Introduction
Head and neck squamous cell carcinomas (HNSCCs),
known for their aggressive growth and propensity to metastasize,
are among the most common tumors that develop in patients with
Fanconi anemia (FA) (1,2). In FA patients, although HNSCC is
morphologically the same, its incidence and course are altered. By
the age of 40 years, FA patients have a 14% chance of developing
HNSCC in contrast to 0.038% in the general population (3). Furthermore, the associated risk
factors of tobacco smoking and alcohol consumption that are
associated with 85% of the non-FA-associated HNSCC (FA HNSCC) cases
do not play as significant a role in FA; approximately 16% of FA
HNSCC cases are associated with these risk factors (3). In patients with FA, HNSCC has been
shown to be more aggressive with early lymph node metastases and
early soft tissue invasion resulting in poorer prognoses than in
HNSCC patients without FA (3).
Secondary primary tumors occur in 63% of FA patients compared to
only 15% in non-FA patients (3).
Furthermore, the 2-year overall survival is only 49% in FA patients
compared to 70% in non-FA patients (3). The most frequent location of HNSCC is
in the oral cavity (65%) compared to the larynx, hypoharynx and
oropharynx, each at 10%, which differs from HNSCC in the general
population. Due to significant toxic sequelae from the use of
radiation therapy and/or chemotherapy in FA patients, surgical
treatment is the main modality used. HNSCC in the general
population is treated with radiation, chemotherapy and surgery. The
highly metastatic potential of HNSCC in FA patients and inadequate
treatment methods, leading to poor outcomes, create an urgent need
to develop more effective and less toxic treatment
alternatives.
We previously developed strategies to inhibit cancer
development and its spread using naturally occurring nutrients,
such as lysine, proline, ascorbic acid and green tea extract. This
nutrient mixture (NM) has exhibited synergistic anticancer activity
in vivo and in vitro in a number of cancer cell lines
by inhibiting cancer cell growth, matrix metalloproteinase (MMP)
secretion, invasion, metastasis and angiogenesis (4–6). In
the present study, we examine the effect of this NM on the human
OHSU-974 FA HNSCC cell line in vivo, in athymic nude mice
bearing HNSCC xenografts, as well as in vitro, evaluating
cell viability, MMP secretion, invasion and migration.
Materials and methods
Cancer cell line and culture
The human OHSU-974 FA HNSCC cell line was obtained
from the Fanconi Anemia Research Fund, Oregon Health and Science
University, Portland, OR, USA. FA HNSCC cells were maintained in
RPMI medium supplemented with 20% fetal bovine serum (FBS), 100
U/ml penicillin and 100 μg/ml streptomycin. The media and
sera used were obtained from the American Type Culture Collection
(ATCC), and the antibiotics (penicillin and streptomycin) were from
Gibco-BRL (Long Island, NY, USA).
Composition of the NM
The NM was composed of the following at the
indicated ratios: vitamin C (as ascorbic acid and as Mg, Ca, and
palmitate ascorbate) 700 mg; L-lysine 1,000 mg; L-proline 750 mg;
L-arginine 500 mg; N-acetylcysteine 200 mg; standardized green tea
extract [derived from green tea leaves, was obtained from US Pharma
Lab Inc. (Santa Clarita, CA, USA); the certificate of analysis
indicated the following characteristics: total polyphenol 80%,
catechins 60%, epigallocatechin gallate (EGCG) 35% and caffeine
1.0%] 1,000 mg; selenium 30 μg; copper 2 mg; and manganese 1
mg.
In vivo studies
Animals
Male athymic mice (NCr-nu/nu), approximately 5 weeks
of age on arrival, were purchased from Simonsen Laboratories
(Gilroy, CA, USA) and maintained in microisolator cages under
pathogen-free conditions on a 12-h light/12-h dark schedule for a
week. All procedures were performed according to humane and
customary care and use of experimental animals and followed a
protocol approved by the internal institutional animal safety
review committee of our institution.
Experimental design
After housing for a week, the mice (n=12) were
inoculated subcutaneously with 3×106 OHSU-974 cells in
0.2 ml phosphate-buffered saline (PBS) and 0.1 ml Matrigel (BD
Bioscience, Bedford, MA, USA). After injection, the mice were
randomly divided into 2 groups; group A mice were fed regular
Purina mouse chow and group B the regular diet supplemented with 1%
NM (w/w). The regular diet was Laboratory Rodent Diet 5001 from
Purina Mills (Gray Summit, MO, USA) LLC/Test Diet. The 1% NM diet
was milled and pressed by Purina Mills, LLC and generated by
Vitatech (Tustin, CA, USA). During the study, the mice consumed, on
average, 4 g of their respective diets per day. Thus, the
supplemented mice received approximately 40 mg of NM per day. After
4 weeks, the mice were sacrificed and their tumors were excised and
processed for histological analysis. Dimensions (length and width)
of tumors were measured using a digital caliper, and the tumor
burden was calculated using the following formula: 0.5 × length ×
width. The mean weight of the mice at the initiation and
termination of the study did not differ significantly between the
groups.
Histological analysis
Tissue samples were fixed in 10% buffered formalin.
All tissues were embedded in paraffin and cut at 4–5 microns thick.
Sections were deparaffinized through xylene and graduated alcohol
series to water and stained with hematoxylin and eosin (H&E)
for evaluation using a standard light microscope.
In vitro studies
Cell culture
The human OHSU-974 HNSCC cells were grown in RPMI,
supplemented with 20% FBS, penicillin (100 U/ml) and streptomycin
(100 mg/ml) in 24-well tissue culture plates (Costar, Cambridge,
MA, USA). Cells were incubated in 1 ml of medium at 37°C in a
tissue culture incubator equilibrated with 95% air and 5%
CO2. At near confluence, the cells were treated with the
NM, dissolved in medium and examined at 0, 50, 100, 250, 500, and
1,000 μg/ml in triplicate at each dose. Phorbol 12-myristate
13-acetate (PMA), 100 ng/ml was added to cells to induce MMP-9
secretion. The plates were then returned to the incubator.
MTT assay
Cell viability was evaluated by
[3-(4,5-dimethylthiazol-2-yl) 2,5-diphenyl tetrazolium bromide]
(MTT) assay, a colorimetric assay based on the ability of viable
cells to reduce a soluble yellow tetrazolium salt MTT to a blue
formazan crystal by mitochondrial succinate dehydrogenase activity
of viable cells. This test is a good index of mitochondrial
activity and thus of cell viability. After 24 h of incubation, the
cells were washed with PBS and 500 μl of MTT (Sigma #M-2128)
0.5 mg/ml in medium was added to each well. After the addition of
MTT (0.5 mg/ml) the plates were covered and returned to the 37°C
incubator for 2 h, the optimal time for formazan product formation.
