Maintaining normal proton concentrations inside and
outside the cell is essential for the normal physiological
functioning of numerous processes throughout the body (1). Acid-sensitive ion channels (ASICs)
as effective proton sensors are sensitive to extracellular
acidification (2). ASICs are
widely expressed throughout the nervous system (2). Relatively more recently, they were
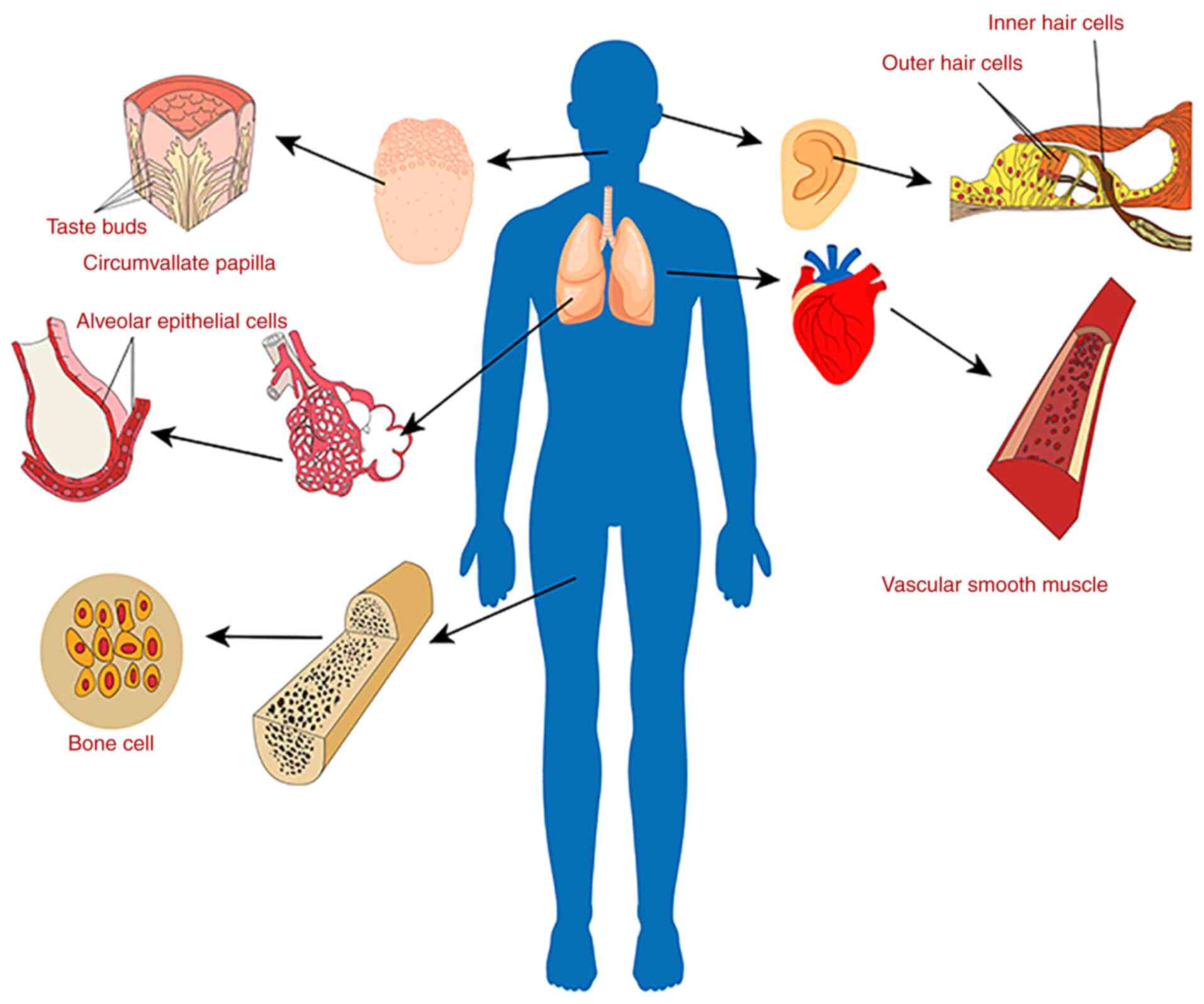
discovered to be expressed in non-excitable tissues, such as in
taste bud cells in the tongue contour nipple, human bone cells,
vascular smooth muscle cells, lung epithelial cells, inner ear, and
cochlear hair cells, among others (Fig. 1) (3). The function of ASICs in the nervous
system has been widely studied (4), but there are considerably fewer
studies assessing their roles in other systems of the body. Recent
studies have found that ASICs are closely related to the
physiological function and pathological development of diseases of
the digestive system. In the digestive system, ASICs are involved
in normal physiological functions such as gastrointestinal
mechanical sensation and duodenal bicarbonate secretion (5,6).
ASICs also underlie the development of gastrointestinal pains,
gastro-intestinal cancer, and other pathological processes that
involve a dysregulated acidic microenvironment or acidosis
(7,8). This article reviews the research
progress concerning ASICs in the digestive system.
The stomach contains an abundance of glands, and the
high concentration of HCl produced plays an important role in the
normal digestive process (81);
however, the strongly acidic environment is also a factor leading
to certain gastrointestinal diseases (82). The erosion of the gastric mucosa
by the excessively high concentration of gastric acid can cause a
series of digestive symptoms (22). To enable gastric acid to play a
physiological role without damaging the gastrointestinal mucosa, a
cell capable of sensing acid is needed for fine regulation of the
pH, in which epithelial cells and acid-sensitive neurons play a
special role (22). Previous
studies suggested that the vagus nerve pathway mainly regulates
autonomic function, but recent studies have shown that the vagus
nerve pathway may contribute to acid-sensing. Molecular and
electrophysiological studies have shown that vagal sensory neurons
projected to the proximal gastrointestinal tract express
proton-gated ion channels (83,84). Krishtal and Pidoplichko (85) observed that sensory neurons could
respond to protons nearby. Later, a large number of experimental
studies confirmed this finding and indicated that acids could
stimulate primary afferent neurons. Proton-gated ASICs are highly
sensitive acid sensors. Although ASICs are rapidly deactivated
after activation, they can still monitor long-term acidosis. This
plays an important role in the gastrointestinal tract, which is
rich in gastric acid. Whole-cell voltage-clamp recordings of dorsal
root ganglia (DRG) and nodular ganglia (NG) neurons showed that
proton-gated currents are related to ASICs (84). ASICs in the gastrointestinal
tract primarily occur in the peripheral fibers of exogenous primary
afferent neurons derived from the DRG and NG, and studies have
shown that NG and DRG neurons in rats express ASIC3 (22,86). ASIC1, ASIC2, and ASIC3 are
expressed in the DRG of thoracolumbar vertebrae that project into
the colons of mice (87). When
the ASIC3 gene was knocked out in mice, acid-induced
excitability of gastric and esophageal vagus nerve fibers decreased
(85). ASIC-mediated currents
can be recorded from DRG neurons (88). ASICs are widely present in the
digestive tract where they act as acid sensors and play an
important role in acid monitoring of the gastrointestinal tract
(2). During this process, ASICs
perceive a decrease in extracellular PH, which stimulates
acid-sensitive neurons and excites acid-sensitive neurons, thereby
initiating the body's acid balance regulation system to maintain
normal physiological functions (22).
Two major groups of mechanoreceptors in the vagus
nerves of the stomach and esophagus of mice elicit signals of
muscle stretching and mucosal touch (89), while two major groups in the
innervation of the colon of mice elicit signals of serosal and
mesenteric deformation (90).
These mechanosensitive groups not only play a role in food intake
and pain, but also in the regulation of digestive reflexes. On the
basis of this experimental conclusion, Page et al (5) determined the expression of ASIC1a,
2, and 3 in the vagus nerve and DRG of mice using ASIC gene
knockout mice, and proved that ASICs were involved in
gastro-intestinal mechanoreception. Their further studies have
shown that ASIC1a contributes to gastroesophageal and colonic
afferent mechanotransduction, and increased sensitivity to all
afferent mechanotransduction sites of visceral colonic afferent and
vagus nerves after ASIC1a destruction. Conversely, in
ASIC-deficient mice, except for gastroesophageal mucosal receptors,
the mechanical sensitivity of all afferent classes was
significantly reduced. For ASIC2, there was a notable difference in
the different afferent subtypes. After ASIC2 was destroyed, the
stomach showed an increase in the mechanical sensitivity of the
gastroesophageal mucosal endings and a decrease in the
gastroesophageal tension receptor, whereas, for the colon, it
showed an increase in the colonic serous endings and no change in
the colonic mesenteric endings (5). This indicates that ASIC subtypes
have different effects on gastro-intestinal mechanical receptors.
