Introduction
Angiogenesis, the formation of new blood vessels,
plays important roles in the normal physiological situations, such
as embryonic growth and wound healing (1,2).
Angiogenesis also has profound impact on pathologic development,
particularly on chronic inflammation (3). Evidence has been gathered regarding
the association between angiogenesis and inflammation in pathologic
conditions. These phenomena have long been coupled together in many
chronic inflammation diseases including psoriasis, diabetes,
Crohn’s disease, rheumatoid arthritis and cancer (4–10).
Many of the cells that play a role during inflammation release
factors that have profound effects on vascular endothelial cells
(11–14). On the other hand, angiogenesis
sustains inflammation. Without angiogenesis, cells that present at
inflammatory sites will be short of oxygen and nutrients to meet
their metabolic needs (15). Thus,
these two processes seem to depend on each other. Common molecular
mechanisms have also been found to support this idea (16,17).
According to this knowledge, direct therapeutic approaches against
both chronic inflammation and angiogenesis will become our pursuit.
Therefore, to further understand the cross-talk between
inflammation and angiogenesis will be an important issue.
Fenamate belongs to a family of non-steroidal
anti-inflammatory drugs (NSAIDs). One of the fenamates, flufenamic
acid (FFA), is an inhibitor of cyclooxygenase (18) and has been shown to modulate
several kinds of ion channels. FFA is commonly used as a blocker of
non-selective cation current. It has been shown to inhibit the
current of several members of TRP channel superfamily, to
potentiate potassium current, to inhibit L-type calcium current,
and to inhibit Ca2+-dependent Cl - current (19–23).
The regulation of FFA on C-type TRP channels (TRPC) appears complex
since FFA blocks currents of TRPC3 and TRPC7 channels whereas it
potentiates the current of TRPC6 channels (24,25).
Interestingly, both TRPC3 and TRPC6 have been shown to mediate
vascular endothelial growth factor (VEGF)-induced current and TRPC6
also mediates VEGF-induced angiogenesis (26–28).
Although FFA affected ion channels have been shown to be involved
in angiogenesis, no report is available on the effect of this
chemical on angiogenesis.
In the present study, we used HUVECs as a cell model
of angiogenesis and the chicken CAM assay as an in vivo
angiogenesis model. We investigated the effect of FFA on HUVECs
proliferation and tube formation and its role in angiogenesis in
chicken CAM.
Materials and methods
Materials
Flufenamic acid was purchased from Sigma-Aldrich
(USA). BrdU monoclonal antibody was obtained from Neomarkers (USA).
Texas-Red-conjugated goat anti-mouse secondary antibody was from
Molecular Probes. Growth factor-free Matrigel was from BD
Biosciences (USA). All cell culture media and reagents were
obtained from Invitrogen (Carlsbad, CA, USA).
Cell culture
The HUVECs purchased from Sciencell (USA) were grown
in ECM supplemented with 5% FBS, ECGs and PS in a humidified
incubator with 5% CO2 at 37°C. The cells were
trypsinized with 0.15% trypsin-EDTA. Passages 3–10 were used for
experiments.
Proliferation assay
Cell proliferation was determined by both counting
the cell numbers and the BrdU incorporation assay. For counting the
cell numbers, HUVECs were seeded at an initial density of
2×105 per well in 6-well plates. The FFA was applied at
a dose of 20, 50 and 100 μM. Cells were harvested and
counted 24, 48 and 72 h after the treatment. Cell numbers were read
in a Beckman Counter. For BrdU incorporation assay, 100 μM
FFA was applied for 24 h. Then, cells were incubated in the medium
with 10 μM BrdU for 3 h and stained with a monoclonal
antibody against BrdU at 4°C for 12 h. A goat anti-mouse IgG
labeled with Texas Red was used as secondary antibody. The results
were expressed as the percentage of BrdU-positive cells over all
the cells.
Tube formation assay
The Matrigel was applied to each well of a 24-well
plate and incubated at 37°C for 60 min. The 6×104 of
endothelial cells was then seeded into each well with the medium
containing 0.8% FCS with or without FFA (100 μM). Images of
representative 10× fields were taken and endothelial cell tubes
were quantified by counting length and branches.
Western blot analysis
The cells were washed twice with PBS and total
cellular protein was then extracted in lysis buffer containing 62.5
mM Tris-HCl, 2% SDS, 10% glycerol with freshly added proteinase
inhibitor cocktail (Sigma, Zwijndrecht, The Netherlands). The
protein concentrations were determined by BCA assay (Pierce,
Waltham, MA, USA). The protein lysates (40 μg/lane) were
separated by SDS-PAGE and transferred onto nitrocellulose
membranes. After blocking with 3% bovine serum albumin in
phosphate-buffered saline, the membranes were incubated with
antiphospho-AMPKα and-AMPKβ, or antiphospho-ACC antibody. After
washing, the membranes were probed with horseradish
peroxidase-conjugated anti-rabbit IgG and the bands were visualized
using an ECL-Plus chemiluminescence detection system (GE
Healthcare, NJ, USA). To confirm equal loading of proteins, the
membranes were probed for β-actin protein.
