1. Introduction
Cholangiocarcinoma (CCA) is a malignant tumour
originating from biliary epithelial cells. This malignancy can be
divided into three subtypes, namely intrahepatic CCA (iCCA),
perihilar CCA (pCCA) and distal CCA (dCCA). The latter two subtypes
have been classified as extrahepatic CCA (eCCA) in the past, but
are now considered to be different entities according to the
biological characteristics of the tumour; they also have different
treatments. Of the three subtypes of CCA, pCCA is the most common
subtype (1). Surgery is the
preferred treatment for CCA, but only ~35% of patients can undergo
surgical removal early in the disease to achieve treatment. For
patients with advanced or unresectable CCA, the effectiveness of
the existing comprehensive chemotherapeutic regimens is limited:
The current standard nursing chemotherapeutic regimen (gemcitabine
and cisplatin) has a median overall survival (OS) of <1 year.
Mesenchymal hyperplasia and genetic heterogeneity are important
factors leading to CCA resistance. The extensive tumour
microenvironment sends strong survival signals for the tumour,
which may limit the efficacy of tumour chemotherapy (2). Therefore, further studies of the
potential biomarkers of CCA for early diagnosis, comprehensive
treatment and prognosis are required.
According to the central principle in the field of
biology, genetic information is transcribed from DNA to RNA and
then translated from RNA to protein, and RNA only serves as a
carrier in the transmission of genetic information. With the rapid
development of life science research, researchers have gradually
realised that the complex biological characteristics of higher
organisms do not depend on the number of genes but rather on the
regulation of gene expression. Noncoding RNA (ncRNAs) are a type of
RNAs that do not code proteins and are produced by genomic
transcription. There are 20,000-25,000 coding genes in the human
genome, 40-90% of which are regulated by microRNAs (miRNAs)
(3). According to nucleotide
length, ncRNAs can be divided into three categories: i) Short
ncRNAs, which are ncRNAs <50 nt in length, mainly including
miRNAs, small interfering RNAs (siRNAs) and RNAs that interact with
Piwi protein; ii) medium ncRNAs, which are ncRNAs 50-200 nt in
length, which mainly include ribosomal RNAs, transfer RNAs, small
nuclear RNAs and small nucleolar RNAs; and iii) long ncRNAs
(lncRNAs), which are ncRNAs of >200 nt (4,5).
Numerous studies (2,3,5) have
confirmed that ncRNAs are not junk genes as originally thought but
are molecules with multiple functions in gene expression regulation
that serve important roles in whole cell systems. The discovery of
the regulatory functions of ncRNAs has refined our knowledge of
heredity. Studies have shown that ncRNAs play an important role in
maintaining the normal function of cells, as well as in the
occurrence and development of diseases, and thus have potential
applications as disease biomarkers.
Due to the rapid development of molecular biology,
especially gene sequencing technology, the mechanism of action of
ncRNAs in CCA has been gradually revealed, and research on the
application of ncRNAs in CCA has substantially expanded. For
example, the expression of lncRNA ECIC1 was shown to be
significantly increased in CCA tissues (6), and silencing of the lncRNA H19 gene
effectively inhibited the proliferation of CCA cells and promoted
apoptosis (7). The present review
partially summarises and describes the physiological and
pathological changes of ncRNAs and their clinical applications in
the diagnosis and treatment of CCA.
2. miRNA
miRNAs are RNA molecules with a length of ~22
nucleotides that are involved in transcriptional gene regulation
and are widely present in eukaryotes (8). In 1993, Lee et al (8) discovered the first miRNA, lin‑4, in
Caenorhabditis elegans (nematodes). In 2000, Bushati and
Cohen (9) found another miRNA,
let-7, thus initiating the study of miRNAs.
miRNAs are usually transcribed by RNA polymerase II,
which carries out the primary transcription of primary (pri)-miRNAs
(5′-cap structure and 3′-polyA tail). Several transcription factors
involved in miRNA transcription can bind to the miRNA gene upstream
of specific sites to influence miRNA expression. Then, pri-miRNAs
undergo two cleavage steps to generate mature miRNAs. Animal
pri-miRNA cleavage first occurs in the nucleus. The RNase III
nuclease Drosha and DGCR8, a cofactor, generate precursors of ~70
nucleotides with an incomplete matching stem structure, called
pre-miRNAs. Pre-miRNAs are transported from the nucleus to the
cytoplasm via Ran-GTP-dependent exportin-5-mediated transfer, and
these molecules undergo a second cleavage in the cytoplasm via the
RNase III nuclease Dicer and its cofactor TRBP; then, PACT cleaves
the segments of double-stranded RNA into 22 nucleotides that form
the mature miRNA and its complementary sequence as dimers (10,11).
There are two modes of interaction between miRNAs and target mRNAs.
When the two are completely complementary, miRNAs act in a manner
similar to RNA interference, that is, they pair and bind with fully
complementary homologous mRNAs, leading to the degradation of the
target mRNAs. However, when the miRNA and target mRNA are not
completely complementary, the miRNA binds to the 3′ UTR of target
mRNA to suppress post-transcriptional translation. Since numerous
miRNAs and target gene mRNAs are not completely complementary, the
main mode of action is post-transcriptional translational
repression (12). In addition,
current research has focused on epigenetic changes in miRNA
expression and miRNA expression via methylation and histone
modification, both of which are markers of epigenetic gene
regulation (13). For example, in
CCA cells, miR-370 is a tumour-suppressor gene that inhibits the
oncogenic protein kinase 8 (MAP3K8) (14). In addition, the overexpression of
interleukin (IL)-6 in CCA reduces the expression of miR-370 through
DNA hypermethylation, while restoring the expression of MAP3K8,
promoting the growth of tumour cells and the progression of CCA
(14). Another study showed that
the expression of immunoglobulin-dimethylformamide via miR‑370
reflected the hypermethylation‑mediated regulation induced by IL-6
in human CCA (15).
miRNAs and CCA
The expression profiles of miRNAs in tumour cells
and normal tissue cells are notably different, and most miRNA genes
are located at key genetic loci associated with tumour formation.
The occurrence of miRNA genes at a high frequency in these regions
is closely associated with tumour genomic variation (16). These findings suggest that miRNAs
may play an important role in tumour formation. However, studies
have found that CCA, a tumour with poor prognosis, is closely
associated with various miRNAs. Different miRNAs and their various
mechanistic targets in CCA are presented in Table I.
 | Table ISpecific expression, target genes and
functions of different types of miRNAs in CCA. |
Table I
Specific expression, target genes and
functions of different types of miRNAs in CCA.
A, Upregulated
|
|---|
| miRNA | Target gene | Function or
mechanism | Refs. |
|---|
| miR-26a | GSK-3β | Activates β-catenin
and promotes proliferation and colony formation of CCA cells | (111) |
| miR-21 | RECK, PDCD4, PTEN,
TIMP3, 15-PGDH | Promotes
proliferation, migration and invasion of CCA cells | (41,112,113) |
| miR-25 | DR4 | Inhibits apoptosis
of CCA cells | (114) |
| miR-31 | RASA1 | Promotes the
proliferation and inhibits the apoptosis of cancer cells | (19) |
| miR-221 | PTEN | Invasion, migration
and EMT | (115) |
| miR-141 | CLOCK | Proliferation and
physiological rhythm | (116) |
| miR-200b | PI3k, PTEN | Multidrug
resistance | (24) |
| miR-210 | Mnt | Proliferation | (116) |
| miR-199a-3p | mTOR | Multidrug
resistance | (117) |
| Let-7a | NF2 | Cell survival,
mitogenic and multidrug resistance | (49) |
| miR-421 | FXR | Proliferation and
migration | (118) |
|
B, Downregulated
|
| miRNA | Target gene | Function or
mechanism | Refs. |
|
| miR-200b/200c | ROCK2, SUZI2 | Inhibits the
migration and invasion of CCA cells and inhibits cell
carcinogenicity | (35) |
| miR-101 | VEGF, COX-2 | Inhibits
angiogenesis and growth of CCA | (37) |
| miR-494 | CDK6 | Blocks the growth
of CCA cells | (119) |
| miR-376c | GRB2 | Attenuates
epidermal growth factor-dependent cell migration | (34) |
| miR-204 | Slug | Inhibits
epithelial-mesenchymal transition and the migration and invasion of
CCA cells | (38) |
| miR-205 | MMP-2 | Multidrug
resistance | (120) |
| miR-34a | Per1, Smad4 | Proliferation,
invasion, migration and the cell cycle | (121,122) |
| miR-320 | Mcl-1/Bcl-2 | Apoptosis | (25) |
| miR-370 | MAP3K8 | Proliferation | (29,127) |
| Let-7a/miR-99a/
miR-125b | IL-6, IL-6R,
IGF1R | Invasion and
migration | (48) |
| miR-410 | XIAP | Proliferation | (123) |
| miR-148a,
miR-152 | DNMT-1 | Proliferation | (124) |
| miR-373 | MBD2 | Proliferation | (125) |
| miR-29b | Mcl-1 | proliferation and
clone formation | (27) |
| miR-214 | TWIST | Invasion and
migration | (30) |
Oncogenic miRNAs
miRNAs can be up- or downregulated in tumours,
either of which may serve a crucial role in different tumours.
Overexpressed miRNAs often affect the occurrence and progression of
CCA through different mechanisms.
By comprehensively analysing the miRNA expression
levels in 241 iCCA tumour tissues, Plieskatt et al (16) found that eight dysregulated miRNAs
were identified in all Ov‑induced ICC plasma samples and not in
control plasma, and seven displaying opposite expression changes in
plasma compared with tissue (miR-1275, miR-193a-5p, miR199b-5p,
miR-320a, miR-483-5p, miR-505-3p and miR-874). In addition,
abnormal miRNA expression in tumour tissues was inconsistent with
that observed in plasma, with 15 highly regulated miRNAs only
detected in the tumour tissue samples. Therefore, this expression
profile in plasma can be used as a biomarker for the early
diagnosis of iCCA. Petrache Voicu et al (17) found that the overexpression of
hsa-miR-21 in the CCA cell lines QBC939 and RBE significantly
promoted the migration and invasion of the cells. In addition, the
overexpression of hsa-miR-21 decreased the expression of E-cadherin
and increased the expression of N-cadherin and vimentin. This
indicates that hsa-miR-21 can induce the epithelial-mesenchymal
transition (EMT) of CCA. Zhu et al (18) found that the expression of the
miR-17-92 cluster in CCA cancer cells was higher than that in
eepithelial cells of normal bile duct, indicating that it was an
oncogenic miRNA.
Further studies have confirmed that the target of
miR-17-92 is PTEN, and identified that a new regulatory axis,
IL-6/STAT3-miR-17-92 cluster-PTEN, is important in the occurrence
and development of CCA. A study found that miR-31 is highly
expressed in CCA tissues and the cell line HCCC‑9810, and
bioinformatics analysis and immunofluores-cence experiments
identified RASA1 as a potential target of miR‑31 (19). Further experiments in that study
confirmed that miR-31 promotes the growth of CCA cells and inhibits
their apoptosis by inhibiting RASA1 expression. Another study
demonstrated that miR-605 inhibits the tumour progression of CCA
cell lines by inhibiting the expression of the oncogene PSMD10
(20). Cheng et al
(21) extracted RNA from bile to
detect the expression levels of miRNAs, and found that miR‑106a
expression in patients with CCA was significantly higher than that
in patients with gallstones, and the diagnostic sensitivity and
specificity were 83.3 and 88%, respectively. In addition,
researchers studied the characteristics of secretory vesicles
extracted from bile, including their size, number and distribution
in the biliary tract, to establish a diagnostic profile of bile
molecules and miRNAs in CCA with potential clinical value (22). Using the TCGA database, Canu et
al (23) found that miR-204
was essential in the pathogenesis of CCA, and on this basis, they
verified this miRNA in CCA cell lines; furthermore, 25 patients
with eCCA had a higher miR-221 level than the average level of 32
controls. The researchers also found that miR-221/β-catenin formed
a positive feedback loop and that PTEN was enhanced, leading to EMT
maintenance. These results confirmed that miR‑221 overexpression
promoted cell migration and invasion (23).
