Introduction
Non-alcoholic fatty liver disease (NAFLD) is a
stress-induced liver disease, which is characterized by excess
accumulation of lipid droplets in hepatocytes and diffused
steatosis without excessive alcohol consumption (1,2).
The incidence of NAFLD as well as that of metabolic syndrome is on
the increase (3,4). NAFLD is considered a major health
burden in adolescents and adults, and is closely associated with
obesity, hyperlipidemia and diabetes (5). In addition, it has been reported
that NAFLD is associated with cardiovascular diseases (6).
Metabolic disturbance and accumulation of lipids in
the liver may result in hepatic steatosis, which further induces
insulin resistance and inflammation, subsequently leading to
diabetes and cardiovascular complications (7,8).
In turn, insulin resistance and inflammation exacerbate hepatic
steatosis, thus forming a vicious cycle (5), which eventually develops into
non-alcoholic steatohepatitis, cirrhosis and liver cancer (9). Day demonstrated that insulin
resistance, oxidative stress and lipid peroxidation are crucial
factors for NAFLD (10).
Therefore, developing strategies to reduce excessive lipid
accumulation in the liver is critical for the treatment of NAFLD
and prevention of potential complications (11).
Although several interventions have been proposed
for patients with NAFLD, none have demonstrated considerable
efficacy for limiting liver damage (12). In recent years, the therapeutic
effects of Traditional Chinese Medicine in patients with NAFLD have
been demonstrated. Green tea polyphenols (GTP) are the effective
components extracted from tea, and primarily include catechins,
flavonoids, anthocyanins and phenolic compounds. Previous findings
have demonstrated the therapeutic effects of GTP in patients with
metabolic syndromes and type 2 diabetes (13,14). It has been reported that GTP
regulates the metabolism of glucose and lipids by enhancing
glycogen synthesis and inhibiting lipogenesis in hepatocytes
(14). In addition, GTP mobilizes
and activates endogenous antioxidant mechanisms, improving the
activity of enzymes such as superoxide dismutase (SOD), decreasing
the levels of lipid peroxide in the plasma, and thus detoxifying
the liver during metabolism.
In the present study, the therapeutic effects of GTP
in a high-fat diet (HFD)-induced NAFLD rat model were examined, and
the underlying mechanisms of GTP were explored. The results of the
present study highlight the potential therapeutic effects of GTP,
and may translate to an effective strategy for protecting the liver
in patients with NAFLD.
Materials and methods
Animals
A total of 30 adult male Sprague Dawley rats
(weight, 180-200 g) were obtained from Hunan SJA Laboratory Animal
Co., Ltd. The rats were housed at 24°C in a standard
specific-pathogen-free environment with a 12/12 h light/dark cycle,
and were acclimatized for a week. The animal experiments were
performed in accordance with The Principles of Laboratory Animal
Care (NIH publication no. 85Y23, revised 1996), and all procedures
were approved by The Experimental Animal Ethics Committee of Wuhan
University Renmin Hospital.
Experimental design and procedures
The rats were randomly assigned to one of following
three experimental groups with ten rats in each: i) Normal control
group (NC); ii) HFD group; and iii) HFD with GTP group (GTP).
Rats in the HFD and GTP groups were fed HFD, and NC
rats were fed standard laboratory chow for 8 weeks. The HFD was
composed of 70% fat, 20% carbohydrate, and 10% protein, with equal
quantities of other components, such as fiber, vitamins, and
minerals, to the standard chow diet. The GTP rats were administered
GTP (Xiya reagent, cat. no. 20875) at 200 mg/kg daily via oral
gavage simultaneously for 8 weeks. The main components of GTP are
catechins compounds, which have been identified as catechin,
citricin, catechin citrate and catechin epicatechin. The NC and HFD
groups were administered the same volume of saline. All the rats
were weighed weekly. The timing and dosage of GTP, which exhibited
beneficial effects against liver injury in rats, were determined in
our preliminary study (data not shown).
After 8 weeks on the assigned diet/treatment, rats
were fasted for 12 h. Rats were anesthetized with isoflurane
(induced with 5% isoflurane and maintaining with 3% in 2 l/min
oxygen flow in a sealed container). Blood samples were collected
from the postcava with vacuum serum separator tubes (BD Vacutainer
SST; Becton, Dickinson and Company), and centrifuged at 4°C, 1,500
× g for 10 min. The serum was collected and stored at −80°C.
Portions of the liver were obtained quickly, washed with ice-cold
PBS and subsequently flash frozen in liquid nitrogen and stored at
−80°C for the molecular assays. The samples were then preserved in
RNA sample preservation solution and stored at −20°C until the RNA
was isolated. Alternatively, the samples were fixed in 4%
phosphate-buffered formaldehyde for histological analysis. All the
rats were sacrificed by cervical dislocation.
GTT
After fasting for 12 h, the fasting blood glucose
and fasting insulin levels of rats were measured using a OneTouch
ultra-glucose meter (LifeScan) and ELISA kit (Elabscience),
respectively. To perform the GTT, rats were intraperitoneally
injected with glucose (2 g/kg body weight), and the blood glucose
was measured at 0, 15, 30, 60, 90 and 120 min with a glucometer
(15). To reflect the glucose
tolerance, the areas under the glucose concentration-time curves of
the glucose concentration after glucose injection during the first
120 min were calculated. Homeostasis model assessment-insulin
resistance index (HOMA-IRI) was calculated to evaluate individual
insulin resistance levels using the following formula: HOMA-IRI =
fasting blood glucose (mmol/l) x fasting insulin (mIU/l)/22.5.
Liver function assay and hepatic lipid
analyses
Liver function was evaluated by determining the
serum concentrations of alanine aminotransferase (ALT) and
aspartate aminotransferase (AST) (ALT, Siemens healthineers, cat.
no. 03036926 and AST, Siemens healthineers, cat. no. 07499718)
using an ADVIA 2400 Chemistry system analyzer. Lipid content of the
liver was measured using the corresponding commercial kits for
triglyceride (TG), total cholesterol (TC) and non-esterified fatty
acid (NEFA) (Nanjing Jiancheng Bioengineering Institute), according
to the manufacturers' protocol.
Histopathological examination
Liver tissues were harvested, cut into small pieces,
fixed in 4% paraformaldehyde, embedded with paraffin and
subsequently stained with hematoxylin and eosin (H&E) to
visualize histopathological changes of the liver. Alternatively,
the tissue samples were embedded with pre-cooled optimal cutting
compound for cryostat sectioning and stained with Oil Red O to
visualize accumulation of hepatic lipid droplets. Morphology was
assessed by two independent pathologists under a light microscope
(Olympus BX63; Olympus Corporation). The degree of liver injury was
scored according to Kalantar et al (16). Indices including inflammatory cell
infiltration, RBC congestion, and nuclear pyknosis were examined.
