Introduction
Osteosarcoma (OS) is a common primary aggressive
tumor affecting the bones, which arises from primitive transformed
cells of mesenchymal origin (1).
It is locally aggressive and is inclined to generate early systemic
metastases (2). Comprehensive data
regarding the epidemiology of OS have revealed that OS is most
prevalent in teenagers and young adults (3,4).
Current therapeutic strategies include pre-operative (neoadjuvant)
chemotherapy followed by the surgical removal of all detectable
cancerous lesions (including metastases) and post-operative
(adjuvant) chemotherapeutic management (1). Patients with high-grade OS have
acquired favorable long-term outcomes in the conjunction of
systemic chemotherapy with surgery (5). A 5-year event-free survival of 60–70%
is achieved in extremity localized, non-metastatic disease
following the introduction of chemotherapy (6,7).
Chemotherapeutic agents that have shown toxicity against OS include
cisplatin, doxorubicin, oxazaphosphorines and high-dose
methotrexate (HDMTX) (8–10).
Methotrexate (MTX), originally known as
4-aminopteroyl-glutamic acid, is a pivotal chemotherapeutic agent
that was discovered in the 1940s and was originally used in the
treatment of childhood leukemia and lymphoma (11). MTX has been used as a
chemotherapeutic agent in the treatment of various types of human
cancer, including breast cancer (12,13),
leukemia (14,15), lung cancer (16), gastric choriocarcinoma (17), lymphoma (18) and OS (19), either alone or in combination with
other agents. However, 35–45% of patients with OS acquire drug
resistance due to the inherent resistance to chemotherapeutic
agents or due to the fact that they become unresponsive to these
drugs during chemotherapy (20,21).
There is evidence to suggest that epithelial-mesenchymal transition
(EMT) is associated with acquired resistance to chemotherapeutic
drugs in human malignancies (22).
EMT is a biological process during which the phenotype of polarized
epithelial cells transforms into one of mesenchymal cells (23). At the molecular level, during the
transition, a decrease in the expression of epithelial cell markers
[such as Zonula occludens-1 (ZO-1) and E-cadherin] is observed, as
well as an increase in the expression of stromal cell markers [such
as N-cadherin, Slug, Snail, Twist, Vimentin, and zinc finger E-box
binding homeobox (ZEB)1 and ZEB2] (24,25).
Chemotherapy has been reported to induce EMT in tumor cells. Fang
et al found that Snail inhibition by transfection with
specific small interfering RNA (siRNA) promoted cisplatin
sensitivity, and cisplatin-induced EMT was significantly blocked
(26). In addition, baicalin has
been shown to inhibit human OS cell invasion, metastasis and
anoikis resistance by suppressing transforming growth factor
(TGF)-β1-induced EMT (27).
Recently, it was reported that catalpol suppresses OS cell
proliferation by blocking EMT and inducing apoptosis (28). Ohbayashi et al found that
lung cancer cells treated with MTX exhibited an EMT-like phenotype
accompanied by the elevation of the expression of interleukin-6
(IL)-6 and TGF-β1, as well as an enhancement of migration (29). However, whether MTX triggers EMT in
OS remains to be fully determined.
F-box E3 ubiquitin ligase S-phase kinase-associated
protein 2 (Skp2) belongs to the ubiquitin proteasome system (UPS).
The deregulation of Skp2-mediated ubiquitination and the
proteolysis of its substrates is involved in tumorigenesis in
various types of human cancer (30). A previous study revealed that Skp2
was overexpressed and was associated with a poor prognosis in
prostate cancer (31), lymphomas
(32), gastric cancer (33), breast cancer (34), liver cancer (35) and nasopharyngeal carcinoma (NPC)
(36), thereby functioning as a
proto-oncogene. Skp2 has been reported to modulate the cell cycle,
cell proliferation, apoptosis and metastasis in a variety of human
cancers by regulating numerous substrates (30,37,38).
Targeting Skp2 suppresses tumorigenesis by Arf-p53-independent
cellular senescence (39). Skp2
has been shown to be highly expressed in NPC specimens and to be
associated with a poor prognosis, and Skp2 inactivation has been
shown to promote cellular senescence in NPC cell lines through
p21cip/WAF and p27Kip (40). Furthermore, Skp2 has been reported
to function as a critical component in the PTEN/PI3-kinase pathway
for the regulation of p27 and cell proliferation in carcinomas
(41). Skp2 has also been shown to
promote the ubiquitin-mediated proteolysis of forkhead box O1
(Foxo1) and to play a key role in tumorigenesis (42). Inuzuka et al found that Skp2
enhanced cellular migration through ubiquitination and the
destruction of E-cadherin (43).
Recently, it was reported that the depletion of Skp2 inhibited cell
growth and triggered the apoptosis of the OS cell lines, MG63 and
SW 1353 cells (44). Therefore,
Skp2 may be an effective therapeutic target in the coming age of
cancer therapy.
In this study, we examined whether Skp2 was
associated with MTX-induced EMT in OS cells. We established
MTX-resistant OS cell lines using the U2OS and MG63 cells. We then
examined whether the MTX-resistant OS cells underwent the
transition from an epithelial into a mesenchymal phenotype.
Finally, we provide evidence that Skp2 is involved in the
resistance of OS cells to MTX and is closely associated with the
acquirement of mesenchymal characteristics.
Materials and methods
Cell culture and reagents
The human osteosarcoma cell lines, U2OS and MG63,
were cultured in Dulbecco's modified Eagle's medium (DMEM; Life
Technologies, Grand Island, NY, USA) medium supplemented with
penicillin (100 U/ml), and streptomycin (100 U/ml) and 10% fetal
bovine serum (FBS). MTX, 3-(4,5-dimethythi-azol- 2-yl)-2,5-diphenyl
tetrazolium bromide (MTT) and anti-α-tubulin (T9028) primary
antibody were purchased from Sigma (St. Louis, MO, USA). Matrigel
was purchased from BD Biosciences (San Jose, CA, USA). Primary
antibodies against ZO-1 (#5406), N-cadherin (#4061), E-cadherin
(#3195), Slug #9585), Vimentin (#5741), Nanog (#4903),
octamer-binding transcription factor 4 (Oct4, #2750), ATP-binding
cassette sub-family B member 1 (ABCB1, #12683), FoxO1 (#2880) and
p21 (#2946) were obtained from Cell Signaling Technology (Danvers,
MA, USA). Anti-Skp2 (sc-7164) antibody was purchased from Santa
Cruz Biotechnology (Santa Cruz, CA, USA). To establish
MTX-resistant cell lines, the U2OS and MG63 cells were cultured at
37°C in 5% CO2 in Dulbecco's modified Eagle's medium
(DMEM; Gibco, Carlsbad, CA, USA) supplemented with 10% fetal bovine
serum (FBS) in increasing concentrations of MTX (10–40 μM)
for >6 months. The MTX-resistant OS cells developed a resistance
to 40 μM MTX.
Cell viability assay
The parental and MTX-resistant OS cells
(4×103 cells/well) were seeded in 96-well plates and
incubated at 37°C overnight. Various concentrations of MTX (10, 20,
30 and 40 μM) were added and cell culture was continued for
48 and 72 h. MTT assay was then performed to measure cell viability
using a microplate reader (Thermo Fisher Scientific, Inc., Waltham,
MA, USA) at 570 nm.
Cell attachment and detachment
For attachment assay, 5×104/well cells
were seeded in a 24-well plate and incubated at 37°C. One hour
later, the unattached cells were removed and the attached cells
were counted using the Countess II FL Automated Cell Counter
(Thermo Fisher Scientific). For cell detachment assay, the cells
were seeded and incubated at 37°C for 24 h. The cells were treated
with 0.05% trypsin for 3 min, and then counted as detached cells
using the Countess II FL Automated Cell Counter.
Viral infection
Skp2 knockdown was performed using Skp2 short
hairpin (Skp2-RNAi; shRNA1, shRNA2, shRNA3, Genechem, Shanghai,
China) or scrambled shRNA (CON054) lentiviral particles (Genechem).
