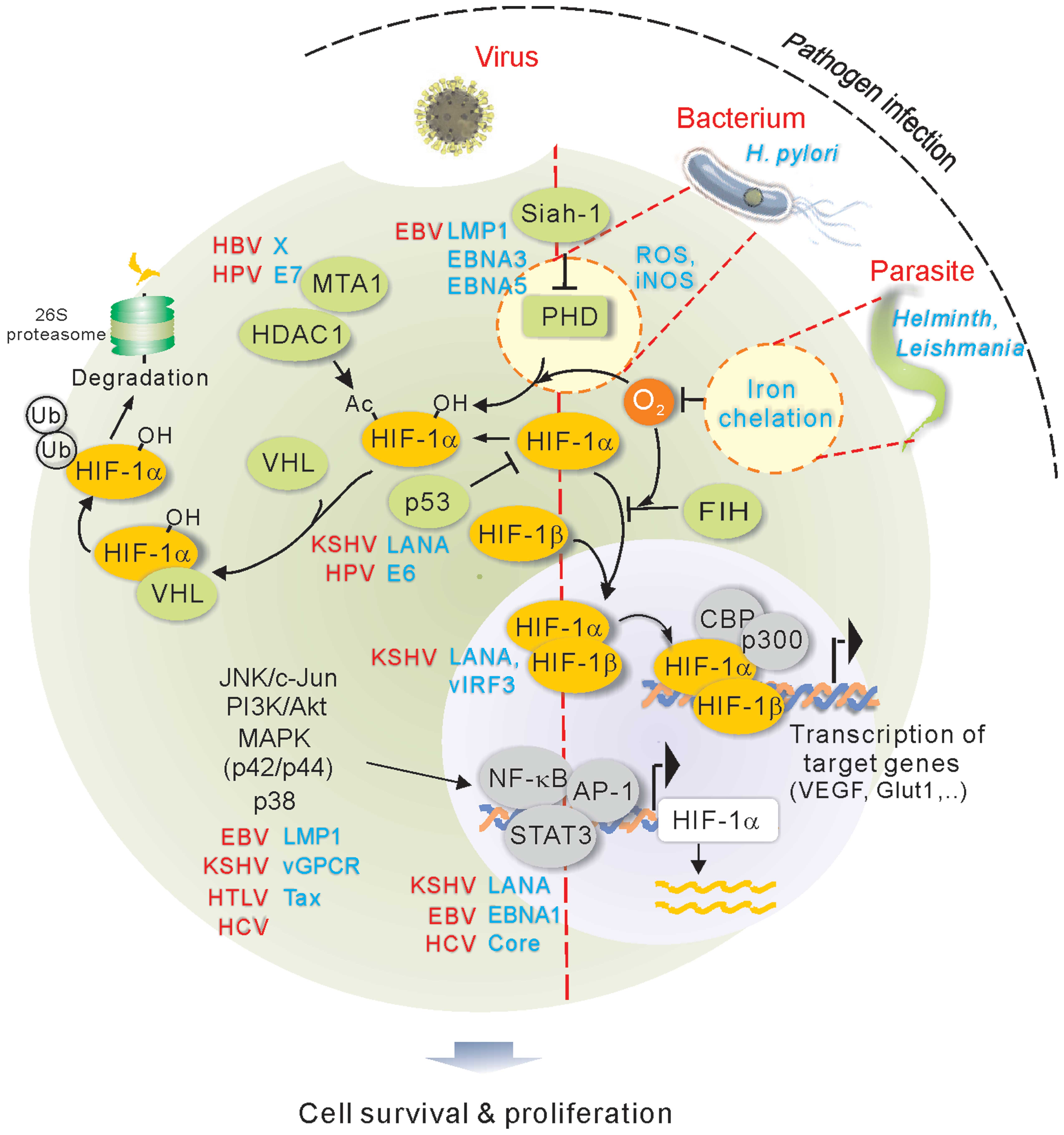
1. Introduction
To date approximately one-sixth of global cancers
are attributable to an infectious agent (1). The fraction of cancers linked to
pathogen infection varies greatly due to geographical location and
socioeconomic factors: approximately 8% in developed countries, up
to 23% in developing countries, and above one-third in Sub-Saharan
Africa (1). Carcinogenesis
associated with infections is a complex process, often mediated by
chronic inflammatory conditions that is a progressive component in
the tumor microenvironment and represents a key hallmark of
cancer.
Hypoxia (low oxygen) is a phenotype of hostile
microenvironment usually formed by cancer cell rapid growth
(2). It has been demonstrated that
hypoxia occur in many types of human malignancies caused by
pathogen infections and tightly associated with chronic
inflammation (3).
Hypoxia-inducible factor (HIF) is the master regulator molecule in
response to hypoxic stress. HIF, that belongs to
basic-helix-loop-helix-PAS family, is a heterodimer transcriptional
factor composed of an inducible α subunit (HIFα, oxygen-dependent)
and a constitutively expressed β subunit (HIFβ) (4). Three HIF isoforms (HIF-1, HIF-2 and
HIF-3) have been identified. Since the majority of studies have
reported on HIF-1, here we focus on HIF-1 not HIF-2 or HIF-3. The
half-life of HIF-1α protein is very short, and the stability of
HIF-1α will determine its effect. The specific proline residue of
HIF-1α is hydroxylated by prolyl hydroxylases (PHDs) in the
presence of oxygen. Upon hydroxylation, HIF-1α is targeted by the
VHL tumor suppressor for ubiquitylation and proteasome-mediated
degradation; while in hypoxia condition, the activity of PHD is
blocked due to the absence of oxygen, and in turn HIF-1α is
stabilized. The stabilized HIF-1α translocates into the nucleus and
form a heterodimer with HIF-1β, where they function as a
transcription factor to regulate many downstream genes that are
critical for various cellular processes including inflammation and
cell survival in hypoxia (5). In
addition, substantial evidence has shown that transcription and
translation of HIF-1 is regulated by many post-translational
modifications including phosphorylation, acetylation and
SUMOylation, as well as complex formation with other molecules
which are important for HIF-1 accumulation and transactivation
under hypoxic conditions (Fig. 1).
