Prostate cancer (PCa) and breast cancer (BCa) are
two common types of malignant tumor with high mortality rates.
According to recent statistical data, the number of new cases of
PCa and BCa accounts for 7.1 and 11.6% of the total cancer cases
worldwide, and the numbers of deaths from PCa and BCa account for
3.8 and 6.6% of all cancer deaths, respectively (1). Particularly in Asian countries like
China, the incidences of PCa and BCa have been constantly
increasing over the last two decades (2). Owing to the improved early diagnosis
and advanced therapeutic strategies, the mortality rates of PCa and
BCa have appreciably decreased. Unfortunately, the majority of
patients eventually develop more aggressive malignant forms that
are resistant to the most common treatments, leading to a poor
prognosis (3,4). Thus, therapeutic resistance still
poses a major challenge on the path to conquer PCa and BCa.
As sex hormone-related cancer types, PCa and BCa
share a common feature; namely, that the interaction between sex
hormones and hormone receptors is required to initiate
tumorigenesis (5,6). In PCa, the androgen response is
thought to be essential for tumorigenesis. Blockage of the
interaction between androgen and the androgen receptor (AR) has
been implicated in the induction of caspase-mediated apoptosis, as
well as the inhibition of cell proliferation by altering cell
cycling (7,8). Like androgen, estrogen is also
essential for cell survival and proliferation, and estrogen
receptor (ER) activation is recognized to play a pivotal role in
BCa progression (9-11). Overall, heightened AR and ER
activities are thought to contribute to the development of PCa and
BCa through AR/ER-mediated signal transduction.
Since sex hormone responses are a key factor for the
initiation of tumorigenesis in both PCa and BCa, hormone
deprivation has become a common therapeutic option for the
treatment of these two types of cancer. However, although most
patients can gain certain therapeutic benefits from hormone
therapies in the early stages, a large number of patients
eventually acquire therapeutic resistance, leading to tumor
recurrence and metastasis in hormone-free conditions (3,12).
Overall, the therapeutic strategies for PCa and BCa are quite
similar (Table I).
For patients with low- and intermediate-risk
localized PCa, local treatment such as prostatectomy and
radiotherapy are efficient to prevent distant organ metastasis
(13-15). Additionally, radiotherapy plus
hormone therapy has been applied to treat patients with high-risk
locally advanced PCa, metastatic PCa that is unsuitable for
surgery, or tumor recurrence after prostatectomy (13,15,16).
Finally, chemotherapy with serious side-effects is still required
to treat malignant PCa when hormone therapy is no longer effective
(13,15). Notably, recent advanced targeted
therapy and immunotherapy for inhibiting malignancy-associated
molecules, as well as specific signaling pathways, have been
successfully used to control hormone-refractory states (17,18).
In BCa treatment, for patients with the early stages of BCa, after
breast-conserving surgery or mastectomy, radiotherapy is essential
to reduce the risk of recurrence (19,20).
Generally, endocrino-therapy is the first-line treatment for
ER-positive BCa (21). Ultimately,
chemotherapy is essential in treating patients with metastatic BCa,
including human epidermal growth factor receptor 2 (HER2)-positive
BCa, high-risk luminal HER2-negative BCa and triple-negative BCa
(TNBC) (22-24). Likewise, targeted therapy for
inhibiting HER2 appears to efficiently treat malignant BCa
(25). Immunotherapy also has also
become a potent therapeutic approach to controlling BCa progression
and reversing drug resistance (26).
Overall, hormone therapy is a powerful tool for the
treatment of the early stages of AR-positive PCa or ER-positive
BCa. Nevertheless, chemotherapy is necessary for treating
late-stage disease that is resistant to hormone therapy.
Unfortunately, advanced disease with a metastatic phenotype remains
incurable, particularly life-threatening metastases to the bones or
brain (27-30). Thus, more efficient targeted
therapy and immunotherapy are needed to more effectively treat
advanced PCa/BCa. To that end, the aim of the present review was to
integrate research on the mechanism by which PCa or BCa gradually
progresses to the AR/ER-negative genotype. Activation of the NF-κB
pathway appears to play a central role in the progression of
hormone-independent malignancies and in endocrinotherapy
resistance; in particular, RelB is a key factor in sustaining NF-κB
activity to replace the function of AR/ER.
The interaction between ligands and receptors is
thought to be essential for normal physiological development, but
also to be involved in cancer progression. Abnormal activation of
AR/ER signaling uniquely contributes toward the tumorigenesis of
PCa/BCa. Nevertheless, unlike PCa with AR alone, the progesterone
receptor (PR) and HER2 are also important receptors, along with ER,
in BCa.
As major sex hormone receptors, AR and ER belong to
the nuclear receptor superfamily, which can be activated by
multiple ligands including steroids, thyroid hormones and retinoic
acid (31-33). AR and ER function as transcription
factors in the regulation of downstream gene expression (8,34).
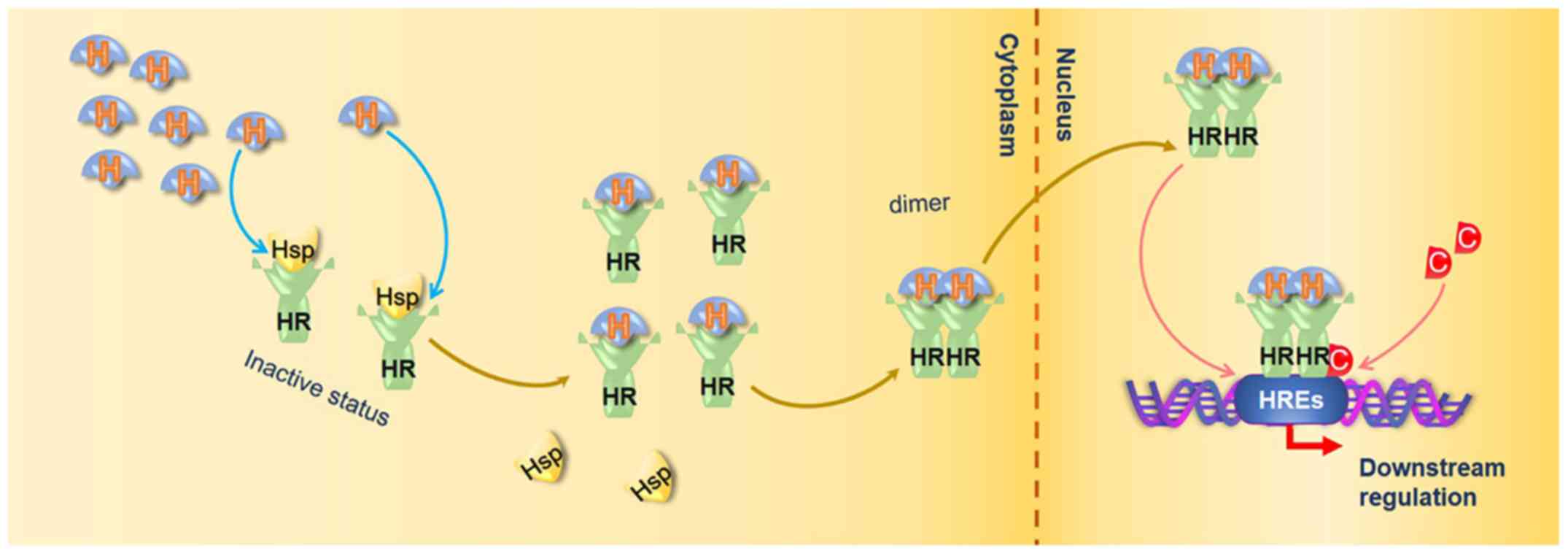
The mechanisms of AR/ER-mediated transcriptional regulation are
illustrated in Fig. 1. AR is
expressed in both androgen-dependent and -independent PCa (5,33);
it can be activated by various steroid hormones, particularly
androgenic hormones including testosterone and dihydrotestosterone
(35,36). Similar to AR, there exist both ERa
and Erβ, which are responsive to estrogen activation (6). In general, AR/ER form heterodimers
with heat shock proteins (HSPs) to remain in an inactive state in
the cytosol. HSP is released when hormone ligands bind to AR/ER,
and subsequently the hormone ligand-receptor complexes transfer
into the nuclei as a dimer, and bind to androgen/estrogen response
elements located in the enhancer regions of the downstream
regulated genes (3,8,37).
Additionally, many co-factors also participate in AR/ER-mediated
transcriptional regulation by interacting with AR/ER (3,6,8,33,37).
Accordingly, multiple endocrine therapeutic approaches focusing on
the suppression of AR/ER activation have been frequently used to
treat PCa and BCa. However, after the initial benefits received
from the AR/ER-targeted treatment, the therapeutic efficacies are
inevitably declined when patients develop more aggressive
AR/ER-independent malignancies (7,33,34,38).
In addition to ER, PR is another important sex
steroid hormone receptor for sexual maturation and gestation, whose
function is also relevant to BCa progression (39-42).
Notably, HER2, a typical proto-oncogene, has been recognized as a
key factor for promoting high risk BCa through a
steroid-independent signaling pathway (43-45).
Thus, HER2 has become an important biomarker for BCa progression as
well as a therapeutic target for ~30% of patients with BCa
(44-46). Increasing evidence has demonstrated
that downregulation of PR and/or upregulation of HER2 in BCa leads
to the acquisition of endocrinotherapy resistance (41,43,44,47).
The functional consequences of cell signaling
modulation are mainly ascribed to gene transcriptional regulation
in PCa and BCa progression (48,49).
AR/ER-mediated transcriptional regulation is thought to be critical
for the development of the early stages of PCa/BCa. Nevertheless,
AR/ER function eventually declines in the late stages of malignant
PCa/BCa, particularly as a consequences of hormone deprivation
therapy (50,51). Notably, other transcription factors
like NF-κB functionally take over AR/ER to substantially reprogram
the cell transcriptome, sustaining PCa/BCa progression under
hormone-free conditions (52-54).
