and 30
μg/ml PFME for 24 h. The cell proliferation was analysed by
MTT (Amnesco, Solon, OH, USA), using the analysis expressed
previously (24). To calculate
actual observance, the background levels of PFME when incubated
only with MTT solution were subtracted. The optical density (OD)
was measured using a multimode microplate reader (Tecan Trading AG,
Mannedorf, Switzerland) at 570 nm.
Prostaglandin E2
(PGE2) assay
In the supernatant, the PGE2
concentration was verified using a commonly available
PGE2 enzyme-linked immunosorbent assay (ELISA) kit
(Cayman Chemical Co., Ann Arbor, MI, USA), according to the
manufacturer's protocols as described previously (25).
ELISA
The levels of pro-inflammatory cytokines were
determined with commercial ELISA kits. IL-6, IL-1β and TNF-α were
purchased from R&D Systems (Minneapolis, MN, USA). The assay
procedure for each item was conducted according to manufacturer's
instructions. The concentrations of mediators were determined at
450 nm using a multimode microplate reader (Tecan Trading AG).
Reverse transcription-polymerase chain
reaction (RT-PCR)
RT-PCR was performed to detect the mRNA expression
of COX-2 and β-actin. Briefly, after LPS (0.5 μg/ml)
stimulation of the RAW264.7 cells for 6 h, the total RNA was
isolated using TRIzol™ reagent (Invitrogen) as recommended by the
producer, and a reverse transcription reaction was accomplished
using an AMPIGENE® cDNA synthesis kit (Enzo Life
Sciences, Farmingdale, NY, USA). The PCR was conducted using a
premix and specific sense and antisense primers in conformity with
the manufacturer's instructions (Bioneer, Daejeon, Korea). The
amplification course consisted of denaturing at 94°C for 5 min,
followed by 94°C for 20 sec, annealing at 60°C for 30 sec, and
extension at 72°C for 45 sec, with a final extension performed at
72°C for 1 min. The primer sequences were as follow: murine COX-2,
5′-GAAGTCTTTGGTCTGGTGCCTG-3′ (sense) and
5′-GTCTGCTGGTTTGGAATAGTTGC-3′ (antisense); and murine β-actin,
5′-TGTTTGAGACCTTCAACACC-3′ (sense) and 5′-CGCTCATTGCCGATAGTGAT-3′
(antisense). β-actin was used as the housekeeping gene when
indicated. PCR products were resolved on 1.5% agarose gels and
stained with ethidium bromide. The images were captured by an
Olympus C4000 Zoom Camera system (Olympus Corp. America, Inc.,
Center Valley, PA, USA).
Immunoblot analysis
Equal amounts of extracted protein (30 μg)
were divided by 11% SDS-polyacrylamide gel electrophoresis
(SDS-PAGE) and transferred to polyvinylidene difluoride (PVDF)