A study found that miR-141 is highly expressed in
CCA cells, and target genes of miR-141 include the CLOCK gene
(24). This gene not only
regulates circadian rhythm but also acts as a tumour suppressor
gene to inhibit cell division and promote cell apoptosis. Thus,
inhibition of miR-141 can increase CLOCK protein expression in CCA
cells, inhibiting the tumour suppressor function of the CLOCK
protein and promoting the development of tumours. Chen et al
(25) found that miR-106a was
elevated 110-fold in CCA tissues compared with normal liver
tissues, suggesting that it is also a specific oncogene in CCA.
miR-106a has also been found to be over-expressed in gastric,
colorectal, colorectal and pancreatic cancer tissues, but
downregulated in glioma in which it was shown to have an anticancer
role (21,25). However, high tissue levels of
miR-106a have been reported to be associated with glioma invasion
through the targeting of metalloproteinase-2, 3 and 4 (26).
Tumour suppressor miRNAs
In contrast to the genes that are highly expressed
in CCA, some miRNAs exhibit low expression in CCA, and the roles of
miRNAs in CCA involve different targets and mechanisms. Okamoto
et al (27) confirmed that
the overexpression of miR-29b significantly inhibited the
proliferation and colony formation of CCA cells through MTT and
colony formation assays. Furthermore, flow cytometric analysis
showed that the overexpression of miR‑29b significantly induced
apoptosis and blocked the cell cycle in the S phase, suggesting
that miR-29b is a tumour suppressor miRNA in CCA. Another miRNA,
miR-373 has been found to target and negatively regulate MBD2,
which is an important modifier of genes silenced by methylation,
and affects the expression of the downstream oncosuppressor gene
RASSFlA (28). CCA cancer cells
transfected with miR-373 precursors exhibited inhibited growth with
downregulated MBD2 and increased expression of RASSFlA, indicating
that miR-373 can act as a tumour suppressor gene in CCA and exert a
corresponding inhibitory effect on tumour development (28).
The expression of miR-370 in CCA specimens and CCA
cell lines has been shown to be downregulated to varying degrees
(29). The target gene of miR-370
is the oncogene MAP3K8, which is negatively regulated by miR-370.
IL-6 regulates miR-370; when IL-6 is overexpressed, miR-370 is
downregulated, and the expression of its target gene MAP3K8 is
increased, thus promoting the proliferation of biliary cancer cells
in vitro and the growth of allogeneic tumours in mice.
Increased expression of miR-370 inhibited the proliferation of CCA
cells. Ngankeu et al (30)
found that the antiapoptotic gene McL-1 was targeted by miR-29b,
and detected McL in various tumours. Abnormal overexpression of
McL-1 was also shown to be associated with tumour prognosis and
recurrence (30). In CCA, the
overexpression of McL-1 led to tumour cell resistance to tumour
necrosis factor-related apoptosis-inducing ligand (TRAIL)-mediated
apoptosis, while reduced expression of this gene increased the
sensitivity of cells to apoptotic mechanisms (30). Although the expression of miR-29b
is downregulated in CCA cells, increased expression of miR-29b was
able to reduce the level of the target gene McL-1, thus indicating
its anticancer role (30).
It has been reported that Hedgehog signalling,
Toll-like receptor and c-Myc can downregulate the expression of
miR-29b, and these three genes allow CCA cells to escape
TRAIL-mediated apoptosis (31).
Kwon et al (32) identified
miR-34a as an important tumour suppressor in human CCA and
disclosed a novel epigenetic regulatory mechanism in which miR-34a
inhibits CCA growth by targeting the Notch pathway. This finding
also suggests that restoration of endogenous miR-34a expression by
targeting the epigenetic machinery, such as the enhancer of zeste
homolog 2 (EZH2) inhibitor, or delivery of an exogenous miR-34a
mimic may represent novel therapeutic strategies for the treatment
of human CCA. Using quantitative PCR (qPCR), Li et al
(33) found that the expression of
miR‑214 in metastatic CCA tissues was significantly lower than that
in non-metastatic CCA tissues. When miR-214 was downregulated in
CCA cells, the transcription level of the mesenchymal-epithelial
transition (MET) regulatory gene TWIST increased, resulting in
decreased E-cadherin expression in epithelial cells. The
researchers concluded that miR-214 regulated the invasion and
metastasis of cells by controlling the transcription of the target
gene TWIST.
Iwaki et al (34) found that another miRNA, miR-376c,
was also associated with the metastasis of CCA; miR-376c was
expressed at low levels in the CCA cell line HuCCT1, which
accelerated the epidermal growth factor-dependent tumour metastatic
process by acting on growth factor-binding receptor 2. In addition,
epigenetic analysis confirmed that the low level of miR-376c in CCA
cell lines was associated with hypermethylation of the CpG island
upstream of the gene (34).
Another study reported the low expression of the miR-200 family,
including miR-200a, miR-200b, miR-200c and miR‑429, in CCA and
verified that miR‑200b/c could inhibit the invasion and metastasis
of CCA cells by directly inhibiting the expression of rho-kinase2
(35). In addition miR-200b/c was
shown to inhibit the formation of tumours, via the target SUZ12
(35). miR‑144 has also been shown
to be significantly downregulated in CCA, inhibiting not only the
growth of tumour cells but also the invasion and metastasis of CCA
cells by acting on platelet-activating factor acetylhydrolase
isoenzyme 1b (36). In another
study, Zhang et al (37)
found that miR-101 exhibited low expression in 43.5% of CCA tissue
specimens and in three CCA cell lines compared with noncancerous
controls. Further analysis of its biological function demonstrated
that miR-101 inhibits tumour angiogenesis in CCA cell lines by
acting on vascular endothelial growth factor to inhibit the growth
of CCA (37). The miR-29 and
miR-320 expression levels in CCA cells or tissues are suppressed,
and the Bcl-2 antiapoptotic genes in the Mcl-1 family are
associated with miR-29 and target genes of miR-320 (25,38),
Mcl-1 plays an important role in resistance to CCA cell apoptosis
(39), and the increased
expression of miR-320 and miR-29 in CCA cells reduces the
expression of Mcl-1, leading to decreased cell resistance. The
expression of miR-204 has been shown to be low in CCA tissue
(25,38). Furthermore, miR-204 is able to
inhibit the expression of Bcl-2, thus increasing the drug
resistance of cells (38).
miR‑21
Numerous studies have shown that miR-21, a miRNA
upregulated in CCA, is overexpressed in numerous types of malignant
tumours, such as nasopharyngeal, oesophageal squamous cell,
gastric, liver, pancreatic, colorectal, lung, breast and ovarian
cancer, and acts as an oncogene. This miRNA is associated with poor
prognosis of malignant tumours (40). Researchers have reported that the
overexpression of miR-21 promoted the proliferation, migration and
metastasis of cancer cells, while its downregulation had the
opposite effect (40). In
addition, miR-21 may be involved in the phenotypic characteristics
of cancer cells, including cell proliferation, apoptosis and the
cell cycle, and may play a crucial role in the expression of gene
products (40,41). Selaru et al (41) found that miR‑21 was significantly
overexpressed in CCA tissues compared with normal tissues, and
revealed that the knockdown of miR-21 can reduce the metastatic
potential of CCA cells by regulating PDCD4 and tissue inhibitor of
metalloproteinases 3 (TIMP3). TIMP3 plays a crucial role in the
invasion and metastasis of cancer (42). In addition, Ars2 protein is
essential for the development of miRNAs (42). Ars2 and miR‑21 have been identified
to be overexpressed in CCAs, with the knockdown of Ars2 resulting
in downregulation of miR-21 expression and inhibiting tumour
formation, while the overexpression of Ars2 did not increase miR-21
expression or promote tumour formation (43). Furthermore, the overexpression of
miR-21 can promote the invasion and metastasis of the RBE CCA cell
line, while inhibition of miR-21 has the opposite effect (44). miR-21 affects the invasion and
metastasis of CCA cells through the tumour suppressor gene PTEN.
The high expression of miR-21 in CCA has been shown to inhibit the
expression of tumour suppressor genes such as PDCD4 and TIMP3, as
well as the expression of PTEN, and influences the invasion and
metastasis of RBE cells by inducing EMT (41).
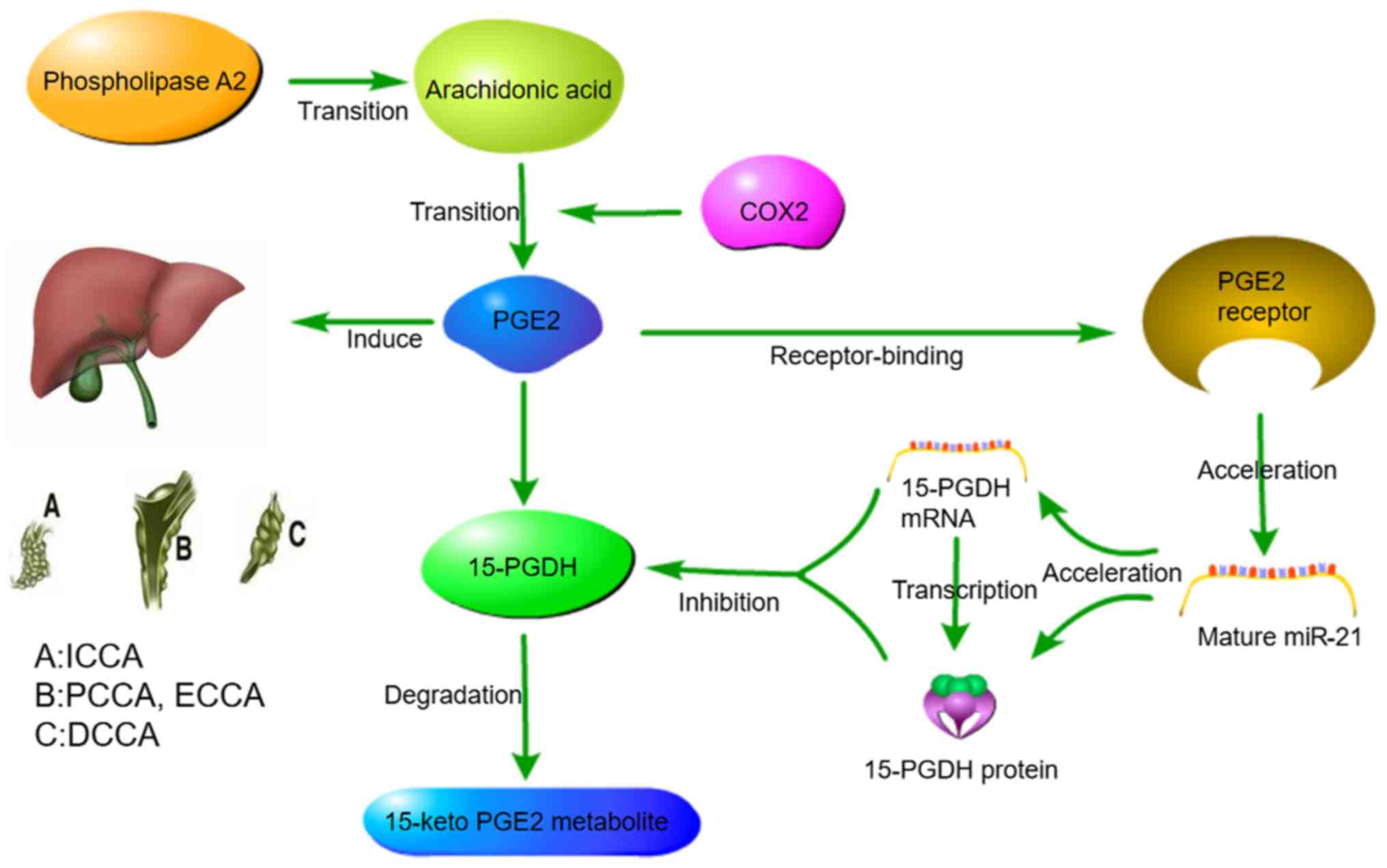
A study revealed that increased prostaglandin E2
(PGE2) signalling pathway activation in CCA enhanced the expression
of miR‑21. Sequence alignment analysis identified the binding site
of miR-21 in the 3′ UTR of 15-hydroxyprostaglandin dehydrogenase
(PGDH), and the overexpression of miR-21 reduced the mRNA and
protein levels of 15-PGDH. miR-21 targets 15-PGDHand leads to
blocked PGE2 degradation. The accumulation of PGE2 further
increased the expression of miR-21. These signals cascade to form a
feed-forward loop that drives the development of CCA and tumour
progression (41), as illustrated
in Fig. 1.
