Introduction
The behavior of cancer cells such as invasion and
metastasis has been proposed to be mediated by the surrounding
microenviroment including extracellular matrix (ECM) proteins and
stromal cells (1–5). Integrins are heterodimeric
transmembrane receptors composed of α and β subunits, which bind a
wide range of ligands such as ECM proteins and cell surface
proteins. There are at least 18 α subunits and eight β subunits,
forming 24 different integrin heterodimers (6,7).
Most of α subunits dimerize with only one β subunit. In contrast,
integrin αv is unique because αv subunit associates with β1, β3,
β5, β6 or β8 subunit and forms five distinct heterodimers (7,8).
Integrin-ECM interaction leads to the activation of
signal transduction pathways, which regulate various biological
events including cell adhesion, migration, proliferation and
differentiation (9,10). Integrins also contribute to tumor
progression by facilitating the proliferation, migration and
survival of cancer cells (11–13).
Altered expression of integrins has been shown in malignant tumors
compared to their normal counterparts (14–17).
Especially, it has been shown that the overexpression of the
integrin αv subfamily such as αvβ1, αvβ3, αvβ5 and αvβ6 correlates
with poor prognosis in malignant tumors such as ovarian cancer
(18,19), lung cancer (20), nasopharyngeal cancer (21), gastric cancer (22) and breast cancer (23). In vitro studies have also
shown the participation of αv integrins in the proliferation
(24), motility (25,26)
and proteolysis (26–28) in various cancer cells. In addition,
integrin αvβ3 is a cell-surface receptor for active matrix
metalloproteinase (MMP-2), indicating that integrin αvβ3 regulates
tumor invasion and metastasis by increasing pericellular
proteolysis (29). However, the
precise mechanism of the progression of squamous cell carcinoma
(SCC) mediated by integrin αv is poorly documented.
In the present study, to elucidate the role of
integrin αv in the progression of oral SCC, the effect of induction
of integrin αv on the proliferation and invasion of oral SCC cells
were examined. The signal transduction via integrin αv that
regulates the proliferation and invasiveness of oral SCC cells was
also examined.
Materials and methods
Cells and culture
The oral SCC cell line, SCCKN (30) was grown in RD medium (a 1:1 mixture
of RPMI-1640 and Dulbecco’s Modified Eagle’s Medium) supplemented
with 5% fetal bovine serum (FBS) in 5% CO2 at 37°C. The
proliferation of the cells on ECM proteins was estimated as
follows: The wells of 24-well tissue culture plates were incubated
with 100 μg/ml type I collagen, type IV collagen, fibronectin,
laminin or vitronectin (all from Sigma, St. Louis, MO, USA)
overnight at 4°C. Poly-L-lysine (100 μg/ml; Sigma) was used as a
non-integrin-dependent adhesion substrate. The cells
(2×104) suspended in RD containing 10 μg/ml bovine
insulin, 5 μg/ml human transferrin, 0.5 mg/ml fatty acid-free
bovine serum albumin (BSA), 10 μM 2-mercaptoethanol, 10 μM
2-aminoethanol and 10 nM sodium selenite (all from Sigma) (31) were seeded in each well of the
culture plates and cultured in 5% CO2 for 6 days at
37°C. The number of cells was measured by the Coulter counter
(Beckman Coulter, Inc., Tokyo, Japan). The measurements were done
in triplicate.
Construction of integrin αv expression
vector
The open reading frame of human integrin αv was
amplified by PCR from plasmid CDM8 containing human αv cDNA, which
was provided by Dr Joseph C. Loftus (Mayo Clinic Arizona,
Scottsdale, AZ, USA). The forward primer (5′-CGGAATTCTTCGGCGATGGCTTTTCCGC-3′)
containing EcoRI site (underlined) and the reverse primer
(5′-TCCCCCGGGTTAAGTTTCTGAGTTTCCTTCACCAT-3′)
containing SmaI site (underlined) were used for the
amplification. The PCR products were double-digested with
EcoRI (New England Bio Labs, Ipswich, MA, USA) and
SmaI (New England Bio Labs) and then ligated into pCI-neo
Mammalian Expression Vector (Promega Corporation, Madison, WI, USA)
digested with both EcoRI and SmaI. The inserted cDNA
sequences were all verified by DNA sequence analysis. The resultant
plasmid was termed as pCI/neo-αv, and pCI/neo without insert was
used as negative control.
Transfection and selection
SCCKN cells were transfected with pCI/neo-αv or
pCI/neo (5 μg per 60-mm dish) using TransFast transfection reagent
(Promega Corporation) according to the manufacturer’s instruction.
Selection was initiated 48 h after transfection by adding 600 μg/ml
G418 (Geneticin; Invitrogen Life Technologies, San Diego, CA, USA)
to the culture medium. The selection medium was changed every 4
days for 2 weeks until all non-transfected cells died. Resistant
cell clones were isolated. Cell clones transfected with pCI/neo-αv
or pCI/neo were termed as KNαv or KNmock, respectively.
Western blotting
To detect integrin αv and β8 proteins, SCCKN, KNmock
and KNαv cells were lysed with lysis buffer [10 mM Tris-HCl, pH
7.4, 150 mM NaCl, 5 mM EDTA, 1% Triton X-100, 1% protease inhibitor
cocktail (Sigma)]. The samples containing 10 μg of total protein
were electrophoresed on 10% SDS-polyacrylamide gels under reducing
condition and transferred to polyvinylidene difluoride (PVDF)
membrane filters (Millipore, Bedford, MA, USA). The filters were
blocked in T-TBS (20 mM Tris-HCl, pH 7.5, 137 mM NaCl, 0.1%
Tween-20) containing 5% skim milk for 1 h at room temperature and
then incubated with rabbit anti-integrin αv polyclonal antibody
(Santa Cruz Biotechnology, Inc., Dallas, TX, USA) or goat
anti-integrin β8 polyclonal antibody (Santa Cruz Biotechnology,
Inc.), followed with the incubation with horseradish peroxidase
(HRP)-conjugated anti-rabbit IgG antibody (Cell Signaling
Technology, Inc., Danvers, MA, USA) or HRP-conjugated anti-goat IgG
antibody (KPL, Gaithersburg, MD, USA), respectively. Rabbit
anti-β-actin polyclonal antibody (Cell Signaling Technology, Inc.)
was used as internal control to confirm equal loading of total
protein. Protein bands were visualized by enhanced
chemiluminescence detection (ECL Plus System; GE Healthcare,
Uppsala, Sweden).
Cell adhesion assay
The wells of 24-well culture plates were incubated
with 100 μg/ml type I collagen, type IV collagen, fibronectin,
laminin or vitronectin overnight at 4°C. Poly-L-lysine (100 μg/ml)
was used as a non-integrin-dependent adhesion substrate. The wells
were washed five times with phosphate-buffered saline (PBS) and
incubated with in PBS containing 1% BSA for 1 h at 37°C to block
non-specific binding. Subconfluent culture of cells was
radiolabeled with 1 μCi/ml [methyl-3H]-thymidine
(Perkin-Elmer, Waltham, MA, USA) for 24 h. The labeled cells
(1×105) suspended in RD medium containing 0.1% BSA were
added to each well of the culture plates. After incubation for 30
min at 37 °C, the medium was aspirated, and the wells were gently
rinsed twice with PBS. The cells adhering to the well were
dissolved in 1 N NaOH, and the radioactivity was measured with a
liquid scintillation counter (LSC903; Aloka, Co., Ltd., Tokyo,
Japan). The adhesion capacity was determined relative to the
radioactivity of seeded cells (1×105) that was
considered to be 100%. Each assay was performed in triplicates and
repeated three times.
