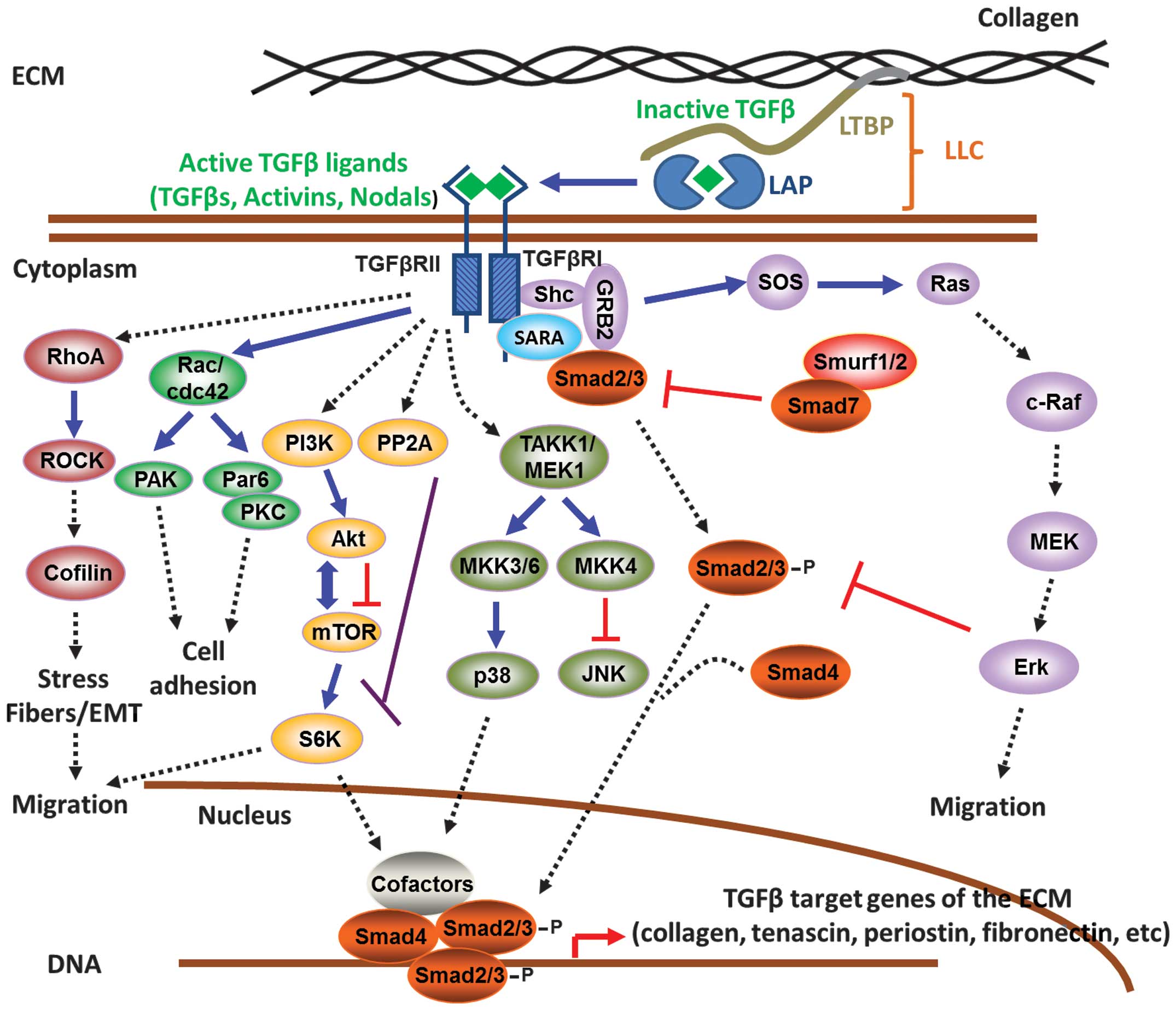
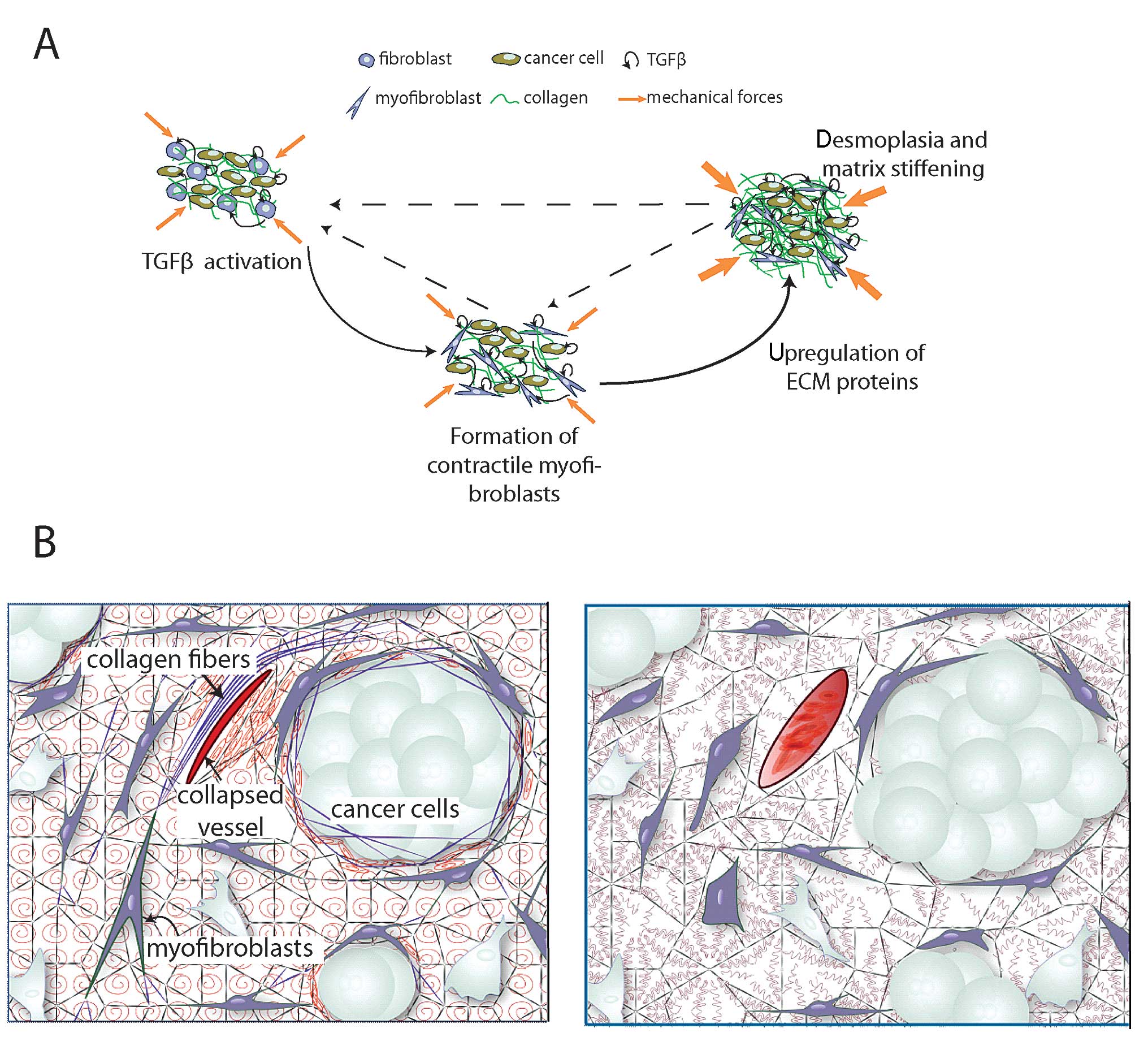
|
1
|
Derynck R and Akhurst RJ: Differentiation
plasticity regulated by TGF-beta family proteins in development and
disease. Nat Cell Biol. 9:1000–1004. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wakefield LM and Hill CS: Beyond TGFβ:
roles of other TGFβ superfamily members in cancer. Nat Rev Cancer.
13:328–341. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Massagué J, Seoane J and Wotton D: Smad
transcription factors. Genes Dev. 19:2783–2810. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Annes JP, Munger JS and Rifkin DB: Making
sense of latent TGFbeta activation. J Cell Sci. 116:217–224. 2003.
View Article : Google Scholar
|
|
5
|
Gleizes PE, Beavis RC, Mazzieri R, Shen B
and Rifkin DB: Identification and characterization of an
eight-cysteine repeat of the latent transforming growth factor-beta
binding protein-1 that mediates bonding to the latent transforming
growth factor-beta1. J Biol Chem. 271:29891–29896. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Miyazono K, Olofsson A, Colosetti P and
Heldin CH: A role of the latent TGF-beta 1-binding protein in the
assembly and secretion of TGF-beta 1. EMBO J. 10:1091–1101.
1991.PubMed/NCBI
|
|
7
|
Saharinen J, Taipale J and Keski-Oja J:
Association of the small latent transforming growth factor-beta
with an eight cysteine repeat of its binding protein LTBP-1. EMBO
J. 15:245–253. 1996.PubMed/NCBI
|
|
8
|
Unsöld C, Hyytiäinen M, Bruckner-Tuderman
L and Keski-Oja J: Latent TGF-beta binding protein LTBP-1 contains
three potential extracellular matrix interacting domains. J Cell
Sci. 114:187–197. 2001.
|
|
9
|
Nunes I, Gleizes PE, Metz CN and Rifkin
DB: Latent transforming growth factor-beta binding protein domains