293T cells were obtained from the American Type Culture Collection
(ATCC; Manassas, VA, USA) and cultured in DMEM supplemented with
10% FBS, 100 U/ml penicillin/streptomycin at 37°C with 5%
CO2. The 293T cells were co-transfected with the
packaging plasmids, pVSV-G, pΔR-rev (Jiran Co., Shanghai,, China)
and shRNA or scrambled shRNA expression plasmids using
Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) to produce
lentivirus particles according to the manufacturer's instructions
(Invitrogen). Supernatant from the 293T cells was collected at 48 h
following transfection. The MTX-resistant OS cells were grown to
40–50% confluency in DMEM with 10% FBS and exposed to the collected
lentivirus particles for 48 h. Subsequently, the cells were
selected by 4 μg/ml puromycin. The puromycin-contained
medium was displaced every 3 days for approximately 2 weeks until
the non-transduced cells disappeared. Single clones were selected,
and inoculated onto a new plate to grow in the presence of
puromycin. The single clone with the stable knockdown of the
Skp2 gene was expanded and passaged for use in subsequent
experiments.
Invasion assay
The MTX-resistant and parental OS cells were
observed and photographed under a microscope (Olympus IX71;
Olympus, Tokyo, Japan) to observe any morphological changes.
Subsequently, the invasive capacity of the MTX-resistant cells was
determined by placing the cells into 24-well Transwell inserts
pre-coated with Matrigel following the manufacturer's instructions.
Briefly, the OS cells, MTX-resistant cells with control
shRNA-transfected or Skp2 shRNA-transfected cells were cultured in
the upper chamber of the inserts with 200 μl FBS-free DMEM.
The bottom chamber contained 500 μl medium with 10% FBS.
Following incubation at 37°C for approximately 24 h, the
non-invading cells on the upper surface of the filter membrane were
removed carefully using a cotton swab, while the invading cells on
the bottom surface of the membrane were stained with Calcein-AM
(C3099, Invitrogen) for 10 min. Subsequently, the membrane was
rinsed with water and photographed and the invading cells were
counted under a fluorescent microscope (Olympus, IX71;
Olympus).
Wound healing assay
The parental, MTX-resistant cells with control shRNA
transfection and MTX-resistant cells in which Skp2 was knocked down
were seeded (1×105 cells/ml) in a 6-well plate. The
supernatant was absorbed after the cells grew to approximately 90%
confluence. The scratch wound was generated by scratching the
monolayers with a 10 μl sterile pipette tip. The cells were
washed carefully with PBS to remove floating cell debris and then
supplemented with DMEM. Following cultured for approximately 20 h,
the cells that had migrated into the wound area were photographed
under an inverted microscope (Olympus, IX71; Olympus).
Reverse transcription-quantitative RT-PCR
(RT-qPCR)
Total RNA was isolated from the parental and
MTX-resistant OS cells using the RNeasy Plus Mini kit (Qiagen China
Co., Ltd, Shanghai, China). The concentrations and purities of the
RNA were determined by an ND-1000 spectrophotometer (NanoDrop
Technologies; Thermo Fisher Scientific, Inc.). First-strand cDNA
was reverse transcribed using the TaqMan Reverse Transcription
Reagents (Applied Biosystems; Thermo Fisher Scientific, Inc.). The
mRNA levels of EMT markers, including Vimentin, Slug, N-cadherin,
ZO-1 and E-cadherin were detected by RT-qPCR assay using the
SYBR-Green assay kit (Applied Biosystems; Thermo Fisher Scientific,
Inc.) on an ABI 7900 HT Fast Real-Time PCR system (Applied
Biosystems; Thermo Fisher Scientific, Inc.). The primer sequences
were as follows: Skp2 forward, 5′-GCT GCT AAA GGT CTC TGG TGT-3′
and reverse, 5′-AGG CTT AGA TTC TGC AAC TTG-3′; E-cadherin forward,
5′-GAA GTG TCC GAG GAC TTT GG-3′ and reverse, 5′-CAG TGT CTC TCC
AAA TCC GAT A-3′; N-cadherin forward, 5′-CCT GCG CGT GAA GGT TTG
CC-3′ and reverse, 5′-CCA AGC CCC GCA CCC ACA AT-3′; Vimentin
forward, 5′-TGT CCA AAT CGA TGT GGA TGT TTC-3′ and reverse, 5′-TTG
TAC CAT TCT TCT GCC TCC TG-3′; Slug forward, 5′-CAT GCC TGT CAT ACC
ACA AC-3′ and reverse, 5′-GGT GTC AGA TGG AGG AGG G-3′; ZO-1
forward, 5′-AGA AGA TAG CCC TGC AGC-3′ and reverse, 5′-AGT CCA TAG
GGA GAT TCC-3′; and GAPDH forward, 5′-ACC CAG AAG ACT GTG GAT GG-3′
and reverse, (5′-CAG TGA GCT TCC CGT TCA G-3′). The expression of
GAPDH was used as an internal control.
Target sequences were amplified at 95°C for 10 min,
followed by 40 cycles at 95°C for 15 sec and 60°C for 1 min.
β-actin was amplified as an endogenous normalization control. The
fold change in the mRNA level was calculated according to
2−ΔΔCq method (45).
Western blot analysis
Total proteins were isolated from the cells with
protein lysis buffer. The concentrations of the protein samples
were determined by bicinchoninic acid (BCA; Thermo Scientific)
protein assay. Proteins samples (40 μg) were run and
separated on a 10% of SDS-polyacrylamide gel (SDS-PAGE), and then
transferred onto PVDF membranes (Millipore, Billerica, MA, USA).
After blocking in blocking buffer (1X TBST with 5% w/v de-fatted
milk powder), the membranes were incubated with specific primary
antibodies at 4°C overnight. Primary antibodies against Skp2
(1:1,000), ZO-1 (1:1,000), N-cadherin (1:1,000), E-cadherin
(1:2,000), Slug (1:1,500), Vimentin (1:1,000), Nanog (1:1,000),
Oct4 (1:1,500), ABCB1 (1:1,500), Foxo1 (1:1,500) and p21 (1:1,000)
were used. The membranes were then washed with TBST and probed with
anti-mouse (Cat. no. #A3682, 1:4,000, Sigma-Aldrich, St. Louis, MO,
USA) or anti-rabbit secondary antibodies (cat. no. A16110 1:3,000,
Thermo Fisher Scientific) at room temperature for 1 h. Finally, the
membranes were washed again and detected using enhanced
chemiluminescence substrate (ECL) (Sigma-Aldrich; EMD Millipore).
Quantitative analysis was carried out using QuantiOne imaging
software with gel imaging equipment (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA).
Statistical analysis
Statistical analysis was carried out using GraphPad
Prism 4.0 (Graph pad Software, La Jolla, CA, USA). The mean,
standard error and P-values were analyzed using the two-tailed
Student's t-test. Data are presented as the means ± SEM. P<0.05
was considered to indicate a statistically significant
difference.