These ASIC gene knockout experiments affected mechanical
conduction, and this resulted in a change in the gastrointestinal
'emptying mode' and affected food digestion.
The above studies show that ASICs are an important
target for regulating gastrointestinal mechanical sensation.
Several gastrointestinal diseases involving mechanical sensation
can be treated by inhibiting or enhancing ASICs. For example, in
irritable bowel syndrome, the stronger the expansion of the colon
and rectum, the stronger the intestinal mechanical sensory signal,
and the higher the perceived pain (91). If the expression of ASICs is
weakened, the colorectal mechanical sensation signal is also
weakened, and the pain will also be reduced. Similarly, in
gastroesophageal reflux disease, if the expression of ASICs in the
proximal stomach is lower, the proximal gastric mechanical sensory
signal is reduced, the trigger of lower esophageal sphincter
relaxation is weakened, and the reflux is also reduced (91).
For example, in duodenal ulcers, by enhancing the
expression of ASICs, the secretion of duodenal bicarbonate also
increases, which is beneficial to the improvement of symptoms in
patients with duodenal ulcers.
It has been well studied that central and peripheral
nervous systems can regulate pain through ASICs (23,31,70,92,93). It has been found that ASICs are
also associated with non-nerve associated pains. A decreased
extracellular pH and inflammation are stimuli for pain. Local
tissue acidification is also involved in the production of pain
under pathological conditions. It may be that the acidic substances
produced during inflammation reduce extracellular pH, activate
proton-sensitive receptors/channels, regulate the function of
pain-sensing neurons, and induce inflammatory pain allergy.
Stimulating the gastric cavity of rats or mice with HCl
concentrations exceeding physiological levels caused a visceral
motor response, indicative of pain (94). Wultsch et al (95) eliminated the effects of
ASIC3 gene disruption on the expression of c-Fos after
gastric acid-induced neuronal excitability under gastric
inflammation, whereas ASIC2 gene knockout did not alter the
inflammatory hyperresponsiveness. Therefore, ASIC3 plays a role in
the inflammatory hyperresponsiveness of gastric acid, which may
also be related to ASIC3 leading to gastroesophageal reflux disease
related to gastric acid reflux. Several studies have shown that
primary neurons that dominate the small intestine and large
intestine express acidic sensors such as ASICs (47,96). Previous experiments have shown
that ASIC3 and TRPV1 contribute to the common functional visceral
hypersensitivity in irritable bowel syndrome (7). After intracolonic injection of
butyric acid (97), the colon of
rats expanded without any inflammatory characteristics. Injection
of the ASIC1a specific antagonist PcTx1 completely prevented the
occurrence of colonic hypersensitivity (98), indicating that ASIC1 and ASIC3
were associated with the resultant colonic hypersensitivity. The
more sensitive the colon expansion, the stronger the pain. A
related study also confirmed that the high reactivity of colon
dilatation was related to the upregulation of ASIC1a in colon DRG
neurons and in the spinal cord (96,98). This established a novel direction
for the treatment of patients with irritable bowel disease;
reducing pain by weakening the expression of ASICs. Together, these
studies suggest that ASICs play an important role in colonic
hypersensitivity and hyperalgesia in irritable bowel patients.
As the symptoms of gastroesophageal reflux disease
are closely related to acid reflux, the role of ASICs in the
esophagus has attracted considerable attention. Studies have shown
that ASIC1, ASIC2, and ASIC3 are expressed in the esophagus and its
innervated nerves (5). The
expression and function of each subtype of ASIC differ in the
esophagus. ASIC1 mainly mediates the inhibitory response to acid
and mechanical stimulation in the esophagus, whereas ASIC3 is
expressed in esophageal spine cells and the mucosal muscle layer
mediates high sensitivity to acid and inflammatory responses
following acid stimulation and stimulation by strong mechanical
expansion (99). Inflammatory
mediators in the esophageal mucosa of gastroesophageal reflux
disease patients can reduce the signal transduction threshold of
ASICs, thereby mediating peripheral sensitization (100). Previous studies have shown that
ASIC3 is closely related to the perception of pain, and more recent
studies have found that they are also related to pain sensitivity.
ASIC3 persistent expression in DRG neurons is involved in the
formation of pain. With a decrease in the pain threshold and the
emergence of pain sensitivity, ASIC3 expression is significantly
upregulated (101,102). Inhibitors of ASICs can inhibit
the pain allergy caused by peripheral acidification in rats and the
pain caused by acidification in the human body (29), which further indicates the role
of ASICs in pain sensitization. Although the pathogenesis of
gastroesophageal reflux disease is complex, through in-depth
studies of gastroesophageal reflux disease, it has been shown that
visceral hypersensitivity and inflammation play an important role
in the pathogenesis of gastroesophageal reflux disease (103). Recent studies have demonstrated
that the upregulation of ASIC1 and ASIC3 expression is a possible
cause of esophageal hypersensitivity to gastroesophageal reflux
disease, providing a potential therapeutic target for patients with
gastroesophageal reflux disease who do not respond to proton pump
inhibitors (104). A
gastroesophageal reflux disease rat model study found that during
inflammation, the expression of ASIC1 and ASIC3 subunits was
increased in the rat DRG (5).
The above experiments show that ASICs are involved in
gastroesophageal reflux disease caused by visceral hypersensitivity
and inflammation. This discovery has provided a novel direction for
the development of ASIC-related therapeutics for the management of
gastroesophageal reflux disease.
Tumor tissues generally grow faster and metabolize
vigorously, so local hypoxia and acidification of solid tumors are
common phenomena and pathological features. An acidic
microenvironment is an intrinsic feature of a tumor, and it
promotes tumor invasion and metastasis (105). Several cancers are associated
with the expression of ASICs, such as liver cancer, breast cancer,
and glioma; ASIC expression in liver cancer is associated with its
clinical stage (106,107). This suggests that ASICs are
involved in the process of various tumor diseases. After activation
of ASICs, extracellular signals can be introduced into cells,
thereby regulating the expression of specific proteins in tumor
cells. As mentioned above, there is an abundance of ASICs in the
gastrointestinal tract, thus it is reasonable to speculate that the
occurrence and development of gastric cancer may be related to
ASICs. Chen et al (8)
found that the expression of ASIC1a at the protein and mRNA level
in gastric cancer tissues was higher than that in normal tissues,
and that the upregulation of ASIC1a expression was positively
correlated with advanced gastric cancer metastasis. ASIC silencing
significantly inhibited the proliferation, migration, and invasion
of gastric cancer cells in vitro, and the inhibition was
affected by the acidity in the cell microenvironment, suggesting
that a change in acidity could alter the tumorigenicity of cancer
cells in vivo. This study also showed that ASIC1a was
involved in the occurrence of gastric cancer, and that ASIC1a
expression could affect the tumorigenicity of gastric cancer cells.
Knockdown of ASIC1a in gastric cancer cells reduced the invasion
and metastasis of gastric cancer. ASICs have been reported to
activate autophagy (108,109). Zhang et al (110) found that ASIC1 and
autophagy-associated protein 5 (ATG5) were expressed in the gastric
cancer cell line SGC-7901. Downregulation of ATG5 or ASIC1 was able
to inhibit the growth of gastric cancer cells, and ASIC1 also
upregulated autophagy by activating ATG5 in gastric cancer cells.
At the same time, it was found in the ASIC1-knockout mouse
model that the downregulation of ASIC1 slowed down the growth of
tumors and increased the survival time of mice. Although the
current research on ASICs and gastric cancer is still in its
infancy, these data show that inhibition of ASIC1 expression or
inhibition of autophagy signaling pathways may serve as a novel
means of targeted therapy for gastric cancer. At the same time, it
may also play such a role in other types of cancers, thus
highlighting novel avenues for the treatment of other types of
cancer as well.
When inflammation is stimulated, oxidative stress
and lipid peroxidation damage occur in the liver, leading to an
imbalance in extracellular matrix (ECM) synthesis and degradation,
and excessive ECM deposition in the liver tissue resulting in the
formation of fibrosis (111).