Collagen gel culture
Five hundred-microliters of 0.21% type I collagen
gel solution (Koken, Co., Ltd, Tokyo, Japan) in RD neutralized with
reconstitution buffer (0.05 N NaOH, 2.2% NaHCO3, 0.2 M
HEPES) was pipetted into each well of 24-well culture plate and
gelled as a basal layer with incubation for 1 h at 37°C.
Thereafter, 500 μl type collagen solution containing cells
(2.5×104) was poured onto the basal layer and gelled,
and received 1 ml RD containing 5% FBS. The cells in the gels were
cultured for 12 days at 37°C. The colonies that formed in the gel
were fixed with phosphate-buffered 10% formalin. Sections were
prepared and stained with hematoxylin and eosin.
Phosphorylation assay
SCCKN, KNmock and KNαv cells suspended in RD
containing 2% BSA were seeded on culture dishes coated with 100
μg/ml type I collagen. At various incubation times, the cells were
washed with ice-cold PBS containing 1 mM sodium vanadate and lysed
with Laemmli sample buffer (62.5 mM Tris-HCl, pH 6.8, 2% SDS, 10%
glycerol, 1% β-mercaptoethanol) supplemented with protease
inhibitor cocktail and 1 mM sodium vanadate. The samples were
separated on 10% SDS-polyacrylamide gels and transferred onto PVDF
membrane filters. The immunoblot analysis was performed using
rabbit anti-phospho-focal adhesion kinase (FAK) monoclonal
antibody, rabbit anti-phospho-mitogen-activated protein kinase
kinase 1/2 (MEK1/2) monoclonal antibody and rabbit
anti-phospho-extracellular signal-regulated kinase 1/2 (ERK1/2)
monoclonal antibody. To detect total FAK, MEK1/2 and ERK1/2
proteins, rabbit anti-FAK monoclonal antibody, rabbit anti-MEK1/2
monoclonal antibody and rabbit anti-ERK1/2 monoclonal antibody were
used, respectively. After incubation with primary antibodies, the
membranes were incubated with HRP-conjugated secondary antibody,
and protein bands were detected using an enhanced chemiluminescence
reagent. All antibodies used for the phosphorylation assay were
purchased from Cell Signaling Technology, Inc.
Integrin β8-specific morpholino antisense
oligonucleotide
To downregulate integrin β8, a morpholino antisense
oligonucleotide specific for integrin β8 obtained from GeneTools
(Philomath, OR, USA) was used. The sequence of the antisense
oligonucleotide is as follows: 5′-AAGCCAGGGCCGAGCCGCACATAAT-3′. A
standard control morpholino oligonucleotide
(5-CCTCTTACCTCAGTTACAATTTATA-3) was used as a negative control.
Delivery of the oligonucleotides into the cells was performed
according to the GeneTools protocol. Briefly, 80–100% confluent
SCCKN, KNmock or KNαv cells were treated with 10 μM of the
morpholino antisense oligonucleotide or the standard control
oligonucleotide, and 6 μM of Endo-Porter reagent (GeneTools). After
24 h, the cells were used for the subsequent experiments.
Northern blot analysis
Total cytoplasmic RNA of SCCKN, KNmock or KNαv cells
in confluent cultures was isolated with using TRIzol reagent (Life
Technologies, Rockville, MD, USA) according to manufacture’s
instruction. Total RNA (20 μg) obtained from the cells were
separated on a 1% agarose gels containing 2.2 M formaldehyde and
transferred directly from the gel to a nylon membrane (Hybond-N+;
GE Healthcare) in 10X SSC (1X SSC is 0.15 M NaCl plus 1.5 mM sodium
citrate) overnight. After transfer, RNA was UV cross-linked
(120,000 μJ of UV), and the membrane was prehybridized with
Rapid-hyb buffer (GE Healthcare) for 15 min at 65°C. The specific
probe for integrin β8 was obtained by reverse transcriptase-PCR as
follows: First-strand cDNA was synthesized from total RNA of SCCKN
cells with ReverTra Ace (Toyobo, Osaka, Japan) and PCR
amplification was performed using forward primer
(5′-GATCAGACGTCTCATCTCGC-3′) and reverse primer
(5′-CTCTTCCACTGCACACTTGG-3′). The PCR products (961 bp) were
subcloned into pGEM-T Easy Vector (Promega Corporation), and the
inserted cDNA sequences were verified by DNA sequence analysis. The
plasmid was digested with EcoRI, and the insert was
gel-purified and radiolabeled with [α-32P]-dCTP
(Perkin-Elmer) using Rediprime II DNA Labeling System (GE
Healthcare). Hybridization was carried out for 2 h at 65°C in
Rapid-hyb buffer containing 1×106 cpm/ml probe. The
membrane was washed with 2X SSC/0.1% SDS for 20 min at room
temperature and washed twice with 0.5X SSC/0.1% SDS for 15 min at
65°C. Hybridization signals were detected by the BAS 2000 image
analyzer (Fujifilm, Tokyo, Japan). Equivalent loading of ribosomal
RNA was confirmed by methylene blue staining.
