Introduction
A variety of treatments have been used for oral
squamous cell carcinoma (OSCC), including surgery, radiotherapy,
and chemotherapy administered alone or in combination. For patients
with locally advanced OSCC that were unresponsive to induction
chemotherapy (1–3), new chemotherapeutic treatment
strategies are needed for improving the treatment outcome and cure
rates (4–6).
5-Fluorouracil (5-FU) is widely used as an
anticancer agent and considered a key drug in chemotherapeutic
treatments for OSCC, colorectal, gastric, and oesophageal cancer
(5–8). Thymidylate synthase (TS),
dihydropyrimidine dehydrogenase (DPD), and ortate phosphoribosyl
transferase (OPRT) are key enzymes in the regulation of 5-FU
metabolism (9). Two main action
mechanisms have been proposed for 5-FU through its active
metabolites, 5-fluorodeoxyuridine monophosphate (FdUMP) and
5-fluorouridine triphosphate (FUTP) (Fig. 1), with the main mode of action
being through FdUMP (10). FdUMP
suppresses TS by forming covalent ternary complexes with
5,10-methylenetetrahydrofolate (CH2THF), which then
inhibits DNA synthesis. RNA function is inhibited when 5-FU is
modified by OPRT to form 5-fluorouridine monophosphate (FUMP),
which is then converted to FUTP. FUTP is incorporated into cellular
RNA, resulting in RNA dysfunction. Thymidine phosphorylase (TP)
anabolizes 5-FU to FdUMP. DPD is the initial enzyme in the
catabolism of 5-FU to 2-fluoro-β-alanine, primarily in the liver.
DPD is also the rate-limiting enzyme of 5-FU catabolism, degrading
85% of the administered dose of 5-FU into inactive metabolites
(10). Therefore, downregulation
of TS and DPD expression and upregulation of OPRT expression
enhance the anti-tumor effect of 5-FU (9–12).
Hence, the pharmacogenetic variability of these enzymes might be a
major determinant of the variations in outcome among cancer
patients treated with 5-FU (9).
The relative expression levels of the TS, DPD, and OPRT genes were
reported as a predictive factor for the prognosis and survival of
oral cancer patients treated with 5-FU (13,14).
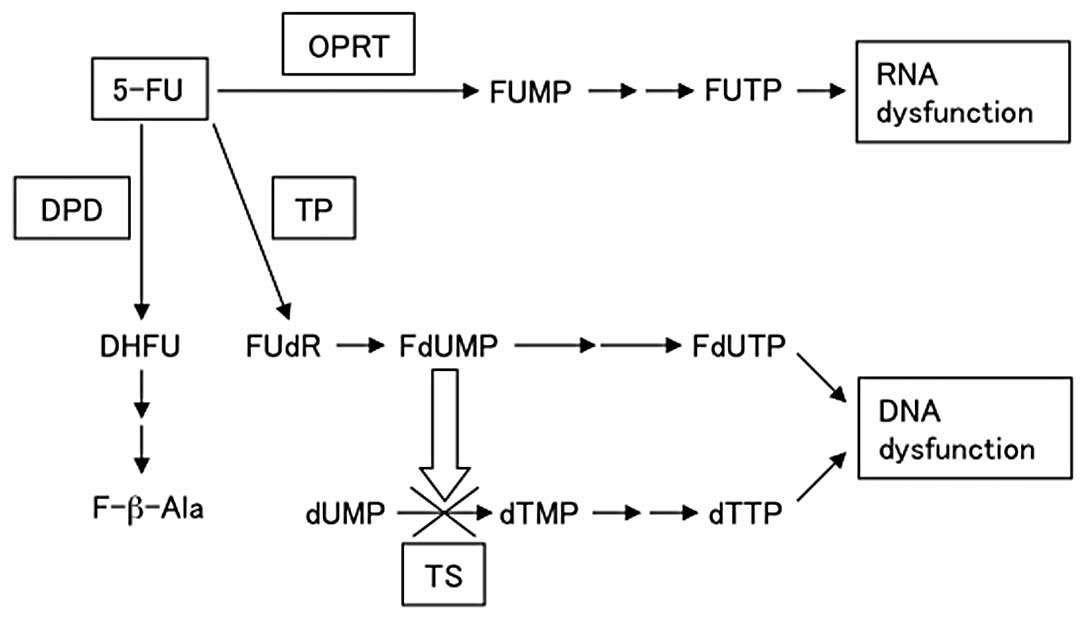 | Figure 15-FU, metabolic pathway of
5-fluorouracil; OPRT, ortate phosphoribosyl transferase; FUMP,
5-fluorouridine monophosphate; FUTP, 5-fluorouridine triphosphate;
TP, thymidine phosphorylase; FUdR, 5-fluorodeoxyuridine; FdUMP,
5-fluorodeoxyuridine monophosphate; FdUTP, 5-fluorodeoxyuridine
triphosphate; TS, thymidylate synthase; dUMP, deoxyuridine
monophosphate; dTMP, deoxythymidine monophosphate; dTTP,
deoxythymidine triphosphate; DPD, dihydropyrimidine dehydrogenase;
DHFU, 5-fluoro-dihydrouracil; F-b-Ala, a-fluoro-b-alanine. |
DOC is also an effective agent against OSCC
(15). We selected DOC as the
combination agent in this study because of its overlapping
antitumor spectrum including breast, oesophageal, gastric, and oral
cancers (15,16). Additionally, DOC has a different
mechanism of action from 5-FU and acts as a potent anti-mitotic
agent by promoting abnormal microtubule stabilization, which
results in inhibition of mitosis between metaphase and anaphase,
and in the initiation of apoptosis (17). The combined treatment of 5-FU and
DOC has been reported to improve response rates (14,15),
however, little is known about sequential treatment with DOC and
5-FU.
The aim of this study was to evaluate the anti-tumor
effects of sequential treatment with DOC and 5-FU against OSCC.
Furthermore, to elucidate the mechanisms underlying the enhanced
growth inhibitory effect of DOC followed by 5-FU, we examined the
expression of the 5-FU metabolic enzymes TS, DPD and OPRT.
Materials and methods
Cell lines and cell culture
B88 cells were previously established from an OSCC
patient in our laboratory (18).
CAL27 cells were obtained from American Type Culture Collection
(Rockville, MD, USA). Both cell lines produce tumors when
subcutaneously inoculated into nude mice. The cells were cultured
and maintained in Dulbecco’s modified Eagle’s medium (DMEM) (Sigma
Aldrich Co., St. Louis, MO, USA) supplemented with 10% fetal bovine
serum (FBS) and 5% antibiotic-antimycotic solution in a humidified
atmosphere containing 5% CO2 at 37°C.
In vitro cell growth assay
Cells (3×103 cells per well) were seeded
in 96-well plates. Twenty-four hours later, cells were treated with
various concentrations of 5-FU or DOC for 24 h. Then, they were
treated either with sequential treatment, 100 pg/ml DOC for 24 h
followed by 4 μg/ml 5-FU for 24 h, 4 μg/ml 5-FU for 24 h followed
by 100 pg/ml DOC for 24 h or with combined treatment, 4 μg/ml 5-FU
and 100 pg/ml DOC at the same time for 48 h. A 10 μl aliquot of 5
mg/ml 3-(4,5-dimethylthiaol-2-yl)-2,5-diphenyltetrazolium bromide
(MTT) (Sigma Aldrich) was added to each well and the cells were
incubated for 4 h. The blue dye taken up by cells was dissolved in
dimethyl sulfoxide, and the absorbance was measured with a
microplate reader (Bio-Rad Laboratories, Hercules, CA, USA) at 540
nm. All assays were run in triplicate.
Flow cytometry
Cells (1×106) were cultured in 100-mm
Petri dishes and treated with 4 μg/ml 5-FU or 100 pg/ml DOC alone,
in combination or in sequence. The cells were collected and fixed
with 70% ethyl alcohol and kept at −20°C until analyzed. Then, they
were treated with propidium iodide (40 μg/ml) and RNase A (1 μg/ml)
at 37°C for 30 min. Samples were kept on ice and the analysis of
the sub G1 population was completed by measuring propidium
iodide-stained DNA content with a Coulter® Epics® XL-MCL
cytometer (Beckman Coulter, Brea, CA, USA).