Let‑7
Members of the let-7 family are highly conserved in
sequence and function from C. elegans to humans, and are
critical regulators of embryonic development, stem cell
maintenance, differentiation, glucose metabolism and the
development of pathological processes, including tumouri-genesis
(45). Analysis using TargetScan
Human 7.0 and the published literature have shown that EZH2 is a
direct target of let-7c in cancer cells (46). Xie et al (47) revealed the complex role of
microporous let-7c in human CCA; overexpression of let-7c inhibited
invasion in vitro but increased distant metastasis in
vivo. In addition, let-7c inhibited the tumourigenic capacity
of CCA cells, including spheroid formation and tumour initiation;
similar results were obtained by regulation the expression of EZH2
and dvl3. These results provide strong experimental evidence for
the involvement of let-7c in the distant metastasis of CCA,
suggesting that miRNAs are novel biomarkers that can be used to
identify patients with metastatic disease (47). A genome‑wide screen identified that
family members of the let-7c/miR-99a/miR-125b cluster are similarly
downregulated in CCA and target the IL-6/STAT3 pathway (49). Their overexpression decreased STAT3
activation and inhibited the malignant transformation of CCA cells
in vitro and their tumourigenicity in vivo. These
results indicate the potential of the let-7c/miR-99a/miR-125b
cluster as a molecular target for CCA therapy (48). Furthermore, treatment of the cells
with let-7c/miR-99a/miR-125b, resulted in prolonged inhibition of
STAT3 phosphorylation (48), which
may counteract many of the biological effects of the STAT3-mediated
tumourigenic pathway (49).
Therefore, the endogenous miRNA targeting STAT3 may provide a
natural and safe treatment option, and these findings suggest a
strategy for the development of a miRNA-based therapy using the
IL-6/STAT3 CCA pathway (48). Meng
et al (49) found that
let-7 expression was upregulated in CCA cells overexpressing IL-6.
Another study (47) indicated that
neurofibromatosis 2 is a target gene of let-7a, and since NF-2 is a
negative regulator of STAT3, let-7a could increase STAT3 pathway
activity by downregulating NF-2 expression, thus enhancing the drug
resistance of CCA cells with overexpression of IL-6. Therefore,
Let-7 may have a dual mechanism of action, with different
regulatory effects on CCA in different states.
miR‑200 family
The miR‑200 family includes five members: miR-200a,
miR-200b, miR-200c, miR-141 and miR-429. A study confirmed that the
miR-200 family can increase the expression of E-cadherin by
inhibiting the expression of zinc finger e‑box‑binding homeobox
(ZEB)1 and ZEB2, thus promoting EMT (50). Another study has shown that the
miR-200 family and the low expression of E-cadherin in a highly
metastatic breast cancer cell line (4TO7) promote cell metastasis
and invasion (51). Furthermore,
miR-141 and miR-200b from the miR-200 family have been found to be
expressed at high levels in CCA cells (24). A target of miR-141, CLOCK, plays an
important role in regulating the physiological rhythm of the body
and also acts as a tumour suppressor gene to inhibit cell division
and promote apoptosis (24).
Therefore, miR-141 may promote the proliferation of CCA cells by
inhibiting the expression of CLOCK. The target gene of miR-200b is
PTPN12, and PTPN12 can inhibit the Ras pathway by dephosphorylation
of c-Abl and Src to achieve tumour suppression. Therefore, the
inhibition of miR-200b expression is likely to lead to a reduction
in the drug resistance of CCA cells (24).
3. Circular RNA
Circular RNAs (circRNAs) are a class of ncRNA
molecules without a 5′-terminal cap and 3′-terminal poly(A) tail,
which form a covalently bonded ring structure and are widely
present in eukaryotic cells (52).
CircRNAs are structurally stable, highly abundant, and expressed in
a tissue‑specific manner. By binding to miRNAs and other molecules,
circRNAs regulate gene expression at the transcriptional and
post-transcriptional levels, thereby affecting various cell
processes (53).
CircRNAs are relatively stable in animal cells and
regulate the expression of target genes through various pathways.
circRNAs have covalent cyclic structures without 5′-3′ polarity or
poly(A) tail. The circular structure includes multiple
miRNA-binding sites named miRNA response elements, via which
circRNAs can act as sponges and thereby serve as candidate RNA
binding proteins to regulate mRNA expression; circRNAs can bind
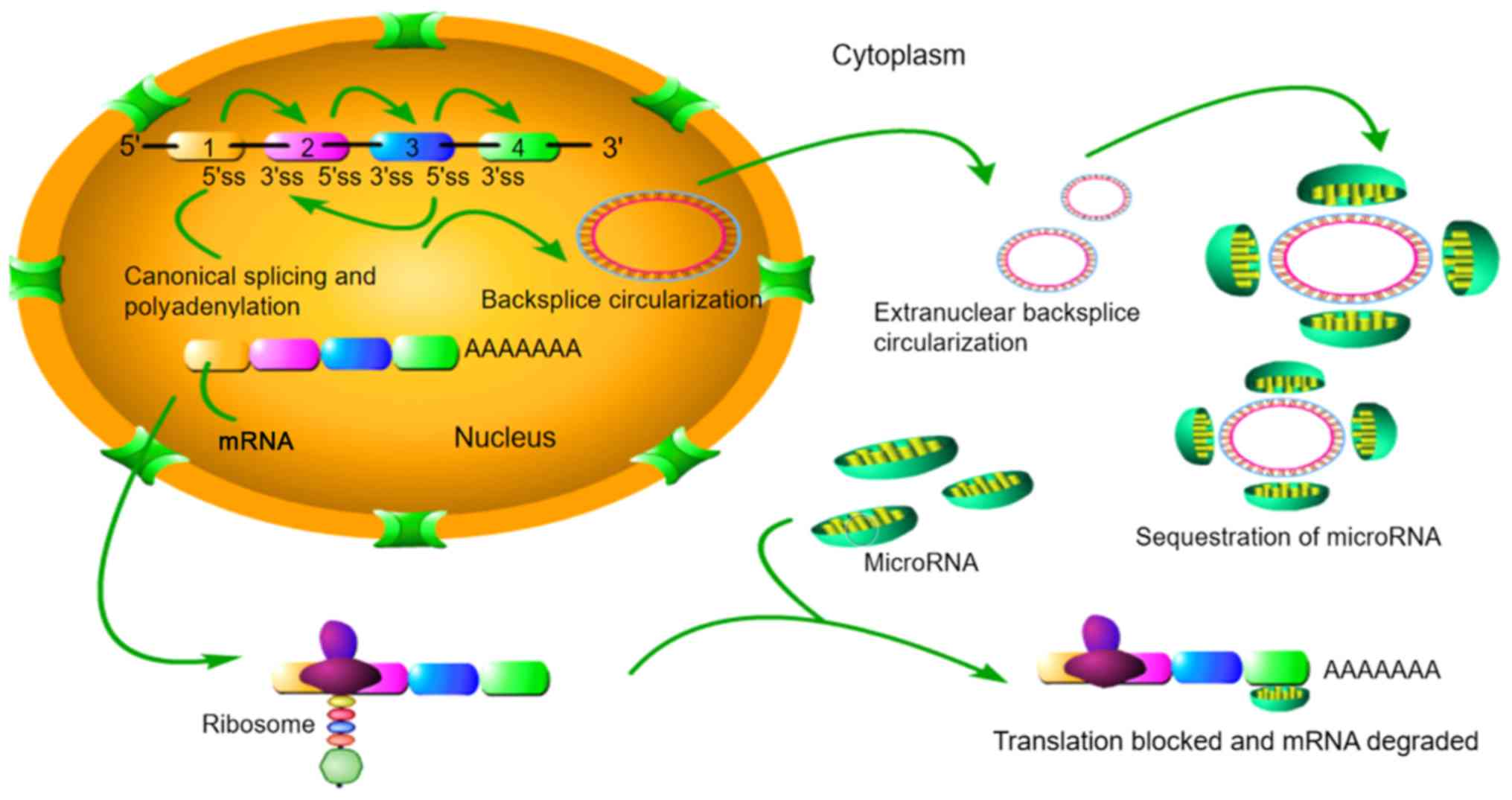
directly to targeted miRNAs to regulate mRNA expression (53), as illustrated in Fig. 2. Therefore, by interacting with
miRNAs, circRNAs play an important role in the regulation of tumour
progression and various tumour signalling pathways (52,54).
Xiong et al (55) reported that circRNA ZNF609
upregulated FOXP4 expression and regulated the progression of renal
cancer cells through sponging miR-138-5p. A study by Xu et
al (56) revealed that the
expression of circ_0005230 was increased in breast cancer, and
could act as a competing endogenous RNA (ceRNA) to increase the
expression of CBX8 through sponging miR-618. However, in another
study by Xu et al (57),
miR-618 was almost unaffected by circ_0005230 in two selected CCA
cell lines, suggesting that circ_0005230 may have a tissue‑specific
mechanism. Of all the predicted miRNAs in that study, only miR-1238
and miR-1299 were negatively correlated with the circ_0005230
levels. Little research has been conducted on the role of miR-1238
in cancer progression. A report by Shi et al (58) identified miR‑1238 as a tumour
suppressor in the development and progression of cancer. The
researchers found that miR-1238 partially inhibited the growth of
non-small cell lung cancer cells by inhibiting LHX2. Xu et
al (57) confirmed that
miR‑1238 was a tumour-suppressive miRNA in CCA, and rescue
experiments showed that the carcinogenic effect of circ_0005230 was
partly due to its inhibition of miR-1238 and miR-1299. Prior to
this, the circRNA Cdr1as was reported to be increased in CCA
(59); however, whether miR-1299
is sponged by Cdr1as in CCA remains unknown. In addition, how
miR-1238 and miR-1299 inhibit the progression of CCA cells and the
downstream targets of these miRNAs remain unclear.
In the study by Jiang et al (59), Cdr1as expression in tumour tissues
was found to be higher than that in adjacent normal tissues. In
addition, the overexpression of Cdr1as exhibited a close
association with advanced TNM stage, lymph node infiltration and
postoperative recurrence. The OS of patients with CCA and high
Cdr1as expression was poorer than that of patients with CCA and low
Cdr1as expression, which further suggests that Cdr1as may serve as
a potential biomarker of malignancy to predict aggressive tumour
progression and poor prognosis in patients with CCA. Xu et
al (60) verified that
circ-0001649 downregulation promoted the migration, proliferation
and invasion of CCA cells. In a study by Wang et al
(61), circ-0000284 was shown to
regulate LY6E expression as a ceRNA by sponging miR-637, and was
suggested to be important in the pathogenesis of CCA. Furthermore,
the study suggested that exosome-transmitted circ-0000284 may
stimulate the malignant behaviours of surrounding normal cells via
the enhancement of migration and proliferation and inhibition of
apoptosis of these cells.
CircRNAs are a new topic of interest in RNA
research, along with miRNA and lncRNAs. Certain circRNAs have been
found to play important regulatory roles in the tumour process, but
their roles in the progression and metastasis of iCCA have not been
fully defined, and further studies are required.
At present, high-throughput sequencing technology
has been used to identify key circRNAs from iCCAs with high and low
metastatic potential, and the let-7/STAT3 signalling pathway has
been used as the entry point to study the mechanism of key
circRNA-key signalling pathway-target gene-cell metastasis
phenotypes. CircRNAs are expected to be markers for the early
diagnosis of iCCA, and research on the pathogenesis and metastasis
of circRNAs in iCCA is ongoing.
4. LncRNA
LncRNAs are a subgroup of ncRNAs >200 nucleotides
in length. LncRNAs are mainly formed by RNA polymerase II
transcription and are widely distributed in the nucleus and
cytoplasm. Similar to mRNAs, lncRNAs have a 5′-cap structure and a
3′-end nucleotide polymer tail structure that allows gene splicing
but lack a complete reading frame, and thus, the lncRNA itself does
not encode a functional protein. Following numerous in-depth
analyses, lncRNAs have been found to regulate gene expression
mainly at the following three levels: i) Transcriptional level; a
lncRNA binds to the adjacent gene promoter region to form a stable
RNA-DNA three-strand structure, thereby inhibiting the binding of
transcription factors and the gene expression of related proteins
(62). ii) Epigenetic modification
level; lncRNA inhibits gene translocation, (the rearrangement of
chromosomes caused by segmental transfer between non-homologous
chromosomes, where genes are transferred from one chromosome to
another) by affecting the chromatin domain methylation, acetylation
and ubiquitin-like status (62).
iii) Post-transcriptional level; lncRNA and target mRNA bind to
each other to form RNA double-stranded bodies, covering up key
elements of the mRNA; affecting the processing, cleavage,
transport, translation and degradation of mRNA precursors; and
regulating the post-transcriptional gene expression (62). LncRNAs can be categorised as sense,
antisense, intronic, intergenic and bidirectional (63). Moreover, lncRNAs can sponge miRNAs,
resulting in post-transcriptional alteration of their target
proteins. Various lncRNAs have been shown to have abnormal
expression or functions in gastric cancer, liver cancer, lung
cancer, CCA, laryngeal cancer, colorectal cancer and other
malignant tumours, and the expression levels vary in different
tumours (44). In 2016, Wang et
al (64) examined the lncRNA
profile of 77 iCCA tissues, and microarray analysis indicated that
2,773 lncRNAs were upregulated in iCCA tissues, while 2,392 lncRNAs
were downregulated.