Results
Effect of overexpression of integrin αv
on cell adhesion to ECM proteins
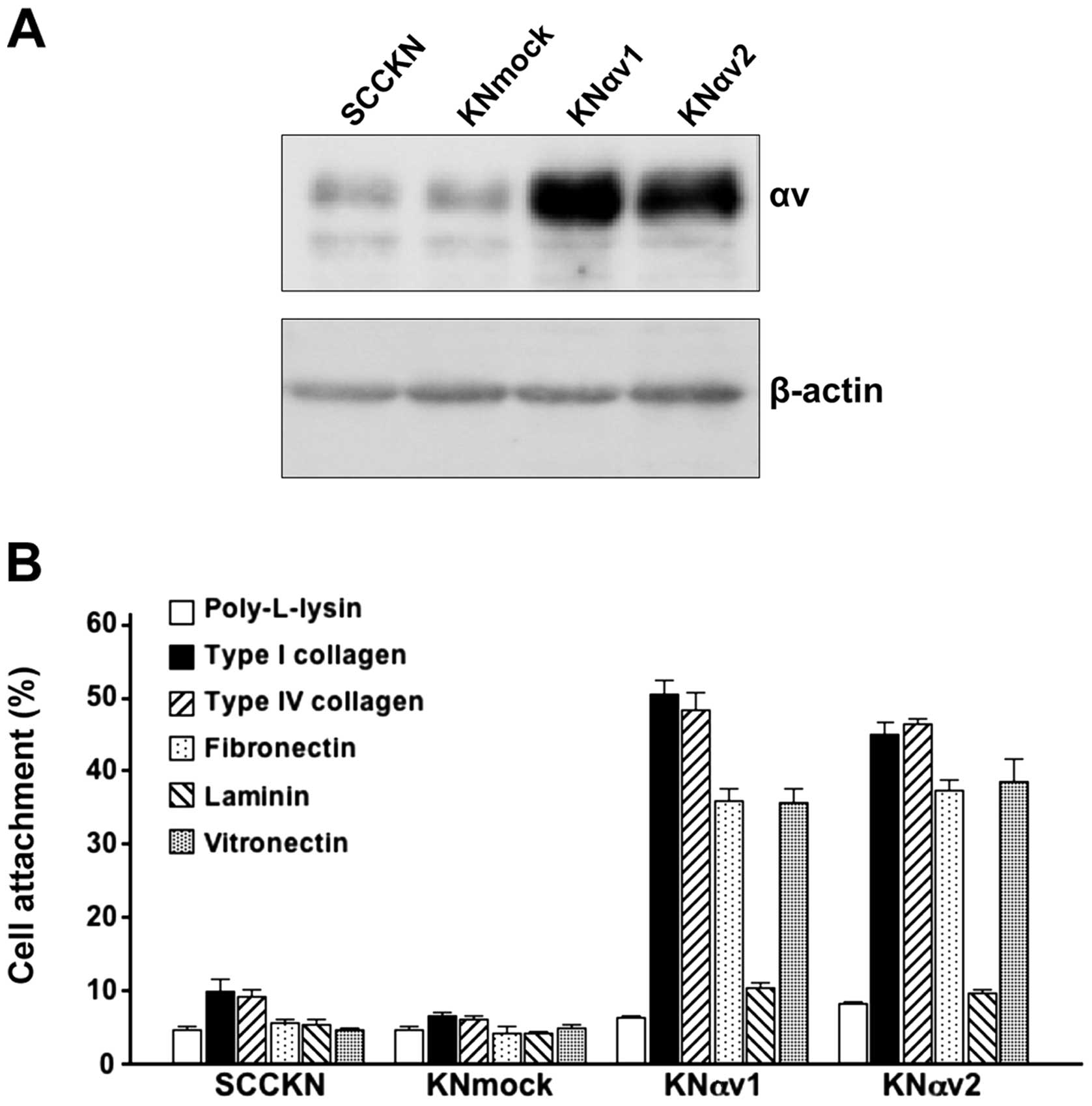
A small amount of integrin αv protein was observed
in SCCKN and KNmock cells. In contrast, integrin αv transfectants
in KNαv cells showed a large amount of integrin αv protein
(Fig. 1A).
To examine the effect of integrin αv on cell
adhesion to ECM proteins, SCCKN, KNmock and KNαv cells were seeded
on various ECM protein-coated wells and incubated for 30 min. Only
5 or 10% of SCCKN and KNmock cells adhered to any ECM protein. In
contrast, over 35% of KNαv cells adhered to type I collagen, type
IV collagen, fibronectin and vitronectin after 30-min incubation
(Fig. 1B).
Effect of overexpression of integrin αv
on the proliferation of SCC cells on ECM proteins
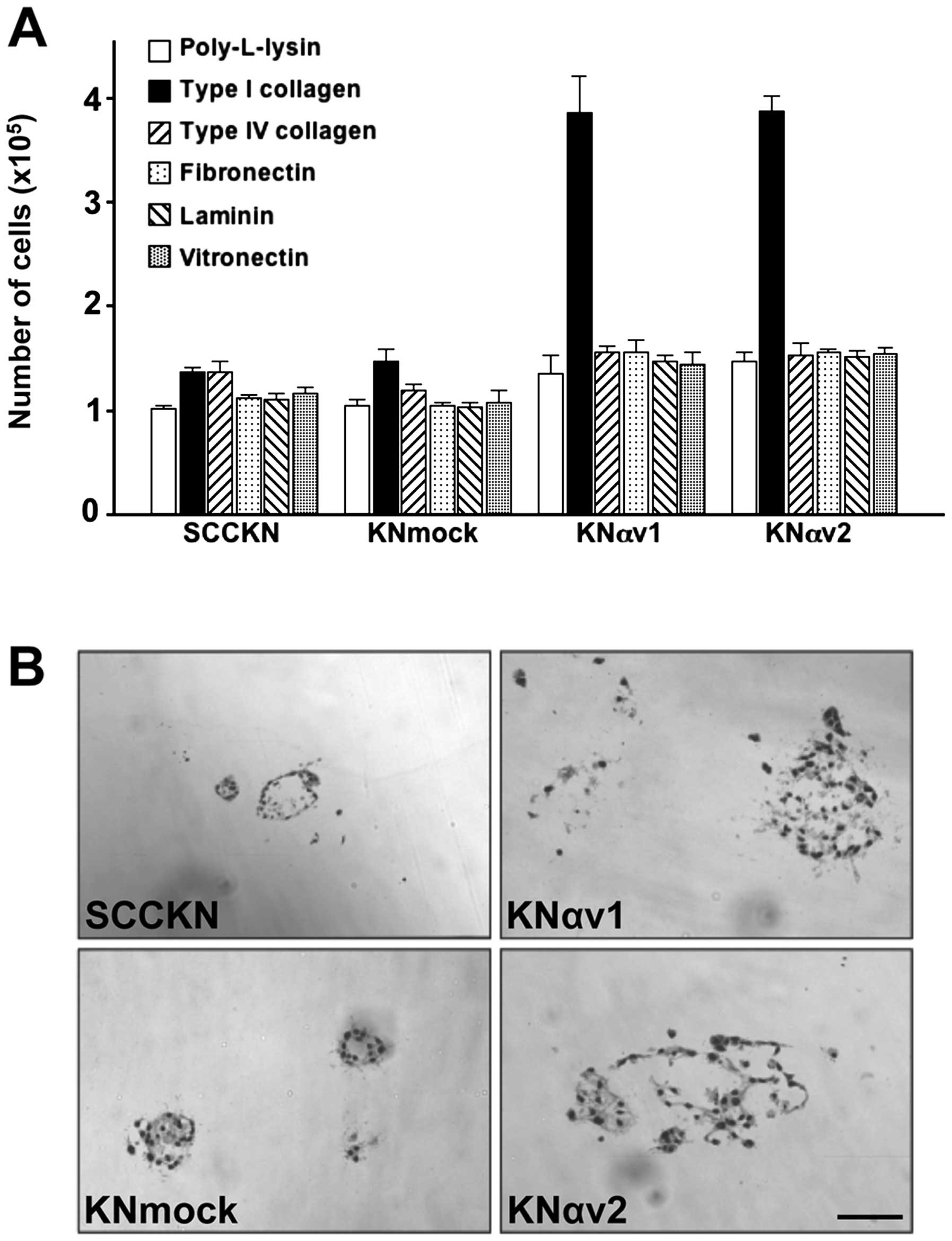
To examine the participation of integrin αv in the
proliferation of SCC cells, SCCKN, KNmock and KNαv cells were grown
on various ECM proteins in the absence of serum for 6 days.
Transfection with integrin αv cDNA led to a marked increase in cell
proliferation on type I collagen. The number of KNαv cells grown on
type I collagen was about 3-fold compared to the number of SCCKN
and KNmock cells on type I collagen. In contrast, other ECM
proteins exhibited no significant effect on the proliferation of
KNαv cells (Fig. 2A).
