Introduction
The pituitary gland is a tiny organ found at the
base of the brain (1). The
pituitary gland is divided into three sections: anterior,
intermediate and posterior lobes (2). The pituitary gland produces many
hormones, including oxytocin, antidiuretic hormone, growth hormone,
thyroid-stimulating hormone (TSH), luteinizing hormone (LH) and
follicle-stimulating hormone (FSH), that travel throughout the body
and direct physiological processes (1,3). The
anterior lobe is mainly involved in the development of the body,
the sexual maturation and the reproduction (4). Hormones produced by the anterior lobe
regulate growth and stimulate the adrenal and thyroid glands as
well as the ovaries and testes.
The pituitary also generates prolactin (PRL), which
is a 23-kDa polypeptide hormone secreted by lactotroph cells of the
anterior pituitary gland (4).
Pituitary PRL secretion is regulated by endocrine neurons in the
hypothalamus, which secrete dopamine to act on dopamine receptors
located on lactotrophs for inhibition of PRL secretion (5). PRL acts in an endocrine, autocrine
and paracrine manner through prolactin receptor (PRLR) and a large
number of cytokine receptors (6).
PRL plays an important role in the reproductive health of both
women and men. Specifically, it stimulates mammary glands to
produce milk (lactation); increased serum concentrations of PRL
during pregnancy cause enlargement of mammary glands of the breasts
and prepare for production of milk. PRL also controls secretion of
sex steroid hormones (4). Highly
elevated levels of PRL reduce levels of estrogen in women and
testosterone in men. The effects of mildly elevated levels of PRL
are much more variable, as estrogen levels in women may either
substantial increase or decrease (7).
Since PRL performs multiple physiological functions,
uncontrolled levels of PRL are associated with many diseases.
Hyperprolactinemia, which is noted in most cases of gonadotroph
pituitary adenoma, is defined as a high concentration of PRL
(8). One common cause of
hyperprolactinemia is tumor growth on the pituitary gland, known as
prolactinoma. If PRL levels are high, a doctor will test thyroid
function and asks about other conditions and medications known to
increase PRL secretion. Diagnosis of prolactinoma by magnetic
resonance imaging (MRI) is the most sensitive method for detecting
pituitary tumors and determining their size (9).
Schisandra chinensis (Turcz.) Baill (S.
chinensis) has a well-recognized history in traditional Chinese
medicine. S. chinensis fruits contain a variety of
pharmacologically active lignans such as gomisin A, B, C, D, E, F,
G, K3, N and J, together with schisandrol B, schisandrin (SS) and
schisandrin C (SC). These compounds have diverse pharmacological
activities, including detoxificant, anti-oxidant,
anti-carcinogenic, anti-hepatotoxic, anti-inflammatory and
anticancer activities (10,11).
Two major lignans, SS and gomisin A (GA), have gained attention due
to their therapeutic effects (11). For instance, SS possesses
biological activities, including hepatoprotective, antiviral and
neuroprotective effects (12,13).
However, the effects of S. chinensis on pituitary and
hormonal regulation have not been addressed. In the present study,
therefore, we examined the effects of S. chinensis and its
single compounds on regulation of PRL and growth hormone production
in the pituitary. In addition, the therapeutic potential of S.
chinensis was evaluated by in vitro and in vivo
experiments.
Materials and methods
Plant material extraction
Dried fruits of S. chinensis were ground to a
fine powder and successively extracted at room temperature with
n-hexane, EtOAc and MeOH. Hexane extract was evaporated in
vacuo and chromatographed on a silica gel column (70×8.0 cm) with a
step gradient of 0, 5, 10, 20 and 30% EtOAc in hexane (each 1 l).
Fraction 11 was separated on a silica gel column (100×3.0 cm) with
25% hexane in CHCl3 to give five subfractions. Fraction
11IA was further purified by column chromatography on silica gel
eluted with CHCl3-acetone (19:1) to give gomisin N (GN).
Fraction 8 was separated on a silica gel column (100×3.0 cm) with
CH2Cl2 to give SC. Fractions 36, 37 and 38
were separated on a silica gel column (100×3.0 cm) with 5%
CH2Cl2 in acetone to give SS.
For extraction of GA and α-iso-cubebene (CU), dried
fruits of S. chinensis were ground and extracted with
n-hexane, CHCl3 and methanol. Hexane extract was
evaporated in vacuo and chromatographed on a silica gel
column (100×10 cm) with a step gradient (0, 5 and 20%) of ethyl
acetate in hexane and 5% methanol in CHCl3 to obtain 38
fractions as described before. (22) Fraction 11 was separated on a silica
gel column (100×3.0 cm) with hexane-chloroform-methanol (75:25:1 by
volume) to obtain four fractions. Fraction 3 was separated on a
Sephadex column (100×3.0 cm) with methanol (KH11ICIC). Finally,
KH11ICIC was separated on a silica gel column (115×3.0 cm) with 5%
acetone in chloroform to yield GA. Fraction 11 was separated on a
silica gel column (100×3.0 cm) with 15% acetone in
CH2Cl2 to obtain nine fractions. Next,
fraction 2 was separated on a silica gel column (100×3.0 cm) with
15% acetone in CH2Cl2 to yield α-iso-cubebene
(CU). Pure CU was identified by HPLC on a Phenomenex Luna C18
column (Phenomenex, 150×4.6 mm ID; 5 µm particle size) with
an acetonitrile-water-reagent alcohol gradient at a flow rate of
1.0 ml/min.
