Cancer is a leading cause of mortality worldwide. In
China, ~4 million new cases of cancer were diagnosed in 2015, and
50% of all mortalities were associated with cancer (1). Surgery, chemotherapy and/or
radiotherapy are used to treat the majority of cancers and to
improve survival of patients. These clinical measures have proven
efficaciousinseveral cases; however, few patients survive >5
years due to the high recurrence and metastasis of tumor cells;
CSCs are considered the root of tumor recurrence and metastasis
(2,3).
CSCs have been identified and characterized in
various tumor types; in particular, CSCs exhibit self-renewal,
multi-lineage differentiation and tumor initiation capacities, and
proliferative potential (4).
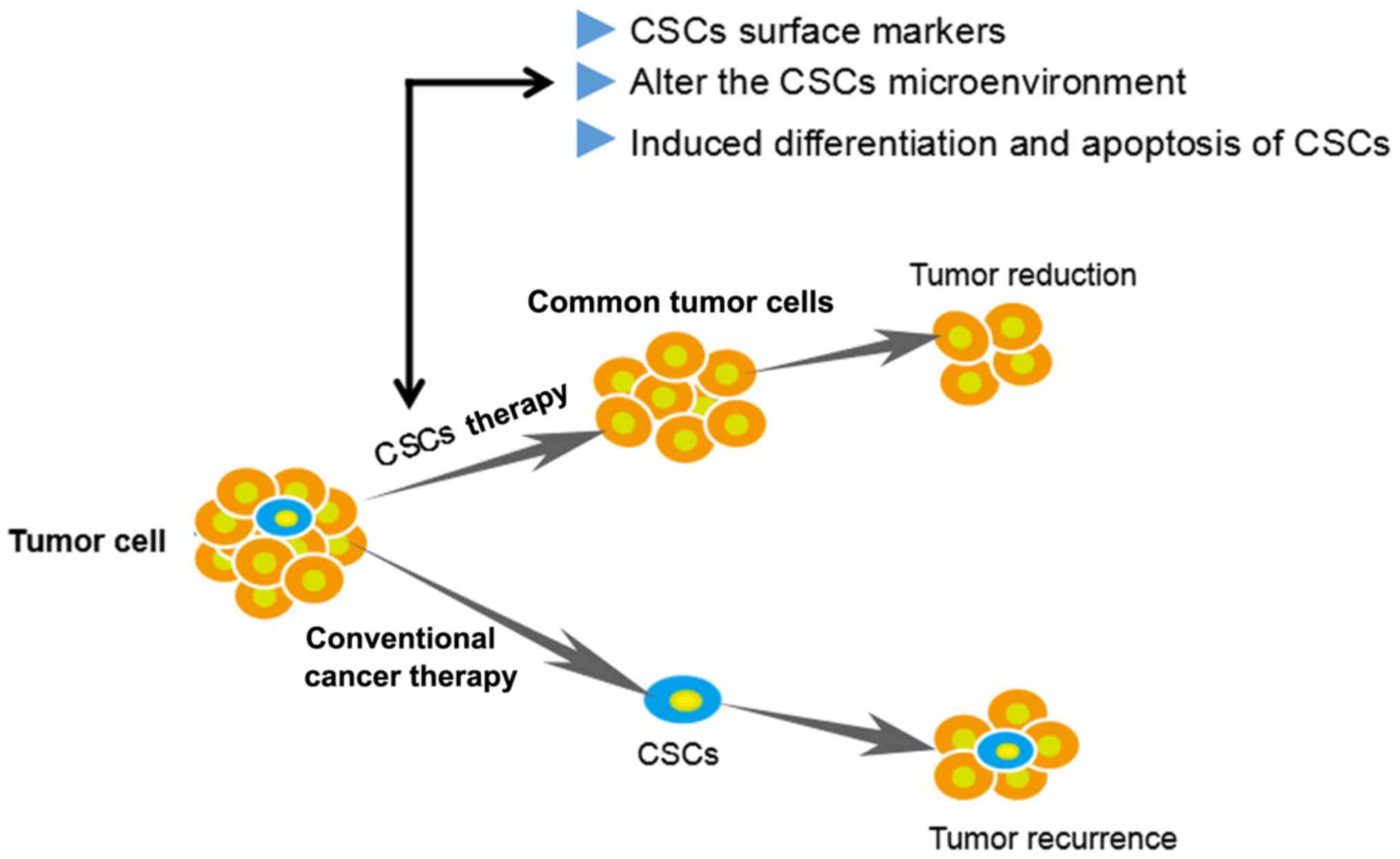
Targeting of CSCs or inhibition of important properties including
self-renewal, differentiation and apoptosis resistance are novel
therapeutic strategies (Fig. 1).
