Introduction
Oral squamous cell carcinoma (OSCC) is the sixth
most common neoplasm in the world, characterized by a poor
prognosis and low survival rates (1,2).
Worldwide, the GLOBOCAN 2002 database estimated that 274,000 new
cases of oral cancer and 127,000 deaths from the disease occur
annually [The GLOBOCAN 2002 database. International Agency for
Cancer Research (IARC), http://www-dep.iarc.fr/]. In patients with locally
advanced disease, the main causes of treatment failure are
locoregional recurrence and metastatic disease. Despite recent
advances in diagnosis and surgical, radiotherapeutic, and
chemotherapeutic management, cervical lymph node involvement is
associated with an approximately 50% lower 5-year survival rate, as
well as an increased risk of distant metastasis (3,4).
Patients with highly invasive carcinomas have poor outcomes because
tumor cells deeply invade surrounding fibrous tissues, metastasize
more frequently to lymph nodes and are less sensitive to
chemotherapeutic agents than lowly invasive carcinomas. Invasion
and metastasis are thus the most crucial characteristics of
malignant tumors.
OSCC cell lines are important preclinical models in
the search for novel targeted therapies for oral cancer. Unlike
many other types of cancer, a wide variety of primary and
metastatic OSCC cell lines are available. In fact, more than 300
cell lines of head and neck cancer have been established, as
compared with approximately 70, 60 and 10 cell lines derived from
breast, colon and prostate cancers, respectively (5,6,7).
Yokoyama et al(8) reported
the establishment of subclonal OSCC cell lines that showed negative
expression of E-cadherin and fibroblastic spindle shape and had
higher invasive activity than the respective parental OSCC cell
lines. Moreover, they demonstrated that OSCC cell lines contained
two kinds of cells: one with an epithelial shape and the other with
a spindle or fibroblastic shape in culture. The cells with
epithelial shape expressed E-cadherin, whereas the cells with
fibroblastic shape did not. Frixen et al(9) reported that carcinoma cell lines with
epithelial phenotype were non-invasive and expressed E-cadherin,
whereas carcinoma cell lines with fibroblastic phenotype were
invasive and had lost E-cadherin expression. These findings
indicate that OSCC is characterized by a heterogeneous cell
population. Fibroblastic type OSCC cells have been acknowledged to
result from epithelial-mesenchymal transition (EMT) in epithelial
cell lines, whereas the relation between cell morphology and tumor
cell motility in OSCC is incompletely understood.
Zyxin is an evolutionary conserved protein that has
been implicated in the regulation of actin assembly and is mainly
localized at focal adhesions. Zyxin is a family of proteins that
also includes lipoma preferred partner (LPP) and thyroid
receptor-interacting protein-6 (TRIP-6) (10,11).
Although Zyxin has been shown to be diffusely distributed in
cytoplasm, it is likely that part of Zyxin enters the nucleus,
binds to h-warts and leads to G2-cell cycle arrest and inhibition
of proliferation, as observed after silencing of LASP-1, a
transcriptional factor of Zyxin (12,13).
Zyxin is characterized by a nuclear-cytoplasmic shuttling of focal
contact proteins and has a potential mechanism for communication
between sites of cell adhesion and the nucleus (14,15,16).
In cancer cells, Zyxin is significantly upregulated in melanoma
cells as compared with melanocytes, and Zyxin expression is
directly related to cell spreading and proliferation and inversely
related to differentiation (17).
However, Sperry et al(18)
found that expression of Zyxin activity is downregulated at
cell-cell junctions during EMT. Therefore, the biological roles of
Zyxin in cancer cells remain controversial. In this study, we
investigated the functions of Zyxin using eight OSCC cell lines
with two different cell morphologies (epithelial type and
fibroblastic type) to clarify the biological roles of Zyxin in
OSCC.
Materials and methods
Cell culture and cell lines
The eight oral squamous carcinoma cell lines [SCCKN
(19), HSC-2, HSC-3 (20), OSC-19 (21), OSC-20 (22), HOC-313, TSU (23–25)
and SCC25] were grown in DMEM containing 10% FBS as growth medium
and subcultured. OSC-19, OSC-20, HOC-313 and TSU were kindly
provided by Professor S. Kawashiri (Department of Oral and
Maxillofacial Surgery, Kanazawa University Graduate School of
Medical Science, Kanazawa, Japan).
RNA extraction and real-time polymerase
chain reaction (PCR)
Total-RNA was isolated using an RNeasy mini kit
(Qiagen, Valencia, CA, USA), and cDNAs were generated by using a
First-Strand cDNA Synthesis kit (Amersham Biosciences, Piscataway,
NJ, USA) with 2 μg of total-RNA and oligo (dT) (GE
Healthcare Japan, Tokyo, Japan). All reagents required for
real-time PCR were from Applied Biosystems (Foster City, CA, USA).
Oligo-nucleotide primers and fluorescent probes for Zyxin and GAPDH
were designed using a primer design program (Primer Express,
Applied Biosystems) and were obtained from Integrated DNA
Technologies (Coralville, IA, USA).
Protein preparation and western blot
analysis
Cell lysates were submitted to western blot analysis
as described previously (26,27).
The following primary antibodies used: rabbit polyclonal antibodies
against Zyxin (Sigma-Aldrich Co., St. Louis, MO, USA), N-cadherin,
RhoA, CDC42 (Santa Cruz Biotechnology, Santa Cruz, CA, USA), and
Rac1/2/3 (Cell Signaling Technology, Boston, MA, USA); mouse
monoclonal antibodies against E-cadherin (Santa Cruz
Biotechnology), LPP (Cell Signaling, Acc, Switzerland), and TRIP-6
(Abnoba, Taipei, Taiwan); and goat polyclonal antibodies against
actin (Santa Cruz Biotechnology). The secondary antibodies used
were anti-goat, anti-mouse or anti-rabbit IgGs conjugated with
alkaline phosphatase (all products, Santa Cruz Biotechnology).
Actin was used as an internal control.
Immunocytochemical analysis
The primary antibodies used in this study included
rabbit anti-Zyxin (Sigma-Aldrich) and mouse polyclonal antibody
against human actin (Santa Cruz Biotechnology). Cultured cells were
washed with PBS (-) twice and fixed in 3.7% paraformaldehyde for 20
min at room temperature. After blocking with 2% bovine serum
albumin, the cells were treated with a primary antibody at 4°C
overnight. The cells were washed and incubated with anti-rabbit
fluorescein isothiocyanate or anti-mouse rhodamine phalloidin
(Cytoskeleton, Denver, CO, USA), followed by counterstaining with
4,6-diamidino-2-phenylindole (DAPI). Finally, fluorescence images
were obtained by using a confocal laser microscope, LSM 510 version
3.2 (Carl Zeiss Co. Ltd., Oberkochen, Germany).
