|
1
|
Soni S and Padwad YS: HIF-1 in cancer
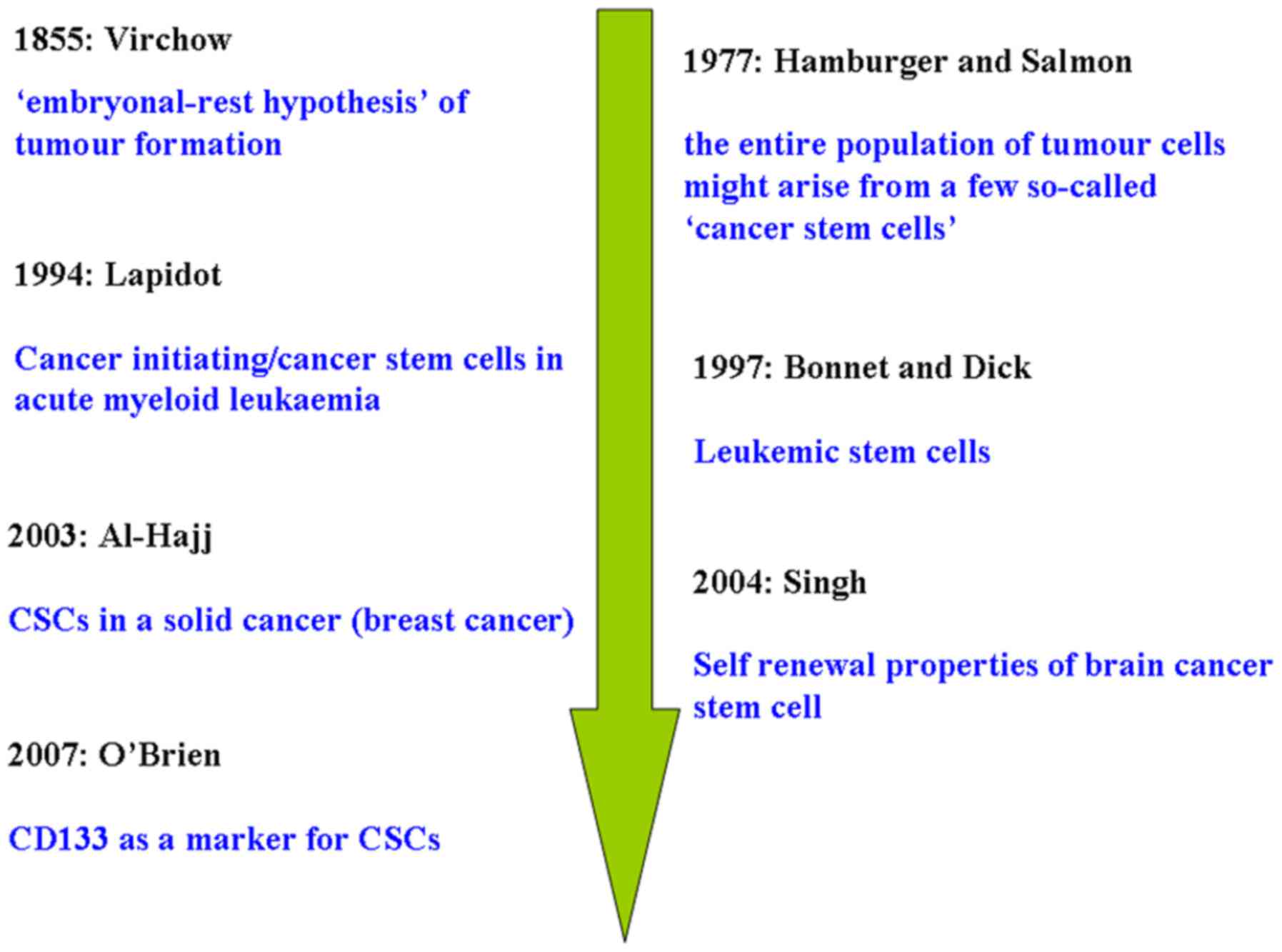
therapy: Two decade long story of a transcription factor. Acta
Oncol. 56:503–515. 2017. View Article : Google Scholar
|
|
2
|
Furth J and Kahn M: The transmission of
leukemia of mice with a single cell. Am J Cancer. 31:276–282.
