Introduction
According to data from the global cancer statistics
in 2018, the most common and malignant form of cancer is that of
lung cancer, which accounts for 11.6% of all cancers and 18.4% of
all cancer-associated mortality globally (1). In China, lung cancer is the most
prominent cause of mortality associate with cancer in both males
and females, with mortality rates of 52.47 and 26.29 per 100,000,
respectively (2). Non-small cell
lung cancer (NSCLC) cases make up ~85% of all lung cancer cases,
with ≤40% of cases not being identified until advanced stages
(III-IV), where treatment options and the probability of survival
become limited (3).
In total, ~15% cancer cases have recently been
suggested to be associated with an infectious origin, accounting
for 1.2 million cases annually (4). Previous clinical and experimental
findings suggest that chronic infection and inflammation are
closely linked with lung cancer (5-7).
Toll-like receptors (TLRs) are key receptors that can detect and
then respond to infections through the innate immune and
inflammatory response mechanisms. TLR4 was the first identified
human toll homolog family of proteins that can be activated by
lipopolysaccharides (LPS) and induces the secretion of
proinflammatory cytokines among others to combat pathogenic
infections (8). Although recent
studies found TLR4 to be expressed in lung cancer cells and tissues
(9,10), the role of TLR4 in lung cancer
remain controversial. The programmed-death 1 receptor/PD-ligand 1
(PD-L1) pathway has been reported to be a key inhibitory mechanism
in lung cancer cells, the activation of which leads to effector T
cell exhaustion and immune escape (11,12).
Previous studies have demonstrated that PD-L1 expression could be
induced by TLR4 in macrophages and colonic stromal cells (13,14),
though the functional relationship between PD-L1 and TLR4 in lung
cancer remains elusive.
In the present study, PD-L1 and TLR4 expression were
measured in lung cancer tissues, where TLR4 and PD-L1 expression
were found to be upregulated in lung cancer tissues, with a
positive correlation being observed between the expression levels
of these two proteins. In addition, the mechanism in which TLR4
influenced the expression of PD-L1 and associated signaling
pathways in the A549 lung cancer cell line was also
investigated.
Materials and methods
Patients
The Ethics Committee of The First Affiliated
Hospital of Nanchang University approved the present study
(Nanchang, China). Informed consent was obtained from all patients.
All patients were pathologically diagnosed as NSCLC and had not
undergone any radio- or chemotherapy and should have a complete set
of clinicopathological and follow-up data. Patients with other
malignant tumors and diseases that may affect survival, including
diabetes and heart failure were excluded. In total, 60 patients
that underwent pulmonary resection from thoracic surgery department
of The First Affiliated Hospital of Nanchang University were
enrolled between January and December 2010. Overall survival (OS)
was defined as the time from diagnosis to mortality or the final
follow-up. Table I highlights the
clinicopathological parameters from all enrolled patients.
 | Table IAssociation between PD-L1 and TLR-4
expression levels and the clinicopathological parameters of
patients with non-small cell lung cancer. |
Table I
Association between PD-L1 and TLR-4
expression levels and the clinicopathological parameters of
patients with non-small cell lung cancer.
| Parameters | Cases | PD-L1
| % | P-valuea | TLR-4
| % | P-valueb |
|---|
| + | − | + | − |
|---|
| Gender | | | | | 0.791 | | | | 0.071 |
| Male | 48 | 30 | 18 | 62.5 | | 22 | 26 | 45.8 | |
| Female | 12 | 7 | 5 | 58.3 | | 9 | 3 | 75 | |
| Age (years) | | | | | 0.559 | | | | 0.123 |
| >60 | 21 | 14 | 7 | 66.7 | | 8 | 13 | 38.1 | |
| ≤60 | 39 | 23 | 16 | 59 | | 23 | 16 | 59.0 | |
| Histology type | | | | | 0.729 | | | | 0.010 |
| SCC | 27 | 16 | 11 | 59.3 | | 9 | 18 | 33.3 | |
| ADC | 33 | 21 | 12 | 63.6 | | 22 | 10 | 69.7 | |
| Grade | | | | | 0.602 | | | | 0.866 |
| High-middle | 42 | 25 | 17 | 35.7 | | 22 | 20 | 52.4 | |
| Low | 18 | 12 | 6 | 66.7 | | 9 | 9 | 50.0 | |
| Lymphatic
invasion | | | | | 0.791 | | | | 0.796 |
| Negative | 30 | 18 | 12 | 60.0 | | 15 | 15 | 50 | |
| Positive | 30 | 19 | 11 | 63.3 | | 16 | 14 | 53.3 | |
| Tumor size
(cm) | | | | | 0.453 | | | | 0.715 |
| ≤3 | 20 | 11 | 9 | 55.0 | | 11 | 9 | 55.0 | |
| >3 | 40 | 26 | 14 | 65.0 | | 20 | 20 | 50.0 | |
| TNM stage | | | | | 0.090 | | | | 0.025 |
| I + II | 39 | 21 | 18 | 53.8 | | 16 | 23 | 41 | |
| III | 21 | 16 | 5 | 76.2 | | 15 | 6 | 71.4 | |
Immunohistochemistry (IHC)
A total of 60 NSCLC samples and 20 matched adjacent
para-cancerous tissues (>2 cm from the edge of tumor tissue)
were collected in this study. Once resected, tissues were fixed in
10% formalin overnight at room temperature (RT), embedded in
paraffin and cut into 4-µm thick tissue sections. Polyclonal
