Introduction
In the development of anticancer approaches,
combined treatments appear to be of great interest (1-5).
The idea of combined treatments is based on the possibility to
obtain the same biological or therapeutic effect with two or more
drugs, using lower concentrations of single drugs. In this case,
the side-effects of the single drug are expected to be limited
(1). In addition, the combined
therapy of cancer may have an impact on acquired resistance
(1,2,5).
Furthermore, patient-to-patient variability and independent drug
action are sufficient to explain the superior efficacy of drug
combinations in the absence of drug synergy or additivity (6). The hypothesis is that in a
combination of drugs exhibiting specific mechanisms of action, each
patient benefits solely from the drug to which the tumor is most
sensitive, with no added benefit from other drugs. In any case, the
impact and the overall interest in anticancer combined therapy are
very high (2).
Combined therapy may be of utmost significance in
the management of glioblastoma (GBM), a lethal malignant tumor
accounting for 42% of the tumors of the central nervous system,
with the median survival being 15 months (7-9).
It should be emphasized that there is currently no effective
pharmacological treatment available, and the first-line drug used,
temozolomide (TMZ), is on average able to prolong the life
expectancy of treated patients by only a few months (10). Additionally, a number of forms of
GBM are or become resistant to TMZ over time (10-12).
Therefore, there is an urgent need to identify and
develop new drugs and novel therapeutic approaches (such as
combined therapy) in order to develop more effective anti-glioma
therapies than those currently available, particularly for
TMZ-resistant cells.
As regards the combined therapy of glioma cells, the
authors have recently reported that antitumor drugs can be combined
with molecules targeting microRNAs (miRNAs or miRs) (5). miRNAs are short non-coding RNA
sequences which, owing to their mechanisms of action, function as
gene regulators by repressing the translation or causing the
cleavage of the RNA transcripts they target (13-15). There is currently ample evidence
to indicate that the altered expression of miRNAs may be involved
in the pathogenesis of cancer (16,17). In particular those miRNAs that are
upregulated in cancer and that cause the downregulation of target
tumor suppressor RNAs are defined as 'oncomiRNAs' and
'metastamiRNAs' (18).
Conversely, tumor suppressor miRNAs may be downregulated in cancer,
leading to the overexpression of target oncogenes (18). In summary, the rationale for this
approach is the following: i) Some miRNAs are deeply involved in
the regulation of cell apoptotic mechanisms and more generally in
carcinogenesis, functioning as 'oncomiRNAs' and 'metastamRNAs'
(13-18); and ii) synergistic effects between
anticancer molecules and antagomiRNA molecules targeting specific
oncomiRNAs have been highlighted by different research groups
(4,19-21). There is strong evidence sustaining
the concept that oncomiRNAs are potent anti-apoptotic agents
(22-25). As regards possible candidates for
anti-miRNA treatment, peptide nucleic acids (PNAs) (26,27) have been proposed as very useful
bioactive molecules (28-34). PNAs are DNA analogues in which the
sugar-phosphate backbone has been replaced by
N-(2-aminoethyl)-glycine units (26,27). These intriguing molecules were
first described by Nielsen et al (26) and, despite the general structure
of the nucleic acid molecule becomes more peptide-like, they can
hybridize with complementary DNA or RNA sequences with exceptional
efficiency and specificity (27).
The present study employed a PNA targeting
miR-221-3p, functionalized with an octaarginine peptide (R8) for
maximizing cellular uptake, as previously reported (32). The reason for selecting miR-221-3p
as PNA-based targeting was based on the following observations: i)
miR-221-3p is overexpressed in glioma patients (35-38); and ii) miR-221-3p targeting
decreases cell migration and metastasis when used in in vivo
glioblastoma model systems (39).
The oncogenic role of miR-221-3p has also been confirmed in other
tumor types, including colon, liver, pancreatic, non-small cell
lung cancer (40-43).
In a recent study, the authors tested and reported
two novel series of active anti-tubulin agents based on the
4,5,6,7-tetrahydrothieno[2,3-c]pyridine and
4,5,6,7-tetrahydrobenzo[b]thiophene molecular skeleton (44). These compounds were found to
interfere with the microtubule-tubulin equilibrium in cancer cells
and were demonstrated to retain anti-proliferative activity on a
panel of cancer cell lines. Compounds targeting tubulin are of
great interest for the treatment of cancer cells (45-48).
The aim of the present study was to verify the
activity on the glioma U251 and T98G tumor cell lines (49,50) of the
2-(3′,4′,5′-trimethoxyanilino)-3-cyano/alkoxycarbonyl-6-substit
uted-4,5,6,7-tetrahydrothieno[2,3-c]pyridine 3b used in combination
with an anti-miR-221-3p PNA, already demonstrated to be able to
induce high levels of apoptosis (32).
Materials and methods
Chemicals and reagents
The anti-tubulin compound 3b and R8-PNA-a221 were
synthesized by the research groups of the author RR (University of
Ferrara) and Professor Roberto Corradini (University of Parma),
respectively; the procedure for the synthesis of both molecules has
been previously reported (32,44). For all cell cultures, RPMI-1640
medium supplemented with 10% FBS and 100 mg/ml streptomycin and 100
IU/ml penicillin was employed. RPMI-1640 medium (cat. no.
BE12-702F) and PBS (cat. no. BE17-516F) were purchased from Lonza
Biosciences, trypsin-EDTA solution (cat. no. 59428C) and 50,000
IU/ml streptomycin and 50 mg/ml penicillin (cat. no. 11074440001)
were from Sigma-Aldrich; Merck KGaA and FBS (cat. no. S1400) was
obtained from Biowest. For flow cytometric assays, the
Muse® Annexin V & Dead Cell kit (cat. no.
MCH100105), Muse® Caspase-3/7 kit (cat. no. MCH100108)
and the Muse® Cell Cycle kit (cat. no. MCH100106) were
purchased from Luminex Corporation.
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
powder (cat. no. M5655) for MTT assay, crystal violet staining
solution (cat. no. V5265) for clonogenic assay, DMSO (cat. no.
D2650) used to resuspend compound 3b and the TRI Reagent (cat. no
T9424) used for RNA extraction were purchased from Sigma-Aldrich;
Merck KGaA, while methanol (cat. no. 309001) was supplied by CARLO
ERBA Reagents.
Cell lines, cell growth conditions and
anti-proliferative assays
The human glioma U251 (51) and T98G (49) cells were employed in the present
study; both cell lines were purchased from Sigma-Aldrich; Merck
KGaA. For anti-proliferative assay using the U251 cell line,
8×104 cells were seeded in a 12-well plate, and after 4
h the cells were treated with a serial dilution of the test
compounds (compound 3b was used at the 1, 2, 4, 6 and 8 µM
concentrations and R8-PNA-a221 at the 1, 2, 4 and 8 µM
concentrations). The cells were incubated for 72 h at 37°C in a
humidified 5% CO2 atmosphere. Following 72 h of
incubation, cells were detached from the plate by trypsinization
and counted using a BECKMAN COULTER® Z2 cell counter
(Beckman Coulter, Inc.). The IC50 value (50% inhibitory
concentration) is defined as the concentration of compound that
inhibits cell proliferation by 50% (44). The IC50 values presented (±
standard deviation) are the average values derived from three
independent experiments.
Morphological analysis
Following each treatment, the cells were observed
and representative images were acquired using a Nikon Eclipse 80i
microscope (Nikon Corporation), in order to observe whether any
morphological changes occurred in the cells following
treatment.
Cytotoxicity assay
For determining the cytotoxic effects of the test
compounds, MTT assay was performed using the U251 cell line
(50). Cells were seeded in a
96-multi-well plate at a density of 8×103 cells/well and
after 4 h, treated with the lower concentrations of compound 3b (4
µM) and R8-PNA-a221 (2 µM) individually and in
combination. Cells were incubated at 37°C for a further 72 h and at
the end of the incubation period, MTT was added to each well at a
final concentration of 0.5 mg/ml. Following 3 h of incubation at
37°C, the medium was discarded, and dimethyl sulfoxide (DMSO) was
added; the plate was stirred for 30 min to fully dissolve the
formazan crystals formed at the bottom of the wells. The absorbance
was measured at 570 nm using the SUNRISE microplate reader (Tecan
Group, Ltd.).
Clonogenic assay
Cells were seeded into 6-well plates (400 cells per
well) in RPMI-1640 medium with 10% FBS and incubated at 37°C for 24
h prior to treatment. The plates were incubated at 37°C for a
further 10 days undisturbed in the incubator. Each well was then
washed twice with PBS and covered with a methanol containing
fixation/staining solution that allows the simultaneous coloration
and fixation of the cells (crystal violet aqueous solution
0.5%/methanol, 1:1 ratio) for 15 min at room temperature and washed
four times with tap water; the plate was air-dried for 1 day prior
to obtaining images. Images were acquired using a Nikon SMZ1000
stereo zoom microscope (Nikon Corporation). Stained colonies
consisting of >50 cells were manually counted, and the number
was recorded (52).
RNA extraction
Cells were detached by trypsinization (cat. no.
59428C; Sigma-Aldrich; Merck KGaA), collected by centrifugation at
1,200 rpm (8 min at room temperature), and lysed with Tri-Reagent
(Sigma-Aldrich; Merck KGaA) according to manufacturer's
instructions. The isolated RNA was washed once with cold 75%
ethanol and stored at −80°C until use. The obtained RNA was dried
and dissolved in nuclease-free water prior to use (32).
Quantitative analyses of miRNAs
The miRNA levels were assayed using the TaqMan
MicroRNA Reverse Transcription kit (cat. no. 43-665-96, Applied
Biosystems; Thermo Fisher Scientific, Inc.) with RT-qPCR and
miRNA-specific primers and probes (listed in Table I) obtained from Thermo Fisher
Scientific, Inc.. All samples were run in duplicate using TaqMan
Universal PCR Master Mix, no AmpErase UNG 2X (cat. no. 4324018;
Thermo Fisher Scientific, Inc.) and the CFX96 Touch Real-Time PCR
Detection System (Bio-Rad Laboratories, Inc.).
 | Table IList of assays employed for miRNA
detection. |
Table I
List of assays employed for miRNA
detection.
| miRNA name | Assay ID (Applied
Biosystems; Thermo Fisher Scientific, Inc.) |
|---|
| hsa-miR-221-3p | 000524 |
| hsa-snRNA U6 | 001973 |
| hsa-let-7c-5p | 000379 |
For PCR reactions, the following protocol was
employed: 95°C for 10 min, 95°C for 15 sec, followed by a step at
60°C for 1 min (last two steps repeated for 50 cycles). Data were
collected and analyzed using Bio-Rad CFX Manager Software (Bio-Rad
Laboratories, Inc.). Relative gene expression was calculated using
the 2−ΔΔCq method and data normalization was performed
using snRNA U6 and hsa-let-7c as reference (53).
