Introduction
The loss of periodontal support tissue caused by
periodontitis and other factors is the primary cause of tooth loss
in adults (1). The treatment of
periodontitis and the restoration of periodontal defects are key
issues that require improvements. Although common periodontal
treatments, such as scaling, curettage and flap surgery can control
the progression of periodontitis and prevent the further loss of
periodontal support tissue, they cannot regenerate the lost tissue
effectively (2). The rapid
development of tissue engineering research brings new hope to the
treatment of periodontitis and makes it possible to regenerate
periodontal tissue by using the methods of tissue engineering
(3). Cell sheet technology is a
novel tissue engineering technology that has rapidly developed in
recent years (4). Cell sheets
play a role in maintaining the biological characteristics and
performing the functions of stem cells (5-7).
Studies have demonstrated that cell sheets have more potent tissue
regenerative effects in the myocardium, liver, cornea, bone tissue
and periodontal regeneration compared with other tissue engineering
methods (8-12). Cell sheets were originally
obtained from a thermosensitive material culture dish invented by
Okano et al, and they were separated from each other by
temperature changes (13). A
previous study demonstrated that cell sheet technology can preserve
the extracellular matrix (ECM) and the connection between cells
in vitro, thus avoiding the application of exogenous
biomaterials and improving the survival rate of cell
transplantation (14).
Traditional temperature-sensitive cell culture dishes are complex
to prepare and expensive to equip. Good biocompatibility,
operability, quantifiable control and adjustment of the size and
thickness of the sheets could make cell sheet technology one of the
preferred methods for cell transplantation.
In recent years, a number of improved methods have
been developed to make it easier to obtain biologically active cell
sheets. It has been shown that cell sheets obtained with
dexamethasone and vitamin C (VC) can promote bone formation
(15). VC, a cofactor in the
biological reaction of the whole body, is an important nutrient for
human health (16). VC
participates in the synthesis and function of immune factors, as
well as collagen synthesis (17).
Studies have demonstrated that VC is essential for the biosynthesis
of ECM and can stimulate the proliferation and differentiation of
stem cells in vitro by mimicking the biological environment
(18,19). In addition, a method of obtaining
cell sheets by VC induction culture was reported in 2012, which
rendered the preparation of cell sheets simpler (20). Compared with traditional
thermosensitive materials, it is easier to obtain complete cell
sheets with VC induction (14,20,21). For the acceleration of the
clinical application of periodontal tissue engineering, the further
investigation of safe, effective and simple cell sheet technology
is necessary.
It has been demonstrated that bioflavonoids exhibit
a variety of biological activities (22), which are expected to be
substitutes for growth factors for the in vitro regulation
of cell biological properties. Rutin is a natural bioflavonoid that
is widely present in plants (23). Rutin exerts antioxidant and
anti-free radical effects, and can be used in the treatment of
cardiovascular and cerebrovascular diseases, tumors and
inflammation (24-26). As a common bioflavonoid, rutin is
inexpensive, safe and easy to obtain (27,28). In vitro, experiments have
identified that rutin can promote cell proliferation and osteogenic
differentiation, which can effectively prevent and treat
osteoporosis (29). Rutin, a type
of vitamin P, is a glycoside of dehydro flavanone and coexists with
VC in food. Vitamin P is a hydrogen transmitter, which can prevent
VC from being oxidized and can enhance the effects of VC (30-33). Based on the aforementioned
studies, the present study proposed the hypothesis that the
addition of rutin and VC during the preparation of cell sheets
could promote the formation of cell sheets and improve their
osteogenic properties.
The present study investigated the effects of rutin
on the formation, proliferation and osteogenic differentiation of
periodontal ligament stem cell (PDLSC) sheets, and provided a
theoretical basis for the improvement of cell sheet technology,
which may accelerate the clinical transformation of periodontal
cell therapy.
Materials and methods
Cell culture
The preparation and culture of PDLSCs was performed
according to previous studies (34-36). All the schemes dealing with human
periodontal tissues were approved by the Ethics Committee of
Shandong University (Shandong, China). Informed consent was
obtained in writing by all donors and their parents. Healthy
premolars extracted due to orthodontic reasons of adolescents aged
12-16 years (3 boys and 3 girls, the boys were 12, 14 and 15 years
old, and the girls were 13, 16 and 16 years old) were selected in
May, 2018. Periodontal ligament tissues of the middle and lower
part of the root were scraped and cut into small sections using a
surgical knife. The sections were digested in α-minimum essential
medium (α-MEM; Gibco, Thermo Fisher Scientific, Inc.) containing 1%
collagenase (Sigma-Aldrich; Merck KGaA) and 1% dispase
(Sigma-Aldrich; Merck KGaA) for 60 min at 37°C. Following
digestion, the tissue was filtered through a 70-µm filter to
obtain suspended single cells. The obtained cells were inoculated
in a flask of 25 cm2 and then cultured in α-MEM
containing 10% fetal bovine serum (FBS; Gibco, Thermo Fisher
Scientific, Inc.), 100 U/ml penicillin (Beyotime, Institute of
Biotechnology) and 100 mg/ml streptomycin (Beyotime Institute of
Biotechnology) at 37°C in 5% CO2.
Flow cytometric identification of cell
surface markers
The BD StemflowTM hMSC Analysis kit (BD Biosciences)
was used to identify the immunophenotype. First generation cells
were cultured in a culture dish (10×1×10 cm). When the cell density
reached 90%, the cells were washed with PBS (Corning, Inc.) twice
and digested by trypsinase and then a single cell suspension was
prepared. The cells were then separated into sterile tubes. A
mesenchymal stem cell (MSC)-positive cocktail (CD90 FITC, CD105
PerCP-Cy5.5, CD73 APC, CD44) and MSC-negative cocktail (CD34,
CD11b, CD19, CD45, HLA-DR) were added to the tubes in the dark at
4°C for 20 min. After the cells were identified by flow cytometry
(BD Biosciences), PDLSCs were passaged to the third generation, and
cells of this generation were used in the subsequent
experiments.
Multiple differentiation analysis
PDLSCs of the third generation were inoculated into
6-well plates at a density of 1×105/well. When the cells
were attached, the medium was replaced with osteogenic induction
medium, namely α-MEM containing 10% FBS, 10 nmol/l dexamethasone
(Beijing Solarbio Science & Technology Co., Ltd.), 50
µg/ml VC (Sigma, Aldrich; Merck KGaA) and 10 mmol/l
β-glycerophosphate (Beijing Solarbio Science & Technology Co.,
Ltd.). Following 4 weeks of induction, the cells were fixed with 4%
paraformaldehyde for 30 min, then stained with Alizarin Red
(Sigma-Aldrich; Merck KGaA) for 15 min at room temperature and
rinsed with PBS 3 times. The nodules were observed under an
inverted microscope (Olympus Corp.) and images were obtained.
Likewise, for adipogenic induction, induction was carried out with
an adipogenic induction solution, which included α-MEM containing
10% FBS, 2 µM dexamethasone, 0.2 mM indomethacin
(Sigma-Aldrich; Merck KGaA), 0.01 mg/ml insulin (Sigma-Aldrich;
Merck KGaA) and 0.5 mM 3-isobutyl-1-methylxanthine (Sigma-Aldrich;
Merck KGaA). The medium was changed every 3 days, after which the
induction and fixation steps were performed, and 0.3% Oil Red O
(Cyagen Biosciences) staining was used for 30 min at room
temperature. The adipogenic droplets were then observed under an
inverted microscope (IX73; Olympus Corp.).
