Introduction
Osteosarcoma (OS) is the major subtype of malignant
bone tumors (1,2). In pediatric patients with localized
disease, ~80–90% of 5-year overall survival is achieved when
treated at experienced centers (3–5);
however, in adult patients (6,7) and
patients with recurrent or metastatic disease (3,8,9),
response to chemotherapy is less satisfactory, and survival of such
patients is generally poor.
Genetically, sarcomas can be classified as
translocation-related and non-translocation-related.
Non-translocation-related sarcomas can be further grouped as
sarcomas with a simple genetic profile based on limited
amplifications, such as dedifferentiated liposarcomas and parosteal
OS (10,11), and major sarcomas with extremely
complex genomic imbalances, such as leiomyosarcomas,
undifferentiated pleomorphic sarcomas, pleomorphic liposarcomas and
conventional OS (12). In
conventional OS, these genomic aberrations cause oncogenic changes
in such diverse processes as cell cycle regulation, cell
death/cytokine pathways, proliferative signaling pathways, telomere
dysfunction, metastasis and tumor suppression (13–20).
Recently, several studies have revealed that the PI3K-mTOR pathway
is crucial in conventional OS (15,16).
However, the result of a clinical study of the mTOR inhibitor in OS
treatment was unsatisfactory (21). Therefore, additional investigations
exploring potential OS targets are necessary.
Polo-like kinase 1 (PLK1), a serine/threonine kinase
and a known oncogene, is crucial in regulating cell cycle
progression (22). Moreover, PLK1
has been demonstrated to be a potential target of OS when short
hairpin RNA libraries were used in lentiviral vectors for protein
kinase screening (23,24). However, few studies have evaluated
PLK1 expression in OS (25). In
our previous study, we revealed that 15-deoxy-Δ12, 14-prostaglandin
J2 (15d-PGJ2), a prostaglandin derivative, exerts cytotoxic effects
against OS cells by downregulating the p-AKT and PKA-PLK1 pathways
through reactive oxygen species-mediated c-Jun-N-terminal kinase
activation (26). Therefore,
inhibiting PLK1 may effectively treat OS.
GSK461364, a potent and selective ATP-competitive
PLK1 inhibitor, has exhibited antiproliferative activity against
multiple tumor cell lines in preclinical studies (27). Elevation of phosphorylated histone
H3 (pHisH3) and suppression of PLK1 were indicators observed in
tumor xenografts (27). In the
present study, we evaluated the expression level of PLK1 in OS and
explored the cytotoxic mechanism of GSK461364 against OS.
Materials and methods
Bioinformatics analysis
In total, 109 microarray and clinicopathological
data sets of sarcoma from Gene Expression Omnibus (GEO) dataset
GSE14827 (OS), GSE13433 (alveolar soft part sarcoma, ASPS), GSE8167
(gastrointestinal stromal tumor, GIST), GSE20196 (synovial sarcoma,
SYN), and GSE20559 (liposarcoma, LPS) and from E-MEXP-1922
(leiomyosarcoma, LMS) were obtained from the NCBI and ArrayExpress
websites, respectively. Nine microarray data sets of normal
osteoblasts (GSE9451 and GSE10311) were retrieved from the NCBI
website. All these datasets were in Affymetrix U133 Plus 2.0
platform. To decrease intrasubtype heterogeneity, not all retrieved
samples were included in the analysis. For OS, only tumors without
subsequent metastasis were selected; for GIST, only those with exon
11 mutation were included; for SYN, only tumor with SYT-SXX type 1
fusion gene and non-poorly differentiated histology were put into
analysis; and for LPS, only well-differentiated (WD) and
dedifferentiated (DD) tumors were chosen due to their similar
genetic background. Gene expression data were normalized using
dChip (28,29). The expression levels of individual
genes were obtained through Z-score transformation, and the
differences between different subtypes were subsequently compared
using the Student's t-test.
Cell lines and reagent
Three OS cell lines, U2OS, MG63 and SJSA were
selected for in vitro study and maintained in either
Dulbecco's modified Eagle's medium (DMEM), Iscove's modified
Dulbecco's medium, or DMEM/F12 base media with 10% fetal bovine
serum. GSK461364 was purchased from Calbiochem (San Diego, CA,
USA). The following antibodies were used for immunoblotting: PLK1
(#4513; 1:1,000), phospho-cdc25C (Ser216) (pCDC25C) (#4901;
1:1,000), phospho-Histone H3 (Ser10) (pHisH3) (#9701; 1:1,000), and
phospho-Histone H2A.X (Ser139) (pH2AX) (#2577; 1:1,000), all from
Cell Signaling Technology, and anti-actin (ABS 24–100;
1:10,000).
Cell viability assay
Two methods were used in the cell viability assay,
the first being the TACS™
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
cell proliferation assay (Trevigen, Gaithersburg, MD, USA).
Approximately 2,000–20,000 cells/100 μl/well were seeded in 96-well
plates overnight. Subsequently, reagents at different
concentrations were added in triplicates. The plates were incubated
for the desired time at 37°C, pulsed with 10 μl MTT reagent, and
incubated for an additional 4 h at 37°C. Furthermore, detergent
reagent of 100 μl/well was added and mixed thoroughly to dissolve
the blue crystals. Absorbance of the converted dye was measured
using a Vmax microplate reader (Molecular Devices, Sunnyvale, CA,
USA) at 570 nm (test) and 650 nm wavelengths. Cell survival was
calculated using the following equation: % survival = (mean
experimental absorbance/mean control absorbance) × 100 (30).
The possible synergistic effect of GSK461364 and
chemotherapy was measured using the combination index (CI)
calculated using CalcuSyn software (Biosoft, Ferguson, MO, USA)
(31). CI >1 was defined as
antagonism, CI = 1 as additivity, and CI <1 as synergy; the
experiment was performed in triplicate.
Cell viability was also measured through a trypan
blue exclusion assay (32). OS
cells were plated at 5×104 cells/well in 24-well plates;
a variable concentration of GSK461364 was added to each well the
following day, and the plates were incubated at 37°C for 72 h.
Subsequently, the cells were trypsinized and mixed with trypan
blue. Viable cells with intact cell membranes and not absorbing
trypan blue were counted using a light microscope. All experiments
were performed in triplicate.
Apoptosis assessment by Annexin V
staining
Annexin V staining was used to detect apoptosis in
cell lines treated with GSK361364. The cells were washed once with
1X phosphate-buffered saline (PBS) following drug treatment and
resuspended in 100 μl staining solution [containing Annexin V
fluorescein and propidium iodide in an Annexin V-binding buffer,
Annexin V-fluorescein isothiocyanate (FITC); BD Biosciences, San
Diego, CA, USA]. Subsequently, the cells were incubated at room
temperature for 15 min and diluted in 1X Annexin V-binding buffer.
The percentages of apoptotic cells were measured through flow
cytometry.
