Precision medicine has evolved in recent years
allowing the incorporation of novel taxonomies and stratification
of patients, and using standardized clinical endpoints, genetic and
other biomarker information (1).
Its role in paediatric healthcare involves the selection of
targeted diagnostic, therapeutic and prevention strategies matched
to precise molecular, epidemiological and clinical profile of each
patient; the management of respiratory syncytial virus (RSV)
infection represents a good paradigm of precision medicine
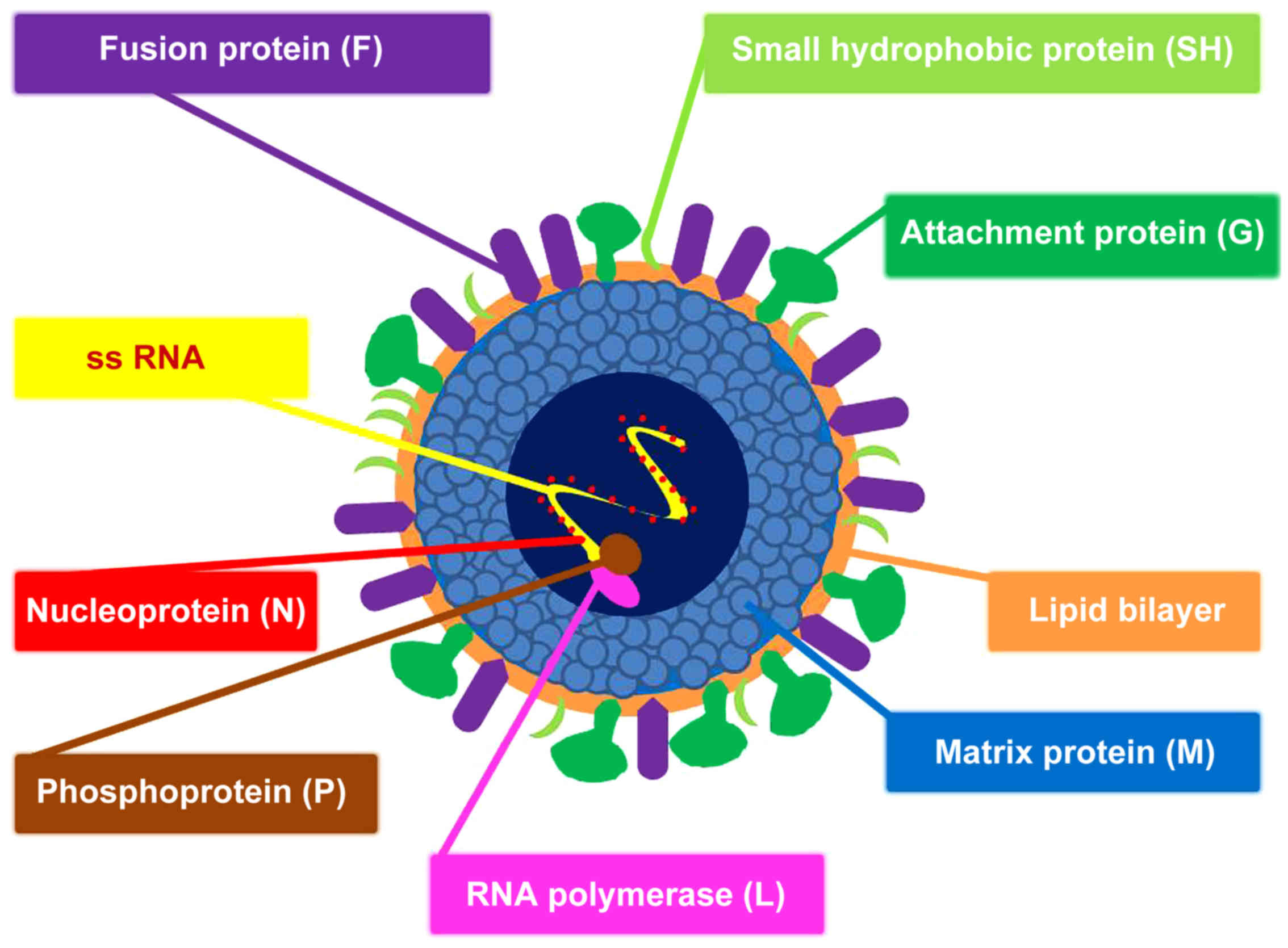
(2). RSV is a single-stranded RNA
virus (Figs. 1 and 2), which represents the most frequent
viral cause of acute lower respiratory tract infection (ALRTI) in
infants, with a worldwide distribution and seasonal occurrence
(2–5). It was first isolated in 1956 from
nasal secretions of chimpanzees with rhinorrhea and coryza; the
novel virus was initially named 'Chimpanzee coryza agent' (CCA)
(6,7). In the following year, when CCA was
also isolated from children with ALRTI, it gained its final name
due to the syncytia observed on electron microscopy; syncytia are
formed by fusion of infected host cells with neighboring cells
leading to the formation of multi-nucleate enlarged cells. Although
the formation of syncytia is the hallmark of the cytopathic effect
of RSV that is associated with host cellular membrane merging,
syncytia are not pathognomonic of RSV (8). Syncytia are also observed in cell
culture with several other viruses, such as parainfluenza, HSV-1,
HIV and MeV. Recently, the International Committee on Taxonomy of
Viruses (ICTV), which authorizes and organizes the classification
and naming of viral species, grouped RSV under the genus
Orthopneumovirus within the family Pneumoviridae (9,10).
Bronchiolitis is the most common clinical
manifestation of RSV infection in infants and although it is
usually self-limiting, in infancy it accounts for a significant
number of hospitalizations and paediatric intensive care unit
(PICU) admissions (3). Despite
its association with relatively high morbidity and mortality in
premature neonates and in certain paediatric populations with
underlying conditions, such as immuno-deficiency and congenital
heart disease, RSV infection may also lead to hospitalization of
previously healthy, full-term infants (11,12). RSV-positive bronchiolitis is
characterized by airway inflammation and oedema, mucus production
and debris leading to airway obstruction and turbulent gas flow.
Even though various therapeutic interventions have been tried, such
as bronchodilators, hypertonic saline and corticosteroids,
supportive care remains the mainstay in most settings, with gentle
suctioning of nasal secretions, prone position, fluid replacement
and oxygen or respiratory support, as necessary. Several clinical
trials on the management and prevention of RSV-positive
bronchiolitis have been recently completed, are underway, or in
development (3). Currently, there
is rapid expansion of RSV vaccine candidate development and there
is hope that one will become available in the near future. Of
course, the safety of vaccines proposed for primary immunization in
an antigen naïve child remains the top priority.
This review article summarizes the key messages of
the plenary lectures, oral presentations and posters of the '5th
Workshop on Paediatric Virology' held in Sparta (Greece) on October
12th, 2019, which was focused on RSV (Table I). This workshop was organized by
the Paediatric Virology Study Group (PVSG) and was co-chaired by Dr
Simon B. Drysdale, Consultant and Honorary Senior Lecturer in
Paediatric Infectious Diseases at St. George's University Hospital
NHS Foundation Trust and St. George's, University of London
(London, UK), Professor Barbara Rath, Co-founder and Chair of the
Vienna Vaccine Safety Initiative (Berlin, Germany), Honorary
Professor of Nottingham School of Medicine (Nottingham, UK) and
Research Director at the University of Bourgogne-Franche-Comté
(Besançon, France), Professor Maria Theodoridou, Professor Emerita
of Paediatrics at the University of Athens School of Medicine
(Athens, Greece), President of the National Immunisation Committee
and f. President of the Hellenic Paediatric Infectious Diseases
Society, Dr Georgia Papaioannou, Head of the Paediatric Radiology
Department at 'Mitera' Children's Hospital in Athens (Greece), Dr
Ioannis N. Mammas, Consultant Paediatrician on the island of Euboea
(Greece) and Coordinator of the PVSG and Professor George P.
Chrousos, Professor Emeritus of Paediatrics and Endocrinology at
the University of Athens School of Medicine (Athens, Greece). The
workshop was held under the auspices of the World Academy of
Sciences (WAS) and was supported by the Laboratory of Clinical
Virology of the University of Crete School of Medicine and the
First Department of Paediatrics of the University of Athens School
of Medicine.
RSV was not recognized as a potentially serious
problem in older adults until the 1970s, when outbreaks of the
virus occurred in long-term care facilities for the elderly
(31-33). Since then, additional studies in
hospitalized adults have suggested that RSV may be an important
cause of illness in adults. Molecular diagnostics suggest that RSV
positive specimens are commonly identified in elderly and high-risk
adults, in a frequency similar to that of seasonal influenza
(31,34). Even though a positive RSV
respiratory panel does not equate pathogenesis, it has been
suggested that RSV may account for as much as 10,000 deaths
annually in the United States among individuals above the age of 65
years (31-33,35). This, in addition to the morbidity
in infants, has stimulated interest in RSV vaccines and antiviral
agents. Additional natural history studies are needed to better
understand the actual burden of RSV infection among the elderly and
high-risk adults.
