Introduction
Lung cancer, the leading cause of cancer related
deaths, is divided into small cell lung carcinoma (SCLC) and
non-small cell lung carcinoma (NSCLC). NSCLC accounts for 85% of
all lung cancer cases, moreover, ~70% of cases are at an advanced
stage with unresectable tumors (1–3).
Radiotherapy is routinely used for treatment of lung cancer.
Ionizing radiation (IR) damage, which is caused indirectly by
radiolysis of intracellular water, leads to formation of ROS. It
has been confirmed that ROS plays a main role in the cytotoxic
action after IR. Excessive ROS results in oxidative stress that
attacks biological macromolecules and leads to cell deaths
(4). Cancer cells possess
antioxidant systems results in low endogenous ROS levels and
protect cells from the cytotoxic effects of radiation. The capacity
of cancer cells is superior to those of normal cells, which leads
to the radio-resistance of cancer cells and radiotherapy failure
(5).
Nrf2 is a crucial transcription factor regulating
the expression of numerous antioxidant genes (6–9).
Under basal conditions, Nrf2 is located in the cytoplasm, where it
is sequestered by its inhibitor Kelch-like ECH-associated protein 1
(Keap1) (10–12). Under oxidative stress, however,
oxidative modification of Keap1 allows Nrf2 to release from Keap1,
and then Nrf2 translocates into the nucleus. Once in the nucleus,
Nrf2 binds the antioxidant response element (ARE) and drives the
expression of several downstream genes such as γ-glutamyl cysteine
synthetase modifier subunit (GCLm), GCLC, HO-1 and NQO1 (13–16).
Both loss of function mutations in Keap1 and gain of function
mutation in Nrf2 lead to Nrf2 overexpression. Accumulated clues
show that Nrf2 is overexpressed in various types of cancer cells,
including lung cancer, esophageal squamous cancer and skin cancer.
Constitutive Nrf2 activation has been implicated in the resistance
of cancer cells to radiation therapy (10). Nrf2 has been reported to cross-talk
with other pathways. Specifically, Nrf2 strongly regulated Notch1
activity. The Notch signaling is involved in proliferation,
differentiation and cell decisions (17–20).
Numerous studies have demonstrated that Notch signaling plays a
critical role in cancer cells (21–24).
It has been reported that Notch1 mediates radio-resistance of
glioma stem cells and loss of Notch sensitizes those cells to
ionizing radiation (25). However,
whether Nrf2 mediated Notch1 downregulation plays a role in
response to ionizing radiation remains elusive.
Thus, the blocking of antioxidant responses could
increase apoptotic death after radiotherapy. This study
investigated the mechanism of the knockdown of Nrf2 enhancing
radiation-induced apoptosis. We identified that Notch1 is strongly
regulated by Nrf2 in response to IR and revealed that Nrf2 enhances
radiation-induced apoptosis through downregulating the expression
of Notch.
Materials and methods
Cell cultures
The human lung cancer cells (A549, NCI-H1299
(H1299), NCI-H460 (H460) were cultured in RPMI-1640 (Gibco Life
Technologies, Carsbad, CA, USA) medium supplemented with 10% fetal
bovine serum (Hyclone, GE Healthcare Life Sciences, Logan, UT, USA)
and incubated at 37°C in a humidified air containing 5%
CO2.
Exposure to ionizing radiation
Cells were exposed to ionizing radiation at room
temperature using Faxitron RX-650 X-rays (Faxitron Bioptics, LLC,
USA). The dose rates were 0.765 Gy/min.
Transfection
siRNA against Nrf2, Notch1 and non-targeting
negative control siRNA were purchased from Invitrogen (Invitrogen
Life Technologies, Carlsbad, CA, USA). Cells were seeded onto new
plates one day prior to transfection. Transfection reagent was
performed with Lipofectamine 2000 (Invitrogen Life Technologies),
following the manufacturer's protocol. The serum-free medium was
replaced with new culture medium for 6 h after transfection.
RNA isolation and reverse
transcription
Total RNA was extracted from cultured cells by using
TRIzol reagent (Takara Biotech Co., Ltd.) and reverse transcription
was carried out using a PrimeScript RT Master Mix (Takara Biotech
Co., Ltd.) in a total volume of 20 μl.
Real-time fluorescent quantitative
PCR
Quantitative PCR was carried out with SYBR Premix Ex
Taq II (Takara Biotech Co., Ltd.), 50 ng DNA and 10 μM of each of
the following primer pairs: Nrf2, 5′-AGCCCAGCACATCCAGTCA-3′
(forward) and 5′-TGCATGCAGTCATCAAAGTACAAAG-3′ (reverse), Hes1,
5′-GTGTCAACACGACACCGGATAAAC-3′ (forward) and
5′-CAGAATGTCCGCCTTCTCCAG-3′ (reverse), β-actin,
5′-TGGCACCCAGCACAATGAA-3′ (forward) and
5′-CTAAGTCATAGTCCGCCTAGAAGCA-3′ (reverse), β-actin for coding
genes. The reaction was conducted on an FTC-3000 qPCR system
(Shanghai Funglyn Biotech Co., Ltd.), with the following cycling
conditions: 95°C for 30 sec, 40 cycles of 95°C for 5 sec and 59°C
for 30 sec. The expression of genes of interest was normalized to
that of β-actin in all samples. Relative quantification approach
(ΔΔCt) was used to calculate the fold change.
Measurement of ROS generation
Intracellular ROS levels were assessed using 10 μM
2′,7′-dichlorofluorescin diacetate (DCFH-DA; Molecular Probes,
Sigma), as described previously (26). Briefly, cells were treated for 30
min at 37°C in the dark. After incubation, cells were gently washed
with PBS to remove the dye. Samples were observed with confocal
microscopy (LSM700; Carl Zeiss) or harvested by trypsinization,
washed, resuspended in 1 ml of PBS, mean intensity was measured at
an excitation wavelength of 488 nm and an emission wavelength of
525 nm using multimode reader (Thermo Varioskan Flash 3001).
Western blot analysis
Cells were washed with cold PBS and lysed with RIPA
buffer (Beyotime, Haimen, China) with protease inhibitor cocktail.
Cells were harvested for 30 min on ice and collected at 10,000 g
for 15 min at 4°C. The total concentrations of samples were
measured using BCA protein assay kit (Pierce, Rockford, IL, USA).
Equal amounts of protein were loaded onto 10% SDS-PAGE and proteins
were transferred to an immobilon PVDF membrane (Roche). The
membranes were then blocked with 0.05% Tween and 5% BSA (BBI Life
Sciences Corp., Canada) in Tris-buffered saline for 2 h at room
temperature and incubated overnight at 4°C with primary antibodies
against Nrf2, GCLC, HO-1, NQO1, Bax, Bcl-2, cleaved (active)
caspase-3, and PARP-1 cleavage (1:500, Cell Signaling Technology,
Danvers, MA, USA). The preparative membranes were reacted with
appropriate secondary antibodies conjugated to HRP. The
immunological complexes were visualized with
electrochemiluminescence (Millipore, Darmstadt, Germany). Band
intensities were analyzed by ImageJ software.
Hoechst 33258 staining
Cells were washed twice with PBS and fixed with 4%
paraformaldehyde for 20 min at room temperature. After washing
twice with PBS, fixed cells were stained with Hoechst 33258 and
incubated for 10 min in the dark and then washed with PBS.
Apoptotic cells were identified by condensation and fragmentation
of nuclei examined by fluorescence microscopy. Apoptotic cells were
counted using cellprofiler 2.1.1 software (Broad Institute,
Cambridge, MA, USA).
Immunofluorescence staining
Cells were pretreated 24 h with siRNA targeting Nrf2
and then exposed to 4 Gy of X-ray radiation. The medium was
aspirated followed by three PBS washes. Using 4% paraformaldehyde,
cells were fixed onto cover slips for 30 min at room temperature.
