Cancer is a major public health concern worldwide.
According to the American Cancer Society, it was estimated that 1.9
million new cases and 610,000 cancer-related deaths occurred in the
United States in 2022, posing a serious threat to human health
(1). Conventional cancer
treatments include surgery, radiotherapy, chemotherapy and targeted
therapy; however, certain types of advanced or metastatic cancer
are difficult to cure using traditional treatments, and novel tools
and approaches are required. Research has shown that the immune
system serves a pivotal role in maintaining the stability of the
internal environment (2); however,
cancer cells often escape surveillance and destruct the host immune
system (3). Therefore, research
has focused on the development of immunotherapy that inhibits
tumor-induced immune tolerance and restores the immune response
against tumor cells. Unlike conventional cancer treatments which
mainly act on cancer cells, immunotherapy indirectly promotes
cancer control through activating the antitumor immune responses of
the patient (4,5). The concept of antitumor immunotherapy
has been around for over a century, starting with Coley's toxins
and Erlich's hypothesis that the immune system suppresses cancer
development (4), but it has
developed rapidly in the past two decades and is currently a major
focus in cancer therapy.
Among the different types of cancer immunotherapy,
immune checkpoint inhibitors (ICIs) demonstrate a broad impact.
ICIs are monoclonal antibodies (mAbs) that specifically target the
inhibitory receptors on T cells, known as immune checkpoint
molecules. These act as negative co-regulators that inhibit further
T-cell activation and are essential for the maintenance of
self-tolerance (4). Tumor cells
often escape host immunity through immune checkpoint dysregulation,
and ICIs are immunomodulators that reinforce antitumor immune
responses (6-10). Moreover, ICIs have demonstrated
notable outcomes in multiple tumor types (11), either as a single treatment or
combined with conventional treatments. ICIs may be used in both
advanced and metastatic cancer, either as adjuvant or neoadjuvant
treatment in the early stage of cancer (12). Notably, ICIs are considered
revolutionary in cancer treatment, highlighted by the 2018 Nobel
Prize in Physiology or Medicine awarded jointly to James Allison
and Tasuku Honjo, for their discovery of cancer treatment via
suppression of negative immune regulation (12). The present review aimed to
summarize the current knowledge and novel advances regarding the
mechanisms and applications of various ICIs. This study may provide
a theoretical basis for further associated research and the
application of immunotherapy.
During the tumor immune response process, T
lymphocytes act as the final effector cell, and the activation of T
cells requires two signals. The first signal originates from the
specific recognition of antigen-major histocompatibility complex
(MHC) complexes by T-cell receptors (TCRs). The second signal
originates from the interaction between co-stimulatory molecules on
the surface of T cells with the corresponding ligands.
Co-stimulatory signals are required to enhance the antigen receptor
signals that induce transcription factor activation and PI3K
activation, thereby ensuring full activation of T cells (13). Compared with these activating
co-stimulatory molecules, certain inhibitory molecules exist on the
surface of T cells that downregulate activation signals. These
inhibitory receptors function to prevent T-cell proliferation and
cytokine production, in order to prevent excessive immune responses
that can lead to destructive inflammatory or autoimmune conditions
(14,15). Moreover, the strict regulation
between co-stimulatory and inhibitory molecules serves a critical
role in immune homeostasis. Co-stimulatory molecules include CD28,
4-1BB and inducible co-stimulator (CD278), and inhibitory molecules
include cytotoxic T lymphocyte associated antigen-4 (CTLA-4) and
programmed death-1 (PD-1), which are both categorized as immune
checkpoint molecules (15).