Maternal antibodies may be able to mitigate RSV
disease severity in young infants (17,36-38). It is assumed that the
transplacental passage of RSV-specific antibodies occurs
predominately during the third trimester of pregnancy. High titers
can potentially protect term infants up to four months of age
(38). In premature infants,
passive immunity may be 'compromised' (39), but still has a role to play. The
degree to which breast feeding may also contribute to passive
immunity and to priming of immune system is currently under
investigation (40). Once
infection is established, the innate immune system plays a dual
role in lowering the viral load and in mounting a secondary immune
response. Prematurity and other conditions that compromise the
immune response may lead to reduced levels of antiviral cytokines,
such as the interferons (41). In
infants, reduced signaling by TLRs and altered antigen-presenting
cell functions, including low interleukin (IL)-12 and enhanced IL-6
and IL-23 production, coupled with reduced activation of regulatory
T cells, may result in an adaptive response that is skewed toward
Th2 and Th17 and away from protective Th1 and CTL responses
(42). The potential specific
role(s) of certain pattern recognition receptors in humans has/ve
been suggested by the fact that certain TLR missense mutations are
associated to a phenotype with propensity to wheezing (43). Apart from the individual genetics
(44), the stage of lung
maturation among term and premature infants also impacts on the Th1
to Th2 switch. In the case of prematurity, the mucosa prior to
alveolarization is being deluged with Th2 inflammatory responses,
even in the earlier stages of bronchopulmonary dysplasia (45). Impaired Th1 activation, coupled
with little or no B cell memory, and inhibition of antibody
production by IFNγ, produces low‑titer, low‑affinity antibody
(46). The result may be a poorly
protective and dysregulated defense mechanism that leads to
bronchiolitis in susceptible infants (43). Later, the host immune response is
permanently oriented to the direction of wheezing exacerbations,
specifically triggered by RSV (47).
MicroRNAs (miRNAs) are involved in
post-transcriptional gene regulation and play significant roles in
the maintenance of the airway epithelial barrier of the respiratory
tract (48-56). miRNAs have been implicated in the
modulation of antiviral defense mounted by host innate and adaptive
immunity, involving not only immune effector and inflammatory
cells, but also parenchymal cells (50). Respiratory viruses, including RSV,
attack, as a primary target, the epithelial cells of the
respiratory tract causing an altered expression of distinct miRNAs
in the airway cells. The human innate immune response inhibits RSV
replication early after inoculation, mainly through the action of
interferons (53). Multiple
miRNAs are induced by infection in a cell‑type‑specific fashion
(51). RSV appears to alter host
cell gene expression also through regulation of expression of
miRNAs related to the interferon response (50,53). Abnormal expression of miRNAs has
been detected in both peripheral blood cells and airway epithelial
cells in RSV-infected infants (54). Understanding alterations in miRNA
expression profiles and identifying miRNA target genes in relation
to the pathogenesis of RSV may help clarify the mechanisms of
virus-host interactions, and immune dysfunction leading to airway
hyper-reactivity and chronic respiratory diseases, such as asthma
(49,50,53,54). There are several methods for the
purification, quantification and characterization of miRNA
expression profiles in biofluids, whole blood samples and tissue
samples obtained in in vivo studies (55). Further research on miRNAs is
expected to clarify their value as biomarkers of RSV infections and
their sequelae (i.e., recurrent wheezing and asthma).
The most frequent causes of secondary thrombocytosis
in childhood are acute respiratory tract infections (57-61). To date, several authors have
reported significantly higher mean platelet counts in patients with
RSV than in patients with other acute respiratory tract infections
(61-64). Thrombocytosis is more likely to
occur in younger patients, who have clinical manifestations of
wheezing and dyspnoea (62,64). Moreover, thrombocytosis has been
suggested as an early marker of RSV infection (62). Excessive thrombocytosis has also
been detected at an early stage in cases of RSV-positive
bronchiolitis (65). It has been
proposed that thrombocytotic patients have a more severe clinical
course and longer duration of hospitalization and that the platelet
count may be a useful clinical marker associated with ALRTI
severity (61,64,66,67). Conversely, other authors have
found that platelet counts do not correlate with disease severity
and clinical outcome (68,69).
Routine prophylactic anti-platelet treatment or further
investigations are not necessary in children with RSV-positive
bronchiolitis and thrombocytosis (61,68,70).
There is compelling evidence that infants with
severe RSV infection in the early months of life have a subsequent
increased risk of developing recurrent wheezing and/or asthma, with
a prevalence of up to 30% compared with non-RSV groups (71-75). Whether this association is causal
has been the subject of considerable debate on the potential role
of RSV infection in the pathogenesis of asthma as well as the
impact of asthma predisposition (genetic, environmental exposure,
etc.) on the clinical course of RSV infection. A recent large
retrospective cohort analysis of Australian children born between
2000 and 2010 suggested that different subgroups of high risk
children, who developed RSV disease within the first 2 years of
life, continued to be at elevated risk of having a first asthma
hospitalization beyond the age of 7 years (76). On the other hand, large
epidemiological observational studies demonstrate that the vast
majority of infants hospitalized for RSV bronchiolitis do not fit
into an 'at-risk' group (atopy, family history, etc.), suggesting
that viral or host factors not thought of as classical risk
factors, may play a role in disease severity (74,77). Prospective studies with
RSV-immunoprophylaxis (e.g., palivizumab) suggest that long‑term
effects of RSV prophylaxis appear less efficacious in infants with
a family history of atopy. In addition, palivizumab decreases
parent-reported recurrent wheeze, however the incidence of
physician-diagnosed asthma is similar (78). A recent single-blind, randomized,
placebo-controlled trial showed that RSV prevention in otherwise
healthy preterm infants, did not have a major effect on asthma or
lung function at the age of 6 years (79).
Considering the above findings, perhaps a more
appropriate conclusion would be that RSV infection is important in
the mechanism of wheezing development, at least in the first few
years of life (80). RSV
possesses the ability to counteract host defense systems through
complex mechanisms that facilitate viral replication. This
significant increase in asthma frequency seems to be predominantly
related to long-term changes in neuroimmune control of airway tone
rather than to allergic sensitization. In contrast to RSV
bronchiolitis, atopy has been clearly associated with childhood
asthma development after RSV-induced early wheezing (81,82). High-risk (parental atopy or
asthma) birth cohort studies from Wisconsin, United States, and
Australia have shown that young children suffering from RSV-induced
wheezing episodes are at high risk of developing school-age asthma
(81,82). Further prospective, follow-up
studies are needed to clarify individual and environmental factors
that promote more severe viral illnesses and long-term adverse
respiratory outcome of children hospitalized for severe RSV
infection. Developing a greater understanding of the
pathophysiological mechanisms through which RSV causes recurrent
wheezing/asthma, will lead to an evidence-based prevention strategy
and perhaps reduce the subsequent risk for asthma (71).
The biomarker CCL5 (previously known as RANTES, a
β-chemoattractant for inflammatory cells including T-lymphocyte
subsets), in the nasal epithelium during RSV bronchiolitis, is
strongly predictive of physician-diagnosed asthma (83,87). Furthermore, it has been suggested
that prematurely born infants have a predisposition to RSV
infection-related respiratory morbidity, including subsequent
respiratory dysfunction (44,85). Single-nucleotide polymorphisms in
genes coding for IL-8, IL-19, IL-20, IL-13, mannose-binding lectin,
IFNG and RANTES, have been associated with wheezing following RSV
LRTI in term-born infants (85).
The site of infection might be another important factor related to
asthma risk, thus viral ALRTI in infancy indicates an increased
risk of subsequent asthma, while gastrointestinal infections might
be protective (86). Asthma after
severe RSV bronchiolitis is positively correlated with maternal
asthma, exposure to high levels of dog allergen, aeroallergen
sensitization and recurrent wheezing; day care attendance and white
race have been associated with decreased asthma risk (87). Several host factors, including
respiratory allergy and virus-induced interferon responses, viral
virulence factors, individual risk factors (e.g., young age,
especially the first 6 months of life, small lung size and
genetics), and environmental exposures (e.g., exposure to tobacco
smoke, airway microbiome) modify the risk of virus-induced wheezing
and promote more severe wheezing illnesses and the risk for
progression to asthma (86,88). The anti-RSV mAb palivizumab
decreases the risk of severe RSV-induced illness and subsequent
recurrent wheeze in prematurely born infants (89). Further understanding of the role
of RSV in asthma pathogenesis may help develop vaccines against RSV
as a way of asthma prevention.
Bronchiolitis obliterans (BO) is a chronic and
irreversible lung disease leading to the obstruction and/or
obliteration of the small airways (90). Most cases of BO in children are
post infectious (PIBO) and are mainly associated with adenovirus
infections, although other viruses may also be implicated,
including measles, influenza, parainfluenza and RSV (91-93). An extensive search of the current
literature in the context of the workshop demonstrated that RSV is
detected in children with PIBO with an incidence ranging from 4.3
to 30% (94–103); however, there are only a few
reports of children with PIBO secondary to RSV as a single
infection. This creates skepticism about the aetiological role of
RSV in PIBO. Further research is required to investigate the
potential impact of RSV co-infection in the severity and outcome of
children with PIBO.