Results
Establishment of MTX-resistant human
osteosarcoma cell lines
MTX-resistant human osteosarcoma cell lines were
established by continuous stepwise selection with increasing
concentrations of MTX in the parental OS cell lines for >6
months. Briefly, the cells were cultured at the exponential phase
and exposed to a low concentration of MTX for 3–4 days. The dead
cells were removed and increasing concentrations of MTX were then
added to the culture medium. After the OS cells were cultured for
more than half a year with increasing concentrations of MTX,
MTX-resistant cells were established. The surviving cells were
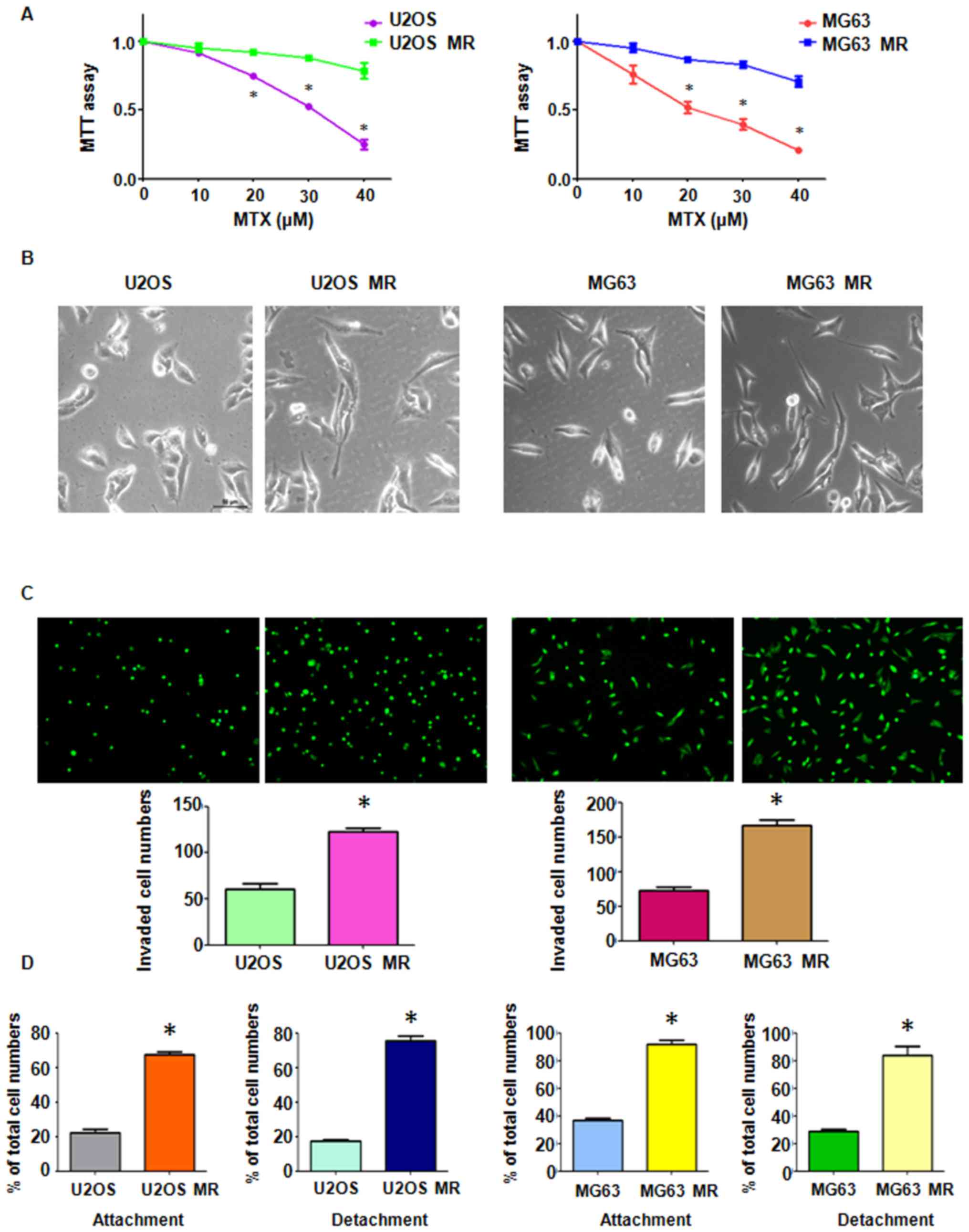
observed to exhibit an enhanced resistance to MTX. MTT assay
revealed that the U2OS and MG63 MTX-resistant cell lines were
successfully established, as the MTX-resistant cells had an
increased viability compared to the parental cells (Fig. 1A). The MTX-resistant OS cells
developed a resistance to 40 μM MTX. During the maintenance
of MTX-resistant OS cells in drug-free medium, the stable
resistance to MTX was guaranteed by continuously measuring the
IC50 value monthly.
MTX treatment promotes mesenchymal-like
properties in MTX-resistant OS cells
Drug-resistant cells always exhibited the EMT
phenotype (46). Cell
morphological changes in the MTX-resistant OS cells were observed
under a light microscope. We found that both the U2OS and MG63
MTX-resistant cell lines appeared to possess the mesenchymal
phenotype, as the cells had developed into elongated and more
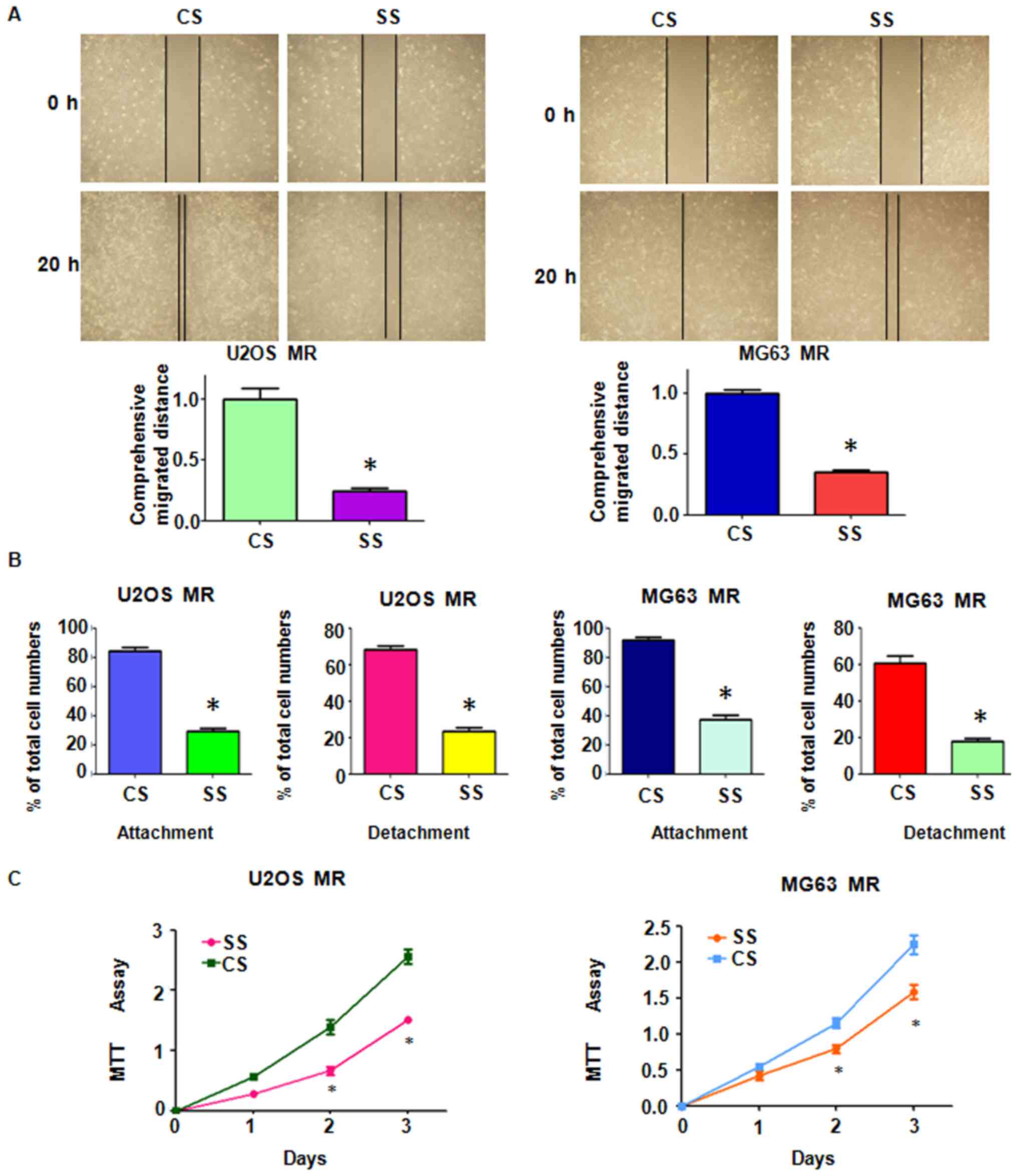
spindle-like shapes (Fig. 1B).
