Immunotherapies utilize monoclonal antibodies
(mAbs), immunological checkpoint blockade (ICB) agents,
cytokine-induced killer cells, tumor-infiltrating lymphocytes
(TILs) and T-cell receptors (TCRs). In recent years, the rapid
development of immunotherapies has produced novel treatment options
for many different types of cancer (1,2). The
most attractive feature of tumor immunotherapy is the ability to
control or eliminate tumors by restarting and maintaining the
tumor-immune cycle in vivo, as well as stimulating and
restoring the body's normal anti-tumor immune response (3). However, in contrast to other adoptive
cell transfer therapies, chimeric antigen receptor (CAR) T-cells
recognize tumor surface-associated antigens directly, independent
of the major histocompatibility complex (MHC) restriction (4). The use of anti-CD19 CAR T-cells for
the treatment of chemotherapy-refractory hematological malignant
tumors has revealed encouraging results, including effective
targeting, killing and persistence (5). Furthermore, its use has provided
novel solutions for immune cell therapy, demonstrating the
tremendous potential for the development and clinical application
of CAR T-cell therapy (6,7). Significant improvements in the
efficacy of CAR T-cell therapy for hematological malignancies have
prompted its development for use in solid tumors (8).
Gastric cancer (GC) is one of the most frequently
diagnosed digestive malignancies and is the third leading cause of
cancer-associated death worldwide (9). According to the CONCORD-3 (10) statistical data of GC obtained from
62 countries in 2010 to 2014 revealed that 29 countries exhibited a
5-year survival rate <30%, occupying 46% of all countries
studied. Furthermore, existing conventional treatments, including
surgery, chemotherapy and radiotherapy, have limited efficacy in
GC; thus, there is an urgent need for novel therapeutic strategies.
In contrast to TCR and ICB immunotherapy, the study of CAR T-cells
is still in its infancy and appears less efficacious for GC.
However, producing an effective CAR T-cell treatment for GC
(11,12) may be possible as the Food and Drug
Administration have approved two second-generation CAR T-cell
therapies, for the treatment of relapsed/refractory B-cell
lymphoma: Kymriah (CD28/CD3ζ costimulatory domain) and Yescarta
(4-1BB/CD3ζ costimulatory domain). Preclinical studies have
demonstrated the anti-tumor efficacy and persistent activity of CAR
T-cells against GC in vitro and in vivo using an
animal xenotransplantation model (13-17).
The current review assessed the potential of CAR
T-cell immunotherapy for patients with GC and discussed the history
of its development, its current status and toxic side effects, as
well as the management of these toxicities.
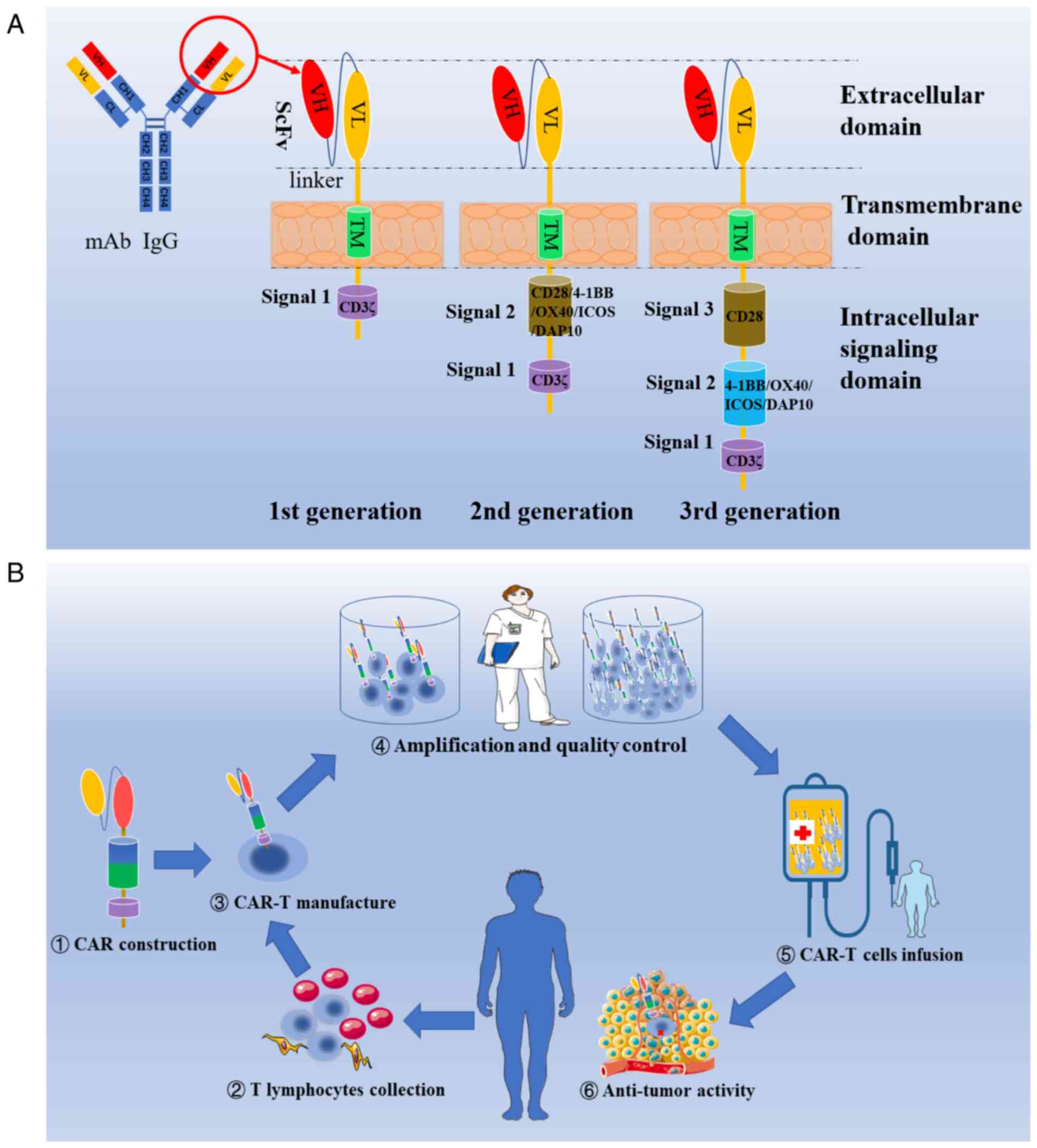
Tumor immunotherapy has been prevalent for >100
years, with CAR T-cell therapy being developed in the last ~30
years. The first-generation CAR, derived from a chimeric TCR, was
pioneered and constructed by Eshhar et al in 1993 (18,19).
First-generation CARs are modular in nature, containing a
single-chain variable fragment (ScFv) and CD3ζ domains, and they
inhibit tumor cell escape by downregulating the expression of MHC
on the surface of tumor cells (20). To address the poor cytokine
production and T-cell expansion observed in first-generation CARs
(21), Finney et al
(22) constructed a
second-generation CAR that incorporated a costimulatory domain. The
superiority of this second-generation CAR in cytokine-secretion and
in T-cell expansion and persistence has been demonstrated in
several studies (23-26) (Fig.
1A). Using second-generation CAR as a foundation, a
third-generation CAR was created, which contained two tandem
costimulatory molecules. The third-generation CAR exhibited
enhanced effector functions and persistence in vivo
(27). However, to further enhance
targeted anti-tumor and trafficking activities of CARs in solid
tumors and to reduce off-target toxicity and immunosuppression,
multiform fourth-generation CARs were constructed using novel
mechanisms, for example, T-cells redirected for universal
cytokine-mediated killing, armored CARs, switchable CARs,
bispecific CARs and CARs incorporating a suicide gene have been
created (28). In addition,
scientists are working to uncover a universal CAR structure to act
against all target cells with an optimal outcome.
