Introduction
Peritoneal dissemination is a common type of
recurrence in patients with gastric cancer (GC) and is associated
with a poor prognosis (1,2). Several treatments, such as
intraperitoneal chemotherapy, have been investigated, but their
efficacy is limited (3,4). Therefore, novel strategies for
treating dissemination are needed to improve treatment outcomes.
The regulation of extracellular osmolality may be a promising
strategy, as hypotonic solutions exert cytocidal effects on cancer
cells (5-9). Peritoneal lavage with distilled water
(DW) has been performed after surgery for various types of cancers,
as the hypotonic shock lyses free cancer cells and prevents
peritoneal seeding.
In order to use the regulation of osmolality for
cancer treatment, a thorough understanding of the physiological
mechanisms of ion and water transport is crucial (5). Under conditions of mild
hypoosmolality, regulatory volume decrease (RVD) occurs after
hypotonicity-induced cell swelling. RVD results from the activation
of ion channels and transporters, which in turn causes
K+, Cl- and H2O efflux, leading to
cell shrinkage (10,11). The osmolality of the lavage water
increases to mild hypotonicity due to intraperitoneal contamination
from ruptured cells (7,12). Under mild hypotonic conditions,
tumor cells avoid rupture through RVD, which decreases the
cytocidal effects of peritoneal lavage with DW.
To improve the efficacy of peritoneal lavage with
DW, inhibition of RVD is necessary. We previously challenged cells
with 5-nitro-2-(3-phenylpropylamino) benzoic acid (NPPB), a
Cl- channel blocker, to increase cell volume by
inhibiting RVD, and found that NPPB enhanced the cytocidal effects
of hypotonic shock on various cancer cells, including GC cells
(12-16). However, NPPB, which blocks multiple
types of chloride channels, is neurotoxic in vivo;
therefore, there is a need for development of a more specific, safe
and simple method to inhibit RVD.
It was recently demonstrated that heat shock
decreases the expression of the water channel aquaporin 5 (AQP5) on
cell membranes by activating autophagic degradation (17). These findings indicate that
temperature may regulate the expression of ion and/or water
transporters on cell membranes, thereby affecting cell volume under
hypotonic shock. Therefore, the aim of the present study was to
investigate the effect of temperature on cell volume and cell death
under conditions of hypoosmolality, and elucidate the underlying
molecular mechanisms, in order to determine whether low temperature
can enhance the cytocidal effect of hypotonic solutions in GC.
Materials and methods
Cell culture and materials
The human GC cell lines NUGC4, MKN45 and KATO-III
were obtained from the RIKEN Cell Bank. Cells were grown in
RPMI-1640 medium (Nacalai Tesque) supplemented with 100 μ g/ml
penicillin, 100 µg/ml streptomycin and 10% fetal bovine serum. The
cells were cultured in flasks or dishes in a humidified incubator
at 37°C under 5% CO2 in air.
Mouse monoclonal anti-LRRC8A (1:1,000 for western
blotting; ab157489), cystic fibrosis transmembrane conductance
regulator (CFTR) (1:1,000 for western blotting; ab2789, rabbit
monoclonal anti-AQP5 (1:1,000 for western blotting; ab92328), and
Na+/K+-ATPase antibodies (1:20,000 for
western blotting; ab76020) were purchased from Abcam. Mouse
monoclonal anti-AQP1 antibody (1:1,000 for western blotting;
sc-32737) was obtained from Santa Cruz Biotechnology, Inc. Mouse
monoclonal β-actin (ACTB) antibody (1:2,000 for western blotting;
A5441) was purchased from Sigma Aldrich; Merck KGaA. Horseradish
peroxidase (HRP)-conjugated anti-rabbit (7074S) or mouse (7076S)
secondary antibodies (1:2,000 for western blotting) were purchased
from Cell Signaling Technology, Inc.
NaCl isotonic and hypotonic
solutions
A 140 mM NaCl isotonic solution, containing 140 mM
NaCl, 5.0 mM KCl, 1.0 mM CaCl2, 1.0 mM MgCl2,
5.0 mM glucose and 10 mM HEPES, was prepared. The pH of each
solution was adjusted to 7.4 with NaOH. Autoclaved Milli-Q water
was used for our DW working solution. To analyze changes in the
volume of cells subjected to hypotonic shock, hypotonic 1/4 NaCl
solution was prepared by diluting stock NaCl solution 4-fold with
DW.
Measurement of cell volume changes in GC
cells after hypotonic shock using a high-resolution flow
cytometer
Cell volume measurements were performed using a
high-resolution flow cytometer (Cell Lab Quanta; Beckman Coulter,
Inc.), as previously described (12-16).
This flow cytometer was designed to measure the electronic volume
(EV) of a cell, and EV data of >10,000 cells were collected and
analyzed using Quanta control software (Beckman Coulter, Inc.). GC
cells grown in culture flasks were detached using trypsin-EDTA and
centrifuged at 180 x g at room temperature for 3 min. A total of
1.0x106 pelleted cells were then suspended in 1 ml of
hypotonic NaCl solution at various temperatures to induce hypotonic
shock. The suspended solution was subsequently displaced into a
Vi-CELLTM Sample Cup (Beckman Coulter, Inc.) and cell volume was
measured at 1, 5, 10, 20 and 30 min after exposure to each
solution. The cell suspension in the isotonic NaCl solution was
used as a control sample without hypotonic shock (0 min).
Re-incubation of GC cells after exposure
to DW
GC cells grown in culture flasks were detached using
trypsin-EDTA and centrifuged at 180 x g at room temperature for 3
min. A total of 2.0x105 pelleted GC cells were then
suspended in DW and incubated for 1 min at 37°C or 24°C.
Subsequently, the suspension was centrifuged at 180 x g at room
temperature for 3 min, and the pelleted cells were re-suspended in
culture medium and seeded on Costar 6-well plates (Corning Inc.).
Approximately 48-72 h after plating, the cells were detached from
the plates in trypsin-EDTA solution, and a viable cell count was
performed using Trypan blue and the Countess Automated Cell Counter
(Invitrogen; Thermo Fisher Scientific, Inc.).
DW was used for the re-incubation experiment, as
severe hypotonicity was required to analyze the cytocidal effects.
On the other hand, mild hypotonicity was used for RVD analysis, as
RVD is a physiological phenomenon observed only in viable cells.
Different conditions were set up according to the purpose of the
experiments, such as functional and survival analyses.
Protein isolation
Cells were lysed with M-PER lysis buffer
supplemented with Halt protease and phosphatase inhibitor cocktail
(Thermo Fisher Scientific, Inc.), sonicated, and centrifuged at
20,000 x g at 4°C for 10 min to obtain supernatants, which
contained total protein. The Pierce Cell Surface Protein Isolation
Kit (Pierce; Thermo Fisher Scientific, Inc.) was used to isolate
cell surface proteins according to the manufacturer's protocol.
Western blotting
Protein concentrations were measured using a Protein
Assay Rapid kit (Wako Pure Chemical Industries, Ltd.). Cell lysates
containing equal amounts of protein were separated by SDS-PAGE with
7.5 or 10% gels and then transferred onto PVDF membranes (Merck
KGaA). The membranes were probed with the indicated antibodies, and
proteins were detected by the ECL Plus Western Blotting Detection
system (GE Healthcare). The primary ACTB antibody was used as a
loading control for whole lysates (17). The primary
Na+/K+-ATPase antibody and the primary
E-cadherin antibody were used as a loading control for cell
membrane proteins (17-20).