In vivo tumor growth assay
The tumorigenic potential of cancer cells was
assessed by inoculation of cells into 5- to 6-week-old female
athymic BALB/c nude mice (Japan Clea Inc., Osaka, Japan). Cells
(5×106) were inoculated subcutaneously into the backs of
mice, 5 mice per group. When tumors reached 50–100 mm3
in volume, they were treated with sterile saline, 15 mg/kg 5-FU,
and 10 mg/kg DOC by intraperitoneal (i.p.) injection. The treatment
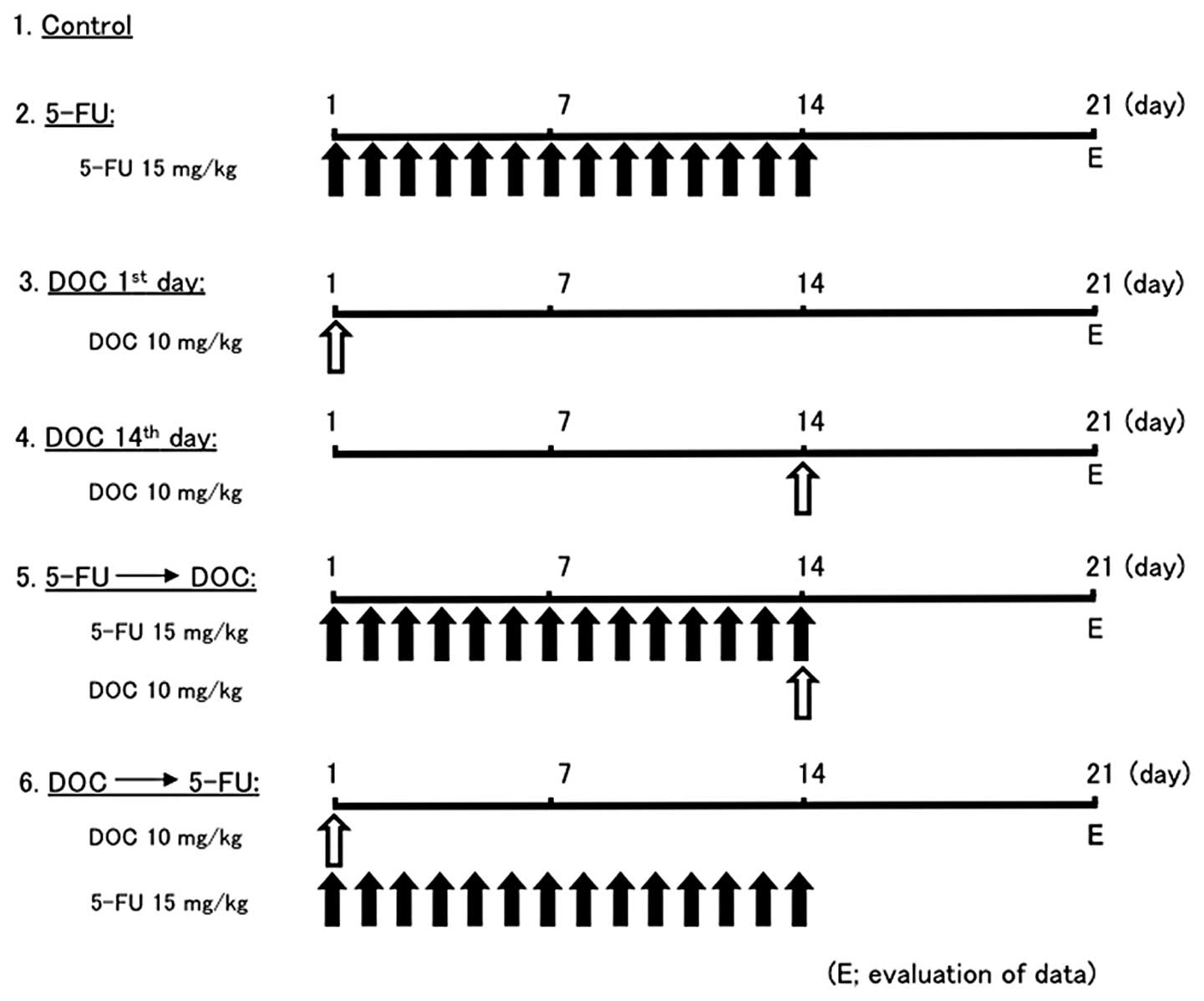
protocol of the six experimental groups of mice is shown in
Fig. 2. Tumor volume and body
weight of mice were measured 3 times a week. The tumor volumes were
calculated by the formula: 0.5 x largest diameter x (smallest
diameter)2. The mice were maintained under pathogen-free
conditions and handled in accordance with the Guidelines for Animal
Experimentation of Tokushima University.
RNA isolation and quantitative real-time
reverse transcriptase-polymerase chain reaction (RT-PCR)
When cells reached subconfluence in culture, they
were treated with 4 μg/ml 5-FU or 100 pg/ml DOC for 3, 6, 12, 24 or
48 h. Total-RNA was extracted by using TRIzol® reagent
(Invitrogen, Carlsbad, CA, USA). The cDNA was synthesized from
total-RNA using the Advantage cDNA PCR kit®
(Invitrogen). For quantitative real-time PCR, equal aliquots of
cDNA were amplified with TaqMan universal (50 μl) PCR master mix
using the ABI prism 7000 Sequence Detection System (Applied
Biosystems, Foster City, CA, USA) according to the manufacturer’s
instructions. The primer set and TaqMan probe used for the
experiments were purchased from TaqMan gene expression assay
systems (TS; Hs00426591_m1, DPD; Hs00559278_m1, and OPRT;
Hs00165978_m1). Data were normalized using RT-PCR
glyceraldehyde-3-phosphate dehydrogenase (GAPDH) primers (Applied
Biosystems).
Western blot analysis
After cells were treated with 4 μg/ml 5-FU or 100
pg/ml DOC alone, in combination or in sequence, they were collected
and lysed. Mice treated with 15 mg/kg 5-FU or 10 mg/kg DOC alone or
in sequence were sacrificed on the 21st day, then tumors were
collected from the mice and proteins were isolated from the tumors.
Whole cell lysate was subjected to electrophoresis on 10%
SDS-polyacrylamide gels and transferred to nitrocellulose
membranes. Membranes were incubated with rabbit polyclonal
antibodies against TS, DPD and OPRT (Taiho Pharma, Tokyo, Japan).
After rinsing membranes, the antibodies were detected using a
chemilumiescent western blotting detection system (Amersham, Tokyo,
Japan) according to the manufacturer’s instructions.
Statistical analysis
Statistical analysis was performed by Mann-Whitney U
test; values of p<0.05 were considered statistically
significant.