Effects on the cell cycle
The cells were treated with the lower and higher
concentrations of compound 3b (4 and 6 µM) and R8-PNA-a221
(2 and 4 µM) individually and in combination at the lower
concentration of both. Following 72 h of incubation at 37°C, the
cells were detached by trypsinization, washed once in PBS and fixed
with 70% EtOH for 24 h. For analysis 5×105 cells were
washed in PBS and resuspended in 200 µl of Muse®
Cell Cycle Reagent and incubated for 30 min at room temperature
protected from light. Finally, the cell suspension was transferred
into a new 1.5 ml tube without cap and the samples were analyzed by
flow cytometry using Guava® Muse® Cell
Analyzer (Luminex Corp.) (44).
Cell apoptosis assay
Apoptosis assays were performed with the
Guava® Muse® Cell Analyzer instrument, and
its relative kits according to the instructions supplied by the
manufacturer. Muse® Annexin V & Dead Cell Kit
utilizes Annexin V to detect Phosphatidyl Serine (PS), a common
apoptotic marker that is exposed out of the external membrane of
apoptotic cells, and 7-ADD (7-aminoactinomycin D) a DNA
intercalating molecule used as an indicator of cell membrane
integrity (it can bind DNA only in cells undergoing late
apoptosis/death stage, when the membrane integrity is lost).
Following 72 h of treatment with compound 3b (4 and 6 µM)
and R8-PNA-a221 (2 and 4 µM) administered individually and
the combination of the lower concentration of both, the cells were
washed with sterile PBS, trypsinized and resuspended in RPMI medium
supplemented with 10% FBS. Finally, 50 µl of cell suspension
were incubated with 100 µl Muse® Annexin V &
Dead Cell reagent at room temperature and protected from light for
20 min. Samples were then analyzed using the Guava®
Muse® Cell Analyzer (Luminex Corp.) and data acquired
utilizing the Annexin V and Dead Cell Software Module (Luminex
Corp.) (54).
Caspase-3/7 activity assay
For the analysis of caspase-3/7 activity following
treatments, the Muse® Caspase-3/7 kit was employed,
which is based on a DNA binding dye linked to a DEVD peptide
substrate. When pro-apoptotic caspase-3/7 are activated, they
cleave the dye and the binding of the dye to DNA results in high
fluorescence signal. This kit also contains a fluorescent DNA
intercalator (7-ADD) as indicator of cell membrane integrity.
Following the same treatments indicated in the Cell apoptosis
assay, trypsinization was performed and 50 µl of cell
suspension were incubated with 5 µl of caspase-3/7 reagent
for 30 min (under strict protection from light). Following 25 min
of incubation at 37°C, 150 µl of 7-AAD working solution were
added to each tube and incubated for 5 min at room temperature
before reading the samples. The samples were then analyzed using
Guava® Muse® Cell Analyzer (Luminex Corp.)
and data acquired utilizing the Caspase-3/7 Software Module
(Luminex Corp.) (54).
Statistical analysis
Results are expressed as the mean ± standard error
of the mean (SEM). Comparisons between groups were made by using
ordinary one-way ANOVA followed by a post hoc multiple comparison
tests. Dunnett's test was used to compare groups against a single
control and Tukey's test was used to make comparisons against more
than one group. P<0.05 was considered to indicate a
statistically significant difference.
Results
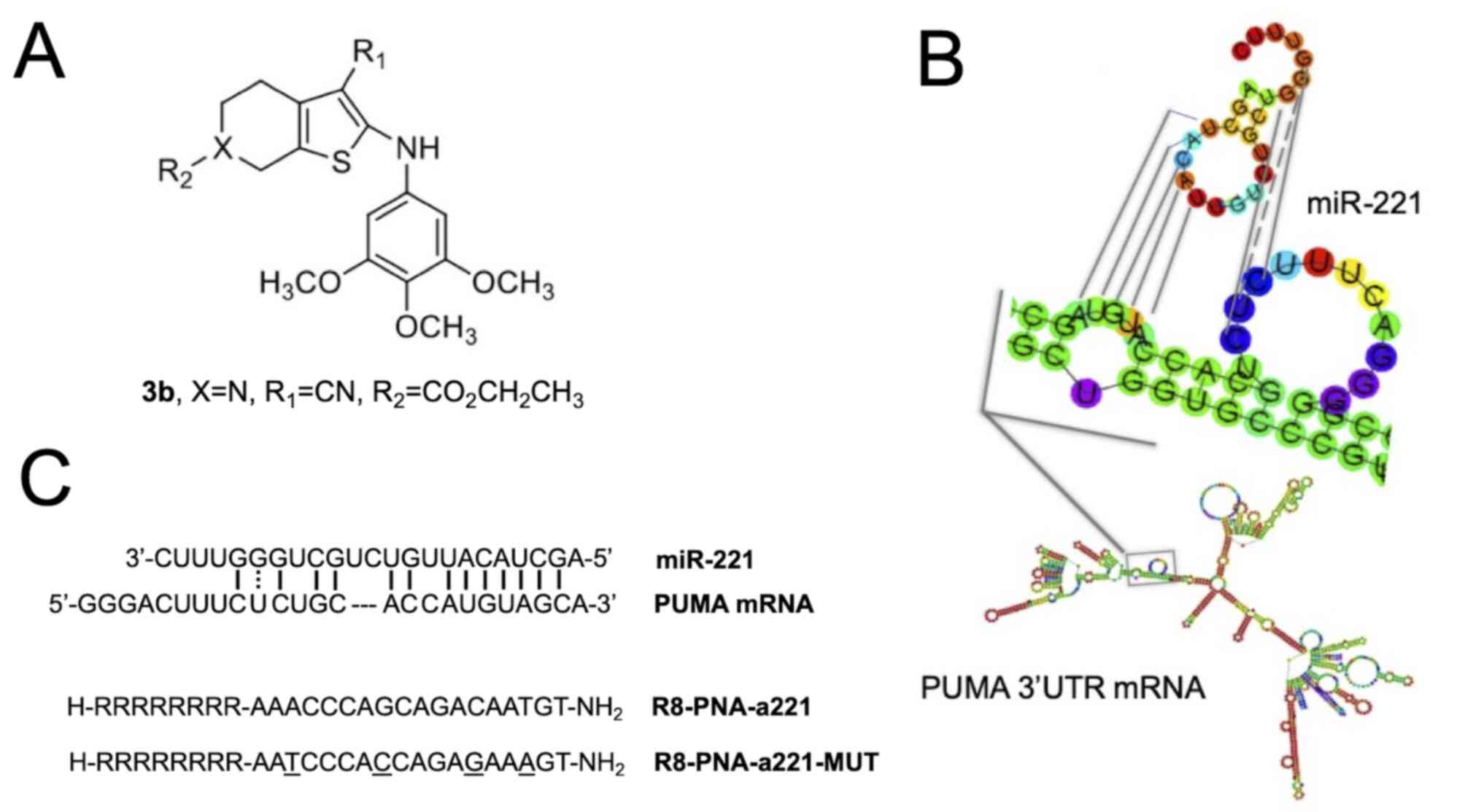
Structure of molecules employed in the
present study
The chemical structures of the compounds used in the
present study are presented in Fig.
1 [tetrahydrothieno[2,3-c]pyridine 3b (Fig. 1A) and PNA-a221 targeting miR-221
(Fig. 1C); these have been shown
to exert a potent anti-apoptotic effect (34). Among the possible
apoptotic-associated mRNAs, ATG10, CDKN1B/p27, BMF, APAF-1, PTEN,
p27(kip1), p57(kip2) and PUMA have been validated as miR-221
targets (55-62). The example of PUMA mRNA is
presented in Fig. 1B, exhibiting
a functional miR-221 binding site in its 3′UTR sequence (61,62).
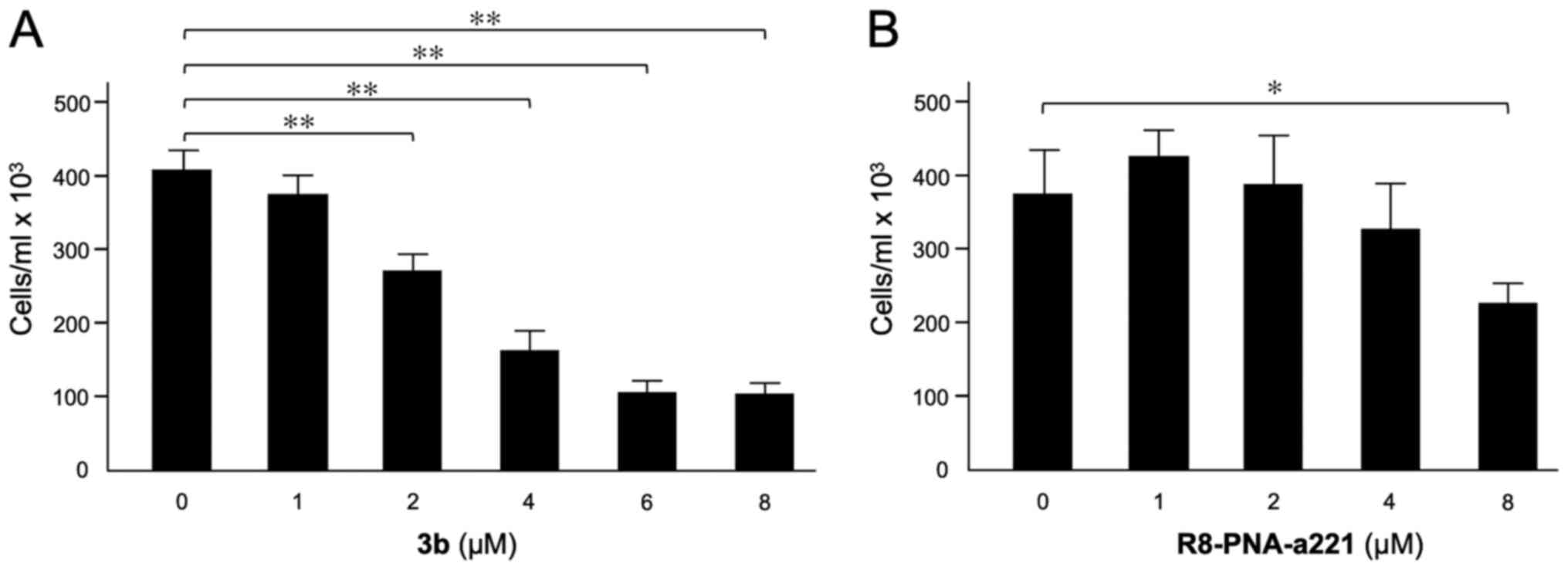
Effects of compound 3b and R8-PNA-a221 on
U251 cell growth
The effects of compound 3b (Fig. 2A) and R8-PNA-a221 (Fig. 2B) on the proliferation of U251
cells were examined. Following 72 h of cell culture in the
indicated experimental conditions, the cell number/ml was
determined. The data indicated that the inhibitory effect of
compound 3b on U251 cell growth reached maximum values when 4-8
µM concentrations of compound 3b were employed. The
anti-proliferative effects of R8-PNA-a221 were lower and detectable
in the same range of concentrations, reaching 50% inhibition of
cell growth only at the 8 µM concentration. In order to
obtain preliminary information on the combinatory effect of the two
drugs, two different concentrations for 3b (4 and 6 µM) and
R8-PNA-a221 (2 and 4 µM) were selected.