In addition, a semiquantitative analysis was carried out using the
following scoring system: 0, normal; 1, mild; 2, moderate; and 3,
severe. The average score for each group was measured to determine
the total score. Lipid droplets were quantified as described by
Jiang et al (17). Results
are presented as area fractions, i.e., the percentage of specific
counts in relation to total number of counted points.
Measurement of serum and liver tissue
indicators of inflammation
The levels of inflammatory cytokines TNF-α and IL-6
in the serum were quantified with corresponding ELISA kits
(Elabscience), according to the manufacturer's protocols. The
frozen liver tissues were homogenized and centrifuged at 4°C,
12,000 × g for 20 min, and the supernatants were then collected.
The oxidative stress markers malondial-dehyde (MDA) and SOD in
liver tissues were measured with biochemical assay kits (Nanjing
Jiancheng Bioengineering Institute), and all procedures were
performed according to the manufacturer's protocol and repeated
three times.
RT-qPCR
Total RNA was extracted from liver samples using
TRIzol® and reverse transcribed to cDNA. RT-qPCR was
performed using the Faststart Universal SYBR Green Master (ROX) kit
(Roche Diagnostics) in an CFX Connect™ Real-Time PCR Detection
system (Bio-Rad Laboratories). The thermocycling conditions were
95°C for 10 mins, followed by 40 cycles of denaturation 95°C for 15
sec and a combined annealing/extension step at 60°C for 1 min. The
primer sequences of the target genes were: GAPDH forward, 5′-CGC
TAA CAT CAA ATG GGG TG-3′ and reverse, 5′-TTG CTG ACA ATC TTG AGG
GAG-3′; and 5′ adenosine monophosphate-activated protein kinase
(AMPK)α forward, 5′-CTC AGG AAG GCT GTA TGC GG-3′ and reverse,
5′-ACG GTT GAG ATA CTC CGG GAT-3′. The mRNA expressions levels were
normalized to the gene GAPDH and analyzed using the
2-ΔΔCq method (18).
All the reactions were performed in triplicate.
Western blotting
Total protein was extracted from liver samples using
a total protein extraction kit (Beyotime Institute of
Biotechnology), and the protein concentrations were determined
using a bicinchoninic acid assay. A total of 20 µg protein
was loaded per well, resolved using a 10% SDS-PAGE gel and
transferred to a PVDF membrane. The membranes were blocked with 5%
skimmed milk in TBST buffer (TBS containing 0.1% Tween-20) at room
temperature for 2 h and subsequently incubated with the following
primary antibodies at 4°C overnight: Anti-phospho-AMPKα (Abcam;
cat. no. ab133448; 1:1,000), anti-AMPKα (Abcam; cat. no. ab80039;
1:1,000) and anti-GAPDH (Abcam; cat. no. ab181602; 1:1,000). After
washing with TBST, the blots were incubated with the corresponding
horseradish peroxidase-conjugated secondary antibodies (Abcam; goat
anti-rabbit antibody, cat. no. ab205718 and goat anti-mouse
antibody, ab205719; 1:2,000) at room temperature for 1 h. The
immunoreactive bands were imaged with a ChemiDoc XRS system
(Bio-Rad Laboratories, Inc.) using enhanced chemiluminescent
reagent. The densities of the bands were quantified with Quantity
One version 4.6 (Bio-Rad Laboratories, Inc.).
Statistical analysis
All statistical analyses were performed using SPSS
software (version 20.0; SPSS, Inc.). Data are presented as the mean
± standard deviation. Differences among multiple groups were
compared with one-way ANOVA followed by Tukey's post hoc test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
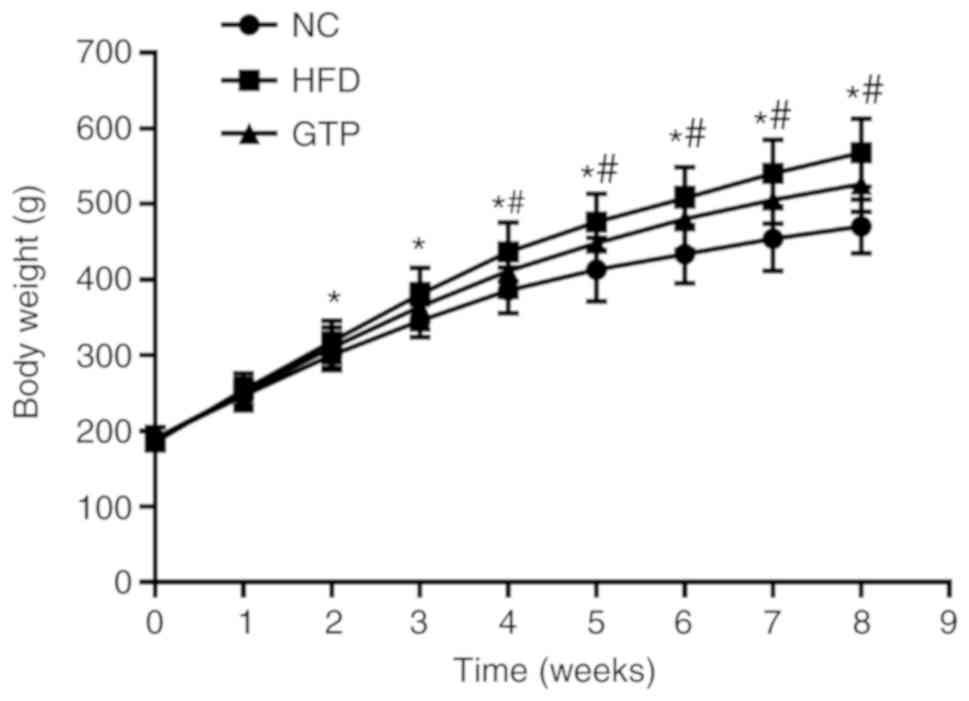
GTP inhibits weight gain in HFD rats
All the rats were fed the same amount of food per
day during the study period and their body weight was monitored
weekly. As shown in Fig. 1, the
weight of HFD rats gradually increased after 2 weeks and was
significantly higher compared with the NC group (P<0.05).
However, when the rats were pretreated with GTP, the weight gain
was slower and the weight was significantly lower compared with the
HFD rats (P<0.05) throughout the study period (8 weeks).