Results
Growth inhibitory effects of sequential
and combined treatment with 5-FU and DOC in oral cancer cells in
vitro
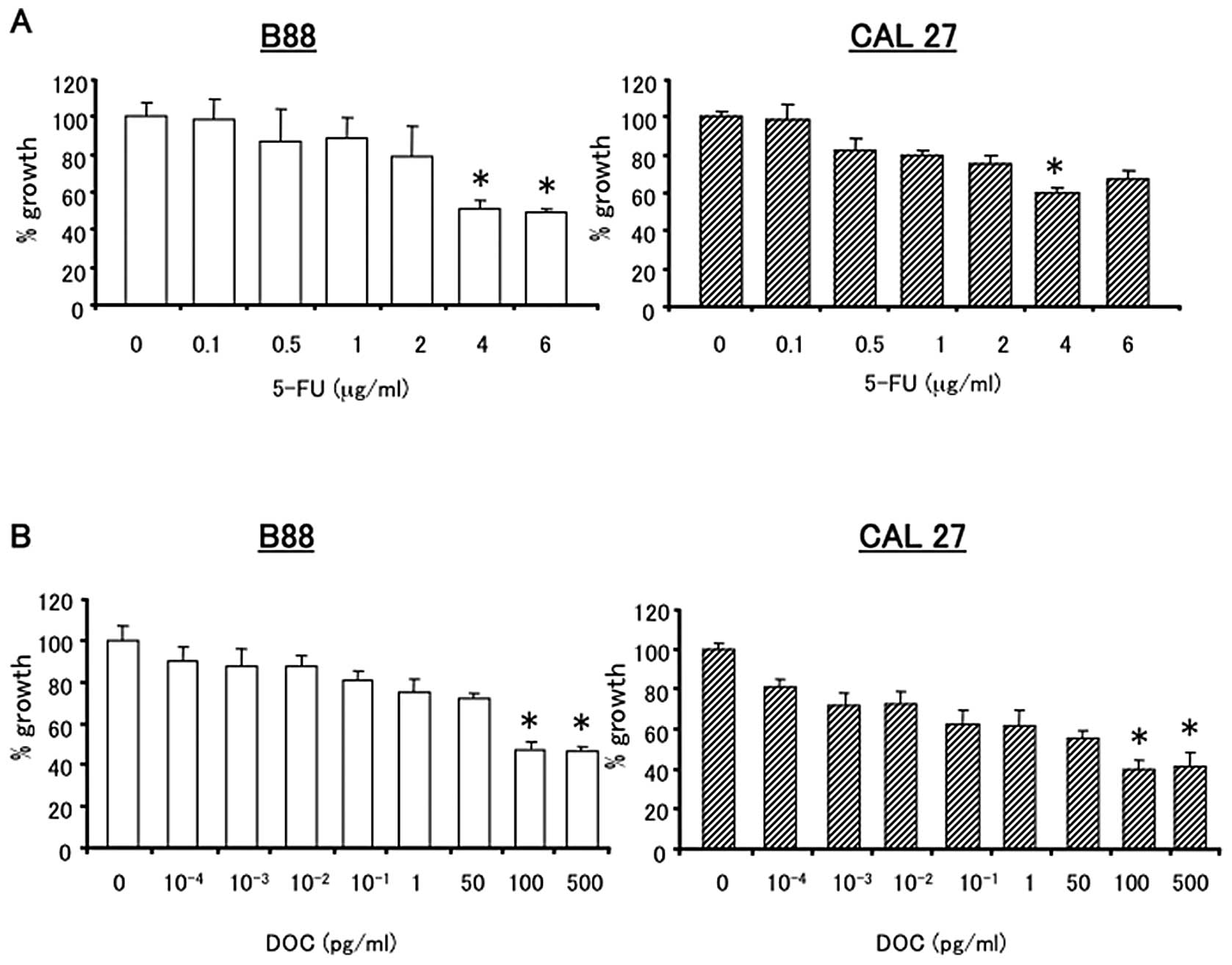
The growth inhibitory effects of 5-FU and DOC on B88
and CAL27 cells were analyzed by the MTT assay. Cells were treated
with various concentrations of 5-FU or DOC alone for 24 h (Fig. 3) and 48 h (data not shown). 5-FU
and DOC inhibited the growth of B88 and CAL27 cells in a
dose-dependent manner. For sequential treatment, a concentration of
4 μg/ml 5-FU and 100 pg/ml DOC were selected. These concentrations
showed a growth inhibitory rate of approximately 40–60% in both
cancer cell lines (Fig. 3A and B).
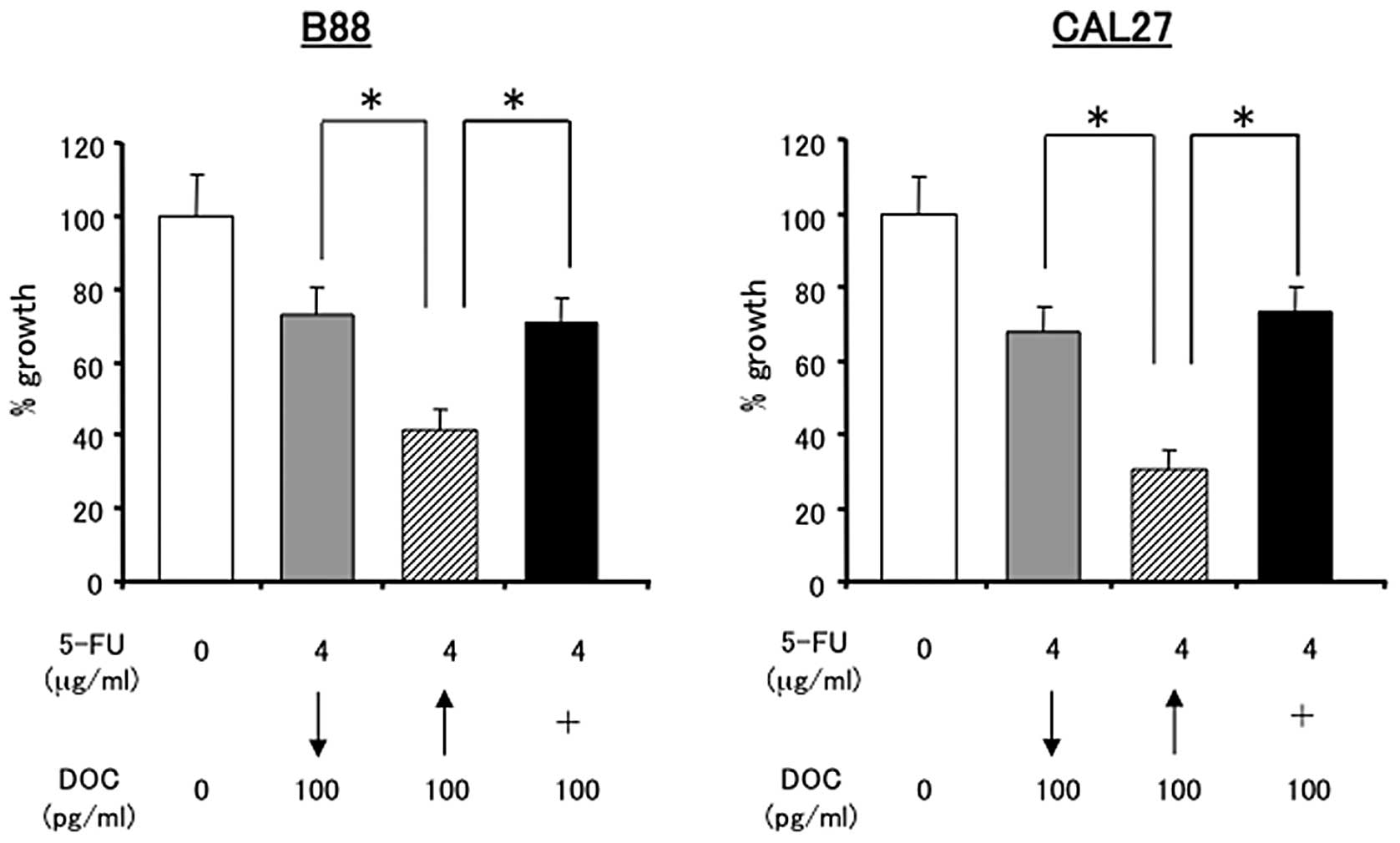
Thereafter, the effects of the sequential treatment with 5-FU and
DOC were evaluated using following sequence. Cells were treated
either with 5-FU (24 h) followed by DOC (24 h), with DOC followed
by 5-FU, or with 5-FU and DOC at the same time (48 h). DOC followed
by 5-FU sequential treatment was more effective in inhibiting
cancer cell growth than 5-FU followed by DOC treatment or combined
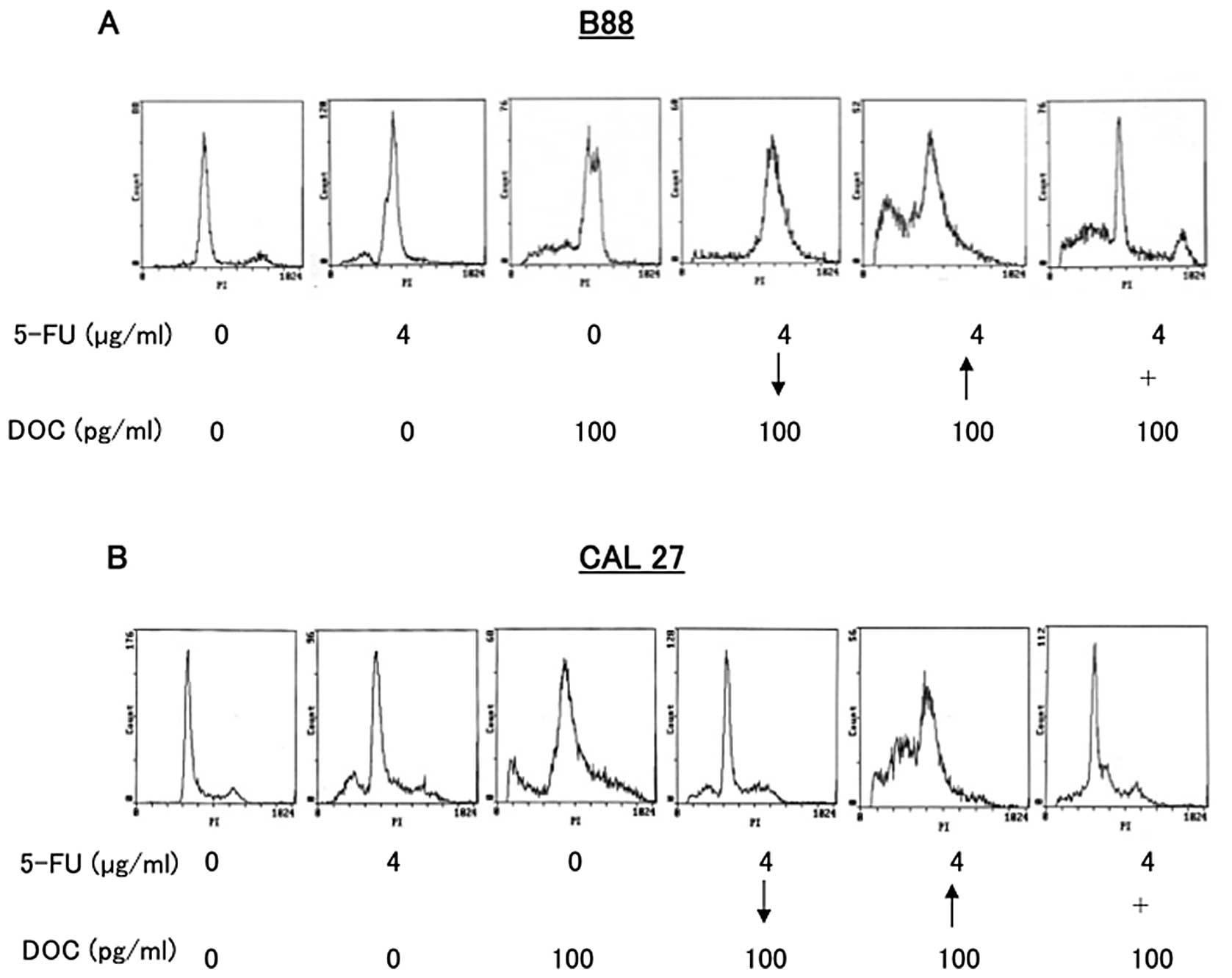
treatment (Fig. 4). To investigate
whether this enhanced cytotoxicity of sequential treatment was due
to apoptosis, the sub G1 population of cancer cells was examined by
flow cytometry (Fig. 5). B88 and