Transfection of siRNAs
Cells were cultured in DMEM supplemented with 10%
FBS for 24 h and then transfected with 5 μM of siRNA, using
Thermo Scientific DharmaFECT Transfection Reagents (Roche,
Indianapolis, IN, USA) according to the manufacturer’s protocol.
SMART pool siRNA targeting Zyxin (L-016734-00-0020) and control
siRNA, On-Target plus GAPDH (D-001830-01-20), were purchased from
Dharmacon Inc. (Lafayette, CO, USA).
Cell growth assay
Cells were plated at 2.5×104 cells/well
in 1-ml volumes in 12-well plates and cultured in growth medium at
37°C. At selected intervals, cell growth was determined by
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay or by using a hemocytometer as described previously (26).
Measurement of apoptosis
Cells were plated at 3×104 cells/well in
100-μl volumes in 96-well plates and cultured in growth
medium at 37°C. Apoptosis induction was examined after 48 h using
an ssDNA Apoptosis ELISA Kit (Chemicon International Inc.,
Temecula, CA, USA).
Flow cytometry
For cell-cycle analysis, cells were harvested 48 h
after Zyxin siRNA transfection. The apoptosis index was measured
using a Cell Cycle Phase Determination kit (Cayman Chemical, Ann
Arbor, MI, USA).
Scratch assay
Cells were plated at 5×105 cells/dish in
60-mm dishes (Asahi Techno Glass Co., Tokyo, Japan) and treated
with 25 nM Zyxin siRNA. Scratch assay was performed by scraping
confluent cell monolayers with a sterile pipette tip after 24 h.
Cell migration was examined by measuring the distance from edge to
edge after 6-h incubation.
Invasion assay
Cell invasion assay was carried out using BioCoat
Matrigel Invasion Chambers (Becton Dickinson, Bedford, MA, USA)
consisting of transwell membrane filter inserts in a 24-well tissue
culture plate. The transwell filter had an 8-μm pore size
membrane coated with Matrigel. One hundred thousand cells were
seeded in the upper chamber of the transwell with serum-free DMEM
and treated with 25 nM Zyxin siRNA. DMEM containing 10% FBS was
added to the lower chamber after 24 h. Non-invading cells were
removed by wiping the upper side of the membrane, and invading
cells were fixed and stained with a Diff-Quick kit (Kokusaishiyaku
Co., Kobe, Japan) after 24-h incubation. The number of invading
cells per membrane was counted in triplicate under a light
microscope at x200 magnification in four fields per membrane.
Expression of Zyxin after Rac1 inhibitor
treatment
To examine the effect of Rac-1 inhibitor on
expression of Zyxin, cells were treated with 50 nM of Rac1