1937.
|
|
3
|
Southam CM and Brunschwig A: Quantitative
studies of auto-transplantation of human cancer. Cancer.
14:971–978. 1961. View Article : Google Scholar
|
|
4
|
Hamburger AW and Salmon SE: Primary
bioassay of human tumor stem cells. Science. 197:461–463. 1977.
View Article : Google Scholar
|
|
5
|
Lapidot T, Sirard C, Vormoor J, Murdoch B,
Hoang T, Caceres-Cortes J, Minden M, Paterson B, Caligiuri MA and
Dick JE: A cell initiating human acute myeloid leukaemia after
transplantation into SCID mice. Nature 3. 67:645–648. 1994.
View Article : Google Scholar
|
|
6
|
Bonnet D and Dick JE: Human acute myeloid
leukemia is organized as a hierarchy that originates from a
primitive hematopoietic cell. Nat Med. 3:730–737. 1997. View Article : Google Scholar
|
|
7
|
Al-Hajj M, Wicha MS, Benito-Hernandez A,
Morrison SJ and Clarke MF: Prospective identification of
tumorigenic breast cancer cells. Proc Natl Acad Sci USA.
100:3983–3988. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Singh SK, Hawkins C, Clarke ID, Squire JA,
Bayani J, Hide T, Henkelman RM, Cusimano MD and Dirks PB:
Identification of human brain tumour initiating cells. Nature.
432:396–401. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ponti D, Costa A, Zaffaroni N, Pratesi G,
Petrangolini G, Coradini D, Pilotti S, Pierotti MA and Daidone MG:
Isolation and in vitro propagation of tumorigenic breast cancer
cells with stem/progenitor cell properties. Cancer Res.
65:5506–5511. 2005. View Article : Google Scholar
|
|
10
|
Patrawala L, Calhoun T,
Schneider-Broussard R, Li H, Bhatia B, Tang S, Reilly JG, Chandra
D, Zhou J, Claypool K, et al: Highly purified CD44+
prostate cancer cells from xenograft human tumors are enriched in
tumorigenic and metastatic progenitor cells. Oncogene.
25:1696–1708. 2006. View Article : Google Scholar
|
|
11
|
López J, Valdez-Morales FJ,
Benítez-Bribiesca L, Cerbón M and Carrancá AG: Normal and cancer
stem cells of the human female reproductive system. Reprod Biol
Endocrinol. 11:532013. View Article : Google Scholar :
|
|
12
|
Takahashi K and Yamanaka S: Induction of
pluripotent stem cells from mouse embryonic and adult fibroblast
cultures by defined factors. Cell. 126:663–676. 2006. View Article : Google Scholar
|
|
13
|
Ohnishi K, Semi K, Yamamoto T, Shimizu M,
Tanaka A, Mitsunaga K, Okita K, Osafune K, Arioka Y, Maeda T, et
al: Premature termination of reprogramming in vivo leads to cancer
development through altered epigenetic regulation. Cell.
156:663–677. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Beck B and Blanpain C: Unravelling cancer
stem cell potential. Nat Rev Cancer. 13:727–738. 2013. View Article : Google Scholar
|
|
15
|
Wang RA, Li ZS, Zhang HZ, Zheng PJ, Li QL,
Shi JG, Yan QG, Ye J, Wang JB, Guo Y, et al: Invasive cancers are
not necessarily from preformed in situ tumours - an alternative way
of carcinogenesis from misplaced stem cells. J Cell Mol Med.
17:921–926. 2013. View Article : Google Scholar
|
|
16
|
Clarke MF, Dick JE, Dirks PB, Eaves CJ,
Jamieson CH, Jones DL, Visvader J, Weissman IL and Wahl GM: Cancer
stem cells - perspectives on current status and future directions:
AACR Workshop on cancer stem cells. Cancer Res. 66:9339–9344. 2006.