rabbit anti-TLR4 (1:100, cat. no. ab13556; Abcam), and polyclonal
rabbit anti-PD-L1 (1:100, cat. no. 13684; Cell signaling
Technologies, Inc.) primary antibodies were used in the present
study for IHC. Matched adjacent para-carcinoma tissues served as
controls. Briefly, the samples were first heated at 70°C for 20 min
prior to de-paraffinization in xylene, followed by rehydration with
a descending ethanol series. Antigen retrieval was performed at
100°C and in citrate buffer (10 mM, pH 6.0) for 2 min and
permeabilized in 0.5% Triton X-100 at RT for 20 min, following
which 0.3% hydrogen peroxide was added to block the activity
endogenous peroxidase for 10 min at RT. The sections were then
blocked with 5% BSA (Beijing Solarbio Science & Technology Co.,
Ltd.) for 30 min at RT before the tissue sections were incubated
with the primary antibodies at 4°C overnight. Samples were then
washed with PBS before the addition of horseradish peroxidase
(HRP)-conjugated secondary antibodies (1:200; cat. no. G1213; Wuhan
Servicebio Technology Co., Ltd.) at 37°C for 30 min. After washing,
the tissues were then treated with 3,3'-diaminobenzidine (MXB
Biotechnologies) for chromogenic development. Nuclei were next
stained using hematoxylin (30 mg/ml; Sigma-Aldrich; Merck KGaA) for
1 min at RT before the sections were then added to cover-slips and
assessed by light microscopy (magnification, ×100; Olympus
Corporation), with all sections assessed by two pathologists
independently in a blinded manner. Sections were deemed to be PD-L1
or TLR4 positive based on the presence of cell membrane or
cytoplasmic staining, with a semi-quantitative system applied for
IHC scoring. Five random areas with typical staining were
identified, with 100 cells were assessed per area to determine the
strength and frequency of staining. Staining strength was
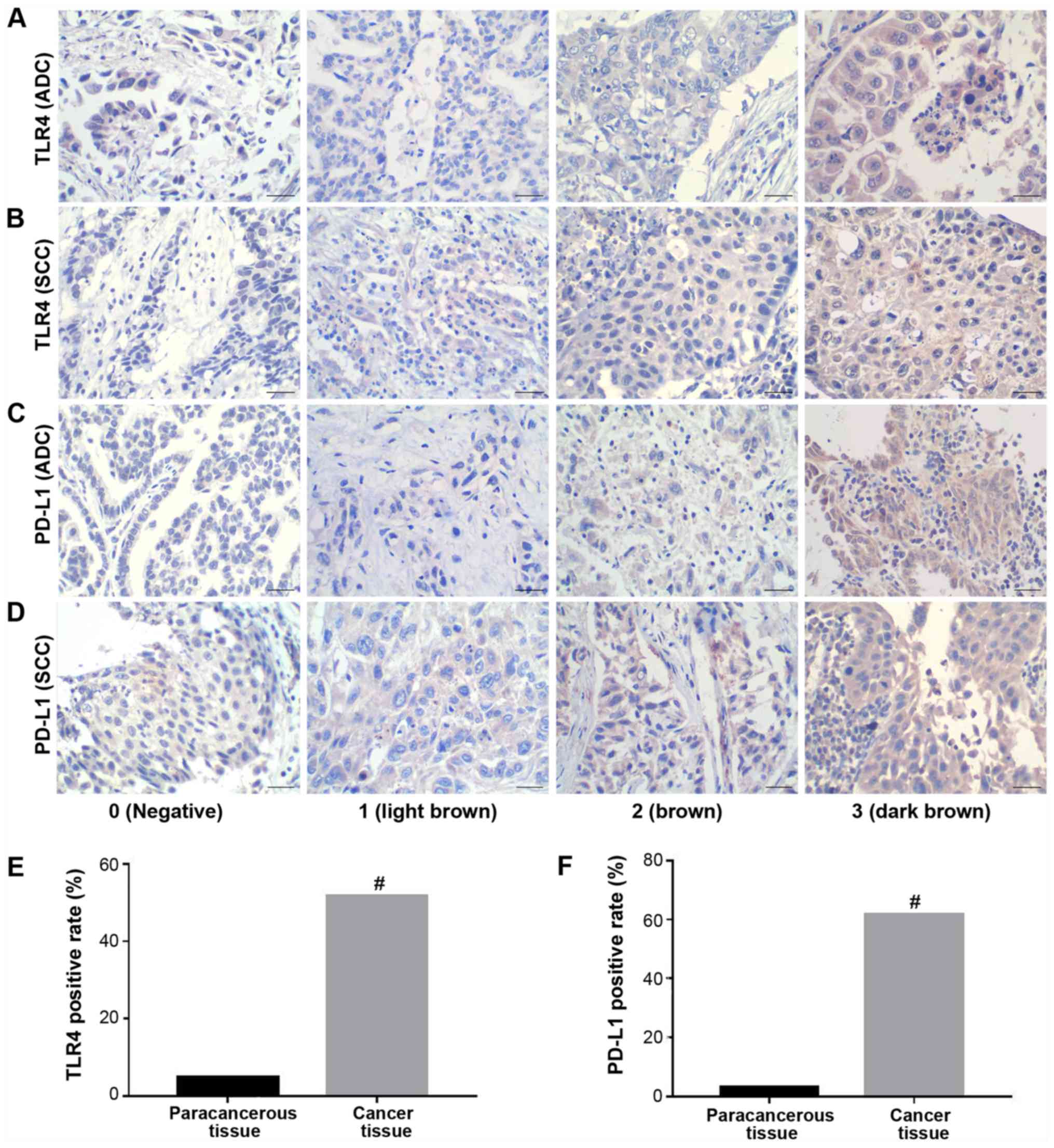
determined based on the following color scale: i) Negative, 0; ii)
light brown, 1; iii) brown, 2; and iv) dark brown, 3. The frequency
of cells staining positive for the indicated antigen was determined
based on the following scale: i) ≤5%, 0; ii) 6-25%, 1; iii) 26-50%,
2; iv) 51-75%, 3; and v) 76-100%, 4. These two scores were then
multiplied together to yield a final staining score, with scores ≤1
being deemed negative (−), whereas those ≥1 were considered
positive (+).
Cell culture
The A549 cell line was a gift from Dr. Jianbin Wang,
Translation Medical Department of Nanchang University (Nanchang,
China). RPMI-1640 (Biological Industries) supplemented with 10%
newborn bovine serum (Biological Industries) and
penicillin/streptomycin (Beijing Solarbio Science & Technology
Co., Ltd.) was used to culture cells at 37°C under a humidified
atmosphere containing 5% CO2. Confluent cells were
collected using trypsin/EDTA for 3 min, after which cells
(3×105 cells/ml) were plated into six-well plates (1
ml/well) for LPS treatment. LPS (Sigma-Aldrich; Merck KGaA) was
used to treat the cells (0.5, 1 and 2 µg/ml) for a range of
time points (15 and 30 min, or 1, 2, 4 or 24 h) at 37°C. PD98059
(cat. no. M1822; Abmole Bioscience Inc.) and LY294002 (cat. no.
9901; Cell Signaling Technology, Inc.) were utilized as inhibitors
for MEK and AKT, respectively. A549 cells were pretreated with
PD98059 or LY294002 at different concentrations for 1 h at 37°C,
following which LPS (1 µg/ml) was added into the medium.
Western blotting
RIPA buffer (Applygen Technologies, Inc.) was used
to lyse the A549 cells for protein extraction, following which a
bicinchoninic acid protein assay kit (Vazyme Biotech Co., Ltd.) was
used to quantify protein concentration. A total of 25 µg
protein of each sample was separated by 10% SDS-PAGE and
transferred onto PVDF membranes, which were then blocked for 1 h
with 5% BSA (Beijing Solarbio Science & Technology Co., Ltd.)
at RT before being probed overnight at 4°C with the following
primary antibodies: Anti-TLR4 (1:500; cat. no. ab13556; Abcam),
anti-PD-L1 (cat. no. 13684; Cell Signaling Technology, Inc.),
anti-phosphorylated (p)-p44/42MAPK (1:1,000; cat. no. 9101; Cell
Signaling Technology, Inc.), anti-p44/42MAPK (1:1,000; cat. no.
4695; Cell Signaling Technology, Inc.), anti-Akt (1:1,000; cat. no.
4691; Cell Signaling Technology, Inc.), anti-p-Akt (Ser473;
1:2,000; cat. no. 4060; Cell Signaling Technology, Inc.) and
anti-β-tubulin (1:2,000; cat. no. TA506805; OriGene Technologies,
Inc.). The membranes were then washed three times and probed again
with HRP-conjugated goat anti-mouse IgG (1:5,000: cat. no. SE131;
Beijing Solarbio Science & Technology Co., Ltd.) or goat
anti-rabbit IgG (1:5,000; cat. no. SE134; Beijing Solarbio Science
& Technology Co., Ltd.) at RT for 1 h.
Electro-chemiluminescence Plus supersensitive luminescent solution
(Beijing Solarbio Science & Technology Co., Ltd.) was used for
protein detection, following which the Image J software (v1.43j;
National Institutes of Health) was used to perform densitometric
analysis using β-tubulin as a loading control for
normalization.