inhibitor (EHT 1864, R&D Systems, IA, USA) for 4 h. Western
blot assay and real-time PCR analysis of Zyxin protein and mRNA in
HOC-313 cells were performed.
Statistical analysis
All values in the figures and text are expressed as
means ± SD. The results were analyzed and individual group means
were compared with the use of Student’s t-test. A p-value of
<0.05 was considered to indicate statistical significance.
Results
Expression of cadherins, Zyxin and Zyxin
family proteins in OSCC cell lines in relation to cell
morphology
To examine the relations between the morphology of
OSCC cell lines and EMT markers, expressions of cadherins, Zyxin
and Zyxin family proteins were examined using western blot
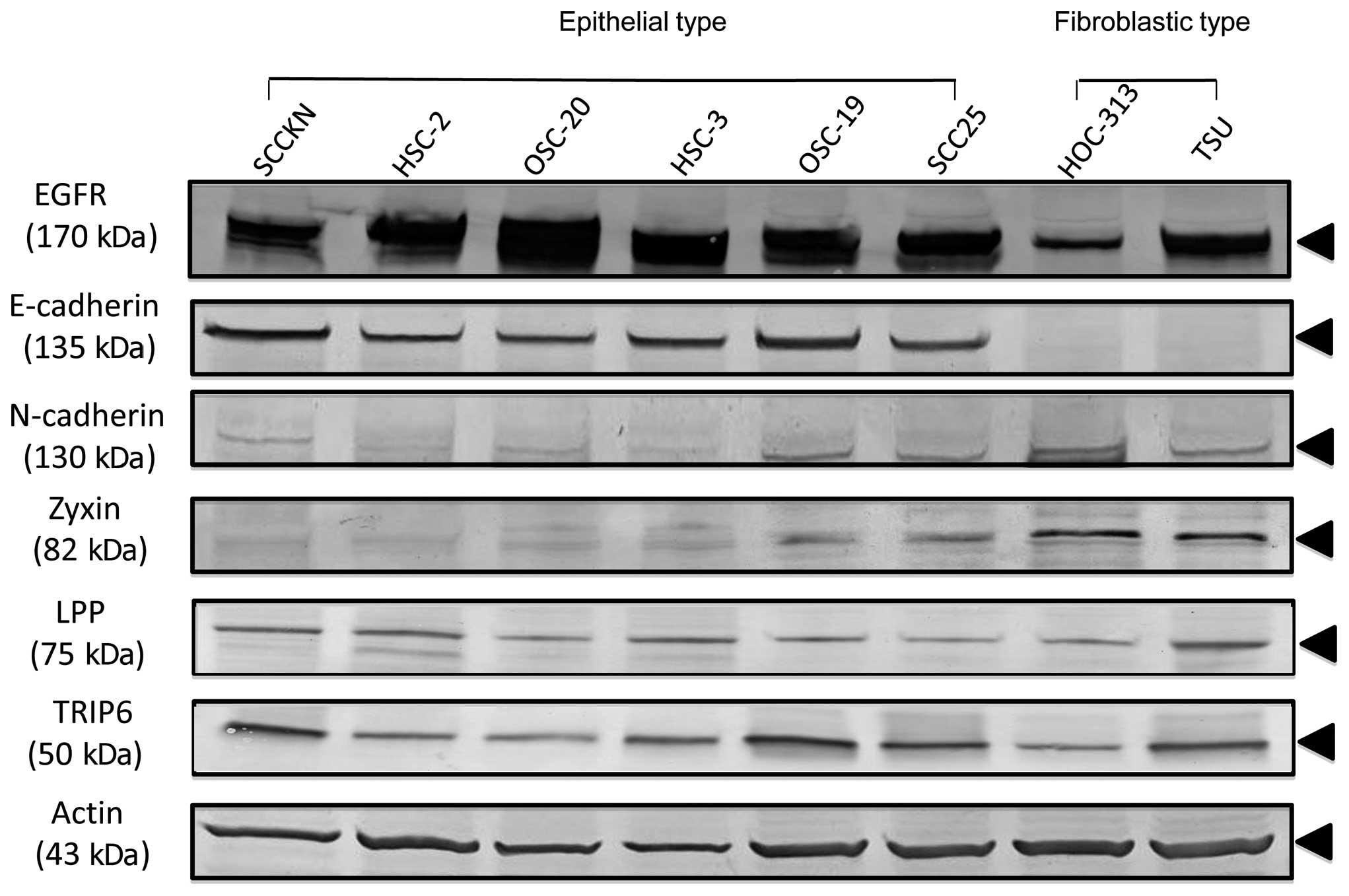
analysis. All eight OSCC cell lines expressed EGFR, indicating that
these cell lines were of epithelial origin and not mesenchymal
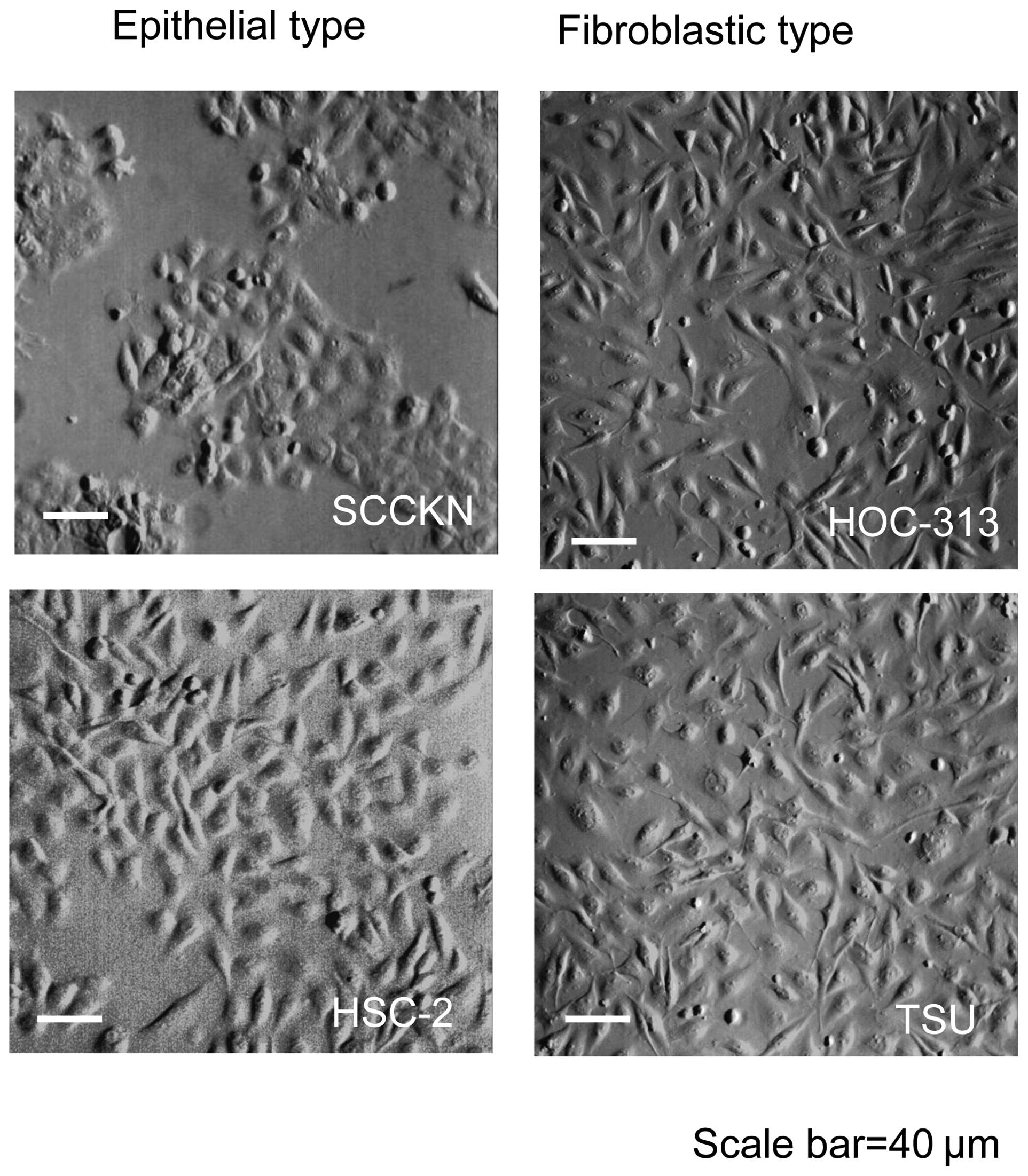
origin. The OSCC cell lines showed two morphological types:
epithelial type cells such as SCCKN and HSC-2; and fibroblastic
type cells such as HOC-313 and TSU (Fig. 1). Western blot analysis revealed
high expression levels of E-cadherin protein in 6 cell lines with
epithelial type morphology, including SCCKN, HSC-2, OSC-20, HSC-3,
OSC-19 and SCC25. These cell lines formed flatter colonies on the
plastic dish surface. In contrast, HOC-313 and TSU showed
fibroblastic morphology and were completely negative for
E-cadherin. These cell lines formed disperse colonies and expressed
N-cadherin, suggesting mesenchymal transition. To gain insight into
the functions of Zyxin family proteins in OSCC cell lines,
expressions of Zyxin and its family members LPP and TRIP-6 were
examined. The cell lines with epithelial type morphology and high
levels of E-cadherin expression showed low levels of Zyxin
expression. High levels of Zyxin and N-cadherin expression were
detected in cell lines with fibroblastic type morphology, such as
HOC-313 and TSU (Fig. 2). When
expressions of Zyxin family members such as LPP and TRIP-6 were
examined in OSCC cell lines, there were no differences between
epithelial and fibroblastic type OSCC cell lines, and all cell
lines showed similar expression. These expression patterns
suggested that Zyxin family members did not compensate for each
other. These results indicated that expression of Zyxin was
suppressed in E-cadherin-expressing epithelial cell lines, while
N-cadherin-expressing cell lines strongly expressed Zyxin.