LncRNAs and CCA
Studies have shown that the occurrence and
development of CCA is closely associated with the abnormal
expression and function of lncRNAs, and the expression of lncRNAs
in tumour tissues of CCA is very different from that in normal
tissues. Hao et al (65)
found through high-throughput sequencing that 230 lncRNAs were
differentially expressed in iCCA tumour tissues compared with
adjacent normal tissues; 97 were upregulated and 133 were
downregulated. Some of the abnormally expressed lncRNAs were
identified to be involved in the proliferation, apoptosis, EMT,
invasion, metastasis, drug resistance and other biological
processes of tumour cells (65).
Different lncRNAs and their various mechanistic targets in CCA are
presented in Table II.
 | Table IIFunctions and targets of some typical
lncRNAs. |
Table II
Functions and targets of some typical
lncRNAs.
| LncRNA | Target | Function | Ref. |
|---|
| H19 | IL‑6 | Key inflammatory
factor IL‑6 is activated to regulate the migration and invasion of
cancer cells | (69) |
| HULC | CXCR4 | Activation of the
chemokine receptor CXCR4 regulates the migration and invasion of
cancer cells | (69) |
| TUG1 | Sirt3 |
TUG1/miR-145/Sirt3/GDH network | (126) |
| CCAT1 | EMT | Leads to EMT
activation of ICC cells | (70) |
| NEAT1 | BAP1, EZH2,
cadherin | Invasion and
migration | (73) |
| MALAT1 | CXCR4 | Growth, migration
and invasion of CCA cells are regulated by miR-204-dependent
CXCR4 | (77) |
| PVT1 | EZH2, ANGPTL4 | Proliferation,
invasion and migration | (71) |
| PCAT1 | miR-122, Wnt,
β-catenin | Promotion of the
Wnt/β-catenin pathway | (127) |
| UCA1 | AKT, GSK-3β,
CCND1 | Proliferation,
invasion and migration | (128) |
| SPRY4-IT1 | EZH2 | Strengthens EZH2
expression | (129) |
| T-UCRs | β-catenin | Wnt/β-catenin
pathway downstream mediators and the development of specific
cancers | (130) |
| LINC01296 | MYCN |
LINC01296/miR-5095/MYCN network | (131) |
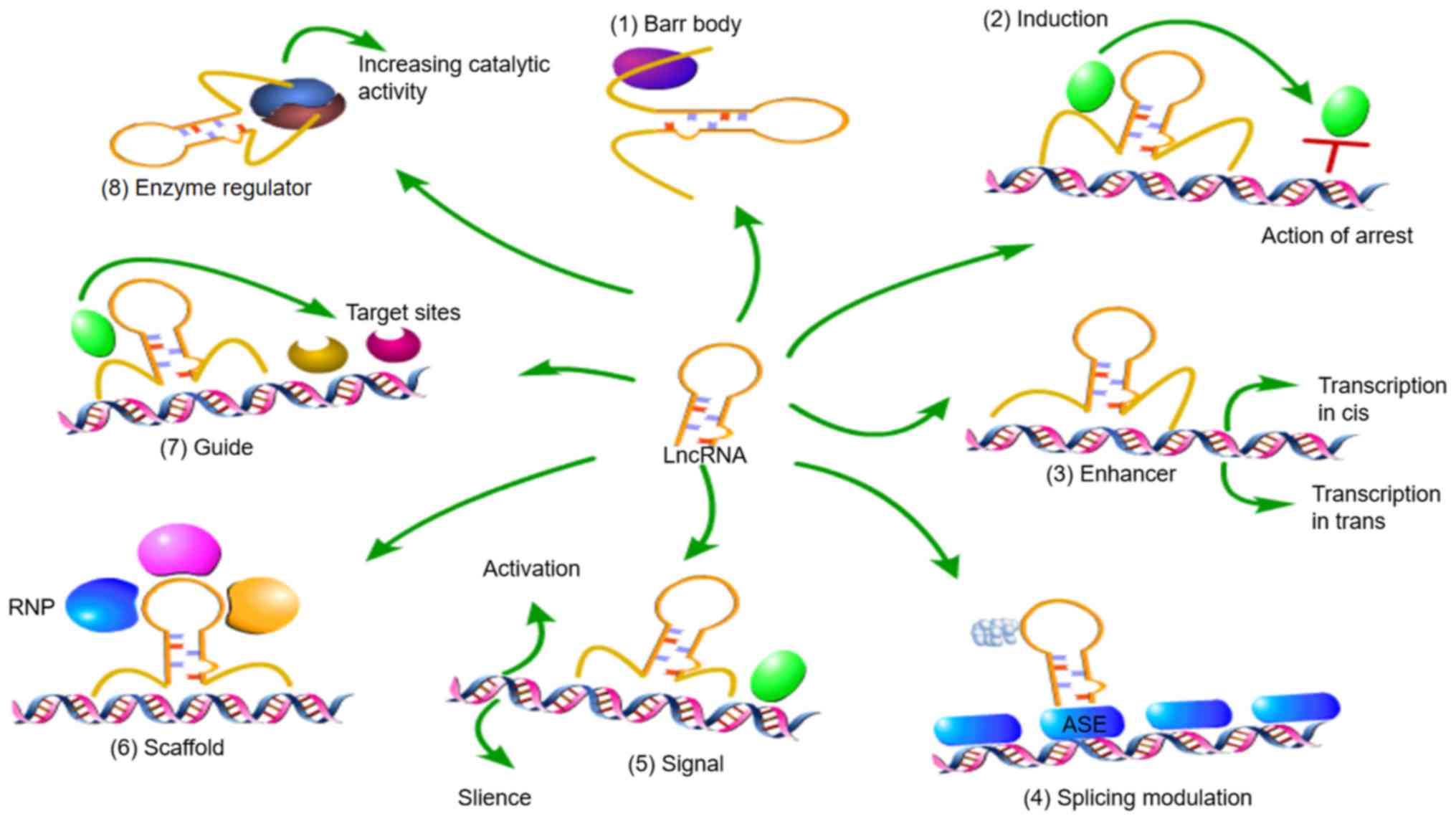
The regulatory mechanisms of lncRNAs in the nucleus
include the following (44,65):
i) lncRNA Xist is a component of the female Barr body; ii) lncRNAs
can inhibit regulatory proteins, such as transcription factors and
chromatin modification factors, to prevent them from binding to
DNA; iii) lncRNAs can act as enhancers, inducing cis-trans
transcription; iv) lncRNAs regulate the selective splicing of
primary transcripts; v) lncRNAs act as a molecular signal to
activate or silence gene expression through regulatory signal
transduction pathways; vi) lncRNAs act as scaffolds by binding with
different proteins to form ribonucleoprotein complexes, which also
affect gene expression; vii) lncRNAs guide proteins, generally
chromatin modifiers, to specific target sites; and viii) lncRNAs
interact with enzymes, such as kinases, to regulate or enhance
their catalytic activity and alter their signal transduction
pathways (Fig. 3).
Oncogenic lncRNAs
Yao et al (66) detected overexpression of the lncRNA
TP73‑AS1 in the tumour tissues and adjacent normal tissues of 75
patients with CCA through reverse transcription (RT)-qPCR, and
found that the degree of upregulation of this gene was closely
associated with tumour size and TNM stage. Silencing TP73-AS1
inhibited the proliferation of CCA cells, activated caspase-3 and
caspase-9, and promoted the apoptosis of tumour cells. Li et
al (67) found that lncRNA
EPIC1 expression was significantly increased in CCA tissues
compared with adjacent normal tissues. EPIC1 knockdown resulted in
growth inhibition and the apoptosis of CCA cells, thereby reducing
their malignant biological behaviours. Furthermore, an interaction
was identified between EPIC1 and myc oncogenes, and the expression
of the myc gene was decreased in EPIC1‑knockout CCA cells. However,
the specific mechanism of myc and ECIC1 in CCA require further
study.
Xu et al (68) found that lncRNA H19 was
overexpressed in the tumour tissues of 56 patients with CCA, and
the expression level of H19 was closely associated with
clinicopathological features. Silencing H19 gene effectively
inhibited the proliferation of CCA cells and promoted apoptosis. In
another study, lncRNA H19 gene silencing was shown to upregulate
E-cadherin, downregulate N-cadherin and vimentin, reverse the EMT
process, and weaken the metastasis and infiltration of CCA cells
(69). Zhang et al
(70) studied the overexpression
of the lncRNA CCAT1 in the tumour tissues of 120 patients with
primary iCCA and adjacent normal tissues, and found that the highly
expressed lncRNA CCAT1 was closely associated with tumour
progression. Silencing CCAT1 in iCCA cells decreased the levels of
N-cadherin, mucin and vimentin and increased those of E-cadherin,
which effectively reversed the EMT process of iCCA. Furthermore, a
luciferase reporter gene assay found that lncRNA CCAT1 and the
oncosuppressive gene miR-152 bind to each other, inducing iCCA cell
metastasis, invasion and EMT (70). EMT is an important process in the
tumour microenvironment. Alteration of lncRNAs in tumour tissues
can reverse the EMT process, and the progression of CCA can be
delayed, suggesting a new strategy for the clinical treatment of
CCA. Ma et al (71) found
that lncRNA AFAP1-AS1 was excessively expressed in the tumour
tissues of iCCA in 56 patients who had not received local or
systemic treatment. After silencing AFAP1-AS1, biological
behaviours such as proliferation, invasion and metastasis of CCA
cells were inhibited. This lncRNA mainly inhibited cell invasion
and metastasis by reducing the expression of matrix
metalloproteinase (MMP)-2 and MMP-9, and decreasing the malignancy
and biological behaviour tumour cells. Zhang et al (72) analysed the tumour tissues of 33
patients with CCA and found that the expression of lncRNA NEAT1 was
significantly increased in tumour tissues compared with adjacent
normal bile duct tissues. Moreover, silencing of NEAT1 resulted in
the invasion and metastasis of CCA cells. Further experiments in
the study showed that lncRNA NEAT1 recruited the E-cadherin
promoter through EZH2, and the downregulation of E-cadherin reduced
the adhesion strength of the cells, resulting in increased cell
activity.
Parasramka et al (73) reported the relationship between
lncRNAs and iCCA, and also noted that CCA cells with low expression
of BRCA-1 associated protein-1 (BAP1) were highly sensitive to
gemcitabine and cisplatin; they also suggested that lncRNA NEAT1
may be a functional downstream molecule of BAP1. Furthermore, NEAT1
knockout in CCA cell lines resulted in significantly higher
gemcitabine cytotoxicity than that of normal CCA cell lines,
suggesting that NEAT1 can be used as a sensitive tissue biomarker
for iCCA and the early diagnosis of CCA. Ma et al (74) found that the expression of
carbamoyl phosphate synthetase 1 (CPS1) and its transcribed lncRNA
(CPS1 intron 1; CPS1‑IT1) were significantly increased in iCCA
tissue compared with normal tissue,. Survival curve analysis showed
that lncRNA-CPS1 expression in tumour tissues was increased in
patients with a poor prognosis, and CPS1 and CPS1-IT1 were closely
associated with abnormal liver function and prognosis, suggesting
that. CPS1-IT1 may be a potential biomarker of iCCA. LncRNA MALAT1
is upregulated in liver, uterine, lung, breast, prostate,
pancreatic and cervical cancer, and other tumours (75). Further studies have confirmed that
MALAT1 is also an independent prognostic factor for some cancers
(76). In CCA, especially in hilar
CCA (hCCA), the expression level of MALAT1 is much higher than that
of adjacent tissues (77). In
vitro and in vivo, MALAT1 has been shown to have
protumourigenic effects on the proliferation, invasion and
migration of CCA cells (77). In
addition, MALAT1 overexpression is associated with low OS, poor TNM
staging, large tumour volume and metastasis in patients with hCCA
(77). Therefore, it may be
concluded that lncRNA MALAT1 is a promising prognostic tissue
biomarker for hCCA.