CAR is an artificially synthesized membrane protein
composed of three domains: An extracellular antigen-recognition
domain, a transmembrane domain and an intracellular signaling
domain (29) (Fig. 1A). The single-chain variable
fragment (ScFv) is a recombinant polypeptide derived from the heavy
and light chains of a monoclonal antibody, which binds directly to
the tumor surface-associated antigens, independently from MHC
restriction (30). The hinge
region provides ScFv flexibility and is associated with the
target-binding capacity of the CAR (31). The transmembrane domain, primarily
consisting of CD8 or immunoglobulin G4 molecules, enhances CAR
stability and provides a connection between the ectodomain and
endodomain (32). In the
intracellular domain, CD3ζ or Fc receptor γ provides the first
signal for T-cell activation (33). Although the B7-CD28 pathway
provides essential signals for T-cell activation, further studies
have revealed that CD3ζ has a more optimal signaling efficacy
(34,35). Additionally, the endodomain
commonly contains costimulatory signal domains that promote T-cell
proliferation, lymphokine secretion and effector function,
including CD28 (36), inducible
T-cell costimulator (34),
DNAX-activating protein 10 (DAP10) (37), CD134 (OX40) (38) or CD137 (4-1BB) (39), which have also been studied
successively in different generations of CARs (27). CD28 promotes the multiplication of
naïve and CD4+ T-cell subsets, whereas costimulatory
CD137 promotes the proliferation of memory and CD8+
T-cell subsets preferentially, improving persistence (40). CD28 has been demonstrated to
promote the ability of CARs to enhance the resistance of modified
T-cells against regulatory T-cells and to reduce antigen-induced
cell death (41). However, CD137
enhances the metabolic adaptability and memory potential of CAR
T-cells to a greater extent than CD28 (42,43).
Despite the aforementioned costimulatory molecules exhibiting
antigen-dependent immune-cytolysis in vitro, there is still
debate over which costimulatory molecule is most optimal (44). Previous evidence has suggested that
the functional activity induced by T-cell-expressed CARs depends on
the interaction of endogenous signaling moieties (45).
Based on previous clinical applications of adoptive
immunotherapies, including TILs, CAR T-cell therapy was designed
for the treatment of various types of cancer. CAR T-cell therapy is
a complex and rigorous multi-step adoptive cell transfer therapy as
indicated in Fig. 1B (46).
Following a decade of study, the curative effect of
CAR T-cells in hematological malignancies has provided valuable
information. First-generation anti-CD19 CAR T-cells were
demonstrated to persist for 6 months at high levels in peripheral
blood and bone marrow. Kochenderfer et al (47) first reported that a
chemotherapy-refractory patient with stage IV B-cell non-Hodgkin
lymphoma (B-NHL) achieved partial remission lasting for 8 months
after receiving anti-CD19 CAR T-cell therapy. Subsequently, a
patient with refractory chronic lymphocytic leukemia achieved a
10-month complete remission (CR) (48). CD20, a second form of CAR T-cell
treatment administered to patients with B-NHL also demonstrated
similar results (32,49). However, a phase II trial of
anti-CD20 CAR T-cell therapy achieved promising effects without
inducing severe toxicities, with an overall objective response rate
(ORR) of 81.8% (8/11) and six patients with B-NHL demonstrating CR
(50). The curative efficacy of
CAR T-cells in hematological malignancies has improved, with ORR
rates increasing from 52 to 92% and CR rates ranging from 43 to 90%
(51-55). Furthermore, encouraging results
from the use of CAR T-cells for the treatment of B cell
malignancies has resulted in the application of this therapy to
solid tumors.
The first CAR T-cell therapy clinical trials were
performed two decades ago in the USA for the treatment of patients
with ovarian cancer and metastatic renal carcinoma (56,57).
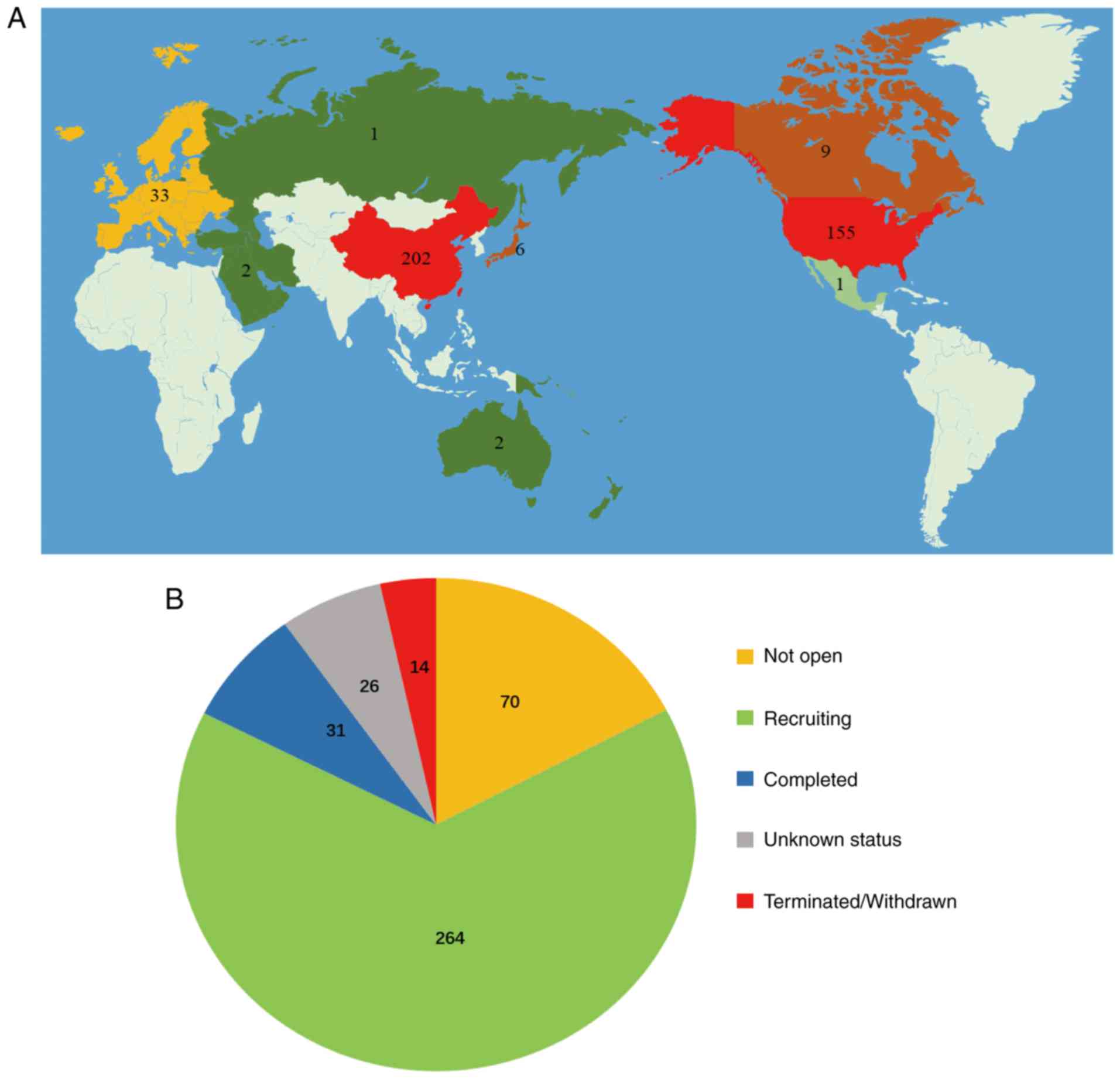
To date, a total of 692 clinical trials have been registered
worldwide on ClinicalTrials.gov, which is over three times the
total number of registrations recorded at the end of 2016 (Fig. 2A). Of these clinical trials,
>400 are associated with cancer therapy. Currently, the majority
of clinical trials are in phase I or II, where appropriate dosage,
safety and efficacy is being established. Only 8% of CAR T-cell
therapy clinical trials have been completed (Fig. 2B).