Reverse transcription polymerase chain
reaction (RT-PCR)
HUVECs were lysed with TRIzol Reagent (Invitrogen)
and total RNA was extracted according to the manufacturer’s
instructions. First-strand cDNA was synthesized from 1.5 μg
of total RNA using Moloney murine leukemia virus reverse
transcriptase (M-MLV RT) according to the manufacturer’s
instructions (Invitrogen). cDNA was amplified by PCR according to
the manufacturer’s instructions with Taq DNA polymerase
(Invitrogen) under the following conditions: 94°C for 5 min,
followed by 40 cycles of 94°C for 30 sec, 56°C for 30 sec and 72°C
for 90 sec, with a final elongation step of 10 min at 72°C. The
primer informations are as follows: VEGF: forward,
5′-CTACCTCCACCATGCCAAGT-3′; reverse, 5′-TTT CTTGCGCTTTCGTTTTT-3′;
AAMP: forward, 5′-CTTTGC ATTGCACTCAGCAT-3′; reverse,
5′-CAGTCACCATTCGGG ACTTT-3′; e-NOS: forward, 5′-GGCTCCCTCCTTCCGG
CTG-3′; reverse, 5′-TAGCCGCACGACGCCCT-3′; GAPDH: forward,
5′-AGCCACTGCTGTGCTTTTAAG-3′; reverse, 5′-CCAAAACCAATGATCTCATCC-3′.
The products were electrophoresed in 2% agarose gel and stained
with ethidium bromide.
Angiogenesis in chick embryo CAM
Angiogenesis was assayed using the chick embryo CAM
assay according to the method described previously (29). Briefly, fertilized chicken eggs
were treated with ethanol (70%) and then incubated at 37°C. On day
3, 2–3-ml albumen was aspirated at the acute pole using a sterile
25-G hypodermic needle in order to allow detachment of the
developing CAM from the eggshell. After the removal of albumen, we
cut a square window ∼10×10 mm into the shell and sealed the window
with transparent tapes. Eggs were then incubated in a horizontal
position. Six days later, we opened the window and implanted a
1-mm3 sterilized gelatin sponge containing DMSO or FFA
onto the CAM. On day 12, the embryos of CAM were fixed by Bouin’s
fluid and the distribution and density of CAM vessels next to the
site of grafting were analyzed.
Statistical analyses
All experiments were repeated three times
independently. Data were presented as mean ± SEM. or as percentage
of control. Statistical comparisons between groups were performed
using the Student’s t-test. p<0.05 was considered statistically
significant.
Results
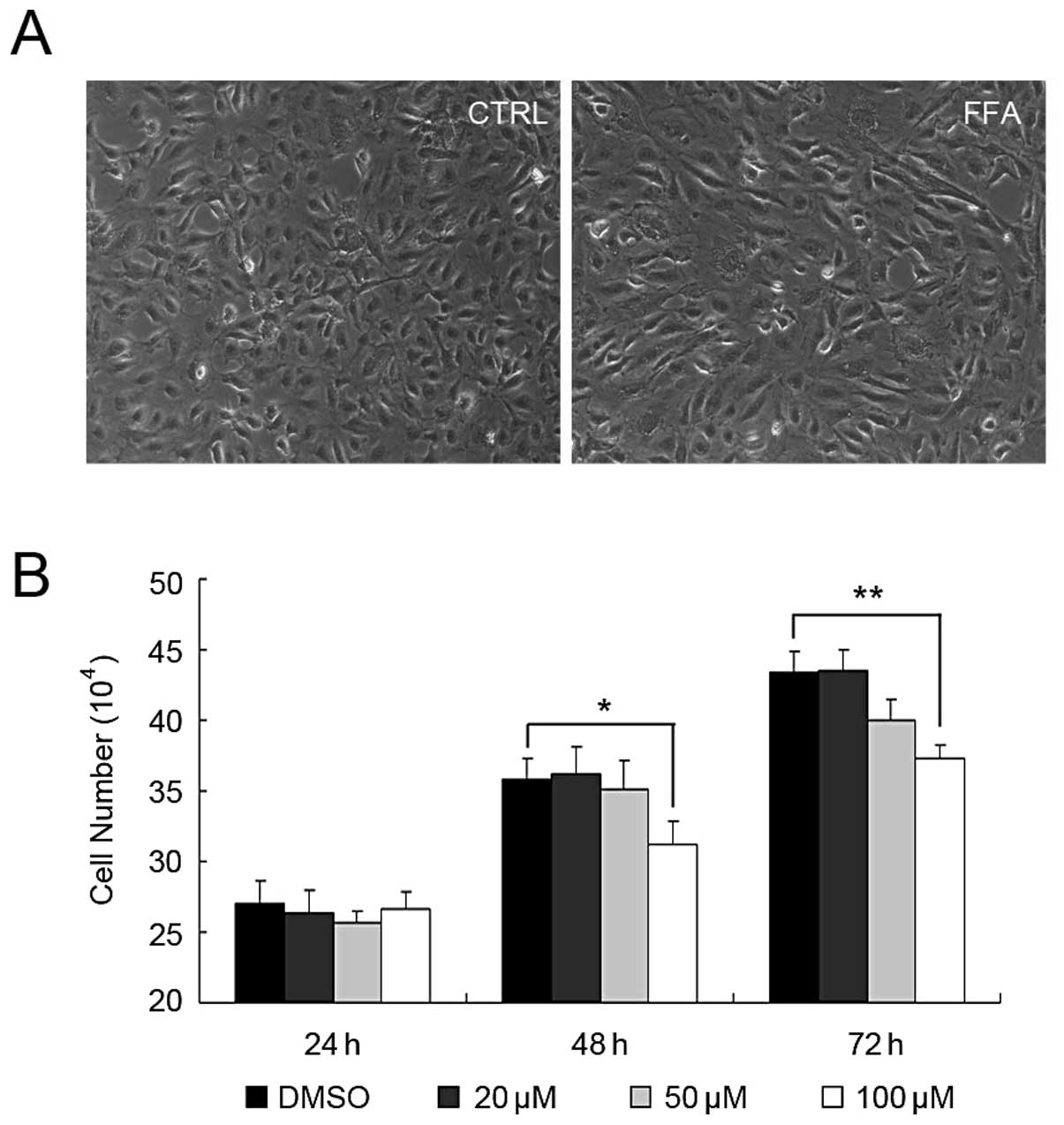
FFA suppressed HUVEC growth
To test whether FFA plays a role in angiogenesis, we
first investigated the effect of FFA on HUVEC growth. The cells
were incubated with DMSO, 20, 50 and 100 μM FFA for 24, 48
and 72 h before the cell numbers were determined. As shown in
Fig. 1, FFA at the concentration
of 20 μM had no effect on HUVEC growth at any time-point.