Tumour suppressor lncRNAs
The results of a study by Qin et al (78) showed that the lncRNA FENDRR had
reduced expression in CCA tissues and cells, and was negatively
associated with the expression of the apoptosis inhibitory protein
survivin. It also suggested that FENDRR may inhibit survivin
through histone methyltransferase, thereby inhibiting the
proliferation, metastasis and invasion of CCA cells. Another study
(79) demonstrated that the lncRNA
maternal gene 3 (MEG3) is consistently expressed in normal tissues,
while its expression is downregulated or absent in tumour cells,
including CCA, hepatocellular carcinoma, nasopharyngeal carcinoma,
colon cancer. Xia et al (80) found that the knockdown of MEG3
could activate the Wnt/β-catenin pathway, and that downregulated
MEG3 expression increased cisplatin resistance in lung
adenocarcinoma cells. Another study revealed that MEG3 may act as a
tumour suppressor gene in CCA cells to downregulate the expression
of ABCG2 and enhance the sensitivity of CCA cells to Adriamycin
(81). These findings provide a
theoretical and experimental basis for the molecular-targeted
therapy of CCA.
Another lncRNA, NEF, has been suggested to be an
upstream inhibitor of Runt-related transcription factor 1 (RUNX1)
in iCCA cells (82). The
regulatory effect of lncRNA NEF on RUNX1 in iCCA may be mediated by
disease-related factors, as the expression levels of lncRNA NEF and
RUNX1 were found to be positively correlated only in tumour tissues
and not in the adjacent healthy tissues of iCCA patients. LncRNA
NEF expression was downregulated while RUNX1 expression was
upregulated in iCCA, and it was suggested that NEF may be involved
in ICCA through interaction with RUNX1. Another study found that
the expression of lncRNA MIR22HG in CCA tissues and cell lines was
significantly decreased (83). In
CCA, the overexpression of MIR22HG inhibited cell proliferation,
migration and invasion, whereas knockdown of MIR22HG had the
opposite effect, negatively regulating the mRNA and protein levels
of the Wnt/β-catenin signalling pathway components (β-catenin,
cyclin D1 and c-myc). The effects of MIR22HG overexpression on CCA
progression were partially rescued by activating the Wnt/β-catenin
signalling pathway. More importantly, the expression of MIR22HG was
found to be associated with TNM stage in CCA patients and thus has
prognostic significance.
H19 and HULC
H19, one of the earliest identified lncRNAs with a
long history of research, serves a crucial role in oesophageal
cancer, breast cancer, bladder cancer, prostate cancer, CCA and
other diseases. This lncRNA is overexpressed in tumour tissues, and
the degree of expression is closely associated with
clinicopathological features.
Increasing evidence indicates that H19 is involved
in tumour proliferation and differentiation together with EMT and
MET, suggesting that it plays an important role in the occurrence
and development of tumours (75).
H19 has an evolutionarily conserved secondary structure, suggesting
its structure-dependent functions, which include binding to EZH2
(84), interacting with
methyl-CpG-binding domain protein 1 and recruiting this protein to
some of its targets that maintain the repressive H3K9me3 histone
marks in their loci (85). H19 has
been shown to interact with and inactivate the P53 protein,
suggesting that H19 plays a role in tumouri-genesis (75). HULC has been reported to promote
hCCA proliferation and regulate the cell cycle by downregulating
the tumour suppressor gene CDKN2C (p18) and participating in the
ATM/ATR, p53 and other signalling pathways (86). One study revealed that the
let-7a/let-7b pathway potentially targets H19, which leads to
partial inactivation of IL-6 in oxidative stress‑mediated CCA
inflammation, and found that HULC is targeted by miR-372 and
miR-373 during the migration and invasion of CCA cells, further
activating the chemokine receptor CXCR4 (69). Furthermore, it found that H19 and
HULC are stimulated by short- and long-term oxidative stress, and
regulate the expression of pivotal genes in the inflammatory
process, suggesting that there is a positive feedback loop between
inflammation and oxidative stress, and the activation of this
feedback loop via lncRNAs might promote tumouri-genesis in CCA
(69).
Studies have found that the imprinting factor H19
controls gene regulation networks via a trans effect in most
malignant tumours and promotes oncogenes, increasing tumour
invasion and metastasis at multiple levels as follows: i)
Maintenance of cancer stem cells (CSCs); most well-differentiated
tumour cells in tumour tissues spread to the target tissues and
organs but cannot form metastatic lesions, and only some have the
self-renewal and multidirectional differentiation potential of
CSCs, which are required for distant metastasis. Therefore, the
maintenance of CSC stem cell characteristics is key to tumour
invasion and metastasis (87,88).
ii) Control of epithelial-interstitial transition; EMT is closely
associated with cell polarity and epithelial cell adhesion. Under
the action of various factors, the epithelial and mesenchymal
characteristics are lost, as shown by cell polarity changes, loss
and reduction of the relationship between surrounding mesenchymal
cells and other cells, cell invasion and random migration (89). iii) Regulation of tumour
neovascularization; research data have shown that the tumour
neovascularization is positively associated with tumour invasion
and metastasis because the formation of tumour blood vessels not
only provides sufficient nutrients and oxygen for tumour growth but
also provides good channels for tumour cell invasion and metastasis
(90). Jia et al (90) studied the influence of H19 on the
biological behaviour of glioma and found that knockout of H19
inhibited the invasion of glioma cells and the formation of tubular
structures. Subsequent dual-luciferase reporter experiments
(90) found that H19 could
directly act on and inhibit the expression of miR-29a, which in
turn targeted the 3′ UTR of the vasohibin 2 (VASH2) gene, inhibited
the expression of VASH2, and blocked the formation of tumour blood
vessels. Therefore, H19 is believed to promote angiogenesis and
invasion and metastasis of glioma cells by reversing the inhibitory
effect of miR-29a on VASH2.
NNT‑AS1
In several studies (91-93),
lncRNA NNT-AS1 was found to be significantly upregulated in CCA,
and the high expression of NNT-AS1 was associated with
differentiation, invasion depth, TNM stage and poor prognosis.
NNT-AS1 downregulation significantly inhibited the proliferation,
migration and invasion of CCA cells, while overexpression of
NNT-AS1 promoted the invasion and survival of cells in vitro
and in vivo. These studies also demonstrated that NNT-AS1
binds to miR-142-5p as a ceRNA, and the expression of miR-142-5p is
negatively correlated with the expression of NNT-AS1 in CCA. These
results suggest that NNT-AS1 plays a key role in the progression of
CCA and is a potential therapeutic target for CCA. Multivariate
analysis (92,94) indicated that NNT-AS1 may be a new
and effective risk biomarker. Several studies have suggested that
upregulated NNT-AS1 could be used as an independent negative
prognostic factor for cancer. For example, NNT-AS1 is increased in
human osteo-sarcoma, cervical cancer, colorectal cancer, breast
cancer, liver cancer and ovarian cancer (95). In addition, Hua et al
(93) indicated that NNT-AS1
increased cell proliferation and invasion by activating the
Wnt/β-catenin pathway in cervical cancer. Liang et al
(82) found that NNT-AS1 could
accelerate the migration and invasion of cells in CCA and bound to
miR-142-3p, thereby acting as a sponge.
SNHG1
A study by Yu et al (96) found that the expression of lncRNA
SNHG1 showed strong upregulation in CCA tissues, and SNHG1
downregulation significantly inhibited cell growth in vivo
and in vitro. SNHG1 also exhibited cancer-promoting
properties in different types of tumours (96). There is evidence to show that SNHG1
interacts with EZH2, which is thought to be a histone‑methylated
modification complex in the nucleus that regulates various target
genes. CDKN1A is one of the genes regulated by SNHG1, as shown by
RNA sequencing (RNA-seq), and acts as a tumour suppressor gene in
different types of cancer (97,98).
In addition, hypermethylation of the CDKN1A promoter region has
been reported to contribute to CDKN1A transcriptional inactivation
in breast cancer (99). SNHG1
binds to EZH2 to inhibit the expression of target genes, including
CDKN1A, in CCA (89). Through
epigenetic regulation of CDKN1A transcription in the nucleus, SNHG1
promotes the malignancy of CCA and the survival and metastasis of
CCA cells (100).
PVT1
Following PVT1 knockdown, an analysis of RNA
sequencing results on the basis of gene ontology revealed that this
genetic change was strongly associated with proliferation and
migration (101). Another study
(102) found that PVT1 binds to
EZH2 to form a histone-methylated modification complex in the
nucleus, thereby potentially regulating various genes in CCA
(103). DNA methylation in the
promoter region of ANGPTL4 has been confirmed to lead to the
inactivation of ANGPTL4 transcription (104). A study showed that PVT1-regulated
histone methylation (H3K27me3) promoted a reduction in ANGPTL4
expression in CCA cell lines, and that PVT1 bound to EZH2 in the
nucleus and mediated the epigenetic silencing of ANGPTL4 (101). Through this mechanism, PVT1
regulated the transcription of ANGPTL4 to promote cell survival and
CCA metastasis, thus enhancing the malignancy of CCA (101). These findings indicate that PVT1,
as a key lncRNA, may be crucial in the diagnosis, treatment and
prognosis of CCA.
AL136359.1
The lncRNA ALl36359.1 plays an important role in
CCA. A study has shown that ALl36359.1 expression leads to
significant increases in the mRNA and protein levels of aldosterone
reductase lB10 (AKR1B10), and when the AKR1B10 gene was highly
expressed, the protein levels of MMP‑2, vimentin, CDK4 and Cyclin
Dl were significantly reduced (105). In another study, lncRNA
ALl36359.1 was significantly decreased in CCA tissues and cell
lines, and the high expression of ALl36359.1 significantly promoted
the expression of the AKRlBIO gene and further inhibited the
proliferation and invasion of CCA cells (106).
Colon cancer‑related transcript 2
(CCAT2)
CCAT2 is a lncRNA that maps to the genomic region of
8q24. This lncRNA was first identified from microsatellite stable
colorectal cancer and was shown to regulate the metastasis and
progression of colon cancer and chromosomal instability (106). CCAT2 can reduce E-cadherin and
increase ZEB2, vimentin and N-cadherin expression, thus stimulating
EMT (107). A study has shown
that CCAT2 is upregulated in CCA tissues and cell lines, and the
transcription level of CCAT2 is upregulated 1.39-3.20-fold in CCA
tissues and cell lines compared with human intrahepatic bile duct
epithelial cells (107). In
vitro experiments have confirmed that CCAT2 silencing can
significantly inhibit cell growth and increase cell apoptosis in
CCA cells (106). Caspase-3 and
-9 may play an important role in mediating the apoptosis induced by
downregulation of CCAT2. Inhibition of CCAT2 expression can inhibit
metastatic properties, reverse EMT, increase E-cadherin expression
and decrease vimentin expression in CCA cells (106).
5. Contribution of next‑generation
sequencing (NGS) to ncRNAs
At present, most quantitative studies of ncRNA rely
on RT-qPCR, in situ hybridization or microarray techniques. These
methods have both advantages and limitations. NGS offers
researchers a powerful tool for detecting RNA molecules in
biological samples. In addition, the length of the protocol,
sequencing time and expense are falling, making NGS an ideal tool
for studying biomarkers (108).
The advantages of NGS include the following: i) Species‑ or
transcript‑specific probes are not required. ii) NGS shows
increased specificity and sensitivity for a wide range of
applications. iii) The high coverage depth of sequencing helps
detect rare or single-cell transcripts and identify weakly
expressed genes. iv) NGS technology is able to detect multiple
splice sites and novel isoforms. v) NGS technology is able to
perform de novo analysis of a sample without a reference genome
(108-110).
The operational process of NGS in ncRNA can be
summarized in the following aspects: i) Library preparation or
sample processing; ii) sequencing; iii) initial quality and raw
data analyses; and iv) variant calling and data interpretation.
This last step is dependent on the specific application (110).
