Neutrophils are innate immune cells that protect the
host from infection by eliminating the invading pathogens. In
recent years, these cells have been shown to play important roles
in other pathological conditions including cancer. Neutrophils make
up a significant portion of the inflammatory cell infiltrate in
cancer, whereby they show high functional plasticity and display
both antitumor and pro-tumor activities (1). The antitumor effects of neutrophils
are related to their cytotoxicity and the regulation of antitumor
immune responses, which has been denominated as N1 neutrophils. In
addition, tumor derived signals can induce a pro-tumor phenotype in
neutrophils, which supports tumor growth and metastasis (N2
neutrophils). N2 polarized neutrophils promote the proliferation,
migration, and invasion of tumor cells, stimulate angiogenesis, as
well as mediate immunosuppression (2,3).
Moreover, increased number of neutrophils in blood and tumors has
been linked to poor clinical outcome. Strategies designed to
inhibit the pro-tumor activities of neutrophils have shown
promising anticancer effects. In this review, we summarize the
recent findings on the functional roles of neutrophils in cancer.
We mainly focus on the molecular mechanisms that modulate the
phenotypic and functional plasticity of neutrophils. The diagnostic
value and therapeutic potential of neutrophils in cancer is also
discussed.
The antitumor activities of neutrophils are
supported by several lines of evidence. Neutrophils limit tumor
growth and metastasis through distinct mechanisms including direct
and antibody-dependent cytotoxic activity as well as the activation
of other innate and adaptive immune cells such as T cells, B cells,
natural killer (NK) cells, and dendritic cells (DCs).
Neutrophils produce a number of antimicrobial
mediators that have potential tumoricidal activity, including
reactive oxygen species (ROS), myeloperoxidase (MPO), hydrogen
peroxide (H2O2), and proteases. Neutrophils
from healthy donors have potent cytotoxicity against tumor cells
(4). The administration of
neutrophils from healthy donors reduces experimental tumor growth
and extends the survival of tumor-bearing animals (5). After stimulation with cytokines,
neutrophils release ROS to trigger oxidative damage and consequent
apoptotic cell death in melanoma cells (6). In addition, neutrophils could inhibit
the metastatic potential of tumor cells. Granot and colleagues
demonstrated that neutrophils generate H2O2
to suppress metastatic seeding of breast cancer cells in the lungs
of mice (7), suggesting that
neutrophils could prevent tumor metastasis via the generation of
cytotoxic substances (8).
Neutrophils are critical effector cells that mediate
the antitumor effects of mAb-mediated immunotherapy.
Antibody-targeting cells could be destroyed by immune cells that
express Fc receptors (FcR). Neutrophils express the family members
of FcγR. The interactions between neutrophils and mAb through FcR
induce the release of tumoricidal mediators (9,10).
In several tumor models mAb-induced tumor reduction is abolished in
mice with depleted neutrophils. In FcR-deficient mice, the transfer
of normal neutrophils or transgenic expression of FcR restore the
antitumor effects of mAb, suggesting that neutrophils are required
for effective, mAb-induced cancer immunotherapy.
In addition to direct and antibody-dependent
cytotoxic effects on tumor cells, neutrophils could also recruit
and activate immune cells to elicit antitumor immune responses
(11–15). Neutrophils release a wide array of
factors including cytokines, chemokines, and proteases that have
promoting roles in the proliferation and cytokine production of T
cells. Neutrophils isolated from the surgically resected human lung
cancer tissues could stimulate T cell proliferation and IFN-γ
release (16). Neutrophils could
efficiently process and present antigens to directly stimulate
immune response. Moreover, TLR-stimulated neutrophils induce
enhanced cytotoxicity and cytokine production in NK cells and
trigger the maturation of dendritic cells, promoting T cell
proliferation and IFN-γ production (17).
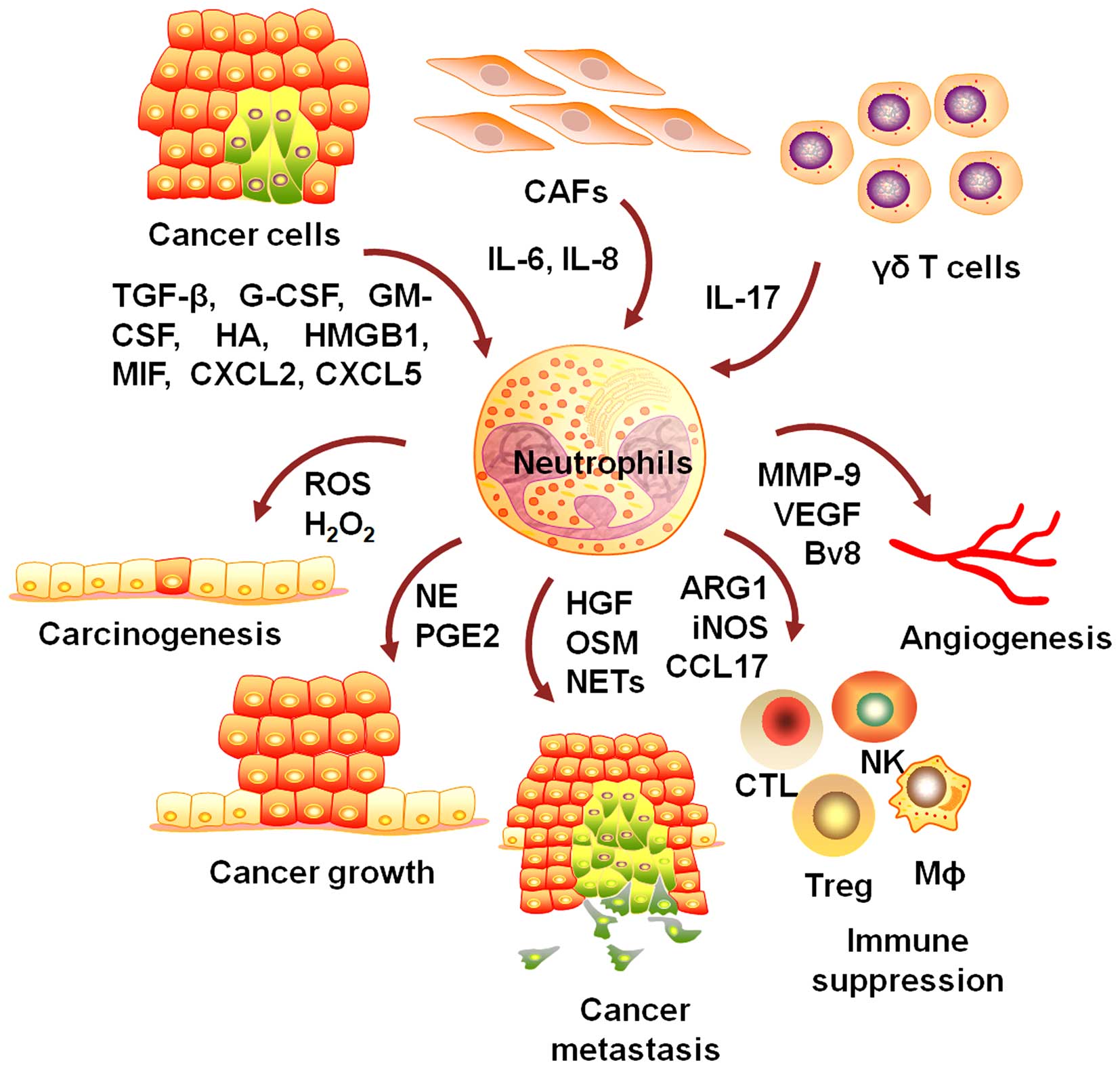
There is mounting evidence showing that neutrophils
are critically involved in the development and progression of
cancer (18). Neutrophils play
important roles in neoplastic transformation, tumor growth and
metastasis, angiogenesis, and the modulation of immunosuppression
(Fig. 1).
The accumulation of genetic instability is
associated with increased cancer risk. Neutrophils release
genotoxic substances to inflict DNA damage on epithelial cells and
initiate carcinogenic response (19–22).
Exposure to activated neutrophils increases the number of
replication errors in colon epithelial cells (23). In colitis-associated colon cancer
(CAC) mouse model, depletion of neutrophils markedly reduces the
number and size of tumors, indicating a crucial role for
neutrophils in the initiation and progression of CAC (24–26).