Morphological changes of HOC-313 induced
by treatment with Zyxin siRNA
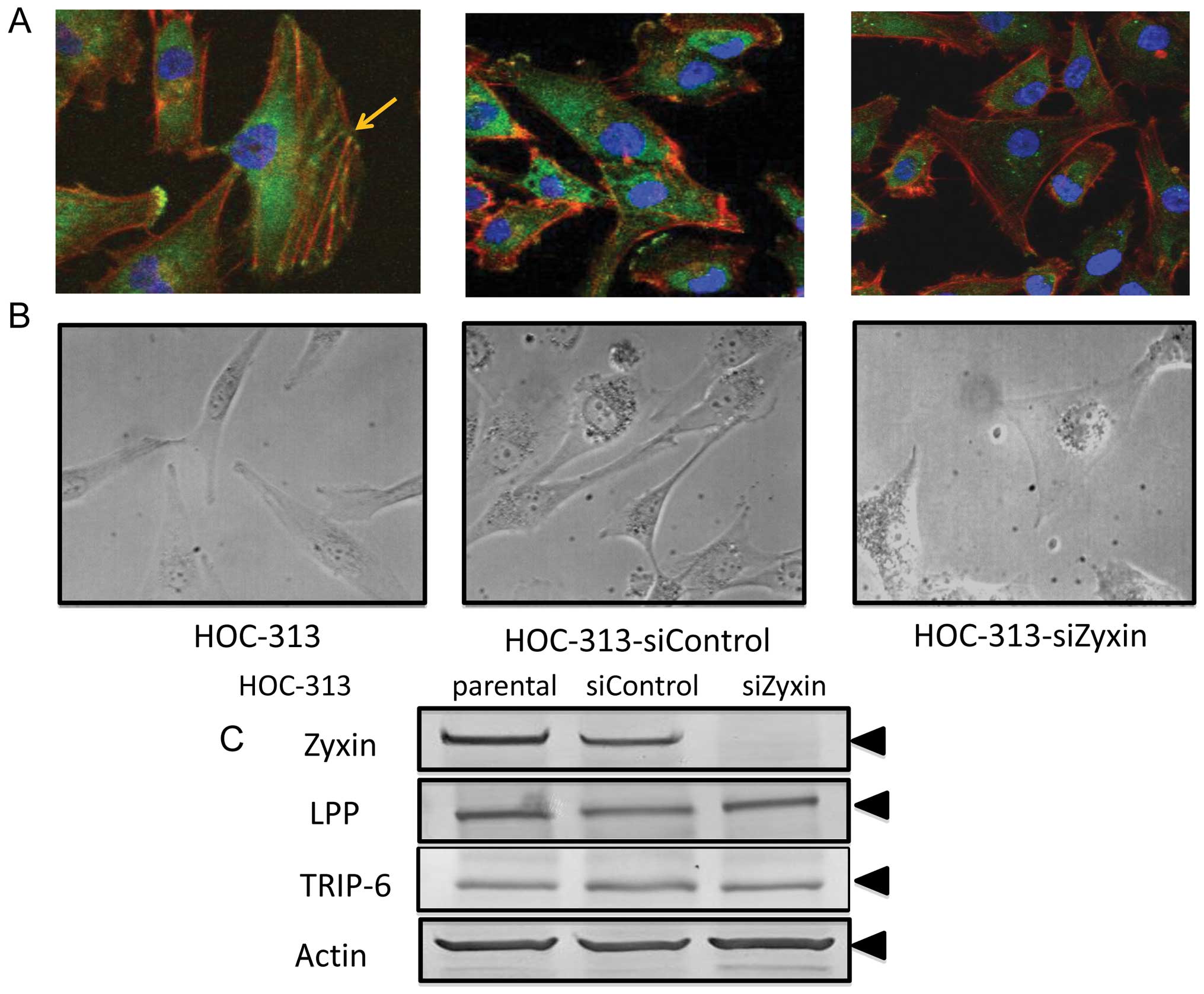
Next, we examined the cellular localization of Zyxin
in HOC-313, a fibroblastic type OSCC cell line maintaining an
invasive character (28).
Endogenous Zyxin was localized at adhesion plaques and some
overlapped with actin stress fibers in HOC-313 (Fig. 3A). To clarify the function of Zyxin
in highly invasive cells, knockdown experiments of Zyxin by siRNA
were performed. On immunocytochemical analysis, a dramatic
reduction in Zyxin expression was observed in Zyxin siRNA-treated
cells as compared with control siRNA-treated cells (Fig. 3A). On western blot analysis, the
protein level of Zyxin was also reduced in Zyxin siRNA-treated
cells (Fig. 3C). These data
indicated that Zyxin siRNA specifically targeted Zyxin expression.
Moreover, Zyxin siRNA-treated HOC-313 showed morphological changes
(Fig. 3B). Zyxin siRNA-treated
HOC-313 showed tripolar or polygonal shape and a large projected
cell area as compared with control siRNA-treated HOC-313.
Therefore, Zyxin was suggested to play important roles in
maintenance of cell morphology via actin re-arrangement. When
expression of Zyxin family members including LPP and TRIP-6 in
HOC-313 were examined after Zyxin siRNA treatment, their expression
was found to be uninhibited by such treatment (Fig. 3C).
Inhibition of cell proliferation, cell
migration and invasive potential in OSCC cell lines with
fibroblastic type morphology after treatment with Zyxin siRNA