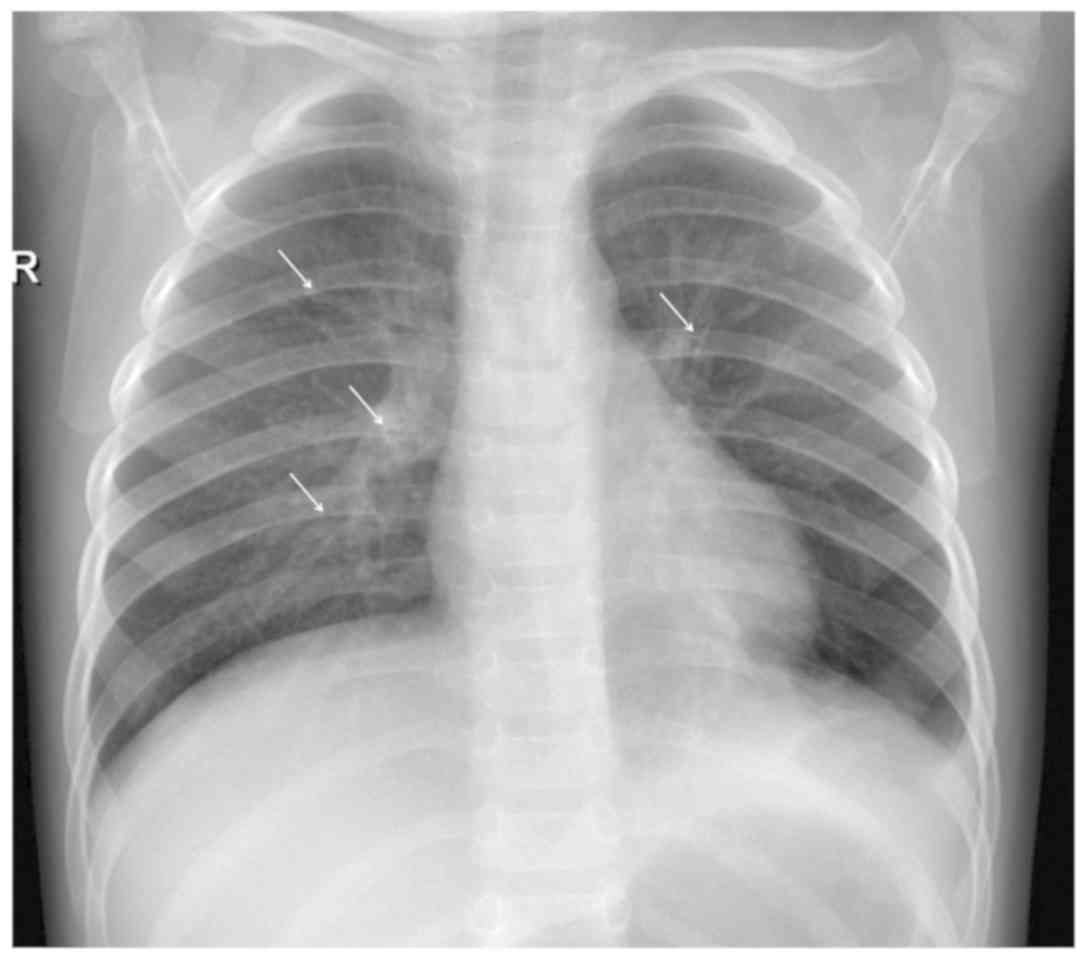
Although imaging cannot confirm the diagnosis of RSV
infection, it is important to identify the possible pattern of
viral disease, in order to avoid unnecessary administration of
antibiotic therapy and to predict possible late effects (Figs. 3-5) (104). The clinical syndrome of
bronchiolitis is commonly diagnosed based on the patient's history
and physical examination; chest radiography is not routinely
recommended to reach the diagnosis due to recommended restriction
of radiation exposure in the paediatric age group (104-106). Chest imaging, however, may be
considered when a child with RSV infection and severe ALRTI is
admitted to intensive care to better under-stand the extent of lung
involvement and atelectasis, which is common in acute RSV infection
(106). It is important to note
that chest radiographs in children with RSV infection may be
entirely normal or reveal non‑specific findings, which are also
encountered in other viral infections: most commonly, perihilar
opacities and hyperinflation, atelectasis and rarely consolidation
and bronchial cuffing or air‑leak (107). Radiography is commonly obtained
to rule out atelectasis and foreign body aspiration. Guidelines
suggest performing a chest radiograph in the presence of
significant respiratory distress or hospitalization (108). In newborns with RSV infection,
the radiological pattern on chest radiography may be a predictor of
clinical outcome (109).
However, it is highlighted that chest radiographs should not be
routinely performed in children with bronchiolitis to avoid
radiation exposure (106). It is
also important to emphasize that chest radiograph is not the right
way to rule out bacterial infection (106); the correct diagnostic approach
for bacterial or ventilator-associated pneumonia in children in the
PICU is to perform respiratory culture or Matrix-assisted laser
desorption ionization time-of-flight (MALDI-TOF) mass spectrometry
from sputum/aspirate or bronchoalveolar lavage (BAL) specimens.
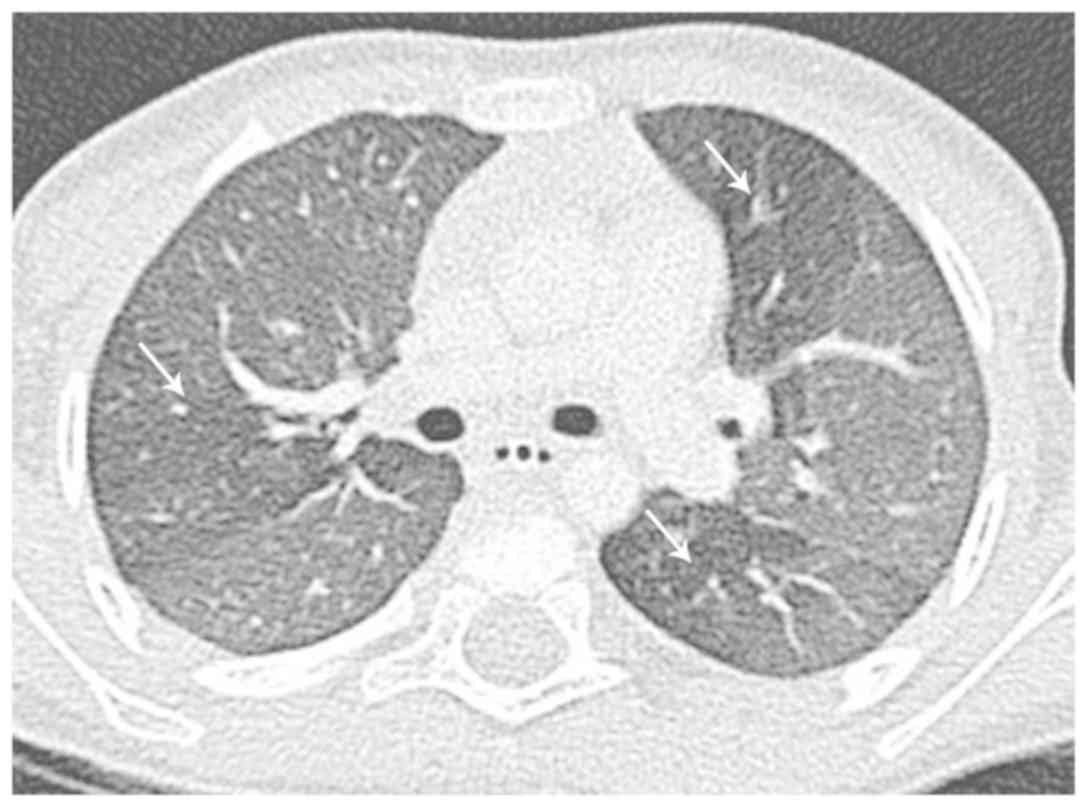
Several studies have revealed that standard
radiological techniques, including computed tomography (CT), are
frequently unable to distinguish between acute bronchiolitis
changes caused by RSV vs. those caused by other respiratory viruses
(104,108). It is interesting that the
radiographic findings, especially in high-resolution computed
tomography (HRCT), reflect the histopathologic changes that RSV
infection provokes: plugging or occlusion of the bronchiolar airway
lumens by sloughed necrotic and irregular epithelium and exudate,
combined with peri‑bronchiolar infiltration and reaction with
inflammatory cells and submucosal oedema. The infiltration is a
combination of neutrophils entering the airway submucosa and
epithelial cell debris in the airway lumens. These cellular
accumulations are likely to result in acute obstruction of the
distal airways, an outcome much more likely to occur in the
extremely narrow bronchioles of infants. Because this is combined
with the inherent loss of mechanical clearance of these small
airways, it likely leads to increased spread of infection,
augmented inflammation and clinical signs of wheezing/obstruction
(110). Consistent with
obstruction, the most common CT findings in RSV pneumonia include
centrilobular nodules, ground-glass opacities, air-space
consolidation, and peribronchial thickening (111). These findings have a bilateral,
usually asymmetric, central and peripheral distribution. Up to 40%
of children with bronchiolitis will develop further wheezing
episodes in the first five years of life. In very severe or
atypical cases, HRCT of the lungs may be required to assess the
extent of bronchial thickening and remodeling, the development of
brochiectases and air-trapping (105).
RSV infection is not always restricted to the
airways. Case reports have also described clinical pictures
resembling viral encephalitis and/or encephalopathic syndromes with
severe sequelae in isolated cases (112). The mechanism of the spread of
the RSV infection to the CNS compartment remains unclear (112). Brain magnetic resonance imaging
(MRI) in infants with CNS involvement has shown predominantly
non-specific findings similar to those also encountered in other
viral and/or limbic system encephalitides (113). In very rare instances,
extra‑pulmonary findings in RSV infection have also included acute
necrotizing encephalopathy (ANE) and acute hepatic failure with
encephalopathy (114).
Clinicians should have a high suspicion of ANE in cases of children
with a respiratory infection and acute neurological
manifestations.