GH3 cell culture and treatments
GH3 rat pituitary epithelial-like tumor cells were
seeded in culture medium and allowed to attach during 24 h of
incubation, after which the seeding medium was removed and replaced
with experimental medium added to 100 U/ml of penicillin and 100
µg/ml of streptomycin for 24 h before treatment. Chemicals
were dissolved in EtOH, diluted with experimental medium and added
to the wells. Cells were treated with crude extract (crude, 0.1
mg/ml) and single compounds, including GN, GA, SC, SS and CU (20
µM) or EtOH as a vehicle control.
Experimental animals and treatments
Immature female Sprague-Dawley (n=19) rats were
acquired from Samtako Bio (Osan, Republic of Korea). Animals were
housed at the Pusan National University Laboratory Animal Resources
Center, which is accredited by the AAALAC and Korea FDA, according
to the National Institutes of Health guidelines. the rats were
housed in cages under a 12-h light/dark cycle and a constant
temperature of 23±1°C. The Ethics Committee of Pusan National
University (Busan, Republic of Korea) approved all experimental
animal procedures (approval no. PNU-2015-0921). Rats were treated
daily with crude (200 mg/kg), GN (20 mg/kg), progesterone (P4, 1
mg/kg) or corn oil (vehicle control) via oral (crude and GN) or
subcutaneous injection (P4) from postnatal days 13–19. Dosage was
adjusted according to changes in body weight (BW). All animals were
sacrificed using CO2 gas for preparation of tissue and
serum samples.
Quantitative real-time PCR (q-PCR)
Total RNA was extracted using TRIzol reagent
(Invitrogen, Carlsbad, CA, USA) according to the manufacturer's
protocol. Concentration of total RNA was measured by a
spectrophotometer. DNA (cDNA) was prepared from total RNA (1
µg) by reverse transcription (RT) using M-MLV reverse
transcriptase (Invitrogen) and random primers (9-mers; Takara Bio,
Shiga, Japan). q-PCR was performed using cDNA template (2
µl) and SYBR-Green (6 µl; Toyobo Co., Ltd., Shiga,
Japan) with specific primers. Primer sequences used for PRL, PRLR,
growth hormone and β-actin are as follows: PRL (left, 5′-AGT CTG
TTC TGG TGG CGA CT-3′ and right, 5′-GAA GTG GGG CAG TCA TTG AT-3′);
PRLR (left, 5′-CCT CTG CAC TTG CTT TCG TC-3′ and right, 5′-ATC GAT
TCC TCC ATC TGT CC-3′); growth hormone (left, 5′-CTG GCT GCT GAC
ACC TAC AA-3′ and right, 5′-AAG CGA AGC AAT TCC ATG TC-3′); β-actin
(left, 5′-ACC AAC TGG GAC GAT ATG GAG AAG-3′ and right, 5′-TAC GAC
CAG AGG CAT ACA GGG ACA-3′). q-PCR was carried out for 40 cycles
using the following parameters: denaturation at 95°C for 15 sec,
annealing and extension at 70°C for 60 sec. Fluorescence intensity
was measured at the end of each extension phase. The threshold
value for the fluorescence intensity of all samples was set
manually. The reaction cycle at which PCR products exceeded this
fluorescence intensity threshold during exponential phase of PCR
amplification was considered to be the cycle of threshold (CT).
Expression of the target gene was quantified relative to that of
β-actin, a housekeeping gene, based on the comparison of CTs at
constant fluorescence intensity.
Western blot analysis
Protein samples were extracted from GH3 cells with
cell lysis buffer (20 mM Tris, 100 mM NaCl, 0.5% NP-40, 0.5 mM EDTA
and 0.5% protease inhibitor cocktail). A total of 25 µg of
protein was separated by 10–12% sodium dodecyl sulfate
polyacrylamide gel electrophoresis (SDS-PAGE) and then transferred
to nitrocellulose membranes (Dogen, Seoul, Korea). Membranes were
subsequently blocked for 2 h with 5% skim milk (Difco™) in
tris-buffered saline (TBS) with 0.05% Tween-20 (TBS-T). After
blocking, membranes were incubated with antibodies specific for PRL
(diluted 1:500) overnight at 4°C as well as horseradish peroxidase
(HRP)-conjugated anti-goat secondary antibodies in 5% skim milk
with TBS-T for 1 h. Luminol reagent (Bio-Rad Laboratories) was used
to visualize antibody binding. Each blot was scanned using Gel Doc
1000, version 1.5 (Bio-Rad Laboratories) and band intensities were
normalized to β-actin levels.