involved in activation and transglutaminase-dependent cross-linking
of latent transforming growth factor-beta. J Cell Biol.
136:1151–1163. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lawrence DA, Pircher R, Krycève-Martinerie
C and Jullien P: Normal embryo fibroblasts release transforming
growth factors in a latent form. J Cell Physiol. 121:184–188. 1984.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Crawford SE, Stellmach V, Murphy-Ullrich
JE, et al: Thrombospondin-1 is a major activator of TGF-beta1 in
vivo. Cell. 93:1159–1170. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ribeiro SM, Poczatek M, Schultz-Cherry S,
Villain M and Murphy-Ullrich JE: The activation sequence of
thrombos-pondin-1 interacts with the latency-associated peptide to
regulate activation of latent transforming growth factor-beta. J
Biol Chem. 274:13586–13593. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Dubois CM, Laprise MH, Blanchette F,
Gentry LE and Leduc R: Processing of transforming growth factor
beta 1 precursor by human furin convertase. J Biol Chem.
270:10618–10624. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sato Y and Rifkin DB: Inhibition of
endothelial cell movement by pericytes and smooth muscle cells:
activation of a latent transforming growth factor-beta 1-like
molecule by plasmin during co-culture. J Cell Biol. 109:309–315.
1989. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yu Q and Stamenkovic I: Cell
surface-localized matrix metalloproteinase-9 proteolytically
activates TGF-beta and promotes tumor invasion and angiogenesis.
Genes Dev. 14:163–176. 2000.PubMed/NCBI
|
|
16
|
Derynck R, Zhang Y and Feng XH: Smads:
transcriptional activators of TGF-beta responses. Cell. 95:737–740.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Massagué J: TGF-beta signal transduction.