It is thought that NF-κB negatively regulates AR
function by competing for transcriptional regulation (55). Previous studies have demonstrated
that androgen-independent PCa exhibits higher constitutive NF-κB
binding activity than its androgen-dependent counterpart. Tumor
necrosis factor (TNF)a induces NF-κB activation via stimulation of
inhibitor of NF-κB (IKK), which is inhibited as an androgen
analogue (56). For example,
prostate-specific antigen (PSA), a common PCa biomarker, is
regulated by AR (57). However,
NF-κB is also able to regulate PSA through binding to a kB response
element located in the promoter region (52). Consistently, inhibition of NF-κB
results in the suppression of castration-resistant prostate cancer
(CRPC) xenograft tumor growth (58). Nevertheless, NF-κB also appears to
positively regulate androgen receptor splicing variant (ARV)
transactivation (59,60). Additionally, AR-negative PCa stem
cells with high constitutive NF-κB activity promote tumor growth
during androgen deprivation therapy, suggesting that NF-κB
gradually substitutes AR during CRPC progression (61). Notably, AR activation results in
the suppression of the canonical NF-κB pathway, but leads to
upregulation of the noncanonical NF-κB pathway (61).
Likewise, NF-κB plays a key role in the promotion of
estrogen-independent growth in both ER-positive and -negative BCa
(62). In particular, the evidence
of low NF-κB activation in ER-positive BCa cells and high NF-κB
activation in ER-negative BCa cells indicates an inverse
relationship between ER and NF-κB in BCa progression (53), suggesting that constitutive NF-κB
activity is consistently increased during ER-independent BCa
progression (63,64). Blockage of NF-κB activation
efficiently inhibits proliferation and reverses therapeutic
resistance in ER-negative cells (54). Mechanistically, NF-κB represses ER
expression, and high levels of NF-κB can cause downregulation of ER
(65). In particular, it has been
noted that levels of RelB are inversely correlated with the status
of ER in BCa cells (66). RelB can
stimulate PR/SET domain 1, which represses ER expression by binding
to the ER promoter (67). However,
in some early-stage ER-positive BCa cells, NF-κB activation has
been shown to recruit ER to p65/estrogen response element motifs,
resulting in increased ER transcriptional responses (68,69).
Taken together, these findings predict that NF-κB gradually
replaces ER, from transcriptional cooperation in inflammatory BCa
states to functional substitution in hormone refractory states. The
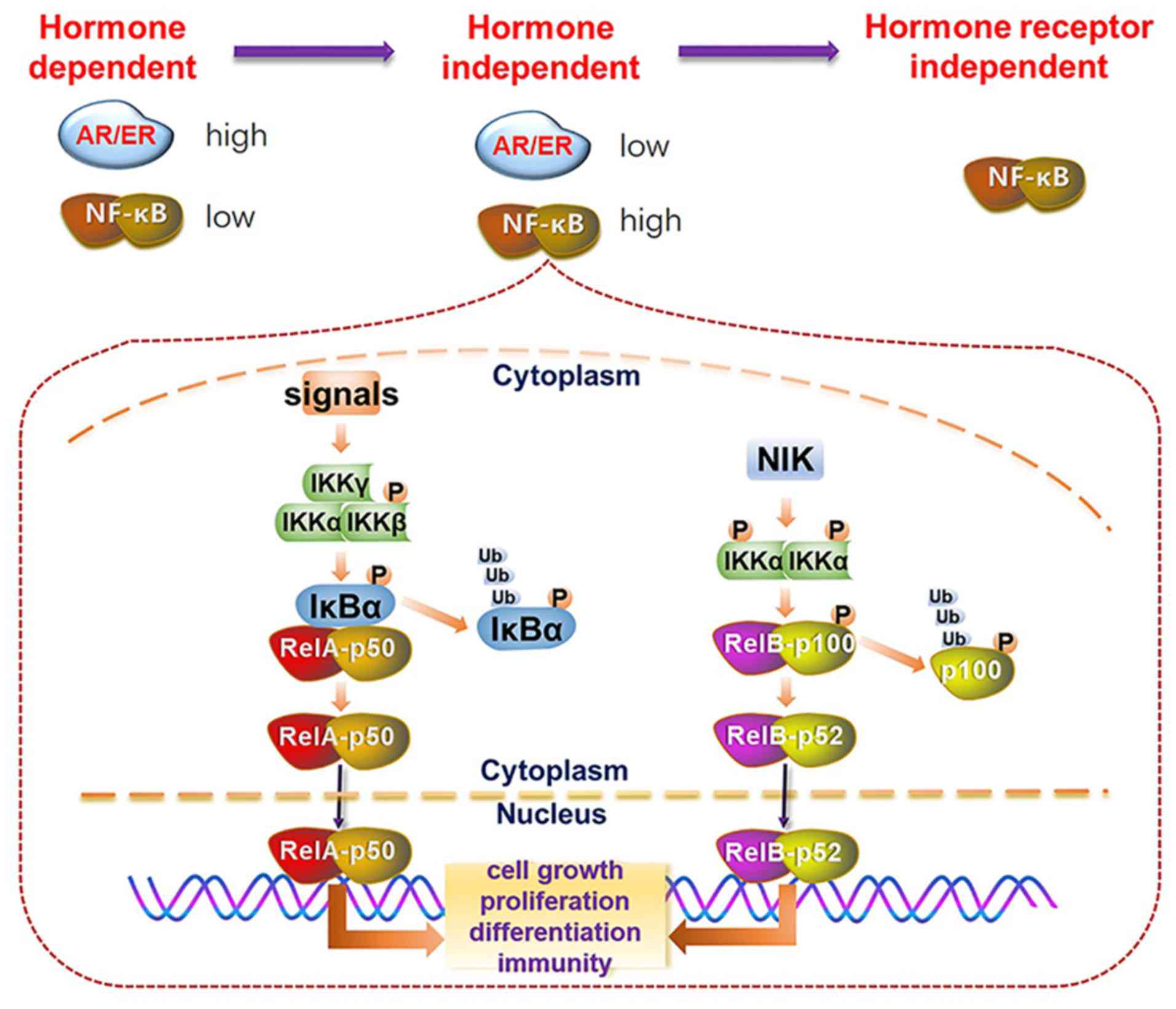
activation of the NF-κB pathway in the progression of
hormone-deprived aggressive PCa and BCa is depicted in Fig. 2.
NF-κB is involved in various biological processes,
such as cell survival, proliferation, differentiation and the
immune response (70). Members of
the NF-κB family have a conserved Rel homology domain at their
N-terminus, including RelA (p65), RelB, c-Rel, NF-κB1 (p50) and
NF-κB2 (p52) (71,72). NF-κB activation is divided into the
canonical NF-κB pathway and the noncanonical NF-κB pathway. In the
canonical NF-κB pathway, stimulating ligands including the
Toll-like superfamily, interleukin (IL)-l, TNF and other antigens
interact with their receptors to recruit adaptors, such as TNF
receptor associated factor (TRAF)2, TRAF3 and nuclear receptor
subfamily 2 group C member 2, which activate the IκB kinase complex
(IKKα, IKKβ and IKKγ/NEMO) to phosphorylate and then ubiquitinate
IKBα, leading to p50:RelA dimer nuclear translocation (73,74).
By contrast, in the activation of the noncanonical NF-κB pathway,
NF-κB-induced kinase stimulates IKKα to phosphorylate pl00,
resulting in the release of p52 and promoting p52:RelB nuclear
translocation (75,76). However, evidence has shown that p50
can also dimerize with RelB to activate the noncanonical NF-κB
pathway (76).
The activation of NF-κB plays a crucial role in PCa
progression. Ras (GTP binding protein) cooperates with NF-κB and
acts as a signal scaffold for metastatic promotion in PCa (77). In this context, it is well
documented that NF-κB-activated inflammation, including
cytokines/chemokines, contributes to CRPC (61). For instance, androgen ablation
results in regression of androgen-dependent PCa, in which
IKKα-activated NF-κB increases cytokine production leading to
androgen-free proliferation (78).
Importantly, constitutive activation of NF-κB is highly associated
with PCa resistance to both chemotherapy and radiotherapy (79).
Mounting evidence highlights that NF-κB promotes BCa
metastasis by activating the epithelial-mesenchymal transition
(EMT) process, partially by upregulating IL-lβ and IL-6 (80). In malignant BCa, epidermal growth
factor receptor is integrated with NF-κB in the activation of IL-l,
which promotes the invasive capacity of BCa cells (8l).
Additionally, IL-8 stimulates the PI3K-AKT-NF-κB signaling axis,
which in turn upregulates integrin βl/β3 expression, leading to
increased motility as well as enhanced chemoresistance and
radioresistance in BCa cells (63). Furthermore, a previous study
demonstrated that the NF-κB-controlled proinflammatory cytokine
network is important for the maintenance of cancer stem cells in
the regulation of BCa plasticity, suggesting that NF-κB-mediated
cytokine activation is critical for the recurrence of BCa after
hormone therapy (82).
Although most patients with PCa and BCa are
responsive to endocrinotherapy initially, treatment resistance and
tumor relapse remains a salient question in clinical supervision.
Indeed, clinical outcomes indicate that hormone deprivation
treatment somehow promotes the development of hormone-independent
malignant tumor types (83,84).
Accordingly, multiple mechanisms have been reported to be relevant
to endocrinotherapy resistance, including activation of the NF-κB
pathway (47,83-87).
