Introduction
Nasopharyngeal carcinoma (NPC) is the most prevalent
malignant cancer in Southeast Asia particularly in South China
(1). Radiotherapy and cisplatin
based chemotherapy are the main therapy for NPC (2,3).
Unfortunately, most patients suffering from NPC do not benefit much
from simultaneous chemoradiotherapy; as 30–40% develop distant
metastases within 4 years (4), and
once metastasis occurs, the prognosis is very poor. Therefore, it
is critical to look for new treatments for NPC.
An increasing amount of attention has been given to
the utilization of complementary and alternative medicine as a part
of the therapy for various cancers associated with current
treatments (5). Curcumin has been
reported to arrest the cell cycle, induce apoptosis and prevent the
propagation and metastasis of tumor cells including NPC (6,7). It
has been reported that curcumin exerts its proapoptotic effects by
producing endoplasmic reticulum (ER) stress in cancer cells
(8,9). Curcumin is notably non-toxic and
possesses promising anticancer activities, however, preclinical and
clinical studies showed its low bioavailability and pharmacokinetic
profiles as a result of its instability under physiological
conditions which have limited its utilization in anticancer
treatments (9,10).
Substantial work was done in modifying curcumin
structure to determine analogues with stronger antitumor activities
and better bioavailability (11,12).
A series of mono-carbonyl analogues of curcumin have been
synthesized by removing the β-diketone moiety (13,14).
Studies have shown that some mono-carbonyl analogues possess
enhanced stability and antitumor activities in vitro as well
as improved pharmacokinetic profiles in vivo. One such
compound, 1,5-bis(2-methoxyphenyl)-penta-1,4-dien-3-one (B63)
(Fig. 1B) was developed as part of
a series of novel curcuminoids (15). Previous studies have shown that B63
exhibited stronger antitumor activtity than curcumin in human lung
cancer cells (15). In this study,
the biological activity of B63 on NPC cells was characterized. The
data obtained demonstrated that B63 prevent cell viability, arrest
cell cycle and induce apoptosis. B63 demonstrated a specificity for
activating ER stress greater than curcumin. B63 therapy also showed
improved growth-suppressive effects in vivo. The data
obtained gave an indication that B63 could be further optimized for
development for NPC therapy.
Materials and methods
Materials
Cell culture reagents were purchased from
Invitrogen. The antibodies: CHOP, XBP-1, ATF-4, Lamin B, and Jab1
were from Santa Cruz, PARP and P27 were from BD Biosciences
Pharmingen, caspase-3 and Cyclin B1 were from Cell Signaling
Technology. Apoptosis (Annexin V/PI) staining kit was purchased
from BD Biosciences. Curcumin and 3-(4,5)-dimethylthiahiazol-(-zy1)-3,5-di-phenyltetra-zolium
bromide (MTT) were from Sigma-Aldrich. B63 was from the School of
Pharmacy, Wenzhou Medical University and stored in DMSO at a
concentration of 50 mM. The final concentration of DMSO in the
experimental system was ≤0.1%.
Cell culture
NPC cells CNE1, CNE2 cells (16) and radioresistant NPC cell line
CNE2R were cultured in RPMI-1640 medium as previously described
(17).
MTT assay
To detect cell viability, we used MTT assay as
previously described (16).
Briefly, the cells seeded in 96-well plates (4,000 cells per well)
were treated with B63 or curcumin for 24 or 48 h. MTT (final
concentration, 0.5 mg/ml) was added to each well and incubated for
3.5 h. The medium was discarded and 150 μl of DMSO was added to
each well, and incubated for 10 min. The absorbance was read at 570
nm. Half-Maximal inhibitory concentrations (IC50), were
used as the drug concentration to acquire half maximal inhibition
of cell viability.
Colony formation assay
The colony formation assay was performed as
described previously (18).
Generally, cells (300 cells per well) seeded in a 6-well plate were
treated with indicated doses of B63 for 24 h. The NPC cells were
cultured for 10 days and then stained with Giemsa dye and colonies
of ≥50 cells were counted by a microscope.
Cell cycle analysis by flow
cytometry
PI staining was performed as previously described
(18). Briefly, after applying 5
μM of B63 or curcumin treatment, NPC cells were collected and fixed
at 4°C in 75% ethanol. Cells were washed two times in PBS, stained
with PI, and analyzed immediately after staining using a flow
cytometer (BD Biosciences).
Apoptosis measurement
To examine viable cells, nuclear staining was
performed. Cells were exposed to 5 μM B63 or curcumin for 48 h and
were washed two times and fixed with methanol. After 15 min, cells
were rewashed and stained with Hoechst 33342 for 15 min and then
detected using a fluorescence microscope (Olympus, DX50).
For Annexin V and PI staining, cells were treated
with 5 μM B63 or curcumin for 48 h, and then collected and
resuspended in 100 μl binding buffer containing Annexin V-FITC and
PI according to the manufacturer's instructions. Flow cytometer was
used for the quantification of Annexin V-FITC and PI binding.
Lentiviral infection for CHOP siRNA
The CHOP siRNA sequence was designed as:
5′-GCAGGCAGGAAATCGAGCGCCTGAC-3′. The recombinant lentiviruses were
produced by transfection of 293T cells with FuGENE 6 Transfection
reagent as described previously (15). Briefly, the subconfluent cells in a
10-cm culture dish were co-transfected with lentiviral vector (10
μg), lentiviral packaging vectors pRSV-REV (2 μg) and pMDLg/pRRE (5
μg) and the vesicular stomatitis virus G glycoprotein (VSVG)
expression vector pMD2G (3 μg). The viruses were collected from the
culture supernatants on days 2 and 3 post-transfection,
concentrated by ultracentrifugation for 1.5 h at 25,000 rpm and
suspended in PBS. After infecting the NPC cells for 48 h, knockdown
of CHOP were further confirmed by immunoblot analysis.