Cell cycle analysis
Cell cycle analysis was performed through flow
cytometry, as previously described (33). Briefly, cells were trypsinized,
washed twice with PBS, and fixed with 70% ethanol at −20°C
overnight, following which the fixed cells were washed twice with
cold PBS, suspended in 420 μl PBS, added with 50 μl 10 mg/ml RNase
A (Sigma), and shaken at 37°C for 15 min. Subsequently, 20 μl of
0.2 mg/ml propidium iodide (PI) was added and cells were retained
at room temperature for 1 h. Flow cytometry was performed using
FACSCalibur (Becton-Dickinson and Co., Oxford, CA, USA) to measure
relative DNA content based on red fluorescence levels. The
percentages of the cells in the different phases of the mitotic
cell cycle were calculated using CellQuest software
(Becton-Dickinson).
Western blotting
Monolayers of cultured cells were rinsed in PBS and
scraped into lysis buffer [25 mM Tris-HCl pH 7.6, 150 mM NaCl, 1%
NP-40, 1% sodium deoxycholate, 0.1% SDS (Thermo Fisher Scientific)]
containing protease and phosphatase inhibitor cocktail (1:100
dilution; Thermo Fisher Scientific). Lysates were incubated for 30
min at 4°C and subsequently clarified through centrifugation for 30
min at 13,200 rpm at 4°C. Protein concentrations were determined
with the Pierce BCA protein assay kit (Thermo Fisher Scientific).
Protein extracts (20–50 μg/lane) were electrophoretically separated
on sodium dodecyl sulfate-polyacrylamide gel electrophoresis gels,
transferred to polyvinyl difluoride membranes (Millipore), and
blotted with specific antibodies. Immunoreactive bands were
detected using enhanced chemiluminescence (Millipore) and exposed
to X-ray film.
Senescence-associated β-galactosidase
assay
Senescence-associated β-galactosidase (SA-β-gal)
activity was detected using the cellular senescence assay kit
(Millipore) according to the manufacturer's instructions. U2OS
cells were treated with GSK461364 for 72 h; the adherent cells were
fixed and stained with X-gal in a staining solution at pH 6.0 and
washed twice with 1X PBS. The percentage of SA-β-gal-positive cells
(the number of positive cells relative to the total number of
cells) was quantified by counting 100 cells in 3 randomly chosen
fields per dish by using an Olympus IX51.
DcR2 expression assayed by flow
cytometry
DcR2 expression was detected through flow cytometry.
OS cells treated with GSK461364 for 72 h were washed twice with 1X
PBS and incubated with Alexa Fluor 488-labeled anti-DcR2 (R&D
Systems, Minneapolis, MN, USA) for 30 min at room temperature in
the dark. After washing twice with 1X PBS and resuspending in 1X
PBS, the mean of fluorescence intensity on the cell surface was
measured through flow cytometry using FACSCalibur
(Becton-Dickinson).
Analysis of interleukin-1α
expression
RNAs were extracted from the cell lines using
TRIzol® reagent (Invitrogen) according to the
manufacturer's instructions and reverse transcribed with 1 μg RNA
by using SuperScript® III First-Strand Synthesis system
(Invitrogen) for reverse transcription polymerase chain reaction
(RT-PCR). The copy number for both interleukin (IL)-1α and
glyceraldehyde 3-phosphate dehydrogenase (GAPDH) mRNA was measured
through qRT-PCR by using Maxima SYBR-Green/ROX qPCR Master Mix
(Thermo Fisher Scientific) and a LightCycler® 480 system
(Roche). The primer sequences used in the reaction were as follows:
IL-1α forward, 5′-CCGTGAGTTTCCCAGAAGAA-3′ and IL-1α reverse,
5′-ACTGCCCAAGATGAAGACCA-3′; GAPDH forward,
5′-GCCAAGGTCATCCATGACAACT-3′ and GAPDH reverse,
5-GAGGGGCCATCCACAGTCTT-3′ (34).
The PCR cycling conditions were as follows: 95°C for 5 min followed
by 45 cycles of 95°C for 30 sec, 55°C for 30 sec followed by 72°C
for 40 sec. The gene expression levels were calculated as
previously described (35).
Results
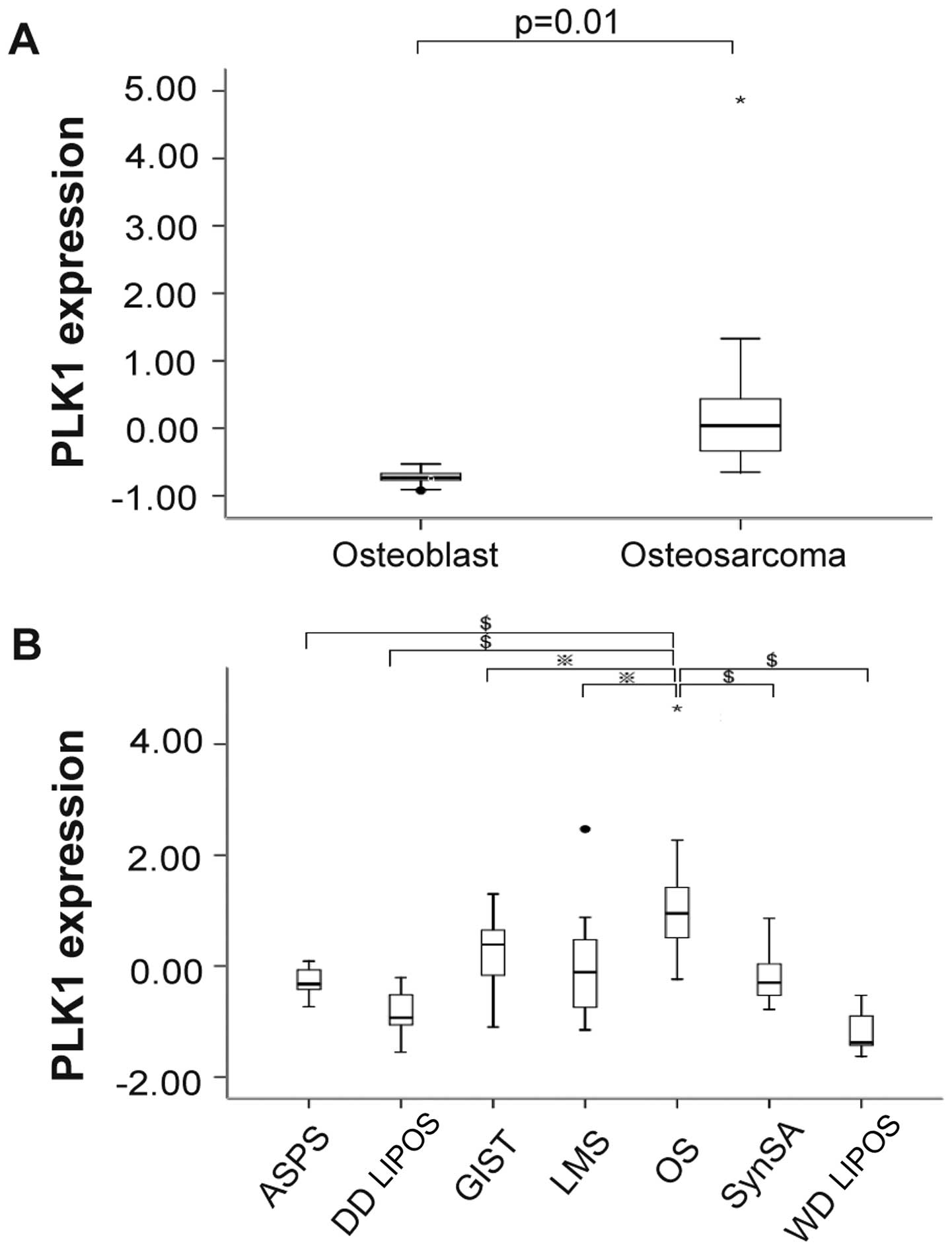
Overexpression of PLK1 in OS
We explored the expression level of PLK1 among
normal osteoblasts and OS as well as among other types of sarcoma.