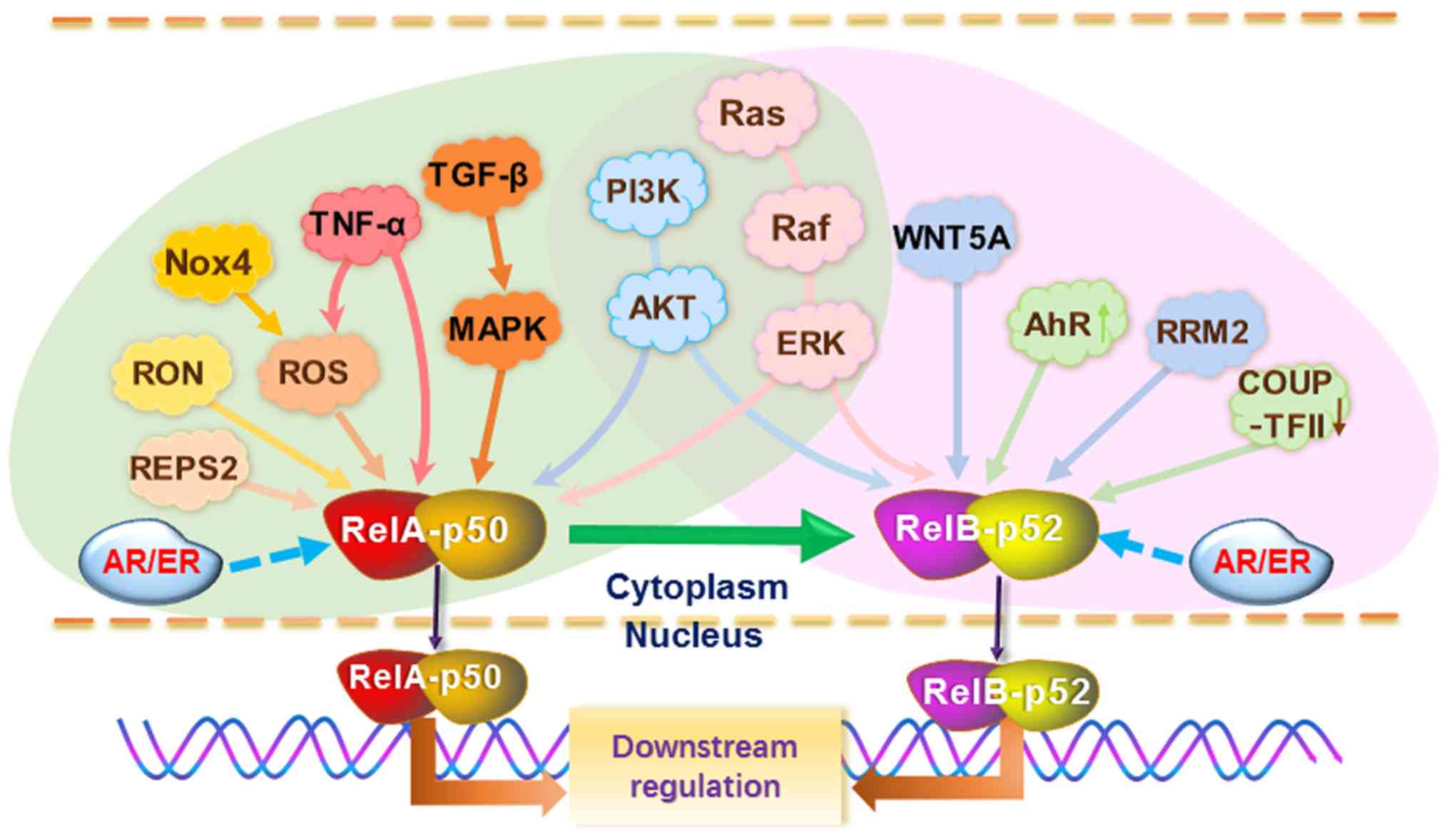
Notably, the TNF-a, WNT5A, PI3K-AKT, Ras-Raf-ERK and transforming
growth factor (TGF)-β1-mitogen activated protein kinase (MAPK)
signaling axes have been demonstrated to be important upstream
signaling pathways of the NF-κB pathway in both PCa and BCa
(47,85,86,88-92).
As a typical redox responsible transcription factor,
NF-κB responds to stimulation with reactive oxygen species (ROS).
In the regard, NADPH oxidase 4 leads to ROS production accompanied
by mitochondrial respiration, thereby stimulating the NF-κB pathway
(88). Anticancer drugs such as
TNF-α and adriamycin adapt to increase ROS, in turn induce
antioxidant enzymes like manganese superoxide dismutase (MnSOD)
through NF-κB activation (88,89).
The PI3K-AKT and Ras-Raf-ERK signaling axes have been shown to play
pivotal roles in PCa/BCa progression by modulating NF-κB in
substitution for AR/ER (47,85,86,90-95).
In particular, the activation of the PI3K-AKT-NF-κB signaling axis
has been well documented for the development of a
hormone-independent phenotype as well as therapeutic resistance in
both PCa and BCa (63,96,97).
Notably, PI3K activation in PTEN-deficient PCa is a hallmark of an
androgen-independent phenotype (17). The results of a previous study
suggested a reciprocal feedback between the two oncogenic pathways
(98). PI3K activation leads to
repression of AR transcriptional output and, consistently, PI3K
inhibition activates AR signaling. Conversely, AR inhibition
promotes PI3K activity in PTEN-deficient PCa. Thus, combined AR and
PI3K inhibition produces improved therapeutic responses (98). Since PI3K is a key upstream
signaling molecule for activation of the NF-κB pathway, this
finding mechanistically elucidated the inverse association between
AR and the NF-κB pathway.
In addition, TGF-β1-induced p38-MAPK signaling
upregulates IL-6 expression due to RelA activation (99-101). RalBP1-associated Eps
domain-containing protein 2 mediates RelA activation, and was also
shown to promote androgen-independent growth (102). Nevertheless, NF-κB has also been
shown to cooperate with AR under androgen deprivation conditions;
for instance, macrophage stimulating 1 receptor, a receptor
tyrosine kinase, is able to activate NF-κB, which is sufficient to
drive AR nuclear localization under androgen deprivation condition
and support CRPC growth (103).
Notably, the canonical NF-κB pathway can actually
induce the noncanonical NF-κB pathway, thereby sustaining high
NF-κB activity (76,104). Additionally, several inducible
agents have been demonstrated to directly activate the noncanonical
NF-κB pathway. WNT5A from bone stromal cells induces bone
morphogenetic protein 6 (BMP-6) via RelB activation; in turn, BMP-6
stimulates PCa cell proliferation via the interaction between Smad5
and β-catenin (28). In addition,
WNT5A activates NF-κB signaling to induce MMP7 expression, thereby
contributing to the invasion of TNBC cells (105). A decrease in chicken ovalbumin
upstream promoter transcription factor II results in
endocrinotherapy resistance in BCa cells by activating the
noncanonical NF-κB pathway (106); whereas, fucoxanthin appears to be
able to reverse BCa endocrinotherapy resistance by suppressing RelB
activation (107). Overexpression
of aryl hydrocarbon receptor (AhR) leads to the activation of RelB,
in turn upregulating IL-8 expression in BCa cells (108,109). Ribonucleotide reductase M2 (RRM2)
leads to increased RelB activity, thereby endowing tamoxifen
resistance due to the upregulation of Bcl-2 in BCa cells (110). Overall, NF-κB functions as a
master switch, changing PCa/BCa from an AR/ER-positive phenotype to
an AR/ER-negative phenotype (Fig.
3).
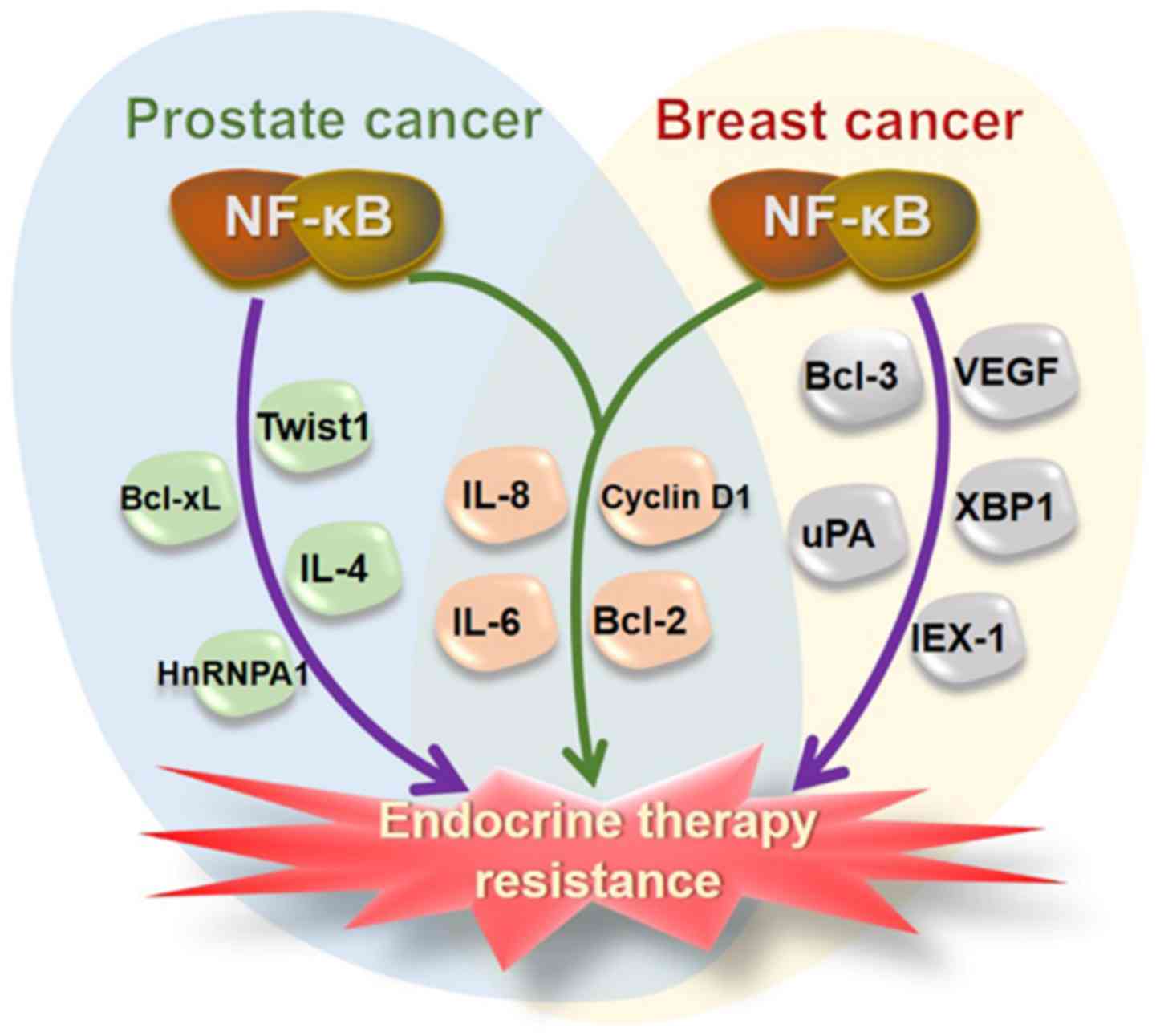
NF-κB regulates a series of genes relevant to
endocrinotherapy resistance. Particularly, it has been widely
recognized that both canonical and noncanonical NF-κB pathways are
vital for resistance to hormone receptor-targeted treatment in PCa
and BCa (52,53,58,60,111). As important NF-κB-regulated
proteins, Bcl-2, cyclin D1, IL-6 and IL-8 appeared to be critical
for endocrinotherapy resistance in both PCa and BCa. The main NF-κB
regulated proteins associated with endocri- notherapy resistance
are summarized in Fig. 4.