Lakritz et al demonstrate that neutrophils are critical for
mammary tumor development because systemic depletion of neutrophils
entirely inhibits tumorigenesis (27). Wilson and colleagues demonstrated
that neutrophils stimulate the production of ROS and telomere DNA
damage in hepatocytes and promote diethylnitrosamine (DEN)-induced
hepatocellular carcinoma (HCC) (28). Yan et al further
demonstrated the promoting role of neutrophils in
hepatocarcinogenesis by using a zebrafish model (29).
Neutrophils generate and release a wide spectrum of
factors to support tumor cell growth in vitro and in
vivo (30). Neutrophil
elastase (NE) was able to enter into tumor cells to degrade insulin
receptor substrate-1 (IRS-1), resulting in increased interaction
between PI3K and PDGFR and accelerated tumor cell proliferation
(31,32). Neutrophils could promote tumor cell
proliferation through COX-2-mediated prostaglandin E2 (PGE2)
synthesis (33,34). Antonio et al suggested that
acute wound (such as tumor biopsy) induces rapid recruitment of
neutrophils to interact with nearby neoplastic cells, leading to
increased proliferation of the neoplastic cells through PGE2
(35). Neutrophils enhanced the
proliferation of glioblastoma-initiating cells through the
upregulation of S100A4 expression (36). Neutrophils could promote the
proliferation of renal cell carcinoma (RCC) cells via modulating
androgen receptor (AR)/c-Myc signals (37). Moreover, neutrophils from B-cell
lymphoma patients induce stromal cell activation to promote the
growth of germinal center B-cell lymphoma cells (38). Neutrophils were able to promote
multiple myeloma (MM) survival from doxorubicin and melphalan by
secretion of soluble factors (39).
Neutrophils recruited by LPS-induced inflammation
could release proteinases such as catheptin G and elastase to
degrade thrombospondin-1 (Tsp-1) and facilitate lung metastasis
(44). When stimulated with GM-CSF
from breast cancer cells, neutrophils release a high level of
oncostatin M (OSM), which in turn promotes the detachment of breast
cancer cells (45). Wu et
al demonstrate that hyaluronan (HA) from tumor cells activates
neutrophils, which in turn effectively enhances the motility of
tumor cells via a cell contact-dependent mechanism (46). Macrophage migration inhibitory
factor (MIF) from human head and neck squamous cell carcinoma
(HNSCC) cells could activate neutrophils, which in turn enhances
the migratory properties of HNSCC cells (47). Moreover, G-CSF from breast cancer
cells expand and mobilize neutrophils to release of Bv8, resulting
in the promotion of metastasis (48).
Neutrophils could induce epithelial-to-mesenchymal
transition (EMT) in tumor cells, which significantly increases the
migratory and invasive capacity of tumor cells (49–52).
Neutrophils increase bladder cancer cell invasion through the
modulation of androgen receptor (AR)/MMP13 signals (53). In addition, neutrophils could
promote renal cell carcinoma cell migration and invasion via the
activation of VEGFa/HIF2α and estrogen receptor β signals (54). Moreover, neutrophils could also
diminish immune protection to promote metastasis. Coffelt et
al demonstrate that gamma delta (γδ) T cell-derived IL-17
induce G-CSF-dependent expansion and activation of neutrophils,
which inhibits cytotoxic CD8+ T lymphocytes and helps
establish metastases (55).
Neutrophils may serve as a carrier to assist tumor
cell extravasation. Tumor-elicited neutrophils bind to tumor cells
and facilitate tumor cell migration, which is dependent on the
expression of intercellular adhesion molecule-1 (ICAM-1) on tumor
cells and CD11b on neutrophils (56,57).
In vivo, neutrophils regulate lung metastasis through
physical interaction and anchoring of circulating tumor cells to
endothelium (58). Neutrophils
promote cancer cell adhesion within liver sinusoids, however,
neutrophil depletion impairs the formation of liver metastasis
(59,60).
Neutrophils synthesize and release a number of
molecules to activate endothelial cells and induce angiogenesis
(61,62). Shojaei et al suggest that
tumor derived G-CSF upregulates Bv8 expression, which mobilizes
neutrophils to promote angiogenesis (63). MMP-9 is implicated in VEGF
activation to induce and maintain angiogenesis. Neutrophils are
found to be the major sources of MMP-9 (64–66).
Human neutrophils uniquely release TIMP-free MMP-9 to provide a
potent stimulator of angiogenesis (67). Tumor infiltrating
neutrophil-derived MMP-9 coordinately regulate tumor angiogenesis
and tumor cell intravasation. Specific inhibition of neutrophil
accumulation results in the coordinated inhibition of tumor
angiogenesis and intravasation (68).
Tumor-elicited neutrophils could inhibit the
proliferation of T cells through the release of arginase 1 (ARG1)
and the modulation of PD-L1/PD-1 signaling (69–72).
Neutrophils isolated from the circulation of tumor-bearing mice
contribute to the survival of tumor cells by suppressing peripheral
leukocyte activation (73). A
subset of neutrophils with low density is enriched in the
peripheral blood of cancer patients and display immature phenotype
similar to that of MDSCs (74).
Fridlender and colleagues have compared tumor-associated
neutrophils (TANs) with granulocytic myeloid-derived suppressor
cells (G-MDSCs) by using transcriptomic analysis and found that the
two cell populations are significantly different in their mRNA
profiles, pointing out the differences between TANs and MDSCs
(75). Hypoxia within the primary
tumor sites induce increased infiltration of immunosuppressive
neutrophils into the lung, where these cells compromise NK cell
cytotoxicity, resulting in a reduced antitumor response that allows
metastasis formation (76).
Neutrophils inhibit NK cell function to increase the intraluminal
survival of tumor cells, facilitating tumor cell extravasation and
metastatic dissemination (77). In
addition, neutrophils isolated from murine tumor tissues secrete
significant amounts of CCL17 to progressively attract Tregs during
tumor development (78). CCL17
secretion is relevant to the number of tumor infiltrating
neutrophils in human lung cancer patients, suggesting that
neutrophils may suppress antitumor immunity and promote tumor
growth by regulating Tregs. Zhou et al demonstrated that
TANs recruit macrophages and Treg cells to promote HCC growth,
progression, and resistance to sorafenib (79). The expansion of myeloid cells
contributes to tumor progression. Using a multistage mouse model of
breast cancer, Casbon et al demonstrate that the invasive
breast cancer reprograms early differentiation of myeloid cells in
the bone marrow to generate immunosuppressive neutrophils (80).
Neutrophil extracellular traps (NETs) is a web-like
structure to trap and kill invading microorganisms (81). The contribution of NETs to tumor
has recently been demonstrated (82–84).
Cancer-associated thrombosis is linked to a poor prognosis and
represents the second-leading cause of death in cancer patients.
Using a murine model of chronic myelogenous leukemia, Demers et
al demonstrated that cancers predispose the release of NETs to
cause cancer-associated thrombosis (85,86).
Cools-Lartigue et al have shown that circulating tumor cells
are trapped within NETs in vitro under both static and
dynamic conditions. In a murine model of lung cancer, deposition of
NETs and consequent trapping of circulating lung carcinoma cells
are associated with increased hepatic metastasis (87). Neutrophils isolated from mouse
models of pancreatic ductal adenocarcinoma (PDA) have shown an
increased ability to form NETs (88). Guglietta et al showed that
increased circulating lipopolysaccharide induces upregulation of
complement C3a receptor on neutrophils and activation of the
complement cascade, which leads to NETosis and N2 polarization of
neutrophils, inducing coagulation and promoting spontaneous
intestinal tumorigenesis (89).
Moreover, neutrophil extracellular traps have been shown to promote
the development and progression of liver metastases after surgical
stress (90).
The origin of the infiltrating neutrophils in tumor
has not been well characterized. Cortez-Retamozo et al
demonstrated that the spleen is an important origin of tumor
associated neutrophils. The precursors of neutrophils relocate from
the spleen to the tumor stroma during tumor progression. Removal of
the spleen reduces the number of the infiltrating neutrophils and
delays tumor growth (91). A large
number of molecules from tumor cells have been shown to recruit
neutrophils. IL-8 is one of the potent neutrophil chemoattractants.
Tumor cells with IL-8 overexpression recruit more neutrophils and
display increased metastatic potential (92). IL-17 recruits blood neutrophils
into the peritumoral stroma of hepatocellular carcinoma by inducing
expression of chemokines in epithelial cells (66). Wu et al also suggest that
tumor-infiltrating DCs induce the activation of IL-17 producing γδT
cells to promote the accumulation and expansion of
immunosuppressive neutrophils in colon cancer (93).