Cell proliferation assay
GH3 cells (3×104 cells/well) were seeded
on 96-well plates in 200 µl of Dulbecco's modified Eagle's
medium (DMEM) containing 10% fetal bovine serum (FBS), 100 U/ml of
penicillin and 100 µg/ml of streptomycin. After 24 h of
incubation, the seeding medium was removed and replaced with
experimental medium (phenol red-free DMEM supplemented with 10%
charcoal-dextran-treated FBS, 100 U/ml of penicillin and 100
µg/ml of streptomycin) for 24 h before treatment. Cells were
treated with all single compounds (20 µM each) or EtOH as a
vehicle control for 24 h, after which 50 µl of MTT solution
(2 mg/ml) was added to each well in 200 µl of medium without
phenol red and the plates incubated for 4 h at 37°C. Dimethyl
sulfoxide (DMSO) was added to all wells and mixed thoroughly to
dissolve the dark blue crystals. After a few minutes at room
temperature to ensure that all crystals were dissolved, absorbance
in the wells was measured at 570 nm with a reference wavelength of
650 nm.
Statistical analyses
Results are presented as the mean ± standard
deviation (SD). Data were analyzed using SigmaPlot 10.0 (Systat
Software, Inc., San Jose, CA, USA). P<0.05 were considered
statistically significant.
Results
Regulation of PRL expression and
secretion by S. chinensis and its compounds in GH3 cells
To investigate the effect of S. chinensis on
pituitary PRL production, GH3 cells were treated with crude, GN,
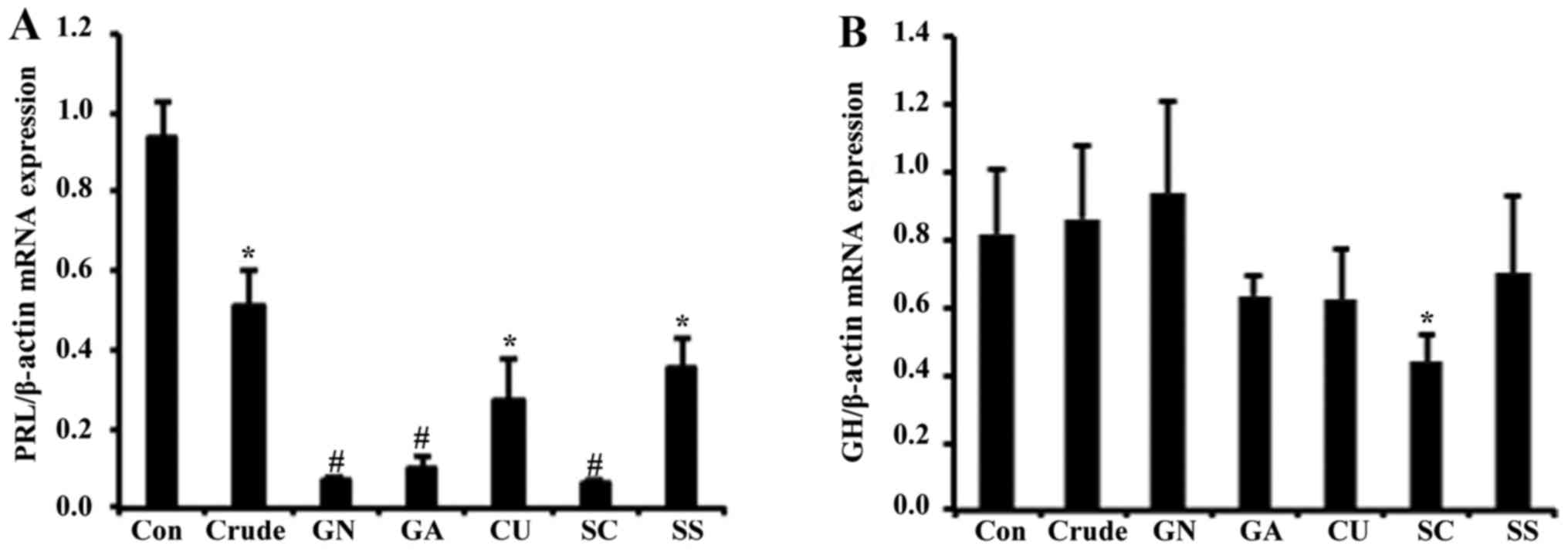
GA, CU, SC and SS for 24 h. Transcription levels of PRL were
reduced in response to crude by ~2-fold (Fig. 1A), and all single compounds of
S. chinensis significantly reduced PRL mRNA levels. As GH3
cells are known to synthesize another critical substance, the
growth hormone, we also analyzed the effect of S. chinensis
on transcriptional regulation of the growth hormone. In contrast to
PRL levels, mRNA expression of growth hormone was not significantly
altered by crude or any single compounds, suggesting that the
effect of S. chinensis is specific to the regulation of PRL
(Fig. 1B).