The expression level of PLK1 was compared between 27 OS and 9
normal osteoblasts. As depicted in Fig. 1A, PLK1 was significantly
overexpressed in OS compared with normal osteoblasts. We further
compared the expression of PLK1 in OS with other types of sarcoma.
As depicted in Fig. 1B, the
transcript level of PLK1 was significantly higher in OS compared
with other types of sarcoma. These results indicated that PLK1 was
overexpressed in OS.
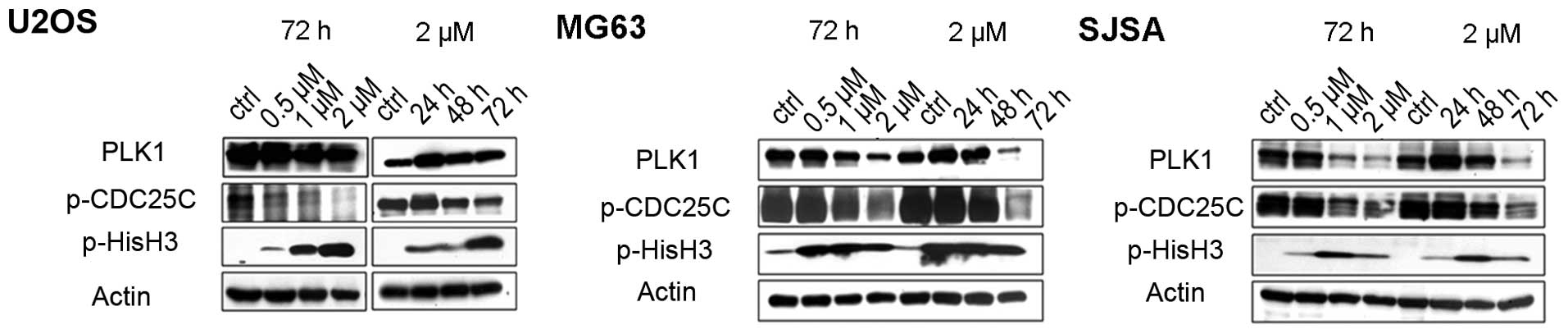
GSK461364-mediated PLK1 inhibition and
mitotic arrest in OS cell lines
An in vitro model was used to explore the
potential of PLK1 as a therapeutic target in OS. GSK461364, a
potent PLK1 inhibitor, has demonstrated favorable activity in
various types of cancers (27). OS
cells were treated with GSK461364 and the levels of PLK1 and
pCDC25C (a downstream effector of PLK1) were measured through
western blotting. Except for U2OS in time-dependent studies,
decreased level of PLK1 and pCDC25C were noted in all three
GSK461364-treated OS cell lines (Fig.
2). Moreover, we demonstrated that all OS cell lines treated
with GSK461364 exhibited a dose- and time-dependent increase in
pHisH3, an indicator of mitotic arrest (Fig. 2).
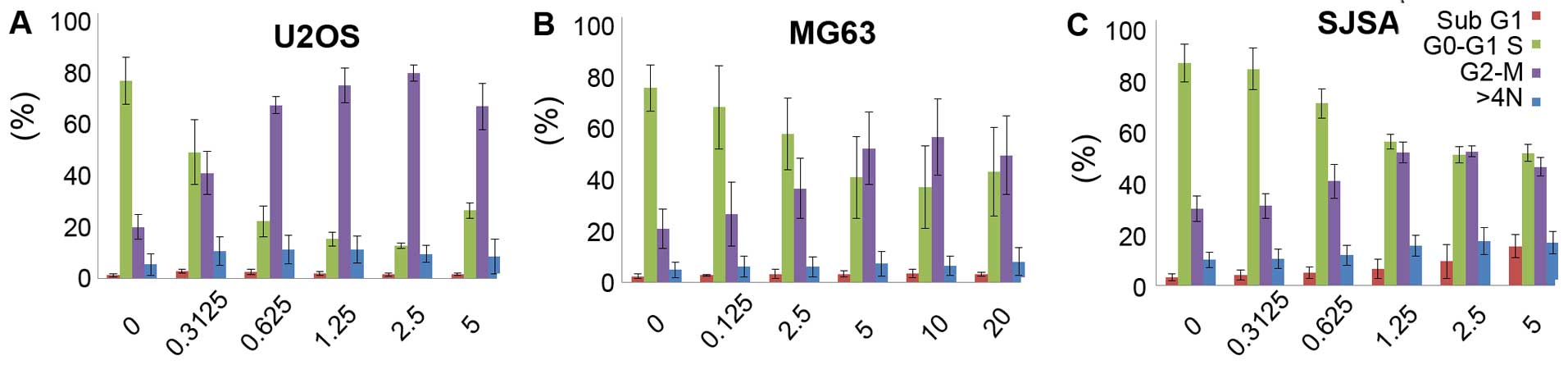
Effects of GSK461364 on cell cycle
progression in OS cell lines
Furthermore, we explored the effects of GSK461364 on
cell cycle progression in OS cell lines. As depicted in Fig. 3, flow cytometric analysis of DNA
content in cells treated with GSK461364 for 24 h demonstrated
marked accumulation of cells at G2/M DNA content in all 3 OS cell
lines.