CAL27 cells were treated with either 5-FU, DOC, or both of the
drugs simultaneously or sequentially. The population of cells in
the sub G1 phase was significantly increased in DOC followed by
5-FU sequential treatment than 5-FU followed by DOC or combined
treatment in both cancer cells (Fig.
5A and B).
Anti-tumor effects of sequential
treatment with DOC and 5-FU on the human tumor xenografts in nude
mice
To investigate the efficacy of the DOC followed by
5-FU sequence in vivo, experiments with B88 and CAL27 tumor
xenografts were performed. The treatment plan is shown in Fig. 2. Control mice were injected with
saline (group 1). The mice in groups 2–6 were injected with 15
mg/kg 5-FU alone, 10 mg/kg DOC alone, or sequential treatment with
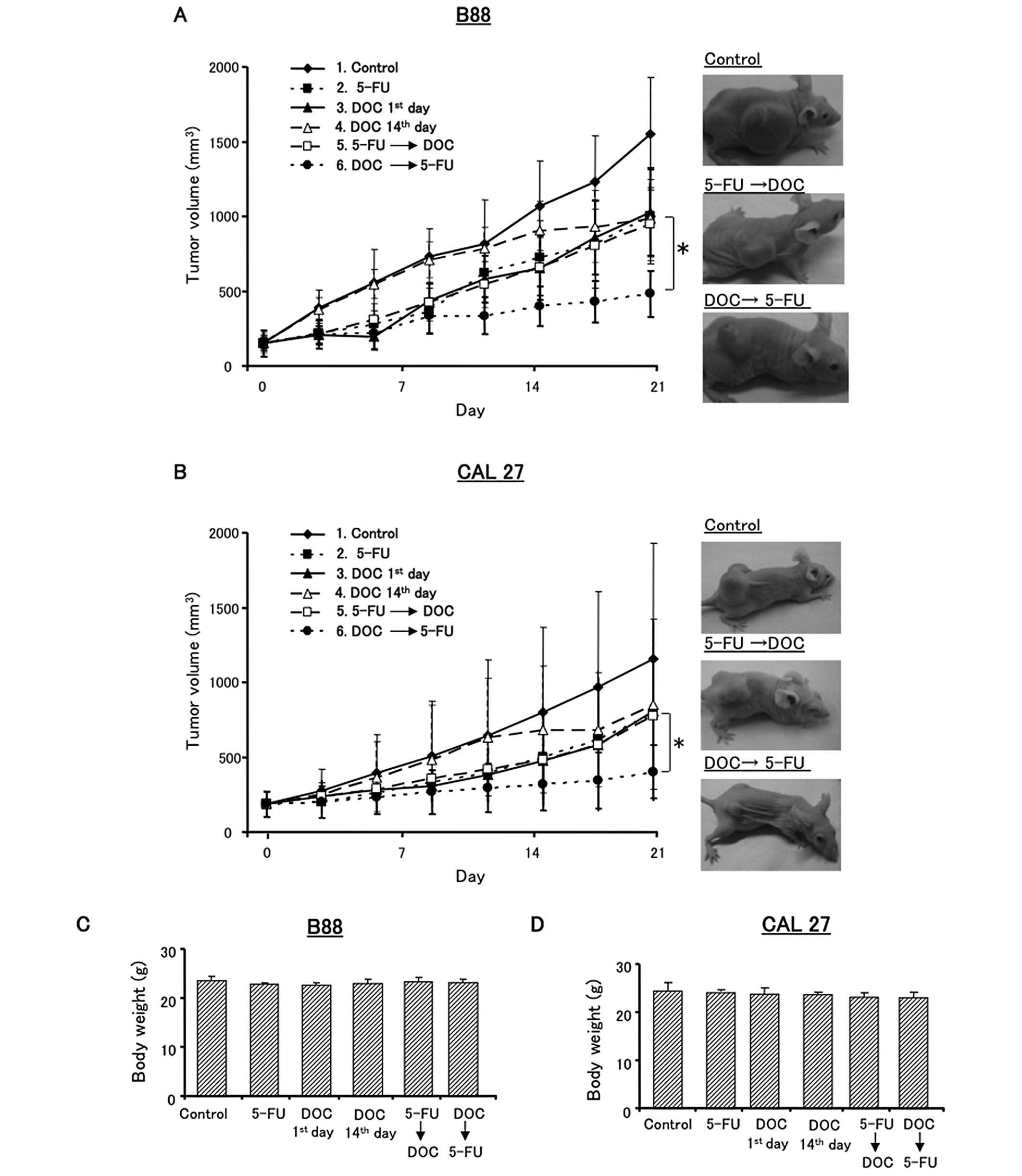
DOC and 5-FU. Fig. 6 shows the
antitumor effects of the various treatment with DOC and/or 5-FU. In
B88 tumor xenografts, DOC followed by 5-FU sequential treatment
significantly reduced tumor growth compared to the control, 5-FU
followed by DOC or other treatment groups (Fig. 6A). However, there was no
significant difference between DOC 1st day (group 3) and DOC 14th
day (group 4). The results for the CAL27 tumor xenografts were
similar to those for the B88 tumors (Fig. 6B). In addition, drug toxicity did
not cause carcass weight loss in any of the treated mice in these
experiments (Fig. 6C and D).