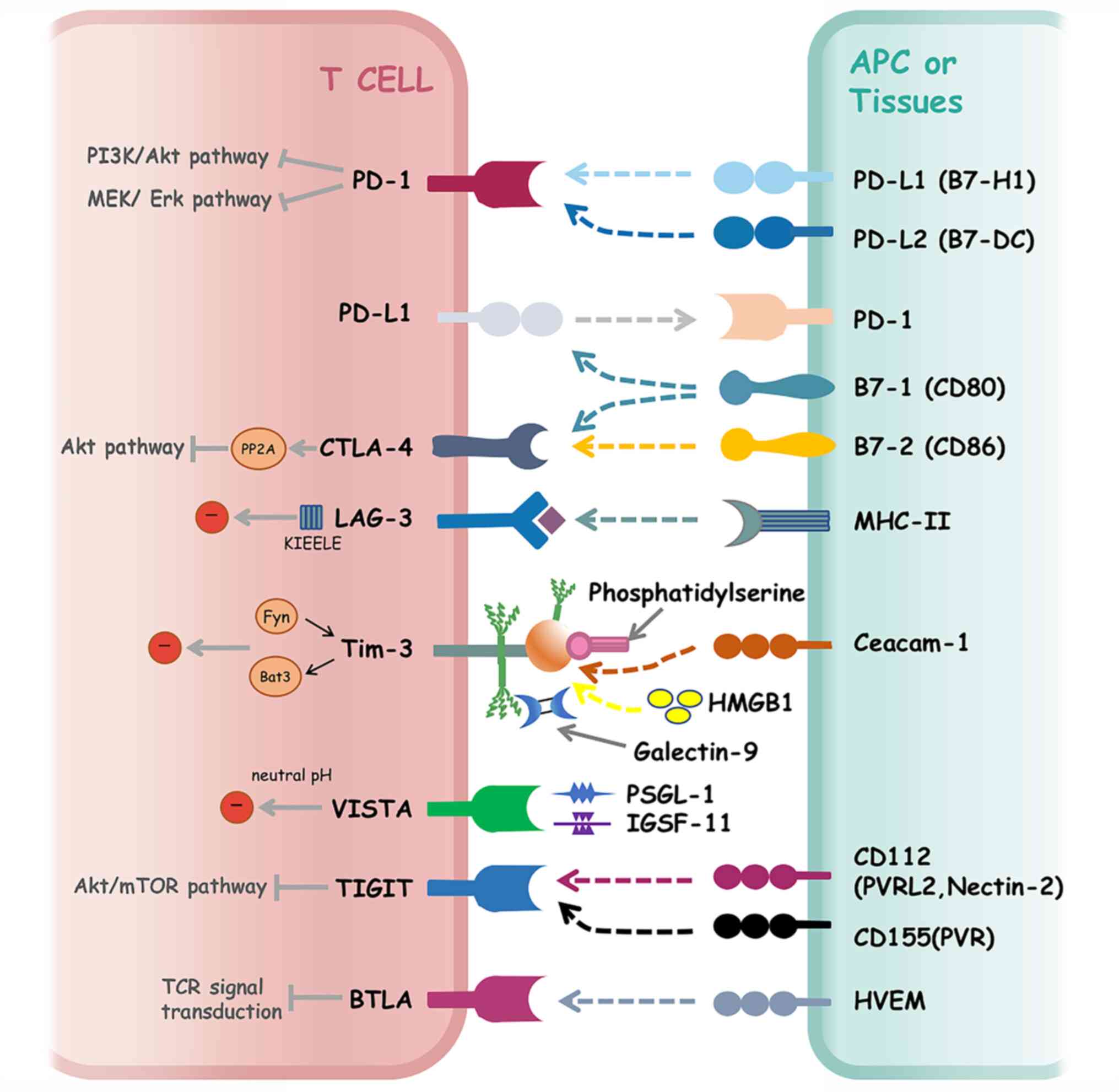
Notably, immune checkpoint molecules refer to 'brake' proteins that
inhibit the activation of immune cells (Fig. 1). Tumor cells often inhibit the
effects of T cells through the immune checkpoint pathway, leading
to immune escape.
CTLA-4 not only prevents the activation of
self-reactive T cells, but also other T cells, by binding to the
ligand for CD28. In addition, CTLA-4 exerts inhibitory effects that
are mediated through regulatory T cells (Tregs). Tregs express high
levels of CTLA-4 on the cell surface, and specific loss of CTLA-4
leads to an increased susceptibility to autoimmune diseases. This
indicates that Treg-derived CTLA-4 is required to maintain
immunologic tolerance (24,25).