Annu Rev Biochem. 67:753–791. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Riggins GJ, Thiagalingam S, Rozenblum E,
et al: Mad-related genes in the human. Nat Genet. 13:347–349. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lagna G, Hata A, Hemmati-Brivanlou A and
Massagué J: Partnership between DPC4 and SMAD proteins in TGF-beta
signalling pathways. Nature. 383:832–836. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Nakao A, Imamura T, Souchelnytskyi S, et
al: TGF-beta receptor-mediated signalling through Smad2, Smad3 and
Smad4. EMBO J. 16:5353–5362. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Heldin CH, Miyazono K and ten Dijke P:
TGF-beta signalling from cell membrane to nucleus through SMAD
proteins. Nature. 390:465–471. 1997. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bierie B and Moses HL: Tumour
microenvironment: TGFbeta: the molecular Jekyll and Hyde of cancer.
Nat Rev Cancer. 6:506–520. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lewis KA, Gray PC, Blount AL, et al:
Betaglycan binds inhibin and can mediate functional antagonism of
activin signalling. Nature. 404:411–414. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wrana JL, Attisano L, Wieser R, Ventura F
and Massagué J: Mechanism of activation of the TGF-beta receptor.
Nature. 370:341–347. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Shi Y and Massagué J: Mechanisms of
TGF-beta signaling from cell membrane to the nucleus. Cell.
113:685–700. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Miyazono K, Maeda S and Imamura T: BMP
receptor signaling: transcriptional targets, regulation of signals,
and signaling cross-talk. Cytokine Growth Factor Rev. 16:251–263.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Derynck R and Zhang YE: Smad-dependent and
Smad-independent pathways in TGF-beta family signalling. Nature.
425:577–584. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Abdollah S, Macías-Silva M, Tsukazaki T,
Hayashi H, Attisano L and Wrana JL: TbetaRI phosphorylation of
Smad2 on Ser465 and Ser467 is required for Smad2-Smad4 complex
formation and signaling. J Biol Chem. 272:27678–27685. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhang Y, Feng X, We R and Derynck R:
Receptor-associated Mad homologues synergize as effectors of the
TGF-beta response. Nature. 383:168–172. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tsukazaki T, Chiang TA, Davison AF,
Attisano L and Wrana JL: SARA, a FYVE domain protein that recruits
Smad2 to the TGFbeta receptor. Cell. 95:779–791. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Feng XH, Zhang Y, Wu RY and Derynck R: The
tumor suppressor Smad4/DPC4 and transcriptional adaptor CBP/p300
are coactivators for smad3 in TGF-beta-induced transcriptional
activation. Genes Dev. 12:2153–2163. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Janknecht R, Wells NJ and Hunter T:
TGF-beta-stimulated cooperation of smad proteins with the
coactivators CBP/p300. Genes Dev. 12:2114–2119. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Itoh S, Ericsson J, Nishikawa J, Heldin CH
and ten Dijke P: The transcriptional co-activator P/CAF potentiates
TGF-beta/Smad signaling. Nucleic Acids Res. 28:4291–4298. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Bai RY, Koester C, Ouyang T, et al: SMIF,
a Smad4-interacting protein that functions as a co-activator in
TGFbeta signalling. Nat Cell Biol. 4:181–190. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Chen CR, Kang Y, Siegel PM and Massagué J:
E2F4/5 and p107 as Smad cofactors linking the TGFbeta receptor to
c-myc repression. Cell. 110:19–32. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kang Y, Chen CR and Massagué J: A
self-enabling TGFbeta response coupled to stress signaling: Smad
engages stress response factor ATF3 for Id1 repression in
epithelial cells. Mol Cell. 11:915–926. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wotton D, Knoepfler PS, Laherty CD,
Eisenman RN and Massagué J: The Smad transcriptional corepressor
TGIF recruits mSin3. Cell Growth Differ. 12:457–463.
2001.PubMed/NCBI
|
|
38
|
Akiyoshi S, Inoue H, Hanai J, et al: c-Ski
acts as a transcriptional co-repressor in transforming growth
factor-beta signaling through interaction with smads. J Biol Chem.
274:35269–35277. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Luo K, Stroschein SL, Wang W, et al: The
Ski oncoprotein interacts with the Smad proteins to repress TGFbeta
signaling. Genes Dev. 13:2196–2206. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Stroschein SL, Wang W, Zhou S, Zhou Q and
Luo K: Negative feedback regulation of TGF-beta signaling by the
SnoN onco-protein. Science. 286:771–774. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Sun Y, Liu X, Eaton EN, Lane WS, Lodish HF
and Weinberg RA: Interaction of the Ski oncoprotein with Smad3
regulates TGF-beta signaling. Mol Cell. 4:499–509. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Seoane J, Le HV, Shen L, Anderson SA and
Massagué J: Integration of Smad and forkhead pathways in the
control of neuroepithelial and glioblastoma cell proliferation.
Cell. 117:211–223. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Pardali K, Kurisaki A, Morén A, ten Dijke
P, Kardassis D and Moustakas A: Role of Smad proteins and
transcription factor Sp1 in p21(Waf1/Cip1) regulation by
transforming growth factor-beta. J Biol Chem. 275:29244–29256.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Zhang Y, Feng XH and Derynck R: Smad3 and
Smad4 cooperate with c-Jun/c-Fos to mediate TGF-beta-induced
transcription. Nature. 394:909–913. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Lin X, Liang YY, Sun B, et al: Smad6
recruits transcription corepressor CtBP to repress bone
morphogenetic protein-induced transcription. Mol Cell Biol.
23:9081–9093. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Peng Y, Zhao S, Song L, Wang M and Jiao K:
Sertad1 encodes a novel transcriptional co-activator of SMAD1 in
mouse embryonic hearts. Biochem Biophys Res Commun. 441:751–756.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Izutsu K, Kurokawa M, Imai Y, Maki K,
Mitani K and Hirai H: The corepressor CtBP interacts with Evi-1 to
repress transforming growth factor beta signaling. Blood.
97:2815–2822. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Xi Q, Wang Z, Zaromytidou AI, et al: A
poised chromatin platform for TGF-β access to master regulators.