Tumor-derived oxysterols could recruit neutrophils
to favor tumor growth by promoting angiogenesis and
immunosuppression (94,95). CXCL5 has a direct chemoattractant
effect on neutrophils. CXCL5 overexpression is positively
correlated with neutrophil infiltration in hepatocellular carcinoma
and intrahepatic cholangiocarcinoma patients (96,97).
UV irradiation-damaged epidermal keratinocytes release high
mobility group box 1 (HMGB1) to recruit and activate neutrophils by
interacting with toll-like receptor 4 (TLR4), which stimulates
angiogenesis and promotes the ability of melanoma cells to
metastasize (98). Leukotriene B4
(LTB4), an inflammation mediator, induces the recruitment of
neutrophils via interaction with BLT1 on neutrophils (30). Neutrophils from HNSCC patients
display a significantly reduced apoptosis compared to those from
healthy donors, which may be associated with the secretion of MIF
by HNSCC cells (99). Hypoxia
induces an HIF-1α-dependent activation of NF-κB to inhibit
neutrophil apoptosis (100). Li
et al demonstrated that the prolonged survival of
neutrophils in tumor is associated with increased autophagy.
Neutrophils in HCC intratumoral regions undergo increased autophagy
and display long-lived phenotypes and sustained production of
pro-metastatic factors (101).
IFN-β negatively regulates the survival and recruitment of
neutrophils. In the absence of endogenous IFN-β the life span of
neutrophils from blood and tumors of IFN-β deficient mice is
remarkably prolonged (102,103). On the contrary, MET is required
for the recruitment of antitumor neutrophils (104). Met deletion in mouse neutrophils
enhances tumor growth and metastasis.
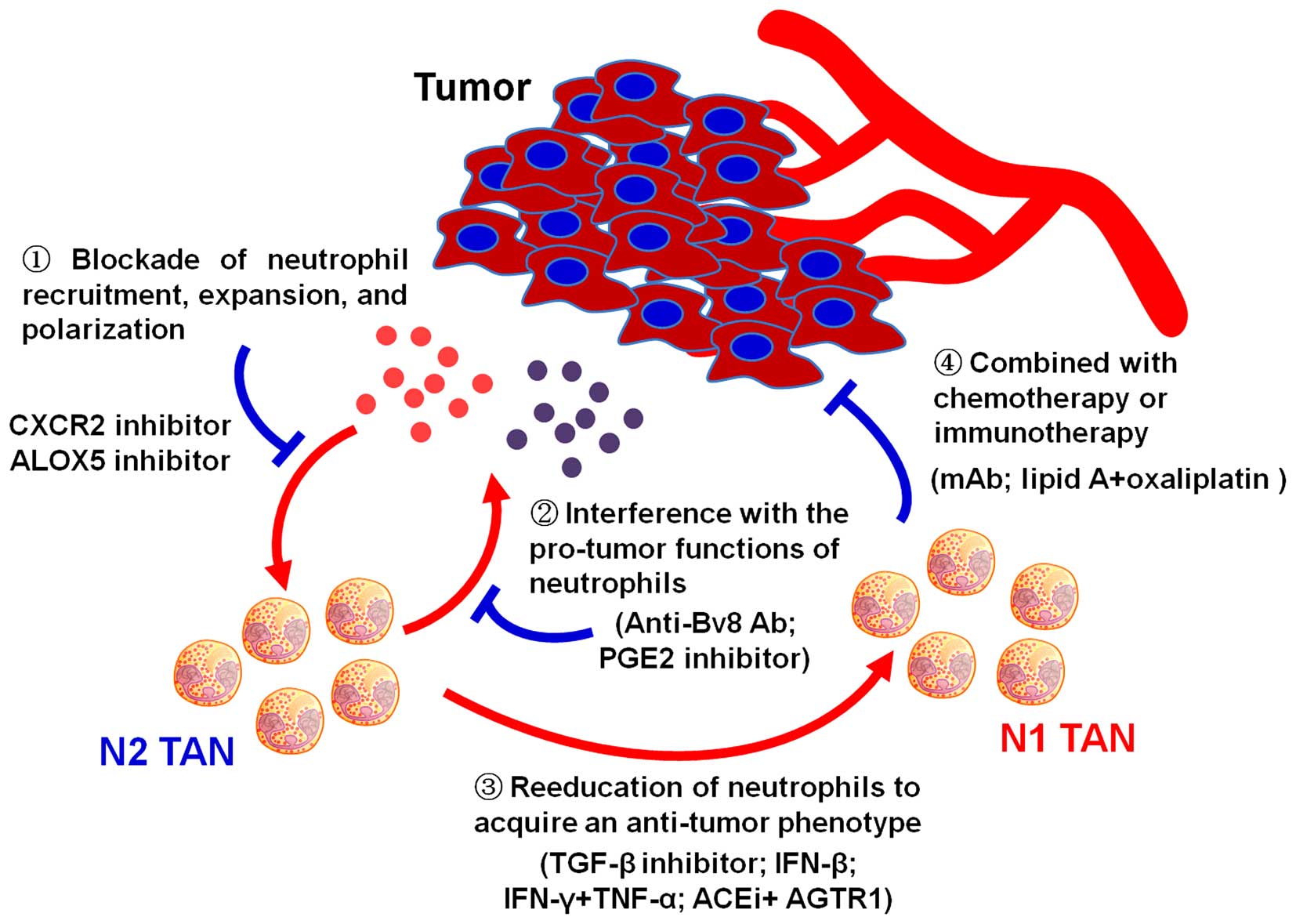
The potent drivers of neutrophil polarization have
recently been demonstrated. Inhibition of TGF-β increases the
expression of chemokines that recruit neutrophils, resulting in an
influx of neutrophils that has strong cytotoxic activity to tumor
cells. Following TGF-β blockade, depletion of these neutrophils
significantly attenuates antitumor effects of treatment and reduces
CD8+ T cell activation. In contrast, in control tumors,
neutrophil depletion decreases tumor growth and results in more
activated CD8+ T cells within tumor, suggesting that
tumor associated neutrophils are driven by TGF-β to acquire N2
protumoral phenotype. In contrast, TGF-β inhibition induces an
antitumor N1 phenotype (107).
The anti- and pro-tumor functions of neutrophils imply its
diversity and plasticity. Sagiv et al have identified a
heterogeneous subset of low density neutrophils (LDNs) that
progressively accumulate in tumors. LDNs consist of both immature
MDSCs and mature cells that are derived from HDNs in a
TGF-β-dependent mechanism (108).
The plasticity of neutrophils has been determined in mouse tumor
models at different time points during tumor progression.
Neutrophils are mainly located at the peritumoral tissues at early
stage of tumor development while these cells are found scattered in
tumor cells at later stage. Neutrophils isolated from tumors at
early stage are more cytotoxic toward tumor cells and produce
higher levels of NO and H2O2. In established
tumors, these functions are decreased and these cells acquire a
more protumorigenic phenotype, suggesting the critical role of
tumor niche in modulating neutrophil phenotype and function. In
line with this phenotype, only depletion of neutrophils at later
stage of tumor development inhibits tumor growth, indicating the
functional changes in neutrophils with tumor progression (109).
In the absence of endogenous IFN-β, mice develop a
fast-growing tumor accompanied with increased infiltration of
neutrophils which produce a large amount of VEGF and MMP-9 to
promote tumor angiogenesis and metastasis. In vitro
treatment with recombinant IFN-β inhibit the activation of STAT3
pathway and the upregulation of VEGF and MMP-9 genes in tumor
infiltrating neutrophils. In addition, the transplantation of
neutrophils from control mice into IFN-β-deficient mice retards
tumor growth, suggesting that IFN-β may be a factor that maintain
the N1 polarization of neutrophils. The conversion of neutrophil
phenotype and function may occur in the bone marrow of
tumor-bearing mice (110).
In addition to tumor cells, the microenvironmental
cells also participate in the regulation of neutrophil biology in
cancer. Tumor-resident mesenchymal stem cell (MSC)-derived IL-6
induced N2 polarized activation of neutrophils (111). Intriguingly, Hu et al
demonstrated that TNFα-primed mouse MSCs could program neutrophils
into an immunosuppressive and tumor-promoting phenotype (112). Moreover, in response to tumor
derived IL-1β signal, tumor infiltrating γδ T cells release IL-17
to recruit, expand, and activate neutrophils to promote cancer
metastasis. Taken together, these findings indicate that
neutrophils are polarized during tumor progression by the signals
from tumor milieu (Table I).