Moreover, Tregs directly remove the co-stimulatory ligands, B7-1
and B7-2, from the surface of APCs via trans-endocytosis. CTLA-4
promotes T-cell motility through antagonizing the formation of
microclusters, thus reducing the T cell/APC dwell times (26). In addition, CTLA-4 negatively
regulates the immune response through inhibiting the maturation and
antigen presentation of APCs (27), and inducing the production of
indolamine-2,3-dioxygenase (IDO) by APCs (28). As CTLA-4 was the first immune
checkpoint molecule to be discovered, the associated regulatory
mechanisms have been extensively studied. However, further
investigations into the function of downstream signal components
are crucial, and future studies should focus on evaluating
CTLA-4-mediated regulation of T-cell activity.
Following the discovery of the role of CTLA-4 in
immune suppression, research has focused on restoring the antitumor
function of T cells through inhibiting the binding of CTLA-4 to B7.
Using animal models (29),
anti-CTLA-4 immunoglobulin (Ig)G1 mAb was developed in 1999, and
later named ipilimumab. In 2010, ipilimumab was successfully used
in the treatment of metastatic melanoma in a phase III clinical
trial. Results of this study demonstrated that the median overall
survival (OS) among patients receiving ipilimumab alone was 10.1
months (30). Subsequently, the
United States Food and Drug Administration (FDA) approved
ipilimumab as a first-line drug in the treatment of advanced
melanoma in 2011; this was the first approved immunotherapy drug
(Table I). After 4 years,
ipilimumab was approved as an adjuvant treatment for stage III
melanoma. In addition, the efficacy of ipilimumab has been
demonstrated in other solid tumors, including lung, renal,
pancreatic and prostate cancer (31-33).
However, the effects of ipilimumab in these solid tumors have been
reported to be limited, and this may be due to low tumor
immunogenicity and a potently immunosuppressive tumor
microenvironment (TME) (34). In
addition to ipilimumab, tremelimumab is currently undergoing
clinical trials in a variety of tumors as an additional CTLA-4
blockade (11,35). In 2015, tremelimumab was granted
orphan drug qualification by the FDA in the treatment of malignant
mesothelioma. In 2020, in combination with durvalumab, tremelimumab
was also used to treat hepatocellular carcinoma (HCC). As well as
regulating T-cell activation, CTLA-4 blockade may limit the
penetration of Tregs into the TME, to prevent Tregs from inhibiting
the activity of cytotoxic T cells and enhancing antitumor activity.
However, associated research is limited at present (36,37).
Unlike CTLA-4, PD-1 expression is transient in the
early stage of T-cell activation and then decreases when the
activating antigen is removed. However, PD-1 expression is high if
the antigen is present for a prolonged period of time; for example,
during chronic infection or in a tumor (47,48).
It has previously been demonstrated that PD-1 expression is
increased on the majority of tumor-infiltrating T lymphocytes
(TILs) in various tumor types, and this is an important cause of
tumor immune escape (49,50). The two ligands of PD-1 are
comparable with other B7 immune regulatory ligand family members,
and the affinity of PD-L2 to PD-1 has been reported to be higher
than that of PD-L1 (46,51). The expression levels of PD-Ls are
different in different human cells. Notably, PD-L1 is expressed on
hematopoietic cells, such as B cells, T cells, macrophages,
dendritic cells (DCs) and mesenchymal stem cells (MSCs) (52), and is also expressed on
nonhematopoietic cells, including vascular endothelial cells,
fibroblastic reticular cells, astrocytes, liver cells, pancreatic
cells and neurons (43). By
contrast, PD-L2 is mainly expressed on APCs, such as macrophages
and DCs, and peritoneal B1 cells (53). In addition, the main PD-1 ligand
expressed on solid tumor cells is PD-L1 (54,55).
Results of previous studies have suggested that PD-L1 is
upregulated in multiple cancer types, including melanoma, ovarian
cancer and lung cancer (56-58).
Activated T cells also produce cytokines that promote the
expression of PD-L1 on cancer cells (56). When PD-L1 on cancer cells binds to
PD-1 on T cells, immunosuppression occurs. In addition, PD-L1
specifically interacts with the B7-1 (CD80) co-stimulatory molecule
to inhibit T-cell responses (59).
Collectively, these mechanisms cause cancer cells to evade the
immune system.