Thus far, ribavirin is the only antiviral agent that
has ever been licensed for the treatment of RSV infection (115). However, its efficacy is not
proven and due to significant toxicity its use has been primarily
restricted to severe cases in immunocompromised patients with
severe RSV-positive ALRTI (116). Several other antiviral
candidates have been developed since, but none have been licensed
as yet. Types of molecules being tested include influenza
antivirals, such as baloxavir, CC-42344, VIS410, immunoglobulin,
hyperimmune plasma, MHAA4549A, pimodivir (JNJ-63623872),
umifenovir, and HA minibinders, RSV antivirals including presatovir
(GS-5806), ziresovir (AK0529), lumicitabine (ALS-008176),
JNJ-53718678, JNJ-64417184, and EDP-938, broad spectrum antivirals
such as favipiravir, VH244, remdesivir, and EIDD-1931/EIDD-2801, as
well as host directed strategies including nitazoxanide, eritoran,
and diltiazem (117-119). Novel molecules disrupt various
stages of the virus life cycle, including cell entry, viral
replication, and polymerization as well as after virus release
through RSV neutralizing anti- or nanobodies (120).
One method used occasionally in phase 2 clinical
testing is human challenge models (115). This method was used in Phase 2a
clinical testing of the non‑fusion inhibitor EDP‑938 (ClinicalTrials.gov Identifier: NCT03691623). All
participants were inoculated with a known strain of RSV and were
then randomized to receive the medication or placebo. The advantage
of this methodology is the removal of the variability in exposure
with natural infection and the collection of samples at precise,
known times after infection, which can aid with the understanding
of the biological mechanisms of the infection and development of
antiviral agents or vaccines (121,122). Due to ethical and technical
constraints, experimental studies are only undertaken in adults,
but if a product is shown to be efficacious at this setting, a
faster move to trials in paediatrics takes place than in
traditional childhood trials.
The current clinical data indicate that RSV disease
dynamics may not be identical in all patients (2,3,115,119). More research is needed to
identify uniform clinical endpoints reflecting how patients
function, thrive, and survive (US Food and Drug Administration) and
to understand inter-individual differences in disease presentation,
with the goal of ultimately selecting the right treatment for the
right patient. A greater understanding of individual differences
may ultimately lead to future personalized treatment strategies.
Individualized approaches and a well-standardized methodology to
assess disease severity at the time of enrolment, as well as during
follow-up visits, will require integration of diagnostic, clinical
and laboratory markers at the point of care (115). Individualized targeted treatment
will constitute an important step in improving outcomes in patients
with RSV infection while minimizing toxicity. A greater
understanding of individual data in newly developed pharmaceutical
agents against RSV will potentially lead to future personalized
treatment regimens. Applying such co-ordinated diagnostic, clinical
and research efforts constitutes an important step in advancing
paediatric care, improving outcomes and limiting global RSV
morbidity and mortality.
Over the last decade, high‑flow nasal cannula (HFNC)
therapy has emerged as a new method to provide respiratory support
in children with RSV-positive bronchiolitis (12,123-126). Its main advantages include its
ease to set up and the fact that it is well tolerated, leading to
better compliance, especially in comparison to other devices of
non-invasive ventilation (125,127). Initially, HFNC was trialed in
infants with moderate to severe bronchiolitis admitted to PICUs,
but nowadays its application has expanded to paediatric wards, even
to emergency departments, in order to avoid a PICU admission
(127,128). Recent data have shown that it
does not significantly reduce time on oxygen compared with standard
therapy, suggesting that early use of HFNC does not modify the
underlying disease process (129). However, the proportion of
children who experience treatment failure is lower in HFNC and many
of those who experience treatment failure on standard therapy can
be rescued by HFNC. Additional studies comparing HFNC with
continuous positive airway pressure (CPAP) in the PICU setting led
to the same conclusion (130,131). Consequently, HFNC may reduce the
need for intubation and invasive respiratory support, thus
potentially lowering costs and adverse effects of mechanical
ventilation, such as ventilator-induced lung injury, infections and
exposure to sedatives. In addition to effectiveness, most studies
have shown no adverse events with HFNC and have concluded that it
is a relative safe method for use even in general wards or
emergency departments (123).
Few cases of pneumothorax have been reported, abdominal distension
has been less significant compared with CPAP, and the majority of
infants have been able to be fed orally or by nasogastric tube
(123,127).
Since RSV-positive bronchiolitis is associated with
airway obstruction and turbulent gas flow, its clinical course can
be improved by Heliox, which facilitates gas flow through
high‑resistance airways (132-138). Heliox is a mixture of
helium-oxygen, which can be administered by all modes of
ventilation in spontaneously breathing patients by face mask, HFNC
or CPAP, and can be adjusted to specific ventilators in intubated
children. Current evidence suggests that the addition of Heliox may
significantly reduce clinical scores evaluating respiratory
distress and the respiratory rate, and may enhance CO2
elimination in the first hour after starting treatment in infants
with acute refractory RSV bronchiolitis (134,139). Recently, Seliem and Sultan
(140) reported that Heliox
results in improvement of oxygenation when used with high flow
nasal cannula in infants with acute RSV bronchiolitis, during the
initial phase of therapy. The combination of Heliox with CPAP also
seems to be beneficial, as the application of CPAP may reduce the
fiO2 needed in these infants. However, no benefit has
been observed in terms of need for intubation and mechanical
ventilation, length of treatment or PICU stay. In addition, its
application in the emergency department does not change the
discharge rate (138,139). More clinical trials are needed
to define the population that may respond to Heliox and its place
in the therapeutic regimens of RSV bronchiolitis.
The prevention of RSV morbidity and mortality
remains a global healthcare priority (115,141). According to the World Health
Organization (WHO), the strategic focus for the prevention of RSV
infection in children and adults includes the passive
administration of immunoglobulins, as well as active immunization.
Passive immunization is currently the only option available to
infants less than 6 months of age, which can be achieved through
administration of antibodies to the infant or through active
immunization of the mother during pregnancy. Passive immunity wanes
fast over time, thus, active immunization is the preferred approach
for infants above six months of age, as well as older children and
adults, including the elderly (141). To date, there is only one
product available for prevention of RSV infection, palivizumab, the
monoclonal antibody (mAb) that has been shown to reduce hospital
admission due to RSV infection in some high-risk infants by up to
80% (142). It is expensive and,
thus, reserved for high risk infants, mainly in high income
countries.
While antibodies are costly and transitory in their
effect, active vaccination would represent the most cost-effective
approach for the prevention of RSV infections and their
transmission to high-risk individuals (115,141). Up to date, several vaccine
candidates are in development, but none have reached licensure yet
(143,144). One of the main barriers for the
development of RSV vaccines has been the fact that the majority of
severe cases in infants occur within the first three months of
life, i.e. at a time when active immunization is not really
possible (145). Additional
caution has been employed during vaccine design because of the
failure of a historical vaccine [formalin inactivated RSV
(FI-RSV)], which triggered a severe adverse effect, enhanced
respiratory disease (ERD) (146). For more than 50 years
live-attenuated vaccine approaches have been unsuccessful because
of the difficulty in balancing immunogenicity and vaccine safety.
It is worth noting that only live-attenuated vaccines have been
tested for active infant immunization.
Recent breakthroughs in determining the structure
and antigenic content of the RSV fusion (F) glycoprotein has
enhanced interest in vaccine development research (115,141,147,148). The general approaches to vaccine
development include engineered viruses that use knowledge of RSV
gene function, naturally attenuated chimeric virus combining genes
from RSV-related viruses, viral vectors encoding RSV surface
antigens, and nucleic acid vaccines using plasmid DNA or messenger
RNA encoding RSV antigens (149,150). As of August 2019, 43 RSV
vaccines were in development (151). Of these, 21 are in clinical
trials in humans; 14 in Phase 1, five in Phase 2 and two (one just
completed) in Phase 3. Twelve vaccines are in trials in children,
four in pregnant women and 10 in older adults (some products are
undergoing trials in more than one target population). Vaccine
types under investigation include live-attenuated/chimeric,
particle-based, subunit and recombinant vector vaccines. This
highlights the variety and breadth of immunization types and
different populations that are being investigated to find an answer
to the 60‑year‑old problem of producing a safe and effective RSV
prophylactic agent.
The most advanced candidate vaccine, ResVax, is an
RSV fusion protein recombinant nanoparticle with aluminum phosphate
as an adjuvant (115,152,153). The new approach to develop this
vaccine is based on engineering small particles that carry altered
RSV proteins. The nanoparticles sensitize the immune system to the
virus so that when a person comes in contact with it the immune
system delivers a robust response. The Phase 3 clinical trial
included more than 4,600 pregnant women examining the efficacy of
prevention of RSV disease in infants through maternal immunization.
Although the trial narrowly missed its primary end point of a
reduction in medically attended RSV-positive ALRTI, it showed a 44%
vaccine efficacy against RSV hospitalization, 25% efficacy against
all respiratory hospitalizations and 39% efficacy against all‑cause
severe hypoxaemia (152). A
possible route to licensure is currently being sought with the US
Food and Drug Administration (FDA) and European licensing agencies,
bringing hope of a vaccine that could save the lives of countless
young infants worldwide.
No funding was received.
Not applicable.
All authors (INM, SBD, BR, MT, GP, AP, EiK, CK,
ElK, VA, PK, GPC and DAS) contributed to the conception and design
of the study, wrote the original draft, edited and critically
revised the manuscript, read and approved the final manuscript.
Not applicable.
Not applicable.
DAS is the Editor-in-Chief for the journal, but had
no personal involvement in the reviewing process, or any influence
in terms of adjudicating on the final decision, for this article.
The other authors declare that they have no competing
interests.