A single high fidelity target antigen is the most
critical factor for the successful clinical application of CAR
T-cell therapy (58). Previous
literature has indicated that an ideal specific antigen must be
expressed on the extracellular surface of cancer cells and be
preferentially selected for its density and differential expression
in tumors rather than in normal tissues (59). If this does not occur, severe or
lethal off-target toxicity, in addition to poor curative effects,
may occur (59). The expression of
surface antigens in GC is highly heterogeneous, providing tumor
cells with the ability to escape host immune surveillance (60). Therefore, the design of CAR T-cell
immunotherapy for GC poses a great challenge.
However, promising results have been obtained using
preclinical models of first-generation CAR T-cells for the
treatment of ovarian cancer (57),
renal cell carcinoma (57,61) and neuroblastoma (62). Furthermore, the durable efficacy of
CAR T-cell therapy has been high in patients with recurrent or
end-stage glioblastoma, demonstrating anti-tumor activity with
acceptable toxicities in subsequent GD2-targeting trials (62,63).
In murine GC models and in vitro experiments, the anti-tumor
activity and persistence of CAR T-cells targeting folate receptor 1
(FOLR1), 3H11 and human epidermal growth factor receptor 2 (HER2)
has been validated (13-17).
In a xenograft subcutaneous mouse model, significant
tumor-killing abilities of CAR-T cell have been demonstrated in
MKN1 cells (16). An additional
HER2-specific CAR T-cell construct has exhibited specific and
persistent anti-tumor efficacy, along with a strong homing ability
against xenografts derived from HER2+ GC cell lines in
mice (16). Similarly, specific
tumor-killing abilities and high affinities were also verified in
primary patient-derived GC cells through intravenous infusion,
which also occurred during HER2 expression knockdown, and these
positive outcomes were further investigated by constructing
humanized chA21-4-1BBz CAR T-cells (13). Additionally, striking tumor
inhibition was observed in an established and advanced
intraperitoneal metastatic GC model (13). As a major component of the ErbB2
(CD340) family, HER2 is highly expressed on gastrointestinal
epithelial cells and has been extensively investigated as a
potential immunotherapy target for various solid tumors (64). The monoclonal antibody,
trastuzumab, has been approved as first-line treatment following
its successful clinical application against advanced GC (65). Furthermore, following the
intravenous injection of HER2-directed CAR T-cells, the
tumorigenicity of cancer stem cells (CSCs) derived from patients
with GC was markedly inhibited in a tumor-bearing mouse model and
was efficiently phagocytized and degraded in vitro via a
sphere-forming assay (16).
Previous studies have indicated that HER2 signaling serves an
important role in maintaining CSC populations in GC (66-68).
Thus, the eradication of CSCs that possess a capacity for clonal
tumor initiation and contribute to carcinogenesis, tumor invasion,
recurrence, metastasis and drug resistance, has been identified as
a promising immunological approach for cancer treatment (69). Luo et al (17) constructed a bifunctional αHER2/CD3
RNA-engineered CAR T-cell with a more effective and specific
tumor-killing capacity to reduce the possibility of tumor antigen
escape and to transfer these attributes to bystander T-cells, which
exhibited similar effects against HER2+ GC cells.
Additionally, the persistence duration of this bispecific αHER2/CD3
CAR T-cell in vivo was 6 days, outlasting other conventional
bispecific CAR T-cells (70).
Third-generation 3H11-directed CAR T-cells also exhibited similar
cytotoxicity and secretion in vitro and in vivo,
while poor trafficking was observed by tail intravenous injection
(14). The HER2-directed CAR
T-cell therapeutic approach has been continually developed and
validated in different types of cancer, including breast cancer
(71), renal cancer (72) and osteosarcoma (73). It is worth noting that adverse
toxicities may occur unnoticed due to the evaluation of therapeutic
effect being implemented on diverse tumor-bearing mouse models.
However, CAR T-cell therapy is still considered to have great
potential in GC treatment and therefore warrants further clinical
development.
A major priority for the development of GC CAR
T-cell immunotherapy is the discovery and validation of authentic
and specific antigens which minimize potential life-threatening
complications. Clinically, various antigens have been targeted for
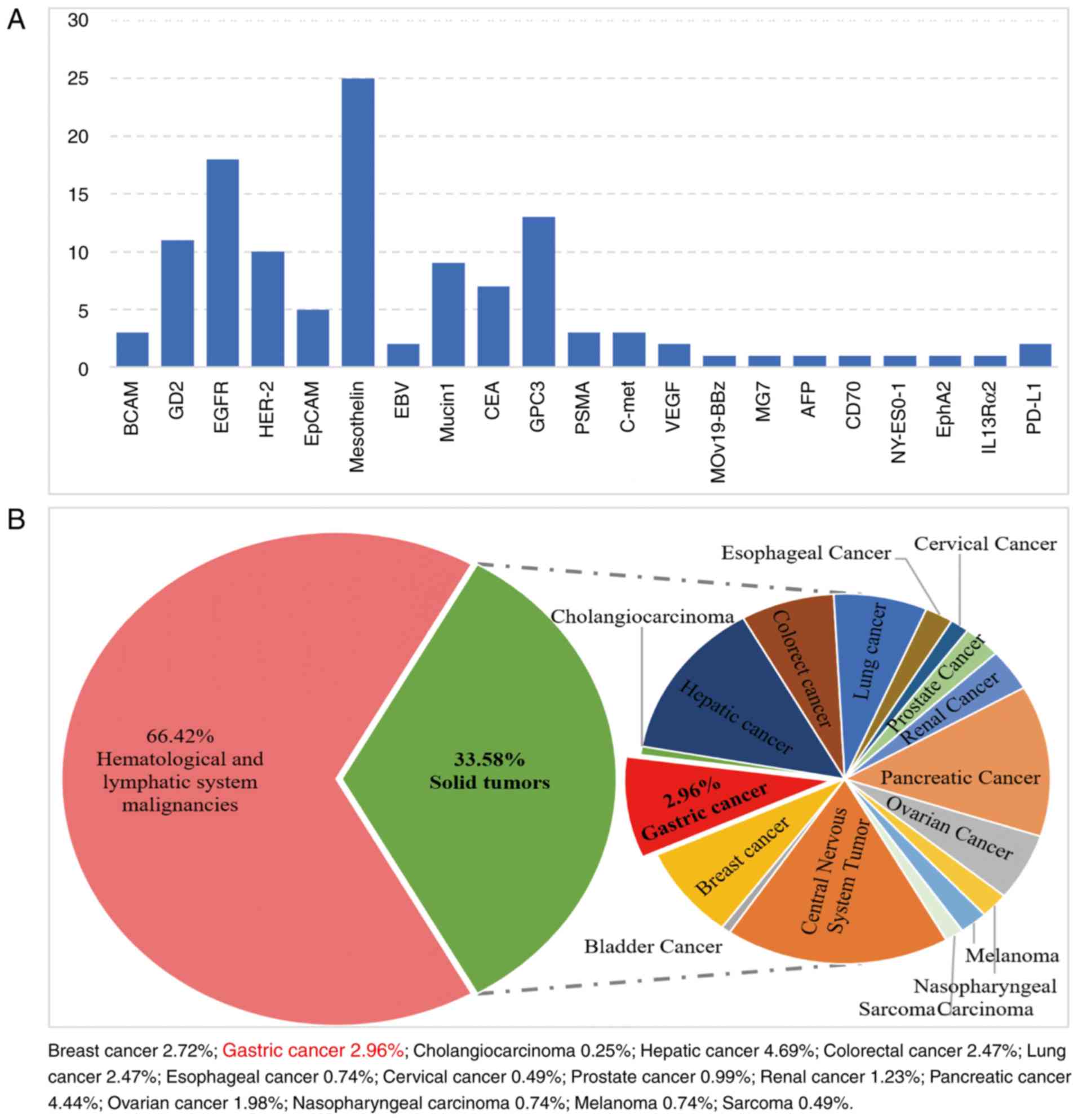
CAR T-cell therapy in solid tumors. These include: Epidermal growth
factor receptor, mesothelin, GPC3, GD2 and HER2 (Fig. 3A). On account of the constraints
applied to the selection of optimizing antigens (74), only 38% of trials are performed on
solid tumors, of which 2.96% are for GC (Fig. 3B). There are still no published
clinical outcomes of CAR T-cells used for GC treatment. Therefore,
the current review summarized the clinical trials registered on
ClinicalTrial.gov. As presented in Table I, a total of 12 registered clinical
trials, utilizing seven different antigens, are distributed in
China and the USA, the majority of which are in the recruitment
phase. The eligibility criteria for participants were as follows:
Individuals aged between 18 to 75 years, without restrictions of
sex or nationality. A good physical condition was required, which
was quantified as an Eastern Cooperative Oncology Group score of ≤2
or a Karnofsky score of ≥60 (75,76).