Bcl-2, an important antiapoptotic protein, was
upregulated in response to ROS-mediated NF-κB activation, promoting
therapeutic resistance (112).
TNF-α-mediated RelA activation contributes to CRPC partially
through upregulation of Bcl-2 (113). In addition, the activation of
NF-κB results in upregulation of IL-6, leading to castration
resistance (114). Induction of
IL-6 is important for hormone resistance, which is positively
regulated by the canonical NF-κB pathway, but negatively regulated
by AP-1 (115). IL-8 also
promotes the progression of CRPC through NF-κB activation (116). NF-κB-activated IL-4 has been
shown to enhance AR function in PCa cells with an absence or low
levels of androgen (117).
Altogether, the feed-forward activation of
NF-κB-cytokines/chemokines is essential for the appearance of CRPC
(118). In androgen-refractory
PCa, the activation of canonical NF-κB pathway significantly
increases the disease-specific death due to AKT-mediated IKK
phosphorylation (119).
Furthermore, NF-κB-enhanced EMT upregulates Twist1 in response to
AR inhibition, leading to CRPC (120).
Estrogen withdrawal leads to increased p50:RelA DNA
binding activity and sustained estrogen-independent growth through
upregulation of cyclin D1 and Bcl-3 (121). Moreover, NF-κB-mediated
upregulation of cyclin D1, urokinase and vascular endothelial
growth factor contributes to endocrino- therapy resistance in
high-risk ER-positive BCa (122).
Immediate early gene X-1 expression is stimulated by tamoxifen
through the binding of NF-κB to the promoter (123). X-box binding protein 1 is a key
factor for antiestrogen resistance, the expression of which is
regulated by modulating RelA (124). In tamoxifen-resistant BCa cells,
NF-κB activation results in an increase in IL-6 (125). In TNBC cells, the
lipoprotein(a)-lysophosphatidic acid receptor 2-enhancer of zeste 2
polycomb repressive complex 2 subunit-NF-κB signaling cascade is
required for the coordinated autocrine effect of IL-6 and IL-8
(126). As expected, Bcl-2 is
upregulated by the canonical NF-κB pathway in response to tamoxifen
(127). Similar to PCa, the
activation of the PI3K-AKT-NF-κB signaling axis is highly
associated with endocrinotherapy resistance in BCa (128).
In contrast to the well-studied p50:RelA activation
described above, the role of p52:RelB in cancer responses to
treatment remains elusive. Indeed, the noncanonical NF-κB pathway
exerts even more effects in metastasis and therapeutic resistance
rather than in tumorigenesis. p52:RelB can activate AR-responsive
genes, such as PSA and NKX3.1 (a prostate-specific tumor
suppressor) in a ligand-independent manner, suggesting that the
noncanonical NF-κB pathway also plays a supporting role in CRPC
progression (129). In addition,
p52:RelB activation increases PCa cell survival and proliferation
by upregulating Bcl-xL and cyclin D1 (130-132). Moreover, p52:RelB activation
contributes to resistance to AR-targeted therapies through
regulation of multiple signaling pathways, such as by modulating AR
(60), upregulating c-Myc-depen-
dent heterogeneous nuclear RNA-binding protein A1 (133), and enhancing glucose flux to the
glycolysis and pentose phosphate pathways (134). Consistent with PCa, RelB is also
highly expressed in hormone therapy-resistant BCa cells (111). Fucoxanthin appears to be able to
reverse hormone therapy resistance by suppressing p52:RelB
(107). Overexpression of AhR and
RRM2 leads to the activation of RelB, thereby endowing tamoxifen
resistance due to the upregulation of Bcl-2 and IL-8 (111). MEK-mediated p52 activation is
required for TNBC growth and drug resistance (135). Overexpression of HOXB13 (a
homeobox protein) enhances RelB nuclear translocation and
contributes to therapeutic resistance (136).
Since NF-κB contributes to endocrinotherapy
resistance in PCa and BCa, NF-κB-targeted therapy has frequently
been applied to enhance endocrinotherapy. Nitric oxide donors
sensitize Trail-mediated apoptosis via inhibition of Bcl-xL through
inactivation of NF-κB (137).
IL-6, a NF-κB-regulated cytokine, contributes to
androgen-independent PCa progression. Inhibition of IL-6 enhances
the sensitivity of PCa to docetaxel (138). NF-κB activation in ARVs
associated with CRPC leads to anti-androgen therapy resistance.
Repression of NF-κB enhances the efficiency of hormone therapy
(59). Notably, Wedelia
chinensis herbal extract has been shown not merely to inhibit
AR activity in androgen-dependent PCa, but also to suppress the
expression of IKKα/β phosphorylation in hormone-independent PCa
cells (139). In BCa, NSC35446, a
hydrochloride salt compound, is able to inhibit anti-estrogenic
tumor growth and reverse antiestrogen resistance by targeting NF-κB
(140). Additionally, ivermectin
reverses chemotherapeutic resistance via suppression of
NF-κB-activated P-gp expression (141). Importantly, a number of compounds
appear to efficiently treat both aggressive PCa and BCa via
repression of NF-κB-mediated transcriptional activation. Curcumin,
an inhibitor of the NF-κB canonical pathway, is able to inhibit the
hormone-mediated invasion of BCa (142). The combination of curcumin and
bicalutamide enhances the growth inhibition of androgen-independent
PCa cells (143), while
1a,25-dihydroxyvitamin D3 has been reported to repress the NF-κB
noncanonical pathway, which strongly reduces the growth of
drug-resistant BCa cells and enhances the radiosensitivity of PCa
cells (144,145). Parthenolide, a native compound
that functions as an NF-κB repressor, has been shown to restore the
sensitivity of tamoxifen to endocrine-resistant BCa cells and to
enhance PCa cell radiosensitivity (146-148).
Endocrinotherapy resistance and tumor relapse are
major challenges in treating advanced PCa and BCa. The progression
from hormone-dependence to hormone-independence has been widely
recognized to be one of main causes of endocrinotherapy resistance.
Therefore, the molecular basis for the failure of treatments
targeting AR or ER has been well investigated. This review
reorganized the molecular mechanisms underlining endocrinotherapy
resistance and concluded that NF-κB is the most important
transcription regulator in activating the expression of a series of
genes, leading to the acquisition of the endocrinotherapy
resistance. In the majority of cases, NF-κB functionally
substitutes AR or ER in transcriptional regulation for sustaining
tumor cell survival and proliferation, by activating a different
set of genes when the efficacy of AR/ER-targeted treatments
declines. It should be noted, however, the inverse association
between AR/ER and NF-κB is not persistent during the progression of
PCa and BCa; in particular, a few case studies have demonstrated
that RelA can cooperate with AR in transcriptional regulation when
androgen deprivation treatment fails (103). Additionally, although the NF-κB
pathway is thought to serve as a key mechanism underlying
endocrinotherapy resistance, other signaling pathways, such as Myc,
Stat3 and Wnt, also play regulatory roles in the acquisition of
therapeutic resistance. Furthermore, NF-κB also plays a crucial
role in the radioresistance of PCa and BCa through upregulation of
antioxidant and antiapoptotic proteins, including MnSOD and Bcl-2
(149).
The present review also outlined that several
upstream signaling pathways engage to trigger the NF-κB pathway; in
particular, PI3K-AKT upstream signaling activates the NF-κB pathway
in response to oxidative stress and inflammatory stimulation.
Importantly, the cytokine/chemokine-NF-κB signaling feed-forward
loop is indispensable for the acquisition of endocrinotherapy
resistance. It was recently concluded that TGF-β, IL-6, IL-8 and
TNF-α are the most important cytokines associated with multidrug
resistance in BCa (150). These
four cytokines are typical NF-κB-regulated proteins, and increased
levels of inflammation in turn activate the NF-κB pathway, which
promotes endocrinotherapy resistance.
Distant organ metastasis associated with multidrug
resistance precludes successful treatment. A myriad of studies have
demonstrated that RelA-activated canonical NF-κB pathway is
critical for cancer progression and therapeutic resistance
(82,103,110,124,151-153). However, the effect of the
RelB-activated noncanonical NF-κB pathway is underestimated.
Indeed, RelA can upregulate RelB, leading to sustained long-term
NF-κB activity in cancer progression (76). Since the function of RelA is
essential for normal physiological development, the failure of
anticancer treatment by targeting RelA may be caused by either low
therapeutic efficacy or unexpected side effects. It has been
demonstrated that RelB is uniquely expressed at a high level in
advanced PCa, which contributes to therapeutic resistance (149,154). Accordingly, blockage of RelB
nuclear translocation has the effect of reversing resistance to
treatment in AR-negative PCa (155). Thus, the inactivation of the
noncanonical NF-κB pathway may provide a promising approach to the
treatment of advanced PCa and BCa when AR/ER-targeted therapeutic
efficiency declines.
In summary, this review emphasized the importance of
NF-κB in the acquisition of endocrinotherapy resistance in PCa and
BCa, suggesting that inhibition of the NF-κB pathway may overcome
endocrinotherapy resistance and should be beneficial in developing
comprehensive treatment strategies to control malignant PCa and
BCa. In addition to the well-documented canonical NF-κB pathway,
the noncanonical NF-κB pathway remains to be fully elucidated.
Emerging evidence predicts that RelB may exert an even greater
effect than RelA on metastasis and therapeutic resistance, based on
its capacity for maintaining NF-κB activity. Of further interest,
therefore, is why and how the noncanonical NF-κB pathway
contributes to cancer progression and therapeutic resistance. To
that end, this review is expected to shed light on future in-depth
investigations into NF-κB function to advance the treatment of
PCa/BCa therapeutic resistance.
This work was supported by National Natural Science
Foundation of China Research Grants (grant no. 81572742) and
National Program Project for Precision Medicine in National
Research and Development Plan, China (grant no. 2016YFC0905900),
and the National Natural Science Foundation of China (grant nos.
81274158 and 81873131).
Not applicable.
XW and YX conceived and wrote the review. XW, YF,
WS, ZX and YZ collected and organized the literature. XW, XD and YX
supervised the work and provided administrative, technical and
material support. All authors read and approved the content of the
review.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
Not applicable.
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Feldman BJ and Feldman D: The development
of androgen-independent prostate cancer. Nat Rev Cancer. 1:34–45.