View Article : Google Scholar
|
|
17
|
Reya T, Morrison SJ, Clarke MF and
Weissman IL: Stem cells, cancer, and cancer stem cells. Nature.
414:105–111. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cabrera MC, Hollingsworth RE and Hurt EM:
Cancer stem cell plasticity and tumor hierarchy. World J Stem
Cells. 7:27–36. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kuroda T, Yasuda S and Sato Y:
Tumorigenicity studies for human pluripotent stem cell-derived
products. Biol Pharm Bull. 36:189–192. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Adorno-Cruz V, Kibria G, Liu X, Doherty M,
Junk DJ, Guan D, Hubert C, Venere M, Mulkearns-Hubert E, Sinyuk M,
et al: Cancer stem cells: Targeting the roots of cancer, seeds of
metastasis, and sources of therapy resistance. Cancer Res.
75:924–929. 2015. View Article : Google Scholar :
|
|
21
|
O'Brien CA, Pollett A, Gallinger S and
Dick JE: A human colon cancer cell capable of initiating tumour
growth in immunodeficient mice. Nature. 445:106–110. 2007.
View Article : Google Scholar
|
|
22
|
Hope KJ, Jin L and Dick JE: Acute myeloid
leukemia originates from a hierarchy of leukemic stem cell classes
that differ in self-renewal capacity. Nat Immunol. 5:738–743. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Xia P, Gou WF, Zhao S and Zheng HC:
Crizotinib may be used in Lewis lung carcinoma: A novel use for
crizotinib. Oncol Rep. 30:139–148. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Vescovi AL, Galli R and Reynolds BA: Brain
tumour stem cells. Nat Rev Cancer. 6:425–436. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Vargo-Gogola T and Rosen JM: Modelling
breast cancer: One size does not fit all. Nat Rev Cancer.
7:659–672. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tsai RY: Balancing self-renewal against
genome preservation in stem cells: How do they manage to have the
cake and eat it too? Cell Mol Life Sci. 73:1803–1823. 2016.
View Article : Google Scholar :
|
|
27
|
Huntly BJ and Gilliland DG: Leukaemia stem
cells and the evolution of cancer-stem-cell research. Nat Rev
Cancer. 5:311–321. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
28
|
Daynac M and Petritsch CK: Regulation of
asymmetric cell division in mammalian neural stem and cancer
precursor cells. Results Probl Cell Differ. 61:375–399. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang L, Bu P and Shen X: Asymmetric
division: An antitumor player? Mol Cell Oncol. 3:e11642792016.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wu L, Wang G, Liu S, Wei J, Zhang S, Li M,
Zhou G and Wang L: Synthesis and biological evaluation of matrine
derivatives containing benzo-α-pyrone structure as potent anti-lung
cancer agents. Sci Rep. 6:359182016. View Article : Google Scholar
|
|
31
|
Ait-Oudhia S and Mager DE: Array of
translational systems pharmacodynamic models of anti-cancer drugs.
J Pharmacokinet Pharmacodyn. 43:549–565. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
O'Connor R, Clynes M, Dowling P, O'Donovan
N and O'Driscoll L: Drug resistance in cancer - searching for
mechanisms, markers and therapeutic agents. Expert Opin Drug Metab
Toxicol. 3:805–817. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yu Z, Pestell TG, Lisanti MP and Pestell
RG: Cancer stem cells. Int J Biochem Cell Biol. 44:2144–2151. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Gottesman MM, Fojo T and Bates SE:
Multidrug resistance in cancer: Role of ATP-dependent transporters.
Nat Rev Cancer. 2:48–58. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ramachandra M, Ambudkar SV, Chen D,
Hrycyna CA, Dey S, Gottesman MM and Pastan I: Human P-glycoprotein
exhibits reduced affinity for substrates during a catalytic
transition state. Biochemistry. 37:5010–5019. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Dean M, Hamon Y and Chimini G: The human
ATP-binding cassette (ABC) transporter superfamily. J Lipid Res.