Currently, the majority of trials are conducted for orthotopic GC
sites via intravenous injection, while only two ongoing trials
(trail nos. NCT03563326 and NCT03682744) have investigated the risk
and potential benefits of CAR T-cell intraperitoneal infusion for
patients with epithelial cell adhesion molecule- and
carcinoembryonic antigen-expressing GC with peritoneal metastasis.
Despite the support of previous research, each clinical trial is
conducted discreetly, with strictly controlled input dosages,
interval times and monitoring indicators, to minimize potentially
life-threatening accompanying side effects.
CAR T-cell therapy has produced a durable remission
in a subset of patients with relapsed or refractory hematological
malignancies (5); however, its
efficacy in GC is yet to be fully elucidated. Severe toxicity is a
main restriction to the promotion and development of CAR T-cell
therapy for patients with GC (47,51).
The most common and serious toxicity is cytokine release syndrome
(CRS), a non-antigen-specific toxicity that leads to respiratory
distress syndrome and multiple organ dysfunction syndrome (MODS).
This toxicity occurs due to the rapid and excessive activation of
various cytokines, including TNF-α, interleukin (IL)-1, IL-6, IL-8,
IL-12, IFN-α, IFN-β and IFN-γ (77). Lymphocyte-depleting chemotherapy
regimens, including fludarabine or cyclophosphamide, enhance the
activation of CAR T-cells in the human body and are associated with
CRS and neurotoxicity (78). In
one instance, a patient with colon cancer immediately developed
rapid respiratory distress and ultimately died of MODS 5 days
following treatment. The death resulted from normal cardiopulmonary
tissue with slight HER2 expression being recognized and attacked by
high-affinity targeting CAR T-cells (79). Additionally, a clinical trial was
suspended due to manufactured anti-CD19-redirected CAR T-cells
inducing CRS, resulting in two deaths (80). Clinical symptomatology of CRS,
on-target off-tumor toxicity and neurotoxicity of CAR T-cells are
summarized in Table II (81-83).
The majority of complications are reversible and self-healing.
However, fatal complications as a result of CRS and neurotoxicity
emphasizes the importance of assessing the preclinical safety of
CAR T-cell therapy (79,84,85).
Biological informatics analyses that predict target protein
distributions in human organs are incomplete and the superior
penetrability of CAR T-cells in solid tissue limits the use of
safety-associated conclusions drawn from studies with mAbs
(86). A patient with chronic
lymphoid leukemia was diagnosed with tumor lysis syndrome on day 22
following anti-CD19-redirected CAR T-cell infusion. However, the
kidney and hepatic function of the patient recovered after fluid
resuscitation and rasburicase treatment (trail no. NCT01029366)
(32). Therefore, accumulating
evidence has indicated that CAR T-cell-associated toxicities may be
minimized or controlled using preventive or protective
interventions (87). Furthermore,
well-controlled liver toxicity may be achieved by blocking
antigenic sites in tumors that are distant to the tumor (88).
Cancer immunotherapy aims to eradicate malignant
cells by harnessing the power of the human immune system. While CAR
T-cells attack targets on the surface of tumor cells to exert its
therapeutic effect, they also cause inevitable harm to normal
tissues in other organs of the body. Therefore, early recognition,
vigilant monitoring and timely intervention are necessary to reduce
CAR T-cell-associated toxicity (82,89).
Thus, based on the National Cancer Institute Common Terminology
Criteria for Adverse Events (version 4.0), toxicity grading systems
are considered to be an important measure for standardized
treatment (90). Furthermore,
according to the Experimental Transplantation and Immunology Branch
of the National Cancer Institute (NCI), a normal cardiovascular
system and a healthy bone marrow function may reduce the incidence
of potential adverse toxicities, demonstrating the necessity for
adequate patient condition assessment before receiving CAR-T
therapy (82). It has been
reported that IL-6 and C-reactive protein can be used as highly
sensitive biomarkers for the diagnosis and potential quantification
of CRS severity (90,91). Previous studies have also indicated
that the IL-6 receptor antagonist, tocilizumab, can attenuate or
eliminate CRS toxicities without affecting the efficacy of CAR
T-cell infusion (44,92). In addition, corticosteroids and
other immunosuppressive drugs (including etanercept, siltuximab and
anakinra) have been effectively applied to reduce CRS-associated
toxicities (93). However, due to
the inhibition of CAR T-cell anti-tumor efficacy and persistence,
these drugs are administered second to tocilizumab (93). Neurotoxicity, which may be
associated with the increased permeability of cerebrospinal fluid,
often occurs concurrently with CRS due to the blood-brain barrier,
resulting in the wide usage of dexamethasone and corticosteroids
instead of tocilizumab (94).
Despite clinical practice experience being derived
from the use of CAR T-cells or treatment against hematological
malignancies, previous studies are valuable for the future
management of CAR T-cell-associated toxicities in GC therapy.
Although CAR T-cell therapy is promising, several
challenges must be overcome to improve its efficacy for the
clinical treatment of GC. Due to the ubiquitous expression of CD19
in the B cell lineage, infections associated with B cell deficiency
or hypoplasia can be prevented or alleviated by immunoglobulin
intervention, providing the rationale for the use of CD19 CAR
T-cells against hematological tumors (95,96).
Similarly, the efficacy of CAR T-cell therapy largely depends on
the selection of an ideal epitope target unique to GC that will
also prevent off-target effects. A single GC-associated surface
neo-antigen is optimal but time-consuming. Thus, a multi-targeted
approach is advocated as a promising solution for CAR T-cell
efficacy and safety in vivo (97). An additional issue to overcome is
the limitation of complex tumor microenvironments (TME): GC cells
generate a physical and metabolic barrier characterized by hypoxia,
nutrient starvation and cytokine secretion, contributing to
tumorigenesis and facilitating CAR T-cell tolerance (98). It has been indicated that combined
pre-condition treatment, including chemotherapy, radiotherapy,
immune checkpoint molecules and other drugs involving small
molecules, may contribute to the removal of regulatory T
lymphocytes. This makes the TME permissive for immunotherapy and
for the improvement of antitumor effects (99,100). However, compared with traditional
cell experiments, GC organoids can simulate the GC microenvironment
in vitro and accurately assess the specific efficacy and
toxicities of CAR T-cells for GC in vitro (101). Traditional subcutaneous tumor
implant and patient-derived xenograft models have the disadvantage
of not simulating human immunity and human-derived tumors,
resulting in different preclinical and clinical study outcomes
(102).
