1. Introduction
Gynecological malignancies, including cervical,
ovarian, uterine, vulvar, vaginal and fallopian tube cancers, are
among the leading cause of mortality among females worldwide; among
these, ovarian, cervical and endometrial cancers are the most
common (1). When these diseases
are detected at an early stage, surgery is the primary and most
effective treatment option. With the development of medical
science, the earlier detection of endometrial cancer (2) and cervical cancer (3) has increased; however, ovarian cancer
is often diagnosed in later stages, at which point, numerous
treatment options are not available. Moreover, the recurrence and
chemical resistance of ovarian cancer leads to poor treatment
outcomes and a poor prognosis (4).
Ovarian cancer causes more deaths than any other
type of cancer of the female reproductive system (5). According to the histological
classification of female genital tumors established by the World
Health Organization (6), the main
histological categories of ovarian cancer are epithelial carcinoma,
malignant ovarian germ cell tumor, sex cord-stromal carcinoma and
metastatic ovarian cancer. Among these, epithelial carcinoma
accounts for the majority of ovarian cancer cases (7). Epithelial carcinoma is also
classified as serous, mucinous, endometrioid or transparent cells
and certain other types of cancer. Notably, the most common type of
epithelial carcinoma is high-grade serous ovarian cancer, which
accounts for 75% of epithelial carcinoma-associated deaths
(6). As ovarian cancer has no
notable symptoms during the early stages, it is often not diagnosed
until it reaches an advanced stage (5). In addition, ~75% of patients develop
extensive peritoneal metastasis by the time of diagnosis, in stages
III or IV. Despite an increased understanding of this type of
cancer in recent years, the associated survival rate has not
improved, due to these difficulties in early diagnosis (4). Notably the 5-year survival rate for
patients with stage III or IV ovarian cancer is <30% (8). By contrast, the 5-year survival rates
of patients diagnosed with stage I or II disease are as high as
70-90% (9). At present, tumor
resection combined with platinum chemotherapy is the standard
treatment option for ovarian cancer (10). Surgical tumor reduction combined
with platinum and taxane chemotherapy may lead to clinical
remission in up to 75% of cases (8). However, the majority of patients with
advanced-stage ovarian cancer relapse or develop drug resistance,
leading to treatment failure and mortality (10).
The results of previous studies indicated that the
incidence and mortality rates of patients with cervical cancer have
decreased with the increase in human papillomavirus (HPV) vaccines,
and increases in global cervical cancer testing facilities. For
women aged 20 to 39 years, cervical cancer is the third leading
cause of cancer-related mortality (11-13).
Notably, the incidence rates of adenocarcinoma have increased in
young females aged <40 years. Moreover, the prevention, early
detection and prognosis of cervical adenocarcinoma remain poor
(14). It is well-established that
pre-cervical or cervical cancers are caused by HPV infection.
Notably, HPV is present in >90% of tumors (15), and is often transmitted through
sexual activity. Cervical cancer progression is often associated
with a persistent high-risk HPV infection (16). Lymph node metastasis is the primary
mode of the distant metastasis of cervical cancer; however, blood
transfer occurs relatively infrequently and during the late stages
(17). The most common metastatic
sites of cervical cancer are the lung and liver. In addition, the
5-year survival rate of patients with cervical cancer without
metastasis is 91.5%, while the 5-year survival rate of patients
with cervical cancer with metastasis is 16.5% (18).
Endometrial carcinoma is the most common
gynecological cancer in developed countries, such as the United
States, and the incidence rate of endometrial cancer continues to
increase. At present, 67% of endometrial cancers are detected and
confirmed in the early stages (19). Moreover, there are two types of
endometrial cancer, namely, types I and II. Type I endometrial
cancer may be caused by obesity, driven by estrogen and associated
with the excessive proliferation of endometrial cells. Type I is
more common, accounting for ~70% of endometrial cancer cases, and
exhibits a lower risk. Patients with type I endometrial cancer
often present with metabolic disorders, such as hyperlipidemia,
hyperestrogenemia, diabetes or anovulation uterine bleeding. By
contrast, type II endometrial cancer is not associated with obesity
or endometrial hyperplasia, and is not associated with metabolic or
endocrine diseases. Type II endometrial cancer is rare and highly
invasive. Type I and II endometrial cancers can be distinguished
histologically. Notably, type I tumor samples are often
endometrioid cancers that are well-differentiated. Type II,
however, is developed through endometrial atrophy, and the most
common types are serous and clear cell adenocarcinoma. Premalignant
forms differ for each type. The premalignant form of type I cancer
is endometrial intraepithelial neoplasia, while the premalignant
form of type II cancer is endometrial intraepithelial carcinoma
(2,20).
Vulvar cancer is considered rare and accounts for
only 5% of cancers of the female genital tract. Vulvar cancers are
divided into a variety of types depending on their histology, among
which, squamous cell carcinoma accounts for >85% of cases. Other
rare histological types include basal cell carcinoma, Bartholin
adenocarcinoma, extra mammal Paget's disease, sweat gland
adenocarcinoma and intestinal adenocarcinoma (21,22).
The results of a previous study demonstrated that risk factors for
vulvar cancer include HPV infection, vulvar lichen sclerosis
disease and vulvar intraepithelial neoplasia in young females.
Imaging techniques, including pelvic magnetic resonance imaging,
computed tomography (CT) or positron emission tomography/CT, and
ultrasound, may be used to evaluate the condition of the patient
(21). Vulvar cancer is often
treated with surgery, followed by radiotherapy and
chemotherapy.
Similarly, uterine sarcomas are rare, accounting for
~1% of all female genital tract malignancies, and 2 to 3% of all
uterine cancers (23). Uterine
sarcomas are categorized into the following two groups: i)
Mesenchymal tumors and ii) mixed epithelial and mesenchymal tumors.
Mesenchymal tumors include endometrial stromal sarcoma,
leiomyosarcoma and undifferentiated endometrial or uterine sarcoma.
Mixed epithelial and mesenchymal tumors include adenosarcoma and
carcinosarcoma (21). The rarity
of uterine sarcoma and its histopathological diversity result in a
lack of agreement on risk factors and available treatment option
(22). Patients with uterine
sarcoma often present with abnormal uterine bleeding or pelvic
pain. However, a definitive diagnosis requires biopsy or
histopathological analysis.
2. miRNAs and the Warburg effect
MicroRNAs (miRNAs/miRs)
miRNAs are small, non-protein-coding RNAs that
produce ~22 nucleotide-long sequences. These mediate
post-transcriptional gene suppression via the inhibition of protein
translation or the destabilization of target transcription, by
recognizing the 3′untranslated region (UTR) of homologous mRNAs.
Among the miRNAs, LIN-4 was the first to be discovered (24). Subsequently, additional miRNAs and
their corresponding functions have been studied, and a miRNA
database has been established (25). This database provides a unique name
to a miRNA prior to its discovery, and the sequences of all
published miRNAs are included. In total, ~25% of human miRNA genes
are located in the introns of precursor miRNAs (pre-mRNAs),
suggesting that the majority of miRNAs are not transcribed from
their own promoters but are instead processed from introns
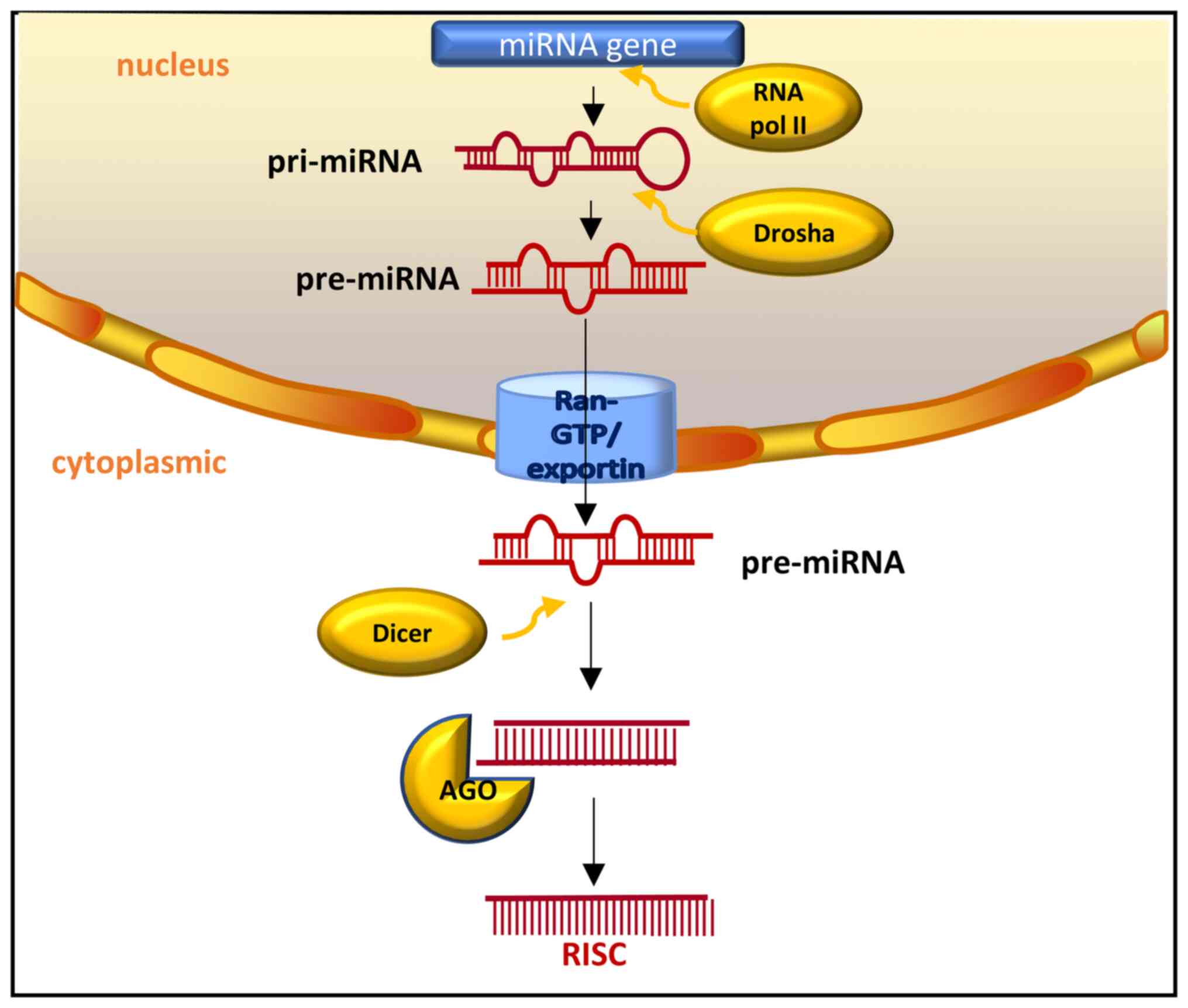
(26). As illustrated in Fig. 1, the transcription of miRNAs is
often carried out by RNA polymerase II, and the transcript is
further processed using capping and polyadenylation (27). Two successive processing reactions
transform transcripts into mature miRNAs (28). In mammals, one of the processing
reactions involves he removal of stem rings from the rest of the
pri-miRNA transcript in the nucleus. This is carried out by nuclear
members of Drosha to create pre-miRNA products (27). The second process includes the
active transportation of pre-miRNAs out of the nucleus via output
receptor and RAS-related nuclear protein (RAN)-GTP. In the
cytoplasm, the terminal ring is removed from the stem of pre-miRNA
by Dicer, to create a mature miRNA double-stranded body with a
length of ~22 base pairs (26,27).
The mature miRNA double strand is an unstable entity; it is at a
high speed when bound to the Argonaute (AGO) protein. Only one
strand is retained, depending on the relative thermodynamic
stability of the ends of each strand (28).
miRNAs bind to AGO and GW182 proteins to form
RNA-induced silencing complexes (RISCs), that mediate gene
expression and participate in various biologically critical
processes (29). Notably, this
process involves two mechanisms. Firstly, the target RNA contains
sequences entirely complementary to miRNA, and is cleaved by
ribonuclease in the RISC complex (30,31).
Moreover, when the target RNA includes sequences that are not
entirely complimentary to the miRNA, these are controlled in
translation (30,32). Through these mechanisms, miRNAs are
involved in multifarious biological impacts, such as cell growth,
cell death, cell differentiation, cell apoptosis, intercellular
signaling and cell metabolism, including fat metabolism (26,33-35).
The present review will focus on the steps of glycolysis in
gynecological tumor cells in which miRNAs are involved.
Warburg effect
Otto Heinrich Warburg (36,37)
initially discovered the Warburg effect in the 1920s. This effect
refers to the tendency of tumor cells to produce adenosine
triphosphate (ATP) through anaerobic glycolysis, even when
sufficient oxygen is present (36,37).
Normal cellular glucose metabolism can be divided into two phases
and ten reactions. The first phase is the production of two
propanose phosphates from glucose and the second phase is the
conversion of propanose phosphate to pyruvate. The specific process
displayed in Fig. 2. Under aerobic
conditions, pyruvate is further oxidized and decomposed to form
acetyl CoA in the mitochondria, which can be wholly oxidized into
H2O and CO2, through the electron transfer
chain and Krebs cycle to generate ATP (38). Pyruvate cannot be further oxidized
in anoxic conditions and is reduced to lactate.