Western blot analysis
After curcumin or B63 treatment whole cell lysate
and nuclear and cytosolic extracts were isolated as described
previously (15). Antibodies
against the following proteins were used: CHOP, ATF-4, XBP-1, Lamin
B. Immunoreactive bands were visualized with a secondary antibody
and Western Lightning Chemiluminescence Plus reagent.
Tumorigenicity assay in nude mouse
NPC xenograft model was used to examine B63's
antitumor activity as previously described (15). Four-week-old athymic nude (nu/nu)
mice were obtained from the Animal Center of Sun Yat-Sen
University. CNE2 and CNE2R were treated with either curcumin (10
μM), B63 (5 and 10 μM), or DMSO for 12 h and then injected
subcutaneously into the right flank of each mouse (3×106
cells/mouse, 6 mice/group). Mice were checked every 2 days for
xenograft development. After tumors became obvious (~0.1
mm3), tumor volumes was calculated using the following
formula: length × width2/2 every 3 days. At the end of
the experiments the mice were sacrificed, and the tumors extracted
for weighing. All the animal work was approved by the Institutional
Animal Care and Use Committee of Sun Yat-sen University.
Statistical analysis
Results are shown as means ± standard deviation.
Statistical analysis for the results was performed using Student's
t-test for only two groups, or one-way analysis of variance for
more than two groups. Differences between groups were considered
statistically significant at P<0.05.
Results
B63 shows stronger antitumor activity
than curcumin in suppressing NPC cell viability
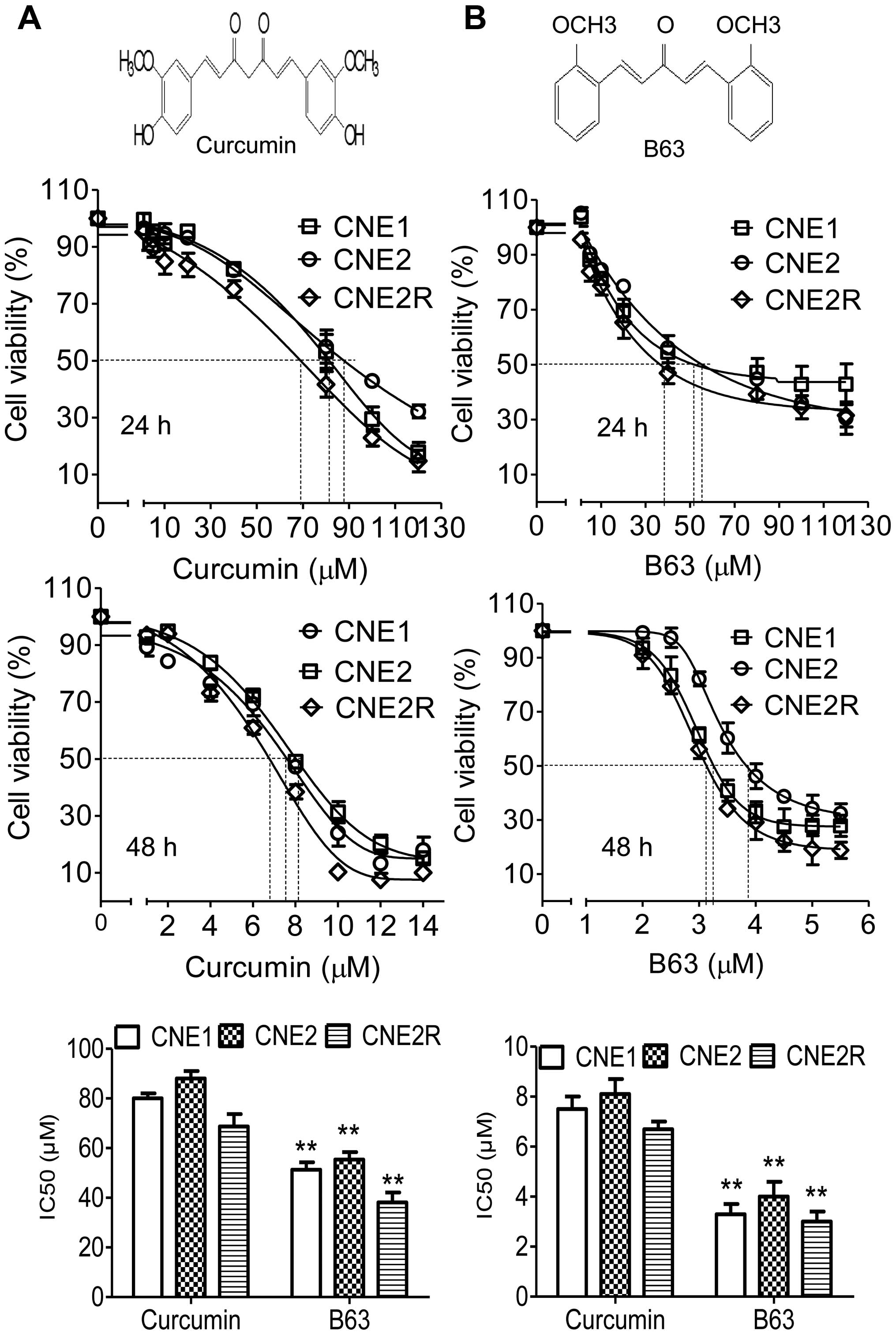
In this study, the antitumor activities of B63 and
curcumin in NPC cells were examined. After treatment for 24 h, both
B63 and curcumin showed suppression of cell viability in all three
NPC cell types (Fig. 1). However,
B63 exhibited greater inhibition than curcumin. IC50
values of B63 were 51, 55 and 38 μM in CNE1, CNE2, and CNE2R,
respectively, which are substantially more powerful than curcumin
(IC50 values 80, 88 and 69 μM). In this study, B63
showed growth-suppressive activity in the NPC cell lines tested in
a dose- and time-dependent manner, after 48-h treatment, the
IC50 values of B63 were much lower, with 3.3, 4.0 and
3.0 μM in CNE1, CNE2, and in CNE2R, respectively, which are also
more powerful than curcumin (IC50 values 7.5, 8.1 and
6.7 μM).