Cells were rinsed three times with PBS and incubated with 0.5%
Triton X-100 for 10 min. The cells were washed three times and
blocked with 0.1% BSA in PBS for 1 h. Primary anti-Nrf2 antibody
(Cell Signaling Technology) was added and incubated overnight at
4°C. After three washes with PBS, the cells were incubated for 1 h
at room temperature with the appropriate secondary antibody. After
PBS washes, the slides were incubated with 0.5 mg/ml DAPI
(4′,6′-diamidino-2-phenylindole) at room temperature for 5 min. All
images were observed under a confocal microscope equipped with a
digital camera (LSM700; Carl Zeiss).
Statistical analysis
Results are presented as mean ± standard deviation
(mean ± SD). The data were analyzed using Student's t-tests. A
p-value of <0.05 was considered statistically significant.
Results
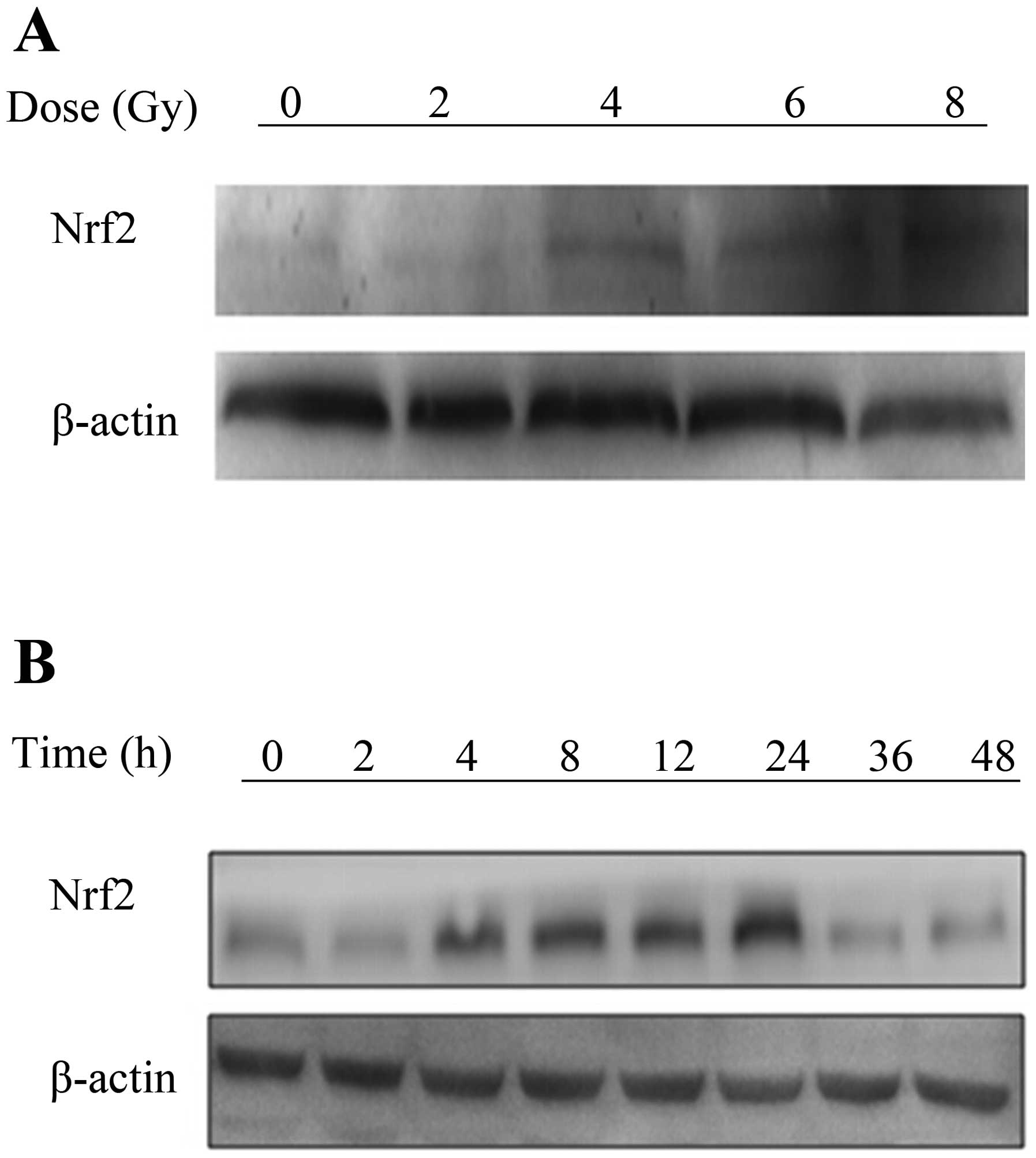
Nrf2 is induced by ionizing radiation in
A549 cells
Nrf2 is induced by external stimulation (15), such as IR. To investigate the
effect of IR on Nrf2, A549 cells were irradiated with varying doses
of X-rays radiation. The protein expression levels of Nrf2 were
measured by western blot analysis. The results showed that Nrf2 was
induced in a dose-dependent manner from 4 to 8 Gy. Furthermore, the
expression of Nrf2 was increasingly elevated from 4 to 24 h after a
single dose of 4 Gy (Fig. 1A and
B). The effective dose was 4 Gy and the maximal effect was
observed at 24 h, therefore, we chose these parameters for our
further experiments.
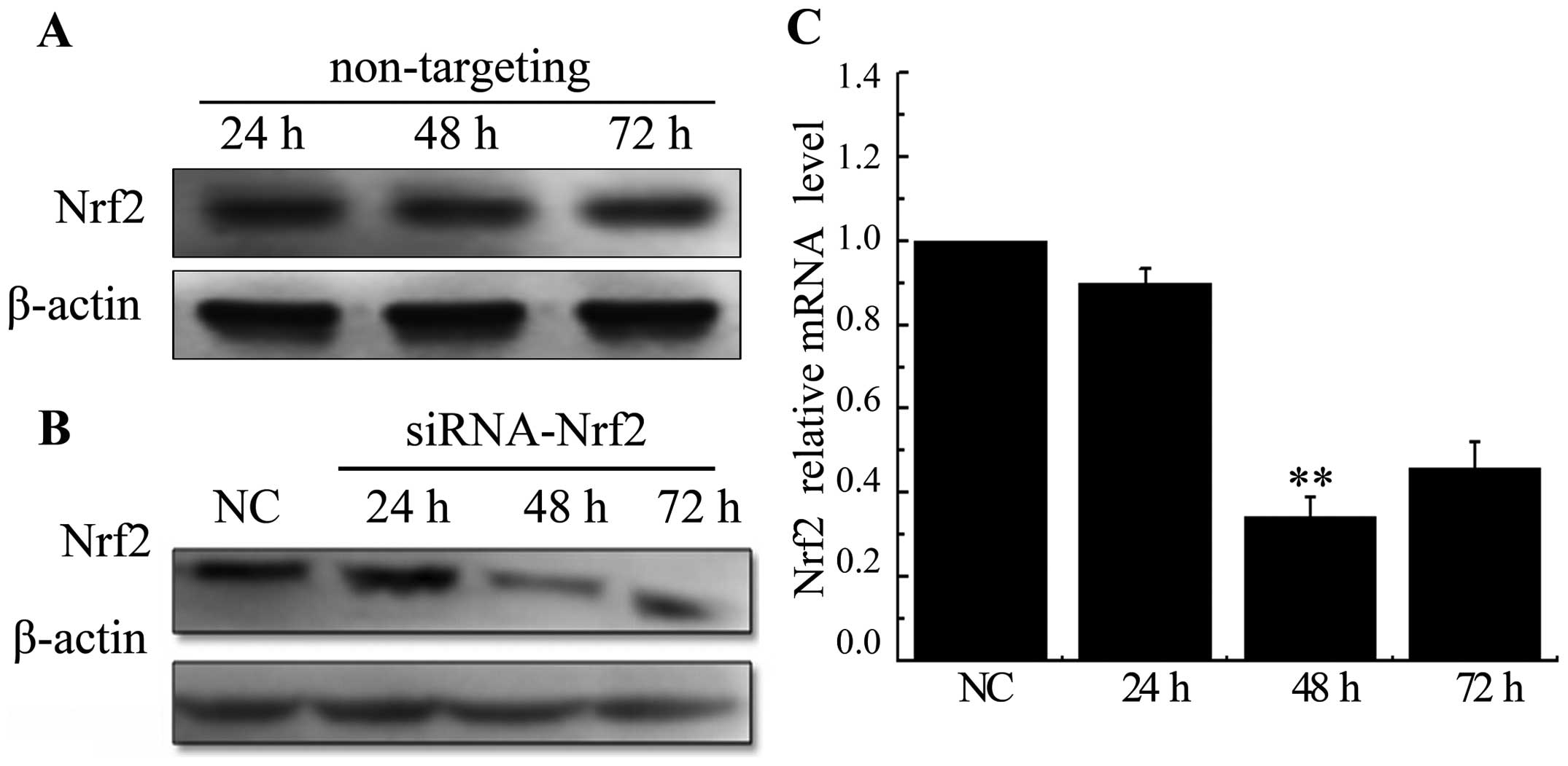
Knockdown of Nrf2 decreases
radiation-upregulated Nrf2 in A549 cells
A549 cells were treated with non-targeting siRNA or
siRNA specific to Nrf2 at 24, 48 and 72. There were no significant
changes in expression levels of the Nrf2 in cells transfected with
a scrambled siRNA sequence (Fig.