Further study assessing GC CAR T-cell therapy should
focus on the following aspects: i) Seeking ideal CAR T-cell
therapeutic targets with higher positive expression rates in GC
tissues; ii) clarifying the specific role of other combined
precondition treatments used in CAR T-cell therapy for GC; and iii)
developing a novel GC organoid model and humanized tumor
implantation model to improve the reliable evaluation of CAR T-cell
efficacy and toxicity in preclinical research. Additionally, the
development of a generic CAR structure may lead to an increase in
the number of patients with GC benefiting from CAR T-cell therapy,
causing a reduction in medical costs.
CAR T-cell immunotherapy is confronted with many
challenges and difficulties; however, it is still recognized as the
most potent cure for GC (103).
Although GC CAR T-cell research is in its infancy, the positive
results of preliminary trials provides a rationale for the further
exploration of its use in clinical practice. This indicates that
CAR T-cell therapeutic models are advancing and may eventually
improve with continued exploration. Combined with a deeper
understanding of the TME, novel target epitopes and
scientific-technical progress, CAR T-cell therapy may improve its
current standing in the near future. Improving the tumor-killing
effect and prolonging the survival time of patients should also be
readily solved with future study. Furthermore, combining CAR T-cell
therapy with precondition treatment may address its current
ineffectiveness. In conclusion, the available evidence strongly
supports the potential of CAR T-cells in the treatment of patients
with GC.
The current review was supported by the Fundamental
Research Funds of the Central Universities (grant no.
lzujbky-2019-cd06), the Cuiying Science and Technology Innovation
Project of Lanzhou City (grant no. CY2017-ZD03) and the National
Natural Science Foundation of China (grant no. 31670847).
Not applicable.
ZJ and BL conceptualized the present review. ZY, LQ
and QL drafted the manuscript. BZ and HY designed and finalized the
figures. LW and GZ collected and analyzed the data. XJ and ZY
designed and finalized the tables. All authors read and approved
the final manuscript.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
Not applicable.
|
1
|
Khalil DN, Budhu S, Gasmi B, Zappasodi R,
Hirschhorn-Cymerman D, Plitt T, De Henau O, Zamarin D, Holmgaard
RB, Murphy JT, et al: The new era of cancer immunotherapy:
Manipulating T-cell activity to overcome malignancy. Adv Cancer
Res. 128:1–68. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rosenberg SA, Yang JC, Sherry RM, Kammula
US, Hughes MS, Phan GQ, Citrin DE, Restifo NP, Robbins PF,
Wunderlich JR, et al: Durable complete responses in heavily
pretreated patients with metastatic melanoma using T-cell transfer
immunotherapy. Clin Cancer Res. 17:4550–4557. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Schuster M, Nechansky A and Kircheis R:
Cancer immunotherapy. Biotechnol J. 1:138–147. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Fesnak AD, June CH and Levine BL:
Engineered T cells: The promise and challenges of cancer
immunotherapy. Nat Rev Cancer. 16:566–581. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chow VA, Shadman M and Gopal AK:
Translating anti-CD19 CAR T-cell therapy into clinical practice for
relapsed/refractory diffuse large B-cell lymphoma. Blood.
132:777–781. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Grupp SA, Michael K, David B, Aplenc R,
Porter DL, Rheingold SR, Teachey DT, Chew A, Hauck B, Wright JF, et
al: Chimeric antigen receptor-modified T cells for acute lymphoid
leukemia. N Engl J Med. 368:1509–1518. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kochenderfer JN, Dudley ME, Kassim SH,
Somerville RP, Carpenter RO, Stetler-Stevenson M, Yang JC, Phan GQ,
Hughes MS, Sherry RM, et al: Chemotherapy-refractory diffuse large
B-cell lymphoma and indolent B-cell malignancies can be effectively
treated with autologous T cells expressing an anti-CD19 chimeric
antigen receptor. J Clin Oncol. 33:540–549. 2015. View Article : Google Scholar :
|
|
8
|
Wang Z, Wu Z, Liu Y and Han W: New
development in CAR-T cell therapy. J Hematol Oncol. 10:532017.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Allemani C, Matsuda T, Di Carlo V,
Harewood R, Matz M, Nikšić M, Bonaventure A, Valkov M, Johnson CJ,
Estève J, et al: Global surveillance of trends in cancer survival
2000-14 (CONCORD-3): Analysis of individual records for 37513025
patients diagnosed with one of 18 cancers from 322 population-based
registries in 71 countries. Lancet. 391:1023–1075. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
FDA approves second CAR T-cell therapy.
Cancer Discov. 8:5–6. 2018. View Article : Google Scholar
|
|
12
|
June CH, O'Connor RS, Kawalekar OU,
Ghassemi S and Milone MC: CAR T cell immunotherapy for human
cancer. Science. 359:1361–1365. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Han Y, Liu C, Li G, Li J, Lv X, Shi H, Liu
J, Liu S, Yan P, Wang S, et al: Antitumor effects and persistence
of a novel HER2 CAR T cells directed to gastric cancer in
preclinical models. Am J Cancer Res. 8:106–119. 2018.PubMed/NCBI
|
|
14
|
Han H, Wang S, Hu Y, Li Z, Yang W, Lv Y,
Wang L, Zhang L and Ji J: Monoclonal antibody 3H11 chimeric antigen
receptors enhance T cell effector function and exhibit efficacy
against gastric cancer. Oncol Lett. 15:6887–6894. 2018.PubMed/NCBI
|
|
15
|
Kim M, Pyo S, Kang CH, Lee CO, Lee HK,
Choi SU and Park CH: Folate receptor 1 (FOLR1) targeted chimeric
antigen receptor (CAR) T cells for the treatment of gastric cancer.
PLoS One. 13:e01983472018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Song Y, Tong C, Wang Y, Gao Y, Dai H, Guo
Y, Zhao X, Wang Y, Wang Z, Han W and Chen L: Effective and
persistent antitumor activity of HER2-directed CAR-T cells against
gastric cancer cells in vitro and xenotransplanted tumors in vivo.
Protein Cell. 9:867–878. 2018. View Article : Google Scholar :
|
|
17
|
Luo F, Qian J, Yang J, Deng Y, Zheng X,
Liu J and Chu Y: Bifunctional αHER2/CD3 RNA-engineered CART-like
human T cells specifically eliminate HER2(+) gastric cancer. Cell
Res. 26:850–853. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gross G, Waks T and Eshhar Z: Expression
of immunoglobulin-T-cell receptor chimeric molecules as functional
receptors with antibody-type specificity. Proc Natl Acad Sci USA.
86:10024–10028. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Eshhar Z, Waks T, Gross G and Schindler
DG: Specific activation and targeting of cytotoxic lymphocytes
through chimeric single chains consisting of antibody-binding
domains and the gamma or zeta subunits of the immunoglobulin and
T-cell receptors. Proc Natl Acad Sci USA. 90:720–724. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jaspers JE and Brentjens RJ: Development
of CAR T cells designed to improve antitumor efficacy and safety.