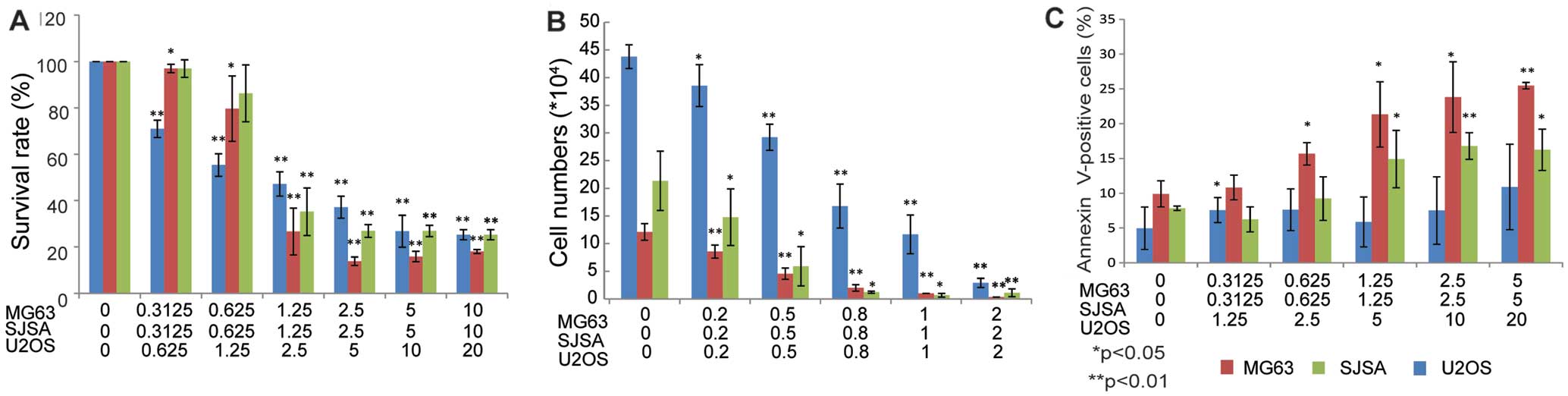
Cell viability assays were used to detect the
possible cytotoxic activity of GSK461364 against OS cell lines. As
depicted in Fig. 4, GSK461364
displayed cytotoxicity against all the three OS cell lines when
assessed using either an MTT assay (Fig. 4A), or a trypan blue exclusion assay
(Fig. 4B). Moreover, a significant
induction of apoptosis in OS cell lines was revealed on co-staining
with PI and FITC-labeled Annexin V (Annexin V-FITC; Fig. 4C).
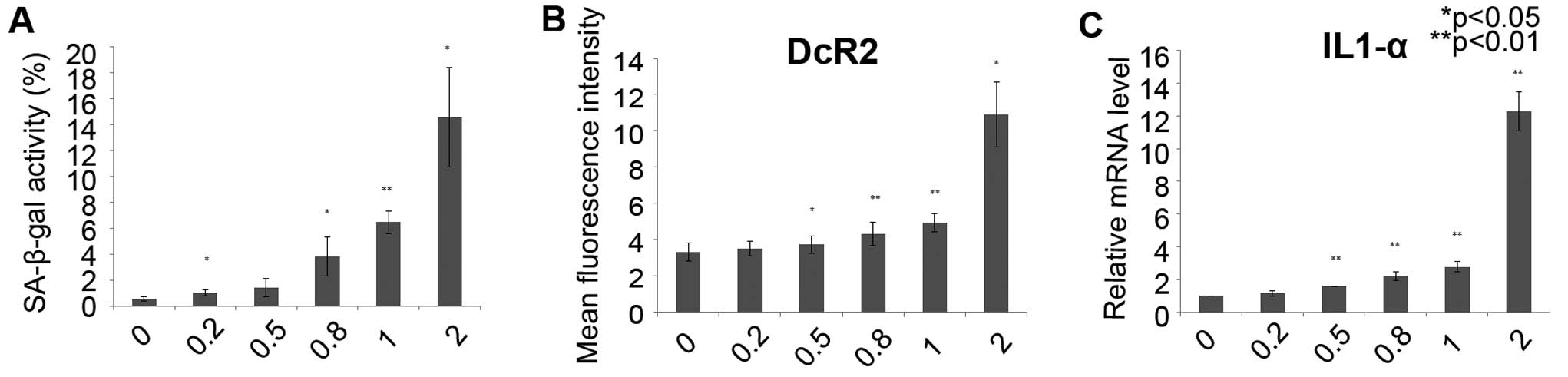
GSK461364 induces cellular senescence in
U2OS cell lines
Cellular senescence can be induced by cell cycle
inhibition. To explore whether GSK461364 induces senescence in OS
cells, a SA-β-gal assay was used in the U2OS cells following
treatment with GSK461364 for 72 h. A significant increase in
SA-β-gal activity in the U2OS cells was revealed following
GSK461364 treatment (Fig. 5A). In
addition, the expression of DcR2, a well-known senescence biomarker
