Introduction
Malignant gliomas are the most common primary brain
tumors with high mortality and morbidity. Despite recent
advancements in chemotherapy, radiotherapy and neurosurgery, the
prognosis of patients with malignant gliomas remains dismal
(1). So there is a urgent need to
develop novel and effective therapeutic strategies for this
disease.
MicroRNAs (miRNAs) are a large family of endogenous
non-protein-coding small RNAs with 20–25 nucleotides in length
(2). miRNAs control gene
expression through binding to the 3′-untranslated regions (UTRs) of
target mRNAs, resulting in translational repression and/or mRNA
degradation (3). Emerging evidence
supports that miRNAs are novel players in carcinogenesis (4,5). In
this regard, miRNAs are dysregulated in most, if not all, types of
human cancer examined so far. Dysregulated miRNAs take part in
tumorigenesis by regulating cellular processes important to cancer
development, including cell proliferation, cell survival, cell
motility and invasiveness, angiogenesis, and drug resistance
(6–8). Targeting upregulated miRNAs have been
shown to suppress malignant phenotypes of cancer cells and may
therefore represent a novel treatment approach (6).
Accumulating evidence suggests that miR-21 and
miR-10b may behave as novel oncogenes in human cancers. miR-21 has
been identified as one of the most upregulated miRNAs in human
cancers. Increased level of plasma miR-21 has also been
demonstrated in some cancer types (9). In human gliomas, miR-21 is
upregulated during the progression of the tumor. Importantly, when
miR-21 is targeted in glioma tissues, inhibition of cell
proliferation and caspase-dependent apoptosis occur (10,11).
Several tumor-suppressors, including programmed cell death 4
(PDCD4) and tropomyosin (TPM1), have been identified as direct
targets of miR-21 (12,13). Regarding miR-10b, this putatively
oncogenic miRNA was initially found to be highly expressed in
metastatic breast cancer cells. miR-10b also positively regulates
the migration and invasion of breast cancer cells (14,15).
Overexpression of miR-10b in otherwise non-metastatic breast tumors
initiates robust invasion and metastasis. Recently, miR-10b was
reported to be upregulated in glioma tissues compared with
non-tumoral brain tissues. Upregulation of miR-10b was also
associated with higher grade gliomas (16). In relation to the intracellular
signaling network, miR-10b has been shown to target the tumor
suppressor HOXD10, which in turn regulates the expression of
metastasis-related genes, such as RhoC and urokinase-type
plasminogen activator receptor (14,17).
Although miR-21 and miR-10b are upregulated in
glioma tissues, their interactions in the regulation of oncogenic
phenotypes and pathways are unknown. Moreover, targeting these two
oncogenic miRNAs simultaneously may exert a more potent anti-cancer
effect as compared with inhibition of either miRNA alone. In the
present study, using synthetic miRNA inhibitors, we demonstrate
that inhibition of miR-21 and miR-10b synergistically induced cell
cycle arrest and apoptosis and inhibited cell invasiveness in a
human glioma cell line. Effects of co-inhibition of miR-21 and
miR-10b on the expression of several putative cellular targets were
also examined.
Materials and methods
Cell line and reagents
The human glioblastoma cell line U87MG was obtained
from the Institute of Biochemistry and Cell Biology, Chinese
Academy of Science (Shanghai, China). Primary antibodies were
purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). The
2′-O-methyl (2′-OMe-) miR-10b and miR-21 inhibitors were chemically
synthesized by Invitrogen (Carlsbad, CA, USA) with the following
sequences: miR-10b inhibitor: 5′-CAC AAA UUC GGU UCU ACA GGG UA-3′;
miR-21 inhibitor: 5′-UCA ACA UCA GUC UGA UAA GCU A-3′; scrambled
control: 5′-UCU UCA UGA GUC AGA UUA CCU A-3′. The sequence of
scrambled control has been analyzed by BLAST search to exclude
potential hits in the human transcriptome. The oligonucleotides
were purified by high-pressure liquid chromatography, dissolved in
diethylpyrocarbonate water, and frozen at −20°C. The final working
concentrations of scrambled control, miR-10b inhibitor, miR-21
inhibitor and combined miRNA inhibitors were 100 nmol/ml.
Cell culture and transfection
U87MG cells were maintained in Dulbecco’s modified
Eagle’s medium (Gibco, Carlsbad, CA, USA) supplemented with 10%
fetal bovine serum (Gibco), 2 mM glutamine (Sigma, St. Louis, USA),
100 units of penicillin/ml (Sigma), and 100 μg of
streptomycin/ml (Sigma). Cells were seeded in 25-cm2
flasks and incubated at 37°C with 5% CO2. Twenty-four
hours before transfection, cells at 70–90% confluence were
detached, transferred to 96-well or 6-well plates, and cultured in
fresh medium without antibiotics. Oligonucleotides (5–100 pmol)
were transfected into U87MG cells at 70% confluence using
Lipofectamine-2000 (0.25–5 μl) according to the
manufacturer’s instructions (Invitrogen). Transfection efficiency
was examined by fluorescence microscopy detection of FAM green
fluorescence signal (Abs 495nm, Em 520nm) that was labeled to the
scrambled control.
RNA extraction and real-time reverse
transcription-PCR for miRNA detection
miRNA was isolated 48 h after transfection with
Ambion mir-Vana™ miRNA isolation kit (Ambion, USA). A nanodrop
spectrophotometer (Gene, USA) was used to measure the concentration
of total RNA. Relative levels of miRNA were examined using SYBR
green real-time quantitative reverse transcription-PCR (qRT-PCR)
(Applied Biosystems) and normalized with U6 snRNA. RT and PCR
primers were purchased from Ambion. The amplification reaction was
performed using MJ real-time PCR (Bio-Rad, Hercules, CA, USA) for
40 cycles. Relative expression was calculated using the
2−ΔΔCt method (18).
