Introduction
Cancer is a world-wide disease urgently requiring
novel therapeutic agents having a new class of structure (1–3). An
oxindole structure possessing a bicyclic aromatic heterocycle with
a benzene ring fused to γ-lactam is one of the structural classes
(4). Diverse derivatives based on
oxindole have been reported for development of new type anticancer
candidates (5–8). Representative natural product
possessing oxindole is spirotryprostatin A, which is well known for
its anticancer activities through blockade of the G2/M progression
in mouse mammary carcinoma cells (4,9). The
other oxindole small molecule, sunitinib was developed as a
multi-targeted receptor tyrosine kinase inhibitor and has been
widely used in the treatment of renal cell carcinoma and
imatinib-resistant gastrointestinal stromal tumors (10,11).
In 2005, Ding et al (13)
developed the MDM2 inhibitor from spiro-oxindole structure of
spirotryprostatin A and the IC50 value of MDM2 inhibitor
was 0.83 μM in LNCaP prostate cancer cells (12–14).
In a previous study, we developed a series of oxindole derivatives
having simplified α-quaternary chiral lactam and evaluated their
anticancer efficacy (2,15). However, the mechanisms of action
underlying its anticancer effects are largely unknown and need to
be better understood to allow this therapy to be used for clinical
trials. This prompted us to study further the anti-tumorigenic
effects of YH-304, including its underlying molecular
mechanisms.
The p53 tumor suppressor mediates growth arrest,
senescence, and apoptosis in response to a broad array of cellular
damage and protects the organism against the propagation of cells
with damaged DNA (16). In
unstressed cells, MDM2 acts as an p53-antagonist by inducing
ubiquitination of p53, which results in p53 degradation (17). Therefore, inhibition of the
p53-MDM2 interaction using chemical or biological inhibitors
becomes an attractive strategy for cancer therapy (18). Many MDM2 inhibitors have been
developed and evaluated in a large panel of tumors, including
lymphomas (19,20). Nutlin-3 is one of the specific
inhibitors of the MDM2-p53 interaction and leads to stabilization
and activation of the p53 (21,22).
However, recently, several studies reported the limitation of the
efficacy of nutlin-3 such as resistance formation (23). Therefore, there is an urgent need
to develop novel MDM2 inhibitors or p53 activators.
Zebrafish has become a widely used model in
pre-clinical drug screening and the mechanistic studies of
angiogenesis, since its embryo is small and optically transparent
to visualize the whole vessels (24,25).
Especially, the use of vascular specific transgene allows for the
detection of individual growing cells or vessel formation (26). To date, many types of cancer models
have been developed in zebrafish and providing excellent tools for
anticancer drug discovery through candidate drug testing (27,28).
In the present study, we used transgenic fluorescent
zebrafish tg(fli1:EGFP) to evaluate the anti-angiogenic
efficacy of YH-304 in live zebrafish embryos. In the present study,
we showed the anti-tumorigenic effect of YH-304 using in
vitro clonogenic assay, TUNEL assay, and cell viability assay,
as well as the effect on bFGF-induced in vivo angiogenesis.
At the molecular level, we found that YH-304 increases the number
of TUNEL-positive cells and the expression level of cleaved PARP,
cleaved caspase-9 and Bax, resulting in apoptotic cell death.
YH-304 did not alter the p53 or its target gene expression levels.
In addition, YH-304 potently inhibited angiogenesis of
bFGF-mediated AKT and ERK phosphorylation. The anti-tumorigenic and
anti-angiogenic potential of YH-304 to induce apoptosis with broad
spectrum and downregulate ERK/AKT activity suggests a putative use
in anticancer therapy.
Materials and methods
Cell cultures
HeLa (human cervical carcinoma cell line), HCT-116
(human colon carcinoma cell line), A549, 226B (human non-small lung
cancer cell lines), and MDA-MB-231 (human breast cancer cell line)
were purchased from the American Type Culture Collection (ATCC;
Manassas, VA, USA) and maintained at 37°C in a humidified 5%
CO2 atmosphere. HeLa, HCT-116 and MDA-MB-231 cells were
cultured in Dulbecco’s modified Eagle’s medium and A549 and 226B
cells were cultured in RPMI-1640 medium supplemented with 10% fetal
bovine serum (FBS) and 1% antibiotics (Invitrogen, Carlsbad, CA,
USA).
Reagents
Recombinant bFGF was purchased from BD Biosciences
(Franklin Lakes, NJ, USA), whereas the anti-GAPDH, α-Tubulin, PARP
and p53 antibodies were purchased from Santa Cruz Biotechnology
(Santa Cruz, CA, USA) and the anti-Akt, p-Akt, cleaved caspase-9,
Bax, ERK and p-ERK antibodies used were purchased from Cell
Signaling Technology (Boston, MA, USA). Nutlin-3 was purchased from
Sigma-Aldrich (St. Louis, MO, USA). YH-304 [(S)-Methyl
1-benzhydryl-3-(4-methylbenzyl)-2-oxopiperidine-3-carboxylate] was
produced as previously described (2).
Clonogenic assay
In brief, A549 and 226B cells were seeded at
2×103 cells/well on a 6-well plate and allowed to attach
overnight. The next day, medium was replaced with fresh medium
containing different concentrations (0.5 and 2 μM) of YH-304 and
incubated for 2 h. Following incubation, cells were harvested by
trypsinization and counted. For clonogenic assay, 4 ml of cell
suspension (50 cells/ml) was seeded in a 6-well plate and were
incubated at 37°C for 14 days without any disturbance. Following
incubation, the medium was removed and colonies were fixed and
stained with 0.01% (w/v) crystal violet for 30 min. A colony
consisting of at least 50 cells was counted with ImageJ software
(NIH, Bethesda, MD, USA). The experiment was carried out twice in
triplicate.