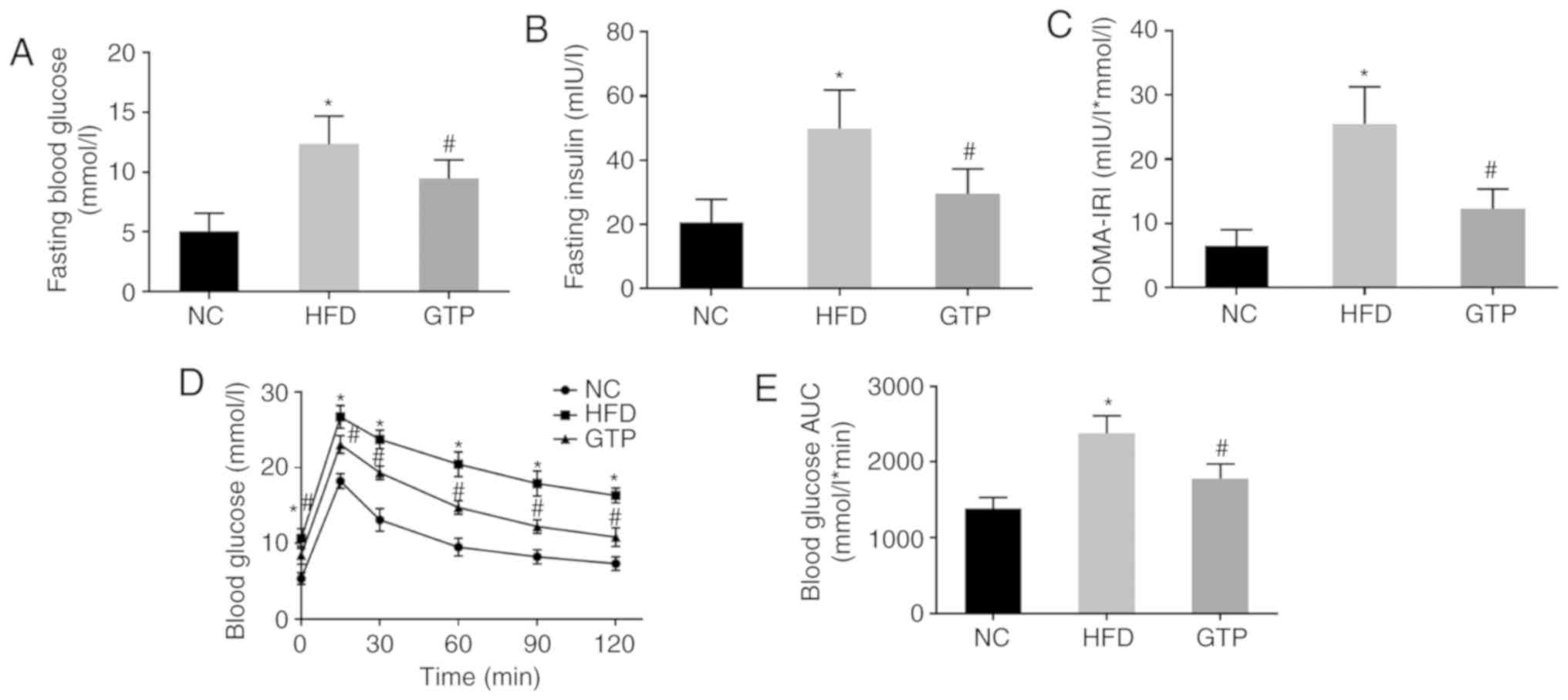
GTP alleviates insulin resistance induced
by HFD
To investigate the effect of GTP on insulin
resistance, fasting glucose and insulin levels were measured, and a
GTT was performed and the HOMA-IRI was calculated. As presented in
Fig. 2A-C, after 8 weeks of HFD,
rats developed notably higher fasting glucose and fasting insulin
levels, and a higher HOMA-IRI, which was indicative of increased
insulin resistance compared with the NC group. GTP intervention
inhibited the increase in fasting glucose and fasting insulin
levels as well as the HOMA-IRI (both P<0.05) in the HFD rats. In
addition, as the results of the glucose tolerance test showed, HFD
impaired glucose tolerance and the area under the curve for glucose
in the GTP group was significantly reduced compared with the HFD
rats (Fig. 2D and E). Taken
together, these results suggest that GTP may improve insulin
resistance in the HFD rats.
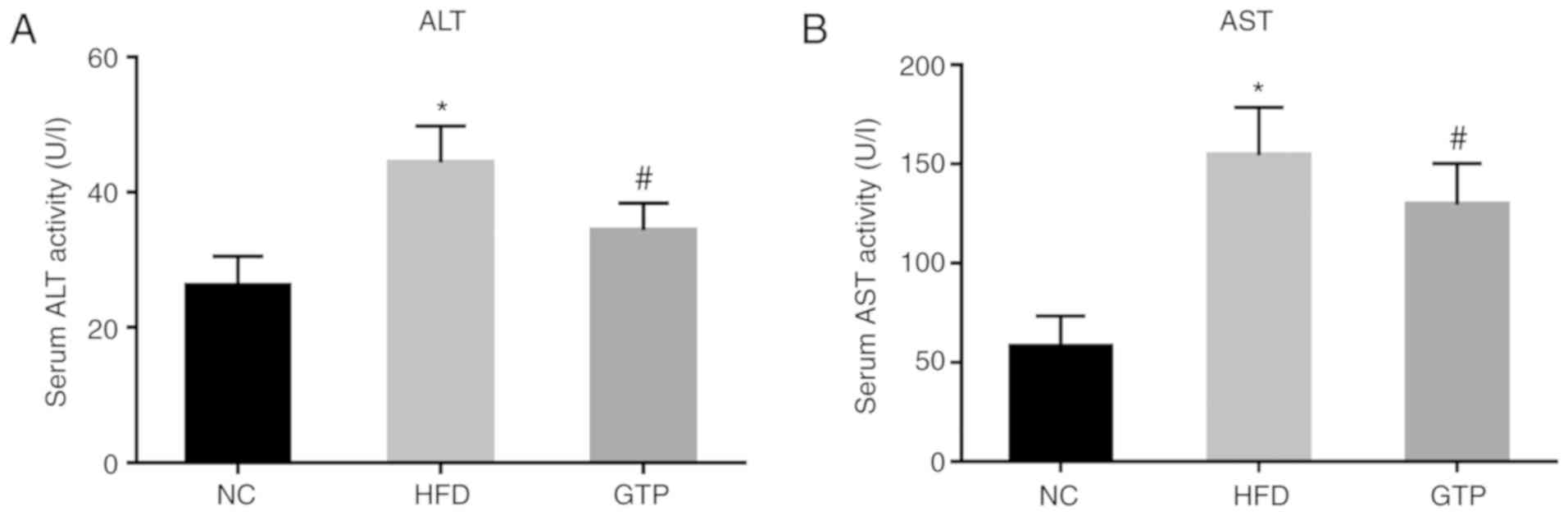
GTP improves hepatic function
The serum ALT and AST levels were measured to
evaluate the effect of GTP on hepatic function. As shown in
Fig. 3, within 8 weeks of the
HFD, the serum ALT and AST levels in HFD rats were significantly
increased compared with the control group. GTP intervention
significantly decreased the levels of ALT and AST (both P<0.05)
compared with the HFD rats (Fig.