Pharmacol Ther. 178:83–91. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Brocker T and Karjalainen K: Signals
through T cell receptor-zeta chain alone are insufficient to prime
resting T lymphocytes. J Exp Med. 181:1653–1659. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Finney HM, Lawson AD, Bebbington CR and
Weir AN: Chimeric receptors providing both primary and
costimulatory signaling in T cells from a single gene product. J
Immunol. 161:2791–2797. 1998.PubMed/NCBI
|
|
23
|
Savoldo B, Ramos CA, Liu E, Mims MP,
Keating MJ, Carrum G, Kamble RT, Bollard CM, Gee AP, Mei Z, et al:
CD28 costimulation improves expansion and persistence of chimeric
antigen receptor-modified T cells in lymphoma patients. J Clin
Invest. 121:1822–1826. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Brentjens RJ, Rivière I, Park JH, Davila
ML, Wang X, Stefanski J, Taylor C, Yeh R, Bartido S, Borquez-Ojeda
O, et al: Safety and persistence of adoptively transferred
autologous CD19-targeted T cells in patients with relapsed or
chemotherapy refractory B-cell leukemias. Blood. 118:4817–4828.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Brentjens RJ, Davila ML, Riviere I, Park
J, Wang X, Cowell LG, Bartido S, Stefanski J, Taylor C, Olszewska
M, et al: CD19-targeted T cells rapidly induce molecular remissions
in adults with chemotherapy-refractory acute lymphoblastic
leukemia. Sci Transl Med. 5:177ra382013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kalos M, Levine BL, Porter DL, Katz S,
Grupp SA, Bagg A and June CH: T cells with chimeric antigen
receptors have potent antitumor effects and can establish memory in
patients with advanced leukemia. Sci Transl Med. 3:95ra732011.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Finney HM, Akbar AN and Lawson AD:
Activation of resting human primary T cells with chimeric
receptors: Costimulation from CD28, inducible costimulator, CD134,
and CD137 in series with signals from the TCR zeta chain. J
Immunol. 172:104–113. 2004. View Article : Google Scholar
|
|
28
|
Knochelmann HM, Smith AS, Dwyer CJ, Wyatt
MM, Mehrotra S and Paulos CM: CAR T cells in solid tumors:
Blueprints for building effective therapies. Front Immunol.
9:17402018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ramos CA and Gianpietro D: Chimeric
antigen receptor (CAR)-engineered lymphocytes for cancer therapy.
Expert Opin Biol Ther. 11:855–873. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Jackson HJ, Rafiq S and Brentjens RJ:
Driving CAR T-cells forward. Nat Rev Clin Oncol. 13:370–383. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Till BG, Jensen MC, Wang J, Qian X, Gopal
AK, Maloney DG, Lindgren CG, Lin Y, Pagel JM, Budde LE, et al:
CD20-specific adoptive immunotherapy for lymphoma using a chimeric
antigen receptor with both CD28 and 4-1BB domains: Pilot clinical
trial results. Blood. 119:3940–3950. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Porter DL, Levine BL, Kalos M, Bagg A and
June CH: Chimeric antigen receptor-modified T cells in chronic
lymphoid leukemia. N Engl J Med. 365:725–733. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Jensen MC and Riddell SR: Designing
chimeric antigen receptors to effectively and safely target tumors.
Curr Opin Immunol. 33:9–15. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
McAdam AJ, Greenwald RJ, Levin MA,
Chernova T, Malenkovich N, Ling V, Freeman GJ and Sharpe AH: ICOS
is critical for CD40-mediated antibody class switching. Nature.
409:102–105. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lipowska-Bhalla G, Gilham DE, Hawkins RE
and Rothwell DG: Targeted immunotherapy of cancer with CAR T cells:
Achievements and challenges. Cancer Immunol Immunother. 61:953–962.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Haynes NM, Trapani JA, Teng MW, Jackson
JT, Cerruti L, Jane SM, Kershaw MH, Smyth MJ and Darcy PK:
Single-chain antigen recognition receptors that costimulate potent
rejection of established experimental tumors. Blood. 100:3155–3163.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wu J, Song Y, Bakker AB, Bauer S, Spies T,
Lanier LL and Phillips JH: An activating immunoreceptor complex
formed by NKG2D and DAP10. Science. 285:730–732. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Pulè MA, Straathof KC, Dotti G, Heslop HE,
Rooney CM and Brenner MK: A chimeric T cell antigen receptor that
augments cytokine release and supports clonal expansion of primary
human T cells. Mol Ther. 12:933–941. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Milone MC, Fish JD and Carmine C: Chimeric
receptors containing CD137 signal transduction domains mediate
enhanced survival of T cells and increased antileukemic efficacy in
vivo. Mol Ther. 17:1453–1464. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Hua Z, Snyder KM, Suhoski MM, Maus MV,
Kapoor V, June CH and Mackall CL: 4-1BB is superior to CD28
costimulation for generating CD8+ cytotoxic lymphocytes for
adoptive immunotherapy. J Immunol. 179:4910–4918. 2007. View Article : Google Scholar
|
|
41
|
Friedmann-Morvinski D, Bendavid A, Waks T,
Schindler D and Eshhar Z: Redirected primary T cells harboring a
chimeric receptor require costimulation for their antigen-specific
activation. Blood. 105:30872005. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Porter DL, Hwang WT, Frey NV, Lacey SF,
Shaw PA, Loren AW, Bagg A, Marcucci KT, Shen A, Gonzalez V, et al:
Chimeric antigen receptor T cells persist and induce sustained
remissions in relapsed refractory chronic lymphocytic leukemia. Sci
Transl Med. 7:303ra1392015. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Kawalekar OU, O'Connor RS, Fraietta JA,
Guo L, McGettigan SE, Posey AD Jr, Patel PR, Guedan S, Scholler J,
Keith B, et al: Distinct signaling of coreceptors regulates
specific metabolism pathways and impacts memory development in CAR
T cells. Immunity. 44:380–390. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Maude SL, Noelle F, Shaw PA, Aplenc R,
Barrett DM, Bunin NJ, Chew A, Gonzalez VE, Zheng Z, Lacey SF, et
al: Chimeric antigen receptor T cells for sustained remissions in
leukemia. N Engl J Med. 371:1507–1517. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Cheadle EJ, Rothwell DG, Bridgeman JS,
Sheard VE, Hawkins RE and Gilham DE: Ligation of the CD2
co-stimulatory receptor enhances IL-2 production from
first-generation chimeric antigen receptor T cells. Gene Ther.
19:1114–1120. 2012. View Article : Google Scholar
|
|
46
|
Han S, Latchoumanin O, Wu G, Zhou G,
Hebbard L, George J and Qiao L: Recent clinical trials utilizing
chimeric antigen receptor T cells therapies against solid tumors.
Cancer Lett. 390:188–200. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Kochenderfer JN, Wilson WH, Janik JE,
Dudley ME, Stetler-Stevenson M, Feldman SA, Maric I, Raffeld M,
Nathan DA, Lanier BJ, et al: Eradication of B-lineage cells and
regression of lymphoma in a patient treated with autologous T cells
genetically engineered to recognize CD19. Blood. 116:4099–4102.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Wang Y, Zhang WY, Han QW, Liu Y, Dai HR,
Guo YL, Bo J, Fan H, Zhang Y, Zhang YJ, et al: Effective response
and delayed toxicities of refractory advanced diffuse large B-cell
lymphoma treated by CD20-directed chimeric antigen
receptor-modified T cells. Clin Immunol. 155:160–175. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Till BG, Jensen MC, Wang J, Chen EY, Wood
BL, Greisman HA, Qian X, James SE, Raubitschek A, Forman SJ, et al:
Adoptive immunotherapy for indolent non-Hodgkin lymphoma and mantle
cell lymphoma using genetically modified autologous CD20-specific T
cells. Blood. 112:2261–2271. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Zhang WY, Wang Y, Guo YL, Dai HR, Yang QM,
Zhang YJ, Zhang Y, Chen MX, Wang CM, Feng KC, et al: Treatment of
CD20-directed Chimeric Antigen Receptor-modified T cells in
patients with relapsed or refractory B-cell non-Hodgkin lymphoma:
An early phase IIa trial report. Signal Transduct Target Ther.