Since we found high levels of Zyxin expression in
OSCC cell lines with fibroblastic type morphology as compared with
cell lines with epithelial type morphology, the effects of
knockdown of Zyxin on cell proliferation, migration, and invasive
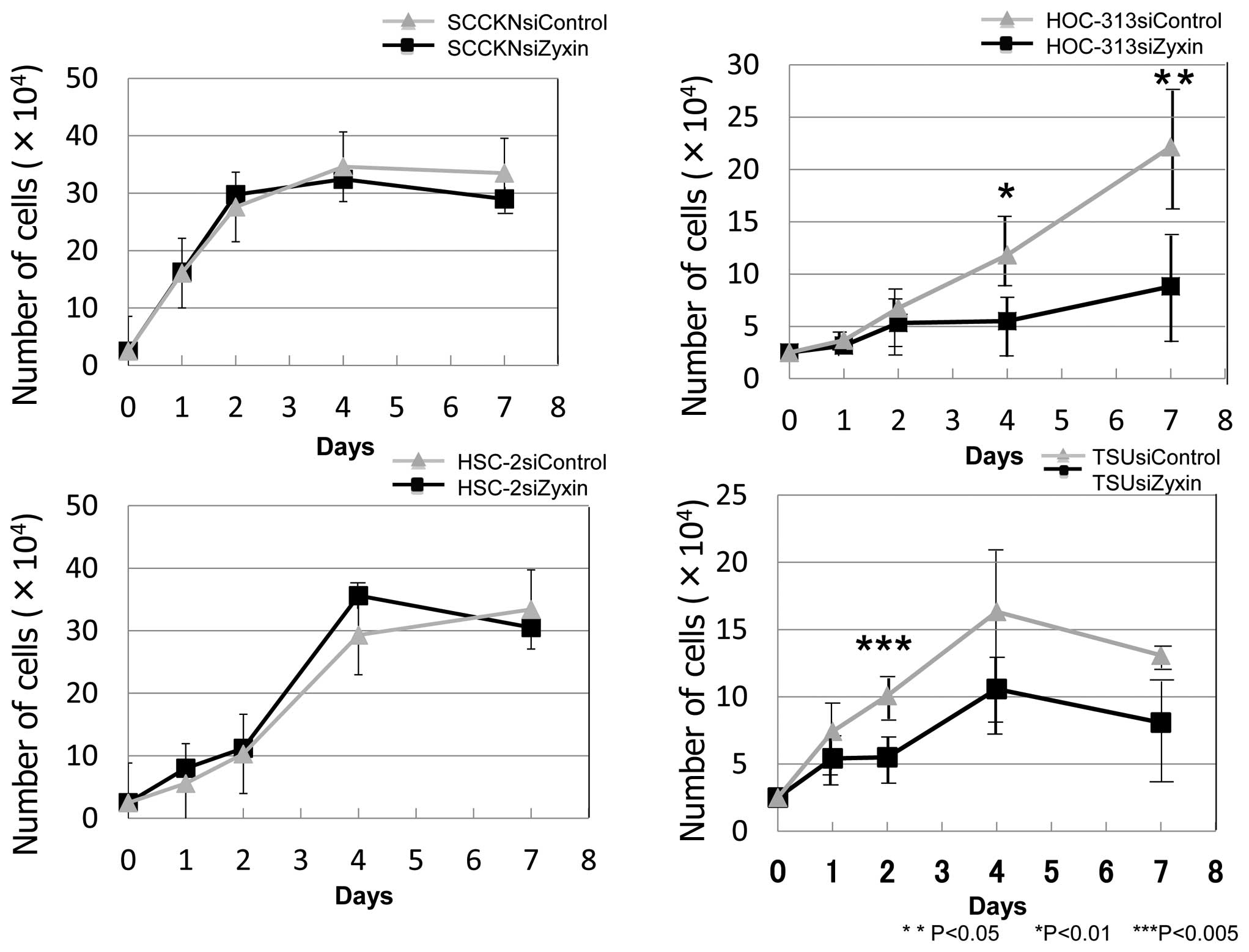
potential were examined in fibroblastic type of OSCC. Growth curves
of HOC-313 and TSU treated with Zyxin siRNA and control siRNA are
shown in Fig. 4. Cell
proliferation was significantly inhibited in Zyxin siRNA-treated
HOC-313 and TSU from day 4 onward, and the reduction rate on day 4
was 53.5 and 35.2%, respectively. Significant difference was
observed only on day 2 in TSU. However, cell proliferation of SCCKN
and HSC-2 lines that do not express Zyxin, was not inhibited by
Zyxin siRNA treatment. Zyxin siRNA treatment of HOC-313 also
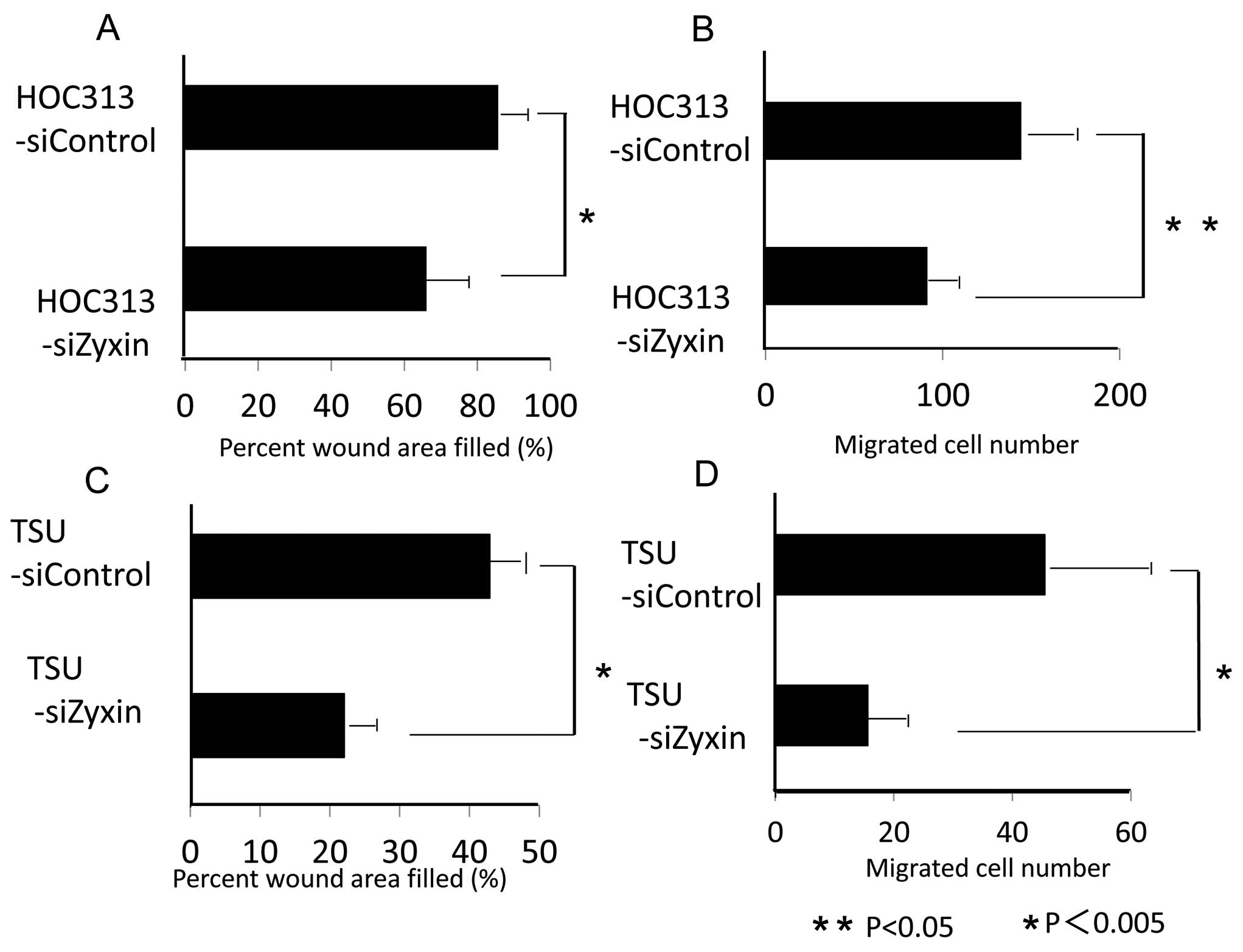
significantly inhibited cell migration and invasion. It inhibited
cell migration by 19.6% and cell invasion by 36.7% as compared with
control siRNA treatment (Fig. 5A and
B). Zyxin siRNA treatment of TSU also significantly inhibited
cell migration by 20.9% and invasion by 65.7% as compared with
control siRNA treatment (Fig. 5C and
D). To investigate why Zyxin siRNA treatment inhibited
proliferation of HOC-313, the apoptosis index and cell cycle
distribution were examined. However, the apoptosis index did not
increase, and the cell cycle distribution was unaffected by Zyxin
siRNA treatment (data not shown).