FFA at the concentration of 50 μM had weak effect on HUVEC
growth. The cell number was slightly reduced at the concentration
of 50 μM after 72 h, however, there was no significant
difference compared with DMSO control. When 100 μM FFA was
applied to HUVEC, the numbers of the HUVEC started to reduce after
48 h and was greatly reduced after 72 h. No apoptosis was found at
this concentration (data not shown).
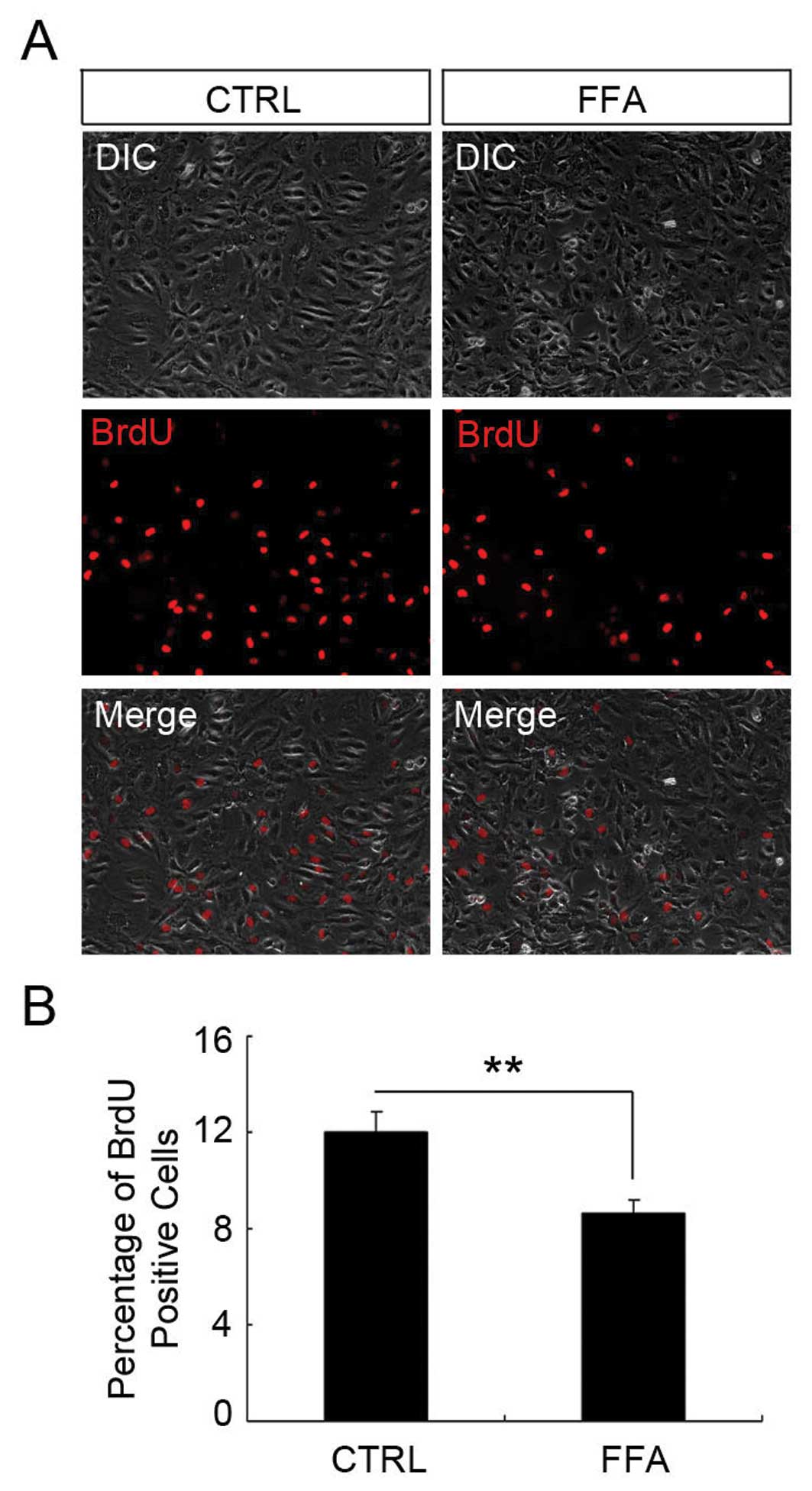
FFA reduced HUVEC proliferation
It has been reported that FFA inhibits cell
proliferation in other cell types (30–32).
We next determined whether the effect of FFA on HUVEC cell number
was due to its influence on cell proliferation. To address this
question, we applied the BrdU incorporation assay. HUVECs were
treated with 100 μM FFA for 24 h before they were incubated
with 10 μM BrdU for another 3 h. BrdU is an analog of DNA
precursor thymidine. When cells are proliferating, BrdU can be
incorporated into DNA similarly to thymidine. In this way, the
amount of the BrdU in the cells reflects the proliferation rate of
the cells. As shown in Fig. 2, the
percentage of BrdU-positive cell in DMSO and FFA-treated group was
12±0.86 and 8.65±0.49%, respectively. There was a significant
reduction of the percentage of BrdU-positive cells in the FFA
treatment group compared with the control group (p<0.05).