Generally, the overexpression of HIF-1 in cancers was primarily
attributed to induction by environmental hypoxia or genetic
mutations in the HIF-1-degradation pathways; however, it has now
become clear that many human oncogenic pathogens directly enhance
HIF-1 stability and activity through various mechanisms. Thus,
elevated expression of HIF-1 represents a common outcome of
comprehensive regulation by human oncogenic pathogens.
Here we will summarize the current works in
understanding the mechanisms of HIF-1 stability and activity
promoted by oncogenic pathogens including viruses, bacteria and
parasites (Table I), to further
address the role of HIF-1 in different oncogenic
pathogen-associated cancers, and highlight the potential diagnostic
markers and therapeutic targets.
 | Table IDeregulation of pathogens-associated
HIF signaling. |
Table I
Deregulation of pathogens-associated
HIF signaling.
| Pathogen | Associated cancers
(28,61,67,68) | Pathogen
molecules | Mechanisms of
pathogenic HIF signaling | Refs. |
|---|
| EBV | Burkitt’s lymphoma,
Hodgkin’s B cell lymphoma, gastric and nasopharyngeal
carcinoma | LMP1 | Degradation of PHD
by Siah1-mediated ubiquitylation; Phosphorylation of CBP by MAPK
(p42/p44); Activation of JNK/c-Jun Phosphorylation by ROS
signaling | (7–9) |
| | EBNA1 | Enhances
transcription of HIF-1α by targeting AP-1 | (10) |
| | EBNA5, EBNA3 | Stabilization of
HIF-1α by blocking interaction with PHD1 and PHD2 | (11) |
| KSHV | Kaposi’s sarcoma,
primary effusion lymphoma, multicentric | vGPCR | Activation of MAPK
(p42, p44), p38 by phosphorylation | (41,42) |
| Castlman’s
disease | LANA | Stabilization and
relocation of HIF-1α; Degradation of VHL through EC5S ubiquitin
complex | (35,37,38) |
| | vIRF3 | Stabilize
HIF-1α | (39,40) |
| HPV | Cervix, anus,
vulva, penis, oropharynx | E6 | NF-κB activation by
inhibiting CYLD deubiquitinase | (15) |
| | E7 | Enhanced activity
of HIF-1 by blocking the association with HDAC1, HDAC4 and
HDAC7 | (13,14) |
| HBV | Liver cancer
(hepatocellular carcinoma) | X | Deactylation by
MTA1 and HDAC1; Phosphorylation of CBP by MAPK (p42/p44); | (18,19) |
| HLTV | T cell
lymphoma | Tax | Phosphorylation by
Akt/PI3K pathway | (24) |
| HCV | Liver cancer
(hepatocellular carcinoma) | Core | Likely through
phosphorylation of NF-κB, STAT3, PI3K/Akt, MAPK (p42/p44) | (29,31,32) |
| H.
pylori | Gastric
adenocarcinoma | ND | Enhanced
transcription of HIF-1 by ROS-induced APE1; Inhibition of HIF-1 by
iNOS | (51,54) |
|
Bartonella | Bacillary
angiomatosis (BA) | BadA | ND | (56,58) |
| Helminth | Bladder carcinoma,
cholangiocarcinoma | ND | Induction of a
hypoxic microenvironment | (62) |
| Leishmania | Skin cancer | ND | Exhaustion cellular
iron pool; Elevated expression of HIF-1 by infected macrophage | (66) |
2. HIF-1 activity is directly enhanced by
oncogenic viruses at transcriptional or translational level
In the past decades, substantial evidence from both
epidemiology and experimental study have accumulated pointing out
seven different human viruses, namely EBV, HPV, HBV, HTLV, HCV,
KSHV and MCV, as causal agents of various human cancers.
Inspiringly, six of them, excluding newly discovered MCV, all have
been clearly indicated involving in the deregulation of cellular
HIF-1 signaling pathway.
EBV
Epstein-Barr virus (EBV) is a ubiquitous human
γ-herpesvirus that is associated with lymphoproliferative disease
in immunosuppressed patients as well as several types of
malignancies, such as Burkitt’s lymphoma, lymphoproliferative
disorders, T-cell lymphomas, Hodgkin’s disease and some gastric
carcinomas (6). Previous studies
from different research groups successively proposed distinct
mechanisms that EBV infection results in accumulation and
activation of HIF-1. In particular, the latent-membrane protein 1
(LMP-1), a principal EBV-encoded oncoprotein, has emerged as one of
the most important viral proteins associated with HIF-1α. LMP1
expression in the EBV positive cell lines KR-4 and KR-1 was found
to lead to increasing expression of HIF-1α, which is through to be
activation of p42/p44 MAPK activity and oxidative stress signaling.
LMP1 could promote HIF-1α DNA binding activity and increase
activation of JNK/c-Jun signaling which in turn enhanced HIF-1α
downstream gene (i.e. VEGF) expression (7,8). In
addition, LMP1 was also shown to stabilize HIF-1α in nasopharyngeal
epithelial cells through upregulating the level of Siah1 E3
ubiquitin ligase, which induces proteasomal degradation of
proline-hydroxylases PHD1 and PHD3 and ultimately inhibits HIF-1
degradation (9). Likewise, EBNA1,
as the key antigen during EBV latency, has also been found to
enhance transcription of HIF-1α through modulating the AP-1
transcriptional factor (10). At
the post-translational level, more recent studies revealed that
HIF-1α is also stabilized by EBV-encoded EBNA-5 and EBNA-3 in the
EBV-transformed lymphoblastoid cells through binding to PHD1 and
PHD2, two of which participate in the degradation of HIF-1α
(11). Interestingly, it has also
been shown that hypoxia stress is able to induce EBV lytic
replication (12).