We would like to thank the participants of the '5th
Workshop on Paediatric Virology' (Sparta, Greece, October 12th,
2019) for their comments, corrections and feedback. We would also
like to thank the organizing committee the '24th World Congress on
Advances in Oncology' and the '24th International Symposium on
Molecular Medicine' for the outstanding hosting of the workshop, as
well as all members of the PVSG and the newly founded Institute of
Paediatric Virology (IPV) based on the island of Euboea for their
valuable contribution in the preparation of the manuscript.
|
1
|
König IR, Fuchs O, Hansen G, von Mutius E
and Kopp MV: What is precision medicine? Eur Respir J.
50:17003912017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Theodoridou M: RSV and precision medicine:
Time for a more precise approach to diagnosis, treatment and
prevention. Int J Mol Med. 44:S322019.
|
|
3
|
Barr R, Green CA, Sande CJ and Drysdale
SB: Respiratory syncytial virus: Diagnosis, prevention and
management. Ther Adv Infect Dis. 6:20499361198657982019.PubMed/NCBI
|
|
4
|
Li Y, Reeves RM, Wang X, Bassat Q, Brooks
WA, Cohen C, Moore DP, Nunes M, Rath B, Campbell H, et al RSV
Global Epidemiology Network; RESCEU investigators: Global patterns
in monthly activity of influenza virus, respiratory syncytial
virus, parainfluenza virus, and metapneumovirus: A systematic
analysis. Lancet Glob Health. 7:e1031–e1045. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Alchikh M, Conrad T, Hoppe C, Ma X,
Broberg E, Penttinen P, Reiche J, Biere B, Schweiger B and Rath B:
Are we missing respiratory viral infections in infants and
children? Comparison of a hospital-based quality management system
with standard of care. Clin Microbiol Infect. 25:380.e9–380.e16.
2019. View Article : Google Scholar
|
|
6
|
Blount RE Jr, Morris JA and Savage RE:
Recovery of cytopathogenic agent from chimpanzees with coryza. Proc
Soc Exp Biol Med. 92:544–549. 1956. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kapikian AZ, Morens DM and Fauci AS: In
Memoriam: Robert M. Chanock, MD, 1924-2010. J Infect Dis. 203:3–5.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gagliardi TB, Criado MF, Proença-Módena
JL, Saranzo AM, Iwamoto MA, de Paula FE, Cardoso RS, Delcaro LS,
Silva ML, Câmara AA, et al: Syncytia induction by clinical isolates
of human respiratory syncytial virus A. Intervirology. 60:56–60.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mammas IN and Spandidos DA: Updating
taxonomy changes: RSV is now known as Orthopneumovirus. Int J Mol
Med. 44:S312019.
|
|
10
|
ICTV Taxonomy history: Human
orthopneumovirus. International Committee on Taxonomy of Viruses
(ICTV). 2019, https://talk.ictvonline.org/taxonomy/.
|
|
11
|
Karampatsas K, Kong J and Cohen J:
Bronchiolitis: An update on management and prophylaxis. Br J Hosp
Med (Lond). 80:278–284. 2019. View Article : Google Scholar
|
|
12
|
Cunningham S: Respiratory Support in
Bronchiolitis: Trial Evidence. Am J Perinatol. 35:553–556. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Rath B, Maltezou HC, Papaevangelou V,
Papagrigoriou-Theodoridou MA, Alchikh M, Myles P and Schweiger B;
PEDSIDEA Network: Partnering for enhanced digital surveillance of
influenza‑like disease and the effect of antivirals and vaccines
(PEDSIDEA). Influenza Other Respir Viruses. 13:309–318. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nair H, Brooks WA, Katz M, Roca A, Berkley
JA, Madhi SA, Simmerman JM, Gordon A, Sato M, Howie S, et al:
Global burden of respiratory infections due to seasonal influenza
in young children: A systematic review and meta-analysis. Lancet.
378:1917–1930. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhou H, Thompson WW, Viboud CG, Ringholz
CM, Cheng PY, Steiner C, Abedi GR, Anderson LJ, Brammer L and Shay
DK: Hospitalizations associated with influenza and respiratory
syncytial virus in the United States, 1993-2008. Clin Infect Dis.
54:1427–1436. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Homaira N, Mallitt KA, Oei JL, Hilder L,
Bajuk B, Lui K, Rawlinson W, Snelling T and Jaffe A: Risk factors
associated with RSV hospitalisation in the first 2 years of life,
among different subgroups of children in NSW: A
whole-of-population-based cohort study. BMJ Open. 6:e0113982016.
View Article : Google Scholar
|
|
17
|
Shi T, McAllister DA, O'Brien KL, Simoes
EAF, Madhi SA, Gessner BD, Polack FP, Balsells E, Acacio S, Aguayo
C, et al: RSV Global Epidemiology Network: Global, regional, and
national disease burden estimates of acute lower respiratory
infections due to respiratory syncytial virus in young children in
2015: A systematic review and modelling study. Lancet. 390:946–958.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Broberg EK, Waris M, Johansen K, Snacken R
and Penttinen P; European Influenza Surveillance Network.
Seasonality and geographical spread of respiratory syncytial virus
epidemics in 15 European countries 2010 to 2016. Euro Surveill.
23:232018. View Article : Google Scholar
|
|
19
|
Boeckh M, Chien J, Daniel G, Daniels S,
Dubovsky F, Cody Meissner H, McClellan M, Munoz FM, Murray JS, Rath
BA, et al: Advancing Drug Development for Respiratory Syncytial
Virus. Duke; Washington, DC: 2016, https://healthpolicy.duke.edu/events/advancing-drug-development-respiratory-syncytial-virus
Accessed May 2, 2016.
|
|
20
|
European Centre for Disease Prevention and
Control (ECDC): Technical Workshop: Burden of RSV disease in
Europe. ECDC; Solna: 2015, https://ecdc.europa.eu/en/news-events/technical-workshop-burden-rsv-disease-europe.
Accessed January 3, 2018.
|
|
21
|
Alchikh M, Hoppe C, Conrad T, Schweiger B
and Rath B: Are we missing respiratory viral infections in infants
and children? Comparison of a hospital-based quality management
system with standard of care. ESCMID. 25:380.e9–380.e16. 2019.
|
|
22
|
Rath B and Penttinen P: Incidence,
severity and impact of influenza: A joint meeting organised by the
ISIRV Epidemiology Group and ECDC, Stockholm, 2019. Euro Surveill.
24:19003482019. View Article : Google Scholar :
|
|
23
|
Ma X, Alchikh M, Conrad T, et al: Can we
Distinguish Respiratory Viral Infections based on Clinical
Symptoms? Lessons from a Pediatric Inception Cohort. In:
Proceedings of the ASM2017 Microbe American Society for
Microbiology Conference; New Orleans, LA. 2017
|
|
24
|
Tuttle R, Weick A, Schwarz WS, Chen X,
Obermeier P, Seeber L, Tief F, Muehlhans S, Karsch K, Peiser C, et
al: Evaluation of novel second‑generation RSV and influenza rapid
tests at the point of care. Diagn Microbiol Infect Dis. 81:171–176.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Rath B: Vienna Vaccine Safety Initiative.
Hum Vaccin Immunother. 14:1038–1041. 2018. View Article : Google Scholar :
|
|
26
|
Rath B, Ali M, Codarini G, Elemuwa C,
Khamesipour A, Maurer W, Mworozi E, Rundblad G, Varughese S and
Kochhar S: Promoting evidence-based vaccine safety research and
communication - the Vienna Vaccine Safety Initiative. J Trop
Pediatr. 58:167–169. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Rath B, Conrad T, Myles P, Alchikh M, Ma
X, Hoppe C, Tief F, Chen X, Obermeier P, Kisler B, et al: Influenza
and other respiratory viruses: Standardizing disease severity in
surveillance and clinical trials. Expert Rev Anti Infect Ther.
15:545–568. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Rath B, Maltezou HC, Papaevangelou V,
Papagrigoriou-Theodoridou MA, Alchikh M, Myles P, Schweiger B,
Asimaki H, Dimopoulou D, Hoppe C, et al: PEDSIDEA Network:
Partnering for enhanced digital surveillance of influenza‑like
disease and the effect of antivirals and vaccines (PEDSIDEA).
Influenza Other Respir Viruses. 13:309–318. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Rath B, Conrad T, Myles P, Alchikh M, Ma
X, Hoppe C, Tief F, Chen X, Obermeier P, Kisler B and Schweiger B:
The ViVI Disease Severity Score: Enabling Real-time Surveillance of
Influenza Disease Severity for Multi-dimensional Mapping. In:
Proceedings of 25th International Biodetection Technologies
Conference 2017, Biodefense World Summit; Alexandria, VA. 2017
|
|
30
|
Griffiths C, Drews SJ and Marchant DJ:
Respiratory syncytial virus: Infection, detection, and new options
for prevention and treatment. Clin Microbiol Rev. 30:277–319. 2017.
View Article : Google Scholar :
|
|
31
|
Kozanidou E and Achtsidis V: RSV
infection: Not for children only. Int J Mol Med. 44:S322019.
|
|
32
|
Falsey AR, Hennessey PA, Formica MA, Cox C
and Walsh EE: Respiratory syncytial virus infection in elderly and
high-risk adults. N Engl J Med. 352:1749–1759. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Branche AR and Falsey AR: Respiratory
syncytial virus infection in older adults: An under-recognized
problem. Drugs Aging. 32:261–269. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Blanco JCG, Boukhvalova MS, Morrison TG
and Vogel SN: A multifaceted approach to RSV vaccination. Hum
Vaccin Immunother. 14:1734–1745. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Mammas IN and Spandidos DA: Overlap
between RSV, influenza viruses and human metapneumovirus in
childhood. Int J Mol Med. 44:S432019.
|
|
36
|
Koutsounaki E: The neonatal immune
response to RSV infection: Advances in our understanding of viral
and host cellular interactions. Int J Mol Med. 44:S432019.
|
|
37
|
Collins PL and Graham BS: Viral and host
factors in human respiratory syncytial virus pathogenesis. J Virol.
