Introduction
Myocardial infarction (MI) is a life-threatening
condition caused by acute or chronic myocardial ischemia and
usually leads to heart failure (1). Necroptosis induced by tumor necrosis
factor (TNF) has been implicated in the development of
cardiovascular diseases, including MI (2). The receptor-interacting protein
(RIP)1 and RIP3 kinases have been identified as the two critical
key signaling molecules required for this type of programmed
necrosis (3). RIP3 mediates
MI-induced myocardial cell death and adverse remodeling, whereas
RIP1 is critically involved in cardiac ischemia/reperfusion injury
(4,5). Therefore, the necroptosis pathway
may be a promising target for the treatment of MI.
Autophagy, which is an intracellular degradation
process, occurs in the healthy myocardium and serves an important
role in maintaining cardiac structure and function. Increasing
evidence has suggested the importance of autophagy in the process
of cardiac remodeling, through limiting infarct size, protecting
cardiomyocytes and maintaining the function of the left ventricle
(6-9). As autophagy complexes cell death by
necroptosis (10-12), the balance between autophagy and
necroptosis may be important for cardiomyocyte survival and cardiac
function.
Garlic possesses antibiotic, antithrombotic and
antineo-plastic effects (13).
S-allyl-cysteine sulfoxide (alliin) is an organosulfur compound and
is considered to be the main component of intact garlic. When raw
garlic is chopped, crushed or chewed, alliin is converted to
allicin (also known as garlicin) by alliinase (14). The medicinal effects of garlic
preparations are mainly due to alliin, which has been shown to
inhibit inflammatory responses, suppress oxidation, protect against
atherogenesis and prevent isoproterenol-induced MI and injury in
rats (15-17). Furthermore, alliin has been shown
to exert cardioprotective effects in a model of
ischemia-reperfusion by inhibiting apoptosis and increasing
autophagy (18). In the present
study, the potentially beneficial effect of alliin on MI was
examined.
Materials and methods
Animal experiments
The present study was approved by the Ethics
Committee of Nanjing Drum Tower Hospital. For the experiments,
8-10-week-old male C57BL/6 mice (18-20 g) were obtained from the
Center of Experimental Animals of Nanjing Medical University. All
animals were fed sterile water and food ad libitum under
specific pathogen-free conditions at 25°C and 70% humidity, with a
12:12-h light/dark cycle. A total of 45 mice were randomly divided
into three groups: Sham group, MI group, and MI + alliin (100
mg/kg) group. The induction of MI was performed as previously
described (19). Briefly, the
mice were pretreated with alliin (100 mg/kg) intraperitoneally 7
days before surgery. The mice were then subjected to permanent
ligation of the left anterior descending coronary artery (LAD)
following anesthesia with pentobar-bital (50 mg/kg). Once the heart
was exposed, a suture was placed 2 mm below the tip of the atrial
appendage. The mice in the sham group underwent the same
thoracotomy procedure without LAD ligation. Following surgery,
alliin (100 mg/kg) was administered every day for 14 days, and
heart tissues were collected 14 days after surgery for subsequent
analysis.
Primary cardiomyocyte isolation and cell
culture
A total of 10 neonatal male C57B6 mice (obtained
from the same supplier) were sacrificed using carbon dioxide
asphyxiation followed by cervical dislocation within 24 h of birth.
The beating hearts were removed, and the atria and great vessels
were carefully dissected and placed in PBS on ice. The hearts were
minced and digested with 0.08% collagenase type II (Worthington
Biochemical Corp.) in PBS at 37°C in 5% CO2 for 30 min.
Single cells were plated in dishes and cultured in DMEM containing
20% FBS and 1% penicillin/streptomycin (all Gibco; Thermo Fisher
Scientific, Inc.) at 37°C in 5% CO2 for 90 min to allow
the adherence of non-myocytes. The supernatant containing
cardiomyocytes was collected and seeded in 6-well plates. The
primary cardiomyocytes were cultured in DMEM containing 20% FBS and
1% penicillin/streptomycin at 37°C in 5% CO2 for 1-5
days. H9c2 cells were obtained from ScienCell Research Laboratories
and were cultured in ECM containing 5% FBS, and 1%
penicillin/streptomycin (all Gibco; Thermo Fisher Scientific, Inc.)
at 37°C in 5% CO2.
Apoptosis assay
Apoptosis of the cells was evaluated using an
Annexin V-FITC/propidium iodide (PI) kit (Beijing Biosea
Biotechnology Co., Ltd.), according to the manufacturer's
instructions. The H9c2 cells (2×106 cells/well in 6-well
plates) were exposed to hypoxia at 37°C for 24 h as previously
described (20), following
pretreatment with alliin at different concentrations (25, 100 and
200 μg/ml) for 4 h at 37°C. The cells were stained with
Annexin V and PI for 30 min at 4°C, and then flow analysis was
conducted using a BD FACScalibur device (BD Biosciences) and
analyzed with FlowJo v10 (FlowJo, LLC).
RNA sequencing and reverse
transcription-quantitative (RT-q) PCR analysis
Following exposure of the primary cardiomyocytes to
hypoxia in the absence or presence of alliin (100 μg/ml) as
described above, total RNA was extracted with TRIzol (Invitrogen;
Thermo Fisher Scientific, Inc.). The transcriptome library was
generated for sequencing using a VAHTS mRNA-seq V2 Library Prep kit
for Illumina® (Vazyme Biotech) according to the
manufacturer's instructions. The quality of the library was checked
via qPCR. The Fastq files were generated by sequencing with HiSeq
2000 (read length: 50 base pairs, single end; Illumina, Inc.).