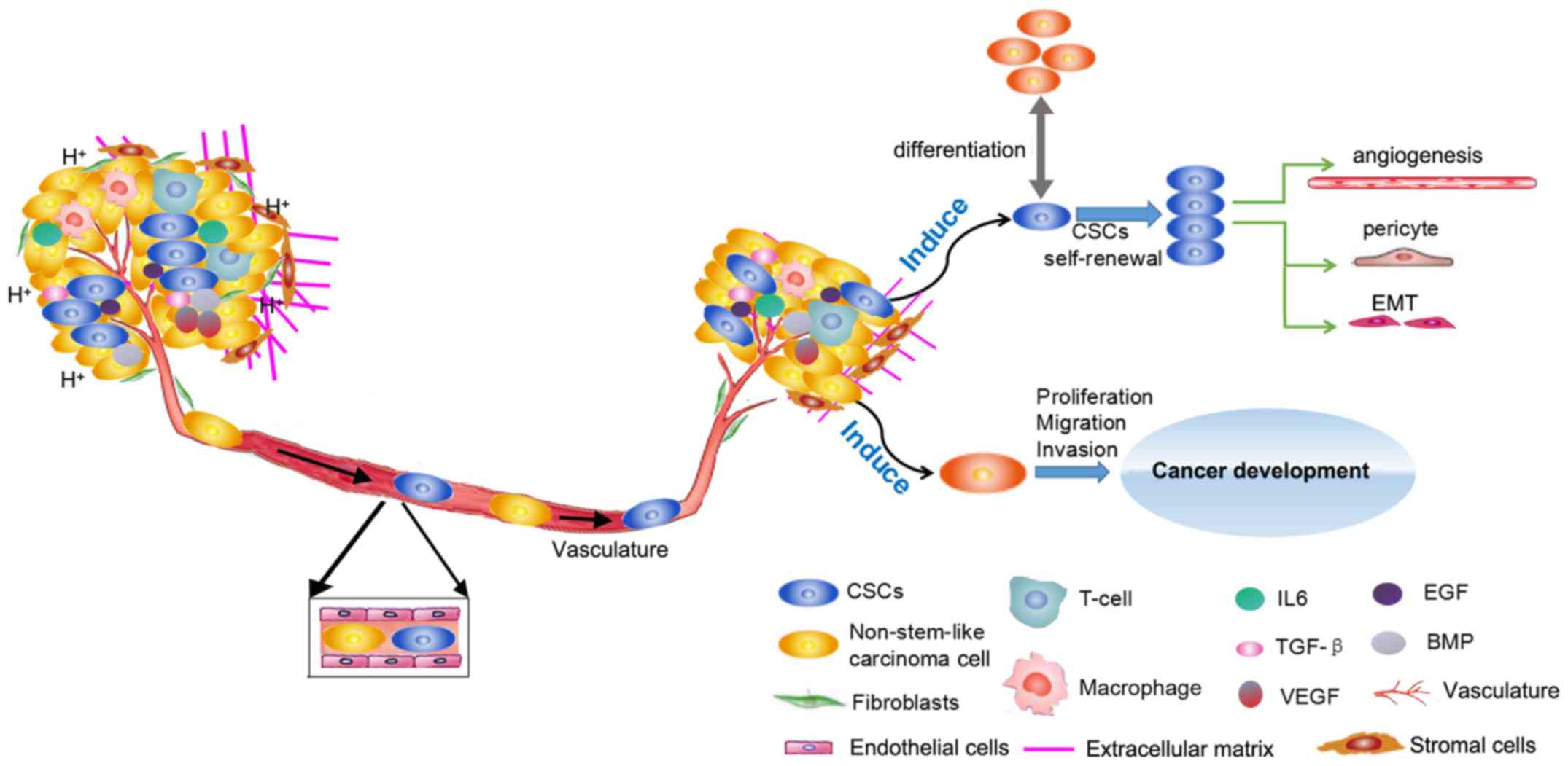
Several lines of evidence have indicated that CSCs serve a key role
in tumorigenesis, recurrence and metastasis (5-7).
When tumors occur, CSCs are considered to be the origin of abnormal
differentiation; uncontrolled self-renewal of CSCs induces
malignant transformation and rapid proliferation of cells. In
advanced tumor stages, once activated, CSCs can promote tumor
development and metastasis by regulating tumor angiogenesis
(8). Notably, the current
anti-tumor drugs mainly target rapidly proliferating mitotic cells;
however, CSCs are usually dormant or quiescent, and can therefore
exhibit immune escape and resist the suppressive effects of
chemotherapy drugs, thereby becoming the root of tumor recurrence
(3). Therefore, CSCs are
considered to be the key to tumor recurrence and metastasis of seed
cells and malignant tumors. Previous studies have suggested that
there are three major sources of CSCs, as follows: i) Normal stem
or progenitor cells are malignantly transformed into CSCs due to
gene mutations; ii) viral infection or formation of CSCs through
intercellular fusion (9,10); iii) mature end-stage tumor cells
regain CSC-like properties induced by ionizing radiation, hypoxia
or the tumor microenvironment (11,12).
In addition, both inflammatory factors [interleukin (IL) 6, and
transforming growth factor (TGF)-β], and cytokines [endothelial
growth factor (EGF) and vascular (EGF)] regulate CSC growth and
maintenance (Fig. 2).
Previous studies have reported that ion channels
serve an important role in cancer development (13,14).
Numerous ion channels have been confirmed to be highly expressed in
various tumor types and are closely associated with tumor cell
biological behaviors (15-17). Ion channels are specific
hydrophilic microporous proteins that exhibit selective
permeability for various ions; they are usually named according to
the ions with the highest permeability, including potassium
(K+) channels, calcium (Ca2+) channels and
chloride (Cl−) channels. These ion channels are
distributed in almost every cell membrane of the body and have an
important role in the physiology and pathology of excitable cells
with regards to the following aspects: i) Determination of cell
excitability, conductivity, contractility and rhythmicity; in
nerve, muscle and other excitable cells, Na+ and
Ca2+ channels mainly regulate depolarization, whereas
K+ channels mainly regulate repolarization and maintain
the resting potential (18,19);
ii) regulation of vasomotor smoothing and contraction activities
(20); iii) participation in
synaptic transmission (21); iv)
maintenance of normal cell volume (22,23);
v) regulation of intracellular cAMP, cGMP, Ca2+ and
other second messenger concentrations, in order to trigger muscle
contraction, glandular secretion, protein kinase activation and
gene expression regulation (24,25).