Immunofluorescence staining
NUGC4 cells were cultured on SPL 8-chamber cell
culture slides (SPL Life Science) for 24 h. To compare protein
expression between cells under normal conditions and those under
low temperature for immunofluorescence staining, the cells were
incubated at 37°C or 24°C for 12 h in 5% CO2. The cells
were subsequently fixed with 4% paraformaldehyde at room
temperature for 20 min, permeabilized in 0.25% Triton X-100 in
phosphate-buffered saline (PBS), and incubated in blocking buffer
containing 1% bovine serum albumin. The cells were then incubated
with anti-LRRC8A or anti-AQP5 antibody at room temperature for 1 h.
After three washes in PBS, the cells were incubated with Alexa
Fluor 488-labeled goat anti-rabbit secondary antibodies at room
temperature for 1 h. After three washes in PBS, the cells were
incubated with rhodamine phalloidin and
40,6-diamidino-2-phenylindole (DAPI) for 30 min. The slides were
then mounted using Vectashield Mounting Medium (Vector
Laboratories, Inc.; Maravai LifeSciences). The distribution of
LRRC8A and AQP5 proteins was examined under a BZ-X700 microscope
(Keyence Corporation).
siRNA transfection
Cells were transfected with 12 nmol/l LRRC8A siRNA
(Stealth RNAiTM siRNA, cat. no. HSS125512, Invitrogen; Thermo
Fisher Scientific, Inc.) using the Lipofectamine RNAiMAX reagent
(Invitrogen; Thermo Fisher Scientific, Inc.) according to the
manufacturer's instructions. Medium containing siRNA was replaced
with fresh medium after 24 h. The control siRNA provided (Stealth
RNAiTM siRNA Negative Control; Invitrogen; Thermo Fisher
Scientific, Inc.) was used as a negative control.
Overexpression study
Control-HaloTagR plasmid (Promega Corporation, cat.
no. G6591) and AQP5-HaloTagR plasmid were transfected into NUGC4
cells using FuGENE HD transfection reagent (Promega Corporation,
cat. no. E2311) according to the manufacturer's instructions.
Transfection of vector was confirmed by fluorescence microscopy for
HaloTag® fusion protein stained with the
tetramethylrhodamine-conjugated HaloTag® ligand (Promega
Corporation, cat. no. G8252) according to the manufacturer's
protocol. After passaging cells, AQP5-expressing cells were used to
measure cell volume.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
Total RNA was extracted using an RNeasy kit
(Qiagen). mRNA expression was measured by qPCR (7300 Real-Time PCR
system; Applied Biosystems; Thermo Fisher Scientific, Inc.) using
TaqMan gene expression assays (Applied Biosystems; Thermo Fisher
Scientific, Inc.) according to the manufacturer's instructions. The
expression levels of LRRC8A (Hs01555916_m1) and AQP5
(Hs00387048_m1) were measured. The expression of each gene was
normalized against the housekeeping gene ACTB (Hs01060665_g1;
Applied Biosystems; Thermo Fisher Scientific, Inc.). Each assay was
performed in triplicate.
Statistical analysis
Results are expressed as means ± standard error of
the mean. Statistical analyses were performed using Student's
t-test. Differences were considered statistically significant when
the P-value was <0.05. These analyses were performed using the
statistical software JMP (version 10, SAS Institute, Inc.).
Results
Cell volume changes in GC cells after
hypotonic shock at various temperatures
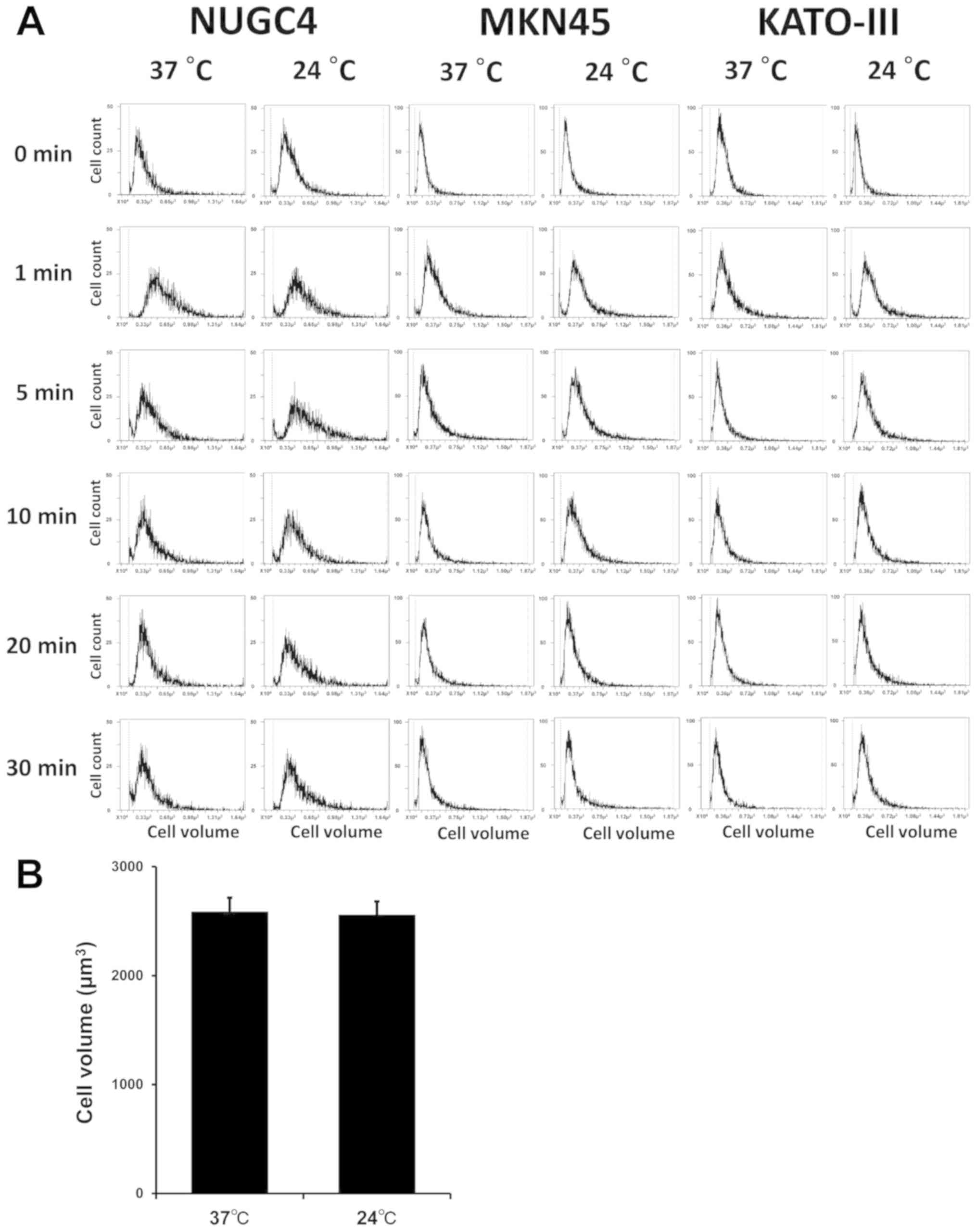
To analyze serial cell volume changes in GC cells
after hypotonic shock at various temperatures, cell volume and cell
counts were simultaneously assessed after exposure to 1/4 NaCl
solution using Cell Lab Quanta. The results for NUGC4, MKN45 and
KATO-III cells before and after hypotonic shock at 37°C (normal
temperature) or 24°C (low temperature) are shown in Fig. 1A. After exposure to the hypotonic
buffers, the population shifted to the right, indicating cell
swelling by water influx. During exposure to hypotonicity, the cell
volume decreased gradually, returning to the initial volume despite
the continued presence of extracellular hypotonicity, which
suggests the occurrence of RVD. It was also confirmed that low
temperature did not affect cell volume in isotonic NaCl solution
(Fig. 1B).