2001. View
Article : Google Scholar
|
|
4
|
Rhodes LV, Short SP, Neel NF, Salvo VA,
Zhu Y, Elliott S, Wei Y, Yu D, Sun M, Muir SE, et al: Cytokine
receptor CXCR4 mediates estrogen-independent tumorigenesis,
metastasis, and resistance to endocrine therapy in human breast
cancer. Cancer Res. 71:603–613. 2011. View Article : Google Scholar :
|
|
5
|
Debes JD and Tindall DJ: The role of
androgens and the androgen receptor in prostate cancer. Cancer
Lett. 187:1–7. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Duffy MJ: Estrogen receptors: Role in
breast cancer. Crit Rev Clin Lab Sci. 43:325–347. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Balk SP and Knudsen KE: AR, the cell
cycle, and prostate cancer. Nucl Recept Signal. 6:e0012008.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lamb AD, Massie CE and Neal DE: The
transcriptional programme of the androgen receptor (AR) in prostate
cancer. BJU Int. 113:358–366. 2014. View Article : Google Scholar
|
|
9
|
Russo J and Russo IH: The role of estrogen
in the initiation of breast cancer. J Steroid Biochem Mol Biol.
102:89–96. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yue W, Wang JP, Li Y, Fan P, Liu G, Zhang
N, Conaway M, Wang H, Korach KS, Bocchinfuso W, et al: Effects of
estrogen on breast cancer development: Role of estrogen receptor
independent mechanisms. Int J Cancer. 127:1748–1757. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Samavat H and Kurzer MS: Estrogen
metabolism and breast cancer. Cancer Lett. 356:231–243. 2015.
View Article : Google Scholar :
|
|
12
|
DeMichele A and Chodosh LA: 'Braking' the
cycle of resistance in endocrine therapy for breast cancer. Clin
Cancer Res. 21:4999–5001. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Horwich A, Parker C, de Reijke T and
Kataja V; ESMO Guidelines Working Group: Prostate cancer: ESMO
Clinical Practice Guidelines for diagnosis, treatment and
follow-up. Ann Oncol. 24(Suppl 6): vi106–vi114. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Heidenreich A, Bastian PJ, Bellmunt J,
Bolla M, Joniau S, van der Kwast T, Mason M, Matveev V, Wiegel T,
Zattoni F, et al European Association of Urology: EAU guidelines on
prostate cancer. Part 1: Screening, diagnosis, and local treatment
with curative intent-update 2013. Eur Urol. 65:124–137. 2014.
View Article : Google Scholar
|
|
15
|
Mohler JL, Armstrong AJ, Bahnson RR,
D'Amico AV, Davis BJ, Eastham JA, Enke CA, Farrington TA, Higano
CS, Horwitz EM, et al: Prostate cancer, version 1.2016. J Natl
Compr Canc Netw. 14:19–30. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Heidenreich A, Bastian PJ, Bellmunt J,
Bolla M, Joniau S, van der Kwast T, Mason M, Matveev V, Wiegel T,
Zattoni F, et al European Association of Urology: EAU guidelines on
prostate cancer: Part II: Treatment of advanced, relapsing, and
castration-resistant prostate cancer. Eur Urol. 65:467–479. 2014.
View Article : Google Scholar
|
|
17
|
Wise HM, Hermida MA and Leslie NR:
Prostate cancer, PI3K, PTEN and prognosis. Clin Sci (Lond).
131:197–210. 2017. View Article : Google Scholar
|
|
18
|
Bilusic M, Madan RA and Gulley JL:
Immunotherapy of prostate cancer: Facts and hopes. Clin Cancer Res.
23:6764–6770. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kim YJ, Jung SY and Kim K: Survival
benefit of radiotherapy after surgery in de novo stage IV breast
cancer: A population-based propensity-score matched analysis. Sci
Rep. 9:85272019. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yao Y, Chu Y, Xu B, Hu Q and Song Q:
Radiotherapy after surgery has significant survival benefits for
patients with triple-negative breast cancer. Cancer Med. 8:554–563.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Rugo HS, Rumble RB, Macrae E, Barton DL,
Connolly HK, Dickler MN, Fallowfield L, Fowble B, Ingle JN,
Jahanzeb M, et al: Endocrine therapy for hormone receptor-positive
metastatic breast cancer: American society of clinical oncology
guideline. J Clin Oncol. 34:3069–3103. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Alvarez Lopez I, de la Haba Rodriguez J,
Ruiz Simon A, Bellet Ezquerra M, Calvo Martinez L, Garcia Estevez
L, Rodriguez Lescure A and Isla Casado D: SEOM (Spanish Society for
Medical Oncology): SEOM clinical guidelines for the treatment of
metastatic breast cancer. Clin Transl Oncol. 12:719–723. 2010.
View Article : Google Scholar
|
|
23
|
Senkus E, Kyriakides S, Ohno S,
Penault-Llorca F, Poortmans P, Rutgers E, Zackrisson S and Cardoso
F; Committee EG: ESMO Guidelines Committee: Primary breast cancer:
ESMO Clinical Practice Guidelines for diagnosis, treatment and
follow-up. Ann Oncol. 26(Suppl 5): v8–v30. 2015. View Article : Google Scholar
|
|
24
|
Goetz Mp, Gradishar WJ, Anderson BO,
Abraham J, Aft R, Allison Kh, Blair SL, Burstein HJ, Dang C, Elias
ad, et al: NCCN guidelines insights: Breast cancer, version 3.2018.
J Natl Compr Canc Netw. 17:118–126. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ahmed S, Sami A and Xiang J: HER2-directed
therapy: Current treatment options for HER2-positive breast cancer.
Breast Cancer. 22:101–116. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Esteva FJ, Hubbard-Lucey VM, Tang J and
Pusztai L: Immunotherapy and targeted therapy combinations in
metastatic breast cancer. Lancet Oncol. 20:e175–e186. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tremont-Lukats IW, Bobustuc G, Lagos GK,
Lolas K, Kyritsis AP and Puduvalli VK: Brain metastasis from
prostate carcinoma: The M.D. Anderson Cancer Center experience.
Cancer. 98:363–368. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lee GT, Kang DI, Ha YS, Jung YS, Chung J,
Min K, Kim TH, Moon KH, Chung JM, Lee DH, et al: Prostate cancer
bone metastases acquire resistance to androgen deprivation via
WNT5A-mediated BMP-6 induction. Br J Cancer. 110:1634–1644. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bergen ES, Berghoff AS, Medjedovic M,
Rudas M, Fitzal F, Bago-Horvath Z, Dieckmann K, Mader RM, Exner R,
Gnant M, et al: Continued endocrine therapy is associated with
improved survival in patients with breast cancer brain metastases.
Clin Cancer Res. 25:2737–2744. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yao B, Wang J, Qu S, Liu Y, Jin Y, Lu J,
Bao Q, Li L, Yuan H and Ma C: Upregulated osterix promotes invasion
and bone metastasis and predicts for a poor prognosis in breast
cancer. Cell Death Dis. 10:282019. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Mangelsdorf DJ, Thummel C, Beato M,
Herrlich P, Schütz G, Umesono K, Blumberg B, Kastner P, Mark M,
Chambon P, et al: The nuclear receptor superfamily: The second
decade. Cell. 83:835–839. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Girdler F and Brotherick I: The oestrogen
receptors (ER alpha and ER beta) and their role in breast cancer: A
review. Breast. 9:194–200. 2000. View Article : Google Scholar
|
|
33
|
Suzuki H, Ueda T, Ichikawa T and Ito H:
Androgen receptor involvement in the progression of prostate
cancer. Endocr Relat Cancer. 10:209–216. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Carroll JS: Mechanisms of oestrogen
receptor (ER) gene regulation in breast cancer. Eur J Endocrinol.
175:R41–R49. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Russell DW and Wilson JD: Steroid 5
alpha-reductase: Two genes/two enzymes. Annu Rev Biochem. 63:25–61.
1994. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Marcelli M and Cunningham GR: Hormonal
signaling in prostatic hyperplasia and neoplasia. J Clin Endocrinol
Metab. 84:3463–3468. 1999.PubMed/NCBI
|
|
37
|
Sommer S and Fuqua SA: Estrogen receptor
and breast cancer. Semin Cancer Biol. 11:339–352. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Alluri PG, Speers C and Chinnaiyan AM:
Estrogen receptor mutations and their role in breast cancer
progression. Breast Cancer Res. 16:4942014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Mc Cormack O, Chung WY, Fitzpatrick P,
Cooke F, Flynn B, Harrison M, Fox E, Gallagher E, McGoldrick A,
Dervan PA, et al: Progesterone receptor B (PRB) promoter
hypermethylation in sporadic breast cancer: Progesterone receptor B
hypermethylation in breast cancer. Breast Cancer Res Treat.
111:45–53. 2008. View Article : Google Scholar
|
|
40
|
Wang H, Lee EW, Zhou L, Leung PC, Ross DD,
Unadkat JD and Mao Q: Progesterone receptor (PR) isoforms PRA and
PRB differentially regulate expression of the breast cancer
resistance protein in human placental choriocarcinoma BeWo cells.
Mol Pharmacol. 73:845–854. 2008. View Article : Google Scholar
|
|
41
|
Wu X, Zhang X, Zhang H, Su P, Li W, Li L,
Wang Y, Liu W, Gao P and Zhou G: Progesterone receptor
downregulates breast cancer resistance protein expression via
binding to the progesterone response element in breast cancer.
Cancer Sci. 103:959–967. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Grimm SL, Hartig SM and Edwards DP:
Progesterone receptor signaling mechanisms. J Mol Biol.
428:3831–3849. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Dowsett M: Overexpression of HER-2 as a
resistance mechanism to hormonal therapy for breast cancer. Endocr
Relat Cancer. 8:191–195. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Kurokawa H and Arteaga CL: ErbB (HER)
receptors can abrogate antiestrogen action in human breast cancer
by multiple signaling mechanisms. Clin Cancer Res. 9:511S–515S.
2003.PubMed/NCBI
|
|
45
|
Hsu JL and Hung MC: The role of HER2,
EGFR, and other receptor tyrosine kinases in breast cancer. Cancer
Metastasis Rev. 35:575–588. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Järvinen TAH, Pelto-Huikko M, Holli K and
Isola J: Estrogen receptor beta is coexpressed with ERalpha and PR
and associated with nodal status, grade, and proliferation rate in
breast cancer. Am J Pathol. 156:29–35. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Fan W, Chang J and Fu P: Endocrine therapy
resistance in breast cancer: Current status, possible mechanisms
and overcoming strategies. Future Med Chem. 7:1511–1519. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Ding J, Wang X, Zhang Y, Sang X, Yi J, Liu
C, Liu Z, Wang M, Zhang N, Xue Y, et al: Inhibition of BTF3
sensitizes luminal breast cancer cells to PI3Ka inhibition through
the transcriptional regulation of ERa. Cancer Lett. 440-441:54–63.