Since the effects of GN, GA and SC were more
dominant compared with those of CU and SS, we conducted further
experiments using these three compounds. When the cells were
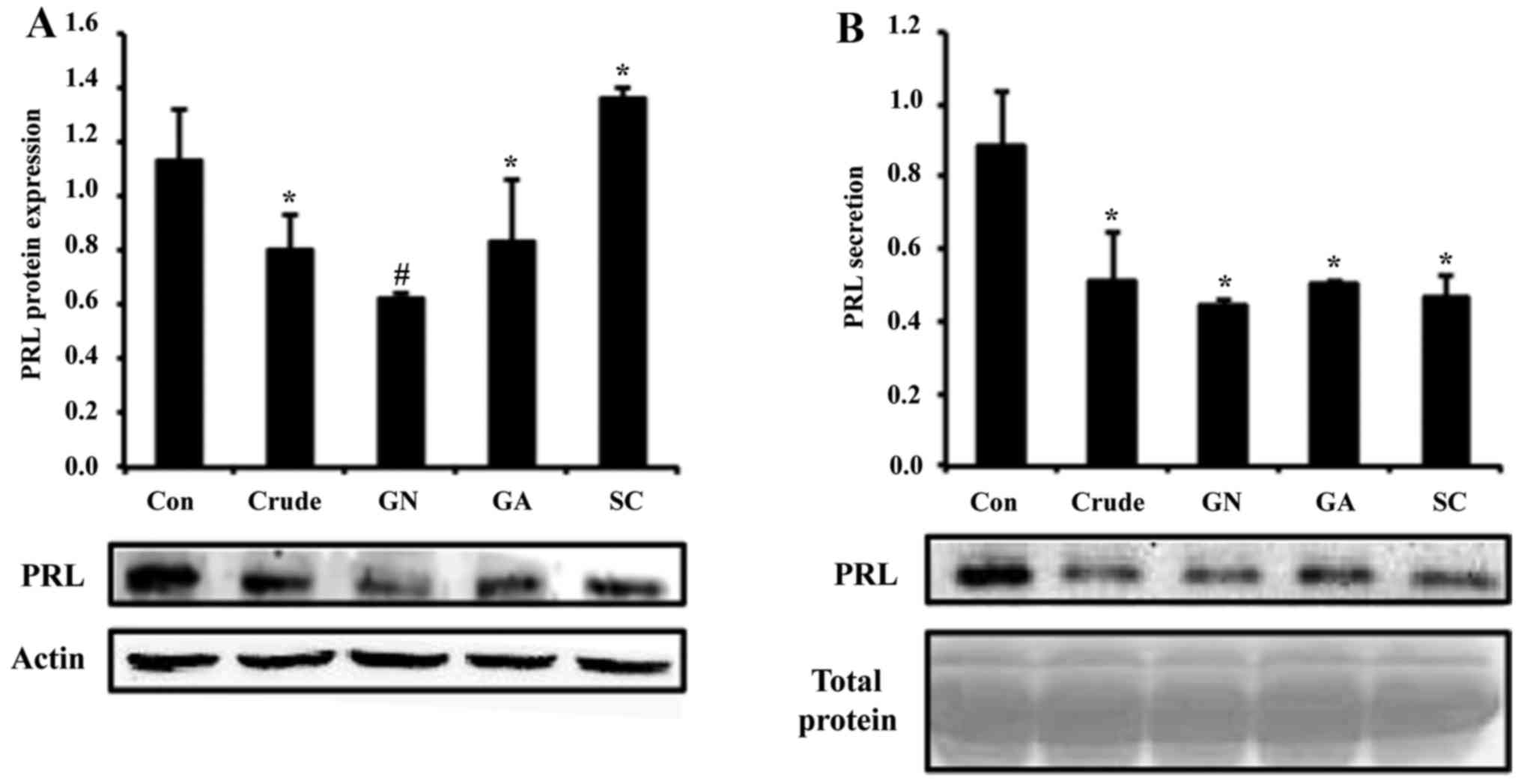
treated with crude and single compounds (GN, GA and SC), protein
levels of PRL were reduced ~2-fold by crude and GN (Fig. 2A). To test secretion of PRL, cell
culture media was collected and subjected to western blot assay. In
the results, PRL secretion was also reduced by all treatment
groups, which is consistent with the mRNA and protein results
(Fig. 2B). In all experiments, GN
showed the most significant alteration of PRL production among
single compounds.
Dose- and time-dependent regulation of
PRL synthesis
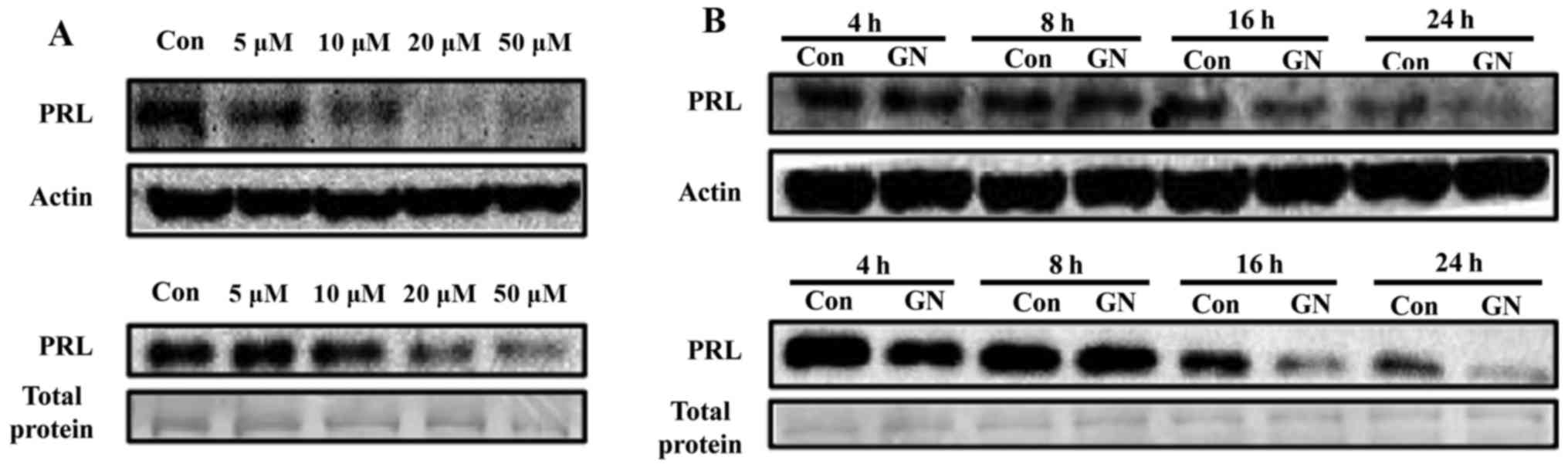
Regulation of PRL by GN, a single compound of S.