3), suggesting that GTP may improve hepatic function.
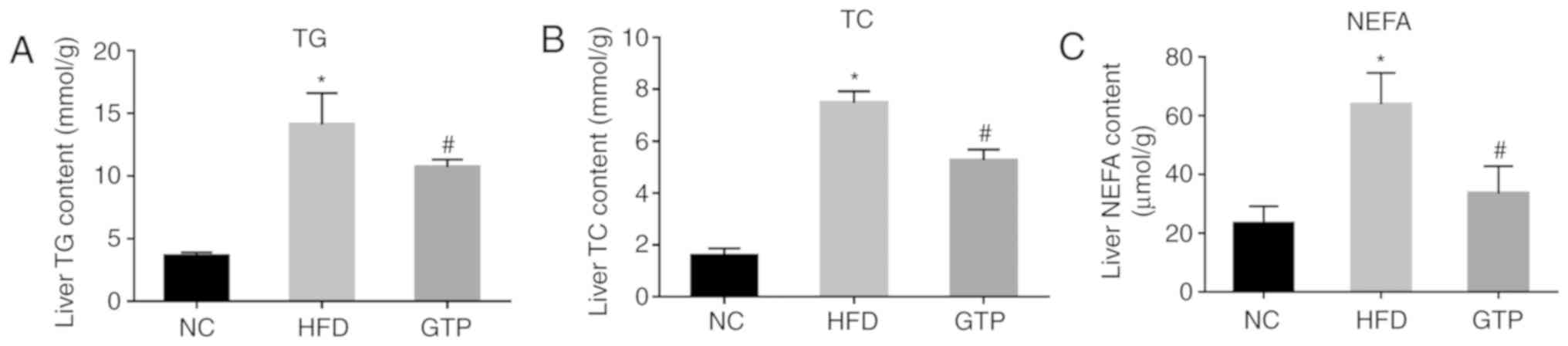
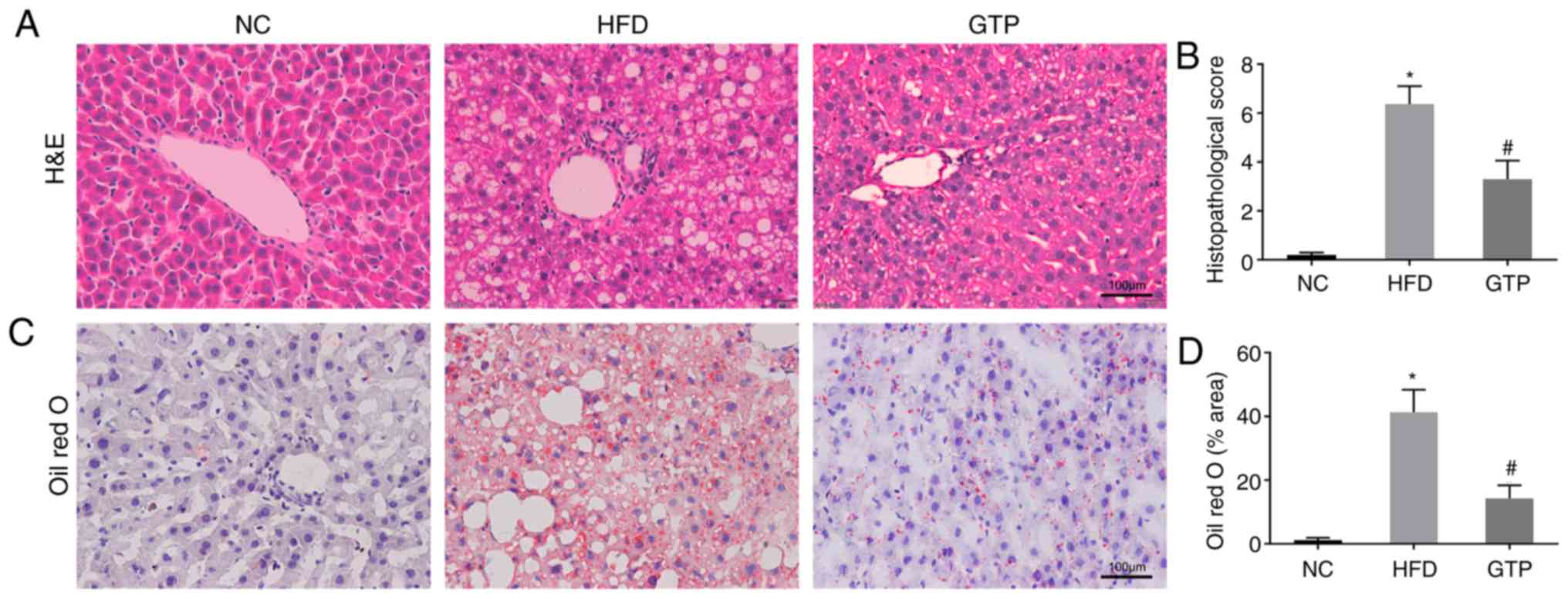
GTP mitigates HFD-induced hepatic
steatosis
A continuous HFD may lead to the excess accumulation
of lipid in the liver, which is termed hepatic steatosis and is one
of the most prominent features of NAFLD (19). Therefore, the effect of GTP on
hepatic steatosis was determined by measuring hepatic lipid
metabolism, and by using H&E and Oil Red O staining. The
concentrations of TG, TC and NEFA were significantly increased in
livers of the HFD rats compared with the NC rats after 8 weeks of a
HFD (Fig. 4A-C). H&E staining
revealed that the substructure of the liver was normal and clear,
and the liver lobules were regular in NC rats. By contrast, the
livers of the HFD rats presented with widespread lipid vacuoles and
the liver cells were damaged (Fig.
5A). Hepatic steatosis and fat droplets were further confirmed
by Oil Red O staining, which is a more sensitive and specific
method for the detection of hepatic steatosis. The Oil Red
O-staining results showed that there were more lipid droplets in
the cryosections of the HFD rats compared with the NC rats
(Fig. 5C). Treatment with GTP
notably reduced the levels of TG, TC and NEFA in the liver
(Fig. 4A-C), alleviated hepatic
histopathological injuries (Fig.