2A). Whereas, both protein and mRNA levels were significantly
decreased after being transfected with siRNA targeting Nrf2
(Fig. 2B and C). These results
conveyed that the selected siRNA efficiently knocked down the
expression of Nrf2, especially at 48 h time-point. Inhibition of
Nrf2 by siRNA significantly decreased radiation-induced Nrf2
expression at mRNA levels (Fig.
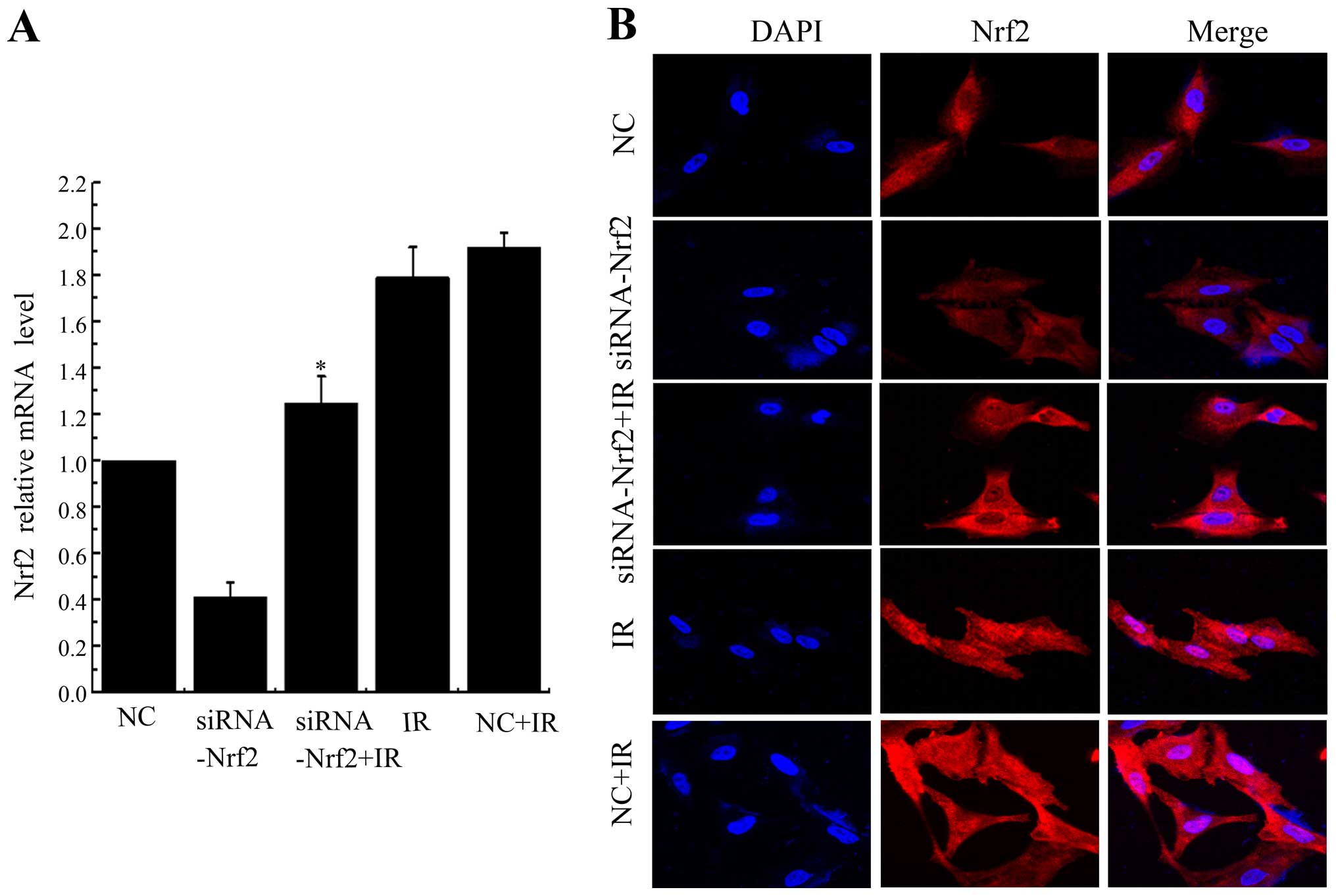
3A). Nrf2 was localized at both the cytoplasm and the nucleus
in control cells, but IR prominently stimulated translocation of
Nrf2 into the nucleus. Knockdown of Nrf2 significantly attenuated
IR-induced Nrf2 nuclear translocation (Fig. 3B). Taken together, these results
indicated that IR induced Nrf2 nuclear localization and this effect
could be counteracted by Nrf2 knockdown.