B63 is more powerful in inhibiting
proliferation and inducing NPC cell cycle arrest
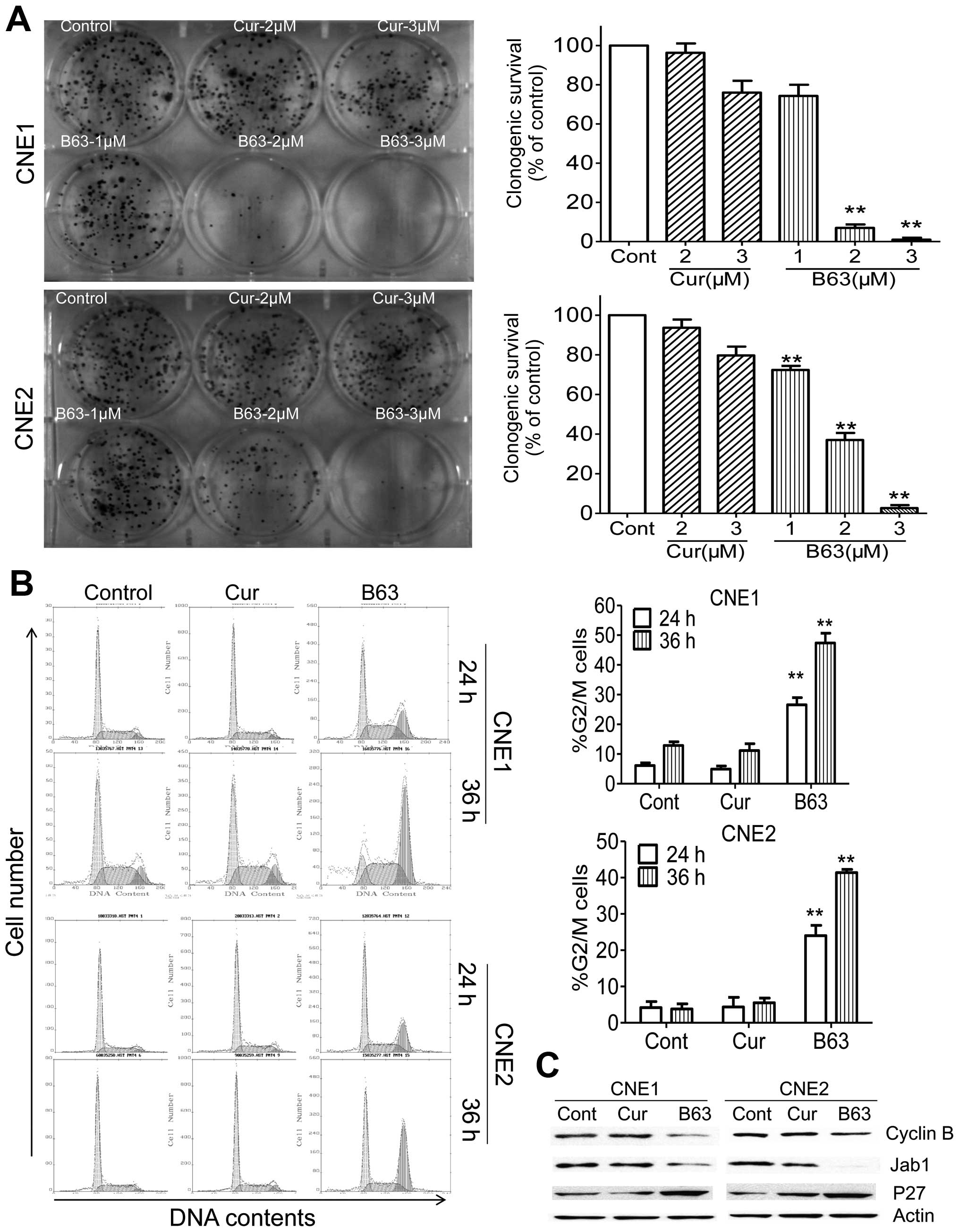
A colony formation assay to test the effect of B63
on NPC cell proliferation was further performed. As expected,
treatment of curcumin or B63 led to decrease in colony formation
when compared to the DMSO control group (Fig. 2A). Three micromolar (3 μM) curcumin
produced a decrease of nearly 24% (CNE1) and 20% (CNE2) in colony
formation, respectively. While 3 μM B63 treatment showed 99% in
CNE1 and 97% in CNE2 reductions in colony numbers. These results
showed that B63 was more potent than curcumin in preventing cell
viability and propagation of NPC cells.
Cell cycle distribution of CNE1 and CNE2 was
accessed following 24-h treatment with B63 (5 μM) or curcumin.
Therapy of B63 led to an increase in the G2/M phase, from 4 to 26%
in CNE1 and from 4 to 24% in CNE2. Furthermore, B63 arrested NPC
cells in G2/M phase in a time-dependent manner, after 36-h therapy,
B63 arrested more cells in G2/M phase (47% in CNE1 and 41% in
CNE2), whereas, curcumin at the same concentration did not induce
significant change in the cell cycle (Fig. 2B). By 36 h, decrease in cyclin B
expression was also observed in B63-treated cells relative to
controls (Fig. 2C). As our
previous studies demonstrated Jab1/ P27 pathway plays an important
role in the pathogenesis of NPC (16), we also evaluated the effect of B63
on this pathway and found that B63 inhibited Jab1 and consequently
increased P27 (Fig. 2C).