5B) and reduced the number and volume of lipid droplets
(Fig. 5D), suggesting that GTP
may prevent lipid accumulation and hepatic steatosis.
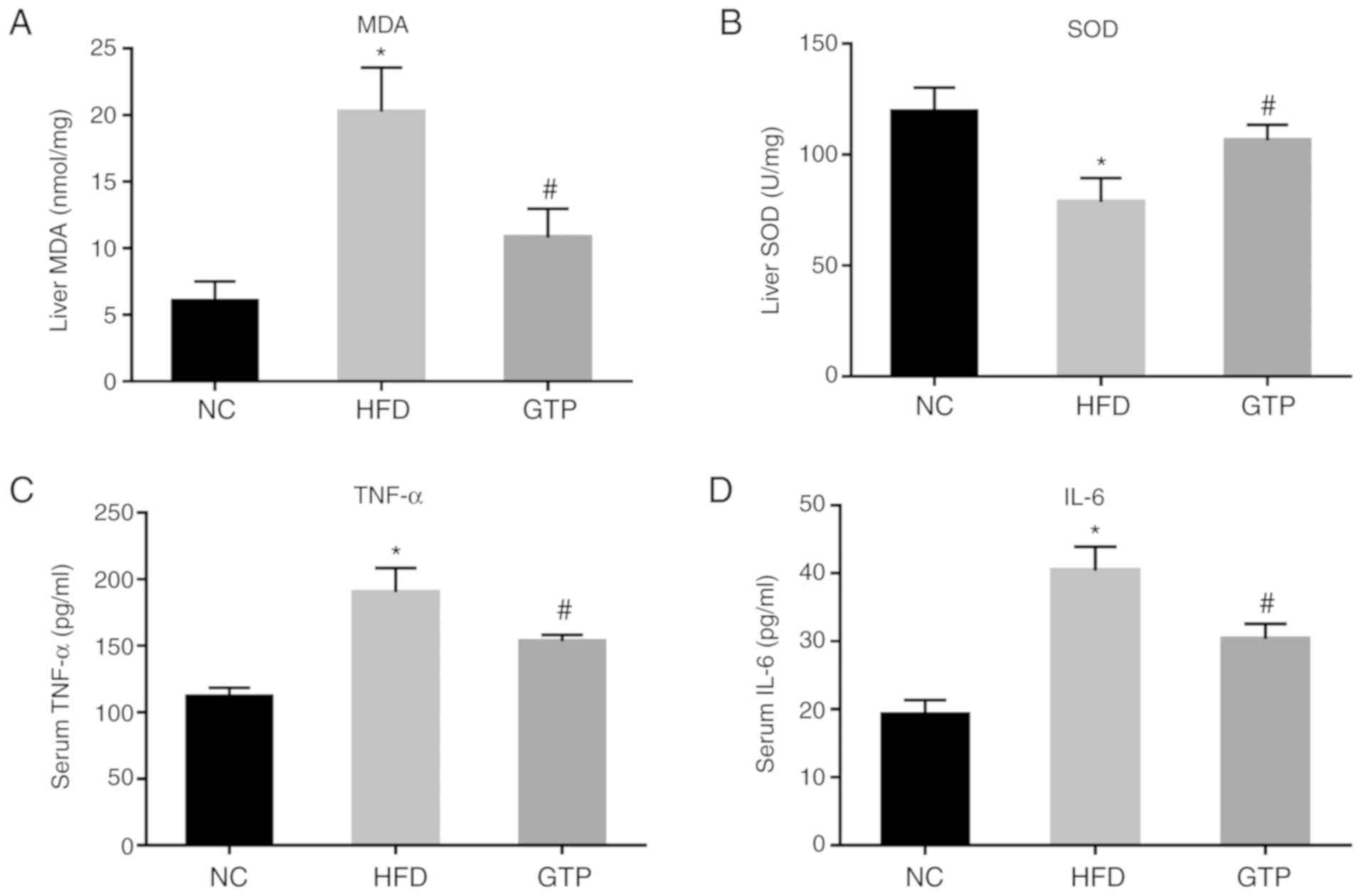
GTP attenuates HFD-induced oxidative
stress and the inflammatory response
One of the most notable features of NAFLD is
oxidative stress. Therefore, the effect of GTP on oxidative stress
was assessed. After 8 weeks of HFD, the level of MDA was
significantly increased in the liver of the HFD rats compared with
the control group (Fig. 6A),
while the activity of SOD was notably decreased (Fig. 6B) and GTP treatment reversed or
prevented these changes.
Another important stage of NAFLD is the inflammatory
response (20). Therefore, the
effect of GTP on HFD-induced inflammatory response was measured
using ELISA. The results showed that the serum levels of the
inflammatory cytokines TNF-α and IL-6 were notably increased in the
HFD rats compared with the control group, and GTP treatment
decreased the levels of TNF-α and IL-6 in the HFD rats (Fig. 6C and D; both P<0.05). Taken
together, the findings suggest that GTP inhibits HFD-induced
oxidative stress and the inflammatory response in the liver of
rats.