The formation of hepatic fibrosis is related to the
activation of hepatic stellate cells (112). ASIC1a is expressed in rat
hepatic stellate cells, suggesting that the formation of liver
fibrosis is related to ASIC1a (112). Zhu et al (113) found that ASIC1a expression was
significantly increased in liver fibrosis, and miR-350 was involved
in the formation of liver fibrosis regulated by ASIC1a and
activation of stellate cells. Further analysis proved that the
mechanism of hepatic fibrosis caused by ASIC1a was via
ASIC1a-mediated regulation of m6A to affect the processing and
modification of miR-350, and participate in hepatic fibrosis
through modulation of the PI3K/AKT and ERK pathways (Fig. 3). This provided powerful insights
into the potential treatment of liver fibrosis, highlighting the
potential value of antagonists of PI3K/AKT and ERK signaling
pathways to prevent and treat liver fibrosis. The early stages of
hepatolenticular degeneration are the same as those for hepatic
fibrosis, which is characterized by fibrosis of stellate cells. It
was hypothesized that hepatolenticular degeneration may also be
related to ASIC1a. Hepatolenticular degeneration is a disorder of
copper metabolism. The disease is caused by a gene mutation of
copper to Golgi body in the transfer cells, which leads to a
disorder of copper metabolism. Excessive copper accumulates in the
liver and brain, causing the corresponding systemic abnormalities
(114). A large amount of
copper accumulates in liver cells and this is toxic, leading to
liver cell necrosis, inflammation, and activation of hepatic
stellate cells to secrete ECM. Therefore, the early occurrence of
hepatolenticular degeneration is the same as that of liver
fibrosis. Kong et al (115) induced HSC-T6 activation with
CuSO4, and found that ASIC1a was highly expressed in
activated HSC-T6 cells. They speculated that ASIC1a played a role
in promoting the process of hepatolenticular degeneration fibrosis.
They also found high expression levels of ASIC1a in liver tissues
of copper-loaded rats in animal experiments. Further analysis
showed that ASIC1a in CuSO4-induced HSC-T6 cells may
have regulated endoplasmic reticulum stress (ERS) via the P38MAPK
pathway, thereby affecting hepatolenticular degeneration fibrosis
and copper transport. Inhibition of ASIC1a-mediated ERS can improve
copper transport, and the accumulation of copper in the liver and
the degree of fibrosis in rats can be alleviated, which has a
protective effect on Wilson's disease fibrosis. In summary, the
above studies showed that liver fibrosis and hepatolenticular
degeneration fibrosis are related to the activation of ASIC1a in an
acidic environment, and the inhibition of ASIC1a may serve as a
means of treatment of liver fibrosis.
Hepatocellular carcinoma (HCC) mortality ranks third
in global cancer mortality rates (116). β-catenin expression is common
in cancer cells due to its ability to bind to intracellular surface
cadherins to regulate cell adhesion. In cancer cells, the
β-catenin/lymphoid enhancer factor (LEF)/T cell enhancer factor
(TCF) axis is readily activated, leading to cancer cell
proliferation (107). Jin et
al (107) showed that
ASIC1a could promote the excessive proliferation of liver cancer
in vitro and in vivo through β-catenin activation and
nuclear accumulation in an acidic environment. Knockdown/knockout
of ASIC1a can inhibit the growth of liver cancer cells in
vivo and in vitro, induce apoptosis of liver cancer
cells, and induce cell cycle arrest of liver cancer by inhibiting
LEF-TCF activity. This suggested that the mechanism by which ASIC1a
resulted in the pathogenesis of liver cancer involved the
activation of the β-catenin/LEF-TCF pathway to induce excessive
proliferation of liver cancer cells. This finding provides a
potential druggable target for the treatment of liver cancer.
Quantitative studies have shown that an acidic microenvironment
promotes cancer cell proliferation and migration (117). Jin et al (106) showed that the expression of
ASIC1a in tumor tissues was significantly higher than that in
non-tumor tissues based on immunohistochemical analysis, and that
the expression in liver cancer tissues that exhibited postoperative
metastasis was higher than that in liver cancer tissues without
metastasis. Transwell assays showed that the mRNA and protein
expression levels of ASIC1a in SMMC-7721 cells were significantly
higher than those at pH 7.4 and 6.0 in a moderately acidic
environment at pH 6.5, while the migration and invasion of
SMMC-7721 cells were significantly inhibited after knockdown of
ASIC1a. Additionally, in the same study, in 90 patients with liver
cancer, it was found that ASIC1a expression in these patients was
upregulated and was significantly correlated with a later clinical
stage and a poorer prognosis. The above studies show that ASIC1a is
significantly correlated with the migration and invasion of HCC, as
well as a later clinical stage and a poorer prognosis. The higher
the expression of ASIC1a in a moderately acidic environment, the
more obvious the liver cancer symptoms were. Therefore, ASIC1a may
serve as a marker for the diagnosis and prognosis of HCC. The
inhibition of ASIC1a expression may highlight a novel direction for
the treatment of liver cancer.
Pancreatic cancer is a common malignant epithelial
tumor of the digestive system, with strong invasive properties.
Epithelial-mesenchymal transition (EMT) plays an important role in
tumor invasion and metastasis. In recent years, several studies
have shown that the occurrence, development, invasion, and
metastasis of pancreatic cancer show active EMT (118-120), but the specific mechanism of
EMT in pancreatic cancer has not been elucidated. It has been
previously reported that melanoma cells in acidic environments
exhibit significant EMT-like characteristics in vitro and
in vivo (121).
An acidic microenvironment induces EMT in pancreatic
cancer cells by regulating the miR-652/ZEB1 pathway (122). An acidic microenvironment is
also associated with ASICs, thus the relationship between ASICs and
pancreatic cancer has attracted attention. Zhu et al
(123) confirmed that ASIC1 and
ASIC3 proteins are expressed in pancreatic cancer cell lines where
they have a regulatory effect on pancreatic cancer cells in an
acidic environment. In further experiments, they knocked out both
ASIC1/ASIC3 and used amiloride to inhibit ASICs to reverse EMT of
pancreatic cancer cells in an acidic environment, indicating that
ASICs were involved in the process of EMT-induced pancreatic
cancer. Calcium, as a second messenger in cells, is widely involved
in various signaling pathways and plays an important role in
regulating the invasion and migration of tumor cells (124). It was confirmed that ASIC1 and
ASIC3 regulate EMT induced by an acidic environment via an increase
in the intracellular Ca2+ concentration. RhoA is a
member of the Rho family of small GTPases and plays a key role in
cell invasion and metastasis (125). Ca2+ can regulate
cytoskeletal remodeling by activating RhoA, thus playing a role in
cell migration (126,127). Based on this theory, they
showed that RhoA was activated by an
ASIC1/ASIC3-[Ca2+]i pathway in an acidic
environment to promote EMT of pancreatic cancer cells. In addition,
stable knockdown of ASIC1 and ASIC3 in a nude mouse subcutaneously
implanted tumor model significantly inhibited the invasion and
metastasis of pancreatic cancer. In conclusion, they elucidated the
complete signaling pathway of ASIC-induced pancreatic cancer; that
is, an acidic environment induces EMT of pancreatic cancer cells
through an ASIC1/3-[Ca2+]i-RhoA signaling
pathway, resulting in increased invasion and metastasis of
pancreatic cancer cells. The inhibition of ASIC1/3 can delay the
progression of pancreatic cancer. It can also be used to manage
pancreatic cancer by decreasing the Ca2+ concentration
or inhibiting the RhoA protein.