Western blot analysis
Western blot analyses were performed as previously
described using the antibodies mentioned above. The respective
protein bands were detected by chemiluminescence (FUSION SL4;
Vilber Lourmat, Marne la Vallée, France).
Real-time PCR analysis
Total RNA was isolated from cells using
TRIzol® reagent (Invitrogen) and transcribed using a
PrimeScript First Strand cDNA Synthesis kit (Takara, Shiga, Japan)
according to the manufacturers’ protocols. Quantitative real-time
PCR was performed in triplicate on Rotor-Gene® Q (Roche
Diagnostics, Indianapolis, IN, USA) using LightCycler®
SYBR-Green I Master (Roche Diagnostics), and data were analyzed on
the basis of threshold cycle values of each sample and normalized
with β-actin. The PCR primers used in this study are listed below
and were purchased from Bioneer: forward p53
5′-GCCCAACAACACCAGCTCCT-3′ and reverse p53
5′-GCCCAACAACACCAGCTCCT-3′; forward p21 5′-GGCCTCCTGACCCACAGCAG-3′
and reverse p21 5′-GGGCTCAACTCAACACCCACC-3′; forward β-actin
5′-AGAGGGAAATCGTGC-3′ and reverse β-actin 5′-GGC
CGTCAGGCAGCTCATAG-3′.
Cell death assays by TUNEL staining
DNA strand breaks during apoptosis were detected by
TUNEL of free 3′-OH ends of cleaved DNA. Following YH-304 treatment
for 24 h, 226B cells were fixed in 4% (v/v) paraformaldehyde for 30
min on ice. Fixed cells were washed once in phosphate-buffered
saline (PBS) and incubated for 60 min at 37°C in 50 μl of
TdT-containing solution (Roche Diagnostics, Mannheim, Germany).
Following TUNEL staining, all samples were washed once in PBS and
nuclei were stained using 4′-6-Diamidino-2-phenylindole (DAPI;
Invitrogen). Images were obtained with an Axiovert M200 microscope
(Carl Zeiss AG, Oberkochen, Germany).
Cell viability assay
HCT-116, HeLa, A549 and MDA-MB-231 cells were
treated with different concentration of YH-304 as indicated. After
24 h, 20 μl of CellTiter 96® Aqueous One solution
reagent (Promega, Madison, WI, USA) was added and then read at 490
nm. Dose-response curves were plotted to determine half-maximal
inhibitory concentrations (IC50) for the compounds with
SigmaPlot software.
Zebrafish angiogenesis assay
Fertilized zebrafish (Danio rerio) eggs of the
transgenic strain expressing green fluorescent protein (GFP) under
the flk-1 promoter (flk-1:GFP) were used for in vivo
imaging of embryonic vascular development. At 24 h
post-fertilization (hpf), EGFP-expressing tg(fli1:EGFP)
embryos were manually dechorionated and anesthetized with 0.003%
tricaine. Anesthetized embryos were then transferred onto a 4%
agarose gel for microinjection. Approximately 5 nl of Matrigel
containing either PBS or basic fibroblast growth factor (bFGF, 25