EMT characteristics of MTX-resistant OS
cells
Multiple biological changes were examined in the
MTX-resistant OS cells. The results of Transwell assay revealed a
significant increase in the invasive ability of both MTX-resistant
OS cell lines (Fig. 1C). Moreover,
the MTX-resistant OS cells developed intensive attachment and
detachment capacities, compared with their parental cell lines
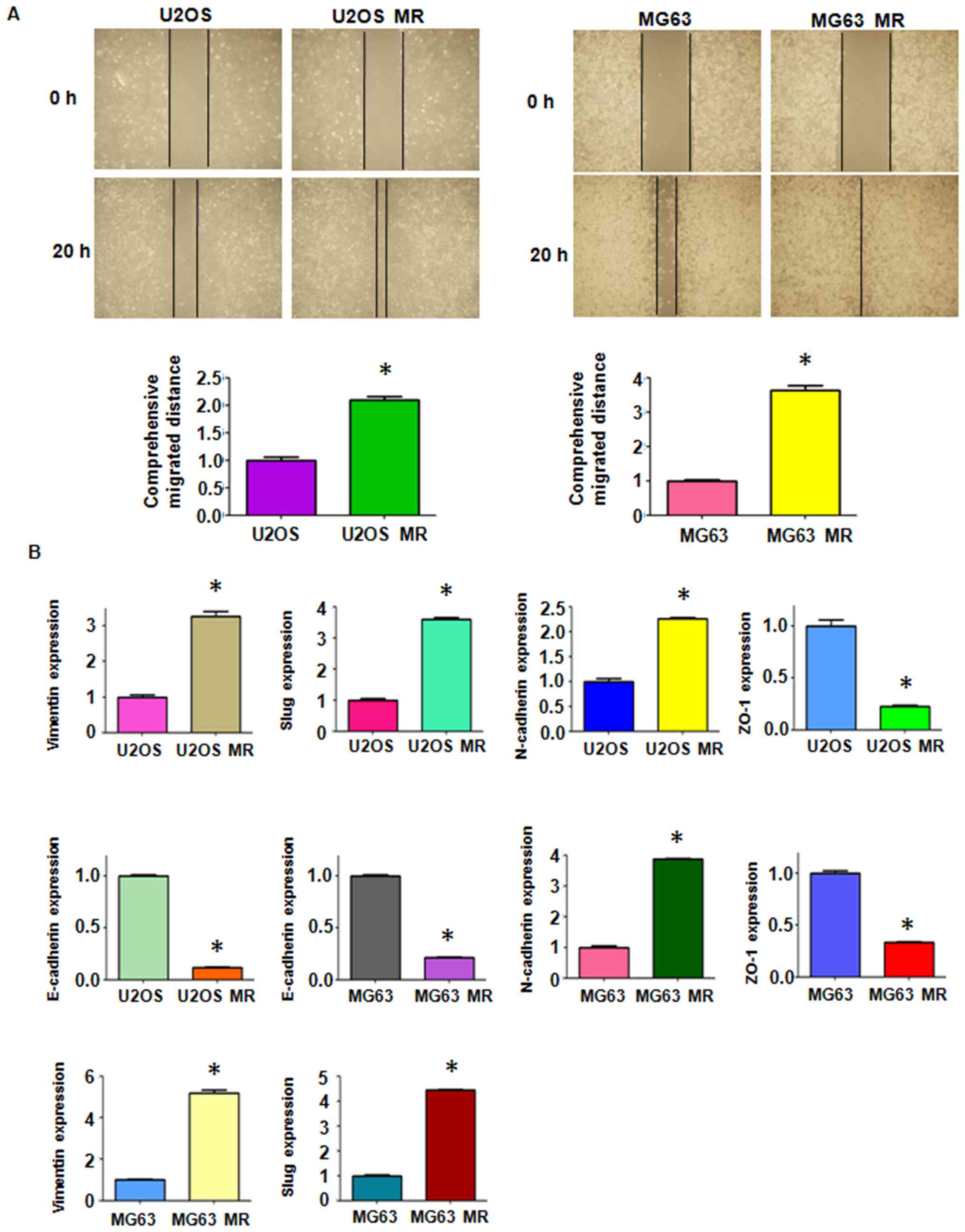
(Fig. 1D). The cell motility
activity was further detected by wound healing assay. We observed
an increased amount of MTX-resistant cells which had migrated into
the wound area, indicating an enhanced motility activity of the
drug-resistant cells (Fig.
2A).
MTX-resistant OS cells undergo EMT and
acquire stem cell molecular markers
To investigate whether drug-resistant cells undergo
EMT-related molecular marker changes, the mRNA and protein levels
of several EMT markers were measured between the resistant cells
and their paired parental cells. RT-qPCR analysis was performed to
detect the expression of mRNAs. The results revealed a significant
increase in the mRNA levels of mesenchymal markers, such as
Vimentin, Slug and N-cadherin in the MTX-resistant OS cells
(Fig. 2B). By contrast, the
expression levels of the epithelial molecules, ZO-1 and E-cadherin,
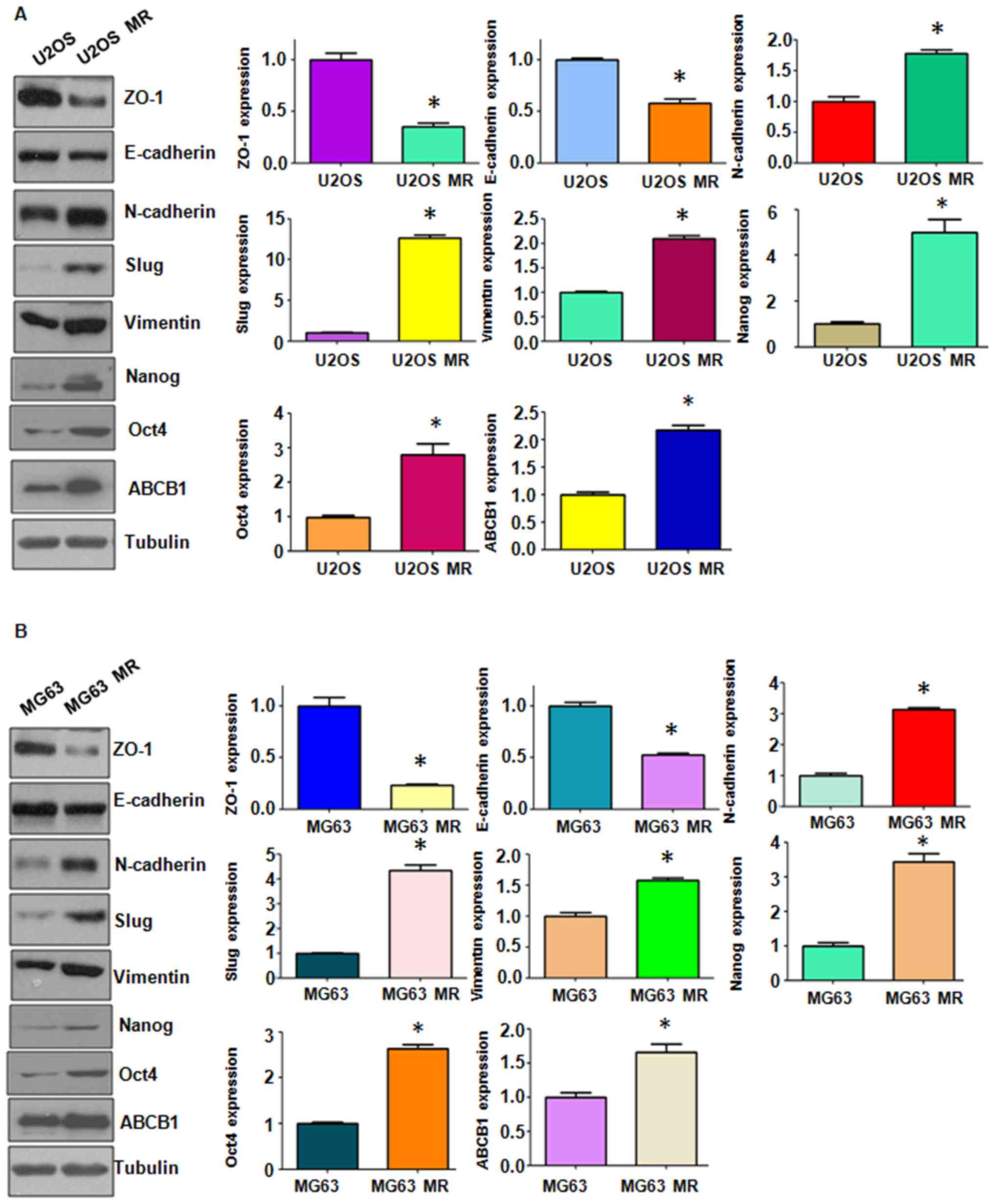
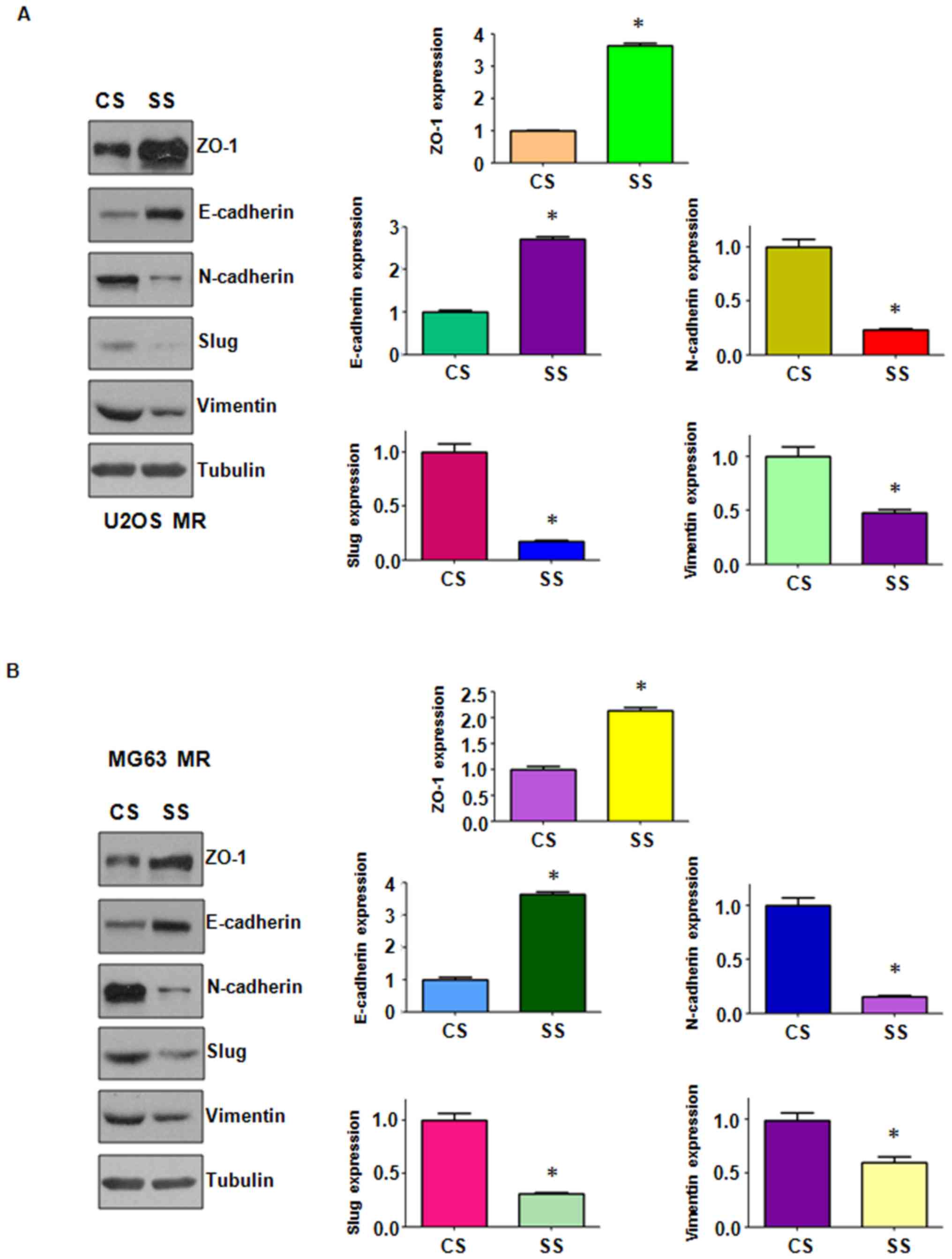
were markedly decreased in the MTX-resistant OS cells (Fig. 2B). We further confirmed the changes