82:2040–2055. 2008. View Article : Google Scholar :
|
|
38
|
Scheltema NM, Kavelaars XM, Thorburn K,
Hennus MP, van Woensel JB, van der Ent CK, Borghans JAM, Bont LJ
and Drylewicz J: Potential impact of maternal vaccination on
life-threatening respiratory syncytial virus infection during
infancy. Vaccine. 36:4693–4700. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Mammas IN, Greenough A, Theodoridou M,
Kramvis A, Rusan M, Melidou A, Korovessi P, Papaioannou G,
Papatheodoropoulou A, Koutsaftiki C, et al: Paediatric Virology and
its interaction between basic science and clinical practice
(Review). Int J Mol Med. 41:1165–1176. 2018.PubMed/NCBI
|
|
40
|
Aranda SS and Polack FP: Prevention of
pediatric respiratory syncytial virus lower respiratory tract
illness: Perspectives for the next decade. Front Immunol.
10:10062019. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Goritzka M, Durant LR, Pereira C,
Salek-Ardakani S, Openshaw PJ and Johansson C: Alpha/beta
interferon receptor signaling amplifies early proinflammatory
cytokine production in the lung during respiratory syncytial virus
infection. J Vir ol. 88:6128–6136. 2014.
|
|
42
|
Sun Y and López CB: The innate immune
response to RSV: Advances in our understanding of critical viral
and host factors. Vaccine. 35:481–488. 2017. View Article : Google Scholar
|
|
43
|
Caballero MT, Serra ME, Acosta PL, Marzec
J, Gibbons L, Salim M, Rodriguez A, Reynaldi A, Garcia A, Bado D,
et al: TLR4 genotype and environmental LPS mediate RSV
bronchiolitis through Th2 polarization. J Clin Invest. 125:571–582.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Drysdale SB, Prendergast M, Alcazar M,
Wilson T, Smith M, Zuckerman M, Broughton S, Rafferty GF, Johnston
SL, Hodemaekers HM, et al: Genetic predisposition of RSV
infection-related respiratory morbidity in preterm infants. Eur J
Pediatr. 173:905–912. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Restori KH, Srinivasa BT, Ward BJ and
Fixman ED: Neonatal immunity, respiratory virus infections, and the
development of asthma. Front Immunol. 9:12492018. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Varricchi G, Harker J, Borriello F, Marone
G, Durham SR and Shamji MH: T follicular helper (Tfh) cells in
normal immune responses and in allergic disorders. Allergy.
71:1086–1094. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Blanken MO, Rovers MM, Molenaar JM,
Winkler-Seinstra PL, Meijer A and Kimpen JL: Respiratory syncytial
virus and recurrent wheeze in healthy preterm infants. N Engl J
Med. 368:1791–1799. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Koutsaftiki C: MicroRNAs as potential
biomarkers in children with RSV infection. Int J Mol Med.
44:S322019.
|
|
49
|
Głobińska A, Pawełczyk M and Kowalski ML:
MicroRNAs and the immune response to respiratory virus infections.
Expert Rev Clin Immunol. 10:963–971. 2014. View Article : Google Scholar
|
|
50
|
Rossi GA, Silvestri M and Colin AA:
Respiratory syncytial virus infection of airway cells: Role of
microRNAs. Pediatr Pulmonol. 50:727–732. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Kozomara A and Griffiths‑Jones S: miRBase:
Annotating high confidence microRNAs using deep sequencing data.
Nucleic Acids Res. 42(D1): D68–D73. 2014. View Article : Google Scholar :
|
|
52
|
Maltby S, Plank M, Tay HL, Collison A and
Foster PS: Targeting microRNA function in respiratory diseases:
Mini-review. Front Physiol. 7:212016. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Thornburg NJ, Hayward SL and Crowe JE Jr:
Respiratory syncytial virus regulates human microRNAs by using
mechanisms involving beta interferon and NF-κB. MBio. 3:e00220–e12.
2012. View Article : Google Scholar
|
|
54
|
Feng S, Zeng D, Zheng J and Zhao D:
MicroRNAs: Mediators and therapeutic targets to airway hyper
reactivity after respiratory syncytial virus infection. Front
Microbiol. 9:21772018. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Anderson L, Jorquera PA and Tripp RA:
MicroRNA profiling from RSV‑infected biofluids, whole blood, and
tissue samples. Methods Mol Biol. 1442:195–208. 2016. View Article : Google Scholar
|
|
56
|
Thibault PA and Wilson JA: Targeting
miRNAs to treat Hepatitis C Virus infections and liver pathology:
Inhibiting the virus and altering the host. Pharmacol Res.
75:48–59. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Mammas IN and Spandidos DA: Thrombocytosis
and RSV infection in hospitalized children with bronchiolitis. Int
J Mol Med. 44:S312019.
|
|
58
|
Chiarello P, Magnolia M, Rubino M, Liguori
SA and Miniero R: Thrombocytosis in children. Minerva Pediatr.
63:507–513. 2011.PubMed/NCBI
|
|
59
|
Schafer AI: Thrombocytosis. N Engl J Med.
350:1211–1219. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Mantadakis E, Tsalkidis A and
Chatzimichael A: Thrombocytosis in childhood. Indian Pediatr.
45:669–677. 2008.PubMed/NCBI
|
|
61
|
Shin J, Lee DH, Jung N, Choi HJ and Shim
YJ: A cross-sectional retrospective study to analyze the underlying
causes and clinical characteristics of children with reactive
thrombocytosis at a Korean tertiary medical center. Blood Res.
53:233–239. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Kubota M, Maeda H, Yoshimoto J, Kobayashi
K, Usami I and Yamaoka K: Thrombocytosis at an early stage of
respiratory tract viral infection. Acta Paediatr. 94:364–366.
2005.PubMed/NCBI
|
|
63
|
Bilavsky E, Yarden-Bilavsky H, Shouval DS,
Fisch N, Garty BZ, Ashkenazi S and Amir J: Respiratory syncytial
virus-positive bronchiolitis in hospitalized infants is associated
with thrombocytosis. Isr Med Assoc J. 12:39–41. 2010.PubMed/NCBI
|
|
64
|
Zheng SY, Xiao QY, Xie XH, Deng Y, Ren L,
Tian DY, Luo ZX, Luo J, Fu Z, Huang AL, et al: Association between
secondary thrombocytosis and viral respiratory tract infections in
children. Sci Rep. 6:229642016. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Mammas I, Koutsaftiki C,
Tapaki-Papadopoulou G and Myriokefalitakis N: Respiratory syncytial
virus (RSV) bronchiolitis and excessive thrombocytosis. Acta
Paediatr. 99:489–490. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Ng KF, Tan KK, Sam ZH, Ting GS and Gan WY:
Epidemiology, clinical characteristics, laboratory findings and
severity of respiratory syncytial virus acute lower respiratory
infection in Malaysian children, 2008-2013. J Paediatr Child
Health. 53:399–407. 2017. View Article : Google Scholar
|
|
67
|
Vlacha V and Feketea G: Thrombocytosis in
pediatric patients is associated with severe lower respiratory
tract inflammation. Arch Med Res. 37:755–759. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Haidopoulou K, Goutaki M, Lemonaki M,
Kavga M and Papa A: Reactive thrombocytosis in children with viral
respiratory tract infections. Minerva Pediatr. 63:257–262.
2011.PubMed/NCBI
|
|
69
|
Indolfi G, Catania P, Bartolini E, Azzari
C, Massai C, Poggi GM, De Martino M and Resti M: Incidence and
clinical significance of reactive thrombocytosis in children aged 1
to 24 months, hospitalized for community-acquired infections.
Platelets. 19:409–414. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Denton A and Davis P: Extreme
thrombocytosis in admissions to paediatric intensive care: No
requirement for treatment. Arch Dis Child. 92:515–516. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Korovessi P: RSV bronchiolitis and
paediatric asthma. Int J Mol Med. 44:S422019.
|
|
72
|
Kabego L and de Beer C: Association
between respiratory syncytial virus infection in infancy and
subsequent asthma: A meta-analysis of observational studies. JSM
Allergy Asthma. 2:10092017.
|
|
73
|
Régnier SA and Huels J: Association
between respiratory syncytial virus hospitalizations in infants and
respiratory sequelae: Systematic review and meta-analysis. Pediatr
Infect Dis J. 32:820–826. 2013.PubMed/NCBI
|
|
74
|
Jartti T, Smits HH, Bønnelykke K, Bircan
O, Elenius V, Konradsen JR, Maggina P, Makrinioti H, Stokholm J,
Hedlin G, et al: EAACI Task Force on Clinical Practice
Recommendations on Preschool Wheeze: Bronchiolitis needs a revisit:
Distinguishing between virus entities and their treatments.
Allergy. 74:40–52. 2019. View Article : Google Scholar
|
|
75
|
Fauroux B, Simões EAF, Checchia PA, Paes
B, Figueras-Aloy J, Manzoni P, Bont L and Carbonell-Estrany X: The
burden and long-term respiratory morbidity associated with
respiratory syncytial virus infection in early childhood. Infect
Dis Ther. 6:173–197. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Homaira N, Briggs N, Pardy C, Hanly M, Oei
JL, Hilder L, Bajuk B, Lui K, Rawlinson W, Snelling T, et al:
Association between respiratory syncytial viral disease and the
subsequent risk of the first episode of severe asthma in different
subgroups of high-risk Australian children: A
whole-of-population-based cohort study. BMJ Open. 7:e0179362017.