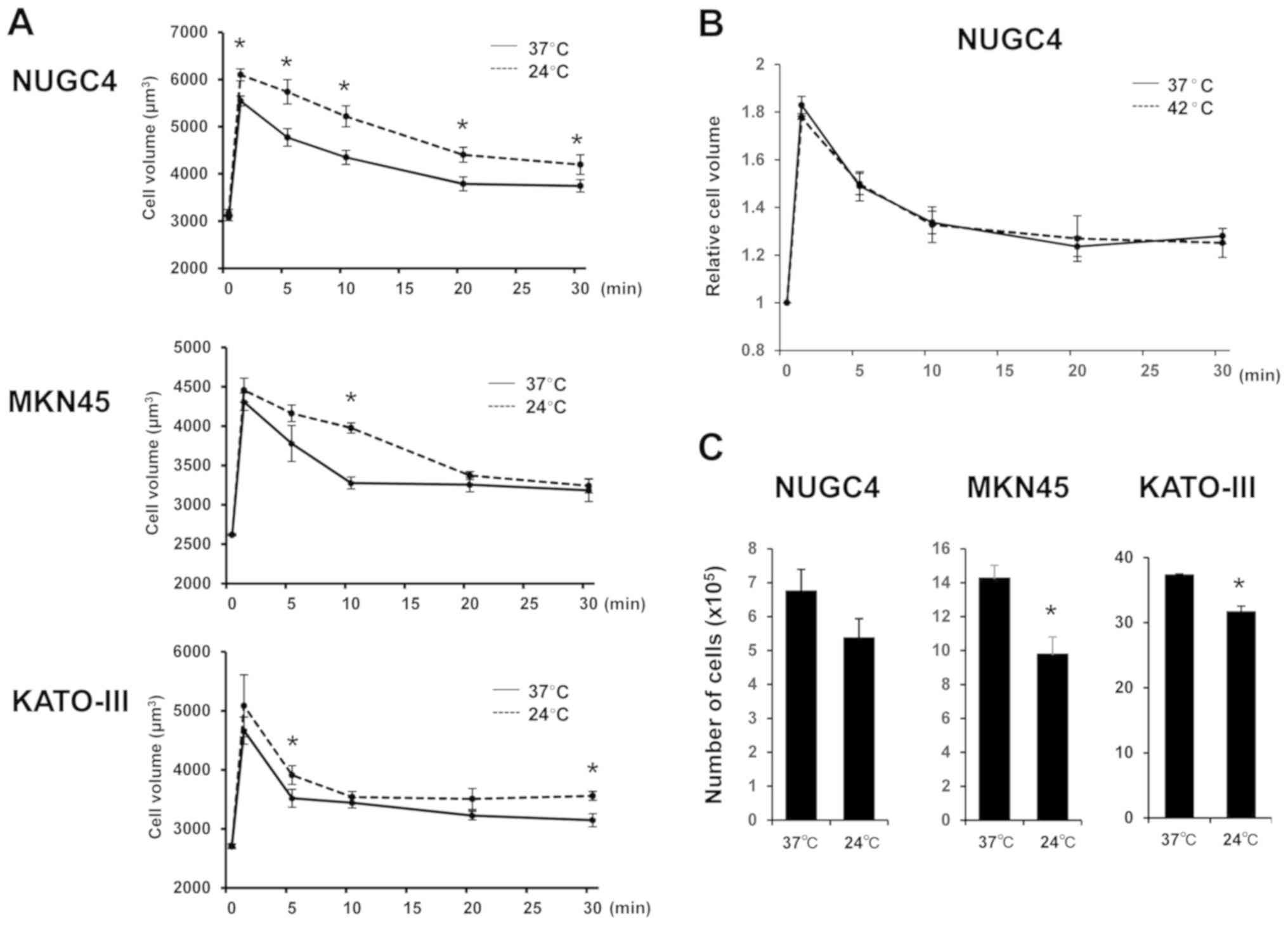
To investigate whether temperature affects cell
volume changes in GC cells after hypotonic shock, mean cell volumes
were measured following exposure to hypotonic NaCl solution at 37°C
or 24°C using Cell Lab Quanta. Serial changes in the mean volume of
GC cells following their exposure to hypotonic solutions at normal
or low temperature are shown in Fig.
2A. The volume of GC cells treated with hypotonic solution at
normal temperature initially increased for 1 min, and subsequently
decreased from 5 min after the start of treatment onwards,
indicating RVD. By contrast, low temperature enhanced initial cell
swelling and markedly slowed the decrease in cell volume following
cell swelling induced by hypotonic shock in NUGC4, MKN45 and
KATO-III cells. The results under low temperature suggested that
these effects were induced by an increase in initial water influx
and inhibition of RVD. On the other hand, high temperature (42°C)
did not significantly affect cell volume after hypotonic shock in
GC cells (Fig. 2B).
To confirm the effect of low temperature on the
cytocidal effects of hypotonic shock induced by DW on GC cells,
suspended GC cell lines were re-incubated following exposure to DW
at 37°C or 24°C, and cultured cell number was counted 48-72 h
later. As shown in Fig. 2C, the
number of surviving cells after severe hypotonic shock at 24°C was
lower compared with that at 37°C, suggesting that low temperature
enhanced the cytocidal effects of hypotonic shock.