in the protein expression levels of EMT markers by western blot
analysis. We observed changes in the protein levels of EMT markers
in the MTX-resistant OS cells (Fig.
3). We also found that the stem cell markers, Nanog and Oct4,
were highly expressed in the MTX-resistant cells (Fig. 3). Importantly, we found that ABCB1
expression was increased in the MTX-resistant cells (Fig. 3). Thus the MTX-resistant OS cells
acquired EMT-like and stem cell-like characteristics; their
drug-resistant capabilities may be attributed to mesenchymal
transition.
Skp2 expression is elevated in
MTX-resistant OS cells
Skp2 enhances tumor metastasis by modulating
molecular markers of EMT (47,48).
In accordance with this finding, in this study, we observed that
Skp2 expression was significantly elevated in the MTX-resistant OS
cells at both the mRNA and protein level (Fig. 4A). Moreover, we found that the
levels of downstream molecules of Skp2, Foxo1 and p21, were
markedly downregulated in the MTX-resistant cells compared with the
parental cells (Fig. 4A and B).
These findings suggest that Skp2 is closely involved in EMT induced
by MTX resistance and may thus play a critical role in human
OS.
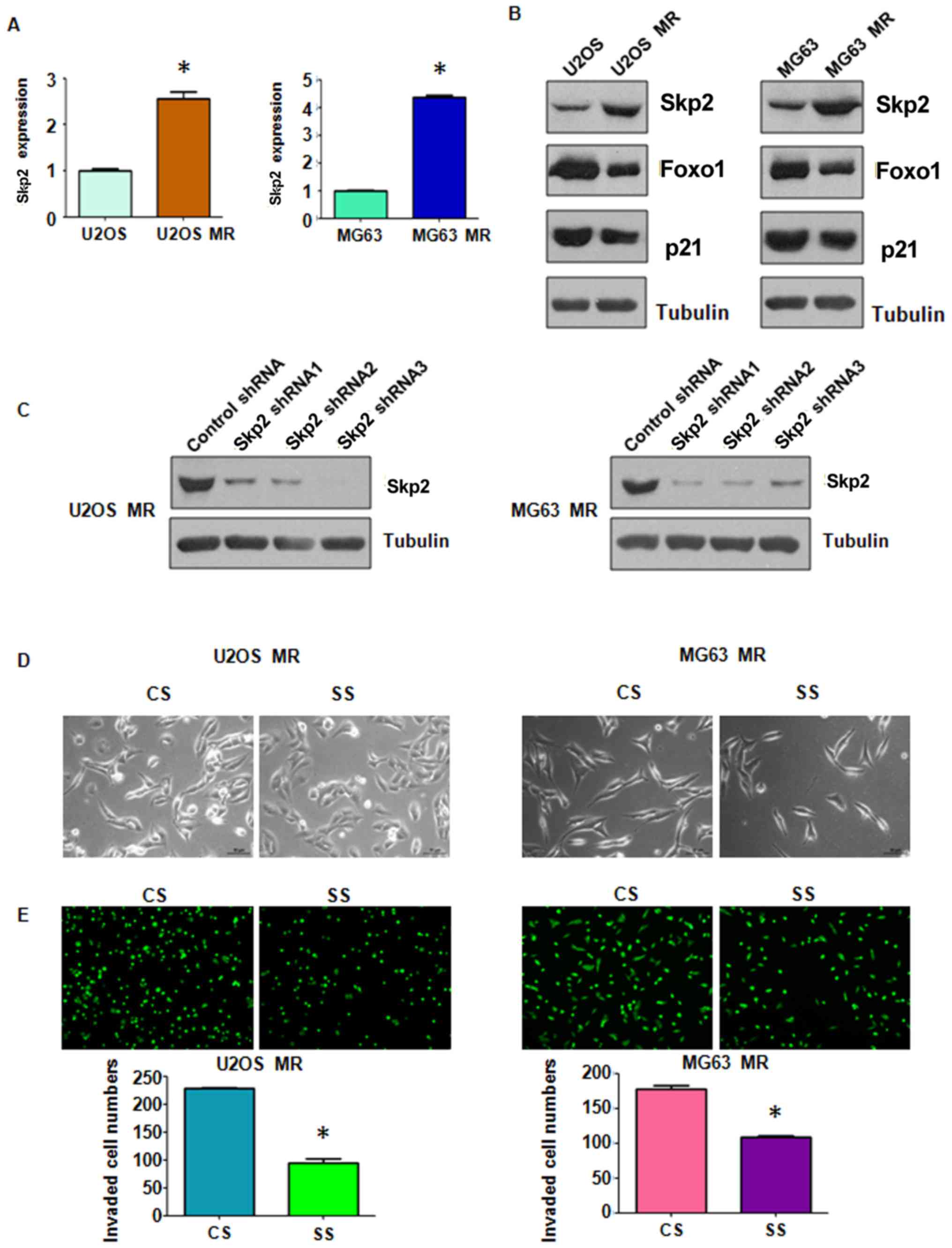
Stable downregulation of Skp2 reverses
EMT to mesenchymal-epithelial transition (MET) in MTX-resistant OS
cells
Stable Skp2 knockdown in the MTX-resistant OS cells
was established by using Skp2 shRNA lentiviral particles infection.