All qRT-PCRs were performed in triplicate, and analyzed initially
using Opticon Monitor Analysis software V2.02 software (MJ
Research, USA).
Protein extraction and western
blotting
After transfection, total proteins were extracted
after solubilization in lysis buffer containing 62.5 mM Tris-HCl
(pH 6.8), 2% SDS, 10% glycerol, 1 mM sodium vanadate, and 1 mM
sodium fluoride. Protein extracts were resolved on a Tris-HCl 11%
gradient gel. Western blotting was performed using standard
methodologies as previously described (19). Blots were developed using the
enhanced chemiluminescence (ECL) reagents (Amersham Pharmacia,
Buckinghamshire, UK) and visualized using the Gene-Genius Imaging
System (Syngene, Frederick, MD, USA).
Cell viability assay
Cell viability was determined by the MTT assay.
Briefly, 5,000 transfected cells per well were seeded in 96-well
plates and allowed to attach overnight. On each day of five
consecutive days, 20 μl of MTT (0.5 mg/ml) was added to the
wells and the cells were incubated at 37°C for 4 h. The reaction
was then stopped by lysing the cells with 200 μl of dimethyl
sulfoxide (DMSO) for 15 min. Measurements of optical density were
obtained at the wavelength of 570 nm using spectrophotometric
analysis. The half maximal inhibitory concentration
(IC50) values were calculated from the linear regression
line of the plot of percentage inhibition versus log inhibitor
concentration.
Cell cycle analysis
Cell cycle was analyzed flow cytometry. In brief,
transfected and control cells in the log phase of growth were
harvested, washed with phosphate-buffered saline, fixed with 90%
ethanol overnight at 4°C, and then incubated with RNase at 37°C for
30 min. The nuclei of cells were stained with propidium iodide (PI)
for another 30 min. A total of 104 cells were examined
in a FACSCalibur flow cytometer (Becton-Dickinson, USA). Samples
were analyzed by flow cytometry for the FL-2 area and DNA
histograms were analyzed by Modifit software. Experiments were
performed in triplicate. Results are presented as percentage of
cells in a particular phase.
Quantitation of apoptosis
To quantify miRNA inhibitor-induced apoptosis,
annexin V/PI staining was performed, and apoptosis was evaluated by
flow cytometry analysis. Briefly, after transfection, both floating
and attached cells were collected and subject to annexin V/PI
staining using an annexin V-FITC Apoptosis Detection kit
(BioVision, Palo Alto, CA), according to the manufacturer’s