2019. View Article : Google Scholar
|
|
49
|
Blessing AM, Rajapakshe K, Reddy Bollu L,
Shi Y, White MA, Pham AH, Lin C, Jonsson P, Cortes CJ, Cheung E, et
al: Transcriptional regulation of core autophagy and lysosomal
genes by the androgen receptor promotes prostate cancer
progression. Autophagy. 13:506–521. 2017. View Article : Google Scholar :
|
|
50
|
McCartan D, Bolger jC, Fagan A, Byrne C,
Hao Y, Qin L, McIlroy M, Xu J, Hill AD, Gaora pO, et al: Global
characterization of the SRC-1 transcriptome identifies ADAM22 as an
ER-independent mediator of endocrine-resistant breast cancer.
Cancer Res. 72:220–229. 2012. View Article : Google Scholar
|
|
51
|
Sahin I, Mega AE and Carneiro BA: Androgen
receptor-independent prostate cancer: An emerging clinical entity.
Cancer Biol Ther. 19:347–348. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Chen CD and Sawyers CL: NF-kappa B
activates prostate-specific antigen expression and is upregulated
in androgen-independent prostate cancer. Mol Cell Biol.
22:2862–2870. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Zhou Y, Eppenberger-Castori S, Eppenberger
U and Benz CC: The NFkappaB pathway and endocrine-resistant breast
cancer. Endocr Relat Cancer. 12(Suppl 1): S37–S46. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Oida K, Matsuda A, Jung K, Xia Y, Jang H,
Amagai Y, Ahn G, Nishikawa S, Ishizaka S, Jensen-Jarolim E, et al:
Nuclear factor-KB plays a critical role in both intrinsic and
acquired resistance against endocrine therapy in human breast
cancer cells. Sci Rep. 4:40572014. View Article : Google Scholar
|
|
55
|
Malinen M, Niskanen EA, Kaikkonen MU and
Palvimo JJ: Crosstalk between androgen and pro-inflammatory
signaling remodels androgen receptor and NF-κB cistrome to
reprogram the prostate cancer cell transcriptome. Nucleic Acids
Res. 45:619–630. 2017. View Article : Google Scholar
|
|
56
|
Péant B, Diallo JS, Lessard L, Delvoye N,
Le Page C, Saad F and Mes-Masson AM: Regulation of IkappaB kinase
epsilon expression by the androgen receptor and the nuclear
factor-kappaB transcription factor in prostate cancer. Mol Cancer
Res. 5:87–94. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Yuan X, Cai C, Chen S, Chen S, Yu Z and
Balk SP: Androgen receptor functions in castration-resistant
prostate cancer and mechanisms of resistance to new agents
targeting the androgen axis. Oncogene. 33:2815–2825. 2014.
View Article : Google Scholar
|
|
58
|
Zhang L, Altuwaijri S, Deng F, Chen L, Lal
P, Bhanot UK, Korets R, Wenske S, Lilja HG, Chang C, et al:
NF-kappaB regulates androgen receptor expression and prostate
cancer growth. Am J Pathol. 175:489–499. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Jin R, Yamashita H, Yu X, Wang J, Franco
OE, Wang Y, Hayward SW and Matusik RJ: Inhibition of NF-kappa B
signaling restores responsiveness of castrate-resistant prostate
cancer cells to anti-androgen treatment by decreasing androgen
receptor-variant expression. Oncogene. 34:3700–3710. 2015.
View Article : Google Scholar
|
|
60
|
Nadiminty N, Tummala R, Liu C, Yang J, Lou
W, Evans CP and Gao AC: NF-κB2/p52 induces resistance to
enzalutamide in prostate cancer: Role of androgen receptor and its
variants. Mol Cancer Ther. 12:1629–1637. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Staal J and Beyaert R: Inflammation and
NF-kappaB signaling in prostate cancer: Mechanisms and clinical
implications. Cells. 7:72018. View Article : Google Scholar
|
|
62
|
Sas L, Lardon F, Vermeulen PB, Hauspy J,
Van Dam P, Pauwels P, Dirix LY and Van Laere SJ: The interaction
between ER and NFkB in resistance to endocrine therapy. Breast
Cancer Res. 14:2122012. View Article : Google Scholar
|
|
63
|
Shao N, Lu Z, Zhang Y, Wang M, Li W, Hu Z,
Wang S and Lin Y: Interleukin-8 upregulates integrin β3 expression
and promotes estrogen receptor-negative breast cancer cell invasion
by activating the PI3K/Akt/NF-κB pathway. Cancer Lett. 364:165–172.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Nakshatri H Jr, Bhat-Nakshatri P, Martin
DA, Goulet RJ Jr and Sledge GW Jr: Constitutive activation of
NF-kappaB during progression of breast cancer to
hormone-independent growth. Mol Cell Biol. 17:3629–3639. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Belguise K and Sonenshein GE: PKCtheta
promotes c-Rel-driven mammary tumorigenesis in mice and humans by
repressing estrogen receptor alpha synthesis. J Clin Invest.
117:4009–4021. 2007.PubMed/NCBI
|
|
66
|
Wang X, Belguise K, Kersual N, Kirsch KH,
Mineva ND, Galtier F, Chalbos D and Sonenshein GE: Oestrogen
signalling inhibits invasive phenotype by repressing RelB and its
target BCL2. Nat Cell Biol. 9:470–478. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Wang X, Belguise K, O'Neill CF,
Sanchez-Morgan N, Romagnoli M, Eddy SF, Mineva ND, Yu Z, Min C,
Trinkaus-Randall V, et al: RelB NF-kappaB represses estrogen
receptor alpha expression via induction of the zinc finger protein
Blimp1. Mol Cell Biol. 29:3832–3844. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Pradhan M, Baumgarten SC, Bembinster LA
and Frasor J: CBP mediates NF-κB-dependent histone acetylation and
estrogen receptor recruitment to an estrogen response element in
the BIRC3 promoter. Mol Cell Biol. 32:569–575. 2012. View Article : Google Scholar :
|
|
69
|
Frasor J, El-Shennawy L, Stender JD and
Kastrati I: NFkB affects estrogen receptor expression and activity
in breast cancer through multiple mechanisms. Mol Cell Endocrinol.
418:235–239. 2015. View Article : Google Scholar
|
|
70
|
Zeligs KP, Neuman MK and Annunziata CM:
Molecular pathways: The balance between cancer and the immune
system challenges the therapeutic specificity of targeting nuclear
factor-kB signaling for cancer treatment. Clin Cancer Res.
22:4302–4308. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Sun SC: Non-canonical NF-κB signaling
pathway. Cell Res. 21:71–85. 2011. View Article : Google Scholar
|
|
72
|
Kastrati I, Siklos MI, Calderon-Gierszal
EL, El-Shennawy L, Georgieva G, Thayer EN, Thatcher GR and Frasor
J: Dimethyl fumarate inhibits the nuclear factor kB pathway in
breast cancer cells by covalent modification of p65 protein. J Biol
Chem. 291:3639–3647. 2016. View Article : Google Scholar
|
|
73
|
Vrábel D, Pour L and Ševčíková S: The
impact of NF-κB signaling on pathogenesis and current treatment
strategies in multiple myeloma. Blood Rev. 34:56–66. 2019.
View Article : Google Scholar
|
|
74
|
Park MH and Hong JT: Roles of NF-κB in
cancer and inflammatory diseases and their therapeutic approaches.
Cells. 5:52016. View Article : Google Scholar
|
|
75
|
Maubach G, Feige MH, Lim MCC and Naumann
M: NF-kappaB-inducing kinase in cancer. Biochim Biophys Acta Rev
Cancer. 1871:40–49. 2019. View Article : Google Scholar
|
|
76
|
Gray CM, Remouchamps C, McCorkell KA, Solt
LA, Dejardin E, Orange JS and May MJ: Noncanonical NF-κB signaling
is limited by classical NF-κB activity. Sci Signal. 7:ra132014.
View Article : Google Scholar
|
|
77
|
Min J, Zaslavsky A, Fedele G, McLaughlin
SK, Reczek EE, De Raedt T, Guney I, Strochlic DE, Macconaill LE,
Beroukhim R, et al: An oncogene-tumor suppressor cascade drives
metastatic prostate cancer by coordinately activating Ras and
nuclear factor-kappaB. Nat Med. 16:286–294. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Ammirante M, Luo JL, Grivennikov S,
Nedospasov S and Karin M: B-cell-derived lymphotoxin promotes
castration-resistant prostate cancer. Nature. 464:302–305. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Mendonca MS, Turchan WT, Alpuche ME,
Watson CN, Estabrook NC, Chin-Sinex H, Shapiro JB, Imasuen-Williams
IE, Rangel G, Gilley DP, et al: DMAPT inhibits NF-κB activity and
increases sensitivity of prostate cancer cells to X-rays in vitro
and in tumor xenografts in vivo. Free Radic Biol Med. 112:318–326.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Kendellen MF, Bradford JW, Lawrence CL,
Clark KS and Baldwin AS: Canonical and non-canonical NF-κB
signaling promotes breast cancer tumor-initiating cells. Oncogene.
33:1297–1305. 2014. View Article : Google Scholar
|
|
81
|
Streicher KL, Willmarth NE, Garcia J,
Boerner JL, Dewey TG and Ethier SP: Activation of a nuclear factor
kappaB/interleukin-1 positive feedback loop by amphiregulin in
human breast cancer cells. Mol Cancer Res. 5:847–861. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Saha S, Mukherjee S, Khan P, Kajal K,
Mazumdar M, Manna A, Mukherjee S, De S, Jana D, Sarkar DK, et al:
Aspirin suppresses the acquisition of chemoresistance in breast
cancer by disrupting an NFkappaB-IL6 signaling axis responsible for
the generation of cancer stem cells. Cancer Res. 76:2000–2012.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Watson PA, Arora VK and Sawyers CL:
Emerging mechanisms of resistance to androgen receptor inhibitors
in prostate cancer. Nat Rev Cancer. 15:701–711. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Stender JD, Nwachukwu JC, Kastrati I, Kim
Y, Strid T, Yakir M, Srinivasan S, Nowak J, Izard T and Rangarajan
ES: et al Structural and molecular mechanisms of cytokine-mediated
endocrine resistance in human breast cancer cells. Mol Cell.