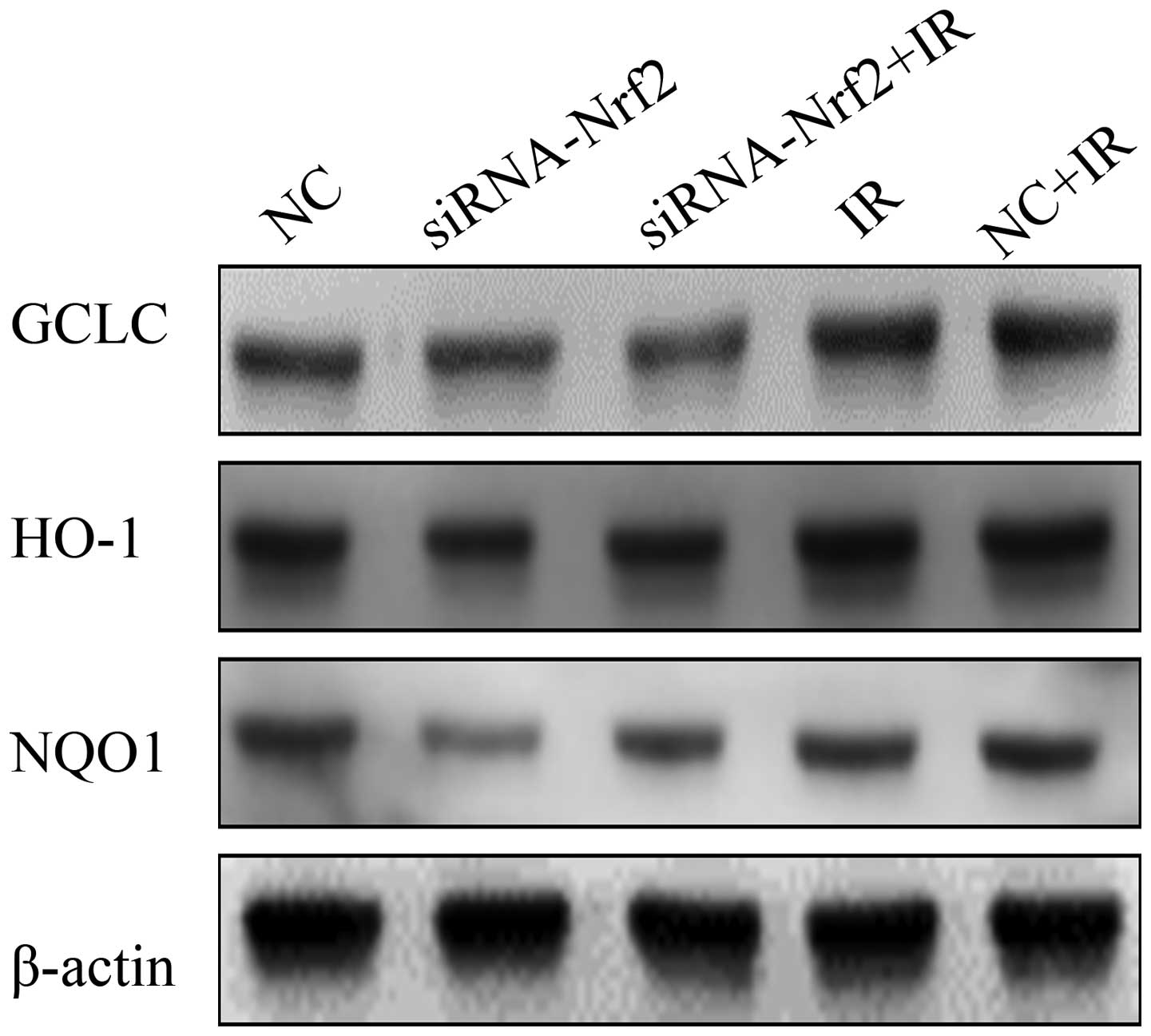
Suppression of Nrf2 on the redox
status
To further confirm the inhibitory effect of Nrf2
knockdown on antioxidant responses in A549 cells, we measured the
function of the Nrf2 knockdown (Fig.
4). Notably, IR significantly increased Nrf2 target proteins
GCLC, HO-1 and NQO1 expression in A549 lung cancer cells. Whereas,
lowering Nrf2 expression levels led to a significantly decreased
expression of Nrf2 target proteins GCLC, HO-1 and NQO1. These
results showed that RNAi-mediated reduction of Nrf2, at least
partly, decreasing the levels of antioxidant proteins induced by
IR.
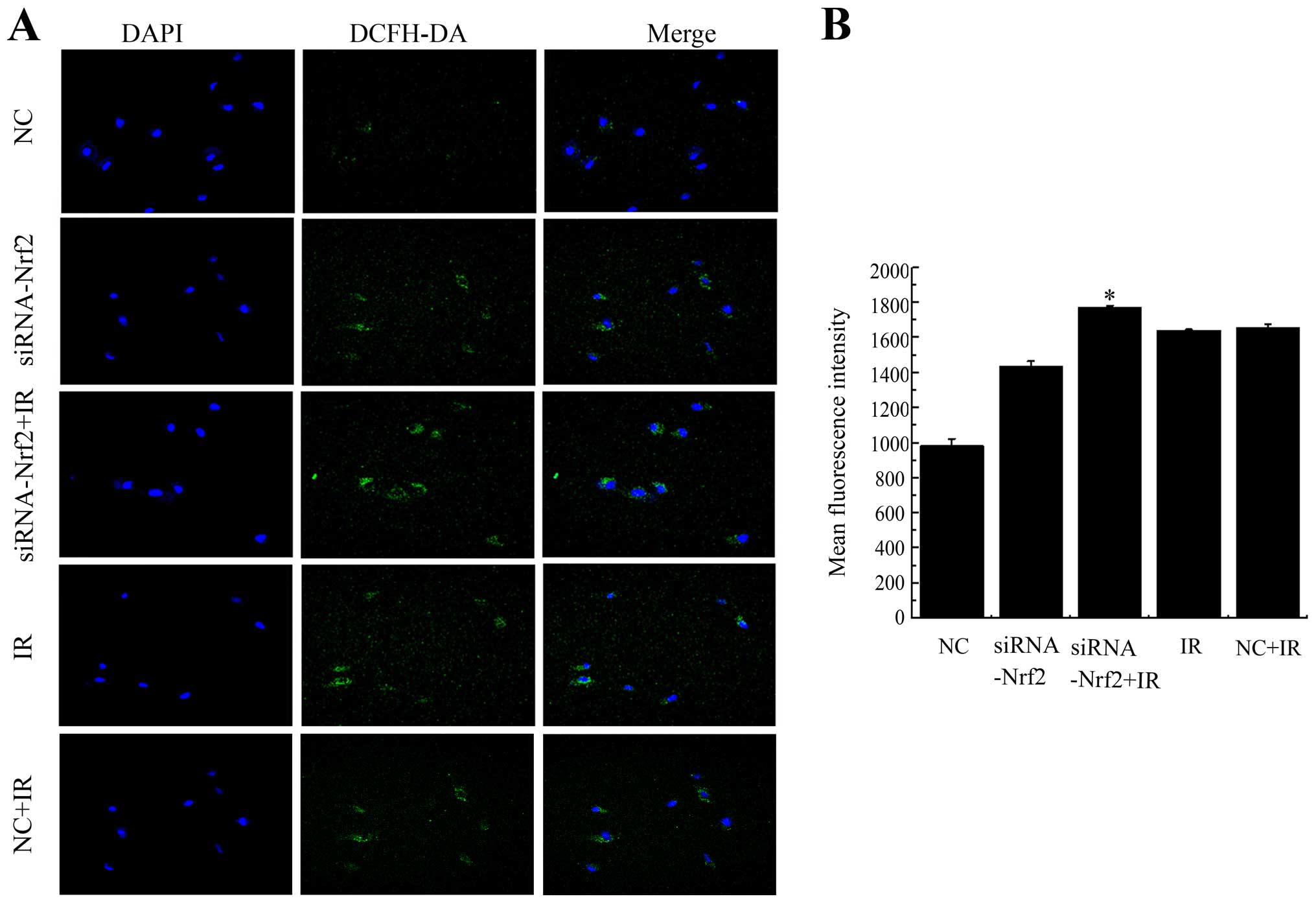
Knockdown of Nrf2 increases ROS
accumulation after exposure
To determine whether the decrease in Nrf2 function
increased ROS generation after exposure to IR, intracellular ROS
levels were monitored using the fluorescent indicator DCFH-DA at 24
h post irradiation in A549 cells. Knockdown of Nrf2 distinctly
increased ROS after X-ray radiation as seen in confocal images and
the mean fluorescent intensity (MFI) compared with NC+IR (Fig. 5). To some extent, lowering Nrf2
also effected the generation of ROS. Exposure to IR combined with
knockdown of Nrf2 further enhanced the ROS production and increased
the MFI compared to unirradiated cells. These results suggested
that decreased Nrf2 activity enhanced the generation of ROS after
irradiation.