The normal structure and function of ion channels are the basis for
cells to carry out their normal activities. Mutations in specific
ion channel sites lead to abnormalities in their activation and
inactivation, causing cell dysfunction and the formation of various
diseases, including epilepsy and arrhythmia, and skeletal muscle
dysfunction (26,27). Disorders associated with aberrant
ion channel functions are commonly known as 'ion channel diseases'
(28,29).
At present, few reports have focused on the
association between ion channels and CSCs. Our recent work
indicated that solute carrier family 8 member A1 and transient
receptor potential cation channel subfamily C member 6 are
expressed in cluster of differentiation (CD)133+ stem
cells in Huh7 hepatic cancer cells, thus indicating that ion
channels may be involved in the occurrence and development of
cancer (30). Furthermore, ion
channel inhibitors can reduce drug resistance of tumor cells via
regulation of CSC function (31,32).
The present review aimed to summarize the roles of ion channels,
and describe their expression and function in CSCs. Further
evaluation of the association between ion channels and CSCs is
critically important to understand malignancy.
Kv1.3 (together with KCa3.1) has been implicated in
the control of cell proliferation in rat MSCs; silencing KCa3.1
inhibits the proliferation of rat bone marrow MSCs by inducing cell
cycle arrest at the G0/G1 phase (51). The voltage-sensitive human ERG
(hERG, Kv11.1) K+ channel acts as a regulator of
proliferation and survival in cancer cells (52,53).
The expression of Kv11.1 has been reported in several cancer types,
as well as cancer cell lines of different lineages, such as
epithelial, leukemic, connective or neuronal cells. Recently, Li
et al reported that hERG (Kv11.1) is highly expressed in
CD34+/CD38−/CD123 leukemia stem cells (LSCs),
interferes with the cell cycle and promotes tumor cell
proliferation. Furthermore, the hERG-specific blocker E-4031
inhibits LSC proliferation, by inhibiting G1/S phase
transition (54). Another hERG
inhibitor, clofilium, destroys the osmotic pressure balance of LSCs
intra- and extracellularly via K+-induced cell swelling
and rupture. These results suggest that hERG channels may be
involved in regulation of the LSC cycle, and that LSCs maintain a
constant volume by adjusting osmotic pressure inside and outside of
the cell (55).
In recent decades, growing scientific evidence has
supported the potential involvement of ion channels in
tumorigenesis and carcinogenesis. Setti et al indicated that
Cl− intracellular channel protein 1 (CLIC1) is
overexpressed in GBM CSCs, where it serves an important role in GBM
CSCs self-renewal and proliferation; CLIC1 is primarily detected in
the nuclear membrane and in the plasma membrane. In addition, Setti
et al demonstrated that overexpression of CLIC1 in GBM CSCs
is negatively correlated with patient survival. Conversely,
silencing CLIC1 inhibits the proliferation, cloning and
tumorigenicity of GBM (61). These
results may indicate a novel therapeutic approach targeted to GBM.
CLIC1 may be considered an attractive target in the CSC population
that could finally cure GBM. Compared with CLIC1, CLIC4 is
expressed in metastatic CSCs and is associated with the prognostic
risks of colorectal cancer (62).
In conclusion, Cl− channels may serve an important role
in tumor cell migration and tumor metastasis; therefore,
Cl− channels may be potential drug targets for the
treatment of tumors.
In recent years, ion channel drugs have been widely
used in clinical practice. It has been reported that various ion
channel blockers can affect the proliferation, differentiation,
apoptosis and metastasis of tumor cells in numerous types of cancer
(58). Inhibiting the
K+ efflux can promote apoptosis, and a K+
channel inhibitor may reverse multidrug resistance (MDR) in tumor
cells (63). Zhao et al
reported that the Ca2+ channel blocker verapamil targets
MDR-associated proteins, inhibits pancreatic CSC
(gemcitabine-resistant) proliferation and promotes apoptosis of
pancreatic cancer cells (64). The
specific inhibitor of the Kv1.3 channel aflatoxin (MgTX) and the
non-specific inhibitor 4-AP can suppress prostate cancer cell
metastasis and lung cancer cell proliferation. Additionally, MgTX
can promote prostate cancer cell apoptosis by regulating the
transition to the G1-S phase (65). Treatment with the KCa3.1 blocker
TRAM-34 and temozolomide (TMZ) is able to significantly reduce DNA
synthesis, as well as GBM and CSC survival, compared with TMZ
alone. Notably, TMZ/TRAM-34 combination therapy can reduce
infiltration of glioma cells (66,67).
CSCs isolated from GBM are highly resistant to
bis-chloroethylnitrosourea (BCNU) in vitro, whereas the
combination of BCNU and a Cl− channel inhibitor
4,4′-diisothiocyanostilbene-2,2′-disulfonic acid inhibits the
proliferation and promotes apoptosis of BCNU-resistant CSCs
(63). CLIC1 is involved in the
resistance of BCNU-resistant CSCs and BCNU/DIDS combined-therapy
can provide valuable insight for promoting apoptosis or sensitizing
glioblastomas to BCNU chemotherapy. These results suggest that
CLIC1 may be a drug efflux channel that participates in the
resistance of GBM CSCs to BCNU (68). In addition, the use of a blocker
[5-nitro-1-(3-phenylpropyl amino) benzoic acid] or small
interfering RNA silencing of CLCN3 Cl− volume sensitive
channel expression, as well as mRNA and protein downregulation of
cyclin D and E, inhibits MSCs proliferation in vitro.