B63 promotes apoptosis in NPC cells
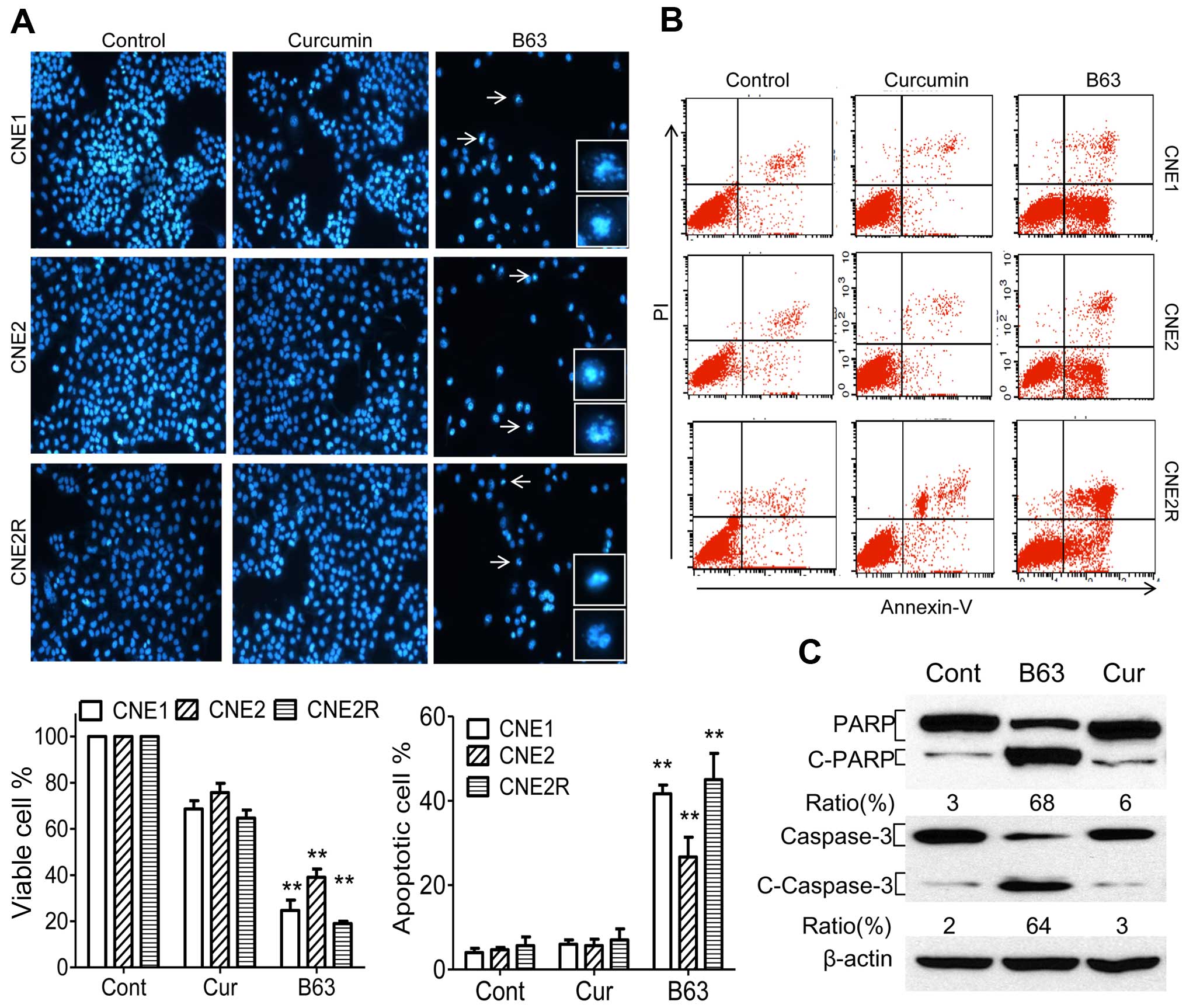
Next, it was determined if the B63-induced cell
viability suppression was followed by increases in apoptosis. The
effect of B63 on the induction of apoptosis using Hoechst
fluorescence and flow cytometry was analyzed. B63 induced
morphological apoptotic characteristic in NPC cells (Fig. 3A). Control cells and curcumin
treated cells showed excellent growth status. Treatment with B63
resulted in a remarkable decrease in the number of viable CNE1
cells (76%), CNE2 cells (61%) and CNE2R cells (81%) compared with
control cells (Fig. 3A).
Flow cytometric analysis confirmed the above
morphological observations. There was no significant difference in
apoptosis induction between curcumin treated group and control
group. However, after the same concentration of B63 treatment, the
ratios of apoptosis were 42% (CNE1), 27% (CNE2) and 45% (CNE2R),
respectively Fig. 3B. Because
cleavage of PARP and caspase-3 activation are indication of the
beginning of apoptosis (19,20),
the influence of B63 on CNE2 cells was further examined. As
expected, B63 induced more significant PARP and caspase-3 cleavage
than curcumin (Fig. 3C). These
data suggested an enhanced activity of B63 in inducing apoptosis in
NPC cells.