A previous study found that the CTLA-4 inhibitor was
effective only for certain cases of advanced melanoma, and serious
side effects may occur in treating other types of tumors (34). Notably, ICIs were not widely
accepted until the use of PD-1/PD-L1 antibodies was established in
the clinic in 2014. Although PD-1/PD-L1 antibodies mainly function
through upregulating T-cell activation, they also block the
host-derived PD-L1 signals from non-tumor cells in the
microenvironment, as well as the interactions between PD-L1 and
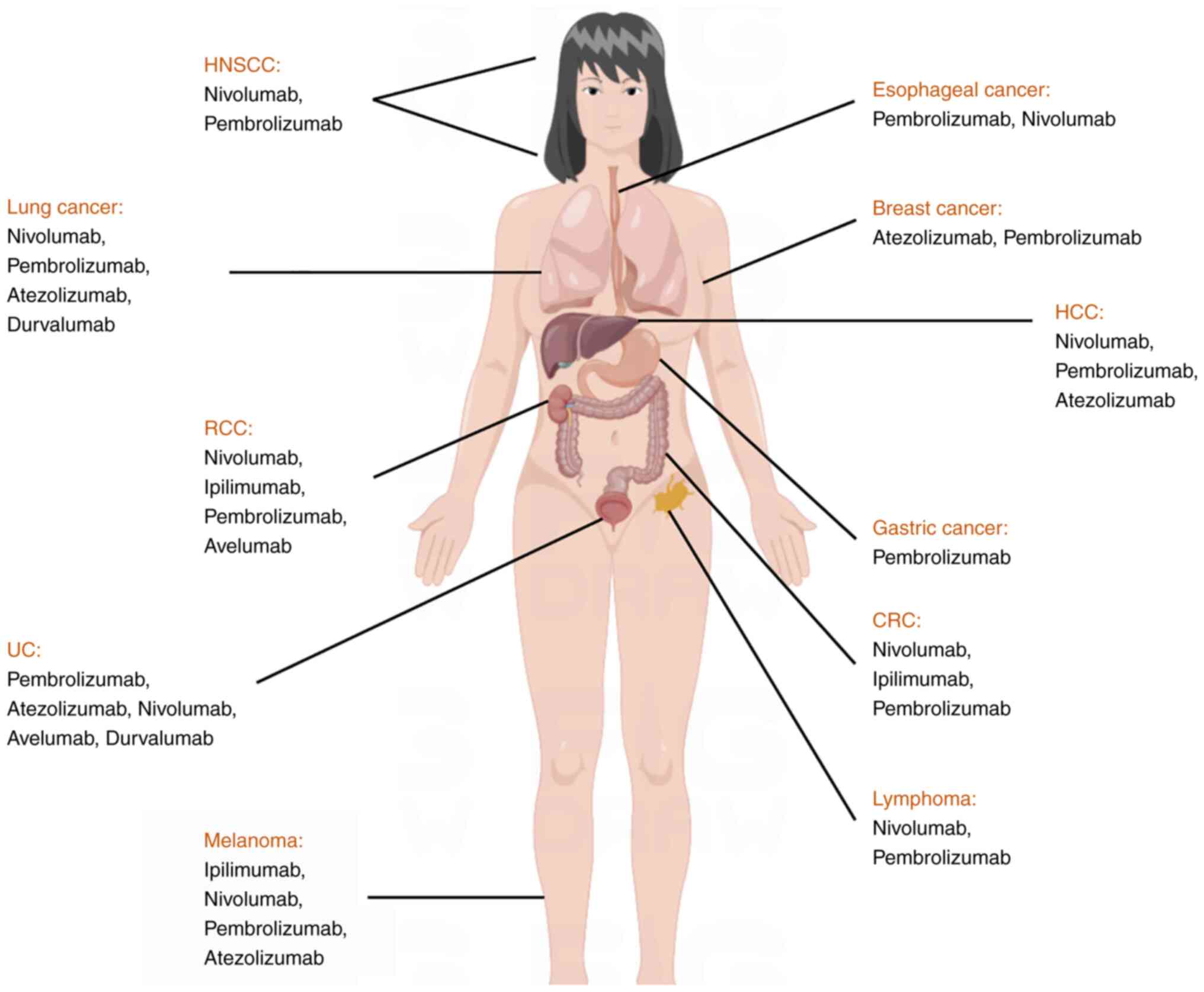
B7-1 (11). At present, a total of
six PD-1/PD-L1 inhibitors have been approved by the FDA, including
nivolumab, pembrolizumab, atezolizumab, avelumab, durvalumab and
cemiplima. The associated indications include melanoma, Hodgkin's
lymphoma, non-small cell lung cancer (NSCLC), renal cell cancer
(RCC), gastric cancer, liver cancer, colorectal cancer (CRC),
cutaneous squamous cell cancer and urothelial cancer (UC). These
indications are displayed in Table
I.