Cell. 147:1511–1524. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Ross S, Cheung E, Petrakis TG, Howell M,
Kraus WL and Hill CS: Smads orchestrate specific histone
modifications and chromatin remodeling to activate transcription.
EMBO J. 25:4490–4502. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Papageorgis P, Lambert AW, Ozturk S, et
al: Smad signaling is required to maintain epigenetic silencing
during breast cancer progression. Cancer Res. 70:968–978. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Nakao A, Afrakhte M, Morén A, et al:
Identification of Smad7, a TGFbeta-inducible antagonist of TGF-beta
signalling. Nature. 389:631–635. 1997. View
Article : Google Scholar : PubMed/NCBI
|
|
52
|
Itóh S, Landström M, Hermansson A, et al:
Transforming growth factor beta1 induces nuclear export of
inhibitory Smad7. J Biol Chem. 273:29195–29201. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Hayashi H, Abdollah S, Qiu Y, et al: The
MAD-related protein Smad7 associates with the TGFbeta receptor and
functions as an antagonist of TGFbeta signaling. Cell.
89:1165–1173. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Ebisawa T, Fukuchi M, Murakami G, et al:
Smurf1 interacts with transforming growth factor-beta type I
receptor through Smad7 and induces receptor degradation. J Biol
Chem. 276:12477–12480. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Kavsak P, Rasmussen RK, Causing CG, et al:
Smad7 binds to Smurf2 to form an E3 ubiquitin ligase that targets
the TGF beta receptor for degradation. Mol Cell. 6:1365–1375. 2000.
View Article : Google Scholar
|
|
56
|
Zhang S, Fei T, Zhang L, et al: Smad7
antagonizes transforming growth factor beta signaling in the
nucleus by interfering with functional Smad-DNA complex formation.
Mol Cell Biol. 27:4488–4499. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Zhang YE: Non-Smad pathways in TGF-beta
signaling. Cell Res. 19:128–139. 2009. View Article : Google Scholar :
|
|
58
|
Hartsough MT and Mulder KM: Transforming
growth factor beta activation of p44mapk in proliferating cultures
of epithelial cells. J Biol Chem. 270:7117–7124. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Frey RS and Mulder KM: TGFbeta regulation
of mitogen-activated protein kinases in human breast cancer cells.
Cancer Lett. 117:41–50. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Papageorgis P, Cheng K, Ozturk S, et al:
Smad4 inactivation promotes malignancy and drug resistance of colon
cancer. Cancer Res. 71:998–1008. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Finlay GA, Thannickal VJ, Fanburg BL and
Paulson KE: Transforming growth factor-beta 1-induced activation of
the ERK pathway/activator protein-1 in human lung fibroblasts
requires the autocrine induction of basic fibroblast growth factor.
J Biol Chem. 275:27650–27656. 2000.PubMed/NCBI
|
|
62
|
Vinals F and Pouysségur J: Transforming
growth factor beta1 (TGF-beta1) promotes endothelial cell survival
during in vitro angiogenesis via an autocrine mechanism implicating
TGF-alpha signaling. Mol Cell Biol. 21:7218–7230. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Ellenrieder V, Hendler SF, Boeck W, et al:
Transforming growth factor beta1 treatment leads to an
epithelial-mesenchymal transdifferentiation of pancreatic cancer
cells requiring extra-cellular signal-regulated kinase 2
activation. Cancer Res. 61:4222–4228. 2001.PubMed/NCBI
|
|
64
|
Xie L, Law BK, Chytil AM, Brown KA, Aakre
ME and Moses HL: Activation of the Erk pathway is required for
TGF-beta1-induced EMT in vitro. Neoplasia. 6:603–610. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Lee MK, Pardoux C, Hall MC, et al:
TGF-beta activates Erk MAP kinase signalling through direct
phosphorylation of ShcA. EMBO J. 26:3957–3967. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Liao JH, Chen JS, Chai MQ, Zhao S and Song
JG: The involvement of p38 MAPK in transforming growth factor
beta1-induced apoptosis in murine hepatocytes. Cell Res. 11:89–94.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Kimura N, Matsuo R, Shibuya H, Nakashima K
and Taga T: BMP2-induced apoptosis is mediated by activation of the
TAK1-p38 kinase pathway that is negatively regulated by Smad6. J
Biol Chem. 275:17647–17652. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Bakin AV, Rinehart C, Tomlinson AK and
Arteaga CL: p38 mitogen-activated protein kinase is required for
TGFbeta-mediated fibroblastic transdifferentiation and cell
migration. J Cell Sci. 115:3193–3206. 2002.PubMed/NCBI
|
|
69
|
Hocevar BA, Brown TL and Howe PH: TGF-beta
induces fibronectin synthesis through a c-Jun N-terminal
kinase-dependent, Smad4-independent pathway. EMBO J. 18:1345–1356.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Yu L, Hébert MC and Zhang YE: TGF-beta
receptor-activated p38 MAP kinase mediates Smad-independent
TGF-beta responses. EMBO J. 21:3749–3759. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Yamaguchi K, Shirakabe K, Shibuya H, et
al: Identification of a member of the MAPKKK family as a potential
mediator of TGF-beta signal transduction. Science. 270:2008–2011.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Shim JH, Xiao C, Paschal AE, et al: TAK1,
but not TAB1 or TAB2, plays an essential role in multiple signaling
pathways in vivo. Genes Dev. 19:2668–2681. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Sorrentino A, Thakur N, Grimsby S, et al:
The type I TGF-beta receptor engages TRAF6 to activate TAK1 in a
receptor kinase-independent manner. Nat Cell Biol. 10:1199–1207.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Yamashita M, Fatyol K, Jin C, Wang X, Liu
Z and Zhang YE: TRAF6 mediates Smad-independent activation of JNK
and p38 by TGF-beta. Mol Cell. 31:918–924. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Zhang L, Wang W, Hayashi Y, et al: A role
for MEK kinase 1 in TGF-beta/activin-induced epithelium movement
and embryonic eyelid closure. EMBO J. 22:4443–4454. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Kim KY, Kim BC, Xu Z and Kim SJ: Mixed
lineage kinase 3 (MLK3)-activated p38 MAP kinase mediates
transforming growth factor-beta-induced apoptosis in hepatoma
cells. J Biol Chem. 279:29478–29484. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Jaffe AB and Hall A: Rho GTPases:
biochemistry and biology. Annu Rev Cell Dev Biol. 21:247–269. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Bhowmick NA, Ghiassi M, Bakin A, et al:
Transforming growth factor-beta1 mediates epithelial to mesenchymal
transdifferentiation through a RhoA-dependent mechanism. Mol Biol
Cell. 12:27–36. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Edlund S, Landström M, Heldin CH and
Aspenström P: Transforming growth factor-beta-induced mobilization
of actin cytoskeleton requires signaling by small GTPases Cdc42 and
RhoA. Mol Biol Cell. 13:902–914. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Ozdamar B, Bose R, Barrios-Rodiles M, Wang
HR, Zhang Y and Wrana JL: Regulation of the polarity protein Par6
by TGFbeta receptors controls epithelial cell plasticity. Science.