B63 specifically activated the ER stress
pathway
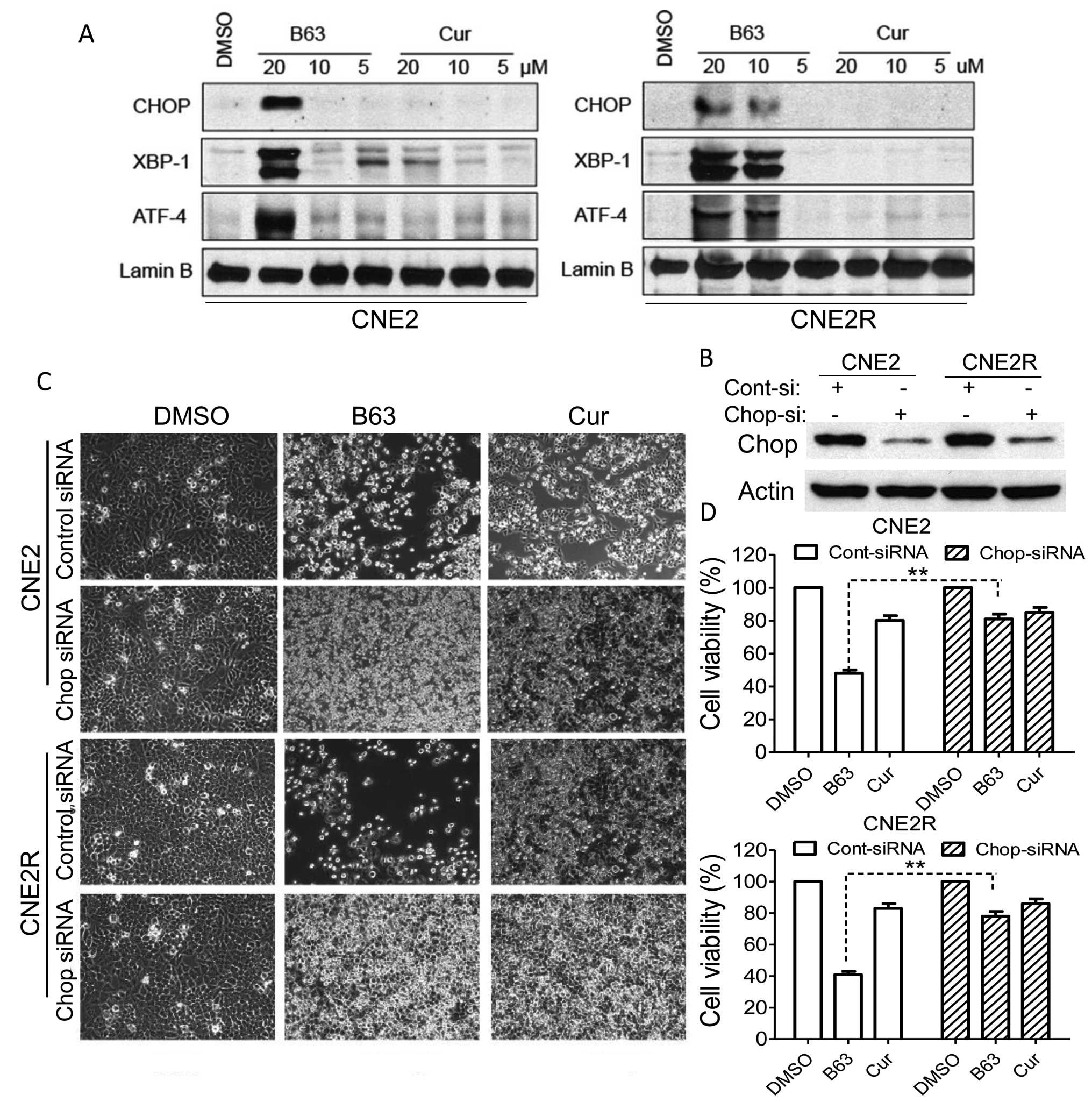
CHOP, ATF-4 and XBP-1 are markers of ER stress
(21) and prolonged activation of
CHOP can trigger apoptosis in cells (22). In this study, B63 significantly
elevated the CHOP, XBP-1, ATF-4 protein levels, whereas curcumin
did not induce any change in these markers (Fig. 4A). The data demonstrated that
B63-induced ER stress may represent a major mechanism of anticancer
function. For further confirmation that ER stress plays a major
role in B63-induced apoptosis, lentiviral siRNA targetting CHOP
gene was produced and used to infect NPC cells. The reduction of
CHOP expression was proven using western blot assay (Fig. 4B). In addition, to prove that CHOP
levels affected B63 activity in NPC cells, CHOP siRNA-transfected
NPC cells were treated with curcumin and B63 and it was found that
cell apoptosis induced by B63 in the CHOP deficient cells was
significantly reduced when compared to the control cells (Fig. 4C). Besides, depletion of CHOP
attenuates the cell viability inhibition induced by B63 as measured
by MTT assay (Fig. 4D). These
results pointed out that B63- induced cell apoptosis is, at least
partly, mediated via the ER stress pathway.