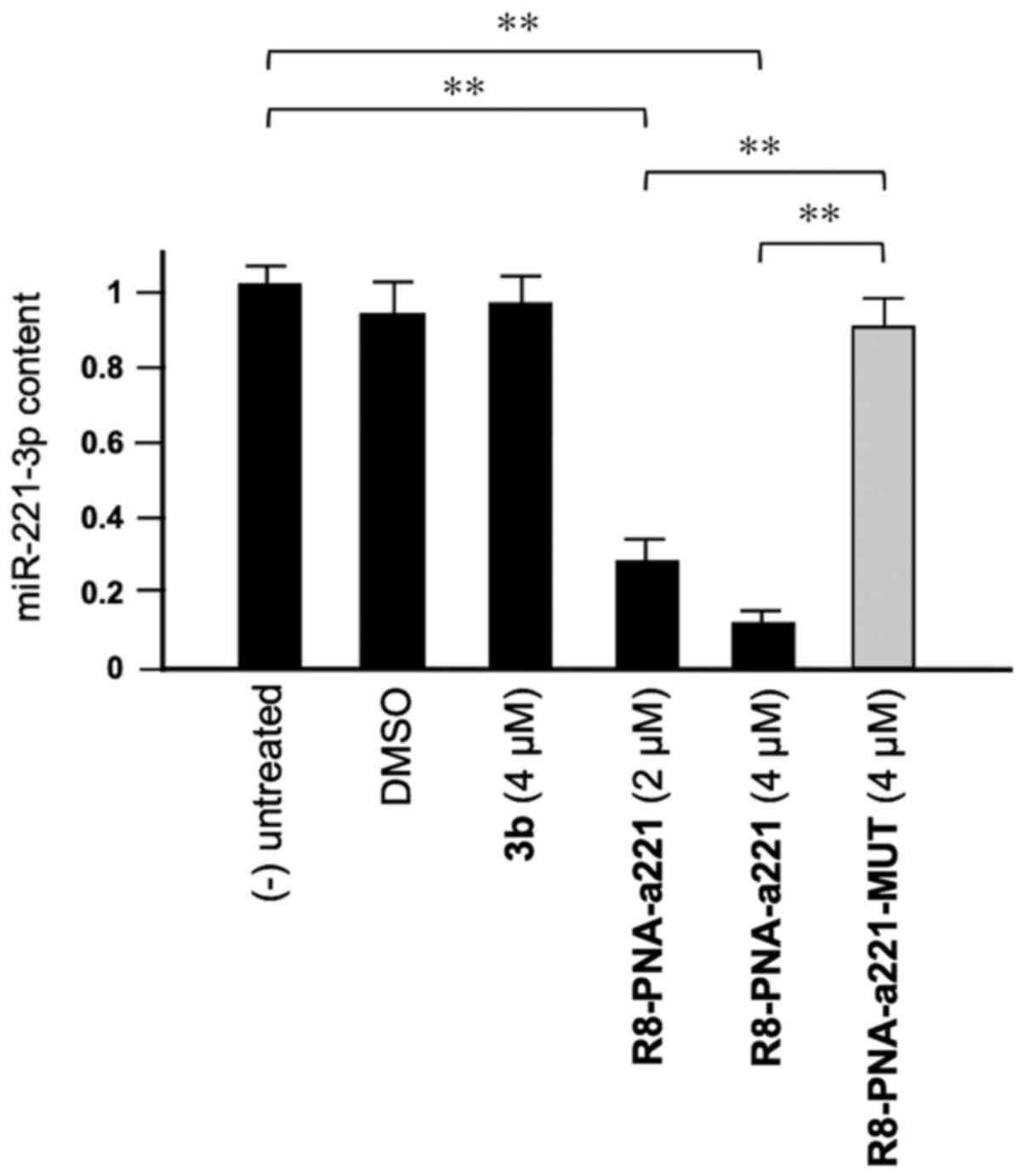
Compound 3b does not affect miR-221-3p
expression
As shown in Fig.
3, tetrahydrothieno[2,3-c]pyridines 3b did not affect the
miR-221-3p content. This experiment was conducted by exposing, for
72 h, human glioma U251 cells to the indicated concentrations of
compound 3b and R8-PNA-a221 and R8-PNA-a221-MUT; the mutated PNA
contains 4 mismatches in the sequence (Fig. 1) which prevents it from
hybridizing with the target mir-221-3p sequence, demonstrating the
enhanced sensitivity of R8-PNA-a221 (32). Following this period of cell
culture, RNA was isolated and miR-221-3p sequences were quantified
by RT-qPCR. As was expected, the R8-PNA-a221 (but not the mutated
R8-PNA-a221-MUT) inhibited the production of miR-221-3p. On the
contrary, no inhibitory effects were observed with compound 3b
treatment at 4 µM.
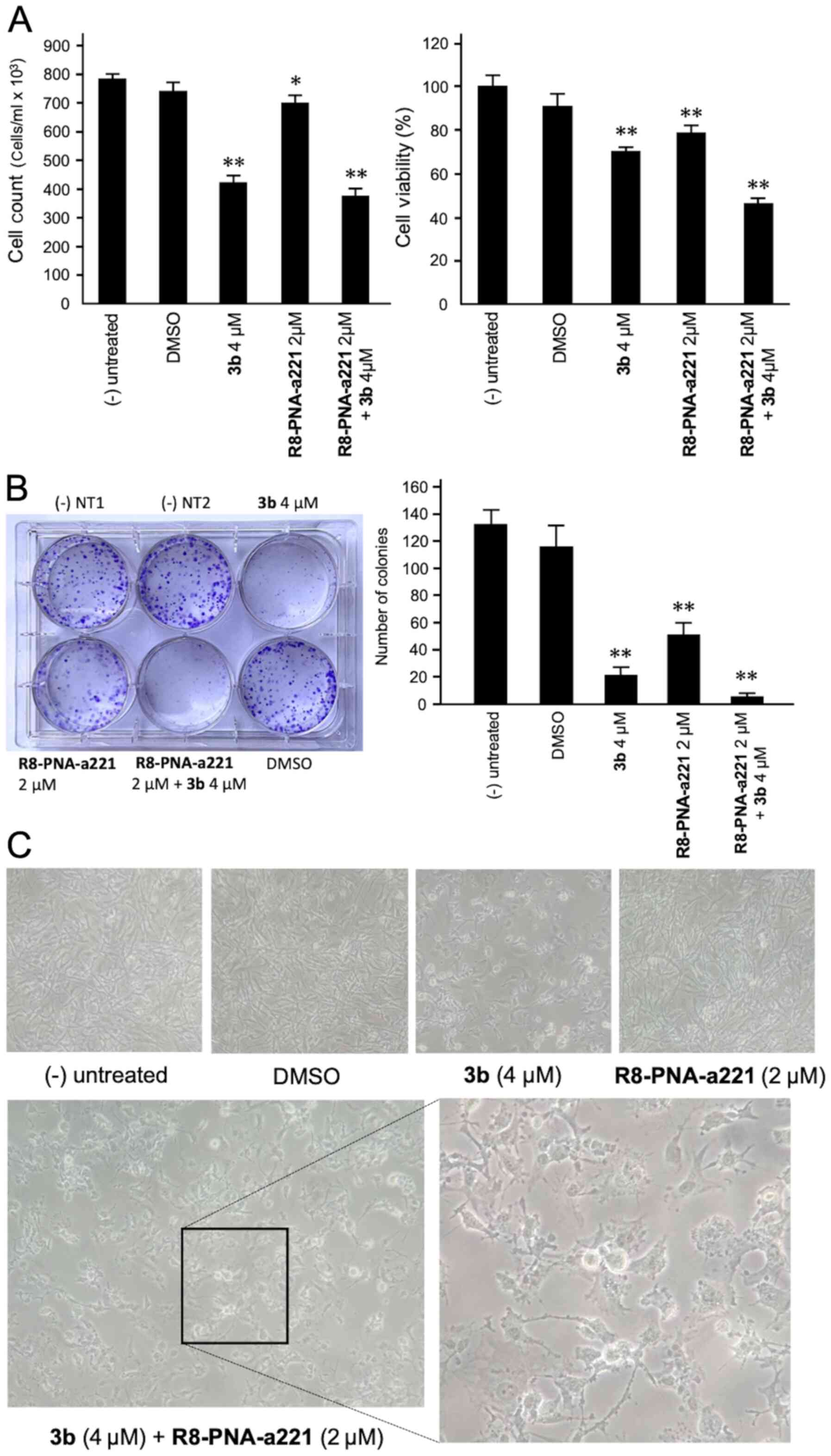
Co-treatment of U251 cells with 3b and
R8-PNA-a221: Effects on cell growth, viability, and colony
formation
The anti-proliferative and cytotoxic effects of
single and combined treatments are shown in Fig. 4. The anti-proliferative effects
were assayed by employing a cell counter (Fig. 4A, left panel), while cytotoxicity
was evaluated by MTT assay (Fig.