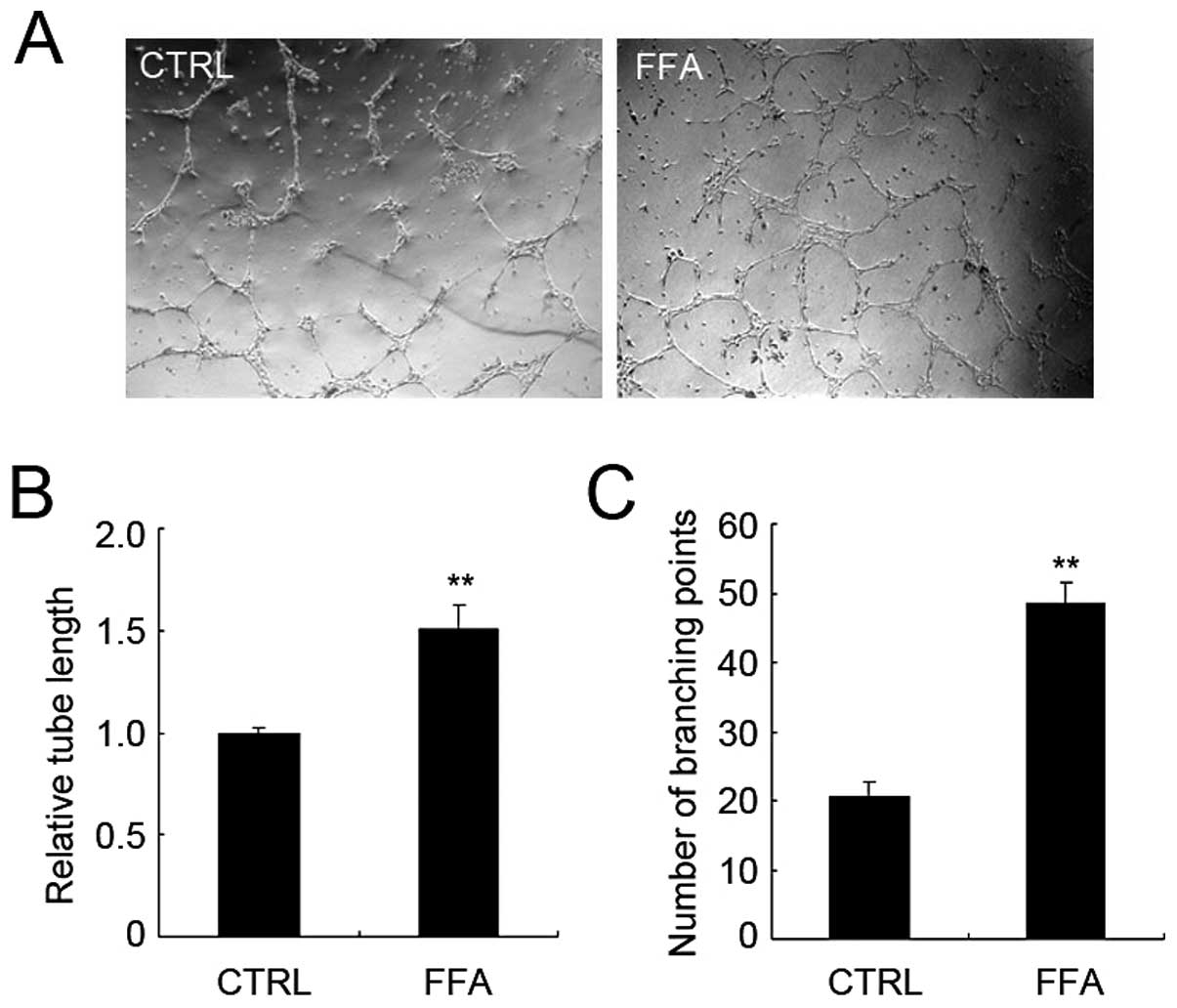
FFA promoted tube formation of
HUVECs
In vitro assays of tube formation using
endothelial cells are commonly used to study critical steps of
angiogenesis (33). We then
examine the effect of FFA on tube formation using HUVECs cultured
on Matrigel. The tube-like structure appeared 12 h after the HUVECs
were seeded. The total tube length and the number of branching
points were analyzed as indexes of angiogenesis.
We applied FFA to HUVECs when they were seeded on
Matrigel. As shown in Fig. 3A,
after 12 h, FFA greatly increased the formation of tube structure.
Both total tube length and branching points were significantly
increased in FFA treatment group. The relative total tube length of
FFA-treated group increased 50.4% compared with that of control
group (Fig. 3B). The average
branching points per field of FFA-treated group increased 135%
compared with that of control group (Fig. 3C). Together, these results suggest
that FFA promotes angiogenesis without promoting endothelial cell
proliferation.