chinensis, was further explored in a dose- and time-dependent
manner. Pituitary cells were treated with various concentrations
(5, 10, 20 and 50 µM) of GN for 24 h, resulting in
significant reduction of PRL protein levels and secretion at
concentrations of 10 to 50 µM in a dose-dependent manner
(Fig. 3A). Basal PRL expression
levels were reduced according to culture time, suggesting that PRL
synthesis may be controlled by autocrine regulation of PRL itself.
When cells were treated with GN, PRL protein expression was
downregulated from 16 to 24 h compared with the negative control
during the same time. GN treatment also reduced secretion of PRL in
culture media of GH3 cells from 16 to 24 h, which is similar to
mRNA and protein expression levels (Fig. 3B).
Effect of S. chinensis and GN on GH3 cell
proliferation and PRL target gene
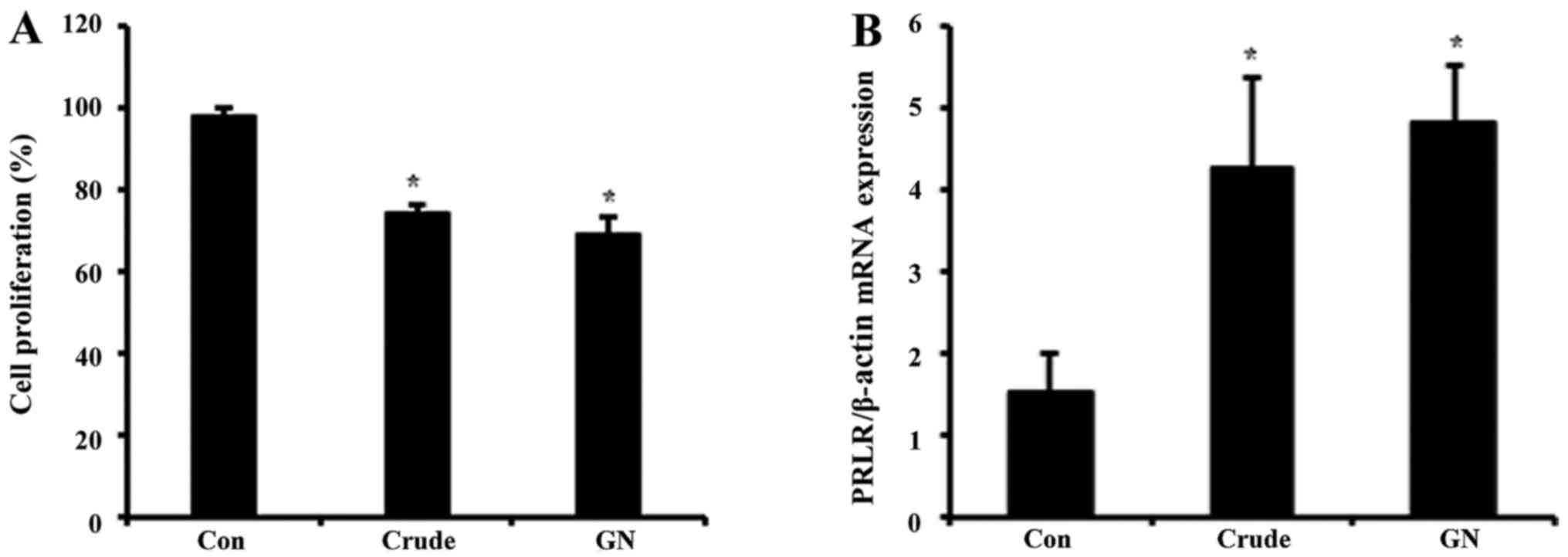
To evaluate whether or not GN regulates viability of
GH3 cells, we performed MTT assay (Fig. 4A). GH3 cells were treated with
crude and GN for 24 h, after which the cell viability was measured.
Cell proliferation was inhibited by ~20% in both crude and GN
groups compared to the control. These results showed that S.
chinensis and its single compound, GN, inhibit not only PRL
production but also viability of PRL-secreting cells. To examine
the expression of PRL target gene after crude and GN treatment,
mRNA levels of the PRL-specific receptor PRLR were tested. Both
crude and GN enhanced expression of PRLR up to 4-fold compared to
the control (Fig. 4B).