The efficiency of RNAi was confirmed by western blot analysis. As
shown in Fig. 4C, Skp2 expression
was effectively suppressed in both the U2OS and MG63 MTX-resistant
cells. We selected Skp2 shRNA2 lentiviral particles to infect the
MTX-resistant OS cells in the subsequent experiments. We observed
that following the exposure of MTX-resistant OS cells in which Skp2
was knocked down (SS group in Fig.
4C) to MTX, they exhibited a less spindle-like shape (Fig. 4D). Thus, Skp2 knockdown partially
reversed EMT to MET. Moreover, the results of Transwell assay
revealed that the invasive ability of the MTX-resistant OS cells
was markedly inhibited following Skp2 knockdown (Fig. 4E). The effects of Skp2 knockdown on
mobility of the MTX-resistant cells were further determined by
wound healing assay. Skp2 knockdown suppressed the migratory
ability of the MTX-resistant OS cells (Fig. 5A). Furthermore, Skp2 knockdown
markedly abrogated the attachment and detachment capacity of the
MTX-resistant OS cells (Fig. 5B).
It is important to note that Skp2 shRNA did not inhibit cell growth
at 24 h (Fig. 5C). However, Skp2
shRNA suppressed cell migration (Fig.
5A) and invasion (Fig. 4E) at
20 and 24 h, respectively, suggesting that the suppression of cell
migration and invasion by Skp2 shRNA was not due to cell growth
inhibition by Skp2 downregulation. Taken together, these results
demonstrate that Skp2 may play an important role in the regulation
of EMT in MTX-resistant OS cells.
Stable downregulation of Skp2 enhances
the sensitivity of resistant OS cells to MTX treatment
MTT assay was carried out to further examine the
effects of stable thje downregulation of Skp2 on sensitivity of OS
cells to the treatment drug. As shown in Fig. 5C, the increased viability of the
MTX-resistant OS cells was antagonized by Skp2 knockdown,
indicating that the sensitivity of the OS cells to MTX was enhanced
following the downregulation of Skp2. This finding suggested that
Skp2 may be used as a credible therapeutic target with which to
combat drug resistance in OS.
Stable downregulation of Skp2 regulates
the expression of EMT markers
We observed changes in EMT-related characteristics
in the MTX-resistant OS cells following Skp2 knockdown and further
performed western blot analysis to detect whether the knockdown of
Skp2 could modulate the expression of EMT-related molecules. The
results revealed that Skp2 knockdown promoted the expression of the
epithelial markers, ZO-1 and E-cadherin, whereas it suppressed the
expression of the mesenchymal markers, N-cadherin, Slug and
Vimentin (Fig. 6). These results
indicated that the EMT-like characteristics of MTX-resistant OS
cells can be abrogated by Skp2 knockdown.
Discussion
Osteosarcoma is the most common aggressive bone
malignancy affecting adolescents and young adults (49). The treatment outcomes have been
greatly improved since the introduction of chemotherapy. MTX is one
of the most widely used and effective anti-neoplastic drugs in the
treatment of various types of solid tumors. Pre-operative high-dose
methotrexate (HD-MTX) with folinic acid (leucovorin) is still a
mainstay in the treatment of patients with OS (19,50,51).
However, multidrug resistance often develops during the late stages
of treatment. The detailed mechanisms responsible for drug
resistance remain to be determined, and novel therapeutic
strategies are required in order to overcome drug resistance in
tumor cells and to prevent tumor progression.
In this study, we established MTX-resistant cells
using U2OS and MG63 cells. These two MTX-resistant cell lines
exhibited a much higher resistant ability to MTX than their
parental cells (Fig. 1A). It may
be of great importance to explore new molecular mechanisms
responsible for MTX resistance in OS cells. Recently,
chemotherapeutic agent-resistant tumor cells, were found to acquire
an EMT-like morphology and molecular markers (26,27,52,53).
Tumor metastasis is a complex process involving vessel formation,
cell attachment, invasion, migration and cell proliferation,
leading to tumor cell growth in other sites of the body (54). EMT has been reported to contribute
to tumor cell invasion and distant metastases in human cancers
(22). In this study, OS cells
were treated with a sublethal dose of MTX, and any surviving cells
presented with enhanced mesenchymal-like characteristics (Fig. 1B). MTX-resistant OS cells also
acquired enhanced invasive, migratory and attachment abilities
(Figs. 1C and D, and 2A). Moreover, alterations in the levels
of EMT markers were observed. The MTX-resistant MR OS cells
exhibited a significantly decreased expression of ZO-1 and
E-cadherin, and a simultaneously increased expression of
mesenchymal markers (Figs. 2B and
3). These findings suggest that
EMT may be essential for the development of MTX resistance in OS
cells and may thus play an important role in tumor metastasis in
OSs.
The effectiveness of chemotherapeutic drugs, such as
MTX in cancer is limited due to drug resistance. Thus, the further
elucidation of the molecular mechanisms responsible for drug
resistance in OS is of utmost importance. To this end, in this
study, we detected cell signaling molecular changes associated with
EMT in the MTX-resistant OS cells. We also found that Skp2
expression was increased in the MTX-resistant OS cells. Skp2
targets cell cycle-negative regulators, such as p27Kip1,
p21Cip1, p130Cas and Foxo1, for
ubiquitination and proteasomal degradation, ultimately positively
maintaining and preserving cell cycle progression (30,55).
In this series of experiments, the expression of Foxo1 and p21 was
also downregulated in the resistant cells (Fig. 4A and B) in which Skp2 was
upregulated. It has been reported that rapamycin resistance is
linked to the defective regulation of Skp2, and that the
RNAi-mediated silencing of Skp2 in human tumor cells enhances their
sensitivity to rapamycin in vitro and inhibits the growth of
tumor xenografts in vivo (56). Skp2 has also been shown to regulate
salinomycin-induced cell cycle arrest and the apoptosis of
drug-resistant cancer cells (57).
The mitotic arrest deficient protein (MAD2B), a well-defined
anaphase-promoting complex/cyclosome (APC/C) inhibitor, promotes
tubular EMT and renal tubulointerstitial fibrosis by inducing Skp2
expression (58). It has been
recently reported that the acquisition of EMT-like characteristics
is associated with Skp2 expression in paclitaxel-resistant breast
cancer cells 48). Skp2 is associated with prostate cancer cell
resistance to paclitaxel (59) and
the pharmacological inhibition of Skp2 has been shown to sensitize
lung cancer cells to paclitaxel (60).
Consistent with the findings of these
above-mentioned previous studies, in this study, the use of
targeted shRNA against Skp2 resulted in an enhancement of the
sensitivity of the resistant OS cells to MTX, evidenced by a
decrease in cell proliferation in the MTX-resistant cells in which
Skp2 was knocked down (Fig. 5).
Importantly, the stable knockdown of Skp2 abrogated the EMT-like
characteristics, and decreased the migratory and attachment
abilities of the MTX-resistant cells (Figs. 4Figure 5–6). These results indicated that Skp2
overexpression is closely associated with the MTX resistance of OS
cells and EMT properties. The silencing of Skp2 probably prevents
EMT and metastasis, and restores the sensitivity of OS cells to
MTX. Thus, the pharmacological inhibition of Skp2 may be used as a
novel therapeutic strategy with which to overcome drug resistance
in OS. Recently, compound 25, a novel Skp2 inhibitor, was shown to
exhibit potent antitumor activities and to cooperate with
chemotherapeutic agents to suppress cancer cell survival (61). Several natural compounds have been
reported to exert their antitumor activities via the inhibition of
Skp2 expression in human cancers (62–64).
It is important to note that natural compounds do not specifically
inhibit Skp2. The current study implied that targeting Skp2 may
prove to be helpful for overcoming MTX resistance in OS. However,
future studies are warranted to investigate other types of cancer
cell lines in order to increase credibility. In addition, the use
of animal models and clinical trials are required to fully assess
the effects of Skp2 targeting on the prevention of cancer relapse,
metastasis and chemoresistance.
Acknowledgments
Not applicable.