Effect of overexpression of integrin αv
on the morphology of colonies of SCC cells in three-dimensional
type I collagen gels
The behavior of SCCKN, KNmock and KNαv cells was
examined by three-dimensional culture using type I collagen gel.
The cells embedded in type I collagen gel were cultured for 12
days. SCCKN and KNmock cells formed small and spherical colonies in
the gel. In contrast, KNαv cells formed dilated colonies with
irregular margins, and some cells migrated into the surrounding
collagen gel, suggesting that transfection with integrin αv cDNA
led to the enhancement of invasiveness of SCC cells (Fig. 2B).
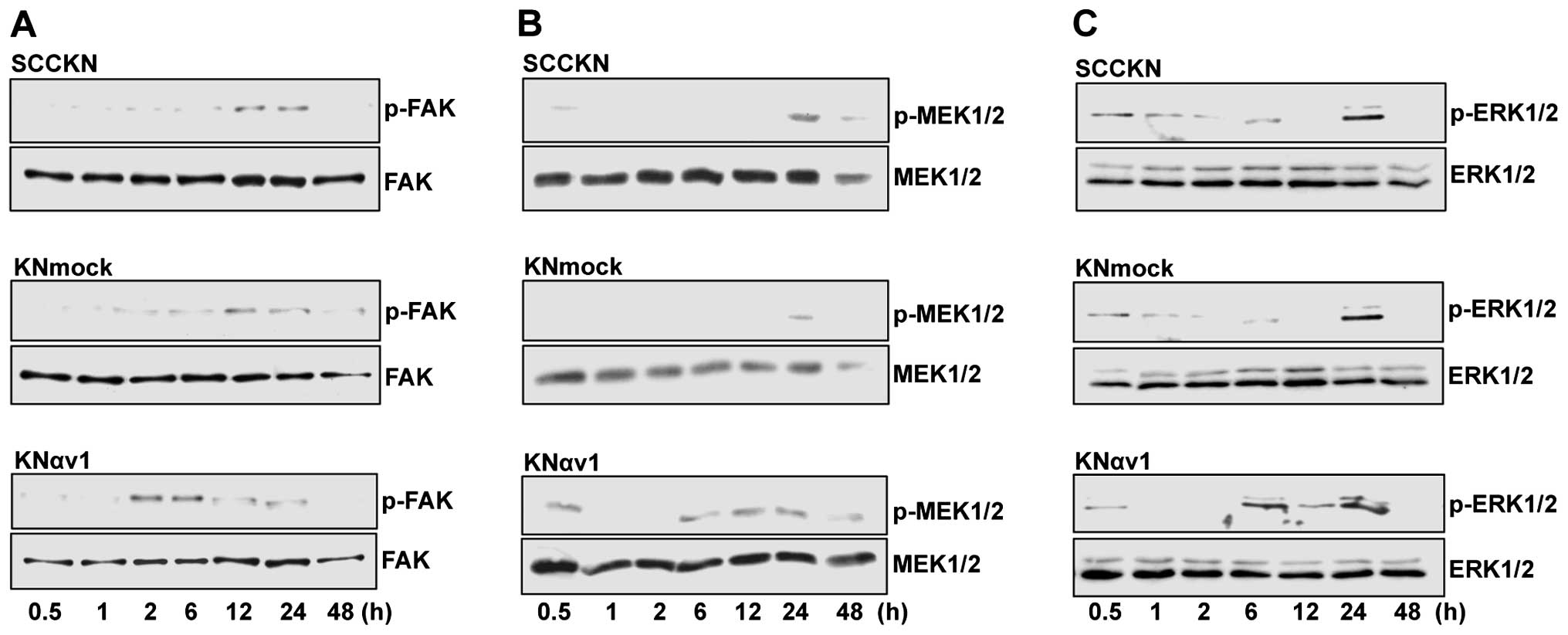
Activation of FAK and the MEK/ERK
signaling pathway by type I collagen
Type I collagen enhanced the proliferation of KNαv
cells, and KNαv cells exhibited the enhanced invasiveness into type
I collagen gel compared to SCCKN and KNmock cells. Several studies
have shown that the MEK/ERK signaling pathway via integrin αv
regulates cell proliferation (32–34).
To clarify the participation of the MEK/ERK signaling pathway in
type I collagen-induced proliferation and invasion of KNαv cells,
SCCKN, KNmock and KNαv cells were detached and replated onto type I
collagen, and the phosphorylation of FAK, MEK1/2 and ERK1/2 in the
cells was investigated after cultivation for various periods. The
phosphorylation of FAK, MEK1/2 and ERK1/2 in KNαv cells was
observed at 2, 6 h and 6 h after replating on type I collagen,
respectively. In contrast, the phosphorylation of FAK, MEK1/2 and
ERK1/2 in SCCKN and KNmock cells was observed at 12, 24 h and 24 h
after replating, respectively (Fig.
3).
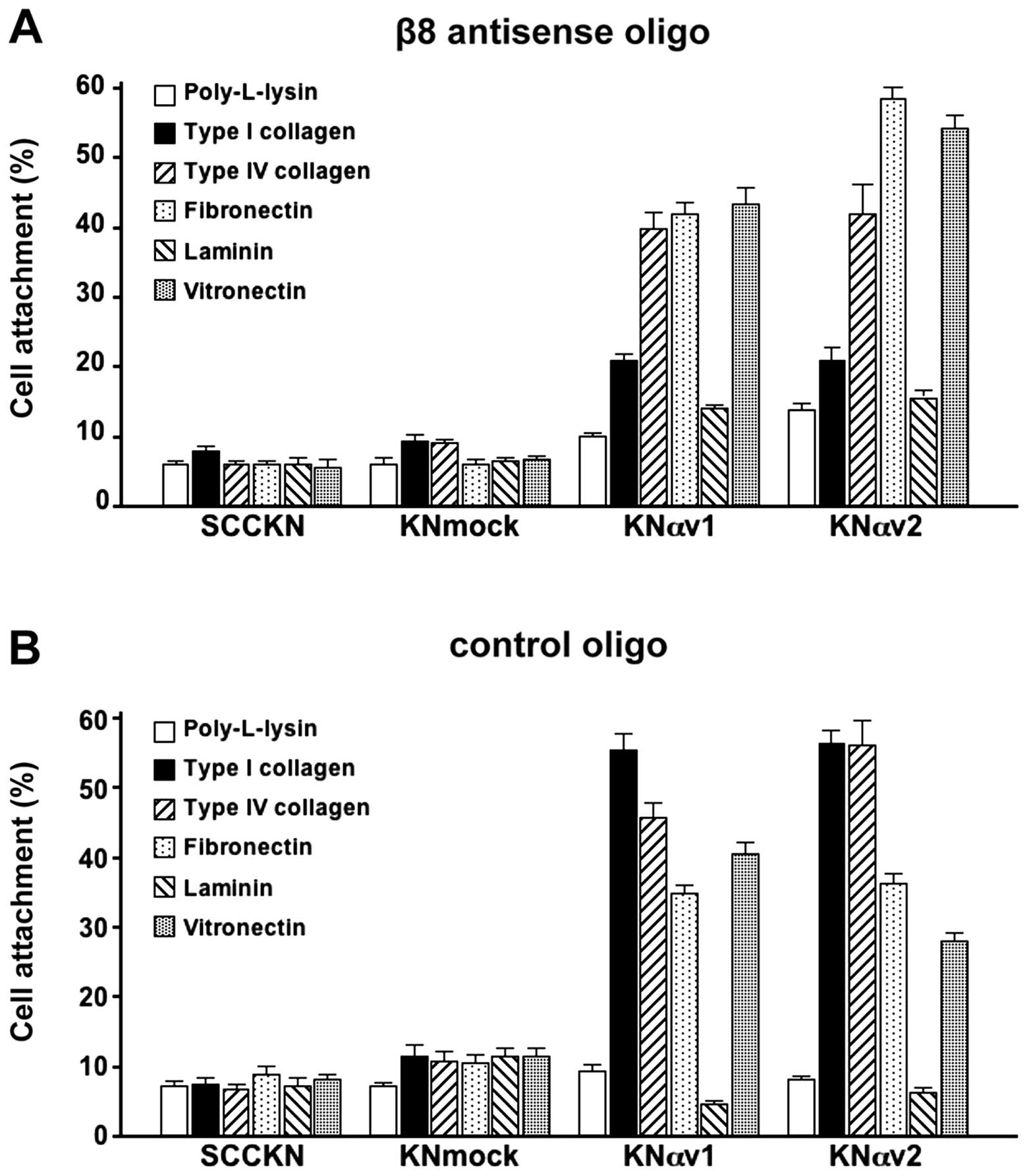
Participation of integrin β8 in integrin
αv-mediated cell adhesion of SCC cells
To examine the effect of the suppression of integrin
β8 on the adhesion of SCC cells to ECM proteins, the cells were
transfected with a morpholino antisense oligonucleotide targeting
integrin β8 subunit (Fig. 4A) or a
control oligonucleotide (Fig. 4B).