Altogether, these results showed that DOC followed by 5-FU was the
most effective treatment sequence in vivo.
Effects of 5-FU or DOC treatment on the
expression of TS, DPD and OPRT
To further identify the mechanisms underlying the
enhanced growth inhibition by the sequential treatment, DOC
followed by 5-FU, the expression levels of 5-FU metabolic enzymes,
TS, DPD and OPRT were examined in cancer cells. These expression
profiles were determined by real-time RT-PCR and western blot
analysis, following treatment of cancer cells with 5-FU or DOC
alone, in combination or in sequence.
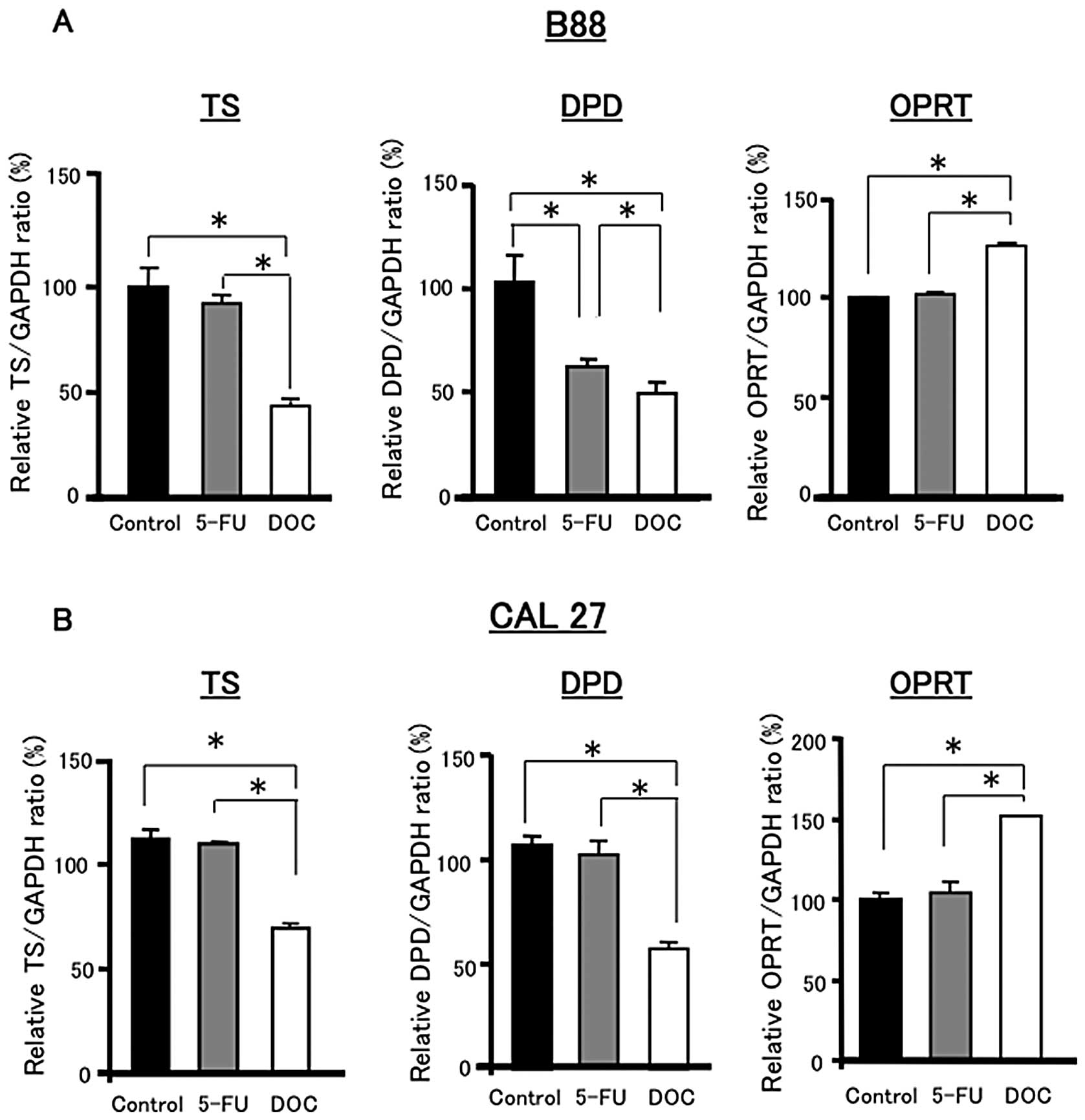
Fig. 7 shows the
mRNA expression levels of TS, DPD and OPRT in B88 and CAL27 cells
after 12 h of treatment with 4 μg/ml 5-FU or 100 pg/ml DOC alone.
There were no significant differences in TS expression between the
control and 5-FU treatment. However, DOC treatment significantly
decreased the expression of TS compared to the control and 5-FU
treatment in B88 and CAL27 cells. The expression of DPD was also
reduced by DOC treatment compared to the control and 5-FU treatment
in both cell lines. In contrast, DOC significantly increased the
expression of OPRT compared to the control and 5-FU treatment in
both cell lines.
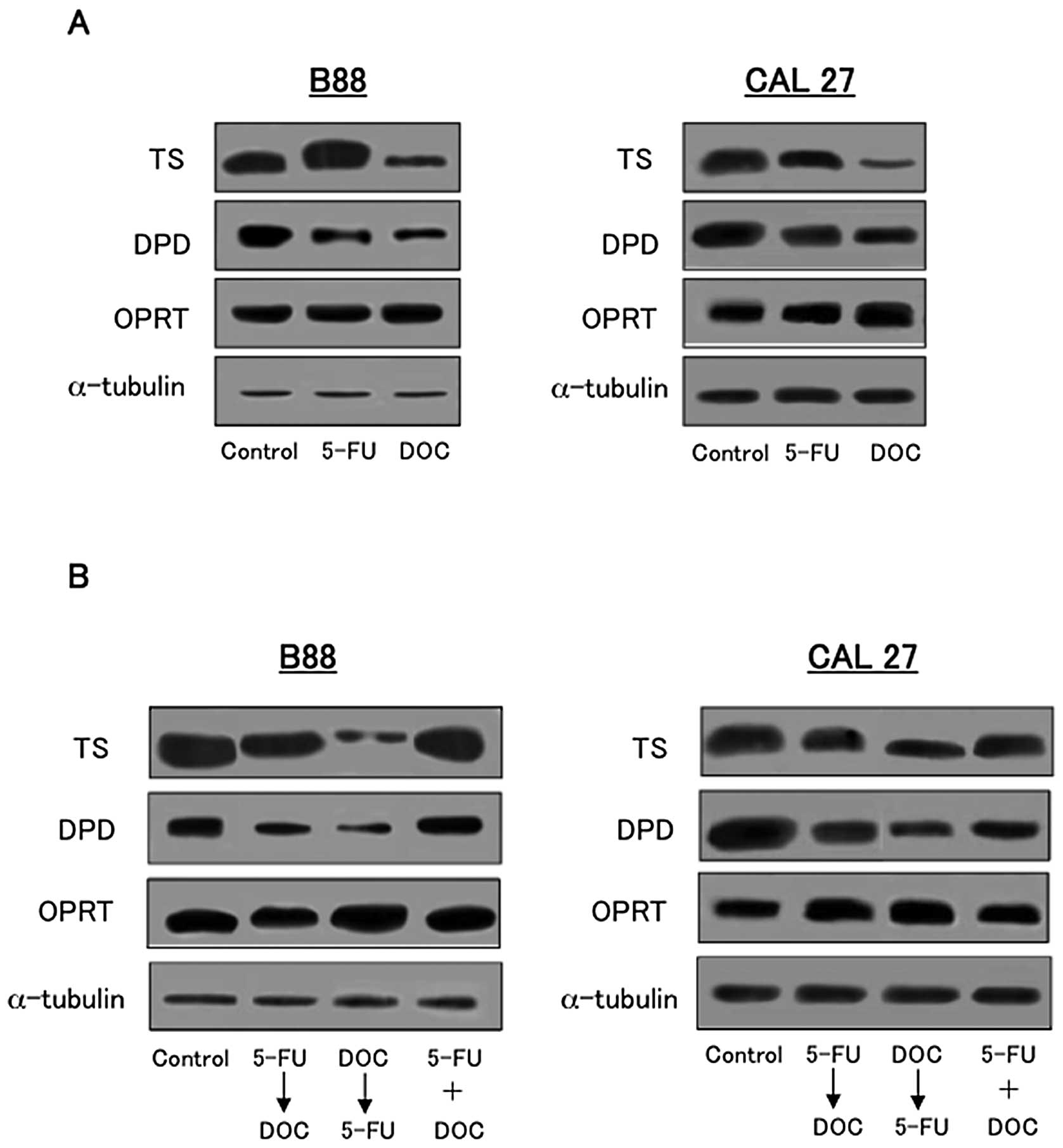
To examine the expression of TS, DPD and OPRT at the
protein level, western blot analysis was performed. As shown in
Fig. 8A, the expression of TS, DPD
and OPRT after 5-FU or DOC treatment in B88 and CAL27 cells was
examined. The expression of TS and DPD was reduced by the treatment
with DOC compared to the control and 5-FU treatment, whereas, the
expression of OPRT was slightly increased by DOC treatment
(Fig. 8A). Fig. 8B shows the expression of TS, DPD
and OPRT in B88 and CAL27 cells after 24 h of 5-FU and DOC combined
and sequential treatment. The expression of TS and DPD was
downregulated by DOC followed by 5-FU compared to 5-FU followed by
DOC or combined treatment. OPRT expression was also upregulated by
DOC followed by 5-FU in both cell lines (Fig. 8B).