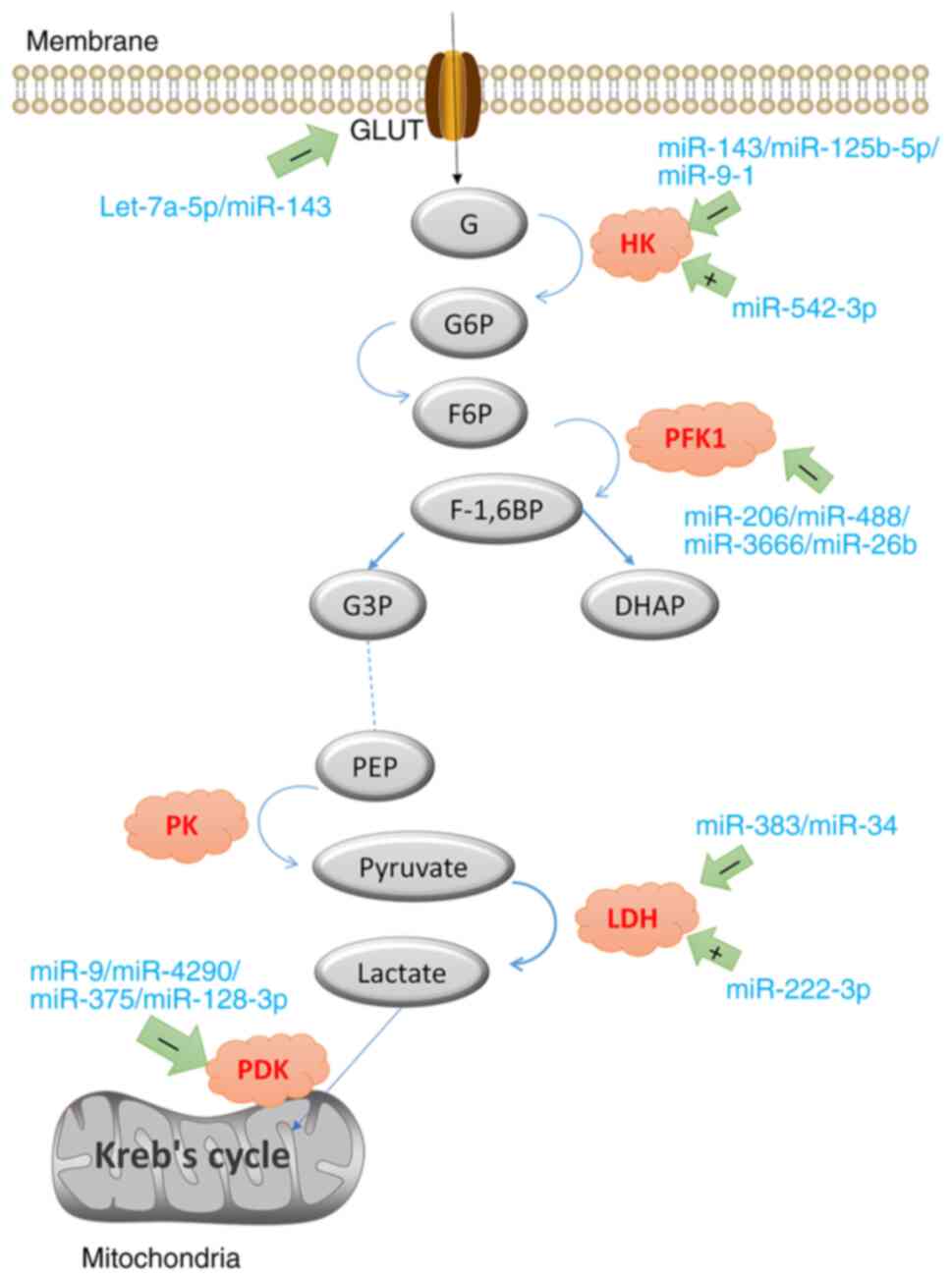 | Figure 2Glucose is transported into the cell
by GLUT, and HK catalyzes the production of G-6-P. Phosphohexose
isomerase catalyzes the production of F-6-P. PFK1 is further
phosphorylated to form F-1, 6-BP, which is catalyzed by aldolase
into DHAP and glyceraldehyde 3-phosphate. PEP is subsequently
generated through multiple reversible reactions, and PEP is
converted to pyruvate with PK catalysis. The + symbol indicates
promotion, and the-symbol indicates inhibition. miR, miRNA; GLUT,
glucose transporter; HK, hexokinase; G-6-P, glucose 6 phosphate;
F-6-P, fructose 6-phosphate; PFK1, phosphofructokinase L; F-1,
6-BP, fructose 1,6-diphosphate; DHAP, dihydroxyacetone phosphate;
PEP, phosphoenolpyruvate; PK, pyruvate kinase; LDH, lactate
dehydrogenase; PDK, pyruvate dehydrogenase. |
Despite sufficient levels of oxygen in tumor cells,
the cells continue to turn pyruvate into lactic acid, which is
known as aerobic glycolysis. In addition, tumor cells exhibit an
increased glucose uptake. Therefore, the production of lactic acid
by pyruvate increases and the pH value of tumor cells l decreases.
The results of a previous study demonstrated that this may be due
to tumor cells living in environments rich in glucose and other
nutrients, indicating that there is no ATP deficiency. On the other
hand, tumor cells may convert all glucose into CO2,
through oxidative phosphorylation, which maximizes ATP production
but does not meet the requirements of rapid cell proliferation.
Notably, the rapid proliferation of tumor cells requires numerous
substances, such as nucleotides, lipids and amino acids (39). In addition, glucose produces acetyl
coenzyme A and ribose, which are used for nucleotide biosynthesis
for rapid DNA replication (40,41).
However, the requirements of the Warburg effect in cancer have yet
to be fully elucidated (41-43).
3. miRNAs and glycolysis
Upstream regulation of miRNAs
Long non-coding RNAs (lncRNAs)
lncRNAs are transcripts >200 nucleotides in
length, that cannot be translated into proteins (44). lncRNAs contain intergene
transcripts and enhancer RNA (45). The results of a previous study
demonstrated that lncRNAs play a role in regulating gene expression
during transcription or post-transcription, such as
cis-trans transcriptional regulation, and the regulation of
the organization of nuclear domain and protein/RNA molecules
(46). On the other hand, lncRNAs
also bind with proteins to regulate protein activity. For example,
complementary pairing with miRNA bases regulates the abundance or
activity of miRNA to affect its function (44). lncRNAs are vital in various
biological processes of carcinogenesis (47), and are closely associated with
numerous human diseases (48,49).
The results of a previous study demonstrated that
the expression levels of lncRNA-TDRG1, determined using reverse
transcription-quantitative PCR (RT-qPCR), were increased in
cervical cancer cells (50). Under
conditions of hypoxia, tests of the trans hole, glucose, lactic
acid and functional loss demonstrated that lncRNA-TDRG1 knockdown
inhibited glycolysis and the progression of cervical cancer. The
results obtained from a bioinformatics database revealed the
association between lncRNA-TDRG1 and miR-214-5p, and miR-214-5p and
signaling protein 4C (SEMA4C), and this was confirmed using a
dual-luciferase reporter assay. The results of that study
demonstrated that lncRNA-TDRG1 regulated the expression of
miR-214-5p by sponging miR-214-5p. Subsequently, miR-214-5p binds
to SEMA4C to regulate the corresponding levels, and regulates
glycolysis and the growth of cervical cancer cells (50).
Similarly, the results of another study demonstrated
that lncRNA-MALAT1 promoted the glycolysis and metastasis of oral
squamous cell carcinoma (OSCC) cells, by targeting miR-101/EZH2
(51). By contrast, lncRNA-CASC2
expression was decreased in OSCC cells, and inhibited tumor cell
replication by targeting miRNA-21 (52). In hepatocellular carcinoma,
lncRNA-MALAT1 has been found to regulate glycolysis and the
progression of hepatocellular carcinoma through sponging miR-142-3p
(53).
Downstream targets of miRNAs
Glucose transporter (GLUT)
The Warburg effect begins with the transport of
extracellular glucose into the cell. Glucose requires specific
membrane transporters, and the GLUT family plays an essential role
in transportation. GLUT provides glucose for cellular metabolism
and maintains a constant blood glucose level (54). A total of 14 glucose transporters
have been identified in mammals using genome sequencing. The 14
subtypes are divided into three groups. GLUT1-4 and GLUT14
constitute the first group, and GLUTs 5, 7, 9 and 11 constitute the
second group. Group 3 includes GLUTs 6, 8, 10, 12 and 13. GLUT1 is
the most crucial subtype, and it exists in the majority of human
cells, particularly at the blood-brain barrier (55). GLUT1 is expressed in numerous types
of cancer (56). GLUT2 mainly
plays a role in the liver and pancreas, GLUT3 primarily exists in
the brain (57), and GLUT4 mainly
exists in fat, heart and skeletal muscle (58).
The results of a previous study demonstrated that
GLUT12 was significantly upregulated in triple-negative breast
cancer (TNBC), compared with other types of breast cancer. In
addition, GLUT12 is key in adjusting TNBC cell proliferation, wound
healing and transportation measurements (59). Notably, five miRNAs were identified
as targets of GLUT12 using the TargetScan and miRanda databases.
Results of western blot analysis and dual-luciferase assays
demonstrated that Let-7a-5p inhibited GLUT12 at the highest levels.
Moreover, Let-7a-5p directly inhibited GLUT12 through binding to
the 3′-UTR of GLUT12. Thus, Let-7a-5p is considered a tumor
inhibitor (60). Similarly,
Let-7a-5p demonstrated an inhibitory effect in TNBC. Following
Let-7a-5p overexpression, both glucose uptake and lactic acid
production were reduced in TNBC cells. In summary, Let-7a-5p
inhibited glycolysis and prevented TNBC cell propagation, transfer
and migration through targeting GLUT12.
In addition to affecting tumor cell proliferation
and migration, miRNA targets GLUT to adjust T-cell polarization.
Notably, miR-143 ultimately inhibits glycolysis in T-cells and
regulates T-cell polarization via directly targeting and inhibiting
GLUT-1 (Fig. 3) (61).
Hexokinase (HK)
HK is the key enzyme in glucose metabolism. It
phosphorylates glucose to form G-6-P, which is an irreversible
process. There are four subtypes of HK; however, only HK2 is
associated with tumor development (62), and increased levels of HK2 are a
characteristic of numerous tumors (63,64).
In several types of cancer, including prostate (65), colon (66) and gallbladder cancer (67), increased HK2 expression levels have
been observed, promoting tumor growth and metastasis. In addition,
miR-143 inhibits HK2 expression through the recognition of specific
sequences in the 3′UTR of HK2 (63), thus inhibiting cellular glucose
metabolism. Moreover, miR-138 also affects HK1 expression through
the identification of a specific motif in HK1 mRNA 3′UTR; however,
HK1 does not play a role in tumor development (63), and studies are limited.
In pancreatic cancer (68), breast cancer (69) and bladder cancer (70), miR-125b-5P plays a role as a
tumor-inhibiting miRNA that targets HK2 and inhibits HK2 expression
(71). Subsequently, this reduces
ATP levels, glucose uptake and lactic acid release from tumor
cells. HK2 also plays a role in the propagation, transfer,
migration and glycolysis of tumor cells. In addition, miR-9-1 was
determined to directly target the 3′UTR of HK2 to inhibit
translation; thus, controlling tumor cell growth, metastasis and
glycolysis (72). At present,
through the determination of dual luciferase reporter genes,
alternate miRNAs may inhibit cancer through targeting HK2 in
different tumors. For example, miR-199a-5p (73), miR-145 (74), miR-223-3p (75), miR-455 (76), miR-1271-5p (77), miR-615 (78), miR-3662 (79), miR-513a-3p (80), miR-188-5p (81), miR-206 (82), miR-202 (83), miR-185 (84), miR-885-5p (85), miR-603 (86), miR-216a-5p (87), miR-98 (88), miR-181B (89) and miR-214 (90) may target HK2.
By contrast, certain miRNAs may promote tumor cells.
For example, miR-542-3p promotes HK2-mediated glycolysis in human
glioma cells, aids tumor cell growth and metastasis, and leads to a
poor prognosis in patients with glioma (91).
6-Phosphofructo-2-kinase/fructose-2,6-biphosphatase 3 (PFKFB3)
Using dual-luciferase and bioinformatics analyses,
the results of previous studies revealed PFKFB3 as a rate-limiting
enzyme that regulates glycolysis. In addition, the levels of PFKFB3
are significantly increased in a variety of human tumors,
ultimately expanding the glycolysis levels of tumor cells, and the
corresponding levels of proliferation and metastasis (92,93).
miR-206 interacts directly with the 3′UTR of PFKFB3 mRNA. The
overexpression of miR-206 obstructs the production of
fructose-2,6-bisphosphatase (F-2, 6-BP), reduces lactic acid
production, and reduces cell propagation and tumor migration
(94). In other types of cancer,
such as colorectal cancer, miR-488 directly targets PFKFB3, and
PFKFB3 mRNA levels are inhibited by miR-488. This results in
decreased glucose uptake and lactate secretion, increased
sensitivity to chemotherapy drugs, and decreased proliferation,
invasion, and migration (95,96).
miR-3666 and miR-26b also reduce the rate of glycolysis through
inhibiting the activity of PFKFB3; thus, contributing to tumor
inhibition (97,98).