HPV
HPV, as a typical human oncogenic non-enveloped DNA
virus, is characterized by definite causal role in a subset of
squamous cell carcinoma, mainly cervical cancer. HPV-encoded E6 and
E7 oncoproteins are shown to continuously express in cervical
cancer lesions, which reveal the critical role of these two
oncoproteins in the development of cervical tumors. Tang and
colleagues (13) have demonstrated
an increased expression of HIF-1α and HIF-1α-dependent VEGF in
cervical cancer cells with the overexpression of E6 and E7. Further
studies identified that E7 protein enhances HIF-1 activity by
blocking the association of HIF-1 with histone deacetylases HDAC1,
HDAC4 and HDAC7 (14). Notably,
HPV-encoded E6 was found to display a distinct mechanism in
regulation of hypoxia signal pathway. For example, E6 protein can
prolong hypoxia-induced NF-κB activation by inhibiting CYLD lysine
63 deubiquitinase, which is a negative regulator of the NF-κB
pathway, and then promote HPV-associated malignance (15). These findings shed light on the
mechanisms of how HPV contributes to the stabilization and
activation of HIF-1.
HBV
Hepatitis B virus (HBV), a small DNA virus belongs
to hepadnaviridae family, is a globally distributed human pathogen
that can cause life-threatening diseases like liver cirrhosis and
hepatocellular carcinoma (HCC) (16). The HBV-encoded X protein (HBx), as
one of the four HBV overlapping open reading frames encoded
proteins, were proven with accumulating evidence to play a primary
function in angiogenesis during the malignant development of HCC
(17). Considering the established
role of HIF-1 in angiogenesis, HBx links to HIF-1 has been
extensively explored. Among these, a research study from Yoo group
depicts a positive association between HBx and the MTA1/HDAC
complex in stabilizing HIF-1α. HBx induces expression of both MTA1
and HDAC1 to enhance deacetylation of HIF-1 within oxygen-dependent
degradation domain (18).
Consistently, Holotnakova et al (19) further found that HBx could also
increase the transcriptional activity of HIF-1, which in turn
enhances a hypoxia-responsive gene CA9 promoter activity and
contributes to the development of HCC.
HTLV
Among human retrovirus, human T-cell leukemia virus
type 1 (HTLV-1), is the only identified retrovirus that directly
linked to certain types of human cancer, such as ATL (adult T-cell
leukemia). Tax protein has been recognized as one of the most
important oncogenic proteins encoded by HTLV-1 (20). Tax has been shown to interact with
several transcription factors and involve in activation of several
oncogenic pathways, including CREB/ATF, AP1, NF-κB and the PI3K/Akt
signaling pathway (21–23). In hypoxia signaling pathway, Tomita
et al (24) showed that
phosphorylation of PI3K/Akt induced by Tax leads to activation of
HIF-1 in HTLV-infected cell lines, and proposed a
PI3K/Akt-dependent mechanism that is responsible for HIF-1 protein
accumulation and DNA-binding activity.
HCV
Hepatitis C virus (HCV) is a positive-strand RNA
virus (25). Since the success of
identification of HCV in 1989 by Houghton and colleagues, similar
to HBV, the relationship between HCV chronic infection and the
development of HCC has been established (26,27).
In contrast to the mechanism by which HBV causes HCC, no
HCV-derived viral protein has been found to function as
oncoprotein. However, chronic inflammation and sustained liver
damage caused by HCV infection could likely account for HCV-related
hepatocellular carcinoma (28).
Also, increasing evidence have shown that a state of oxidative
stress is a characteristic manifestation induced by HCV infection
(28). Stabilization of HIF-1α
induced by HCV is potentially attributable to oxidative stress
(29,30). For example, Nasimuzzaman et
al (29) showed that the
activation of NF-κB, STAT-3, PI3K/Akt and p42/44 mitogen-activated
protein kinase under oxidative stress strongly link to the HIF-1α
stabilization and VEGF synthesis. In contrast, Ripoli et al
(30) provided another explanation
that HCV infection causes severe impairment of mitochondrial
oxidative phosphorylation, which results in HIF-1α stabilization
under normoxic condition. Increased HIF-1α further stimulates the
expression of HIF-controlled genes including glycolytic enzymes.
More recent studies carried by different research groups disclose
that HCV core protein plays a role in upregulation of HIF-1α both
in transcription and protein level (31,32).
However, the related mechanism remains to be further determined.
Taken together, all these findings provide new insights into the
role of HIF-1 signaling on the carcinogenesis of HCV-related
HCC.
KSHV
Kaposi’s sarcoma-associated herpesvirus (KSHV), as a
member of the γ-herpesviruses, also referred to as human
herpesvirus 8 (HHV-8). It is well known that KSHV is tightly
associated with Kaposi’s sarcoma (KS), primary effusion lymphoma
(PEL) and multicentric Castleman’s disease (MCD) (33,34).
Notably, Kaposi’s sarcoma, commonly occurred in untreated AIDS, is
an angioproliferative and endothelial cell-derived tumor (35). Given the close link between active
HIF-1 and angiogenesis, extensive studies indicate that KSHV has
developed multiple distinct mechanisms to regulate HIF-1 signaling
in KSHV-infected cells. The latency-associated nuclear antigen
(LANA), as a key antigen in KSHV latency state, plays a crucial
role not only in KSHV episomal persistence, but also in modulating
viral and cellular gene expression (36,37).
Research from our group revealed the role of LANA in regulating
HIF-1 signaling. LANA is capable of stabilizing HIF-1α by targeting
its suppressor von Hippel-Lindau (VHL) protein and p53 for
degradation. This process depends on its suppressor of cytokine
signaling (SOCS)-box motif that can recruit the (Elongin BC-Cullin
5-SOCS) EC5S ubiquitin complex (38). In addition, a potential α-helical
amino-terminal domain of LANA was found responsible for inducing
nuclear accumulation of HIF-1α in normoxic condition (35). In the transcriptional level, LANA
is also shown to augment HIF-1α mRNA level (37). Remarkably, LANA also presents to
directly interact with HIF-1α, and binds to the hypoxia-responsive
element (HRE) motifs of the viral replication transcription
activator (RTA) promoter, which partly explain the mechanism of
hypoxia-induced KSHV lytic replication (37).