Reverse transcription-quantitative-PCR
(RT-qPCR)
RNAsimple Total RNA Kit (Tiangen Biotech Co., Ltd.)
was used for RNA isolation according to manufacturer's protocols.
Subsequently, reverse transcription was performed using FastQuant
RT kit (Tiangen Biotech Co., Ltd.) according to manufacturer's
protocols. The temperature protocol was 42°C for 15 min followed by
95°C for 3 min for cDNA synthesis. SuperReal PreMix Plus (SYBR
Green; Tiangen Biotech Co., Ltd.) was then used for qPCR in an ABI
7500 system (Applied Biosystems; Thermo Fisher Scientific, Inc.)
using the following thermocycling conditions: Initial denaturation
at 95°C for 15 min, followed by 40 cycles of 95°C for 10 sec and
60°C for 30 sec. Sequences of the primers were as follows: PD-L1
forward, 5′-GCC GAC TAC AAG CGA ATT AC-3′ and reverse, 5′-TCT CAG
TGT GCT GGT CAC AT-3′ and β-actin forward, 5′-CGG GAA ATC GTG CGT
GAC-3′ and reverse, 5′-TAG AAG CAT TTG CGG TGG-3′. The
2−ΔΔCq method was utilized when assessing relative
expression (15).
Statistical analysis
SPSS statistical software (version 19.0; IBM Corp.)
and GraphPad Prism 5.0 (GraphPad Software, Inc.) were utilized when
performing statistical assessments. All experiments were repeated 3
times and data were presented as the mean ± SD or SEM. Spearman
rank correlation coefficient was used for correlation analyzes.
χ2-test was used for comparisons between categorical
variables and the Kaplan-Meier method was used when assessing
overall survival based on the IHC scores with log-rank tests used
for comparisons of significance. A Cox proportional hazard model
was applied for multivariate analyses of the independent factors
associated with survival. Statistical comparisons between >2
groups were performed using one-way ANOVA followed by Tukey's test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
TLR4 and PD-L1 expression are both
increased in NSCLC tissues, which exhibit positive association with
each other
To assess TLR4 and PD-L1 expression in NSCLC
tissues, IHC was performed in 60 cases of NSCLC tissues and 20
matched adjacent para-cancerous tissues. Positive TLR4 and PD-L1
staining was mainly observed at the membrane and cytoplasm of the
cancerous tissues (Figs. 1 and
S1). The rate of TLR4-positive
expression was observed in 31/60 (51.7%) NSCLC tissues, compared
with 1/20 (5%) observed in adjacent para-cancerous tissues, which
was found to be significant (P<0.001; Table SI). The rate of PD-L1-positive
expression was observed in 37/60 (61.7%) NSCLC tissues and 2/20
(10%) adjacent para-cancerous tissues, with the difference found to
be significant (P<0.001; Table
SI). Both TLR4 and PD-L1 positive rates were found to be
significantly higher in NSCLC tissues compared with those in
para-cancerous tissues (Fig. 1). A
total of 24 tissues exhibited positive staining for both TLR4 and
PD-L1, whilst 16 tissues were tested negative for both TLR4 and
PD-L1 staining among the 60 lung cancer tissues (Table SII). χ2-test revealed a
positive association between the incidence of positive TLR4 and
that of positive PD-L1 expression based on the IHC scores (χ=6.733,
P=0.0095).
To assess the clinical relevance of TLR4 and PD-L1,
the association between the clinicopathological characteristics,
including age, gender, histological type, stages of pathological
differentiation, lymphatic invasion, tumor size and TNM stage, and
the expression of TLR4/PD-L1 was analyzed in Table I. TLR4 expression was found to
associate with the histological type (P=0.01) and TNM stages
(P=0.025) but PD-L1 expression did not associate with any of the
clinicopathological parameters tested.
Elevated TLR4 and PD-L1 correspond to
poorer prognoses in patients with NSCLC
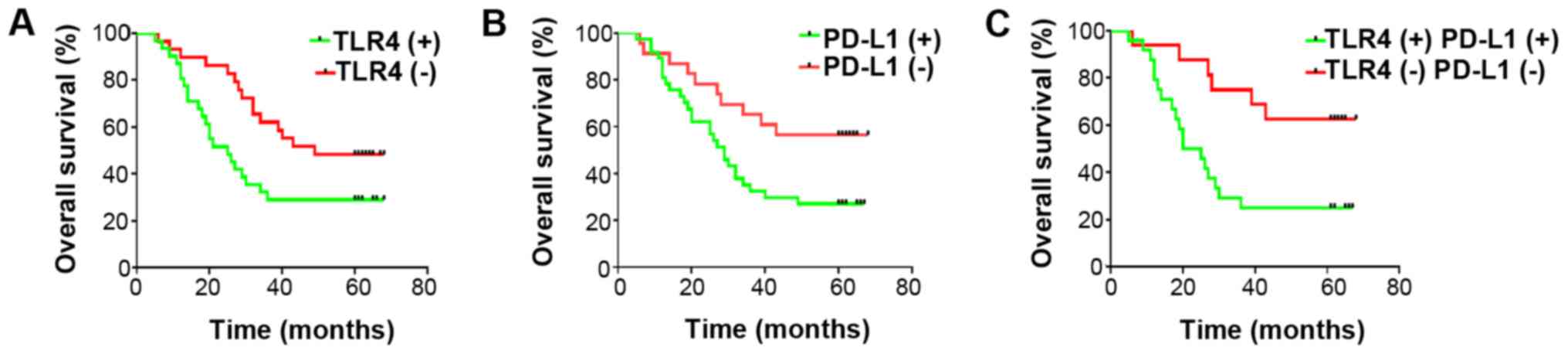
The average follow-up time for the patients was
38.33±2.814 months (range, 5-68 months), where 37 died and 23
surviving to the final follow-up on 31st December, 2015. The 1-, 3-
and 5-year OS rates for these individuals were found to be 88, 45
and 38.3%, respectively. Patients with positive TLR4 and/or PD-L1
staining were found to associate significantly with lower OS
compared with those with negative staining at each time point
(Fig. 2). Although it was found by
univariate analysis that the OS rate was significantly associated
with lymphatic invasion (P=0.01), tumor stage (P=0.01), TLR4
(P=0.02) and PD-L1 expression (P=0.01), subsequent multivariate
analysis did not demonstrate these to be independent prognostic
factors (Table II).
 | Table IIUnivariate and multivariate analysis
of the clinicopathologic factors in patients with NSCLC with
respect to overall survival. |
Table II
Univariate and multivariate analysis
of the clinicopathologic factors in patients with NSCLC with
respect to overall survival.