ng/ml) was injected into the yolk sac of each embryo using
microinjector (Narishige International, Inc., East Meadow, NY,
USA). Non-filamentous borosilicate glass capillary needles were
used for the microinjection (0.75 mm internal- and 1.0 mm
external-diameter). Embryos were transferred into housing-keeping
water immediately after injection and incubated with 0.003%
1-phenyl-2-thiourea (Sigma-Aldrich) at 28.5°C to prevent
pigmentation. YH-304 treatment was carried out immediately after
Matrigel injection and embryos were examined for angiogenesis using
a fluorescent microscope (Carl Zeiss) 24 h later.
Statistical analysis
All values are expressed as means ± SE. Statistical
significance was set at P<0.05; P<0.05; P<0.001.
Results
YH-304 inhibits proliferation and colony
formation of A549 and 226B NSCLC cells
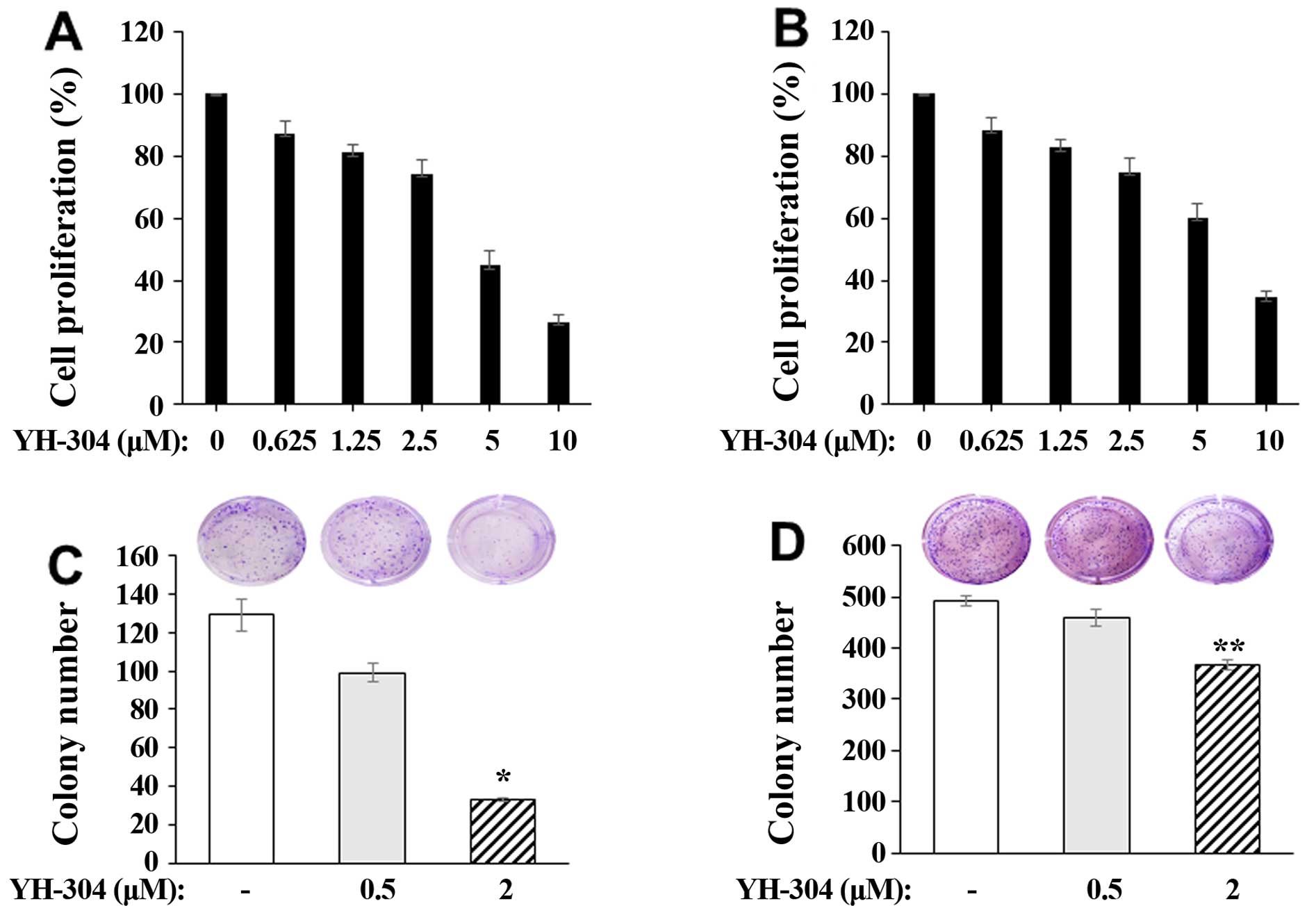
To investigate the effect of YH-304 on the cancer
cell proliferation, A549 and 226B NSCLC cells were pretreated with
or without YH-304 ranging from 0.625 to 10 μM for 24 h and then
measured by 3-[4,5-methylthiazol-2-yl]-2,5-diphenyl-tetrazolium
bromide (MTT) assay. We found that YH-304 inhibited cancer cell
proliferation in both A549 (Fig.
1A) and 226B (Fig. 1B) NSCLC
cells in a dose-dependent manner. The anticancer effect of YH-304
was subsequently confirmed by clonogenic assay in A549 and 226B
NSCLC cells. As observed above, we found that YH-304 suppresses
colony formation ability of both A549 (Fig. 1C) and 226B (Fig. 1D) NSCLC cells. This suggests that
YH-304 has potential for anticancer efficacy in NSCLC cells.
YH-304 induces apoptosis of A549 NSCLC
cells
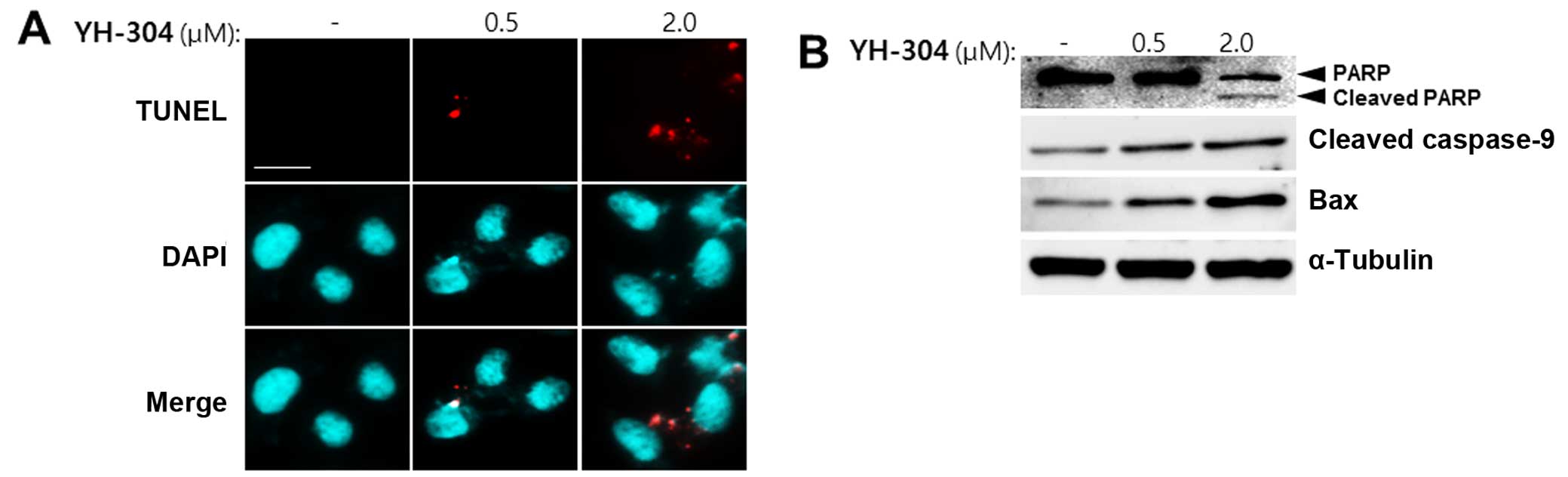
We next set out to determine whether YH-304 is
involved in apoptosis using TUNEL assay. When A549 cells were
treated with 0.5 or 2.0 μM YH-304, TUNEL-positive cells were
increased as measured by fluorescence microscopy at individual cell
level (Fig. 2A). From these cell
images, it is clear that the red fluorescence intensity of
individual cells increased in a concentration-dependent manner
indicating apoptosis by YH-304. Additionally, we evaluated whether
YH-304 can induce apoptotic cell death using apoptotic markers such
as cleaved poly(ADP-ribose) polymerase (PARP), cleaved caspase-9
and Bax. When A549 cells were treated with 0.5 or 2.0 μM YH-304 for
24 h, the expression of intact PARP was decreased and that of
cleaved PARP was detected as compared with control group (Fig. 2B). Also, cleaved caspase-9 and Bax
were increased by YH-304 treatment in a dose-dependent manner
(Fig. 2B). Together, these results
suggest that YH-304 may induce apoptosis of A549 cells.