Regulation of PRL and PRLR production by
S. chinensis and GN in immature rats
Based on our in vitro findings, we next
examined the effects of S. chinensis and GN in vivo
using immature female rats. Rats were orally injected with crude,
GN, or corn oil as a vehicle control from postnatal days 16 to 18
and sacrificed on day 19. Rats were also treated with P4 by
subcutaneous injection as a positive control for reduction of PRL.
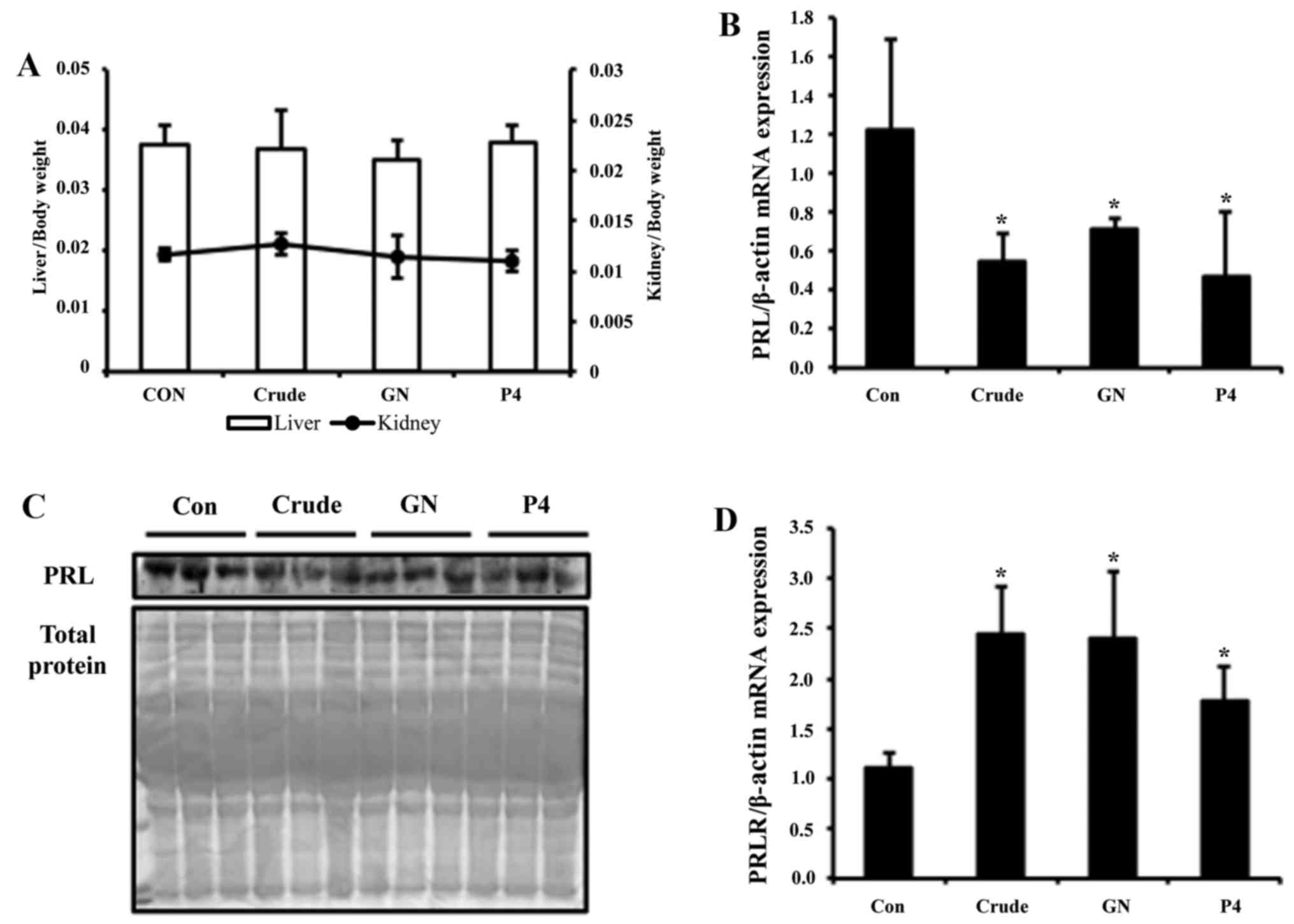
The ratio of organ weight to body weight indicated no significant
alteration of liver and kidney weights in all animals, suggesting
that there is no toxic effect by the treatment (Fig. 5A). Transcription level of PRL in
the pituitary of immature female rats was examined using real-time
PCR. Expression of PRL was reduced ~3-fold by crude and 2-fold by
GN treatment (Fig. 5B). To test
PRL secretion levels, serum of immature female rats was analyzed by
western blot assay. Crude group showed more strong reduction of PRL
secretion, whereas the GN group showed moderate reduction (Fig. 5C). For the next experiment, PRLR
mRNA levels were analyzed by real-time PCR. The mRNA expression
level of PRLR was significantly elevated upon crude and GN
treatment, which is consistent with the in vitro study
(Fig. 5D).
Discussion
Homeostatic maintenance of PRL is essential since
this hormone performs multiple physiological functions (14). General guidelines for
hyperprolactinemia define the upper threshold of normal PRL at 25
µg/l for women and 20 µg/l for men, whereas
hypoprolacinemia is defined as PRL levels below 3 µg/l in
women and 5 µg/l in men (15-17).