An acidic extracellular microenvironment, namely
acidosis caused by the Warburg effect (aerobic glycolysis) and a
poor vasculature, is a biochemical feature of cancer (128). Acidosis changes the
transcriptome characteristics of tumor cells, resulting in tumor
cells suitable for survival, growth, and even metastasis in acidic
environments (129). An
increasing number of studies have shown that acidosis affects
cancer progression by promoting tumor cell migration, invasion,
metastasis, and angiogenesis (130-132). Previous studies have shown that
acidosis promotes the invasion of CRC cells (131). On this basis, it is speculated
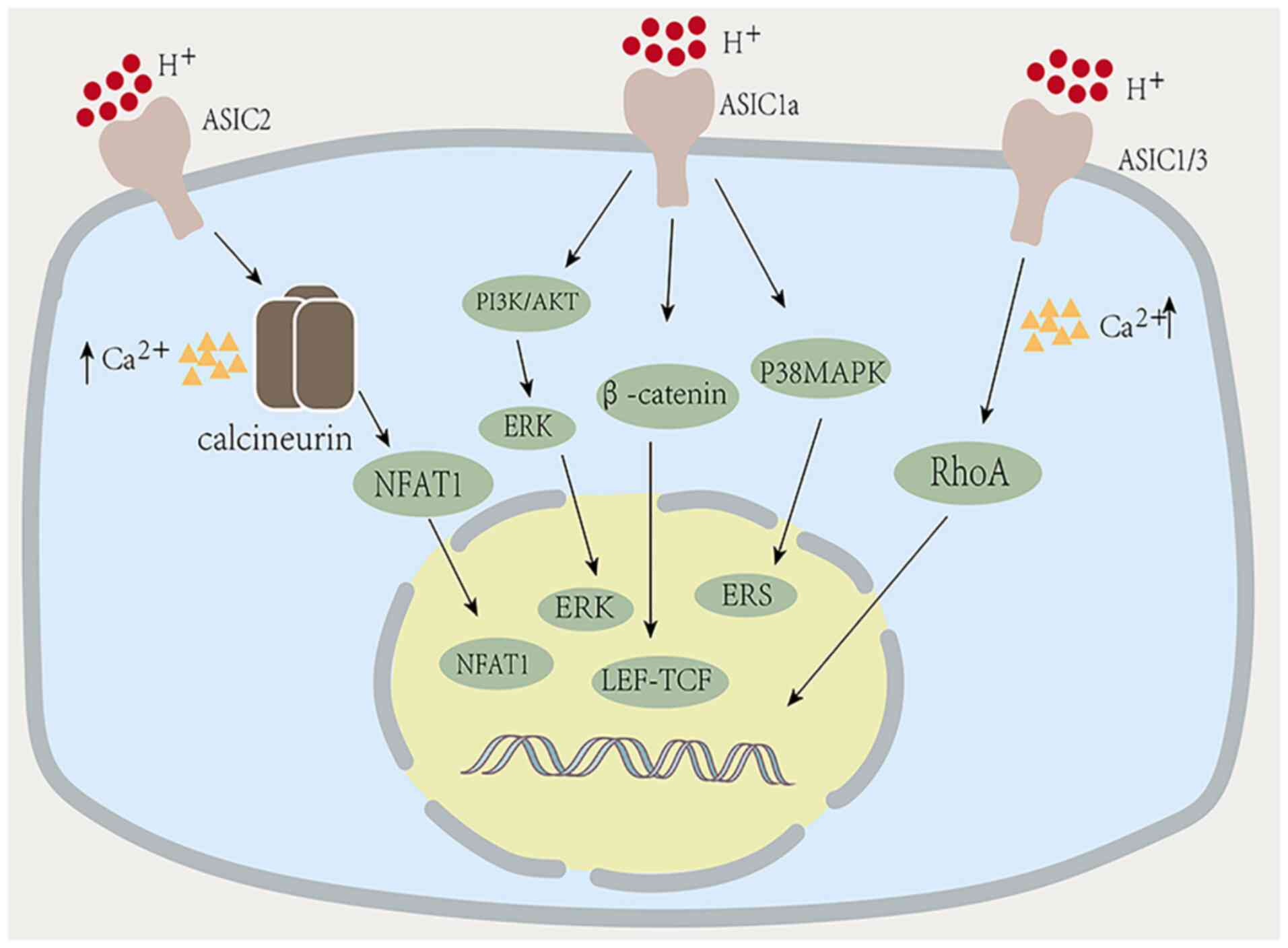
that CRC may be related to ASICs. Zhou et al (80) verified the conjecture that ASIC2
promotes the invasion of CRC cells during acidosis. In further
experiments, it was found that the average weight of tumors
expressing ASIC2 was significantly higher than that of tumors in
which ASIC2 expression was knocked down, indicating that ASIC2
promoted the proliferation of CRC cells in vitro and in
vivo. At the same time, ASIC2 was also found to promote liver
metastasis of CRC cells in vivo. Calcineurin is a
Ca2+-dependent serine/threonine phosphatase with the
immune central function that promotes the development of intestinal
tumors by regulating the function of mouse tumor stem cells
(133). When activated by
intracellular Ca2+, calcineurin dephosphorylates the
activated T nuclear factor (NFAT) protein, leading to nuclear
translocation of NFAT (133).
The transcription factors of the NFAT family play a key role in
T-cell activation (134). The
expression of IL-6 in NFAT1-deficient mice was significantly
decreased, resulting in the occurrence and development of CRC
(135,136). The results showed that ASIC2
overexpression significantly increased NFAT1 nuclear translocation
induced by acidosis, while ASIC2 gene knockout had the
opposite effect. In addition, CsA (a calcineurin inhibitor)
inhibited calcineurin/NFAT signaling in a dose-dependent manner,
which significantly reduced the invasion of SW480 cells induced by
ASIC2 acidosis, and NFAT1 knockdown also inhibited the invasion of
CRC cells. These results suggest that NFAT1 plays an important role
in the invasion, migration, and metastasis of CRC by regulating
gene transcription. Based on the above experimental results, ASIC2
promotes the invasion of CRC cells by activating the
calcineurin/NFAT1 signaling pathway under acidosis. Further studies
also showed that ASIC2 expression is related to CRC recurrence,
tumor staging, distant metastasis, a poorer prognosis, and NFAT1
expression. If ASIC2 expression is inhibited, CRC can be delayed to
a certain extent, highlighting a novel direction for the treatment
of CRC. In summary, ASICs are associated with the occurrence,
development, invasion, and metastasis of several digestive system
tumors in acidosis, and this may underlie treatments for the
prevention and treatment of digestive system tumors, and also open
up avenues for novel research directions for tumors of other
systems.
ASICs are widely distributed throughout the body
where they function as acid sensors, widely involved in a variety
of pathophysiological processes that involve acidosis. This review
provides a basic and systematic summary of the physiological and
pathological roles of ASICs in the digestive system, and shows that
different subtypes of ASICs participate in the occurrence,
development, invasion, and metastasis of digestive diseases and
tumors in the acidic microenvironments, which is of great
significance for further exploring the physiological role of ASICs
in the body and developing targeted ASIC drug therapies. However,
the extensive physiological and pathological mechanisms of ASICs in
the human body have not been elucidated and require further study.
The physiological and pathological significance of ASICs in the
digestive system should be further studied and their relevance as
molecular markers for the diagnosis and treatment of human-related
diseases should be assessed.
Not applicable.
LZ and LZ made substantial contributions to the
conception and design of the article. XY, SY, HW, JA, HJ, GW, and
BT were involved in revising the manuscript critically for
important intellectual content. Data authentication is not
applicable. All authors read and approved the final manuscript for
publication.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
Not applicable.
The present study was supported by grants from the National
Natural Science Foundation of China (grant nos. 81960507, 82073087
and 82160112), the Science and Technology Bureau fund of Zunyi City
[grant no. ZUN SHI KE HE HZ ZI (2019)93-Hao] and the Science and
Technology Plan Project of Guizhou Province [grant nos. QIAN KE HE
JI CHU-ZK(2021)YI BAN451 and QIAN KE HE LH ZI(2017)7095 HAO].
|
1
|
Levin LR and Buck J: Physiological roles
of acid-base sensors. Annu Rev Physiol. 77:347–362. 2015.
View Article : Google Scholar
|
|
2
|
Cheng YR, Jiang BY and Chen CC:
Acid-sensing ion channels: Dual function proteins for chemo-sensing
and mechano-sensing. J Biomed Sci. 25:462018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Deval E and Lingueglia E: Acid-Sensing Ion
Channels and nociception in the peripheral and central nervous
systems. Neuropharmacology. 94:49–57. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chu XP and Xiong ZG: Physiological and
pathological functions of acid-sensing ion channels in the central
nervous system. Curr Drug Targets. 13:263–271. 2012. View Article : Google Scholar :
|
|
5
|
Page AJ, Brierley SM, Martin CM, Price MP,
Symonds E, Butler R, Wemmie JA and Blackshaw LA: Different
contributions of ASIC channels 1a, 2, and 3 in gastrointestinal
mechanosensory function. Gut. 54:1408–1415. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Dong X, Ko KH, Chow J, Tuo B, Barrett KE
and Dong H: Expression of acid-sensing ion channels in intestinal
epithelial cells and their role in the regulation of duodenal
mucosal bicarbonate secretion. Acta Physiol (Oxf). 201:97–107.