The adhesion of SCCKN and KNmock cells to ECM proteins were not
greatly affected by the suppression of integrin β8. In contrast,
the suppression of integrin β8 by the antisense oligonucleotide led
to the remarkable decrease in the attachment of KNαv cells to type
I collagen compare to the attachment of KNαv cells transfected with
the control oligonucleotide. However, the suppression of integrin
β8 did not reduce the adhesion of KNαv cells to any ECM protein
except type I collagen.
Participation of integrin αvβ8 on the
proliferation of SCC cells via the MEK/ERK signaling pathway
We examined the participation of integrin αvβ8 in
type I collagen-induced proliferation of KNαv cells. The
suppression of integrin β8 by a morpholino antisense
oligonucleotide targeting integrin β8 led to remarkable decrease in
the proliferation of KNαv cells cultured on type I collagen.
However, transfection with the antisense oligonucleotide did not
have strong effect on the proliferation of SCCKN and KNmock cells
on type I collagen (Fig. 5A).
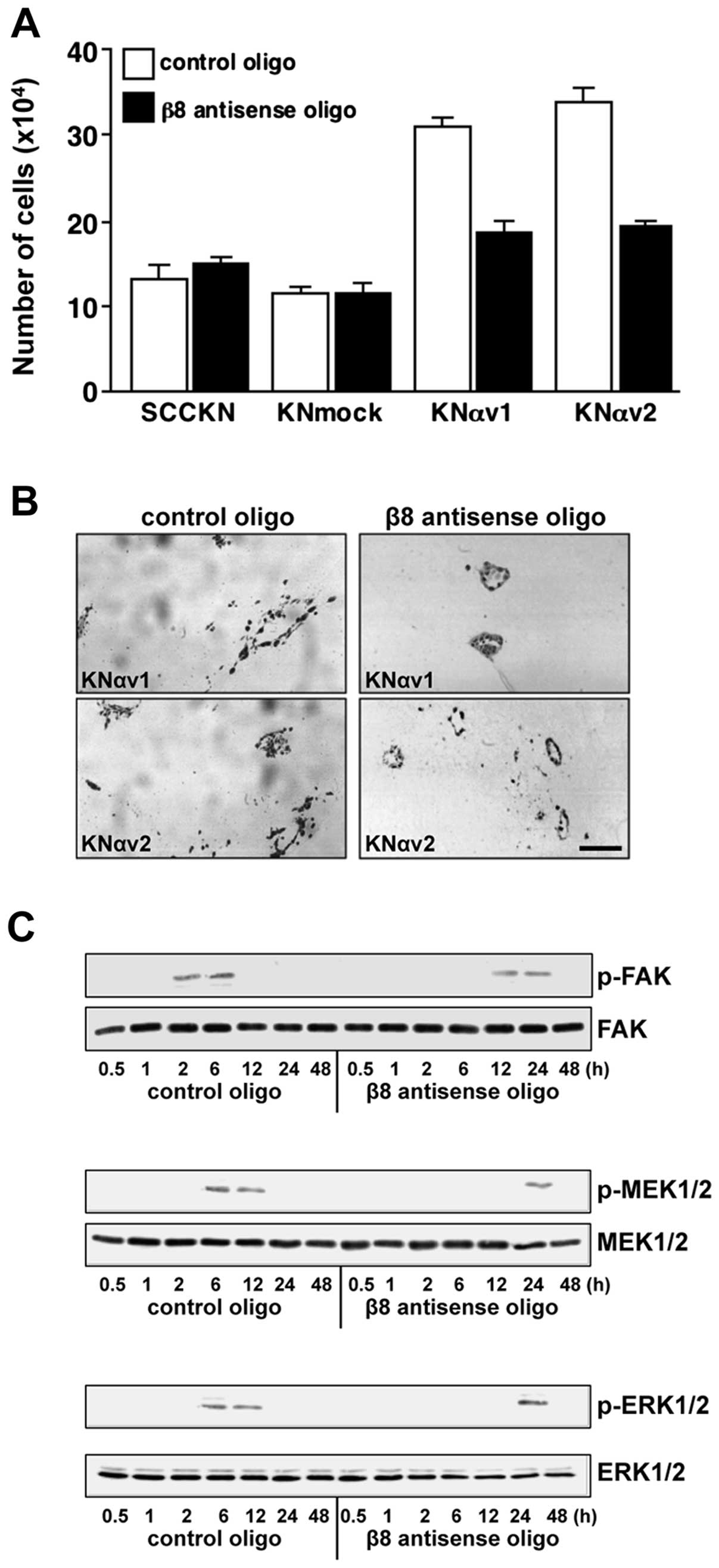 | Figure 5Suppression of integrin β8 reduces
type I collagen-induced growth and phosphorylation of focal
adhesion kinase (FAK), mitogen-activated protein kinase kinase 1/2
(MEK1/2) and extracellular signal-regulated kinase 1/2 (ERK1/2) of
squamous cell carcinoma (SCC) cells. (A) SCCKN, KNmock and KNαv
cells (2×104) transfected with β8 antisense oligo or
control oligo were suspended in RD containing 10 μg/ml bovine
insulin, 5 μg/ml human transferrin, 0.5 mg/ml fatty acid-free
bovine serum albumin (BSA), 10 μM 2-mercaptoethanol, 10 μM
2-aminoethanol and 10 nM sodium selenite, and were seeded in each
well of 24-well tissue culture plates coated with type I collagen.