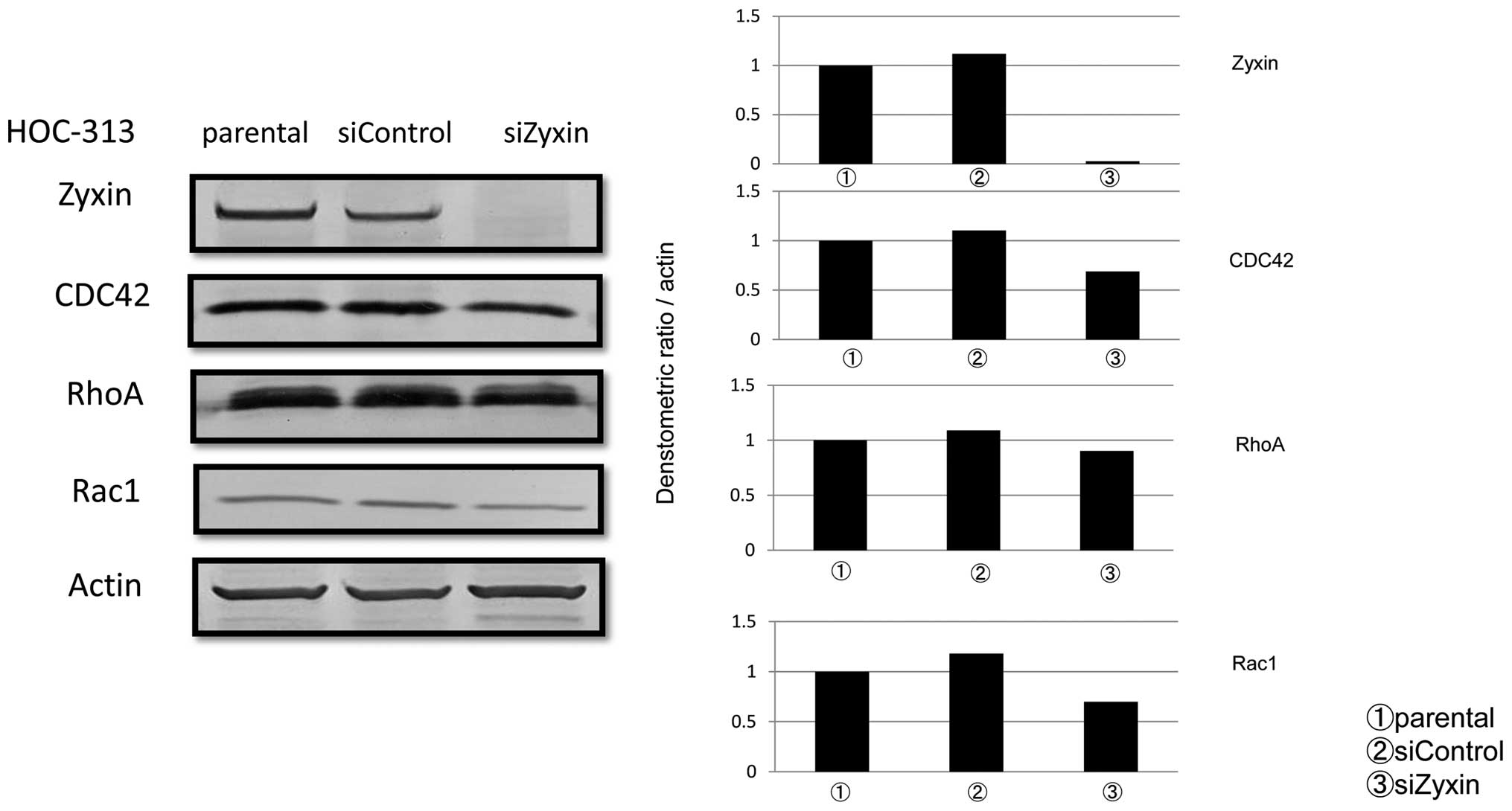
Expression of Rho family proteins in
Zyxin siRNA-treated HOC-313 cells
Because Rho family small GTPases such as RhoA, Rac1
and CDC42, are involved in cell migration and invasive potential,
as well as in regulation of EMT (29), functional interactions between Rho
family and Zyxin were examined in HOC-313. Although there was no
significant difference in expression of RhoA between control siRNA-
and siZyxin-treated cells, expression of CDC42 and Rac1 was reduced
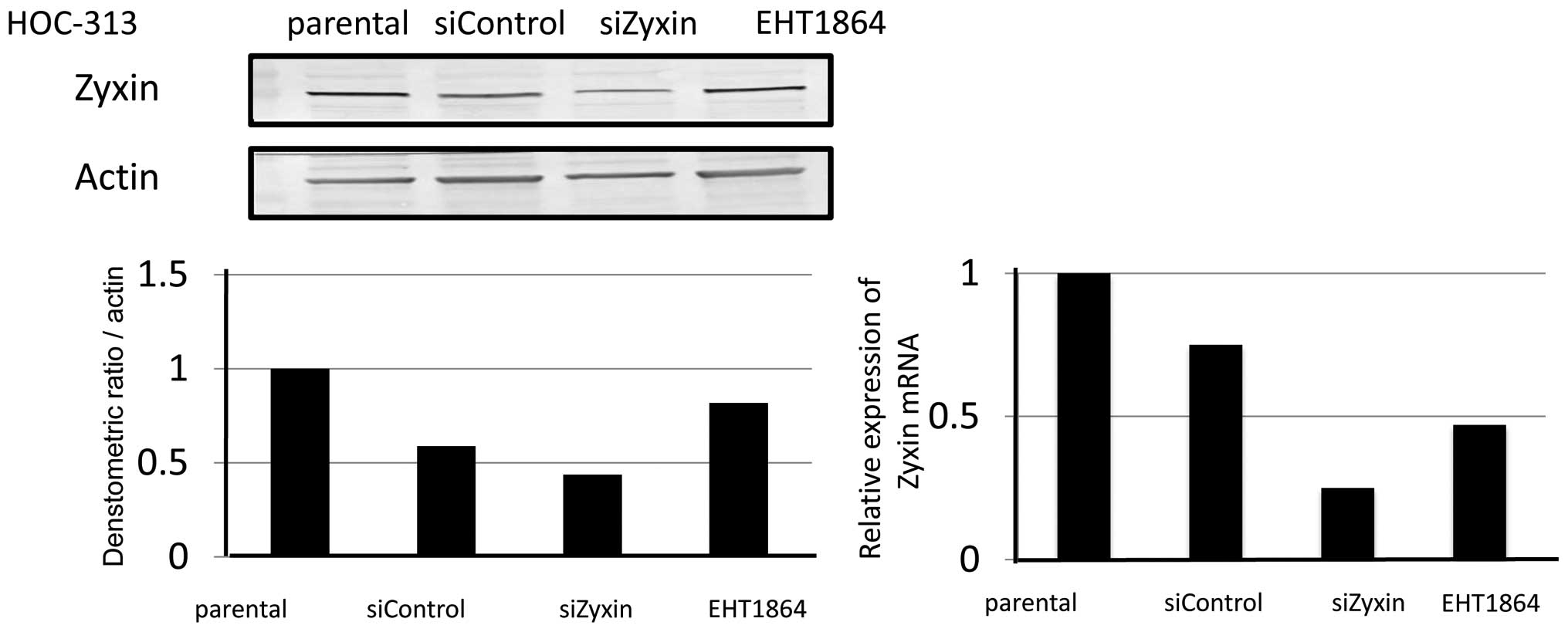
by 37.6 and 40.9%, respectively, on western blot analysis (Fig. 6). Since it is well known that CDC42
mainly forms filopodia that act as sensors of the cancer
microenvironment and Rac1 forms lamellipodia that induce motogenic
activity and resolve extracellular matrix, the effect of the Rac1
inhibitor EHT1864 on expression of Zyxin in HOC-313 was examined.