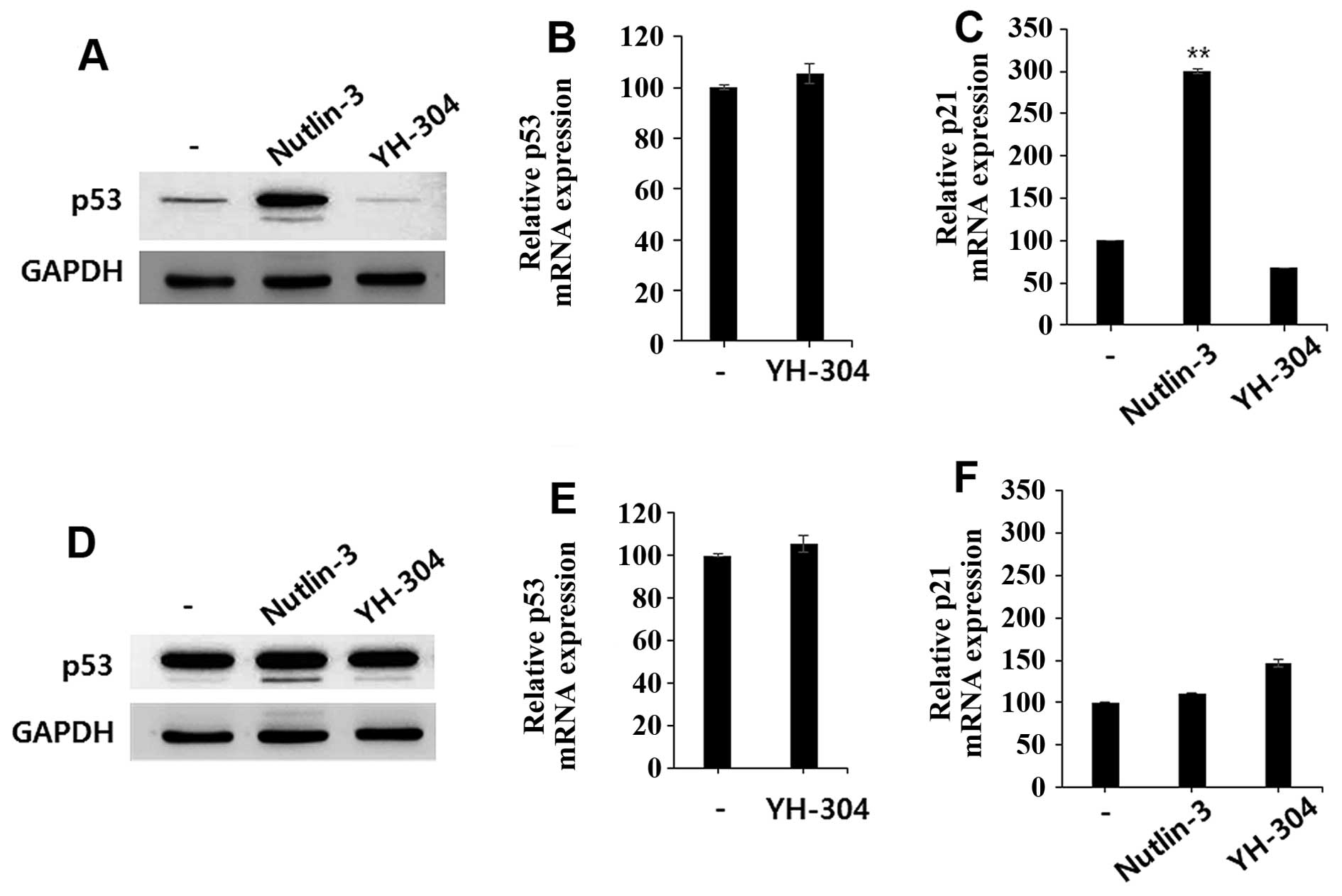
YH-304 does not affect p53 expression
levels in HCT-116 and MDA-MB-231 cells
We previously reported that YH-304 is a simplified
spiro-oxindole alkaloid which shares structural similarity with
MDM2 inhibitor (an active anticancer agent) (2). Based on structural characteristics,
we hypothesized that YH-304 might show the anticancer activity
through MDM2 inhibition, resulting in p53 stabilization. To
investigate whether the cytotoxic effect of YH-304 is dependent on
MDM2-mediated p53 modulation, we determined whether YH-304 induces
p53 expression through inhibition of MDM2. We treated either YH-304
or nutlin-3 (a well-known MDM2 inhibitor) in p53 wild-type cells
(HCT-116) as well as p53 mutant cells (MDA-MB-231) and then
evaluated the expression levels of p53 protein and p53-dependent
mRNAs including p21. As expected, nutlin-3 induced both p53 protein
expression level (Fig. 3A) and p21
mRNA levels (Fig. 3B)
significantly in HCT-116 cells, but not in MDA-MB-231 cells
(Fig. 3C and D). However, YH-304
did not alter the mRNA or protein expression levels of p53 in the
HCT-116 (Fig. 3A and E) or
MDA-MB-231 cells (Fig. 3C and F).