View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Thomsen SF, van der Sluis S, Stensballe
LG, Posthuma D, Skytthe A, Kyvik KO, Duffy DL, Backer V and
Bisgaard H: Exploring the association between severe respiratory
syncytial virus infection and asthma: A registry-based twin study.
Am J Respir Crit Care Med. 179:1091–1097. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Mochizuki H, Kusuda S, Okada K, Yoshihara
S, Furuya H, Simões EAF, Asanuma H, Yoshida H, Katayose M, Imamura
T, et al: Scientific Committee for Elucidation of Infantile Asthma:
Palivizumab prophylaxis in preterm infants and subsequent recurrent
wheezing. Six-year follow-up study. Am J Respir Crit Care Med.
196:29–38. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Scheltema NM, Nibbelke EE, Pouw J, Blanken
MO, Rovers MM, Naaktgeboren CA, Mazur NI, Wildenbeest JG, van der
Ent CK and Bont LJ: Respiratory syncytial virus prevention and
asthma in healthy preterm infants: A randomised controlled trial.
Lancet Respir Med. 6:257–264. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Carbonell-Estrany X, Pérez-Yarza EG,
García LS, Guzmán Cabañas JM and Bòria EV: Long-term burden and
respiratory effects of respiratory syncytial virus hospitalization
in preterm infants - The SPRING Study. PLoS One. 10:e01254222015.
View Article : Google Scholar
|
|
81
|
Lukkarinen M, Koistinen A, Turunen R,
Lehtinen P, Vuorinen T and Jartti T: Rhinovirus‑induced first
wheezing episode predicts atopic but not nonatopic asthma at school
age. J Allergy Clin Immunol. 140:988–995. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Rubner FJ, Jackson DJ, Evans MD, Gangnon
RE, Tisler CJ, Pappas TE, Gern JE and Lemanske RF Jr: Early life
rhinovirus wheezing, allergic sensitization, and asthma risk at
adolescence. J Allergy Clin Immunol. 139:501–507. 2017. View Article : Google Scholar
|
|
83
|
Koutsaftiki C: Predicting asthma following
RSV-positive bronchiolitis in early childhood. Int J Mol Med.
44:S312019.
|
|
84
|
Drysdale SB, Alcazar-Paris M, Wilson T,
Smith M, Zuckerman M, Peacock JL, Johnston SL and Greenough A:
Viral lower respiratory tract infections and preterm infants'
healthcare utilisation. Eur J Pediatr. 174:209–215. 2015.
View Article : Google Scholar
|
|
85
|
Drysdale SB, Milner AD and Greenough A:
Respiratory syncytial virus infection and chronic respiratory
morbidity - is there a functional or genetic predisposition? Acta
Paediatr. 101:1114–1120. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Gern JE: Viral respiratory infection and
the link to asthma. Pediatr Infect Dis J. 27(Suppl): S97–S103.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Bacharier LB, Cohen R, Schweiger T,
Yin-Declue H, Christie C, Zheng J, Schechtman KB, Strunk RC and
Castro M: Determinants of asthma after severe respiratory syncytial
virus bronchiolitis. J Allergy Clin Immunol. 130:91–100.e3. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Lu S, Hartert TV, Everard ML, Giezek H,
Nelsen L, Mehta A, Patel H, Knorr B and Reiss TF: Predictors of
asthma following severe respiratory syncytial virus (RSV)
bronchiolitis in early childhood. Pediatr Pulmonol. 51:1382–1392.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Jartti T and Gern JE: Role of viral
infections in the development and exacerbation of asthma in
children. J Allergy Clin Immunol. 140:895–906. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Grivas G, Lymperatou C and Korovessi P:
Post infectious bronchiolitis obliterans caused by respiratory
syncytial virus (RSV) in children. Int J Mol Med. 44:S422019.
|
|
91
|
Kavaliunaite E and Aurora P: Diagnosing
and managing bronchiolitis obliterans in children. Expert Rev
Respir Med. 13:481–488. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Champs NS, Lasmar LM, Camargos PA, Marguet
C, Fischer GB and Mocelin HT: Post-infectious bronchiolitis
obliterans in children. J Pediatr (Rio J). 87:187–198. 2011.
|
|
93
|
Rodríguez DA, Rodríguez-Martínez CE,
Cárdenas AC, Quilaguy IE, Mayorga LY, Falla LM and Nino G:
Predictors of severity and mortality in children hospitalized with
respiratory syncytial virus infection in a tropical region. Pediatr
Pulmonol. 49:269–276. 2014. View Article : Google Scholar :
|
|
94
|
Wu XY, Luo ZX, Fu Z, Liu EM, Luo J and He
L: Clinical analysis of 28 cases of bronchiolitis obliterans.
Zhongguo Dang Dai Er Ke Za Zhi. 15:845–849. 2013.In Chinese.
PubMed/NCBI
|
|
95
|
Yanagisawa J, Shiraishi T, Okamatsu Y and
Iwasaki A: Successful lung volume reduction surgery in an infant
with emphysema after respiratory syncytial virus-induced
obliterative bronchiolitis. J Thorac Cardiovasc Surg. 145:e47–e49.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Chen DH, Lin YN, Lan SL, Pan XA, Zeng QS,
He ZT, Liang M, Zhang BY, Wu SZ, Xu JX, et al: Clinical
characteristics of bronchiolitis obliterans in pediatric patients.
Zhonghua Er Ke Za Zhi. 50:98–102. 2012.In Chinese. PubMed/NCBI
|
|
97
|
Giovannini-Chami L, Khirani S, Thouvenin
G, Ramirez A and Fauroux B: Work of breathing to optimize
noninvasive ventilation in bronchiolitis obliterans. Intensive Care
Med. 38:722–724. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Sardón O, Pérez-Yarza EG, Aldasoro A,
Corcuera P, Mintegui J and Korta J: Bronchiolitis obliterans:
Outcome in the medium term. An Pediatr (Barc). 76:58–64. 2012.In
Spanish. View Article : Google Scholar
|
|
99
|
Wang W, Shen KL and Zeng JJ: Clinical
studies of children with bronchiolitis obliterans. Zhonghua Er Ke
Za Zhi. 46:732–738. 2008.In Chinese. PubMed/NCBI
|
|
100
|
Lobo AL, Guardiano M, Nunes T, Azevedo I
and Vaz LG: Pos-infectious bronchiolitis obliterans in children.
Rev Port Pneumol. 13:495–509. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Hirschheimer M, Silva PS, Giudici R,
Carrilho M, Mauad T and Ishida M: Simultaneous viral infection and
childhood bronchiolitis obliterans. Braz J Infect Dis. 6:146–148.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Massie R and Armstrong D: Bronchiectasis
and bronchiolitis obliterans post respiratory syncytial virus
infection: Think again. J Paediatr Child Health. 35:497–498. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Yalçin E, Doğru D, Haliloğlu M, Ozçelik U,
Kiper N and Göçmen A: Postinfectious bronchiolitis obliterans in
children: Clinical and radiological profile and prognostic factors.
Respiration. 70:371–375. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Papaioannou G: Imaging in children with
RSV infection. Int J Mol Med. 44:S302019.
|
|
105
|
Dawson-Caswell M and Muncie HL Jr:
Respiratory syncytial virus infection in children. Am Fam
Physician. 83:141–146. 2011.PubMed/NCBI
|
|
106
|
National Institute for Health and Care
Excellence (NICE): Bronchiolitis in children: diagnosis and
management, NICE guideline [NG9]. https://www.nice.org.uk/guidance/ng9.
Accessed June 1, 2015.
|
|
107
|
Kern S, Uhl M, Berner R, Schwoerer T and
Langer M: Respiratory syncytial virus infection of the lower
respiratory tract: Radiological findings in 108 children. Eur
Radiol. 11:2581–2584. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Hilmes MA, Daniel Dunnavant F, Singh SP,
Ellis WD, Payne DC, Zhu Y, Griffin MR, Edwards KM and Williams JV:
Chest radiographic features of human metapneumovirus infection in
pediatric patients. Pediatr Radiol. 47:1745–1750. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Alkan Ozdemir S, Ozer EA, Pekcevik Y,
Ilhan O and Sutcuoglu S: Is radiological appearance of lower
respiratory tract infection due to respiratory syncytial virus a
predictor of clinical outcome? J Matern Fetal Neonatal Med.
28:1660–1663. 2015. View Article : Google Scholar
|
|
110
|
Pickles R and DeVincenzo J: PSV and its
propensity for causing bronchioloitis. J Pathol. 235:266–267. 2015.
View Article : Google Scholar
|
|
111
|
Franquet T: Imaging of pulmonary viral
pneumonia. Radiology. 260:18–39. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Xu L, Gao H, Zeng J, Liu J, Lu C, Guan X,
Qian S and Xie Z: A fatal case associated with respiratory
syncytial virus infection in a young child. BMC Infect Dis.
18:2172018. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Park A, Suh SI, Son GR, Lee YH, Seo HS,
Eun BL, Lee NJ and Seol HY: Respiratory syncytial virus-related
encephalitis: Magnetic resonance imaging findings with
diffusion‑weighted study. Neuroradiology. 56:163–168. 2014.
View Article : Google Scholar
|
|
114
|
Al-Maskari N, Mohsin J, Al-Maani A,
Al-Macki N and Al-Ismaili S: Atypical presentations of respiratory
syncytial virus infection: Case series. Sultan Qaboos Univ Med J.