membranes. Each membrane was blocked for 1 h with 5% skim milk in
TBS/T buffer (0.1% Tween-20, 0.1 M Tris-HCl, 0.9% NaCl, pH 7.4) to
block non-specific binding and was then incubated with primary
antibodies that recognized COX-2 (1:1,000), the total forms of
extracellular signal-regulated kinase 1/2 (ERK1/2), p38 MAPK, and
JNK1/3 (1:1,000), the phosphorylated forms of p38 MAPK (1:1,000)
(all from Santa Cruz Biotechnology, Santa Cruz, CA, USA) and the
phosphorylated forms of ERK1/2 and JNK1/2 (1:1,000), in addition to
those for β-actin (1:4,000) and PARP (1:1,000) (all from Cell
Signaling Technology, Beverly, MA, USA). Secondary antibodies were
goat anti-rabbit or anti-mouse antibodies (1:5,000; Santa Cruz
Biotechnology, Inc.). The enhanced chemiluminescence reaction was
performed using a Clarity™ Western ECL Substrate (Bio-Rad
Laboratories, Hercules, CA, USA), and the positive bands were
detected on radiographic film.
Statistical analyses
From the statistical analyses, the values are
expressed as the mean ± SEM of the sample determinations. The
statistical significance was determined using a two-tailed
Student's t-test for independent means, with P-values of <0.05
statistically significant.
Results
Effects of PFME on cell viability in
RAW264.7 macrophages
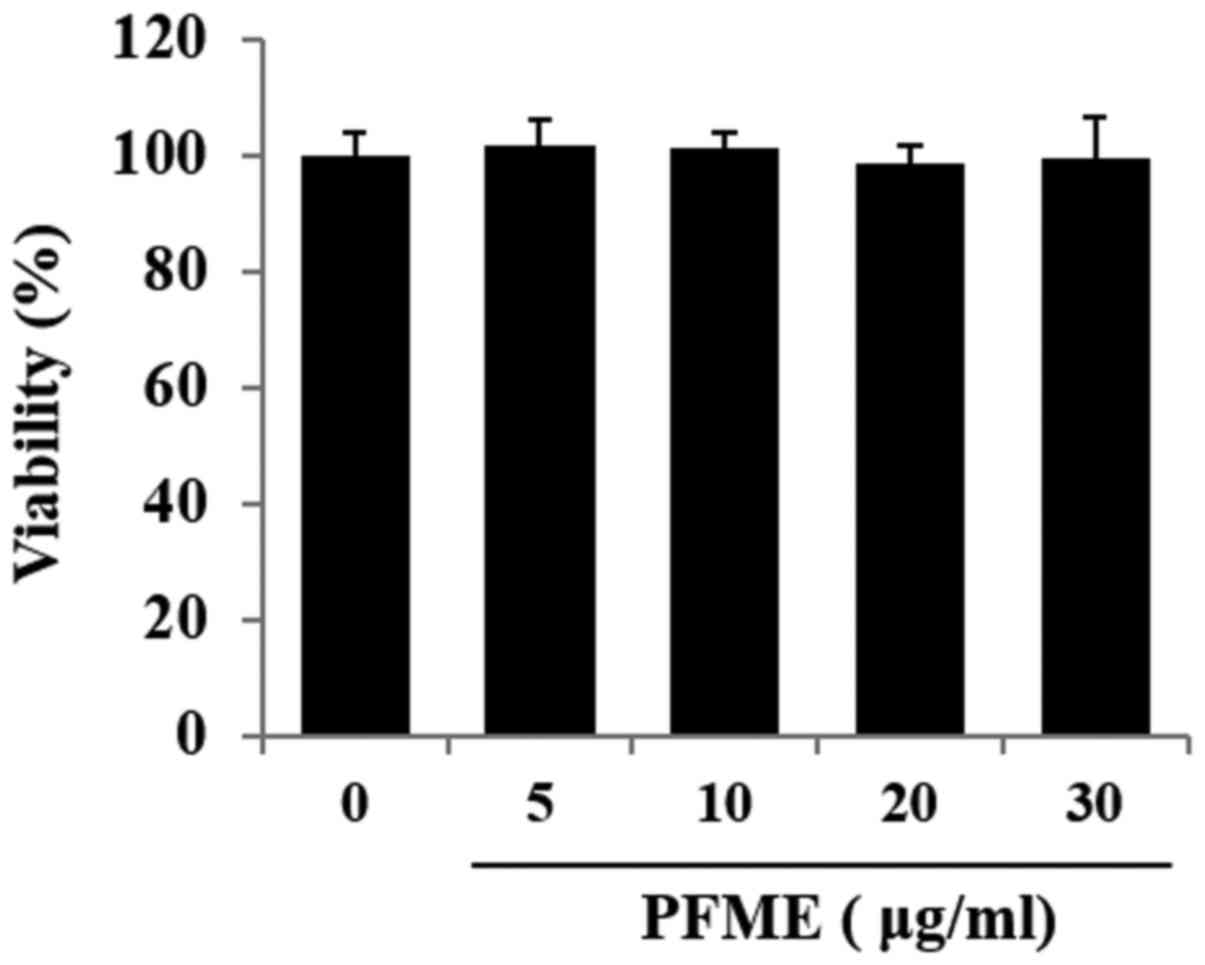
Fig. 1 shows the
effect of PFME on cell viability as determined by the MTT assay,
with morphology of the cells confirmed with microscopic pictures.
Cell viability did not decrease significantly at concentrations of
PFME up to 30 μg/ml. Accordingly, for all ensuing
experiments, the concentration range used was 0-30 μg/ml
PFME.
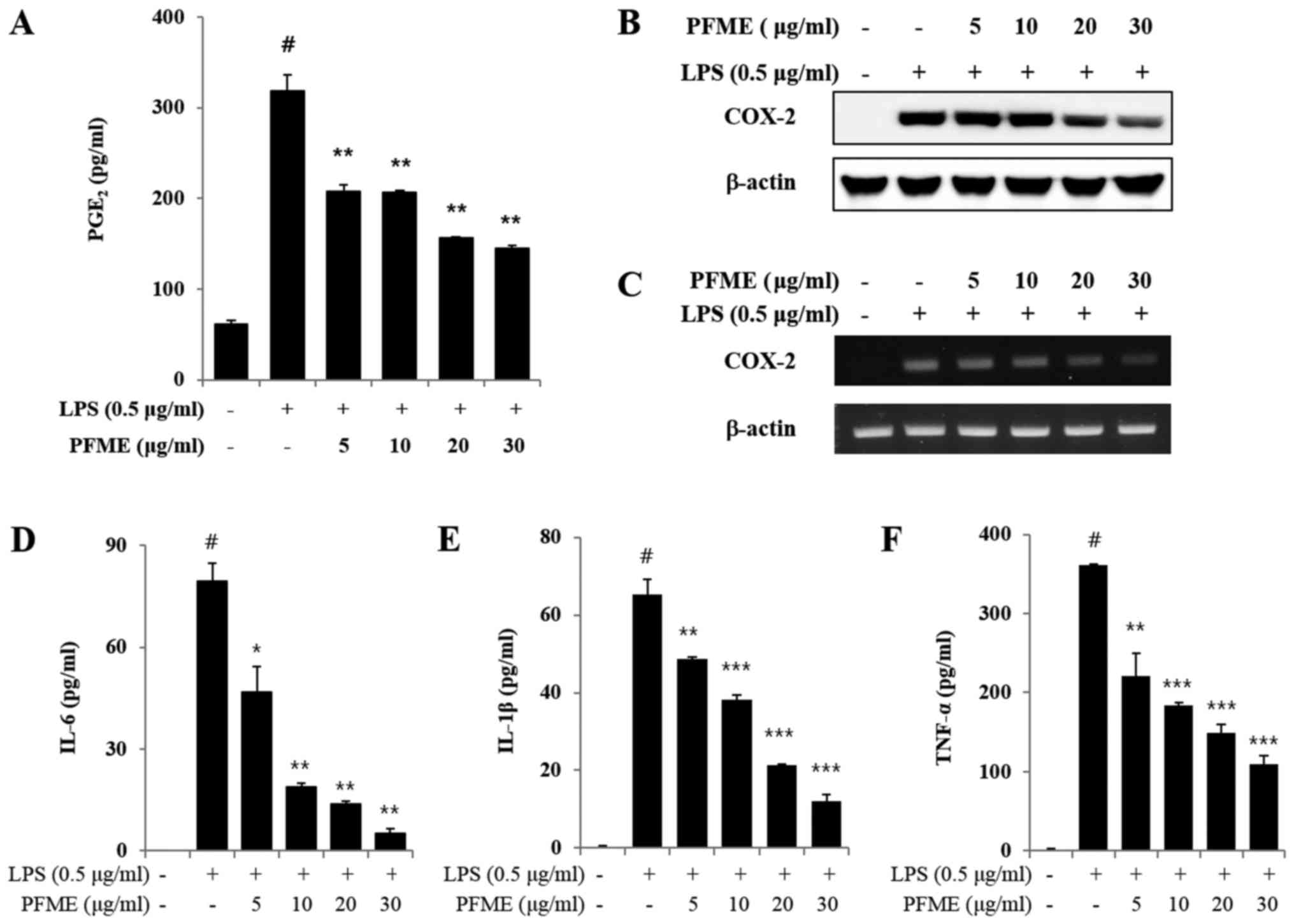
PFME decreases the production of
PGE2 by suppressing COX-2 and pro-inflammatory cytokine
expression in LPS-stimulated RAW264.7 cells
Unstimulated RAW264.7 cells secreted basal levels of
PGE2, whereas PGE2 production increased with
LPS stimulation. We determined that PFME decreased the
LPS-stimulated production of PGE2 and inhibited the
expression of COX-2 in the macrophage cells. Moreover, PFME
substantially decreased the LPS-stimulated PGE2
production in a concentration-dependent manner (Fig. 2A). The LPS-stimulated RAW264.7
cells overexpressed COX-2, whereas the PFME-treated cells
stimulated with LPS suppressed expression of COX-2 protein
(Fig. 2B). The expression of
COX-2 protein was consistent with the mRNA results, and the PFME
suppressed the expression of COX-2 mRNA in the LPS-stimulated
RAW264.7 cells (Fig. 2C).