RT-qPCR analysis was also performed; the total RNA was reverse
transcribed into cDNA with AMV reverse transcriptase (Takara Bio,
Inc.) according to the manufacturer's instructions. qPCR was
performed with a TaqMan PCR kit (Invitrogen; Thermo Fisher
Scientific, Inc.) and an Applied Biosystems 7900 Sequence Detection
system (Applied Biosystems; Thermo Fisher Scientific, Inc.). qPCR
was performed with the following cycling profile: 95°C for 30 sec,
then 40 cycles of 95°C for 5 sec and 60°C for 30 sec, and a melting
curve analysis protocol (60-95°C with temperature increment of
0.2°C every 10 sec). A comparative quantitation cycle (ΔCq) method
was used to compare each condition with the controls, and values
are expressed as 2-ΔΔCq values as previously described
(21). On completion of the
reaction, the mRNA expression in cells or tissues was normalized to
the expression of GAPDH. Sequences of primers used are as follows:
ATG4A, forward 5′-GCT GGT ATG GAT TC TGG GG-3′, reverse 5′-TCC CAG
TTC CAA TCC CTT CC-3′; ATG4B, forward 5′-TGG AGC TGA AGT CAC CA A
CA-3′, reverse 5′-CTC CTC CCC AAC ATA GCC AA-3′; ATG4C, forward
5′-GCG GCA AAC CTA AAC AGT CA-3′, reverse 5′-GTG ATT TCT TCA GAC
GCT CGT TC-3′; ATG4D, forward 5′-CGT ATC CCG GAT CCT AGC AA-3′,
reverse 5′-CTG CCC AAC CAT ACT TGA CG-3′; ATG16L2, forward 5′-TCA
GCG AGA TCC CAA ACA CT-3′, reverse 5′-CTT CAC CAC ATC CAC ACA
GC-3′; ATG9A, forward 5′-CTT CTT CGC TGG CTC TAT CCT-3′, reverse
5′-GTG CAA GAA TCA CTC GGA GC-3′; GAPDH, forward 5′-AGG TCG GTG TGA
ACG GAT TTG-3′, reverse 5′-TGT AGA CCA TGT AGT TGA GGT CA-3′.
Echocardiography
For echocardiography, the mice were anesthetized
with 2% isoflurane and scanning was performed with a VisualSonics
Vevo 2100 imaging system (VisualSonics; SonoSite, Inc.). The M-mode
ventricular dimensions were averaged over 3-5 cycles. Fractional
shortening (FS) was calculated based on the ventricular dimensions
at the end of systole and diastole (LVES and LVED, respectively)
using the following formula: FS = [(LVED-LVES)/LVED] x100 (%). The
surgical procedure for MI in the mice with permanent ligation of
the LAD has been described above.
Histological analysis
The mouse hearts were fixed with 10% formalin in PBS
for 24 h at room temperature and dehydrated for paraffin embedding.
Sagittal sections (5-μm thickness) were collected from each
heart and fibrosis was detected with Masson's trichrome staining
using a Masson's Trichrome Stain Kit according to the
manufacturer's protocols (Nanjing KeyGen Biotech Co., Ltd.). Blue
collagen staining was quantified using MetaMorph 6.1 software
(Molecular Devices, LLC) as described previously (22).
A TUNEL assay was performed with an ApopTag
Peroxidase In Situ Apoptosis Detection kit (EMD Millipore),
according to the manufacturer's instructions, to analyze myocardial
cell death. The nucleus was stained using DAPI (EMD Millipore;
1:1,000) and samples were mounted with antifade solution
(Invitrogen; Thermo Fisher Scientific, Inc.). The specimens were
sampled from five individual fields, and the rate of cell death was
defined as follows: (Number of positively stained nuclei/total
number of nuclei in the field) x100.
Western blot analysis
The cells and mouse heart tissues were homogenized
and lysed using RIPA buffer (Cell Signaling Technology, Inc.) with
protease and phosphatase inhibitors (Cell Signaling Technology,
Inc.). The protein concentration was determined by using a
Bicinchoninic Acid Assay kit (Pierce; Thermo Fisher Scientific,
Inc.). A total of 40 μg of total protein was resolved on 10%
SDS-PAGE gels and then electrotransferred onto polyvinylidene
difluoride membranes (EMD Millipore). The membranes were blocked
with 5% nonfat milk at room temperature for 30 min and then probed
with antibodies targeting the following proteins overnight at 4°C:
Cleaved caspase 3 (1:1,000; cat. no. 9664; Cell Signaling
Technology, Inc.); GAPDH (1:1,000; cat. no. ab8245; Abcam); RIP1
(1:1,000; cat. no. ab72139; Abcam); RIP3 (1:1,000; cat. no.
ab56164; Abcam); TNF receptor-associated factor 2 (TRAF2; 1:1,000;
cat. no. ab126758; Abcam); Beclin 1 (1:1,000; cat. no. ab207612;
Abcam); microtubule-associated protein 1 light chain 3 (LC3;
1:1,000; cat. no. ab51520; Abcam); autophagy-related (ATG)7
(1:1,000; cat. no. ab133528; Abcam), P62 (1:1,000; cat. no.
ab56416; Abcam) and peroxisome prolif-erator-activated receptor γ
(PPARγ; 1:1,000; cat. no. ab45036; Abcam). After 3 15-min washes,
the blots were incubated with the appropriate horseradish
peroxidase-conjugated secondary antibody [goat anti-rabbit IgG
(1:1,000; cat. no. ab6721; Abcam) and goat anti-mouse IgG (1:1,000;
cat. no. ab6789; Abcam)] and detected with an enhanced
chemiluminescence reagent (Cell Signaling Technology, Inc.). The
autoradiographic intensity of each protein band was quantified by
using ImageJ software (version 1.52a; National Institutes of
Health) and normalized against GAPDH.
Bioinformatic analysis
Gene Ontology (GO) analysis and Kyoto Encyclopedia
of Genes and Genomes (KEGG) pathway enrichment analysis was
performed using R Programming Language (R version 3.5.2) (23-28). The clusterProfiler package
(version 3.10.1) was used according to the reference manual
(29).
Statistical analysis
All figures showing western blot, immunofluorescence
labeling and flow cytometry data are representative of at least
three independent experiments. The statistical analysis was
conducted using GraphPad Prism software 5.01 (GraphPad Software,
Inc.) and SPSS 22 (IBM Corp.). Data are presented as the mean ± SEM
of at least three independent experiments. Differences among groups
were analyzed by one-way analysis of variance (ANOVA). P<0.05
was considered to indicate a statistically significant difference.
If ANOVA was significant, the Student-Newman-Keuls post hoc test
was used for pairwise multiple comparisons.
Results
Cardioprotective effect of alliin against
MI
To investigate the effect of alliin on myocytes
following MI, mice were administered with alliin (100 mg/kg) by
intraperitoneal injection before and after surgery (Figs. 1A and S1A). Following infarction, the
untreated MI group exhibited ~50% mortality, which generally
occurred within the first 13 days. By contrast, the alliin-treated
mice were protected (1/15 mice died; P<0.05; Fig. 1B). Quantitative analysis of the
fibrosis using Masson's trichrome staining revealed decreased
fibrosis in the myocardium of the alliin-treated group compared
with that in the MI control mice (Fig. 1C). Representative M-mode
echocardiograms are shown in Fig.