Pyruvate dehydrogenase kinase (PDK)
PDK phosphorylates pyruvate dehydrogenase and
converts glucose metabolism from OXPHOS to glycolysis, resulting in
increased lactate production. There are four subtypes of PDK in
human cells (PDK1, PDK2, PDK3 and PDK4), each highly expressed in
different tumors. The results of a dual-luciferase reporter gene
assay demonstrated that miRNA-9, as a tumor suppressor, directly
targets PDK1, reduces the levels of glycolysis and inhibits the
development of prostate cancer (99). miR-4290 has been shown to enhance
cisplatin sensitivity in gastric cancer cells by inhibiting
PDK1-mediated glycolysis (100).
miRNA-375 was the most downregulated miRNA in gastric cancer cells,
and its ectopic expression significantly inhibited cell
survivability through a caspase-mediated apoptosis pathway.
Moreover, high miR-375 expression levels inhibited the expression
of PDK1 (101). In nasopharynx
cancer, PDK1 is the target of miR-375, and tumor development is
negatively associated with miR-375 expression levels (102). The results obtained from the
Interactive Analysis of Gene Expression Profiling database
demonstrated that PDK1 levels were markedly increased in glioma
tissues. The downstream target of miR-128-3p is PDK1, and
miR-128-3p overexpression interfered with the Warburg effect in
neuroglioma cells by decreasing PDK1 expression, and inhibiting the
occurrence and development of glioma (103).
In TNBC cells, PDK4, as a downstream target of
miR-136-5p, has been found to be downregulated, subsequently
reducing the Warburg effect and inhibiting the proliferation and
metastasis of TNBC cells (104).
In cervical cancer, miR-16-5p targets PDK4 to reduce glycolysis
levels and chemical resistance (105). Other human tumor cells that also
target PDK4 and inhibit expression include miR-21 (106), miR-9-5p (107) and miR-5683 (108).
In gastric cancer tissues, PDK3 levels are
increased, and miR-497-5p directly downregulates PDK3, thereby
inhibiting the proliferation and growth of cells (109).
p53
p53 is one of the most common tumor suppressor
genes. In the process of normal development, p53 activity is often
low. p53 pathway deactivation occurs in tumors, as it functions in
inhibiting cell proliferation and in controlling metabolism. Thus,
p53 plays a role in resisting high levels of glycolysis of tumor
cells, and aiding in the adaptation to metabolic stress. p53
activity aids in preventing the occurrence and development of
cancer, and provides a basis for the development of novel tumor
therapies (110-112).
The activation of p53 induces a variety of
non-coding miRNAs, including miR-34a. The results of previous
studies demonstrated that p53 overexpression reduces the expression
levels of HK2, pyruvate kinase M (PKM), phosphofructokinase 1
(PFKP), PFKFB3 and hypoxia-inducible factor-1α (HIF-1α) in cells,
by inducing the upregulation of miR-34a expression; thus,
inhibiting the Warburg effect. This ultimately impacts the
proliferation and metastasis of tumor cells (113). TP53-inducible glycolysis and
apoptosis regulator (TIGAR), an apoptosis and glycolysis regulatory
factor, is a downstream target of p53, that controls cell
metabolism and prevents programmed cell death. TIGAR primarily
functions as a F-2, 6-BP to inhibit the Warburg effect. In liver
tumors, miR-885-5p and the associated precursors bind to TIGAR
promoter binding sites, alter local chromatins construction, and
subsequently adjust the levels of TIGAR (114,115). miR-125b is a negative regulator
of p53 (112). Other miRNAs that
act on p53, such as miR-504, miR-25 and miR-30d, inhibit its
expression by directly binding to the 3′UTR of p53 mRNA. By
contrast, miR-34, miR-215, miR-194, miR-605, miR-192 and miR-29
affect the regulation of p53, leading to the indirect activation of
p53 (116).
c-myc
C-myc is an oncogenic transcription factor that is
overexpressed in numerous cancer types, and is involved in cell
metabolism and proliferation processes, including nucleotide
metabolism, glucose metabolism and, glutamine metabolism. Notably,
c-myc also participates in ribosomal and mitochondrial biogenesis.
Myc functions as an oncogene in the development of numerous human
cancers (117,118). Therefore, the inhibition of c-myc
may exhibit potential in glucose metabolism in tumor cells, thus
providing a basis for the development of novel treatment option
(118).
The results of previous studies have demonstrated an
association between c-myc and miRNAs. For example, miR-222-3p
indirectly activates c-myc and c-myc target genes, such as GULT1,
HK2 and lactate dehydrogenase A (LDHA) (119). miR-644a directly inhibits c-myc,
thereby inhibiting the Warburg effect and proliferation (120). Through miRNA target prediction,
c-myc was identified as a potential target of Let-7a, which
inhibits the glucose metabolism activity of tumor cells through
inhibiting c-myc (121).
miR-3679-5p indirectly regulates c-myc by inhibiting the
transcription of neuronal precursor cell-expressed developmentally
downregulated 4, an E3 ligase, leading to c-myc stability and
Warburg effect increases, thus driving chemical resistance in lung
cancer (122). The results of a
previous study demonstrated that miR-155-deficient cells possess
reduced genes that play a role in the Warburg effect, and exhibit
reduced HK2, LDHA and PKM2 expression levels. Further results
demonstrated that the downregulation of c-myc controls the
PIK3R1-PDK1/Akt-Foxo3a pathway; however, there is no miR-155
binding domain in c-myc. These associations have yet to be fully
elucidated (123).
AMP kinase (AMPK)
AMPK plays a role as a metabolic master switch,
regulating three major types of metabolisms in metabolic tissues,
such as muscles and the liver. AMPK is also a stabilizer of organic
energy in cells. AMPK harmonizes and balances various metabolic
pathways, including glucose uptake and mitochondrial biogenesis,
and ultimately regulates cell and organ tissue growth (124).
The results of previous studies demonstrated that
the human tumor suppressor, liver kinase B1 (LKB1), directly
activates AMPK by encoding serine/threonine kinases, suggesting
that the LKB1-AMPK axis may be an essential cancer inhibitory
pathway (125,126). Epstein Barr virus
(EBV)-miR-bart1-5p, a miRNA encoded by EBV-Barts, binds to the α1
telomerase of AMPK (AMPKα1). This activates the mTOR/HIF1 pathway
to affect angiogenesis, leading to angiogenesis and glycolysis in
tumor cells. This leads to the propagation, transfer and migration
of tumor cells (127). The
results of a previous study demonstrated that miR-27a inhibited the
AMPK pathway, enhanced mTOR signaling, and functioned
synergistically with oncogenes and tumor cell metabolic regulators,
leading to enhanced glycolysis, unrestricted growth and chemical
resistance (128). By contrast,
aryl camphor flavone increased miR-124-3p expression in glioma
cells by activating the reactive oxygen species (ROS)/AMPK
signaling pathway, inducing apoptosis and inhibiting cell
glycolysis (129).
AKT
AKT, also known as protein kinase B, is a
serine/threonine kinase with three subtypes in mammalian cells. AKT
is stimulated by various growth factors to become phosphorylated
(p-)AKT, which affects multiple cellular functions and is a crucial
regulator of pro-growth signals (130). The results of a previous study
demonstrated that increased AKT and p-AKT expression levels were
associated with the appearance, development and prognosis of
various human tumors (131).
Another study verified that proteins of the PI3K/AKT/mTOR signaling
pathway were highly expressed in tumors (130). This pathway regulates essential
processes in tumor cell development, such as substance metabolism
and cytoskeletal remodeling metastasis. The reduced activity of the
PI3K/AKT/mTOR signaling pathway decreases the levels of glycolysis
in tumor cells. Notably, miR-21 and miR-520a-3p act by reducing the
expression levels of PKM2 and LDHA in the glycolysis pathway to
reduce glycolysis in tumor cells (132,133). In addition, miR-485-3p binds to
AKT3 mRNA and downregulates glycol- and migration-associated
proteins expression by suppressing the activation of the AKT3/mTOR
pathway, and inhibiting cell glycolysis, propagation and migration
(134).
As a PTEN binding protein, N-myc
downstream-regulated gene 2 (NDRG2) regulates PTEN phosphatase
through dephosphorylation. In addition, PIP3, the primary substrate
of PTEN, phosphorylates AKT. Thus, PTEN/PI3K/AKT is an essential
axis for controlling propagation, migration and metabolism
(135). miR-181a-5p binds to
NDRG2 and promotes migration and glycolysis through stimulating the
PTEN/AKT axis, ultimately leading to a poor prognosis (136).
Insulin-like growth factor-1 receptor (IGF-1R) is a
carcinogen that stimulates cell propagation and metabolism and is
upregulated in numerous tumors. miRNA-342-3p and miR-7 inhibit the
IGF-1R-mediated PI3K/AKT/GLUT1 signaling pathway through directly
binding with the 3′UTR of IGF-1R; thus, reducing glucose uptake
(137,138).
4. miRNAs and glycolysis in gynecological
cancers
Ovarian cancer
As presented in Table
I, various miRNAs play differential roles in the glycolysis of
ovarian cancer cells. The results of a previous study demonstrated
that the levels of lncRNA-NEAT1 in ovarian cancer were notably
increased, and the proliferation, invasion and glycolysis of
ovarian cancer were markedly inhibited following NEAT1 knockdown
(139). In addition, StarBase
database prediction and a dual-luciferase reporter assay were used
to verify that NEAT1 was the sponge of miR-4500, and alkaline
leucine zipper and w2-domain protein 1 (BZW1) were direct targets
of miR-4500. miR-4500 silencing reversed the suppressive effects of
NEAT1 knockdown on ovarian cancer, whereas the overexpression of
BZW1 reversed the suppressive effects of miR-4500 on the tumor.
Notably, NEAT1 accelerated the occurrence and development of
ovarian cancer via the miR-4500/BZW1 axis. Thus, NEAT1 may exhibit
potential in the treatment of ovarian cancer; however, further
investigations are required (139). lncRNA OPA-interacting protein 5
antisense transcript 1 (lncRNA-OIP5-AS1) is also elevated in
ovarian cancer. As previously demonstrated, OIP5-AS1 knockdown
inhibited the migration and glycolysis of ovarian cancer, and
accelerated programmed cell death. The results obtained using
StarBase, TargetScan and a dual-luciferase reporter assay
demonstrated that OIP5-AS1 indirectly regulated cyclin G1 (CCNG1)
levels by sponging miR-128-3p. Moreover, miR-128-3p suppressed the
progression of numerous cancers (140,141). Subsequent experiments
demonstrated that miR-128-3p knockdown alleviated the decrease in
glucose consumption and lactic acid production, enhanced apoptosis,
and prevented cell migration caused by CCNG1 knockdown. These
results demonstrated that OIP5-AS1 functions as a carcinogen in the
development of ovarian cancer through the miR-128-3p/CCNG1 axis,
which may act as a basis for the development of novel therapeutic
options for ovarian cancer (142). The results of an RT-qPCR analysis
demonstrated that lncRNA-LINC00504 expression was upregulated in
ovarian cancer. In addition, the results of further assays
demonstrated that LINC00504 was overexpressed in ovarian cancer,
which promoted cell proliferation and upregulated PDK1, PKM2 and
HK2, thereby increasing the levels of glycolysis in cells.
Moreover, LINC00504 reduced the expression of miR-1244 through
sponging. In summary, LNC00504 promotes ovarian cancer cell
development and glycolysis through targeting miR-1244, suggesting
that LINC00504 may function as a therapeutic target for ovarian
cancer (143).
 | Table ImiRNAs and glycolysis in ovarian
cancer. |
Table I
miRNAs and glycolysis in ovarian
cancer.
| Authors, year | miRNA | Role | Expression | Upstream | Downstream | Glycolysis | Value | (Refs.) |
|---|
| Xu et al,
2020 | miR-4500 | Tumor promoter | Up | lncRNA-NEAT1 | BZW1 | Increased | Potential
treatment | (139) |
| Liu et al,
2021 | miR-128-3p | Tumor promoter | Up |
lncRNA-OIP5-AS1 | CCNG1 | Increased | Potential
treatment | (142) |
| Liu et al,
2020 | miR-1244 | Tumor promoter | Up | LINC00504 | | Increased
treatment | Potential | (143) |
| Liu et al,
2021 | miR-486-5p | Tumor promoter | Up | LINC00857 | YAP1 | Increased | Potential
treatment | (146) |
| Li et al,
2022 | miR-580-3p | Tumor promoter | Up | lncRNA-RMRP | MICU1 | Increased | Potential treatment
for chemical resistance | (157) |
| Teng et al,
2015 | miR-29b | Tumor
inhibitor | Down | | AKT2/ AKT3 | Reduced | Potential
treatment | (147) |
| Boscaro et
al, 2022 | miR-206 | Tumor
inhibitor | Down | | PFKFB3 | Reduced | Potential treatment
for chemical resistance | (93) |
| Rao et al,
2020 | miR-195 | Tumor
inhibitor | Down | | MICU1 | Reduced | Potential
treatment | (154) |
| Han et al,
2017 | miR-383 | Tumor
inhibitor | Down | | LDHA | Reduced | Potential
treatment | (158) |
| Lu et al,
2019 | miR-603 | Tumor
inhibitor | Down | | HK2 | Reduced | Potential
treatment | (86) |
| Rafat et al,
2021 | miR-132 | Tumor
inhibitor | Down | | PI3K, TGFβ, Ras,
AKT, mTOR | Reduced | Potential
treatment | (159) |
| Xiaohong et
al, 2016 | miR-203 | Tumor promoter | Up | | PDHB | Increased | Potential
treatment | (163) |
| Hu et al,
2019 | miR-1180 | Tumor promoter | Up | | SFRP1 | Increased | Potential
treatment | (161) |
Previous research has demonstrated that the Hippo
signaling pathway is associated with tumor development. As a
critical oncogene of the Hippo pathway (144,145), yes1-associated transcriptional
regulator (YAP1) is targeted by miR-486-5p, and miR-486-5p binds to
LINC00857 in ovarian cancer, and inhibits the progression and
glycolysis of ovarian cancer. Thus, LINC00857 inactivates the Hippo
pathway. Notably, LINC00857 regulates ovarian cancer development
through sponging miR-486-5p and upregulating YAP1, through
regulation of the Hippo signaling pathway (146).
miR-29b functions as a tumor inhibitor in numerous
types of cancer, and the expression of this miRNA was reduced in
epithelial ovarian cancer (147).