After cultivation for 6 days, the number of cells was measured. The
results are the means of triplicated determinations ± SD. (B) KNαv
cells transfected with β8 antisense oligo or control oligo were
embedded in type I collagen gel and cultured for 12 days. The cells
were fixed in buffered formalin and embedded in paraffin, and the
sections stained with hematoxylin and eosin. Scale bar, 250 μm. (C)
KNαv cells transfected with the β8 antisense oligo or control oligo
were detached and replated onto type I collagen. The cells were
lysed at the indicated times after replating, and the
phosphorylation of FAK, MEK1/2 and ERK1/2 was analysed by western
blotting. |
We next examined the effect of the suppression of
integrin β8 on the morphology of colonies of KNαv cells in type I
collagen gels. KNαv cells transfected with the control
oligonucleotide formed dilated colonies with irregular margin, and
some cells migrated into the surrounding collagen gel. In contrast,
the colonies of KNαv cells transfected with the antisense
oligonucleotide were small and spherical colonies (Fig. 5B).
The phosphorylation of FAK, MEK1/2 and ERK1/2 in
KNαv cells transfected with the control oligonucleotide was
observed at 2, 6 h and 6 h after replating on type I collagen,
respectively. In contrast, the phosphorylation of FAK, MEK1/2 and
ERK1/2 in KNαv cells transfected with the antisense oligonucleotide
was observed at 12, 24 h and 24 h after replating, respectively
(Fig. 5C).
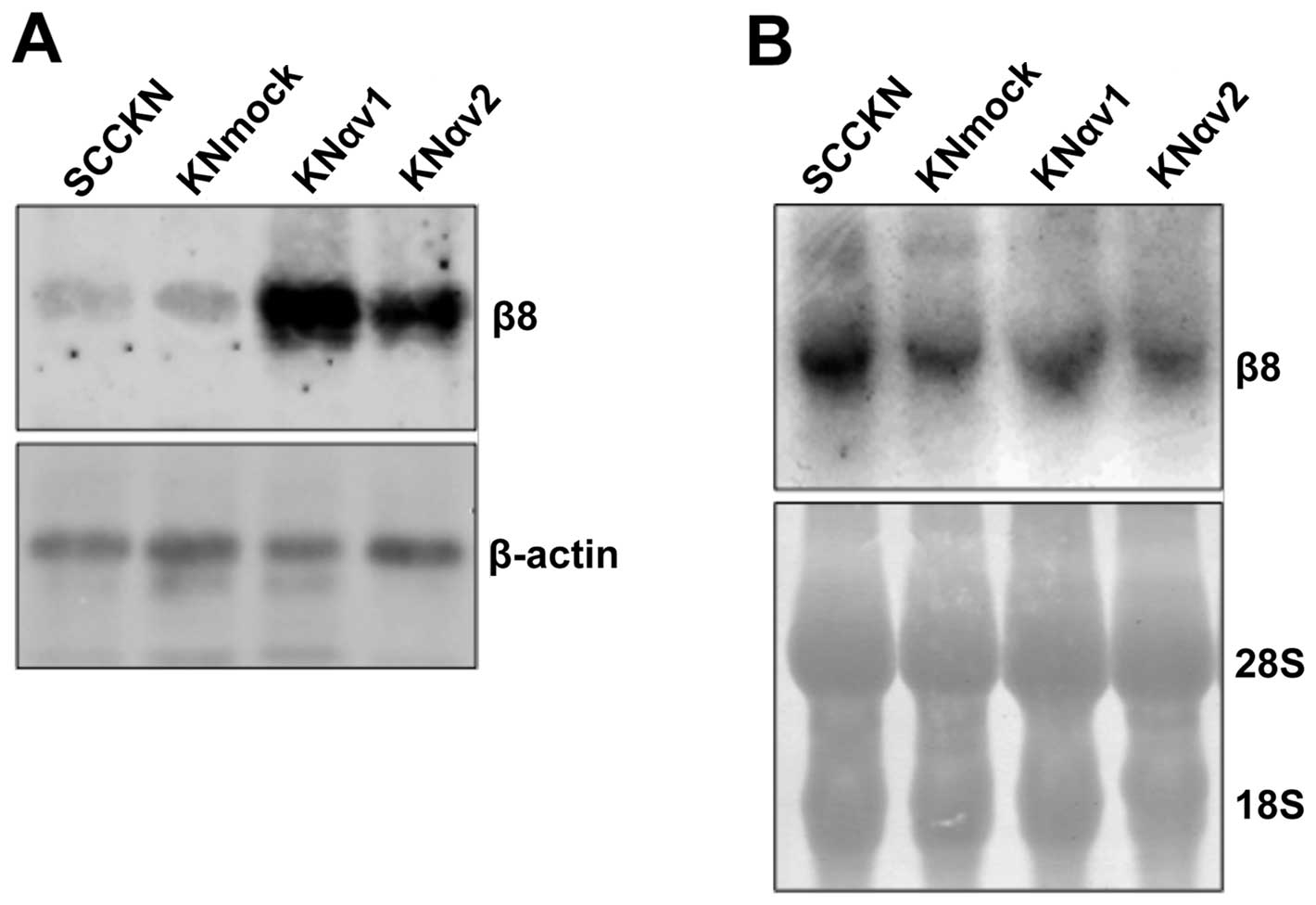
Expression of integrin β8 in integrin αv
transfectants
The proliferation of KNαv cells on type I collagen
was reduced by the suppression of integrin β8. Moreover, type I
collagen-stimulated phosphorylation of FAK, MEK1/2 and ERK1/2 in
KNαv cells was also inhibited by the suppression of integrin β8.
These findings suggest that the interaction of integrin αvβ8 with
type I collagen might activate FAK and the MEK/ERK signaling
pathway and induce the proliferation of SCC cells. Therefore, the
expression of integrin β8 protein and mRNA in SCCKN, KNmock and
KNαv cells was examined by western blotting and northern blotting,
respectively. The expression of integrin β8 protein in KNαv cells
was enhanced compared to SCCKN and KNmock cells. In contrast, there
was no remarkable difference in the expression of integrin β8 mRNA
between SCCKN, KNmock and KNαv cells (Fig. 6).
Discussion
Integrin αv, which heterodimerizes with β1, β3, β5,
β6 or β8, regulates several biological events such as cell
adhesion, proliferation and differentiation (7,8).
Several studies have shown that integrin αvβ3, αvβ5 and αvβ6 are
implicated in carcinogenesis, tumor invasion and metastasis
(34–37). Our previous study has also shown
that integrin αv mediates the proteolytic activity of SCC cells by
anchoring active MMP-2 on the cell surfaces, suggesting that
integrin αv might promote the progression of oral SCC (38).