1D. Compared with those in the MI mice, the ejection fraction
(EF) percentage and the percentage of fractional shortening (FS)
were markedly elevated in the alliin-treated mice (Fig. 1E). Furthermore, TUNEL staining
showed that alliin protected cardiomyocytes from cell death. As
shown, the percentage of TUNEL-positive cells in the infarction
zone increased to 40.56±4.7%, whereas alliin significantly reduced
the index of cardiac cell death to 12.10±2.5% (Fig. 1F). Taken together, these data
suggest that alliin may alleviate MI by regulating cardiomyocyte
cell death.
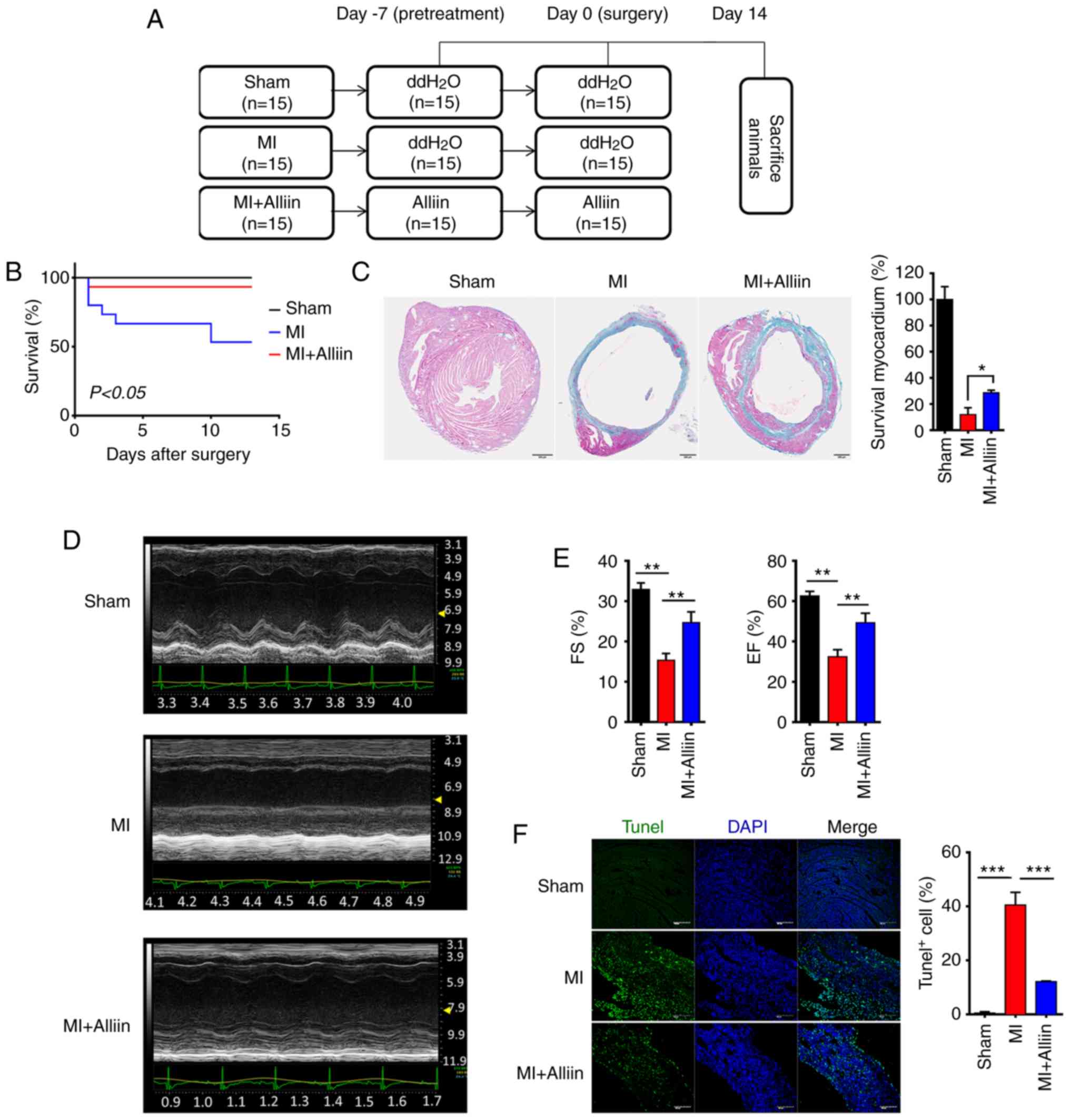 | Figure 1Alliin protects cardiomyocytes
against MI injury. (A) Experimental design. The mice were treated
with alliin (100 mg/kg) or water 7 days before surgery and for 14
days after surgery. On day 0, surgery was performed, and the mice
were sacrificed on day 14. (B) Kaplan-Meier survival curves
following MI. (C) Representative Masson's trichrome-stained
sections from the sham, MI and alliin-treated MI groups
(magnification, x10). (D) Representative echo-cardiography M-mode
images from the sham, MI and alliin-treated MI groups. (E) EF and
FS percentages in the sham, MI and alliin-treated MI groups. (F)
Representative TUNEL staining images (magnification, x40). The data
presented in each panel are representative of at least three
independent experiments and are presented as the mean ± SEM.