Detection of cell proliferative
activity
The Cell Counting Kit-8 (CCK-8; Dojindo Molecular
Technologies, Inc.) was used to detect the cell proliferative
activity. In total, 2,000 cells/well were seeded into a 96-well
culture plate and divided into 6 groups with 5 multiple wells in
each group. Cell proliferation was continuously examined for 3
days, and the culture medium was changed every day. After the cells
had attached, PDLSCs were cultured with various concentrations of
rutin (0, 1×10−8, 1×10−7, 1×10−6,
1×10−5 and 1×10−4 mol/l) in α-MEM containing
a certain concentration of VC (20 µg/ml). At the indicated
time point, each well was incubated with 10 µl CCK-8
solution for 1 h. The absorbance was measured at 450 nm by a
microplate reader (SPECTROstar Nano).
Formation and observation of cell
sheets
A total of 1×105 cells per well were
seeded into a 6-well plate. Following cell attachment, 20
µg/ml VC and 1×10−6 mol/l rutin were added to the
medium as the experimental group. In addition, 20 µg/ml VC
were added to the medium in the control group. Following 10 days of
culture, the cell sheets were formed, and the morphology of the
cell sheets was then observed under a microscope (Olympus Corp.).
For hematoxylin and eosin (H&E) staining (Beijing Solarbio
Science & Technology Co., Ltd.), the cell sheets of each group
were uncovered by cell scraping and fixed with 4% paraformaldehyde
solution. Following routine dehydration, paraffin embedding and
serial sections, H&E staining was performed for 1 h at room
temperature. For observation through scanning electron microscopy
(SEM) (Phenom G2 Pro, Holland), the sheets were fixed with 3%
glutaraldehyde (pH 7.4), dehydrated, critical point dried and
sprayed with gold, and then the extension of PDLSCs in the sheets
was observed.
Alkaline phosphatase (ALP) activity assay
and ALP staining
Following the formation of cell sheets, osteogenic
induction medium with various rutin concentrations was used for 7
days, and the cell sheets were then fixed with 4% paraformaldehyde
for 30 min at room temperature. ALP staining was performed with an
alkaline phosphatase staining kit (Nanjing Jiancheng Bioengineering
Institute) for 15 min at room temperature. The sheets were washed 3
times with distilled water and observed under an inverted
microscope (IX73; Olympus Corp.). For ALP activity detection,
following 7 days of osteogenic induction, the cells were rinsed
with PBS, lysed with RIPA buffer (Beyotime Institute of
Biotechnology) and centrifuged at 4°C and 12,000 × g for 15 min.
According to the manufacturer's protocol of the ALP kit, the
reaction solution was added to each test well. Subsequently, after
30 min, a spectrophotometer (SPECTROstar Nano) was used to detect
the absorbance value (OD value) at 520 nm.
Alizarin Red staining and Alizarin Red
semi-quantitative detection
Following the formation of cell sheets, the cells
were cultured in osteogenic induction medium for 14 days and then
fixed in 4% paraformaldehyde for 30 min, followed by staining with
Alizarin Red solution for 30 min at room temperature. Subsequently,
the cells were washed with PBS, dried at room temperature and
observed under an inverted microscope. Finally, mineralized nodules
were dissolved in 2% cetylpyridinium chloride (Sigma-Aldrich; Merck
KGaA) and OD values were measured at 562 nm using a
spectrophotometer (SPECTROstar Nano) for statistical analysis.
Reverse transcription-quantitative PCR
(RT-qPCR)
PDLSCs (1×105/well) were seeded into
6-well paltes. Following cell adherence, the culture medium was
replaced with sheet culture medium. Following 10 days of sheet
formation, the formed cell sheets was further induced in osteogenic
induction medium, and the osteogenic genes were then detected by
RT-qPCR on days 7 and 14. This time point of detecting osteogenic
genes was selected based on the published literature (14,20,37). Total RNA was extracted using
TRIzol® reagent (Takara Bio, Inc.) according to the
manufacturer's protocol. A total of 1 µg RNA was reverse
transcribed into complementary DNA (cDNA) using a RevertAid First
Strand cDNA Synthesis kit (Thermo Fisher Scintific, Inc). qPCR was
performed using a Roche Light Cycler®480II. The qPCR
parameters used were as follows: 95°C for 15 sec, 40 cycles of 95°C
for 5 sec, followed by 60°C for 60 sec. Relative gene expression
data was calculated through the 2−ΔΔCq method (38). The gene primers used are listed in
Table I. GAPDH was used for
normalization.
 | Table ISequences of primers used for
RT-qPCR. |
Table I
Sequences of primers used for
RT-qPCR.
| Genes | Forward
sequence | Reverse
sequence |
|---|
| GAPDH |
5′-GGAGCGAGATCCCTCCAAAAT-3′ |
5′-GGCTGTTGTCATACTTCTCATGG-3′ |
| ALP |
5′-GTGAACCGCAACTGGTACTC-3′ |
5′-GAGCTGCGTAGCGATGTCC-3′ |
| COL1 |
5′-GCTGATGATGCCAATGTGGTT-3′ |
5′-CCAGTCAGAGTGGCACATCTTG-3′ |
| RUNX2 |
5′-GTTTCACCTTGACCATAACCGT-3′ |
5′-GGGACACCTACTCTCATACTGG-3′ |
| OPN |
5′-CAGTTGTCCCCACAGTAGACAC-3′ |
5′-GTGATGTCCTCGTCTGTAGCATC-3′ |
Western blot analysis
The PDLSCs were seeded into 6-well plates at a
density of 1×105/well. Following 10 days of sheet
formation, total protein was extracted to detect the expression of
ECM-related proteins. Following the formation of the sheets, cells
were cultured in osteogenic induction medium for 7 or 14 days.
Briefly, the sheets in the 6-well plate were scraped off using a
cell scraper and transferred to sterile tubes. Cells were lysed
using RIPA buffer (Beyotime Institute of Biotechnology),
centrifuged at 4°C and 12,000 × g for 15 min, and the supernatant
was removed and transferred to different sterile tubes. The total
protein of each group was extracted and the protein concentration
was determined using a BCA Protein assay kit (Beijing Solarbio
Science & Technology Co., Ltd.). Subsequently, 10% SDS-PAGE was
performed with a suitable amount of protein. The protein was
transferred to a PVDF membrane (EMD Millipore), blocked with 5%
skim milk powder, and incubated with primary antibodies overnight
at 4°C. The following day, the membrane was then incubated with the
secondary antibody (goat anti-rabbit IgG, cat. no. 7074S; Santa
Cruz Biotechnology, Inc.) for 1 h at room temperature. The
following primary antibodies were used: Anti-human GAPDH monoclonal
(1:20,000, cat. no. HRP-60004; ProteinTech Group, Inc.), rabbit
anti-human ALP (1:10,000, cat. no. ab108337; Abcam), rabbit
anti-human osteopontin (OPN; 1:500, cat. no. ab8448; Abcam), rabbit
anti-human runt-related transcription factor 2 (RUNX2; 1:1,000,
cat. no. cst12556; Cell Signaling Technology), rabbit anti-human
collagen type I (COL1; 1:500, cat. no. wl0088; Wanlei Biotechnology
Co., Ltd.), rabbit anti-human fibronectin (1:500, cat. no. wl00712;
Wanlei Biotechnology Co., Ltd.), and rabbit anti-human integrin β1
(1:500, cat. no. wl01615; Wanlei Biotechnology Co., Ltd.). The
protein bands were visual-ized using enhanced chemiluminescence
reagents (EMD Millipore) and protein levels were analyzed using
ImageJ (1.47V; National Institutes of Health).
Statistical analysis
The experimental data are expressed as the means ±
standard deviation, and were analyzed using SPSS17.0 software. A
paired Student's t-test was used for comparisons between 2 groups,
and one-way ANOVA with Tukey's post hoc test was used for
comparisons between multiple groups. A value of P<0.05 was
considered to indicate a statistically significant difference.