Cytokines and pro-inflammatory mediators play significant roles in
an immune reaction (26,27). Therefore, we estimated the
influence of PFME on the production of cytokines, such as IL-6,
TNF-α and IL-1β, and pro-inflammatory mediators in RAW264.7
macrophage cells (Fig. 2D–F). As
shown in Fig. 2D–F as measured by
ELISA, PFME suppressed production of IL-6, TNF-α and IL-1β in a
concentration-dependent manner.
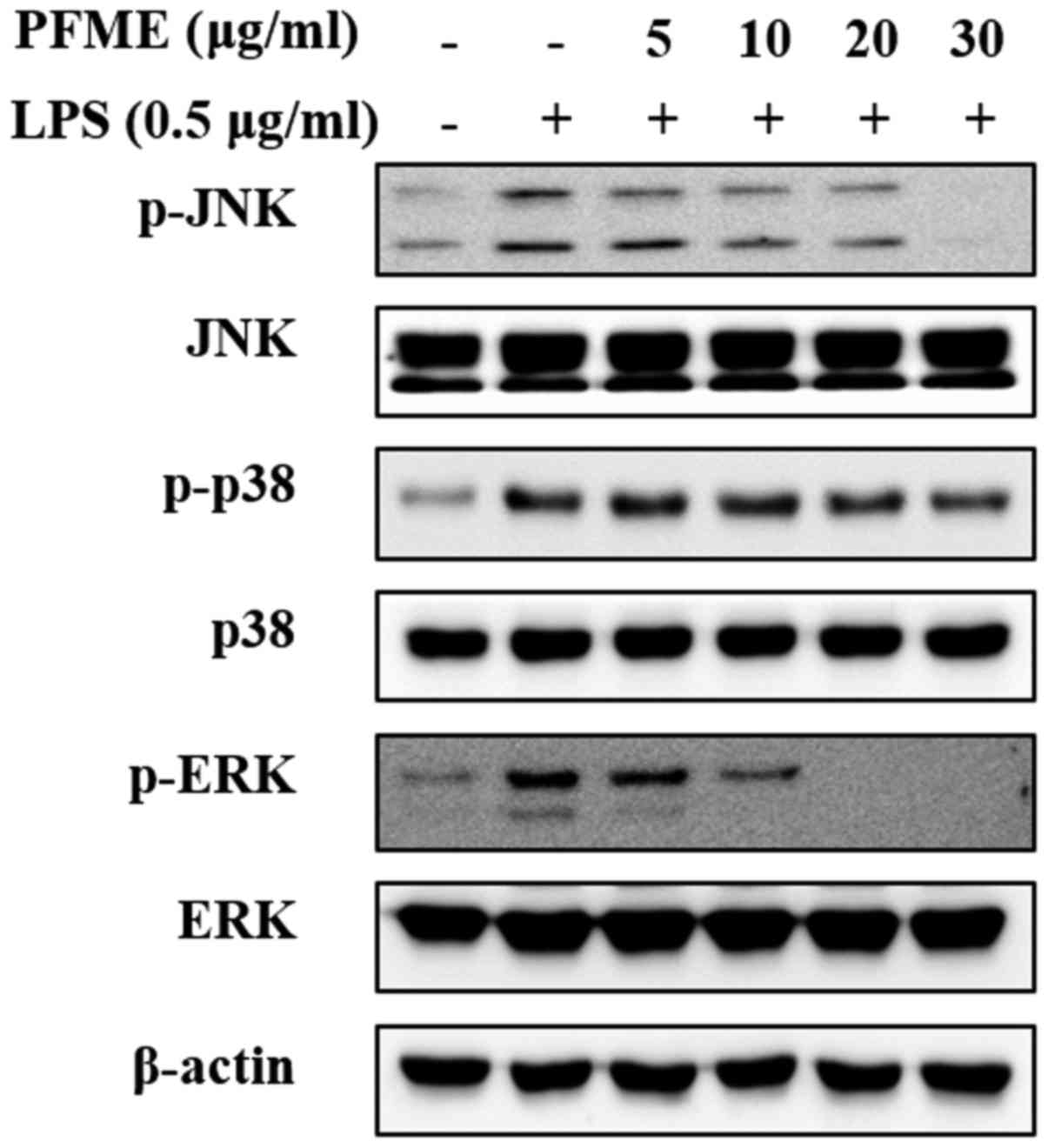
PFME inhibits the phosphorylation of the
MAPKs in LPS-stimulated RAW264.7 cells
MAPKs play a vital role in the regulation of
differentiation and cell growth and also control cellular responses
to cytokines and stress (28). To
determine the suppression of PFME on MAPKs signalling of
inflammation in RAW264.7 cells, the regulation of the
phosphorylation of the mediators JNK, p38 and ERK1/2 was
investigated. The whole cell lysates were then examined with
phospho-specific antibodies for JNK, p38 and ERK. The RAW264.7
cells treated with LPS alone showed increased phosphorylation of
JNK, p38 and ERK1/2; however, PFME treatment decreased levels of
phosphorylation of JNK, p38 and ERK1/2 in LPS-stimulated RAW264.7
cells in a concentration-dependent manner (Fig. 3). The level of expression of
non-phosphorylated JNK, p38 and ERK1/2 did not change in cells
treated with LPS or LPS and PFME. These experiments indicated that
suppression of the phosphorylation of p38 and ERK1/2 kinases may
explain the dramatic inhibitory effect of PFME on LPS-stimulated
inflammatory mediator activation in RAW264.7 cells, although the
phosphorylation of JNK was only slightly inhibited by PFME in the
macrophage cells.