Cancer-related inflammation plays a key role in
tumor progression. The increased neutrophil infiltration in tumor
is associated with poor outcome in renal cell carcinoma (113), head and neck squamous cell
carcinoma (114), melanoma
(115), lung carcinoma (116,117), colorectal carcinoma (118), gastric carcinoma (119), cholangiocarcinoma (97), hepatocellular carcinoma (120), tongue squamous cell carcinoma
(121), and esophageal squamous
cell carcinoma (122,123). High intratumoral neutrophil is
positively correlated with lymph node metastasis, tumor grade, and
tumor stage. High densities of neutrophils in tumor are identified
as an independent risk factor for poor prognosis (124). In addition, a high
neutrophil-to-lymphocyte ratio (NLR) has also been suggested as a
poor prognostic indicator in cancer (125). Moreover, the high numbers of
neutrophils and NLR in cancer patients are associated with poor
response to chemotherapy and immunotherapy (126).
The roles of neutrophils in the pathogenesis of
cancer have recently become an intense research area. Neutrophils
have both pro-tumor and antitumor activities. Neutrophils are
frequently recruited to local tumor sites, whereby neutrophils can
be polarized towards distinct phenotypes by tumor derived signals.
In turn, neutrophils suppress or promote tumor development and
progression by cell contact-dependent mechanism or secretion of
soluble factors. Herein, we summarize the roles of neutrophils in
cancer and their potential as cancer diagnosis biomarker and
therapy target. Although early studies indicate that neutrophils
have direct cytotoxicity against tumor cells and regulate the
functions of innate and adaptive immune cells, more recent reports
have shown that neutrophils promote tumor development and
progression by enhancing tumor cell growth and metastasis,
stimulating tumor angiogenesis, and mediating immunosuppression.
Previous studies have mainly focused on experimental animal models
of cancer (135), however, more
studies are needed to elucidate the cellular and molecular
mechanisms that modulate the phenotype and function of neutrophils
in human tumor, such as recruitment to the tumor site, prolonged
survival and enhanced release of tumor-promoting factors. In
addition, further studies are needed to elucidate the relationship
between heterogeneous neutrophil subsets. Moreover, novel
strategies to reeducate the tumor-promoting neutrophils to activate
the host’s innate and adaptive immune responses will provide new
approaches for tumor therapy.
This study was supported by the National Natural
Science Foundation of China (81201660), the Natural Science
Foundation of the Jiangsu Province (BK20141303), the Key Research
and Development Project of Zhenjiang (SH2015034), the Jiangsu Key
Laboratory of Medical Science and Laboratory Medicine Project
(JSKLM-2014-006), Jiangsu Province’s Qing Lan project, Foundation
for Young Academic Leader of Jiangsu University, Starting
Foundation for Senior Talents of Jiangsu University (13JDG086).
|
1
|
Piccard H, Muschel RJ and Opdenakker G: On
the dual roles and polarized phenotypes of neutrophils in tumor
development and progression. Crit Rev Oncol Hematol. 82:296–309.
2012. View Article : Google Scholar
|
|
2
|
Brandau S, Dumitru CA and Lang S: Protumor
and antitumor functions of neutrophil granulocytes. Semin
Immunopathol. 35:163–176. 2013. View Article : Google Scholar
|
|
3
|
Dumitru CA, Lang S and Brandau S:
Modulation of neutrophil granulocytes in the tumor
microenvironment: Mechanisms and consequences for tumor
progression. Semin Cancer Biol. 23:141–148. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yan J, Kloecker G, Fleming C, Bousamra M
II, Hansen R, Hu X, Ding C, Cai Y, Xiang D, Donninger H, et al:
Human polymorphonuclear neutrophils specifically recognize and kill
cancerous cells. OncoImmunology. 3:e9501632014. View Article : Google Scholar
|
|
5
|
Jaganjac M, Poljak-Blazi M, Kirac I,
Borovic S, Joerg Schaur R and Zarkovic N: Granulocytes as effective
anticancer agent in experimental solid tumor models. Immunobiology.
215:1015–1020. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Dissemond J, Weimann TK, Schneider LA,
Schneeberger A, Scharffetter-Kochanek K, Goos M and Wagner SN:
Activated neutrophils exert antitumor activity against human
melanoma cells: Reactive oxygen species-induced mechanisms and
their modulation by granulocyte-macrophage-colony-stimulating
factor. J Invest Dermatol. 121:936–938. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Granot Z, Henke E, Comen EA, King TA,
Norton L and Benezra R: Tumor entrained neutrophils inhibit seeding
in the premetastatic lung. Cancer Cell. 20:300–314. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
López-Lago MA, Posner S, Thodima VJ,
Molina AM, Motzer RJ and Chaganti RS: Neutrophil chemokines
secreted by tumor cells mount a lung antimetastatic response during
renal cell carcinoma progression. Oncogene. 32:1752–1760. 2013.
View Article : Google Scholar
|
|
9
|
Stockmeyer B, Beyer T, Neuhuber W, Repp R,
Kalden JR, Valerius T and Herrmann M: Polymorphonuclear
granulocytes induce antibody-dependent apoptosis in human breast
cancer cells. J Immunol. 171:5124–5129. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Albanesi M, Mancardi DA, Jönsson F,
Iannascoli B, Fiette L, Di Santo JP, Lowell CA and Bruhns P:
Neutrophils mediate antibody-induced antitumor effects in mice.
Blood. 122:3160–3164. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mayadas TN, Cullere X and Lowell CA: The
multifaceted functions of neutrophils. Annu Rev Pathol. 9:181–218.
2014. View Article : Google Scholar :
|
|
12
|
Amulic B, Cazalet C, Hayes GL, Metzler KD
and Zychlinsky A: Neutrophil function: From mechanisms to disease.
Annu Rev Immunol. 30:459–489. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kolaczkowska E and Kubes P: Neutrophil
recruitment and function in health and inflammation. Nat Rev
Immunol. 13:159–175. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Scapini P and Cassatella MA: Social
networking of human neutrophils within the immune system. Blood.
124:710–719. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mantovani A, Cassatella MA, Costantini C
and Jaillon S: Neutrophils in the activation and regulation of
innate and adaptive immunity. Nat Rev Immunol. 11:519–531. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Eruslanov EB, Bhojnagarwala PS, Quatromoni
JG, Stephen TL, Ranganathan A, Deshpande C, Akimova T, Vachani A,
Litzky L, Hancock WW, et al: Tumor-associated neutrophils stimulate
T cell responses in early-stage human lung cancer. J Clin Invest.
124:5466–5480. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Riise RE, Bernson E, Aurelius J, Martner
A, Pesce S, Della Chiesa M, Marcenaro E, Bylund J, Hellstrand K,
Moretta L, et al: TLR-stimulated neutrophils instruct NK cells to
trigger dendritic cell maturation and promote adaptive T cell
responses. J Immunol. 195:1121–1128. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Powell DR and Huttenlocher A: Neutrophils
in the tumor micro-environment. Trends Immunol. 37:41–52. 2016.
View Article : Google Scholar
|
|
19
|
Haqqani AS, Sandhu JK and Birnboim HC:
Expression of inter-leukin-8 promotes neutrophil infiltration and
genetic instability in mutatect tumors. Neoplasia. 2:561–568. 2000.
View Article : Google Scholar
|
|
20
|
Sandhu JK, Privora HF, Wenckebach G and
Birnboim HC: Neutrophils, nitric oxide synthase, and mutations in
the mutatect murine tumor model. Am J Pathol. 156:509–518. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Knaapen AM, Güngör N, Schins RP, Borm PJ
and Van Schooten FJ: Neutrophils and respiratory tract DNA damage
and mutagenesis: A review. Mutagenesis. 21:225–236. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Güngör N, Knaapen AM, Munnia A, Peluso M,
Haenen GR, Chiu RK, Godschalk RW and van Schooten FJ: Genotoxic
effects of neutrophils and hypochlorous acid. Mutagenesis.
25:149–154. 2010. View Article : Google Scholar
|
|
23
|
Campregher C, Luciani MG and Gasche C:
Activated neutrophils induce an hMSH2-dependent G2/M checkpoint
arrest and replication errors at a (CA)13-repeat in colon
epithelial cells. Gut. 57:780–787. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Shang K, Bai YP, Wang C, Wang Z, Gu HY, Du
X, Zhou XY, Zheng CL, Chi YY, Mukaida N, et al: Crucial involvement
of tumor-associated neutrophils in the regulation of chronic
colitis-associated carcinogenesis in mice. PLoS One. 7:e518482012.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang Y, Wang K, Han GC, Wang RX, Xiao H,
Hou CM, Guo RF, Dou Y, Shen BF, Li Y, et al: Neutrophil
infiltration favors colitis-associated tumorigenesis by activating
the interleukin-1 (IL-1)/ IL-6 axis. Mucosal Immunol. 7:1106–1115.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ning C, Li YY, Wang Y, Han GC, Wang RX,
Xiao H, Li XY, Hou CM, Ma YF, Sheng DS, et al: Complement
activation promotes colitis-associated carcinogenesis through
activating intestinal IL-1β/IL-17A axis. Mucosal Immunol.