| Parameters | Cases | Univariate analysis
| Multivariate
analysis
|
|---|
| Survive time | P-value | HR (95% CI) | P-value |
|---|
| Gender | | | 0.609 | | |
| Male | 48 | 36.06±3.29 | | | |
| Female | 12 | 35.42±5.16 | | | |
| Age (years) | | | 0.845 | | |
| >60 | 21 | 39.1±4.75 | | | |
| ≤60 | 39 | 37.92±3.54 | | | |
| Histology type | | | 0.293 | | |
| SCC | 27 | 41.63±4.48 | | | |
| ADC | 33 | 35.64±3.57 | | | |
| Grade | | | 0.177 | | |
| High-middle | 42 | 40.83±3.36 | | | |
| Low | 18 | 32.5±5.03 | | | |
| Lymphatic
invasion | | | 0.013 | | |
| Negative | 30 | 31.47±3.73 | | 0.557
(0.246-1.258) | 0.159 |
| Positive | 30 | 45.2±3.88 | | | |
| Tumor size
(cm) | | | 0.355 | | |
| ≤3 | 20 | 42.05±4.79 | | | |
| >3 | 40 | 36.48±3.48 | | | |
| TNM stage | | | 0.009 | | |
| I +II | 39 | 43.67±3.33 | | 1.24
(0.538-2.86) | 0.614 |
| III | 21 | 28.43±4.47 | | | |
| PD-L1 | | | 0.023 | | |
| Positive | 37 | 33.32±3.36 | | 0.663
(0.31-1.422) | 0.291 |
| Negative | 23 | 46.39±4.57 | | | |
| TLR4 | | | 0.014 | | |
| Positive | 31 | 31.71±3.85 | | 0.591
(0.298-1.17) | 0.131 |
| Negative | 29 | 45.41±3.75 | | | |
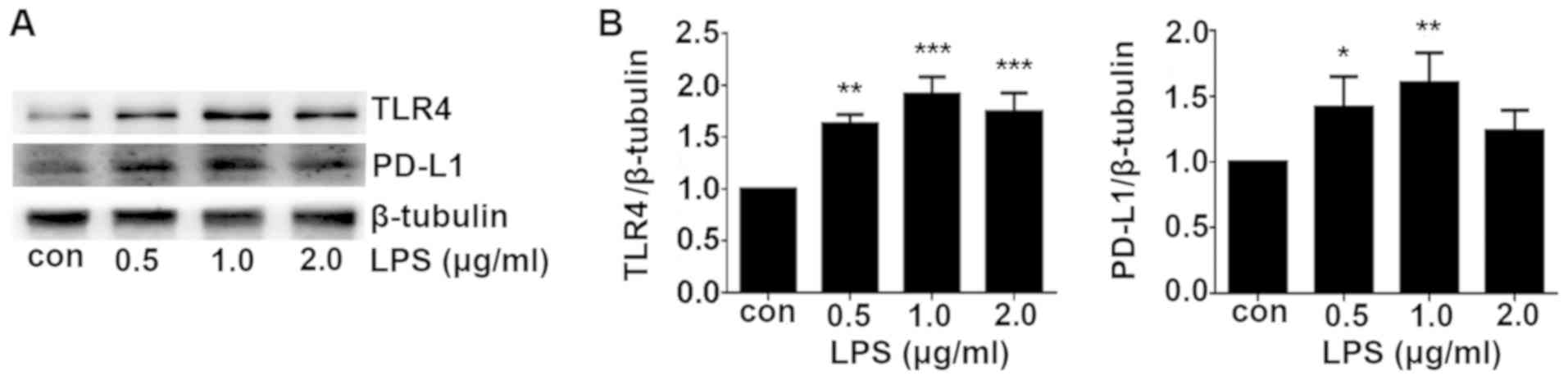
LPS induces TLR4 and PD-L1 expression in
a dose-dependent manner
LPS is a potent agonist for TLR4 (9). In the present study, A549 cells were
treated with different concentrations (0.5, 1 and 2 µg/ml)
of LPS for 24 h. TLR4 expression was demonstrated to be
significantly increased by LPS treatment in a dose-dependent manner
compared with that in the control group, which peaked at 1.0
µg/ml (Fig. 3). PD-L1
expression was also increased after LPS treatment compared with
that in control group with the optimal concentration found to be
1.0 µg/ml (Fig. 3). A
higher concentration of LPS (2.0 µg/ml) was not able to