protocol. The resulting fluorescence was measured by flow cytometry
using a FACS flow cytometer (BD Biosciences, San Jose, CA).
Measurement of cell invasiveness
Cell invasiveness was examined using six-well
transwell chambers and a reconstituted extracellular matrix
membrane (Matrigel, USA). The cell invasion chambers were prepared
by placing 100 μl of a 1:5 dilution of Matrigel onto the
filter, and incubating the filter at 37°C for 30 min to allow
Matrigel polymerization. Transfected cells were resuspended at
5×105 cells/ml in serum-free medium. Afterwards, 200
μl of cell suspension from each treatment group was added to
the upper chambers. The chambers were incubated for 48 h in 37°C
with 5% CO2. The filters were then fixed in 95% ethanol
and stained with hematoxylin. The upper surfaces of the filters
were scraped twice with cotton swabs to remove non-migrated cells.
The experiments were repeated in triplicate wells, and the migrated
cells were counted microscopically (200×) in five different fields
per filter.
Statistical analysis
Results were analyzed using SPSS software 11.0 and
compared using one-way analysis of variance (ANOVA) with Fisher’s
post hoc test. Data were presented as mean ± standard deviation
(SD) of separate experiments (n≥3). P-values <0.05 were
considered to be significant.
Results
miR-10b and miR-21 inhibitors reduced the
expression of their miRNA targets in U87 glioma cells
FAM-labeled scrambled control was used to determine
the transfection efficiency of Lipofectamine-mediated entry of RNA
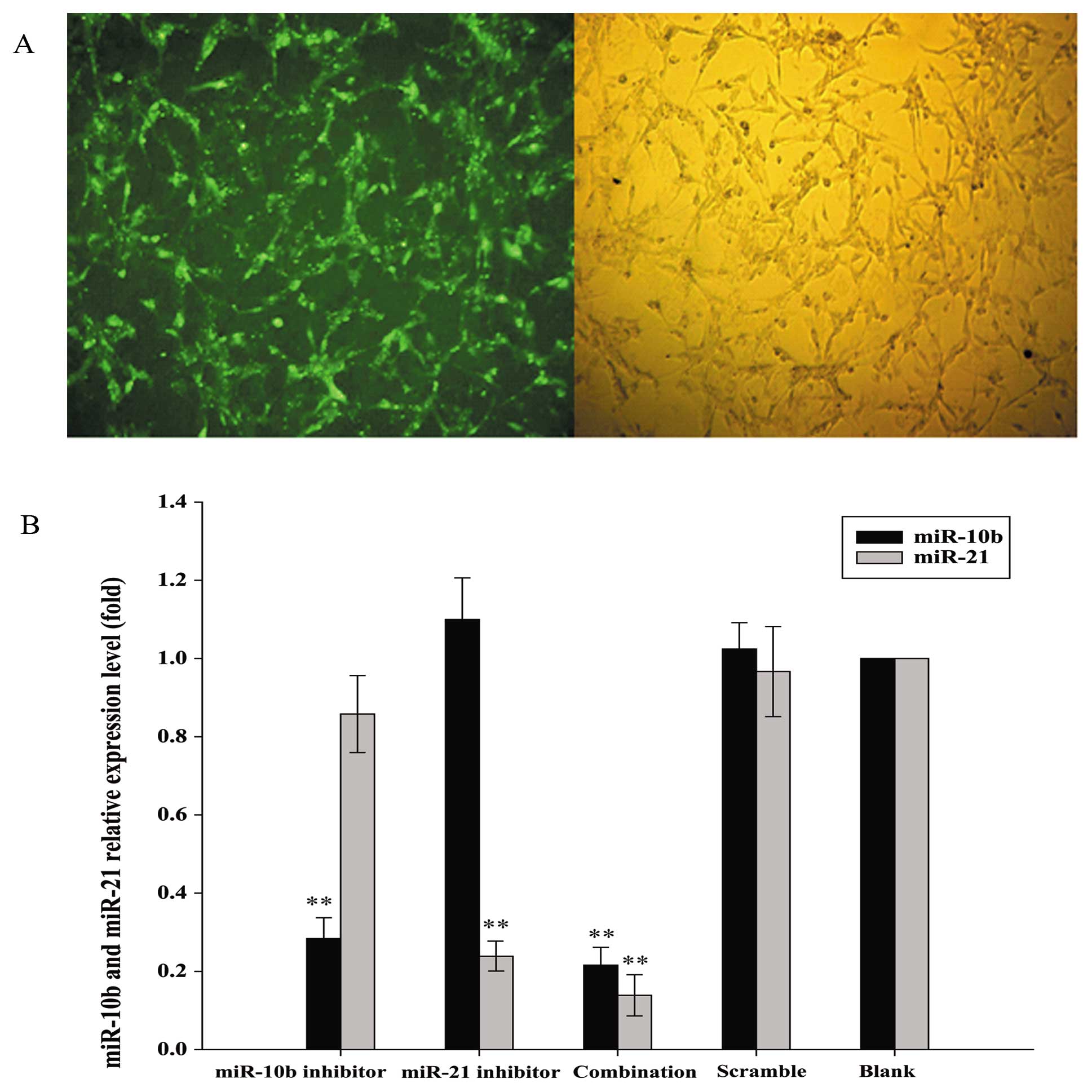
oligos into human glioma U87 cells. As shown in Fig. 1A, green fluorescent signals were
detected in more than 95% FAM-labeled scrambled control-transfected
U87 cells, indicating that RNA oligonucleotides could readily gain
access to the cells. RNA sequences that showed complementarity with
the sequences of miR-10b and miR-21 were then transfected into U87
cells to inhibit the functions of these two miRNAs. Since miRNA
inhibitors have been shown to induce the degradation of their
endogenous miRNA targets (20), we
determined if U87 cells transfected with miR-10b and miR-21
inhibitors, alone or in combination, showed downregulation of
miR-10b and miR-21 by qRT-PCR. Inhibitors of miR-10b and miR-21
specifically reduced the levels of their respective target miRNA in
U87 cells when compared with scrambled control. Moreover,
co-transfection of miR-10b and miR-21 inhibitors effectively
reduced the levels of both miRNAs (Fig. 1B).