6. Conclusions
The prognosis of carcinoma of the bile tract is
poor, and it presents early and systemic metastasis lymph node
involvement. Currently, iCCA lacks effective drug and chemotherapy
regimens, and surgical resection is the most effective method to
treat this malignancy. However, due to its specific anatomical
site, hidden onset of disease and the lack of effective early
diagnostic markers, patients with iCCA are often in the late stage
of disease when they are diagnosed, and have lost the opportunity
for radical surgery. CCA carcinogenesis is the result of various
oncogenes, tumour suppressor genes and associated molecular
disorders. Elucidation of the molecular mechanisms may help to
identify effective therapeutic targets and biomarkers. Therefore,
ncRNAs have gradually become an important topic of research. In
this review, the biological characteristics of different ncRNAs,
such as miRNAs, circRNAs and lncRNAs, are introduced, as well as
their roles and mechanisms in CCA, and the clinical applications of
relevant ncRNAs in CCA. An improved understanding of CCA biology
has revealed the key role of ncRNAs in influencing CCA initiation,
progression and therapeutic tolerance. Under these conditions,
ncRNAs are being further studied as promising tumour biomarkers to
be incorporated into clinical management and improve the prognosis
of CCA patients. The initial results are encouraging; however,
further studies are required to confirm their reliability and
clinical reproducibility.
Funding
No funding was received.
Availability data and materials
Not applicable.
Authors' contributions
YL and YZ conceived the study. YL, ZW, KZ and GZ
conducted the literature search. YL, ZW, KZ and GZ wrote and
prepared the original draft of the manuscript. All authors
participated in the writing and reviewing of the article. YL, YZ
and SH edited the article. All authors have read and approved the
final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
References
|
1
|
Blechacz B: Cholangiocarcinoma: Current
Knowledge and New Developments. Gut Liver. 11:13–26. 2017.
View Article : Google Scholar :
|
|
2
|
Rizvi S, Khan SA, Hallemeier CL, Kelley RK
and Gores GJ: Cholangiocarcinoma - evolving concepts and
therapeutic strategies. Nat Rev Clin Oncol. 15:95–111. 2018.
View Article : Google Scholar
|
|
3
|
Lv Y and Huang S: Role of non-coding RNA
in pancreatic cancer. Oncol Lett. 18:3963–3973. 2019.PubMed/NCBI
|
|
4
|
Wang KC and Chang HY: Molecular mechanisms
of long noncoding RNAs. Mol Cell. 43:904–914. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ponting CP, Oliver PL and Reik W:
Evolution and functions of long noncoding RNAs. Cell. 136:629–641.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang Z, Yang B, Zhang M, Guo W, Wu Z, Wang
Y, Jia L, Li S, Xie W, Yang D, et al: Cancer Genome Atlas Research
Network: LncRNA epigenetic landscape analysis identifies EPIC1 as
an oncogenic lncRNA that interacts with MYC and promotes cell cycle
progression in cancer. Cancer Cell. 33:706–720.e9. 2018. View Article : Google Scholar
|
|
7
|
Han D, Gao X, Wang M, Qiao Y, Xu Y, Yang
J, Dong N, He J, Sun Q, Lv G, et al: Long noncoding RNA H19
indicates a poor prognosis of colorectal cancer and promotes tumor
growth by recruiting and binding to eIF4A3. Oncotarget.
7:22159–22173. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lee RC, Feinbaum RL and Ambros V: The C.
elegans heter-ochronic gene lin-4 encodes small RNAs with antisense
complementarity to lin-14. Cell. 75:843–854. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bushati N and Cohen SM: microRNA
functions. Annu Rev Cell Dev Biol. 23:175–205. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lin S and Gregory RI: MicroRNA biogenesis
pathways in cancer. Nat Rev Cancer. 15:321–333. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Pandey P, Srivastava PK and Pandey SP:
Prediction of Plant miRNA Targets. Methods Mol Biol. 1932:99–107.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Borges F and Martienssen RA: The expanding
world of small RNAs in plants. Nat Rev Mol Cell Biol. 16:727–741.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ushijima K, Yamada Y, Yano T and Tashiro
M: An electrosurgical burn possibly caused by radio-frequency
leakage current through a stainless forceps. Masui. 49:909–912.
2000.In Japanese. PubMed/NCBI
|
|
14
|
Piontek K and Selaru FM: MicroRNAs in the
biology and diagnosis of cholangiocarcinoma. Semin Liver Dis.
35:55–62. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nakaoka T, Saito Y and Saito H: Aberrant
DNA Methylation as a Biomarker and a Therapeutic Target of
Cholangiocarcinoma. Int J Mol Sci. 18:182017.
|
|
16
|
Plieskatt J, Rinaldi G, Feng Y, Peng J,
Easley S, Jia X, Potriquet J, Pairojkul C, Bhudhisawasdi V, Sripa
B, et al: A microRNA profile associated with Opisthorchis
viverrini-induced cholangiocarcinoma in tissue and plasma. BMC
Cancer. 15:3092015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Petrache Voicu SN, Dinu D, Sima C,
Hermenean A, Ardelean A, Codrici E, Stan MS, Zărnescu O and
Dinischiotu A: Silica Nanoparticles Induce Oxidative Stress and
Autophagy but Not Apoptosis in the MRC-5 Cell Line. Int J Mol Sci.
16:29398–29416. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhu H, Han C, Lu D and Wu T: miR-17-92
cluster promotes cholangiocarcinoma growth: Evidence for PTEN as
downstream target and IL-6/Stat3 as upstream activator. Am J
Pathol. 184:2828–2839. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hu C, Huang F, Deng G, Nie W, Huang W and
Zeng X: miR-31 promotes oncogenesis in intrahepatic
cholangiocarcinoma cells via the direct suppression of RASA1. Exp
Ther Med. 6:1265–1270. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li J, Tian F, Li D, Chen J, Jiang P, Zheng
S, Li X and Wang S: miR-605 represses PSMD10/Gankyrin and inhibits
intrahepatic cholangiocarcinoma cell progression. FEBS Lett.
588:3491–3500. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cheng Q, Feng F, Zhu L, Zheng Y, Luo X,
Liu C, Yi B and Jiang X: Circulating miR-106a is a Novel Prognostic
and Lymph Node Metastasis Indicator for Cholangiocarcinoma. Sci
Rep. 5:161032015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Patel T: Extracellular vesicle noncoding
RNA: New players in the diagnosis and pathogenesis of
cholangiocarcinoma. Hepatology. 60:782–784. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Canu V, Sacconi A, Lorenzon L, Biagioni F,
Lo Sardo F, Diodoro MG, Muti P, Garofalo A, Strano S, D'Errico A,
et al: miR-204 down-regulation elicited perturbation of a gene
target signature common to human cholangiocarcinoma and gastric
cancer. Oncotarget. 8:29540–29557. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Meng F, Henson R, Lang M, Wehbe H,
Maheshwari S, Mendell JT, Jiang J, Schmittgen TD and Patel T:
Involvement of human micro-RNA in growth and response to
chemotherapy in human cholangiocarcinoma cell lines.
Gastroenterology. 130:2113–2129. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chen L, Yan HX, Yang W, Hu L, Yu LX, Liu
Q, Li L, Huang DD, Ding J, Shen F, et al: The role of microRNA
expression pattern in human intrahepatic cholangiocarcinoma. J
Hepatol. 50:358–369. 2009. View Article : Google Scholar
|
|
26
|
Yang G, Zhang R, Chen X, Mu Y, Ai J, Shi
C, Liu Y, Shi C, Sun L, Rainov NG, et al: miR-106a inhibits glioma
cell growth by targeting E2F1 independent of p53 status. J Mol Med
(Berl). 89:1037–1050. 2011. View Article : Google Scholar
|
|
27
|
Okamoto K, Miyoshi K and Murawaki Y:
miR-29b, miR-205 and miR-221 enhance chemosensitivity to
gemcitabine in HuH28 human cholangiocarcinoma cells. PLoS One.
8:e776232013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chen Y, Gao W, Luo J, Tian R, Sun H and
Zou S: Methyl-CpG binding protein MBD2 is implicated in
methylation-mediated suppression of miR-373 in hilar
cholangiocarcinoma. Oncol Rep. 25:443–451. 2011. View Article : Google Scholar
|
|
29
|
An F, Yamanaka S, Allen S, Roberts LR,
Gores GJ, Pawlik TM, Xie Q, Ishida M, Mezey E, Ferguson-Smith AC,
et al: Silencing of miR-370 in human cholangiocarcinoma by allelic
loss and interleukin-6 induced maternal to paternal epigenotype
switch. PLoS One. 7:e456062012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ngankeu A, Ranganathan P, Havelange V,
Nicolet D, Volinia S, Powell BL, Kolitz JE, Uy GL, Stone RM,
Kornblau SM, et al: Discovery and functional implications of a
miR-29b-1/miR-29a cluster polymorphism in acute myeloid leukemia.
Oncotarget. 9:4354–4365. 2017. View Article : Google Scholar
|
|
31
|
Mott JL, Kurita S, Cazanave SC, Bronk SF,
Werneburg NW and Fernandez-Zapico ME: Transcriptional suppression
of mir-29b1/mir-29a promoter by c-Myc, hedgehog, and NF-kappaB. J
Cell Biochem. 110:1155–1164. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kwon H, Song K, Han C, Zhang J, Lu L, Chen
W and Wu T: Epigenetic Silencing of miRNA-34a in Human
Cholangiocarcinoma via EZH2 and DNA Methylation: Impact on
Regulation of Notch Pathway. Am J Pathol. 187:2288–2299. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Li B, Han Q, Zhu Y, Yu Y, Wang J and Jiang
X: Down-regulation of miR-214 contributes to intrahepatic
cholangiocarcinoma metastasis by targeting Twist. FEBS J.
279:2393–2398. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Iwaki J, Kikuchi K, Mizuguchi Y,
Kawahigashi Y, Yoshida H, Uchida E and Takizawa T: miR-376c
down-regulation accelerates EGF-dependent migration by targeting
GRB2 in the HuCCT1 human intrahepatic cholangiocarcinoma cell line.
PLoS One. 8:e694962013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Peng F, Jiang J, Yu Y, Tian R, Guo X, Li
X, Shen M, Xu M, Zhu F, Shi C, et al: Direct targeting of
SUZ12/ROCK2 by miR-200b/c inhibits cholangiocarcinoma
tumourigenesis and metastasis. Br J Cancer. 109:3092–3104. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yang R, Chen Y, Tang C, Li H, Wang B, Yan
Q, Hu J and Zou S: MicroRNA-144 suppresses cholangiocarcinoma cell
proliferation and invasion through targeting platelet activating
factor acetylhydrolase isoform 1b. BMC Cancer. 14:9172014.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhang J, Han C, Zhu H, Song K and Wu T:
miR-101 inhibits cholangiocarcinoma angiogenesis through targeting
vascular endothelial growth factor (VEGF). Am J Pathol.
182:1629–1639. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Qiu YH, Wei YP, Shen NJ, Wang ZC, Kan T,
Yu WL, Yi B and Zhang YJ: miR-204 inhibits epithelial to
mesenchymal transition by targeting slug in intrahepatic
cholangiocarcinoma cells. Cell Physiol Biochem. 32:1331–1341. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kobayashi S, Werneburg NW, Bronk SF,
Kaufmann SH and Gores GJ: Interleukin-6 contributes to Mcl-1
up-regulation and TRAIL resistance via an Akt-signaling pathway in
cholangiocar-cinoma cells. Gastroenterology. 128:2054–2065. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Xiong B, Cheng Y, Ma L and Zhang C: miR-21
regulates biological behavior through the PTEN/PI-3 K/Akt signaling
pathway in human colorectal cancer cells. Int J Oncol. 42:219–228.
2013. View Article : Google Scholar
|
|
41
|
Selaru FM, Olaru AV, Kan T, David S, Cheng
Y, Mori Y, Yang J, Paun B, Jin Z, Agarwal R, et al: MicroRNA-21 is
overexpressed in human cholangiocarcinoma and regulates programmed
cell death 4 and tissue inhibitor of metalloproteinase 3.
Hepatology. 49:1595–1601. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Jiang Y, Goldberg ID and Shi YE: Complex
roles of tissue inhibitors of metalloproteinases in cancer.
Oncogene. 21:2245–2252. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
He Q, Cai L, Shuai L, Li D, Wang C, Liu Y,
Li X, Li Z and Wang S: Ars2 is overexpressed in human
cholangiocarcinomas and its depletion increases PTEN and PDCD4 by
decreasing microRNA-21. Mol Carcinog. 52:286–296. 2013. View Article : Google Scholar
|
|
44
|
Lu L, Byrnes K, Han C, Wang Y and Wu T:
miR-21 targets 15-PGDH and promotes cholangiocarcinoma growth. Mol
Cancer Res. 12:890–900. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Triboulet R, Pirouz M and Gregory RI: A
Single Let-7 MicroRNA Bypasses LIN28-Mediated Repression. Cell Rep.