Following incubation, the supernatant was carefully removed from
the wells, the formazan product was dissolved in 1 ml
dimethylsulphoxide (DMSO), and absorbance was measured at 570 nm in
a BioSpec 1601, Shimadzu spectrometer. The OD570 of the
DMSO solution in each well was considered to be proportional to the
number of cells. The OD570 of the control (treatment
without supplement) was considered 100%.
Gelatinase zymography
Gelatinase zymography was performed in 10% Novex
Pre-Cast SDS polyacrylamide gel (Invitrogen) in the presence of
0.1% gelatin under non-reducing conditions. The culture media (20
μl) were mixed with sample buffer and loaded for SDS-PAGE
with Trisglycine-SDS buffer, as suggested by the manufacturer
(Novex). Samples were not boiled prior to electrophoresis.
Following electrophoresis, the gels were washed twice in 2.5%
Triton X-100 for 30 min at room temperature to remove SDS. The gels
were then incubated at 37°C overnight in substrate buffer
containing 50 mM Tris-HCl and 10 mM CaCl2 at pH 8.0 and
stained with 0.5% Coomassie Blue R250 in 50% methanol and 10%
glacial acetic acid for 30 min and destained. Upon renaturation of
the enzyme, the gelatinases digested the gelatin in the gel,
producing clear bands against an intensely stained background.
Protein standards were run concurrently and approximate molecular
weights were determined by plotting the relative mobilities of
known proteins.
Matrigel invasion
Invasion experiments were conducted using Matrigel
(Becton-Dickinson) inserts in 24-well plates. Suspended in medium,
OHSU-974 cells were supplemented with nutrients, as specified in
the design of the experiment and seeded on the insert in the well.
Thus both the medium on the insert and in the well contained the
same supplements. The plates with the inserts were then incubated
in a culture incubator equilibrated with 95% air and 5%
CO2 for 24 h. After incubation, the medium was withdrawn
from the wells. The cells on the upper surface of the inserts were
gently scrubbed away with cotton swabs. The cells that had
penetrated the Matrigel membrane and migrated onto the lower
surface of the Matrigel were stained with H&E and visually
counted under a microscope.
Cell migration: scratch test
To examine cell migration, a 2-mm wide single
uninterrupted scratch was made from the top to bottom of culture
plates of OHSU-947 cells grown to confluence. Culture plates were
washed with PBS and incubated with NM in medium and examined at 0,
50, 100, 250 and 500 μg/ml, in triplicate at each dose for
24 h. Cells were washed with PBS, fixed and stained with H&E
and photomicrographs were obtained.
Morphology: H&E
The morphology of the cells cultured for 24 h in the
test concentrations of NM was evaluated by H&E staining and
observed and photographed under a microscope.
Statistical analysis
The results are expressed as the means ± SD. Data
were analyzed by an independent sample t-test. Pearson’s
correlation co-efficients were determined for toxicity and invasion
correlations to the NM concentration using MedCalc software
(Mariakerke, Belgium).
Results
In vivo studies
Tumor growth and burden
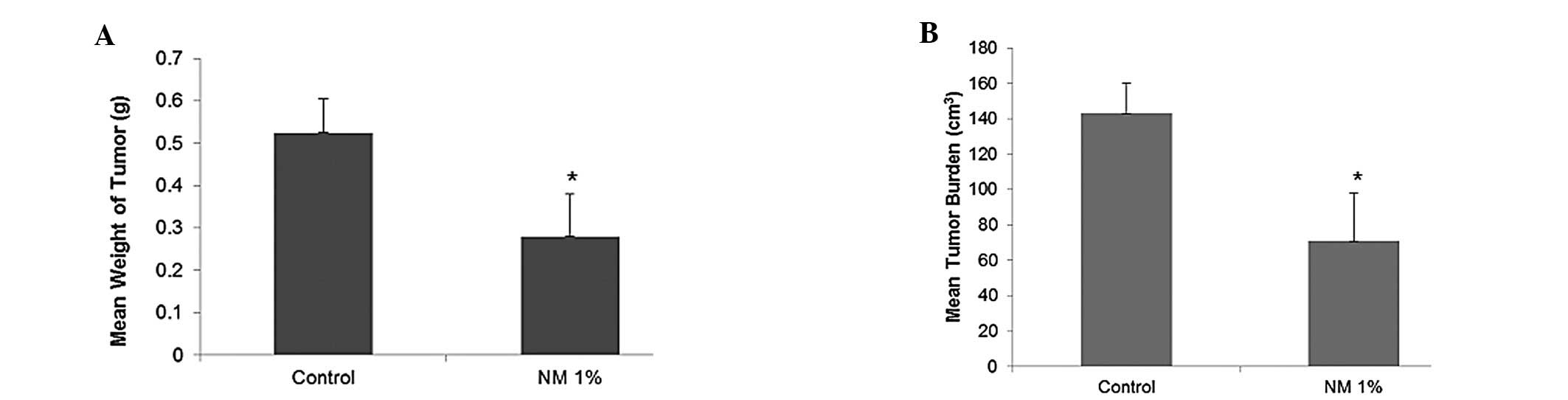
NM strongly inhibited the growth of OHSU-974
xenografts in nude mice. Mean tumor weight was inhibited by 47%
(p=0.0009) with NM 1% dietary supplementation, as shown in Fig. 1A and tumor burden was inhibited by
50% (p=0.0003), as shown in Fig.
1B.