2011. View Article : Google Scholar
|
|
7
|
Jones RC III, Otsuka E, Wagstrom E, Jensen
CS, Price MP and Gebhart GF: Short-term sensitization of colon
mechanoreceptors is associated with long-term hypersensitivity to
colon distention in the mouse. Gastroenterology. 133:184–194. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chen X, Sun X, Wang Z, Zhou X, Xu L, Li F,
Zhang X, Pan J, Qi L, Qian H and Mao Z: Involvement of acid-sensing
ion channel 1a in gastric carcinoma cell migration and invasion.
Acta Biochim Biophys Sin (Shanghai). 50:440–446. 2018. View Article : Google Scholar
|
|
9
|
Krishtal OA and Pidoplichko VI: A receptor
for protons in the nerve cell membrane. Neuroscience. 5:2325–2327.
1980. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Waldmann R, Champigny G, Bassilana F,
Heurteaux C and Lazdunski M: A proton-gated cation channel involved
in acid-sensing. Nature. 386:173–177. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Garty H and Palmer LG: Epithelial sodium
channels: Function, structure, and regulation. Physiol Rev.
77:359–396. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Waldmann R and Lazdunski M: H (+)-gated
cation channels: Neuronal acid sensors in the NaC/DEG family of ion
channels. Curr Opin Neurobiol. 8:418–424. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kellenberger S and Schild L: Epithelial
sodium channel/degenerin family of ion channels: A variety of
functions for a shared structure. Physiol Rev. 2:735–767. 2002.
View Article : Google Scholar
|
|
14
|
Benos DJ and Stanton BA: Functional
domains within the degenerin/epithelial sodium channel (Deg/ENaC)
super-family of ion channels. J Physiol. 520(Pt 3): 631–644. 1999.
View Article : Google Scholar
|
|
15
|
Kellenberger S and Schild L: International
union of basic and clinical pharmacology. XCI. structure, function,
and pharmacology of acid-sensing ion channels and the epithelial
Na+ channel. Pharmacol Rev. 67:1–35. 2015. View Article : Google Scholar
|
|
16
|
Sherwood TW, Frey EN and Askwith CC:
Structure and activity of the acid-sensing ion channels. Am J
Physiol Cell Physiol. 303:C699–C710. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Grunder S and Chen X: Structure, function,
and pharmacology of acid-sensing ion channels (ASICs): Focus on
ASIC1a. Int J Physiol Pathophysiol Pharmacol. 2:73–94.
2010.PubMed/NCBI
|
|
18
|
Jasti J, Furukawa H, Gonzales EB and
Gouaux E: Structure of acid-sensing ion channel 1 at 1.9 A
resolution and low pH. Nature. 449:316–323. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gonzales EB, Kawate T and Gouaux E: Pore
architecture and ion sites in acid-sensing ion channels and P2X
receptors. Nature. 460:599–604. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Krishtal O: The ASICs: Signaling
molecules? modulators? Trends Neurosci. 26:477–483. 2003.
View Article : Google Scholar
|
|
21
|
Wemmie JA, Taugher RJ and Kreple CJ:
Acid-sensing ion channels in pain and disease. Nat Rev Neurosci.
14:461–471. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Holzer P: Acid-sensing ion channels in
gastrointestinal function. Neuropharmacology. 94:72–79. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wemmie JA, Price MP and Welsh MJ:
Acid-sensing ion channels: Advances, questions and therapeutic
opportunities. Trends Neurosci. 29:578–586. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Holzer P: Acid-sensitive ion channels and
receptors. Handb Exp Pharmacol. 283–332. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sherwood TW, Lee KG, Gormley MG and
Askwith CC: Heteromeric acid-sensing ion channels (ASICs) composed
of ASIC2b and ASIC1a display novel channel properties and
contribute to acidosis-induced neuronal death. J Neurosci.
31:9723–9734. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Waldmann R, Bassilana F, de Weille J,
Champigny G, Heurteaux C and Lazdunski M: Molecular cloning of a
non-inactivating proton-gated Na+ channel specific for sensory
neurons. J Biol Chem. 272:20975–20978. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yu Y, Chen Z, Li WG, Cao H, Feng EG, Yu F,
Liu H, Jiang H and Xu TL: A nonproton ligand sensor in the
acid-sensing ion channel. Neuron. 68:61–72. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Noel J, Salinas M, Baron A, Diochot S,
Deval E and Lingueglia E: Current perspectives on acid-sensing ion
channels: New advances and therapeutic implications. Expert Rev
Clin Pharmacol. 3:331–346. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Diochot S, Salinas M, Baron A, Escoubas P
and Lazdunski M: Peptides inhibitors of acid-sensing ion channels.
Toxicon. 49:271–284. 2007. View Article : Google Scholar
|
|
30
|
Wemmie JA, Askwith CC, Lamani E, Cassell
MD, Freeman JH Jr and Welsh MJ: Acid-sensing ion channel 1 is
localized in brain regions with high synaptic density and
contributes to fear conditioning. J Neurosci. 23:5496–5502. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chen CC and Zimmer A, Sun WH, Hall J,
Brownstein MJ and Zimmer A: A role for ASIC3 in the modulation of
high-intensity pain stimuli. Proc Natl Acad Sci USA. 99:8992–8997.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Baron A, Voilley N, Lazdunski M and
Lingueglia E: Acid sensing ion channels in dorsal spinal cord
neurons. J Neurosci. 28:1498–1508. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wu LJ, Duan B, Mei YD, Gao J, Chen JG,
Zhuo M, Xu L, Wu M and Xu TL: Characterization of acid-sensing ion
channels in dorsal horn neurons of rat spinal cord. J Biol Chem.
279:43716–43724. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Su X, Li Q, Shrestha K, Cormet-Boyaka E,
Chen L, Smith PR, Sorscher EJ, Benos DJ, Matalon S and Ji HL:
Interregulation of proton-gated Na(+) channel 3 and cystic fibrosis
transmembrane conductance regulator. J Biol Chem. 281:36960–36968.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Jahr H, van Driel M, van Osch GJ, Weinans
H and van Leeuwen JP: Identification of acid-sensing ion channels
in bone. Biochem Biophys Res Commun. 337:349–354. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Richter TA, Dvoryanchikov GA, Roper SD and
Chaudhari N: Acid-sensing ion channel-2 is not necessary for sour
taste in mice. J Neurosci. 24:4088–4091. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Grifoni SC, Jernigan NL, Hamilton G and
Drummond HA: ASIC proteins regulate smooth muscle cell migration.
Microvasc Res. 75:202–210. 2008. View Article : Google Scholar
|
|
38
|
Berdiev BK, Xia J, McLean LA, Markert JM,
Gillespie GY, Mapstone TB, Naren AP, Jovov B, Bubien JK, Ji HL, et
al: Acid-sensing ion channels in malignant gliomas. J Biol Chem.
278:15023–15034. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Chen CC, England S, Akopian AN and Wood
JN: A sensory neuron-specific, proton-gated ion channel. Proc Natl
Acad Sci USA. 95:10240–10245. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Page AJ, Brierley SM, Martin CM,
Martinez-Salgado C, Wemmie JA, Brennan TJ, Symonds E, Omari T,
Lewin GR, Welsh MJ and Blackshaw LA: The ion channel ASIC1
contributes to visceral but not cutaneous mechanoreceptor function.
Gastroenterology. 127:1739–1747. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Liu L and Simon SA: Acidic stimuli
activates two distinct pathways in taste receptor cells from rat
fungiform papillae. Brain Res. 923:58–70. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Tan ZY, Lu Y, Whiteis CA, Benson CJ,
Chapleau MW and Abboud FM: Acid-sensing ion channels contribute to
transduction of extracellular acidosis in rat carotid body glomus
cells. Circ Res. 101:1009–1019. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Ugawa S, Inagaki A, Yamamura H, Ueda T,
Ishida Y, Kajita K, Shimizu H and Shimada S: Acid-sensing ion
channel-1b in the stereocilia of mammalian cochlear hair cells.
Neuroreport. 17:1235–1239. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Garcia-Anoveros J, Derfler B,
Neville-Golden J, Hyman BT and Corey DP: BNaC1 and BNaC2 constitute
a new family of human neuronal sodium channels related to
degenerins and epithelial sodium channels. Proc Natl Acad Sci USA.