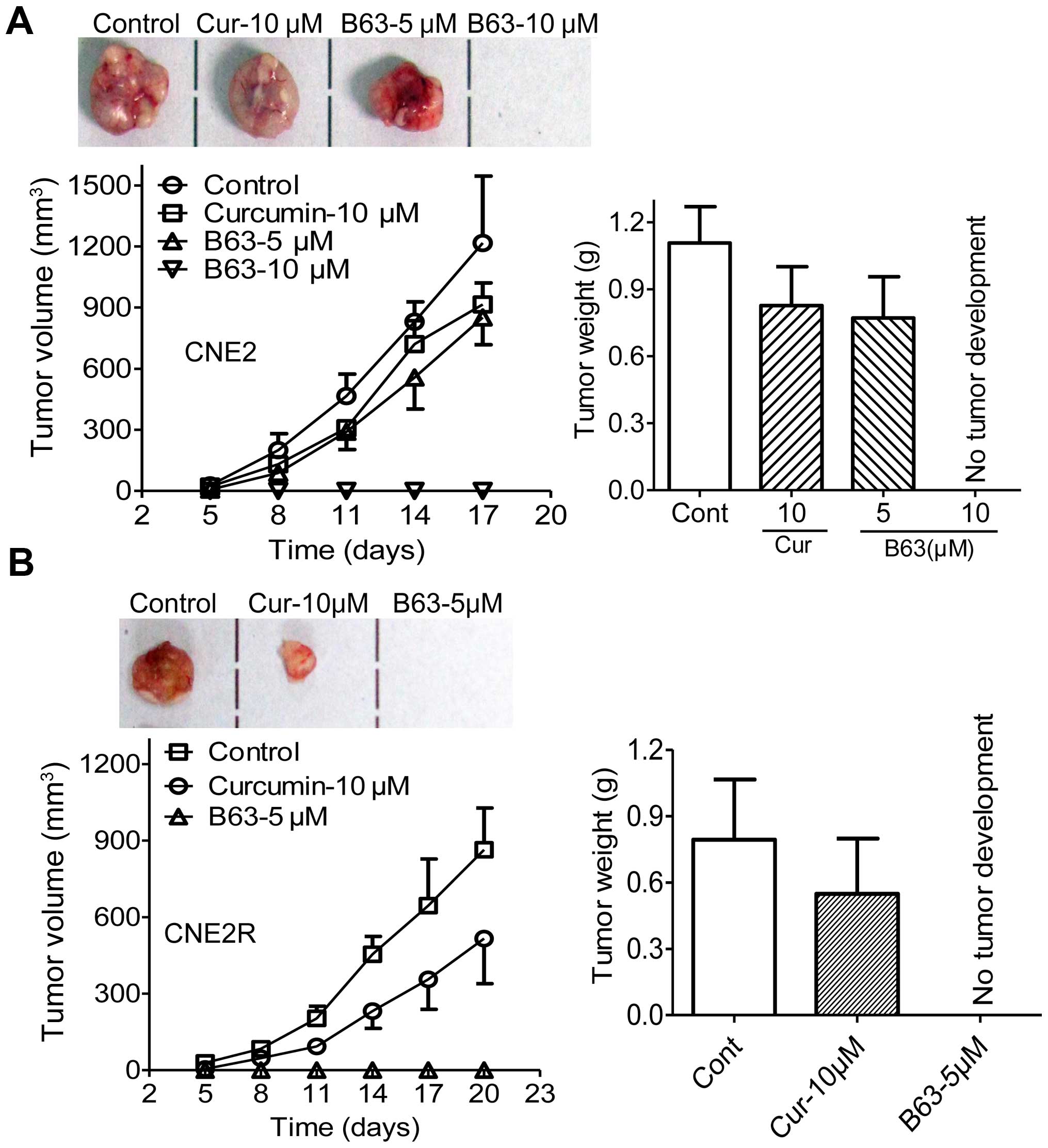
B63 represses tumorigenicity of NPC
cells
Tumorigenicity assay in nude mice was performed to
validate the B63 anti-tumor activity, CNE2 and CNE2R cells were
treated with B63 (5 or 10 μM), curcumin (10 μM) or vehicle (DMSO)
for 12 h and were then injected subcutaneously into the flank of
nude mice, and tumor formation was monitored. B63 effectively
suppressed the tumor growth in mice bearing CNE2 and CNE2R cells
(Fig. 5), reducing the tumor
weight by 30% compared with the CNE2 control group. Furthermore, we
did not observe tumor development in mice bearing CNE2 cells with
10 μM B63 pretreatment or CNE2R cells with 5 μM B63 pretreatment,
supporting that B63 is more powerful than curcumin in preventing
NPC tumorigenicity.
Discussion
Curcumin, commonly called turmeric, is a natural
polyphenol derived from the Curcuma longa, and is capable of
inhibiting and treating different cancers (23,24).
Nevertheless, the anti-tumor activity of curcumin is severely
affected by its rapid metabolism (10,25,26).
Studies have reported the structural modification of curcumin and
the anticancer activity of its analogs (27). Curcumin is unstable at pH >6.5
because of its highly reactive β-diketone moiety (28). A series of monocarbonyl analogs of
curcumin have been designed by removing the highly reactive
β-diketone moiety in the structure of curcumin. One such compound
is B63 which displayed a higher chemical stability in culture
medium (15). B63 exerted
antitumor activity on lung cancer cells by the induction of
apoptosis, which involves the ER stress signaling pathway (15). In this study, it was demonstrated
that B63 is more powerful than curcumin in preventing cell
viability and propagation and further inducing G2/M arrest and
apoptosis in NPC cells. The data obtained in this study also
demonstrated that B63-induced apoptosis promoted PARP and caspase-3
activation. Although curcumin-induced apoptosis through the
activation of caspase-3 has been previously reported (29), we did not see cleavage of PARP and
caspase-3 changes in the curcumin treated NPC cells, which may due
to the low concentration (5 μM).
ER regulates the protein synthesis, folding and
trafficking. When signals disturb the ER function and cause ER
stress, the ER stress response is a balance between prosurvival and
proapoptotic signaling pathways (21). Cells undergo apoptosis when the
prosurvival responses fail (21,30),
which is consistant with our study that B63-induced apoptosis in
NPC cells. It was found that B63 could increase XBP1, ATF-4 and
CHOP, triggering ER stress-specific cascade. The upregulations of
CHOP, XBP-1 and ATF-1 in the nuclei of NPC cells after B63 therapy
(Fig. 4A) suggest that the
B63-induced ER stress was developed into the commitment phase
toward apoptosis. Cleavage and activation of procaspase-3 have been
noted in different studies on ER stress-induced human cancer cell
apoptosis (22,31,32).
Our data also showed that B63 induced caspase-3 cleavage.
Collectively, our data demonstrated that ER stress markers were
induced by B63, suggesting that B63-induced apoptosis is associated
with ER stress.
CHOP is considered to be a marker of commitment of
ER stress-induced apoptosis (33).
Knockdown of CHOP decreased ER stress-mediated apoptosis in human
cancer cell (34–36). In agreement with these reports, our
data also showed that depletion of CHOP attenuated the B63
antitumor activity. Besides the cellular effects, B63 prevented
tumor growth in the nude mouse model.
However, other apoptotic pathways may also
participate in the B63-induced apoptosis. For example,
mitochondria-mediated apoptotic pathway where caspase-3 activation
plays an important role (37).