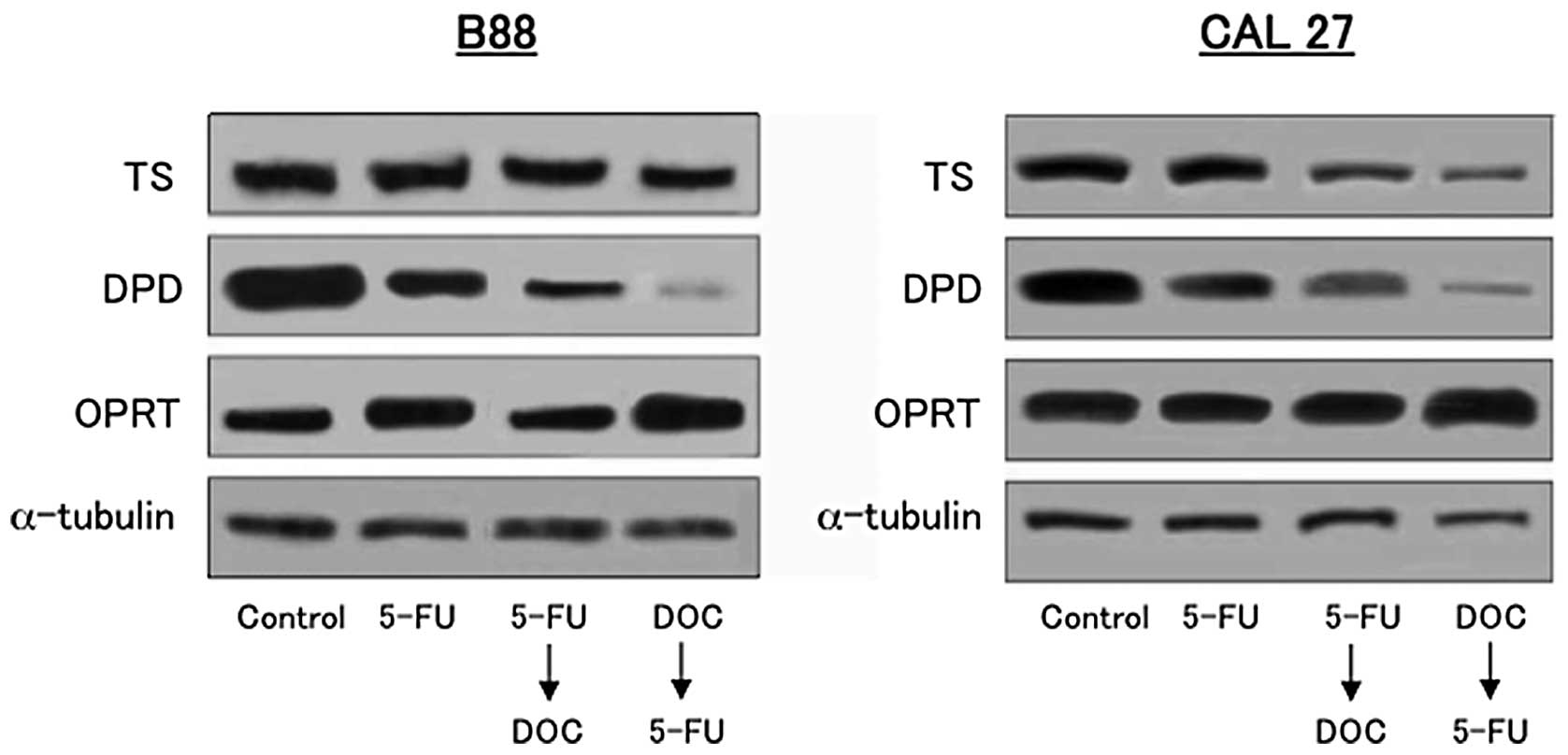
To investigate the expression of TS, DPD and OPRT
in vivo, western blot analysis was performed with tumors
extirpated from mice used in the xenograft experiment shown in
Figs. 2 and 6. As shown in Fig. 9, DOC followed by 5-FU downregulated
the expression of TS and DPD and upregulated the expression of OPRT
compared to 5-FU followed by DOC or 5-FU alone. These results show
that downregulation of TS and DPD expression and upregulation of
OPRT expression were induced by DOC treatment in vitro and
in vivo.
Discussion
In this study, the anti-tumor effects of sequential
treatment with DOC and 5-FU against OSCC were investigated. It was
clearly demonstrated that DOC followed by 5-FU treatment more
effectively inhibited tumor growth in vitro and in
vivo compared to 5-FU followed by DOC treatment. Furthermore,
to elucidate the mechanisms underlying the enhanced growth
inhibitory effect of DOC followed by 5-FU, the expression of the
5-FU metabolic enzymes TS, DPD, and OPRT was examined. Thus, DOC
downregulated the expression of TS and DPD and upregulated OPRT
expression in cancer cells, and these alterations of 5-FU metabolic
enzyme expression could enhance anti-tumor effects of 5-FU in DOC
followed by 5-FU treatment.
5-FU metabolic enzymes regulate the anti-tumor
efficacy of 5-FU (9–11). High expression of TS and DPD in
tumors has been associated with its resistance to 5-FU (19–21).
TS, of these enzymes, is the most important regulator of the
sensitivity of cancer cells to 5-FU. TS plays important roles in
cellular proliferation and growth, catalyzing the methylation of
FdUMP to deoxythymidine monophosphate (dTMP), an essential
precursor for DNA synthesis (22).
Therefore, TS inhibiting drugs could augment the efficacy of 5-FU.
The present study demonstrated that the expression of TS protein
and mRNA was decreased by DOC, however, the expression of TS
protein was enhanced by 5-FU in B88 cells. The precise mechanisms
responsible for the induction of TS expression by 5-FU and
downregulation of TS expression by DOC are not fully understood
(19). It was reported that the
transcriptional activator E2F1, a cell cycle regulatory protein
forming complexes with Rb, encodes a representative transcriptional
enzyme that transcribes the messages of TS (23). In addition, several studies using
cDNA microarray demonstrated that the expression of E2F1 and Rb was
decreased by DOC in head and neck squamous cell carcinoma (HNSCC)
(24,25). Therefore, DOC could lead to
suppression of TS expression via inhibition of E2F1/Rb expression.
On the other hand, DPD expression was also downregulated by DOC.
However, the mechanisms behind this downregulation of DPD have not
been fully analyzed. Recently, Ukon et al (26) reported that activation of protein
(AP)-1 accelerated DPD gene transcription in gastric cancer cells.