Furthermore, Gritti et al revealed that metformin can
inhibit CLIC1 channel function and reduce the survival of human GBM
CSCs, and short hairpin RNA against CLIC1 significantly increases
the inhibitory effects of metformin on human GBM CSC activity
(69). In addition to
K+ and Cl− channel inhibitors,
Ca2+ channel inhibitors may reverse cancer cell MDR
(70,71).
The novel concept of CSCs was introduced in the late
1990s, and numerous research efforts have aimed to elucidate its
role over the past decades (72,73).
This concept may influence all approaches of cancer biology, since
CSCs have an important role in tumorigenesis, drug resistance
(74,75), invasion, metastasis and recurrence.
The function of CSCs is predominantly regulated by
microenvironmental factors that provide an adaptive landscape for
relapsed tumor cells (76-78). Therefore, identifying novel methods
for preventing CSC drug resistance could improve the long-term
survival of patients. The main factors controlling CSCs include
epithelial-mesenchymal transition and the niche environment
(79,80). In recent years, the potential
regulatory role of ion channels in the tumor microenvironment has
been widely recognized, due to the abnormal expression of ion
channels in CSCs, and various mechanisms regulating tumorigenesis,
malignant transformation and metastasis (81-84).
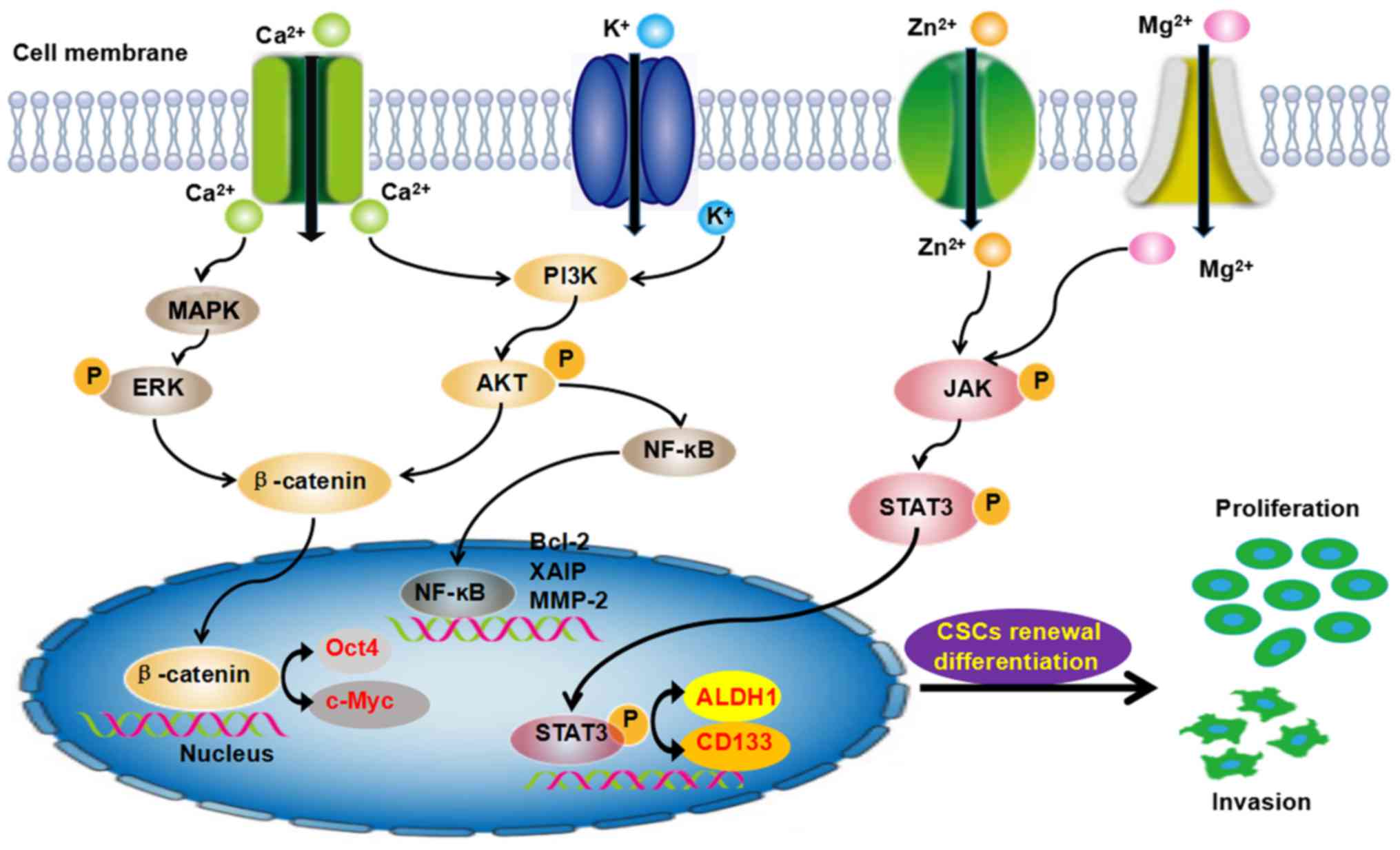
Moreover, those ion channels further induced the aberrant
activation of signaling pathways and play important roles in the
evolution of cancer development. The PI3K/Akt, JNK, STAT3, Wnt and
NF-KB pathways are involved in the self-renewal of CSCs (Fig. 3). These findings have provided
novel information, which may aid the eradication of CSCs, improve
the efficacy of antitumor drugs and result in a potential cure.