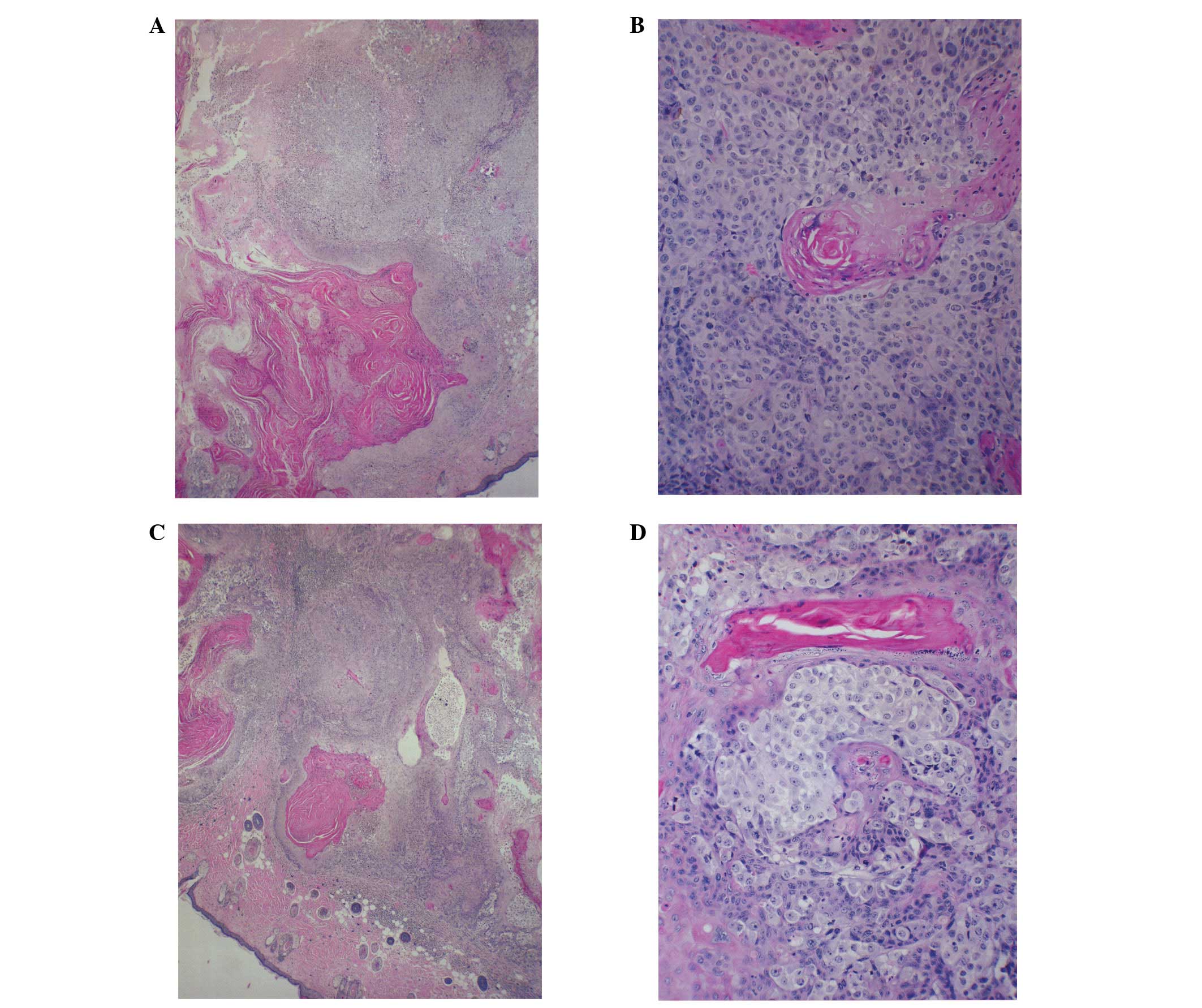
Histological analysis
Histologically, the tumors from both groups were
composed of solid nests of large, irregularly round, ulcerated,
skin subcutaneous masses, consistent with squamous cell carcinoma.
Tumors from the control and NM-supplemented mice were similar
morphologically, although the tumors from the NM-supplemented mice
were significantly smaller in size (Fig. 2).
In vitro studies
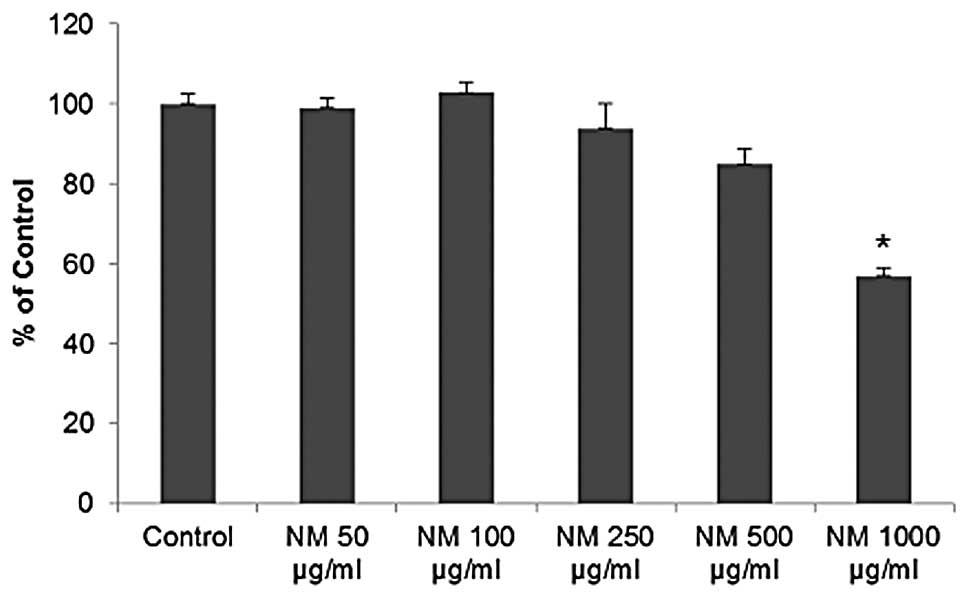
Cytotoxicity
NM exhibited no significant toxicity to OHSU-974
HNSCC cells in vitro at lower concentrations. However, a
cytotoxicity of 15 (p=0.005) and 40% (p<0.001) at 500 and 1,000
μg/ml was observed, respectively, compared to the control,
as shown in Fig. 3.