13:260–266. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Au SL, Wong CC, Lee JM, Fan DN, Tsang FH,
Ng IO and Wong CM: Enhancer of zeste homolog 2 epigenetically
silences multiple tumor suppressor microRNAs to promote liver
cancer metastasis. Hepatology. 56:622–631. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Xie Y, Zhang H, Guo XJ, Feng YC, He RZ, Li
X, Yu S, Zhao Y, Shen M, Zhu F, et al: Let-7c inhibits
cholangiocarcinoma growth but promotes tumor cell invasion and
growth at extrahepatic sites. Cell Death Dis. 9:2492018. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Lin KY, Ye H, Han BW, Wang WT, Wei PP, He
B, Li XJ and Chen YQ: Genome-wide screen identified
let-7c/miR-99a/miR-125b regulating tumor progression and stem-like
properties in cholan-giocarcinoma. Oncogene. 35:3376–3386. 2016.
View Article : Google Scholar
|
|
49
|
Meng F, Henson R, Wehbe-Janek H, Smith H,
Ueno Y and Patel T: The MicroRNA let-7a modulates
interleukin-6-dependent STAT-3 survival signaling in malignant
human cholangiocytes. J Biol Chem. 282:8256–8264. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Gregory PA, Bert AG, Paterson EL, Barry
SC, Tsykin A, Farshid G, Vadas MA, Khew-Goodall Y and Goodall GJ:
The miR-200 family and miR-205 regulate epithelial to mesenchymal
transition by targeting ZEB1 and SIP1. Nat Cell Biol. 10:593–601.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Korpal M, Lee ES, Hu G and Kang Y: The
miR-200 family inhibits epithelial-mesenchymal transition and
cancer cell migration by direct targeting of E-cadherin
transcriptional repressors ZEB1 and ZEB2. J Biol Chem.
283:14910–14914. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Greene J, Baird AM, Brady L, Lim M, Gray
SG, McDermott R and Finn SP: Circular RNAs: Biogenesis, Function
and Role in Human Diseases. Front Mol Biosci. 4:382017. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Zhang Z, Xie Q, He D, Ling Y, Li Y, Li J
and Zhang H: Circular RNA: new star, new hope in cancer. BMC
Cancer. 18:8342018. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Werfel S, Nothjunge S, Schwarzmayr T,
Strom TM, Meitinger T and Engelhardt S: Characterization of
circular RNAs in human, mouse and rat hearts. J Mol Cell Cardiol.
98:103–107. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Xiong Y, Zhang J and Song C: CircRNA
ZNF609 functions as a competitive endogenous RNA to regulate FOXP4
expression by sponging miR-138-5p in renal carcinoma. J Cell
Physiol. 234:10646–10654. 2019. View Article : Google Scholar
|
|
56
|
Xu Y, Yao Y, Leng K, Ji D, Qu L, Liu Y and
Cui Y: Increased Expression of Circular RNA circ_0005230 Indicates
Dismal Prognosis in Breast Cancer and Regulates Cell Proliferation
and Invasion via miR-618/CBX8 Signal Pathway. Cell Physiol Biochem.
51:1710–1722. 2018. View Article : Google Scholar
|
|
57
|
Xu Y, Yao Y, Liu Y, Wang Z, Hu Z, Su Z, Li
C, Wang H, Jiang X, Kang P, et al: Elevation of circular RNA
circ_0005230 facilitates cell growth and metastasis via sponging
miR-1238 and miR-1299 in cholangiocarcinoma. Aging (Albany NY).
11:1907–1917. 2019. View Article : Google Scholar
|
|
58
|
Shi X, Zhan L, Xiao C, Lei Z, Yang H, Wang
L, Zhao J and Zhang HT: miR-1238 inhibits cell proliferation by
targeting LHX2 in non-small cell lung cancer. Oncotarget.
6:19043–19054. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Jiang XM, Li ZL, Li JL, Xu Y, Leng KM, Cui
YF and Sun DJ: A novel prognostic biomarker for cholangiocarcinoma:
circRNA Cdr1as. Eur Rev Med Pharmacol Sci. 22:365–371.
2018.PubMed/NCBI
|
|
60
|
Xu Y, Yao Y, Zhong X, Leng K, Qin W, Qu L,
Cui Y and Jiang X: Downregulated circular RNA hsa_circ_0001649
regulates proliferation, migration and invasion in
cholangiocarcinoma cells. Biochem Biophys Res Commun. 496:455–461.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Wang S, Hu Y, Lv X, Li B, Gu D, Li Y, Sun
Y and Su Y: Circ-0000284 arouses malignant phenotype of
cholangiocarcinoma cells and regulates the biological functions of
peripheral cells through cellular communication. Clin Sci (Lond).
133:1935–1953. 2019. View Article : Google Scholar
|
|
62
|
Sun M and Kraus WL: From discovery to
function: The expanding roles of long noncoding RNAs in physiology
and disease. Endocr Rev. 36:25–64. 2015. View Article : Google Scholar :
|
|
63
|
Chen J, Miao Z, Xue B, Shan Y, Weng G and
Shen B: Long Non-coding RNAs in Urologic Malignancies: Functional
Roles and Clinical Translation. J Cancer. 7:1842–1855. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Wang J, Xie H, Ling Q, Lu D, Lv Z, Zhuang
R, Liu Z, Wei X, Zhou L, Xu X, et al: Coding-noncoding gene
expression in intra-hepatic cholangiocarcinoma. Transl Res.
168:107–121. 2016. View Article : Google Scholar
|
|
65
|
Hao S, Yao L, Huang J, He H, Yang F, Di Y,
Jin C and Fu D: Genome‑Wide Analysis Identified a Number of
Dysregulated Long Noncoding RNA (lncRNA) in Human Pancreatic Ductal
Adenocarcinoma. Technol Cancer Res Treat. 17:15330346177484292018.
View Article : Google Scholar
|
|
66
|
Yao Y, Sun Y, Jiang Y, Qu L and Xu Y:
Enhanced expression of lncRNA TP73-AS1 predicts adverse phenotypes
for cholangio-carcinoma and exerts oncogenic properties in vitro
and in vivo. Biomed Pharmacother. 106:260–266. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Li Y, Cai Q, Li W, Feng F and Yang L: Long
non-coding RNA EPIC1 promotes cholangiocarcinoma cell growth.
Biochem Biophys Res Commun. 504:654–659. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Xu Y, Wang Z, Jiang X and Cui Y:
Overexpression of long noncoding RNA H19 indicates a poor prognosis
for cholangio-carcinoma and promotes cell migration and invasion by
affecting epithelial-mesenchymal transition. Biomed Pharmacother.
92:17–23. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Wang WT, Ye H, Wei PP, Han BW, He B, Chen
ZH and Chen YQ: LncRNAs H19 and HULC, activated by oxidative
stress, promote cell migration and invasion in cholangiocarcinoma
through a ceRNA manner. J Hematol Oncol. 9:1172016. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Zhang S, Xiao J, Chai Y, Du YY, Liu Z,
Huang K, Zhou X and Zhou W: LncRNA-CCAT1 Promotes Migration,
Invasion, and EMT in Intrahepatic Cholangiocarcinoma Through
Suppressing miR-152. Dig Dis Sci. 62:3050–3058. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Ma F, Wang SH, Cai Q, Zhang MD, Yang Y and
Ding J: Overexpression of LncRNA AFAP1-AS1 predicts poor prognosis
and promotes cells proliferation and invasion in gallbladder
cancer. Biomed Pharmacother. 84:1249–1255. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Zhang C, Li JY, Tian FZ, Zhao G, Hu H, Ma
YF and Yang YL: Long Noncoding RNA NEAT1 Promotes Growth and
Metastasis of Cholangiocarcinoma Cells. Oncol Res. 26:879–888.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Parasramka M, Yan IK, Wang X, Nguyen P,
Matsuda A, Maji S, Foye C, Asmann Y and Patel T: BAP1 dependent
expression of long non-coding RNA NEAT-1 contributes to sensitivity
to gemcitabine in cholangiocarcinoma. Mol Cancer. 16:222017.
View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Ma SL, Li AJ, Hu ZY, Shang FS and Wu MC:
Co expression of the carbamoyl phosphate synthase 1 gene and its
long non coding RNA correlates with poor prognosis of patients with
intrahepatic cholangiocarcinoma. Mol Med Rep. 12:7915–7926. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Wang C, Mao ZP, Wang L, Wu GH, Zhang FH,
Wang DY and Shi JL: Long non-coding RNA MALAT1 promotes
cholangio-carcinoma cell proliferation and invasion by activating
PI3K/Akt pathway. Neoplasma. 64:725–731. 2017. View Article : Google Scholar
|
|
76
|
Shi X, Sun M, Liu H, Yao Y and Song Y:
Long non-coding RNAs: A new frontier in the study of human
diseases. Cancer Lett. 339:159–166. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Tan X, Huang Z and Li X: Long Non-Coding
RNA MALAT1 Interacts With miR-204 to Modulate Human Hilar
Cholangiocarcinoma Proliferation, Migration, and Invasion by
Targeting CXCR4. J Cell Biochem. 118:3643–3653. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Qin X, Lu M, Zhou Y, Li G and Liu Z:
LncRNA FENDRR represses proliferation, migration and invasion
through suppression of survivin in cholangiocarcinoma cells. Cell
Cycle. 18:889–897. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Zhang X, Zhou Y, Mehta KR, Danila DC,
Scolavino S, Johnson SR and Klibanski A: A pituitary-derived MEG3
isoform functions as a growth suppressor in tumor cells. J Clin
Endocrinol Metab. 88:5119–5126. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Xia Y, He Z, Liu B, Wang P and Chen Y:
Downregulation of Meg3 enhances cisplatin resistance of lung cancer
cells through activation of the WNT/β-catenin signaling pathway.
Mol Med Rep. 12:4530–4537. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Sribenja S, Natthasirikul N,
Vaeteewoottacharn K, Sawanyawisuth K, Wongkham C, Jearanaikoon P
and Wongkham S: Thymosin β10 as a predictive biomarker of response
to 5‑fluorouracil chemotherapy in cholangiocarcinoma. Ann Hepatol.
15:577–585. 2016.PubMed/NCBI
|
|
82
|
Liang Z, Zhu B, Meng D, Shen X, Li X, Wang
Z and Li L: Down-regulation of lncRNA-NEF indicates poor prognosis
in intrahepatic cholangiocarcinoma. Biosci Rep. 39:392019.
View Article : Google Scholar
|
|
83
|
Hu X, Tan Z, Yang Y and Yang P: Long
non-coding RNA MIR22HG inhibits cell proliferation and migration in
cholangio-carcinoma by negatively regulating the Wnt/β-catenin
signaling pathway. J Gene Med. 21:e30852019. View Article : Google Scholar
|
|
84
|
Luo M, Li Z, Wang W, Zeng Y, Liu Z and Qiu
J: Long non-coding RNA H19 increases bladder cancer metastasis by
associating with EZH2 and inhibiting E-cadherin expression. Cancer
Lett. 333:213–221. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Monnier P, Martinet C, Pontis J, Stancheva
I, Ait-Si-Ali S and Dandolo L: H19 lncRNA controls gene expression
of the Imprinted Gene Network by recruiting MBD1. Proc Natl Acad
Sci USA. 110:20693–20698. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Du Y, Kong G, You X, Zhang S, Zhang T, Gao
Y, Ye L and Zhang X: Elevation of highly up-regulated in liver
cancer (HULC) by hepatitis B virus X protein promotes hepatoma cell
proliferation via down-regulating p18. J Biol Chem.
287:26302–26311. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
King CE, Cuatrecasas M, Castells A,
Sepulveda AR, Lee JS and Rustgi AK: LIN28B promotes colon cancer
progression and metastasis. Cancer Res. 71:4260–4268. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Viswanathan SR and Daley GQ: Lin28: A
microRNA regulator with a macro role. Cell. 140:445–449. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Cao MX, Jiang YP, Tang YL and Liang XH:
The crosstalk between lncRNA and microRNA in cancer metastasis:
Orchestrating the epithelial-mesenchymal plasticity. Oncotarget.