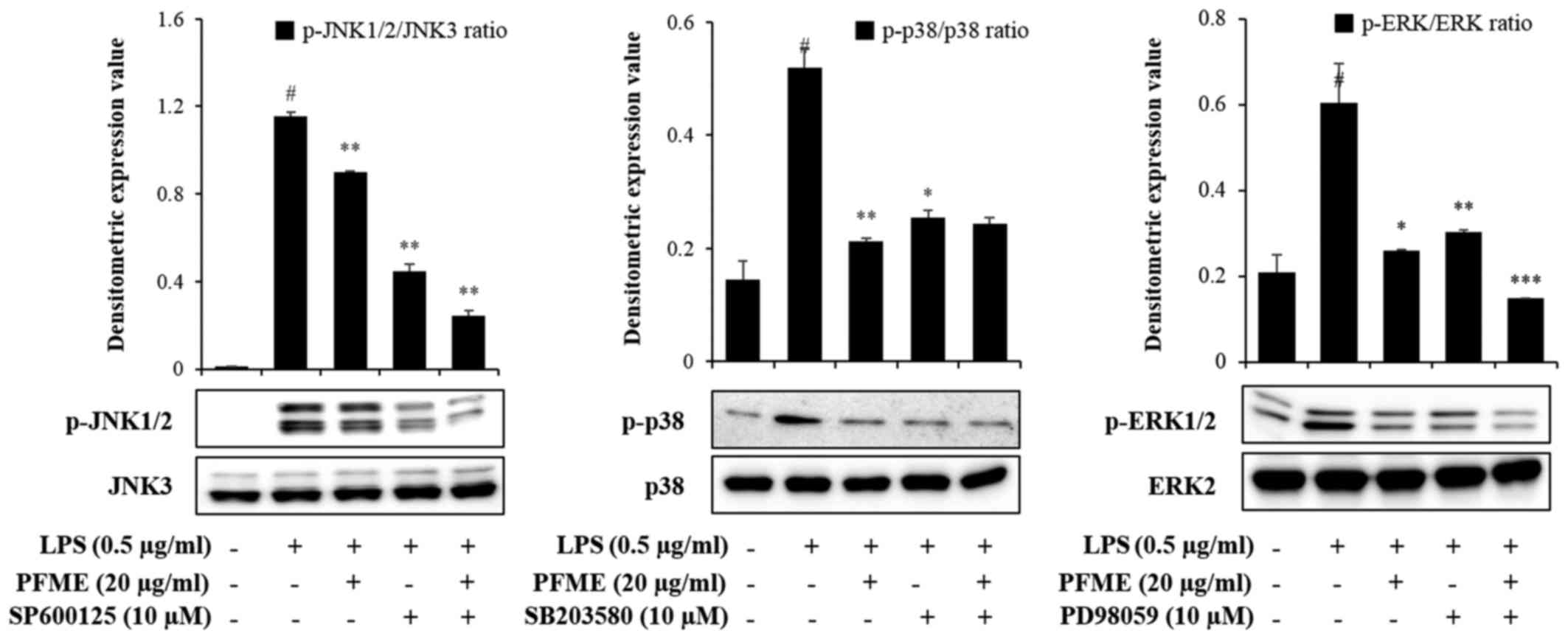
PFME and an inhibitor of MAPK
phosphorylation inhibit the phosphorylation of the activation of
MAPKs and the expression of NO and pro-inflammatory cytokines in
LPS-stimulated RAW264.7 cells
To investigate the molecular mechanism of
inflammatory mediator inhibition by PFME and MAPK inhibitors in
LPS-stimulated RAW264.7 cells, we studied the inhibition of the
phosphorylation of JNK, p38 and ERK1/2. In cells treated with LPS
alone, phosphorylation of JNK, p38 and ERK1/2 increased. However,
PFME, SP600125 (JNK), SB203580 (p38), and PD98059 (ERK) treatment
decreased levels of phosphorylated JNK, p38 and ERK1/2 in
LPS-stimulated RAW264.7 cells (Fig.
4). We further investigated the effect of the PFME, SP600125,
SB203580, and PD98059 on the inhibition of the NO assay and ELISA
(IL-6 and TNF-α) (Fig. 5). These
results suggested that suppression of phosphorylation of JNK, p38
and ERK1/2 may be involved in the inhibitory effect of PFME,
SP600125, SB203580 and PD98059 on LPS-stimulated inflammatory
mediator activation in RAW264.7 cells.