Funding
This study was supported by a grant from the
National Natural Science Foundation of China (no. 81760468) and the
program for graduate innovation research of Xingjiang Medical
University (no. CXCY2017033).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
LD, JB and RL were involved in the conceptualization
of the study; LD, CW and YC were involved in data curation; XH and
YZ were involved in formal analysis; LD, CW, YC, XH and YZ were
involved in the investigative aspects of the study; JB and RL were
involved in project administration; JB and RL supervised the study;
LD, JB and RL wrote and edited the manuscript. All authors have
read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Luetke A, Meyers PA, Lewis I and Juergens
H: Osteosarcoma treatment - where do we stand? A state of the art
review. Cancer Treat Rev. 40:523–532. 2014. View Article : Google Scholar
|
|
2
|
Raymond AK and Jaffe N: Osteosarcoma
multidisciplinary approach to the management from the pathologist's
perspective. Cancer Treat Res. 152:63–84. 2009. View Article : Google Scholar
|
|
3
|
Moore DD and Luu HH: Osteosarcoma. Cancer
Treat Res. 162:65–92. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ottaviani G and Jaffe N: The epidemiology
of osteosarcoma. Cancer Treat Res. 152:3–13. 2009. View Article : Google Scholar
|
|
5
|
Ta HT, Dass CR, Choong PF and Dunstan DE:
Osteosarcoma treatment: State of the art. Cancer Metastasis Rev.
28:247–263. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sampo MM, Tarkkanen M, Kivioja AH,
Taskinen MH, Sankila R and Böhling TO: Osteosarcoma in Finland from
1971 through 1990: A nationwide study of epidemiology and outcome.
Acta Orthop. 79:861–866. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Allison DC, Carney SC, Ahlmann ER,
Hendifar A, Chawla S, Fedenko A, Angeles C and Menendez LR: A
meta-analysis of osteosarcoma outcomes in the modern medical era.
Sarcoma. 2012:7048722012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Link MP, Goorin AM, Miser AW, Green AA,
Pratt CB, Belasco JB, Pritchard J, Malpas JS, Baker AR, Kirkpatrick
JA, et al: The effect of adjuvant chemotherapy on relapse-free
survival in patients with osteosarcoma of the extremity. N Engl J
Med. 314:1600–1606. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Meyers PA, Schwartz CL, Krailo M,
Kleinerman ES, Betcher D, Bernstein ML, Conrad E, Ferguson W,
Gebhardt M, Goorin AM, et al: Osteosarcoma: A randomized,
prospective trial of the addition of ifosfamide and/or muramyl
tripeptide to cisplatin, doxorubicin, and high-dose methotrexate. J
Clin Oncol. 23:2004–2011. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jaffe N, Puri A and Gelderblom H:
Osteosarcoma: Evolution of treatment paradigms. Sarcoma.
2013:2035312013. View Article : Google Scholar :
|
|
11
|
Farber S, Diamond LK, Mercer RD, Sylvester
RF Jr and Wolff JA: Temporary remissions in acute leukemia in
children produced by folic acid antagonist, 4-aminopteroyl-glutamic
acid. N Engl J Med. 238:787–793. 1948. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jain A, Sharma G, Kushwah V, Garg NK,
Kesharwani P, Ghoshal G, Singh B, Shivhare US, Jain S and Katare
OP: Methotrexate and beta-carotene loaded-lipid polymer hybrid
nanoparticles: A preclinical study for breast cancer. Nanomedicine
(Lond). 12:1851–1872. 2017. View Article : Google Scholar
|
|
13
|
Fizazi K, Asselain B, Vincent-Salomon A,
Jouve M, Dieras V, Palangie T, Beuzeboc P, Dorval T and Pouillart
P: Meningeal carcinomatosis in patients with breast carcinoma.
Clinical features, prognostic factors, and results of a high-dose
intrathecal methotrexate regimen. Cancer. 77:1315–1323. 1996.
View Article : Google Scholar
|
|
14
|
Koga S, Fujimoto T, Hasegawa K and Sueishi
K: Disseminated necrotizing leukoencephalopathy following
intrathecal methotrexate in childhood leukemia (author's transl).
Fukuoka Igaku Zasshi. 67:24–31. 1976.In Japanese. PubMed/NCBI
|
|
15
|
Kim JY, Kim ST, Nam DH, Lee JI, Park K and
Kong DS: Leukoencephalopathy and disseminated necrotizing
leukoencephalopathy following intrathecal methotrexate chemotherapy
and radiation therapy for central nerve system lymphoma or
leukemia. J Korean Neurosurg Soc. 50:304–310. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Shan W, Zhang X, Li M, Deng F and Zhang J:
Over expression of miR-200c suppresses invasion and restores
methotrexate sensitivity in lung cancer A549 cells. Gene.
593:265–271. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Takahashi K, Tsukamoto S, Saito K,
Ohkohchi N and Hirayama K: Complete response to multidisciplinary
therapy in a patient with primary gastric choriocarcinoma. World J
Gastroenterol. 19:5187–5194. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cornejo CM, Novoa RA, Krisch RE and Kim
EJ: Low-dose radiotherapy for primary cutaneous anaplastic
large-cell lymphoma while on low-dose methotrexate. Cutis.
98:253–256. 2016.PubMed/NCBI
|
|
19
|
Comandone A, Passera R, Boglione A, Tagini
V, Ferrari S and Cattel L: High dose methotrexate in adult patients
with osteosarcoma: Clinical and pharmacokinetic results. Acta
Oncol. 44:406–411. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hattinger CM, Pasello M, Ferrari S, Picci
P and Serra M: Emerging drugs for high-grade osteosarcoma. Expert
Opin Emerg Drugs. 15:615–634. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chou AJ and Gorlick R: Chemotherapy
resistance in osteosarcoma: Current challenges and future
directions. Expert Rev Anticancer Ther. 6:1075–1085. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Polyak K and Weinberg RA: Transitions
between epithelial and mesenchymal states: Acquisition of malignant
and stem cell traits. Nat Rev Cancer. 9:265–273. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kalluri R and Weinberg RA: The basics of
epithelial-mesenchymal transition. J Clin Invest. 119:1420–1428.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ksiazkiewicz M, Markiewicz A and Zaczek
AJ: Epithelialmesenchymal transition: a hallmark in metastasis
formation linking circulating tumor cells and cancer stem cells.
Pathobiology. 79:195–208. 2012. View Article : Google Scholar
|
|
25
|
Samatov TR, Tonevitsky AG and Schumacher
U: Epithelialmesenchymal transition: Focus on metastatic cascade,
alternative splicing, non-coding RNAs and modulating compounds. Mol
Cancer. 12:1072013. View Article : Google Scholar
|
|
26
|
Fang S, Yu L, Mei H, Yang J, Gao T, Cheng
A, Guo W, Xia K and Liu G: Cisplatin promotes mesenchymal-like
characteristics in osteosarcoma through Snail. Oncol Lett.
12:5007–5014. 2016. View Article : Google Scholar
|
|
27
|
Wang Y, Wang H, Zhou R, Zhong W, Lu S, Ma
Z and Chai Y: Baicalin inhibits human osteosarcoma cells invasion,
metastasis, and anoikis resistance by suppressing the transforming
growth factor-β1-induced epithelial-to-mesenchymal transition.
Anticancer Drugs. 28:581–587. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wang L and Xue GB: Catalpol suppresses
osteosarcoma cell proliferation through blocking
epithelial-mesenchymal transition (EMT) and inducing apoptosis.
Biochem Biophys Res Commun. 495:27–34. 2018. View Article : Google Scholar
|
|
29
|
Ohbayashi M, Kubota S, Kawase A, Kohyama
N, Kobayashi Y and Yamamoto T: Involvement of
epithelial-mesenchymal transition in methotrexate-induced pulmonary
fibrosis. J Toxicol Sci. 39:319–330. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Frescas D and Pagano M: Deregulated
proteolysis by the F-box proteins SKP2 and beta-TrCP: Tipping the
scales of cancer. Nat Rev Cancer. 8:438–449. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Arbini AA, Greco M, Yao JL, Bourne P,
Marra E, Hsieh JT, di Sant'agnese PA and Moro L: Skp2
overexpression is associated with loss of BRCA2 protein in human
prostate cancer. Am J Pathol. 178:2367–2376. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lim MS, Adamson A, Lin Z, Perez-Ordonez B,
Jordan RC, Tripp S, Perkins SL and Elenitoba-Johnson KS: Expression
of Skp2, a p27 (Kip1) ubiquitin ligase, in malignant lymphoma:
Correlation with p27 (Kip1) and proliferation index. Blood.