Results
Isolation and identification of
PDLSCs
Following 10 days of culture, the fusion degree of
the cultured cells reached 90%. The cells were spindle-shaped,
fibroblast-like and spiral-like (Fig.
1A). Following 28 days of osteogenic induction, scattered milky
white sandy nodules at the bottom of the Petri dish were visible to
the eye, which varied in size. Alizarin Red staining and
microscopic observation demonstrated that the milky white nodules
were stained red (Fig. 1B). Under
the action of adipogenic induction solution, cell proliferation
decreased and the cells gradually changed from a spindle-like shape
to an ellipse shape. Furthermore, 4 weeks later, some of the cells
had beaded adipogenic droplets in the cytoplasm, and the Oil Red O
staining result was red (Fig.
1C). Flow cytometric analysis revealed that CD90 FITC, CD105
PerCP-Cy5.5, CD73 APC and CD44 PE were highly expressed, while CD34
PE, CD11b PE, CD19 PE, CD45 PE and HLA-DR PE were almost not
expressed (Fig. 1D). Thus, PDLSCs
were successfully obtained.
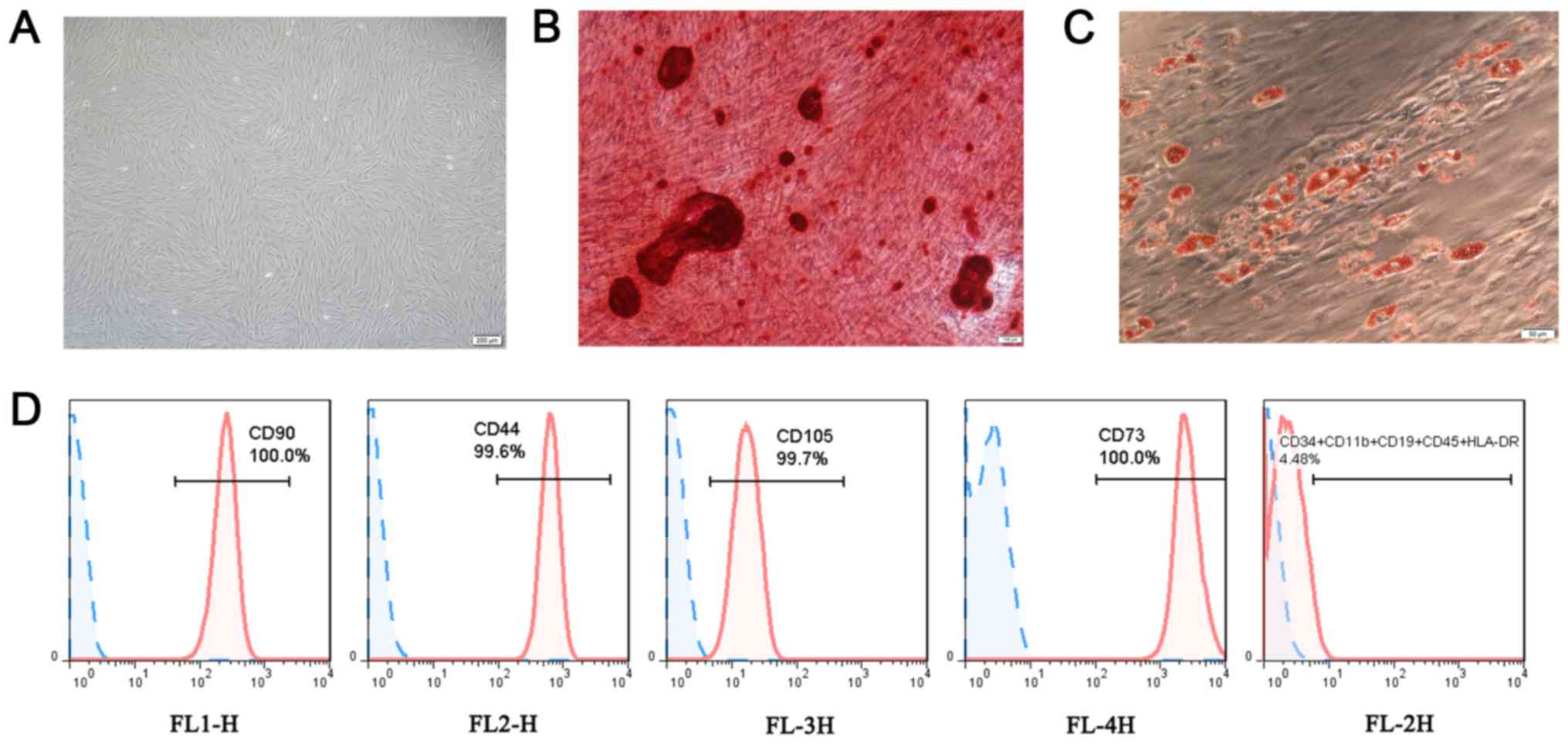 | Figure 1Characterization of PDLSCs. (A)
PDLSCs presented a spindle-shaped morphology (scale bar, 200
µm). (B) Osteogenic differentiation of PDLSCs was
demonstrated as red mineralized nodules (scale bar, 100 µm).
(C) Adipogenic differentiation of PDLSCs was demonstrated as red
oil drops (scale bar, 50 µm). (D) PDLSCs were positive for
CD90, CD44, CD105, CD73 and negative for CD34, CD11b, CD19, CD45
and HLA-DR. PDLSC, periodontal ligament stem cell. |
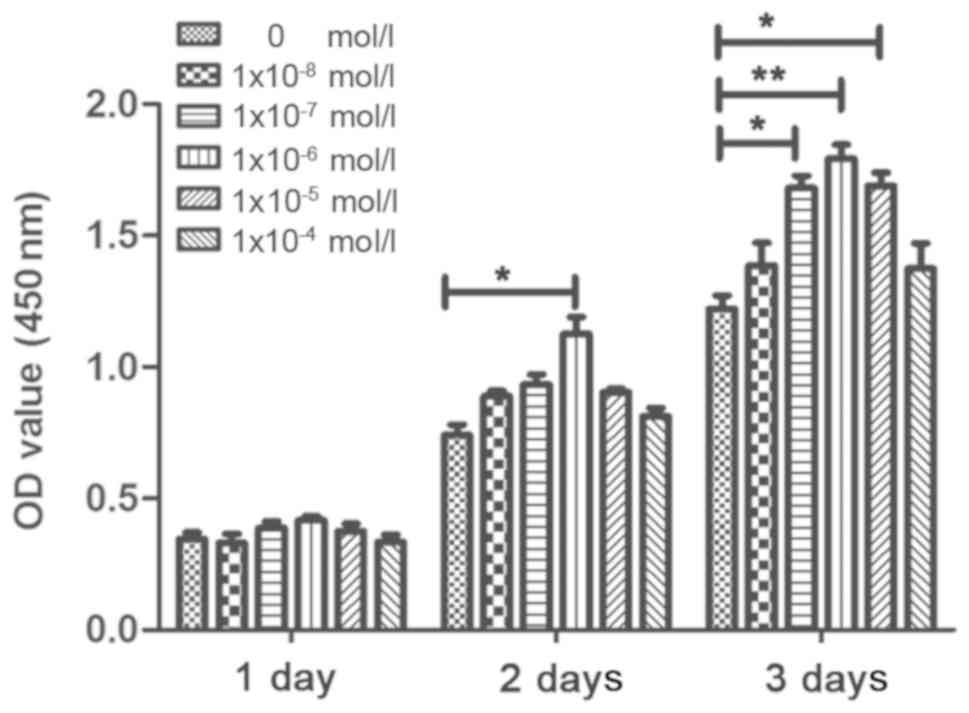
Cell proliferation
CCK-8 was used to determine the effects of rutin on
the proliferation of PDLSCs. In the VC medium with a certain
concentration (20 µg/ml), the PDLSCs were treated with
various concentrations of rutin, which had different effects on the
proliferation of the PDLSCs. The OD values of rutin at
concentrations of 1×10−5, 1×10−6 and
1×10−7 mol/l were higher compared with those of the
control group, which demonstrated that all 3 concentrations
promoted cell proliferation; however, the 1×10−6 mol/l
group promoted the proliferation of PDLSC sheets most significantly
(Figs. 2 and S1A). Therefore, 1×10−6 mol/l
rutin was determined to be the optimal concentration for the
culture of cell sheets.