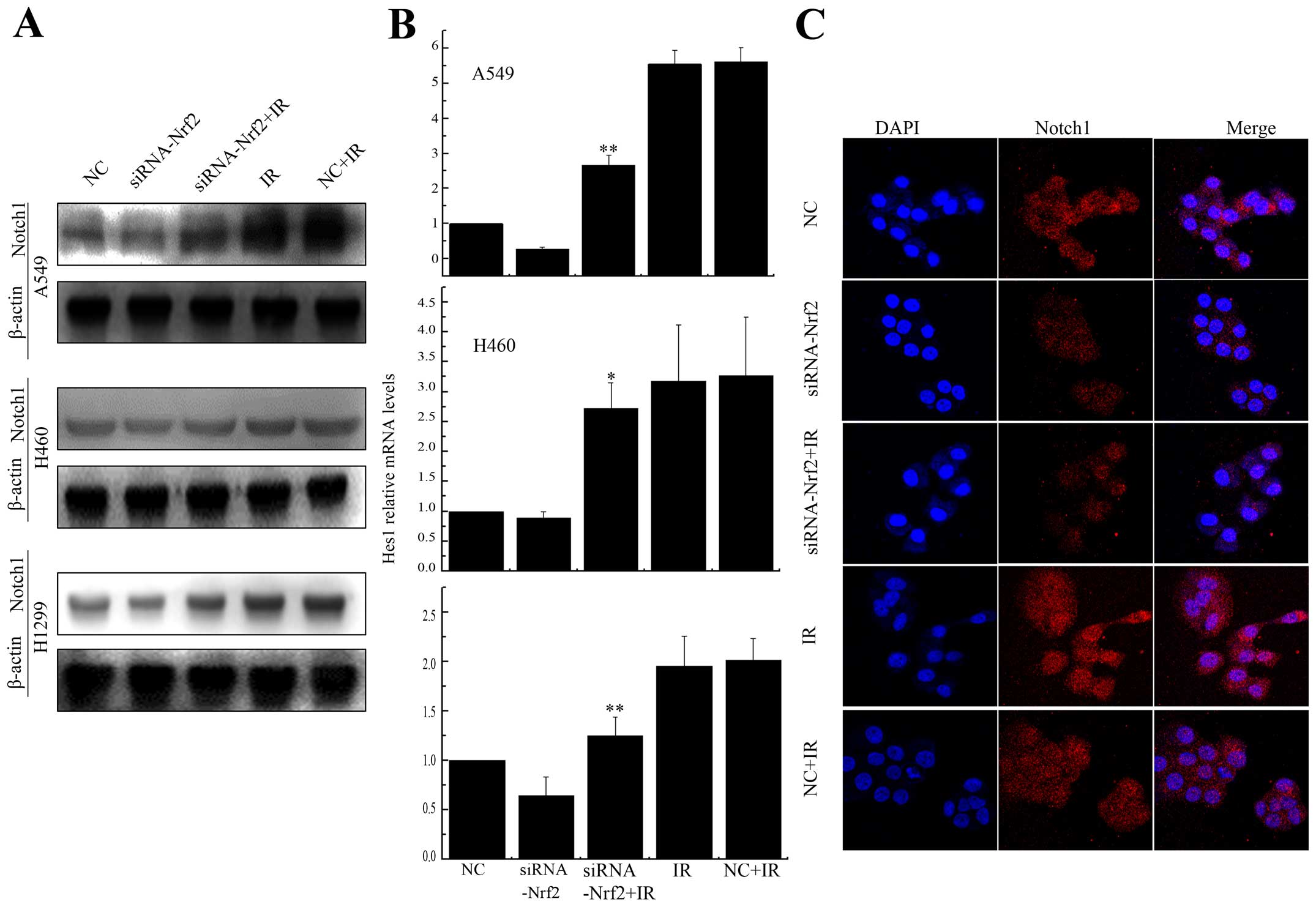
Knockdown of Nrf2 decreased Notch1
expression after IR
Previous studies have shown cross-talk between Nrf2
and the Notch pathway (27–31).
To test whether Notch1 expression could be regulated by Nrf2 after
irradiation, we treated A549 and H460 cells, which express Keap1
protein with different domain mutations, and H1299 cells, which
express no mutation in Keap1. The expression of Notch1 was
upregulated after radiation, while, in Nrf2 knockdown cells, Notch1
was debilitated (Fig. 6A).
Furthermore, the expression of Hes1, a downstream gene in the
Notch1 pathway (32), was reduced
substantially after X-rays radiation in Nrf2 knockdown cells
(Fig. 6B). We also demonstrated
Notch1 was accumulated in A549 cells, but was reduced in Nrf2
expressing cells after irradiation. Collectively, these findings
confirmed that Notch1 activation was abolished by the knockdown of
Nrf2 in A549, H460 and H1299 cells.
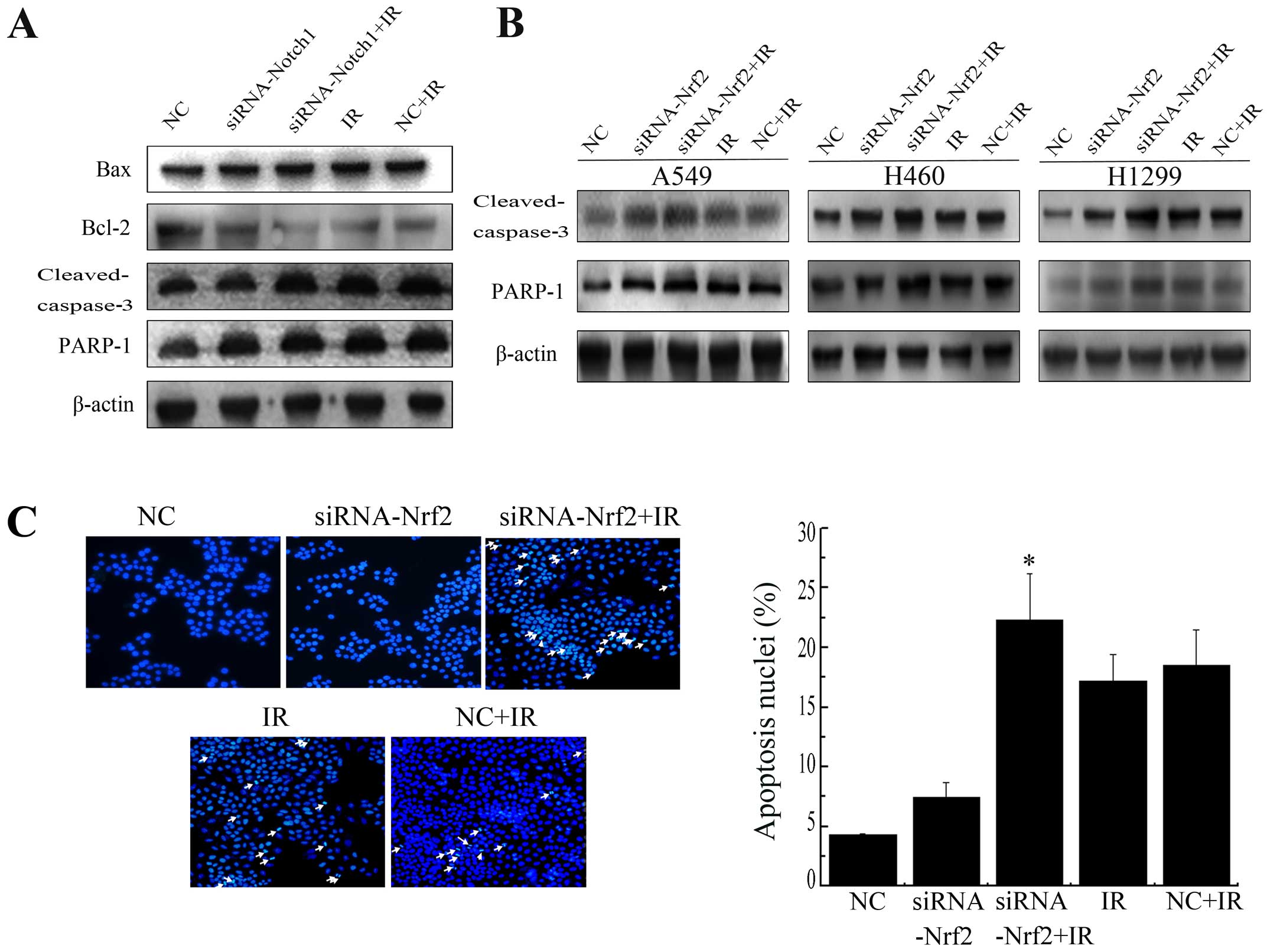
Decrease in Nrf2 and Notch1 increases
IR-induced apoptosis
Bcl-2 family members, caspase-3 and PARP-1 are
crucial mediators of apoptosis. To determine whether downregulation
of Nrf2 and Notch1 following radiation induce apoptosis, we
examined the expression of these mediators. Knockdown of Notch1
induced a sharp increase in the protein level of Bax, cleaved
caspase-3 and PARP-1 cleavage and a sharp decrease in the protein
level of Bcl-2 after IR in A549 cells (Fig. 7A). Similarly, knockdown of Nrf2
enhanced radiation-induced cleaved caspase-3 and PARP-1 cleavage
expression in all three cell lines (Fig. 7B). Furthermore, the nuclear
morphological changes were also measured. Apoptotic cells showed a
number of common features, such as cell shrinkage, nuclear
condensation, and formation of pyknotic bodies of condensed
chromatin. Suppression of Nrf2 resulted in a pronounced increase in
cellular apoptosis after irradiation compared with NC+IR alone
(Fig. 7C). Together, these
findings suggest that lowering Nrf2 expression clearly facilitated
IR induced apoptosis in NSCLC cell lines.