*P<0.05, **P<0.01,
***P<0.001. MI, myocardial infarction; EF, ejection
fraction; FS, fractional shortening. |
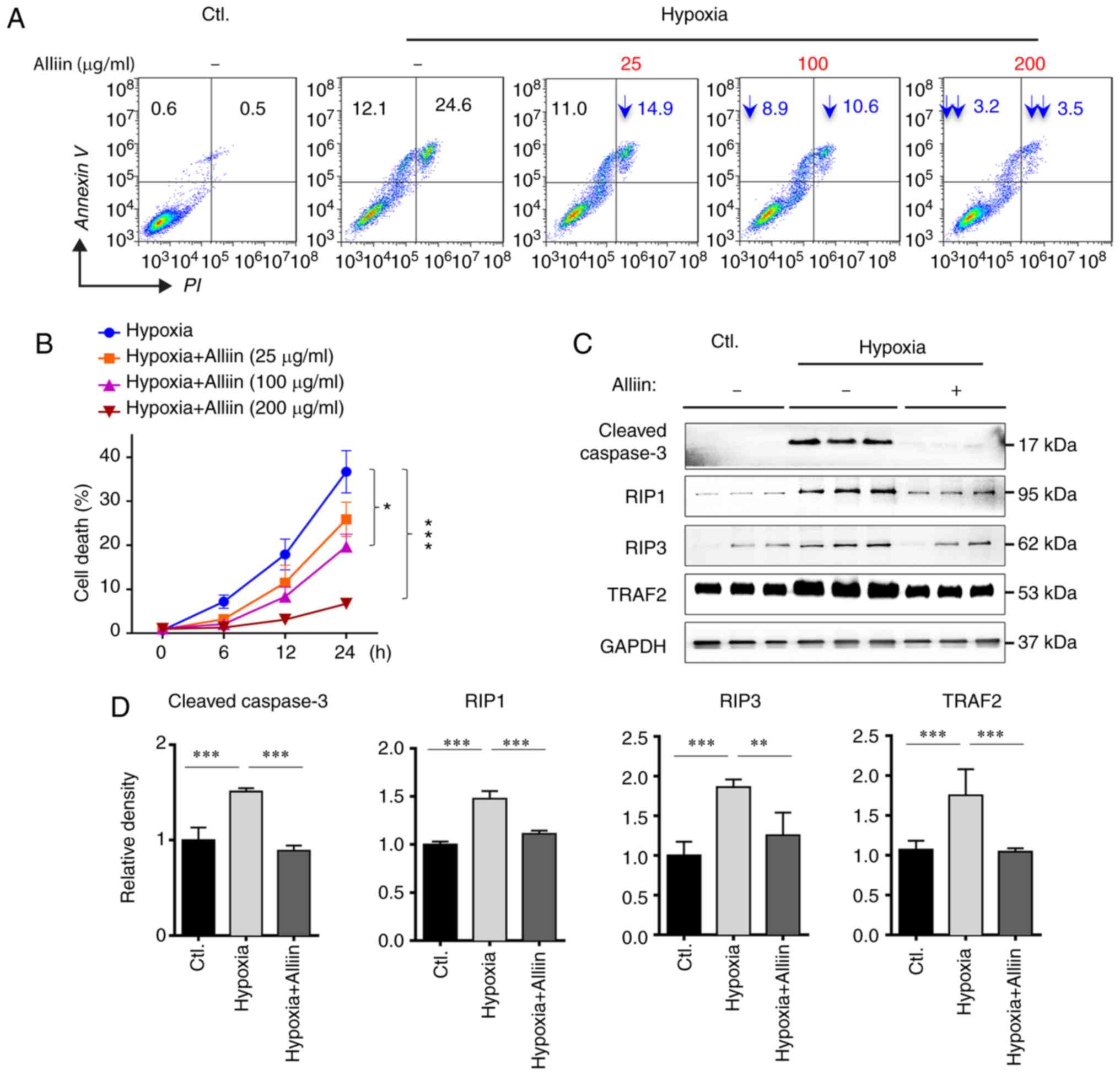
Alliin decreases hypoxia-induced
necroptosis in H9c2 cells
To confirm the protective effect of alliin,
cardiomyocytes were treated with different concentrations of alliin
in vitro under hypoxic conditions, following which a cell
apoptosis assay was performed. As shown in Fig. 2A and B, compared with the cells
without alliin treatment, treatment with alliin significantly
decreased the apoptosis and necroptosis of H9c2 cells in a
dose-dependent manner. Similar results were observed in detecting
the level of cleavage of caspase-3 (Fig. 2C and D). As the TNFα-dependent
formation of the RIP1 and RIP3 complex is a key signal for the
initiation of necroptosis (30),
the expression levels of RIP1, RIP3 and TRAF2 were analyzed in H9c2
cells under hypoxia by western blotting. It was found that alliin
treatment significantly inhibited the expression of RIP1, RIP3 and
TRAF2 (Fig. 2C and D), suggesting
that alliin protects cardiomyocytes against hypoxia-induced
necroptosis.
Role of alliin in autophagy during
hypoxia
To investigate the mechanism by which alliin
regulates cardiomyocyte apoptosis and necrosis, RNA sequencing
analysis was performed on primary cardiomyocytes under hypoxic
conditions in the absence or presence of alliin. Molecular
enrichment analysis based specifically on KEGG pathways and GO
molecular function terms revealed that alliin may regulate
autophagy and cell survival in cardiomyocytes (Figs. 3A and S1B). Notably, alliin upregulated a
number of genes in the autophagy pathway, including Atg4A, Atg4C
and Atg4D, under hypoxic conditions (Fig. 3B). RT-qPCR analysis was performed
to confirm these results. As shown in Fig. 3C, the levels of ATG4A, ATG4C,
ATG4D and ATG9A were decreased, whereas the level of ATG16L2 was
increased following hypoxia. However, the presence of alliin
significantly increased the levels of autophagy-related genes
ATG4A, ATG4C, ATG4D, ATG9A and ATG16L2 following hypoxia. Notably,
hypoxia and alliin treatment had no effect on ATG4B. These results
further confirmed the role of alliin in autophagy.