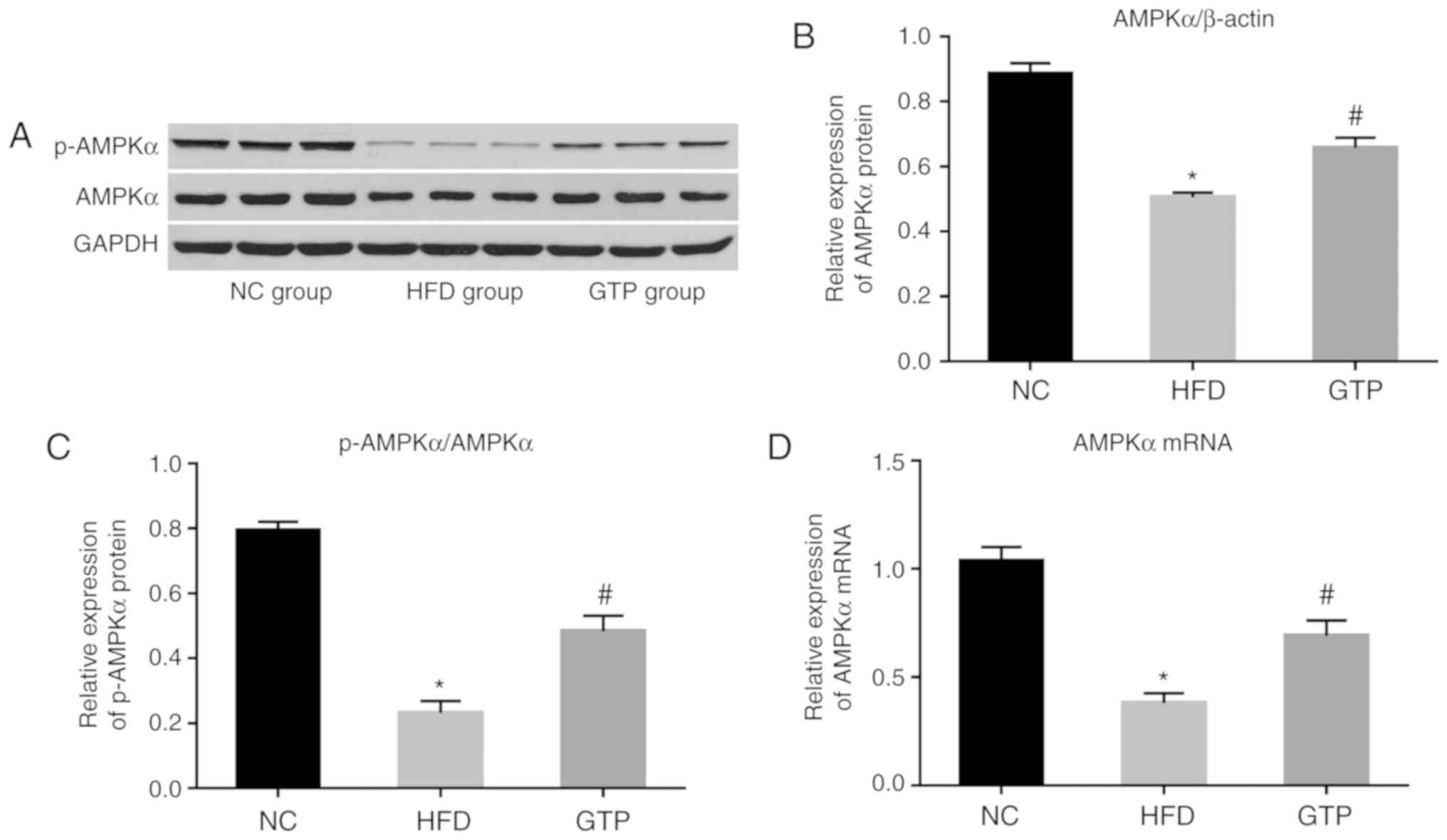
GTP enhances AMPK activation in the liver
of HFD rats
AMPK is one of the key enzymes regulating the
metabolism of lipids. Therefore, it was hypothesized that GTP may
exert its protective effects by modulating the AMPK signaling
pathway. As shown in Fig. 7,
western blotting revealed that the levels of AMPKα and
phospho-AMPKα were significantly lower in the liver of HFD rats
compared with the control rats, and GTP treatment significantly
upregulated the levels of AMPKα and phospho-AMPKα. Consistent with
these results, the mRNA expression levels of AMPKα in liver of HFD
rats were significantly lower compared with the NC rats, and GTP
treatment increased its expression. These results suggest that the
hepatoprotective role of GTP may be mediated by upregulation of the
AMPK signaling pathway.
Discussion
NAFLD is closely associated with obesity,
hyperlipidemia and diabetes mellitus. NAFLD is increasingly
prevalent and is a leading risk factor of hepatic cirrhosis and
liver cancer (21). However,
there are no effective therapeutic strategies for treating NAFLD at
present owing to an incomplete understanding of the underlying
molecular mechanisms. In the present study, the effectiveness of
GTP treatment in protecting against HFD-induced NAFLD in rats was
demonstrated. Treatment with GTP alleviated obesity, hepatic
steatosis, insulin resistance and inflammation in HFD-induced NAFLD
rats. The results suggest that GTP may serve as a promising
therapeutic treatment for prevention of NAFLD and metabolic
syndrome.
In the present study, HFD-fed rats were used to
mimic the key metabolic characteristics of humans with NAFLD. After
8 weeks of a HFD, the NAFLD rat model was successfully established
as evidenced by the significantly increased weight of HFD rats,
elevated ALT and AST levels, hepatic steatosis, insulin resistance
and inflammation, which are known to aggravate the progression of
NAFLD (22,23). Therefore, the effects of GTP on
these conditions were examined to determine its effects on
NAFLD.
GTP intervention significantly inhibited the weight
gain of HFD rats, and rats in the GTP group weighed significantly
less compared with the rats in the HFD group, suggesting that GTP
may reduce obesity. Furthermore, GTP treatment significantly
improved hepatic function, indicated by ALT and AST levels, and
decreased the levels of TG, TC and NEFA in the liver of HFD rats.
The elevated serum ALT and AST levels are the primary abnormalities
reported in patients with NAFLD and other hepatic diseases
(24). Histopathological analysis
showed that GTP also reduced the number and volume of lipid
droplets and reduced the HFD-induced histopathological changes in
the liver, which indicates that GTP protects against HFD-induced
hepatic steatosis.
The excess accumulation of lipids in the body and
organs promotes oxidative stress and induces an inflammatory
response, which are considered the two primary causes of
pathogenesis of NAFLD (25).
Oxidative stress and lipid peroxidation are part of the second
stage of NAFLD. Oxidative stress is the consequence of the
imbalance between the generation of reactive oxygen species and the
antioxidant defense responses (26). SOD and MDA are the primary
indicators of oxidative stress. The activity of SOD indirectly
reflects the capacity to remove oxygen-free radicals, whereas MDA,
a lipid peroxidation product, reflects the degree of lipid
peroxidation and indirectly represents the degree of hepatic cell
injury. In the present study, after 8 weeks of HFD, the activity of
SOD in the liver was significantly decreased, whereas the content
of MDA was increased. In addition, GTP treatment significantly
enhanced the activity of SOD and decreased the levels of MDA,
indicating that GTP can inhibit oxidation through the upregulation
of SOD activity and downregulation of MDA content. However, the
exact mechanism of how GTP upregulates SOD activity and
downregulates MDA content need to be further explored.
Previous findings have confirmed that the
inflammatory response is another stage of NAFLD. IL-6 and TNF-α are
the most common cytokines in the inflammatory response. IL-6 is
regarded as a potential mediator leading to NAFLD, and TNF-α has
been implicated in liver fibrosis and the advanced stages of NAFLD
in humans (27,28). In the present study, HFD-induced
NAFLD rats exhibited significantly higher serum TNF-α and IL-6
levels, and the changes were reversed by GTP treatment. In
agreement with the present study, a previous study reported that
epigallocatchin-3-gallate, a major active compound of GTP, may
protect against HFD-induced liver inflammation in rats (29). The results suggest that GTP
treatment may effectively prevent or attenuate the HFD-induced
inflammatory response in the liver of rats.