Using dual-luciferase reporter gene detection, the results
demonstrated that miR-29b bound directly to AKT2/AKT3 3′UTR and
inhibited the corresponding expression. Activated AKT promotes GLUT
and HK expression, to increase the activity of phosphofructokinase
(148); thus, stimulating the
glucose to lactic acid metabolism pathway. This ultimately promotes
the replication and migration of tumor cells. Therefore, it was
hypothesized that miR-29b may negatively regulate AKT to reduce
glycolysis levels and inhibit tumor development in epithelial
ovarian cancer. The results of a previous study demonstrated that
miR-29b silencing upregulated the mRNA expression of AKT2/3, PKM2
and HK2, and increased glucose intake and lactate production in
ovarian cancer cells. On the other hand, miR-29b overexpression
demonstrated the reverse effects (147). Thus, these results demonstrated
that miR-29b plays a key role in regulating the Warburg effect, and
in the replication and migration of ovarian cancer cells through
impacting the expression of AKT. This may provide a theoretical
basis for the development of novel treatment options for epithelial
ovarian cancer.
The results of previous studies demonstrated that
PFKFB3 was overexpressed in human cancers, such as in ovarian
cancer, where the upregulation of PFKFB3 expression promoted cell
growth and migration and enhanced chemotherapeutic resistance
(149,150). Using a dual-luciferase assay, the
results of a previous study demonstrated that miR-206 reduced the
levels of PFKFB3 by binding with the 3′UTR, miR-206 overexpression
prevented the proliferation and migration of ovarian cancer by
downregulating the PFKFB3 levels. In addition, the results of
another study demonstrated that miR-206 downregulated PFKFB3
expression and negatively regulated focal adhesion kinase (FAK)
post-transcription. These results indicated that miR-206 exhibits
numerous roles, and may be used to control ovarian cancer
characterized by FAK overexpression and resistance to chemotherapy
(93).
Mitochondrial calcium uptake 1 (MICU1) is a protein
in the inner membrane of the mitochondria that inhibits calcium
ions from entering. However, in ovarian cancer, MICU1
overexpression increases glycolysis and enhances cellular
chemotherapy resistance. Moreover, MICU1 plays a role in the low
survival rates of patients with ovarian cancer (151), highlighting its potential use as
a target in the treatment of ovarian cancer. miR-195 is a tumor
inhibitor in various types of cancer (152,153), and its expression is reduced in
seven different ovarian cancer cell lines (OVCAR4, A2780-CP20,
TYK-NU(JCRB0234.0), TYK-NU.CPr(JCRB0234.1), OVSAHO(JCRB1046), ATCC
CRL-11732™ and FTE188). Notably, MICU1 expression was significantly
increased in six of these cancer cells, compared with standard cell
lines (152). It was demonstrated
that miR-195 targeted the MICU1 3′UTR in healthy cells, leading to
decreased MICU1 expression. Moreover, intracellular lactic acid
levels were significantly reduced in ovarian cancer cell lines
following miR-195 overexpression or MICU1 knockdown, suggesting
that glycolysis was inhibited (154). It was also demonstrated that
miR-195 expression levels maintained calcium homeostasis and
reduced the glycolysis of ovarian cancer cells by regulating MICU1;
thus, inhibiting cell growth and migration. Tumor growth
experiments in female athymic mice revealed that miR-195 prolonged
the in vivo survival of ovarian cancer models in mice, and
the re-expression of MICU1 in cells reversed the tumor
growth-inhibiting phenotype caused by miR-195 overexpression. These
results suggest that miR-195 extends overall survival in ovarian
cancer patients by targeting MICU1 (154). In addition, lncRNA component of
mitochondrial RNA processing endoribonuclease (lncRNA-RMRP)
functions as a tumor activator in various types of cancer (155,156), and also increases MICU1
expression by sponging miR-580-3p, thus promoting glycolysis and
paclitaxel resistance in ovarian cancer (157).
Similarly, miR-383, miR-603 and miR-132 are
inhibitors of several human cancers, and their expression levels
are significantly decreased. The results of previous studies
demonstrated that the ectopic levels of miR-383 inhibit LDHA mRNA
expression by binding with the 3′UTR of LDHA. Moreover, miR-603
directly targets HK2 expression. miR-132 affects PI3K and TGFβ
signaling pathways or Ras, AKT and mTOR oncogene expression.
Notably, these miRNAs promote cell apoptosis, reduce glucose
consumption and lactic acid production, and inhibit cell
proliferation and invasion (86,158,159).
By contrast, miR-203 and miR-1180 expression have
been shown to be increased in ovarian cancer tissues, compared with
healthy tissues. miR-203 mainly targets the 3′UTR of pyruvate
dehydrogenase B, while secreted frizzled-related protein 1, a tumor
inhibitor (160), acts as a
direct target gene of miR-1180, and activates the Wnt signaling
pathway following miR-1180-induced inhibition (161,162). Notably, miRNAs promote glucose
consumption and lactate production in tumor cells to increase
glycolysis. This leads to cell colony growth, migration and
invasion (163).
Cervical cancer
The roles of numerous miRNAs in the development of
cervical cancer are presented in Table II. The results of a previous study
demonstrated that hypoxic-responsive lncRNA-TDRG1 expression was
increased in cervical cancer. Subsequently, the interactive
association between miR-214-5p, lncRNA-TDRG1 and signaling SEMA4C
was analyzed. The levels of lncRNA-TDRG1, miR-214-5p, SEMA4C and
HK2 were evaluated using western blot analysis and RT-qPCR. Under
conditions of hypoxia, lncRNA-TDRG1 knockdown inhibited glycolysis
and the migration of cervical cancer. Moreover, miR-214-5p
knockdown or SEMA4C overexpression reversed tumor inhibition. In
conclusion, lncRNA-TDRG1 acts on miR-214-5p to affect SEMA4C
expression, glycolysis and the migration of cervical cancer cells
(50). Compared with healthy
cells, NEAT1 expression levels have been found to be increased in
cervical cancer cells, which affect the levels of miR-34a, leading
to dysglycolysis and 5-fluorouracil resistance. Bioinformatics
prediction using TargetScan revealed that LDHA was a close target
of miR-34a-5p, and miR-34a and LDHA mRNA expression were negatively
correlated. The NEAT1-mediated miR-34a/LDHA axis may act as a
latent treatment for chemotherapy-resistant cervical cancer
(164). lncRNA-OIP5-AS1 is
considered a hypoxic-responsive lncRNA. In addition, OIP5-AS1
inhibits miR-124-5p to promote Isocitrate dehydrogenase 2 (IDH2)
expression, and IDH2 promotes the Warburg effect in cervical cancer
cells through regulating HIF-1α expression (165).
 | Table IImiRNAs and glycolysis in cervical
cancer. |
Table II
miRNAs and glycolysis in cervical
cancer.
| Authors, year | miRNA | Role | Expression | Upstream | Downstream | Glycolysis | Value | (Refs.) |
|---|
| Li, et al,
2021 | miR-214-5p | Tumor promoter | Up | lncRNA-TDRG1 | SEMA4C | Increased | Potential
treatment | (50) |
| Shao et al,
2021 | miR-34a | Tumor promoter | Up | lncRNA-NEAT1 | LDHA | Increased | Potential
treatment | (164) |
| Li et al,
2021 | miR-124-5p | Tumor promoter | Up |
lncRNA-OIP5-AS1 | IDH2/ HIF-1α | Increased | Potential
treatment | (165) |
| Li et al,
2019 | miR-27a | Tumor promoter | Up | | INPP1 | Increased | Potential
treatment | (169) |
| Zhang et al,
2016 | miR-34a | Tumor
inhibitor | Down | | LDHA | Reduced | Potential
treatment | (173) |
| Yang et al,
2016 | miR-497 | Tumor promoter | Up | | TKT | Increased | Potential treatment
for chemical resistance | (174) |
| Zhao et al,
2020 | miR-16-5p | Tumor
inhibitor | Down | | PDK4 | Reduced | Potential treatment
for chemical resistance | (105) |
Inositol polyphosphate 1-phosphatase (INPP1) is an
enzyme involved in glycolysis and lipid metabolism. Using RT-qPCR,
the results demonstrated that INPP1 mRNA expression was increased
in cervical cancer. In addition, INPP1 overexpression enhanced cell
viability and facilitated cell irradiation. Results of wound
healing and translocation assays demonstrated that migration and
invasion rates were significantly increased following INPP1 was
overexpression. Moreover, results of a dual-luciferase reporter
assay demonstrated that miR-27a binds with the 3′UTR of INPP.
miR-27a is highly expressed in a variety of cancer cells and is
essential in the invasion of cancer (166-168). The results of a previous study
demonstrated that miR-27a expression was increased cervical cancer,
and was positively associated with INPP1. Notably, miR-27a is
upregulated in cervical cancer cells and promotes the
overexpression of INPP1. This regulates cell metabolic
reprogramming and promotes aerobic glycolysis, ultimately
increasing the viability of cervical cancer cells (169). Thus, miR-27a and INPP1 may
function as potential biomarkers for cervical cancer.
By contrast, miR-34a expression is often reduced in
tumors (170,171), and plays a crucial role in tumor
suppression. Notably, miR-34a regulates a variety of target genes
involved in cell replication, differentiation and apoptosis, and
interferes with the processes involved in cancer cell metastasis
and chemical tolerance (172).
The results of the measurement of lactate production and glucose
consumption demonstrated that cervical cancer cells with exogenous
miR-34a consume less glucose, highlighting that miR-34a prevents
the aerobic glycolysis of cervical cancer cells (173). The results obtained using the
miRNA target prediction program, miRbase, demonstrated that LDHA is
a downstream target of miR-34a. In addition, the ectopic expression
of miR-34a or LDHA knockdown reduced tumor growth and invasion
(173). Thus, it was hypothesized
that miR-34a may play an inhibitory role in cervical cancer, and
may exhibit potential in the treatment of cervical cancer.
The results of a previous study demonstrated that
miRNA-497 regulated ROS and glutathione levels by targeting the
transketolase enzyme involved in the pentose phosphate pathway and
subsequently promoting cisplatin chemical resistance in cervical
cancer (174). miR-16-5p directly
binds to PDK4. The inhibition of miR-16-5p has been shown to lead
to an increased expression of PDK4, which activates glycolytic
metabolic activity and enhances chemoresistance in cervical cancer
(105). Therefore, miR-497 and
miR-16-5p may exhibit potential as targets in the treatment of
chemical resistance in cervical cancer.
Endometrial carcinoma
The roles of miRNAs in endometrial cancer are
presented in Table III. Notably,
HK2 is upregulated in various types of cancer, such as endometrial
cancer. HK2 expression is significantly increased in high-risk
endometrial cancer cells, suggesting that HK2 expression is
associated with the prognosis of endometrial cancer. Numerous
prediction algorithms have revealed that miR-455 and miR-181a may
bind to the 3′UTR of HK2, and miR-455 directly inhibits HK2
expression. In addition, lncRNA deleted in lymphocytic leukemia 2
(lncRNA-DLEU2) binds EZH2 and increases the corresponding
expression while silencing miR-181a. Subsequently, this increases
HK2 levels and promotes glycolysis (76). In conclusion, DLEU2 affects the
progression of endometrial cancer through targeting the miR-455/HK2
and EZH2/miR-181a/HK2 axis, which may exhibit potential in the
treatment of endometrial cancer.
 | Table IIImiRNAs and glycolysis in endometrial
carcinoma. |
Table III
miRNAs and glycolysis in endometrial
carcinoma.
| Authors, year | miRNA | Role | Expression | Upstream | Downstream | Glycolysis | Value | (Refs.) |
|---|
| Dong et al,
2021 | miR-455 | Tumor promoter | Up | lncRNA-DLEU2 | HK2 | Increased | Potential
treatment | (76) |
| Dong et al,
2021 | miR-181a | Tumor promoter | Up | HK2 | HK2 | Increased | Potential
treatment | (76) |
Other gynecological tumors
At present, research focused on the expression of
miRNAs in vulvar carcinoma is limited. The results of a previous
study demonstrated that miR-182-5p, miR-183-5p and miR-590-5p were
upregulated, and miR-103a-3p, miR-107 and miR-603 were
downregulated in vulvar squamous cell carcinoma (175). Moreover, miR-19-b1-5p and
miR-223-5p downregulation were previously found to be associated
with the presence of lymph node metastasis (176). However, the role and molecular
mechanisms of miRNAs in glycolysis, and their potential to predict
treatment outcomes in patients with uterine sarcoma have yet to be
fully elucidated. The results of a previous study demonstrated that
the downregulation of miR-10a-5p and miR-125a-5p, and the
upregulation of miR-34c-5p and miR-196a-5p were associated with the
prognosis of patients with uterine leiomyosarcoma (177). In endometrial stromal sarcoma,
the downregulation of miR-23-3p and let-7b-5p, and the upregulation
of miR-372-3p and miR-373-3p were associated with poor survival
outcomes. The expression levels of miR-210-3p, miR-301a-3p and
miR-335-5p were increased in patients with tumor metastasis and
recurrence (177).