42:1007–1017. 2001.PubMed/NCBI
|
|
37
|
Kort A, van Hoppe S, Sparidans RW,
Wagenaar E, Beijnen JH and Schinkel AH: Brain accumulation of
ponatinib and its active metabolite, N-desmethyl ponatinib, is
limited by P-glycoprotein (P-GP/ABCB1) and breast cancer resistance
protein (BCRP/ABCG2). Mol Pharm. 14:3258–3268. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Eckford PD and Sharom FJ: ABC efflux
pump-based resistance to chemotherapy drugs. Chem Rev.
109:2989–3011. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Trock BJ, Leonessa F and Clarke R:
Multidrug resistance in breast cancer: A meta-analysis of
MDR1/gp170 expression and its possible functional significance. J
Natl Cancer Inst. 89:917–931. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zhou DC, Zittoun R and Marie JP:
Expression of multidrug resistance-associated protein (MRP) and
multidrug resistance (MDR1) genes in acute myeloid leukemia.
Leukemia. 9:1661–1666. 1995.PubMed/NCBI
|
|
41
|
Grogan TM, Spier CM, Salmon SE, Matzner M,
Rybski J, Weinstein RS, Scheper RJ and Dalton WS: P-glycoprotein
expression in human plasma cell myeloma: Correlation with prior
chemotherapy. Blood. 81:490–495. 1993.PubMed/NCBI
|
|
42
|
Chan HS, Grogan TM, Haddad G, DeBoer G and
Ling V: P-glycoprotein expression: Critical determinant in the
response to osteosarcoma chemotherapy. J Natl Cancer Inst.
89:1706–1715. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Gillet JP and Gottesman MM: Mechanisms of
multidrug resistance in cancer. Methods Mol Biol. 596:47–76. 2010.
View Article : Google Scholar
|
|
44
|
Leslie EM, Deeley RG and Cole SP:
Multidrug resistance proteins: Role of P-glycoprotein, MRP1, MRP2,
and BCRP (ABCG2) in tissue defense. Toxicol Appl Pharmacol.
204:216–237. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Rohwer N and Cramer T: Hypoxia-mediated
drug resistance: Novel insights on the functional interaction of
HIFs and cell death pathways. Drug Resist Updat. 14:191–201. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
da Costa KM, Valente RC, Salustiano EJ,
Gentile LB, Freire-de-Lima L, Mendonça-Previato L and Previato JO:
Functional Characterization of ABCC proteins from trypanosoma cruzi
and their involvement with thiol transport. Front Microbiol.
9:2052018. View Article : Google Scholar :
|
|
47
|
Yin JY, Han LF, Huang Q, Xu XJ, Zhou HH
and Liu ZQ: ABCC1 polymorphism Arg723Gln (2168G> A) is
associated with lung cancer susceptibility in a Chinese population.
Clin Exp Pharmacol Physiol. 38:632–637. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Arumugam P and Song JM: Quantitative
evaluation of ABC transporter-mediated drug resistance based on the
determination of the anticancer activity of camptothecin against
breast cancer stem cells using TIRF. Integr Biol. 8:704–711. 2016.
View Article : Google Scholar
|
|
49
|
Liu C, Li Z, Bi L, Li K, Zhou B, Xu C,
Huang J and Xu K: NOTCH1 signaling promotes chemoresistance via
regulating ABCC1 expression in prostate cancer stem cells. Mol Cell
Biochem. 393:265–270. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Johnson ZL and Chen J: ATP binding enables
substrate release from multidrug resistance protein 1. Cell.
172:81–89.e10. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Castilho L and Reid ME: A review of the JR
blood group system. Immunohematology. 29:63–68. 2013.PubMed/NCBI
|
|
52
|
Fujita K and Ichida K: ABCG2 as a
therapeutic target candidate for gout. Expert Opin Ther Targets.
22:123–129. 2018. View Article : Google Scholar
|
|
53
|
D'Ignazio L, Batie M and Rocha S: Hypoxia
and inflammation in cancer, focus on HIF and NF-κB. Biomedicines.