4A, right panel). A representative image of the clonogenic
assay is presented in Fig. 4B.
The results obtained clearly indicated that co-treatment with
compound 3b and R8-PNA-a221 was associated with the lowest number
of colonies, and the highest anti-proliferative and cytotoxic
effects.
A morphological analysis was also performed
(Fig. 4C) demonstrating that, in
agreement with the enhanced effects on cell growth and vitality,
the alterations of the morphology of the U251 glioma cells occurred
following co-treatment with compound 3b and R8-PNA-a221. In
particular the combined use of the two compounds led to major
morphology alterations, including the loss of the original
'astrocytic' shape, and typical hallmarks of apoptosis, such as
membrane blebbing.
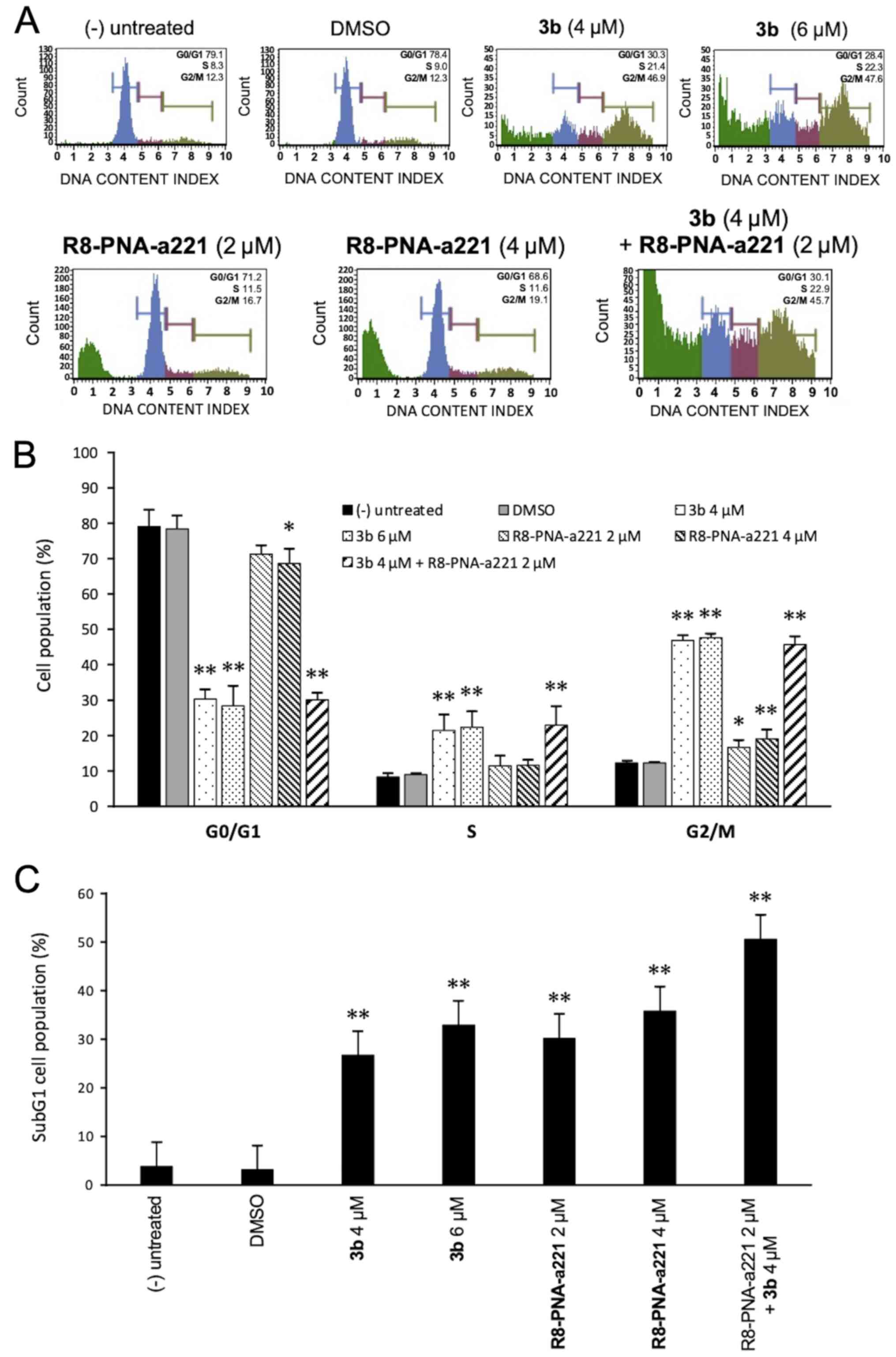
Cell cycle effects
Since the mechanisms of action of anti-tubulin
compounds, such as compound 3b involved causing cell cycle
alterations, the effects of compound 3b and PNA-a221 on the cell
cycle were investigated by flow cytometric analysis in the present
study. The cells were treated and incubated for 72 h as indicated
in the experiment illustrated in Fig.
5A. Compound 3b induced a marked increase in the number of
cells in the G2/M phase of the cell cycle, associated with a
decrease in the number of cells in the G0/G1 phase. As shown in
Fig. 5A, treatment of the U251
cells with compound 3b at 4 and 6 µM increased the
percentage of cells in the G2/M phase from 12.3% (control group) to
46.9 and 47.6%, respectively. This finding is in agreement with
data obtained using human K562 leukemia cells previously reported
(44) and confirms that compound
3b affects cell growth through cell cycle blockade. On the
contrary, the R8-PNA-a221 exerted only minor effects on cell
growth, inducing a very low increase in the percentage of cells in
the G2/M phase from 12.3% (control group) to 16.7-19.1%. The
minimal effect of 8-PNA-a221 on the cell cycle was confirmed by the
fact that combined treatment with 4 µM compound 3b and 2
µM R8-PNA-a221 induced a cell cycle distribution very
similar to that induced by treatment with compound 3b alone. The
quantitative data of the effects of the treatments on the cell
cycle are presented in Fig. 5B.
When the results of FACS analyses shown in Fig. 5A were considered, it was evident
that an increase in the sub-G1 cell population occurred when the
cells were treated with compound 3b, R8-PNA-a221 or the combination