5. Conclusions and future perspectives
It has been demonstrated that miRNAs are involved in
the glycolysis in ovarian, cervical and endometrial cancers.
However, there is a lack of consensus on the involvement of miRNAs
in vulvar cancer, uterine sarcoma, or other rare gynecological
tumors. The incidence of cancer, development mechanisms and the
early diagnosis of ovarian cancer are complex (178). Recurrence and challenges in both
diagnosis and treatment processes affect invasive cervical cancer
and endometrial carcinoma, and lead to a poor prognosis (179). Although previous studies have
focused on improving potential treatment options and prognosis
(180-182), further investigations are
required.
The consumption and metabolism of glucose during
glycolysis in tumor cells leads to an increased glucose uptake,
lactic acid production, changes in the tumor microenvironment and
an increased ATP production. All of the aforementioned factors may
promote tumor progression, invasion, metastasis and chemical
resistance (183,184). Therefore, glycolysis may exhibit
potential as a novel drug target. The results of previous studies
have demonstrated that miRNAs participate in the glycolysis
process, and inhibit or promote glycolysis through lncRNA-mediated
regulation. miRNAs act on transporters and critical enzymes in the
glycolysis process, and also target multiple cellular signaling
pathways, tumor suppressor genes and transcription factors
(1,185-187). However, the interaction between
various factors during glycolysis remains to be fully
elucidated.
In conclusion, miRNAs are vital in the glycolysis
of tumor cells. Future investigations will aim to explore
additional miRNA-targeting molecules and mechanisms and continue to
explore metabolic reprogramming in other gynecological tumors.
Currently, a number of preclinical trials have indicated the
potential of targeted miRNAs for cancer treatment. For example,
tumor growth experiments in female athymic mice revealed that
miR-195 prolonged the in vivo survival of ovarian cancer
models in mice, and the re-expression of MICU1 in cells reversed
the tumor growth-inhibiting phenotype caused by miR-195
overexpression. These results suggest that miR-195 extends the
overall survival of patients with ovarian cancer by targeting MICU1
(154). There is currently a lack
of clinical trials to verify the feasibility and effectiveness of
miRNA-targeted drugs in gynecological cancer patients. Further
studies are also required to aim at exploring potential biomarkers,
and provide novel theoretical platforms for the treatment of
cancer.
At the same time, targeting miRNAs in the diagnosis
of gynecological tumors also warrants attention. miRNAs whose
expression is clearly dysregulated in serum and tissues, and whose
target genes and pathways are associated with tumors, are screened
by enrichment analysis may play a key role in cancer diagnosis
(188).
Availability of data and materials
Not applicable.
Authors' contributions
QC drafted and revised the manuscript. JT designed
and supervised the study. SS and NL collected the data for
inclusion in the review and designed the tables. All authors have
read and approved the final manuscript. Data authentication is not
applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
Funding
No funding was received.
References
|
1
|
Dwivedi SKD, Rao G, Dey A, Mukherjee P,
Wren JD and Bhattacharya R: Small non-coding-RNA in gynecological
malignancies. Cancers. 13:10852021. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Brooks RA, Fleming GF, Lastra RR, Lee NK,
Moroney JW, Son CH, Tatebe K and Veneris JL: Current
recommendations and recent progress in endometrial cancer. CA
Cancer J Clin. 69:258–279. 2019.PubMed/NCBI
|
|
3
|
Tsikouras P, Zervoudis S, Manav B, Tomara
E, Iatrakis G, Romanidis C, Bothou A and Galazios G: Cervical
cancer: Screening, diagnosis and staging. J BUON. 21:320–325.
2016.PubMed/NCBI
|
|
4
|
Stewart C, Ralyea C and Lockwood S:
Ovarian cancer: An integrated review. Semin Oncol Nurs. 35:151–156.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Shafabakhsh R and Asemi Z: Quercetin: A
natural compound for ovarian cancer treatment. J Ovarian Res.
12:552019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cree IA, White VA, Indave BI and Lokuhetty
D: Revising the WHO classification: Female genital tract tumours.
Histopathology. 76:151–156. 2020. View Article : Google Scholar
|
|
7
|
Devouassoux-Shisheboran M and Genestie C:
Pathobiology of ovarian carcinomas. Chin J Cancer. 34:50–55. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Vergote I, González-Martín A, Ray-Coquard
I, Harter P, Colombo N, Pujol P, Lorusso D, Mirza MR, Brasiuniene
B, Madry R, et al: European experts consensus: BRCA/homologous
recombination deficiency testing in first-line ovarian cancer. Ann
Oncol. 33:276–287. 2022. View Article : Google Scholar
|
|
9
|
Steinberga I, Jansson K and Sorbe B:
Quality indicators and survival outcome in Stage IIIB-IVB
epithelial ovarian cancer treated at a single institution. In Vivo.
33:1521–1530. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang C and Liu N: Noncoding RNAs in the
glycolysis of ovarian cancer. Front Pharmacol. 13:8554882022.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Saslow D, Solomon D, Lawson HW, Killackey
M, Kulasingam SL, Cain J, Garcia FA, Moriarty AT, Waxman AG, Wilbur
DC, et al: American Cancer Society, American Society for Colposcopy
and cervical pathology, and American Society for Clinical Pathology
screening guidelines for the prevention and early detection of
cervical cancer. CA Cancer J Clin. 62:147–172. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Siegel RL, Miller KD, Fuchs HE and Jemal
A: Cancer statistics, 2022. CA Cancer J Clin. 72:7–33. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Xu HH, Wang K, Feng XJ, Dong SS, Lin A,
Zheng LZ and Yan WH: Prevalence of human papillomavirus genotypes
and relative risk of cervical cancer in China: A systematic review
and meta-analysis. Oncotarget. 9:15386–15397. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bray F, Carstensen B, Møller H, Zappa M,
Zakelj MP, Lawrence G, Hakama M and Weiderpass E: Incidence trends
of adenocarcinoma of the cervix in 13 European countries. Cancer
Epidemiol Biomarkers Prev. 14:2191–2199. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li H, Wu X and Cheng X: Advances in
diagnosis and treatment of metastatic cervical cancer. J Gynecol
Oncol. 27:e432016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Crosbie EJ, Einstein MH, Franceschi S and
Kitchener HC: Human papillomavirus and cervical cancer. Lancet.
382:889–899. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Han X, Wen H, Ju X, Chen X, Ke G, Zhou Y,
Li J, Xia L, Tang J, Liang S and Wu X: Predictive factors of
para-aortic lymph nodes metastasis in cervical cancer patients: A
retrospective analysis based on 723 para-aortic lymphadenectomy
cases. Oncotarget. 8:51840–51847. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ferlay J, Colombet M, Soerjomataram I,
Dyba T, Randi G, Bettio M, Gavin A, Visser O and Bray F: Cancer
incidence and mortality patterns in Europe Estimates for 40
countries and 25 major cancers in 2018. Eur J Cancer. 103:356–387.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Koh WJ, Abu-Rustum NR, Bean S, Bradley K,
Campos SM, Cho KR, Chon HS, Chu C, Cohn D, Crispens MA, et al:
Uterine neoplasms, version 1.2018, NCCN clinical practice
guidelines in oncology. J Natl Compr Canc Netw. 16:170–199. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kajo K, Vallová M, Biró C, Bognár G,
Macháleková K, Závodná K, Galbavý Š and Žúbor P: Molecular
pathology of endometrial carcinoma-a review. Cesk Patol. 51:65–73.
2015.In Czech.
|
|
21
|
Miccò M, Sala E, Lakhman Y, Hricak H and
Vargas HA: Imaging features of uncommon gynecologic cancers. AJR Am
J Roentgenol. 205:1346–1359. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Di Fiore R, Suleiman S, Pentimalli F,
O'Toole SA, O'Leary JJ, Ward MP, Conlon NT, Sabol M, Ozretić P,
Erson-Bensan AE, et al: Could MicroRNAs be useful tools to improve
the diagnosis and treatment of rare gynecological cancers? A Brief
overview. Int J Mol Sci. 22:38222021. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Mbatani N, Olawaiye AB and Prat J: Uterine
sarcomas. Int J Gynaecol Obstet. 143(Suppl 2): S51–S58. 2018.
View Article : Google Scholar
|
|
24
|
Lee RC, Feinbaum RL and Ambros V: The C.
elegans heterochronic gene lin-4 encodes small RNAs with antisense
complementarity to lin-14. Cell. 75:843–854. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Griffiths-Jones S: The microRNA registry.
Nucleic Acids Res. 32(Database Issue): D109–D111. 2004. View Article : Google Scholar :
|
|
26
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kim VN: MicroRNA biogenesis: Coordinated
cropping and dicing. Nat Rev Mol Cell Biol. 6:376–385. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Carthew RW and Sontheimer EJ: Origins and
mechanisms of miRNAs and siRNAs. Cell. 136:642–655. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Riedmann LT and Schwentner R: miRNA,
siRNA, piRNA and argonautes: News in small matters. RNA Biol.
7:133–139. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Brennecke J, Hipfner DR, Stark A, Russell
RB and Cohen SM: Bantam encodes a developmentally regulated
microRNA that controls cell proliferation and regulates the
proapoptotic gene hid in Drosophila. Cell. 113:25–36. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zeng Y, Wagner EJ and Cullen BR: Both
natural and designed micro RNAs can inhibit the expression of
cognate mRNAs when expressed in human cells. Mol Cell. 9:1327–1333.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Doench JG, Petersen CP and Sharp PA:
siRNAs can function as miRNAs. Genes Dev. 17:438–442. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Xu P, Vernooy SY, Guo M and Hay BA: The
drosophila microRNA Mir-14 suppresses cell death and is required
for normal fat metabolism. Curr Biol. 13:790–795. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Krützfeldt J and Stoffel M: MicroRNAs: A
new class of regulatory genes affecting metabolism. Cell Metab.
4:9–12. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Gao P, Tchernyshyov I, Chang TC, Lee YS,
Kita K, Ochi T, Zeller KI, De Marzo AM, Van Eyk JE, Mendell JT and
Dang CV: c-Myc suppression of miR-23a/b enhances mitochondrial
glutaminase expression and glutamine metabolism. Nature.
458:762–765. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Warburg O: On the origin of cancer cells.
Science. 123:309–314. 1956. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Warburg O: The chemical constitution of
respiration ferment. Science. 68:437–443. 1928. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Rolfe DF and Brown GC: Cellular energy
utilization and molecular origin of standard metabolic rate in
mammals. Physiol Rev. 77:731–758. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Vander Heiden MG, Cantley LC and Thompson
CB: Understanding the Warburg effect: The metabolic requirements of
cell proliferation. Science. 324:1029–1033. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
DeBerardinis RJ, Mancuso A, Daikhin E,
Nissim I, Yudkoff M, Wehrli S and Thompson CB: Beyond aerobic
glycolysis: Transformed cells can engage in glutamine metabolism
that exceeds the requirement for protein and nucleotide synthesis.
Proc Natl Acad Sci USA. 104:19345–19350. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Fantin VR, St-Pierre J and Leder P:
Attenuation of LDH-A expression uncovers a link between glycolysis,
mitochondrial physiology, and tumor maintenance. Cancer Cell.
9:425–434. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Shim H, Chun YS, Lewis BC and Dang CV: A
unique glucose-dependent apoptotic pathway induced by c-Myc. Proc
Natl Acad Sci USA. 95:1511–1516. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Birsoy K, Wang T, Chen WW, Freinkman E,
Abu-Remaileh M and Sabatini DM: An essential role of the
mitochondrial electron transport Chain in cell proliferation is to
enable aspartate synthesis. Cell. 162:540–551. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Kopp F and Mendell JT: Functional
classification and experimental dissection of long noncoding RNAs.
Cell. 172:393–407. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Derrien T, Johnson R, Bussotti G, Tanzer
A, Djebali S, Tilgner H, Guernec G, Martin D, Merkel A, Knowles DG,
et al: The GENCODE v7 catalog of human long noncoding RNAs:
Analysis of their gene structure, evolution, and expression. Genome
Res. 22:1775–1789. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Ulitsky I and Bartel DP: lincRNAs:
Genomics, evolution, and mechanisms. Cell. 154:26–46. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Chen F, Chen J, Yang L, Liu J, Zhang X,
Zhang Y, Tu Q, Yin D, Lin D, Wong PP, et al: Extracellular
vesicle-packaged HIF-1α-stabilizing lncRNA from tumour-associated
macrophages regulates aerobic glycolysis of breast cancer cells.