Although LANA represents a critical role in the
deregulation of hypoxia signaling, several other viral proteins
encoded by KSHV have also been implicated to associate with HIF-1α.
For example, LANA2 (also named vIRF3), which is reported
exclusively expressed in KSHV-infected B cells (39), has been shown to stabilize and
stimulate HIF-1α transcriptional activity. LANA2 also interacts
with HIF-1α via a double α-helix motif, which could inhibit HIF-1α
degradation under normoxia (40).
G protein-coupled receptor (vGPCR), which is encoded by KSHV with
the nature of potent transformation and proangiogenesis in KS
development (28). Earlier studies
have documented the effect of vGPCR in HIF-1α activity is involved
by several intercellular pathways. Sodhi et al (41) elucidated that the phosphorylation
of the inhibitory domain of HIF-1α induced by vGPCR is through the
activation of p38 and MAPK signaling pathways, which leads to
increased transcriptional activity of HIF-1α and VEGF protein
levels. In addition, it is worth mentioning that cytokines
secretion induced by vGPCR also activate several kinase pathways
including AKT, p38 and IKKβ, which ultimately stimulate HIF
activity and VEGF secretion in a mTOR-independent manner (42). Recently, lytic proteins PF-8 and
gpK8.1 and vIL-6 encoded by KSHV are also involved in HIF pathways.
In both chronic and acute hypoxia, lytic proteins PF-8 and gpK8.1
are strongly expressed in KSHV-infected primary effusion lymphoma
(PEL) cells (43). vIL-6 as an
important cytokine in the pathogenesis of KS, is also higher
expressed in hypoxia than in normoxia. Collectively, this evidence
imply a critical role of HIF-1α in promoting KSHV latency and lytic
replication (43).
3. HIF-1 is indirectly activated by
bacteria-induced oxidative stress
Although it is still debated whether bacterial
infection can directly cause human cancer (44), two bacteria Helicobacter pylori
(H. pylori) and Bartonella have been widely demonstrated
to highly associate with different human cancer development, and
their infection can indirectly increase the expression levels of
HIF in host cells in innate immune response (45).
H. pylori
Since the success of isolating Helicobacter
pylori in 1984, many studies have focused on the casual
relationship between the pathogen and peptic ulcer disease as well
as gastric cancer (46). H.
pylori infection is believed to elevate the risk of gastric
cancer development (47,48). Subsequently research work of
Griffiths et al has shown an increased expression of
hypoxia-inducible protein, like HIF-1α, HIF-2α and VEGF, in the
Barrett’s metaplasia-dysplasia-adenocarcinoma sequence (49). Also, they found that the expression
of HIF-1α in gastric cancer development is increasing and is linked
to a poor prognosis (48). The
evidence together suggest that HIF-1α is associated with malignant
progression of gastric cancer and may play a critical role in
carcinogenesis. HIF-1α has been implicated in proliferation,
apoptosis events and the inflammatory process that as a result of
H. pylori infection, is believed to potentially transform
H. pylori-induced chronic gastritis into intestinal-type
carcinoma. The study of Park et al (50) revealed that HIF-1α protein is
aberrantly expressed in gastric cancer cells under normoxia, and
this phenomenon is correlated with endogenous ROS generation.
Importantly, they found that H. pylori-stimulated gastric
epithelial non-mitochondrial ROS can induce HIF-1α expression as
well as activate HIF-1α-mediated transcription. Therefore, they
proposed a novel mechanism of stabilization of HIF-1α, which is
separate from genetic abnormalities, such as functional loss of VHL
tumor suppressor. A recent study from Bhattacharyya et al
(51) further explains the
underlying mechanism of HIF-1α stabilization and accumulation in
H. pylori-infected gastric epithelia under normoxic
condition. ROS generation, due to neutrophil infiltration in
response to H. pylori infection, induces the expression of
APE1 in human gastric epithelial cells. Subsequently, APE1 not only
augments HIF-1α expression, but also interacts with transcriptional
co-activator p300 enhancing the transcriptional activity of
HIF-1α.
In addition to ROS, exogenous reactive nitrogen
species has been extensively indicated in gastric carcinogenesis as
well as chronic inflammation relevant to gastric cancer (52). A previous study by Mannick et
al (53) has shown an
increased expression of iNOS and sustained formation of nitric
oxide in gastric mucosa in response to H. pylori infection.
To unravel the molecular mechanism of HIF-1α accumulation under
normoxia by nitric oxide, another group has found that nitric oxide
impairs the degradation of HIF-1α under normoxia by inhibiting
S-nitrosoglutathione (GSNO)-mediated prolyl hydroxylases (54). Collectively, the evidence provides
us with a novel approach that can be employed by H. pylori
to regulate the accumulation and activation of HIF-1α under
normoxic condition.
Bartonella
Bartonella species is a gram-negative,
fastidious, facultative intracellular bacteria that can infect many
different mammalian hosts, and is considered as the only known
bacterial pathogen causing vasculoproliferative disorders in humans
(55,56). Among of more than 20 species, B.
henselae and B. Quintana are the major causative agents
of bacillary angiomatosis (BA) and bacillary peliosis (BP) in
immunocompromised patients (57).
Kempf et al (56) showed
that in BA lesions and B. henselae infected host cells, the
expression of HIF-1 is very high, and B. henselae infection
could result in the activation of cellular genes targeted by
hypoxia. Further studies showed that Bartonella adhesin A (BadA)
could be crucial for this bacteria to induce angiogenic
reprogramming of the host cells via activation of HIF-1 (56,58),
however, the related mechanism remains to be further
investigated.