miR-10b and miR-21 inhibitors lowered
cell proliferation in U87
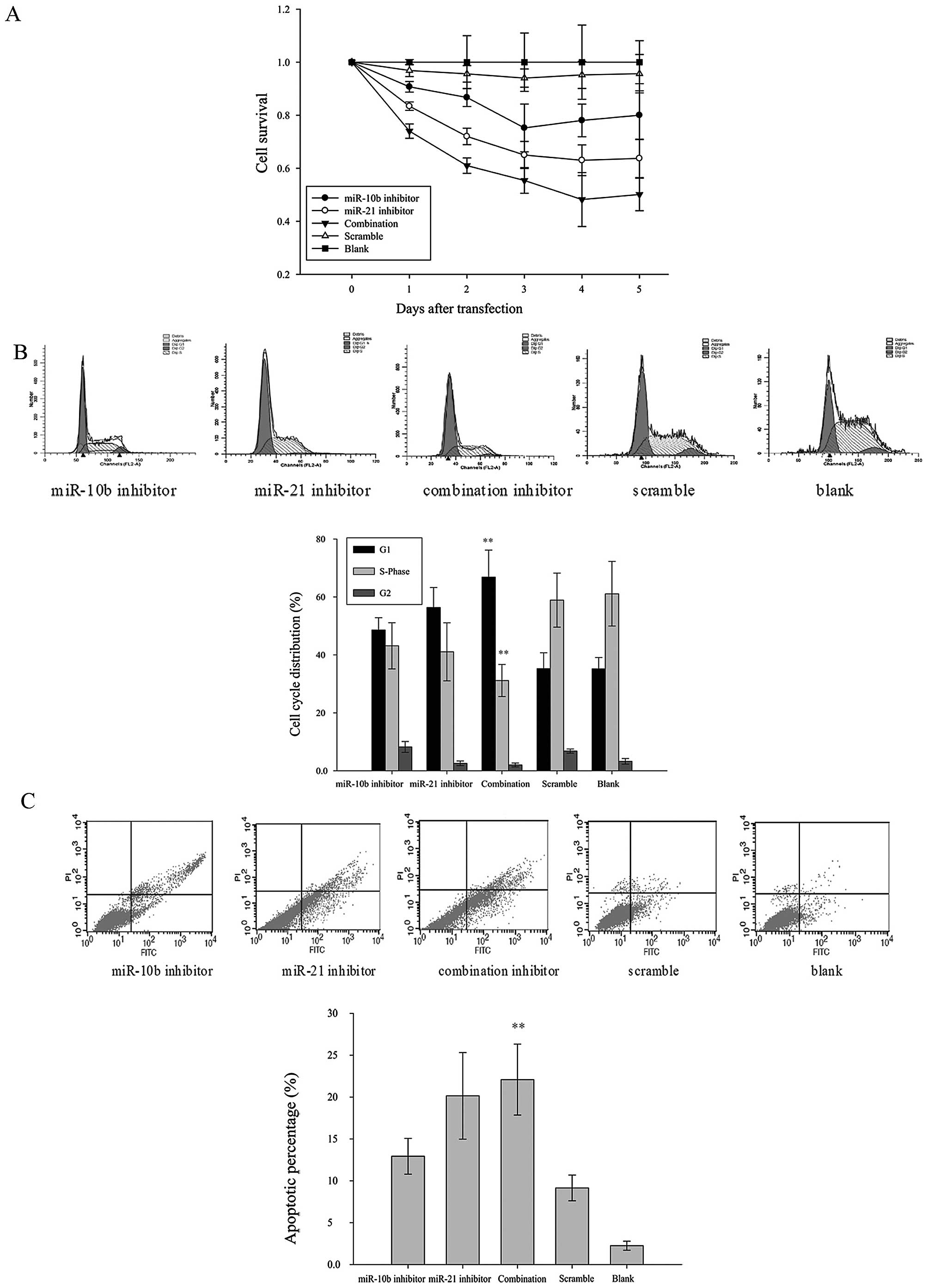
Cell viability was measured in miRNA
inhibitor-transfected U87 cells up to 5 days after transfection by
MTT assay. Results revealed that miR-10b and miR-21 inhibitors,
alone or in combination, exerted inhibitory effect on U87 cell
viability. The growth-inhibitory effect peaked on day 3–4
post-transfection (Fig. 2A). The
inhibitory effect of miR-21 was more prominent than that of miR-10b
(35.00±5.12% inhibition vs 24.80±9.00% inhibition on day 3).
Notably, when U87 cells were co-transfected with both miR-10b and
miR-21 inhibitors, cell viability was further reduced (48.20±10.18%
inhibition on day 3). These data suggest that inhibiting the
functions of miR-10b and miR-21 reduced the cell proliferation in
glioma cells.
miR-10b and miR-21 inhibitors induced
G0/G1-phase cell cycle arrest in U87
To determine if decreased cell viability was a
result of cell cycle arrest, we analyzed the cell cycle
distribution of miRNA inhibitor- or scrambled control-transfected
U87 cells by flow cytometry. At 48 h post-transfection, miR-21
inhibitor and miR-10b inhibitor, alone or in combination, increased
the proportion of U87 cells in G0/G1-phase
when compared with the scrambled control-transfected group. A
reciprocal reduction of cells in S-phase was also observed in these
treatment groups. The effect on cell cycle was more prominent in
U87 cells co-transfected with miR-10b and miR-21 inhibitors as
compared with those transfected with either miRNA inhibitor. These
data suggest that miR-10b and miR-21 inhibitors, especially in
combination, induced G0/G1-phase cell cycle
arrest in glioma cells (Fig.
2B).
miR-10b and miR-21 inhibitors induce
apoptosis in U87
Loss of phosphatidylserine asymmetry is a molecular
hallmark of apoptotic cell death. To determine if miR-10b and
miR-21 inhibitors induced apoptosis in addition to cell cycle
arrest, phosphatidylserine externalization was assayed by flow
cytometry of Annexin V/PI double-stained U87 cells. As shown in
Fig. 2C, the percentage of the
Annexin V-positive apoptotic cells were significantly higher in
cells transfected with inhibitors of miR-10b and miR-21, alone or
in combination, when compared with the scrambled control
transfected group. The pro-apoptotic effect of combined
transfection of miR-10b and miR-21 inhibitors was significantly
stronger than that of miR-10b inhibitor alone (p<0.05).