Nat Cell Biol. 21:498–510. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Gao YL, Zhao ZS, Zhang MY, Han LJ, Dong YJ
and Xu B: Long noncoding RNA PVT1 facilitates cervical cancer
progression via negative regulating of miR-424. Oncol Res.
25:1391–1398. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Wang S, Hui Y, Li X and Jia Q: Silencing
of lncRNA CCDC26 restrains the growth and migration of glioma cells
in vitro and in vivo via targeting miR-203. Oncol Res.
26:1143–1154. 2018. View Article : Google Scholar
|
|
50
|
Li X, Zhang C and Tian Y: Long non-coding
RNA TDRG1 promotes hypoxia-induced glycolysis by targeting the
miR-214-5p/SEMA4C axis in cervical cancer cells. J Mol Histol.
52:245–256. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Xiao L, Wang W, Zhao J, Xu H, Li S and
Yang X: lncRNA MALAT1 promotes cell proliferation and invasion by
regulating the miR-101/EZH2 axis in oral squamous cell carcinoma.
Oncol Lett. 20:1642020. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Erratum: Long Non-coding RNA CASC2 serves
as A ceRNA of microRNA-21 to promote pdcd4 expression in oral
squamous cell carcinoma [Corrigendum]. Onco Targets Ther.
12:95692019. View Article : Google Scholar
|
|
53
|
Yu Q, Xiang L, Chen Z, Liu X, Ou H, Zhou J
and Yang D: MALAT1 functions as a competing endogenous RNA to
regulate SMAD5 expression by acting as a sponge for miR-142-3p in
hepatocellular carcinoma. Cell Biosci. 9:392019. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Holman GD: Chemical biology probes of
mammalian GLUT structure and function. Biochem J. 475:3511–3534.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Simpson IA, Appel NM, Hokari M, Oki J,
Holman GD, Maher F, Koehler-Stec EM, Vannucci SJ and Smith QR:
Blood-brain barrier glucose transporter: Effects of hypo- and
hyperglycemia revisited. J Neurochem. 72:238–247. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Yu M, Yongzhi H, Chen S, Luo X, Lin Y,
Zhou Y, Jin H, Hou B, Deng Y, Tu L and Jian Z: The prognostic value
of GLUT1 in cancers: A systematic review and meta-analysis.
Oncotarget. 8:43356–43367. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Maher F, Vannucci SJ and Simpson IA:
Glucose transporter proteins in brain. FASEB J. 8:1003–1011. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
James DE, Strube M and Mueckler M:
Molecular cloning and characterization of an insulin-regulatable
glucose transporter. Nature. 338:83–87. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Shi Y, Zhang Y, Ran F, Liu J, Lin J, Hao
X, Ding L and Ye Q: Let-7a-5p inhibits triple-negative breast tumor
growth and metastasis through GLUT12-mediated warburg effect.
Cancer Lett. 495:53–65. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Tang R, Yang C, Ma X, Wang Y, Luo D, Huang
C, Xu Z, Liu P and Yang L: MiR-let-7a inhibits cell proliferation,
migration, and invasion by down-regulating PKM2 in gastric cancer.
Oncotarget. 7:5972–5984. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Zhang T, Zhang Z, Li F, Ping Y, Qin G,
Zhang C and Zhang Y: Correction: miR-143 regulates memory T cell
differentiation by reprogramming T cell metabolism. J Immunol.
201:2165–2175. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Ciscato F, Ferrone L, Masgras I, Laquatra
C and Rasola A: Hexokinase 2 in cancer: A prima donna playing
multiple characters. Int J Mol Sci. 22:47162021. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Peschiaroli A, Giacobbe A, Formosa A,
Markert EK, Bongiorno-Borbone L, Levine AJ, Candi E, D'Alessandro
A, Zolla L, Finazzi Agrò A and Melino G: miR-143 regulates
hexokinase 2 expression in cancer cells. Oncogene. 32:797–802.
2013. View Article : Google Scholar
|
|
64
|
Wolf A, Agnihotri S, Micallef J, Mukherjee
J, Sabha N, Cairns R, Hawkins C and Guha A: Hexokinase 2 is a key
mediator of aerobic glycolysis and promotes tumor growth in human
glioblastoma multiforme. J Exp Med. 208:313–326. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Zhou P, Chen WG and Li XW: MicroRNA-143
acts as a tumor suppressor by targeting hexokinase 2 in human
prostate cancer. Am J Cancer Res. 5:2056–2063. 2015.PubMed/NCBI
|
|
66
|
Gregersen LH, Jacobsen A, Frankel LB, Wen
J, Krogh A and Lund AH: MicroRNA-143 down-regulates Hexokinase 2 in
colon cancer cells. BMC Cancer. 12:2322012. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Chen J, Yu Y, Li H, Hu Q, Chen X, He Y,
Xue C, Ren F, Ren Z, Li J, et al: Long non-coding RNA PVT1 promotes
tumor progression by regulating the miR-143/HK2 axis in gallbladder
cancer. Mol Cancer. 18:332019. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Yu T, Li G, Wang C, Gong G, Wang L, Li C,
Chen Y and Wang X: MIR210HG regulates glycolysis, cell
proliferation, and metastasis of pancreatic cancer cells through
miR-125b-5p/HK2/PKM2 axis. RNA Biol. 18:2513–2530. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Li Y, Wang Y, Fan H, Zhang Z and Li N:
miR-125b-5p inhibits breast cancer cell proliferation, migration
and invasion by targeting KIAA1522. Biochem Biophys Res Commun.
504:277–282. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Liu S, Chen Q and Wang Y: MiR-125b-5p
suppresses the bladder cancer progression via targeting HK2 and
suppressing PI3K/AKT pathway. Hum Cell. 33:185–194. 2020.
View Article : Google Scholar
|
|
71
|
Hui L, Zhang J and Guo X: MiR-125b-5p
suppressed the glycolysis of laryngeal squamous cell carcinoma by
down-regulating hexokinase-2. Biomed Pharmacother. 103:1194–1201.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Xu QL, Luo Z, Zhang B, Qin GJ, Zhang RY,
Kong XY, Tang HY and Jiang W: Methylation-associated silencing of
miR-91 promotes nasopharyngeal carcinoma progression and glycolysis
via HK2. Cancer Sci. 112:4127–4138. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Guo W, Qiu Z, Wang Z, Wang Q, Tan N, Chen
T, Chen Z, Huang S, Gu J, Li J, et al: MiR-199a-5p is negatively
associated with malignancies and regulates glycolysis and lactate
production by targeting hexokinase 2 in liver cancer. Hepatology.
62:1132–1144. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Yoshino H, Enokida H, Itesako T, Kojima S,
Kinoshita T, Tatarano S, Chiyomaru T, Nakagawa M and Seki N:
Tumor-suppressive microRNA-143/145 cluster targets hexokinase-2 in
renal cell carcinoma. Cancer Sci. 104:1567–1574. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Zhao Y, Zhong R, Deng C and Zhou Z: Circle
RNA circABCB10 modulates PFN2 to promote breast cancer progression,
as well as aggravate radioresistance through facilitating
glycolytic metabolism via miR-223-3p. Cancer Biother Radiopharm.
36:477–490. 2021.
|
|
76
|
Dong P, Xiong Y, Konno Y, Ihira K,
Kobayashi N, Yue J and Watari H: Long non-coding RNA DLEU2 drives
EMT and glycolysis in endometrial cancer through HK2 by
competitively binding with miR-455 and by modulating the
EZH2/miR-181a pathway. J Exp Clin Cancer Res. 40:2162021.
View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Zhang B, Chen J, Cui M and Jiang Y: LncRNA
ZFAS1/miR-1271-5p/HK2 promotes glioma development through
regulating proliferation, migration, invasion and apoptosis.
Neurochem Res. 45:2828–2839. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Sun L, Wang P, Zhang Z, Zhang K, Xu Z, Li
S and Mao J: MicroRNA-615 functions as a tumor suppressor in
osteosarcoma through the suppression of HK2. Oncol Lett.
20:2262020. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Ye J, Xiao X, Han Y, Fan D, Zhu Y and Yang
L: MiR-3662 suppresses cell growth, invasion and glucose metabolism
by targeting HK2 in hepatocellular carcinoma cells. Neoplasma.
67:773–781. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Li C, Yu Z and Ye J: MicroRNA-513a-3p
regulates colorectal cancer cell metabolism via targeting
hexokinase 2. Exp Ther Med. 20:572–580. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Ding Z, Guo L, Deng Z and Li P: Circ-PRMT5
enhances the proliferation, migration and glycolysis of hepatoma
cells by targeting miR-188-5p/HK2 axis. Ann Hepatol. 19:269–279.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Jia KG, Feng G, Tong YS, Tao GZ and Xu L:
miR-206 regulates non-small-cell lung cancer cell aerobic
glycolysis by targeting hexokinase 2. J Biochem. 167:365–370. 2020.
View Article : Google Scholar
|
|
83
|
Wang J, Chen J, Sun F, Wang Z, Xu W, Yu Y,
Ding F and Shen H: miR-202 functions as a tumor suppressor in
hepatocellular carcinoma by targeting HK2. Oncology Lett.
19:2265–2271. 2020.
|
|
84
|
Liu C, Cai L and Li H: miR-185 regulates
the growth of osteosarcoma cells via targeting Hexokinase 2. Mol
Med Rep. 20:2774–2782. 2019.PubMed/NCBI
|
|
85
|
Xu F, Yan JJ, Gan Y, Chang Y, Wang HL, He
XX and Zhao Q: miR-885-5p negatively regulates warburg effect by
silencing hexokinase 2 in liver cancer. Mol Ther Nucleic Acids.
18:308–319. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Lu J, Wang L, Chen W, Wang Y, Zhen S, Chen
H, Cheng J, Zhou Y, Li X and Zhao L: miR-603 targeted hexokinase-2
to inhibit the malignancy of ovarian cancer cells. Arch Biochem
Biophys. 661:1–9. 2019. View Article : Google Scholar
|
|
87
|
Liu Y, Huo Y, Wang D, Tai Y, Li J, Pang D,
Zhang Y, Zhao W, Du N and Huang Y: MiR-216a-5p/Hexokinase 2 axis
regulates uveal melanoma growth through modulation of Warburg
effect. Biochem Biophys Res Commun. 501:885–892. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Zhu W, Huang Y, Pan Q, Xiang P, Xie N and
Yu H: MicroRNA-98 Suppress Warburg Effect by Targeting HK2 in Colon
Cancer Cells. Dig Dis Sci. 62:660–668. 2017. View Article : Google Scholar
|
|
89
|
Li LQ, Yang Y, Chen H, Zhang L, Pan D and
Xie WJ: MicroRNA-181b inhibits glycolysis in gastric cancer cells
via targeting hexokinase 2 gene. Cancer Biomark. 17:75–81. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Zhang K, Zhang M, Jiang H, Liu F, Liu H
and Li Y: Down-regulation of miR-214 inhibits proliferation and
glycolysis in non-small-cell lung cancer cells via down-regulating
the expression of hexokinase 2 and pyruvate kinase isozyme M2.
Biomed Pharmacother. 105:545–552. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Kim J, Park MW, Park YJ, Ahn JW, Sim JM,
Kim S, Heo J, Jeong JH, Lee M, Lim J and Moon JS: miR-542-3p
Contributes to the HK2-mediated high glycolytic phenotype in human
glioma cells. Genes. 12:6332021. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Atsumi T, Chesney J, Metz C, Leng L,
Donnelly S, Makita Z, Mitchell R and Bucala R: High expression of
inducible 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase
(iPFK-2; PFKFB3) in human cancers. Cancer Res. 62:5881–5887.
2002.PubMed/NCBI
|
|
93
|
Boscaro C, Baggio C, Carotti M, Sandonà D,
Trevisi L, Cignarella A and Bolego C: Targeting of PFKFB3 with
miR-206 but not mir-26b inhibits ovarian cancer cell proliferation
and migration involving FAK downregulation. FASEB J. 36:e221402022.
View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Ge X, Lyu P, Cao Z, Li J, Guo G, Xia W and
Gu Y: Overexpression of miR-206 suppresses glycolysis,
proliferation and migration in breast cancer cells via PFKFB3
targeting. Biochem Biophys Res Commun. 463:1115–1121. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Deng X, Li D, Ke X, Wang Q, Yan S, Xue Y,
Wang Q and Zheng H: Mir-488 alleviates chemoresistance and
glycolysis of colorectal cancer by targeting PFKFB3. J Clin Lab
Anal. 35:e235782021. View Article : Google Scholar
|
|
96
|
Wang J, Li X, Xiao Z, Wang Y, Han Y, Li J,
Zhu W, Leng Q, Wen Y and Wen X: MicroRNA-488 inhibits proliferation
and glycolysis in human prostate cancer cells by regulating PFKFB3.
FEBS Open Bio. 9:1798–1807. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Chen L and Cao Y, Wu B and Cao Y:
MicroRNA-3666 suppresses cell growth in head and neck squamous cell
carcinoma through inhibition of PFKFB3-mediated warburg effect.