307:1603–1609. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Bakin AV, Tomlinson AK, Bhowmick NA, Moses
HL and Arteaga CL: Phosphatidylinositol 3-kinase function is
required for transforming growth factor beta-mediated epithelial to
mesenchymal transition and cell migration. J Biol Chem.
275:36803–36810. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Shin I, Bakin AV, Rodeck U, Brunet A and
Arteaga CL: Transforming growth factor beta enhances epithelial
cell survival via Akt-dependent regulation of FKHRL1. Mol Biol
Cell. 12:3328–3339. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Hidalgo M and Rowinsky EK: The
rapamycin-sensitive signal transduction pathway as a target for
cancer therapy. Oncogene. 19:6680–6686. 2000. View Article : Google Scholar
|
|
84
|
Lamouille S and Derynck R: Cell size and
invasion in TGF-beta-induced epithelial to mesenchymal transition
is regulated by activation of the mTOR pathway. J Cell Biol.
178:437–451. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Roberts AB and Wakefield LM: The two faces
of transforming growth factor beta in carcinogenesis. Proc Natl
Acad Sci USA. 100:8621–8623. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Tang B, Vu M, Booker T, et al: TGF-beta
switches from tumor suppressor to prometastatic factor in a model
of breast cancer progression. J Clin Invest. 112:1116–1124. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Wakefield LM and Roberts AB: TGF-beta
signaling: positive and negative effects on tumorigenesis. Curr
Opin Genet Dev. 12:22–29. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Siegel PM, Shu W, Cardiff RD, Muller WJ
and Massagué J: Transforming growth factor beta signaling impairs
Neu-induced mammary tumorigenesis while promoting pulmonary
metastasis. Proc Natl Acad Sci USA. 100:8430–8435. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Siegel PM and Massagué J: Cytostatic and
apoptotic actions of TGF-beta in homeostasis and cancer. Nat Rev
Cancer. 3:807–821. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Choi ME and Ballermann BJ: Inhibition of
capillary morphogenesis and associated apoptosis by dominant
negative mutant transforming growth factor-beta receptors. J Biol
Chem. 270:21144–21150. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Hyman KM, Seghezzi G, Pintucci G, et al:
Transforming growth factor-beta1 induces apoptosis in vascular
endothelial cells by activation of mitogen-activated protein
kinase. Surgery. 132:173–179. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Rich JN, Zhang M, Datto MB, Bigner DD and
Wang XF: Transforming growth factor-beta-mediated p15(INK4B)
induction and growth inhibition in astrocytes is SMAD3-dependent
and a pathway prominently altered in human glioma cell lines. J
Biol Chem. 274:35053–35058. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Yang X, Letterio JJ, Lechleider RJ, et al:
Targeted disruption of SMAD3 results in impaired mucosal immunity
and diminished T cell responsiveness to TGF-beta. EMBO J.
18:1280–1291. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Laiho M, DeCaprio JA, Ludlow JW,
Livingston DM and Massagué J: Growth inhibition by TGF-beta linked
to suppression of retinoblastoma protein phosphorylation. Cell.
62:175–185. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Hannon GJ and Beach D: p15INK4B is a
potential effector of TGF-beta-induced cell cycle arrest. Nature.
371:257–261. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Datto MB, Li Y, Panus JF, Howe DJ, Xiong Y
and Wang XF: Transforming growth factor beta induces the
cyclin-dependent kinase inhibitor p21 through a p53-independent
mechanism. Proc Natl Acad Sci USA. 92:5545–5549. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Polyak K, Kato JY, Solomon MJ, et al:
p27Kip1, a cyclin-Cdk inhibitor, links transforming growth
factor-beta and contact inhibition to cell cycle arrest. Genes Dev.
8:9–22. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Pietenpol JA, Stein RW, Moran E, et al:
TGF-beta 1 inhibition of c-myc transcription and growth in
keratinocytes is abrogated by viral transforming proteins with pRB
binding domains. Cell. 61:777–785. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Norton JD: ID helix-loop-helix proteins in
cell growth, differentiation and tumorigenesis. J Cell Sci.