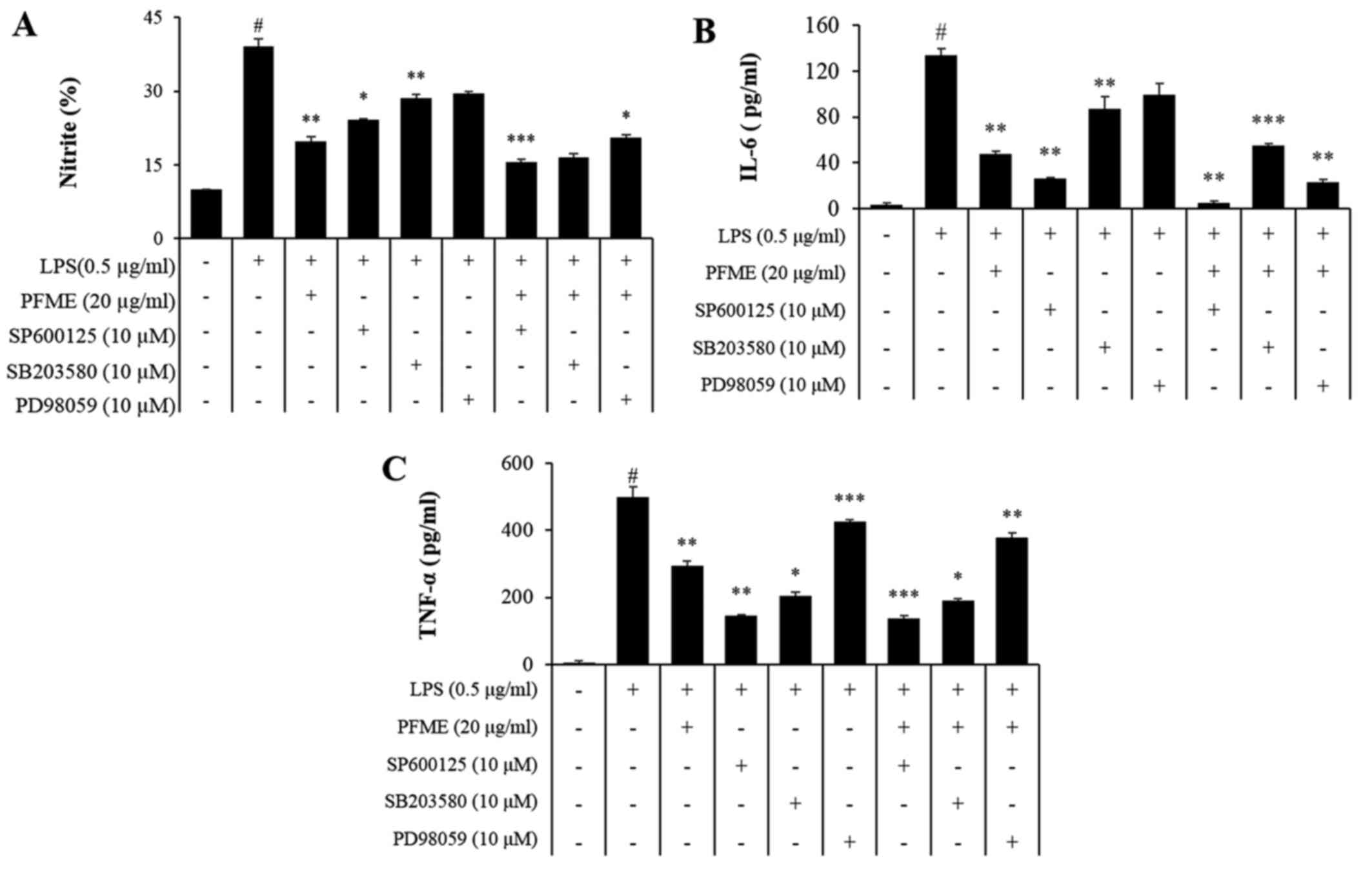 | Figure 5Passiflora foetida L. (PFME)
and MAPK inhibitors suppress NO and pro-inflammatory cytokines in
lipopolysaccharide (LPS)-stimulated RAW264.7 cells. (A) RAW264.7
cells were pretreated with increasing concentrations of PFME (30
μg/ml), SP600125, SB203580 and PD98059 for 1 h and then
stimulated with LPS (0.5 μg/ml) for 24 h. The effect of
PFME, SP600125, SB203580 and PD98059 on the production of (A) NO,
(B) IL-6 and (C) TNF-α in LPS-stimulated RAW264.7 macrophages. The
values are expressed as the mean ± SEM of three independent
experiments, each performed in triplicate. $P<0.05, significant
difference from unstimulated cells; *P<0.05 and
**P<0.01, significant difference from LPS-treated
cells. Control, DMSO (0.1%); LPS, LPS only (0.5 μg/ml),
LPS+PFME: PFME (30 μg/ml) and LPS treatment. |
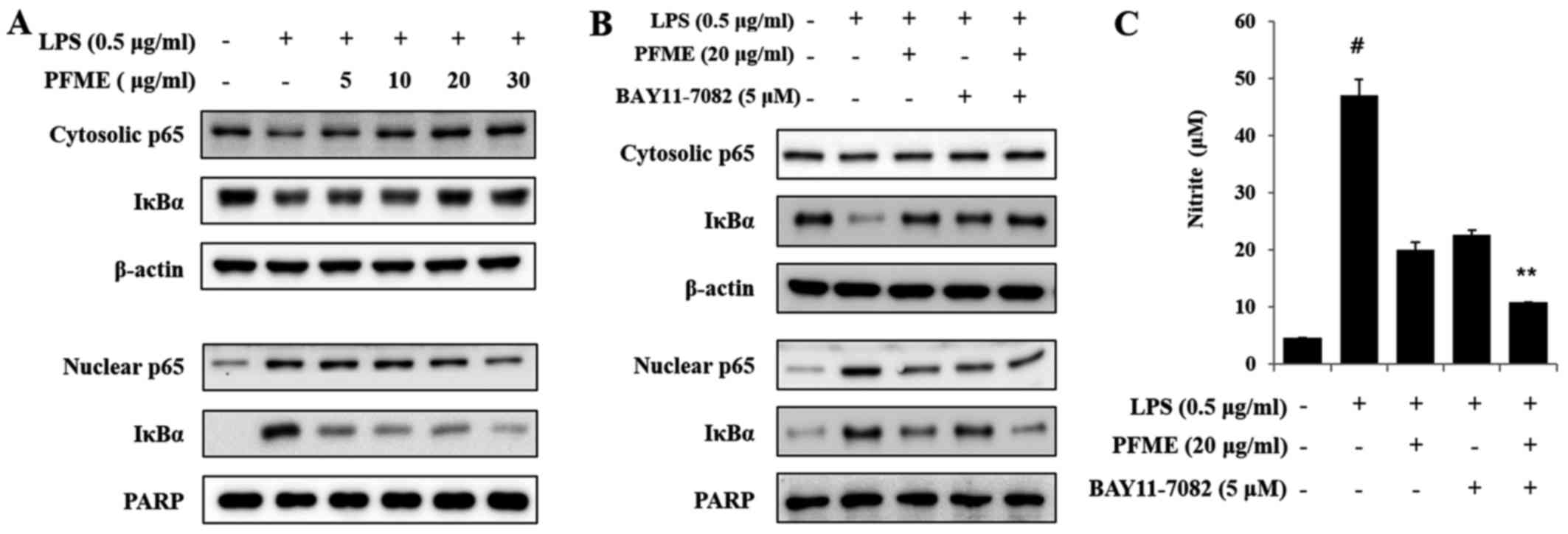
PFME and an inhibitor of IκBα
phosphorylation inhibits the phosphorylation of the activation of
NF-κB in LPS-stimulated RAW264.7 cells
The levels of IκBα, a molecular marker in the NF-κB
mechanism, were examined by immunoblot analysis. As shown in
Fig. 6A, the basal level of IκBα
and p65 in resting cells was high, but LPS treatment led to
reduction in the translocation of p65 and IκBα degradation through
the ubiq-uitin-proteasome pathway, with decreases in levels of p65
and IκBα. IκBα sequestered NF-κB in unstimulated RAW264.7 cells,
but in LPS-induced cells, degradation of IκBα allowed NF-κB to
translocate to the nucleus, with subsequent activation of gene
expression resulting from the interaction of NF-κB and the
corresponding cis-acting element. Therefore, we examined the
levels of p65, the major subunit of NF-κB, in nuclear and
cytoplasmic fractions, using PARP as a nuclear loading control. In
this study, the inhibitor of cytokine-induced IκBα phosphorylation
was BAY11-7082 (IC50, 10 μM), which was used to
inhibit the NF-κB pathway as an irreversible inhibitor of IκBα
phosphorylation that increases stabilization of IκBα and
specifically blocks NF-κB signalling. As shown in Fig. 6B, the NF-κB p65 subunit decreased
in the cytoplasm and increased in nuclear extracts after treatment
with LPS. Treatment with PFME and BAY11-7082 reversed these trends
in a concentration-dependent manner. We further investigated the
effect of PFME and BAY11-7082 on the inhibition of NO. The
LPS-stimulated translocation of the NF-κB p65 subunit from the
cytosol to the nucleus in RAW264.7 cells (Fig. 6C) was inhibited by PFME. The NF-κB
p65 and IκBα protein levels were significantly correlated with the
reduced nuclear accumulations. Thus, we suggest that the PFME
inhibited the translocation of the NF-κB p65 subunit and IκBα via
the regulation of a signal transduction mechanism related to NF-κB
activation.