In addition to anti-oxidative stress and
inflammatory response effects, GTP also reduced insulin resistance
in HFD-induced NAFLD rats. The elevated levels of inflammatory
cytokines inhibit insulin signaling and result in insulin
resistance (30,31). Along with the inflammatory
response, HFD-induced rats presented significantly upregulated
fasting glucose and insulin levels, as well as HOMA-IRI, indicating
that the rats developed insulin resistance. GTP treatment
alleviated these pathophysiological changes as evidenced by the
decreased fasting glucose and insulin levels.
In NAFLD, owing to increased adipose lipolysis,
excess free fatty acids/NEFAs are delivered to the liver and
converted into TGs, leading to the accumulation of lipids and thus
lipotoxicity to hepatocytes (32). In the present study, NEFA levels
were significantly increased in the liver of HFD rats when compared
with the control, and after 8 weeks of GTP treatment, the elevated
NEFA levels were significantly decreased, which may reduce the
delivery of NEFAs to the liver for hepatic TG synthesis and
accumulation, thus preventing lipid peroxidation-induced hepatic
injury (32,33). Furthermore, reduction of hepatic
lipids may be attributed to improved hyperglycemia, as GTP
treatment decreased glucose influx to the liver and thus ultimately
decreased lipogenesis in the liver.
AMPK serves a critical role in the regulation of
hepatic lipogenesis (34,35) and it is also involved in the
insulin signaling pathway. AMPK is activated through promoting its
phosphorylation at Thr172 (36),
and further induces phosphorylation of acteyly-CoA-carboxylase
(ACC), causing a reduction in ACC activity, which leads to a
reduction in hepatic TG synthesis (37,38). In the present study, the level of
phosphorylated AMPK in the liver of rats exposed to HFD was
significantly reduced compared with the NC rats, and GTP treatment
significantly increased phosphorylation of AMPK. However, how GTP
upregulates AMPK activity needs to be further explored. The lack of
the identification of the underlying mechanism is a limitation to
the present study. Further experiments should be conducted to
clarify the mechanism.
In conclusion, the results of the present study
suggest that GTP treatment may protect against HFD-induced NAFLD in
rats by inhibiting hepatic steatosis, insulin resistance and
inflammation through enhancing AMPK activity. These findings
represent promising therapeutic possibilities of GTP for the
treatment of NAFLD and related metabolic pathologies in human.
Acknowledgments
The authors are grateful to the Hubei Key Laboratory
of Digestive System Disease of Renmin Hospital of Wuhan University
for providing relevant experimental facilities and technical
support.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
HMX and SQT contributed to the conception and design
of the study. HMX performed the experiments and wrote the
manuscript. JW and XJX established the rat model and administered
the GTP treatment. HMX, JW and XJX performed ELISA, western
blotting and reverse transcription-quantitative PCR analyses. LJX
analyzed the data. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Animal use and care were in accordance with The
Principles of Laboratory Animal Care (NIH publication no. 85Y23,
revised 1996) and all procedures were approved by the Experimental
Animal Ethics Committee of Wuhan University Renmin Hospital.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Lee JH, Baek SY, Jang EJ, Ku SK, Kim KM,
Ki SH, Kim CE, Park KI, Kim SC and Kim YW: Oxyresveratrol
ameliorates nonalcoholic fatty liver disease by regulating hepatic
lipogenesis and fatty acid oxidation through liver kinase B1 and
AMP-activated protein kinase. Chem Biol Interact. 289:68–74. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chalasani N, Younossi Z, Lavine JE,
Charlton M, Cusi K, Rinella M, Harrison SA, Brunt EM and Sanyal AJ:
The diagnosis and management of nonalcoholic fatty liver disease:
Practice guidance from the American Association for the Study of
Liver Diseases. Hepatology. 67:328–357. 2018. View Article : Google Scholar
|
|
3
|
Williams CD, Stengel J, Asike MI, Torres
DM, Shaw J, Contreras M, Landt CL and Harrison SA: Prevalence of
nonalcoholic fatty liver disease and nonalcoholic steatohepatitis
among a largely middle-aged population utilizing ultrasound and
liver biopsy: A prospective study. Gastroenterology. 140:124–131.
2011. View Article : Google Scholar
|
|
4
|
Wong VW, Chu WC, Wong GL, Chan RS, Chim
AM, Ong A, Yeung DK, Yiu KK, Chu SH, Woo J, et al: Prevalence of
non-alcoholic fatty liver disease and advanced fibrosis in Hong
Kong Chinese: A population study using proton-magnetic resonance
spectroscopy and transient elastography. Gut. 61:409–415. 2012.
View Article : Google Scholar
|
|
5
|
Luo P, Qin C, Zhu L, Fang C, Zhang Y,
Zhang H, Pei F, Tian S, Zhu XY, Gong J, et al: Ubiquitin-specific
peptidase 10 (USP10) inhibits hepatic steatosis, insulin
resistance, and inflammation through Sirt6. Hepatology.
68:1786–1803. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Anstee QM, Targher G and Day CP:
Progression of NAFLD to diabetes mellitus, cardiovascular disease
or cirrhosis. Nat Rev Gastroenterol Hepatol. 10:330–344. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Samuel VT, Liu ZX, Qu X, Elder BD, Bilz S,
Befroy D, Romanelli AJ and Shulman GI: Mechanism of hepatic insulin
resistance in non-alcoholic fatty liver disease. J Biol Chem.
279:32345–32353. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Savage DB, Petersen KF and Shulman GI:
Disordered lipid metabolism and the pathogenesis of insulin
resistance. Physiol Rev. 87:507–520. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
El-Sherbiny M, Eldosoky M, El-Shafey M,
Othman G, Elkattawy HA, Bedir T and Elsherbiny NM: Vitamin D
nano-emulsion enhances hepatoprotective effect of conventional
vitamin D in rats fed with a high-fat diet. Chem Biol Interact.
288:65–75. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Day CP: Non-alcoholic fatty liver disease:
A massive problem. Clin Med (Lond). 11:176–178. 2011. View Article : Google Scholar
|
|
11
|
Ding S, Jiang J, Zhang G, Bu Y, Zhang G
and Zhao X: Resveratrol and caloric restriction prevent hepatic
steatosis by regulating SIRT1-autophagy pathway and alleviating
endoplasmic reticulum stress in high-fat diet-fed rats. PLoS One.