upregulate the TLR4 and PD-L1 expression further. Therefore, 1.0
µg/ml was used as the concentration for subsequent LPS
stimulation experiments.
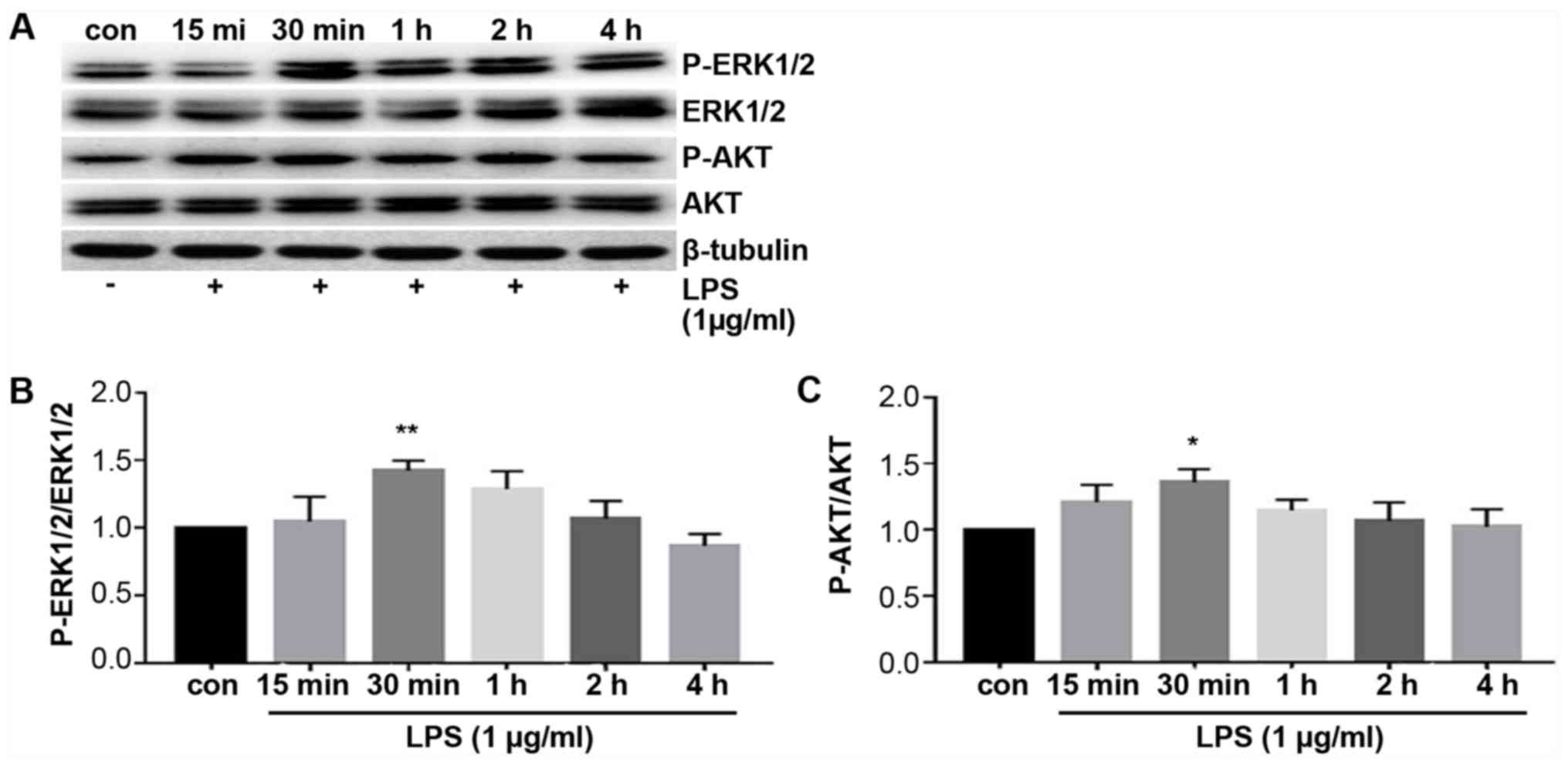
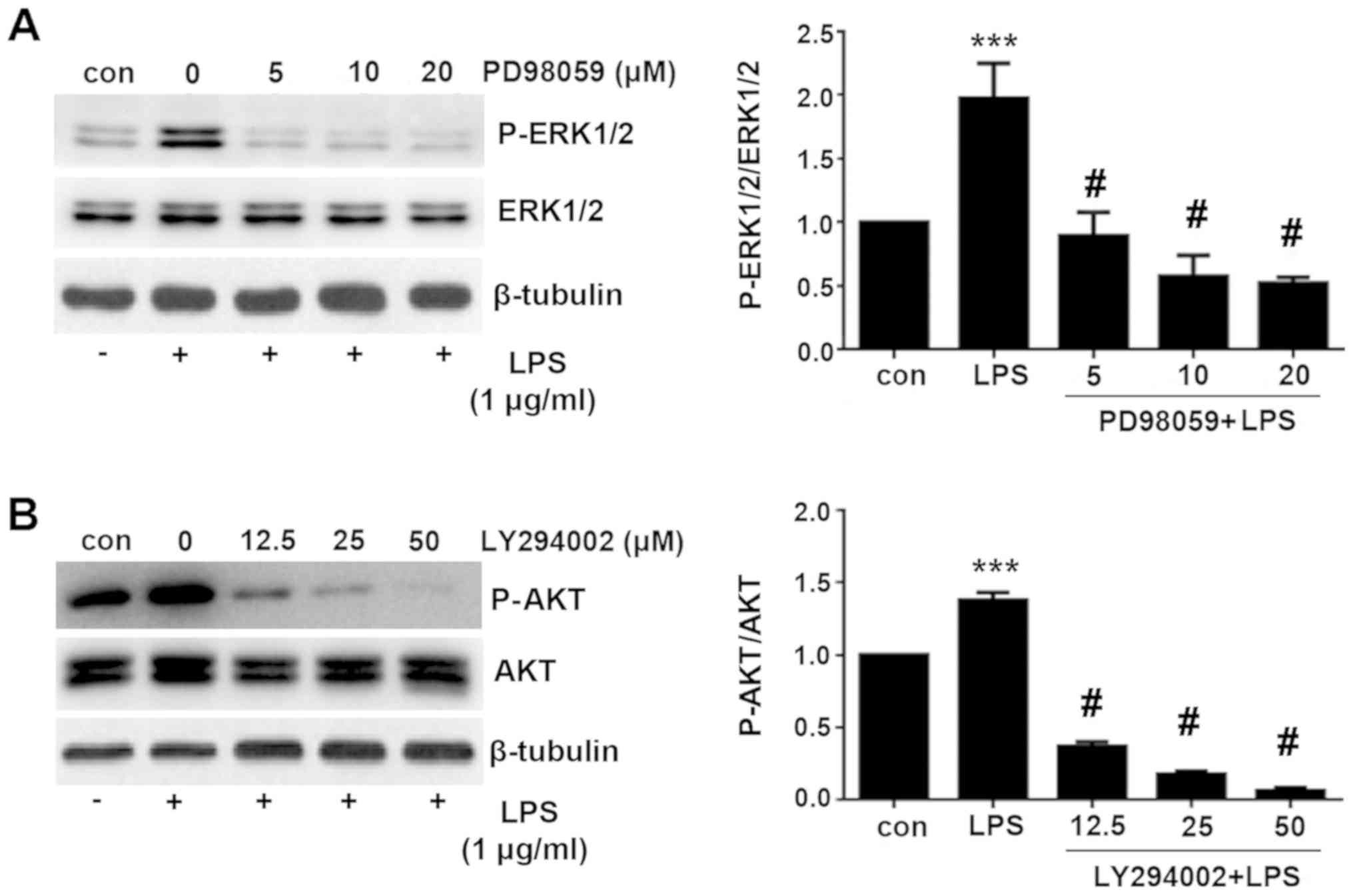
ERK and PI3K/AKT signaling pathway are
activated by LPS stimulation
To explore the mechanism underlying the TLR4 and
PD-L1 upregulation by LPS treatment, expression of proteins
associated with the ERK and PI3K/AKT signaling pathway were
measured using western blotting. ERK and AKT phosphorylation were
found to be significantly increased following treatment with LPS
compared with those in control cells (Fig. 4). The levels of phosphorylation
peaked at 30 min after LPS treatment, which then decreased
thereafter (Fig. 4). These results
suggest that the ERK and PI3K/AKT signaling pathway was activated
by LPS stimulation.
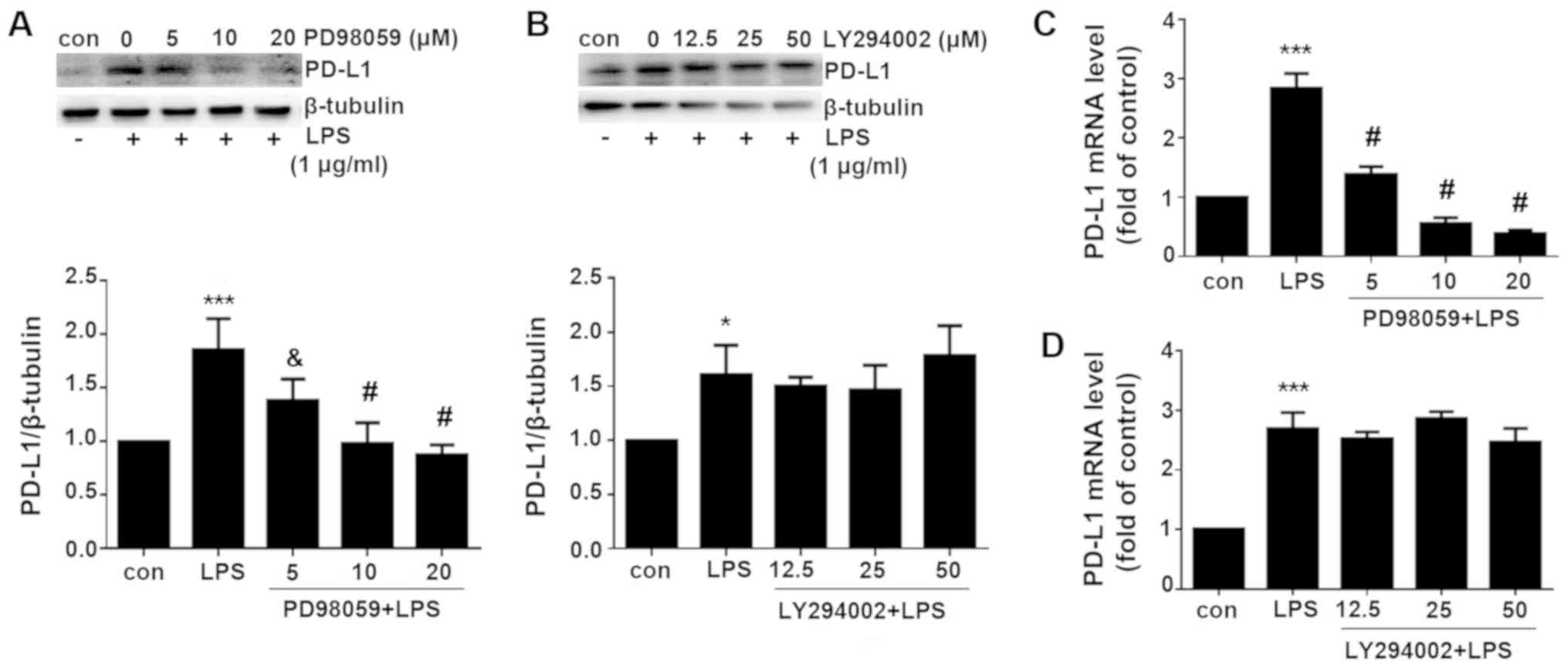
LPS-induced PD-L1 expression is mediated
via ERK signaling but not the PI3K/AKT pathway
Since both ERK and PI3K/AKT signaling pathway were
activated by LPS treatment, pharmacological inhibitors of the ERK
and PI3K/AKT signaling pathway applied in the present study to
investigate which pathway is necessary for the induction of PD-L1
expression. PD98059 is a selective inhibitor of MAPK/ERK kinase
(MEK), which binds to the inactive form of MEK to prevent the
activation of MEK1 and MEK2 by upstream kinases (16). PD98059 was therefore used to
inhibit ERK activity. By contrast, LY294002 is a broad-spectrum
PI3K inhibitor that has been demonstrated to block PI3K-dependent
AKT phosphorylation and kinase activity (17). As shown in Fig. 5, PD98059 significantly inhibited
ERK phosphorylation, whilst LY294002 significantly inhibited AKT
phosphorylation compared with cells treated with LPS alone.