65:1122–1135.e1125. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Osborne CK and Schiff R: Mechanisms of
endocrine resistance in breast cancer. Annu Rev Med. 62:233–247.
2011. View Article : Google Scholar
|
|
86
|
Karantanos T, Evans CP, Tombal B, Thompson
TC, Montironi R and Isaacs WB: Understanding the mechanisms of
androgen deprivation resistance in prostate cancer at the molecular
level. Eur Urol. 67:470–479. 2015. View Article : Google Scholar
|
|
87
|
Crona DJ and Whang YE: Androgen
receptor-dependent and -independent mechanisms involved in prostate
cancer therapy resistance. Cancers (Basel). 9:92017. View Article : Google Scholar
|
|
88
|
Lee JW, Kim GY and Kim JH: Androgen
receptor is up-regulated by a BLT2-linked pathway to contribute to
prostate cancer progression. Biochem Biophys Res Commun.
420:428–433. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Penney RB and Roy D: Thioredoxin-mediated
redox regulation of resistance to endocrine therapy in breast
cancer. Biochim Biophys Acta. 1836:60–79. 2013.PubMed/NCBI
|
|
90
|
Bakin RE, Gioeli D, Bissonette EA and
Weber MJ: Attenuation of Ras signaling restores androgen
sensitivity to hormone-refractory C4-2 prostate cancer cells.
Cancer Res. 63:1975–1980. 2003.PubMed/NCBI
|
|
91
|
Mulholland DJ, Kobayashi N, Ruscetti M,
Zhi A, Tran LM, Huang J, Gleave M and Wu H: Pten loss and RAS/MAPK
activation cooperate to promote EMT and metastasis initiated from
prostate cancer stem/progenitor cells. Cancer Res. 72:1878–1889.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Heckler MM, Thakor H, Schafer CC and
Riggins RB: ERK/MAPK regulates ERRγ expression, transcriptional
activity and receptor-mediated tamoxifen resistance in
ER+ breast cancer. FEBS J. 281:2431–2442. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Hong SK, Jeong JH, Chan AM and Park JI:
AKT upregulates B-Raf Ser445 phosphorylation and ERK1/2 activation
in prostate cancer cells in response to androgen depletion. Exp
Cell Res. 319:1732–1743. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Wang H, Zhang L, Fu Y, Fang F, Jiang Y,
Dong Y and Zhu W: CSL regulates AKT to mediate androgen
independence in prostate cancer progression. Prostate. 76:140–150.
2016. View Article : Google Scholar
|
|
95
|
Lu S, Ren C, Liu Y and Epner DE: PI3K-Akt
signaling is involved in the regulation of p21(WAF/CIP) expression
and androgen-independent growth in prostate cancer cells. Int J
Oncol. 28:245–251. 2006.
|
|
96
|
Lee SO, Lou W, Nadiminty N, Lin X and Gao
AC: Requirement for NF-(kappa)B in interleukin-4-induced androgen
receptor activation in prostate cancer cells. Prostate. 64:160–167.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Rodriguez M, Luo W, Weng J, Zeng L, Yi Z,
Siwko S and Liu M: PSGR promotes prostatic intraepithelial
neoplasia and prostate cancer xenograft growth through NF-κB.
Oncogenesis. 3:e1142014. View Article : Google Scholar
|
|
98
|
Carver BS, Chapinski C, Wongvipat J,
Hieronymus H, Chen Y, Chandarlapaty S, Arora VK, Le C, Koutcher J,
Scher H, et al: Reciprocal feedback regulation of PI3K and androgen
receptor signaling in PTEN-deficient prostate cancer. Cancer Cell.
19:575–586. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Park JI, Lee MG, Cho K, Park BJ, Chae KS,
Byun DS, Ryu BK, Park YK and Chi SG: Transforming growth
factor-beta1 activates interleukin-6 expression in prostate cancer
cells through the synergistic collaboration of the Smad2,
p38-NF-kappaB, JNK, and Ras signaling pathways. Oncogene.
22:4314–4332. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Holloway JN, Murthy S and El-Ashry D: A
cytoplasmic substrate of mitogen-activated protein kinase is
responsible for estrogen receptor-alpha down-regulation in breast
cancer cells: The role of nuclear factor-kappaB. Mol Endocrinol.
18:1396–1410. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Tian M and Schiemann WP: TGF-beta
stimulation of EMT programs elicits non-genomic ER-alpha activity
and anti-estrogen resistance in breast cancer cells. J Cancer
Metastasis Treat. 3:150–160. 2017. View Article : Google Scholar :
|
|
102
|
Penninkhof F, Grootegoed JA and Blok LJ:
Identification of REPS2 as a putative modulator of NF-kappaB
activity in prostate cancer cells. Oncogene. 23:5607–5615. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Brown NE, Paluch AM, Nashu MA, Komurov K
and Waltz SE: Tumor cell autonomous RON receptor expression
promotes prostate cancer growth under conditions of androgen
deprivation. Neoplasia. 20:917–929. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
House CD, Jordan E, Hernandez L, Ozaki M,
James JM, Kim M, Kruhlak MJ, Batchelor E, Elloumi F, Cam MC, et al:
NFkappaB promotes ovarian tumorigenesis via classical pathways that
support proliferative cancer cells and alternative pathways that
support ALDH(+) cancer stem-like cells. Cancer Res. 77:6927–6940.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Han B, Zhou B, Qu Y, Gao B, Xu Y, Chung S,
Tanaka H, Yang W, Giuliano AE and Cui X: FOXC1-induced
non-canonical WNT5A-MMP7 signaling regulates invasiveness in
triple-negative breast cancer. Oncogene. 37:1399–1408. 2018.
View Article : Google Scholar :
|
|
106
|
Litchfield LM, Appana SN, Datta S and
Klinge CM: COUP-TFII inhibits NFkappaB activation in
endocrine-resistant breast cancer cells. Mol Cell Endocrinol.
382:358–367. 2014. View Article : Google Scholar
|
|
107
|
Rwigemera A, Mamelona J and Martin LJ:
Inhibitory effects of fucoxanthinol on the viability of human
breast cancer cell lines MCF-7 and MDA-MB-231 are correlated with
modulation of the NF-kappaB pathway. Cell Biol Toxicol. 30:157–167.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Vogel CF, Li W, Wu D, Miller JK, Sweeney
C, Lazennec G, Fujisawa Y and Matsumura F: Interaction of aryl
hydrocarbon receptor and NF-κB subunit RelB in breast cancer is
associated with interleukin-8 overexpression. Arch Biochem Biophys.
512:78–86. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Bekki K, Vogel H, Li W, Ito T, Sweeney C,
Haarmann- Stemmann T, Matsumura F and Vogel CF: The aryl
hydrocarbon receptor (AhR) mediates resistance to apoptosis induced
in breast cancer cells. Pestic Biochem Physiol. 120:5–13. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Shah KN, Wilson EA, Malla R, Elford HL and
Faridi JS: Targeting ribonucleotide reductase M2 and NF-kappaB
activation with didox to circumvent tamoxifen resistance in breast
cancer. Mol Cancer Ther. 14:2411–2421. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Yde CW, Emdal KB, Guerra B and Lykkesfeldt
AE: NFkB signaling is important for growth of antiestrogen
resistant breast cancer cells. Breast Cancer Res Treat. 135:67–78.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Catz SD and Johnson JL: Transcriptional
regulation of bcl-2 by nuclear factor kappa B and its significance
in prostate cancer. Oncogene. 20:7342–7351. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Srinivasan S, Kumar R, Koduru S,
Chandramouli A and Damodaran C: Inhibiting TNF-mediated signaling:
A novel therapeutic paradigm for androgen independent prostate
cancer. Apoptosis. 15:153–161. 2010. View Article : Google Scholar :
|
|
114
|
Liu VWS, Yau WL, Tam CW, Yao KM and Shiu
SY: Melatonin inhibits androgen receptor splice variant-7
(AR-V7)-induced nuclear factor-kappa B (NF-kappaB) activation and
NF-kappaB activator-induced AR-V7 expression in prostate cancer
cells: Potential implications for the use of melatonin in
castration-resistant prostate cancer (CRPC) therapy. Int J Mol Sci.
18:182017. View Article : Google Scholar
|
|
115
|
Zerbini LF, Wang Y, Cho JY and Libermann
TA: Constitutive activation of nuclear factor kappaB p50/p65 and
Fra-1 and JunD is essential for deregulated interleukin 6
expression in prostate cancer. Cancer Res. 63:2206–2215.
2003.PubMed/NCBI
|
|
116
|
Araki S, Omori Y, Lyn D, Singh RK,
Meinbach DM, Sandman Y, Lokeshwar VB and Lokeshwar BL:
Interleukin-8 is a molecular determinant of androgen independence
and progression in prostate cancer. Cancer Res. 67:6854–6862. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Lee SO, Pinder E, Chun JY, Lou W, Sun M
and Gao AC: Interleukin-4 stimulates androgen-independent growth in
LNCaP human prostate cancer cells. Prostate. 68:85–91. 2008.
View Article : Google Scholar
|
|
118
|
Jeong JH, Park SJ, Dickinson SI and Luo
JL: A constitutive intrinsic inflammatory signaling circuit
composed of miR-196b, Meis2, PPP3CC, and p65 drives prostate cancer
castration resistance. Mol Cell. 65:154–167. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
McCall P, Bennett L, Ahmad I, Mackenzie
LM, Forbes IW, Leung HY, Sansom OJ, Orange C, Seywright M,
Underwood MA, et al: NFκB signalling is upregulated in a subset of
castrate-resistant prostate cancer patients and correlates with
disease progression. Br J Cancer. 107:1554–1563. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Shiota M, Yokomizo A, Takeuchi A,
Kashiwagi E, Dejima T, Inokuchi J, Tatsugami K, Uchiumi T and Eto
M: Protein kinase C regulates Twist1 expression via NF-κB in
prostate cancer. Endocr Relat Cancer. 24:171–180. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Pratt MAC, Bishop TE, White D, Yasvinski
G, Menard M, Niu MY and Clarke R: Estrogen withdrawal-induced
NF-kappaB activity and bcl-3 expression in breast cancer cells:
Roles in growth and hormone independence. Mol Cell Biol.