12:e01835412017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Rotman Y and Sanyal AJ: Current and
upcoming pharmacotherapy for non-alcoholic fatty liver disease.
Gut. 66:180–190. 2017. View Article : Google Scholar
|
|
13
|
Sabu MC, Smitha K and Kuttan R:
Anti-diabetic activity of green tea polyphenols and their role in
reducing oxidative stress in experimental diabetes. J
Ethnopharmacol. 83:109–116. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kim JJ, Tan Y, Xiao L, Sun YL and Qu X:
Green tea polyphenol epigallocatechin-3-gallate enhance glycogen
synthesis and inhibit lipogenesis in hepatocytes. Biomed Res Int.
2013:9201282013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lubaczeuski C, Gonçalves LM, Vettorazzi
JF, Kurauti MA, Santos-Silva JC, Bonfleur ML, Boschero AC,
Costa-Júnior JM and Carneiro EM: Vagotomy reduces insulin clearance
in obese mice programmed by low-protein diet in the adolescence.
Neural Plast. 2017:96529782017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kalantar M, Kalantari H, Goudarzi M,
Khorsandi L, Bakhit S and Kalantar H: Crocin ameliorates
methotrexate-induced liver injury via inhibition of oxidative
stress and inflammation in rats. Pharmacol Rep. 71:746–752. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jiang S, Tang X, Wang K, Liang Y, Qian Y,
Lu C and Cai L: Hepatic functional and pathological changes of type
1 diabetic mice in growing and maturation time. J Cell Mol Med. Jun
20–2019, (Epub ahead of print). http://doi.org/10.1111/jcmm.14504urisimpledoi.org/10.1111/jcmm.14504.
View Article : Google Scholar
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
19
|
Tilg H and Moschen AR: Insulin resistance,
inflammation, and non-alcoholic fatty liver disease. Trends
Endocrinol Metab. 19:371–379. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gong J, Fang C, Zhang P, Wang PX, Qiu Y,
Shen LJ, Zhang L, Zhu XY, Tian S, Li F, et al: Tumor progression
locus 2 in hepatocytes potentiates both liver and systemic
metabolic disorders in mice. Hepatology. 69:524–544. 2019.
View Article : Google Scholar
|
|
21
|
Zhao GN, Zhang P, Gong J, Zhang XJ, Wang
PX, Yin M, Jiang Z, Shen LJ, Ji YX, Tong J, et al: Tmbim1 is a
multivesicular body regulator that protects against non-alcoholic
fatty liver disease in mice and monkeys by targeting the lysosomal
degradation of Tlr4. Nat Med. 23:742–752. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kitade H, Chen G, Ni Y and Ota T:
Nonalcoholic fatty liver disease and insulin resistance: New
insights and potential new treatments. Nutrients. 9:pii: E387.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Samuel VT and Shulman GI: Mechanisms for
insulin resistance: Common threads and missing links. Cell.
148:852–871. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Arora A and Sharma P: Non-invasive
diagnosis of fibrosis in non-alcoholic fatty liver disease. J Clin
Exp Hepatol. 2:145–155. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Podrini C, Borghesan M, Greco A, Pazienza
V, Mazzoccoli G and Vinciguerra M: Redox homeostasis and
epigenetics in non-alcoholic fatty liver disease (NAFLD). Curr
Pharm Des. 19:2737–2746. 2013. View Article : Google Scholar
|
|
26
|
Reddy VP, Zhu X, Perry G and Smith MA:
Oxidative stress in diabetes and Alzheimer's disease. J Alzheimers
Dis. 16:763–774. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kishimoto T: IL-6: From its discovery to
clinical applications. Int Immunol. 22:347–352. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lesmana CR, Hasan I, Budihusodo U, Gani
RA, Krisnuhoni E, Akbar N and Lesmana LA: Diagnostic value of a
group of biochemical markers of liver fibrosis in patients with
non-alcoholic steatohepatitis. J Dig Dis. 10:201–206. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Xiao J, Ho CT, Liong EC, Nanji AA, Leung
TM, Lau TY, Fung ML and Tipoe GL: Epigallocatechin gallate
attenuates fibrosis, oxidative stress, and inflammation in
non-alcoholic fatty liver disease rat model through TGF/SMAD, PI3
K/Akt/FoxO1, and NF-kappa B pathways. Eur J Nutr. 53:187–199. 2014.
View Article : Google Scholar
|
|
30
|
Wen H, Ting JP and O'Neill LA: A role for
the NLRP3 inflam-masome in metabolic diseases-did Warburg miss
inflammation? Nat Immunol. 13:352–357. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Park MH, Kim DH, Lee EK, Kim ND, Im DS,
Lee J, Yu BP and Chung HY: Age-related inflammation and insulin
resistance: A review of their intricate interdependency. Arch Pharm
Res. 37:1507–1514. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Guturu P and Duchini A: Etiopathogenesis
of nonalcoholic steatohepatitis: Role of obesity, insulin
resistance and mechanisms of hepatotoxicity. Int J Hepatol.
2012:2128652012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Jones JG: Hepatic glucose and lipid
metabolism. Diabetologia. 59:1098–1103. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lambert JE, Ramos-Roman MA, Browning JD
and Parks EJ: Increased de novo lipogenesis is a distinct
characteristic of individuals with nonalcoholic fatty liver
disease. Gastroenterology. 146:726–735. 2014. View Article : Google Scholar
|
|
35
|
Smith BK, Marcinko K, Desjardins EM, Lally
JS, Ford RJ and Steinberg GR: Treatment of nonalcoholic fatty liver
disease: Role of AMPK. Am J Physiol Endocrinol Metab.
311:E730–E740. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Chiu CY, Chan IL, Yang TH, Liu SH and
Chiang MT: Supplementation of chitosan alleviates high-fat
diet-enhanced lipogenesis in rats via adenosine monophosphate
(AMP)-activated protein kinase activation and inhibition of
lipogenesis-associated genes. J Agric Food Chem. 63:2979–2988.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Cho YS, Lee JI, Shin D, Kim HT, Jung HY,
Lee TG, Kang LW, Ahn YJ, Cho HS and Heo YS: Molecular mechanism for
the regulation of human ACC2 through phosphorylation by AMPK.
Biochem Biophys Res Commun. 391:187–192. 2010. View Article : Google Scholar
|
|
38
|
Choi S, Choi Y, Choi Y, Kim S, Jang J and
Park T: Piperine reverses high fat diet-induced hepatic steatosis
and insulin resistance in mice. Food Chem. 141:3627–3635. 2013.
View Article : Google Scholar : PubMed/NCBI
|