113:3897–3905. 2000.PubMed/NCBI
|
|
100
|
Grotendorst GR: Connective tissue growth
factor: a mediator of TGF-beta action on fibroblasts. Cytokine
Growth Factor Rev. 8:171–179. 1997. View Article : Google Scholar
|
|
101
|
Park K, Kim SJ, Bang YJ, et al: Genetic
changes in the transforming growth factor beta (TGF-beta) type II
receptor gene in human gastric cancer cells: correlation with
sensitivity to growth inhibition by TGF-beta. Proc Natl Acad Sci
USA. 91:8772–8776. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Kim IY, Ahn HJ, Zelner DJ, et al: Genetic
change in transforming growth factor beta (TGF-beta) receptor type
I gene correlates with insensitivity to TGF-beta 1 in human
prostate cancer cells. Cancer Res. 56:44–48. 1996.PubMed/NCBI
|
|
103
|
Markowitz S, Wang J, Myeroff L, et al:
Inactivation of the type II TGF-beta receptor in colon cancer cells
with microsatellite instability. Science. 268:1336–1338. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Riggins GJ, Kinzler KW, Vogelstein B and
Thiagalingam S: Frequency of Smad gene mutations in human cancers.
Cancer Res. 57:2578–2580. 1997.PubMed/NCBI
|
|
105
|
Schutte M, Hruban RH, Hedrick L, et al:
DPC4 gene in various tumor types. Cancer Res. 56:2527–2530.
1996.PubMed/NCBI
|
|
106
|
Eppert K, Scherer SW, Ozcelik H, et al:
MADR2 maps to 18q21 and encodes a TGFbeta-regulated MAD-related
protein that is functionally mutated in colorectal carcinoma. Cell.
86:543–552. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Hahn SA, Hoque AT, Moskaluk CA, et al:
Homozygous deletion map at 18q21.1 in pancreatic cancer. Cancer
Res. 56:490–494. 1996.PubMed/NCBI
|
|
108
|
Hahn SA, Schutte M, Hoque AT, et al: DPC4,
a candidate tumor suppressor gene at human chromosome 18q21.1.
Science. 271:350–353. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Thiagalingam S, Lengauer C, Leach FS, et
al: Evaluation of candidate tumour suppressor genes on chromosome
18 in colorectal cancers. Nat Genet. 13:343–346. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Schwarte-Waldhoff I, Volpert OV, Bouck NP,
et al: Smad4/DPC4-mediated tumor suppression through suppression of
angiogenesis. Proc Natl Acad Sci USA. 97:9624–9629. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Kretzschmar M, Doody J, Timokhina I and
Massagué J: A mechanism of repression of TGFbeta/Smad signaling by
oncogenic Ras. Genes Dev. 13:804–816. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Kretzschmar M, Doody J and Massagué J:
Opposing BMP and EGF signalling pathways converge on the TGF-beta
family mediator Smad1. Nature. 389:618–622. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Massagué J: Integration of Smad and MAPK
pathways: a link and a linker revisited. Genes Dev. 17:2993–2997.
2003. View Article : Google Scholar
|
|
114
|
Gomis RR, Alarcón C, Nadal C, Van Poznak C
and Massagué J: C/EBPbeta at the core of the TGFbeta cytostatic
response and its evasion in metastatic breast cancer cells. Cancer
Cell. 10:203–214. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Padua D, Zhang XH, Wang Q, et al: TGFbeta
primes breast tumors for lung metastasis seeding through
angiopoietin-like 4. Cell. 133:66–77. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Thiery JP: Epithelial-mesenchymal
transitions in tumour progression. Nat Rev Cancer. 2:442–454. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Yang J, Mani SA, Donaher JL, et al: Twist,
a master regulator of morphogenesis, plays an essential role in
tumor metastasis. Cell. 117:927–939. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Xu J, Lamouille S and Derynck R:
TGF-beta-induced epithelial to mesenchymal transition. Cell Res.
19:156–172. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Cano A, Pérez-Moreno MA, Rodrigo I, et al:
The transcription factor snail controls epithelial-mesenchymal
transitions by repressing E-cadherin expression. Nat Cell Biol.
2:76–83. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Savagner P, Yamada KM and Thiery JP: The
zinc-finger protein slug causes desmosome dissociation, an initial
and necessary step for growth factor-induced epithelial-mesenchymal
transition. J Cell Biol. 137:1403–1419. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Eger A, Aigner K, Sonderegger S, et al:
DeltaEF1 is a transcriptional repressor of E-cadherin and regulates
epithelial plasticity in breast cancer cells. Oncogene.
24:2375–2385. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Comijn J, Berx G, Vermassen P, et al: The
two-handed E box binding zinc finger protein SIP1 downregulates
E-cadherin and induces invasion. Mol Cell. 7:1267–1278. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Thuault S, Valcourt U, Petersen M,
Manfioletti G, Heldin CH and Moustakas A: Transforming growth
factor-beta employs HMGA2 to elicit epithelial-mesenchymal
transition. J Cell Biol. 174:175–183. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Mani SA, Yang J, Brooks M, et al:
Mesenchyme Forkhead 1 (FOXC2) plays a key role in metastasis and is
associated with aggressive basal-like breast cancers. Proc Natl
Acad Sci USA. 104:10069–10074. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Derynck R, Akhurst RJ and Balmain A:
TGF-beta signaling in tumor suppression and cancer progression. Nat
Genet. 29:117–129. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Yang J and Weinberg RA:
Epithelial-mesenchymal transition: at the crossroads of development
and tumor metastasis. Dev Cell. 14:818–829. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Zhu B, Fukada K, Zhu H and Kyprianou N:
Prohibitin and cofilin are intracellular effectors of transforming
growth factor beta signaling in human prostate cancer cells. Cancer
Res. 66:8640–8647. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Deckers M, van Dinther M, Buijs J, et al:
The tumor suppressor Smad4 is required for transforming growth
factor beta-induced epithelial to mesenchymal transition and bone
metastasis of breast cancer cells. Cancer Res. 66:2202–2209. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Kang Y and Massagué J:
Epithelial-mesenchymal transitions: twist in development and
metastasis. Cell. 118:277–279. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Grande JP: Role of transforming growth
factor-beta in tissue injury and repair. Proc Soc Exp Biol Med.
214:27–40. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Singer AJ and Clark RA: Cutaneous wound
healing. N Engl J Med. 341:738–746. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Pickup M, Novitskiy S and Moses HL: The
roles of TGFβ in the tumour microenvironment. Nat Rev Cancer.