5:E212017. View Article : Google Scholar
|
|
54
|
Zhang P, Yao Q, Lu L, Li Y, Chen PJ and
Duan C: Hypoxia-inducible factor 3 is an oxygen-dependent
transcription activator and regulates a distinct transcriptional
response to hypoxia. Cell Reports. 6:1110–1121. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Wang GL, Jiang BH, Rue EA and Semenza GL:
Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS
heterodimer regulated by cellular O2 tension. Proc Natl Acad Sci
USA. 92:5510–5514. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Kallio PJ, Okamoto K, O'Brien S, Carrero
P, Makino Y, Tanaka H and Poellinger L: Signal transduction in
hypoxic cells: Inducible nuclear translocation and recruitment of
the CBP/p300 coactivator by the hypoxia-inducible factor-1alpha.
EMBO J. 17:6573–6586. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Dayan F, Roux D, Brahimi-Horn MC,
Pouyssegur J and Mazure NM: The oxygen sensor factor-inhibiting
hypoxia-inducible factor-1 controls expression of distinct genes
through the bifunctional transcriptional character of
hypoxia-inducible factor-1alpha. Cancer Res. 66:3688–3698. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Jiang BH, Semenza GL, Bauer C and Marti
HH: Hypoxia-inducible factor 1 levels vary exponentially over a
physiologically relevant range of O2 tension. Am J Physiol.
271:C1172–C1180. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Hu CJ, Sataur A, Wang L, Chen H and Simon
MC: The N-terminal transactivation domain confers target gene
specificity of hypoxia-inducible factors HIF-1alpha and HIF-2alpha.
Mol Biol Cell. 18:4528–4542. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Badowska-Kozakiewicz AM, Sobol M and
Patera J: Expression of multidrug resistance protein P-glycoprotein
in correlation with markers of hypoxia (HIF-1α, EPO, EPO-R) in
invasive breast cancer with metastasis to lymph nodes. Arch Med
Sci. 13:1303–1314. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Rodríguez ME, Catrinacio C, Ropolo A,
Rivarola VA and Vaccaro MI: A novel HIF-1α/VMP1-autophagic pathway
induces resistance to photodynamic therapy in colon cancer cells.
Photochem Photobiol Sci. 16:1631–1642. 2017. View Article : Google Scholar
|
|
62
|
Shao JS, Sun J, Wang S, Chung K, Du JT,
Wang J, Qiu XS, Wang EH and Wu GP: HPV16 E6/E7 upregulates HIF-2α
and VEGF by inhibiting LKB1 in lung cancer cells. Tumour Biol.
39:1010428317717137. 2017. View Article : Google Scholar
|
|
63
|
Zhang Q, Lou Y, Zhang J, Fu Q, Wei T, Sun
X, Chen Q, Yang J, Bai X and Liang T: Hypoxia-inducible factor-2α
promotes tumor progression and has crosstalk with Wnt/β-catenin
signaling in pancreatic cancer. Mol Cancer. 16:1192017. View Article : Google Scholar
|
|
64
|
Raspaglio G, Petrillo M, Martinelli E, Li
Puma DD, Mariani M, De Donato M, Filippetti F, Mozzetti S, Prislei
S, Zannoni GF, et al: Sox9 and Hif-2α regulate TUBB3 gene
expression and affect ovarian cancer aggressiveness. Gene.
542:173–181. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Jun JC, Rathore A, Younas H, Gilkes D and
Polotsky VY: Hypoxia-Inducible Factors and Cancer. Curr Sleep Med
Rep. 3:1–10. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Vandyke K, Zeissig MN, Hewett DR, Martin
SK, Mrozik KM, Cheong CM, Diamond P, To LB, Gronthos S, Peet DJ, et
al: HIF-2α promotes dissemination of plasma cells in multiple
myeloma by regulating CXCL12/CXCR4 and CCR1. Cancer Res.
77:5452–5463. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Ma X, Zhang H, Xue X and Shah YM:
Hypoxia-inducible factor 2α (HIF-2α) promotes colon cancer growth
by potentiating Yes-associated protein 1 (YAP1) activity. J Biol
Chem. 292:17046–17056. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Garziera M, Scarabel L and Toffoli G:
Hypoxic modulation of HLA-G expression through the metabolic sensor
HIF-1 in human cancer cells. J Immunol Res. 2017:45875202017.