In 2006, the first clinical trial of a PD-1
inhibitor, nivolumab, was conducted in refractory solid tumors.
Notably, nivolumab was known as IgG4 anti-PD-1 mAb and Opdivo
(MDX-1106, ONO-4538, BMS-936558), and was developed by
Bristol-Myers Squibb. In December 2014, nivolumab was approved by
the FDA for the treatment of metastatic melanoma, with the results
of the CheckMate 037 clinical trial demonstrating an objective
response rate of 31.7% in the nivolumab group, and 10.6% in the
chemotherapy group (dacarbazine orcarboplatin) (54). At present, the indications have
extended to various advanced solid tumors, including NSCLC, RCC,
UC, squamous cell carcinoma of head and neck, Hodgkin's lymphoma,
HCC, CRC and esophageal squamous cell carcinoma (59-63).
In September 2014, an additional PD-1 inhibitor,
pembrolizumab (Keytruda, lambrolizumab, MK-3475), was approved by
the FDA for the treatment of unresectable or metastatic melanoma.
Pembrolizumab, a fully humanized IgG4 with high-affinity and
high-selectivity, demonstrated an objective response rate of
>30% in patients with advanced melanoma (NCT01866319) (63). At present, pembrolizumab is
approved for use in >14 indications of 10 tumor types (64-69).
In September 2018, another PD-1 inhibitor,
cemiplimab (Libtayo), was approved by the FDA for treatment of
metastatic or locally advanced non-resectable cutaneous squamous
cell carcinoma. Notably, this was the first drug approved by the
FDA that was specifically for the treatment of patients with
advanced cutaneous squamous cell carcinoma (70).
Atezolizumab (Tecentriq), a human IgG1 anti-PD-L1
mAb, was the first PD-L1 inhibitor approved by the FDA for the
treatment of advanced or metastatic UC. Results of a phase II
clinical trial demonstrated an objective response rate of 15% in
all patients and 26% in patients with the highest levels of PD-L1
expression following treatment with atezolizumab (71). The indications of atezolizumab have
subsequently extended to NSCLC, small cell lung cancer, breast
cancer, HCC and melanoma (71-75).
An additional PD-L1 inhibitor, avelumab (Bavencio),
not only functions via blocking PD-1/PD-L1 interactions, but also
through antibody-dependent cell-mediated cytotoxicity (76). In March 2017, avelumab was approved
by the FDA for the treatment of metastasis Merkel cell carcinoma in
adolescents and adults >12 years of age, and the indication was
subsequently expanded to UC and RCC.
In May 2017, the FDA-approved durvalumab (Imfinzi),
an additional PD-L1 antibody, for the treatment of advanced UC,
using accelerated approval. Results of a previous study
demonstrated that durvalumab substantially improved the
progression-free survival of patients with NSCLC, compared with the
placebo (16.8 months vs. 5.6 months) (77). Subsequently, the FDA expanded the
indications to include Stage III NSCLC in February 2018.
A novel PD-L1 inhibitor, envafolimab (KN035), was
awarded Orphan Drug Designation by the FDA for the treatment of
biliary tract cancer. Notably, the PD-1/PD-L1 inhibitors approved
by the FDA may continue to be approved for additional indications.
However, alternative anti-PD-1/PD-L1 axis-targeted therapies, such
as pidilizumab (CT-011) and BMS-936559 (MDX-1105) are under
investigation in clinical trials, and these treatment options may
exhibit potential in a broad range of tumor types (78,79).