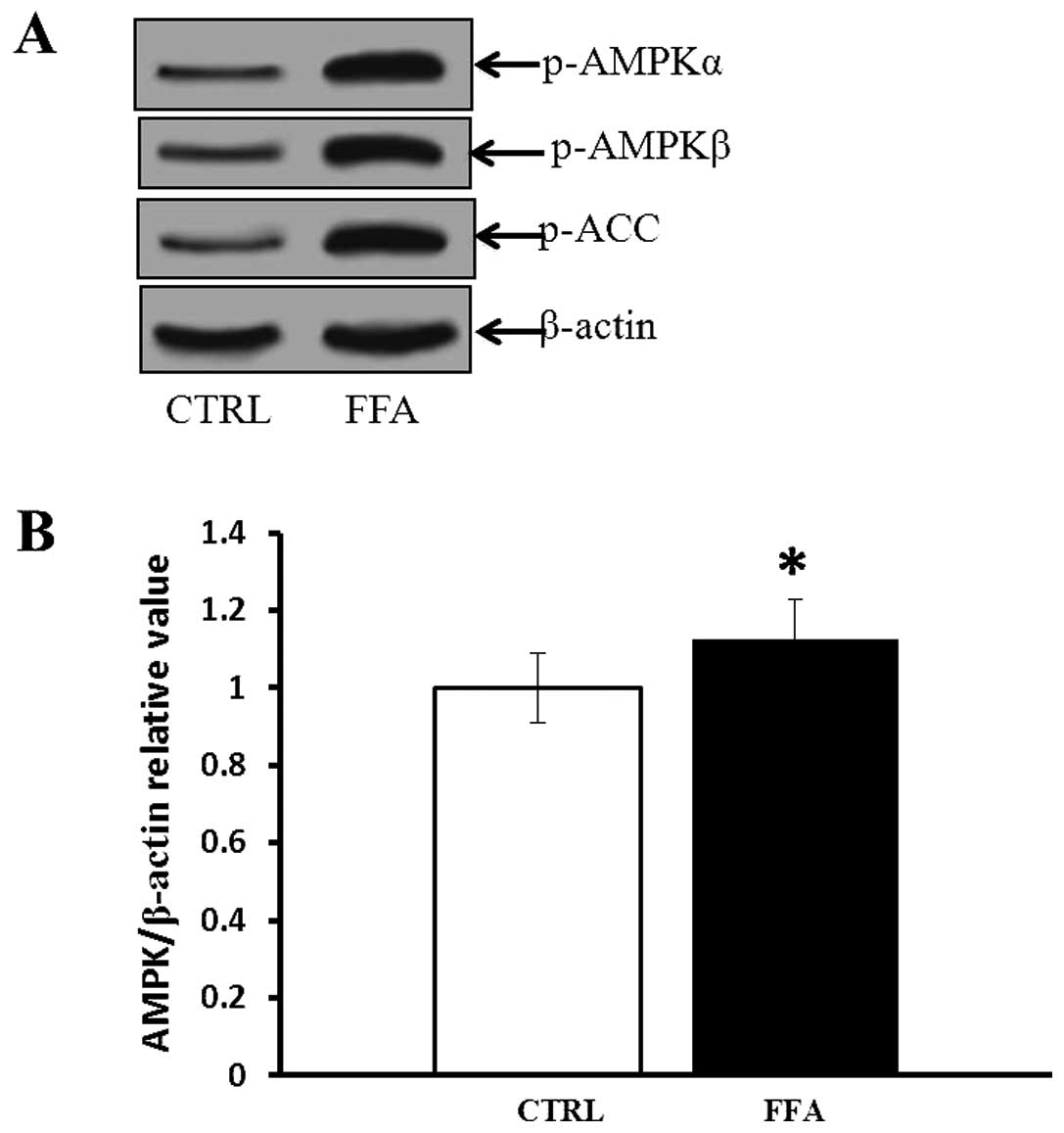
FFA induced AMPK activation
FFA is one of the Nary-anthranilic acid derivatives,
belonging to the fenamate group of NSAIDs (34). To examine whether FFA regulates
angiogenesis through AMPK activation, phospho-AMPKα and-AMPKβ and
phospho-ACC were measured by western blotting. As shown in Fig. 4, incubation of HUVECs with FFA
resulted in increased levels of phosphorylated AMPKα and AMPKβ,
which was associated with paralleled elevation of phosphorylated
ACC, one of the AMPK substrates (35). Compared with control, FFA-treated
group had significantly higher phosphorylated AMPK levels
(p<0.05).
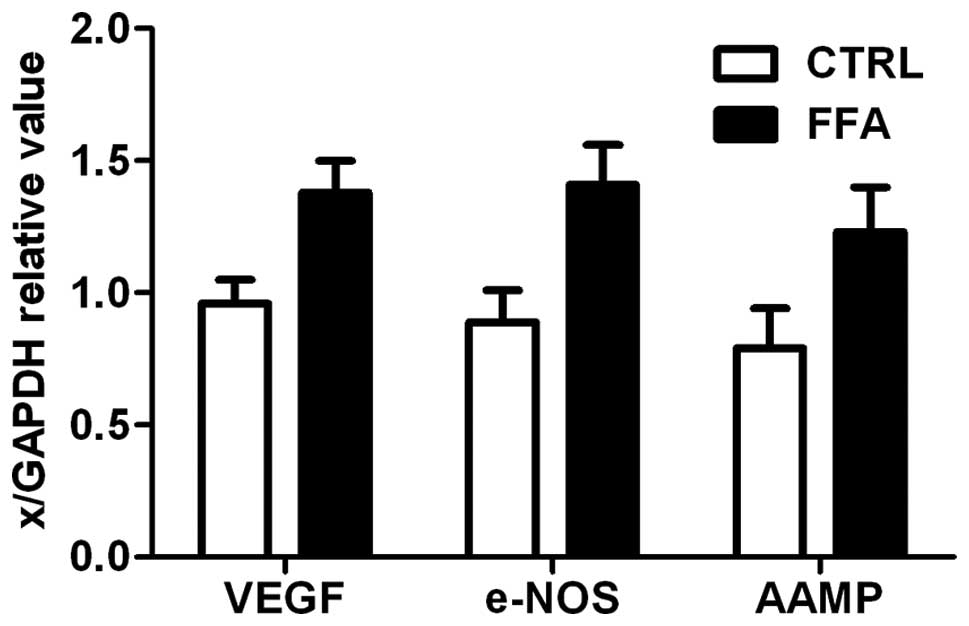
Expression of angiogenesis markers
Vascular endothelial growth factor (VEGF),
endothelial NO synthase (e-NOS), the angio-associated migratory
cell protein (AAMP) are three angiogenesis related genes, which
were strongly expressed in endothelial cells (36). The mRNA levels of VEGF, e-NOS and
AAMP were measured by RT-PCR. Expression of all three angiogenesis
related genes is shown in Fig. 5.