PD-L1 expression was subsequently measured. Western
blotting and RT-qPCR analysis revealed that the LPS-induced PD-L1
upregulation was significantly reversed by the MEK inhibitor
PD98059 but not by the PI3K inhibitor PD294002 on both protein and
mRNA levels (Fig. 6). These data
indicated that LPS induced PD-L1 upregulation via ERK signaling
pathway.
Discussion
Lung cancer ranks number one in the number of
mortalities associated with cancer in men and second in women, with
1.8 million newly diagnosed cases and 1.6 million deaths resulting
from this disease globally each year (18). In total, ~80-85% lung cancer cases
are of the NSCLC type. Since clinical manifestations and symptoms
are nonspecific, the majority of patients with NSCLC are diagnosed
after the occurrence of metastasis (19), greatly diminishing the efficacy of
surgery. Although the introduction of targeted therapies such as
immunotherapy have improved survival to a certain degree, the
overall survival rate remains unsatisfactory (19-21).
Therefore, early diagnosis and treatment are crucial in preventing
tumor progression and reducing the mortality of patients.
There is accumulating evidence demonstrating that
cancer is associated with infectious agents and inflammation
(4,22-26).
It is estimated that ≤20% of all cancers are preceded by
inflammation as a result of pathogenic infection, with
hepatocellular carcinoma and hepatitis B virus-induced hepatitis,
gastric cancer and H. pylori-induced gastritis, cervical
cancer and human papillomavirus infection among the well documented
examples (27-29). Denholm et al (30) previously found the incidence of
lung cancer to be significantly associated with chronic bronchitis
and emphysema, with the presence of both conditions associating
more strongly with lung cancer compared with chronic bronchitis
alone. Interestingly, a growing body of evidence are supporting an
association between H. pylori infection with lung cancer
(31). However, the mechanisms
through which inflammation promotes cancer are not fully
understood.
TLR4 is a key mediator of innate immunity, which
specifically recognizes conserved motifs expressed by pathogens to
mediate immune responses (32).
Huang et al (33)
previously demonstrated that TLR4 is expressed by many types of
cancer cells, including colon, breast, prostate and lung cancer
cells. Following TLR4 activation, tumor cells can synthesize a
number of factors, including interleukin-6, interleukin-12 and
PD-L1, which is a co-stimulator of T cell function. Interaction
between PD-L1 and PD-1 expressed on cytolytic T cells leads to the
negative co-stimulation of TCR signaling, resulting in effector T
cell exhaustion (34).
PD1/PD-L1-induced immune evasion by tumor cells is an important
mechanism for NSCLC, the blockade of which has improved the
survival of a small percentage of patients with NSCLC (35,36).
However, the majority of patients showed little to no response or
acquire resistance during treatment (37).
A number of studies have previously reported that
TLR4 and PD-L1 were aberrantly expressed in cancer tissues or cell
lines (13,14,38-40).
PD-L1 expression can be induced by extracellular vesicles from
melanoma cells via TLR4 signaling (41). Both TLR4 and PD-L1 were upregulated
in ~50% of peripheral T-cell lymphomas, which were found to be
associated with poor prognoses (42). Therefore, in the present study it
was hypothesized that TLR4-induced PD-L1 expression could be the
mechanism underlying lung cancer progression. TLR4 and PD-L1
expression levels were first measured in NSCLC tissues, which
demonstrated that both TLR4 and PD-L1 were significantly more
prominent in NSCLC tissues compared with those in para-cancerous
tissues. In addition, a statistically significant positive
correlation was observed between TLR4 and PD-L1 expression, whilst
overall survival was also revealed to associate significantly with
TLR4 and PD-L1 expression. However, none were demonstrated to be
independent prognostic factors for NSCLC. Wang et al
(40) reported different findings,
who determined that higher expression of TLR4 in lung cancer
tissues was significantly associated with poorer OS and
disease-free survival, where TLR4 was found to be a independent
prognostic factor for NSCLC through multivariate analysis. There
are several studies that revealed contradictory results. Wei et
al (43) assessed the
relevance of serum levels of soluble TLR4 (sTLR4) in NSCLC, who
found lower sTLR4 levels to be indicative of reduced survival among
patients with early-stage NSCLC that had recently undergone tumor
resection surgery. Another previous study by Bauer et al
(10) also supported the notion
that increasing TLR4 expression may improve outcomes, but no
significance was found. A possible explanation for this
inconsistency may be due to different sample sizes, whilst another
explanation could be that the complex formed by sTLR4 and the
adaptor protein myeloid differentiation factor-2 (MD-2) may
attenuate TLR4-mediated signaling, since TLR4 requires MD-2 to
respond efficiently to LPS (44,45).
Although the present study didn't uncover a
prognostic value of TLR4 and PD-L1. It is believed that
inflammation can lead to carcinogenesis (46,47).