View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Anam MT, Ishika A, Hossain MB and Jesmin:
A meta-analysis of hypoxia inducible factor 1-alpha (HIF1A) gene
polymorphisms: Association with cancers. Biomark Res. 3:292015.
View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Frank B, Hoffmeister M, Klopp N, Illig T,
Chang-Claude J and Brenner H: Single nucleotide polymorphisms in
Wnt signaling and cell death pathway genes and susceptibility to
colorectal cancer. Carcinogenesis. 31:1381–1386. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Guo X, Li D, Chen Y, An J, Wang K, Xu Z,
Chen Z and Xing J: SNP rs2057482 in HIF1A gene predicts clinical
outcome of aggressive hepatocellular carcinoma patients after
surgery. Sci Rep. 5:118462015. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Han SS, Yeager M, Moore LE, Wei MH,
Pfeiffer R, Toure O, Purdue MP, Johansson M, Scelo G, Chung CC, et
al: The chromosome 2p21 region harbors a complex genetic
architecture for association with risk for renal cell carcinoma.
Hum Mol Genet. 21:1190–1200. 2012. View Article : Google Scholar :
|
|
73
|
Yamamoto Y, Kiyohara C, Ogata-Suetsugu S,
Hamada N and Nakanishi Y: Association between genetic polymorphisms
involved in the hypoxia-inducible factor pathway and lung cancer
risk: A case-control study in Japan. Asia Pac J Clin Oncol.
13:234–242. 2017. View Article : Google Scholar
|
|
74
|
Haja Mohideen AM, Hyde A, Squires J, Wang
J, Dicks E, Younghusband B, Parfrey P, Green R and Savas S:
Examining the polymorphisms in the hypoxia pathway genes in
relation to outcome in colorectal cancer. PLoS One. 9:e1135132014.
View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Autour A, Jeng S C Y, Cawte A D,
Abdolahzadeh A, Galli A, Panchapakesan SSS, Rueda D, Ryckelynck M
and Unrau PJ: Fluorogenic RNA Mango aptamers for imaging small
non-coding RNAs in mammalian cells. Nat Commun. 9:6562018.
View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Camps C, Saini HK, Mole DR, Choudhry H,
Reczko M, Guerra-Assunção JA, Tian YM, Buffa FM, Harris AL,
Hatzigeorgiou AG, et al: Integrated analysis of microRNA and mRNA
expression and association with HIF binding reveals the complexity
of microRNA expression regulation under hypoxia. Mol Cancer.
13:282014. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Ho JJ, Metcalf JL, Yan MS, Turgeon PJ,
Wang JJ, Chalsev M, Petruzziello-Pellegrini TN, Tsui AK, He JZ,
Dhamko H, et al: Functional importance of Dicer protein in the
adaptive cellular response to hypoxia. J Biol Chem.
287:29003–29020. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
van den Beucken T, Koch E, Chu K,
Rupaimoole R, Prickaerts P, Adriaens M, Voncken JW, Harris AL,
Buffa FM, Haider S, et al: Hypoxia promotes stem cell phenotypes
and poor prognosis through epigenetic regulation of DICER. Nat
Commun. 5:52032014. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Montoya MM, Maul J, Singh PB, Pua HH,
Dahlström F, Wu N, Huang X, Ansel KM and Baumjohann D: A distinct
inhibitory function for miR-18a in Th17 cell differentiation. J
Immunol. 199:559–569. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Li JY, Zhang Y, Zhang WH, Jia S, Kang Y
and Zhu XY: Differential distribution of miR-20a and miR-20b may
underly metastatic heterogeneity of breast cancers. Asian Pac J
Cancer Prev. 13:1901–1906. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Kunej T, Obsteter J, Pogacar Z, Horvat S
and Calin GA: The decalog of long non-coding RNA involvement in
cancer diagnosis and monitoring. Crit Rev Clin Lab Sci. 51:344–357.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Im JH and Muschel RJ: New evidence of
lncRNA role in tumor progression and metastasis. Hepatobiliary Surg
Nutr. 1:55–56. 2012.PubMed/NCBI
|
|
83
|
Schito L and Semenza GL: Hypoxia-inducible
factors: Master regulators of cancer progression. Trends Cancer.