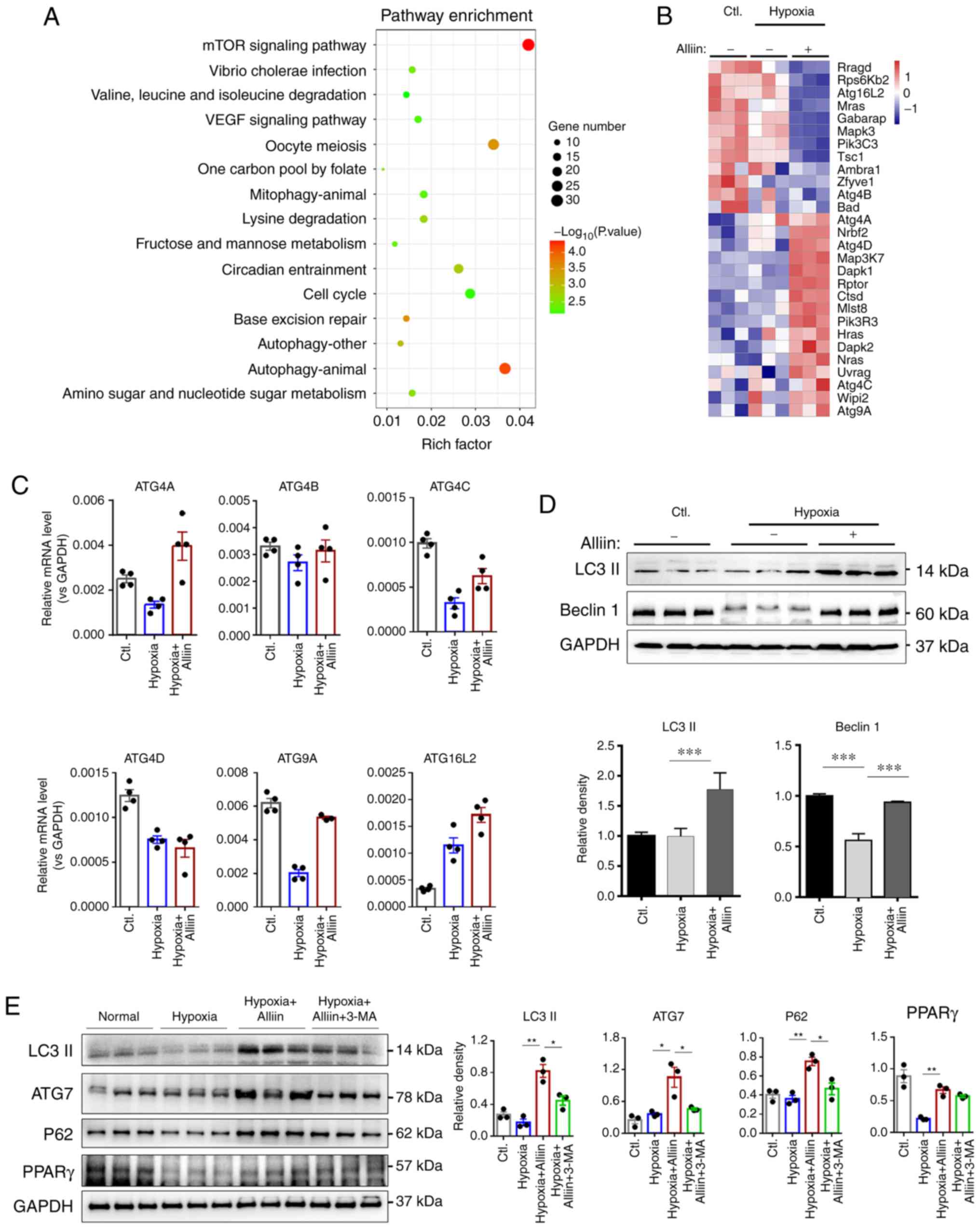 | Figure 3RNA sequencing analysis reveals a
role for alliin in autophagy. (A) KEGG pathway analysis of proteins
regulated by alliin during hypoxia. (B) Autophagy-related genes
regulated by alliin identified by RNA sequencing analysis. (C)
Levels of ATG4A, ATG4B, ATG4C, ATG4D, ATG9A and ATG16L2 in primary
cardiomyocytes were detected by reverse transcription-quantitative
PCR analysis. (D) Expression levels of Beclin 1, LC3, ATG7, p62 and
PPARγ in H9c2 cells under hypoxia were analyzed by western
blotting, and the (E) effect of alliin was reversed by autophagy
inhibitor 3-MA. The data presented in each panel are representative
of at least three independent experiments and are presented as the
mean ± SEM. *P<0.05, **P<0.01 and
***P<0.001. Ctl, control; LC3, microtubule-associated
protein 1 light chain 3; ATG, autophagy-related; PPARγ, peroxisome
proliferator-activated receptor γ. |
As autophagy is induced by the activation of Beclin
1 and its triggering of LC3 lipidation (31), the levels of Beclin 1 and LC3 were
analyzed. Alliin reversed the hypoxia-induced reduction in the
expression of Beclin 1 (Fig. 3D).
In addition, alliin induced higher levels of LC3, ATG7 and p62
following hypoxia, suggesting that alliin promoted autophagy during
hypoxia (Fig. 3D and E). Notably,
the alliin-induced activation of autophagy was reversed by
administration of the autophagy inhibitor 3-MA (Fig. 3E). Recently, alliin was shown to
protect against LPS-induced acute lung injury by directly
activating PPARγ (32). The
expression of PPARγ was detected to further investigate the
protective mechanism of alliin during hypoxia. As shown in Fig. 3E, hypoxia induced a decrease in
the expression of PPARγ, whereas alliin treatment reversed the
hypoxia-induced reduction of PPARγ. However, 3-MA had no effect on
the expression of PPARγ.