8:12472–12483. 2017. View Article : Google Scholar :
|
|
90
|
Jia P, Cai H, Liu X, Chen J, Ma J, Wang P,
Liu Y, Zheng J and Xue Y: Long non-coding RNA H19 regulates glioma
angiogenesis and the biological behavior of glioma-associated
endothelial cells by inhibiting microRNA-29a. Cancer Lett.
381:359–369. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Gu Y, Li C, Xiao L, Li J, Pei H, Xu D,
Jiang Y, Zhang X, Zhang L, Li K, et al: High expression of long
non-coding RNA NNT-AS1 facilitates progression of
cholangiocarcinoma through promoting epithelial-mesenchymal
transition. Am J Transl Res. 11:5438–5456. 2019.PubMed/NCBI
|
|
92
|
Wang X, Ren M, Li Y, Hu J, Lu G, Ma W, Guo
D, Lu X and He S: Long noncoding RNA NNT-AS1 promotes gastric
cancer proliferation and invasion by regulating microRNA-363
expression. J Cell Biochem. 120:5704–5712. 2019. View Article : Google Scholar
|
|
93
|
Hua F, Liu S, Zhu L, Ma N, Jiang S and
Yang J: Highly expressed long non-coding RNA NNT-AS1 promotes cell
proliferation and invasion through Wnt/β-catenin signaling pathway
in cervical cancer. Biomed Pharmacother. 92:1128–1134. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Huang Y, Shi J and Xu Y: Long non-coding
RNA NNT-AS1 contributes to cell proliferation, metastasis and
apoptosis in human ovarian cancer. Oncol Lett. 15:9264–9270.
2018.PubMed/NCBI
|
|
95
|
Merry CR, Forrest ME, Sabers JN, Beard L,
Gao XH, Hatzoglou M, Jackson MW, Wang Z, Markowitz SD and Khalil
AM: DNMT1-associated long non-coding RNAs regulate global gene
expression and DNA methylation in colon cancer. Hum Mol Genet.
24:6240–6253. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Yu Y, Zhang M, Wang N, Li Q, Yang J, Yan
S, He X, Ji G and Miao L: Epigenetic silencing of tumor suppressor
gene CDKN1A by oncogenic long non-coding RNA SNHG1 in
cholangiocarcinoma. Cell Death Dis. 9:7462018. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Jalili A, Wagner C, Pashenkov M, Pathria
G, Mertz KD, Widlund HR, Lupien M, Brunet JP, Golub TR, Stingl G,
et al: Dual suppression of the cyclin-dependent kinase inhibitors
CDKN2C and CDKN1A in human melanoma. J Natl Cancer Inst.
104:1673–1679. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Guo H, Xu Y and Fu Q: Curcumin inhibits
growth of prostate carcinoma via miR-208-mediated CDKN1A
activation. Tumour Biol. 36:8511–8517. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Askari M, Sobti RC, Nikbakht M and Sharma
SC: Aberrant promoter hypermethylation of p21 (WAF1/CIP1) gene and
its impact on expression and role of polymorphism in the risk of
breast cancer. Mol Cell Biochem. 382:19–26. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Yu Y, Zhang M, Liu J, Xu B, Yang J, Wang
N, Yan S, Wang F, He X, Ji G, et al: Long Non-coding RNA PVT1
Promotes Cell Proliferation and Migration by Silencing ANGPTL4
Expression in Cholangiocarcinoma. Mol Ther Nucleic Acids.
13:503–513. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Kong R, Zhang EB, Yin DD, You LH, Xu TP,
Chen WM, Xia R, Wan L, Sun M, Wang ZX, et al: Long noncoding RNA
PVT1 indicates a poor prognosis of gastric cancer and promotes cell
proliferation through epigenetically regulating p15 and p16. Mol
Cancer. 14:822015. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Tsai MC, Manor O, Wan Y, Mosammaparast N,
Wang JK, Lan F, Shi Y, Segal E and Chang HY: Long noncoding RNA as
modular scaffold of histone modification complexes. Science.
329:689–693. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Okochi-Takada E, Hattori N, Tsukamoto T,
Miyamoto K, Ando T, Ito S, Yamamura Y, Wakabayashi M, Nobeyama Y
and Ushijima T: ANGPTL4 is a secreted tumor suppressor that
inhibits angiogenesis. Oncogene. 33:2273–2278. 2014. View Article : Google Scholar
|
|
104
|
Taskoparan B, Seza EG, Demirkol S, Tuncer
S, Stefek M, Gure AO and Banerjee S: Opposing roles of the
aldo‑keto reductases AKR1B1 and AKR1B10 in colorectal cancer. Cell
Oncol (Dordr). 40:563–578. 2017. View Article : Google Scholar
|
|
105
|
Sinreih M, Štupar S, Čemažar L, Verdenik
I, Frković Grazio S, Smrkolj Š and Rižner TL: STAR and AKR1B10 are
down-regulated in high-grade endometrial cancer. J Steroid Biochem
Mol Biol. 171:43–53. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Xu Y, Yao Y, Qin W, Zhong X, Jiang X and
Cui Y: Long non-coding RNA CCAT2 promotes cholangiocarcinoma cells
migration and invasion by induction of epithelial-to-mesenchymal
transition. Biomed Pharmacother. 99:121–127. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Bai JG, Tang RF, Shang JF, Qi S, Yu GD and
Sun C: Upregulation of long non coding RNA CCAT2 indicates a poor
prognosis and promotes proliferation and metastasis in intrahepatic
cholangio-carcinoma. Mol Med Rep. 17:5328–5335. 2018.PubMed/NCBI
|
|
108
|
Le Gallo M, Lozy F and Bell DW:
Next-Generation Sequencing. Adv Exp Med Biol. 943:119–148. 2017.
View Article : Google Scholar
|
|
109
|
Levy SE and Myers RM: Advancements in
Next-Generation Sequencing. Annu Rev Genomics Hum Genet. 17:95–115.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Slatko BE, Gardner AF and Ausubel FM:
Overview of Next-Generation Sequencing Technologies. Curr Protoc
Mol Biol. 122:e592018. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Zhang J, Han C and Wu T: MicroRNA-26a
promotes cholangio-carcinoma growth by activating β-catenin.
Gastroenterology. 143:246–56.e8. 2012. View Article : Google Scholar
|
|
112
|
Namwat N, Chusorn P, Loilome W, Techasen
A, Puetkasichonpasutha J, Pairojkul C, Khuntikeo N and Yongvanit P:
Expression profiles of oncomir miR‑21 and tumor suppressor let-7a
in the progression of opisthorchiasis-associated
cholangiocarcinoma. Asian Pac J Cancer Prev. 13(Suppl): 65–69.
2012.
|
|
113
|
Meng F, Henson R, Wehbe-Janek H, Ghoshal
K, Jacob ST and Patel T: MicroRNA-21 regulates expression of the
PTEN tumor suppressor gene in human hepatocellular cancer.
Gastroenterology. 133:647–658. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Razumilava N, Bronk SF, Smoot RL, Fingas
CD, Werneburg NW, Roberts LR and Mott JL: miR-25 targets
TNF-related apoptosis inducing ligand (TRAIL) death receptor-4 and
promotes apoptosis resistance in cholangiocarcinoma. Hepatology.
55:465–475. 2012. View Article : Google Scholar
|
|
115
|
Khapre RV, Samsa WE and Kondratov RV:
Circadian regulation of cell cycle: Molecular connections between
aging and the circadian clock. Ann Med. 42:404–415. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Yang H, Li TW, Peng J, Tang X, Ko KS, Xia
M and Aller MA: A mouse model of cholestasis-associated
cholangiocar-cinoma and transcription factors involved in
progression. Gastroenterology. 141:378–388.e3884. 2011. View Article : Google Scholar
|
|
117
|
Li Q, Xia X, Ji J, Ma J, Tao L, Mo L and
Chen W: miR-199a-3p enhances cisplatin sensitivity of
cholangiocarcinoma cells by inhibiting mTOR signaling pathway and
expression of MDR1. Oncotarget. 8:33621–33630. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Zhong XY, Yu JH, Zhang WG, Wang ZD, Dong
Q, Tai S, Cui YF and Li H: MicroRNA-421 functions as an oncogenic
miRNA in biliary tract cancer through down-regulating farnesoid X
receptor expression. Gene. 493:44–51. 2012. View Article : Google Scholar
|
|
119
|
Olaru AV, Ghiaur G, Yamanaka S, Luvsanjav
D, An F, Popescu I, Alexandrescu S, Allen S, Pawlik TM, Torbenson
M, et al: MicroRNA down-regulated in human cholangiocarcinoma
control cell cycle through multiple targets involved in the G1/S
checkpoint. Hepatology. 54:2089–2098. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Cermakian N and Sassone-Corsi P:
Multilevel regulation of the circadian clock. Nat Rev Mol Cell
Biol. 1:59–67. 2000. View Article : Google Scholar
|
|
121
|
Han Y, Meng F, Venter J, Wu N, Wan Y,
Standeford H, Francis H, Meininger C, Greene J Jr, Trzeciakowski
JP, et al: miR-34a-dependent overexpression of Per1 decreases
cholangiocarcinoma growth. J Hepatol. 64:1295–1304. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Qiao P, Li G, Bi W, Yang L, Yao L and Wu
D: microRNA-34a inhibits epithelial mesenchymal transition in human
cholangio-carcinoma by targeting Smad4 through transforming growth
factor-beta/Smad pathway. BMC Cancer. 15:4692015. View Article : Google Scholar
|
|
123
|
Palumbo T, Poultsides GA, Kouraklis G,
Liakakos T, Drakaki A, Peros G, Hatziapostolou M and Iliopoulos D:
A functional microRNA library screen reveals miR-410 as a novel
anti-apoptotic regulator of cholangiocarcinoma. BMC Cancer.
16:3532016. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Braconi C, Huang N and Patel T:
MicroRNA-dependent regulation of DNA methyltransferase-1 and tumor
suppressor gene expression by interleukin-6 in human malignant
cholangiocytes. Hepatology. 51:881–890. 2010.PubMed/NCBI
|
|
125
|
Chen Y, Luo J, Tian R, Sun H and Zou S:
miR-373 negatively regulates methyl-CpG-binding domain protein 2
(MBD2) in hilar cholangiocarcinoma. Dig Dis Sci. 56:1693–1701.
2011. View Article : Google Scholar
|
|
126
|
Zeng B, Ye H, Chen J, Cheng D, Cai C, Chen
G, Chen X, Xin H, Tang C and Zeng J: LncRNA TUG1 sponges miR-145 to
promote cancer progression and regulate glutamine metabolism via
Sirt3/GDH axis. Oncotarget. 8:113650–113661. 2017. View Article : Google Scholar
|
|
127
|
Zhang F, Wan M, Xu Y, Li Z, Leng K, Kang
P, Cui Y and Jiang X: Long noncoding RNA PCAT1 regulates
extrahepatic chol-angiocarcinoma progression via the
Wnt/β-catenin-signaling pathway. Biomed Pharmacother. 94:55–62.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Kong L, Wu Q, Zhao L, Ye J, Li N and Yang
H: Upregulated lncRNA-UCA1 contributes to metastasis of bile duct
carcinoma through regulation of miR-122/CLIC1 and activation of the
ERK/MAPK signaling pathway. Cell Cycle. 18:1212–1228. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Xu Y, Yao Y, Jiang X, Zhong X, Wang Z, Li
C, Kang P, Leng K, Ji D, Li Z, et al: SP1-induced upregulation of
lncRNA SPRY4-IT1 exerts oncogenic properties by scaffolding
EZH2/LSD1/DNMT1 and sponging miR-101-3p in cholangio-carcinoma. J
Exp Clin Cancer Res. 37:812018. View Article : Google Scholar
|
|
130
|
Carotenuto P, Fassan M, Pandolfo R, Lampis
A, Vicentini C, Cascione L, Paulus-Hock V, Boulter L, Guest R,
Quagliata L, et al: Wnt signalling modulates
transcribed-ultra-conserved regions in hepatobiliary cancers. Gut.
66:1268–1277. 2017. View Article : Google Scholar
|
|
131
|
Zhang D, Li H, Xie J, Jiang D, Cao L, Yang
X, Xue P and Jiang X: Long noncoding RNA LINC01296 promotes tumor
growth and progression by sponging miR-5095 in human
chol-angiocarcinoma. Int J Oncol. 52:1777–1786. 2018.PubMed/NCBI
|