2:758–770. 2016. View Article : Google Scholar
|
|
84
|
Mohlin S, Wigerup C, Jögi A and Påhlman S:
Hypoxia, pseudo-hypoxia and cellular differentiation. Exp Cell Res.
356:192–196. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Shi QY, Zhang SJ, Liu L, Chen QS, Yu LN,
Zhang FJ and Yan M: Sevoflurane promotes the expansion of glioma
stem cells through activation of hypoxia-inducible factors in
vitro. Br J Anaesth. 114:825–830. 2015. View Article : Google Scholar
|
|
86
|
Lee G, Auffinger B, Guo D, Hasan T,
Deheeger M, Tobias AL, Kim JY, Atashi F, Zhang L, Lesniak MS, et
al: Dedifferentiation of glioma cells to glioma stem-like cells by
therapeutic stress-induced HIF signaling in the recurrent GBM
model. Mol Cancer Ther. 15:3064–3076. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Zhang S, Luo X, Wan F and Lei T: The roles
of hypoxia-inducible factors in regulating neural stem cells
migration to glioma stem cells and determinating their fates.
Neurochem Res. 37:2659–2666. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Semenza GL: Regulation of the breast
cancer stem cell phenotype by hypoxia-inducible factors. Clin Sci
(Lond). 129:1037–1045. 2015. View Article : Google Scholar
|
|
89
|
Samanta D, Gilkes DM, Chaturvedi P, Xiang
L and Semenza GL: Hypoxia-inducible factors are required for
chemotherapy resistance of breast cancer stem cells. Proc Natl Acad
Sci USA. 111:E5429–E5438. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Deynoux M, Sunter N, Hérault O and
Mazurier F: Hypoxia and hypoxia-inducible factors in leukemias.
Front Oncol. 6:412016. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Heddleston JM, Wu Q, Rivera M, Minhas S,
Lathia JD, Sloan AE, Iliopoulos O, Hjelmeland AB and Rich JN:
Hypoxia-induced mixed-lineage leukemia 1 regulates glioma stem cell
tumorigenic potential. Cell Death Differ. 19:428–439. 2012.
View Article : Google Scholar
|
|
92
|
Sun JC, He F, Yi W, Wan MH, Li R, Wei X,
Wu R and Niu DL: High expression of HIF-2α and its
anti-radiotherapy effect in lung cancer stem cells. Genet Mol Res.
14:18110–18120. 2015. View Article : Google Scholar
|
|
93
|
Dhatwalia SK, Kumar M and Dhawan DK: Role
of EGCG in containing the progression of lung tumorigenesis - A
multistage targeting approach. Nutr Cancer. 70:334–349. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Vadde R, Vemula S, Jinka R, Merchant N,
Bramhachari PV and Nagaraju GP: Role of hypoxia-inducible factors
(HIF) in the maintenance of stemness and malignancy of colorectal
cancer. Crit Rev Oncol Hematol. 113:22–27. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Thomas S, Harding MA, Smith SC, Overdevest
JB, Nitz MD, Frierson HF, Tomlins SA, Kristiansen G and Theodorescu
D: CD24 is an effector of HIF-1-driven primary tumor growth and
metastasis. Cancer Res. 72:5600–5612. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Bhagat M, Palanichamy JK, Ramalingam P,
Mudassir M, Irshad K, Chosdol K, Sarkar C, Seth P, Goswami S, Sinha
S, et al: HIF-2α mediates a marked increase in migration and
stemness characteristics in a subset of glioma cells under hypoxia
by activating an Oct-4/Sox-2-Mena (INV) axis. Int J Biochem Cell
Biol. 74:60–71. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Johansson E, Grassi ES, Pantazopoulou V,
Tong B, Lindgren D, Berg TJ, Pietras EJ, Axelson H and Pietras A:
CD44 interacts with HIF-2α to modulate the hypoxic phenotype of
perinecrotic and perivascular glioma cells. Cell Reports.
20:1641–1653. 2017. View Article : Google Scholar
|