Additionally, curcumin has been reported to exert anticancer
activity through multi-targeting mechanisms (12,23,38).
Further studies are necessary to detect the in vivo
pharmacodynamics of B63 as a candidate for inhibiting and treating
different cancers.
Since many tumor cells are deficient in G1/S cell
cycle checkpoint, they are more dependent on G2/M checkpoints to
allow time for DNA repair. Previous studies demonstrated that tumor
cells in the G2/M phase are more sensitive to radiotherapy
(39,40). In this study, the number of NPC
cells in the G2/M phase increased significantly after exposure to
B63. Thus, the link between B63-induced G2/M arrest and
hyper-radiosensitivity needs to be investigated in the future. A
more detailed understanding of the mechanisms of B63 induced-G2/M
checkpoint activation is thus essential for the development of
radiotherapy sensitizer.
In conclusion, our data indicated that B63 displayed
enhanced anticancer effects on NPC through an ER stress-mediated
pathway, suggested B63 could become part of an effective
therapeutic regimen for NPC.
Acknowledgements
This study was supported by funds from the National
Natural Science Foundation of China (81270597) and the Fundamental
Research Funds for the Central Universities (JUSRP115A31).
References
|
1
|
Lo KW, Chung GT and To KF: Deciphering the
molecular genetic basis of NPC through molecular, cytogenetic, and
epigenetic approaches. Semin Cancer Biol. 22:79–86. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hui EP, Ma BB, Leung SF, King AD, Mo F,
Kam MK, Yu BK, Chiu SK, Kwan WH, Ho R, et al: Randomized phase II
trial of concurrent cisplatin-radiotherapy with or without
neoadjuvant docetaxel and cisplatin in advanced nasopharyngeal
carcinoma. J Clin Oncol. 27:242–249. 2009. View Article : Google Scholar
|
|
3
|
Chan AT: Nasopharyngeal carcinoma. Ann
Oncol. 21(Suppl 7): vii308–vii312. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yip KW, Mocanu JD, Au PY, Sleep GT, Huang
D, Busson P, Yeh W-C, Gilbert R, O'Sullivan B, Gullane P, et al:
Combination bcl-2 antisense and radiation therapy for
nasopharyngeal cancer. Clin Cancer Res. 11:8131–8144. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lev-Ari S, Lichtenberg D and Arber N:
Compositions for treatment of cancer and inflammation. Recent
Patents Anticancer Drug Discov. 3:55–62. 2008. View Article : Google Scholar
|
|
6
|
Gandhy SU, Kim K, Larsen L, Rosengren RJ
and Safe S: Curcumin and synthetic analogs induce reactive oxygen
species and decreases specificity protein (Sp) transcription
factors by targeting microRNAs. BMC Cancer. 12:5642012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Pan Y, Xiao J, Liang G, Wang M, Wang D,
Wang S and Yang H: A new curcumin analogue exhibits enhanced
antitumor activity in nasopharyngeal carcinoma. Oncol Rep.
30:239–245. 2013.PubMed/NCBI
|
|
8
|
Lin SS, Huang HP, Yang JS, Wu JY, Hsia TC,
Lin CC, Lin CW, Kuo CL, Gibson Wood W and Chung JG: DNA damage and
endoplasmic reticulum stress mediated curcumin-induced cell cycle
arrest and apoptosis in human lung carcinoma A-549 cells through
the activation caspases cascade- and mitochondrial-dependent
pathway. Cancer Lett. 272:77–90. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bakhshi J, Weinstein L, Poksay KS,
Nishinaga B, Bredesen DE and Rao RV: Coupling endoplasmic reticulum
stress to the cell death program in mouse melanoma cells: effect of
curcumin. Apoptosis. 13:904–914. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Anand P, Kunnumakkara AB, Newman RA and
Aggarwal BB: Bioavailability of curcumin: Problems and promises.
Mol Pharm. 4:807–818. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yogosawa S, Yamada Y, Yasuda S, Sun Q,
Takizawa K and Sakai T: Dehydrozingerone, a structural analogue of
curcumin, induces cell-cycle arrest at the G2/M phase and
accumulates intracellular ROS in HT-29 human colon cancer cells. J
Nat Prod. 75:2088–2093. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Subramaniam D, May R, Sureban SM, Lee KB,
George R, Kuppusamy P, Ramanujam RP, Hideg K, Dieckgraefe BK,
Houchen CW, et al: Diphenyl difluoroketone: A curcumin derivative
with potent in vivo anticancer activity. Cancer Res. 68:1962–1969.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liang G, Yang S, Zhou H, Shao L, Huang K,
Xiao J, Huang Z and Li X: Synthesis, crystal structure and
anti-inflammatory properties of curcumin analogues. Eur J Med Chem.
44:915–919. 2009. View Article : Google Scholar
|
|
14
|
Liang G, Shao L, Wang Y, Zhao C, Chu Y,
Xiao J, Zhao Y, Li X and Yang S: Exploration and synthesis of
curcumin analogues with improved structural stability both in vitro
and in vivo as cytotoxic agents. Bioorg Med Chem. 17:2623–2631.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Xiao J, Wang Y, Peng J, Guo L, Hu J, Cao
M, Zhang X, Zhang H, Wang Z, Li X, et al: A synthetic compound,
1,5-bis(2-methoxyphenyl)penta-1,4-dien-3-one (B63), induces
apoptosis and activates endoplasmic reticulum stress in non-small
cell lung cancer cells. Int J Cancer. 131:1455–1465. 2012.
View Article : Google Scholar
|
|
16
|
Pan Y, Zhang Q, Tian L, Wang X, Fan X,
Zhang H, Claret FX and Yang H: Jab1/CSN5 negatively regulates p27
and plays a role in the pathogenesis of nasopharyngeal carcinoma.