Some ion channel agonists or antagonists demonstrate antitumor
activity in specific CSCs, which provides a theoretical basis for
clinical implementation (83).
Additional in-depth research regarding the relationship between ion
channels and MDR may lay the foundation for the development of
novel agents through drug design and development. Novel
perspectives will be gained from the characterization of various
ion channel structures and may promote the development of anti-CSC
drug targets. It has been hypothesized that through further
exploration of the relationship between ion channels and CSCs, ion
channels may be revealed to participate in the regulation of CSC
pathways, and their inhibitors may provide more information
regarding clinical targets in CSC-targeted therapy.
The authors would like to thank Professor Biguang
Tuo (Department of Gastroenterology, Affiliated Hospital of Zunyi
Medical College) for highly professional services.
The present study was supported by research grants
from the National Natural Science Foundation of China (grant no.
816660412 to RX, grant no. 81160265 to JYX, grant no. 81360311 to
HJ).
Not applicable.
QC, and AC wrote the manuscript; QD, QL, ZS, CC, XY,
YH, JZ, SL, GW, JA and HJ collect the literature; BT and RX.
primarily revised and finalized manuscript. JX revised the
manuscript for clarity and style.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hardingham JE, Grover P, Winter M, Hewett
PJ, Price TJ and Thierry B: Detection and clinical significance of
circulating tumor cells in colorectal cancer - 20 years of
progress. Mol Med. 21(Suppl 1): S25–S31. 2015. View Article : Google Scholar :
|
|
3
|
Shiozawa Y, Nie B, Pienta KJ, Morgan TM
and Taichman RS: Cancer stem cells and their role in metastasis.
Pharmacol Ther. 138:285–293. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Dalerba P, Cho RW and Clarke MF: Cancer
stem cells: Models and concepts. Annu Rev Med. 58:267–284. 2007.
View Article : Google Scholar
|
|
5
|
Fan ST, Yang ZF, Ho DW, Ng MN, Yu WC and
Wong J: Prediction of posthepatectomy recurrence of hepatocellular
carcinoma by circulating cancer stem cells: A prospective study.
Ann Surg. 254:569–576. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chinn SB, Darr OA, Peters RD and Prince
ME: The role of head and neck squamous cell carcinoma cancer stem
cells in tumorigenesis, metastasis, and treatment failure. Front
Endocrinol (Lausanne). 3:902012.
|
|
7
|
Nandy SB and Lakshmanaswamy R: Cancer stem
cells and metastasis. Prog Mol Biol Transl Sci. 151:137–176. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ribatti D: Cancer stem cells and tumor
angiogenesis. Cancer Lett. 321:13–17. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Marusyk A, Almendro V and Polyak K:
Intra-tumour heterogeneity: A looking glass for cancer? Nat Rev
Cancer. 12:323–334. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ansieau S: EMT in breast cancer stem cell
generation. Cancer Lett. 338:63–68. 2013. View Article : Google Scholar
|
|
11
|
Wei J, Wu A, Kong LY, Wang Y, Fuller G,
Fokt I, Melillo G, Priebe W and Heimberger AB: Hypoxia potentiates
glioma-mediated immunosuppression. PLoS One. 6:e161952011.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Salnikov AV, Liu L, Platen M, Gladkich J,
Salnikova O, Ryschich E, Mattern J, Moldenhauer G, Werner J,
Schemmer P, et al: Hypoxia induces EMT in low and highly aggressive
pancreatic tumor cells but only cells with cancer stem cell
characteristics acquire pronounced migratory potential. PLoS One.
7:e463912012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Takanami I, Inoue Y and Gika M: G-protein
inwardly rectifying potassium channel 1 (GIRK 1) gene expression
correlates with tumor progression in non-small cell lung cancer.
BMC Cancer. 4:79–85. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Stringer BK, Cooper AG and Shepard SB:
Overexpression of the G-protein inwardly rectifying potassium
channel 1 (GIRK1) in primary breast carcinomas correlates with
axillary lymph node metastasis. Cancer Res. 61:582–588.
2001.PubMed/NCBI
|
|
15
|
Zhang Y, Wang H, Qian Z, Feng B, Zhao X,
Jiang X and Tao J: Low-voltage-activated T-type Ca2+
channel inhibitors as new tools in the treatment of glioblastoma:
The role of endostatin. Pflugers Arch. 466:811–818. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang XT, Nagaba Y, Cross HS, Wrba F, Zhang
L and Guggino SE: The mRNA of L-type calcium channel elevated in
colon cancer: Protein distribution in normal and cancerous colon.
Am J Pathol. 157:1549–1562. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Vanden Abeele F, Lemonnier L, Thébault S,
Lepage G, Parys JB, Shuba Y, Skryma R and Prevarskaya N: Two types
of store-operated Ca2+ channels with different
activation modes and molecular origin in LNCaP human prostate
cancer epithelial cells. J Biol Chem. 279:30326–30337. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Catterall WA and Zheng N: Deciphering
voltage-gated Na(+) and Ca(2+) channels by studying prokaryotic
ancestors. Trends Biochem Sci. 40:526–534. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
González C, Baez-Nieto D, Valencia I,
Oyarzún I, Rojas P, Naranjo D and Latorre R: K(+) channels:
Function-structural overview. Compr Physiol. 2:2087–2149. 2012.
|
|
20
|
Firth AL, Remillard CV, Platoshyn O,
Fantozzi I, Ko EA and Yuan JX: Functional ion channels in human
pulmonary artery smooth muscle cells: Voltage-dependent cation
channels. Pulm Circ. 1:48–71. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Voglis G and Tavernarakis N: The role of
synaptic ion channels in synaptic plasticity. EMBO Rep.