TLR4 is a component of the innate and adaptive immune response to
infection and inflammation, whilst PD-L1 also has a pivotal role in
immune escape by lung cancer. Therefore, the relationship between
TLR4 and PD-L1 was explored further in vitro in the present
study, using LPS as the inflammatory stimulator. TLR4 activation by
LPS was found to induce PD-L1 expression in A549 cells. LPS
stimulation can induce TLR4 pathway activation, in turn activating
the NF-κB, MAPKs, p38, ERK and PI3K⁄AKT signaling pathways
(48,49). Data from the present study
confirmed that the ERK and PI3K/AKT signaling pathways were
activated by LPS treatment. By using the MEK inhibitor to inhibit
ERK activity and PI3K inhibitor to inhibit AKT activity
respectively, it was revealed that LPS-induced PD-L1 upregulation
was dependent on the TLR4/ERK but not the TLR4/PI3K/AKT signaling
pathway. These results are consistent with those previously
reported by Qian et al (38) and Wang et al (39) on bladder cancer tissues and
cells.
A number of limitations remain associated with the
present study. The cancer tissue sample size obtained for IHC is
relatively small, whilst the in vitro part of the present
study is restricted to A549 cell line. Although the underlying
mechanism between TLR4 and PD-L1 was mainly focused on ERK and
PI3K/AKT signaling pathway in the present study, other signaling
pathways downstream of TLR4 may also be involved in the process,
such as the NF-κB and interferon regulatory factor 5 (IRF5)
pathway. The NF-κB pathway activation has been demonstrated to
contribute to PD-L1 upregulation in LPS-treated gastric cancer
cells (50), but whether the same
phenomenon exists in lung cancer cells remain poorly understood and
require further investigations.
Despite its limitations, the present study
contributed to the understanding of the functional relationship
between TLR4 and PD-L1 in NSCLC. TLR4 and PD-L1 expression are
found to be significantly associated with OS, whilst TLR4 can
induce PD-L1 expression through the ERK signaling pathway following
stimulation by LPS. Taken together, during conditions of chronic
inflammation, TLR4 induced PD-L1 expression may contribute to NSCLC
initiation.
Supplementary Data
Funding
The present study was supported by the Jiangxi
Provincial Education Department Key Project (grant no. GJJ17024),
the Natural Science Foundation of Jiangxi Science and Technology
Department (grant no. 20181BAB205058) and Project of Jiangxi
Provincial Health Department (grant no. 20185144).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
GW contributed to the design of the study and
editing of the manuscript. XK, PL, CZ processed the experiments. XK
also responsible for manuscript writing. YZ was responsible for
collecting and organizing the clinical data. HH was responsible for
following up the patients and revising the manuscript. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
The Ethical Committee of The First Affiliated
Hospital of Nanchang University (Nanchang, China) approved the
present study. All patients gave informed consents to
participate.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
The authors would like to acknowledge the assistance
of pathologists, Dr Shanshan Wang and Dr Luxia Tu, Department of
Pathology, The First Affiliated Hospital of Nanchang University
(Nanchang, China). The authors would also like to thank Dr Jianbin
Wang, Translation Medical Department of Nanchang University
(Nanchang, China), for the gift of the A549 cell line.
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen W, Zheng R, Zeng H, Zhang S and He J:
Annual report on status of cancer in China, 2011. Chin J Cancer
Res. 27:2–12. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bocanegra A, Fernandez-Hinojal G,
Zuazo-Ibarra M, Arasanz H, Garcia-Granda MJ, Hernandez C, Ibañez M,
Hernandez-Marin B, Martinez-Aguillo M, Lecumberri MJ, et al: PD-L1
expression in systemic immune cell populations as a potential
predictive biomarker of responses to PD-L1/PD-1 blockade therapy in
lung cancer. Int J Mol Sci. 20:pp. E16312019, View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pisani P, Parkin DM, Muñoz N and Ferlay J:
Cancer and infection: Estimates of the attributable fraction in
1990. Cancer Epidemiol Biomarkers Prev. 6:387–400. 1997.PubMed/NCBI
|
|
5
|
Gomes M, Teixeira AL, Coelho A, Araujo A
and Medeiros R: The role of inflammation in lung cancer. Adv Exp
Med Biol. 816:1–23. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Houghton AM, Mouded M and Shapiro SD:
Common origins of lung cancer and COPD. Nat Med. 14:1023–1024.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lin TY, Huang WY, Lin JC, Lin CL, Sung FC,
Kao CH and Yeh JJ: Increased lung cancer risk among patients with
pneumococcal pneumonia: A nationwide population-based cohort study.
Lung. 192:159–165. 2014. View Article : Google Scholar
|
|
8
|
De Nardo D: Toll-like receptors:
Activation, signalling and transcriptional modulation. Cytokine.
74:181–189. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
He W, Liu Q, Wang L, Chen W, Li N and Cao
X: TLR4 signaling promotes immune escape of human lung cancer cells
by inducing immunosuppressive cytokines and apoptosis resistance.
Mol Immunol. 44:2850–2859. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bauer AK, Upham BL, Rondini EA, Tennis MA,
Velmuragan K and Wiese D: Toll-like receptor expression in human
non-small cell lung carcinoma: Potential prognostic indicators of
disease. Oncotarget. 8:91860–91875. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
He J, Hu Y, Hu M and Li B: Development of
PD-1/PD-L1 pathway in tumor immune microenvironment and treatment
for non-small cell lung cancer. Sci Rep. 5:131102015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Johnson DB, Rioth MJ and Horn L: Immune
checkpoint inhibitors in NSCLC. Curr Treat Options Oncol.
15:658–669. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Loke P and Allison JP: PD-L1 and PD-L2 are
differentially regulated by Th1 and Th2 cells. Proc Natl Acad Sci
USA. 100:5336–5341. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Beswick EJ, Johnson JR, Saada JI, Humen M,
House J, Dann S, Qiu S, Brasier AR, Powell DW, Reyes VE and Pinchuk
IV: TLR4 activation enhances the PD-L1-mediated tolerogenic
capacity of colonic CD90+ stromal cells. J Immunol. 193:2218–2229.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
16
|
Dudley DT, Pang L, Decker SJ, Bridges AJ
and Saltiel AR: A synthetic inhibitor of the mitogen-activated
protein kinase cascade. Proc Natl Acad Sci USA. 92:7686–7689. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Vlahos CJ, Matter WF, Hui KY and Brown RF:
A specific inhibitor of phosphatidylinositol 3-kinase,
2-(4-morpholinyl)- 8-phenyl-4H-1-benzopyran-4-one (LY294002). J
Biol Chem. 269:5241–5248. 1994.PubMed/NCBI
|
|
18
|
Schwartz AG and Cote ML: Epidemiology of
lung cancer. Adv Exp Med Biol. 893:21–41. 2016. View Article : Google Scholar
|
|
19
|
Zhu J, Luo J, Li Y, Jia M, Wang Y, Huang Y
and Ke S: HMGB1 induces human non-small cell lung cancer cell
motility by activating integrin αvβ3/FAK through TLR4/NF-κB
signaling pathway. Biochem Biophys Res Commun. 480:522–527. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Dong J, Li B, Lin D, Zhou Q and Huang D:
Advances in targeted therapy and immunotherapy for non-small cell
lung cancer based on accurate molecular typing. Front Pharmacol.