Discussion
The Keap1-Nrf2 signaling is the main cellular
antioxidant system that regulates a broad spectrum of
cytoprotective gene expression against oxidative injure,
inflammation and apoptosis (33–36).
Keap1 negatively regulates Nrf2 activity by promoting proteasomal
degradation of Nrf2. Several reports demonstrated that Keap1 is
present in somatic mutations leading to aberrant constitutive Nrf2
activation in NSCLC cells (37–39).
Activation of the Nrf2 pathway has been reported to mediate the
radio-resistance of lung cancer (40). However, Notch1 regulates cell
proliferation, invasion and apoptosis and its dysregulation leads
to lung cancer initiation (41)
and progression (42). Herein, we
demonstrated that Nrf2 regulated the Notch signaling of NSCLC
cells. The important finding was that Nrf2 and Notch1 promoted lung
cancer cells to apoptosis in coordination.
Nrf2 can be stimulated by ionizing radiation.
Several reports have shown that Nrf2 can be activated by
137Cs exposure only after five day in MCF7 cell line
(40) and was also induced at 0.1
Gy in Raw 264.7 cells (43).
Recently, Nrf2 activation was observed within 6 h in H1299 cells
(37). Various types of cells
affect the activation of Nrf2. In this study, our results showed a
dose-dependent increase of Nrf2 expression after irradiation and
the minimum radiation dose inducing accumulation of Nrf2 was 4 Gy.
The level of Nrf2 increased at 4–24 h and was strongly elevated at
24 h after exposure to 4 Gy X-rays in A549 cells.
It is well accepted that IR enhance cell apoptosis,
which is a major type of cell death. IR produces substantial
amounts of intracellular ROS. In cancer cells, it is believed that
ROS induced genetic instability and is associated with oncogenic
transformation (44). However, ROS
must be eliminated to some extent, to avoid potential damage to
DNA, and various cellular responses, including apoptosis and death.
Diehn et al reported that enhanced ROS scavenger results in
low ROS levels, and tumor radio-resistance (45). GCLC, HO-1 and NQO1 are
Nrf2-regulated genes and protect against oxidative and xenobiotic
stress by mopping up ROS (46–49).
In our study, inhibition of Nrf2 significantly attenuated
IR-induced Nrf2 nuclear translocation and decreased GCLC, HO-1 and
NQO1 expression after irradiation. Our results indicated that
decreased level of GCLC, HO-1 and NQO1 lead to increased ROS levels
through weak antioxidant capacity. In response to external stimuli,
a series of events lead to the stabilization of Nrf2 and its
translocation into the nucleus, where Nrf2 exerts its function and
controls the expression of antioxidants. However, antioxidants
could also regulate Nrf2 activation through feedback effects
(50). In a Keap1 mutant cell
line, Keap1 hardly regulates Nrf2 activation, and Nrf2 protein
levels were high. When introducing exogenous siRNAs, Nrf2 is
silenced and few Nrf2 activated, which suggest that Nrf2 nuclear
translocation is at low level.
Nrf2 can cross-talk with other critical molecular
pathways. Notch signaling is a candidate. Notch pathway influences
cell fate determination (21,51),
and its activation is a mechanism of radiation resistance in breast
cancer (52). Knockdown of Notch1
inhibited the self-renewal capacity in glioma cells (53,54),
induced apoptosis in NSCLC (41),
and significantly increased cell death after X-ray irradiation
(25). Interestingly, Notch1
signaling could be regulated by Nrf2 (16,27,28,30).
Several reports have showed that Notch1 expression was reduced in
Nrf2 knockout cells (27,28). Nrf2 activated Notch1 signaling and
regulated the repopulating capacity of hematopoietic stem
progenitor cell (55). Thus, we
speculated that there exists a mechanism with a different type of
Nrf2 expressing lung cancer cells. For example, A549 and H460 cell
lines express high level of Nrf2 (16,39),
while H1299 express wild-type Keap1 (37). Our results confirmed our
speculation that decrease of Nrf2 mitigates the expression of
Notch1 in NSCLC. Notch1 dramatically accumulates after exposure to
X-rays. Loss of Nrf2 also reduced radiation-induced Notch1 and Hes1
activation. Our results showed that Notch1 ablation or IR activated
the expression of cleaved caspase-3, PARP-1 and pro-apoptotic
protein Bax, and decreased the expression of anti-apoptotic protein
Bcl-2. Knockdown of Notch1 in A549 cells strongly increased the
apoptosis proteins after irradiation. This indicated that loss of
Notch1 increased irradiation-induced apoptosis. Similarly, lowering
Nrf2 expression induced apoptosis after exposure through increased
production of ROS (16,56). RNAi-mediated reduction of Nrf2
could effectively promote cleaved caspase-3 and PARP-1 cleavage
activation compared with IR alone in NSCLC cells. Nrf2-Notch
pathway is a conserved pathway that is designed to allow cells to
respond to the challenge of IR. It coincides with substantial
research results, ROS, as inducer of Notch signaling, could
activate Nrf2 signaling, which might play a dual trigger role in
Nrf2-Notch axis (57–59). It is reasonable to suggest that
knockdown of Nrf2 induced production of ROS, thus promoted cellular
apoptosis. Downregulation of Nrf2 reduced Notch1 expression, Notch1
also facilitated apoptosis. The double regulation impelled cells to
undergo apoptosis.
No information exists regarding the role of Notch in
Nrf2 knockdown in NSCLC cells under irradiation. Further
investigation to examine the association between Nrf2 and Notch1 is
required.
In conclusion, we have shown that knockdown of Nrf2
facilitated ROS generation by blocking cellular antioxidant
abilities. Suppression of Nrf2 shows weaker expression of Notch1.
These synergistic effect promoted IR-induced apoptosis. This study
contributes to future strategies against lung cancer.
Acknowledgements
This study was supported by the Key Program of
National Natural Science Foundation of China (U1432248) and the
National Natural Science Foundation of China (11105203,
11305224).