of compound 3b and R8-PNA-a221. As shown in Fig. 5C, a marked increase in the number
of cells in the sub-G1 phase was observed when the U251 cells were
treated with compound 3b. As in the case of alteration of cell
growth and cytotoxicity, the highest proportion of sub-G1 cells was
observed when the cells were treated with compound 3b plus
R8-PNA-a221. Since the appearance of a sub-G1 population is a
hallmark of the activation of apoptosis, an analysis was performed
using two apoptosis-associated assays, Annexin V and caspase-3/7
assays.
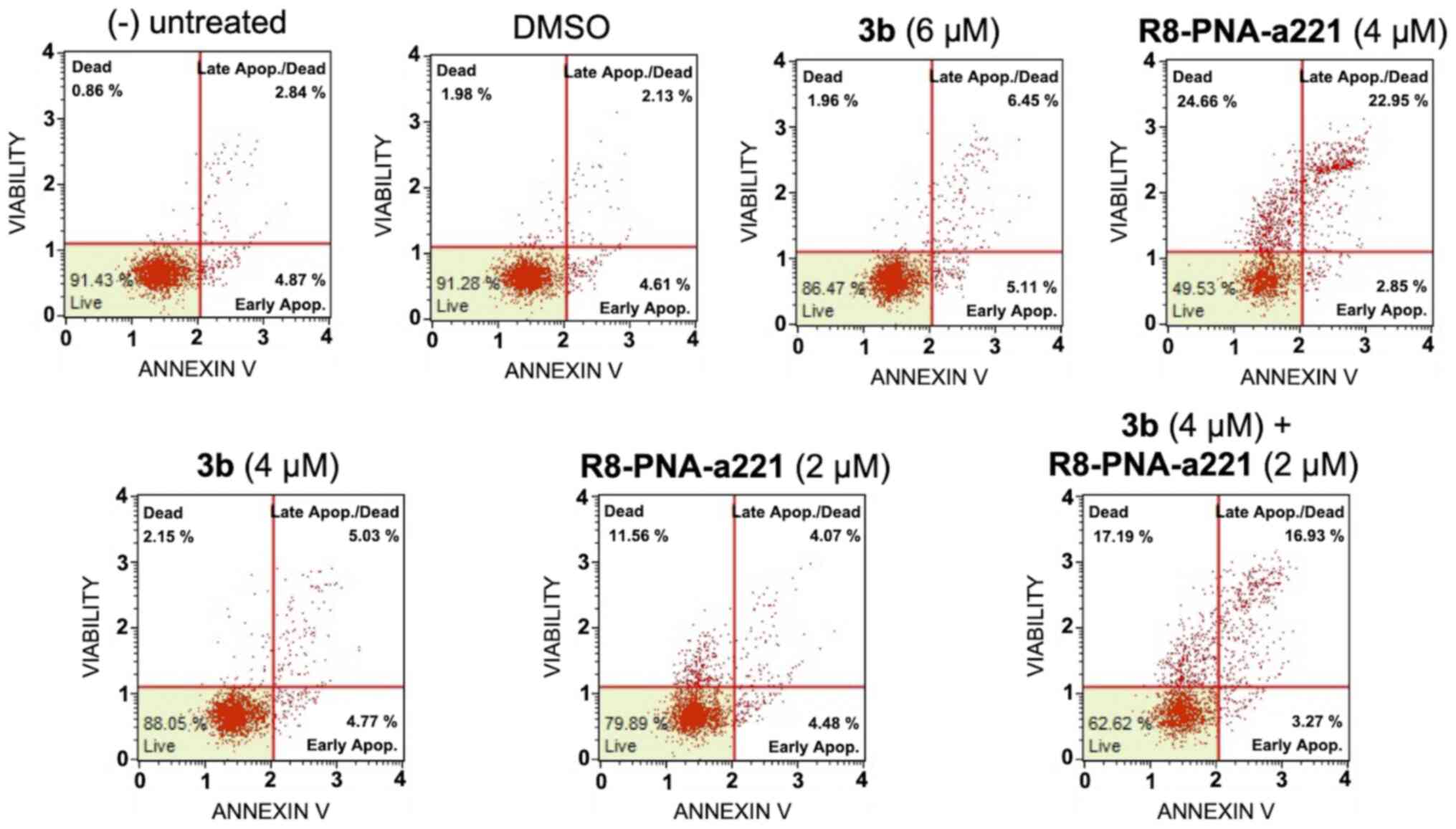
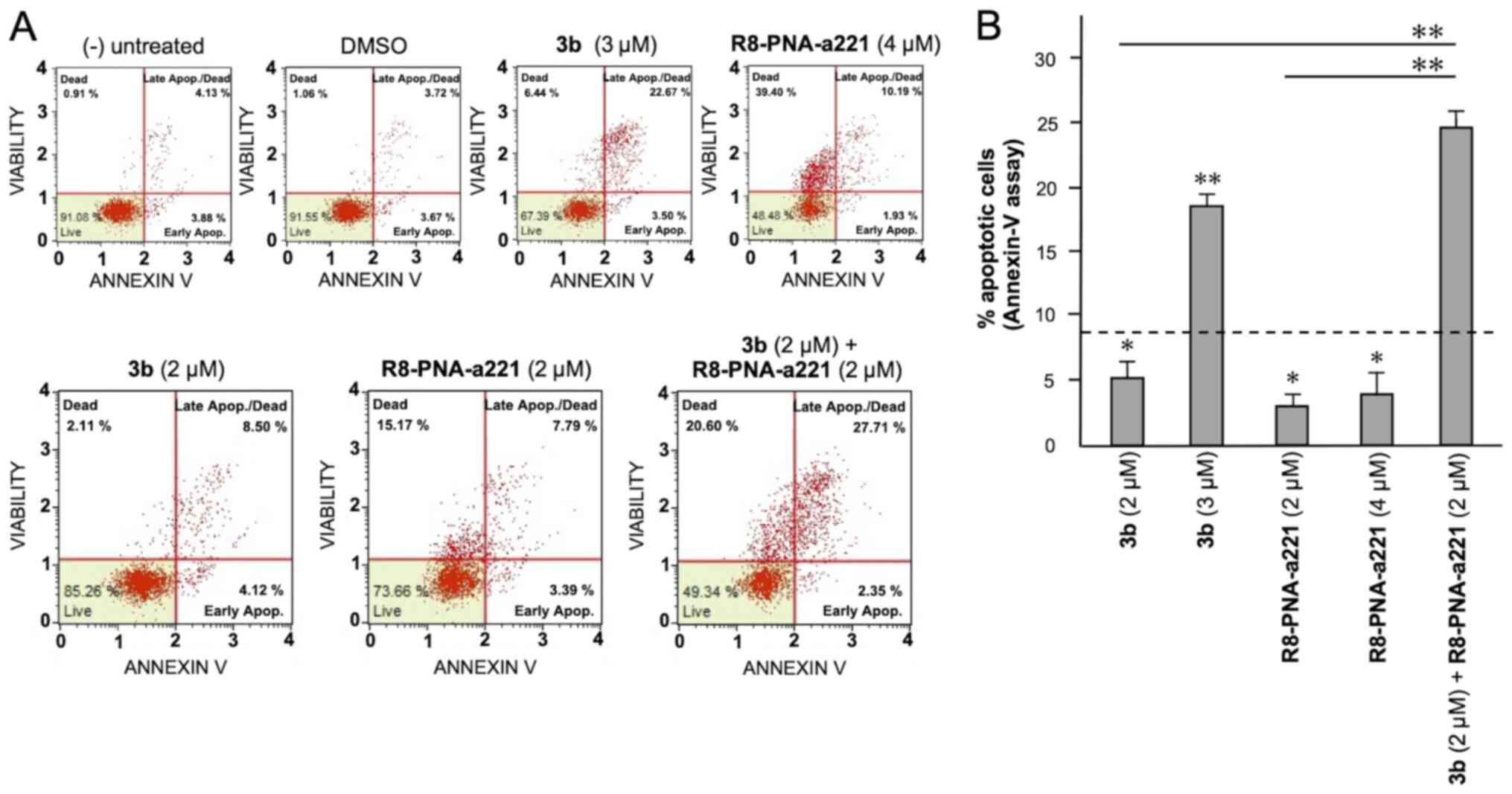
Apoptosis study of U251 glioma cells
In order to examine the induction potential of
compound 3b and R8-PNA-a221 to induce cell apoptosis and death when
used individually and to verify a possible synergistic effect when
used in combination, two different apoptosis detection kits were
used. Representative plots demonstrating the effects of various
concentrations of compound 3b and R8-PNA-a221 administered alone or
in combination are shown in Fig.
6. Considering the increase in total apoptotic cells (early and
late apoptotic cells of the treated minus the early and late
apoptotic cells of the untreated sample or DMSO-treated cells),
both agents induced an increase in the number of Annexin V-positive
cells in comparison with the controls after 3 days, and this
increase occurred in a concentration-dependent manner, reaching a
maximum of a 4.82% increase with respect to the control
DMSO-treated cells with 6 µM compound 3b and 18.09% with 4
µM R8-PNA-a221 (compared to the untreated cells). Combined
treatments with sub-optimal concentrations of compound 3b and
R8-PNA-a221 (4 and 2 µM, respectively) led to a sharp
induction of apoptosis (13.46%), a proportion which was much higher
than the sum of the effects of the singularly added agents
(3.06+0.84=4.70%). It should be noted that the increase in the
proportion of apoptotic cells was particularly evident in the 'late
apoptotic cell' fraction. The increase of the proportion of the
'dead cell' fraction was fully in agreement with the cytotoxicity
data depicted in Fig. 4.
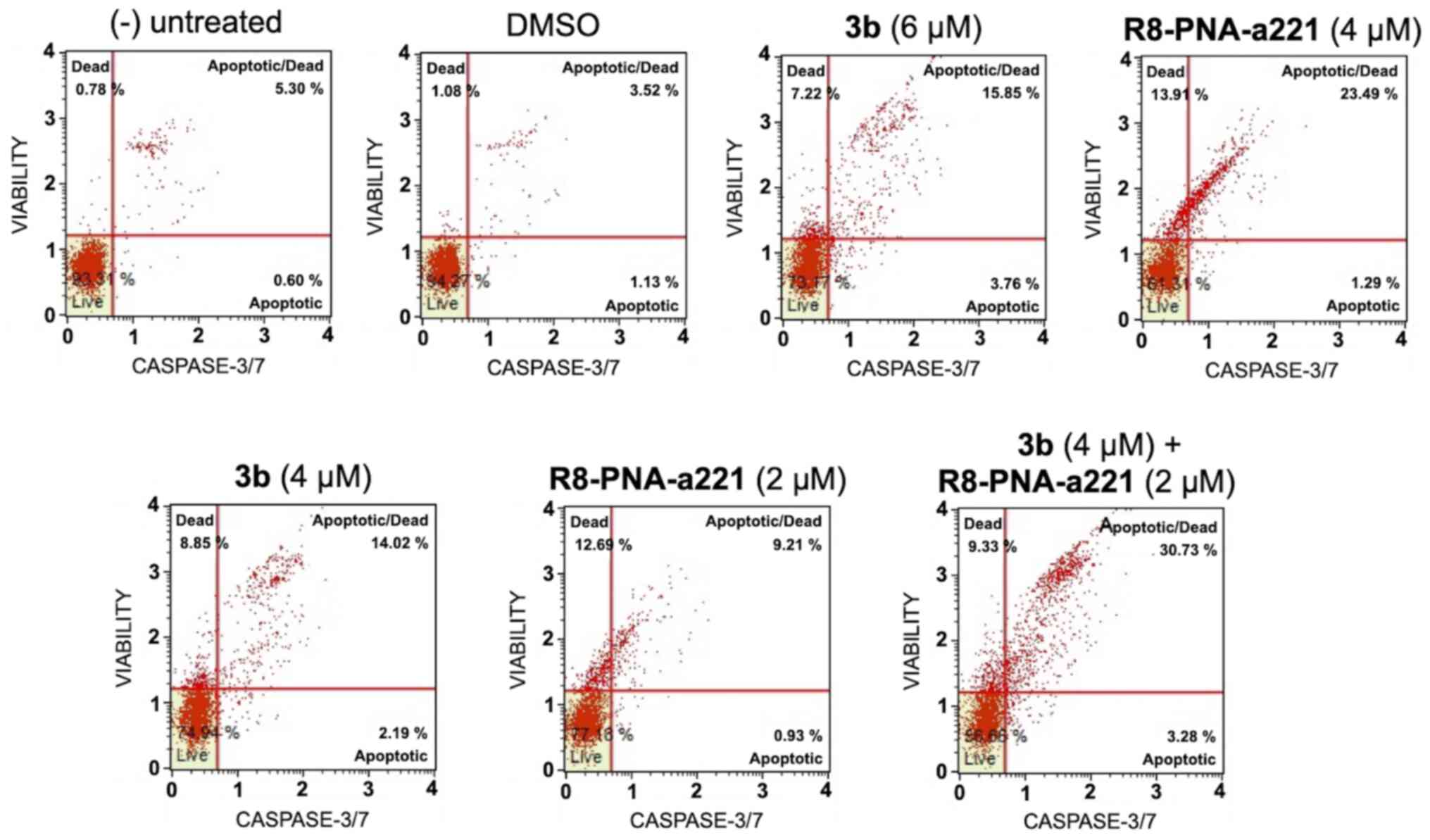
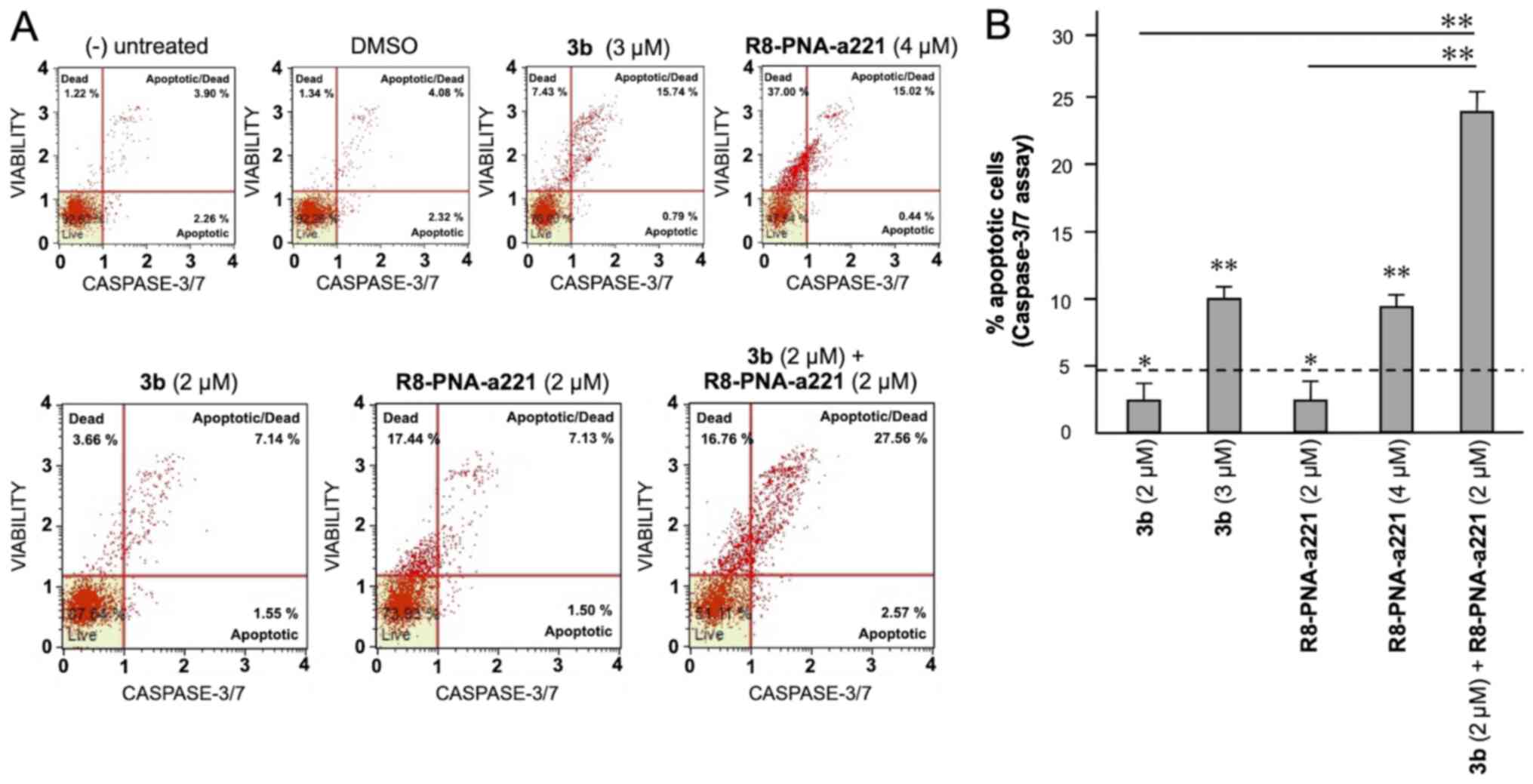
The same conclusion derived from the experiments
performed using the Annexin V assay can be gathered on the basis of
the caspase-3/7 assay shown in Fig.