In this study, the role of integrin αv in the
progression of SCC cells was examined. Induction of integrin αv
expression led to the enhancement of cell attachment of SCCKN cells
to any ECM proteins used in the present study. Especially, integrin
αv transfectants, KNαv cells had a high ability to bind type I
collagen, type IV collagen and fibronectin. We next examined the
proliferation of SCC cells on ECM proteins. The proliferation of
SCCKN and KNmock cells was not greatly influenced by any ECM
proteins. In contrast, the proliferation of KNαv cells on type I
collagen was significantly enhanced. Moreover, the effect of
integrin αv on the behavior of SCC cells using three-dimensional
type I collagen gel culture system was examined. SCCKN and KNmock
cells embedded in type I collagen gel formed small and spherical
colonies. In contrast, KNαv cells in type I collagen gel formed
dilated colonies with irregular margins, and some cells migrated
into the surrounding gel. These findings suggest that the binding
of αv integrins to type I collagen activates the signaling pathway
involved in the proliferation and invasion of SCC cells.
The binding of integrins to ECM proteins leads to
the activation of FAK, and results in the activation of several
signaling pathways such as Akt/PI3 kinase signaling,
mitogen-activated protein (MAP) kinase signaling and Rho family
GTPase signaling (39–42). It is well known that cell
proliferation via the integrin αv subfamily such as αvβ3 and αvβ5
is mediated by the MEK/ERK signaling pathway, which is
characterized firstly in MAP kinase cascades (32–34).
Therefore, the participation of the MEK/ERK signaling pathway in
type I collagen-induced growth of integrin αv transfectants was
examined. Rapid phosphorylation of FAK, MEK1/2 and ERK1/2 was
observed in KNαv cells cultured on type I collagen. In contrast,
the phosphorylation of these molecules was delayed in SCCKN and
KNmock cells on type I collagen. These findings indicate that type
I collagen induces the activation of FAK and the MEK/ERK signaling
pathway via αv integrins.
Formation of α/β heterodimer is essential for the
expression and function of integrins. Integrin αv subunit
associates with β1, β3, β5, β6 or β8 subunit and forms five
distinct heterodimers (7,8). Integrin αv transfectants, KNαv cells
had a high ability to bind type I collagen, and exhibited a
remarkable proliferative response to type I collagen. These
findings suggest that some of the five αv integrins of KNαv cells
interact with type I collagen and regulate cell proliferation as
well as cell adhesion. The five αv integrins bind various ECM
proteins such as fibronectin, laminin, vitronectin, fibrinogen and
osteopontin. The binding of the αv integrins to ECM proteins is
dependent upon the β subunit counterpart. Some studies have shown
that only integrin αvβ8 in the integrin αv subfamily is a potential
receptor for collagens (43,44).
We therefore examined the participation of integrin αvβ8 in type I
collagen-induced proliferation of KNαv cells. The suppression of
integrin β8 by its antisense oligonucleotide led to a remarkable
decrease in the adhesion of KNαv cells to type I collagen. The
treatment of integrin β8 antisense oligonucleotide also reduced the
proliferation of KNαv cells on type I collagen and the invasiveness
of KNαv cells into type I collagen gel. In addition, the activation
of FAK and the MEK/ERK signaling pathway of KNαv cells on type I
collagen was inhibited by the treatment of integrin β8 antisense
oligonucleotide. These findings indicate that the binding of
integrin αvβ8 to type I collagen might induce the proliferation and
invasion of SCC cells via the MEK/ERK signaling pathway.
We next examined the expression of integrin β8 in
SCCKN, KNmock and KNαv cells. Interestingly, KNαv cells expressed a
large amount of integrin β8 protein compared to SCCKN and KNmock
cells, whereas there is no significant difference in the expression
of integrin β8 mRNA between SCCKN, KNmock and KNαv cells. At
present, the mechanism of the enhanced expression of integrin β8
protein following transfection with integrin αv cDNA is unclear. A
previous study showed that the expression of mouse integrin β1 and
β7 was induced following transfection with human integrin α4
subunit in mouse fibroblasts (45). The possibility should be considered
that integrin β8 subunit dimerizes with integrin αv subunit
expressed abundantly in KNαv cells, and integrin β8 dimerized with
integrin αv might be stable compared to integrin β8 monomer. Most
of integrin β8 subunits in SCCKN and KNmock might exist as monomer
because of the insufficiency of integrin αv subunits available for
forming αvβ8 heterodimers.
In conclusion, the overexpression of integrin αv led
to the enhancement of the proliferation of oral SCC cells via
interaction with type I collagen. The expression of integrin β8
subunit is induced following transfection with integrin αv subunit
in SCC cells. Interaction of integrin αvβ8 with type I collagen
activates the MEK/ERK signaling pathway in SCC cells, resulting in
the enhancement of the proliferation and invasiveness. These
findings suggest that integrin αvβ8 might be a prognostic factor
for oral SCC and could serve as a therapeutic target to prevent the
progression of oral SCC.
References
|
1
|
Bosman FT, de Bruïne A, Flohil C, van der
Wurff A, ten Kate J and Dinjens WW: Epithelial-stromal interactions
in colon cancer. Int J Dev Biol. 37:203–211. 1993.PubMed/NCBI
|
|
2
|
Ziober BL, Lin CS and Kramer RH:
Laminin-binding integrins in tumor progression and metastasis.
Semin Cancer Biol. 7:119–128. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chrenek MA, Wong P and Weaver VM:
Tumour-stromal interactions. Integrins and cell adhesions as
modulators of mammary cell survival and transformation. Breast
Cancer Res. 3:224–229. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Labat-Robert J: Fibronectin in malignancy.
Semin Cancer Biol. 12:187–195. 2002. View Article : Google Scholar
|
|
5
|
Huang CY, Lee CY, Chen MY, et al: Stromal
cell-derived factor-1/CXCR4 enhanced motility of human osteosarcoma
cells involves MEK1/2, ERK and NF-kappaB-dependent pathways. J Cell
Physiol. 221:204–212. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hynes RO: Integrins: bidirectional,
allosteric signaling machines. Cell. 110:673–687. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Barczyk M, Carracedo S and Gullberg D:
Integrins. Cell Tissue Res. 339:269–280. 2010. View Article : Google Scholar
|
|
8
|
Takada Y, Ye X and Simon S: The integrins.
Genome Biol. 8:2152007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Howe A, Aplin AE, Alahari SK and Juliano
RL: Integrin signaling and cell growth control. Curr Opin Cell
Biol. 10:220–231. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Dedhar S: Cell-substrate interactions and
signaling through ILK. Curr Opin Cell Biol. 12:250–256. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Schwartz MA and Assoian RK: Integrins and
cell proliferation: regulation of cyclin-dependent kinases via
cytoplasmic signaling pathways. J Cell Sci. 114:2553–2560.
2001.PubMed/NCBI
|
|
12
|
Hood JD and Cheresh DA: Role of integrins
in cell invasion and migration. Nat Rev Cancer. 2:91–100. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chung J and Mercurio AM: Contributions of
the α6 integrins to breast carcinoma survival and progression. Mol
Cells. 17:203–209. 2004.
|
|
14
|
Gleason B, Adley B, Rao MS and Diaz LK:
Immunohistochemical detection of the β4 integrin subunit in
pancreatic adenocarcinoma. J Histochem Cytochem. 53:799–801.