Cancer Res. 72:1890–1900. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Pan Y, Wang M, Bu X, Zuo Y, Wang S, Wang
D, Liu Q, Su B, Xu T, Wang C, et al: Curcumin analogue T83 exhibits
potent antitumor activity and induces radiosensitivity through
inactivation of Jab1 in nasopharyngeal carcinoma. BMC Cancer.
13:3232013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Pan Y, Zhang Q, Atsaves V, Yang H and
Claret FX: Suppression of Jab1/CSN5 induces radio- and
chemo-sensitivity in nasopharyngeal carcinoma through changes to
the DNA damage and repair pathways. Oncogene. 32:2756–2766. 2013.
View Article : Google Scholar :
|
|
19
|
Lazebnik YA, Kaufmann SH, Desnoyers S,
Poirier GG and Earnshaw WC: Cleavage of poly(ADP-ribose) polymerase
by a proteinase with properties like ICE. Nature. 371:346–347.
1994. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Nicholson DW, Ali A, Thornberry NA,
Vaillancourt JP, Ding CK, Gallant M, Gareau Y, Griffin PR, Labelle
M, Lazebnik YA, et al: Identification and inhibition of the
ICE/CED-3 protease necessary for mammalian apoptosis. Nature.
376:37–43. 1995. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tabas I and Ron D: Integrating the
mechanisms of apoptosis induced by endoplasmic reticulum stress.
Nat Cell Biol. 13:184–190. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Szegezdi E, Logue SE, Gorman AM and Samali
A: Mediators of endoplasmic reticulum stress-induced apoptosis.
EMBO Rep. 7:880–885. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hatcher H, Planalp R, Cho J, Torti FM and
Torti SV: Curcumin: From ancient medicine to current clinical
trials. Cell Mol Life Sci. 65:1631–1652. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Strimpakos AS and Sharma RA: Curcumin:
preventive and therapeutic properties in laboratory studies and
clinical trials. Antioxid Redox Signal. 10:511–545. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sharma RA, Euden SA, Platton SL, Cooke DN,
Shafayat A, Hewitt HR, Marczylo TH, Morgan B, Hemingway D, Plummer
SM, et al: Phase I clinical trial of oral curcumin: biomarkers of
systemic activity and compliance. Clin Cancer Res. 10:6847–6854.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Dhillon N, Aggarwal BB, Newman RA, Wolff
RA, Kunnumakkara AB, Abbruzzese JL, Ng CS, Badmaev V and Kurzrock
R: Phase II trial of curcumin in patients with advanced pancreatic
cancer. Clin Cancer Res. 14:4491–4499. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Padhye S, Chavan D, Pandey S, Deshpande J,
Swamy KV and Sarkar FH: Perspectives on chemopreventive and
therapeutic potential of curcumin analogs in medicinal chemistry.
Mini Rev Med Chem. 10:372–387. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tomren MA, Másson M, Loftsson T and
Tønnesen HH: Studies on curcumin and curcuminoids XXXI. Symmetric
and asymmetric curcuminoids: Stability, activity and complexation
with cyclodextrin. Int J Pharm. 338:27–34. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Moragoda L, Jaszewski R and Majumdar AP:
Curcumin induced modulation of cell cycle and apoptosis in gastric
and colon cancer cells. Anticancer Res. 21A:873–878. 2001.
|
|
30
|
Dolai S, Pal S, Yadav RK and Adak S:
Endoplasmic reticulum stress-induced apoptosis in Leishmania
through Ca2+-dependent and caspase-independent
mechanism. J Biol Chem. 286:13638–13646. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Rasheva VI and Domingos PM: Cellular
responses to endoplasmic reticulum stress and apoptosis. Apoptosis.
14:996–1007. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Schleicher SM, Moretti L, Varki V and Lu
B: Progress in the unraveling of the endoplasmic reticulum
stress/autophagy pathway and cancer: Implications for future
therapeutic approaches. Drug Resist Updat. 13:79–86. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Oyadomari S and Mori M: Roles of
CHOP/GADD153 in endoplasmic reticulum stress. Cell Death Differ.
11:381–389. 2004. View Article : Google Scholar
|
|
34
|
Huang SM, Cheung CW, Chang CS, Tang CH,
Liu JF, Lin YH, Chen JH, Ko SH, Wong KL and Lu DY: Phloroglucinol
derivative MCPP induces cell apoptosis in human colon cancer. J
Cell Biochem. 112:643–652. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ma J, Qiu Y, Yang L, Peng L, Xia Z, Hou
LN, Fang C, Qi H and Chen HZ: Desipramine induces apoptosis in rat
glioma cells via endoplasmic reticulum stress-dependent CHOP
pathway. J Neurooncol. 101:41–48. 2011. View Article : Google Scholar
|
|
36
|
Wali VB, Bachawal SV and Sylvester PW:
Endoplasmic reticulum stress mediates gamma-tocotrienol-induced
apoptosis in mammary tumor cells. Apoptosis. 14:1366–1377. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Shelton SN, Dillard CD and Robertson JD:
Activation of caspase-9, but not caspase-2 or caspase-8, is
essential for heat-induced apoptosis in Jurkat cells. J Biol Chem.
285:40525–40533. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Freudlsperger C, Greten J and Schumacher
U: Curcumin induces apoptosis in human neuroblastoma cells via
inhibition of NFkappaB. Anticancer Res. 28A:209–214. 2008.
|
|
39
|
Krueger SA, Wilson GD, Piasentin E, Joiner
MC and Marples B: The effects of G2-phase enrichment and checkpoint
abrogation on low-dose hyper-radiosensitivity. Int J Radiat Oncol
Biol Phys. 77:1509–1517. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Fernet M, Mégnin-Chanet F, Hall J and
Favaudon V: Control of the G2/M checkpoints after exposure to low
doses of ionising radiation: Implications for
hyper-radiosensitivity. DNA Repair (Amst). 9:48–57. 2010.
View Article : Google Scholar
|