7. In this case, always considering the increase in total
apoptotic cells (early and late apoptotic cells of the treated
minus the early and late apoptotic cells of the untreated sample or
DMSO-treated cells) obtained following combined treatments with
sub-optimal concentrations of compound 3b and R8-PNA-a221 (4 and 2
µM, respectively) was 29.36%, a proportion which was much
higher than the sum of the effects of singularly added agents
(11.56+4.24%=15.80).
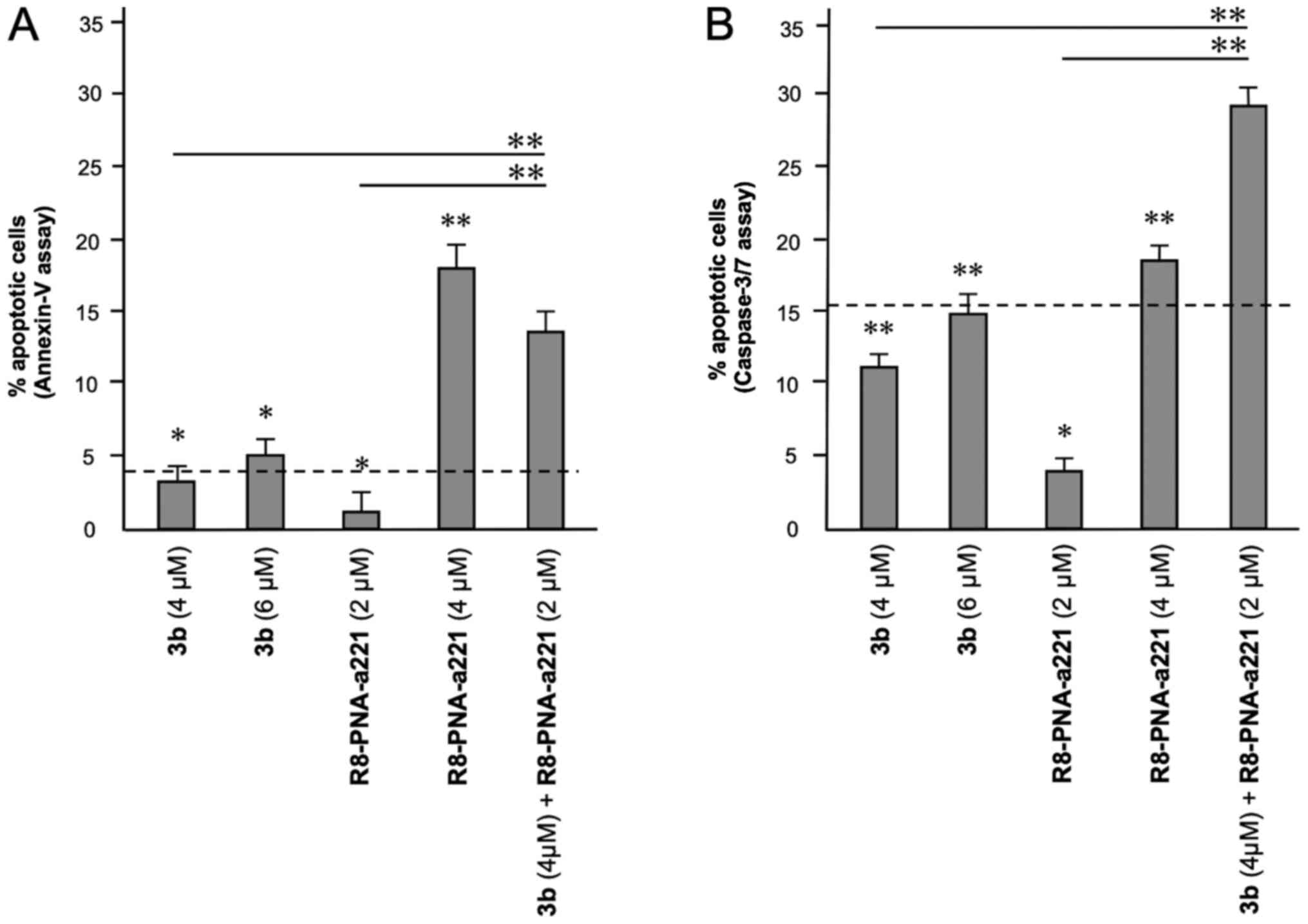
A summary of three independent experiments analyzing
the effects of the tetrahydrothieno[2,3-c]pyridine compound 3b and
PNA-a221 on cell apoptosis is presented in Fig. 8. In the histogram, only apoptotic
cells (early and late) were reported, without including
necrotic/dead cells in the calculations; moreover, the percentage
of apoptotic cells already present in the untreated samples or in
the samples treated with DMSO only was subtracted, in order to
better demonstrate the exact increase in apoptotic cells following
the treatments. It is evident that the combined treatment led to an
increase in apoptosis, which was found to be higher than the sum of
the effects obtained using single administration of compound 3b and
R8-PNA-a221 (outlined by the dashed lines in the graphs in Fig. 8).
Apoptosis of TMZ-resistant T98G glioma
cells
To verify whether the synergistic effect between
compound 3b (IC50, 2 µM) and R8-PNA-a221 can also occur in
TMZ-resistant T98G cells, the protocol employed on U251 cells and
presented in Figs. 6-8 was used. The results shown in Figs. 9 and 10 demonstrated that a synergistic
effect between compound 3b and R8-PNA-a221 was also observed on
T98G cells. In fact, in both Annexin V (Fig. 9) and caspase-3/7 (Fig. 10) assays, combined treatment led
to an increase in apoptosis which was found to be higher than the
sum of the effects obtained using single treatment with compound 3b
or R8-PNA-a221 (outlined by the dashed lines in Figs. 9B and 10B).
Discussion
Patients with GBM express high levels of miR-221-3p,
which exerts anti-apoptotic effects and promotes malignant
progression (35-39). The involvement of miR-221-3p in
GBM is supported by the study by Xu et al (39), which demonstrated that the
inhibition of both miR-221 and miR-222 diminished the
proliferation, invasion, migration and angiogenesis of GBM cells
in vitro and in vivo. The inhibition of the
miR-221/222 cluster reduced the activation of the p-JAK2/JAK2 and
p-STAT3/STAT3 pathway (39), the
levels of different matrix metalloproteinases (MMPs; MMP-2 and
MMP-9) and the levels of vascular endothelial growth factor
(VEGF).
Considering these findings, it appears evident that
the inhibition of the miR-221/222 cluster is an interesting
therapeutic approach aimed at inhibiting the tumorigenesis and
invasiveness of GBM cells. On the other hand, compounds interfering
with the microtubule-tubulin equilibrium in glioma cells have been
demonstrated to retain a potent anti-proliferative activity,
suggesting that compounds targeting tubulin are of great interest
for the treatment of GBM (63,64). In this context, in a recent study,
the authors described two novel series of compounds based on the
4,5,6,7-tetrahydrothieno[2,3-c] pyridine and
4,5,6,7-tetrahydrobenzo[b]thiophene molecular skeleton exerting
potent anti-proliferative effects and capable of inducing a
preferential block of the cell cycle in the G2/M phase (44).
It should be underlined that combined treatments
appear of great interest in the development of anticancer
approaches (1-5), since they are expected to obtain the
same biological or therapeutic effect using lower concentrations of
two or more drugs, thereby limiting side effects (1). Notably, combined therapy may be of
significance in the management of GBM, a lethal malignant tumor
needing of novel therapeutic options. This is mainly due to the
fact that, at present, there is no effective pharmacological
approach in the treatment of glioblastoma. The first-line drug
currently used is TMZ; however, this is able to extend the life
expectancy of patients with GBM by only an average of a few months;
moreover, very often, this type of tumor is or becomes resistant to
this chemotherapeutic agent (7-10).