16:e86–e91. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Drysdale SB: Management of RSV infection
in children: New advances and challenges. Int J Mol Med.
44:S262019.
|
|
116
|
Krilov LR: Safety issues related to the
administration of ribavirin. Pediatr Infect Dis J. 21:479–481.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Behzadi MA and Leyva-Grado VH: Overview of
current therapeutics and novel candidates against influenza,
respiratory syncytial virus, and Middle East respiratory syndrome
coronavirus infections. Front Microbiol. 10:13272019. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Xing Y and Proesmans M: New therapies for
acute RSV infections: Where are we? Eur J Pediatr. 178:131–138.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Beigel JH, Nam HH, Adams PL, Krafft A,
Ince WL, El-Kamary SS and Sims AC: Advances in respiratory virus
therapeutics - A meeting report from the 6th isirv Antiviral Group
conference. Antiviral Res. 167:45–67. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Nicholson EG and Munoz FM: A review of
therapeutics in clinical development for respiratory syncytial
virus and influenza in children. Clin Ther. 40:1268–1281. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Sherman AC, Mehta A, Dickert NW, Anderson
EJ and Rouphael N: The future of flu: A review of the human
challenge model and systems biology for advancement of influenza
vaccinology. Front Cell Infect Microbiol. 9:1072019. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Jozwik A, Habibi MS, Paras A, Zhu J,
Guvenel A, Dhariwal J, Almond M, Wong EHC, Sykes A, Maybeno M, et
al: RSV‑specific airway resident memory CD8+ T cells and
differential disease severity after experimental human infection.
Nat Commun. 6:102242015. View Article : Google Scholar
|
|
123
|
Papatheodoropoulou A: High‑flow warm
humidified oxygen via nasal cannula and RSV-positive bronchiolitis
among children admitted to PICU. Int J Mol Med. 44:S302019.
|
|
124
|
Beggs S, Wong ZH, Kaul S, Ogden KJ and
Walters JA: High‑flow nasal cannula therapy for infants with
bronchiolitis. Cochrane Database Syst Rev. 2014:CD0096092014.
|
|
125
|
Franklin D, Dalziel S, Schlapbach LJ, Babl
FE, Oakley E, Craig SS, Furyk JS, Neutze J, Sinn K, Whitty JA, et
al: PARIS and PREDICT: Early high flow nasal cannula therapy in
bronchiolitis, a prospective randomised control trial (protocol): A
Paediatric Acute Respiratory Intervention Study (PARIS). BMC
Pediatr. 15:1832015. View Article : Google Scholar
|
|
126
|
van Miert C, Fernandes RM, Eccleson H,
Bedson E, Lane S, Peak M, Thorburn K, Compton V, Woolfall K, Lacy
D, et al: Non-invasive ventilation for the management of children
with bronchiolitis (NOVEMBR): A feasibility study and core outcome
set development protocol. Trials. 19:6272018. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Kepreotes E, Whitehead B, Attia J,
Oldmeadow C, Collison A, Searles A, Goddard B, Hilton J, Lee M and
Mattes J: High‑flow warm humidified oxygen versus standard low‑flow
nasal cannula oxygen for moderate bronchiolitis (HFWHO RCT): An
open, phase 4, randomised controlled trial. Lancet. 389:930–939.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Franklin D, Babl FE, Schlapbach LJ, Oakley
E, Craig S, Neutze J, Furyk J, Fraser JF, Jones M, Whitty JA, et
al: A randomized trial of high‑flow oxygen therapy in infants with
bronchiolitis. N Engl J Med. 378:1121–1131. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Goh CT, Kirby LJ, Schell DN and Egan JR:
Humidified high‑flow nasal cannula oxygen in bronchiolitis reduces
need for invasive ventilation but not intensive care admission. J
Paediatr Child Health. 53:897–902. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Clayton JA, McKee B, Slain KN, Rotta AT
and Shein SL: Outcomes of children with bronchiolitis treated with
high‑flow nasal cannula or noninvasive positive pressure
ventilation. Pediatr Crit Care Med. 20:128–135. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Milési C, Essouri S, Pouyau R, Liet JM,
Afanetti M, Portefaix A, Baleine J, Durand S, Combes C, Douillard
A, et al: Groupe Francophone de Réanimation et d'Urgences
Pédiatriques (GFRUP): High flow nasal cannula (HFNC) versus nasal
continuous positive airway pressure (nCPAP) for the initial
respiratory management of acute viral bronchiolitis in young
infants: A multicenter randomized controlled trial (TRAMONTANE
study). Intensive Care Med. 43:209–216. 2017. View Article : Google Scholar
|
|
132
|
Papatheodoropoulou A: Heliox and
RSV-positive bronchiolitis. Int J Mol Med. 44:S322019.
|
|
133
|
Hess DR, Fink JB, Venkataraman ST, Kim IK,
Myers TR and Tano BD: The history and physics of heliox. Respir
Care. 51:608–612. 2006.PubMed/NCBI
|
|
134
|
Nascimento MS, Santos É and Prado CD:
Helium-oxygen mixture: Clinical applicability in an intensive care
unit. Einstein (Sao Paulo). 16:eAO41992018. View Article : Google Scholar
|
|
135
|
Barach AL: The therapeutic use of helium.
JAMA. 107:1273–1280. 1936. View Article : Google Scholar
|
|
136
|
Moraa I, Sturman N, McGuire TM and van
Driel ML: Heliox for croup in children. Cochrane Database Syst Rev.
10:CD0068222018.PubMed/NCBI
|
|
137
|
Morgan SE, Vukin K, Mosakowski S, Solano
P, Stanton L, Lester L, Lavani R, Hall JB and Tung A: Use of heliox
delivered via high-flow nasal cannula to treat an infant with
coronavirus‑related respiratory infection and severe acute air-flow
obstruction. Respir Care. 59:e166–e170. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Martinón-Torres F: Noninvasive ventilation
with helium-oxygen in children. J Crit Care. 27:220.e1–220.e9.
2012. View Article : Google Scholar
|
|
139
|
Liet JM, Ducruet T, Gupta V and Cambonie
G: Heliox inhalation therapy for bronchiolitis in infants. Cochrane
Database Syst Rev. (9): CD0069152015.PubMed/NCBI
|
|
140
|
Seliem W and Sultan AM: Heliox delivered
by high flow nasal cannula improves oxygenation in infants with
respiratory syncytial virus acute bronchiolitis. J Pediatr (Rio J).
94:56–61. 2018. View Article : Google Scholar
|
|
141
|
Theodoridou M: Prevention of RSV
infection: What is new with the vaccines? J Mol Med (Berl).
44:S292019.
|
|
142
|
Wang D, Cummins C, Bayliss S, Sandercock J
and Burls A: Immunoprophylaxis against respiratory syncytial virus
(RSV) with palivizumab in children: A systematic review and
economic evaluation. Health Technol Assess. 12:iiiix–x. 1–86. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Modjarrad K, Giersing B, Kaslow DC and
Smith PG: WHO consultation on Respiratory Syncytial Virus Vaccine
Development Report from a World Health Organization Meeting held on
23-24 March 2015. Vaccine. 34:190–197. 2016. View Article : Google Scholar
|
|
144
|
Nair H, Nokes DJ, Gessner BD, Dherani M,
Madhi SA, Singleton RJ, O'Brien KL, Roca A, Wright PF, Bruce N, et
al: global burden of acute lower respiratory infections due to
respiratory syncytial virus in young children: A systematic review
and meta-analysis. Lancet. 375:1545–1555. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
145
|
Gerretsen HE and Sande CJ: Development of
respiratory syncytial virus (RSV) vaccines for infants. J Infect.
74(Suppl 1): S143–S146. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Prince GA, Jenson AB, Hemming VG, Murphy
BR, Walsh EE, Horswood RL and Chanock RM: Enhancement of
respiratory syncytial virus pulmonary pathology in cotton rats by
prior intramuscular inoculation of formalin-inactivated virus. J
Virol. 57:721–728. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
147
|
Graham BS: Vaccine development for
respiratory syncytial virus. Curr Opin Virol. 23:107–112. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
148
|
Graham BS: Vaccines against respiratory
syncytial virus: The time has finally come. Vaccine. 34:3535–3541.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
149
|
Widjojoatmodjo MN, Bogaert L, Meek B, Zahn
R, Vellinga Custers J, Serroyen J, Radošević K and Schuitemaker H:
Recombinant low-seroprevalent adenoviral vectors Ad26 and Ad35
expressing the respiratory syncytial virus (RSV) fusion protein
induce protective immunity against RSV infection in cotton rats.
Vaccine. 33:5406–5414. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
150
|
Pardi N and Weissman D: Nucleoside
modified mRNA vaccines for infections diseases. Methods Mol Biol.
1499:109–121. 2017. View Article : Google Scholar
|
|
151
|
PATH: RSV vaccine mAb snapshot. https://path.azureedge.net/media/documents/RSV-snapshot-2019_08_28_High_Resolution_PDF.pdf.
Accessed August 28, 2019.
|
|
152
|
Novavax: Prepare™ Trial Topline Results.
https://novavax.com/2019_02_27_ResVax_Draft.pdf.
Accessed February 28, 2019.
|
|
153
|
Patrick M: Will the ResVax vaccine be key
revenue driver for Novavax. Market Realist; 2019, https://marketrealist.com/2019/05/will-the-resvax-vaccine-be-key-revenue-driver-for-novavax/.
Accessed May 6, 2019.
|