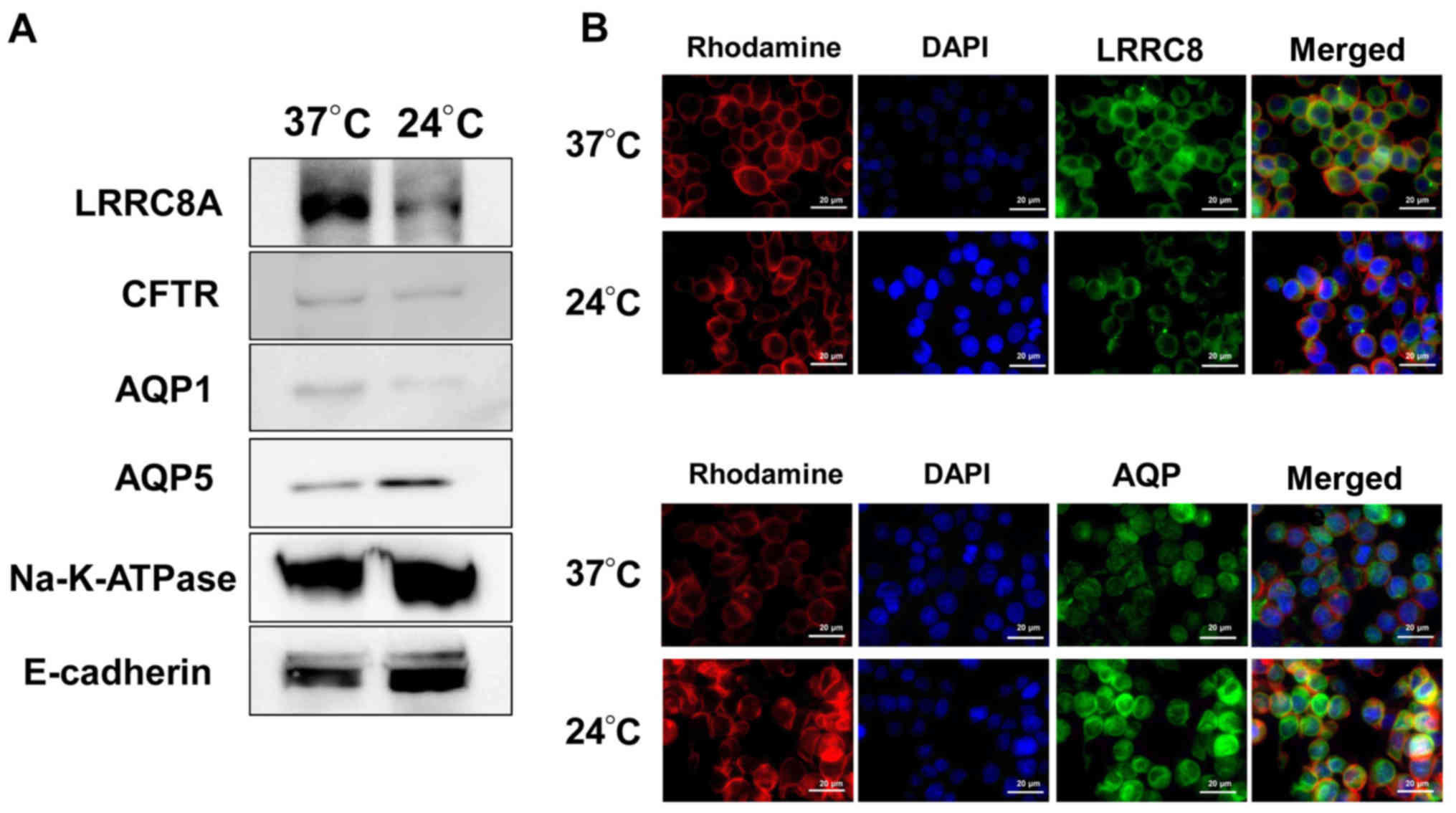
Effect of low temperature on the
expression of membrane transporters in GC cells
To examine the mechanism by which low temperature
affects cell volume change after hypotonic shock, key membrane
transporters, such as Cl- channels and water channels,
that are the major cell volume regulators in hypotonicity, were
investigated. Two Cl- channels (CFTR and LRRC8A) and two
water channels (AQP1 and AQP5) were analyzed. The cell surface
proteins of cells incubated at 37°C or 24°C for 12 h were isolated,
and western blotting of cellular membrane proteins was performed.
Na+/K+-ATPase and E-cadherin were examined as
loading controls in the cell membrane. The expression of LRRC8A in
the cell membrane fraction of cells incubated at low temperature
was lower compared with that in cells at normal temperature
(Fig. 3A). By contrast, the
expression of AQP5 in the cell membrane was higher at low
temperature (Fig. 3A). The
expression levels of CFTR and AQP1 in the cell membrane did not
differ between high and low temperatures.
The expression and distribution of LRRC8A and AQP5
in NUGC4 cells treated at low temperature were examined using
immunofluorescence. In order to identify their localizations more
clearly, the cytoskeleton was labeled with rhodamine and the
nucleus was labeled with DAPI. The staining intensity of LRRC8A in
the cell membrane and cytoplasm of cells incubated at low
temperature was lower compared with that in cells at normal
temperature (Fig. 3B). By
contrast, the expression of AQP5 in the cell membrane and cytoplasm
was higher at low temperature (Fig.
3B). These results suggested that low temperature regulated the
expression and distribution of the membrane transport proteins
LRRC8A and AQP5.
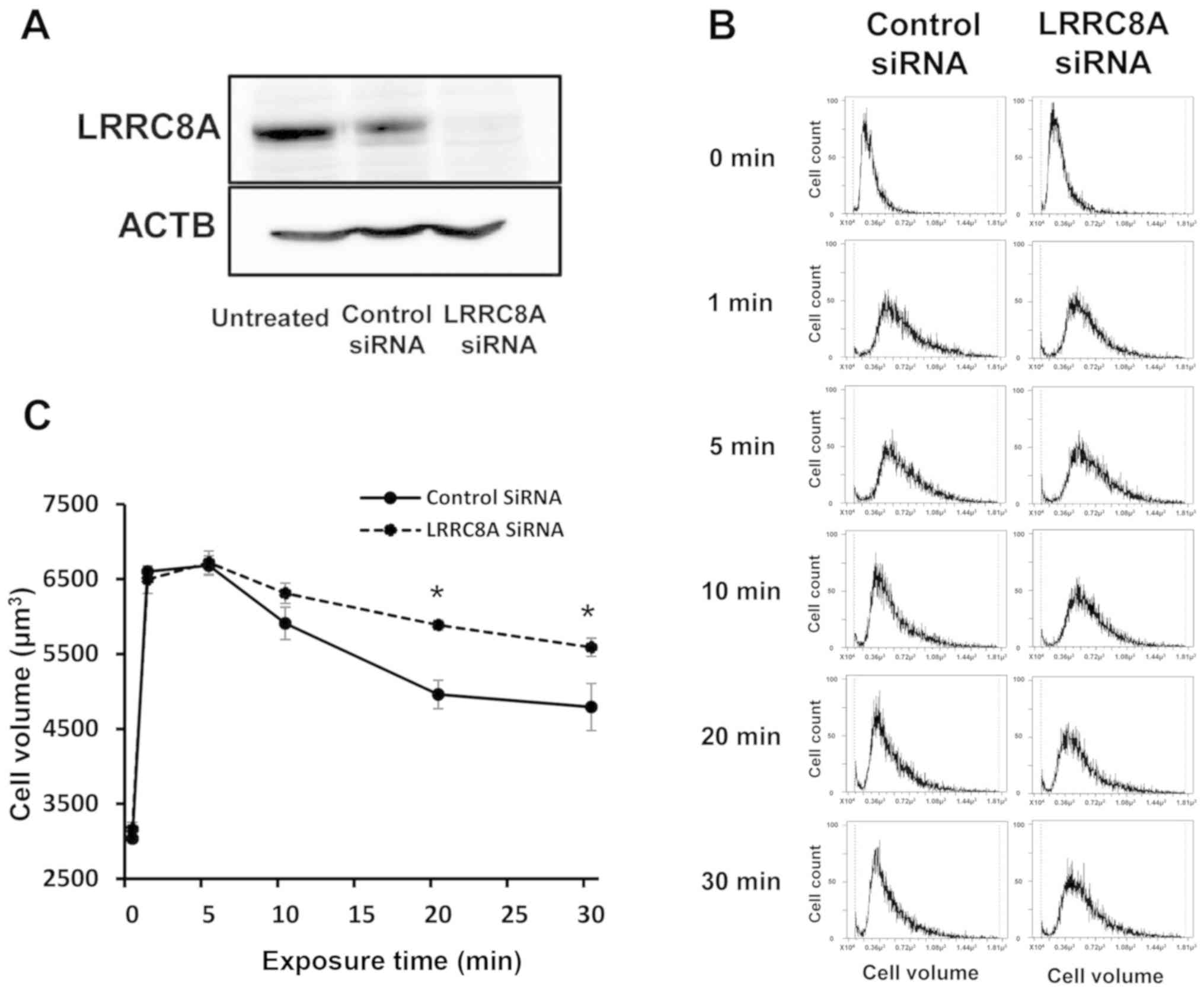
Role of LRRC8A on hypotonicity-induced
volume changes in GC cells
Knockdown experiments using LRRC8A siRNA were
conducted in NUGC4 cells to investigate the effects of LRRC8A
depletion on volume change after hypotonic shock. LRRC8A siRNA
effectively reduced LRRC8A protein levels in NUGC4 cells (Fig. 4A). The primary ACTB antibody was
used as a loading control for whole lysates (Fig. 4A). The depletion of LRRC8A markedly
slowed the decrease in cell volume following cell swelling by
hypotonic shock (Fig. 4B and C),
which suggests that this effect was induced by the inhibition of
RVD. These results indicate that low temperature may suppress RVD
after hypotonic shock via inhibition of LRRC8A expression.