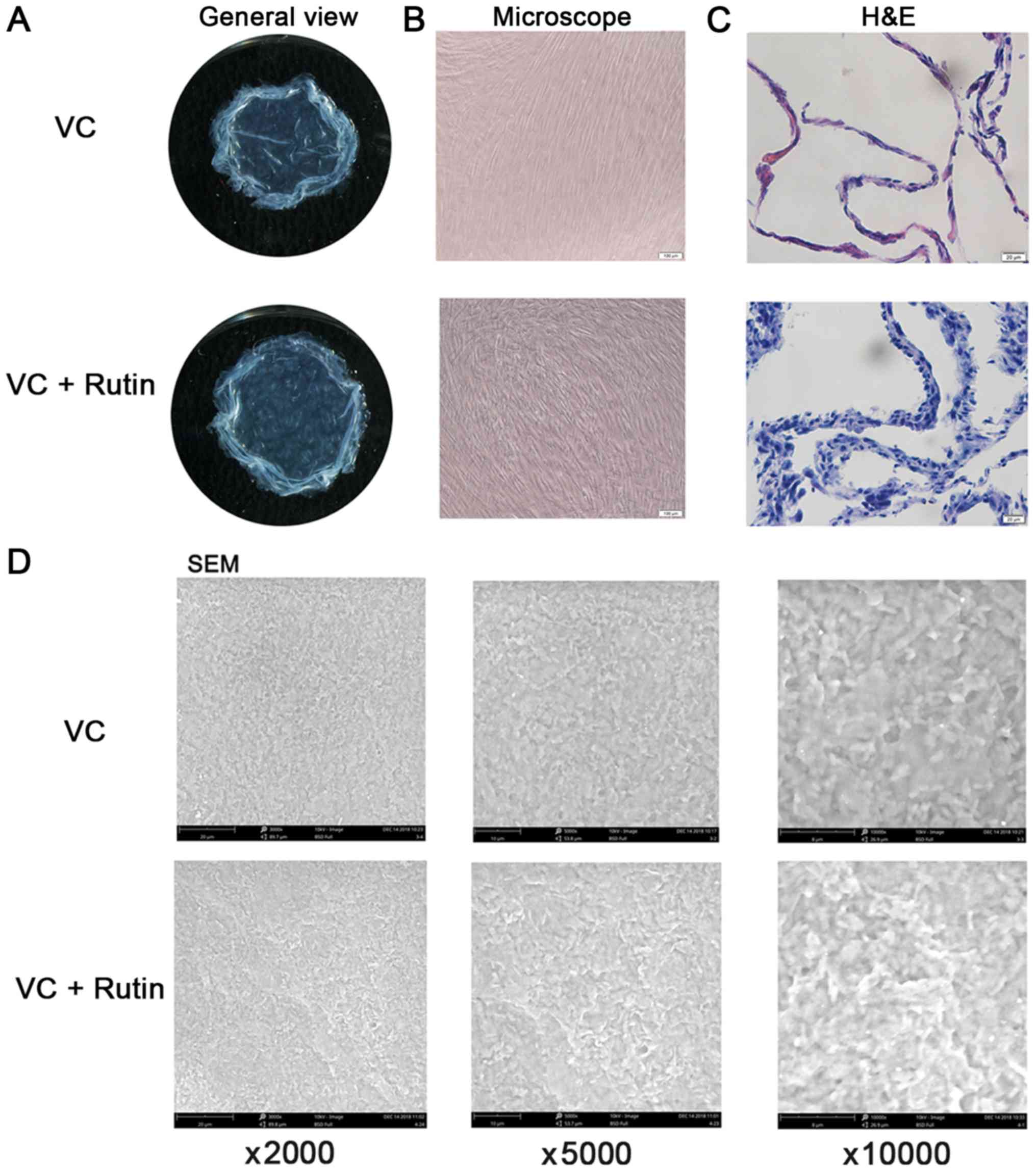
Morphological observation of cell
sheets
Following 10 days of continuous culture, milky white
membrane-like substances were observed at the bottom of the Petri
dish in all groups of PDLSCs. The cell sheets of each group were
carefully peeled off along the petri dish. It was found that the
cell sheets were membrane-like, with a certain thickness and
toughness (Fig. 3A). Under the
inverted microscope, the cells in the rutin-induced group were
denser and thicker (Figs. 3B and
S1B). The results of H&E
staining revealed that the cell membrane was uniform and the
nucleus was stained blue. There was abundant powdered ECM between
cells. In addition, the results revealed that the cell sheet of the
rutin-induced group consisted of 3-4 layers, while that of the
VC-induced group consisted of 1-2 layers (Fig. 3C). The results of SEM revealed
that in the rutin-induced group, the cell sheet cells arranged more
closely and orderly, and the cells extended well and had abundant
ECM (Fig. 3D).
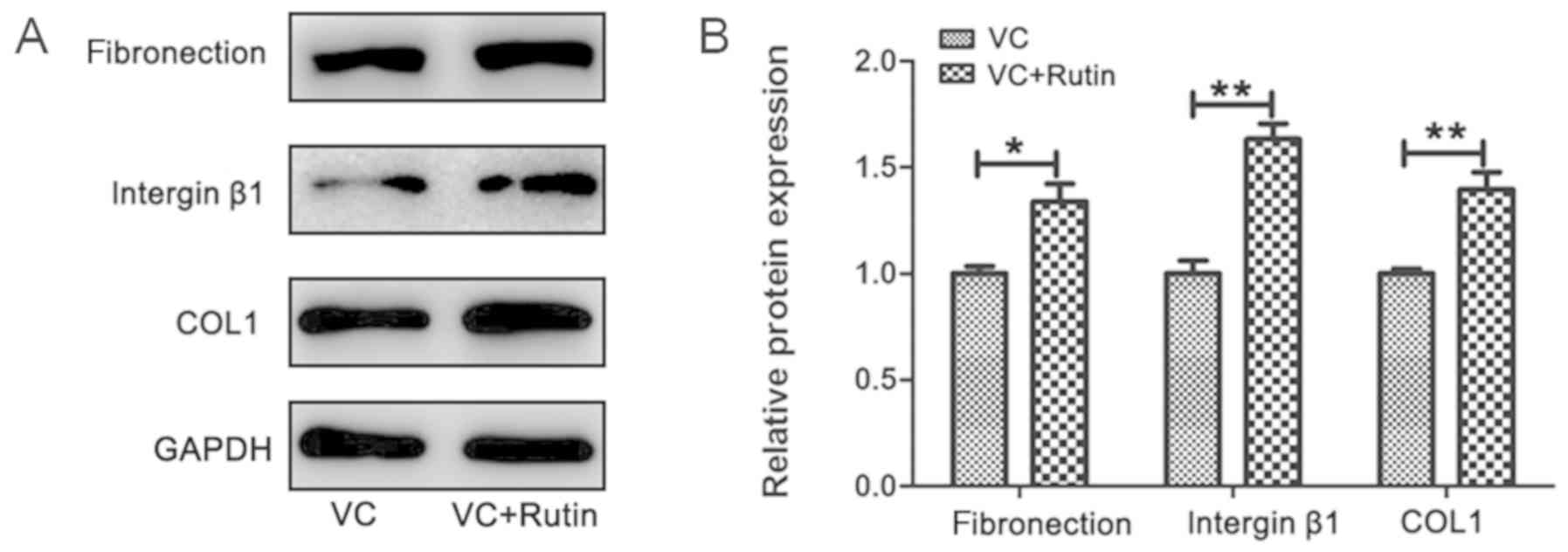
Expression of ECM-related proteins in
PDLSC sheets
Following 10 days of induction, the cell sheets were
formed in the rutin and control groups. The results of western blot
analysis revealed that the protein expression of fibronectin,
integrin β1 and COL1 in the PDLSC sheet was higher in the
experimental group compared with the control group (Fig. 4). Following the addition of rutin,
the expression of ECM-related proteins was increased.