Discussion
Natural products play an important role in both drug
discovery and chemical biology (29). PFME is traditionally used as an
herbal medicine in several countries (21,30), although the anti-inflammatory
effect of PFME is not fully understood. Therefore, we investigated
the molecular mechanism and anti-inflammatory effect of PFME in
LPS-stimulated RAW264.7 macrophage cells. We found that PFME
decreased the release of the inflammatory mediators NO (data not
shown) and PGE2 and suppressed pro-inflammatory cytokine
production, including that of IL-1β, IL-6 and TNF-α (Fig. 2C-2). Additionally, iNOS (data not shown)
and COX-2 protein expression in LPS-stimulated RAW264.7 cells was
downregulated by PFME. We also discovered that PFME considerably
inhibited inflammatory process signalling pathways, including NF-κB
nuclear translocation and phosphorylation of MAPKs. Therefore, we
suggest that PFME increased the anti-inflammatory effect in
LPS-stimulated macrophages.
During inflammation, excess levels of
PGE2, NO, and pro-inflammatory cytokines are caused by
activated inflammatory cells, which result in damaging effects on
tissues and cells and the activation in inflammation-associated
sickness (31). Through the
inflammatory pathways, iNOS and COX-2 expression remarkably
increases the generation of NO and PGE2, respectively,
and accordingly, the inhibition of PGE2 and NO
production can be a critical marker for anti-inflammatory responses
(32). The fundamental role of
the NF-κB signalling pathway is regulation of the transcription
factors that control expression of iNOS and COX-2. In
LPS-unstimulated macrophages, NF-κB was suppressed by IκB in the
cytoplasm. Then, NF-κB can freely translocate from the cytosol to
the nucleus in which it promotes transcription of target genes and
further induces the transcription of pro-inflammatory mediators,
such as IL-6, IL-1β, TNF-α and COX-2 (9,33,34). Many anti-inflammatory agents
express their potencies by suppressing NF-κB signalling (35). The molecular signalling of the
extract-mediated emaciation in macrophage cells had a close
relationship with the inhibition of p65 subunit translocation into
the nucleus. Our results indicated that PFME downregulated the
expression of COX-2 and also the production of PGE2 in
LPS-stimulated cells (Fig. 2).
Additionally, PFME reduced the secretions of IL-6, IL-1β and TNF-α
(Fig. 2). The decrement of
PFME-mediated molecular signalling in the macrophage cells was
closely associated with the oppression of IκB kinase activation
followed by inhibition of the translocation of NF-κB p65 subunits
into the nucleus (Fig. 6). These
results suggest that the PFME inhibited the expression of
pro-inflammatory mediators by downregulating the NF-κB pathway in
stimulated macrophage cells.
MAPKs, including p38, ERK and JNK, are a syndicate
of signalling molecules that likely have an important function in
inflammatory mechanisms (36).
Zhang et al (37)
suggested that LPSs induced MAPK cascades and the pathways leading
to activation of NF-κB p65. Additionally, MAPKs are complicated by
the expression of iNOS in the LPS-induced signalling pathway
(38). In this study, LPS induced
the phosphorylation of MAPKs. The treatment with PFME significantly
inhibited the phosphorylation of LPS-induced ERK, p38 and JNK at 1
h. Consequently, these results suggest that ERK, p38 and JNK were
complicated in the inhibition by PFME of LPS-stimulated NF-κB p65
binding in RAW264.7 macrophage cells. In this study, the treatment
with PFME blocked the activation of ERK, p38 and JNK, which
suggested that PFME suppressed LPS-induced NF-κB translocation by
inhibiting the activation of these intracellular signalling
cascades, leading to decreased protein levels of iNOS and COX-2
(Fig. 4).
The inhibitory activity of PFME on the MAPKs (JNK,
ERK and p38) is shown in Fig. 3.
The PFME showed excellent inhibitory activity of the MAPK pathway.
When the major MAPK signal in the primary inflammatory response is
suppressed, related diseases are affected. Regarding these effects,
a comparative analysis was performed using inhibitors known to be
actual inhibitors of MAPK. The importance of this experiment was to
show that PFME had some influence on the inhibitory activity of
each inhibitor. As confirmed in Fig.
4, the MAPK was inhibited with an inhibitor and PFME for each
target. As shown in Fig. 5, the
inhibitory activity of MAPK against NO, TNF-α and IL-6 was
confirmed, which was the result of treating with inhibitors of MAPK
and PFME, indicating that the signal-affected targets were
inhibited. As a result, the activity of PFME was confirmed to be
better than that of the actual MAPK inhibitors. As with the
inhibitors, PFME affected the target and was more effective when
using two bells simultaneously. Additionally, the NF-κB inhibitor
BAY11-7082 decreased translocation of NF-κB p65 and IκBα and iNOS
production in LPS-stimulated macrophages. BAY11-7082 with PFME also
inhibited p65 and IκBα expression in this result. The results
suggested that blocking of the MAPKs pathway was involved in the
suppression of LPS-stimulated NF-κB bound by PFME in RAW264.7 cells
(Fig. 6).