Role of AQP5 on hypotonicity-induced
volume changes in GC cells
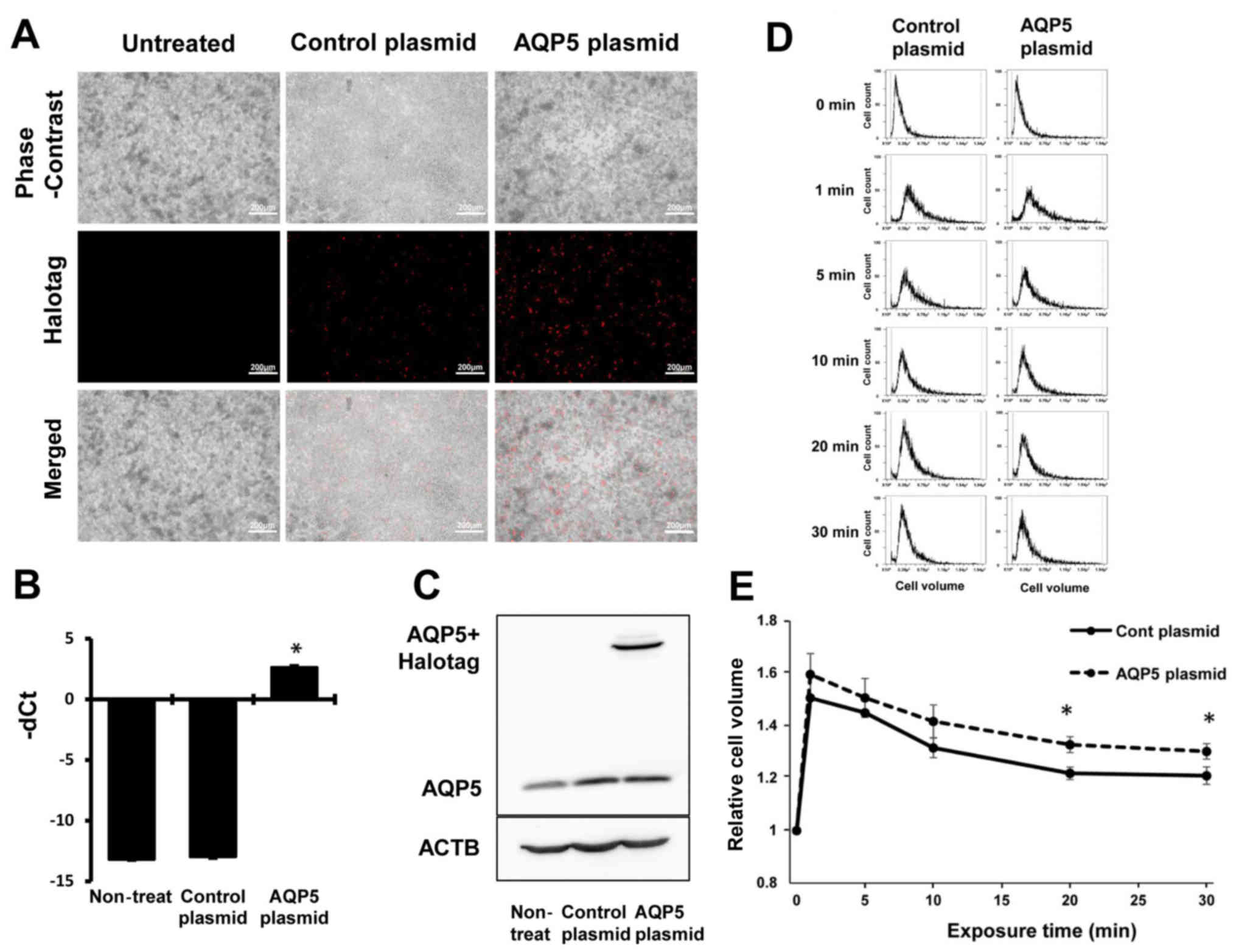
AQP5 was overexpressed in NUGC4 cells and the
effects of AQP5 overexpression on cell volume change after
hypotonic shock were investigated. Cells transfected with
Control-HaloTag® plasmid and AQP5-HaloTag®
plasmid were stained red (Fig.
5A), and AQP5 plasmid increased the AQP5 mRNA levels (Fig. 5B) and AQP5 protein levels plus
HaloTag (Fig. 5C). The primary
ACTB antibody was used as a loading control for whole lysates
(Fig. 5C).
Cell volume changes after hypotonic shock were
analyzed in GC cells transfected with Control-HaloTag®
plasmid or AQP5-HaloTag® plasmid. AQP5 overexpression
increased the initial cell swelling from 1 min after hypotonic
shock and significantly increased the final cell volume (Fig. 5DE), suggesting that this effect was
induced by an increased water influx. These results indicated that
low temperature may increase water influx immediately after
hypotonic shock via an increase in AQP5 expression.