4. HIF-1 is indirectly upregulated by
parasite-mediated iron exhaustion
Although the association of parasite infection with
human cancers is not very clear, some evidence has shown that
infection of some parasites including Helminth and
Leishmania also indirectly impair HIF-1α signaling in their
associated cancers.
Helminth
The prevalence of Helminth infections has a high
correlation with geographic region and sanitary condition. Current
evidence from epidemiological investigation strongly support the
link between a certain type of cancer and a specific parasite
(59). Among these, the
relationship between bladder cancer and schistosomiasia is
relatively explicit, which is mainly based on the observation of
superficial transition cell carcinomas in animals infected with
S. haematobium as well as case-control study (59–61).
In addition, the other two trematodes, Opisthorchis
viverrini and Clonorchis sinensis, are also believed to
be the etiology of cholangiocarcinoma (61). Generally, the development of
helminth-related cancer is a chronic process, often require
exposure to the infection for many years. Both granulomas formation
and inflammatory cell infiltration that responsible for much of the
symptom of schistosomiasis, are important inflammation forms in
response to S. mansoni infection (62,63).
Recently, a research group found that Schistosomal granulomas are
hypoxic, accompanied by the expression of HIF-1α and VEGF. The
findings for the first time reveal a strong positive correlation
between hypoxic tissue microenvironment produced by S.
mansoni infection and HIF-1α expression. HIF-1α expression, in
turn, promotes the development and growth of the granulomas
(62). Considering the role of
chronic inflammation in the development of cancer, hypoxia
microenvironment induced HIF-1α expression is possibly another
mechanism employed by a parasite, S. mansoni, to involve in
the transformation of helminth-infected tissues.
Leishmania
To date, the links between HIF-1α and parasites were
rarely reported, wherein Leishmania in association with
HIF-1α expand our knowledge on the molecular strategy used by the
parasite to deregulate the HIF-1α signal pathway. Studies from the
same group showed that the overexpression of HIF-1α was found in
both Leishmania amazonensis-infected mono-nuclear phagocytes
and cutaneous lesions of BALB/c mice (64,65).
However, the related mechanism of activation needs to be further
discovered. Recently, Singh et al (66) showed there are two potential
distinct mechanisms in activation of HIF-1α in Leishmania
donovani (LD)-infected macrophages. One is that LD can exhaust
host cellular iron pool and then affect prolyl hydroxylase
activity, which contributes to the stabilization of HIF-1α protein.
On the other hand, an elevated expression of HIF-1α may due to the
transcriptional regulation in the LD-infected macrophage.
5. Conclusions
Distinct from other non-pathogen associated cancers,
elucidation of the molecular events underlying the carcinogenesis
of human tumors associated with pathogen will facilitate to develop
a specific pathogen-targeted therapeutic strategy. In the view of
the fact that HIF-1 acts as a central regulator in the adaptation
process of cells and organisms to hypoxia, and plays an important
role in human pathogen-associated inflammatory cancers, it is
possible to prevent pathogenic infection by using HIF-1-specific
inhibitors. Hence, we highlight the molecular mechanisms of HIF-1
and HIF-1-dependent downstream target gene activation directly
controlled by pathogenic infections, while exploring the effect of
hypoxic stress on pathogenic life cycle and chromosome instability
in infected host cells. In the present review, we enumerate at
least six oncogenic viruses that have clearly been implicated in
the modulation of HIF-1 signaling. Compared to other viruses, KSHV
and EBV, two members of γ-herpesvirus family, develop multiple
mechanisms and encode more than one viral protein to shape the
HIF-1 activity in hypoxic, even normoxic condition. For example,
KSHV LANA, vIRF-3 and vGPCR adopt a unique method, respectively,
which ultimately leads to increased HIF-1 activity, including
enhanced HIF-1 protein level and transcription activity. From this
view, more strategies and resources from virus itself reflect the
important role of HIF-1 signaling in the development of virus
associated tumors. With regard to bacteria and parasites, no direct
link between HIF-1 and bacteria or parasite derived molecular
mechanisms was reported. The regulation of HIF-1 by bacteria or
parasite is mainly attributable to indirect effect of infection,
like iNOS and ROS induced by H. pylori. Thus, it remains to
be further investigated. Collectively, understanding the role of
HIF-1 in the progression of different oncogenic pathogen-associated
cancers, and the mechanisms by which oncogenic pathogens including
viruses, bacteria and parasites promote HIF-1 stability and
activity, will provide us with more diagnostic markers and
potential viral targets for therapeutic application.
Acknowledgements
The authors would like to apologize to the many
researchers who have contributed to this area of research but have
not been cited in this review due to space limitations. The present
study is supported by the Research and Innovation Program of the
Shanghai Municipal Education (13zz011), the Shanghai Sailing
Program (15YF1400900), the National Natural Science Foundation of
China (81471930, 81402542, 81501739), and the National Key Basic
Research ‘973’ program of China (2012CB519001). F.W. is a scholar
of Pujiang Talents in Shanghai. Q.C. is a scholar of New Century
Excellent Talents in University of China.
References
|
1
|
de Martel C, Ferlay J, Franceschi S,
Vignat J, Bray F, Forman D and Plummer M: Global burden of cancers
attributable to infections in 2008: A review and synthetic
analysis. Lancet Oncol. 13:607–615. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ackerman D and Simon MC: Hypoxia, lipids,
and cancer: Surviving the harsh tumor microenvironment. Trends Cell
Biol. 24:472–478. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Carmeliet P and Jain RK: Principles and
mechanisms of vessel normalization for cancer and other angiogenic
diseases. Nat Rev Drug Discov. 10:417–427. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Minet E, Michel G, Remacle J and Michiels
C: Role of HIF-1 as a transcription factor involved in embryonic
development, cancer progression and apoptosis (Review). Int J Mol
Med. 5:253–259. 2000.PubMed/NCBI
|
|
5
|
Kurihara T, Westenskow PD and Friedlander
M: Hypoxia-inducible factor (HIF)/vascular endothelial growth
factor (VEGF) signaling in the retina. Adv Exp Med Biol.