To conclude, PFME exhibited anti-inflammatory
activities that were related to the suppression of NO,
PGE2, IL-1β, IL-6 and TNF-α and reduced expression of
COX-2 and iNOS via activation of the NF-κB p65 pathway in
macrophage cells. The anti-inflammatory effect of PFME was
particularly maximized when macrophages were pre-treated with PFME
and an extract containing a mixture of ten plant-based food groups.
Combined, these results suggest that PFME can help diminish the
inflammation mediated by activated macrophages, which could
potentially improve the response of a host to inflammation-mediated
illnesses.
In conclusion, the results of this study indicated
that PFME inhibited the LPS-induced inflammatory and oxidative
responses, with these effects likely closely related with the
suppression of NF-κB activation. Therefore, we propose PFME for
possible use as a therapeutic for treating inflammatory
diseases.
Acknowledgments
Not applicable.
Notes
[1]
Funding
The present study was supported by grants from the
Bio & Medical Technology Development Program of the National
Research Foundation and funded by the Korean government (MSIT;
grant no. NRF-2016K1A1A8A01939075) and from the Korea Research
Institute of Bioscience and Biotechnology Research Initiative
Program of the Republic of Korea (grant no. KGM1221814).
[2] Availability
of data and material
The analyzed datasets generated during the study are
available from the corresponding author upon reasonable
request.
[3] Authors'
contributions
JWP analyzed the data and wrote the manuscript. OKK,
HWR, JHP, IP and PY prepared the Passiflora foetida L., and
analyzed and edited the manuscript. SC, SRO and KSA designed the
study and edited the manuscript. All authors critically revised the
article and have approved the final version of the manuscript.
[4] Ethics
approval and consent to participate
Not applicable.
[5] Consent for
publication
Not applicable.
[6] Competing
interests
The authors declare that they have no competing
interests.
References
|
1
|
Mantovani A, Allavena P, Sica A and
Balkwill F: Cancer-related inflammation. Nature. 454:436–444. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Brevetti G, Giugliano G, Brevetti L and
Hiatt WR: Inflammation in peripheral artery disease. Circulation.
122:1862–1875. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hummasti S and Hotamisligil GS:
Endoplasmic reticulum stress and inflammation in obesity and
diabetes. Circ Res. 107:579–591. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kim HS, Park JW, Kwon OK, Kim JH, Oh SR,
Lee HK, Bach TT, Quang BH and Ahn KS: Anti-inflammatory activity of
a methanol extract from Ardisia tinctoria on mouse macrophages and
paw edema. Mol Med Rep. 9:1388–1394. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lambrecht BN and Hammad H: The role of
dendritic and epithelial cells as master regulators of allergic
airway inflammation. Lancet. 376:835–843. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Christaki E, Opal SM, Keith JC Jr,
Kessinian N, Palardy JE, Parejo NA, Lavallie E, Racie L, Mounts W,
Malamas MS, et al: Estrogen receptor beta agonism increases
survival in experimentally induced sepsis and ameliorates the
genomic sepsis signature: A pharmacogenomic study. J Infect Dis.
201:1250–1257. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Park JW, Shin IS, Ha UH, Oh SR, Kim JH and
Ahn KS: Pathophysiological changes induced by Pseudomonas
aeruginosa infection are involved in MMP-12 and MMP-13 upregulation
in human carcinoma epithelial cells and a pneumonia mouse model.
Infect Immun. 83:4791–4799. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Corriveau CC and Danner RL: Endotoxin as a
therapeutic target in septic shock. Infect Agents Dis. 2:35–43.
1993.PubMed/NCBI
|
|
9
|
Park JW, Kwon OK, Jang HY, Jeong H, Oh SR,
Lee HK, Han SB and Ahn KS: A leaf methanolic extract of Wercklea
insignis attenuates the lipopolysaccharide-induced inflammatory
response by blocking the NF-κB signaling pathway in RAW 264.7
macrophages. Inflammation. 35:321–331. 2012. View Article : Google Scholar
|
|
10
|
Pouliot M, Baillargeon J, Lee JC, Cleland
LG and James MJ: Inhibition of prostaglandin endoperoxide
synthase-2 expression in stimulated human monocytes by inhibitors
of p38 mitogen-activated protein kinase. J Immunol. 158:4930–4937.
1997.PubMed/NCBI
|
|
11
|
Zhang G and Ghosh S: Toll-like
receptor-mediated NF-kappaB activation: A phylogenetically
conserved paradigm in innate immunity. J Clin Invest. 107:13–19.
2001. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Baeuerle PA and Baltimore D: I kappa B: A
specific inhibitor of the NF-kappa B transcription factor. Science.
242:540–546. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Baker JR Jr and Baldwin JL: Allergy and
immunology. JAMA. 275:1794–1795. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Park JW, Kwon OK, Kim JH, Oh SR, Kim JH,
Paik JH, Marwoto B, Widjhati R, Juniarti F, Irawan D, et al:
Rhododendron album Blume inhibits iNOS and COX-2 expression in
LPS-stimulated RAW264.7 cells through the downregulation of NF-κB
signaling. Int J Mol Med. 35:987–994. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Riches DW, Chan ED, Zahradka EA, Winston
BW, Remigio LK and Lake FR: Cooperative signaling by tumor necrosis
factor receptors CD120a (p55) and CD120b (p75) in the expression of
nitric oxide and inducible nitric oxide synthase by mouse
macrophages. J Biol Chem. 273:22800–22806. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ajizian SJ, English BK and Meals EA:
Specific inhibitors of p38 and extracellular signal-regulated
kinase mitogen-activated protein kinase pathways block inducible
nitric oxide synthase and tumor necrosis factor accumulation in
murine macrophages stimulated with lipopolysaccharide and
interferon-gamma. J Infect Dis. 179:939–944. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Asadujjaman M, Mishuk AU, Hossain MA and
Karmakar UK: Medicinal potential of Passiflora foetida L. plant
extracts: Biological and pharmacological activities. J Integr Med.