(34,36), was dose-dependently enhanced
following GSK461364 administration (Fig. 5B). Furthermore, the expression of
IL-1α, a cytokine associated with the senescence-associated
secretory phenotype (SASP) (34,36),
was upregulated in the U2OS cells treated with GSK461364, as
measured through qRT-PCR (Fig.
5C). These results indicate that GSK461364 treatment induces
cellular senescence in OS cells.
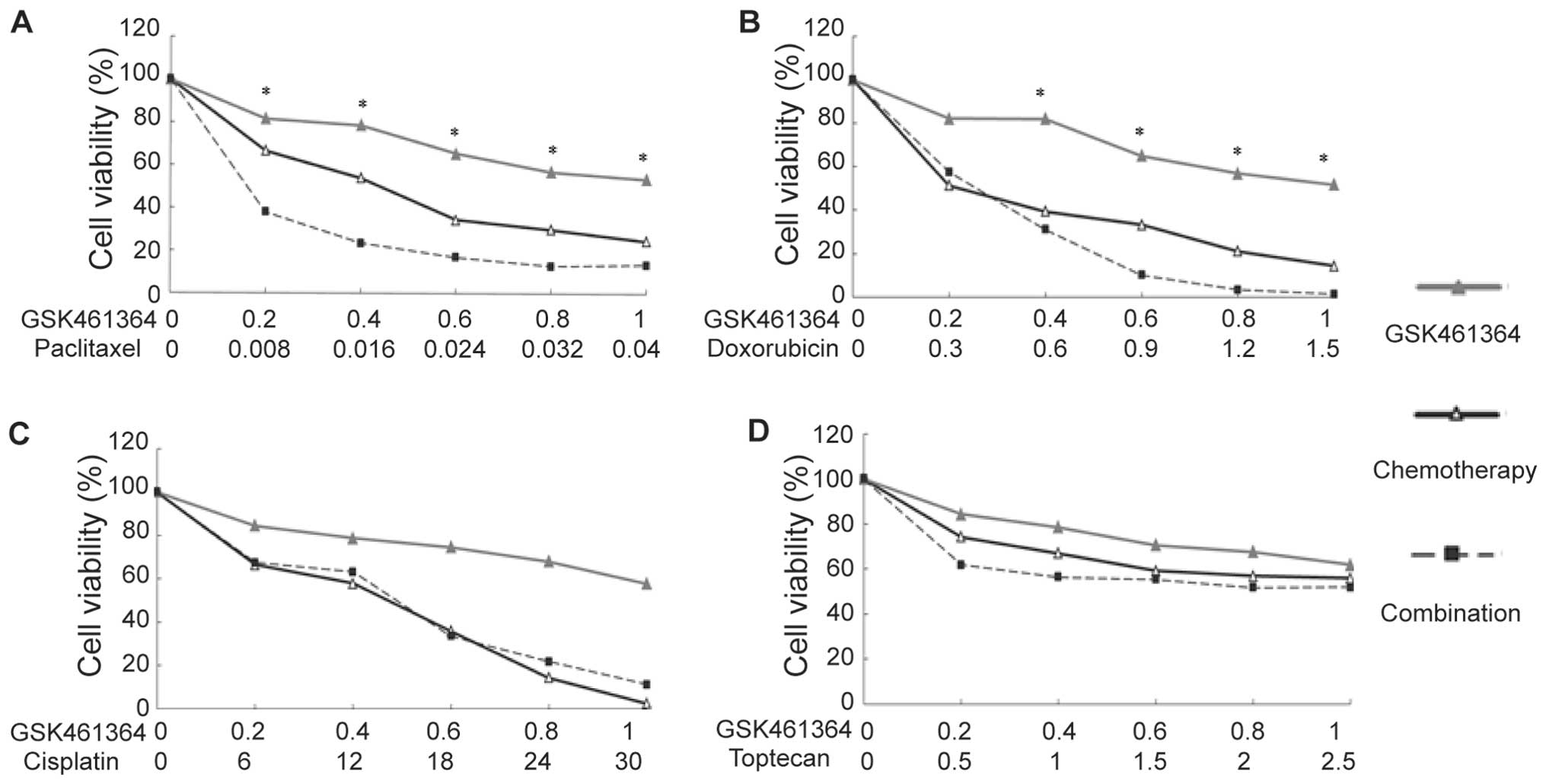
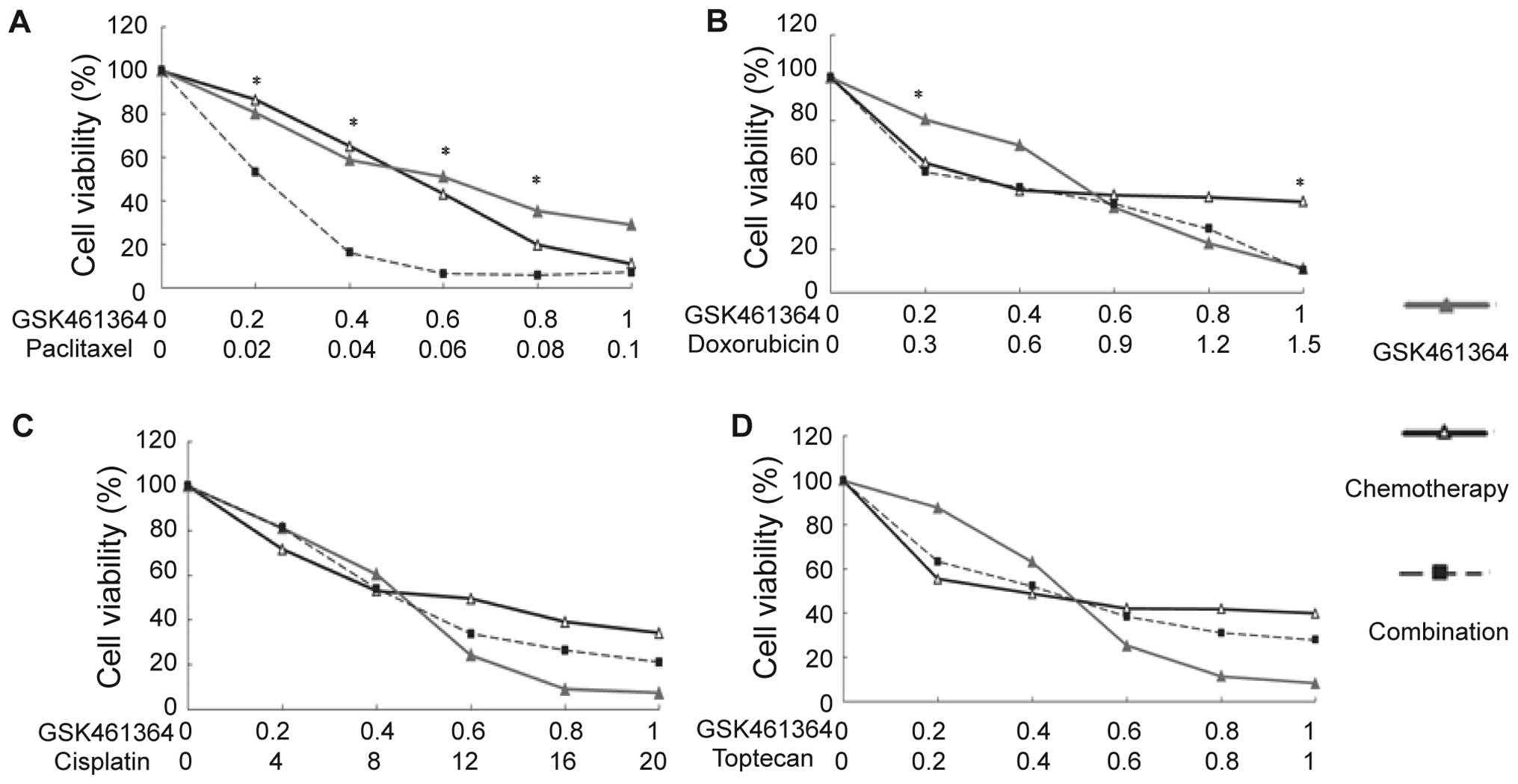
Synergistic effect of GSK461364 with
paclitaxel
We evaluated the possible synergistic effect of
GSK461364 with other chemotherapies. As depicted in Figs. 6 and 7, except for the U2OS cells treated with
doxorubicin, GSK461364 showed no significant synergistic effect
with DNA damaging agents, such as doxorubicin or cisplatin, or with
the topoisomerase inhibitor topotecan. However, it showed a
significant synergistic effect with paclitaxel in both U2OS and
MG63 cells.