In addition, Yoo et al (24,25)
reported that DOC downregulated the expression of c-Jun N-terminal
kinase (JNK) and phosphorylated JNK in HNSCC cells. Therefore, DPD
expression could be downregulated by DOC via inhibition of the
JNK-AP-1 pathway.
It was reported that combined treatment with DOC and
5-FU had synergistic inhibitory effects on the growth of breast and
gastric cancer cells (16),
however, sequential treatment with DOC and 5-FU was not examined.
In the present study, the effects of administration sequence on
drug efficacy with DOC and 5-FU were evaluated. In in vivo
study, it is clearly demonstrated that DOC followed by 5-FU
treatment more effectively inhibited tumor growth compared to 5-FU
followed by DOC. But, the possibility was considered that this
result was affected by the different timing of the DOC injection
into the mice in DOC followed by 5-FU and 5-FU followed by DOC
treatment. Thus, DOC was injected on day 1 in DOC followed by 5-FU
treatment, whereas on day 14 in 5-FU followed by DOC treatment. It
means that the enhanced anti-tumor effect of DOC followed by 5-FU
may be caused by the difference in duration of DOC action. However,
there was no significant difference in tumor growth rates between
DOC 1st and DOC 14th groups on the evaluated day. Therefore, this
result suggested that timing of the DOC injection did not appear to
affect the anti-tumor effect of those two sequential treatments.
Thus, the enhanced efficacy of DOC followed by 5-FU could be caused
by the effect of DOC, which directly regulated 5-FU metabolic
enzymes.
The mechanisms underlying the enhanced growth
inhibitory effect of DOC followed by 5-FU, compared to 5-FU
followed by DOC could be explained by considering two
possibilities. One is that DOC affects the expression of 5-FU
metabolic enzymes or 5-FU regulated genes. The other is that 5-FU
provides the effects on DOC regulated genes. Yoo et al
(25) reported that DOC induced
the expression of the cell cycle regulator proteins p19 and
cyclin-dependent kinase 2, but reduced the expression of cyclin A,
B, C, D2 and D3, E2F1 and bcl-2. Among these genes, the
overexpression of bcl-2 is correlated with upregulation of TS
expression and resistance of colorectal cancer cells to DOC
(27). On the other hand,
resistance to DOC also appears to be caused by the high expression
of P-glycoproteins, thioredoxin, and ribophorin 2 (RPN2) (19) and by the low expression of p27
(19,28). However, effects of 5-FU on the
expression of DOC resistance related genes, RPN2, P-glycoprotein,
bcl-2, and DOC induced genes were not examined in this study.
Studies on effects of 5-FU on genes related to DOC resistance will
be important to understand the mechanisms of DOC and 5-FU
sequential treatment.
A more effective chemotherapy based on 5-FU and DOC
may be developed by using various modulators for metabolic enzymes
of 5-FU and resistance related genes of DOC. Several pathways could
be considered, including the phosphatidylinositol 3-kinases
(PI3K)-Akt mammalian target of rapamycin (mTOR) pathway, which is
related with various types of malignancies (28). It has been reported that activation
of the PI3K-Akt-mTOR pathway induces TS expression and could be
responsible for the incomplete response of cancer for DOC and 5-FU
(28,29). Moreover, Shigematsu et al
(28) reported that an mTOR
inhibitor, rapamycin, downregulated the expression of TS and showed
enhanced anti-tumor effects in combination with DOC and 5-FU in
gastric cancer. The TS inhibitors TOM and Thymitaq, and the DPD
inhibitor eniluracil, have been used in combination with 5-FU for
their enhanced anti-tumor effects against various types of cancers
(29–31). Insufficient inhibition of TS and
DPD could be the cause of poor outcomes of 5-FU and DOC based
treatments, therefore, novel combinations of TS or DPD inhibitors
with 5-FU and DOC could provide important new opportunities for
improving the clinical outcome for oral cancer patients. Moreover,
the overexpression of bcl-2 and constitutive activation of NF-κB
have been reported to cause the resistance to 5-FU and DOC in
cancer cells (32–34). Therefore, understanding the effects
of these modulators on the efficacy of 5-FU and DOC treatment, and
inhibition of the signaling pathways related to bcl-2 or NF-κB
would facilitate the development of new therapeutic strategies
based on 5-FU and DOC.
In conclusion, this study clearly showed that
sequential treatment with DOC followed by 5-FU more effectively
inhibited the tumor growth of oral cancer cells. The mechanisms
underlying the growth inhibitory effect of DOC followed by 5-FU
sequential treatment could be downregulation of TS and DPD
expression, and upregulation of OPRT expression induced by DOC
treatment. Thereby, anti-tumor effect of 5-FU could be enhanced in
DOC followed by 5-FU treatment. These findings demonstrated that
sequential treatment with DOC followed by 5-FU can be more
effective for the patients with OSCC than that with 5-FU followed
by DOC.
Acknowledgements
This work was supported in part by
Grants-in-Aid for young Scientists (B)(23792351).
References
|
1.
|
Azuma M, Harada K, Suprianto, Tamatani T,
Motegi K, Ashida Y and Sato M: Potentiation of induction of
apoptosis by sequential treatment with cisplatin followed by
5-fluorouracil in human oral cancer cells. Int J Oncol.
24:1449–1455. 2004.PubMed/NCBI
|
|
2.
|
Inagi K, Takahashi H, Okamoto M, Nakayama
M, Makoshi T and Nagai H: Treatment effects in patients with
squamous cell carcinoma of the oral cavity. Acta Otolaryngol
(Suppl). 547:25–29. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Shingaki S, Takada M, Sasai K, Bibi R,
Kobayashi T, Nomura T and Saito C: Impact of lymph node metastasis
on the pattern of failure and survival in oral carcinomas. Am J
Surg. 185:278–284. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
LoTempio MM, Veena MS, Steele HL,
Ramamurthy B, Ramalingam TS, Cohen AN, Chakrabarti R, Srivatsan ES
and Wang MB: Curcumin suppresses growth of head and neck squamous
cell carcinoma. Clin Cancer Res. 11:6994–7002. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Janinis J, Papadakou M, Panagos G,
Panousaki A, Georgoulias V, Hatzidaki D, Lefantzis D and Dokianakis
G: Sequential chemoradiotherapy with docetaxel, cisplatin and
5-fluorouracil in patients with locally advanced head and neck
cancer. Am J Clin Oncol. 24:227–231. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Schrijvers D, Herpen CV, Kerger J, Joosens
E, Laer CV, Awada A, Weyngaert VD, Nguyen H, Bouder CL, Castelijns
JA, Kaanders J, Mulder PD and Vermorken JB: Docetaxel, cisplatin
and 5-fluorouracil in patients with locally advanced unresectable
head and neck cancer: a phase I–II feasibility study. Ann Oncol.
15:638–645. 2004.
|
|
7.
|
Yamamoto S, Kurebayashi J, Kurosomi M,
Kunisue H, Otsuki T, Tanaka K and Sonoo H: Combined effects of
docetaxel and fluoropyrimidines on tumor growth and expression of
interleukin-6 and thymidine phosphorylase in breast cancer
xenografts. Cancer Chemother Pharmacol. 48:233–238. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Yoshida K, Ninomiya M, Takakura N,
Hirabayashi N, Takiyama W, Sato Y, Todo S, Terashima M, Gotoh M,
Sakamoto J and Nishiyama M: Phase II study of docetaxel and S-1
combination therapy for advanced or recurrent gastric cancer. Clin
Cancer Res. 12:3402–3407. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Ishikawa M, Miyauchi T and Kashiwagi Y:
Clinical implications of thymidylate synthetase, dihydropyrimidine
dehydrogenase and ortate phosphoribosyl transferase activity level
in colorectal carcinoma following radical resection and
administration of adjuvant 5-FU chemotherapy. BMC Cancer.
8:188–194. 2008. View Article : Google Scholar
|
|
10.
|
Ando T, Ishiguro H, Kuwabara Y, Kimura M,
Mitsui A, Sugito N, Mori R, Ogawa R, Katada T and Fujii Y:
Relationship between expression of 5-fluorouracil metabolic enzymes
and 5-fluorouracil sensitivity in esophageal carcinoma cell lines.