10:2302019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Boeri M, Milione M, Proto C, Signorelli D,
Lo Russo G, Galeone C, Verri C, Mensah M, Centonze G, Martinetti A,
et al: Circulating miRNAs and PD-L1 tumor expression are associated
with survival in advanced NSCLC patients treated with
immunotherapy: A prospective study. Clin Cancer Res. 25:2166–2173.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Oikonomopoulou K, Brinc D, Kyriacou K and
Diamandis EP: Infection and cancer: Revaluation of the hygiene
hypothesis. Clin Cancer Res. 19:2834–2841. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Jacqueline C, Tasiemski A, Sorci G, Ujvari
B, Maachi F, Missé D, Renaud F, Ewald P, Thomas F and Roche B:
Infections and cancer: The 'fifty shades of immunity' hypothesis.
BMC Cancer. 17:2572017. View Article : Google Scholar
|
|
24
|
Islami F, Chen W, Yu XQ, Lortet-Tieulent
J, Zheng R, Flanders WD, Xia C, Thun MJ, Gapstur SM, Ezzati M and
Jemal A: Cancer deaths and cases attributable to lifestyle factors
and infections in China, 2013. Ann Oncol. 28:2567–2574. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hattori N and Ushijima T: Epigenetic
impact of infection on carcinogenesis: Mechanisms and applications.
Genome Med. 8:102016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ewald PW and Swain Ewald HA: Infection and
cancer in multi-cellular organisms. Philos Trans R Soc Lond B Biol
Sci. 370:pp. 201402242015, View Article : Google Scholar
|
|
27
|
Francescone R, Hou V and Grivennikov SI:
Microbiome, inflammation, and cancer. Cancer J. 20:181–189. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Grivennikov SI, Greten FR and Karin M:
Immunity, inflammation, and cancer. Cell. 140:883–899. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wardak S: Human papillomavirus (HPV) and
cervical cancer. Med Dosw Mikrobiol. 68:73–84. 2016.
|
|
30
|
Denholm R, Schüz J, Straif K, Stücker I,
Jöckel KH, Brenner DR, De Matteis S, Boffetta P, Guida F, Brüske I,
et al: Is previous respiratory disease a risk factor for lung
cancer? Am J Respir Crit Care Med. 190:549–559. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
González I, Araya P and Rojas A:
Helicobacter pylori infection and lung cancer: New insights and
future challenges. Zhongguo Fei Ai Za Zhi. 21:658–662. 2018.
|
|
32
|
Ve T, Vajjhala PR, Hedger A, Croll T,
DiMaio F, Horsefield S, Yu X, Lavrencic P, Hassan Z, Morgan GP, et
al: Structural basis of TIR-domain-assembly formation in MAL- and
MyD88-dependent TLR4 signaling. Nat Struct Mol Biol. 24:743–751.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Huang B, Zhao J, Li H, He KL, Chen Y, Chen
SH, Mayer L, Unkeless JC and Xiong H: Toll-like receptors on tumor
cells facilitate evasion of immune surveillance. Cancer Res.
65:5009–5014. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hui E, Cheung J, Zhu J, Su X, Taylor MJ,
Wallweber HA, Sasmal DK, Huang J, Kim JM, Mellman I and Vale RD: T
cell costimulatory receptor CD28 is a primary target for
PD-1-mediated inhibition. Science. 355:1428–1433. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ,
Topalian SL, Hwu P, Drake CG, Camacho LH, Kauh J, Odunsi K, et al:
Safety and activity of anti-PD-L1 antibody in patients with
advanced cancer. N Engl J Med. 366:2455–2465. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Koyama S, Akbay EA, Li YY, Herter-Sprie
GS, Buczkowski KA, Richards WG, Gandhi L, Redig AJ, Rodig SJ,
Asahina H, et al: Adaptive resistance to therapeutic PD-1 blockade
is associated with upregulation of alternative immune checkpoints.
Nat Commun. 7:105012016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Konen JM, Rodriguez BL, Fradette JJ,
Gibson L, Davis D, Minelli R, Peoples MD, Kovacs J, Carugo A,
Bristow C, et al: Ntrk1 promotes resistance to PD-1 checkpoint
blockade in mesenchymal Kras/p53 mutant lung cancer. Cancers.
11:pp. E4622019, View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Qian Y, Deng J, Geng L, Xie H, Jiang G,
Zhou L, Wang Y, Yin S, Feng X, Liu J, et al: TLR4 signaling induces
B7-H1 expression through MAPK pathways in bladder cancer cells.
Cancer Invest. 26:816–821. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wang YH, Cao YW, Yang XC, Niu HT, Sun LJ,
Wang XS and Liu J: Effect of TLR4 and B7-H1 on immune escape of
urothelial bladder cancer and its clinical significance. Asian Pac
J Cancer Prev. 15:1321–1326. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Wang K, Wang J, Wei F, Zhao N, Yang F and
Ren X: Expression of TLR4 in non-small cell lung cancer is
associated with PD-L1 and poor prognosis in patients receiving
pulmonectomy. Front Immunol. 8:4562017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Fleming V, Hu X, Weller C, Weber R, Groth
C, Riester Z, Hüser L, Sun Q, Nagibin V, Kirschning C, et al:
Melanoma extracellular vesicles generate immunosuppressive myeloid
cells by upregulating PD-L1 via TLR4 SIgnaling. Cancer Res.
79:4715–4728. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Zhao S, Sun M, Meng H, Ji H, Liu Y, Zhang
M, Li H, Li P, Zhang Y and Zhang Q: TLR4 expression correlated with
PD-L1 expression indicates a poor prognosis in patients with
peripheral T-cell lymphomas. Cancer Manag Res. 11:4743–4756. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Wei F, Yang F, Li J, Zheng Y, Yu W, Yang L
and Ren X: Soluble Toll-like receptor 4 is a potential serum
biomarker in non-small cell lung cancer. Oncotarget. 7:40106–40114.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Hyakushima N, Mitsuzawa H, Nishitani C,
Sano H, Kuronuma K, Konishi M, Himi T, Miyake K and Kuroki Y:
Interaction of soluble form of recombinant extracellular TLR4
domain with MD-2 enables lipopolysaccharide binding and attenuates
TLR4-mediated signaling. J Immunol. 173:6949–6954. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Shimazu R, Akashi S, Ogata H, Nagai Y,
Fukudome K, Miyake K and Kimoto M: MD-2, a molecule that confers
lipopolysaccharide responsiveness on Toll-like receptor 4. J Exp
Med. 189:1777–1782. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Kuper H, Adami HO and Trichopoulos D:
Infections as a major preventable cause of human cancer. J Intern
Med. 248:171–183. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Coussens LM and Werb Z: Inflammation and
cancer. Nature. 420:860–867. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Chen CY, Kao CL and Liu CM: The Cancer
prevention, anti-inflammatory and anti-oxidation of bioactive
phytochemicals targeting the TLR4 signaling pathway. Int J Mol Sci.
19:pp. E27292018, View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Chen CW, Chen CC, Jian CY, Lin PH, Chou
JC, Teng HS, Hu S, Lieu FK, Wang PS and Wang SW: Attenuation of
exercise effect on inflammatory responses via novel role of
TLR4/PI3K/Akt signaling in rat splenocytes. J Appl Physiol (1985).
121:870–877. 2016. View Article : Google Scholar
|
|
50
|
Li H, Xia JQ, Zhu FS, Xi ZH, Pan CY, Gu LM
and Tian YZ: LPS promotes the expression of PD-L1 in gastric cancer
cells through NF-κB activation. J Cell Biochem. 119:9997–10004.
2018. View Article : Google Scholar : PubMed/NCBI
|