801:275–281. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Calderwood MA, Venkatesan K, Xing L, Chase
MR, Vazquez A, Holthaus AM, Ewence AE, Li N, Hirozane-Kishikawa T,
Hill DE, et al: Epstein-Barr virus and virus human protein
interaction maps. Proc Natl Acad Sci USA. 104:7606–7611. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wakisaka N, Kondo S, Yoshizaki T, Murono
S, Furukawa M and Pagano JS: Epstein-Barr virus latent membrane
protein 1 induces synthesis of hypoxia-inducible factor 1 alpha.
Mol Cell Biol. 24:5223–5234. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yang L, Liu L, Xu Z, Liao W, Feng D, Dong
X, Xu S, Xiao L, Lu J, Luo X, et al: EBV-LMP1 targeted DNAzyme
enhances radiosensitivity by inhibiting tumor angiogenesis via the
JNKs/HIF-1 pathway in nasopharyngeal carcinoma. Oncotarget.
6:5804–5817. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kondo S, Seo SY, Yoshizaki T, Wakisaka N,
Furukawa M, Joab I, Jang KL and Pagano JS: EBV latent membrane
protein 1 up-regulates hypoxia-inducible factor 1alpha through
Siah1-mediated down-regulation of prolyl hydroxylases 1 and 3 in
nasopharyngeal epithelial cells. Cancer Res. 66:9870–9877. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
O’Neil JD, Owen TJ, Wood VH, Date KL,
Valentine R, Chukwuma MB, Arrand JR, Dawson CW and Young LS:
Epstein-Barr virus-encoded EBNA1 modulates the AP-1 transcription
factor pathway in nasopharyngeal carcinoma cells and enhances
angiogenesis in vitro. J Gen Virol. 89:2833–2842. 2008. View Article : Google Scholar
|
|
11
|
Darekar S, Georgiou K, Yurchenko M,
Yenamandra SP, Chachami G, Simos G, Klein G and Kashuba E:
Epstein-Barr virus immortalization of human B-cells leads to
stabilization of hypoxia-induced factor 1 alpha, congruent with the
Warburg effect. PLoS One. 7:e420722012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jiang JH, Wang N, Li A, Liao WT, Pan ZG,
Mai SJ, Li DJ, Zeng MS, Wen JM and Zeng YX: Hypoxia can contribute
to the induction of the Epstein-Barr virus (EBV) lytic cycle. J
Clin Virol. 37:98–103. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tang X, Zhang Q, Nishitani J, Brown J, Shi
S and Le AD: Overexpression of human papillomavirus type 16
oncoproteins enhances hypoxia-inducible factor 1 alpha protein
accumulation and vascular endothelial growth factor expression in
human cervical carcinoma cells. Clin Cancer Res. 13:2568–2576.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bodily JM, Mehta KP and Laimins LA: Human
papillomavirus E7 enhances hypoxia-inducible factor 1-mediated
transcription by inhibiting binding of histone deacetylases. Cancer
Res. 71:1187–1195. 2011. View Article : Google Scholar
|
|
15
|
An J, Mo D, Liu H, Veena MS, Srivatsan ES,
Massoumi R and Rettig MB: Inactivation of the CYLD deubiquitinase
by HPV E6 mediates hypoxia-induced NF-kappaB activation. Cancer
Cell. 14:394–407. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Roman S, Jose-Abrego A, Fierro NA,
Escobedo-Melendez G, Ojeda-Granados C, Martinez-Lopez E and Panduro
A: Hepatitis B virus infection in Latin America: A genomic medicine
approach. World J Gastroenterol. 20:7181–7196. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ali A, Abdel-Hafiz H, Suhail M, Al-Mars A,
Zakaria MK, Fatima K, Ahmad S, Azhar E, Chaudhary A and Qadri I:
Hepatitis B virus, HBx mutants and their role in hepatocellular
carcinoma. World J Gastroenterol. 20:10238–10248. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yoo YG, Na TY, Seo HW, Seong JK, Park CK,
Shin YK and Lee MO: Hepatitis B virus X protein induces the
expression of MTA1 and HDAC1, which enhances hypoxia signaling in
hepatocellular carcinoma cells. Oncogene. 27:3405–3413. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Holotnakova T, Tylkova L, Takacova M,
Kopacek J, Petrik J, Pastorekova S and Pastorek J: Role of the HBx
oncoprotein in carbonic anhydrase 9 induction. J Med Virol.
82:32–40. 2010. View Article : Google Scholar
|
|
20
|
Matsuoka M and Jeang KT: Human T-cell
leukaemia virus type 1 (HTLV-1) infectivity and cellular
transformation. Nat Rev Cancer. 7:270–280. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Grassmann R, Aboud M and Jeang KT:
Molecular mechanisms of cellular transformation by HTLV-1 Tax.
Oncogene. 24:5976–5985. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Jeong SJ, Dasgupta A, Jung KJ, Um JH,
Burke A, Park HU and Brady JN: PI3K/AKT inhibition induces
caspase-dependent apoptosis in HTLV-1-transformed cells. Virology.
370:264–272. 2008. View Article : Google Scholar
|
|
23
|
Peloponese JM Jr and Jeang KT: Role for
Akt/protein kinase B and activator protein-1 in cellular
proliferation induced by the human T-cell leukemia virus type 1 tax
oncoprotein. J Biol Chem. 281:8927–8938. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tomita M, Semenza GL, Michiels C, Matsuda
T, Uchihara JN, Okudaira T, Tanaka Y, Taira N, Ohshiro K and Mori
N: Activation of hypoxia-inducible factor 1 in human T-cell
leukaemia virus type 1-infected cell lines and primary adult T-cell
leukaemia cells. Biochem J. 406:317–323. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Appel N, Schaller T, Penin F and
Bartenschlager R: From structure to function: New insights into
hepatitis C virus RNA replication. J Biol Chem. 281:9833–9836.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tan A, Yeh SH, Liu CJ, Cheung C and Chen
PJ: Viral hepatocarcinogenesis: From infection to cancer. Liver
Int. 28:175–188. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bergonzini V, Salata C, Calistri A,
Parolin C and Palù G: View and review on viral oncology research.