Dis Esophagus. 21:15–20. 2008.PubMed/NCBI
|
|
11.
|
Ichikawa W, Uetake H, Shirota Y, Yamada H,
Nishi N, Nihei Z, Sugihara K and Hirayama R: Combination of
dihydropyrimidine dehydrogenase and thymidylate synthase gene
expressions in primary tumors as predictive parameters for the
efficacy of fluoropyrimidine-based chemotherapy for metastatic
colorectal cancer. Clin Cancer Res. 9:786–791. 2003.
|
|
12.
|
Taomoto J, Yoshida K, Wada Y, Tanabe K,
Konishi K, Tahara H and Fukushima M: Overexpression of the ortate
phosphoribosyl transferase gene enhances the effect of
5-fluorouracil on gastric cancer cell lines. Oncology. 70:458–464.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Sakakura K, Chikamatsu K, Shino M, Sakurai
T and Furuya N: Expression of thymidylate synthase and
dihydropyrimidine dehydrogenase in oral squamous cell carcinoma:
possible markers as predictors of clinical outcome. Acta
Otolaryngol. 126:1295–1302. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Kobayashi H, Koike T, Nakatsuka A, Kurita
H, Sagara J, Taniguchi S and Kurashina K: Dihydropyrimidine
dehydrogenase expression predicts survival outcome and
chemosensitivity to 5-fluorouracil in patients with oral squamous
cell carcinoma. Oral Oncol. 41:38–47. 2005. View Article : Google Scholar
|
|
15.
|
Catimel G, Verwii J, Mattijssen V,
Hanauska A, Piccart M, Wanders J, Franklin H, Le Bail N, Clavel M
and Kaye SB: Docetaxel (taxotere): an active drug for treatment of
patients with advanced squamous cell carcinoma of the head and
neck. EORTC Early Clinical Trials Group. Ann Oncol. 5:533–537.
1994.
|
|
16.
|
Wada Y, Yoshida K, Suzuki T, Mizuiri H,
Konishi K, Ukon K, Tanabe K, Sakata Y and Fukushima M: Synergistic
effects of docetaxel and S-1 by modulating the expression of
metabolic enzymes of 5-fluorouracil in human gastric cancer cell
lines. Int J Cancer. 119:783–791. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Ravdin PM, Burris HA, Cook G, Eisenberg P,
Kane M, Bierman WA, Mortimer J, Genevois E and Bellet RE: Phase II
trial of docetaxel in advanced anthracycline-resistant or
anthracenedione-resistant breast cancer. J Clin Oncol.
13:2879–2885. 1995.PubMed/NCBI
|
|
18.
|
Tamatani T, Azuma M, Ashida Y, Yoshida H
and Sato M: Enhanced radiosensitization and chemosensitization in
NF-κB suppressed human oral cancer cells via the inhibition of
γ-irradiation- and 5-FU-induced production of IL-6 and IL-8. Int J
Cancer. 108:912–921. 2004.
|
|
19.
|
Honma K, Koizumi K, Takeshita F, Yamamoto
Y, Yoshida T, Nishio K, Nagahara S, Kato K and Ochiya T: RPN2 gene
confers docetaxel resistance in breast cancer. Nat Med. 14:939–948.
2008. View
Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Longley DB, Harkin DP and Johnston PG:
5-fluorouracil: mechanisms of action and clinical strategies. Nat
Rev Cancer. 3:330–338. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Van Kuilenburg AB: Dihydropyrimidine
dehydrogenase and the efficacy and toxicity of 5-fluorouracil. Eur
J Cancer. 40:939–950. 2004.PubMed/NCBI
|
|
22.
|
Ceppi P, Volante M, Ferrero A, Righi L,
Rapa I, Rosas R, Berruti A, Dogliotti L, Scagliotti GV and Papotti
M: Thymidylate synthase expression in gastroenteropancreatic and
pulmonary neuroendocrine tumors. Clin Cancer Res. 14:1059–1064.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Yoshinare K, Kubota T, Watanabe M, Wada N,
Nishibori H, Hasegawa H, Kitajima M, Takechi T and Fukushima M:
Gene expression in colorectal cancer and in vitro chemosensitivity
of 5-fluorouracil: a study of 88 surgical specimen. Cancer Sci.
94:633–638. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Yoo GH, Lin HS, Iskander AJ, Piechocki MP,
Oliver J, Kewson D, Lonardo F, Tainsky MA, Kim HR, Kim H and Ensley
JF: Docetaxel associated pathways in cisplatin resistant head and
neck squamous cell carcinoma: a pilot study. Laryngoscope.
115:1938–1946. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Yoo GH, Piechocki MP, Ensley JF, Nguyen T,
Oliver J, Meng H, Kewson D, Shibuya TY, Lonardo F and Tainsky MA:
Docetaxel induced gene expression patterns in head and neck
squamous cell carcinoma using cDNA microarray and powerblot. Clin
Cancer Res. 8:3910–3921. 2002.PubMed/NCBI
|
|
26.
|
Ukon K, Tanimoto K, Shimokuni T, Noguchi
T, Hiyama K, Tsujimoto H, Fukushima M, Toge T and Nishiyama M:
Activator protein accelerates dihydropyrimidine dehydrogenase gene
transcription in cancer cells. Cancer Res. 65:1055–1062.
2005.PubMed/NCBI
|
|
27.
|
Bendardaf R, Ristamäki R, Syrjänen K and
Pyrhönen S: Bcl-2 expression significantly correlates with
thymidylate synthase expression in colorectal cancer patients.
World J Gastroenterol. 14:6218–6223. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Shigematsu H, Yoshida K, Sanada Y, Osada
S, Takahashi T, Wada Y, Konishi K, Okada M and Fukushima M:
Rapamycin enhances chemotherapy-induced cytotoxicity by inhibiting
the expressions of TS and ERK in gastric cancer cells. Int J
Cancer. 126:2716–2725. 2010.PubMed/NCBI
|
|
29.
|
Chang JC, Wooten EC, Tsimelzon A,
Hilsenbeck SG, Gutierrez MC, Tham YL, Kalidas M, Elledge R, Mohsin
S, Osborne CK, Chamness GC, Allred DC, Lewis MT, Wong H and
O’Connell P: Patterns of resistance and incomplete response to
docetaxel by gene expression profiling in breast cancer patients. J
Clin Oncol. 23:1169–1177. 2005. View Article : Google Scholar
|
|
30.
|
Ford HER, Mitchell F, Cunningham D,
Farrugia DC, Hill ME, Rees C, Calvert AH, Judson IR and Jackman AL:
Patterns of elevation of plasma 2′-deoxyuridine, a surrogate marker
of thymidylate synthase (TS) inhibition, after administration of
two different schedules of 5-fluorouracil and the specific TS
inhibitors raltitrexed (Tomudex) and ZD93311. Clin Cancer Res.
8:103–109. 2002.
|
|
31.
|
Ahmed FY, Johnston SJ, Cassidy J, Kelly T,
Binnie N, Murray GI, Gennip AH, Abeling NG, Knight S and McLeod HL:
Eniluracil treatment completely inactivates dihydropyrimidine
dehydrogenase in colorectal tumours. J Clin Oncol. 17:2439–2445.
1999.
|
|
32.
|
Mirjolet JF, Heyob MB, Didelot C, Peyrat
JP, Abecassis J, Millon R and Merlin JL: Bcl-2/Bax protein ratio
predicts 5-fluorouracil sensitivity independently of p53 status. Br
J Cancer. 83:1380–1386. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
33.
|
Domenech JD, Oliva C, Rovira A, Servat JC,
Bosch M, Filella X, Montagut C, Tapia M, Campas C, Dang L, Rolfe M,
Ross JS, Gascon P, Albanell J and Mellado B: Interleukin 6, a
nuclear factor-κB target, predicts resistance to docetaxel in
hormone-independent prostate cancer and nuclear factor-κB
inhibition by PS-1145 enhances docetaxel antitumor activity. Clin
Cancer Res. 12:5578–5586. 2006.
|
|
34.
|
Li J, Minnich DJ, Camp ER, Brank A, MacKay
S and Hochwald SN: Enhanced sensitivity to chemotherapy in
esophageal cancer through inhibition of NF-κB. J Surg Res.
132:112–120. 2006.
|