Our preliminary mechanistic studies of YH-304 suggested that it has
anticancer activities via p53-independent pathway. Further
investigations of mechanistic studies for anticancer activities are
now in progress.
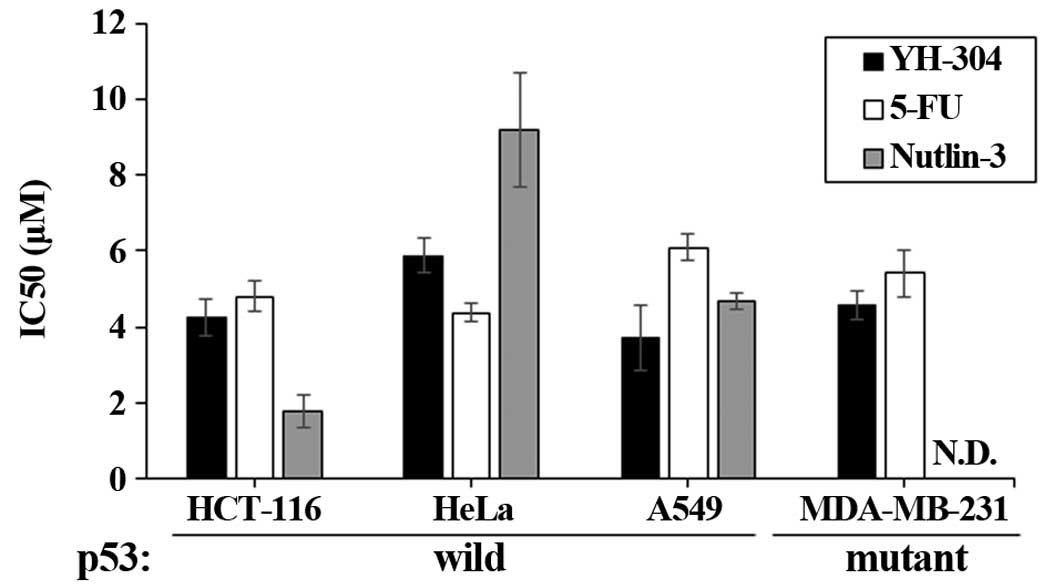
YH-304 shows broad spectrum anticancer
activity in a MDM2-p53-independent manner
The efficacy of YH-304 was investigated in four
different cancer cell lines including HCT-116 (colon cancer), HeLa
(cervix cancer), A549 (lung cancer), and MDA-MB-231 (breast cancer)
cells and compared with a well-known chemotherapy agent, 5-FU and
nutlin-3. Compared to 5-FU, YH-304 showed potent efficacy in four
different cancer cells lines, showing broad spectrum anticancer
effect (Fig. 4). Especially,
IC50 values of YH-304 were significantly lower than 5-FU
in HCT-116, A549 and MDA-MB-231 cells (Fig. 4). Nutlin-3, a specific MDM-2
inhibitor, exerted anticancer effect in p53 wild-type cancer cells
having wild-type p53 but did not affect cell viability of
MDA-MB-231 cells possessing mutant p53. However, YH-304 also showed
good efficacy in both p53 wild-type and p53-mutant cancer cells,
suggesting that YH-304 might be a potent anticancer agent with
broad-spectrum in a p53-independent fashion.
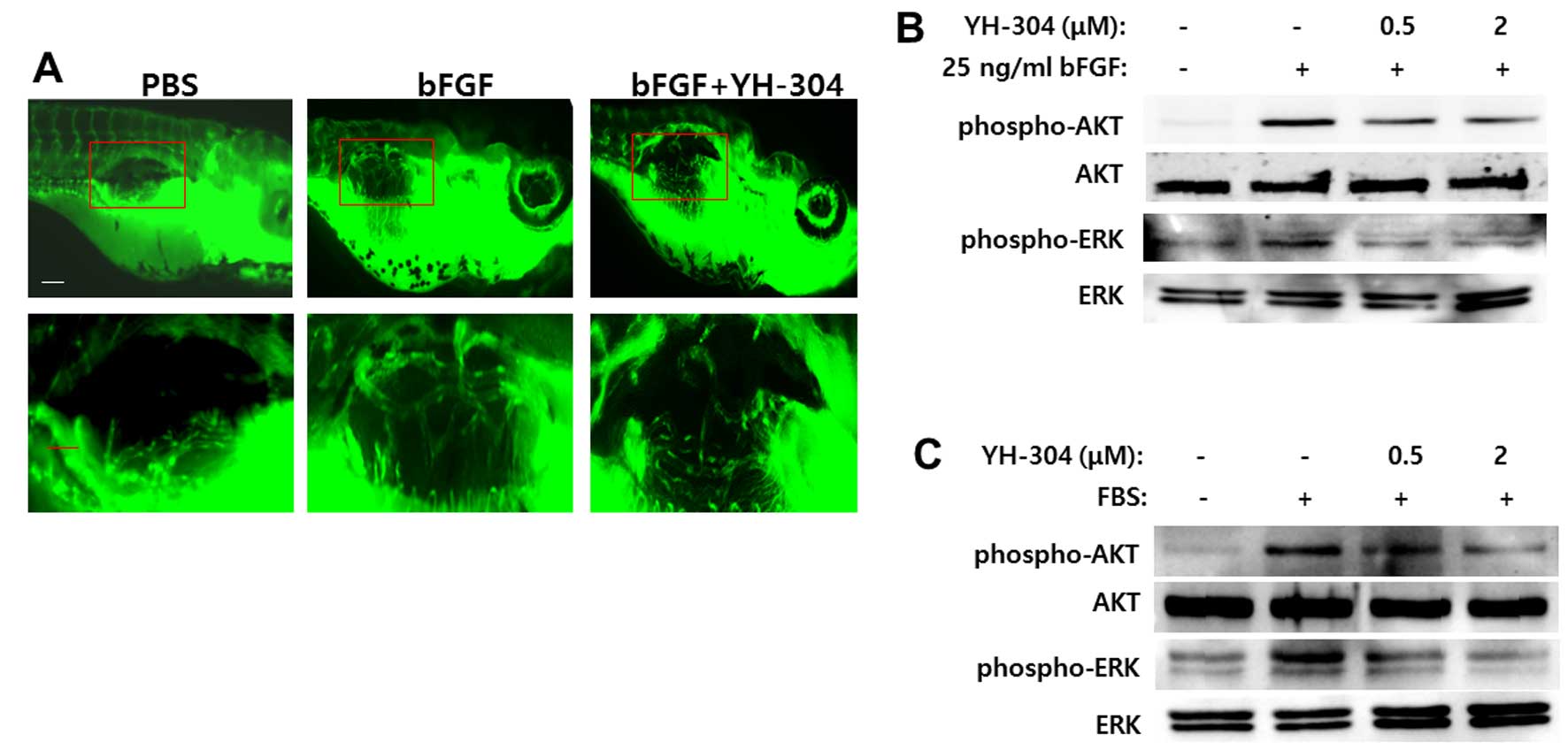
YH-304 inhibits in vivo bFGF-induced
angiogenesis through suppression of AKT and ERK
To investigate the effect of YH-304 on angiogenesis
in vivo, we performed matrigel plug assay with transgenic
fluorescent zebrafish tg(fli1:EGFP) which expresses enhanced
GFP in the entire vasculature under the control of the fli1
promoter to visualize vascular formation in live zebrafish embryos
(26). In plugs with bFGF (200
ng/ml) appeared newly formed vessels (Fig. 5A). In contrast, there was less or
no blood vessel formation in plugs with Matrigel alone or mixed
with YH-304 (2 μM) (Fig. 5A).
To further assess how YH-304 blocks bFGF-mediated
angiogenesis, we tested whether YH-304 can affect AKT and/or ERK
activation, two proteins known to play an important role in the
bFGF signaling pathway. As shown in Fig. 5B, bFGF increased the
phosphorylation of both AKT and ERK, whereas the addition of 0.5 or
2.0 μM YH-304 significantly reduced the phosphorylated AKT and
phosphorylated ERK. Instead of bFGF, when treated with 10% FBS,
YH-304 showed similar inhibition of phosphorylation of both AKT and
ERK (Fig. 5C). Therefore, these
data suggest that YH-304 suppresses bFGF-induced angiogenesis in
vivo, at least partly through blocking AKT/ERK activation.
Discussion
Cancer is now one of the leading causes of mortality
worldwide, causing 7.6 million deaths in 2008 (3). Anticancer drugs are required with
novel structural features to overcome multidrug resistance and
severe side-effects and to enhance the effectiveness of cancer
treatment (29,30). Oxindole derivatives are specialized
structures and exerted various biological activities, ranging from
MDM2 antagonists (31,32), ion channel blockers (33) and anti-inflammatory agents