Infect Agent Cancer. 5:112010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Martin D and Gutkind JS: Human
tumor-associated viruses and new insights into the molecular
mechanisms of cancer. Oncogene. 27(Suppl 2): S31–S42. 2008.
View Article : Google Scholar
|
|
29
|
Nasimuzzaman M, Waris G, Mikolon D,
Stupack DG and Siddiqui A: Hepatitis C virus stabilizes
hypoxia-inducible factor 1alpha and stimulates the synthesis of
vascular endothelial growth factor. J Virol. 81:10249–10257. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ripoli M, D’Aprile A, Quarato G,
Sarasin-Filipowicz M, Gouttenoire J, Scrima R, Cela O, Boffoli D,
Heim MH, Moradpour D, et al: Hepatitis C virus-linked mitochondrial
dysfunction promotes hypoxia-inducible factor 1 alpha-mediated
glycolytic adaptation. J Virol. 84:647–660. 2010. View Article : Google Scholar
|
|
31
|
Liu XH, Zhou X, Zhu CL, Song H and Liu F:
Effects of HCV core protein on the expression of hypoxia-inducible
factor 1 alpha and vascular endothelial growth factor. Zhonghua Gan
Zang Bing Za Zhi. 19:751–754. 2011.(In Chinese).
|
|
32
|
Abe M, Koga H, Yoshida T, Masuda H,
Iwamoto H, Sakata M, Hanada S, Nakamura T, Taniguchi E, Kawaguchi
T, et al: Hepatitis C virus core protein upregulates the expression
of vascular endothelial growth factor via the nuclear
factor-κB/hypoxia-inducible factor-1α axis under hypoxic
conditions. Hepatol Res. 42:591–600. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Mesri EA, Cesarman E and Boshoff C:
Kaposi’s sarcoma and its associated herpesvirus. Nat Rev Cancer.
10:707–719. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Cesarman E, Chang Y, Moore PS, Said JW and
Knowles DM: Kaposi’s sarcoma-associated herpesvirus-like DNA
sequences in AIDS-related body-cavity-based lymphomas. N Engl J
Med. 332:1186–1191. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Cai Q, Murakami M, Si H and Robertson ES:
A potential alpha-helix motif in the amino terminus of LANA encoded
by Kaposi’s sarcoma-associated herpesvirus is critical for nuclear
accumulation of HIF-1alpha in normoxia. J Virol. 81:10413–10423.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Cotter MA II and Robertson ES: The
latency-associated nuclear antigen tethers the Kaposi’s
sarcoma-associated herpesvirus genome to host chromosomes in body
cavity-based lymphoma cells. Virology. 264:254–264. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Cai Q, Lan K, Verma SC, Si H, Lin D and
Robertson ES: Kaposi’s sarcoma-associated herpesvirus latent
protein LANA interacts with HIF-1 alpha to upregulate RTA
expression during hypoxia: Latency control under low oxygen
conditions. J Virol. 80:7965–7975. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Cai QL, Knight JS, Verma SC, Zald P and
Robertson ES: EC5S ubiquitin complex is recruited by KSHV latent
antigen LANA for degradation of the VHL and p53 tumor suppressors.
PLoS Pathog. 2:e1162006. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Rivas C, Thlick AE, Parravicini C, Moore
PS and Chang Y: Kaposi’s sarcoma-associated herpesvirus LANA2 is a
B-cell-specific latent viral protein that inhibits p53. J Virol.
75:429–438. 2001. View Article : Google Scholar
|
|
40
|
Shin YC, Joo CH, Gack MU, Lee HR and Jung
JU: Kaposi’s sarcoma-associated herpesvirus viral IFN regulatory
factor 3 stabilizes hypoxia-inducible factor-1 alpha to induce
vascular endothelial growth factor expression. Cancer Res.
68:1751–1759. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Sodhi A, Montaner S, Patel V, Zohar M,
Bais C, Mesri EA and Gutkind JS: The Kaposi’s sarcoma-associated
herpes virus G protein-coupled receptor up-regulates vascular
endothelial growth factor expression and secretion through
mitogen-activated protein kinase and p38 pathways acting on
hypoxia-inducible factor 1alpha. Cancer Res. 60:4873–4880.
2000.PubMed/NCBI
|
|
42
|
Jham BC, Ma T, Hu J, Chaisuparat R,
Friedman ER, Pandolfi PP, Schneider A, Sodhi A and Montaner S:
Amplification of the angiogenic signal through the activation of
the TSC/mTOR/HIF axis by the KSHV vGPCR in Kaposi’s sarcoma. PLoS
One. 6:e191032011. View Article : Google Scholar
|
|
43
|
Davis DA, Rinderknecht AS, Zoeteweij JP,
Aoki Y, Read-Connole EL, Tosato G, Blauvelt A and Yarchoan R:
Hypoxia induces lytic replication of Kaposi sarcoma-associated
herpesvirus. Blood. 97:3244–3250. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Samaras V, Rafailidis PI, Mourtzoukou EG,
Peppas G and Falagas ME: Chronic bacterial and parasitic infections
and cancer: A review. J Infect Dev Ctries. 4:267–281.
2010.PubMed/NCBI
|
|
45
|
Nizet V and Johnson RS: Interdependence of
hypoxic and innate immune responses. Nat Rev Immunol. 9:609–617.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Dorer MS, Talarico S and Salama NR:
Helicobacter pylori’s unconventional role in health and disease.