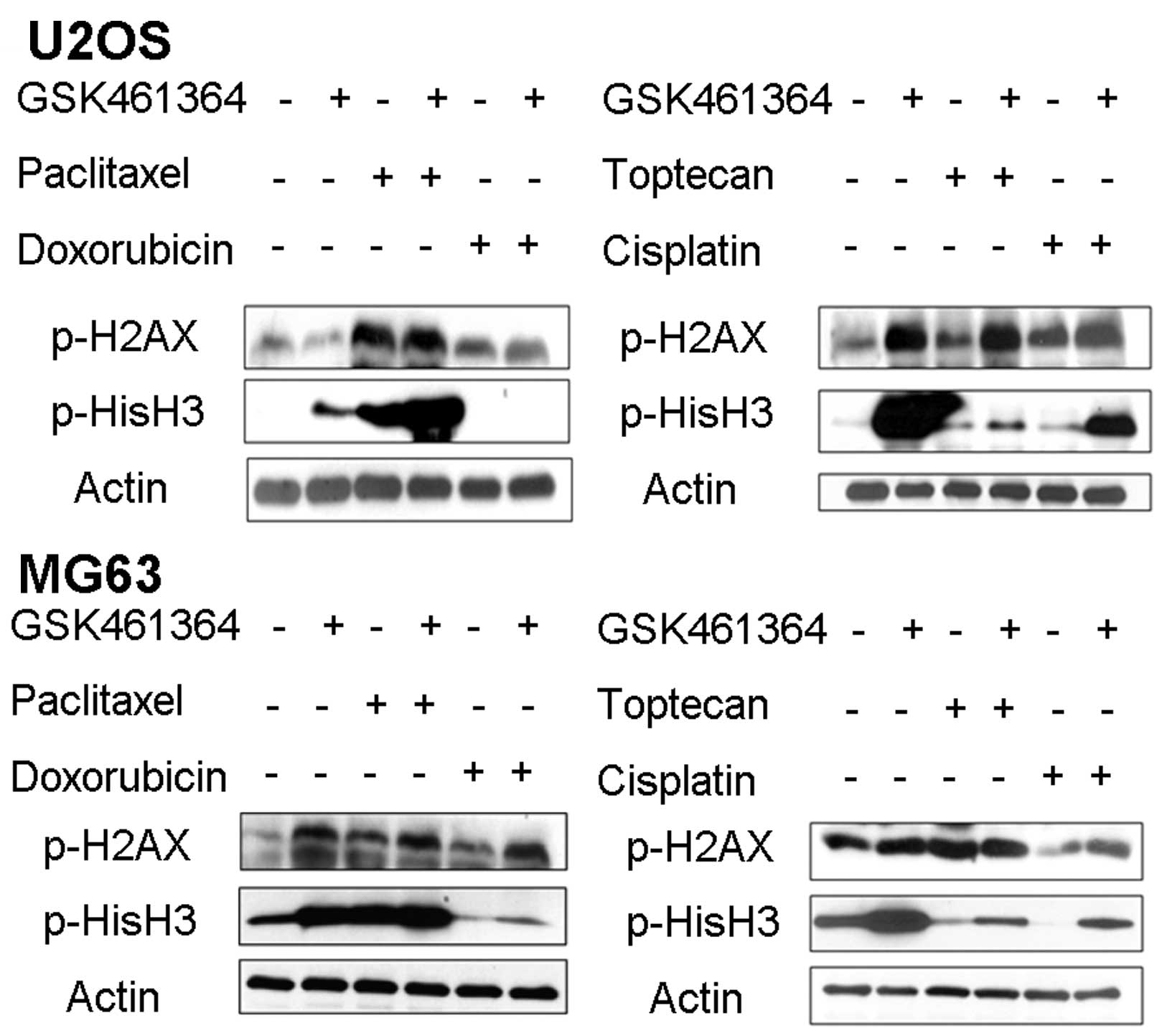
Furthermore, we explored the underlying mechanism of
the synergistic effect of GSK461364 and paclitaxel. As depicted in
Fig. 8, a combination of GSK461364
and paclitaxel produced significantly increased mitotic arrest, as
indicated by the increased pHisH3 level. By contrast, a combination
of GSK461364 with other chemotherapeutic agents reduced mitotic
arrest. No significant changes were observed in the pH2AX level, an
indicator of DNA damage.
Discussion
In the present study, we revealed that PLK1 was
significantly overexpressed in OS compared with normal osteoblasts
or other types of sarcoma. GSK461364, a PLK1 inhibitor, inhibited
PLK1 and induced mitotic arrest through G2/M arrest in OS cell
lines, with subsequent apoptosis. In addition, GSK461364 induced
cellular senescence in OS cell lines and showed a synergistic
effect with paclitaxel.
Identifying treatment targets in sarcoma is
difficult because of its complex genomic background. However, using
genomic and expression profiling in 183 soft tissue sarcoma, Chibon
et al (37) established a
prognostic gene expression signature, namely, the Complexity index
in sarcomas (CINSARC), comprising 67 genes related to mitosis and
chromosome management. CINSARC had predicted metastasis outcome in
an independent validation set of 127 sarcomas. Moreover, by
reanalyzing a dataset of GIST, our group identified aurora kinase A
(AURKA), along with other cell cycle and mitosis genes, as a
prognostic factor for recurrence. In addition, AURKA is a potential
treatment target (38,39). Therefore, cell cycle regulation
genes are a crucial group of potential biomarkers or targets in
sarcoma.
PLK1, essential for regulation of mitosis and
maintenance of genomic stability, is overexpressed in human tumors
and has prognostic potential in cancers, indicating its involvement
in carcinogenesis and its potential as a therapeutic target
(22). Sero et al (25) demonstrated enhanced PLK1 expression
in clinical OS samples and cell lines compared with normal human
tissues. In addition, an enhanced PLK1 expression at diagnosis
appeared to be associated with an unfavorable clinical outcome. In
the present study, we demonstrated significant PLK1 overexpression
in OS by comparing the transcription levels of different subtypes
of sarcoma in a microarray database. However, PLK1 was not found to
have a prognostic influence in OS (data not shown), and its role in
oncogenesis of OS requires further investigation.
Several PLK1 inhibitors have been demonstrated to
exert a cytotoxic effect on OS. BI 2536 suppresses cell line growth
and clonogenicity (40), and
decreases the xenograft tumor size of OS (41). Bogado et al (42) demonstrated that both BI 6727 and
GSK461364 induce cell growth arrest, apoptosis and
radiosensitization in OS cell lines. Sero et al (25) revealed that another PLK1 inhibitor,
NMS-P937, was highly active in both drug-sensitive and
drug-resistant OS cell lines.
We revealed that GSK461364 exerts significant
cytotoxic activity on 3 OS cell lines. We discovered that GSK461364
targets and downregulates PLK1 and pCDC25C in the OS cell line and
induces mitotic arrest, as indicated by an enhanced pHisH3
expression level and a marked G2/M arrest. Our results demonstrated
that GSK461364 is cytotoxic to all 3 OS cells when assessed either
through an MTT assay or a trypan blue exclusion assay. In addition,
a significant induction of apoptosis in OS cell lines was detected
by co-staining with PI and Annexin V-FITC, indicating an
apoptosis-inducing effect. These data are consistent with previous
reports.
Cell cycle inhibitor induces cellular senescence,
wherein cells remain viable but typically arrested at the G1 or
G2/M phases of the cell cycle, failing to proceed even after
mitogen stimulation. Senescence cells are usually characterized by
specific cellular phenotypes (such as increased SA-β-gal activity),
secretory phenotype (SASP, usually a cytokine, such as IL-1α), and
apoptosis-regulatory protein (such as DcR2) (36). In the present study, GSK461364
treatment significantly increased SA-β-gal activity and enhanced
the expression of DcR2 and IL-1α in OS cell lines. In addition,
similar findings were revealed in our previous study of AURKA
inhibitor MLN8237 in the treatment of GIST cell line (39). Our studies indicated that cellular
senescence is a crucial phenotype of PLK1, or in treatment of other
cell cycle inhibitors and that senescence-associated markers may be
valuable biomarkers for therapy with these compounds.
Furthermore, GSK461364 and paclitaxel were
demonstrated to act synergistically in inducing a cytotoxic effect
on OS, probably because of enhanced mitotic arrest. A previous OS
study failed to demonstrate any synergistic effect of GSK461364
with chemotherapy (42). However,
in a breast cancer study, PLK1-specific antisense oligonucleotides
acted synergistically with paclitaxel in inducing cell cycle
arrest, apoptosis, and reduction of tumor growth of xenograft
(43). Therefore, a combination of
GSK461364 and paclitaxel deserves further investigation in a
clinical setting.