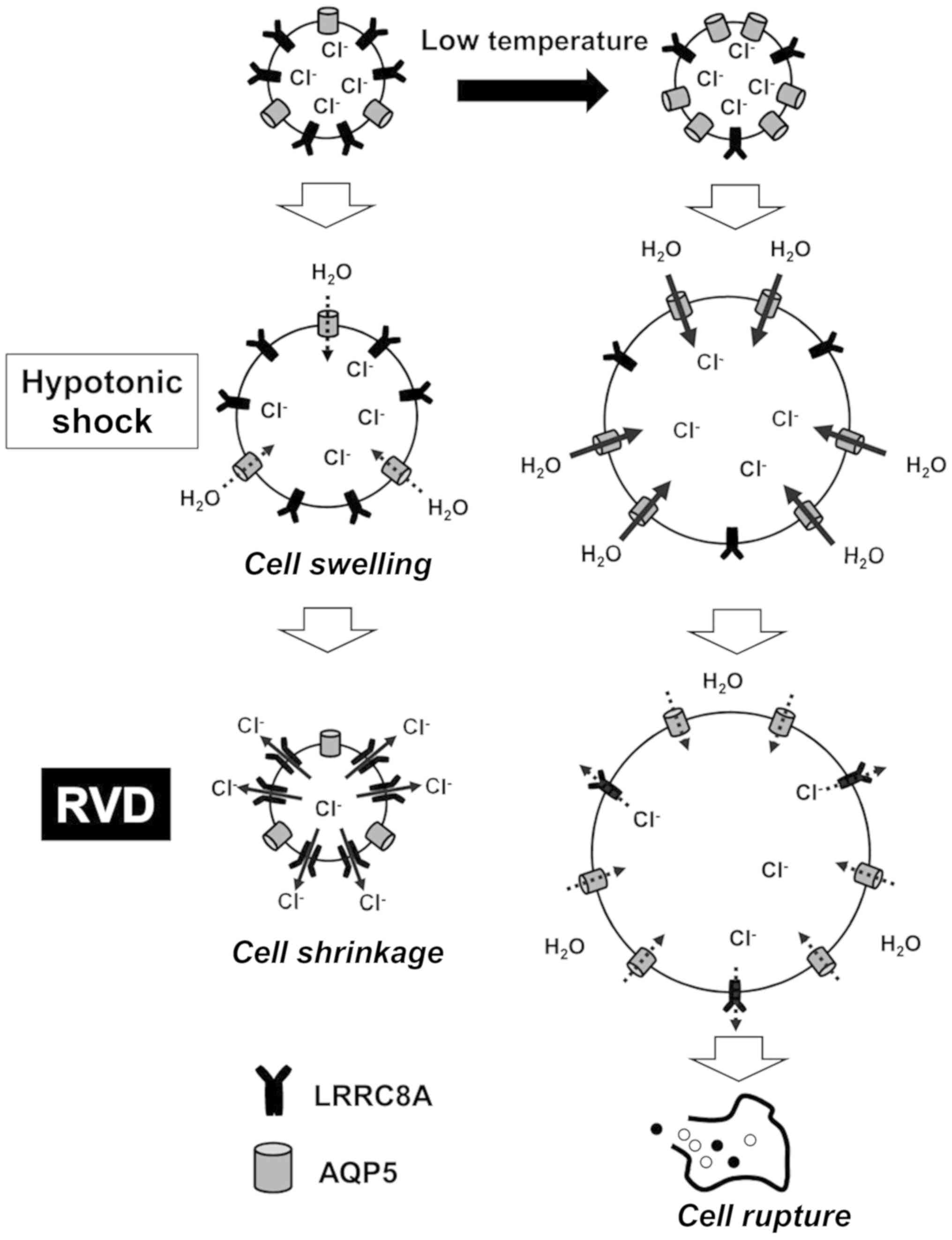
These mechanisms are summarized in Fig. 6. Low temperature was shown to
affect the expression and distribution of membrane transport
proteins in GC cells. In particular, LRRC8A expression was
decreased, and AQP5 expression was increased at low temperature. In
the initial phase at low temperature, hypotonicity-induced cell
swelling was enhanced by increased water influx via AQP5. In the
next phase, low temperature blocked RVD by inhibiting
Cl- efflux via LRRC8A, which suggests that low
temperature enhanced the cytocidal effects of hypotonic
solutions.
Discussion
Several in vitro and in vivo studies
have investigated the cytocidal effects of hypotonic stress on
cancer cells (6-9). We examined the changes in the
morphology and volume of cancer cells subjected to hypotonic shock
using several methods, such as a differential interference contrast
microscope connected to a digital video camera, and revealed that
DW exposure rapidly increases cell volume followed by cell rupture
in esophageal, gastric, colonic, pancreatic and liver cancer cell
lines (12-16). Our research group also determined
the therapeutic effects of a peritoneal injection of DW into nude
mice for the treatment of peritoneal dissemination of GC (21). Furthermore, several clinical
studies have indicated that the use of DW lavage during surgery for
cancer may delay tumor recurrence and improve survival, with
minimal cost (8,22-24).
In addition, several clinical trials, including some for gastric
cancer, have demonstrated the efficacy of the administration of
hypotonic intraperitoneal cisplatin during surgery (25-27).
Recently, Ohki et al reported that washing with DW during
endoscopic examination reduces free GC cell exfoliation into the
stomach lumen (28). The results
of these studies clearly demonstrate the importance of osmolality
in cancer treatments.
Even under hypotonic stress, cells can regulate
their own volume after transient osmotic swelling by a mechanism
referred to as RVD, which mainly occurs by KCl efflux induced by
parallel activation of K+ and Cl- channels
(10,11,29,30).
In the present study, it was demonstrated that low temperature
inhibited RVD in GC cells. Similarly, Souza and Boyle reported that
a decrease in temperature inhibited RVD in chick embryo
cardiomyocytes (31). Although
those studies demonstrated that calcium signaling is important, we
herein focused on the regulatory mechanism via the expression of
membrane transporters, such as Cl- channels and water
channels. A particular type of Cl- channel,
volume-regulated anion channel (VRAC) is involved in RVD. LRRC8A is
a component of VRAC, and is essential for cell volume regulation
(32). We observed that low
temperature regulated RVD by decreasing the expression of LRRC8A in
the cell membrane, and that depletion of LRRC8A with siRNA markedly
slowed RVD in GC cells. A number of anion channel types associated
with cell volume changes are classified into volume-activated anion
channels (VAACs) and volume-correlated anion channels (VCACs)
(33). VAACs can be directly
activated by cell swelling, and include VRAC and the maxi-anion
channel (Maxi-Cl) (33). Maxi-Cl
is also directly involved in the RVD process by providing the
volume-regulatory pathway for anion efflux or indirectly by
releasing ATP (33). Although
further investigations are required, it is suggested that other
types of Cl- channels, such as Maxi-Cl, may also involve
the mechanism found in the present study.
AQPs are a family of transmembrane proteins that
regulate transcellular water movement and play a role in cell
volume regulation, and 13 of these subtypes are expressed in
mammals (34-36). Mola et al demonstrated that
AQP-mediated fast swelling kinetics trigger RVD using biophysical
techniques to measure water flux through the plasma membrane of
wild-type and AQP-knockout astrocytes and in an astrocyte cell line
transfected with AQPs (37). They
found that swelling in the presence of AQP is fast, whereas
swelling in the absence of AQP is slow and depends on the
composition of the lipid bilayer through which water influx occurs
by simple diffusion. AQP5 overexpression was found to enhance
initial cell swelling via increased water influx; we previously
investigated heat shock-induced changes in AQP5 expression on
cellular membranes and found that AQP5 expression was decreased via
autophagic degradation in hepatocellular carcinoma cell lines
(17). In addition, AQP5 knockdown
and heat shock similarly decreased cell volume (17). However, we found completely
opposite results investigating the effect of low temperature, which
suggests that low temperature-induced overexpression of AQP5 may
increase cell volume under hypotonic stress.
Ion channels play critical roles in various cancer
cells, and physiological factors in cells represent novel targets
for cancer therapy (38). Ion
channels and transporters are important in GC cells (38-42),
and Cl- transport is particularly important for the
regulation of osmolality (5,12,18,43).