94:1459–1464. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Lingueglia E, de Weille JR, Bassilana F,
Heurteaux C, Sakai H, Waldmann R and Lazdunski M: A modulatory
subunit of acid sensing ion channels in brain and dorsal root
ganglion cells. J Biol Chem. 272:29778–29783. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Price MP, Lewin GR, McIlwrath SL, Cheng C,
Xie J, Heppenstall PA, Stucky CL, Mannsfeldt AG, Brennan TJ,
Drummond HA, et al: The mammalian sodium channel BNC1 is required
for normal touch sensation. Nature. 407:1007–1011. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Hughes PA, Brierley SM, Young RL and
Blackshaw LA: Localization and comparative analysis of acid-sensing
ion channel (ASIC1, 2, and 3) mRNA expression in mouse colonic
sensory neurons within thoracolumbar dorsal root ganglia. J Comp
Neurol. 500:863–875. 2007. View Article : Google Scholar
|
|
48
|
Ettaiche M, Guy N, Hofman P, Lazdunski M
and Waldmann R: Acid-sensing ion channel 2 is important for retinal
function and protects against light-induced retinal degeneration. J
Neurosci. 24:1005–1012. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Lilley S, LeTissier P and Robbins J: The
discovery and characterization of a proton-gated sodium current in
rat retinal ganglion cells. J Neurosci. 24:1013–1022. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Brockway LM, Zhou ZH, Bubien JK, Jovov B,
Benos DJ and Keyser KT: Rabbit retinal neurons and glia express a
variety of ENaC/DEG subunits. Am J Physiol Cell Physiol.
283:C126–C134. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Peng BG, Ahmad S, Chen S, Chen P, Price MP
and Lin X: Acid-sensing ion channel 2 contributes a major component
to acid-evoked excitatory responses in spiral ganglion neurons and
plays a role in noise susceptibility of mice. J Neurosci.
24:10167–10175. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Huang C, Hu ZL, Wu WN, Yu DF, Xiong QJ,
Song JR, Shu Q, Fu H, Wang F and Chen JG: Existence and distinction
of acid-evoked currents in rat astrocytes. Glia. 58:1415–1424.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Yu XW, Hu ZL, Ni M, Fang P, Zhang PW, Shu
Q, Fan H, Zhou HY, Ni L, Zhu LQ, et al: Acid-sensing ion channels
promote the inflammation and migration of cultured rat microglia.
Glia. 63:483–496. 2015. View Article : Google Scholar
|
|
54
|
Ugawa S, Yamamoto T, Ueda T, Ishida Y,
Inagaki A, Nishigaki M and Shimada S: Amiloride-Insensitive
currents of the acid-sensing ion Channel-2a (ASIC2a)/ASIC2b
heteromeric sour-taste receptor channel. J Neurosci. 23:3616–3622.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Delaunay A, Gasull X, Salinas M, Noël J,
Friend V, Lingueglia E and Deval E: Human ASIC3 channel dynamically
adapts its activity to sense the extracellular pH in both acidic
and alkaline directions. Proc Natl Acad Sci USA. 109:13124–13129.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Voilley N, de Weille J, Mamet J and
Lazdunski M: Nonsteroid Anti-Inflammatory Drugs Inhibit Both the
Activity and the Inflammation-Induced Expression of Acid-Sensing
Ion Channels in Nociceptors. J Neurosci. 21:8026–8033. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Price MP, McIlwrath SL, Xie J, Cheng C,
Qiao J, Tarr DE, Sluka KA, Brennan TJ, Lewin GR and Welsh MJ: The
DRASIC cation channel contributes to the detection of cutaneous
touch and acid stimuli in mice. Neuron. 32:1071–1083. 2001.
View Article : Google Scholar
|
|
58
|
Dusenkova S, Ru F, Surdenikova L,
Nassenstein C, Hatok J, Dusenka R, Banovcin P Jr, Kliment J, Tatar
M and Kollarik M: The expression profile of acid-sensing ion
channel (ASIC) subunits ASIC1a, ASIC1b, ASIC2a, ASIC2b, and ASIC3
in the esophageal vagal afferent nerve subtypes. Am J Physiol
Gastrointest Liver Physiol. 307:G922–G930. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Babinski K, Le KT and Seguela P: Molecular
cloning and regional distribution of a human proton receptor
subunit with biphasic functional properties. J Neurochem. 72:51–57.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Ettaiche M, Deval E, Pagnotta S, Lazdunski
M and Lingueglia E: Acid-sensing ion channel 3 in retinal function
and survival. Invest Ophthalmol Vis Sci. 50:2417–2426. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Ikeuchi M, Kolker SJ, Burnes LA, Walder RY
and Sluka KA: Role of ASIC3 in the primary and secondary
hyperalgesia produced by joint inflammation in mice. Pain.
137:662–669. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Kolker SJ, Walder RY, Usachev Y, Hillman
J, Boyle DL, Firestein GS and Sluka KA: Acid-sensing ion channel 3
expressed in type B synoviocytes and chondrocytes modulates
hyaluronan expression and release. Ann Rheum Dis. 69:903–909. 2010.
View Article : Google Scholar
|
|
63
|
Meng QY, Wang W, Chen XN, Xu TL and Zhou
JN: Distribution of acid-sensing ion channel 3 in the rat
hypothalamus. Neuroscience. 159:1126–1134. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Huang SJ, Yang WS, Lin YW, Wang HC and
Chen CC: Increase of insulin sensitivity and reversal of
age-dependent glucose intolerance with inhibition of ASIC3. Biochem
Biophys Res Commun. 371:729–734. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Sole-Magdalena A, Revuelta EG,
Menénez-Díaz I, Calavia MG, Cobo T, García-Suárez O, Pérez-Piñera
P, De Carlos F, Cobo J and Vega JA: Human odontoblasts express
transient receptor protein and acid-sensing ion channel
mechanosensor proteins. Microsc Res Tech. 74:457–463. 2011.
View Article : Google Scholar
|
|
66
|
Hildebrand MS, de Silva MG, Klockars T,
Rose E, Price M, Smith RJ, McGuirt WT, Christopoulos H, Petit C and
Dahl HH: Characterisation of DRASIC in the mouse inner ear. Hear
Res. 190:149–160. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Grunder S, Geissler HS, Bassler EL and
Ruppersberg JP: A new member of acid-sensing ion channels from
pituitary gland. Neuroreport. 11:1607–1611. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Friese MA, Craner MJ, Etzensperger R,
Vergo S, Wemmie JA, Welsh MJ, Vincent A and Fugger L: Acid-sensing
ion channel-1 contributes to axonal degeneration in autoimmune
inflammation of the central nervous system. Nat Med. 13:1483–1489.
2007. View
Article : Google Scholar : PubMed/NCBI
|
|
69
|
Omerbasic D, Schuhmacher LN, Bernal Sierra
YA, Smith ES and Lewin GR: ASICs and mammalian mechanoreceptor
function. Neuropharmacology. 94:80–86. 2015. View Article : Google Scholar
|
|
70
|
Deval E, Gasull X, Noël J, Salinas M,
Baron A, Diochot S and Lingueglia E: Acid-sensing ion channels
(ASICs): Pharmacology and implication in pain. Pharmacol Ther.
128:549–558. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Vann KT and Xiong ZG: Acid-sensing ion
channel 1 contributes to normal olfactory function. Behav Brain
Res. 337:246–251. 2018. View Article : Google Scholar
|
|
72
|
Ettaiche M, Deval E, Cougnon M, Lazdunski
M and Voilley N: Silencing acid-sensing ion channel 1a alters
cone-mediated retinal function. J Neurosci. 26:5800–5809. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Wemmie JA, Chen J, Askwith CC,
Hruska-Hageman AM, Price MP, Nolan BC, Yoder PG, Lamani E, Hoshi T,
Freeman JH Jr and Welsh MJ: The acid-activated ion channel ASIC
contributes to synaptic plasticity, learning, and memory. Neuron.