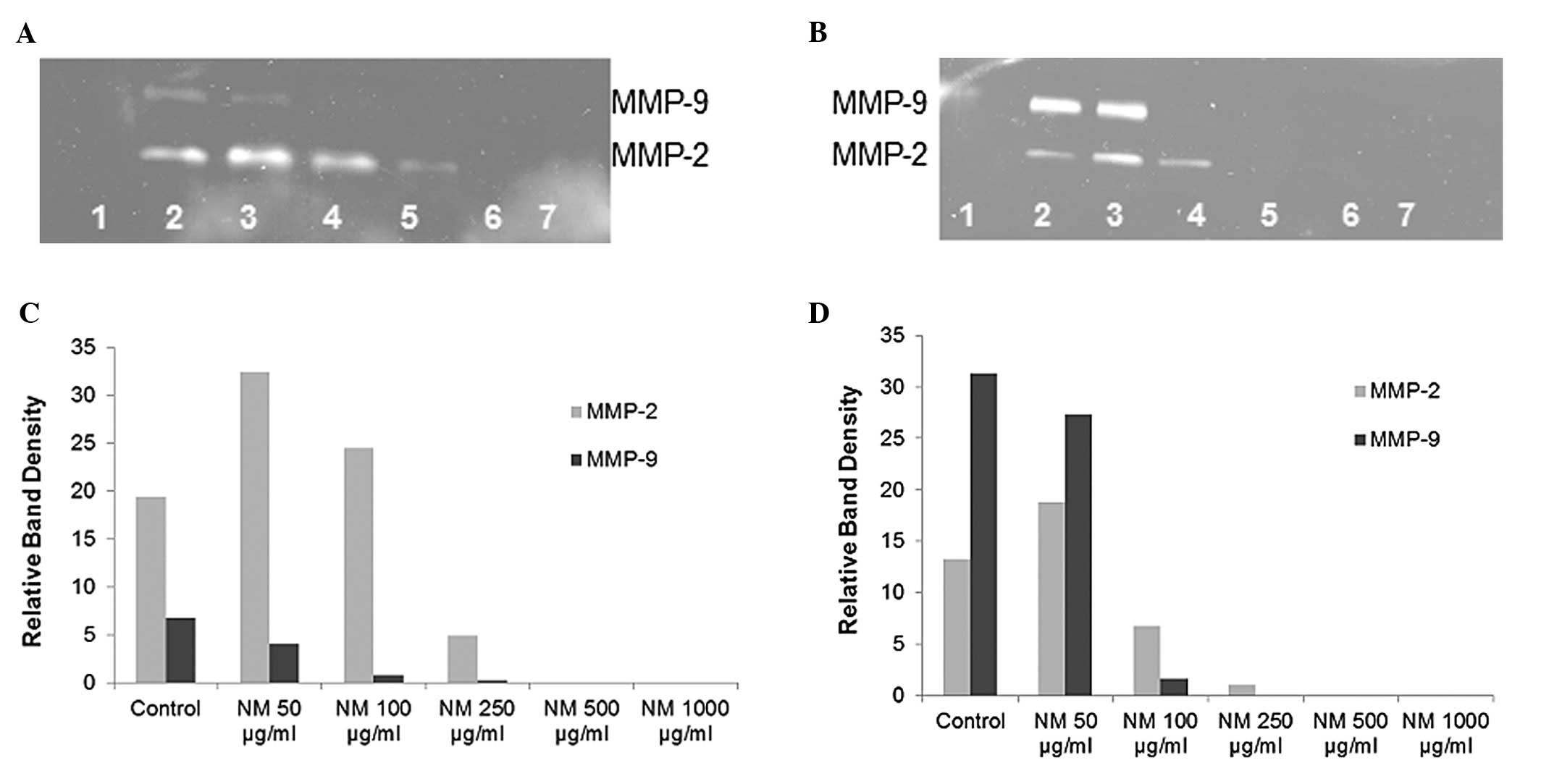
Gelatinase zymography
Gelatinase zymography demonstrated MMP-2 and MMP-9
secretion by normal and PMA-treated OHSU-947 cells. NM inhibited
the secretion of both MMPs in a dose-dependent manner with virtual
total inhibition of MMP-9 and MMP-2 at 500 μg/ml, as shown
in Fig. 4. MMP-2 secretion by
normal OHSU-947 cells was inhibited by 75% at 250 μg/ml NM
and by 100% at 500 μg/ml and 1,000 μg/ml NM (linear
trend, R2=0.6863) and the secretion of MMP-2 by
PMA-treated cells was inhibited by 50% at 100 μg/ml NM, 99%
by 250 μg/ml NM and by 100% at 500 μg/ml and 1,000
μg/ml NM (linear trend, R2=0.7578). MMP-9
secretion by normal OHSU-947 cells was inhibited by 88% at 100
μg/ml NM, 96% by 250 μg/ml NM and by 100% at 500
μg/ml and 1,000 μg/ml NM (linear trend,
R2=0.7898) and the secretion of PMA-treated cells was
inhibited by 95% at 100 μg/ml NM and by 100% at 250, 500 and