The rep resentative cause of hyperprolactinemia is prolactinoma,
and increased PRL levels can cause infertility and bone loss in
both women and men. The goal of treatment for prolactinoma is to
return PRL levels to normal, reduce tumor size, and correct any
visual abnormalities (15). In
some cases, surgical correction of prolacinoma has been shown to
lower PRL levels to less than 250 ng/ml in 80% of patients. Even in
patients with large tumors that cannot be completely removed, drug
therapy may be able to reduce serum PRL levels back to the normal
range (18). Dopamine is the
chemical that normally inhibits PRL secretion, and dopamine
agonists such as bromocriptine and cabergoline are effective
medicines for treatment of hyperprolactinemia. However,
bromocriptine and carbergoline are associated with side-effects
such as nausea, vomiting, dizziness and hypotension (18,19).
To solve these problems, development of a treatment with fewer
side-effects is required.
Natural products that generally show less
side-effects than chemical drugs have become the main focus for the
treatment of prolactinemia. Ginseng treatment was shown to reduce
PRL secretion via a direct nitric oxide-mediated effect on the
anterior pituitary (20). In
another study, Ginkgo biloba extract improved sexual
performance in young sexually experienced male rats via reduction
of serum PRL levels (21).
However, these studies focused on crude extracts of natural
products and not on single compounds.
In the present study, we have for the first time
investigated the therapeutic effects of extract and single
compounds of S. chinensis on pituitary PRL regulation in
vivo and in vitro. GH3 cells, which synthesize and
secrete PRL, were treated with crude and single compounds, and GN
remarkably reduced PRL mRNA and protein levels. PRL secretion was
also reduced by GN in a dose- and time-dependent manner compared
with the control. These results suggest that S. chinensis
and its single compounds, especially GN, have potential as a
natural medicine to treat hyperprolactinemia. In addition, we
examined expression of the representative prolactin target gene,
PRLR, in GH3 cells. As expected, PRL significantly reduced
expression of its receptor. A previous study reported the rapid and
prolonged downregulation of PRLR by PRL. In this study, PRL also
caused degradation of PRLR, which is blocked by inhibitors of
proteasomes and lysosomes (22).
To further examine whether or not S. chinensis is effective
for prolactinoma, we performed cell biability assay and observed
that crude and GN successfully reduced pituitary carcinoma cell
growth.
Following the in vitro study, we investigated
the effect of S. chinensis crude and GN on PRL synthesis and
secretion in immature female rats. In the present study, we
employed sexually immature animals since sex steroid hormones are
reported to regulate production of PRL. Specifically, P4
upregulates PRL synthesis in the endometrium but downregulates it
in the myometrium and breast glandular tissue (23). Estrogen (E2) and P4 inhibit the
stimulatory effects of PRL on milk production. More directly, it
has been reported that E2 increases serum and pituitary PRL in
ovariectomized rats, whereas P4 has inhibitory effects (24). In addition, precursor of E2 and P4,
pregnenolone sulfate also increases prolactin production in the rat
pituitary (25). In our
experiments, we treated immature rats with crude, GN and P4. P4 was
used as a positive control for the reduction of PRL based on
previous studies (24). Similar to
the in vitro study, crude and GN inhibited PRL but increased
PRLR expression that is a target gene of PRL. Therefore, both in
vivo and in vitro studies suggest that S.
chinensis has a therapeutic effect on hyperprolactinoma.
In a previous study, S. chinensis was
confirmed to possess various bioactivities and pharmacological
applications. However, the mechanisms and bioactivities of its
single compounds have not been well studied. GN is known to induce
cellular apoptosis in hepatoma cells and leukemia cells (26,27).
Additionally, studies on the mechanism of GN-induced anticancer
activity against two human tumor cell lines, ovary carcinoma cells
and colon adenocarcinoma cells, have been shown (28). GN successfully inhibited growth of
these cell lines through induction of different types of cell
death. Although there are several studies available on the
therapeutic potentials of GN and S. chinensis in other
tissues, the effects of S. chinensis and its single
compounds in the pituitary have not been addressed at all.
In summary, we examined the suppressive effects of
S. chinensis on the synthesis and secretion of pituitary PRL
both in vivo and in vitro. These findings demonstrate
that S. chinensis can be applied to patients with
PRL-related disorders such as hyperprolactinemia and
prolactinoma.
Acknowledgments
The present study was supported by the National
Research Foundation of Korea (NRF) grant funded by the Korea
government (MOE) (no. 2014R1A1A2057387).
References
|
1
|
Hong GK, Payne SC and Jane JA Jr: Anatomy,
physiology, and laboratory evaluation of the pituitary gland.
Otolaryngol Clin North Am. 49:21–32. 2016. View Article : Google Scholar
|
|
2
|
Amar AP and Weiss MH: Pituitary anatomy
and physiology. Neurosurg Clin N Am. 14:11–23. v2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Blackwell RE, Rodgers-Neame NT, Bradley EL
Jr and Asch RH: Regulation of human prolactin secretion by
gonadotropin-releasing hormone in vitro. Fertil Steril. 46:26–31.
1986. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Freeman ME, Kanyicska B, Lerant A and Nagy
G: Prolactin: Structure, function, and regulation of secretion.