In conclusion, the present study revealed that PLK1
is overexpressed in OS. GSK461364, a PLK1 inhibitor, exerted its
cytotoxic effect on OS through the induction of mitotic arrest and
subsequent apoptosis and induced cellular senescence; therefore,
senescence-associated markers can be used as probable treatment
biomarkers, and a combination of GSK461364 and paclitaxel may be
effective in OS treatment.
Acknowledgements
The present study was jointly supported by the
grants from the National Science Council (NSC 100-2314-B-075-081
and NSC 101-2314-B-075-029) and the Ministry of Science and
Technology, Taiwan (MOST 103-2314-B-075-066), the Taipei Veterans
General Hospital (V102E8-003, V103E8-001, V101C-133, V102C-034,
V103C-188, V104C-099, V104E16-001-MY3-1) and from the Yen Tjing
Ling Medical Foundation (grant number CI-100-19 and CI-103-6)
designated to Dr Chueh-Chuan Yen. This study was also supported by
the Taiwan Clinical Oncology Research Foundation, and the Chong Hin
Loon Memorial Cancer and Biotherapy Research Center of National
Yang-Ming University.
References
|
1
|
Hung GY, Horng JL, Yen HJ, Yen CC, Chen
WM, Chen PC, Wu HT and Chiou HJ: Incidence patterns of primary bone
cancer in Taiwan (2003–2010): A population-based study. Ann Surg
Oncol. 21:2490–2498. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2012. CA Cancer J Clin. 62:10–29. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hung GY, Yen HJ, Yen CC, Chen WM, Chen PC,
Wu HT, Chiou HJ, Chang WH and Hsu HE: Experience of pediatric
osteosarcoma of the extremity at a single institution in Taiwan:
Prognostic factors and impact on survival. Ann Surg Oncol.
22:1080–1087. 2015. View Article : Google Scholar
|
|
4
|
Ferrari S, Smeland S, Mercuri M, Bertoni
F, Longhi A, Ruggieri P, Alvegard TA, Picci P, Capanna R, Bernini
G, et al; Italian and Scandinavian Sarcoma Groups. Neoadjuvant
chemotherapy with high-dose Ifosfamide, high-dose methotrexate,
cisplatin, and doxorubicin for patients with localized osteosarcoma
of the extremity: A joint study by the Italian and Scandinavian
Sarcoma Groups. J Clin Oncol. 23:8845–8852. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Iwamoto Y, Tanaka K, Isu K, Kawai A,
Tatezaki S, Ishii T, Kushida K, Beppu Y, Usui M, Tateishi A, et al:
Multiinstitutional phase II study of neoadjuvant chemotherapy for
osteosarcoma (NECO study) in Japan: NECO-93J and NECO-95J. J Orthop
Sci. 14:397–404. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Harting MT, Lally KP, Andrassy RJ,
Vaporciyan AA, Cox CS Jr, Hayes-Jordan A and Blakely ML: Age as a
prognostic factor for patients with osteosarcoma: An analysis of
438 patients. J Cancer Res Clin Oncol. 136:561–570. 2010.
View Article : Google Scholar
|
|
7
|
Joo MW, Shin SH, Kang YK, Kawai A, Kim HS,
Asavamongkolkul A, Jeon DG, Kim JD, Niu X, Tsuchiya H, et al:
Osteosarcoma in Asian populations over the age of 40 years: A
Multicenter study. Ann Surg Oncol. 22:3557–3564. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Janeway KA, Barkauskas DA, Krailo MD,
Meyers PA, Schwartz CL, Ebb DH, Seibel NL, Grier HE, Gorlick R and
Marina N: Outcome for adolescent and young adult patients with
osteosarcoma: A report from the Children's Oncology Group. Cancer.
118:4597–4605. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Whelan JS, Jinks RC, McTiernan A, Sydes
MR, Hook JM, Trani L, Uscinska B, Bramwell V, Lewis IJ, Nooij MA,
et al: Survival from high-grade localised extremity osteosarcoma:
Combined results and prognostic factors from three European
Osteosarcoma Intergroup randomised controlled trials. Ann Oncol.
23:1607–1616. 2012. View Article : Google Scholar :
|
|
10
|
Mejia-Guerrero S, Quejada M, Gokgoz N,
Gill M, Parkes RK, Wunder JS and Andrulis IL: Characterization of
the 12q15 MDM2 and 12q13-14 CDK4 amplicons and clinical
correlations in osteosarcoma. Genes Chromosomes Cancer. 49:518–525.
2010.PubMed/NCBI
|
|
11
|
Crago AM, Socci ND, DeCarolis P, O'Connor
R, Taylor BS, Qin LX, Antonescu CR and Singer S: Copy number losses
define subgroups of dedifferentiated liposarcoma with poor
prognosis and genomic instability. Clin Cancer Res. 18:1334–1340.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wunder JS, Nielsen TO, Maki RG, O'Sullivan
B and Alman BA: Opportunities for improving the therapeutic ratio
for patients with sarcoma. Lancet Oncol. 8:513–524. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ladanyi M, Cha C, Lewis R, Jhanwar SC,
Huvos AG and Healey JH: MDM2 gene amplification in metastatic
osteosarcoma. Cancer Res. 53:16–18. 1993.PubMed/NCBI
|
|
14
|
Koshkina NV, Kleinerman ES, Li G, Zhao CC,
Wei Q and Sturgis EM: Exploratory analysis of Fas gene
polymorphisms in pediatric osteosarcoma patients. J Pediatr Hematol
Oncol. 29:815–821. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Choy E, Hornicek F, MacConaill L, Harmon
D, Tariq Z, Garraway L and Duan Z: High-throughput genotyping in
osteosarcoma identifies multiple mutations in
phosphoinositide-3-kinase and other oncogenes. Cancer.
118:2905–2914. 2012. View Article : Google Scholar :
|
|
16
|
Perry JA, Kiezun A, Tonzi P, Van Allen EM,
Carter SL, Baca SC, Cowley GS, Bhatt AS, Rheinbay E, Pedamallu CS,
et al: Complementary genomic approaches highlight the PI3K/mTOR
pathway as a common vulnerability in osteosarcoma. Proc Natl Acad
Sci USA. 111:E5564–E5573. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Scheel C, Schaefer KL, Jauch A, Keller M,
Wai D, Brinkschmidt C, van Valen F, Boecker W, Dockhorn-Dworniczak
B and Poremba C: Alternative lengthening of telomeres is associated
with chromosomal instability in osteosarcomas. Oncogene.