1,000 μg/ml NM (linear trend, R2=0.7324).
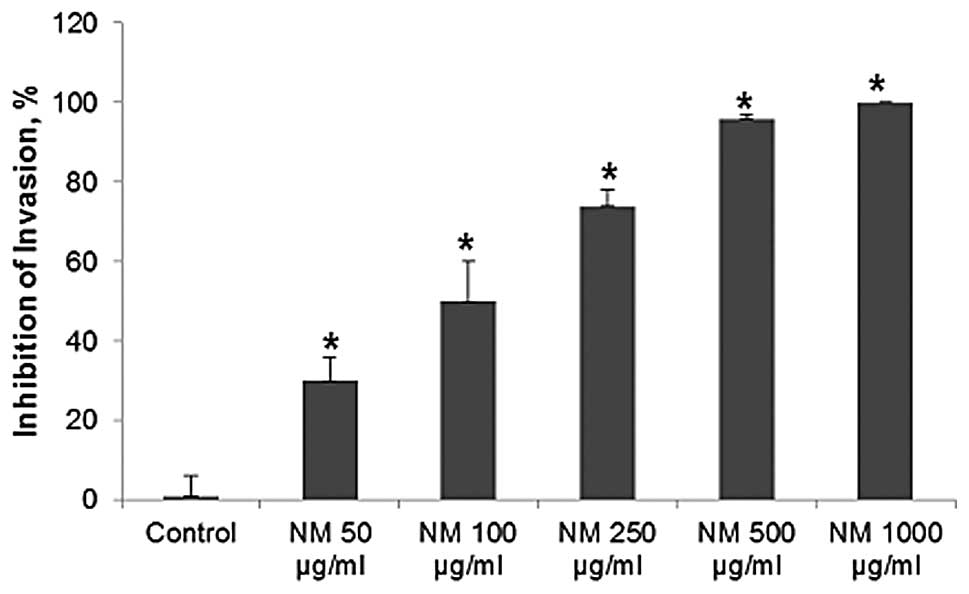
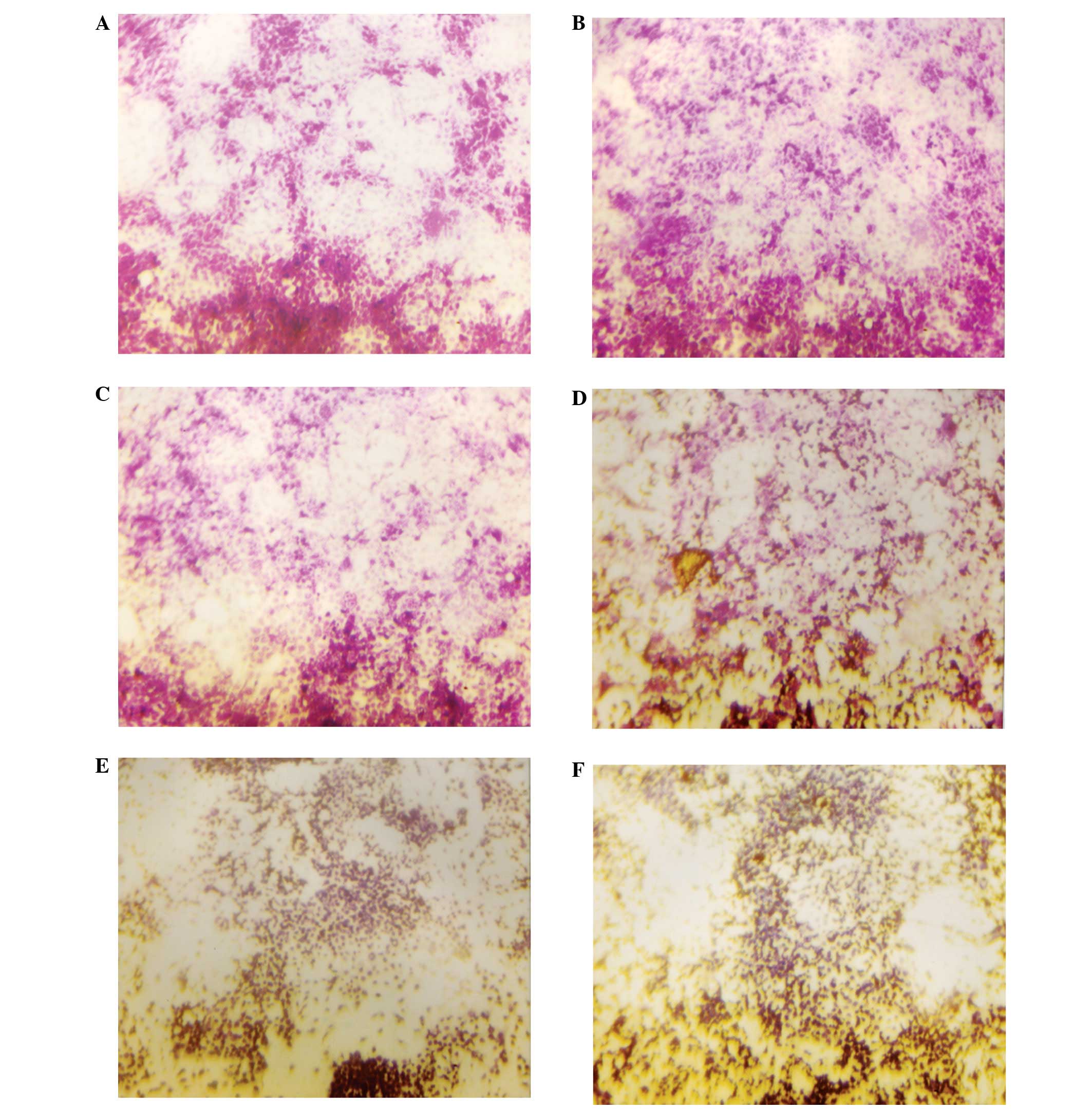
Matrigel invasion
NM significantly inhibited OHSU-974 cell invasion
through Matrigel in a dose-dependent manner, with 30% (p=0.003)
inhibition at 50 μg/ml, 50% (p=0.002) at 100 μg/ml,
74% (p<0.0001) at 250 μg/ml, 96% (p<0.0001) at 500
μg/ml and 100% (p<0.0001) at 1,000 μg/ml, as shown
in Figs. 5 and 6. There was a significant negative
correlation between the NM concentration and the number of OHSU-974
cells that had invaded/migrated through Matrigel (r=−0.9715,
p<0.0001).
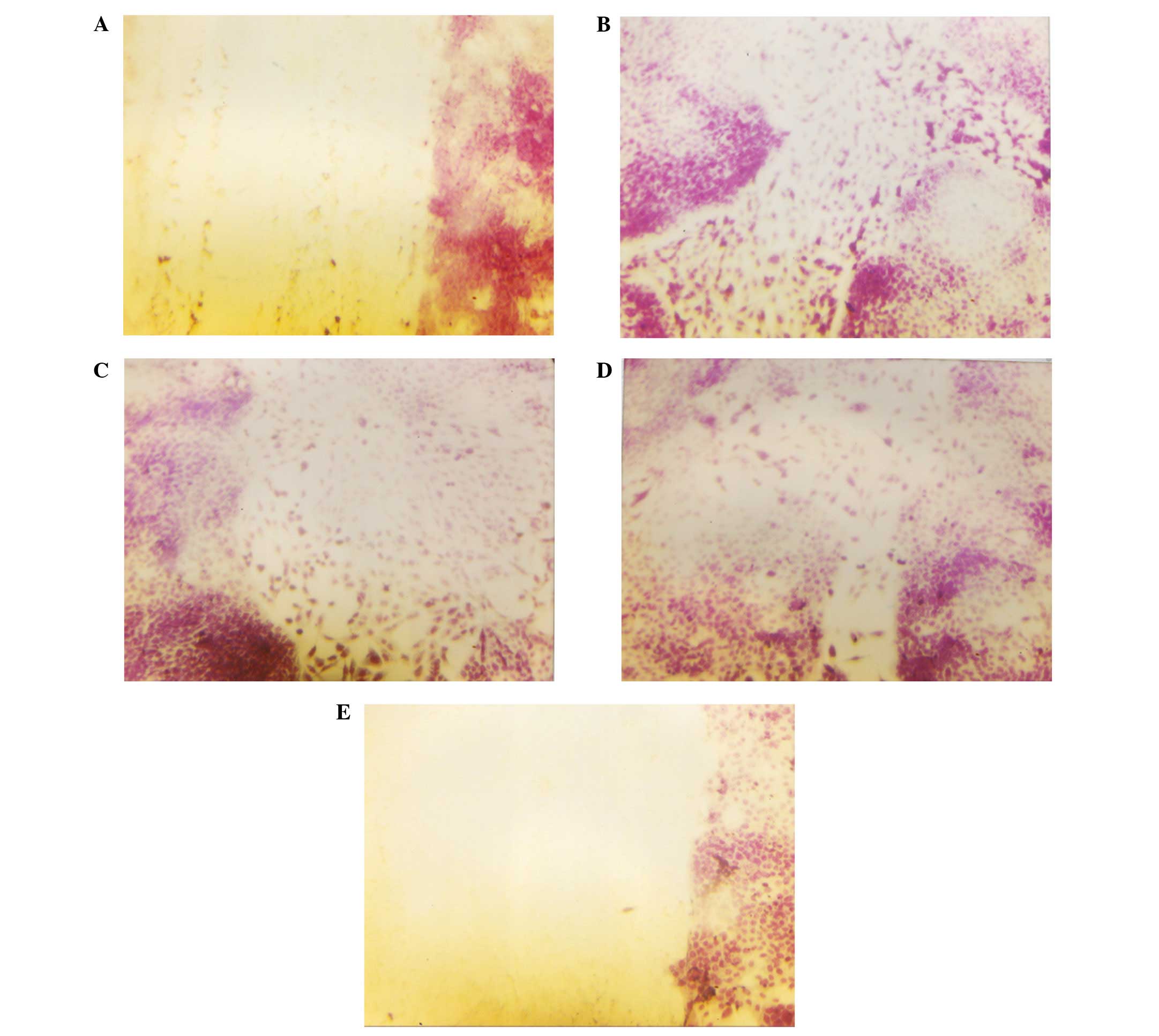
Cell migration: scratch test
NM reduced cell migration in a dose-dependent
manner, with a complete block of OHSU-974 cells at 250
μg/ml. Photomicrographs of the results from the scratch
tests of OHSU-974 cells are shown in Fig. 7.