7:1104–1110. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hua SZ, Gottlieb PA, Heo J and Sachs F: A
mechanosensitive ion channel regulating cell volume. Am J Physiol
Cell Physiol. 298:C1424–C1430. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sardini A, Amey JS, Weylandt KH, Nobles M,
Valverde MA and Higgins CF: Cell volume regulation and
swelling-activated chloride channels. Biochim Biophys Acta.
1618:153–162. 2003. View Article : Google Scholar
|
|
24
|
Subramanyam P and Colecraft HM: Ion
channel engineering: Perspectives and strategies. J Mol Biol.
427:190–204. 2015. View Article : Google Scholar :
|
|
25
|
Grosse W, Essen LO and Koert U: Strategies
and perspectives in ion-channel engineering. ChemBioChem.
12:830–839. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yang B, Cao L, Liu J, Xu Y, Milne G, Chan
W, Heys SD, McCaig CD and Pu J: Low expression of chloride channel
accessory 1 predicts a poor prognosis in colorectal cancer. Cancer.
121:1570–1580. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ievglevskyi O, Isaev D, Netsyk O, Romanov
A, Fedoriuk M, Maximyuk O, Isaeva E, Akaike N and Krishtal O:
Acid-sensing ion channels regulate spontaneous inhibitory activity
in the hippocampus: Possible implications for epilepsy. Philos
Trans R Soc Lond B Biol Sci. 371:1–9. 2016. View Article : Google Scholar
|
|
28
|
Feske S, Wulff H and Skolnik EY: Ion
channels in innate and adaptive immunity. Annu Rev Immunol.
33:291–353. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bulman DE: Phenotype variation and
newcomers in ion channel disorders. Hum Mol Genet. 6:1679–1685.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Xu J, Yang Y, Xie R, Liu J, Nie X, An J,
Wen G, Liu X, Jin H and Tuo B: The NCX1/TRPC6 complex mediates
TGFβ-driven migration and invasion of human hepatocellular
carcinoma cells. Cancer Res. 78:2564–2576. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Moharil RB, Dive A, Khandekar S and
Bodhade A: Cancer stem cells: An insight. J Oral Maxillofac Pathol.
21:4632017. View Article : Google Scholar
|
|
32
|
Reya T, Morrison SJ, Clarke MF and
Weissman IL: Stem cells, cancer, and cancer stem cells. Nature.
414:105–111. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Peters T: Calcium in physiological and
pathological cell function. Eur Neurol. 25(Suppl 1): 27–44. 1986.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Monteith GR, Davis FM and Roberts-Thomson
SJ: Calcium channels and pumps in cancer: Changes and consequences.
J Biol Chem. 287:31666–31673. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
D'Ascenzo M, Piacentini R, Casalbore P,
Budoni M, Pallini R, Azzena GB and Grassi C: Role of L-type
Ca2+ channels in neural stem/progenitor cell
differentiation. Eur J Neurosci. 23:935–944. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Stewart TA, Yapa KT and Monteith GR:
Altered calcium signaling in cancer cells. Biochim Biophys Acta.
1848B:2502–2511. 2015. View Article : Google Scholar
|
|
37
|
Xie J, Pan H, Yao J, Zhou Y and Han W:
SOCE and cancer: Recent progress and new perspectives. Int J
Cancer. 138:2067–2077. 2016. View Article : Google Scholar :
|
|
38
|
Prakriya M and Lewis RS: Store-operated
calcium channels. Physiol Rev. 95:1383–1436. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Lee SH, Rigas NK, Lee CR, Bang A, Srikanth
S, Gwack Y, Kang MK, Kim RH, Park NH and Shin KH: Orai1 promotes
tumor progression by enhancing cancer stemness via NFAT signaling
in oral/oropharyngeal squamous cell carcinoma. Oncotarget.
7:43239–43255. 2016.PubMed/NCBI
|
|
40
|
Zhao W, Wang L, Han H, Jin K, Lin N, Guo
T, Chen Y, Cheng H, Lu F, Fang W, et al: 1B50-1, a mAb raised
against recurrent tumor cells, targets liver tumor-initiating cells
by binding to the calcium channel α2δ1 subunit. Cancer Cell.
23:541–556. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Liu M, Inoue K, Leng T, Guo S and Xiong
ZG: TRPM7 channels regulate glioma stem cell through STAT3 and
Notch signaling pathways. Cell Signal. 26:2773–2781. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Morelli MB, Nabissi M, Amantini C,
Farfariello V, Ricci-Vitiani L, di Martino S, Pallini R, Larocca
LM, Caprodossi S, Santoni M, et al: The transient receptor
potential vanilloid-2 cation channel impairs glioblastoma stem-like
cell proliferation and promotes differentiation. Int J Cancer.