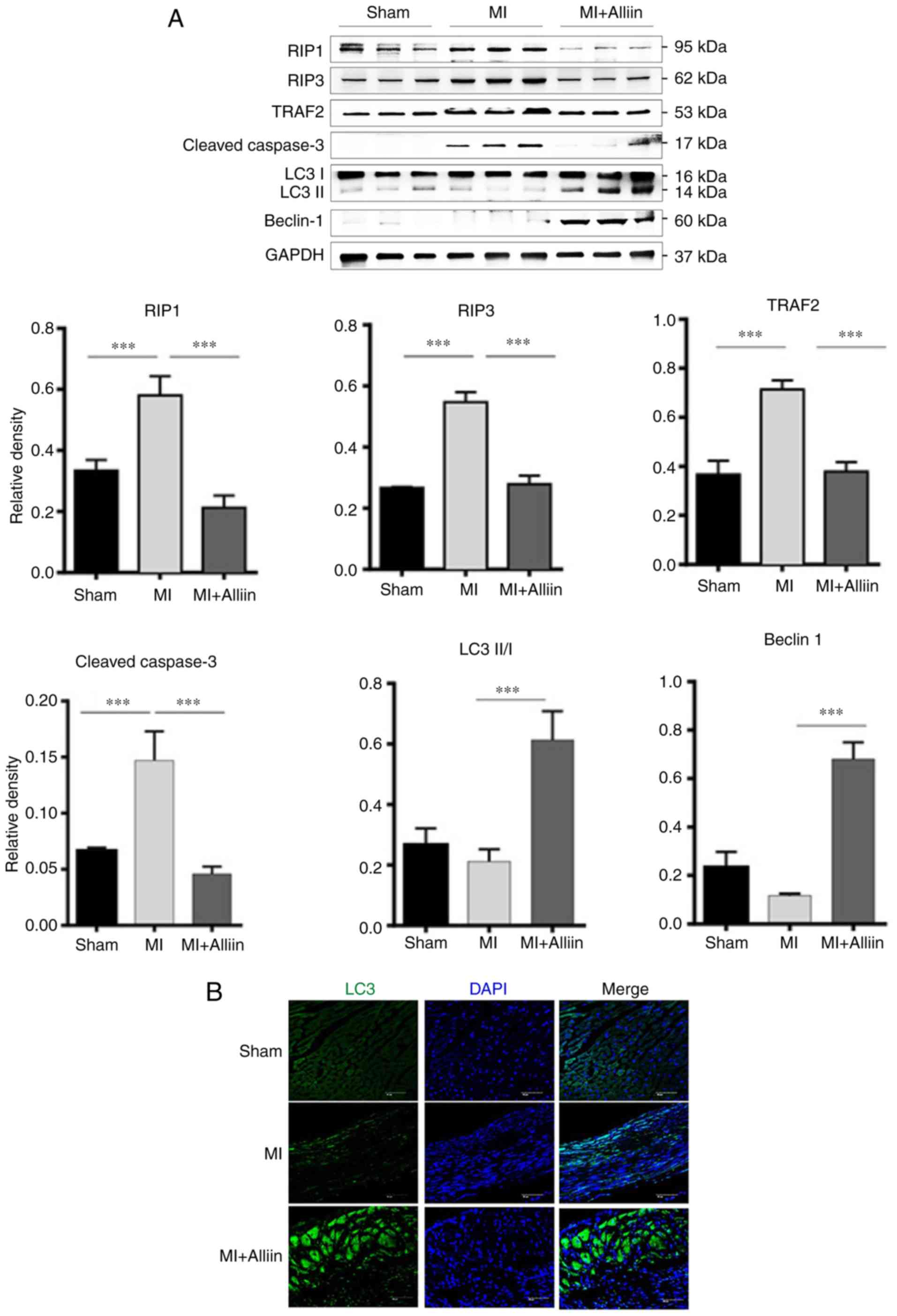
Alliin promotes autophagy during MI
The cardiac expression of necroptosis- and
autophagy-related proteins was also analyzed in vivo
following MI. As shown in Fig.
4A, alliin decreased the cleavage of caspase-3 in the heart.
Alliin also significantly decreased the induction of RIP1, RIP3 and
TRAF2 in the heart tissues following MI. The expression levels of
Beclin 1 and LC3-I/II were also increased. Immunofluorescence
staining confirmed that the expression of LC3 was also improved by
alliin following MI (Fig. 4B).
Taken together, these results suggest that alliin has a protective
effect following MI by promoting autophagy.
Discussion
Cardiomyocyte loss induced by hypoxia is the major
mechanism resulting in heart failure (33,34); therefore, inhibiting cardiomyocyte
death following MI is important for designing effective therapies
for heart failure (35,36). The present study evaluated the
protective role of alliin on cardiac function following MI in a
mouse model and investigated the underlying mechanisms. Alliin is
one of the critical bioactive organosulfur compounds in garlic. It
has been shown to have a number of bioactivities, including
antioxidant and immuno-modulatory effects (37,38). The results of the present study
showed that alliin significantly attenuated the myocardial infarct
area, improved pathological myocardial changes and reduced
cardiomyocyte loss, indicating that alliin has a multifaceted
effect in improving myocardial injury following MI.
The connection between necroptosis and MI was first
observed by Luedde et al (4), who reported that RIP3 was
upregulated in an ischemic model. Necroptosis differs from other
forms of cell death, such as apoptosis and necrosis, as it is
defined by specific signaling triggered by death receptors in the
TNF superfamily, rather than non-specific damage (39,40). To date, few studies have described
the involvement of alliin in MI and its effects on necroptosis. The
present study is the first, to the best of our knowledge, to
observe that treatment with alliin protects cardiomyo-cytes from
hypoxia-induced necroptosis. Previous studies have shown that
necroptosis is elicited by the TNF-induced interaction between RIP1
and RIP3, which leads to the formation of necrosomes (30,41). RIP1-RIP3-MLKL necroptotic
signaling is crucially regulated by Traf2 and adaptor proteins,
including TNFRI-associated death domain (TRADD) (42,43). The western blotting results
confirmed that alliin decreased the protein levels of RIP1, RIP3
and TRAF2 following hypoxic injury. Taken together, these findings
suggest that alliin possesses a cardioprotective effect that may be
associated with the inhibition of necroptosis, which was consistent
with the observations in vivo.
Autophagy is generally defined as a survival program
occurring under stress conditions. The ability of autophagy to
clear damaged proteins or organelles and maintain mitochondrial
function is frequently associated with cyto-protection and
homeostasis (44,45). Autophagy is commonly observed in
acute/chronic MI and heart failure (46-48). The results of the present study
revealed that alliin enhanced autophagy in cardiomyocytes in
vitro and in vivo. Alliin treatment not only promoted
the expression of LC3 and Beclin 1 in cardiomyocytes under hypoxia
but also increased the levels of these proteins in the heart
following MI. In particular, it has been shown that alliin exhibits
concentration-dependent PPARγ ligand-binding activity and
upregulates the expression of PPARγ in a dose-dependent manner
(32). There is accumulating
evidence that PPARγ is involved in the regulation of autophagy and
apoptosis (49,50), and serves a protective role in
ischemia/reperfusion injury by regulating cardiac glucose, lipid
metabolism, inflammatory responses, oxidative stress and apoptosis
(51-54). In the present study, it was shown
that alliin treatment promoted autophagy by targeting PPARγ and
thus protected cardiomyocytes against hypoxia-induced
necroptosis.
In conclusion, these findings demonstrate that
alliin protects mice against MI-induced heart injury via activating
autophagy and exerting anti-necroptotic activity. Therefore, as a
PPARγ agonist, alliin may be a promising therapeutic candidate for
the clinical treatment of MI and other cardiovascular diseases.
Supplementary Data
Acknowledgments
Not applicable.
Funding
This study was supported by grants from the Jiangsu
Provincial Key Medical Discipline Laboratory (grant no.