8:1275–1284. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lakritz JR, Poutahidis T, Mirabal S,
Varian BJ, Levkovich T, Ibrahim YM, Ward JM, Teng EC, Fisher B,
Parry N, et al: Gut bacteria require neutrophils to promote mammary
tumorigenesis. Oncotarget. 6:9387–9396. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wilson CL, Jurk D, Fullard N, Banks P,
Page A, Luli S, Elsharkawy AM, Gieling RG, Chakraborty JB, Fox C,
et al: NFκB1 is a suppressor of neutrophil-driven hepatocellular
carcinoma. Nat Commun. 6:68182015. View Article : Google Scholar
|
|
29
|
Yan C, Huo X, Wang S, Feng Y and Gong Z:
Stimulation of hepatocarcinogenesis by neutrophils upon induction
of oncogenic kras expression in transgenic zebrafish. J Hepatol.
63:420–428. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Satpathy SR, Jala VR, Bodduluri SR,
Krishnan E, Hegde B, Hoyle GW, Fraig M, Luster AD and Haribabu B:
Crystalline silica-induced leukotriene B4-dependent inflammation
promotes lung tumour growth. Nat Commun. 6:70642015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Houghton AM, Rzymkiewicz DM, Ji H, Gregory
AD, Egea EE, Metz HE, Stolz DB, Land SR, Marconcini LA, Kliment CR,
et al: Neutrophil elastase-mediated degradation of IRS-1
accelerates lung tumor growth. Nat Med. 16:219–223. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Gong L, Cumpian AM, Caetano MS, Ochoa CE,
De la Garza MM, Lapid DJ, Mirabolfathinejad SG, Dickey BF, Zhou Q
and Moghaddam SJ: Promoting effect of neutrophils on lung
tumorigenesis is mediated by CXCR2 and neutrophil elastase. Mol
Cancer. 12:1542013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hattar K, Franz K, Ludwig M, Sibelius U,
Wilhelm J, Lohmeyer J, Savai R, Subtil FS, Dahlem G, Eul B, et al:
Interactions between neutrophils and non-small cell lung cancer
cells: Enhancement of tumor proliferation and inflammatory mediator
synthesis. Cancer Immunol Immunother. 63:1297–1306. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ma X, Aoki T, Tsuruyama T and Narumiya S:
Definition of prostaglandin E2-EP2 signals in the colon tumor
microenvironment that amplify inflammation and tumor growth. Cancer
Res. 75:2822–2832. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Antonio N, Bønnelykke-Behrndtz ML, Ward
LC, Collin J, Christensen IJ, Steiniche T, Schmidt H, Feng Y and
Martin P: The wound inflammatory response exacerbates growth of
pre-neoplastic cells and progression to cancer. EMBO J.
34:2219–2236. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Liang J, Piao Y, Holmes L, Fuller GN,
Henry V, Tiao N and de Groot JF: Neutrophils promote the malignant
glioma phenotype through S100A4. Clin Cancer Res. 20:187–198. 2014.
View Article : Google Scholar
|
|
37
|
Song W, Li L, He D, Xie H, Chen J, Yeh CR,
Chang LS, Yeh S and Chang C: Infiltrating neutrophils promote renal
cell carcinoma (RCC) proliferation via modulating androgen receptor
(AR) → c-Myc signals. Cancer Lett. 368:71–78. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Grégoire M, Guilloton F, Pangault C,
Mourcin F, Sok P, Latour M, Amé-Thomas P, Flecher E, Fest T and
Tarte K: Neutrophils trigger a NF-κB dependent polarization of
tumor-supportive stromal cells in germinal center B-cell lymphomas.
Oncotarget. 6:16471–16487. 2015. View Article : Google Scholar
|
|
39
|
Ramachandran IR, Condamine T, Lin C,
Herlihy SE, Garfall A, Vogl DT, Gabrilovich DI and Nefedova Y: Bone
marrow PMN-MDSCs and neutrophils are functionally similar in
protection of multiple myeloma from chemotherapy. Cancer Lett.
371:117–124. 2016. View Article : Google Scholar
|
|
40
|
Liang W and Ferrara N: The complex role of
neutrophils in tumor angiogenesis and metastasis. Cancer Immunol
Res. 4:83–91. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Psaila B and Lyden D: The metastatic
niche: Adapting the foreign soil. Nat Rev Cancer. 9:285–293. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Tazawa H, Okada F, Kobayashi T, Tada M,
Mori Y, Une Y, Sendo F, Kobayashi M and Hosokawa M: Infiltration of
neutrophils is required for acquisition of metastatic phenotype of
benign murine fibrosarcoma cells: Implication of
inflammation-associated carcinogenesis and tumor progression. Am J
Pathol. 163:2221–2232. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Welch DR, Schissel DJ, Howrey RP and Aeed
PA: Tumor-elicited polymorphonuclear cells, in contrast to ‘normal’
circulating poly-morphonuclear cells, stimulate invasive and
metastatic potentials of rat mammary adenocarcinoma cells. Proc
Natl Acad Sci USA. 86:5859–5863. 1989. View Article : Google Scholar
|
|
44
|
El Rayes T, Catena R, Lee S, Stawowczyk M,
Joshi N, Fischbach C, Powell CA, Dannenberg AJ, Altorki NK, Gao D,
et al: Lung inflammation promotes metastasis through neutrophil
protease-mediated degradation of Tsp-1. Proc Natl Acad Sci USA.
112:16000–16005. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Queen MM, Ryan RE, Holzer RG, Keller-Peck
CR and Jorcyk CL: Breast cancer cells stimulate neutrophils to
produce oncostatin M: Potential implications for tumor progression.
Cancer Res. 65:8896–8904. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Wu Y, Zhao Q, Peng C, Sun L, Li XF and
Kuang DM: Neutrophils promote motility of cancer cells via a
hyaluronan-mediated TLR4/PI3K activation loop. J Pathol.
225:438–447. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Dumitru CA, Gholaman H, Trellakis S,
Bruderek K, Dominas N, Gu X, Bankfalvi A, Whiteside TL, Lang S and
Brandau S: Tumor-derived macrophage migration inhibitory factor
modulates the biology of head and neck cancer cells via neutrophil
activation. Int J Cancer. 129:859–869. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Kowanetz M, Wu X, Lee J, Tan M, Hagenbeek
T, Qu X, Yu L, Ross J, Korsisaari N, Cao T, et al:
Granulocyte-colony stimulating factor promotes lung metastasis
through mobilization of Ly6G+Ly6C+ granulocytes. Proc Natl Acad Sci
USA. 107:21248–21255. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Gaida MM, Steffen TG, Günther F,
Tschaharganeh DF, Felix K, Bergmann F, Schirmacher P and Hänsch GM:
Polymorphonuclear neutrophils promote dyshesion of tumor cells and
elastase-mediated degradation of E-cadherin in pancreatic tumors.
Eur J Immunol. 42:3369–3380. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Grosse-Steffen T, Giese T, Giese N,
Longerich T, Schirmacher P, Hänsch GM and Gaida MM:
Epithelial-to-mesenchymal transition in pancreatic ductal
adenocarcinoma and pancreatic tumor cell lines: The role of
neutrophils and neutrophil-derived elastase. Clin Dev Immunol.
2012:7207682012. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Freisinger CM and Huttenlocher A: Live
imaging and gene expression analysis in zebrafish identifies a link
between neutrophils and epithelial to mesenchymal transition. PLoS
One. 9:e1121832014. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Hu P, Shen M, Zhang P, Zheng C, Pang Z,
Zhu L and Du J: Intratumoral neutrophil granulocytes contribute to
epithelial-mesenchymal transition in lung adenocarcinoma cells.
Tumour Biol. 36:7789–7796. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Lin C, Lin W, Yeh S, Li L and Chang C:
Infiltrating neutrophils increase bladder cancer cell invasion via
modulation of androgen receptor (AR)/MMP13 signals. Oncotarget.