(34,35) to various protein kinase inhibitors.
A number of indole-based compounds inhibit various protein kinase
families such as receptor tyrosine kinases (RTKs) and
serine/threonine-specific protein kinases (5,10,36,37).
As a kinase inhibitor, oxindole sunitinib was used for the
treatment of advanced renal carcinoma and gastrointestinal stromal
tumors (10,11,36,37).
We previously reported the potential use of oxindole derivatives
having simplified α-quaternary chiral lactam as anticancer agents.
Among them, YH-304 had the strongest cell cytotoxicity against
several cancer cell lines. However, the mechanisms of action
underlying its anticancer effects are largely unknown and need to
be better understood to allow this therapy to be used for clinical
applications. This prompted us to study further the
anti-tumorigenic effects of YH-304, including its underlying
molecular mechanisms. Therefore, the aim of the present study was
to further clarify the anticancer mechanism of YH-304 itself, which
may offer an opportunity to design effective safe drugs with
minimal toxicity for the treatment of carcinoma.
The p53 tumor suppressor has been implicated as a
mediator of programmed cell death (16). Loss of wild-type p53 activity is
regarded as a major predictor of failure to respond to chemotherapy
in various human cancers (17,19,38).
Therefore, patients with mutated p53 tend to be less responsive to
common chemotherapeutics (39).
Since p53 is the most frequently mutated in malignant cancers,
therapies that do not depend on functional p53 are in general
clinically preferable (39).
Notably, although the structure of YH-304 is designed as a kind of
oxindole derivatives similar to that of MDM2 inhibitor (a p53
activator), it did not alter the expression of p53 in p53 wild-type
(HCT-116) or p53 mutant cells (MDA-MB-231), unlike nutlin-3 (MDM2
inhibitor) (Fig. 3A, C, E and F).
Also, YH-304 showed similar or better efficacy in four different
cancer cell lines compared to 5-FU (Fig. 4), indicating a p53-independent
broad spectrum. Based on p53-independency and broad spectrum,
therefore, YH-304 might be clinically preferable after further
studies such as toxicity and clinical trials.
Our findings also indicate that YH-304 interferes
with AKT and ERK phosphorylation in response to angiogenic factors
such as bFGF or serum. Since AKT and ERK activities are required
for proliferation and tube formation of vascular endothelial cells,
the AKT and ERK pathways are considered as one of the most
promising targets for anti-angiogenic therapy (40,41).
Taken together, it is reasonable to assume that YH-304-mediated
inhibition of AKT and ERK phosphorylation may be responsible for
the inhibitory effect of YH-304 on angiogenesis as well as on
clonogenic growth. Moreover, growing knowledge on cellular
signaling networks in cancer opens up the opportunity to combine
AKT and ERK inhibitors with other pathway specific inhibitors.