1:160022016. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Kochenderfer JN, Dudley ME, Feldman SA,
Wilson WH, Spaner DE, Maric I, Stetler-Stevenson M, Phan GQ, Hughes
MS, Sherry RM, et al: B-cell depletion and remissions of malignancy
along with cytokine-associated toxicity in a clinical trial of
anti-CD19 chimeric-antigen-receptor-transduced T cells. Blood.
119:2709–2720. 2012. View Article : Google Scholar :
|
|
52
|
Locke FL, Neelapu SS, Bartlett NL, Siddiqi
T, Chavez JC, Hosing CM, Ghobadi A, Budde LE, Bot A, Rossi JM, et
al: Phase 1 results of ZUMA-1: A multicenter study of KTE-C19
anti-CD19 CAR T cell therapy in refractory aggressive lymphoma. Mol
Ther. 25:285–295. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Kochenderfer JN, Somerville RPT, Lu T, Shi
V, Bot A, Rossi J, Xue A, Goff SL, Yang JC, Sherry RM, et al:
Lymphoma remissions caused by anti-CD19 chimeric antigen receptor T
cells are associated with high serum interleukin-15 levels. J Clin
Oncol. 35:1803–1813. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Neelapu SS, Locke FL, Bartlett NL, Lekakis
LJ, Miklos DB, Jacobson CA, Braunschweig I, Oluwole OO, Siddiqi T,
Lin Y, et al: Axicabtagene ciloleucel CAR T-cell therapy in
refractory large B-cell lymphoma. N Engl J Med. 377:2531–2544.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Schuster SJ, Svoboda J, Chong EA, Nasta
SD, Mato AR, Anak Ö, Brogdon JL, Pruteanu-Malinici I, Bhoj V,
Landsburg D, et al: Chimeric antigen receptor T cells in refractory
B-cell lymphomas. N Engl J Med. 377:2545–2554. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Kershaw MH, Westwood JA, Parker LL, Wang
G, Eshhar Z, Mavroukakis SA, White DE, Wunderlich JR, Canevari S,
Rogers-Freezer L, et al: A phase I study on adoptive immuno-therapy
using gene-modified T cells for ovarian cancer. Clin Cancer Res.
12:6106–6115. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Lamers CH, Sleijfer S, Vulto AG, Kruit WH,
Kliffen M, Debets R, Gratama JW, Stoter G and Oosterwijk E:
Treatment of metastatic renal cell carcinoma with autologous
T-lymphocytes genetically retargeted against carbonic anhydrase IX:
First clinical experience. J Clin Oncol. 24:e20–e22. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Guo Y, Wang Y and Han W: Chimeric antigen
receptor-modified T cells for solid tumors: Challenges and
prospects. J Immunol Res. 2016:38508392016. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Sadelain M, Brentjens R and Rivière I: The
promise and potential pitfalls of chimeric antigen receptors. Curr
Opin Immunol. 21:215–223. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Fousek K and Ahmed N: The evolution of
T-cell therapies for solid malignancies. Clin Cancer Res.
21:3384–3392. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Lamers CH, Langeveld SC, Grootvan Ruijven
CM, Debets R, Sleijfer S and Gratama JW: Gene-modified T cells for
adoptive immunotherapy of renal cell cancer maintain
transgene-specific immune functions in vivo. Cancer Immunol
Immunother. 56:1875–1883. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Pule MA, Savoldo B, Myers GD, Rossig C,
Russell HV, Dotti G, Huls MH, Liu E, Gee AP, Mei Z, et al:
Virus-specific T cells engineered to coexpress tumor-specific
receptors: Persistence and antitumor activity in individuals with
neuroblastoma. Nat Med. 14:1264–1270. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Brown CE, Badie B, Barish ME, Weng L,
Ostberg JR, Chang WC, Naranjo A, Starr R, Wagner J, Wright C, et
al: Bioactivity and safety of IL13Rα2-redirected chimeric antigen
receptor CD8+ T cells in patients with recurrent glioblastoma. Clin
Cancer Res. 21:4062–4072. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Whilding LM and Maher J: ErbB-targeted CAR
T-cell immunotherapy of cancer. Immunotherapy. 7:229–241. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Fanotto V, Ongaro E, Rihawi K, Avallone A,
Silvestris N, Fornaro L, Vasile E, Antonuzzo L, Leone F, Rosati G,
et al: HER-2 inhibition in gastric and colorectal cancers: Tangible
achievements, novel acquisitions and future perspectives.
Oncotarget. 7:69060–69074. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Jiang J, Zhang Y, Chuai S, Wang Z, Zheng
D, Xu F, Zhang Y, Li C, Liang Y and Chen Z: Trastuzumab (herceptin)
targets gastric cancer stem cells characterized by CD90 phenotype.
Oncogene. 31:671–682. 2012. View Article : Google Scholar
|
|
67
|
Lo PK and Chen H: Cancer stem cells and
cells of origin in MMTV-Her2/neu-induced mammary tumorigenesis.
Oncogene. 32:1338–1340. 2013. View Article : Google Scholar
|
|
68
|
Shah D, Wyatt D, Baker AT, Simms P,
Peiffer DS, Fernandez M, Rakha E, Green A, Filipovic A, Miele L and
Osipo C: Inhibition of HER2 increases JAGGED1-dependent breast
cancer stem cells: Role for membrane JAGGED1. Clin Cancer Res.
24:4566–4578. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Plaks V, Kong N and Werb Z: The cancer
stem cell niche: How essential is the niche in regulating stemness
of tumor cells? Cell Stem Cell. 16:225–238. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Nagorsen D, Kufer P, Baeuerle PA and
Bargou R: Blinatumomab: A historical perspective. Pharmacol Ther.
136:334–342. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Sun M, Shi H, Liu C, Liu J, Liu X and Sun
Y: Construction and evaluation of a novel humanized HER2-specific
chimeric receptor. Breast Cancer Res. 16:1–10. 2014. View Article : Google Scholar
|
|
72
|
Schönfeld K, Sahm C, Zhang C, Naundorf S,
Brendel C, Odendahl M, Nowakowska P, Bönig H, Köhl U, Kloess S, et
al: Selective inhibition of tumor growth by clonal NK cells
expressing an ErbB2/HER2-specific chimeric antigen receptor. Mol
Ther. 23:330–338. 2015. View Article : Google Scholar :
|
|
73
|
Rainusso N, Brawley VS, Ghazi A, Hicks MJ,
Gottschalk S, Rosen JM and Ahmed N: Immunotherapy targeting HER2
with genetically modified T cells eliminates tumor-initiating cells
in osteosarcoma. Adv Exp Med Biol. 19:212–217. 2012.
|
|
74
|
Hartmann J, Schüßler-Lenz M, Bondanza A
and Buchholz CJ: Clinical development of CAR T cells-challenges and
opportunities in translating innovative treatment concepts. EMBO
Mol Med. 9:1183–1198. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Oken MM, Creech RH, Tormey DC, Horton J,
Davis TE, McFadden ET and Carbone PP: Toxicity and response
criteria of the Eastern Cooperative Oncology Group. Am J Clin
Oncol. 5:649–655. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Schag CC, Heinrich RL and Ganz PA:
Karnofsky performance status revisited: Reliability, validity, and
guidelines. J Clin Oncol. 2:187–193. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Frey NV and Porter DL: Cytokine release
syndrome with novel therapeutics for acute lymphoblastic leukemia.