13:788–799. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Dalal BI, Keown PA and Greenberg AH:
Immunocytochemical localization of secreted transforming growth
factor-beta 1 to the advancing edges of primary tumors and to lymph
node metastases of human mammary carcinoma. Am J Pathol.
143:381–389. 1993.PubMed/NCBI
|
|
134
|
Kingsley LA, Fournier PG, Chirgwin JM and
Guise TA: Molecular biology of bone metastasis. Mol Cancer Ther.
6:2609–2617. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Prud’homme GJ: Pathobiology of
transforming growth factor beta in cancer, fibrosis and immunologic
disease, and therapeutic considerations. Lab Invest. 87:1077–1091.
2007. View Article : Google Scholar
|
|
136
|
Wrzesinski SH, Wan YY and Flavell RA:
Transforming growth factor-beta and the immune response:
implications for anti-cancer therapy. Clin Cancer Res.
13:5262–5270. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Akhurst RJ and Hata A: Targeting the TGFβ
signalling pathway in disease. Nat Rev Drug Discov. 11:790–811.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Flavell RA, Sanjabi S, Wrzesinski SH and
Licona-Limón P: The polarization of immune cells in the tumour
environment by TGFbeta. Nat Rev Immunol. 10:554–567. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Laouar Y, Sutterwala FS, Gorelik L and
Flavell RA: Transforming growth factor-beta controls T helper type
1 cell development through regulation of natural killer cell
interferon-gamma. Nat Immunol. 6:600–607. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Rubtsov YP and Rudensky AY: TGFbeta
signalling in control of T-cell-mediated self-reactivity. Nat Rev
Immunol. 7:443–453. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Mantovani A, Sozzani S, Locati M, Allavena
P and Sica A: Macrophage polarization: tumor-associated macrophages
as a paradigm for polarized M2 mononuclear phagocytes. Trends
Immunol. 23:549–555. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
142
|
Gong D, Shi W, Yi SJ, Chen H, Groffen J
and Heisterkamp N: TGFβ signaling plays a critical role in
promoting alternative macrophage activation. BMC Immunol.
13:312012. View Article : Google Scholar
|
|
143
|
Fridlender ZG, Sun J, Kim S, et al:
Polarization of tumor-associated neutrophil phenotype by TGF-beta:
“N1” versus “N2” TAN. Cancer Cell. 16:183–194. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Yamaguchi Y, Tsumura H, Miwa M and Inaba
K: Contrasting effects of TGF-beta 1 and TNF-alpha on the
development of dendritic cells from progenitors in mouse bone
marrow. Stem Cells. 15:144–153. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
145
|
Ramesh S, Wildey GM and Howe PH:
Transforming growth factor beta (TGFbeta)-induced apoptosis: the
rise & fall of Bim. Cell Cycle. 8:11–17. 2009. View Article : Google Scholar
|
|
146
|
Marcoe JP, Lim JR, Schaubert KL, et al:
TGF-β is responsible for NK cell immaturity during ontogeny and
increased susceptibility to infection during mouse infancy. Nat
Immunol. 13:843–850. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
147
|
Wipff PJ, Rifkin DB, Meister JJ and Hinz
B: Myofibroblast contraction activates latent TGF-beta1 from the
extracellular matrix. J Cell Biol. 179:1311–1323. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
148
|
Wipff PJ and Hinz B: Myofibroblasts work
best under stress. J Bodyw Mov Ther. 13:121–127. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
149
|
Tomasek JJ, Gabbiani G, Hinz B, Chaponnier
C and Brown RA: Myofibroblasts and mechano-regulation of connective
tissue remodelling. Nat Rev Mol Cell Biol. 3:349–363. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
150
|
Karagiannis GS, Poutahidis T, Erdman SE,
Kirsch R, Riddell RH and Diamandis EP: Cancer-associated
fibroblasts drive the progression of metastasis through both
paracrine and mechanical pressure on cancer tissue. Mol Cancer Res.
10:1403–1418. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
151
|
Paszek MJ, Zahir N, Johnson KR, et al:
Tensional homeostasis and the malignant phenotype. Cancer Cell.
8:241–254. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
152
|
Samuel MS, Lopez JI, McGhee EJ, et al:
Actomyosin-mediated cellular tension drives increased tissue
stiffness and β-catenin activation to induce epidermal hyperplasia
and tumor growth. Cancer Cell. 19:776–791. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
153
|
Branton MH and Kopp JB: TGF-beta and
fibrosis. Microbes Infect. 1:1349–1365. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
154
|
Egeblad M, Rasch MG and Weaver VM: Dynamic
interplay between the collagen scaffold and tumor evolution. Curr
Opin Cell Biol. 22:697–706. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
155
|
Smith NR, Baker D, Farren M, et al: Tumor
stromal architecture can define the intrinsic tumor response to
VEGF-targeted therapy. Clin Cancer Res. 19:6943–6956. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
156
|
Stylianopoulos T and Jain RK: Combining
two strategies to improve perfusion and drug delivery in solid
tumors. Proc Natl Acad Sci USA. 110:18632–18637. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
157
|
Stylianopoulos T, Martin JD, Chauhan VP,
et al: Causes, consequences, and remedies for growth-induced solid
stress in murine and human tumors. Proc Natl Acad Sci USA.
109:15101–15108. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
158
|
Demou ZN: Gene expression profiles in 3D
tumor analogs indicate compressive strain differentially enhances
metastatic potential. Ann Biomed Eng. 38:3509–3520. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
159
|
Tse JM, Cheng G, Tyrrell JA, et al:
Mechanical compression drives cancer cells toward invasive
phenotype. Proc Natl Acad Sci USA. 109:911–916. 2012. View Article : Google Scholar :
|
|
160
|
Chauhan VP, Martin JD, Liu H, et al:
Angiotensin inhibition enhances drug delivery and potentiates
chemotherapy by decompressing tumor blood vessels. Nat Commun.