References
|
1
|
Molina JR1, Yang P, Cassivi SD, Schild SE
and Adjei AA: Non-small cell lung cancer: Epidemiology, risk
factors, treatment, and survivorship. Mayo Clin Proc. 83:584–594.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ferlay J, Steliarova-Foucher E,
Lortet-Tieulent J, Rosso S, Coebergh JW, Comber H, Forman D and
Bray F: Cancer incidence and mortality patterns in Europe:
Estimates for 40 countries in 2012. Eur J Cancer. 49:1374–1403.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bareschino MA, Schettino C, Rossi A,
Maione P, Sacco PC, Zeppa R and Gridelli C: Treatment of advanced
non small cell lung cancer. J Thorac Dis. 3:122–133. 2011.
|
|
4
|
Hensley K and Floyd RA: Reactive oxygen
species and protein oxidation in aging: A look back, a look ahead.
Arch Biochem Biophys. 397:377–383. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nadkar A, Pungaliya C, Drake K, Zajac E,
Singhal SS and Awasthi S: Therapeutic resistance in lung cancer.
Expert Opin Drug Metab Toxicol. 2:753–777. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kensler TW and Wakabayashi N: Nrf2: Friend
or foe for chemo-prevention? Carcinogenesis. 31:90–99. 2010.
View Article : Google Scholar :
|
|
7
|
Kovac S, Angelova PR, Holmström KM, Zhang
Y, Dinkova-Kostova AT and Abramov AY: Nrf2 regulates ROS production
by mitochondria and NADPH oxidase. Biochim Biophys Acta.
1850:794–801. 2015. View Article : Google Scholar :
|
|
8
|
Zhou R, Lin J and Wu D: Sulforaphane
induces Nrf2 and protects against CYP2E1-dependent binge
alcohol-induced liver steatosis. Biochim Biophys Acta.
1840:209–218. 2014. View Article : Google Scholar
|
|
9
|
Huber S, Valente S, Chaimbault P and
Schohn H: Evaluation of Δ2-pioglitazone, an analogue of
pioglitazone, on colon cancer cell survival: Evidence of drug
treatment association with autophagy and activation of the
Nrf2/Keap1 pathway. Int J Oncol. 45:426–438. 2014.PubMed/NCBI
|
|
10
|
Shibata T, Ohta T, Tong KI, Kokubu A,
Odogawa R, Tsuta K, Asamura H, Yamamoto M and Hirohashi S: Cancer
related mutations in NRF2 impair its recognition by Keap1-Cul3 E3
ligase and promote malignancy. Proc Natl Acad Sci USA.
105:13568–13573. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang P, Peng X, Wei ZF, Wei FY, Wang W, Ma
WD, Yao LP, Fu YJ and Zu YG: Geraniin exerts cytoprotective effect
against cellular oxidative stress by upregulation of Nrf2-mediated
anti-oxidant enzyme expression via PI3K/AKT and ERK1/2 pathway.
Biochim Biophys Acta. 1850:1751–1761. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ding M, Zhao J, Bowman L, Lu Y and Shi X:
Inhibition of AP-1 and MAPK signaling and activation of Nrf2/ARE
pathway by quercitrin. Int J Oncol. 36:59–67. 2010.
|
|
13
|
Alam J, Stewart D, Touchard C, Boinapally
S, Choi AM and Cook JL: Nrf2, a Cap'n'Collar transcription factor,
regulates induction of the heme oxygenase-1 gene. J Biol Chem.
274:26071–26078. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kansanen E, Kuosmanen SM, Leinonen H and
Levonen A-L: The Keap1-Nrf2 pathway: Mechanisms of activation and
dysregulation in cancer. Redox Biol. 1:45–49. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Rana T, Schultz MA, Freeman ML and Biswas
S: Loss of Nrf2 accelerates ionizing radiation-induced bone loss by
upregulating RANKL. Free Radic Biol Med. 53:2298–2307. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Singh A, Boldin-Adamsky S, Thimmulappa RK,
Rath SK, Ashush H, Coulter J, Blackford A, Goodman SN, Bunz F,
Watson WH, et al: RNAi-mediated silencing of nuclear factor
erythroid-2-related factor 2 gene expression in non-small cell lung
cancer inhibits tumor growth and increases efficacy of
chemotherapy. Cancer Res. 68:7975–7984. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Greenwald I: LIN-12/Notch signaling:
Lessons from worms and flies. Genes Dev. 12:1751–1762. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Artavanis-Tsakonas S, Rand MD and Lake RJ:
Notch signaling: Cell fate control and signal integration in
development. Science. 284:770–776. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang X, Zhang J, Zhou L, Sun W, Zheng ZG,
Lu P, Gao Y, Yang XS, Zhang ZC, Tao KS, et al: Fbxw7 regulates
hepatocellular carcinoma migration and invasion via Notch1
signaling pathway. Int J Oncol. 47:231–243. 2015.PubMed/NCBI
|
|
20
|
Xue TC, Zou JH, Chen RX, Cui JF, Tang ZY
and Ye SL: Spatial localization of the JAG1/Notch1/osteopontin
cascade modulates extrahepatic metastasis in hepatocellular
carcinoma. Int J Oncol. 45:1883–1890. 2014.PubMed/NCBI
|
|
21
|
Ayaz F and Osborne BA: Non-canonical notch
signaling in cancer and immunity. Front Oncol. 4:3452014.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Swiatek PJ, Lindsell CE, del Amo FF,
Weinmaster G and Gridley T: Notch1 is essential for
postimplantation development in mice. Genes Dev. 8:707–719. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fre S, Huyghe M, Mourikis P, Robine S,
Louvard D and Artavanis-Tsakonas S: Notch signals control the fate
of immature progenitor cells in the intestine. Nature. 435:964–968.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ersvaer E, Hatfield KJ, Reikvam H and
Bruserud O: Future perspectives: Therapeutic targeting of notch
signalling may become a strategy in patients receiving stem cell
transplantation for hematologic malignancies. Bone Marrow Res.
2011:5707962011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang J, Wakeman TP, Lathia JD, Hjelmeland
AB, Wang XF, White RR, Rich JN and Sullenger BA: Notch promotes
radioresistance of glioma stem cells. Stem Cells. 28:17–28.
2010.
|
|
26
|
Bataller R, Schwabe RF, Choi YH, Yang L,
Paik YH, Lindquist J, Qian T, Schoonhoven R, Hagedorn CH, Lemasters
JJ, et al: NADPH oxidase signal transduces angiotensin II in
hepatic stellate cells and is critical in hepatic fibrosis. J Clin
Invest. 112:1383–1394. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wakabayashi N, Skoko JJ, Chartoumpekis DV,
Kimura S, Slocum SL, Noda K, Palliyaguru DL, Fujimuro M, Boley PA,
Tanaka Y, et al: Notch-Nrf2 axis: Regulation of Nrf2 gene
expression and cytoprotection by notch signaling. Mol Cell Biol.
34:653–663. 2014. View Article : Google Scholar :
|
|
28
|
Wakabayashi N, Shin S, Slocum SL, Agoston
ES, Wakabayashi J, Kwak MK, Misra V, Biswal S, Yamamoto M and
Kensler TW: Regulation of notch1 signaling by Nrf2: Implications
for tissue regeneration. Sci Signal. 3:ra522010.PubMed/NCBI
|
|
29
|
Paul MK, Bisht B, Darmawan DO, Chiou R, Ha
VL, Wallace WD, Chon AT, Hegab AE, Grogan T, Elashoff DA, et al:
Dynamic changes in intracellular ROS levels regulate airway basal
stem cell homeostasis through Nrf2-dependent Notch signaling. Cell
Stem Cell. 15:199–214. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kim JH, Thimmulappa RK, Kumar V, Cui W,
Kumar S, Kombairaju P, Zhang H, Margolick J, Matsui W, Macvittie T,
et al: NRF2-mediated Notch pathway activation enhances
hematopoietic reconstitution following myelosuppressive radiation.