The present study described for the first time, to
the best of our knowledge, a 'combo-therapy' performed by the
combined use of a PNA targeting miR-221 and tetrahydrothieno[2,3-c]
pyridine 3b, one of the most active compounds described by
Romagnoli et al (44). The
combined treatments were associated with more pronounced
anti-proliferative and cytotoxic effects, and a major increase in
the sub-G1 cell population. In agreement, the combined treatment
induced the highest level of 'late apoptotic' and 'dead' cells
following Annexin V analysis. The low level of 'early apoptotic'
cells can be tentatively explained by a rapid transition to the
late stage of apoptosis. The data from Annexin V assay were fully
confirmed by the functional caspase-3/7 assay, which employs a
specific substrate (N-Ac-DEVD-AFC), cleaved by active caspase-3 to
contribute in generating a highly fluorescent activity. It has been
well-established that this assay generates data consistent with
data generated by western blot analyses focusing on the
quantification of cleaved PARP (65,66). With respect to western blot
analysis, caspase-3/7 activity assay allows researchers to obtain a
distribution of cells in different classes, facilitating a
comparison with the data obtained by the Annexin V assay.
In conclusion, the results of the present study
support the concept that the combined treatment of GBM cells with a
PNA targeting a specific upregulated 'oncomiRNA' (in the present
study, miR-221-3p) and an anticancer agent (in the present study,
the anti-tubulin agent, tetrahydrothiene [2,3-c] pyridine 3b) is a
promising strategy in the field of developing effective anti-GBM
therapeutic approaches.
Availability of data and materials
The datasets generated and/or analyzed during the
present study are available from the corresponding author upon
reasonable request.
Authors' contributions
RG, AF and RR were involved in the
conceptualization, supervision and experimental design of the
study. MZ, JG, RR and PO performed the experiments. MZ and JG were
involved in the conceptualization and production of the figures.
RG, AF and RR were involved in the writing, reviewing and editing
of the manuscript. RG and AF were involved in project
administration and funding acquisition. RG and MZ confirm the
authenticity of all the raw data. All authors have read and
approved the final version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
Funding
The authors wish to thank the 'Associazione Tutti per Chiara'
(Montagnana, Italy) and AIRC/FIRC for supporting MZ and JG,
respectively with research fellowships. The present study was
supported by the Associazione Italiana per la Ricerca sul Cancro
(AIRC) (IG #13575 to RG), FAR (University Fund for Scientific
Research) and FIR (University Fund for the Incentivation of
Research). The present study was also supported by the European
Union (EU) Horizon 2020 Research and Innovation Programme [GA
#633937, project ULTRAsensitive PLAsmonic devices for early CAncer
Diagnosis (ULTRAPLACAD)] and by the Interuniversity Consortium for
the Biotechnology, Italy.
References
|
1
|
Bayat Mokhtari R, Homayouni TS, Baluch N,
Morgatskaya E, Kumar S, Das B and Yeger H: Combination therapy in
combating cancer. Oncotarget. 23:38022–38043. 2017. View Article : Google Scholar
|
|
2
|
Tolcher AW and Mayer LD: Improving
combination cancer therapy: The CombiPlex® development
platform. Future Oncol. 14:1317–1332. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bozic I, Reiter JG, Allen B, Antal T,
Chatterjee K, Shah P, Moon YS, Yaqubie A, Kelly N, Le DT, et al:
Evolutionary dynamics of cancer in response to targeted combination
therapy. Elife. 2:e007472013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sun X, Xu H, Huang T, Zhang C, Wu J and
Luo S: Simultaneous delivery of anti-miRNA and docetaxel with
supramolecular self-assembled 'chitosome' for improving
chemosensitivity of triple negative breast cancer cells. Drug Deliv
Transl Res. 11:192–204. 2021. View Article : Google Scholar
|
|
5
|
Gasparello J, Gambari L, Papi C, Rozzi A,
Manicardi A, Corradini R, Gambari R and Finotti A: High Levels of
apoptosis are induced in the human colon cancer HT-29 cell line by
co-administration of sulforaphane and a peptide nucleic acid
targeting miR-15b-5p. Nucleic Acid Ther. 30:164–174. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Palmer AC and Sorger PK: Combination
cancer therapy can confer benefit via patient-to-patient
variability without drug additivity or synergy. Cell.
171:1678–1691.e13. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
von Neubeck C, Seidlitz A, Kitzler HH,
Beuthien-Baumann B and Krause M: Glioblastoma multiforme: Emerging
treatments and stratification markers beyond new drugs. Br J
Radiol. 88:201503542015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Buczkowicz P and Hawkins C: Pathology,
molecular genetics, and epigenetics of diffuse intrinsic pontine
glioma. Front Oncol. 5:1472015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Pace A, Dirven L, Koekkoek JAF, Golla H,
Fleming J, Rudà R, Marosi C, Le Rhun E, Grant R, Oliver K, et al:
European association for neuro-oncology (EANO) guidelines for
palliative care in adults with glioma. Lancet Oncol. 18:e330–e340.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Anjum K, Shagufta BI, Abbas SQ, Patel S,
Khan I, Shah SAA, Akhter N and Hassan SSU: Current status and
future therapeutic perspectives of glioblastoma multiforme (GBM)
therapy: A review. Biomed Pharmacother. 92:681–689. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Santangelo A, Rossato M, Lombardi G,
Benfatto S, Lavezzari D, De Salvo GL, Indraccolo S, Dechecchi MC,
Prandini P, Gambari R, et al: A molecular signature associated with
prolonged survival in glioblastoma patients treated with
regorafenib. Neuro Oncol. 23:264–276. 2021. View Article : Google Scholar :
|
|
12
|
Touat M, Idbaih A, Sanson M and Ligon KL:
Glioblastoma targeted therapy: Updated approaches from recent
biological insights. Ann Oncol. 28:1457–1472. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sontheimer EJ and Carthew RW: Silence from
within: Endogenous siRNAs and miRNAs. Cell. 122:9–12. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Alvarez-Garcia I and Miska EA: MicroRNA
functions in animal development and human disease. Development.
132:4653–4662. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
He L and Hannon GJ: MicroRNAs: Small RNAs
with a big role in gene regulation. Nat Rev Genet. 5:522–531. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Fabbri M, Ivan M, Cimmino A, Negrini M and
Calin GA: Regulatory mechanisms of microRNAs involvement in cancer.
Expert Opin Biol Ther. 7:1009–1019. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Taylor MA and Schiemann WP: Therapeutic
opportunities for targeting microRNAs in cancer. Mol Cell Ther.
2:1–13. 2014. View Article : Google Scholar
|
|
18
|
Gambari R, Brognara E, Spandidos DA and
Fabbri E: Targeting oncomiRNAs and mimicking tumor suppressor
miRNAs: New trends in the development of miRNA therapeutic
strategies in oncology (review). Int J Oncol. 49:5–32. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Miroshnichenko S and Patutina O: Enhanced
inhibition of tumorigenesis using combinations of miRNA-targeted
therapeutics. Front Pharmacol. 10:4882019. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gajda E, Godlewska M, Mariak Z, Nazaruk E
and Gawel D: Combinatory treatment with miR-7-5p and drug-loaded
cubosomes effectively impairs cancer cells. Int J Mol Sci.
21:50392020. View Article : Google Scholar :
|
|
21
|
Ghasabi M, Majidi J, Mansoori B, Mohammadi
A, Shomali N, Shirafkan N, Baghbani E, Kazemi T and Baradaran B:
The effect of combined miR-200c replacement and cisplatin on
apoptosis induction and inhibition of gastric cancer cell line
migration. J Cell Physiol. 234:22581–22592. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
He JQ, Zheng MX, Ying HZ, Zhong YS, Zhang
HH, Xu M and Yu CH: PRP1, a heteropolysaccharide from platycodonis
radix, induced apoptosis of HepG2 cells via regulating
miR-21-mediated PI3K/AKT pathway. Int J Biol Macromol. 158:542–551.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tao Y, Zhan S, Wang Y, Zhou G, Liang H,
Chen X and Shen H: Baicalin, the major component of traditional
Chinese medicine Scutellaria baicalensis induces colon cancer cell
apoptosis through inhibition of oncomiRNAs. Sci Rep. 8:144772018.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhang H, Duan J, Qu Y, Deng T, Liu R,
Zhang L, Bai M, Li J, Ning T, Ge S, et al: Onco-miR-24 regulates
cell growth and apoptosis by targeting BCL2L11 in gastric cancer.
Protein Cell. 7:141–151. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wu Z, Liu K, Wang Y, Xu Z, Meng J and Gu
S: Upregulation of microRNA-96 and its oncogenic functions by
targeting CDKN1A in bladder cancer. Cancer Cell Int. 15:1072015.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Nielsen PE, Egholm M, Berg RH and Buchardt
O: Sequence-selective recognition of DNA by strand displacement
with a thymine-substituted polyamide. Science. 254:1497–1500. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Egholm M, Buchardt O, Christensen L,
Behrens C, Freier SM, Driver DA, Berg RH, Kim SK, Norden B and
Nielsen PE: PNA hybridizes to complementary oligonucleotides
obeying the watson-crick hydrogen-bonding rules. Nature.
365:566–568. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Fabani MM and Gait MJ: miR-122 targeting
with LNA/2′-O-methyl oligonucleotide mixmers, peptide nucleic acids
(PNA), and PNA-peptide conjugates. RNA. 14:336–346. 2008.
View Article : Google Scholar :
|
|
29
|
Brown PN and Yin H: PNA-based microRNA
inhibitors elicit anti-inflammatory effects in microglia cells.
Chem Commun (Camb). 49:4415–4417. 2013. View Article : Google Scholar
|
|
30
|
Fabani MM, Abreu-Goodger C, Williams D,
Lyons PA, Torres AG, Smith KG, Enright AJ, Gait MJ and Vigorito E:
Efficient inhibition of miR-155 function in vivo by peptide nucleic
acids. Nucleic Acids Res. 38:4466–4475. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Fabbri E, Manicardi A, Tedeschi T, Sforza
S, Bianchi N, Brognara E, Finotti A, Breveglieri G, Borgatti M,
Corradini R, et al: Modulation of the biological activity of
microRNA-210 with peptide nucleic acids (PNAs). ChemMedChem.