6:43081–43089. 2015.PubMed/NCBI
|
|
54
|
Song W, Yeh CR, He D, Wang Y, Xie H, Pang
ST, Chang LS, Li L and Yeh S: Infiltrating neutrophils promote
renal cell carcinoma progression via VEGFa/HIF2α and estrogen
receptor β signals. Oncotarget. 6:19290–19304. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Coffelt SB, Kersten K, Doornebal CW,
Weiden J, Vrijland K, Hau CS, Verstegen NJ, Ciampricotti M,
Hawinkels LJ, Jonkers J, et al: IL-17-producing γδ T cells and
neutrophils conspire to promote breast cancer metastasis. Nature.
522:345–348. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Wu QD, Wang JH, Condron C, Bouchier-Hayes
D and Redmond HP: Human neutrophils facilitate tumor cell
transendothelial migration. Am J Physiol Cell Physiol.
280:C814–C822. 2001.PubMed/NCBI
|
|
57
|
Strell C, Lang K, Niggemann B, Zaenker KS
and Entschladen F: Neutrophil granulocytes promote the migratory
activity of MDA-MB-468 human breast carcinoma cells via ICAM-1. Exp
Cell Res. 316:138–148. 2010. View Article : Google Scholar
|
|
58
|
Huh SJ, Liang S, Sharma A, Dong C and
Robertson GP: Transiently entrapped circulating tumor cells
interact with neutrophils to facilitate lung metastasis
development. Cancer Res. 70:6071–6082. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Spicer JD, McDonald B, Cools-Lartigue JJ,
Chow SC, Giannias B, Kubes P and Ferri LE: Neutrophils promote
liver metastasis via Mac-1-mediated interactions with circulating
tumor cells. Cancer Res. 72:3919–3927. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Tabariès S, Ouellet V, Hsu BE, Annis MG,
Rose AA, Meunier L, Carmona E, Tam CE, Mes-Masson AM and Siegel PM:
Granulocytic immune infiltrates are essential for the efficient
formation of breast cancer liver metastases. Breast Cancer Res.
17:452015. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Tazzyman S, Lewis CE and Murdoch C:
Neutrophils: Key mediators of tumour angiogenesis. Int J Exp
Pathol. 90:222–231. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Tazzyman S, Niaz H and Murdoch C:
Neutrophil-mediated tumour angiogenesis: Subversion of immune
responses to promote tumour growth. Semin Cancer Biol. 23:149–158.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Shojaei F, Wu X, Zhong C, Yu L, Liang XH,
Yao J, Blanchard D, Bais C, Peale FV, van Bruggen N, et al: Bv8
regulates myeloid-cell-dependent tumour angiogenesis. Nature.
450:825–831. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Nozawa H, Chiu C and Hanahan D:
Infiltrating neutrophils mediate the initial angiogenic switch in a
mouse model of multistage carcinogenesis. Proc Natl Acad Sci USA.
103:12493–12498. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Deryugina EI, Zajac E, Juncker-Jensen A,
Kupriyanova TA, Welter L and Quigley JP: Tissue-infiltrating
neutrophils constitute the major in vivo source of
angiogenesis-inducing MMP-9 in the tumor microenvironment.
Neoplasia. 16:771–788. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Kuang DM, Zhao Q, Wu Y, Peng C, Wang J, Xu
Z, Yin XY and Zheng L: Peritumoral neutrophils link inflammatory
response to disease progression by fostering angiogenesis in
hepatocellular carcinoma. J Hepatol. 54:948–955. 2011. View Article : Google Scholar
|
|
67
|
Ardi VC, Kupriyanova TA, Deryugina EI and
Quigley JP: Human neutrophils uniquely release TIMP-free MMP-9 to
provide a potent catalytic stimulator of angiogenesis. Proc Natl
Acad Sci USA. 104:20262–20267. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Bekes EM, Schweighofer B, Kupriyanova TA,
Zajac E, Ardi VC, Quigley JP and Deryugina EI: Tumor-recruited
neutrophils and neutrophil TIMP-free MMP-9 regulate coordinately
the levels of tumor angiogenesis and efficiency of malignant cell
intravasation. Am J Pathol. 179:1455–1470. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Rodriguez PC, Ernstoff MS, Hernandez C,
Atkins M, Zabaleta J, Sierra R and Ochoa AC: Arginase I-producing
myeloid-derived suppressor cells in renal cell carcinoma are a
subpopulation of activated granulocytes. Cancer Res. 69:1553–1560.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Rotondo R, Barisione G, Mastracci L,
Grossi F, Orengo AM, Costa R, Truini M, Fabbi M, Ferrini S and
Barbieri O: IL-8 induces exocytosis of arginase 1 by neutrophil
polymorphonuclears in nonsmall cell lung cancer. Int J Cancer.
125:887–893. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
He G, Zhang H, Zhou J, Wang B, Chen Y,
Kong Y, Xie X, Wang X, Fei R, Wei L, et al: Peritumoural
neutrophils negatively regulate adaptive immunity via the
PD-L1/PD-1 signalling pathway in hepatocellular carcinoma. J Exp
Clin Cancer Res. 34:1412015. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Koyama S, Akbay EA, Li YY, Aref AR,
Skoulidis F, Herter-Sprie GS, Buczkowski KA, Liu Y, Awad MM,
Denning WL, et al: STK11/LKB1 deficiency promotes neutrophil
recruitment and proinflammatory cytokine production to suppress
T-cell activity in the lung tumor microenvironment. Cancer Res.
76:999–1008. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Zhang J, Qiao X, Shi H, Han X, Liu W, Tian
X and Zeng X: Circulating tumor-associated neutrophils (cTAN)
contribute to circulating tumor cell survival by suppressing
peripheral leukocyte activation. Tumour Biol. 37:5397–5404. 2016.
View Article : Google Scholar
|
|
74
|
Brandau S, Trellakis S, Bruderek K,
Schmaltz D, Steller G, Elian M, Suttmann H, Schenck M, Welling J,
Zabel P, et al: Myeloid-derived suppressor cells in the peripheral
blood of cancer patients contain a subset of immature neutrophils
with impaired migratory properties. J Leukoc Biol. 89:311–317.
2011. View Article : Google Scholar
|
|
75
|
Fridlender ZG, Sun J, Mishalian I, Singhal
S, Cheng G, Kapoor V, Horng W, Fridlender G, Bayuh R, Worthen GS,
et al: Transcriptomic analysis comparing tumor-associated
neutrophils with granulocytic myeloid-derived suppressor cells and
normal neutrophils. PLoS One. 7:e315242012. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Sceneay J, Chow MT, Chen A, Halse HM, Wong
CS, Andrews DM, Sloan EK, Parker BS, Bowtell DD, Smyth MJ, et al:
Primary tumor hypoxia recruits
CD11b+/Ly6Cmed/Ly6G+ immune suppressor cells
and compromises NK cell cytotoxicity in the premetastatic niche.
Cancer Res. 72:3906–3911. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Spiegel A, Brooks MW, Houshyar S,
Reinhardt F, Ardolino M, Fessler E, Chen MB, Krall JA, DeCock J,
Zervantonakis IK, et al: Neutrophils suppress intraluminal NK-cell
mediated tumor cell clearance and enhance extravasation of
disseminated carcinoma cells. Cancer Discov. 6:630–649. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Mishalian I, Bayuh R, Eruslanov E,
Michaeli J, Levy L, Zolotarov L, Singhal S, Albelda SM, Granot Z
and Fridlender ZG: Neutrophils recruit regulatory T-cells into
tumors via secretion of CCL17 a new mechanism of impaired antitumor
immunity. Int J Cancer. 135:1178–1186. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Zhou SL, Zhou ZJ, Hu ZQ, Huang XW, Wang Z,
Chen EB, Fan J, Cao Y, Dai Z and Zhou J: Tumor-associated
neutrophils recruit macrophages and T-regulatory cells to promote
progression of hepatocellular carcinoma and resistance to
sorafenib. Gastroenterology. S0016-5085(16)00231-6. 2016.
|
|
80
|
Casbon AJ, Reynaud D, Park C, Khuc E, Gan
DD, Schepers K, Passegué E and Werb Z: Invasive breast cancer
reprograms early myeloid differentiation in the bone marrow to
generate immunosuppressive neutrophils. Proc Natl Acad Sci USA.