When cells were treated with EHT1864, expression of Zyxin protein
was reduced slightly, but Zyxin mRNA decreased by half on real-time
PCR analysis (Fig. 7). These
results indicated that interactions between Zyxin and Rac1 were one
of the factors related to migration and invasiveness of
fibroblastic type OSCC cells.
Discussion
An important hallmark of metastasis is increased
cell motility accompanied by actin cytoskeletal re-arrangement. To
gain insight into the relation between tumor cell morphology and
motility in OSCC, we analyzed the roles of Zyxin in cell motility
and cell morphology, using eight OSCC cell lines with different
morphological phenotypes. We found loss of E-cadherin expression
and increased N-cadherin expression on transition from epithelial
type to fibroblastic type. Reduced E-cadherin expression in OSCC
cells is associated with more aggressive tumor behavior and worse
outcomes (30,31). Moreover, cadherin switching (i.e.,
the loss of E-cadherin expression and gain of N-cadherin
expression) is a crucial event of EMT in human cancers, which
correlates with histologic differentiation, invasion pattern and
lymph node metastasis. Head and neck cancer cells also show
cadherin switching associated with EMT features and EMT cancer
cells show increased invasiveness (32). However, molecular relations among
cell morphology, cell motility and EMT in OSCC cells are
incompletely understood.
Zyxin, a focal adhesion-associated LIM protein
family, harbors distinct actin polymerization activity (33) and is located primarily at focal
adhesions and regulates actin cytoskeleton dynamics, cell movement,
and signal transduction (34,35).
When we reduced Zyxin expression levels in fibroblastic type OSCC
cells by treatment with Zyxin siRNA, cell growth was inhibited
significantly as compared with control siRNA-treated cells. To
clarify why Zyxin siRNA treatment inhibited the growth of
fibroblastic type OSCC cells, cell cycles and apoptosis induction
were examined. However, cell cycles were unaffected, and the
apoptosis index was not increased by Zyxin siRNA treatment. We
therefore speculated that the involvement of cell growth factor was
decreased by nutrient availability, hypoxia, heat shock, DNA damage
and osmotic stress (36).
Cells with inhibited Zyxin expression display
reduced adhesive properties, reduced migration, reduced capacity to
build robust actin stress fibers in response to a chemical
stimulus, and disturbed focal adhesion accumulation of actin
regulators in the Zyxin family. Shinto et al(37) reported that stable expression of
the oncoprotein associated with scirrhous gastric cancer cells
resulted in decreased Zyxin levels and a corresponding increase in
motility. Furthermore, Amsellem et al(38) reported that knockout of Zyxin in
Ewing’s sarcoma cells is associated with enhanced cell motility.
However, knockdown of Zyxin by siRNA in SKOV-3 cells, a human
ovarian cancer cell line, had no influence on cell migration
(39). To date, these disparate
effects have not been fully elucidated, but may result from
specific cellular features.
When Rho family protein expression was examined in
Zyxin knockdown cells, reduced expression of Rac1 and CDC42 was
found. This finding was consistent with the results of Pratt et
al(40), who suggested that
the Ajuba/Zyxin family of LIM proteins leads to activation of Rac
during cell migration. These results suggest that Zyxin is a
potential EMT marker and that overexpression of Zyxin promotes cell
growth and invasion via up-regulation of Rac1, CDC42 or both in
OSCC cells. Although we have not yet found an inhibitor of CDC42,
EHT1864 decreased Zyxin mRNA and protein in HOC-313. Our results
suggest a mechanism by which reduced Zyxin expression might
contribute to tumor regression by affecting cytoarchitecture and
motility.
Abbreviations:
|
OSCC
|
oral squamous cell carcinoma
|
|
LPP
|
lipoma preferred partner
|
|
TRIP6
|
thyroid receptor-interacting protein
6
|
Acknowledgements
This study was supported by JSPS
KAKENHI Grant no. 22592249 (to K.N.) and Grant-in-Aid for Young
Scientists (B) 23792399 (to E.S.).
References
|
1
|
Pereira MC, Oliveira DT, Landman G and
Kowalski LP: Histologic subtypes of oral squamous cell carcinoma:
prognostic relevance. J Can Dent Assoc. 73:339–344. 2007.PubMed/NCBI
|
|
2
|
Sudbo J, Bryne M, Mao L, Lotan R, et al:
Molecular based treatment of oral cancer. Oral Oncol. 39:749–758.
2003. View Article : Google Scholar
|
|
3
|
Leemans CR, Tiwari R, Nauta JJ, van der
Waal I and Snow GB: Regional lymph node involvement and
significance in the development of distant metastases in head and
neck carcinoma. Cancer. 71:452–456. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Leemans CR, Tiwari R, Nauta JJ, van der
Waal I and Snow GB: Recurrence at the primary site in head and neck
cancer and the significance of neck lymph node metastases as a
prognostic factor. Cancer. 73:187–190. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Brattain MG, Willson J, Koterba A, Patil S
and Venkateswarlu S: Colorectal cancer. Human Cell Culture. Cancer
Cell Lines, Part 2. Masters JR and Palsson B: 2. Kluwer Academic
Publishers; Norwell, MA: pp. 293–304. 2002
|
|
6
|
Kozlowski J and Sensibar JA: Prostate
cancer. Human Cell Culture. Cancer Cell Lines, Part 2. Masters JR
and Palsson B: 2. Kluwer Academic Publishers; Norwell, MA: pp.
305–332. 2002
|
|
7
|
Sutherland R, Watts CK, Lee CS and
Musgrove EA: Breast cancer. Human Cell Culture. Cancer Cell Lines,
Part 2. Masters JR and Palsson B: 2. Kluwer Academic Publishers;
Norwell, MA: pp. 79–106. 2002
|
|
8
|
Yokoyama K, Kamata N, Hayashi E, Hoteiya
T, Ueda N, Fujimoto R and Nagayama M: Reverse correlation of
E-cadherin and snail expression in oral squamous cell carcinoma
cells in vitro. Oral Oncol. 37:65–71. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Frixen UH, Behrens J, Sachs M, et al:
E-cadherin-mediated cell-cell adhesion prevents invasiveness of
human carcinoma cells. J Cell Biol. 113:173–185. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Petit MM, Fradelizi J, Golsteyn RM, et al:
LPP, an actin cytoskeleton protein related to zyxin, harbors a
nuclear export signal and transcriptional activation capacity. Mol
Biol Cell. 11:117–129. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yi J and Beckerle MC: The human TRIP6 gene
encodes a LIM domain protein and maps to chromosome 7q22, a region
associated with tumorigenesis. Genomics. 49:314–316. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Grunewald TG, Kammerer U, Schulze E,
Schindler D, Hong A, Zimmer M and Butt E: Silencing of LASP-1
influences zyxin localization, inhibits proliferation and reduces
migration in breast cancer cells. Exp Cell Res. 312:974–982. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Grunewald TG, Kammerer U, Winkler C,
Schindler D, Sickmann A, Honig A and Butt E: Overexpression of
LASP-1 mediates migration and proliferation of human ovarian cancer
cells and influences zyxin localisation. Br J Cancer. 96:296–305.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hervy M, Hoffman L and Beckerle MC: From
the membrane to the nucleus and back again: bifunctional focal
adhesion proteins. Curr Opin Cell Biol. 18:524–532. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nix DA and Beckerle MC:
Nuclear-cytoplasmic shuttling of the focal contact protein, zyxin:
a potential mechanism for communication between sites of cell
adhesion and the nucleus. J Cell Biol. 138:1139–1147. 1997.