34:463–477. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Yermolaieva O, Leonard AS, Schnizler MK,
Abboud FM and Welsh MJ: Extracellular acidosis increases neuronal
cell calcium by activating acid-sensing ion channel 1a. Proc Natl
Acad Sci USA. 101:6752–6757. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Wemmie JA, Coryell MW, Askwith CC, Lamani
E, Leonard AS, Sigmund CD and Welsh MJ: Overexpression of
acid-sensing ion channel 1a in transgenic mice increases acquired
fear-related behavior. Proc Natl Acad Sci USA. 101:3621–3626. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Dwyer JM, Rizzo SJ, Neal SJ, Lin Q, Jow F,
Arias RL, Rosenzweig-Lipson S, Dunlop J and Beyer CE: Acid sensing
ion channel (ASIC) inhibitors exhibit anxiolytic-like activity in
preclinical pharmacological models. Psychopharmacology (Berl).
203:41–52. 2009. View Article : Google Scholar
|
|
77
|
Gibbons DD, Kutschke WJ, Weiss RM and
Benson CJ: Heart failure induces changes in acid-sensing ion
channels in sensory neurons innervating skeletal muscle. J Physiol.
593:4575–4587. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Storozhuk M, Cherninskyi A, Maximyuk O,
Isaev D and Krishtal O: Acid-sensing ion channels: Focus on
physiological and some pathological roles in the brain. Curr
Neuropharmacol. 19:1570–1589. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Lee CY, Huang TJ, Wu MH, Li YY and Lee KD:
High expression of acid-sensing ion channel 2 (ASIC2) in bone cells
in osteoporotic vertebral fractures. Biomed Res Int.
2019:47142792019. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Zhou ZH, Song JW, Li W, Liu X, Cao L, Wan
LM, Tan YX, Ji SP, Liang YM and Gong F: The acid-sensing ion
channel, ASIC2, promotes invasion and metastasis of colorectal
cancer under acidosis by activating the calcineurin/NFAT1 axis. J
Exp Clin Cancer Res. 36:1302017. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Holzer P: Acid sensing by visceral
afferent neurones. Acta Physiol (Oxf). 201:63–75. 2011. View Article : Google Scholar
|
|
82
|
Kang JY and Yap I: Acid and gastric ulcer
pain. J Clin Gastroenterol. 13:514–516. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Dang K, Bielfeldt K, Lamb K and Gebhart
GF: Gastric ulcers evoke hyperexcitability and enhance P2X receptor
function in rat gastric sensory neurons. J Neurophysiol.
93:3112–3119. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Sugiura T, Dang K, Lamb K, Bielefeldt K
and Gebhart GF: Acid-sensing properties in rat gastric sensory
neurons from normal and ulcerated stomach. J Neurosci.
25:2617–2627. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Krishtal OA and Pidoplichko VI: A receptor
for protons in the membrane of sensory neurons may participate in
nociception. Neuroscience. 6:2599–2601. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Schicho R, Florian W, Liebmann I, Holzer P
and Lippe IT: Increased expression of TRPV1 receptor in dorsal root
ganglia by acid insult of the rat gastric mucosa. Eur J Neurosci.
19:1811–1818. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Bielefeldt K and Davis BM: Differential
effects of ASIC3 and TRPV1 deletion on gastroesophageal sensation
in mice. Am J Physiol Gastrointest Liver Physiol. 294:G130–G138.
2008. View Article : Google Scholar
|
|
88
|
Leffler A, Monter B and Koltzenburg M: The
role of the capsaicin receptor TRPV1 and acid-sensing ion channels
(ASICS) in proton sensitivity of subpopulations of primary
nociceptive neurons in rats and mice. Neuroscience. 139:699–709.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Page AJ, Martin CM and Blackshaw LA: Vagal
mechanoreceptors and chemoreceptors in mouse stomach and esophagus.
J Neurophysiol. 87:2095–2103. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Brierley SM, Jones RC III, Gebhart GF and
Blackshaw LA: Splanchnic and pelvic mechanosensory afferents signal
different qualities of colonic stimuli in mice. Gastroenterology.
127:166–178. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Ruan N, Tribble J, Peterson AM, Jiang Q,
Wang JQ and Chu XP: Acid-sensing ion channels and mechanosensation.
Int J Mol Sci. 22:48102021. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Bohlen CJ, Chesler AT, Sharif-Naeini R,
Medzihradszky KF, Zhou S, King D, Sánchez EE, Burlingame AL,
Basbaum AI and Julius D: A heteromeric Texas coral snake toxin
targets acid-sensing ion channels to produce pain. Nature.
479:410–414. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Kang S, Jang JH, Price MP, Gautam M,
Benson CJ, Gong H, Welsh MJ and Brennan TJ: Simultaneous disruption
of mouse ASIC1a, ASIC2 and ASIC3 genes enhances cutaneous
mechanosensitivity. PLoS One. 7:e352252012. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Lamb K, Kang YM, Gebhart GF and Bielefeldt
K: Gastric inflammation triggers hypersensitivity to acid in awake
rats. Gastroenterology. 125:1410–1418. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Wultsch T, Painsipp E, Shahbazian A,
Mitrovic M, Edelsbrunner M, Lazdunski M, Waldmann R and Holzer P:
Deletion of the acid-sensing ion channel ASIC3 prevents
gastritis-induced acid hyperresponsiveness of the stomach-brainstem
axis. Pain. 134:245–253. 2008. View Article : Google Scholar
|
|
96
|
Matricon J, Muller E, Accarie A, Meleine
M, Etienne M, Voilley N, Busserolles J, Eschalier A, Lazdunski M,
Bourdu S, et al: Peripheral contribution of NGF and ASIC1a to
colonic hypersensitivity in a rat model of irritable bowel
syndrome. Neurogastroenterol Motil. 25:e740–e754. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Bourdu S, Dapoigny M, Chapuy E, Artigue F,
Vasson MP, Dechelotte P, Bommelaer G, Eschalier A and Ardid D:
Rectal instillation of butyrate provides a novel clinically
relevant model of noninflammatory colonic hypersensitivity in rats.
Gastroenterology. 128:1996–2008. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Matricon J, Gelot A, Etienne M, Lazdunski
M, Muller E and Ardid D: Spinal cord plasticity and acid-sensing
ion channels involvement in a rodent model of irritable bowel
syndrome. Eur J Pain. 15:335–343. 2011. View Article : Google Scholar
|
|
99
|
Miwa H, Kondo T, Oshima T, Fukui H, Tomita
T and Watari J: Esophageal sensation and esophageal
hypersensitivity-overview from bench to bedside. J
Neurogastroenterol Motil. 16:353–362. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Guarino MP, Cheng L, Ma J, Harnett K,
Biancani P, Altomare A, Panzera F, Behar J and Cicala M: Increased
TRPV1 gene expression in esophageal mucosa of patients with
non-erosive and erosive reflux disease. Neurogastroenterol Motil.
22:746–751 e219. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Omori M, Yokoyama M, Matsuoka Y, Kobayashi
H, Mizobuchi S, Itano Y, Morita K and Ichikawa H: Effects of
selective spinal nerve ligation on acetic acid-induced nociceptive
responses and ASIC3 immunoreactivity in the rat dorsal root
ganglion. Brain Res. 1219:26–31. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Staniland AA and McMahon SB: Mice lacking
acid-sensing ion channels (ASIC) 1 or 2, but not ASIC3, show
increased pain behaviour in the formalin test. Eur J Pain.
13:554–563. 2009. View Article : Google Scholar
|
|
103
|
Yang M, Li ZS, Chen DF, Zou DW, Xu XR,
Fang DC, Xu GM, Stephens RL and Wang ZG: Quantitative assessment
and characterization of visceral hyperalgesia evoked by esophageal
balloon distention and acid perfusion in patients with functional
heartburn, nonerosive reflux disease, and erosive esophagitis. Clin
J Pain. 26:326–331. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Han X, Zhang Y, Lee A, Li Z, Gao J, Wu X,
Zhao J, Wang H, Chen D, Zou D and Owyang C: Upregulation of acid
sensing ion channels is associated with esophageal hypersensitivity
in GERD. FASEB J. 36:e220832022. View Article : Google Scholar
|
|
105
|
Webb BA, Chimenti M, Jacobson MP and
Barber DL: Dysregulated pH: A perfect storm for cancer progression.
Nat Rev Cancer. 11:671–677. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Jin C, Ye QH, Yuan FL, Gu YL, Li JP, Shi
YH, Shen XM, Bo-Liu and Lin ZH: Involvement of acid-sensing ion
channel 1alpha in hepatic carcinoma cell migration and invasion.