23:6887–6900. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Zhou Y, Yau C, Gray JW, Chew K, Dairkee
SH, Moore DH, Eppenberger U, Eppenberger-Castori S and Benz CC:
Enhanced NF kappa B and AP-1 transcriptional activity associated
with antiestrogen resistant breast cancer. BMC Cancer. 7:592007.
View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Semlali A, Oliva J, Badia E, Pons M and
Duchesne MJ: Immediate early gene X-1 (IEX-1), a hydroxytamoxifen
regulated gene with increased stimulation in MCF-7 derived
resistant breast cancer cells. J Steroid Biochem Mol Biol.
88:247–259. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Hu R, Warri A, Jin L, Zwart A, Riggins RB,
Fang HB and Clarke R: NF-κB signaling is required for XBP1
(unspliced and spliced)-mediated effects on antiestrogen
responsiveness and cell fate decisions in breast cancer. Mol Cell
Biol. 35:379–390. 2015. View Article : Google Scholar
|
|
125
|
Yamaguchi N, Nakayama Y and Yamaguchi N:
Down-regulation of Forkhead box protein A1 (FOXA1) leads to cancer
stem cell-like properties in tamoxifen-resistant breast cancer
cells through induction of interleukin-6. J Biol Chem.
292:8136–8148. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Hartman ZC, Poage GM, den Hollander P,
Tsimelzon A, Hill J, Panupinthu N, Zhang Y, Mazumdar A, Hilsenbeck
SG, Mills GB, et al: Growth of triple-negative breast cancer cells
relies upon coordinate autocrine expression of the proinflammatory
cytokines IL-6 and IL-8. Cancer Res. 73:3470–3480. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Nehra R, Riggins RB, Shajahan AN, Zwart A,
Crawford AC and Clarke R: BCL2 and CASP8 regulation by NF-kappaB
differentially affect mitochondrial function and cell fate in
antiestrogen-sensitive and -resistant breast cancer cells. FASEB J.
24:2040–2055. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Riggins RB, Zwart A, Nehra R and Clarke R:
The nuclear factor kB inhibitor parthenolide restores ICI 182,780
(Faslodex; fulvestrant)-induced apoptosis in antiestrogen-resistant
breast cancer cells. Mol Cancer Ther. 4:33–41. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Nadiminty N, Lou W, Sun M, Chen J, Yue J,
Kung HJ, Evans CP, Zhou Q and Gao AC: Aberrant activation of the
androgen receptor by NF-kappaB2/p52 in prostate cancer cells.
Cancer Res. 70:3309–3319. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Nadiminty N, Chun JY, Lou W, Lin X and Gao
AC: NF-kappaB2/p52 enhances androgen-independent growth of human
LNCaP cells via protection from apoptotic cell death and cell cycle
arrest induced by androgen-deprivation. Prostate. 68:1725–1733.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Nadiminty N, Dutt S, Tepper C and Gao AC:
Microarray analysis reveals potential target genes of
NF-kappaB2/p52 in LNCaP prostate cancer cells. Prostate.
70:276–287. 2010.
|
|
132
|
Mehraein-Ghomi F, Church DR, Schreiber CL,
Weichmann MA, Basu HS and Wilding G: Inhibitor of p52 NF-κB subunit
and androgen receptor (AR) interaction reduces growth of human
prostate cancer cells by abrogating nuclear translocation of p52
and phosphorylated ARser81. Genes Cancer. 6:428–444.
2015.PubMed/NCBI
|
|
133
|
Nadiminty N, Tummala R, Liu C, Lou W,
Evans CP and Gao AC: NF-kappaB2/p52:c-Myc:hnRNPA1 pathway regulates
expression of androgen receptor splice variants and enzalutamide
sensitivity in prostate cancer. Mol Cancer Ther. 14:1884–1895.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Cui Y, Nadiminty N, Liu C, Lou W, Schwartz
CT and Gao AC: Upregulation of glucose metabolism by NF-κB2/p52
mediates enzalutamide resistance in castration-resistant prostate
cancer cells. Endocr Relat Cancer. 21:435–442. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
House CD, Grajales V, Ozaki M, Jordan E,
Wubneh H, Kimble DC, James JM, Kim MK and Annunziata CM: Ikke
cooperates with either MEK or non-canonical NF-κB driving growth of
triple-negative breast cancer cells in different contexts. BMC
Cancer. 18:5952018. View Article : Google Scholar
|
|
136
|
Kim YR, Kim IJ, Kang TW, Choi C, Kim KK,
Kim MS, Nam KI and Jung C: HOXB13 downregulates intracellular zinc
and increases NF-κB signaling to promote prostate cancer
metastasis. Oncogene. 33:4558–4567. 2014. View Article : Google Scholar
|
|
137
|
Huerta-Yepez S, Vega M, Jazirehi A, Garban
H, Hongo F, Cheng G and Bonavida B: Nitric oxide sensitizes
prostate carcinoma cell lines to TRAIL-mediated apoptosis via
inactivation of NF-kappa B and inhibition of Bcl-xl expression.
Oncogene. 23:4993–5003. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Domingo-Domenech J, Oliva C, Rovira A,
Codony-Servat J, Bosch M, Filella X, Montagut C, Tapia M, Campás C,
Dang L, et al: Interleukin 6, a nuclear factor-kappaB target,
predicts resistance to docetaxel in hormone-independent prostate
cancer and nuclear factor-kappaB inhibition by PS-1145 enhances
docetaxel antitumor activity. Clin Cancer Res. 12:5578–5586. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Tsai CH, Tzeng SF, Hsieh SC, Yang YC,
Hsiao YW, Tsai MH and Hsiao PW: A standardized herbal extract
mitigates tumor inflammation and augments chemotherapy effect of
docetaxel in prostate cancer. Sci Rep. 7:156242017. View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Nathan S, Ma Y, Tomita YA, De Oliveira E,
Brown ML and Rosen EM: BRCA1-mimetic compound NSC35446. HCl
inhibits IKKB expression by reducing estrogen receptor-a occupancy
in the IKKB promoter and inhibits NF-κB activity in
antiestrogen-resistant human breast cancer cells. Breast Cancer Res
Treat. 166:681–693. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Jiang L, Wang P, Sun YJ and Wu YJ:
Ivermectin reverses the drug resistance in cancer cells through
EGFR/ERK/Akt/NF-κB pathway. J Exp Clin Cancer Res. 38:2652019.
View Article : Google Scholar
|
|
142
|
Coker-Gurkan A, Celik M, Ugur M, Arisan
ED, Obakan-Yerlikaya P, Durdu ZB and Palavan-Unsal N: Curcumin
inhibits autocrine growth hormone-mediated invasion and metastasis
by targeting NF-κB signaling and polyamine metabolism in breast
cancer cells. Amino Acids. 50:1045–1069. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Li J, Xiang S, Zhang Q, Wu J, Tang Q, Zhou
J, Yang L, Chen Z and Hann SS: Combination of curcumin and
bicalutamide enhanced the growth inhibition of androgen-independent
prostate cancer cells through SAPK/JNK and MEK/ERK1/2-mediated
targeting NF-κB/p65 and MUC1-C. J Exp Clin Cancer Res. 34:462015.
View Article : Google Scholar
|
|
144
|
Xu Y, Fang F, St Clair DK, Josson S,
Sompol P, Spasojevic I and St Clair WH: Suppression of
RelB-mediated manganese superoxide dismutase expression reveals a
primary mechanism for radiosensitization effect of
1alpha,25-dihydroxyvitamin D(3) in prostate cancer cells. Mol
Cancer Ther. 6:2048–2056. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
145
|
Lundqvist J, Yde CW and Lykkesfeldt AE:
1a,25-dihydroxyvitamin D3 inhibits cell growth and NFκB signaling
in tamoxifen-resistant breast cancer cells. Steroids. 85:30–35.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
146
|
deGraffenried LA, Chandrasekar B,
Friedrichs WE, Donzis E, Silva J, Hidalgo M, Freeman JW and Weiss
GR: NF-kappa B inhibition markedly enhances sensitivity of
resistant breast cancer tumor cells to tamoxifen. Ann Oncol.
15:885–890. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
147
|
Lobanova YS, Scherbakov AM, Shatskaya VA,
Evteev VA and Krasil'nikov MA: NF-kappaB suppression provokes the
sensitization of hormone-resistant breast cancer cells to estrogen
apoptosis. Mol Cell Biochem. 324:65–71. 2009. View Article : Google Scholar
|
|
148
|
Xu Y, Fang F, Miriyala S, Crooks PA,
Oberley TD, Chaiswing L, Noel T, Holley AK, Zhao Y, Kiningham KK,
et al: KEAP1 is a redox sensitive target that arbitrates the
opposing radiosensitive effects of parthenolide in normal and
cancer cells. Cancer Res. 73:4406–4417. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
149
|
Josson S, Xu Y, Fang F, Dhar SK, St Clair
DK and St Clair WH: RelB regulates manganese superoxide dismutase
gene and resistance to ionizing radiation of prostate cancer cells.
Oncogene. 25:1554–1559. 2006. View Article : Google Scholar
|
|
150
|
Tan C, Hu W, He Y, Zhang Y, Zhang G, Xu Y
and Tang J: Cytokine-mediated therapeutic resistance in breast
cancer. Cytokine. 108:151–159. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
151
|
Khurana N and SIKKa SC: Targeting
crosstalk between Nrf-2, NF-κB and androgen receptor signaling in
prostate cancer. Cancers (Basel). 10:102018. View Article : Google Scholar
|
|
152
|
Ahmed KM, Zhang H and Park CC: NF-κB
regulates radioresistance mediated by β1-integrin in
three-dimensional culture of breast cancer cells. Cancer Res.
73:3737–3748. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
153
|
Chaturvedi MM, Sung B, Yadav VR, Kannappan
R and Aggarwal BB: NF-κB addiction and its role in cancer: 'one
size does not fit all'. Oncogene. 30:1615–1630. 2011. View Article : Google Scholar
|
|
154
|
Lessard L, Begin LR, Gleave ME, Mes-Masson
AM and Saad F: Nuclear localisation of nuclear factor-kappaB
transcription factors in prostate cancer: An immunohistochemical
study. Br J Cancer. 93:1019–1023. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
155
|
Zhang Y, Xu Z, Ding J, Tan C, Hu W, Li Y,
Huang W and Xu Y: HZ08 suppresses RelB-activated MnSOD expression
and enhances Radiosensitivity of prostate Cancer cells. J Exp Clin
Cancer Res. 37:1742018. View Article : Google Scholar : PubMed/NCBI
|