Effects of rutin on the osteogenesis of
PDLSC sheets
In order to further detect the osteogenic effects of
rutin on PDLSC sheets, the PDLSCs were induced in 6-well plates for
10 days with a fixed concentration of 20 µg/ml VC and
10−6 mol/l rutin, and were then induced with various
concentrations of rutin. After 7 days, ALP staining and ALP
activity assay were performed. After 14 days, Alizarin Red staining
and Alizarin Red semi-quantitative assay were performed. ALP
staining (Fig. 5A) and the ALP
activity assay (Fig. 5C) revealed
that 1×10−6 mol/l rutin significantly increased the ALP
activity in PDLSC sheets. The results of Alizarin Red staining
(Fig. 5B) and Alizarin Red
semi-quantitative assay (Fig. 5D)
revealed that the rutin-induced group exhibited an increase in
mineralized nodules, and that rutin at 1×10−6 mol/l
demonstrated the most potent effect. These results suggest that
rutin promotes the osteogenic differentiation of PDLSC sheets, and
that rutin at 1×10−6 mol/l promotes the osteogenic
differentiation of PDLSC sheets most significantly.
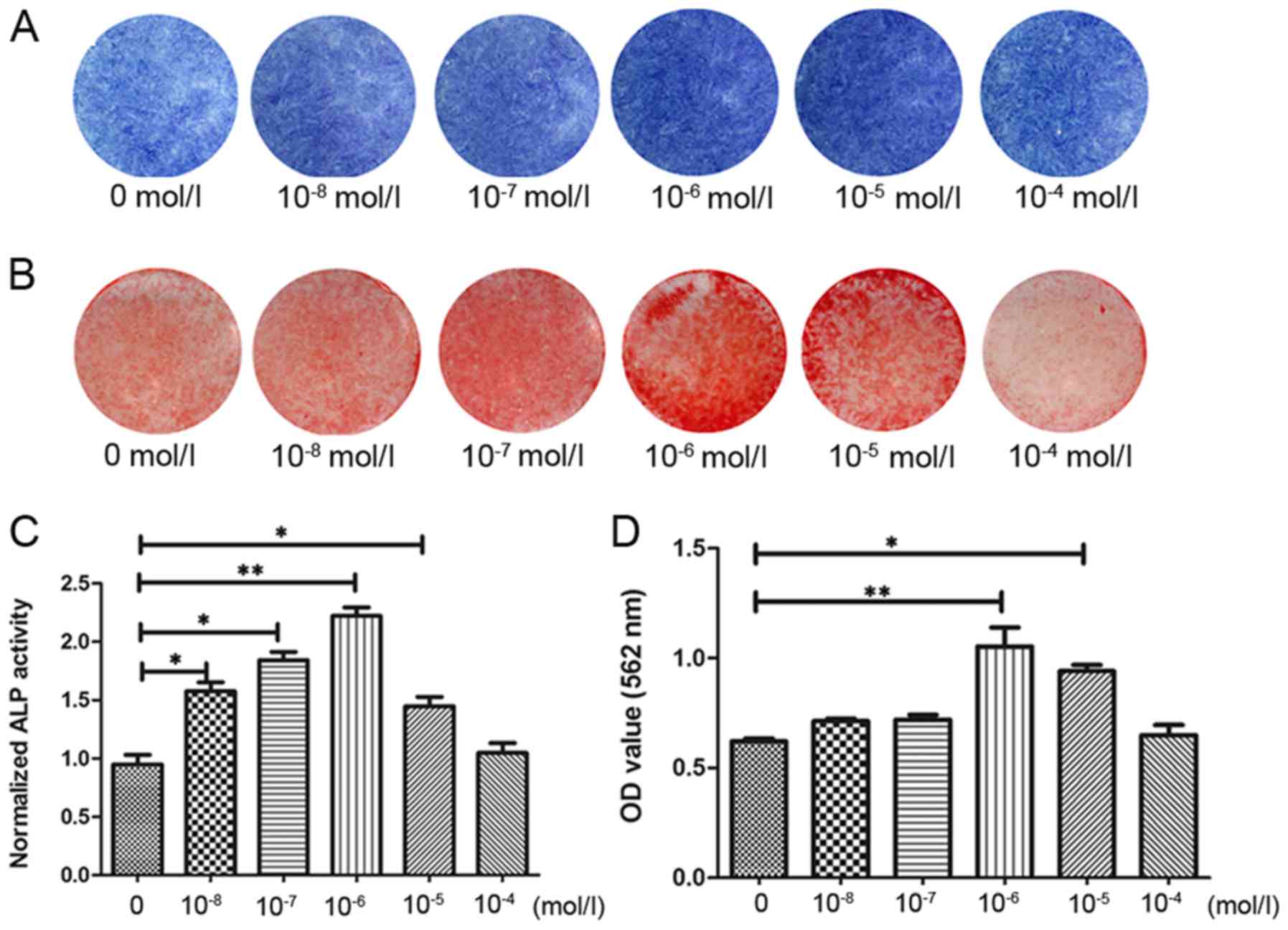 | Figure 5Rutin promotes the osteogenic
differentiation of PDLSC cell sheets. Following the formation of
cell sheets, PDLSCs were cultured with various concentrations of
rutin (0, 1×10−8, 1×10−7, 1×10−6,
1×10−5 and 1×10−4 mol/l) in osteogenic
induction medium. It was found that 1×10−6 mol/l rutin
significantly increased the ALP activity in the PDLSC sheets. (A)
Following osteogenic induction for 7 days, ALP staining was
performed. (B) Following osteogenic induction for 14 days, Alizarin
Red staining was performed. (C) Result of the ALP activity assay
following osteogenic induction for 7 days. (D) Result of Alizarin
Red semi-quantitative analysis after osteogenic induction for 14
days. *P<0.05, **P<0.01. PDLSC,
periodontal ligament stem cell; ALP, alkaline phosphatase. |
Osteogenic-related gene and protein
expression
The expression of osteogenic-related genes and
proteins were detected by RT-qPCR and western blot analysis at 7
and 14 days following osteogenic induction. The results revealed
that the protein (Fig. 6A-C) and
mRNA (Fig. 6D and E) expression
levels of COL1, ALP, RUNX2, and OPN were higher in rutin-induced
group compared with the control group.