PLoS Pathog. 5:e10005442009. View Article : Google Scholar
|
|
47
|
Nardone G, Rocco A and Malfertheiner P:
Review article: Helicobacter pylori and molecular events in
precancerous gastric lesions. Aliment Pharmacol Ther. 20:261–270.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Griffiths EA, Pritchard SA, Valentine HR,
Whitchelo N, Bishop PW, Ebert MP, Price PM, Welch IM and West CM:
Hypoxia-inducible factor-1alpha expression in the gastric
carcinogenesis sequence and its prognostic role in gastric and
gastro-oesophageal adenocarcinomas. Br J Cancer. 96:95–103. 2007.
View Article : Google Scholar
|
|
49
|
Griffiths EA, Pritchard SA, McGrath SM,
Valentine HR, Price PM, Welch IM and West CM: Increasing expression
of hypoxia-inducible proteins in the Barrett’s
metaplasia-dysplasia-adenocarcinoma sequence. Br J Cancer.
96:1377–1383. 2007.PubMed/NCBI
|
|
50
|
Park JH, Kim TY, Jong HS, Kim TY, Chun YS,
Park JW, Lee CT, Jung HC, Kim NK and Bang YJ: Gastric epithelial
reactive oxygen species prevent normoxic degradation of
hypoxia-inducible factor-1alpha in gastric cancer cells. Clin
Cancer Res. 9:433–440. 2003.PubMed/NCBI
|
|
51
|
Bhattacharyya A, Chattopadhyay R, Hall EH,
Mebrahtu ST, Ernst PB and Crowe SE: Mechanism of hypoxia-inducible
factor 1 alpha-mediated Mcl1 regulation in Helicobacter
pylori-infected human gastric epithelium. Am J Physiol Gastrointest
Liver Physiol. 299:G1177–G1186. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Ohshima H and Bartsch H: Chronic
infections and inflammatory processes as cancer risk factors:
Possible role of nitric oxide in carcinogenesis. Mutat Res.
305:253–264. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Mannick EE, Bravo LE, Zarama G, Realpe JL,
Zhang XJ, Ruiz B, Fontham ET, Mera R, Miller MJ and Correa P:
Inducible nitric oxide synthase, nitrotyrosine, and apoptosis in
Helicobacter pylori gastritis: Effect of antibiotics and
antioxidants. Cancer Res. 56:3238–3243. 1996.PubMed/NCBI
|
|
54
|
Metzen E, Zhou J, Jelkmann W, Fandrey J
and Brüne B: Nitric oxide impairs normoxic degradation of
HIF-1alpha by inhibition of prolyl hydroxylases. Mol Biol Cell.
14:3470–3481. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Yuan C, Zhu C, Wu Y, Pan X and Hua X:
Bacteriological and molecular identification of Bartonella species
in cats from different regions of China. PLoS Negl Trop Dis.
5:e13012011. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Kempf VA, Lebiedziejewski M, Alitalo K,
Wälzlein JH, Ehehalt U, Fiebig J, Huber S, Schütt B, Sander CA,
Müller S, et al: Activation of hypoxia-inducible factor-1 in
bacillary angiomatosis: Evidence for a role of hypoxia-inducible
factor-1 in bacterial infections. Circulation. 111:1054–1062. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Relman DA, Falkow S, LeBoit PE, Perkocha
LA, Min KW, Welch DF and Slater LN: The organism causing bacillary
angiomatosis, peliosis hepatis, and fever and bacteremia in
immunocompromised patients. N Engl J Med. 324:15141991. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Kaiser PO, Riess T, Wagner CL, Linke D,
Lupas AN, Schwarz H, Raddatz G, Schäfer A and Kempf VA: The head of
Bartonella adhesin A is crucial for host cell interaction of
Bartonella henselae. Cell Microbiol. 10:2223–2234. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Porta C, Riboldi E and Sica A: Mechanisms
linking pathogens-associated inflammation and cancer. Cancer Lett.
305:250–262. 2011. View Article : Google Scholar
|
|
60
|
Mostafa MH, Sheweita SA and O’Connor PJ:
Relationship between schistosomiasis and bladder cancer. Clin
Microbiol Rev. 12:97–111. 1999.PubMed/NCBI
|
|
61
|
Vennervald BJ and Polman K: Helminths and
malignancy. Parasite Immunol. 31:686–696. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Araújo AP, Frezza TF, Allegretti SM and
Giorgio S: Hypoxia, hypoxia-inducible factor-1α and vascular
endothelial growth factor in a murine model of Schistosoma mansoni
infection. Exp Mol Pathol. 89:327–333. 2010. View Article : Google Scholar
|
|
63
|
Wilson MS, Mentink-Kane MM, Pesce JT,
Ramalingam TR, Thompson R and Wynn TA: Immunopathology of
schistosomiasis. Immunol Cell Biol. 85:148–154. 2007. View Article : Google Scholar
|
|
64
|
Arrais-Silva WW, Paffaro VA Jr, Yamada AT
and Giorgio S: Expression of hypoxia-inducible factor-1alpha in the
cutaneous lesions of BALB/c mice infected with Leishmania
amazonensis. Exp Mol Pathol. 78:49–54. 2005. View Article : Google Scholar
|
|
65
|
Degrossoli A, Bosetto MC, Lima CB and
Giorgio S: Expression of hypoxia-inducible factor 1alpha in
mononuclear phagocytes infected with Leishmania amazonensis.
Immunol Lett. 114:119–125. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Singh AK, Mukhopadhyay C, Biswas S, Singh
VK and Mukhopadhyay CK: Intracellular pathogen Leishmania donovani
activates hypoxia inducible factor-1 by dual mechanism for survival
advantage within macrophage. PLoS One. 7:e384892012. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Morsy TA: Cutaneous leishmaniasis
predisposing to human skin cancer: Forty years local and regional
studies. J Egypt Soc Parasitol. 43:629–648. 2013. View Article : Google Scholar
|
|
68
|
Suzuki H, Iwasaki E and Hibi T:
Helicobacter pylori and gastric cancer. Gastric Cancer. 12:79–87.
2009. View Article : Google Scholar : PubMed/NCBI
|