There was a significant difference between FFA group and control,
FFA group had a significantly higher mRNA accumulation level of all
the three angiogenesis related genes (p<0.05).
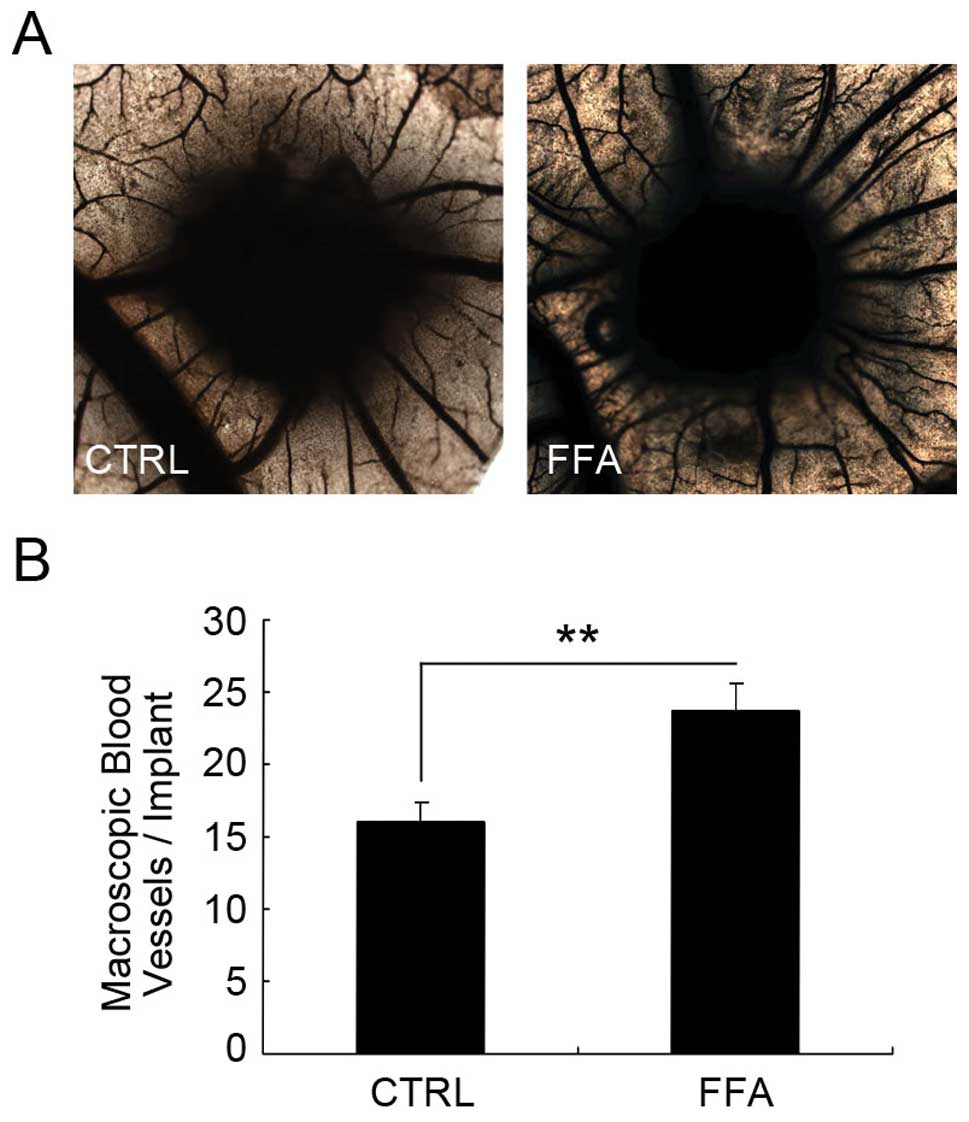
The effect of FFA on angiogenesis in
vivo
We further examined the possible effect of FFA on
angiogenesis in vivo. In order to explore the role of FFA
in vivo, we applied the chicken chorioallantoic membrane
(CAM) assay. We implanted a 1-mm3 sterilized gelatin
sponge which contained PBS or FFA onto the chorioallantoic membrane
for ∼72 h for the blood vessel to grow into the sponge. Then the
sponge was fixed and the distribution and density of CAM vessels
next to the site of grafting were analyzed.
As shown in Fig. 6,
the vessel density in the CAM implanted with the gelatin sponge
containing PBS was 15.99±1.30. The vessel density in the CAM
implanted with the gelatin sponge containing FFA was 23.74±1.82,
which was a significant increase compared with the control group.
These results suggest that FFA promotes angiogenesis in
vivo.
Discussion
Our results indicate that FFA treatment promotes
angiogenesis. In the tube formation assay of HUVEC cells, both
total tube length and the number of branching points were increased
in the FFA treatment group compared to the control group and cell
proliferation was not significantly affected at the time when tube
formation assay was performed. AMP-activated protein kinase (AMPK)
is a key regulator of metabolic homeostasis (35) and has anti-inflammatory effects
(37,38). In addition, it promotes
angiogenesis, and protects cells from apoptosis (39–41).
RT-PCR was used to analyse the expression of VEGF, AAMP, e-NOS and
the results showed significantly increased expression in response
to FFA-stimulation. These results indicated that FFA promoted
angiogenesis in vitro. Moreover, FFA can also promote
angiogenesis in vivo. In the chicken CAM assay FFA
significantly increased the number of vessels that grow into the
gelatin sponge. In addition, we observed that FFA can significantly
increase the phosphorylated levels of AMPK. Our results thus
support the notion that FFA promotes angiogenesis both in
vitro and in vivo through AMPK activation.