112:E566–E575. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Branzk N and Papayannopoulos V: Molecular
mechanisms regulating NETosis in infection and disease. Semin
Immunopathol. 35:513–530. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Demers M and Wagner DD: NETosis: A new
factor in tumor progression and cancer-associated thrombosis. Semin
Thromb Hemost. 40:277–283. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Cools-Lartigue J, Spicer J, Najmeh S and
Ferri L: Neutrophil extracellular traps in cancer progression. Cell
Mol Life Sci. 71:4179–4194. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Fuchs TA, Brill A, Duerschmied D,
Schatzberg D, Monestier M, Myers DD Jr, Wrobleski SK, Wakefield TW,
Hartwig JH and Wagner DD: Extracellular DNA traps promote
thrombosis. Proc Natl Acad Sci USA. 107:15880–15885. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Demers M, Krause DS, Schatzberg D,
Martinod K, Voorhees JR, Fuchs TA, Scadden DT and Wagner DD:
Cancers predispose neutrophils to release extracellular DNA traps
that contribute to cancer-associated thrombosis. Proc Natl Acad Sci
USA. 109:13076–13081. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Demers M and Wagner DD: Neutrophil
extracellular traps: A new link to cancer-associated thrombosis and
potential implications for tumor progression. OncoImmunology.
2:e229462013. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Cools-Lartigue J, Spicer J, McDonald B,
Gowing S, Chow S, Giannias B, Bourdeau F, Kubes P and Ferri L:
Neutrophil extracellular traps sequester circulating tumor cells
and promote metastasis. J Clin Invest. 674842013.PubMed/NCBI
|
|
88
|
Cedervall J, Zhang Y, Huang H, Zhang L,
Femel J, Dimberg A and Olsson AK: Neutrophil extracellular traps
accumulate in peripheral blood vessels and compromise organ
function in tumor-bearing animals. Cancer Res. 75:2653–2662. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Guglietta S, Chiavelli A, Zagato E, Krieg
C, Gandini S, Ravenda PS, Bazolli B, Lu B, Penna G and Rescigno M:
Coagulation induced by C3aR-dependent NETosis drives protumorigenic
neutrophils during small intestinal tumorigenesis. Nat Commun.
7:110372016. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Tohme S, Yazdani HO, Al-Khafaji AB, Chidi
AP, Loughran P, Mowen K, Wang Y, Simmons RL, Huang H and Tsung A:
Neutrophil extracellular traps promote the development and
progression of liver metastases after surgical stress. Cancer Res.
76:1367–1380. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Cortez-Retamozo V, Etzrodt M, Newton A,
Rauch PJ, Chudnovskiy A, Berger C, Ryan RJ, Iwamoto Y, Marinelli B,
Gorbatov R, et al: Origins of tumor-associated macrophages and
neutrophils. Proc Natl Acad Sci USA. 109:2491–2496. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
92
|
De Larco JE, Wuertz BR and Furcht LT: The
potential role of neutrophils in promoting the metastatic phenotype
of tumors releasing interleukin-8. Clin Cancer Res. 10:4895–4900.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Wu P, Wu D, Ni C, Ye J, Chen W, Hu G, Wang
Z, Wang C, Zhang Z, Xia W, et al: γδT17 cells promote the
accumulation and expansion of myeloid-derived suppressor cells in
human colorectal cancer. Immunity. 40:785–800. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Raccosta L, Fontana R, Maggioni D,
Lanterna C, Villablanca EJ, Paniccia A, Musumeci A, Chiricozzi E,
Trincavelli ML, Daniele S, et al: The oxysterol-CXCR2 axis plays a
key role in the recruitment of tumor-promoting neutrophils. J Exp
Med. 210:1711–1728. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Raccosta L, Fontana R, Traversari C and
Russo V: Oxysterols recruit tumor-supporting neutrophils within the
tumor micro-environment: The many facets of tumor-derived
oxysterols. OncoImmunology. 2:e264692013. View Article : Google Scholar
|
|
96
|
Zhou SL, Dai Z, Zhou ZJ, Wang XY, Yang GH,
Wang Z, Huang XW, Fan J and Zhou J: Overexpression of CXCL5
mediates neutrophil infiltration and indicates poor prognosis for
hepatocellular carcinoma. Hepatology. 56:2242–2254. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Zhou SL, Dai Z, Zhou ZJ, Chen Q, Wang Z,
Xiao YS, Hu ZQ, Huang XY, Yang GH, Shi YH, et al: CXCL5 contributes
to tumor metastasis and recurrence of intrahepatic
cholangio-carcinoma by recruiting infiltrative intratumoral
neutrophils. Carcinogenesis. 35:597–605. 2014. View Article : Google Scholar
|
|
98
|
Bald T, Quast T, Landsberg J, Rogava M,
Glodde N, Lopez-Ramos D, Kohlmeyer J, Riesenberg S, van den
Boorn-Konijnenberg D, Hömig-Hölzel C, et al:
Ultraviolet-radiation-induced inflammation promotes angiotropism
and metastasis in melanoma. Nature. 507:109–113. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Trellakis S, Farjah H, Bruderek K, Dumitru
CA, Hoffmann TK, Lang S and Brandau S: Peripheral blood neutrophil
granulocytes from patients with head and neck squamous cell
carcinoma functionally differ from their counterparts in healthy
donors. Int J Immunopathol Pharmacol. 24:683–693. 2011.PubMed/NCBI
|
|
100
|
Walmsley SR, Print C, Farahi N,
Peyssonnaux C, Johnson RS, Cramer T, Sobolewski A, Condliffe AM,
Cowburn AS, Johnson N, et al: Hypoxia-induced neutrophil survival
is mediated by HIF-1alpha-dependent NF-kappaB activity. J Exp Med.
201:105–115. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Li XF, Chen DP, Ouyang FZ, Chen MM, Wu Y,
Kuang DM and Zheng L: Increased autophagy sustains the survival and
pro-tumourigenic effects of neutrophils in human hepatocellular
carcinoma. J Hepatol. 62:131–139. 2015. View Article : Google Scholar
|
|
102
|
Andzinski L, Wu CF, Lienenklaus S, Kröger
A, Weiss S and Jablonska J: Delayed apoptosis of tumor associated
neutrophils in the absence of endogenous IFN-β. Int J Cancer.
136:572–583. 2015.
|
|
103
|
Jablonska J, Wu CF, Andzinski L, Leschner
S and Weiss S: CXCR2-mediated tumor-associated neutrophil
recruitment is regulated by IFN-β. Int J Cancer. 134:1346–1358.
2014. View Article : Google Scholar
|
|
104
|
Finisguerra V, Di Conza G, Di Matteo M,
Serneels J, Costa S, Thompson AA, Wauters E, Walmsley S, Prenen H,
Granot Z, et al: MET is required for the recruitment of
anti-tumoural neutrophils. Nature. 522:349–353. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Galli SJ, Borregaard N and Wynn TA:
Phenotypic and functional plasticity of cells of innate immunity:
Macrophages, mast cells and neutrophils. Nat Immunol. 12:1035–1044.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Gabrilovich DI, Ostrand-Rosenberg S and
Bronte V: Coordinated regulation of myeloid cells by tumours. Nat
Rev Immunol. 12:253–268. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Fridlender ZG, Sun J, Kim S, Kapoor V,
Cheng G, Ling L, Worthen GS and Albelda SM: Polarization of
tumor-associated neutrophil phenotype by TGF-beta: ‘N1’ versus ‘N2’
TAN. Cancer Cell. 16:183–194. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Sagiv JY, Michaeli J, Assi S, Mishalian I,
Kisos H, Levy L, Damti P, Lumbroso D, Polyansky L, Sionov RV, et
al: Phenotypic diversity and plasticity in circulating neutrophil
subpopulations in cancer. Cell Rep. 10:562–573. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Mishalian I, Bayuh R, Levy L, Zolotarov L,
Michaeli J and Fridlender ZG: Tumor-associated neutrophils (TAN)
develop pro-tumorigenic properties during tumor progression. Cancer
Immunol Immunother. 62:1745–1756. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Yan B, Wei JJ, Yuan Y, Sun R, Li D, Luo J,
Liao SJ, Zhou YH, Shu Y, Wang Q, et al: IL-6 cooperates with G-CSF
to induce protumor function of neutrophils in bone marrow by
enhancing STAT3 activation. J Immunol. 190:5882–5893. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Zhu Q, Zhang X, Zhang L, Li W, Wu H, Yuan
X, Mao F, Wang M, Zhu W, Qian H, et al: The IL-6-STAT3 axis
mediates a reciprocal crosstalk between cancer-derived mesenchymal
stem cells and neutrophils to synergistically prompt gastric cancer
progression. Cell Death Dis. 5:e12952014. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Hu X, Zhou Y, Dong K, Sun Z, Zhao D, Wang
W, Yu G, Liu W, Xu G, Han Z, et al: Programming of the development
of tumor-promoting neutrophils by mesenchymal stromal cells. Cell
Physiol Biochem. 33:1802–1814. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Jensen HK, Donskov F, Marcussen N,
Nordsmark M, Lundbeck F and von der Maase H: Presence of
intratumoral neutrophils is an independent prognostic factor in
localized renal cell carcinoma. J Clin Oncol. 27:4709–4717. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Trellakis S, Bruderek K, Dumitru CA,
Gholaman H, Gu X, Bankfalvi A, Scherag A, Hütte J, Dominas N,
Lehnerdt GF, et al: Polymorphonuclear granulocytes in human head
and neck cancer: Enhanced inflammatory activity, modulation by
cancer cells and expansion in advanced disease. Int J Cancer.