miR-10b and miR-21 inhibitors impair U87
cell invasiveness
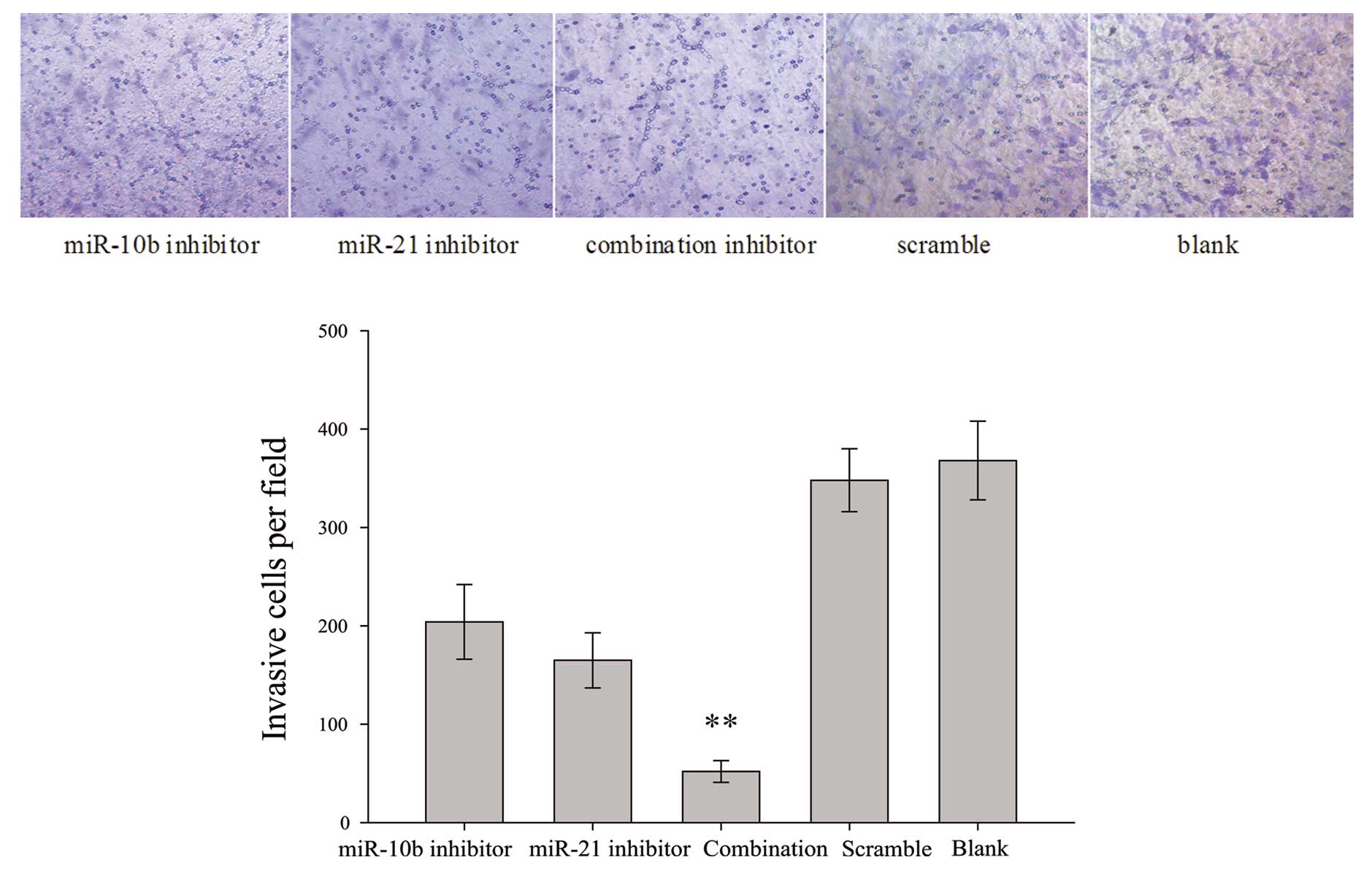
To measure the effects of miR-10b and miR-21
inhibitors, alone or in combination, on glioma cell invasiveness, a
trans-well invasion assay was employed. The system consists of two
fluid-filled stacked compartments, separated by a porous membrane
filter coated with Matrigel. Cells were grown in the upper chamber
and assessed for invasion through the Matrigel toward a
chemo-attractant (10% serum) in the lower chamber. The number of
invasive U87 cells co-transfected with both miR-10b and miR-21
inhibitors was substantially reduced when compared with the
scrambled control-transfected cells (Fig. 3). miR-10b inhibitor or miR-21
inhibitor also exerted minimal-to-moderate inhibitory effect on U87
cell invasiveness.
miR-10b and miR-21 inhibitors increase
the protein expression of respective miRNA targets and
synergistically repress the expression of EGFR and MMP-2
PDCD4 and TPM1 have been reported to be the direct
targets of miR-21 in other cell types (12,13).
We therefore determined if these two proteins could be upregulated
by miR-21 inhibitor in U87 cells. Results revealed that miR-21
inhibitor alone upregulated the protein expression of PDCD4, but
not TPM1. Unexpectedly, miR-10b inhibitor exerted
minimal-to-moderate stimulatory effect on PDCD4 and TPM1 protein
expression. In this regard, miR-21 inhibitor potentiated the
stimulatory effect of miR-10b inhibitor on TPM1 protein
expression.
miR-10b has been shown to target HoxD10 to induce
RhoC protein expression in breast cancer cells to enhance tumor
cell invasiveness and metastasis (14). We therefore determined whether
miR-10b inhibitor could reverse this metastatic cascade. Results
showed that miR-10b inhibitor but not miR-21 inhibitor increased
HoxD10 and reduced RhoC protein expression. Similar effect could be
observed in U87 cells co-transfected with both miR-10b and miR-21
inhibitors. In addition to the abovementioned mediators, we
measured the protein expression levels of matrix metalloproteinase
(MMP)-2 and epidermal growth factor receptor (EGFR), both of which
have been reported to be associated with malignant phenotypes of
glioma cells (21,22). As shown in Fig. 4 and 5, both miR-10b inhibitor and miR-21
inhibitor reduced the protein expression of both MMP-2 and EGFR.
When these miRNA inhibitors were co-transfected into U87 cells, a
synergistic inhibitory effect on MMP-2 and EGFR protein expression
was observed.