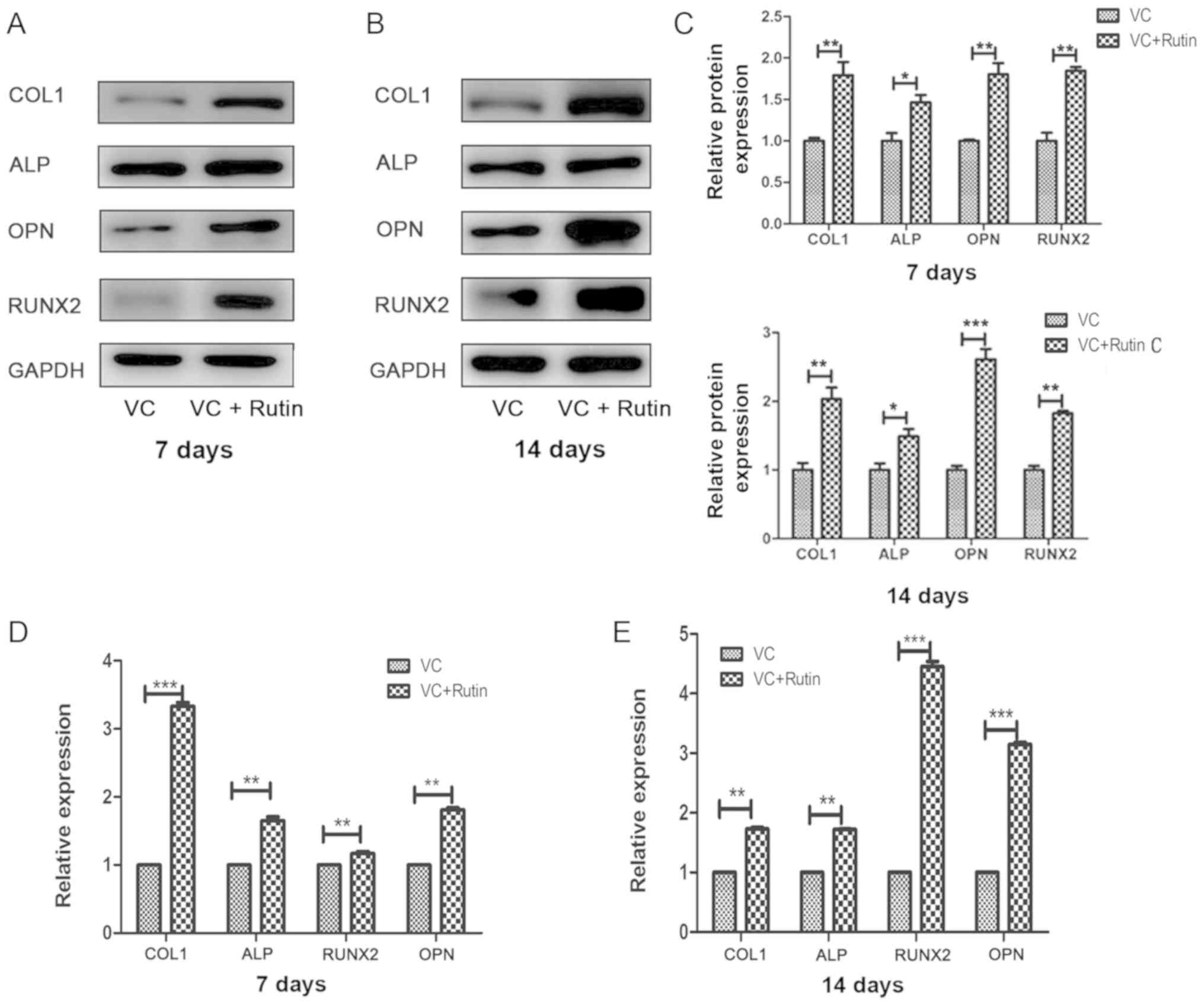 | Figure 6Osteogenic gene and protein
expression. The protein and mRNA expression of COL1, ALP, OPN and
RUNX2 in PDLSC sheets of the rutin and control groups following
osteogenic induction for 7 and 14 days. The protein expression of
COL1, ALP, RUNX2 and OPN after osteogenic induction for (A) 7 days
and (B) 14 days. (C) Relative quantitative analysis of COL1, ALP,
RUNX2 and OPN proteins on days 7 and 14. The mRNA expression of
COL1, ALP, RUNX2 and OPN after osteogenic induction for (D) 7 days
and (E) 14 days. *P<0.05, **P<0.01,
***P<0.001. PDLSC, periodontal ligament stem cell;
COL1, collagen, type I; ALP, alkaline phosphatase; RUNX2,
runt-related transcription factor 2; OPN, osteopontin; VC, vitamin
C. |
Discussion
In recent years, cell sheet technology has widely
been used in periodontal tissue regeneration and has achieved good
results (39,40). In the present study, we designed
rutin-modified PDLSC sheets for periodontal tissue regeneration
in vitro. First, PDLSCs were isolated and identified
according to previously published articles (14,37,41). Multilineage differentiation
potential is one of the distinguishing features of MSCs. Thus far,
a number of studies have used the osteogenic induction method and
adipogenic induction method to detect the multiple differentiation
ability of MSCs (36,42). In the current study, the ability
of PDLSCs to differentiate into osteoblasts was demonstrated by
Alizarin Red staining. On the other hand, the ability of PDLSCs to
differentiate into adipocytes was demonstrated by Oil Red O
staining. In flow cytometric analysis, the isolated cells highly
expressed CD90 FITC, CD105 PerCP-Cy5.5, CD73 APC and CD44 PE, and
almost did not express CD34 PE, CD11b PE, CD19PE, CD45 PE and
HLA-DR PE, which was consistent with the standards formulated by
the International Society for Cellular Therapy (43). Additionally, the results of the
present study were consistent with those of previous studies
(35,42,44). Thus, the present study
successfully obtained PDLSCs.
Developing and optimizing cell sheet technology can
help promote periodontal regeneration via stem-cell therapy. In
order to obtain more efficient cell sheets, bioactive substances
can be added to the sheet culture medium to promote the function of
PDLSCs. Previous studies have demonstrated that rutin can enhance
the effects of vitamin C (31,45). Applying this characteristic to
stem cell sheet formation may contribute to cultivating cell
sheets. The results of the present study first confirmed that a
combination of rutin at 1×10−6 mol/l and VC (20
µg/ml) effectively promoted the formation of PDLSC sheets,
and the proliferation of PDLSCs was accelerated in the
rutin-induced group. However, a perfect cell sheet not only needs
to form rapidly, but also requires a certain thickness, toughness
and abundant ECM. Therefore, H&E staining, SEM observation and
ECM-related protein detection were used to observe the effects of
rutin on cell sheet formation. The results revealed that following
10 consecutive days of culture, the cells in each group grew in
multiple layers. H&E staining revealed that the number of cell
layers in the control group was 1-2 layers, while the number of
cell layers in the rutin-induced group was up to 3-4 layers. ECM
was abundant in the cell sheets of the 2 cell groups, and the cell
arrangement was regular. SEM observation revealed cell extension
and cell-to-cell junctions in the 2 groups, and the surface
structure of the rutin-induced cells was more compact. These
results indicated that the addition of rutin further promoted the
formation of cell sheets. In addition, the regulation of the ECM on
cells depends on proteins in the ECM, which determine the shape of
cells, control cell differentiation and participate in cell
migration (46,47). Firstly, fibronectin can promote
the binding of cells to the matrix (48). COL1 and proteoglycan are the basic
skeletons that form a fibrous reticular complex on the cell surface
(49). The majority of the
receptors are membrane integrins, which are connected to the
cytoskeleton proteins in the cell. The ECM connects the
extracellular and intracellular regions via integrin, which is
conducive to the transmission of intracellular and extracellular
signals (50). Therefore, the
expression of the ECM-related proteins, COL1, fibronectin and
integrin β1, was observed in the present study. The results
revealed that rutin promoted the expression of fibronectin, COL1
and integrin β1.