Since synergistic effects between such inhibitors are known to
exist, further studies are warranted combining YH-304 with other
targeted inhibitors.
Our current data provide experimental evidence for a
novel anticancer activity of YH-304 through suppression of AKT and
ERK phosphorylation and, thereby, their downstream signaling
cascade. Further studies are required to delineate the exact role
and mode of action of YH-304, i.e., whether and how blood vessels
regress in YH-304-treated Matrigel plugs. The present study
provides a proof-of-principle for the development of YH-304 as an
anti-angiogenic agent.
Acknowledgements
The present study was supported by the Basic Science
Research Program through the National Research Foundation of Korea
funded by the Ministry of Science, ICT and Future Planning
(NRF-2016R1C1B2012270, NRF-2013R1A1A1059709 and
NRF-2014R1A1A1002151).
References
|
1
|
Chen L, Yang J, Zheng M, Kong X, Huang T
and Cai YD: The use of chemical-chemical interaction and chemical
structure to identify new candidate chemicals related to lung
cancer. PLoS One. 10:e01286962015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lee H, Hwang SJ, Jung J, Hong S, Lee M,
Park HG, Lee HJ and Park Y: Asymmetric synthesis and evaluation of
α-quaternary chiral lactam derivatives as novel anticancer agents.
Arch Pharm Res. 37:1264–1270. 2014. View Article : Google Scholar
|
|
3
|
Jemal A, Siegel R, Ward E, Hao Y, Xu J,
Murray T and Thun MJ: Cancer statistics, 2008. CA Cancer J Clin.
58:71–96. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Galliford CV and Scheidt KA:
Pyrrolidinyl-spirooxindole natural products as inspirations for the
development of potential therapeutic agents. Angew Chem Int Ed
Engl. 46:8748–8758. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chen G, Weng Q, Fu L, Wang Z, Yu P, Liu Z,
Li X, Zhang H and Liang G: Synthesis and biological evaluation of
novel oxindole-based RTK inhibitors as anti-cancer agents. Bioorg
Med Chem. 22:6953–6960. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Natarajan A, Guo Y, Harbinski F, Fan YH,
Chen H, Luus L, Diercks J, Aktas H, Chorev M and Halperin JA: Novel
arylsulfoanilide-oxindole hybrid as an anticancer agent that
inhibits translation initiation. J Med Chem. 47:4979–4982. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Silva BV, Ribeiro NM, Vargas MD,
Lanznaster M, Carneiro JW, Krogh R, Andricopulo AD, Dias LC and
Pinto AC: Synthesis, electrochemical studies and anticancer
activity of ferrocenyl oxindoles. Dalton Trans. 39:7338–7344. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kamal A, Ramakrishna G, Raju P, Rao AV,
Viswanath A, Nayak VL and Ramakrishna S: Synthesis and anticancer
activity of oxindole derived imidazo[1,5-a]pyrazines. Eur J Med
Chem. 46:2427–2435. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cui CB, Kakeya H and Osada H:
Spirotryprostatin B, a novel mammalian cell cycle inhibitor
produced by Aspergillus fumigatus. J Antibiot (Tokyo). 49:832–835.
1996. View Article : Google Scholar
|
|
10
|
Papaetis GS and Syrigos KN: Sunitinib: A
multitargeted receptor tyrosine kinase inhibitor in the era of
molecular cancer therapies. BioDrugs. 23:377–389. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang WL, Conley A, Reynoso D, Nolden L,
Lazar AJ, George S and Trent JC: Mechanisms of resistance to
imatinib and sunitinib in gastrointestinal stromal tumor. Cancer
Chemother Pharmacol. 67(Suppl 1): S15–S24. 2011. View Article : Google Scholar
|
|
12
|
Kussie PH, Gorina S, Marechal V, Elenbaas
B, Moreau J, Levine AJ and Pavletich NP: Structure of the MDM2
oncoprotein bound to the p53 tumor suppressor transactivation
domain. Science. 274:948–953. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ding K, Lu Y, Nikolovska-Coleska Z, Qiu S,
Ding Y, Gao W, Stuckey J, Krajewski K, Roller PP, Tomita Y, et al:
Structure-based design of potent non-peptide MDM2 inhibitors. J Am
Chem Soc. 127:10130–10131. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ding K, Lu Y, Nikolovska-Coleska Z, Wang
G, Qiu S, Shangary S, Gao W, Qin D, Stuckey J, Krajewski K, et al:
Structure-based design of spiro-oxindoles as potent, specific
small-molecule inhibitors of the MDM2-p53 interaction. J Med Chem.
49:3432–3435. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Park Y, Lee YJ, Hong S, Lee M and Park HG:
Highly enantioselective total synthesis of (+)-isonitramine. Org
Lett. 14:852–854. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Reed SM and Quelle DE: p53 Acetylation:
Regulation and consequences. Cancers (Basel). 7:30–69. 2014.
View Article : Google Scholar
|
|
17
|
Melvin AT, Dumberger LD, Woss GS, Waters
ML and Allbritton NL: Identification of a p53-based portable degron
based on the MDM2-p53 binding region. Analyst (Lond). 141:570–578.