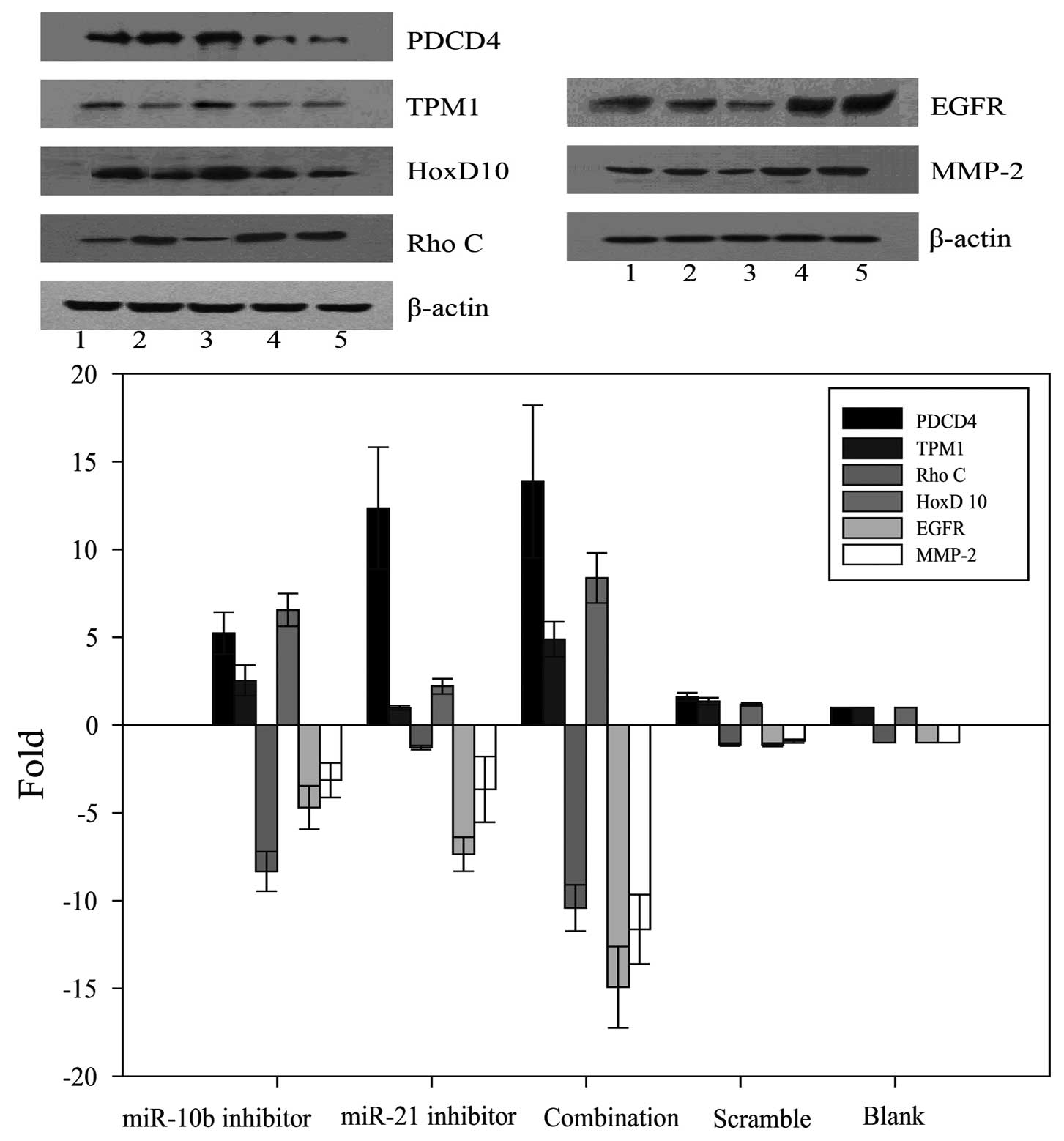 | Figure 4Valuation of the expression of PDCD4,
TPM1, RhoC, HoxD10, EGFR and MMP-2 in human glioblastoma U87 cells.
Western blot analysis of protein extracts from cells treated with
the miR-10b inhibitor, miR-21 inhibitor alone or combination. The
expression of β-actin was examined to ensure uniform protein
loading in the lanes. (line: 1, miR-10b inhibitor; 2, miR-21
inhibitor; 3, combination inhibitor; 4, scramble; 5, blank). |
Discussion
miRNAs have been considered as emerging key players
in carcinogenesis due to their widespread dysregulation in cancer
and important roles in the control of gene expression. The
mechanism by which miRNAs are dysregulated in cancer is complex and
may involve genetic and epigenetic abnormalities as well as altered
activity of certain transcription factors. Dysregulated miRNAs
contribute to tumorigenesis by regulating the levels of their
target genes post-transcriptionally. Importantly, a single miRNA
very often represses multiple targets to mediate its biological
function. The orchestrated alterations of gene expression then
modulates cancer-related phenotypes thereby promoting or
suppressing tumor formation.
Using high-throughput profiling of miRNA expression,
miR-10b and miR-21 were identified as two of the most strongly
upregulated miRNAs in a large proportion of human glioma specimens
(17,23). By miRNA microarray, miR-10b was
shown to be upregulated 1.97-fold to 13.6-fold in 5 out of 9
glioblastomas samples. The finding was also confirmed by northern
blotting (16). miR-21 was also
identified as one of the most overexpressed miRNAs in a large-scale
profiling experiments designed for the characterization of miRNA
expression in human cancers. Aberrant upregulation of miR-21 in
human glioblastoma has been reported (10). In this regard, compared with normal
brain tissue, miR-21 expression was elevated 7- to 11-fold in
low-grade astrocytomas, anaplastic astrocytomas, and glioblastoma
multiforme (24). In the present
study, miR-10b and miR-21 antagonized by their respective
inhibitors in U87 glioma cells, in which their downregulation was
confirmed by qRT-PCR. Functionally, inhibition of miR-10b and
miR-21 exert potent anti-glioma effects, as evidenced by inhibition
of cell cycle progression, enhanced apoptosis and reduced cell
invasion. The anticancer actions of anti-miR-10b and anti-miR-21
are accompanied by the upregulation of their respective targets,
including PDCD4, TPM1 and HOXD10.
PDCD4, a novel tumor suppressor, is downregulated in
various types of cancer. It has been shown that
post-transcriptional downregulation of PDCD4 by miR-21 in T98G
glioma cells stimulates cell proliferation and inhibits apoptosis
(12). Besides PDCD4, TPM1 has
been identified as a potential miR-21 target in tumors (13). TPM1 exhibits an anti-oncogenic
function through binding microfilament and regulating cytoskeleton
(25). Downregulation of TPM1 is
consistently observed in transformed breast epithelial cells.