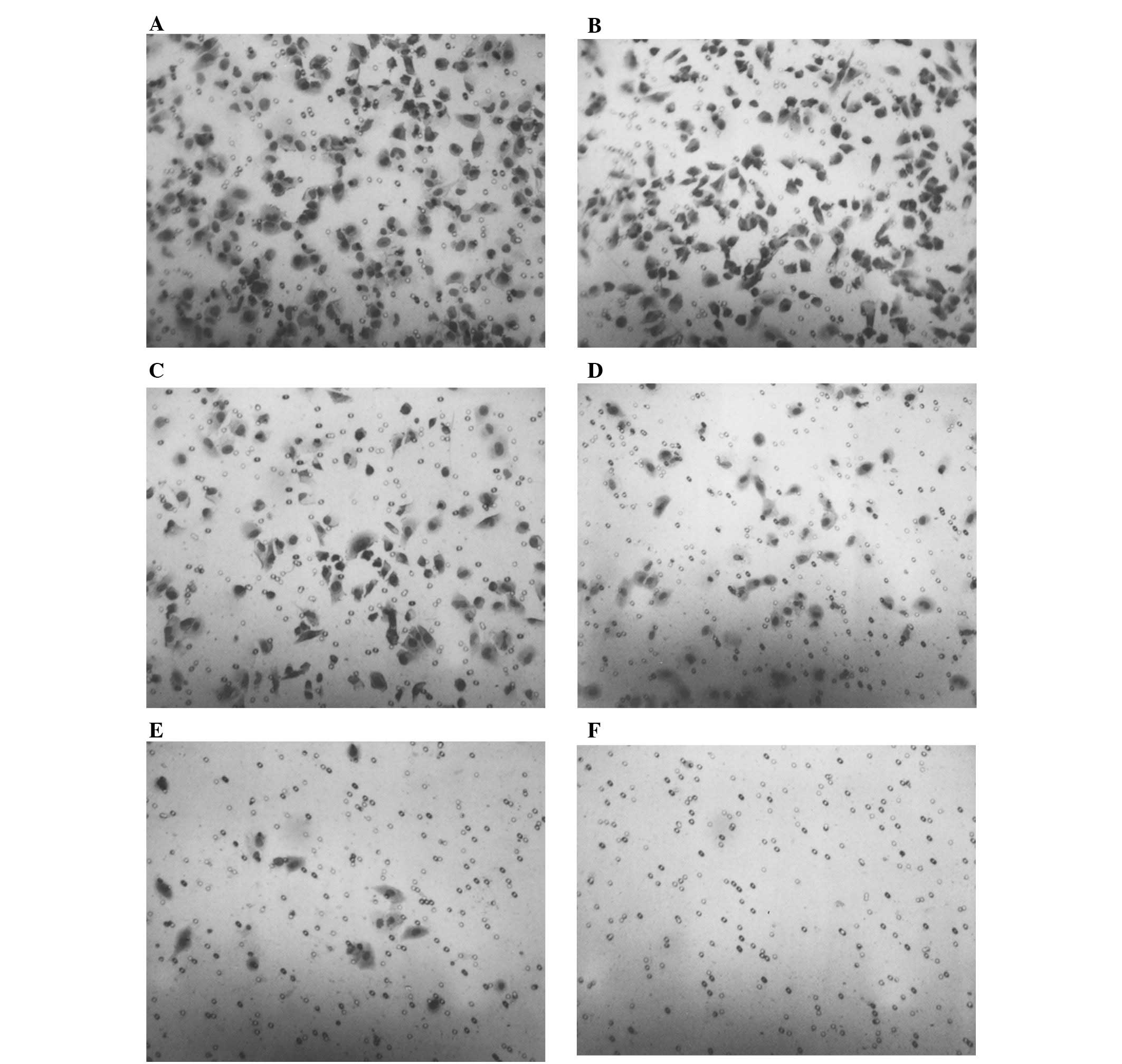
Morphology: H&E staining
No morphological changes were observed following
H&E staining below 500 μg/ml, as shown in Fig. 8.
Discussion
The results of the in vivo study of human
HNSCC xenografts in immune impaired (athymic) nude mice
demonstrated a significant suppression of HNSCC OHSU-974 tumor
growth (47% inhibition of mean tumor weight and 50% inhibition of
mean tumor burden with 1% NM dietary supplementation). The results
from the cellular proliferation study support the in vivo
findings, as NM showed an increased toxicity in OHSU-974 cells in a
dose-dependent manner, with 40% inhibition of cell growth exposed
to 1,000 μg/ml NM.
Growing tumors become hypoxic and acidotic beyond
the size of 2 mm and secrete several growth factors to stimulate
local angiogenesis. In a previous study, we demonstrated that NM
significantly (p<0.05) reduced bFGF-induced angiogenesis
[utilizing a chorioallantoic membrane (CAM) assay in chick
embryos], and decreased the human U2OS osteosarcoma cell expression
of VEGF, angiopoietin-2, bFGF, PDGF and TGFβ-1 (4).