129:2183–2193. 2011. View Article : Google Scholar
|
|
115
|
Jensen TO, Schmidt H, Møller HJ, Donskov
F, Høyer M, Sjoegren P, Christensen IJ and Steiniche T:
Intratumoral neutrophils and plasmacytoid dendritic cells indicate
poor prognosis and are associated with pSTAT3 expression in AJCC
stage I/II melanoma. Cancer. 118:2476–2485. 2012. View Article : Google Scholar
|
|
116
|
Chen X, Sun J, Song Y, Gao P, Zhao J,
Huang X, Liu B, Xu H and Wang Z: The novel long noncoding RNA
AC138128.1 may be a predictive biomarker in gastric cancer. Med
Oncol. 31:2622014. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Yang SZ, Ji WH, Mao WM and Ling ZQ:
Elevated levels of preoperative circulating CD44+
lymphocytes and neutrophils predict poor survival for non-small
cell lung cancer patients. Clin Chim Acta. 439:172–177. 2015.
View Article : Google Scholar
|
|
118
|
Rao HL, Chen JW, Li M, Xiao YB, Fu J, Zeng
YX, Cai MY and Xie D: Increased intratumoral neutrophil in
colorectal carcinomas correlates closely with malignant phenotype
and predicts patients’ adverse prognosis. PLoS One. 7:e308062012.
View Article : Google Scholar
|
|
119
|
Zhao JJ, Pan K, Wang W, Chen JG, Wu YH, Lv
L, Li JJ, Chen YB, Wang DD, Pan QZ, et al: The prognostic value of
tumor-infiltrating neutrophils in gastric adenocarcinoma after
resection. PLoS One. 7:e336552012. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Li YW, Qiu SJ, Fan J, Zhou J, Gao Q, Xiao
YS and Xu YF: Intra-tumoral neutrophils: A poor prognostic factor
for hepatocellular carcinoma following resection. J Hepatol.
54:497–505. 2011. View Article : Google Scholar
|
|
121
|
Wang N, Feng Y, Wang Q, Liu S, Xiang L,
Sun M, Zhang X, Liu G, Qu X and Wei F: Neutrophils infiltration in
the tongue squamous cell carcinoma and its correlation with CEACAM1
expression on tumor cells. PLoS One. 9:e899912014. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Hu P, Pang Z, Shen H, Wang G, Sun H and Du
J: Tumor-infiltrating neutrophils predict poor outcome in
adenocarcinoma of the esophagogastric junction. Tumour Biol.
36:2965–2971. 2015. View Article : Google Scholar
|
|
123
|
Wang J, Jia Y, Wang N, Zhang X, Tan B,
Zhang G and Cheng Y: The clinical significance of
tumor-infiltrating neutrophils and neutrophil-to-CD8+
lymphocyte ratio in patients with resectable esophageal squamous
cell carcinoma. J Transl Med. 12:72014. View Article : Google Scholar
|
|
124
|
Shen M, Hu P, Donskov F, Wang G, Liu Q and
Du J: Tumor-associated neutrophils as a new prognostic factor in
cancer: A systematic review and meta-analysis. PLoS One.
9:e982592014. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Templeton AJ, McNamara MG, Šeruga B,
Vera-Badillo FE, Aneja P, Ocaña A, Leibowitz-Amit R, Sonpavde G,
Knox JJ, Tran B, et al: Prognostic role of neutrophil-to-lymphocyte
ratio in solid tumors: A systematic review and meta-analysis. J
Natl Cancer Inst. 106:dju1242014. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Ferrucci PF, Gandini S, Battaglia A,
Alfieri S, Di Giacomo AM, Giannarelli D, Cappellini GC, De Galitiis
F, Marchetti P, Amato G, et al: Baseline neutrophil-to-lymphocyte
ratio is associated with outcome of ipilimumab-treated metastatic
melanoma patients. Br J Cancer. 112:1904–1910. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Gregory AD and Houghton AM:
Tumor-associated neutrophils: New targets for cancer therapy.
Cancer Res. 71:2411–2416. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Sun R, Luo J, Li D, Shu Y, Luo C, Wang SS,
Qin J, Zhang GM and Feng ZH: Neutrophils with protumor potential
could efficiently suppress tumor growth after cytokine priming and
in presence of normal NK cells. Oncotarget. 5:12621–12634. 2014.
View Article : Google Scholar
|
|
129
|
Pang Y, Gara SK, Achyut BR, Li Z, Yan HH,
Day CP, Weiss JM, Trinchieri G, Morris JC and Yang L: TGF-β
signaling in myeloid cells is required for tumor metastasis. Cancer
Discov. 3:936–951. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Andzinski L, Kasnitz N, Stahnke S, Wu CF,
Gereke M, von Köckritz-Blickwede M, Schilling B, Brandau S, Weiss S
and Jablonska J: Type I IFNs induce anti-tumor polarization of
tumor associated neutrophils in mice and human. Int J Cancer.
138:1982–1993. 2016. View Article : Google Scholar
|
|
131
|
Jamieson T, Clarke M, Steele CW, Samuel
MS, Neumann J, Jung A, Huels D, Olson MF, Das S, Nibbs RJ, et al:
Inhibition of CXCR2 profoundly suppresses inflammation-driven and
spontaneous tumorigenesis. J Clin Invest. 122:3127–3144. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Tazzyman S, Barry ST, Ashton S, Wood P,
Blakey D, Lewis CE and Murdoch C: Inhibition of neutrophil
infiltration into A549 lung tumors in vitro and in vivo using a
CXCR2-specific antagonist is associated with reduced tumor growth.
Int J Cancer. 129:847–858. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Wculek SK and Malanchi I: Neutrophils
support lung colonization of metastasis-initiating breast cancer
cells. Nature. 528:413–417. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Shrestha S, Noh JM, Kim SY, Ham HY, Kim
YJ, Yun YJ, Kim MJ, Kwon MS, Song DK and Hong CW: Angiotensin
converting enzyme inhibitors and angiotensin II receptor antagonist
attenuate tumor growth via polarization of neutrophils toward an
antitumor phenotype. OncoImmunology. 5:e10677442015. View Article : Google Scholar
|
|
135
|
Hagerling C and Werb Z: Neutrophils:
Critical components in experimental animal models of cancer. Semin
Immunol. 28:197–204. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Zhou SL, Zhou ZJ, Hu ZQ, Li X, Huang XW,
Wang Z, Fan J, Dai Z and Zhou J: CXCR2/CXCL5 axis contributes to
epithelial-mesenchymal transition of HCC cells through activating
PI3K/ Akt/GSK-3β/Snail signaling. Cancer Lett. 358:124–135. 2015.
View Article : Google Scholar
|
|
137
|
Benevides L, da Fonseca DM, Donate PB,
Tiezzi DG, De Carvalho DD, de Andrade JM, Martins GA and Silva JS:
IL17 Promotes mammary tumor progression by changing the behavior of
tumor cells and eliciting tumorigenic neutrophils recruitment.
Cancer Res. 75:3788–3799. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Wislez M, Rabbe N, Marchal J, Milleron B,
Crestani B, Mayaud C, Antoine M, Soler P and Cadranel J: Hepatocyte
growth factor production by neutrophils infiltrating
bronchioloalveolar subtype pulmonary adenocarcinoma: Role in tumor
progression and death. Cancer Res. 63:1405–1412. 2003.PubMed/NCBI
|
|
139
|
Ibrahim SA, Katara GK, Kulshrestha A,
Jaiswal MK, Amin MA and Beaman KD: Breast cancer associated a2
isoform vacuolar ATPase immunomodulates neutrophils: Potential role
in tumor progression. Oncotarget. 6:33033–33045. 2015.PubMed/NCBI
|