Onco Targets Ther. 13:9029–9041. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Du JY, Wang LF, Wang Q and Yu LD: miR-26b
inhibits proliferation, migration, invasion and apoptosis induction
via the downregulation of
6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase-3 driven
glycolysis in osteosarcoma cells. Oncol Rep. 33:1890–1898. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Sheng W, Xu W, Ding J, Li L, You X, Wu Y
and He Q: Curcumol inhibits the malignant progression of prostate
cancer and regulates the PDK1/AKT/mTOR pathway by targeting miR-9.
Oncol Rep. 46:2462021. View Article : Google Scholar :
|
|
100
|
Qian Y, Wu X, Wang H, Hou G, Han X and
Song W: MicroRNA-4290 suppresses PDK1-mediated glycolysis to
enhance the sensitivity of gastric cancer cell to cisplatin. Braz J
Med Biol Res. 53:e93302020. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Tsukamoto Y, Nakada C, Noguchi T, Tanigawa
M, Nguyen LT, Uchida T, Hijiya N, Matsuura K, Fujioka T, Seto M and
Moriyama M: MicroRNA-375 is downregulated in gastric carcinomas and
regulates cell survival by targeting PDK1 and 14-3-3zeta. Cancer
Res. 70:2339–2349. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Jia-Yuan X, Wei S, Fang-Fang L, Zhi-Jian
D, Long-He C and Sen L: miR-375 inhibits the proliferation and
invasion of nasopharyngeal carcinoma cells by suppressing PDK1.
Biomed Res Int. 2020:97042452020. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Qu C, Yan C, Cao W, Li F, Qu Y, Guan K, Si
C, Yu Z and Qu Z: miR-128-3p contributes to mitochondrial
dysfunction and induces apoptosis in glioma cells via targeting
pyruvate dehydrogenase kinase 1. IUBMB Life. 72:465–475. 2020.
View Article : Google Scholar
|
|
104
|
Huang Y, Zheng S, Lin Y and Ke L: Circular
RNA circ-ERBB2 elevates the warburg effect and facilitates
triple-negative breast cancer growth by the MicroRNA
136-5p/pyruvate dehydrogenase Kinase 4 axis. Mol Cell Biol.
41:e00609202021. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Zhao Z, Ji M, Wang Q, He N and Li Y:
miR-16-5p/PDK4-mediated metabolic reprogramming is involved in
chemoresistance of cervical cancer. Mol Ther Oncolytics.
17:509–517. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Guda MR, Asuthkar S, Labak CM, Tsung AJ,
Alexandrov I, Mackenzie MJ, Prasad DV and Velpula KK: Targeting
PDK4 inhibits breast cancer metabolism. Am J Cancer Res.
8:1725–1738. 2018.PubMed/NCBI
|
|
107
|
Si T, Ning X, Zhao H, Zhang M, Huang P, Hu
Z, Yang L and Lin L: microRNA-9-5p regulates the mitochondrial
function of hepatocellular carcinoma cells through suppressing
PDK4. Cancer Gene Ther. 28:706–718. 2021. View Article : Google Scholar
|
|
108
|
Miao Y, Li Q, Sun G, Wang L, Zhang D, Xu H
and Xu Z: MiR-5683 suppresses glycolysis and proliferation through
targeting pyruvate dehydrogenase kinase 4 in gastric cancer. Cancer
Med. 9:7231–7243. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Feng L, Cheng K, Zang R, Wang Q and Wang
J: miR-497-5p inhibits gastric cancer cell proliferation and growth
through targeting PDK3. Biosci Rep. 39:BSR201906542019. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Cheung EC and Vousden KH: The role of p53
in glucose metabolism. Curr Opin Cell Biol. 22:186–191. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Yang L, Zhang B, Wang X, Liu Z, Li J,
Zhang S, Gu X, Jia M, Guo H, Feng N, et al: P53/PANK1/miR-107
signalling pathway spans the gap between metabolic reprogramming
and insulin resistance induced by high-fat diet. J Cell Mol Med.
24:3611–3624. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Le MT, Teh C, Shyh-Chang N, Xie H, Zhou B,
Korzh V, Lodish HF and Lim B: MicroRNA-125b is a novel negative
regulator of p53. Genes Dev. 23:862–876. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Gopu V, Fan L, Shetty RS, Nagaraja MR and
Shetty S: Caveolin-1 scaffolding domain peptide regulates glucose
metabolism in lung fibrosis. JCI Insight. 5:e1379692020. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Zou S, Rao Y and Chen W: miR-885-5p plays
an accomplice role in liver cancer by instigating TIGAR expression
via targeting its promoter. Biotechnol Appl Biochem. 66:763–771.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Tang J, Chen L, Qin ZH and Sheng R:
Structure, regulation, and biological functions of TIGAR and its
role in diseases. Acta Pharmacol Sin. 42:1547–1555. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Jones M and Lal A: MicroRNAs, wild-type
and mutant p53: More questions than answers. RNA Biol. 9:781–791.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Dang CV: MYC on the path to cancer. Cell.
149:22–35. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Broecker-Preuss M, Becher-Boveleth N,
Bockisch A, Dührsen U and Müller S: Regulation of glucose uptake in
lymphoma cell lines by c-MYC- and PI3K-dependent signaling pathways
and impact of glycolytic pathways on cell viability. J Transl Med.
15:1582017. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Zhai S, Xu Z, Xie J, Zhang J, Wang X, Peng
C, Li H, Chen H, Shen B and Deng X: Epigenetic silencing of LncRNA
LINC00261 promotes c-myc-mediated aerobic glycolysis by regulating
miR-222-3p/HIPK2/ERK axis and sequestering IGF2BP1. Oncogene.
40:277–291. 2021. View Article : Google Scholar :
|
|
120
|
Ebron JS, Shankar E, Singh J, Sikand K,
Weyman CM, Gupta S, Lindner DJ, Liu X, Campbell MJ and Shukla GC:
MiR-644a disrupts oncogenic transformation and warburg effect by
direct modulation of multiple genes of tumor-promoting pathways.
Cancer Res. 79:1844–1856. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Luan W, Wang Y, Chen X, Shi Y, Wang J,
Zhang J, Qian J, Li R, Tao T, Wei W, et al: PKM2 promotes glucose
metabolism and cell growth in gliomas through a mechanism involving
a let-7a/ c-Myc/hnRNPA1 feedback loop. Oncotarget. 6:13006–13018.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Wang H, Wang L, Pan H, Wang Y, Shi M, Yu
H, Wang C, Pan X and Chen Z: Exosomes derived from macrophages
enhance aerobic glycolysis and chemoresistance in lung cancer by
stabilizing c-Myc via the Inhibition of NEDD4L. Front Cell Dev
Biol. 8:6206032020. View Article : Google Scholar
|
|
123
|
Kim S, Lee E, Jung J, Lee JW, Kim HJ, Kim
J, Yoo HJ, Lee HJ, Chae SY, Jeon SM, et al: microRNA-155 positively
regulates glucose metabolism via PIK3R1-FOXO3a-cMYC axis in breast
cancer. Oncogene. 37:2982–2991. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Ojuka EO: Role of calcium and AMP kinase
in the regulation of mitochondrial biogenesis and GLUT4 levels in
muscle. Proc Nutr Soc. 63:275–278. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Shackelford DB and Shaw RJ: The LKB1-AMPK
pathway: Metabolism and growth control in tumour suppression. Nat
Rev Cancer. 9:563–575. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Hardie DG: AMP-activated/SNF1 protein
kinases: Conserved guardians of cellular energy. Nat Rev Mol Cell
Biol. 8:774–785. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Lyu X, Wang J, Guo X, Wu G, Jiao Y, Faleti
OD, Liu P, Liu T, Long Y, Chong T, et al: EBV-miR-BART1-5P
activates AMPK/mTOR/HIF1 pathway via a PTEN independent manner to
promote glycolysis and angiogenesis in nasopharyngeal carcinoma.
PLoS Pathog. 14:e10074842018. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Barisciano G, Colangelo T, Rosato V,
Muccillo L, Taddei ML, Ippolito L, Chiarugi P, Galgani M,
Bruzzaniti S, Matarese G, et al: miR-27a is a master regulator of
metabolic reprogramming and chemoresistance in colorectal cancer.
Br J Cancer. 122:1354–1366. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Zhaohui W, Yingli N, Hongli L, Haijing W,
Xiaohua Z, Chao F, Liugeng W, Hui Z, Feng T, Linfeng Y and Hong J:
Amentoflavone induces apoptosis and suppresses glycolysis in glioma
cells by targeting miR-124-3p. Neurosci Lett. 686:1–9. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Wang L, Guo W, Ma J, Dai W, Liu L, Guo S,
Chen J, Wang H, Yang Y, Yi X, et al: Aberrant SIRT6 expression
contributes to melanoma growth: Role of the autophagy paradox and
IGF-AKT signaling. Autophagy. 14:518–533. 2018. View Article : Google Scholar :
|
|
131
|
Vivanco I and Sawyers CL: The
phosphatidylinositol 3-Kinase AKT pathway in human cancer. Nat Rev
Cancer. 2:489–501. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
132
|
Sun Y, Liu W, Zhao Q, Zhang R, Wang J, Pan
P, Shang H, Liu C and Wang C: Down-regulating the expression of
miRNA-21 inhibits the glucose metabolism of A549/DDP cells and
promotes cell death through the PI3K/AKT/mTOR/HIF-1α pathway. Front
Oncol. 11:6535962021. View Article : Google Scholar
|
|
133
|
Pan C, Liu Q and Wu X:
HIF1α/miR-520a-3p/AKT1/mTOR feedback promotes the proliferation and
glycolysis of gastric cancer cells. Cancer Manage Res.
11:10145–10156. 2019. View Article : Google Scholar
|
|
134
|
Wang Q, Liu MJ, Bu J, Deng JL, Jiang BY,
Jiang LD and He XJ: miR-485-3p regulated by MALAT1 inhibits
osteosarcoma glycolysis and metastasis by directly suppressing
c-MET and AKT3/mTOR signalling. Life Sci. 268:1189252021.
View Article : Google Scholar
|
|
135
|
Carnero A, Blanco-Aparicio C, Renner O,
Link W and Leal JF: The PTEN/PI3K/AKT signalling pathway in cancer,
therapeutic implications. Curr Cancer Drug Targets. 8:187–198.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Zhai Z, Mu T, Zhao L, Li Y, Zhu D and Pan
Y: MiR-181a-5p facilitates proliferation, invasion, and glycolysis
of breast cancer through NDRG2-mediated activation of PTEN/AKT
pathway. Bioengineered. 13:83–95. 2022. View Article : Google Scholar :
|
|
137
|
Liu W, Kang L, Han J, Wang Y, Shen C, Yan
Z, Tai Y and Zhao C: miR-342-3p suppresses hepatocellular carcinoma
proliferation through inhibition of IGF-1R-mediated Warburg effect.
Onco Targets Ther. 11:1643–1653. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Wang B, Sun F, Dong N, Sun Z, Diao Y,
Zheng C, Sun J, Yang Y and Jiang D: MicroRNA-7 directly targets
insulin-like growth factor 1 receptor to inhibit cellular growth
and glucose metabolism in gliomas. Diagn Pathol. 9:2112014.
View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Xu H, Sun X, Huang Y, Si Q and Li M: Long
non-coding RNA NEAT1 modifies cell proliferation, colony formation,
apoptosis, migration and invasion via the miR-4500/BZW1 axis in
ovarian cancer. Mol Med Rep. 22:3347–3357. 2020.PubMed/NCBI
|
|
140
|
Xu M, Zhou K, Wu Y, Wang L and Lu S:
Linc00161 regulated the drug resistance of ovarian cancer by
sponging microRNA-128 and modulating MAPK1. Mol Carcinog.
58:577–587. 2019. View Article : Google Scholar
|
|
141
|
Zhao J, Li D and Fang L: MiR-128-3p
suppresses breast cancer cellular progression via targeting LIMK1.
Biomed Pharmacother. 115:1089472019. View Article : Google Scholar : PubMed/NCBI
|
|
142
|
Liu Y, Fu X, Wang X, Liu Y and Song X:
Long non-coding RNA OIP5-AS1 facilitates the progression of ovarian
cancer via the miR-128-3p/CCNG1 axis. Mol Med Rep. 23:3882021.
View Article : Google Scholar :
|
|
143
|
Liu Y, He X, Chen Y and Cao D: Long
non-coding RNA LINC00504 regulates the Warburg effect in ovarian
cancer through inhibition of miR-1244. Mol Cell Biochem. 464:39–50.
2020. View Article : Google Scholar
|
|
144
|
Goto H, Nishio M, To Y, Oishi T, Miyachi
Y, Maehama T, Nishina H, Akiyama H, Mak TW, Makii Y, et al: Loss of
Mob1a/b in mice results in chondrodysplasia due to YAP1/
TAZ-TEAD-dependent repression of SOX9. Development.
145:dev1592442018. View Article : Google Scholar
|
|
145
|
Wu DW, Wang YC, Wang L, Chen CY and Lee H:
A low microRNA-630 expression confers resistance to tyrosine kinase
inhibitors in EGFR-mutated lung adenocarcinomas via
miR-630/YAP1/ERK feedback loop. Theranostics. 8:1256–1269. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Lin X, Feng D, Li P and Lv Y: LncRNA
LINC00857 regulates the progression and glycolysis in ovarian
cancer by modulating the Hippo signaling pathway. Cancer Med.