The invasion of host tissues is dependent on tumor
cell adhesion, cell migration and the proteolytic degradation of
the extracellular matrix (ECM) by MMPs (7). MMPs, particularly MMP-2 and MMP-9,
are prognostic markers for survival and metastatic potential in
head and neck squamous carcinomas. In an examination of genolytic
activity in human oral squamous cell carcinoma tissues, Kawamata
et al(8) observed increased
activity of pro-MMP-9 and active MMP-2 in cancer cell nests
compared with normal surrounding gingival tissue and significantly
higher MMP-2 activity in metastatic cancer cell nests. Patel et
al(9) reported a significant
elevation of latent, active and total forms of MMP-2 and MMP-9 in
malignant tissue compared with adjacent normal tissues in oral
cancer patients. In addition, MMP-2 correlated with lymph node
metastatic development (9). In an
examination of a group of patients with early stage oral squamous
cell carcinoma, Katayama et al(10) found that patients who developed
regional lymph node and/or distant metastasis showed significantly
increased MMP-9 and TIMP metallopeptidase inhibitor-2 (TIMP-2)
expression compared to patients without any tumor metastasis. In
addition, the expression of MMP-9 and TIMP-2 correlated with worst
cause-specific survival. Reidel et al(11) found that MMP-9 expression in
patients with HNSCC correlated with poor survival, high VEGF
expression and higher mean vessel density compared to
MMP-9-negative tumors, suggesting that MMP-9 functions as a
regulator of tumor angiogenesis supporting endothelial cell
invasion in human head and neck cancer. Kurahara et
al(12) demonstrated a
significant decrease in ECM staining (indicating loss of ECM) in
invasive and metastatitc cases of oral squamous cell carcinoma with
increased expression of MMP-1, MMP-2 and MMP-9.
The results from our in vitro study of
OSH-947 HNSCC cells demonstrated a potent, significant suppression
of invasive parameters by the NM. NM inhibited MMP-2 and MMP-9
secretion with a complete block of both at 500 μg/ml and
100% inhibition of invasion of cells through Matrigel at 1,000
μg/ml. The migration of cells using a scratch test showed
total block at 250 μg/ml NM. In a previous study of HNSCC
FaDu cells, NM was found to inhibit xenograft tumor growth and
invasive parameters (13).
NM was formulated by defining critical physiological
targets in cancer progression and metastasis, such as ECM integrity
and MMP activity. Adequate supplies of ascorbic acid and the amino
acids, lysine and proline, ensure proper synthesis and
hydroxylation of collagen fibers for optimal ECM structure.
Manganese and copper are also essential for collagen formation.
Lysine, a natural inhibitor of plasmin-induced proteolysis, plays
an important role in ECM stability (14,15).
Green tea extract has been shown to modulate cancer cell growth,
metastasis, angiogenesis and other aspects of cancer progression
(16–20). N-acetylcysteine has been shown to
modulate MMP-9 and the invasive activities of tumor cells (21,22).
Selenium has been shown to inhibit MMP secretion, tumor invasion,
and the migration of endothelial cells through the ECM (23). Ascorbic acid demonstrates cytotoxic
and antimetastatic effects on malignant cell lines (25–28)
and cancer patients have been found to have low levels of ascorbic
acid (29,30). Low levels of arginine, a precursor
of nitric oxide (NO), can limit the production of NO, which has
been shown to predominantly act as an inducer of apoptosis
(31).
Current treatment methods available for
FA-associated cancers are generally ineffective and particularly
toxic to these patients. Thus, there is a need for development of
effective therapeutic agents for these cancers with minimal
toxicity. In this study, our results demonstrated that NM
significantly inhibited the growth and tumor burden of the OHSU-974
FA HNSCC cell line in vivo. In addition, invasive
parameters, such as OHSU-974 cell line MMP-2 and -9 secretion and
invasion were significantly inhibited by NM in vitro. These
findings suggest the potential use of NM in the treatment of FA
HNSCC. Furthermore, in contrast to the toxic side-effects of
current chemotherapy, the NM has been shown to be a safe
therapeutic agent. In a previous in vivo study addressing
safety issues, we found that gavaging adult female ODS rats
(weighing 250–300 g) with the NM (at 30, 90 or 150 mg per day for 7
days), had no adverse effects on vital organs (heart, liver and
kidney), and on associated functional serum enzymes, indicating
that this mixture is safe to use even at these high doses, which
far exceed the normal equivalent dosage of the nutrient (32).
Acknowledgements
The present study was funded by the Dr
Rath Health Foundation (Santa Clara, CA, USA), a non-profit
organization. Consulting pathologist Dr Alexander de Paoli, IDEXX
Reference Laboratories, provided the histopathological slides of
the OHSU-974 HNSCC tumors.
References
|
1.
|
BP AlterMH GreeneI VelasquezPS
RosenbergCancer in Fanconi
anemiaBlood10120722073200310.1182/blood-2002-11-359712584146
|
|
2.
|
DI KutlerAD AuerbachJH SatagopanHigh
incidence of head and neck squamous cell carcinoma in patients with
Fanconi anemiaArch Otolaryngol Head Neck
Surg192106112200310.1001/archotol.129.1.106
|
|
3.
|
B SinghHead and neck squamous carcinoma in
Fanconi anemia patientsFanconi Anemia: Guidelines for Diagnosis and
ManagementME EilerD FohnmayerL ForhnmayerK LarsenJ Owen3rd
editionFanconi Anemia Research Fund, IncEugene, OR2502632008
|
|
4.
|
MW RoomiN RoomiV IvanovT KalinovskyA
NiedzwieckiM RathInhibitory effect of a mixture containing ascorbic
acid, lysine, proline and green tea extract on critical parameters
in angiogenesisOncol Rep14807815200516142336
|
|
5.
|
MW RoomiV IvanovT KalinovskyA NiedzwieckiM
RathInhibition of pulmonary metastasis of melanoma B16FO cells in
C57BL/6 mice by a nutrient mixture consisting of ascorbic acid,
lysine, proline, arginine, and green tea extractExp Lung
Res32517530200610.1080/0190214060109855217169857
|
|
6.
|
A NiedzwieckiMW RoomiT KalinovskyM
RathMicronutrient synergy - a new tool in effective control of
metastasis and other key mechanisms of cancerCancer Metastasis
Rev29529543201010.1007/s10555-010-9244-120717705
|
|
7.
|
MJ DuffyThe role of proteolytic enzymes in
cancer invasion and metastasisClin Exp
Metastasis10145155199210.1007/BF001327461582084
|
|
8.
|
H KawamataD UchidaH HamanoT
Kimura-YanagawaKI NakashiroS HinoF OmoteharaH YoshidaM SatoActive
MMP-2 in cancer cell nests of oral cancer patients: Correlation
with lymph node metastasisInt J Oncol1369970419989735398
|
|
9.