Hematology Am Soc Hematol Educ Program. 2016:567–572. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Ali SA, Shi V, Maric I, Wang M, Stroncek
DF, Rose JJ, Brudno JN, Stetler-Stevenson M, Feldman SA, Hansen BG,
et al: T cells expressing an anti-B-cell maturation antigen
chimeric antigen receptor cause remissions of multiple myeloma.
Blood. 128:1688–1700. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Morgan RA, Yang JC, Kitano M, Dudley ME,
Laurencot CM and Rosenberg SA: Case report of a serious adverse
event following the administration of T cells transduced with a
chimeric antigen receptor recognizing ERBB2. Mol Ther. 18:843–851.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Kim MG, Kim D, Suh SK, Park Z, Choi MJ and
Oh YK: Current status and regulatory perspective of chimeric
antigen receptor-modified T cell therapeutics. Arch Pharm Res.
39:437–452. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Teachey DT, Lacey SF, Shaw PA, Melenhorst
JJ, Maude SL, Frey N, Pequignot E, Gonzalez VE, Chen F, Finklestein
J, et al: Identification of predictive biomarkers for cytokine
release syndrome after chimeric antigen receptor T-cell therapy for
acute lymphoblastic leukemia. Cancer Discov. 6:664–679. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Brudno JN and Kochenderfer JN: Toxicities
of chimeric antigen receptor T cells: Recognition and management.
Blood. 127:3321–3330. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Brudno JN and Kochenderfer JN: Recent
advances in CAR T-cell toxicity: Mechanisms, manifestations and
management. Blood Rev. 34:45–55. 2019. View Article : Google Scholar :
|
|
84
|
Park JH, Rivière I, Gonen M, Wang X,
Sénéchal B, Curran KJ, Sauter C, Wang Y, Santomasso B, Mead E, et
al: Long-term follow-up of CD19 CAR therapy in acute lymphoblastic
leukemia. N Engl J Med. 378:449–459. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Thistlethwaite FC, Gilham DE, Guest RD,
Rothwell DG, Pillai M, Burt DJ, Byatte AJ, Kirillova N, Valle JW,
Sharma SK, et al: The clinical efficacy of first-generation
carcinoembryonic antigen (CEACAM5)-specific CAR T cells is limited
by poor persistence and transient pre-conditioning-dependent
respiratory toxicity. Cancer Immunol Immunothe. 66:1425–1436. 2017.
View Article : Google Scholar
|
|
86
|
Gross G and Eshhar Z: Therapeutic
potential of T cell chimeric antigen receptors (CARs) in cancer
treatment: Counteracting off-tumor toxicities for safe CAR T cell
therapy. Annu Rev Pharmacol Toxicol. 56:59–83. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Kenderian SS, Ruella M, Shestova O,
Klichinsky M, Aikawa V, Morrissette JJ, Scholler J, Song D, Porter
DL, Carroll M, et al: CD33-specific chimeric antigen receptor T
cells exhibit potent preclinical activity against human acute
myeloid leukemia. Leukemia. 29:1637–1647. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Lamers CH, Sleijfer S, van Steenbergen S,
van Elzakker P, van Krimpen B, Groot C, Vulto A, den Bakker M,
Oosterwijk E, Debets R and Gratama JW: Treatment of metastatic
renal cell carcinoma with CAIX CAR-engineered T cells: Clinical
evaluation and management of on-target toxicity. Mol Ther.
21:904–912. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Mei H, Jiang H, Wu Y, Guo T, Xia L, Jin R
and Hu Y: Neurological toxicities and coagulation disorders in the
cytokine release syndrome during CAR-T therapy. Br J Haematol.
181:689–692. 2018. View Article : Google Scholar
|
|
90
|
Davila ML, Riviere I, Wang X, Bartido S,
Park J, Curran K, Chung SS, Stefanski J, Borquez-Ojeda O, Olszewska
M, et al: Efficacy and toxicity management of 19-28z CAR T cell
therapy in B cell acute lymphoblastic leukemia. Sci Transl Med.
6:224ra252014. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Schultz DR and Arnold PI: Properties of
four acute phase proteins: C-reactive protein, serum amyloid A
protein, alpha 1-acid glycoprotein, and fibrinogen. Semin Arthritis
Rheum. 20:129–147. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Lee DW, Kochenderfer JN, Stetlerstevenson
M, Cui YK, Delbrook C, Feldman SA, Fry TJ, Orentas R, Sabatino M,
Shah NN, et al: T cells expressing CD19 chimeric antigen receptors
for acute lymphoblastic leukaemia in children and young adults: A
phase 1 dose-escalation trial. Lancet. 385:517–528. 2015.
View Article : Google Scholar
|
|
93
|
Lee DW, Rebecca G, Porter DL, Louis CU,
Ahmed N, Jensen M, Grupp SA and Mackall CL: Current concepts in the
diagnosis and management of cytokine release syndrome. Blood.
124:188–195. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Pardridge WM: CNS drug design based on
principles of blood-brain barrier transport. J Neurochem.
70:1781–1792. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Brudno JN, Maric I, Hartman SD, Rose JJ,
Wang M, Lam N, Stetler-Stevenson M, Salem D, Yuan C, Pavletic S, et
al: T cells genetically modified to express an anti-B-cell
maturation antigen chimeric antigen receptor cause remissions of
poor-prognosis relapsed multiple myeloma. J Clin Oncol.
36:2267–2280. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Maude SL, Laetsch TW, Buechner J, Rives S,
Boyer M, Bittencourt H, Bader P, Verneris MR, Stefanski HE, Myers
GD, et al: Tisagenlecleucel in children and young adults with
B-cell lymphoblastic leukemia. N Engl J Med. 378:439–448. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Beatty GL and O'Hara M: Chimeric antigen
receptor-modified T cells for the treatment of solid tumors:
Defining the challenges and next steps. Pharmacol Ther. 166:30–39.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Sharma P and Allison JP: The future of
immune checkpoint therapy. Science. 348:56–61. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Kang TH, Mao CP, Lee SY, Chen A, Lee JH,
Kim TW, Alvarez RD, Roden RB, Pardoll D, Hung CF and Wu TC:
Chemotherapy acts as an adjuvant to convert the tumor
microenvironment into a highly permissive state for
vaccination-induced antitumor immunity. Cancer Res. 73:2493–2504.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Shahabi V, Postow MA, Tuck D and Wolchok
JD: Immune-priming of the tumor microenvironment by radiotherapy:
Rationale for combination with immunotherapy to improve anticancer
efficacy. Am J Clin Oncol. 38:90–97. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Nanki K, Toshimitsu K, Takano A, Fujii M,
Shimokawa M, Ohta Y, Matano M, Seino T, Nishikori S, Ishikawa K, et
al: Divergent routes toward Wnt and R-spondin niche independency
during human gastric carcinogenesis. Cell. 174:856–869.e17. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Hidalgo M, Amant F, Biankin AV, Budinská
E, Byrne AT, Caldas C, Clarke RB, de Jong S, Jonkers J, Mælandsmo
GM, et al: Patient-derived xenograft models: An emerging platform
for translational cancer research. Cancer Discov. 4:998–1013. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Lv J, Zhao R, Wu D, Zheng D, Wu Z, Shi J,
Wei X, Wu Q, Long Y, Lin S, et al: Mesothelin is a target of
chimeric antigen receptor T cells for treating gastric cancer. J
Hematol Oncol. 12:182019. View Article : Google Scholar : PubMed/NCBI
|