We previously investigated hypotonicity-induced cell volume changes
by controlling RVD. In particular, we challenged GC cells with
NPPB, a Cl- channel blocker, to increase cell volume by
inhibiting RVD, and revealed that the cytocidal effects of a
hypotonic solution were enhanced in GC cells (12). Furthermore, RVD was inhibited by
quinine hydrochloride (Quin), which blocks K+ channels.
Treatment of GC cells with Quin enhanced the cytocidal effects of
hypotonic shock by inhibiting RVD (43). The present study demonstrated that
low temperature enhanced the cytocidal effects of hypotonic shock
by inhibiting RVD. This novel and simple strategy for peritoneal
lavage using low-temperature DW may overcome several problems, such
as drug toxicity, at a low cost. Although the effects of peritoneal
lavage with saline at low temperature have been investigated using
an in vivo model (44),
there are no reports on peritoneal hypothermia under hypotonic
conditions. We consider that our findings, derived from a cell
culture model, are also applicable in animal models, and further
in vivo investigations may lead to the use of peritoneal
hypotonic hypothermia as a prevention against peritoneal
dissemination.
In summary, the present study demonstrated that low
temperature during hypotonic stimulation inhibited RVD and enhanced
the cytocidal effects on GC cells. The analysis of membrane
transporters indicated that LRRC8A expression was decreased,
whereas AQP5 expression was increased by stimulation with low
temperature. Knockdown and overexpression experiments suggested
that the effects of low temperature were induced by an initial
increase of water influx via AQP5 and inhibition of Cl-
efflux via LRRC8A. Further investigation of the physiological and
molecular roles of low temperature under hypotonic conditions may
uncover its potential as a novel lavage method to reduce peritoneal
recurrence of GC following curative surgery.
Acknowledgments
Not applicable.
Funding
The present study was supported by Grants-in-Aid for
Scientific Research (C) (17K10602, 17K10710, 18K08628, 18K08689)
from the Japan Society for the Promotion of Science.
Availability of data and materials
All data generated or analyzed during the present
study are included in this published article.
Authors' contributions
AS and YY designed the study, wrote the manuscript
and performed the majority of the experiments; TK, MK, KS, TA, HK,
SK, TK, HF and KO performed cellular physiological and molecular
biological experiments; EO and YM were involved in editing the
manuscript. All the authors have read and approved the final
version of this manuscript for publication.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Lim L, Michael M, Mann GB and Leong T:
Adjuvant therapy in gastric cancer. J Clin Oncol. 23:6220–6232.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rajdev L: Treatment options for surgically
resectable gastric cancer. Curr Treat Options Oncol. 11:14–23.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hartgrink HH, Jansen EP, van Grieken NC
and van de Velde CJ: Gastric cancer. Lancet. 374:477–490. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Emoto S, Yamaguchi H, Kishikawa J,
Yamashita H, Ishigami H and Kitayama J: Antitumor effect and
pharmacokinetics of intraperitoneal NK105, a nanomicellar
paclitaxel formulation for peritoneal dissemination. Cancer Sci.
103:1304–1310. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Shiozaki A, Ichikawa D, Kosuga T, Marunaka
Y and Otsuji E: Regulation of osmolality for cancer treatment. J
Physiol Sci. 67:353–360. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Park KG, Chetty U, Scott W and Miller W:
The activity of locally applied cytotoxics to breast cancer cells
in vitro. Ann R Coll Surg Engl. 73:96–99. 1991.PubMed/NCBI
|
|
7
|
Huguet EL and Keeling NJ: Distilled water
peritoneal lavage after colorectal cancer surgery. Dis Colon
Rectum. 47:2114–2119. 2004. View Article : Google Scholar
|
|
8
|
Zhou SJ, Zhang EL, Liang BY, Zhang ZY,
Chen XP and Huang ZY: Distilled water lavage during surgery
improves long-term outcomes of patients with ruptured
hepatocellular carcinoma. J Gastrointest Surg. 19:1262–1270. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fechner G, Pocha K, Schmidt D and Müller
SC: Reducing recurrence and costs in superficial bladder cancer:
Preclinical evaluation of osmotic cytolysis by distilled water vs.
mitomycin. Int J Clin Pract. 60:1178–1180. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Caplanusi A, Kim KJ, Lariviere E, Van
Driessche W and Jans D: Swelling-activated K+ efflux and
regulatory volume decrease efficiency in human bronchial epithelial
cells. J Membr Biol. 214:33–41. 2006. View Article : Google Scholar
|
|
11
|
Miyazaki H, Shiozaki A, Niisato N and
Marunaka Y: Physiological significance of hypotonicity-induced
regulatory volume decrease: Reduction in intracellular
Cl- concentration acting as an intracellular signaling.
Am J Physiol Renal Physiol. 292:F1411–F1417. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Iitaka D, Shiozaki A, Ichikawa D, Kosuga
T, Komatsu S, Okamoto K, Fujiwara H, Ishii H, Nakahari T, Marunaka
Y, et al: Blockade of chloride ion transport enhances the cytocidal
effect of hypotonic solution in gastric cancer cells. J Surg Res.
176:524–534. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kosuga T, Shiozaki A, Ichikawa D, Fujiwara
H, Komatsu S, Iitaka D, Tsujiura M, Morimura R, Takeshita H, Nagata
H, et al: Pleural lavage with distilled water during surgery for
esophageal squamous cell carcinoma. Oncol Rep. 26:577–586.
2011.PubMed/NCBI
|
|
14
|
Nako Y, Shiozaki A, Ichikawa D, Komatsu S,
Konishi H, Iitaka D, Ishii H, Ikoma H, Kubota T, Fujiwara H, et al:
Enhancement of the cytocidal effects of hypotonic solution using a
chloride channel blocker in pancreatic cancer cells. Pancreatology.
12:440–448. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Takemoto K, Shiozaki A, Ichikawa D,
Komatsu S, Konishi H, Nako Y, Murayama Y, Kuriu Y, Nakanishi M,
Fujiwara H, et al: Evaluation of the efficacy of peritoneal lavage
with distilled water in colorectal cancer surgery: In vitro and in
vivo study. J Gastroenterol. 50:287–297. 2015. View Article : Google Scholar
|
|
16
|
Kudou M, Shiozaki A, Kosuga T, Ichikawa D,
Konishi H, Morimura R, Komatsu S, Ikoma H, Fujiwara H, Okamoto K,
et al: Inhibition of regulatory volume decrease enhances the
cytocidal effect of hypotonic shock in hepatocellular carcinoma. J
Cancer. 7:1524–1533. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kudou M, Shiozaki A, Kosuga T, Shimizu H,
Ichikawa D, Konishi H, Morimura R, Komatsu S, Ikoma H, Fujiwara H,
et al: Heat shock exerts anticancer effects on liver cancer via
autophagic degradation of aquaporin 5. Int J Oncol. 50:1857–1867.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bünger S, Roblick UJ and Habermann JK:
Comparison of five commercial extraction kits for subsequent
membrane protein profiling. Cytotechnology. 61:153–159. 2009.
View Article : Google Scholar
|
|
19
|
Liu Y, Belkina NV, Park C, Nambiar R,
Loughhead SM, Patino-Lopez G, Ben-Aissa K, Hao JJ, Kruhlak MJ, Qi
H, et al: Constitutively active ezrin increases membrane tension,
slows migration, and impedes endothelial transmigration of
lymphocytes in vivo in mice. Blood. 119:445–453. 2012. View Article : Google Scholar :
|
|
20
|
Kédinger V, Alpy F, Baguet A, Polette M,
Stoll I, Chenard MP, Tomasetto C and Rio MC: Tumor necrosis factor
receptor-associated factor 4 is a dynamic tight junction-related
shuttle protein involved in epithelium homeostasis. PLoS One.