2016. View Article : Google Scholar
|
|
18
|
Huang KY, Weng JT, Lee TY and Weng SL: A
new scheme to discover functional associations and regulatory
networks of E3 ubiquitin ligases. BMC Syst Biol. 10(Suppl 1):
32016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Richmond J, Carol H, Evans K, High L,
Mendomo A, Robbins A, Meyer C, Venn NC, Marschalek R, Henderson M,
et al: Effective targeting of the P53-MDM2 axis in preclinical
models of infant MLL-rearranged acute lymphoblastic leukemia. Clin
Cancer Res. 21:1395–1405. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Saiki AY, Caenepeel S, Cosgrove E, Su C,
Boedigheimer M and Oliner JD: Identifying the determinants of
response to MDM2 inhibition. Oncotarget. 6:7701–7712. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Deben C, Wouters A, Op de Beeck K, van Den
Bossche J, Jacobs J, Zwaenepoel K, Peeters M, Van Meerbeeck J,
Lardon F, Rolfo C, et al: The MDM2-inhibitor Nutlin-3 synergizes
with cisplatin to induce p53 dependent tumor cell apoptosis in
non-small cell lung cancer. Oncotarget. 6:22666–22679. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zanjirband M, Edmondson RJ and Lunec J:
Pre-clinical efficacy and synergistic potential of the MDM2-p53
antagonists, Nutlin-3 and RG7388, as single agents and in combined
treatment with cisplatin in ovarian cancer. Oncotarget. May
20–2016.(Epub ahead of print). View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Michaelis M, Rothweiler F, Barth S, Cinatl
J, van Rikxoort M, Löschmann N, Voges Y, Breitling R, von Deimling
A, Rödel F, et al: Adaptation of cancer cells from different
entities to the MDM2 inhibitor nutlin-3 results in the emergence of
p53-mutated multi-drug-resistant cancer cells. Cell Death Dis.
2:e2432011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Rennekamp AJ and Peterson RT: 15 years of
zebrafish chemical screening. Curr Opin Chem Biol. 24:58–70. 2015.
View Article : Google Scholar
|
|
25
|
Sun Y, Sheng Z, Ma C, Tang K, Zhu R, Wu Z,
Shen R, Feng J, Wu D, Huang D, et al: Combining genomic and network
characteristics for extended capability in predicting synergistic
drugs for cancer. Nat Commun. 6:84812015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Liu Z and Liu F: Cautious use of
fli1a:EGFP transgenic zebrafish in vascular research. Biochem
Biophys Res Commun. 427:223–226. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Barriuso J, Nagaraju R and Hurlstone A:
Zebrafish: A new companion for translational research in oncology.
Clin Cancer Res. 21:969–975. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Eden CJ, Ju B, Murugesan M, Phoenix TN,
Nimmervoll B, Tong Y, Ellison DW, Finkelstein D, Wright K, Boulos
N, et al: Orthotopic models of pediatric brain tumors in zebrafish.
Oncogene. 34:1736–1742. 2015. View Article : Google Scholar
|
|
29
|
Gonçalves AS, Macedo AS and Souto EB:
Therapeutic nanosystems for oncology nanomedicine. Clin Transl
Oncol. 14:883–890. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yin Q, Shen J, Zhang Z, Yu H, Chen L, Gu W
and Li Y: Multi-functional nanoparticles improve therapeutic effect
for breast cancer by simultaneously antagonizing multiple
mechanisms of multidrug resistance. Biomacromolecules.
14:2242–2252. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ribeiro CJ, Amaral JD, Rodrigues CM,
Moreira R and Santos MM: Synthesis and evaluation of
spiroisoxazoline oxindoles as anticancer agents. Bioorg Med Chem.
22:577–584. 2014. View Article : Google Scholar
|
|
32
|
Shangary S, Ding K, Qiu S,
Nikolovska-Coleska Z, Bauer JA, Liu M, Wang G, Lu Y, McEachern D,
Bernard D, et al: Reactivation of p53 by a specific MDM2 antagonist
(MI-43) leads to p21-mediated cell cycle arrest and selective cell
death in colon cancer. Mol Cancer Ther. 7:1533–1542. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Jensen BS: BMS-204352: A potassium channel
opener developed for the treatment of stroke. CNS Drug Rev.
8:353–360. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Chen G, Jiang L, Dong L, Wang Z, Xu F,
Ding T, Fu L, Fang Q, Liu Z, Shan X, et al: Synthesis and
biological evaluation of novel indole-2-one and 7-aza-2-oxindole
derivatives as anti-inflammatory agents. Drug Des Devel Ther.
8:1869–1892. 2014.PubMed/NCBI
|
|
35
|
Sun Y, Liu J, Jiang X, Sun T, Liu L, Zhang
X, Ding S, Li J, Zhuang Y, Wang Y, et al: One-step synthesis of
chiral oxindole-type analogues with potent anti-inflammatory and
analgesic activities. Sci Rep. 5:136992015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ho HK, Chua BT, Wong W, Lim KS, Teo V, Ong
HT, Chen X, Zhang W, Hui KM, Go ML, et al: Benzylidene-indolinones
are effective as multi-targeted kinase inhibitor therapeutics
against hepatocellular carcinoma. Mol Oncol. 8:1266–1277. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Izzedine H, Buhaescu I, Rixe O and Deray
G: Sunitinib malate. Cancer Chemother Pharmacol. 60:357–364. 2007.
View Article : Google Scholar
|
|
38
|
Engeland K: Simplify p53: Just an
activator. Oncotarget. 6:3–4. 2015.PubMed/NCBI
|
|
39
|
Koshino A, Goto-Koshino Y, Setoguchi A,
Ohno K and Tsujimoto H: Mutation of p53 gene and its correlation
with the clinical outcome in dogs with lymphoma. J Vet Intern Med.
30:223–229. 2016. View Article : Google Scholar :
|
|
40
|
Hellesøy M and Lorens JB: Cellular
context-mediated Akt dynamics regulates MAP kinase signaling
thresholds during angiogenesis. Mol Biol Cell. 26:2698–2711. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Huang JJ, Shi YQ, Li RL, Hu A, Lu ZY, Weng
L, Wang SQ, Han YP, Zhang L, Li B, et al: Angiogenesis effect of
therapeutic ultrasound on HUVECs through activation of the
PI3K-Akt-eNOS signal pathway. Am J Transl Res. 7:1106–1115.
2015.PubMed/NCBI
|