Physiol Rev. 80:1523–1631. 2000.PubMed/NCBI
|
|
5
|
Ben-Jonathan N and Hnasko R: Dopamine as a
prolactin (PRL) inhibitor. Endocr Rev. 22:724–763. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rillema JA: Mechanism of prolactin action.
Fed Proc. 39:2593–2598. 1980.PubMed/NCBI
|
|
7
|
McMurray RW: Estrogen, prolactin, and
autoimmunity: Actions and interactions. Int Immunopharmacol.
1:995–1008. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Capozzi A, Scambia G, Pontecorvi A and
Lello S: Hyperprolactinemia: Pathophysiology and therapeutic
approach. Gynecol Endocrinol. 31:506–510. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Glezer A and Bronstein MD: Prolactinomas.
Endocrinol Metab Clin North Am. 44:71–78. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Panossian A and Wikman G: Pharmacology of
Schisandra chinensis Bail: An overview of Russian research and uses
in medicine. J Ethnopharmacol. 118:183–212. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hancke JL, Burgos RA and Ahumada F:
Schisandra chinensis (Turcz) Baill. Fitoterapia. 70:451–471. 1999.
View Article : Google Scholar
|
|
12
|
Chang HM and But P: Pharmacology and
applications of Chinese Materia Edica Volume 1. World Scientific;
Singapore: pp. 199–209. 1986, View Article : Google Scholar
|
|
13
|
Bao T-T, Tu G-F, Liu G-T, Sun R-H and Song
Z-Y: A comparison of the pharmacological actions of seven
constituents isolated from fructus schizadrae (author's transl).
Yao Xue Xue Bao. 14:1–7. 1979.In Chinese.
|
|
14
|
Cowie AT: Physiological actions of
prolactin. Proc R Soc Med. 66:861–862. 1973.PubMed/NCBI
|
|
15
|
Mancini T, Casanueva FF and Giustina A:
Hyperprolactinemia and prolactinomas. Endocrinol Metab Clin North
Am. 37:67–99. viii2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Schwärzler P, Untergasser G, Hermann M,
Dirnhofer S, Abendstein B and Berger P: Prolactin gene expression
and prolactin protein in premenopausal and postmenopausal human
ovaries. Fertil Steril. 68:696–701. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ufearo CS and Orisakwe OE: Restoration of
normal sperm characteristics in hypoprolactinemic infertile men
treated with metoclopramide and exogenous human prolactin. Clin
Pharmacol Ther. 58:354–359. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bronstein MD: Potential for long-term
remission of microprolactinoma after withdrawal of dopamine-agonist
therapy. Nat Clin Pract Endocrinol Metab. 2:130–131. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Auriemma RS, Pivonello R, Ferreri L,
Priscitelli P and Colao A: Cabergoline use for pituitary tumors and
valvular disorders. Endocrinol Metab Clin North Am. 44:89–97. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Murphy LL and Lee TJ: Ginseng, sex
behavior, and nitric oxide. Ann N Y Acad Sci. 962:372–377. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yeh KY, Pu HF, Kaphle K, Lin SF, Wu LS,
Lin JH and Tsai YF: Ginkgo biloba extract enhances male copulatory
behavior and reduces serum prolactin levels in rats. Horm Behav.
53:225–231. 2008. View Article : Google Scholar
|
|
22
|
Lu JC, Piazza TM and Schuler LA:
Proteasomes mediate prolactin-induced receptor down-regulation and
fragment generation in breast cancer cells. J Biol Chem.
280:33909–33916. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zinger M, McFarland M and Ben-Jonathan N:
Prolactin expression and secretion by human breast glandular and
adipose tissue explants. J Clin Endocrinol Metab. 88:689–696. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chen CL and Meites J: Effects of estrogen
and progesterone on serum and pituitary prolactin levels in
ovariectomized rats. Endocrinology. 86:503–505. 1970. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kang EJ, Hong SH, Lee JE, Kim SC, Yang HS,
Yi PI, Lee SM and An BS: Pregnenolone sulfate regulates prolactin
production in the rat pituitary. J Endocrinol. 230:339–346. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yim SY, Lee YJ, Lee YK, Jung SE, Kim JH,
Kim HJ, Son BG, Park YH, Lee YG, Choi YW, et al: Gomisin N isolated
from Schisandra chinensis significantly induces anti-proliferative
and pro-apoptotic effects in hepatic carcinoma. Mol Med Rep.
2:725–732. 2009.PubMed/NCBI
|
|
27
|
Kim JH, Choi YW, Park C, Jin CY, Lee YJ,
Park DJ, Kim SG, Kim GY, Choi IW, Hwang WD, et al: Apoptosis
induction of human leukemia U937 cells by gomisin N, a
dibenzocyclooctadiene lignan, isolated from Schizandra chinensis
Baill. Food Chem Toxicol. 48:807–813. 2010. View Article : Google Scholar
|
|
28
|
Casarin E, Dall'Acqua S, Smejkal K,
Slapetova T, Innocenti G and Carrara M: Molecular mechanisms of
antiproliferative effects induced by Schisandra-derived
dienzocyclooctadiene lignin ()-deoxychisandrin and (-)-gomisin N in
human tumor cell lines. Fitoterapia. 98:241–247. 2014. View Article : Google Scholar : PubMed/NCBI
|