131:E1067–E1077. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Curci A, Mele A, Camerino GM, Dinardo MM
and Tricarico D: The large conductance Ca(2+) -activated K(+)
(BKCa) channel regulates cell proliferation in SH-SY5Y
neuroblastoma cells by activating the staurosporine-sensitive
protein kinases. Front Physiol. 5:4762014. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Rosa P, Sforna L, Carlomagno S, Mangino G,
Miscusi M, Pessia M, Franciolini F, Calogero A and Catacuzzeno L:
Overexpression of large-conductance calcium-activated potassium
channels in human glioblastoma stem-like cells and their role in
cell migration. J Cell Physiol. 232:2478–2488. 2017. View Article : Google Scholar
|
|
45
|
Zhang YY, Yue J, Che H, Sun HY, Tse HF and
Li GR: BKCa and hEag1 channels regulate cell proliferation and
differentiation in human bone marrow-derived mesenchymal stem
cells. J Cell Physiol. 229:202–212. 2014. View Article : Google Scholar
|
|
46
|
Nio K, Yamashita T and Kaneko S: The
evolving concept of liver cancer stem cells. Mol Cancer. 16:42017.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Heubach JF, Graf EM, Leutheuser J, Bock M,
Balana B, Zahanich I, Christ T, Boxberger S, Wettwer E and Ravens
U: Electrophysiological properties of human mesenchymal stem cells.
J Physiol. 554:659–672. 2004. View Article : Google Scholar
|
|
48
|
Li G-R, Sun H, Deng X and Lau CP:
Characterization of ionic currents in human mesenchymal stem cells
from bone marrow. Stem Cells. 23:371–382. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Wang S-P, Wang J-A, Luo R-H, Cui W-Y and
Wang H: Potassium channel currents in rat mesenchymal stem cells
and their possible roles in cell proliferation. Clin Exp Pharmacol
Physiol. 35:1077–1084. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Pardo LA and Stühmer W: The roles of K(+)
channels in cancer. Nat Rev Cancer. 14:39–48. 2014. View Article : Google Scholar
|
|
51
|
Wang ZH, Shen B, Yao HL, Jia YC, Ren J,
Feng YJ and Wang YZ: Blockage of intermediate-conductance-Ca(2+)
-activated K(+) channels inhibits progression of human endometrial
cancer. Oncogene. 26:5107–5114. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Rao VR, Perez-Neut M, Kaja S and Gentile
S: Voltage-gated ion channels in cancer cell proliferation. Cancers
(Basel). 7:849–875. 2015. View Article : Google Scholar
|
|
53
|
Šatková J and Bébarová M: Functional
impact of hERG: From physiological role to target of anticancer
therapy. Vnitr Lek. 63:114–123. 2017.In Czech.
|
|
54
|
Li H, Liu L, Guo L, Zhang J, Du W, Li X,
Liu W, Chen X and Huang S: HERG K+ channel expression in
CD34+/CD38−/CD123(high) cells and primary
leukemia cells and analysis of its regulation in leukemia cells.
Int J Hematol. 87:387–392. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Jehle J, Schweizer PA, Katus HA and Thomas
D: Novel roles for hERG K(+) channels in cell proliferation and
apoptosis. Cell Death Dis. 2:e1932011. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Kubota D, Orita H, Yoshida A, Gotoh M,
Kanda T, Tsuda H, Hasegawa T, Katai H, Shimada Y, Kaneko K, et al:
Pfetin as a prognostic biomarker for gastrointestinal stromal
tumor: Validation study in multiple clinical facilities. Jpn J Clin
Oncol. 41:1194–1202. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Li L, Duan T, Wang X, Zhang RH, Zhang M,
Wang S, Wang F, Wu Y, Huang H and Kang T: KCTD12 Regulates
colorectal cancer cell stemness through the ERK pathway. Sci Rep.
6:204602016. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Jentsch TJ, Stein V, Weinreich F and
Zdebik AA: Molecular structure and physiological function of
chloride channels. Physiol Rev. 82:503–568. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Nako Y, Shiozaki A, Ichikawa D, Komatsu S,
Konishi H, Iitaka D, Ishii H, Ikoma H, Kubota T, Fujiwara H, et al:
Enhancement of the cytocidal effects of hypotonic solution using a
chloride channel blocker in pancreatic cancer cells. Pancreatology.
12:440–448. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Soroceanu L, Manning TJ Jr and Sontheimer
H: Modulation of glioma cell migration and invasion using Cl(−) and
K(+) ion channel blockers. J Neurosci. 19:5942–5954. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Setti M, Savalli N, Osti D, Richichi C,
Angelini M, Brescia P, Fornasari L, Carro MS, Mazzanti M and
Pelicci G: Functional role of CLIC1 ion channel in
glioblastoma-derived stem/progenitor cells. J Natl Cancer Inst.
105:1644–1655. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Deng YJ, Tang N, Liu C, Zhang JY, An SL,
Peng YL, Ma LL, Li GQ, Jiang Q, Hu CT, et al: CLIC4, ERp29, and
Smac/DIABLO derived from metastatic cancer stem-like cells stratify
prognostic risks of colorectal cancer. Clin Cancer Res.
20:3809–3817. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Higgins CF: Volume-activated chloride
currents associated with the multidrug resistance P-glycoprotein. J
Physiol. 482(Suppl): 31S–36S. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Zhao L, Zhao Y, Schwarz B, Mysliwietz J,
Hartig R, Camaj P, Bao Q, Jauch KW, Guba M, Ellwart JW, et al:
Verapamil inhibits tumor progression of chemotherapy-resistant
pancreatic cancer side population cells. Int J Oncol. 49:99–110.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Comes N, Bielanska J, Vallejo-Gracia A,
Serrano-Albarrás A, Marruecos L, Gómez D, Soler C, Condom E, Ramón
Y, Cajal S, Hernández-Losa J, et al: The voltage-dependent K(+)
channels Kv1.3 and Kv1.5 in human cancer. Front Physiol. 4:2832013.