|
BP PatelSV ShahSN ShuklaPM ShabPS
PatelClinical significance of MMP-2 and MMP-9 in patients with oral
cancerHead Neck29564572200710.1002/hed.2056117252594
|
|
10.
|
A KatayamaN BandohK KishibeM TakaharaT
OginoS NonakaY HarabuchiExpression of matrix metalloproteinases in
early-stage oral squamous cell carcinoma as predictive indicators
of tumor metastases and prognosisClin Cancer
Res10634640200410.1158/1078-0432.CCR-0864-0214760086
|
|
11.
|
F ReidelK GötteJ SchwalbW BerglerK
HörmannExpression of 92-kDa type IV collagenase correlates with
angiogenic markers and poor survival in head and neck squamous cell
carcinomaInt J Oncol1710991105200011078794
|
|
12.
|
S KuraharaM ShinoharaT IkebeS NakamuraM
BeppuA HirakiH TakeuchiK ShirasunaExpression of MMPs, MT-MMP, and
TIMPs in squamous cell carcinoma of the oral cavity: correlation
with tumor invasion and metastasisHead
Neck21627638199910.1002/(SICI)1097-0347(199910)21:7%3C627::AID-HED7%3E3.0.CO;2-210487950
|
|
13.
|
MW RoomiN RoomiT KalinovskyM RathA
NiedzwieckiMarked inhibition of growth and invasive parameters of
head and neck squamous carcinoma FaDu by a nutrient mixtureIntegr
Cancer Ther8168176200910.1177/153473540833463219679626
|
|
14.
|
M RathL PaulingPlasmin-induced proteolysis
and the role of apoprotein(a), lysine and synthetic
analogsOrthomolecular Med717231992
|
|
15.
|
Z SunYH ChenP WangJ ZhangV GurewichP
ZhangJN LiuThe blockage of high-affinity lysine binding sites of
plasminogen by EACA significantly inhibits prourokinase-induced
plasminogen activationBiochem Biophys Acta15961821922002
|
|
16.
|
S ValcicBN TimmermannDS AlbertsGA WachterM
KrutzschJ WymerJM GuillenInhibitory effect of six green tea
catechins and caffeine on the growth of four selected human tumor
cell linesAnticancer
Drugs7461468199610.1097/00001813-199606000-000118826614
|
|
17.
|
H MukhtarN AhmedTea polyphenols:
prevention of cancer and optimizing healthAm J Clin
Nutr711698S1702S200010837321
|
|
18.
|
GY YangJ LiaoK KimEJ YurtowCS
YangInhibition of growth and induction of apoptosis in human cancer
cell lines by tea
polyphenolsCarcinogenesis19611616199810.1093/carcin/19.4.6119600345
|
|
19.
|
S TaniguchiH FujikiH KobayashiH GoK
MiyadoH SadanoR ShimikawaEffect of (−)-epigallocatechin gallate,
the main constituent of green tea, on lung metastasis with mouse
B16 melanoma cell linesCancer Lett6551541992
|
|
20.
|
Y HaraGreen tea: Health Benefits and
ApplicationsMarcel DekkerNew York200110.1201/9780203907993
|
|
21.
|
S KawakamiY KageyamaY FujiiK KiharaH
OshimaInhibitory effects of N-acetylcysteine on invasion and MMP-9
production of T24 human bladder cancer cellsAnticancer
Res21213219200111299737
|
|
22.
|
M MoriniT CaiMG AluigiDM NoonanL MasielloS
De FloroF D’AgostininA AlbiniG FassimaThe role of the thiol
N-acetylcysteine in the prevention of tumor invasion and
angiogenesisInt J Biol Markers14268271199910669958
|
|
23.
|
SO YoonMM KimAS ChungInhibitory effects of
selenite on invasion of HT1080 tumor cellsJ Biol
Chem2762008520092200110.1074/jbc.M10114320011274215
|
|
24.
|
C MaramagM MenonKC BalajiPG ReddyS
LaxmananEffect of vitamin C on prostate cancer cells in vitro:
effect on cell number, viability and DNA
synthesisProstate32188195199710.1002/(SICI)1097-0045(19970801)32:3%3C188::AID-PROS5%3E3.0.CO;2-H9254898
|
|
25.
|
KA NaiduRC KarlD CoppolaAntiproliferative
and proapoptotic effect of ascorbyl stearate in human pancreatic
cancer cells: association with decreased expression of insulin-like
growth factor 1 receptorDig Dis
Sci48230237200310.1023/A:1021779624971
|
|
26.
|
WS KohSJ LeeH LeeC ParkMH ParkWS KimSS
YoonK ParkSI HongMH ChungCH ParkDifferential effects and transport
kinetics of ascorbate derivatives in leukemic cell linesAnticancer
Res82487249319989703897
|
|
27.
|
Q ChenMG EspeyMC KrishnaJB MitchellCP
CorpeGR BuettnerE ShacterM LevinePharmacologic ascorbic acid
concentrations selectively kill cancer cells: Action as a pro-drug
to deliver hydrogen peroxide to tissuesProc Natl Acad Sci
USA1021360413609200510.1073/pnas.050639010216157892
|
|
28.
|
CM KurbacherU WagnerB KolsterPE AndreottiD
KrebsHW BrucknerAscorbic acid (vitamin C) improves the
antineoplastic activity of doxorubicin, cisplatin and paclitaxel in
human breast carcinoma cells in vitroCancer
Lett103183189199610.1016/0304-3835(96)04212-78635156
|
|
29.
|
HM AnthonyCJ SchorahSevere hypovitaminosis
C in lung-cancer patients: The utilization of vitamin C in surgical
repair and lymphocyte-related host resistanceBr J
Cancer46354367198210.1038/bjc.1982.2117126425
|
|
30.
|
C Núñez MartínA Ortiz de Apodaca y
RuizAscorbic acid in the plasma and blood cells of women with
breast cancer. The effect of consumption of food with an elevated
content of this vitaminNutr Hosp103683721995(In Spanish).
|
|
31.
|
JP CookeVJ DzauNitric oxide synthase: Role
in the genesis of vascular diseaseAnnu Rev
Med48489509199710.1146/annurev.med.48.1.4899046979
|
|
32.
|
MW RoomiV IvanovSP NetkeA NiedzwieckiM
RathSerum markers of the liver, heart, and kidney and lipid profile
and histopathology in ODS rats treated with nutrient synergyJ AM
Coll Nutr22477abs. 862003
|