6:2192–2202. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Brognara E, Fabbri E, Bazzoli E, Montagner
G, Ghimenton C, Eccher A, Cantù C, Manicardi A, Bianchi N, Finotti
A, et al: Uptake by human glioma cell lines and biological effects
of a peptide-nucleic acids targeting miR-221. J Neurooncol.
118:19–28. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Gambari R, Fabbri E, Borgatti M, Lampronti
I, Finotti A, Brognara E, Bianchi N, Manicardi A, Marchelli R and
Corradini R: Targeting microRNAs involved in human diseases: A
novel approach for modification of gene expression and drug
development. Biochem Pharmacol. 82:1416–1429. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Fabbri E, Tamanini A, Jakova T, Gasparello
J, Manicardi A, Corradini R, Sabbioni G, Finotti A, Borgatti M,
Lampronti I, et al: A peptide nucleic acid against MicroRNA
miR-145-5p enhances the expression of the cystic fibrosis
transmembrane conductance regulator (CFTR) in Calu-3 cells.
Molecules. 23:712017. View Article : Google Scholar
|
|
35
|
Swellam M, Ezz El Arab L, Al-Posttany AS
and B Said S: Clinical impact of circulating oncogenic MiRNA-221
and MiRNA-222 in glioblastoma multiform. J Neurooncol. 144:545–551.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Chen YY, Ho HL, Lin SC, Ho TD and Hsu CY:
Upregulation of miR-125b, miR-181d, and miR-221 predicts poor
prognosis in MGMT promoter-unmethylated glioblastoma patients. Am J
Clin Pathol. 149:412–417. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Yang JK, Yang JP, Tong J, Jing SY, Fan B,
Wang F, Sun GZ and Jiao BH: Exosomal miR-221 targets DNM3 to induce
tumor progression and temozolomide resistance in glioma. J
Neurooncol. 131:255–265. 2017. View Article : Google Scholar
|
|
38
|
Xie Q, Yan Y, Huang Z, Zhong X and Huang
L: MicroRNA-221 targeting PI3-K/Akt signaling axis induces cell
proliferation and BCNU resistance in human glioblastoma.
Neuropathology. 34:455–464. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Xu CH, Liu Y, Xiao LM, Chen LK, Zheng SY,
Zeng EM, Li DH and Li YP: Silencing microRNA-221/222 cluster
suppresses glioblastoma angiogenesis by suppressor of cytokine
signaling-3-dependent JAK/STAT pathway. J Cell Physiol.
234:22272–22284. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Tao K, Yang J, Guo Z, Hu Y, Sheng H, Gao H
and Yu H: Prognostic value of miR-221-3p miR-342-3p and miR-491-5p
expression in colon cancer. Am J Transl Res. 6:391–401. 2014.
|
|
41
|
Dong Y, Zhang N, Zhao S, Chen X, Li F and
Tao X: miR-221-3p and miR-15b-5p promote cell proliferation and
invasion by targeting Axin2 in liver cancer. Oncol Lett.
18:6491–6500. 2019.PubMed/NCBI
|
|
42
|
Yin G, Zhang B and Li J: miR-221-3p
promotes the cell growth of non-small cell lung cancer by targeting
p27. Mol Med Rep. 20:604–612. 2019.PubMed/NCBI
|
|
43
|
Li F, Xu JW, Wang L, Liu H, Yan Y and Hu
SY: MicroRNA-221-3p is up-regulated and serves as a potential
biomarker in pancreatic cancer. Artif Cells Nanomed Biotechnol.
46:482–487. 2018. View Article : Google Scholar
|
|
44
|
Romagnoli R, Prencipe F, Oliva P, Cacciari
B, Balzarini J, Liekens S, Hamel E, Brancale A, Ferla S, Manfredini
S, et al: Synthesis and biological evaluation of new antitubulin
agents containing
2-(3′,4′,5′-trimethoxyanilino)-3,6-disubstituted-4,5,6,7-tetrahydrothieno[2,3-c]pyridine
scaffold. Molecules. 25:16902020. View Article : Google Scholar
|
|
45
|
Khodyuk RGD, Bai R, Hamel E, Lourenço EMG,
Barbosa EG, Beatriz A, Dos Santos EDA and de Lima DP: Diaryl
disulfides and thiosulfonates as combretastatin A-4 analogues:
Synthesis, cytotoxicity and antitubulin activity. Bioorg Chem.
101:1040172020. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Liu H, Fu Q, Lu Y, Zhang W, Yu P, Liu Z
and Sun X: Anti-tubulin agent vinorelbine inhibits metastasis of
cancer cells by regulating epithelial-mesenchymal transition. Eur J
Med Chem. 200:1123322020. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Wang G, Liu W, Gong Z, Huang Y, Li Y and
Peng Z: Design, synthesis, biological evaluation and molecular
docking studies of new chalcone derivatives containing diaryl ether
moiety as potential anticancer agents and tubulin polymerization
inhibitors. Bioorg Chem. 95:1035652020. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Yang F, Yu LZ, Diao PC, Jian XE, Zhou MF,
Jiang CS, You WW, Ma WF and Zhao PL: Novel
[1,2,4]triazolo[1,5-a]pyrimidine derivatives as potent antitubulin
agents: Design, multicomponent synthesis and antiproliferative
activities. Bioorg Chem. 92:1032602019. View Article : Google Scholar
|
|
49
|
Pen A, Durocher Y, Slinn J, Rukhlova M,
Charlebois C, Stanimirovic DB and Moreno MJ: Insulin-like growth
factor binding protein 7 exhibits tumor suppressive and vessel
stabilization properties in U87MG and T98G glioblastoma cell lines.
Cancer Biol Ther. 12:634–646. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Twentyman PR and Luscombe M: A study of
some variables in a tetrazolium dye (MTT) based assay for cell
growth and chemosensitivity. Br J Cancer. 56:279–285. 1987.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Cao X, Gu Y, Jiang L, Wang Y, Liu F, Xu Y,
Deng J, Nan Y, Zhang L, Ye J and Li Q: A new approach to screening
cancer stem cells from the U251 human glioma cell line based on
cell growth state. Oncol Rep. 29:1013–1018. 2013. View Article : Google Scholar
|
|
52
|
Munshi A, Hobbs M and Meyn RE: Clonogenic
cell survival assay. Methods Mol Med. 110:21–28. 2005.PubMed/NCBI
|
|
53
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
54
|
Milani R, Brognara E, Fabbri E, Manicardi
A, Corradini R, Finotti A, Gasparello J, Borgatti M, Cosenza LC,
Lampronti I, et al: Targeting miR-155-5p and miR-221-3p by peptide
nucleic acids induces caspase-3 activation and apoptosis in
temozolomide-resistant T98G glioma cells. Int J Oncol. 55:59–68.
2019.PubMed/NCBI
|
|
55
|
Shen H, Lin Z, Shi H, Wu L, Ma B, Li H,
Yin B, Tang J, Yu H and Yin X: MiR-221/222 promote migration and
invasion, and inhibit autophagy and apoptosis by modulating ATG10
in aggressive papillary thyroid carcinoma. 3 Biotech. 10:3392020.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Hu XH, Zhao ZX, Dai J, Geng DC and Xu YZ:
MicroRNA-221 regulates osteosarcoma cell proliferation, apoptosis,
migration, and invasion by targeting CDKN1B/p27. J Cell Biochem.
120:4665–4674. 2019. View Article : Google Scholar
|
|
57
|
Xie X, Huang Y, Chen L and Wang J: miR-221
regulates proliferation and apoptosis of ovarian cancer cells by
targeting BMF. Oncol Lett. 16:6697–6704. 2018.PubMed/NCBI
|
|
58
|
Li J, Li Q, Huang H, Li Y, Li L, Hou W and
You Z: Overexpression of miRNA-221 promotes cell proliferation by
targeting the apoptotic protease activating factor-1 and indicates
a poor prognosis in ovarian cancer. Int J Oncol. 50:1087–1096.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Zhou L, Jiang F, Chen X, Liu Z, Ouyang Y,
Zhao W and Yu D: Downregulation of miR-221/222 by a microRNA sponge
promotes apoptosis in oral squamous cell carcinoma cells through
upregulation of PTEN. Oncol Lett. 12:4419–4426. 2016. View Article : Google Scholar
|
|
60
|
Sarkar S, Dubaybo H, Ali S, Goncalves P,
Kollepara SL, Sethi S, Philip PA and Li Y: Down-regulation of
miR-221 inhibits proliferation of pancreatic cancer cells through
up-regulation of PTEN, p27(kip1), p57(kip2), and PUMA. Am J Cancer
Res. 3:465–477. 2013.PubMed/NCBI
|
|
61
|
Zhang CZ, Zhang JX, Zhang AL, Shi ZD, Han
L, Jia ZF, Yang WD, Wang GX, Jiang T, You YP, et al: MiR-221 and
miR-222 target PUMA to induce cell survival in glioblastoma. Mol
Cancer. 9:2292010. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Zhang C, Zhang J, Zhang A, Wang Y, Han L,
You Y, Pu P and Kang C: PUMA is a novel target of miR-221/222 in
human epithelial cancers. Int J Oncol. 37:1621–1626.
2010.PubMed/NCBI
|
|
63
|
Döbber A, Phoa AF, Abbassi RH, Stringer
BW, Day BW, Johns TG, Abadleh M, Peifer C and Munoz L: Development
and biological evaluation of a photoactivatable small molecule
microtubule-targeting agent. ACS Med Chem Lett. 8:395–400. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Cherry AE, Haas BR, Naydenov AV, Fung S,
Xu C, Swinney K, Wagenbach M, Freeling J, Canton DA, Coy J, et al:
ST-11: A new brain-penetrant microtubule-destabilizing agent with
therapeutic potential for glioblastoma multiforme. Mol Cancer Ther.
15:2018–2029. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Nam GH, Jo KJ, Park YS, Kawk HW, Kim SY
and Kim YM: In vitro and in vivo induction of p53-dependent
apoptosis by extract of euryale ferox salisb in A549 human
caucasian lung carcinoma cancer cells is mediated through Akt
signaling pathway. Front Oncol. 9:4062019. View Article : Google Scholar :
|
|
66
|
Kim EJ, Kim GT, Kim BM, Lim EG, Kim SY and
Kim YM: Apoptosis-induced effects of extract from artemisia annua
linné by modulating PTEN/p53/PDK1/Akt/signal pathways through
PTEN/p53-independent manner in HCT116 colon cancer cells. BMC
Complement Altern Med. 17:2362017. View Article : Google Scholar
|