ZDXKA2016019 to DJW) and Jiangsu Provincial Medical Youth Talent
(grant no. QNRC2016035 to QZ).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
QZ, TSX and DJW designed the research. LJY, XYZ,
RSL, HJC, BG, CCT, CL and YXX performed the experiments and
analyzed data. LJY, QZ, TSX and DJW drafted the manuscript. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Nanjing Drum Tower Hospital.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Cahill TJ and Kharbanda RK: Heart failure
after myocardial infarction in the era of primary percutaneous
coronary intervention: Mechanisms, incidence and identification of
patients at risk. World J Cardiol. 9:407–415. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhang T, Zhang Y, Cui M, Jin L, Wang Y, Lv
F, Liu Y, Zheng W, Shang H, Zhang J, et al: CaMKII is a RIP3
substrate mediating ischemia- and oxidative stress-induced
myocardial necroptosis. Nat Med. 22:175–182. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
He S, Huang S and Shen Z: Biomarkers for
the detection of necroptosis. Cell Mol Life Sci. 73:2177–2181.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Luedde M, Lutz M, Carter N, Sosna J,
Jacoby C, Vucur M, Gautheron J, Roderburg C, Borg N, Reisinger F,
et al: RIP3, a kinase promoting necroptotic cell death, mediates
adverse remodelling after myocardial infarction. Cardiovasc Res.
103:206–216. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Oerlemans MI, Liu J, Arslan F, den Ouden
K, van Middelaar BJ, Doevendans PA and Sluijter JP: Inhibition of
RIP1-dependent necrosis prevents adverse cardiac remodeling after
myocardial ischemia-reperfusion in vivo. Basic Res Cardiol.
107:2702012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tian XF, Yang SW and Zhou YJ: Autophagy,
dysglycemia and myocardial infarction. Ijc Metab Endocr. 14:40–44.
2017. View Article : Google Scholar
|
|
7
|
Nakai A, Yamaguchi O, Takeda T, Higuchi Y,
Hikoso S, Taniike M, Omiya S, Mizote I, Matsumura Y, Asahi M, et
al: The role of autophagy in cardiomyocytes in the basal state and
in response to hemodynamic stress. Nat Med. 13:619–624. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sciarretta S, Zhai PY, Shao D, Maejima Y,
Robbins J, Volpe M, Condorelli G and Sadoshima J: Rheb is a
critical regulator of autophagy during myocardial ischemia:
Pathophysiological implications in obesity and metabolic syndrome.
Circulation. 125:1134–1166. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sengupta A, Molkentin JD, Paik JH, DePinho
RA and Yutzey KE: FoxO transcription factors promote cardiomyocyte
survival upon induction of oxidative stress. J Biol Chem.
9:7468–7478. 2011. View Article : Google Scholar
|
|
10
|
Goodall ML, Cramer SD and Thorburn A:
Autophagy complexes cell death by necroptosis. Oncotarget.
7:50818–50819. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ogasawara M, Yano T, Tanno M, Abe K,
Ishikawa S, Miki T, Kuno A, Tobisawa T, Muratsubaki S, Ohno K, et
al: Suppression of autophagic flux contributes to cardiomyocyte
death by activation of necroptotic pathways. J Mol Cell Cardiol.
108:203–213. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Nikoletopoulou V, Markaki M, Palikaras K
and Tavernarakis N: Crosstalk between apoptosis, necrosis and
autophagy. Biochim Biophys Acta. 1833.3448–3459. 2013.
|
|
13
|
Rana SV, Pal R, Vaiphei K, Sharma SK and
Ola RP: Garlic in health and disease. Nutr Res Rev. 24:60–71. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chan JY, Yuen AC, Chan RY and Chan SW: A
review of the cardiovascular benefits and antioxidant properties of
allicin. Phytother Res. 27:637–646. 2013. View Article : Google Scholar
|
|
15
|
Quintero-Fabián S, Ortuño-Sahagún D,
Vázquez-Carrera M and López-Roa RI: Alliin, a garlic (Allium
sativum) compound, prevents LPS-induced inflammation in 3T3-L1
adipocytes. Mediators Inflamm. 2013.381815:2013.
|
|
16
|
Augusti KT and Sheela CG: Antiperoxide
effect of S-allyl cysteine sulfoxide, an insulin secretagogue, in
diabetic rats. Experientia. 52:115–120. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sangeetha T and Darlin Quine S: Preventive
effect of S-allyl cysteine sulfoxide (alliin) on cardiac marker
enzymes and lipids in isoproterenol-induced myocardial injury. J
Pharm Pharmacol. 58:617–623. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhao R, Xie E, Yang X and Gong B: Alliin
alleviates myocardial ischemia-reperfusion injury by promoting
autophagy. Biochem Biophys Res Commun. 512:236–243. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gao E, Lei YH, Shang X, Huang ZM, Zuo L,
Boucher M, Fan Q, Chuprun JK, Ma XL and Koch WJ: A novel and
efficient model of coronary artery ligation and myocardial
infarction in the mouse. Circ Res. 107:1445–1453. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sun MY, Ma DS, Zhao S, Wang L, Ma CY and
Bai Y: Salidroside mitigates hypoxia/reoxygenation injury by
alleviating endoplasmic reticulum stress-induced apoptosis in H9c2
cardiomyocytes. Mol Med Rep. 18:3760–3768. 2018.PubMed/NCBI
|
|
21
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
22
|
Banerjee I, Fuseler JW, Intwala AR and
Baudino TA: IL-6 loss causes ventricular dysfunction, fibrosis,
reduced capillary density, and dramatically alters the cell
populations of the developing and adult heart. Am J Physiol Heart
Circ Physiol. 296:H1694–H1704. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ashburner M, Ball CA, Blake JA, Botstein
D, Butler H, Cherry JM, Davis AP, Dolinski K, Dwight SS, Eppig JT,
et al: Gene Ontology: Tool for the unification of biology. The Gene
Ontology Consortium Nat Genet. 25:25–29. 2000.
|
|
24
|
The Gene Ontology: Consortium: The Gene
Ontology Resource: 20 years and still GOing strong. Nucleic Acids
Res. 47:D330–D338. 2019. View Article : Google Scholar
|
|
25
|
Kanehisa M and Goto S: KEGG: Kyoto
encyclopedia of genes and genomes. Nucleic Acids Res. 28:27–30.
2000. View Article : Google Scholar
|
|
26
|
Kanehisa M, Sato Y, Furumichi M, Morishima
K and Tanabe M: New approach for understanding genome variations in
KEGG. Nucleic Acids Res. 47:D590–D595. 2019. View Article : Google Scholar :
|
|
27
|
Kanehisa M: Toward understanding the
origin and evolution of cellular organisms. Protein Sci. Aug
22–2019.Epub ahead of print. View
Article : Google Scholar : PubMed/NCBI
|
|
28
|
R Core Team: R: A language and environment
for statistical computing. R Foundation for Statistical Computing.