9:8122–8132. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
147
|
Teng Y, Zhang Y, Qu K, Yang X, Fu J, Chen
W and Li X: MicroRNA-29B (mir-29b) regulates the Warburg effect in
ovarian cancer by targeting AKT2 and AKT3. Oncotarget.
6:40799–40814. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
148
|
Young CD, Nolte EC, Lewis A, Serkova NJ
and Anderson SM: Activated Akt1 accelerates MMTV-c-ErbB2 mammary
tumourigenesis in mice without activation of ErbB3. Br Cancer Res.
10:R702008. View Article : Google Scholar
|
|
149
|
Kotowski K, Rosik J, Machaj F, Supplitt S,
Wiczew D, Jabłońska K, Wiechec E, Ghavami S and Dzięgiel P: Role of
PFKFB3 and PFKFB4 in cancer: Genetic basis, impact on disease
development/progression, and potential as therapeutic targets.
Cancers. 13:9092021. View Article : Google Scholar : PubMed/NCBI
|
|
150
|
Mondal S, Roy D, Sarkar Bhattacharya S,
Jin L, Jung D, Zhang S, Kalogera E, Staub J, Wang Y, Xuyang W, et
al: Therapeutic targeting of PFKFB3 with a novel glycolytic
inhibitor PFK158 promotes lipophagy and chemosensitivity in
gynecologic cancers. Int J Cancer. 144:178–189. 2019. View Article : Google Scholar
|
|
151
|
Chakraborty PK, Mustafi SB, Xiong X,
Dwivedi SKD, Nesin V, Saha S, Zhang M, Dhanasekaran D, Jayaraman M,
Mannel R, et al: MICU1 drives glycolysis and chemoresistance in
ovarian cancer. Nat Commun. 8:146342017. View Article : Google Scholar : PubMed/NCBI
|
|
152
|
Singh R, Yadav V, Kumar S and Saini N:
MicroRNA-195 inhibits proliferation, invasion and metastasis in
breast cancer cells by targeting FASN, HMGCR, ACACA and CYP27B1.
Sci Rep. 5:174542015. View Article : Google Scholar : PubMed/NCBI
|
|
153
|
Yang Y, Li M, Chang S, Wang L, Song T, Gao
L, Hu L, Li Z, Liu L, Yao J and Huang C: MicroRNA-195 acts as a
tumor suppressor by directly targeting Wnt3a in HepG2
hepatocellular carcinoma cells. Mol Med Rep. 10:2643–2648. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
154
|
Rao G, Dwivedi SKD, Zhang Y, Dey A,
Shameer K, Karthik R, Srikantan S, Hossen MN, Wren JD, Madesh M, et
al: MicroRNA-195 controls MICU1 expression and tumor growth in
ovarian cancer. EMBO Rep. 21:e484832020. View Article : Google Scholar : PubMed/NCBI
|
|
155
|
Cao HL, Liu ZJ, Huang PL, Yue YL and Xi
JN: lncRNA-RMRP promotes proliferation, migration and invasion of
bladder cancer via miR-206. Eur Rev Med Pharmacol Sci.
23:1012–1021. 2019.PubMed/NCBI
|
|
156
|
Hongfeng Z, Andong J, Liwen S, Mingping B,
Xiaowei Y, Mingyong L and Aimin Y: lncRNA RMRP knockdown suppress
hepatocellular carcinoma biological activities via regulation
miRNA-206/TACR1. J Cell Biochem. 121:1690–1702. 2020. View Article : Google Scholar
|
|
157
|
Li L, Zeng S, Guo L, Huang P, Xi J, Feng
J, Li Q, Li Y, Xiao X, Yan R and Zhang J: Long noncoding RNA RMRP
contributes to paclitaxel sensitivity of ovarian cancer by
regulating miR-580-3p/MICU1 signaling. J Oncol.
2022:83019412022.PubMed/NCBI
|
|
158
|
Han RL, Wang FP, Zhang PA, Zhou XY and Li
Y: miR-383 inhibits ovarian cancer cell proliferation, invasion and
aerobic glycolysis by targeting LDHA. Neoplasma. 64:244–252. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
159
|
Rafat M, Moraghebi M, Afsa M and
Malekzadeh K: The outstanding role of miR-132-3p in carcinogenesis
of solid tumors. Hum Cell. 34:1051–1065. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
160
|
Cooper SJ, von Roemeling CA, Kang KH,
Marlow LA, Grebe SK, Menefee ME, Tun HW, Colon-Otero G, Perez EA
and Copland JA: Reexpression of tumor suppressor, sFRP1, leads to
antitumor synergy of combined HDAC and methyltransferase inhibitors
in chemoresistant cancers. Mol Cancer Ther. 11:2105–2115. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
161
|
Hu J, Zhao W, Huang Y, Wang Z, Jiang T and
Wang L: MiR-1180 from bone marrow MSCs promotes cell proliferation
and glycolysis in ovarian cancer cells via SFRP1/Wnt pathway.
Cancer Cell Int. 19:662019. View Article : Google Scholar : PubMed/NCBI
|
|
162
|
Gu ZW, He YF, Wang WJ, Tian Q and Di W:
MiR-1180 from bone marrow-derived mesenchymal stem cells induces
glycolysis and chemoresistance in ovarian cancer cells by
upregulating the Wnt signaling pathway. J Zhejiang Univ Sci B.
20:219–237. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
163
|
Xiaohong Z, Lichun F, Na X, Kejian Z,
Xiaolan X and Shaosheng W: MiR-203 promotes the growth and
migration of ovarian cancer cells by enhancing glycolytic pathway.
Tumour Biol. 37:14989–14997. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
164
|
Shao X, Zheng X, Ma D, Liu Y and Liu G:
Inhibition of lncRNA-NEAT1 sensitizes 5-Fu resistant cervical
cancer cells through de-repressing the microRNA-34a/LDHA axis.
Biosci Rep. 41:BSR202005332021. View Article : Google Scholar : PubMed/NCBI
|
|
165
|
Li L, Ma Y, Maerkeya K, Reyanguly D and
Han L: LncRNA OIP5-AS1 regulates the warburg effect through
miR-124-5p/IDH2/HIF-1α pathway in cervical cancer. Front Cell Dev
Biol. 9:6550182021. View Article : Google Scholar
|
|
166
|
Luo W, Zhang D, Ma S, Wang C, Zhang Q,
Wang H, He K and Liu Z: miR-27a is highly expressed in H1650 cancer
stem cells and regulates proliferation, migration, and invasion. J
Cancer Res Ther. 14(Suppl): S1004–S1011. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
167
|
Jiang G, Shi W, Fang H and Zhang X:
miR-27a promotes human breast cancer cell migration by inducing EMT
in a FBXW7-dependent manner. Mol Med Rep. 18:5417–5426.
2018.PubMed/NCBI
|
|
168
|
Li W, Yu ZX and Ma BF: The increase of
miR-27a affects the role of cisplatin on proliferation and
migration capacities of liver cancer cells. Eur Rev Med Pharmacol
Sci. 22:5490–5498. 2018.PubMed/NCBI
|
|
169
|
Li P, Zhang Q and Tang H: INPP1
up-regulation by miR-27a contributes to the growth, migration and
invasion of human cervical cancer. J Cell Mol Med. 23:7709–7716.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
170
|
Zhang S, Chen P, Huang Z, Hu X, Chen M, Hu
S, Hu Y and Cai T: Sirt7 promotes gastric cancer growth and
inhibits apoptosis by epigenetically inhibiting miR-34a. Sci Rep.
5:97872015. View Article : Google Scholar : PubMed/NCBI
|
|
171
|
Gougelet A, Sartor C, Bachelot L, Godard
C, Marchiol C, Renault G, Tores F, Nitschke P, Cavard C, Terris B,
et al: Antitumour activity of an inhibitor of miR-34a in liver
cancer with β-catenin-mutations. Gut. 65:1024–1034. 2016.
View Article : Google Scholar
|
|
172
|
Hermeking H: The miR-34 family in cancer
and apoptosis. Cell Death Differ. 17:193–199. 2010. View Article : Google Scholar
|
|
173
|
Zhang R, Su J, Xue SL, Yang H, Ju LL, Ji
Y, Wu KH, Zhang YW, Zhang YX, Hu JF and Yu MM: HPV E6/p53 mediated
down-regulation of miR-34a inhibits Warburg effect through
targeting LDHA in cervical cancer. Am J Cancer Res. 6:312–320.
2016.PubMed/NCBI
|
|
174
|
Yang H, Wu XL, Wu KH, Zhang R, Ju LL, Ji
Y, Zhang YW, Xue SL, Zhang YX, Yang YF and Yu MM: MicroRNA-497
regulates cisplatin chemosensitivity of cervical cancer by
targeting transketolase. Am J Cancer Res. 6:2690–2699.
2016.PubMed/NCBI
|
|
175
|
Yang X and Wu X: miRNA expression profile
of vulvar squamous cell carcinoma and identification of the
oncogenic role of miR-590-5p. Oncol Rep. 35:398–408. 2016.
View Article : Google Scholar
|
|
176
|
de Melo Maia B, Lavorato-Rocha AM,
Rodrigues LS, Coutinho-Camillo CM, Baiocchi G, Stiepcich MM, Puga
R, de A Lima L, Soares FA and Rocha RM: microRNA portraits in human
vulvar carcinoma. Cancer Prev Res (Phila). 6:1231–1241. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
177
|
Gonzalez Dos Anjos L, de Almeida BC, Gomes
de Almeida T, Mourão Lavorato Rocha A, De Nardo Maffazioli G,
Soares FA, Werneck da Cunha I, Baracat EC and Carvalho KC: Could
miRNA signatures be useful for predicting uterine sarcoma and
carcinosarcoma prognosis and treatment? Cancers (Basel).
10:3152018. View Article : Google Scholar : PubMed/NCBI
|
|
178
|
Tyagi K, Mandal S and Roy A: Recent
advancements in therapeutic targeting of the Warburg effect in
refractory ovarian cancer: A promise towards disease remission.
Biochim Biophys Acta Rev Cancer. 1876:1885632021. View Article : Google Scholar : PubMed/NCBI
|
|
179
|
Hietanen S, Lain S, Krausz E, Blattner C
and Lane DP: Activation of p53 in cervical carcinoma cells by small
molecules. Proc Natl Acad Sci USA. 97:8501–8506. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
180
|
Guan XY, Guan XL and Jiao ZY: Improving
therapeutic resistance: Beginning with targeting the tumor
microenvironment. J Chemother. 34:492–516. 2022. View Article : Google Scholar
|
|
181
|
Arvizo RR, Moyano DF, Saha S, Thompson MA,
Bhattacharya R, Rotello VM, Prakash YS and Mukherjee P: Probing
novel roles of the mitochondrial uniporter in ovarian cancer cells
using nanoparticles. J Biol Chem. 288:17610–17618. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
182
|
Khan S, Chib R, Shah BA, Wani ZA, Dhar N,
Mondhe DM, Lattoo S, Jain SK, Taneja SC and Singh J: A cyano
analogue of boswellic acid induces crosstalk between p53/PUMA/Bax
and telomerase that stages the human papillomavirus type 18
positive HeLa cells to apoptotic death. Eur J Pharmacol.
660:241–248. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
183
|
Yuan Y, Li H, Pu W, Chen L, Guo D, Jiang
H, He B, Qin S, Wang K, Li N, et al: Cancer metabolism and tumor
microenvironment: Fostering each other? Sci China Life Sci.
65:236–279. 2022. View Article : Google Scholar
|
|
184
|
Hashemian SM, Pourhanifeh MH, Fadaei S,
Velayati AA, Mirzaei H and Hamblin MR: Non-coding RNAs and
exosomes: Their role in the pathogenesis of sepsis. Mol Ther
Nucleic Acids. 21:51–74. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
185
|
Razavi ZS, Tajiknia V, Majidi S, Ghandali
M, Mirzaei HR, Rahimian N, Hamblin MR and Mirzaei H: Gynecologic
cancers and non-coding RNAs: Epigenetic regulators with emerging
roles. Crit Rev Oncol Hematol. 157:1031922021. View Article : Google Scholar
|
|
186
|
Bonneau E, Neveu B, Kostantin E, Tsongalis
GJ and De Guire V: How close are miRNAs from clinical practice? A
perspective on the diagnostic and therapeutic market. EJIFCC.
30:114–127. 2019.PubMed/NCBI
|
|
187
|
Fatmi A, Chabni N, Cernada M, Vento M,
González-López M, Aribi M, Pallardó FV and García-Giménez JL:
Clinical and immunological aspects of microRNAs in neonatal sepsis.
Biomed Pharmacother. 145:1124442022. View Article : Google Scholar
|
|
188
|
Gumusoglu-Acar E, Gunel T, Hosseini MK,
Dogan B, Tekarslan EE, Gurdamar B, Cevik N, Sezerman U, Topuz S and
Aydinli K: Metabolic pathways of potential miRNA biomarkers derived
from liquid biopsy in epithelial ovarian cancer. Oncol Lett.
25:1422023. View Article : Google Scholar : PubMed/NCBI
|