3:e35182008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Shiozaki A, Ichikawa D, Takemoto K, Nako
Y, Nakashima S, Shimizu H, Kitagawa M, Kosuga T, Konishi H, Komatsu
S, et al: Efficacy of a hypotonic treatment for peritoneal
dissemination from gastric cancer cells: An in vivo evaluation.
BioMed Res Int. 2014:7070892014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Whiteside OJ, Tytherleigh MG, Thrush S,
Farouk R and Galland RB: Intra-operative peritoneal lavage--who
does it and why? Ann R Coll Surg Engl. 87:255–258. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lin CH, Hsieh HF, Yu JC, Chen TW, Yu CY
and Hsieh CB: Peritoneal lavage with distilled water during liver
resection in patients with spontaneously ruptured hepatocellular
carcinomas. J Surg Oncol. 94:255–256. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chang YM, Hsu KF, Yu JC, Chan DC, Chen CJ,
Chen TW, Hsieh CB and Hsieh HF: Distilled water peritoneal lavage
in patients with rupture hepatocellular carcinoma.
Hepatogastroenterology. 60:140–143. 2013.PubMed/NCBI
|
|
25
|
Ichinose Y, Tsuchiya R, Koike T, Yasumitsu
T, Nakamura K, Tada H, Yoshimura H, Mitsudomi T, Nakagawa K, Yokoi
K, et al: A prematurely terminated phase III trial of
intraoperative intra-pleural hypotonic cisplatin treatment in
patients with resected non-small cell lung cancer with positive
pleural lavage cytology: The incidence of carcinomatous pleuritis
after surgical intervention. J Thorac Cardiovasc Surg. 123:695–699.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tsujitani S, Fukuda K, Saito H, Kondo A,
Ikeguchi M, Maeta M and Kaibara N: The administration of hypotonic
intraperitoneal cisplatin during operation as a treatment for the
peritoneal dissemination of gastric cancer. Surgery. 131(Suppl):
S98–S104. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Seto T, Ushijima S, Yamamoto H, Ito K,
Araki J, Inoue Y, Semba H and Ichinose Y; Thoracic Oncology Group:
Intrapleural hypotonic cisplatin treatment for malignant pleural
effusion in 80 patients with non-small-cell lung cancer: A
multi-institutional phase II trial. Br J Cancer. 95:717–721. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ohki A, Abe N, Yoshimoto E, Hashimoto Y,
Takeuchi H, Nagao G, Masaki T, Mori T, Ohkura Y and Sugiyama M:
Gastric washing by distilled water can reduce free gastric cancer
cells exfoliated into the stomach lumen. Gastric Cancer.
21:998–1003. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
McManus ML, Churchwell KB and Strange K:
Regulation of cell volume in health and disease. N Engl J Med.
333:1260–1266. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Strange K, Emma F and Jackson PS: Cellular
and molecular physiology of volume-sensitive anion channels. Am J
Physiol. 270:C711–C730. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Souza MM and Boyle RT: A moderate decrease
in temperature inhibits the calcium signaling mechanism(s) of the
regulatory volume decrease in chick embryo cardiomyocytes. Braz J
Med Biol Res. 34:137–141. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Qiu Z, Dubin AE, Mathur J, Tu B, Reddy K,
Miraglia LJ, Reinhardt J, Orth AP and Patapoutian A: SWELL1, a
plasma membrane protein, is an essential component of
volume-regulated anion channel. Cell. 157:447–458. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Okada Y, Okada T, Sato-Numata K, Islam MR,
Ando-Akatsuka Y, Numata T, Kubo M, Shimizu T, Kurbannazarova RS,
Marunaka Y, et al: Cell volume-activated and volume-correlated
anion channels in mammalian cells: Their biophysical, molecular,
and pharmacological properties. Pharmacol Rev. 71:49–88. 2019.
View Article : Google Scholar
|
|
34
|
Ishibashi K, Hara S and Kondo S: Aquaporin
water channels in mammals. Clin Exp Nephrol. 13:107–117. 2009.
View Article : Google Scholar
|
|
35
|
Day RE, Kitchen P, Owen DS, Bland C,
Marshall L, Conner AC, Bill RM and Conner MT: Human aquaporins:
Regulators of transcellular water flow. Biochim Biophys Acta.
1840:1492–1506. 2014. View Article : Google Scholar
|
|
36
|
Hoffmann EK, Lambert IH and Pedersen SF:
Physiology of cell volume regulation in vertebrates. Physiol Rev.
89:193–277. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Mola MG, Sparaneo A, Gargano CD, Spray DC,
Svelto M, Frigeri A, Scemes E and Nicchia GP: The speed of swelling
kinetics modulates cell volume regulation and calcium signaling in
astrocytes: A different point of view on the role of aquaporins.
Glia. 64:139–154. 2016. View Article : Google Scholar :
|
|
38
|
Shiozaki A, Ichikawa D, Otsuji E and
Marunaka Y: Cellular physiological approach for treatment of
gastric cancer. World J Gastroenterol. 20:11560–11566. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Shiozaki A, Miyazaki H, Niisato N,
Nakahari T, Iwasaki Y, Itoi H, Ueda Y, Yamagishi H and Marunaka Y:
Furosemide, a blocker of
Na+/K+/2Cl- cotransporter,
diminishes proliferation of poorly differentiated human gastric
cancer cells by affecting G0/G1 state. J Physiol Sci. 56:401–406.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Miyazaki H, Shiozaki A, Niisato N, Ohsawa
R, Itoi H, Ueda Y, Otsuji E, Yamagishi H, Iwasaki Y, Nakano T, et
al: Chloride ions control the G1/S cell-cycle checkpoint by
regulating the expression of p21 through a p53-independent pathway
in human gastric cancer cells. Biochem Biophys Res Commun.
366:506–512. 2008. View Article : Google Scholar
|
|
41
|
Shiozaki A, Otsuji E and Marunaka Y:
Intracellular chloride regulates the G(1)/S cell cycle progression
in gastric cancer cells. World J Gastrointest Oncol. 3:119–122.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Shiozaki A, Ariyoshi Y, Iitaka D, Kosuga
T, Shimizu H, Kudou M, Konishi T, Shoda K, Arita T, Konishi H, et
al: Functional analysis and clinical significance of sodium iodide
symporter expression in gastric cancer. Gastric Cancer. 22:473–485.
2019. View Article : Google Scholar
|
|
43
|
Kosuga T, Shiozaki A, Kudou M, Yamazato Y,
Ichikawa D, Komatsu S, Konishi H, Okamoto K, Shoda K, Arita T, et
al: Blockade of potassium ion transports enhances
hypoto-nicity-induced cytocidal effects in gastric cancer.
Oncotarget. 8:101394–101405. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Koca YS, Tarhan OR, Kaya S and Gökçe
Ceylan B: Effects of saline lavage temperature on peritoneal
fibrinolysis and adhesion formation. Ulus Travma Acil Cerrahi Derg.
22:1–6. 2016.PubMed/NCBI
|