20:3835–3844. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wan X, Mendoza A, Khanna C and Helman LJ:
Rapamycin inhibits ezrin-mediated metastatic behavior in a murine
model of osteosarcoma. Cancer Res. 65:2406–2411. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yen CC, Chen WM, Chen TH, Chen WY, Chen
PC, Chiou HJ, Hung GY, Wu HT, Wei CJ, Shiau CY, et al:
Identification of chromosomal aberrations associated with disease
progression and a novel 3q13.31 deletion involving LSAMP gene in
osteosarcoma. Int J Oncol. 35:775–788. 2009.PubMed/NCBI
|
|
20
|
Pasic I, Shlien A, Durbin AD, Stavropoulos
DJ, Baskin B, Ray PN, Novokmet A and Malkin D: Recurrent focal
copy-number changes and loss of heterozygosity implicate two
noncoding RNAs and one tumor suppressor gene at chromosome 3q13.31
in osteosarcoma. Cancer Res. 70:160–171. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Grignani G, Palmerini E, Ferraresi V,
D'Ambrosio L, Bertulli R, Asaftei SD, Tamburini A, Pignochino Y,
Sangiolo D, Marchesi E, et al; Italian Sarcoma Group. Sorafenib and
everolimus for patients with unresectable high-grade osteosarcoma
progressing after standard treatment: A non-randomised phase 2
clinical trial. Lancet Oncol. 16:98–107. 2015. View Article : Google Scholar
|
|
22
|
Strebhardt K and Ullrich A: Targeting
polo-like kinase 1 for cancer therapy. Nat Rev Cancer. 6:321–330.
2006. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Duan Z, Ji D, Weinstein EJ, Liu X, Susa M,
Choy E, Yang C, Mankin H and Hornicek FJ: Lentiviral shRNA screen
of human kinases identifies PLK1 as a potential therapeutic target
for osteosarcoma. Cancer Lett. 293:220–229. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yamaguchi U, Honda K, Satow R, Kobayashi
E, Nakayama R, Ichikawa H, Shoji A, Shitashige M, Masuda M, Kawai
A, et al: Functional genome screen for therapeutic targets of
osteosarcoma. Cancer Sci. 100:2268–2274. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sero V, Tavanti E, Vella S, Hattinger CM,
Fanelli M, Michelacci F, Versteeg R, Valsasina B, Gudeman B, Picci
P, et al: Targeting polo-like kinase 1 by NMS-P937 in osteosarcoma
cell lines inhibits tumor cell growth and partially overcomes drug
resistance. Invest New Drugs. 32:1167–1180. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yen CC, Hsiao CD, Chen WM, Wen YS, Lin YC,
Chang TW, Yao FY, Hung SC, Wang JY, Chiu JH, et al: Cytotoxic
effects of 15d-PGJ2 against osteosarcoma through ROS-mediated AKT
and cell cycle inhibition. Oncotarget. 5:716–725. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gilmartin AG, Bleam MR, Richter MC,
Erskine SG, Kruger RG, Madden L, Hassler DF, Smith GK, Gontarek RR,
Courtney MP, et al: Distinct concentration-dependent effects of the
polo-like kinase 1-specific inhibitor GSK461364A, including
differential effect on apoptosis. Cancer Res. 69:6969–6977. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li C and Hung Wong W: Model-based analysis
of oligonucleotide arrays: model validation, design issues and
standard error application. Genome Biol. 2:Research0032.
2001.PubMed/NCBI
|
|
29
|
Li C and Wong WH: Model-based analysis of
oligonucleotide arrays: Expression index computation and outlier
detection. Proc Natl Acad Sci USA. 98:31–36. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Mosmann T: Rapid colorimetric assay for
cellular growth and survival: Application to proliferation and
cytotoxicity assays. J Immunol Methods. 65:55–63. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chou TC and Talalay P: Quantitative
analysis of dose-effect relationships: The combined effects of
multiple drugs or enzyme inhibitors. Adv Enzyme Regul. 22:27–55.
1984. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Schmit TL, Nihal M, Ndiaye M, Setaluri V,
Spiegelman VS and Ahmad N: Numb regulates stability and
localization of the mitotic kinase PLK1 and is required for transit
through mitosis. Cancer Res. 72:3864–3872. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Tuveson DA, Willis NA, Jacks T, Griffin
JD, Singer S, Fletcher CD, Fletcher JA and Demetri GD: STI571
inactivation of the gastrointestinal stromal tumor c-KIT
oncoprotein: Biological and clinical implications. Oncogene.
20:5054–5058. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhu Y, Xu L, Zhang J, Hu X, Liu Y, Yin H,
Lv T, Zhang H, Liu L, An H, et al: Sunitinib induces cellular
senescence via p53/Dec1 activation in renal cell carcinoma cells.
Cancer Sci. 104:1052–1061. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Müller-Tidow C, Metzger R, Kügler K,
Diederichs S, Idos G, Thomas M, Dockhorn-Dworniczak B, Schneider
PM, Koeffler HP, Berdel WE, et al: Cyclin E is the only
cyclin-dependent kinase 2-associated cyclin that predicts
metastasis and survival in early stage non-small cell lung cancer.
Cancer Res. 61:647–653. 2001.PubMed/NCBI
|
|
36
|
Ewald JA, Desotelle JA, Wilding G and
Jarrard DF: Therapy-induced senescence in cancer. J Natl Cancer
Inst. 102:1536–1546. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Chibon F, Lagarde P, Salas S, Pérot G,
Brouste V, Tirode F, Lucchesi C, de Reynies A, Kauffmann A, Bui B,
et al: Validated prediction of clinical outcome in sarcomas and
multiple types of cancer on the basis of a gene expression
signature related to genome complexity. Nat Med. 16:781–787. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Yen CC, Yeh CN, Cheng CT, Jung SM, Huang
SC, Chang TW, Jan YY, Tzeng CH, Chao TC, Chen YY, et al:
Integrating bioinformatics and clinicopathological research of
gastrointestinal stromal tumors: Identification of aurora kinase A
as a poor risk marker. Ann Surg Oncol. 19:3491–3499. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Yeh CN, Yen CC, Chen YY, Cheng CT, Huang
SC, Chang TW, Yao FY, Lin YC, Wen YS, Chiang KC, et al:
Identification of aurora kinase A as an unfavorable prognostic
factor and potential treatment target for metastatic
gastrointestinal stromal tumors. Oncotarget. 5:4071–4086. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Morales AG, Brassesco MS, Pezuk JA,
Oliveira JC, Montaldi AP, Sakamoto-Hojo ET, Scrideli CA and Tone
LG: BI 2536-mediated PLK1 inhibition suppresses HOS and MG-63
osteosarcoma cell line growth and clonogenicity. Anticancer Drugs.
22:995–1001. 2011.PubMed/NCBI
|
|
41
|
Liu X, Choy E, Harmon D, Yang S, Yang C,
Mankin H, Hornicek FJ and Duan Z: Inhibition of polo-like kinase 1
leads to the suppression of osteosarcoma cell growth in vitro and
in vivo. Anticancer Drugs. 22:444–453. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Bogado RF, Pezuk JA, de Oliveira HF, Tone
LG and Brassesco MS: BI 6727 and GSK461364 suppress growth and
radiosensitize osteosarcoma cells, but show limited cytotoxic
effects when combined with conventional treatments. Anticancer
Drugs. 26:56–63. 2015. View Article : Google Scholar
|
|
43
|
Spankuch B, Heim S, Kurunci-Csacsko E,
Lindenau C, Yuan J, Kaufmann M and Strebhardt K: Down-regulation of
Polo-like kinase 1 elevates drug sensitivity of breast cancer cells
in vitro and in vivo. Cancer Res. 66:5836–5846. 2006. View Article : Google Scholar : PubMed/NCBI
|