100:2950–2956. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wei Z, Jiang X, Liu F, Qiao H, Zhou B,
Zhai B, Zhang L, Zhang X, Han L, Jiang H, et al: Downregulation of
Skp2 inhibits the growth and metastasis of gastric cancer cells in
vitro and in vivo. Tumour Biol. 34:181–192. 2013. View Article : Google Scholar
|
|
34
|
Chan CH, Li CF, Yang WL, Gao Y, Lee SW,
Feng Z, Huang HY, Tsai KK, Flores LG, Shao Y, et al: The Skp2-SCF
E3 ligase regulates Akt ubiquitination, glycolysis, herceptin
sensitivity, and tumorigenesis. Cell. 149:1098–1111. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Huang Y, Tai AW, Tong S and Lok AS: HBV
core promoter mutations promote cellular proliferation through
E2F1-mediated upregulation of S-phase kinase-associated protein 2
transcription. J Hepatol. 58:1068–1073. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Xu HM, Liang Y, Chen Q, Wu QN, Guo YM,
Shen GP, Zhang RH, He ZW, Zeng YX, Xie FY, et al: Correlation of
Skp2 overexpression to prognosis of patients with nasopharyngeal
carcinoma from South China. Chin J Cancer. 30:204–212. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wang Z, Liu P, Inuzuka H and Wei W: Roles
of F-box proteins in cancer. Nat Rev Cancer. 14:233–247. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Chan CH, Morrow JK, Zhang S and Lin HK:
Skp2: A dream target in the coming age of cancer therapy. Cell
Cycle. 13:679–680. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Lin HK, Chen Z, Wang G, Nardella C, Lee
SW, Chan CH, Yang WL, Wang J, Egia A, Nakayama KI, et al: Skp2
targeting suppresses tumorigenesis by Arf-p53-independent cellular
senescence. Nature. 464:374–379. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Wang J, Huang Y, Guan Z, Zhang JL, Su HK,
Zhang W, Yue CF, Yan M, Guan S and Liu QQ: E3-ligase Skp2 predicts
poor prognosis and maintains cancer stem cell pool in
nasopharyngeal carcinoma. Oncotarget. 5:5591–5601. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Mamillapalli R, Gavrilova N, Mihaylova VT,
Tsvetkov LM, Wu H, Zhang H and Sun H: PTEN regulates the
ubiquitin-dependent degradation of the CDK inhibitor p27(KIP1)
through the ubiquitin E3 ligase SCF(SKP2). Curr Biol. 11:263–267.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Huang H, Regan KM, Wang F, Wang D, Smith
DI, van Deursen JM and Tindall DJ: Skp2 inhibits FOXO1 in tumor
suppression through ubiquitin-mediated degradation. Proc Natl Acad
Sci USA. 102:1649–1654. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Inuzuka H, Gao D, Finley LW, Yang W, Wan
L, Fukushima H, Chin YR, Zhai B, Shaik S, Lau AW, et al:
Acetylation-dependent regulation of Skp2 function. Cell.
150:179–193. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Ding L, Li R, Han X, Zhou Y, Zhang H, Cui
Y, Wang W and Bai J: Inhibition of Skp2 suppresses the
proliferation and invasion of osteosarcoma cells. Oncol Rep.
38:933–940. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
46
|
Wang Y, Shi J, Chai K, Ying X and Zhou BP:
The role of Snail in EMT and tumorigenesis. Curr Cancer Drug
Targets. 13:963–972. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Wei Z, Jiang X, Qiao H, Zhai B, Zhang L,
Zhang Q, Wu Y, Jiang H and Sun X: STAT3 interacts with Skp2/p27/p21
pathway to regulate the motility and invasion of gastric cancer
cells. Cell Signal. 25:931–938. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Yang Q, Huang J, Wu Q, Cai Y, Zhu L, Lu X,
Chen S, Chen C and Wang Z: Acquisition of epithelial-mesenchymal
transition is associated with Skp2 expression in
paclitaxel-resistant breast cancer cells. Br J Cancer.
110:1958–1967. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Picci P: Osteosarcoma (osteogenic
sarcoma). Orphanet J Rare Dis. 2:62007. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Dupuis C, Mercier C, Yang C,
Monjanel-Mouterde S, Ciccolini J, Fanciullino R, Pourroy B, Deville
JL, Duffaud F, Bagarry-Liegey D, et al: High-dose methotrexate in
adults with osteosarcoma: A population pharmacokinetics study and
validation of a new limited sampling strategy. Anticancer Drugs.
19:267–273. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Zhang W, Zhang Q, Tian X, Zhao H, Lu W,
Zhen J and Niu X: Population pharmacokinetics of high-dose
methotrexate after intravenous administration in Chinese
osteosarcoma patients from a single institution. Chin Med J (Engl).
128:111–118. 2015. View Article : Google Scholar
|
|
52
|
Wang Z, Li Y, Kong D, Banerjee S, Ahmad A,
Azmi AS, Ali S, Abbruzzese JL, Gallick GE and Sarkar FH:
Acquisition of epithelial-mesenchymal transition phenotype of
gemcitabine-resistant pancreatic cancer cells is linked with
activation of the notch signaling pathway. Cancer Res.
69:2400–2407. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Liang SQ, Marti TM, Dorn P, Froment L,
Hall SR, Berezowska S, Kocher G, Schmid RA and Peng RW: Blocking
the epithelial-to-mesenchymal transition pathway abrogates
resistance to anti-folate chemotherapy in lung cancer. Cell Death
Dis. 6:e18242015. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Fidler IJ: The organ microenvironment and
cancer metastasis. Differentiation. 70:498–505. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Zhang H, Kobayashi R, Galaktionov K and
Beach D: p19Skp1 and p45Skp2 are essential elements of the cyclin
A-CDK2 S phase kinase. Cell. 82:915–925. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Totary-Jain H, Sanoudou D, Dautriche CN,
Schneller H, Zambrana L and Marks AR: Rapamycin resistance is
linked to defective regulation of Skp2. Cancer Res. 72:1836–1843.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Koo KH, Kim H, Bae YK, Kim K, Park BK, Lee
CH and Kim YN: Salinomycin induces cell death via inactivation of
Stat3 and downregulation of Skp2. Cell Death Dis. 4:e6932013.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Tang H, Fan D, Lei CT, Ye C, Gao P, Chen
S, Meng XF, Su H and Zhang C: MAD2B promotes tubular
epithelial-to-mesenchymal transition and renal tubulointerstitial
fibrosis via Skp2. J Mol Med (Berl). 94:1297–1307. 2016. View Article : Google Scholar
|
|
59
|
Yang Y, Lu Y, Wang L, Mizokami A, Keller
ET, Zhang J and Fu J: Skp2 is associated with paclitaxel resistance
in prostate cancer cells. Oncol Rep. 36:559–566. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Huang T, Yang L, Wang G, Ding G, Peng B,
Wen Y and Wang Z: Inhibition of Skp2 sensitizes lung cancer cells
to paclitaxel. OncoTargets Ther. 10:439–446. 2017. View Article : Google Scholar
|
|
61
|
Chan CH, Morrow JK, Li CF, Gao Y, Jin G,
Moten A, Stagg LJ, Ladbury JE, Cai Z, Xu D, et al: Pharmacological
inactivation of Skp2 SCF ubiquitin ligase restricts cancer stem
cell traits and cancer progression. Cell. 154:556–568. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Mou H, Guo P, Li X, Zhang C, Jiang J, Wang
L, Wang Q and Yuan Z: Nitidine chloride inhibited the expression of
S phase kinase-associated protein 2 in ovarian cancer cells. Cell
Cycle. 16:1366–1375. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Su J, Wang L, Yin X, Zhao Z, Hou Y, Ye X,
Zhou X and Wang Z: Rottlerin exhibits anti-cancer effect through
inactivation of S phase kinase-associated protein 2 in pancreatic
cancer cells. Am J Cancer Res. 6:2178–2191. 2016.PubMed/NCBI
|
|
64
|
Feng S, Wang Y, Zhang R, Yang G, Liang Z,
Wang Z and Zhang G: Curcumin exerts its antitumor activity through
regulation of miR-7/Skp2/p21 in nasopharyngeal carcinoma cells.
OncoTargets Ther. 10:2377–2388. 2017. View Article : Google Scholar
|