12:121–126. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Huang WC, Wu SJ, Tu RS, Lai YR and Liou
CJ: Phloretin inhibits interleukin-1β-induced COX-2 and ICAM-1
expression through inhibition of MAPK, Akt, and NF-κB signaling in
human lung epithelial cells. Food Funct. 6:1960–1967. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Krishnaveni A and Thaakur SR:
Pharmacognostical and preliminary phytochemical studies of
Passiflora foetida. Anc Sci Life. 27:19–23. 2008.PubMed/NCBI
|
|
20
|
Nunes ES, Brown JK, Moreira AG, Watson G,
Lourenção AL, Piedade SM, Rezende JA and Vieira ML: First report
and differential colonization of Passiflora species by the B
biotype of Bemisia tabaci (Gennadius) (Hemiptera: Aleyrodidae) in
Brazil. Neotrop Entomol. 37:744–746. 2008. View Article : Google Scholar
|
|
21
|
Sathish R, Sahu A and Natarajan K:
Antiulcer and antioxidant activity of ethanolic extract of
Passiflora foetida L. Indian J Pharmacol. 43:336–339. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sasikala V, Saravanan S and Parimelazhagan
T: Analgesic and anti-inflammatory activities of Passiflora foetida
L. Asian Pac J Trop Med. 4:600–603. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Nguyen TY, To DC, Tran MH, Lee JS, Lee JH,
Kim JA, Woo MH and Min BS: Anti-inflammatory flavonoids isolated
from Passiflora foetida. Nat Prod Commun. 10:929–931.
2015.PubMed/NCBI
|
|
24
|
Lee JW, Shin NR, Park JW, Park SY, Kwon
OK, Lee HS, Hee Kim J, Lee HJ, Lee J, Zhang ZY, et al: Callicarpa
japonica Thunb. attenuates cigarette smoke-induced neutrophil
inflammation and mucus secretion. J Ethnopharmacol. 175:1–8. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Park JW, Kwon OK, Yuniato P, Marwoto B,
Lee J, Oh SR, Kim JH and Ahn KS: Amelioration of an LPS-induced
inflammatory response using a methanolic extract of Lagerstroemia
ovalifolia to suppress the activation of NF-κB in RAW264.7
macrophages. Int J Mol Med. 38:482–490. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Shin IS, Park JW, Shin NR, Jeon CM, Kwon
OK, Lee MY, Kim HS, Kim JC, Oh SR and Ahn KS: Melatonin inhibits
MUC5AC production via suppression of MAPK signaling in human airway
epithelial cells. J Pineal Res. 56:398–407. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lee BH, Kushwah R, Wu J, Ng P, Palaniyar
N, Grinstein S, Philpott DJ and Hu J: Adenoviral vectors stimulate
innate immune responses in macrophages through cross-talk with
epithelial cells. Immunol Lett. 134:93–102. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Park JW, Lee IC, Shin NR, Jeon CM, Kwon
OK, Ko JW, Kim JC, Oh SR, Shin IS and Ahn KS: Copper oxide
nanoparticles aggravate airway inflammation and mucus production in
asthmatic mice via MAPK signaling. Nanotoxicology. 10:445–452.
2016. View Article : Google Scholar
|
|
29
|
Newman DJ and Cragg GM: Natural products
as sources of new drugs over the 30 years from 1981 to 2010. J Nat
Prod. 75:311–335. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Puricelli L, Dell'Aica I, Sartor L,
Garbisa S and Caniato R: Preliminary evaluation of inhibition of
matrix-metalloprotease MMP-2 and MMP-9 by Passiflora edulis and P.
foetida aqueous extracts. Fitoterapia. 74:302–304. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Xiao YQ, Malcolm K, Worthen GS, Gardai S,
Schiemann WP, Fadok VA, Bratton DL and Henson PM: Cross-talk
between ERK and p38 MAPK mediates selective suppression of
pro-inflammatory cytokines by transforming growth factor-beta. J
Biol Chem. 277:14884–14893. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Navarro C, Bravo ML, Carulla C and Bulbena
O: Gastrotoxic activity and inhibitory effects on gastric mucosal
PGE2 production with different non-steroidal
anti-inflammatory drugs: Modifications induced by pretreatment with
zinc acexamate. Prostaglandins Leukot Essent Fatty Acids.
50:305–310. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Jung WK, Heo SJ, Jeon YJ, Lee CM, Park YM,
Byun HG, Choi YH, Park SG and Choi IW: Inhibitory effects and
molecular mechanism of dieckol isolated from marine brown alga on
COX-2 and iNOS in microglial cells. J Agric Food Chem.
57:4439–4446. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kim HG, Shrestha B, Lim SY, Yoon DH, Chang
WC, Shin DJ, Han SK, Park SM, Park JH, Park HI, et al: Cordycepin
inhibits lipopolysaccharide-induced inflammation by the suppression
of NF-kappaB through Akt and p38 inhibition in RAW 264.7 macrophage
cells. Eur J Pharmacol. 545:192–199. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Umezawa K and Chaicharoenpong C: Molecular
design and biological activities of NF-kappaB inhibitors. Mol
Cells. 14:163–167. 2002.PubMed/NCBI
|
|
36
|
An H, Xu H, Yu Y, Zhang M, Qi R, Yan X,
Liu S, Wang W, Guo Z, Qin Z, et al: Up-regulation of TLR9 gene
expression by LPS in mouse macrophages via activation of NF-kappaB,
ERK and p38 MAPK signal pathways. Immunol Lett. 81:165–169. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhang JS, Feng WG, Li CL, Wang XY and
Chang ZL: NF-kappaB regulates the LPS-induced expression of
interleukin 12 p40 in murine peritoneal macrophages: Roles of PKC,
PKA, ERK, p38 MAPK, and proteasome. Cell Immunol. 204:38–45. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Cañas N, Gorina R, Planas AM, Vergés J,
Montell E, García AG and López MG: Chondroitin sulfate inhibits
lipopolysaccharide-induced inflammation in rat astrocytes by
preventing nuclear factor kappa B activation. Neuroscience.
167:872–879. 2010. View Article : Google Scholar : PubMed/NCBI
|