Inhibition of TPM1 function caused by miR-21 overexpression leads
to enhanced cell migration and invasion (13). MiR-10b has been reported to inhibit
the translation of HOXD10, which in turn modulates expression of
downstream targets involving in cell invasion, migration,
extracellular matrix remodeling and tumor progression, including
uPAR, RhoC, integrins, and MMP-14 (26,27).
MiR-10b is known to be induced by TWIST, a master regulator of
morphogenesis and tumor metastasis. TWIST has been detected in a
large proportion of human gliomas and increased TWIST mRNA level is
associated with increased glioma grading.
About 30–50% of gliomas show dysregulated EGFR
signaling, including aberrant amplification of the EGFR gene and/or
auto-secretion of EGFR ligands (28). Enhanced EGFR signaling results in
increased cell proliferation, angiogenesis and cell invasion. The
latter involves the infiltration of tumor cells through the
extracellular matrix by local proteolysis mediated by MMPs. In this
study, inhibition of miR-10b and miR-21 reduces the expression of
EGFR and MMP-2. These two mediators may be important for the
anti-proliferative and anti-metastatic effects of anti-miR-10b and
anti-miR-21 in glioma cells.
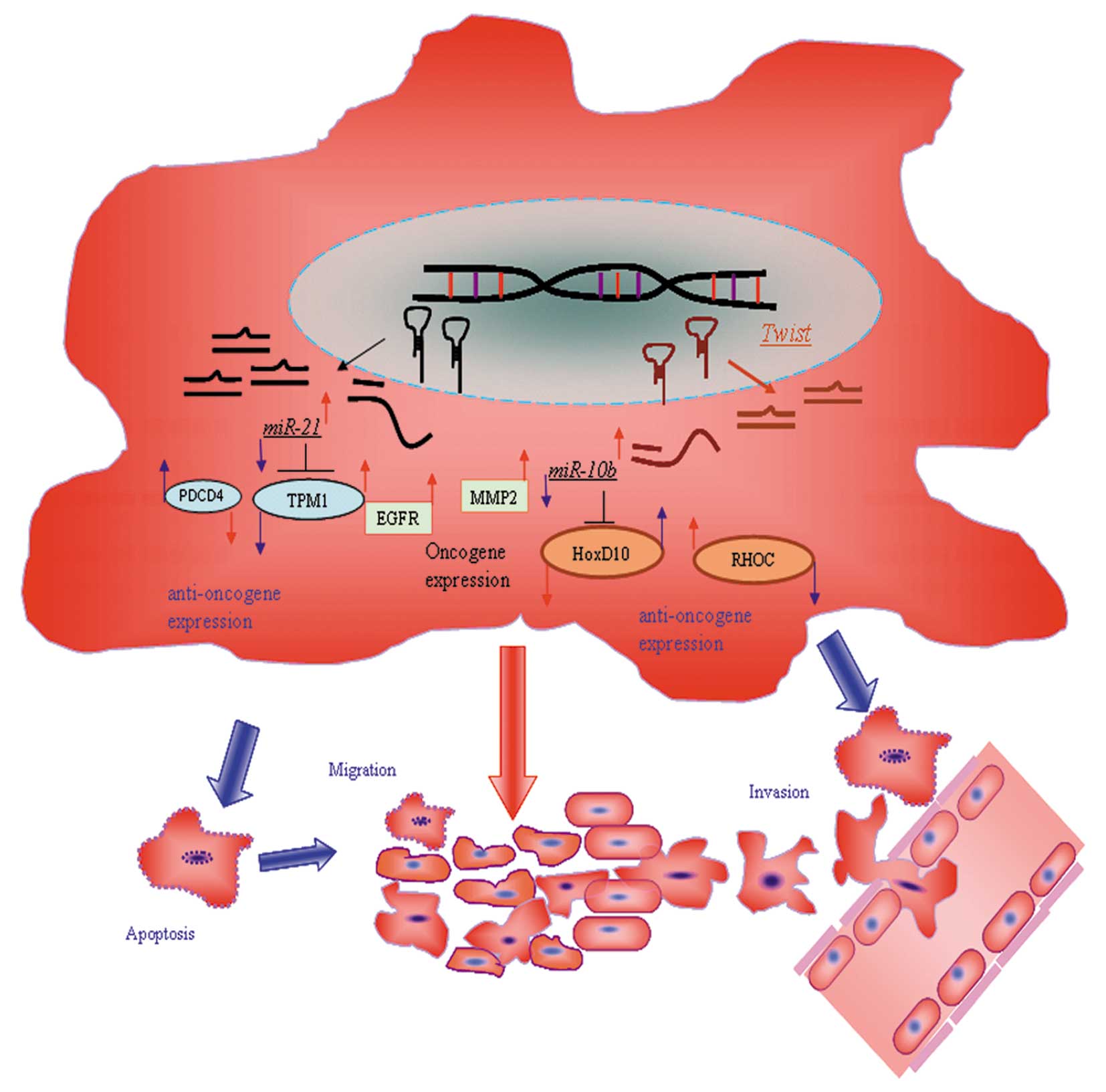
In conclusion, inhibition of miR-10b and miR-21,
alone and in combination, can effectively retard the growth of
glioma through inhibition of cell cycle progression and induction
of apoptosis. Inhibition of these two oncogenic microRNAs also
impairs the invasiveness of glioma cells. Our findings provide a
proof-of-concept that microRNA inhibitors could serve as
therapeutic agents to exert their anticancer effects on glioma
cells. Importantly, besides derepressing the expression of their
verified targets, we here demonstrate that inhibition of miR-10b
and miR-21 synergistically suppress the expression of MMP-2 and
EGFR. These findings suggest that inhibition of miR-10b and miR-21
could affect common downstream signaling components, which may be
important for the synergism between these two microRNA inhibitors.