J Clin Invest. 124:730–741. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wakabayashi N, Chartoumpekis DV and
Kensler TW: Crosstalk between Nrf2 and Notch signaling. Free Radic
Biol Med. 88B:158–167. 2015. View Article : Google Scholar
|
|
32
|
Iso T, Kedes L and Hamamori Y: HES and
HERP families: Multiple effectors of the Notch signaling pathway. J
Cell Physiol. 194:237–255. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yang H, Ramani K, Xia M, Ko KS, Li TW, Oh
P, Li J and Lu SC: Dysregulation of glutathione synthesis during
cholestasis in mice: Molecular mechanisms and therapeutic
implications. Hepatology. 49:1982–1991. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Tan Y, Ichikawa T, Li J, Si Q, Yang H,
Chen X, Goldblatt CS, Meyer CJ, Li X, Cai L, et al: Diabetic
downregulation of Nrf2 activity via ERK contributes to oxidative
stress-induced insulin resistance in cardiac cells in vitro and in
vivo. Diabetes. 60:625–633. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Goldring CE, Kitteringham NR, Elsby R,
Randle LE, Clement YN, Williams DP, McMahon M, Hayes JD, Itoh K,
Yamamoto M, et al: Activation of hepatic Nrf2 in vivo by
acetaminophen in CD-1 mice. Hepatology. 39:1267–1276. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Rizvi F, Mathur A and Kakkar P: Morin
mitigates acetaminophen-induced liver injury by potentiating Nrf2
regulated survival mechanism through molecular intervention in
PHLPP2-Akt-Gsk3beta axis. Apoptosis. 20:12962015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lee S, Lim MJ, Kim MH, Yu CH, Yun YS, Ahn
J and Song JY: An effective strategy for increasing the
radiosensitivity of Human lung Cancer cells by blocking
Nrf2-dependent antioxidant responses. Free Radic Biol Med.
53:807–816. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Singh A, Bodas M, Wakabayashi N, Bunz F
and Biswal S: Gain of Nrf2 function in non-small-cell lung cancer
cells confers radioresistance. Antioxid Redox Signal. 13:1627–1637.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Singh A, Misra V, Thimmulappa RK, Lee H,
Ames S, Hoque MO, Herman JG, Baylin SB, Sidransky D, Gabrielson E,
et al: Dysfunctional KEAP1-NRF2 interaction in non-small-cell lung
cancer. PLoS Med. 3:e4202006. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
McDonald JT, Kim K, Norris AJ, Vlashi E,
Phillips TM, Lagadec C, Della Donna L, Ratikan J, Szelag H, Hlatky
L, et al: Ionizing radiation activates the Nrf2 antioxidant
response. Cancer Res. 70:8886–8895. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Licciulli S, Avila JL, Hanlon L, Troutman
S, Cesaroni M, Kota S, Keith B, Simon MC, Puré E, Radtke F, et al:
Notch1 is required for Kras-induced lung adenocarcinoma and
controls tumor cell survival via p53. Cancer Res. 73:5974–5984.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Donnem T, Andersen S, Al-Shibli K, Al-Saad
S, Busund LT and Bremnes RM: Prognostic impact of Notch ligands and
receptors in nonsmall cell lung cancer: Coexpression of Notch-1 and
vascular endothelial growth factor-A predicts poor survival.
Cancer. 116:5676–5685. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Tsukimoto M, Tamaishi N, Homma T and
Kojima S: Low-dose gamma-ray irradiation induces translocation of
Nrf2 into nuclear in mouse macrophage RAW264.7 cells. J Radiat Res
(Tokyo). 51:349–353. 2010. View Article : Google Scholar
|
|
44
|
Ettinger DS, Wood DE, Akerley W, Bazhenova
LA, Borghaei H, Camidge DR, Cheney RT, Chirieac LR, D'Amico TA,
Demmy TL, et al: Non-small cell lung cancer, version 6.2015. J Natl
Compr Cancer Netw. 13:515–524. 2015.
|
|
45
|
Diehn M, Cho RW, Lobo NA, Kalisky T, Dorie
MJ, Kulp AN, Qian D, Lam JS, Ailles LE, Wong M, et al: Association
of reactive oxygen species levels and radioresistance in cancer
stem cells. Nature. 458:780–783. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Caro AA, Commissariat A, Dunn C, Kim H,
García SL, Smith A, Strang H, Stuppy J, Desrochers LP and Goodwin
TE: Prooxidant and antioxidant properties of salicylaldehyde
isonicotinoyl hydrazone iron chelators in HepG2 cells. Biochim
Biophys Acta. 1850:2256–2264. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Hwang GH, Ryu JM, Jeon YJ, Choi J, Han HJ,
Lee YM, Lee S, Bae JS, Jung JW, Chang W, et al: The role of
thioredoxin reductase and glutathione reductase in
plumbagin-induced, reactive oxygen species-mediated apoptosis in
cancer cell lines. Eur J Pharmacol. 765:384–393. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Ernst IM, Schuemann C, Wagner AE and
Rimbach G: 3,3′-Diindolylmethane but not indole-3-carbinol
activates Nrf2 and induces Nrf2 target gene expression in cultured
murine fibroblasts. Free Radic Res. 45:941–949. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Zhang Y, Guan L, Wang X, Wen T, Xing J and
Zhao J: Protection of chlorophyllin against oxidative damage by
inducing HO-1 and NQO1 expression mediated by PI3K/Akt and Nrf2.
Free Radic Res. 42:362–371. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Schmidt EE: Interplay between cytosolic
disulfide reductase systems and the Nrf2/Keap1 pathway. Biochem Soc
Trans. 43:632–638. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Bi P and Kuang S: Notch signaling as a
novel regulator of metabolism. Trends Endocrinol Metab. 26:248–255.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Phillips TM, McBride WH and Pajonk F: The
response of CD24(−/low)/CD44+ breast cancer-initiating
cells to radiation. J Natl Cancer Inst. 98:1777–1785. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Purow BW, Haque RM, Noel MW, Su Q, Burdick
MJ, Lee J, Sundaresan T, Pastorino S, Park JK, Mikolaenko I, et al:
Expression of Notch-1 and its ligands, Delta-like-1 and Jagged-1,
is critical for glioma cell survival and proliferation. Cancer Res.
65:2353–2363. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Farnie G, Clarke RB, Spence K, Pinnock N,
Brennan K, Anderson NG and Bundred NJ: Novel cell culture technique
for primary ductal carcinoma in situ: Role of Notch and epidermal
growth factor receptor signaling pathways. J Natl Cancer Inst.
99:616–627. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Kim JH, Thimmulappa RK, Kumar V, Cui W,
Kumar S, Kombairaju P, Zhang H, Margolick J, Matsui W, Macvittie T,
et al: NRF2-mediated Notch pathway activation enhances
hematopoietic reconstitution following myelosuppressive radiation.
J Clin Invest. 124:730–741. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Kim W, Youn H, Kang C and Youn B:
Inflammation-induced radioresistance is mediated by ROS-dependent
inactivation of protein phosphatase 1 in non-small cell lung cancer
cells. Apoptosis. 20:1242–1252. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Servettaz A, Goulvestre C, Kavian N, Nicco
C, Guilpain P, Chéreau C, Vuiblet V, Guillevin L, Mouthon L, Weill
B, et al: Selective oxidation of DNA topoisomerase 1 induces
systemic sclerosis in the mouse. J Immunol. 182:5855–5864. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Kavian N, Servettaz A, Mongaret C, Wang A,
Nicco C, Chéreau C, Grange P, Vuiblet V, Birembaut P, Diebold MD,
et al: Targeting ADAM-17/notch signaling abrogates the development
of systemic sclerosis in a murine model. Arthritis Rheum.
62:3477–3487. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Kavian N, Servettaz A, Weill B and Batteux
F: New insights into the mechanism of notch signalling in fibrosis.
Open Rheumatol J. 6:96–102. 2012. View Article : Google Scholar : PubMed/NCBI
|