4:25162013. View Article : Google Scholar
|
|
161
|
Facciabene A, Peng X, Hagemann IS, et al:
Tumour hypoxia promotes tolerance and angiogenesis via CCL28 and
T(reg) cells. Nature. 475:226–230. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
162
|
Wilson WR and Hay MP: Targeting hypoxia in
cancer therapy. Nat Rev Cancer. 11:393–410. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
163
|
Jain RK, Martin JD and Stylianopoulos T:
The role of mechanical forces in tumor growth and therapy. Annu Rev
Biomed Eng. 16:321–346. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
164
|
Jain RK and Stylianopoulos T: Delivering
nanomedicine to solid tumors. Nat Rev Clin Oncol. 7:653–664. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
165
|
Chauhan VP and Jain RK: Strategies for
advancing cancer nanomedicine. Nat Mater. 12:958–962. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
166
|
Popovi Z, Liu W, Chauhan VP, et al: A
nanoparticle size series for in vivo fluorescence imaging. Angew
Chem Int Ed Engl. 49:8649–8652. 2010. View Article : Google Scholar
|
|
167
|
Stylianopoulos T, Poh MZ, Insin N, et al:
Diffusion of particles in the extracellular matrix: the effect of
repulsive electrostatic interactions. Biophys J. 99:1342–1349.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
168
|
Zhong Z, Carroll KD, Policarpio D, et al:
Anti-transforming growth factor beta receptor II antibody has
therapeutic efficacy against primary tumor growth and metastasis
through multi-effects on cancer, stroma, and immune cells. Clin
Cancer Res. 16:1191–1205. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
169
|
Uhl M, Aulwurm S, Wischhusen J, et al:
SD-208, a novel transforming growth factor beta receptor I kinase
inhibitor, inhibits growth and invasiveness and enhances
immunogenicity of murine and human glioma cells in vitro and in
vivo. Cancer Res. 64:7954–7961. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
170
|
Kim S, Buchlis G, Fridlender ZG, et al:
Systemic blockade of transforming growth factor-beta signaling
augments the efficacy of immunogene therapy. Cancer Res.
68:10247–10256. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
171
|
Chakrabarti R, Subramaniam V, Abdalla S,
Jothy S and Prud’homme GJ: Tranilast inhibits the growth and
metastasis of mammary carcinoma. Anticancer Drugs. 20:334–345.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
172
|
Achyut BR, Bader DA, Robles AI, et al:
Inflammation-mediated genetic and epigenetic alterations drive
cancer development in the neighboring epithelium upon stromal
abrogation of TGF-β signaling. PLoS Genet. 9:e10032512013.
View Article : Google Scholar
|
|
173
|
Bragado P, Estrada Y, Parikh F, et al:
TGF-β2 dictates disseminated tumour cell fate in target organs
through TGF-β-RIII and p38α/β signalling. Nat Cell Biol.
15:1351–1361. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
174
|
Biswas T, Gu X, Yang J, Ellies LG and Sun
LZ: Attenuation of TGF-β signaling supports tumor progression of a
mesenchymal-like mammary tumor cell line in a syngeneic murine
model. Cancer Lett. 346:129–138. 2014. View Article : Google Scholar
|
|
175
|
Stockmann C, Doedens A, Weidemann A, et
al: Deletion of vascular endothelial growth factor in myeloid cells
accelerates tumorigenesis. Nature. 456:814–818. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
176
|
Rhim AD, Mirek ET, Aiello NM, et al: EMT
and dissemination precede pancreatic tumor formation. Cell.
148:349–361. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
177
|
Diop-Frimpong B, Chauhan VP, Krane S,
Boucher Y and Jain RK: Losartan inhibits collagen I synthesis and
improves the distribution and efficacy of nanotherapeutics in
tumors. Proc Natl Acad Sci USA. 108:2909–2914. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
178
|
Wilop S, von Hobe S, Crysandt M, Esser A,
Osieka R and Jost E: Impact of angiotensin I converting enzyme
inhibitors and angiotensin II type 1 receptor blockers on survival
in patients with advanced non-small-cell lung cancer undergoing
first-line platinum-based chemotherapy. J Cancer Res Clin Oncol.
135:1429–1435. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
179
|
Keizman D, Huang P, Eisenberger MA, et al:
Angiotensin system inhibitors and outcome of sunitinib treatment in
patients with metastatic renal cell carcinoma: a retrospective
examination. Eur J Cancer. 47:1955–1961. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
180
|
Nakai Y, Isayama H, Ijichi H, et al: Phase
I trial of gemcitabine and candesartan combination therapy in
normotensive patients with advanced pancreatic cancer: GECA1.
Cancer Sci. 103:1489–1492. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
181
|
Liu J, Liao S, Diop-Frimpong B, et al:
TGF-β blockade improves the distribution and efficacy of
therapeutics in breast carcinoma by normalizing the tumor stroma.
Proc Natl Acad Sci USA. 109:16618–16623. 2012. View Article : Google Scholar
|
|
182
|
Kozono S, Ohuchida K, Eguchi D, et al:
Pirfenidone inhibits pancreatic cancer desmoplasia by regulating
stellate cells. Cancer Res. 73:2345–2356. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
183
|
Bouquet F, Pal A, Pilones KA, et al: TGFβ1
inhibition increases the radiosensitivity of breast cancer cells in
vitro and promotes tumor control by radiation in vivo. Clin Cancer
Res. 17:6754–6765. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
184
|
Zhang M, Kleber S, Röhrich M, et al:
Blockade of TGF-β signaling by the TGFβR-I kinase inhibitor
LY2109761 enhances radiation response and prolongs survival in
glioblastoma. Cancer Res. 71:7155–7167. 2011. View Article : Google Scholar : PubMed/NCBI
|