View Article : Google Scholar
|
|
16
|
Nix DA, Fradelizi J, Bockholt S, Menichi
B, Louvard D, Friedich E and Beckerle MC: Targeting of zyxin to
sites of actin membrane interaction and to the nucleus. J Biol
Chem. 276:34759–34767. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
van der Gaag EJ, Leccica MT, Dekker SK,
Jalbert NL, Amodeo DM and Byers HR: Role of Zyxin in differential
cell spreading and proliferation of melanoma cells and melanocytes.
J Invest Dermatol. 118:246–254. 2002.PubMed/NCBI
|
|
18
|
Sperry RB, Bishop SN, Bramwell JJ, et al:
Zyxin controls migration in epithelial-mesenchymal transition by
mediating actin-membrane linkages at cell-cell junctions. J Cell
Physiol. 222:612–624. 2010.PubMed/NCBI
|
|
19
|
Urade M, Sugi M and Miyazaki T:
Establishment of three bleomycin-resistant human carcinoma cell
lines and their cross resistance to other antitumor agents. Cancer.
61:1501–1507. 1998. View Article : Google Scholar
|
|
20
|
Kamata N, Chida K, Rikimaru K, Horikoshi
M, Enomoto S and Kuroki T: Growth inhibitory effects of epidermal
growth factor and overexpression of its receptors on human squamous
cell carcinomas in culture. Cancer Res. 46:1648–1653.
1986.PubMed/NCBI
|
|
21
|
Kawahara E, Okada Y, Nakanishi I, Iwata K,
Kojima S, Kumagai S and Yamamoto E: The expression of invasive
behavior of differentiated squamous carcinoma cell line evaluated
by an in vitro invasion model. Jpn J Cancer Res. 84:409–418. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kojima S: Experimenral study of invasive
activity of oral squamous cell carcinoma. J Juzen Med Soc.
101:266–281. 1992.
|
|
23
|
Hoteiya T, Hayashi E, Satomura K, Kamata N
and Nagayama N: Expression of E-cadherin in oral cancer cell lines
and its relationship to invasiveness in SCID mice in vivo. J Oral
Pathol Med. 28:107–111. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kawashiri S, Tanaka A, Noguchi N, et al:
Invasion and metastasis models of oral squamous carcinoma cell
lines. Jpn J Tissue Cult Dent Res. 12:1–13. 2004.(in Japanese).
|
|
25
|
Moriyama M: Development of diffuse
invasive (grade4D) human oral squamous cell carcinoma model in
severe combined immunodeficiency mice: microangioarchitectual
analysis and immunohistochemical study. Oral Oncol. 35:395–400.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hashitani S, Urade M, Nishimura N, Maeda
N, Takaoka K, Noguchi K and Sakurai K: Apoptosis induction
enhancement of anticancer drugs by celecoxib, a selective
cyclooxygenase-2 inhibitor, in human head neck carcinoma cell
lines. Int J Oncol. 23:665–672. 2003.
|
|
27
|
Hiromoto T, Noguchi K, Yamamura M, et al:
Up-regulation of neurophil gelatinase-associated lipocalin in oral
squaamous cell carcinoma: relation to cell differentiation. Oncol
Rep. 26:1415–1421. 2011.PubMed/NCBI
|
|
28
|
Higashikawa K, Yoneda S, Tobiume K, et al:
ΔNp63α-dependent expression of Id-3 distinctively suppresses the
invasiveness of human squamous cell carcinoma. Int J Cancer.
124:2837–2844. 2009.
|
|
29
|
Bendris N, Arsic N, Lemmers B and
Bianchard JM: Cyclin A2, Rho GTPases and EMT. Small GTPases. Oct
1–2012.(Epub ahead of print).
|
|
30
|
Diniz-Freitas M, García-Caballero T,
Antúnez-López J, Gándara-Rey JM and García-García A: Reduced
E-cadherin expression is an indicator of unfavourable prognosis in
oral squamous cell carcinoma. Oral Oncol. 42:190–200. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Tanaka N, Odajima T, Ogi K, Ikeda T and
Satoh M: Expression of E-cadherin, alpha-catenin, and beta-catenin
in the process of lymph node metastasis in oral squamous cell
carcinoma. Br J Cancer. 89:557–563. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Krisanaprakornkit S and Iamaroon A:
Epithelial-mesenchymal transition in oral squamous cell carcinoma.
ISRN Oncol. 681469. 2012. View Article : Google Scholar
|
|
33
|
Fradelizi j, Noireaux V, Plastino J, et
al: ActA and human zyxin harbor Arp2/3-independent
actin-polymerization activity. Nat Cell Biol. 3:699–707. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Beckerle MC: Spatial control of action
filament assembly: lessons from Listeria. Cell. 95:741–748. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kadrmas JL and Beckerle MC: The LIM
domain: from the cytoskeleton to the nucleus. Nat Rev Mol Cell
Biol. 5:920–931. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
36
|
Reiling JH and Sabatini DM: Stress and
mTORture signaling. Oncogene. 25:6373–6383. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Shinto O, Yashiro M, Kawajiri H, Shimizu
K, Shimizu T, Miwa A and Hirakawa K: Inhibitory effect of a TGFbeta
receptor type-I inhibitor, Ki26894, on invasiveness of scirrhous
gastric cancer cells. Br J Cancer. 102:844–851. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Amsellem V, Kryszke MH, Hervy M, et al:
The actin cytoskeleton-associated protein zyxin acts as a tumor
suppressor in Ewing tumor cells. Exp Cell Res. 304:443–456. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wendt MK, Allington TM and Schiemann WP:
Mechanisms of the epithelial-mesenchymal transition by TGF-beta.
Future Oncol. 5:1145–1168. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Pratt S J, Epple H, Ward M, Feng Y, Braga
VM and Longmore GD: The LIM protein Ajuba influences p130Cas
localization and Rac1 activity during cell migration. J Cell Biol.
168:813–824. 2005.PubMed/NCBI
|