The process of angiogenesis includes the
proliferation of endothelial cells and migration of these cells to
form tube-like structures. At the concentration of 100 μM,
12-h FFA treatment did not affect HUVEC proliferation whereas
promoted the formation of tube-like structures and it was not until
24 h that FFA treatment began to inhibit cell proliferation. The
differential effect of FFA on tube formation and cell proliferation
is probably due to the multiple targets of FFA. FFA has been
reported to inhibit cell proliferation in several cell types
(30,42). As a non-selective cation blocker,
FFA blocks several channels that have been shown to be involved in
the process of cell proliferation. For example, TRPM7 is required
for MCF-7 cell proliferation (43). TRPC3 has been shown to be necessary
for proliferation of SKOV3 cells (44). Whether the effect of FFA on HUVEC
proliferation is the result of its inhibitory effect on these
channels remains uncertain. The way FFA promotes HUVEC tube
formation is probably through its other targets. For instance,
FFA-potentiated non-selective cation channel is required for
lysophosphatidylcholine-induced monocyte migration (45). FFA can potentiate the current of
TRPC6 and TRPC6 has been shown to promote HUVECs tube formation
(28). It is thus possible that
TRPC6 participates in FFA-induced tube formation.
Common molecular mechanisms have already been shown
to regulate both chronic inflammation and angiogenesis (46,47).
These two processes seem to depend on each other based on
literature (3,48). However, as an anti-inflammatory
agent, FFA promotes angiogenesis in our system. Therefore, a
careful understanding of the cross-talk between angiogenesis and
chronic inflammation is very important for more effective
therapies.
In conclusion, our data show that FFA treatment
promotes HUVEC tube formation in vitro. In the in
vivo experiment using chick CAM assay, FFA also promotes
vessels to grow into the gelatin sponge. Moreover, the
phosphorylated AMPK levels were significantly higher in FFA-treated
group. These data suggest that FFA promotes angiogenesis both in
vitro and in vivo.
References
|
1
|
Tonnesen MG, Feng X and Clark RA:
Angiogenesis in wound healing. J Investig Dermatol Symp Proc.
5:40–46. 2000. View Article : Google Scholar
|
|
2
|
Breier G: Angiogenesis in embryonic
development - a review. Placenta. 21(Suppl A): S11–S15. 2000.
View Article : Google Scholar
|
|
3
|
Costa C, Incio J and Soares R:
Angiogenesis and chronic inflammation: cause or consequence?
Angiogenesis. 10:149–166. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Coussens LM and Werb Z: Inflammation and
cancer. Nature. 420:860–867. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Carmeliet P: Angiogenesis in life, disease
and medicine. Nature. 438:932–936. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lusis AJ: Atherosclerosis. Nature.
407:233–241. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Trayhurn P and Wood IS: Adipokines:
inflammation and the pleiotropic role of white adipose tissue. Br J
Nutr. 92:347–355. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wubben DP and Adams AK: Metabolic
syndrome: what’s in a name? WMJ. 105:17–20. 2006.
|
|
9
|
Tan TT and Coussens LM: Humoral immunity,
inflammation and cancer. Curr Opin Immunol. 19:209–216. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Otani A, Takagi H, Oh H, Koyama S,
Matsumura M and Honda Y: Expressions of angiopoietins and Tie2 in
human choroidal neovascular membranes. Invest Ophthalmol Vis Sci.
40:1912–1920. 1999.PubMed/NCBI
|
|
11
|
Benelli R, Lorusso G, Albini A and Noonan
DM: Cytokines and chemokines as regulators of angiogenesis in
health and disease. Curr Pharm Des. 12:3101–3115. 2006. View Article : Google Scholar
|
|
12
|
Nathan C: Points of control in
inflammation. Nature. 420:846–852. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Folkman J: Angiogenesis in cancer,
vascular, rheumatoid and other disease. Nat Med. 1:27–31. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mrowietz U and Boehncke WH: Leukocyte
adhesion: a suitable target for anti-inflammatory drugs. Curr Pharm
Des. 12:2825–2831. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lee FH, Haskell C, Charo IF and Boettiger
D: Receptor-ligand binding in the cell-substrate contact zone: a
quantitative analysis using CX3CR1 and CXCR1 chemokine receptors.
Biochemistry. 43:7179–7186. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Pacifico F and Leonardi A: NF-kappaB in
solid tumors. Biochem Pharmacol. 72:1142–1152. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Nam NH: Naturally occurring NF-kappaB
inhibitors. Mini Rev Med Chem. 6:945–951. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Flower RJ: Drugs which inhibit
prostaglandin biosynthesis. Pharmacol Rev. 26:33–67. 1974.
|
|
19
|
Naziroglu M, Luckhoff A and Jungling E:
Antagonist effect of flufenamic acid on TRPM2 cation channels
activated by hydrogen peroxide. Cell Biochem Funct. 25:383–387.
2007. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Peppiatt-Wildman CM, Albert AP, Saleh SN
and Large WA: Endothelin-1 activates a Ca2+-permeable
cation channel with TRPC3 and TRPC7 properties in rabbit coronary
artery myocytes. J Physiol. 580:755–764. 2007.PubMed/NCBI
|
|
21
|
Farrugia G, Rae JL, Sarr MG and
Szurszewski JH: Potassium current in circular smooth muscle of
human jejunum activated by fenamates. Am J Physiol. 265:G873–G879.
1993.PubMed/NCBI
|
|
22
|
Doughty JM, Miller AL and Langton PD:
Non-specificity of chloride channel blockers in rat cerebral
arteries: block of the L-type calcium channel. J Physiol.