Tumour Biol. 36:4309–4317. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Jin C, Yuan FL, Gu YL, Li X, Liu MF, Shen
XM, Liu B and Zhu MQ: Over-expression of ASIC1a promotes
proliferation via activation of the β-catenin/LEF-TCF axis and is
associated with disease outcome in liver cancer. Oncotarget.
8:25977–25988. 2017. View Article : Google Scholar
|
|
108
|
Sun X, Cao YB, Hu LF, Yang YP, Li J, Wang
F and Liu CF: ASICs mediate the modulatory effect by paeoniflorin
on α-synuclein autophagic degradation. Brain Res. 1396:77–87. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Zhou RP, Wu XS, Wang ZS, Xie YY, Ge JF and
Chen FH: Novel insights into acid-sensing ion channels:
Implications for degenerative diseases. Aging Dis. 7:491–501. 2015.
View Article : Google Scholar
|
|
110
|
Zhang Q, Wu S, Zhu J, Chai D and Gan H:
Down-regulation of ASIC1 suppressed gastric cancer via inhibiting
autophagy. Gene. 608:79–85. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Lee UE and Friedman SL: Mechanisms of
hepatic fibrogenesis. Best Pract Res Clin Gastroenterol.
25:195–206. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Wu FR, Pan CX, Rong C, Xia Q, Yuan FL,
Tang J, Wang XY, Wang N, Ni WL and Chen FH: Inhibition of
acid-sensing ion channel 1a in hepatic stellate cells attenuates
PDGF-induced activation of HSCs through MAPK pathway. Mol Cell
Biochem. 395:199–209. 2014. View Article : Google Scholar
|
|
113
|
Zhu Y, Pan X, Du N, Li K, Hu Y, Wang L,
Zhang J, Liu Y, Zuo L, Meng X, et al: ASIC1a regulates
miR-350/SPRY2 by N6 -methyladenosine to promote liver
fibrosis. FASEB J. 34:14371–14388. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
de Bie P, van de Sluis B, Burstein E, van
de Berghe PV, Muller P, Berger R, Gitlin JD, Wijmenga C and Klomp
LW: Distinct Wilson's disease mutations in ATP7B are associated
with enhanced binding to COMMD1 and reduced stability of ATP7B.
Gastroenterology. 133:1316–1326. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Kong L, Huang H, Luan S, Liu H, Ye M and
Wu F: Inhibition of ASIC1a-Mediated ERS improves the activation of
HSCs and copper transport under copper load. Front Pharmacol.
12:6532722021. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Yu LX and Schwabe RF: The gut microbiome
and liver cancer: Mechanisms and clinical translation. Nat Rev
Gastroenterol Hepatol. 14:527–539. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Andersen AP, Moreira JM and Pedersen SF:
Interactions of ion transporters and channels with cancer cell
metabolism and the tumour microenvironment. Philos Trans R Soc Lond
B Biol Sci. 369:201300982014. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Javle MM, Gibbs JF, Iwata KK, Pak Y,
Rutledge P, Yu J, Black JD, Tan D and Khoury T:
Epithelial-mesenchymal transition (EMT) and activated extracellular
signal-regulated kinase (p-Erk) in surgically resected pancreatic
cancer. Ann Surg Oncol. 14:3527–3533. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
von Burstin J, Eser S, Paul MC, Seidler B,
Brandl M, Messer M, von Werder A, Schmidt A, Mages J, Pagel P, et
al: E-cadherin regulates metastasis of pancreatic cancer in vivo
and is suppressed by a SNAIL/HDAC1/HDAC2 repressor complex.
Gastroenterology. 137:361–371. 371.e1–5. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Deng S, Zhu S, Wang B, Li X, Liu Y, Qin Q,
Gong Q, Niu Y, Xiang C, Chen J, et al: Chronic pancreatitis and
pancreatic cancer demonstrate active epithelial-mesenchymal
transition profile, regulated by miR-217-SIRT1 pathway. Cancer
Lett. 355:184–191. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Peppicelli S, Bianchini F, Torre E and
Calorini L: Contribution of acidic melanoma cells undergoing
epithelial-to-mesenchymal transition to aggressiveness of
non-acidic melanoma cells. Clin Exp Metastasis. 31:423–433. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Deng S, Li X, Niu Y, Zhu S, Jin Y, Deng S,
Chen J, Liu Y, He C, Yin T, et al: MiR-652 inhibits acidic
microenvironment-induced epithelial-mesenchymal transition of
pancreatic cancer cells by targeting ZEB1. Oncotarget.
6:39661–39675. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Zhu S, Zhou HY, Deng SC, Deng SJ, He C, Li
X, Chen JY, Jin Y, Hu ZL, Wang F, et al: ASIC1 and ASIC3 contribute
to acidity-induced EMT of pancreatic cancer through activating
Ca2+/RhoA pathway. Cell Death Dis. 8:e28062017.
View Article : Google Scholar
|
|
124
|
Prevarskaya N, Skryma R and Shuba Y:
Calcium in tumour metastasis: New roles for known actors. Nat Rev
Cancer. 11:609–618. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Jaffe AB and Hall A: Rho GTPases:
Biochemistry and biology. Annu Rev Cell Dev Biol. 21:247–269. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Gulhati P, Bowen KA, Liu J, Stevens PD,
Rychahou PG, Chen M, Lee EY, Weiss HL, O'Connor KL, Gao T and Evers
BM: mTORC1 and mTORC2 regulate EMT, motility, and metastasis of
colorectal cancer via RhoA and Rac1 signaling pathways. Cancer Res.
71:3246–3256. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Fernandez-Tenorio M, Porras-González C,
Castellano A, Del Valle-Rodríguez A, López-Barneo J and Ureña J:
Metabotropic regulation of RhoA/Rho-associated kinase by L-type
Ca2+ channels: New mechanism for depolarization-evoked mammalian
arterial contraction. Circ Res. 108:1348–1357. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Fais S, De Milito A, You H and Qin W:
Targeting vacuolar H+-ATPases as a new strategy against cancer.
Cancer Res. 67:10627–10630. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Chen JL, Lucas JE, Schroeder T, Mori S, Wu
J, Nevins J, Dewhirst M, West M and Chi JT: The genomic analysis of
lactic acidosis and acidosis response in human cancers. PLoS Genet.
4:e10002932008. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Moellering RE, Black KC, Krishnamurty C,
Baggett BK, Stafford P, Rain M, Gatenby RA and Gillies RJ: Acid
treatment of melanoma cells selects for invasive phenotypes. Clin
Exp Metastasis. 25:411–425. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Estrella V, Chen T, Lloyd M, Wojtkowiak J,
Cornnell HH, Ibrahim-Hashim A, Bailey K, Balagurunathan Y, Rothberg
JM, Sloane BF, et al: Acidity generated by the tumor
microenvironment drives local invasion. Cancer Res. 73:1524–1535.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Fukumura D, Xu L, Chen Y, Gohongi T, Seed
B and Jain RK: Hypoxia and acidosis independently up-regulate
vascular endothelial growth factor transcription in brain tumors in
vivo. Cancer Res. 61:6020–6024. 2001.PubMed/NCBI
|
|
133
|
Peuker K, Muff S, Wang J, Künzel S, Bosse
E, Zeissig Y, Luzzi G, Basic M, Strigli A, Ulbricht A, et al:
Epithelial calcineurin controls microbiota-dependent intestinal
tumor development. Nat Med. 22:506–515. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Chuvpilo S, Jankevics E, Tyrsin D,
Akimzhanov A, Moroz D, Jha MK, Schulze-Luehrmann J, Santner-Nanan
B, Feoktistova E, König T, et al: Autoregulation of NFATc1/A
expression facilitates effector T cells to escape from rapid
apoptosis. Immunity. 16:881–895. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Weigmann B, Lehr HA, Yancopoulos G,
Valenzuela D, Murphy A, Stevens S, Schmidt J, Galle PR, Rose-John S
and Neurath MF: The transcription factor NFATc2 controls
IL-6-dependent T cell activation in experimental colitis. J Exp
Med. 205:2099–2110. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Gerlach K, Daniel C, Lehr HA, Nikolaev A,
Gerlach T, Atreya R, Rose-John S, Neurath MF and Weigmann B:
Transcription factor NFATc2 controls the emergence of colon cancer
associated with IL-6-dependent colitis. Cancer Res. 72:4340–4350.
2012. View Article : Google Scholar : PubMed/NCBI
|