In order to further examine whether rutin can
improve the osteogenic efficiency of stem cell sheets, the
expression of osteogenic-related genes was investigated.
Osteoblasts predominantly express COL1, ALP, RUNX2, OPN and other
ECM-related proteins during cell proliferation, matrix maturation
and mineralization stages. Observing the expression of these marker
genes and protein levels can indicate the differentiation and
maturation stage of MSCs into osteoblasts to a certain extent.
Previous studies have indicated that ALP and RUNX2 are secreted in
the early stages of osteogenic differentiation, and OPN is a
necessary factor for bone calcification and mineralization
(51). The results of the present
study demonstrated that rutin increased the expression of ALP,
RUNX2 and OPN in the cell sheets. Furthermore, ALP staining, the
ALP activity assay, Alizarin Red staining and the Alizarin Red
semi-quantitative results confirmed that rutin enhanced the
osteogenic differentiation ability of PDLSC sheets compared with
those induced with VC alone. Therefore, rutin and vitamin C may
exert a more significant effect in promoting the formation and
osteogenic differentiation of cell sheets. The present experimental
results confirmed that rutin, a bioflavonoid, can effectively
enhance the effects of VC and accelerate the formation of cell
sheets. This induction method may provide higher quality and more
effective cell sheets.
In conclusion, the results of the present study
demonstrated that rutin, a natural bioflavonoid, promotes the
formation of PDLSC sheets and bone regeneration, which is expected
to become an important tool in the improvement and optimization of
cell sheet technology. The method based on ruitn and VC
co-treatment is effective and potentially valuable. Therefore, the
present study provides a theoretical basis for the future
application of periodontal ligament stem cell sheet in the study of
periodontal tissue regeneration, and provides a novel proposition
for tissue engineering to regenerate periodontal tissue.
Supplementary Data
Acknowledgments
The authors would like to thank the Director of
Shandong Provincial Key Laboratory of Oral Tissue Regeneration for
providing technical support.
Funding
The present study was supported by the Construction
Engineering Special Fund of Taishan Scholars (grant no.
ts201511106).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article or are available from the
corresponding author on reasonable request.
Authors' contributions
XX designed the experiments. BZ wrote the
manuscript. BZ and YX performed the experiments. BZ and YZ analyzed
the data. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
This study was approved by the Medical Ethical
Committee of School of Stomatology, Shandong University (Shandong,
China). Informed consent was obtained in writing by all donors and
their parents.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Scapoli L, Girardi A, Palmieri A,
Martinelli M, Cura F, Lauritano D and Carinci F: Quantitative
analysis of periodontal pathogens in periodontitis and gingivitis.
J Biol Regul Homeost Agents. 29(Suppl 1): 101–110. 2015.PubMed/NCBI
|
|
2
|
Chen FM, Zhang J, Zhang M, An Y, Chen F
and Wu ZF: A review on endogenous regenerative technology in
periodontal regenerative medicine. Biomaterials. 31:7892–7927.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chen FM and Jin Y: Periodontal tissue
engineering and regeneration: Current approaches and expanding
opportunities. Tissue Eng Part B Rev. 16:219–255. 2010. View Article : Google Scholar
|
|
4
|
Matsuda N, Shimizu T, Yamato M and Okano
T: Tissue Engineering Based on Cell Sheet Technology. Adv Mater.
19:3089–3099. 2007. View Article : Google Scholar
|
|
5
|
Nakamura A, Akahane M, Shigematsu H,
Tadokoro M, Morita Y, Ohgushi H, Dohi Y, Imamura T and Tanaka Y:
Cell sheet transplantation of cultured mesenchymal stem cells
enhances bone formation in a rat nonunion model. Bone. 46:418–424.
2010. View Article : Google Scholar
|
|
6
|
Sukho P, Cohen A, Hesselink JW,
Kirpensteijn J, Verseijden F and Bastiaansen-Jenniskens YM: Adipose
Tissue-Derived Stem Cell Sheet Application for Tissue Healing In
Vivo: A Systematic Review. Tissue Eng Part B Rev. 24:37–52. 2018.
View Article : Google Scholar
|
|
7
|
Matsuura K, Utoh R, Nagase K and Okano T:
Cell sheet approach for tissue engineering and regenerative
medicine. J Control Release. 190:228–239. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Miyagawa S, Saito A, Sakaguchi T,
Yoshikawa Y, Yamauchi T, Imanishi Y, Kawaguchi N, Teramoto N,
Matsuura N, Iida H, et al: Impaired myocardium regeneration with
skeletal cell sheets - a preclinical trial for tissue-engineered
regeneration therapy. Transplantation. 90:364–372. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Itaba N, Matsumi Y, Okinaka K, Ashla AA,
Kono Y, Osaki M, Morimoto M, Sugiyama N, Ohashi K, Okano T, et al:
Human mesenchymal stem cell-engineered hepatic cell sheets
accelerate liver regeneration in mice. Sci Rep. 5:161692015.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Nishida K, Yamato M, Hayashida Y, Watanabe
K, Yamamoto K, Adachi E, Nagai S, Kikuchi A, Maeda N, Watanabe H,
et al: Corneal reconstruction with tissue-engineered cell sheets
composed of autologous oral mucosal epithelium. N Engl J Med.
351:1187–1196. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Shimizu K, Ito A, Yoshida T, Yamada Y,
Ueda M and Honda H: Bone tissue engineering with human mesenchymal
stem cell sheets constructed using magnetite nanoparticles and
magnetic force. J Biomed Mater Res B Appl Biomater. 82:471–480.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tsumanuma Y, Iwata T, Washio K, Yoshida T,
Yamada A, Takagi R, Ohno T, Lin K, Yamato M, Ishikawa I, et al:
Comparison of different tissue-derived stem cell sheets for
periodontal regeneration in a canine 1-wall defect model.
Biomaterials. 32:5819–5825. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Okano T, Yamada N, Okuhara M, Sakai H and
Sakurai Y: Mechanism of cell detachment from temperature-modulated,
hydrophilic-hydrophobic polymer surfaces. Biomaterials. 16:297–303.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang Z, Feng Z, Wu G, Bai S, Dong Y and
Zhao Y: In vitro studies on human periodontal ligament stem cell
sheets enhanced by enamel matrix derivative. Colloids Surf B
Biointerfaces. 141:102–111. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Akahane M, Shimizu T, Kira T, Onishi T,
Uchihara Y, Imamura T and Tanaka Y: Culturing bone marrow cells
with dexamethasone and ascorbic acid improves osteogenic cell sheet
structure. Bone Joint Res. 5:569–576. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Weber P, Bendich A and Schalch W: Vitamin
C and human health - a review of recent data relevant to human
requirements. Int J Vitam Nutr Res. 66:19–30. 1996.
|
|
17
|
Naidu KA: Vitamin C in human health and
disease is still a mystery? An overview. Nutr J. 2:7. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang Y, Sun GF, Jin YP, Jia LH and Wang
Y: Effects of fluoride on proliferation and differentiation of rat
osteoblasts in vitro and the antagonistic action of vitamin C.
Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi. 21:250–252. 2003.In
Chinese.
|
|
19
|
Van Pham P, Tran NY, Phan NL, Vu NB and
Phan NK: Vitamin C stimulates human gingival stem cell
proliferation and expression of pluripotent markers. In Vitro Cell
Dev Biol Anim. 52:218–227. 2016. View Article : Google Scholar
|
|
20
|
Wei F, Qu C, Song T, Ding G, Fan Z, Liu D,
Liu Y, Zhang C, Shi S and Wang S: Vitamin C treatment promotes
mesenchymal stem cell sheet formation and tissue regeneration by
elevating telomerase activity. J Cell Physiol. 227:3216–3224. 2012.