2018.
|
|
29
|
Yu GC, Wang LG, Han YY and He QY:
clusterProfiler: An R package for comparing biological themes among
gene clusters. OMICS. 16:284–287. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Vandenabeele P, Declercq W, Van Herreweghe
F and Vanden Berghe T: The role of the kinases RIP1 and RIP3 in
TNF-induced necrosis. Sci Signal. 3:re42010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
He C and Klionsky DJ: Regulation
mechanisms and signaling pathways of autophagy. Annu Rev Genet.
43:67–93. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wang YL, Guo XY, He W, Chen RJ and Zhuang
R: Effects of alliin on LPS-induced acute lung injury by activating
PPARgamma. Microb Pathog. 110:375–379. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Konstantinidis K, Whelan RS and Kitsis RN:
Mechanisms of cell death in heart disease. Arterioscler Thromb Vasc
Biol. 32:1552–1562. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Sabbah HN: Apoptotic cell death in heart
failure. Cardiovasc Res. 45:704–712. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Briasoulis A, Androulakis E, Christophides
T and Tousoulis D: The role of inflammation and cell death in the
pathogenesis, progression and treatment of heart failure. Heart
Fail Rev. 21:169–176. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Moe GW and Marín-García J: Role of cell
death in the progression of heart failure. Heart Fail Rev.
21:157–167. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kourounakis PN and Rekka EA: Effect on
active oxygen species of alliin and Allium sativum (garlic) powder.
Res Commun Chem Pathol Pharmacol. 74:249–252. 1991.PubMed/NCBI
|
|
38
|
Salman H, Bergman M, Bessler H, Punsky I
and Djaldetti M: Effect of a garlic derivative (alliin) on
peripheral blood cell immune responses. Int J Immunopharmacol.
21:589–597. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Hanson B: Necroptosis: A new way of dying?
Cancer Biol Ther. 17:899–910. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Smith CC and Yellon DM: Necroptosis,
necrostatins and tissue injury J Cell Mol Med. 15:1797–1806.
2011.
|
|
41
|
Sun L, Wang H, Wang Z, He S, Chen S, Liao
D, Wang L, Yan J, Liu W, Lei X and Wang X: Mixed lineage kinase
domain-like protein mediates necrosis signaling downstream of RIP3
kinase. Cell. 148:213–227. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Zhao J, Jitkaew S, Cai ZY, Choksi S, Li Q,
Luo J and Liu ZG: Mixed lineage kinase domain-like is a key
receptor interacting protein 3 downstream component of TNF-induced
necrosis. Proc Natl Acad Sci USA. 109:5322–5327. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Guo XY, Yin HF, Li L, Chen Y, Li J, Doan
J, Steinmetz R and Liu Q: Cardioprotective role of tumor necrosis
factor receptor-associated factor 2 by suppressing apoptosis and
necroptosis. Circulation. 136:729–742. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Ryter SW, Mizumura K and Choi AM: The
impact of autophagy on cell death modalities. International journal
of cell biology. 2014:5026762014. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Anding AL and Baehrecke EH: Cleaning
house: Selective autophagy of organelles. Dev Cell. 41:10–22. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Wu X, He L, Chen F, He X, Cai Y, Zhang G,
Yi Q, He M and Luo J: Impaired autophagy contributes to adverse
cardiac remod-eling in acute myocardial infarction. PLoS One.
9:e1128912014. View Article : Google Scholar
|
|
47
|
Kaplan O and Demircan G: Relationship of
autophagy and apop-tosis with total occlusion of coronary arteries.
Med Sci Monit. 24:6984–6988. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Kanamori H, Takemura G, Goto K, Maruyama
R, Tsujimoto A, Ogino A, Takeyama T, Kawaguchi T, Watanabe T,
Fujiwara T, et al: The role of autophagy emerging in postinfarction
cardiac remodelling. Cardiovasc Res. 91:330–339. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Wu J, Wu JJ, Yang LJ, Wei LX and Zou DJ:
Rosiglitazone protects against palmitate-induced pancreatic
beta-cell death by activation of autophagy via 5′-AMP-activated
protein kinase modulation. Endocrine. 44:87–98. 2013. View Article : Google Scholar
|
|
50
|
Vasheghani F, Zhang Y, Li YH, Blati M,
Fahmi H, Lussier B, Roughley P, Lagares D, Endisha H, Saffar B, et
al: PPARγ deficiency results in severe, accelerated osteoarthritis
associated with aberrant mTOR signalling in the articular
cartilage. Ann Rheum Dis. 74:569–578. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Yue TL, Bao WK, Gu JL, Cui J, Tao L, Ma
XL, Ohlstein EH and Jucker BM: Rosiglitazone treatment in Zucker
diabetic fatty rats is associated with ameliorated cardiac insulin
resistance and protection from ischemia/reperfusion-induced
myocardial injury. Diabetes. 54:554–562. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Nakano Y, Matoba T, Tokutome M, Funamoto
D, Katsuki S, Ikeda G, Nagaoka K, Ishikita A, Nakano K, Koga J, et
al: Nanoparticle-mediated delivery of irbesartan induces
cardio-protection from myocardial ischemia-reperfusion injury by
antagonizing monocyte-mediated inflammation. Sci Rep. 6:296012016.
View Article : Google Scholar
|
|
53
|
Kim YJ, Park KJ, Song JK, Shim TJ, Islam
KN, Bae JW, Kim SM, Lee SY, Hwang KK, Kim DW, et al: The PPARγ
agonist protects cardiomyocytes from oxidative stress and apoptosis
via thiore-doxin overexpression. Biosci Biotechnol Biochem.
76:2181–2187. 2012. View Article : Google Scholar
|
|
54
|
Taniguchi Y, Ooie T, Takahashi N,
Shinohara T, Nakagawa M, Yonemochi H, Hara M, Yoshimatsu H and
Saikawa T: Pioglitazone but not glibenclamide improves cardiac
expression of heat shock protein 72 and tolerance against
ischemia/reper-fusion injury in the heredity insulin-resistant rat.
Diabetes. 55:2371–2378. 2006. View Article : Google Scholar : PubMed/NCBI
|