View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Fraser SP, Grimes JA and Djamgoz MB:
Effects of voltage-gated ion channel modulators on rat prostatic
cancer cell proliferation: Comparison of strongly and weakly
metastatic cell lines. Prostate. 44:61–76. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
D'Alessandro G, Grimaldi A, Chece G,
Porzia A, Esposito V, Santoro A, Salvati M, Mainiero F, Ragozzino
D, Di Angelantonio S, et al: KCa3.1 channel inhibition sensitizes
malignant gliomas to temozolomide treatment. Oncotarget.
7:30781–30796. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Kang MK and Kang SK: Pharmacologic
blockade of chloride channel synergistically enhances apoptosis of
chemotherapeutic drug-resistant cancer stem cells. Biochem Biophys
Res Commun. 373:539–544. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Gritti M, Würth R, Angelini M, Barbieri F,
Peretti M, Pizzi E, Pattarozzi A, Carra E, Sirito R, Daga A, et al:
Metformin repositioning as antitumoral agent: Selective
antiproliferative effects in human glioblastoma stem cells, via
inhibition of CLIC1-mediated ion current. Oncotarget.
5:11252–11268. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Chiu LY, Ko JL, Lee YJ, Yang TY, Tee YT
and Sheu GT: L-type calcium channel blockers reverse docetaxel and
vincristine-induced multidrug resistance independent of ABCB1
expression in human lung cancer cell lines. Toxicol Lett.
192:408–418. 2010. View Article : Google Scholar
|
|
71
|
Firuzi O, Javidnia K, Mansourabadi E, Saso
L, Mehdipour AR and Miri R: Reversal of multidrug resistance in
cancer cells by novel asymmetrical 1,4-dihydropyridines. Arch Pharm
Res. 36:1392–1402. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Lapidot T, Sirard C, Vormoor J, Murdoch B,
Hoang T, Caceres-Cortes J, Minden M, Paterson B, Caligiuri MA and
Dick JE: A cell initiating human acute myeloid leukaemia after
transplantation into SCID mice. Nature. 367:645–648. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Bonnet D and Dick JE: Human acute myeloid
leukemia is organized as a hierarchy that originates from a
primitive hema-topoietic cell. Nat Med. 3:730–737. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Hu G, Li F, Ouyang K, Xie F, Tang X, Wang
K, Han S, Jiang Z, Zhu M, Wen D, et al: Intrinsic gemcitabine
resistance in a novel pancreatic cancer cell line is associated
with cancer stem cell-like phenotype. Int J Oncol. 40:798–806.
2012.
|
|
75
|
de la Mare JA, Sterrenberg JN, Sukhthankar
MG, Chiwakata MT, Beukes DR, Blatch GL and Edkins AL: Assessment of
potential anti-cancer stem cell activity of marine algal compounds
using an in vitro mammosphere assay. Cancer Cell Int. 13:392013.
View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Kise K, Kinugasa-Katayama Y and Takakura
N: Tumor micro-environment for cancer stem cells. Adv Drug Deliv
Rev. 99B:197–205. 2016. View Article : Google Scholar
|
|
77
|
Broz ML, Binnewies M, Boldajipour B,
Nelson AE, Pollack JL, Erle DJ, Barczak A, Rosenblum MD, Daud A,
Barber DL, et al: Dissecting the tumor myeloid compartment reveals
rare activating antigen-presenting cells critical for T cell
immunity. Cancer Cell. 26:638–652. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Ishiwata T: Cancer stem cells and
epithelial-mesenchymal transition: Novel therapeutic targets for
cancer. Pathol Int. 66:601–608. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Plaks V, Kong N and Werb Z: The cancer
stem cell niche: How essential is the niche in regulating stemness
of tumor cells? Cell Stem Cell. 16:225–238. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Wang L, Fan J, Hitron JA, Son YO, Wise JT,
Roy RV, Kim D, Dai J, Pratheeshkumar P, Zhang Z, et al: Cancer
stem-like cells accumulated in nickel-induced malignant
transformation. Toxicol Sci. 151:376–387. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Oskarsson T, Batlle E and Massagué J:
Metastatic stem cells: Sources, niches, and vital pathways. Cell
Stem Cell. 14:306–321. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Tian Y, Bresenitz P, Reska A, El Moussaoui
L, Beier CP and Gründer S: Glioblastoma cancer stem cell lines
express functional acid sensing ion channels ASIC1a and ASIC3. Sci
Rep. 7:136742017. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Shiozaki A, Kudou M, Ichikawa D, Fujiwara
H, Shimizu H, Ishimoto T, Arita T, Kosuga T, Konishi H, Komatsu S,
et al: Esophageal cancer stem cells are suppressed by tranilast, a
TRPV2 channel inhibitor. J Gastroenterol. 53:197–207. 2018.
View Article : Google Scholar
|
|
84
|
Bao B, Azmi AS, Li Y, Ahmad A, Ali S,
Banerjee S, Kong D and Sarkar FH: Targeting CSCs in tumor
microenvironment: The potential role of ROS-associated miRNAs in
tumor aggressiveness. Curr Stem Cell Res Ther. 9:22–35. 2014.
View Article : Google Scholar
|