View Article : Google Scholar :
|
|
21
|
Zhou S, Zou Q, Zhang K, Yang R, Zhao W and
Fu Q: A Novel Experimental Study on Establish a Myoblasts
Differentiated Cell Sheet Using Induced Adipose-Derived Stem Cell
Technology. J Biomater Tissue Eng. 7:371–378. 2017. View Article : Google Scholar
|
|
22
|
Zhang G, Wang Z and Yan H: The
relationship between chemical structure and bioactivity of
bioflavonoids. J Biol. 22:4–7. 2005.
|
|
23
|
Sharma S, Ali A, Ali J, Sahni JK and
Baboota S: Rutin: Therapeutic potential and recent advances in drug
delivery. Expert Opin Investig Drugs. 22:1063–1079. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
La Casa C, Villegas I, Alarcón de la
Lastra C, Motilva V and Martín Calero MJ: Evidence for protective
and antioxidant properties of rutin, a natural flavone, against
ethanol induced gastric lesions. J Ethnopharmacol. 71:45–53. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Guardia T, Rotelli AE, Juarez AO and
Pelzer LE: Anti-inflammatory properties of plant flavonoids.
Effects of rutin, quercetin and hesperidin on adjuvant arthritis in
rat. Farmaco. 56:683–687. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Diwan V, Brown L and Gobe GC: The
flavonoid rutin improves kidney and heart structure and function in
an adenine-induced rat model of chronic kidney disease. J Funct
Foods. 33:85–93. 2017. View Article : Google Scholar
|
|
27
|
Piller NB: A comparison of the
effectiveness of some anti-inflammatory drugs on thermal oedema. Br
J Exp Pathol. 56:554–560. 1975.PubMed/NCBI
|
|
28
|
Hosseinzadeh H and Nassiri-Asl M: Review
of the protective effects of rutin on the metabolic function as an
important dietary flavonoid. J Endocrinol Invest. 37:783–788. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hyun H, Park H, Jeong J, Kim J, Kim H, Oh
HI, Hwang HS and Kim HH: Effects of Watercress Containing Rutin and
Rutin Alone on the Proliferation and Osteogenic Differentiation of
Human Osteoblast-like MG-63 Cells. Korean J Physiol Pharmacol.
18:347–352. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Crampton EW and Lloyd LE: A quantitative
estimation of the effect of rutin on the biological potency of
vitamin C. J Nutr. 41:487–498. 1950. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Guo R, Wei P and Liu W: Combined
antioxidant effects of rutin and vitamin C in Triton X-100
micelles. J Pharm Biomed Anal. 43:1580–1586. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Alsaif MA: Beneficial effects of rutin and
vitamin C coadminis-tration in a streptozotocin-induced diabetes
rat model of kidney nephrotoxicity. Pak J Nutr. 8:745–754. 2009.
View Article : Google Scholar
|
|
33
|
Alsaif MA: Combined treatment of rutin and
vitamin C improves the antioxidant status in streptozotocin-induced
diabetic rats. J Med Sci. 9:1–9. 2009. View Article : Google Scholar
|
|
34
|
Seo BM, Miura M, Gronthos S, Bartold PM,
Batouli S, Brahim J, Young M, Robey PG, Wang CY and Shi S:
Investigation of multi-potent postnatal stem cells from human
periodontal ligament. Lancet. 364:149–155. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wada N, Menicanin D, Shi S, Bartold PM and
Gronthos S: Immunomodulatory properties of human periodontal
ligament stem cells. J Cell Physiol. 219:667–676. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yin L, Cheng W, Qin Z, Yu H, Yu Z, Zhong
M, Sun K and Zhang W: Effects of Naringin on Proliferation and
Osteogenic Differentiation of Human Periodontal Ligament Stem Cells
In Vitro and In Vivo. Stem Cells Int. 2015:7587062015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Li M, Feng C, Gu X, He Q and Wei F: Effect
of cryopreservation on proliferation and differentiation of
periodontal ligament stem cell sheets. Stem Cell Res Ther.
8:772017. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
39
|
Panduwawala CP, Zhan X, Dissanayaka WL,
Samaranayake LP, Jin L and Zhang C: In vivo periodontal tissue
regeneration by periodontal ligament stem cells and endothelial
cells in three-dimensional cell sheet constructs. J Periodontal
Res. 52:408–418. 2017. View Article : Google Scholar
|
|
40
|
Hu J, Cao Y, Xie Y, Wang H, Fan Z, Wang J,
Zhang C, Wang J, Wu CT and Wang S: Periodontal regeneration in
swine after cell injection and cell sheet transplantation of human
dental pulp stem cells following good manufacturing practice. Stem
Cell Res Ther. 7:1302016. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Kim JH, Ko SY, Lee JH, Kim DH and Yun JH:
Evaluation of the periodontal regenerative properties of patterned
human periodontal ligament stem cell sheets. J Periodontal Implant
Sci. 47:402–415. 2017. View Article : Google Scholar
|
|
42
|
Deng C, Sun Y, Liu H, Wang W, Wang J and
Zhang F: Selective adipogenic differentiation of human periodontal
ligament stem cells stimulated with high doses of glucose. PLoS
One. 13:e01996032018. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Dominici M, Le Blanc K, Mueller I,
Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A,
Prockop Dj and Horwitz E: Minimal criteria for defining multipotent
mesenchymal stromal cells. The International Society for Cellular
Therapy position statement. Cytotherapy. 8:315–317. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Xing Y, Zhang Y, Jia L and Xu X:
Lipopolysaccharide from Escherichia coli stimulates osteogenic
differentiation of human periodontal ligament stem cells through
Wnt/β-catenin-induced TAZ elevation. Mol Oral Microbiol.
34:2019.
|
|
45
|
Je HD, Shin CY, Park SY, Yim SH, Kum C,
Huh IH, Kim JH and Sohn UD: Combination of vitamin C and rutin on
neuropathy and lung damage of diabetes mellitus rats. Arch Pharm
Res. 25:184–190. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Adams JC and Watt FM: Regulation of
development and differentiation by the extracellular matrix.
Development. 117:1183–1198. 1993.PubMed/NCBI
|
|
47
|
Raines EW: The extracellular matrix can
regulate vascular cell migration, proliferation, and survival:
Relationships to vascular disease. Int J Exp Pathol. 81:173–182.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Thant AA, Nawa A, Kikkawa F, Ichigotani Y,
Zhang Y, Sein TT, Amin AR and Hamaguchi M: Fibronectin activates
matrix metal-loproteinase-9 secretion via the MEK1-MAPK and the
PI3K-Akt pathways in ovarian cancer cells. Clin Exp Metastasis.
18:423–428. 2000. View Article : Google Scholar
|
|
49
|
Soikkeli J, Podlasz P, Yin M, Nummela P,
Jahkola T, Virolainen S, Krogerus L, Heikkilä P, von Smitten K,
Saksela O, et al: Metastatic outgrowth encompasses COL-I, FN1, and
POSTN up-regulation and assembly to fibrillar networks regulating
cell adhesion, migration, and growth. Am J Pathol. 177:387–403.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Wilkins JA, Stupack D, Stewart S and
Caixia S: Beta 1 integrin-mediated lymphocyte adherence to
extracellular matrix is enhanced by phorbol ester treatment. Eur J
Immunol. 21:517–522. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Deng Y, Wu S, Zhou H, Bi X, Wang Y, Hu Y,
Gu P and Fan X: Effects of a miR-31, Runx2, and Satb2 regulatory
loop on the osteogenic differentiation of bone mesenchymal stem
cells. Stem Cells Dev. 22:2278–2286. 2013. View Article : Google Scholar : PubMed/NCBI
|