507:433–439. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Greenwood IA and Large WA: Comparison of
the effects of fenamates on Ca-activated chloride and potassium
currents in rabbit portal vein smooth muscle cells. Br J Pharmacol.
116:2939–2948. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Inoue R, Okada T, Onoue H, et al: The
transient receptor potential protein homologue TRP6 is the
essential component of vascular alpha(1)-adrenoceptor-activated
Ca(2+)-permeable cation channel. Circ Res. 88:325–332.
2001.PubMed/NCBI
|
|
25
|
Jung S, Strotmann R, Schultz G and Plant
TD: TRPC6 is a candidate channel involved in receptor-stimulated
cation currents in A7r5 smooth muscle cells. Am J Physiol Cell
Physiol. 282:C347–C359. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Poteser M, Graziani A, Eder P, et al:
Identification of a rare subset of adipose tissue-resident
progenitor cells, which express CD133 and TRPC3 as a VEGF-regulated
Ca2+ entry channel. FEBS Lett. 582:2696–2702. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hamdollah Zadeh MA, Glass CA, Magnussen A,
Hancox JC and Bates DO: VEGF-mediated elevated intracellular
calcium and angiogenesis in human microvascular endothelial cells
in vitro are inhibited by dominant negative TRPC6.
Microcirculation. 15:605–614. 2008.PubMed/NCBI
|
|
28
|
Ge R, Tai Y, Sun Y, et al: Critical role
of TRPC6 channels in VEGF-mediated angiogenesis. Cancer Lett.
283:43–51. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ribatti D, Nico B, Vacca A and Presta M:
The gelatin sponge-chorioallantoic membrane assay. Nat Protoc.
1:85–91. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Schober W, Wiskirchen J, Kehlbach R, et
al: Flufenamic acid: growth modulating effects on human aortic
smooth muscle cells in vitro. J Vasc Interv Radiol. 13:89–96. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Tiemann U, Neels P, Pohland R, Walzel H
and Lohrke B: Influence of inhibitors on increase in intracellular
free calcium and proliferation induced by platelet-activating
factor in bovine oviductal cells. J Reprod Fertil. 116:63–72. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Weiser T and Wienrich M: Investigations on
the mechanism of action of the antiproliferant and ion channel
antagonist flufenamic acid. Naunyn Schmiedebergs Arch Pharmacol.
353:452–460. 1996.PubMed/NCBI
|
|
33
|
Wilson BD, Ii M, Park KW, et al: Netrins
promote developmental and therapeutic angiogenesis. Science.
313:640–644. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Chi Y, Li K, Yan Q, et al: Nonsteroidal
anti-inflammatory drug flufenamic acid is a potent activator of
AMP-activated protein kinase. J Pharmacol Exp Ther. 339:257–266.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Towler MC and Hardie DG: AMP-activated
protein kinase in metabolic control and insulin signaling. Circ
Res. 100:328–341. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Del Carratore R, Carpi A, Beffy P, et al:
Itraconazole inhibits HMEC-1 angiogenesis. Biomed Pharmacother.
66:312–317. 2012.PubMed/NCBI
|
|
37
|
Aoki C, Hattori Y, Tomizawa A, Jojima T
and Kasai K: Anti-inflammatory role of cilostazol in vascular
smooth muscle cells in vitro and in vivo. J Atheroscler Thromb.
7:503–509. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
38
|
Shin MJ, Lee YP, Kim DW, et al: Transduced
PEP-1-AMPK inhibits the LPS-induced expression of COX-2 and iNOS in
Raw264. 7 cells. BMB Rep. 43:40–45. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kongsuphol P, Cassidy D, Hieke B, et al:
Mechanistic insight into control of CFTR by AMPK. J Biol Chem.
284:5645–5653. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kréneisz O, Benoit JP, Bayliss DA and
Mulkey DK: AMP-activated protein kinase inhibits TREK channels. J
Physiol. 587:5819–5830. 2009.PubMed/NCBI
|
|
41
|
Klein H, Garneau L, Trinh NTN, et al:
Inhibition of the KCa3. 1 channels by AMP-activated protein kinase
in human airway epithelial cells. Am J Physiol Cell Physiol.
296:C285–C295. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Schlichter LC, Sakellaropoulos G, Ballyk
B, Pennefather PS and Phipps DJ: Properties of K+ and
Cl− channels and their involvement in proliferation of
rat microglial cells. Glia. 17:225–236. 1996.
|
|
43
|
Guilbert A, Gautier M, Dhennin-Duthille I,
Haren N, Sevestre H and Ouadid-Ahidouch H: Evidence that TRPM7 is
required for breast cancer cell proliferation. Am J Physiol Cell
Physiol. 297:C493–C502. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Yang SL, Cao Q, Zhou KC, Feng YJ and Wang
YZ: Transient receptor potential channel C3 contributes to the
progression of human ovarian cancer. Oncogene. 28:1320–1328. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Schilling T and Eder C: Non-selective
cation channel activity is required for
lysophosphatidylcholine-induced monocyte migration. J Cell Physiol.
221:325–334. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Fiedler U, Reiss Y, Scharpfenecker M, et
al: Angiopoietin-2 sensitizes endothelial cells to TNF-alpha and
has a crucial role in the induction of inflammation. Nat Med.
12:235–239. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
47
|
Fiedler U and Augustin HG: Angiopoietins:
a link between angiogenesis and inflammation. Trends Immunol.
27:552–558. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Noonan DM, De Lerma Barbaro A, Vannini N,
Mortara L and Albini A: Inflammation, inflammatory cells and
angiogenesis: decisions and indecisions. Cancer Metastasis Rev.
27:31–40. 2008. View Article : Google Scholar : PubMed/NCBI
|