Taken together, our study provides functional and mechanistic
insights into the anti-glioma effects of anti-miR-10b and
anti-miR-21. Whether our findings can be translated into clinical
benefits of glioma patients, however, awaits further
investigation.
Acknowledgements
We thank Dr M. Scala for editing the
manuscript. This study was supported by the Jiangsu Natural
Scientific Fund (SBK200921106) and by the Yangtz River Scholar and
Innovation Research Team Development Program (Project no.
IRT0945).
References
|
1.
|
Stewart LA: Chemotherapy in adult
high-grade glioma: a systematic review and meta-analysis of
individual patient data from 12 randomised trials. Lancet.
359:1011–1018. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Ambros V: The functions of animal
microRNAs. Nature. 431:350–355. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Alvarez-Garcia I and Miska EA: MicroRNA
functions in animal development and human disease. Development.
132:4653–4662. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Brennecke J, Hipfner DR, Stark A, et al:
Bantam encodes a developmentally regulated microRNA that controls
cell proliferation and regulates the proapoptotic gene hid in
Drosophila. Cell. 113:25–36. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Calin GA and Croce CM: MicroRNA signatures
in human cancers. Nat Rev Cancer. 6:857–866. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Gaur A, Jewell DA, Liang Y, et al:
Characterization of microRNA expression levels and their biological
correlates in human cancer cell lines. Cancer Res. 67:2456–2468.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Lu J, Getz G, Miska EA, et al: MicroRNA
expression profiles classify human cancers. Nature. 435:834–838.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Kumar MS, Lu J, Mercer KL, et al: Impaired
micro-RNA processing enhances cellular transformation and
tumorigenesis. Nat Genet. 39:673–677. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Mathupala SP, Mittal S, Guthikonda M and
Sloan AE: MicroRNA and brain tumors: a cause and a cure? DNA Cell
Biol. 26:301–310. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Lu Z, Liu M, Stribinskis V, et al:
MicroRNA-21 promotes cell transformation by targeting the
programmed cell death 4 gene. Oncogene. 27:4373–4379. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Frankel LB, Christoffersen NR, Jacobsen A,
et al: Programmed cell death 4 (PDCD4) is an important functional
target of the microRNA miR-21 in breast cancer cells. J Biol Chem.
283:1026–1033. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Asangani A, Rasheed SAK, Nikolova DA, et
al: MicroRNA-21 (miR-21) post-transcriptionally downregulates tumor
suppressor Pdcd4 and stimulates invasion, intravasation and
metastasis in colorectal cancer. Oncogene. 27:2128–2136. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Zhu S, Si ML, Wu H and Mo YY: MicroRNA-21
targets the tumor suppressor gene tropomyosin 1 (TPM1). J Biol
Chem. 282:14328–14336. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Ma L, Teruya-Feldstein J and Weinberg RA:
Tumour invasion and metastasis initiated by microRNA-10b in breast
cancer. Nature. 449:682–688. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Ladeiro Y, Couchy G and Balabaud C:
MicroRNA profiling in hepatocellular tumors is associated with
clinical features and oncogene/tumor suppressor gene mutations.
Hepatology. 47:1807–1809. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Ciafrè SA, Galardi S, Mangiola A, et al:
Extensive modulation of a set of microRNAs in primary glioblastoma.
Biochem Biophys Res Commun. 334:1351–1358. 2005.PubMed/NCBI
|
|
17.
|
Sasayama T, Nishihara M and Kondoh T:
MicroRNA-10b is overexpressed in malignant glioma and associated
with tumor invasive factors, uPAR and RhoC. Int J Cancer.
125:1407–1413. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Hildeman D, Jorgensen T, Kappler J and
Marrack P: Apoptosis and the homeostatic control of immune
responses. Curr Opin Immunol. 19:516–521. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Cheng AM, Byrom MW, Shelton J and Ford LP:
Antisense inhibition of human miRNAs and indications for an
involvement of miRNA in cell growth and apoptosis. Nucleic Acids
Res. 33:1290–1297. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Gabriely G, Wurdinger T, Kesari S, et al:
MicroRNA 21 promotes glioma invasion by targeting matrix
metalloproteinase regulators. Mol Cell Biol. 28:5369–5380. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Guillamo JS, de Boüard S, Valable S, et
al: Molecular mechanisms underlying effects of epidermal growth
factor receptor inhibition on invasion, proliferation, and
angiogenesis in experimental glioma. Clin Cancer Res. 15:3697–3704.
2009. View Article : Google Scholar
|
|
23.
|
Chan JA, Krichevsky AM and Kosik KS:
MicroRNA-21 is an antiapoptotic factor in human glioblastoma cells.
Cancer Res. 65:6029–6033. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Conti A, Aguennouz M, La Torre D, et al:
miR-21 and 221 upregulation and miR-181b downregulation in human
grade II–IV astrocytic tumors. J Neurooncol. 3:325–332.
2009.PubMed/NCBI
|
|
25.
|
Mahadev K, Raval G, Bharadwaj S, et al:
Suppression of the transformed phenotype of breast cancer by
tropomyosin-1. Exp Cell Res. 279:40–51. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Yang J, Mani SA and Donaher JL: Twist, a
master regulator of morphogenesis, plays an essential role in tumor
metastasis. Cell. 117:927–939. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Myers C, Charboneau A, Cheung I, Hanks D
and Boudreau N: Sustained expression of homeobox D10 inhibits
angiogenesis. Am J Pathol. 161:2099–2109. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Chakravarti A, Chakladar A and Delaney MA:
The epidermal growth factor receptor pathway mediates resistance to
sequential administration of radiation and chemotherapy in primary
human glioblastoma cells in a RAS dependent manner. Cancer Res.
62:4307–4315. 2002.
|