2005.
|
|
15
|
Valea FA, Haskill S, Moore DH and Fowler
WC Jr: Immunohistochemical analysis of α1-integrins in cervical
cancer. Am J Obstet Gynecol. 173:808–813. 1995.
|
|
16
|
Boudjadi S, Carrier JC and Beaulieu JF:
Integrin α1 subunit is up-regulated in colorectal cancer. Biomark
Res. 1:162013.
|
|
17
|
Bartolazzi A, Cerboni C, Flamini G,
Bigotti A, Lauriola L and Natali PG: Expression of α3β1 integrin
receptor and its ligands in human lung tumors. Int J Cancer.
64:248–252. 1995.
|
|
18
|
Goldberg I, Davidson B, Reich R, et al: αv
integrin expression is a novel marker of poor prognosis in
advanced-stage ovarian carcinoma. Clin Cancer Res. 7:4073–4079.
2001.
|
|
19
|
Davidson B, Goldberg I, Reich R, et al:
αv- and β1-integrin subunits are commonly expressed in malignant
effusions from ovarian carcinoma patients. Gynecol Oncol.
90:248–257. 2003.
|
|
20
|
Jin Y, Tong DY, Chen JN, et al:
Overexpression of osteopontin, αvβ3 and Pim-1 associated with
prognostically important clinicopathologic variables in non-small
cell lung cancer. PLoS One. 7:e485752012.
|
|
21
|
Xuan SH, Zhou YG, Pan JQ, Zhu W and Xu P:
Overexpression of integrin αv in the human nasopharyngeal carcinoma
associated with metastasis and progression. Cancer Biomark.
13:323–328. 2013.
|
|
22
|
Kawashima A, Tsugawa S, Boku A, et al:
Expression of αv integrin family in gastric carcinomas: increased
αvβ6 is associated with lymph node metastasis. Pathol Res Pract.
199:57–64. 2003.
|
|
23
|
Vogetseder A, Thies S, Ingold B, et al:
αv-Integrin isoform expression in primary human tumors and brain
metastases. Int J Cancer. 133:2362–2371. 2013.
|
|
24
|
Cruet-Hennequart S, Maubant S, Luis J,
Gauduchon P, Staedel C and Dedhar S: αv integrins regulate cell
proliferation through integrin-linked kinase (ILK) in ovarian
cancer cells. Oncogene. 22:1688–1702. 2003.
|
|
25
|
Wong NC, Mueller BM, Barbas CF, et al: αv
integrins mediate adhesion and migration of breast carcinoma cell
lines. Clin Exp Metastasis. 16:50–61. 1998.
|
|
26
|
Samanna V, Wei H, Ego-Osuala D and
Chellaiah MA: Alpha-V-dependent outside-in signaling is required
for the regulation of CD44 surface expression, MMP-2 secretion, and
cell migration by osteopontin in human melanoma cells. Exp Cell
Res. 312:2214–2230. 2006. View Article : Google Scholar
|
|
27
|
Khatib AM, Nip J, Fallavollita L, Lehmann
M, Jensen G and Brodt P: Regulation of urokinase plasminogen
activator/plasmin-mediated invasion of melanoma cells by the
integrin vitronectin receptor αvβ3. Int J Cancer. 91:300–308.
2001.PubMed/NCBI
|
|
28
|
Dutta A, Li J, Lu H, et al: Integrin αvβ6
promotes an osteolytic program in cancer cells by upregulating
MMP2. Cancer Res. 74:1598–1608. 2014.
|
|
29
|
Brooks PC, Strömblad S, Sanders LC, et al:
Localization of matrix metalloproteinase MMP-2 to the surface of
invasive cells by interaction with integrin αvβ3. Cell. 85:683–693.
1996.
|
|
30
|
Hayashido Y, Shirasuna K, Sugiura T,
Nakashima M and Matsuya T: Effect of dexamethasone on invasion of
human squamous cell carcinoma cells into collagen gel. Cancer Lett.
108:81–86. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yamanaka T, Sakamoto A, Tanaka Y, et al:
Isolation and serum-free culture of epithelial cells derived from
epithelial rests of Malassez in human periodontal ligament. In
Vitro Cell Dev Biol Anim. 36:548–553. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hood JD, Frausto R, Kiosses WB, Schwartz
MA and Cheresh DA: Differential αv integrin-mediated Ras-ERK
signaling during two pathways of angiogenesis. J Cell Biol.
162:933–943. 2003.
|
|
33
|
Kobayashi-Sakamoto M, Isogai E, Hirose K
and Chiba I: Role of αv integrin in osteoprotegerin-induced
endothelial cell migration and proliferation. Microvasc Res.
76:139–144. 2008.
|
|
34
|
Bianchi-Smiraglia A, Paesante S and Bakin
AV: Integrin β5 contributes to the tumorigenic potential of breast
cancer cells through the Src-FAK and MEK-ERK signaling pathways.
Oncogene. 32:3049–3058. 2013.
|
|
35
|
Natali PG, Hamby CV, Felding-Habermann B,
et al: Clinical significance of αvβ3 integrin and intercellular
adhesion molecule-1 expression in cutaneous malignant melanoma
lesions. Cancer Res. 57:1554–1560. 1997.
|
|
36
|
Fabricius EM, Wildner GP,
Kruse-Boitschenko U, Hoffmeister B, Goodman SL and Raguse JD:
Immunohistochemical analysis of integrins αvβ3, αvβ5 and α5β1, and
their ligands, fibrinogen, fibronectin, osteopontin and
vitronectin, in frozen sections of human oral head and neck
squamous cell carcinomas. Exp Ther Med. 2:9–19. 2011.
|
|
37
|
Enns A, Korb T, Schlüter K, et al:
αvβ5-integrin mediate early steps of metastasis formation. Eur J
Cancer. 41:1065–1072. 2005.
|
|
38
|
Hayashido Y, Urabe K, Yoshioka Y, Kitano
H, Okamoto T and Matsuya T: Participation of fibroblasts in MMP-2
binding and activation on the surface of oral squamous cell
carcinoma cells. Int J Oncol. 22:657–662. 2003.PubMed/NCBI
|
|
39
|
Wang S and Basson MD: Integrin-linked
kinase: a multi-functional regulator modulating extracellular
pressure-stimulated cancer cell adhesion through focal adhesion
kinase and AKT. Cell Oncol. 31:273–289. 2009.
|
|
40
|
Cary LA, Han DC and Guan JL:
Integrin-mediated signal transduction pathways. Histol Histopathol.
14:1001–1009. 1999.PubMed/NCBI
|
|
41
|
Cabodi S, Di Stefano P, Leal Mdel P, et
al: Integrins and signal transduction. Adv Exp Med Biol. 674:43–54.
2010. View Article : Google Scholar
|
|
42
|
Huveneers S and Danen EH: Adhesion
signaling-crosstalk between integrins, Src and Rho. J Cell Sci.
122:1059–1069. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Metlapally R, Jobling AI, Gentle A and
McBrien NA: Characterization of the integrin receptor subunit
profile in the mammalian sclera. Mol Vis. 12:725–734.
2006.PubMed/NCBI
|
|
44
|
Nemeth JA, Nakada MT, Trikha M, et al:
Alpha-v integrins as therapeutic targets in oncology. Cancer
Invest. 25:632–646. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Webb DL, Conrad PJ, Ma L and Blue ML:
Induction of mouse β integrin expression following transfection
with human α4 chain. J Cell Biochem. 61:127–138. 1996.
|