PD-1/PD-L1 inhibitors are used in the treatment of
various types of cancer. However, results may differ between
patients, and the mechanistic basis for the variation in response
patterns are multifaceted. For example, a previous study reported
that patients with melanoma that respond to these inhibitors had a
higher proportion of BRCA2 mutations (80). In addition, innately resistant
tumors display a transcriptional signature, indicating concurrent
increased expression of genes involved in the regulation of
mesenchymal transition, cell adhesion, extracellular matrix
remodeling, angiogenesis and wound healing (80). Moreover, PD-1 inhibitor monotherapy
for patients with NSCLC accompanied by EGFR mutations exhibit low
response rates and unsatisfactory efficacy. PD-1 inhibitors may be
ineffective in microsatellite stable type carcinoma (81), and PD-1/PD-L1 inhibitors exert
minimal effects on certain types of tumors, such as multiple
myeloma and uveal melanoma (78).
However, further investigations into the specific molecular and
cellular mechanisms of PD-1/PD-L1 inhibitors in antitumor immune
enhancement are required.
Following the discovery of CTLA-4 and PD-1/PD-L1 in
antitumor treatment, further preclinical and clinical studies have
focused on additional immune checkpoint molecules.
Lymphocyte activation gene-3 (LAG-3), also known as
CD223, is a member of the Ig superfamily and a homologous protein
of CD4+, which can bind to MHC-II molecules with high
affinity. LAG-3 is expressed on active NK cells, T cells, B cells,
TILs, Tregs and DCs, and is required for immune homeostasis
(82-84). However, persistent antigen
stimulation in cancer may lead to chronic LAG-3 expression,
promoting T-cell exhaustion. Results of previous studies have
demonstrated that LAG-3 is highly expressed on the TILs of multiple
tumors (85-88). The signaling pathways downstream of
LAG-3 responsible for its inhibitory function are still unclear,
but the KIEELE motif of the LAG-3 cytoplasmic tail has been shown
to be essential for the inhibitory function (84). At present, research is focused on
numerous therapeutic agents targeting LAG-3, for the treatment of
multiple types of human cancer (89,90).
T-cell Ig mucin (Tim)-3, a member of the Tim family,
is a type I transmembrane glycoprotein, which was initially
recognized in CD4+ T helper and CD8+ T
cytotoxic cells, and was subsequently shown to be expressed in
Tregs, NK cells, monocytes and DCs (84,91).
Several ligands of Tim-3 have been recognized, including galectin-9
(Gal-9), phosphatidyl serine (PS), high-mobility group box 1
(HMGB1) and carcinoembryonic antigen cell adhesion molecule 1
(Ceacam-1) (92). Notably, the
binding of Tim-3 and Gal-9 induces T-cell death and reduces the
immune response (92). The binding
of Tim-3 and PS mediates the phagocytosis of apoptotic cells and
cross-presentation (93). The
binding of Tim-3 and HMGB1 inhibits antitumor immune responses
mediated by HMGB1 (94). Ceacam-1
was identified as a novel ligand for Tim-3. In the absence of
Ceacam-1, the negative regulatory function of Tim-3 is defective,
indicating that the interaction between Ceacam-1 and Tim-3 is
required for optimal Tim-3 function (95). Bat-3 and Fyn bind to the same
region on the cytoplasmic tail of Tim-3, the binding of Gal-9 or
Ceacam-1 to Tim-3 can trigger the dissociation of Bat-3 from the
cytoplasmic tail of Tim-3, thus allowing Fyn to bind, which is
implicated in the induction of T-cell anergy (84). Tim-3 expression is indicative of
dysfunctional or exhausted T cells in cancer, and is elevated in
various tumors (96-99). In addition, the co-blockade of
Tim-3/PD-1 demonstrated increased efficacy in treating tumors,
compared with blocking Tim-3 or PD-1 alone (100).
T-cell Ig and ITIM domain (TIGIT) is a member of the
Ig superfamily, and is expressed on NK cells, activated T cells,
memory T cells, follicular T helper cells and Tregs (101-104). The ligands of TIGIT, including
CD155 (PVR) and CD112 (PVRL2, nectin-2), are expressed on APCs, T
cells, nonhematopoietic cells and tumor cells (101,105). CD155/TIGIT can suppress the
function of NK cells through inhibiting PI3K/MAPK and NF-κB
signaling, and can suppress the function of T cells through
inhibiting AKT/mTOR signaling. In addition, TIGIT can reduce the
activity of the co-stimulatory molecule CD226 during the antitumor
T-cell response (84). At present,
three phase I/II clinical studies targeting TIGIT for cancer
immunotherapy are ongoing (NCT05130177, NCT04354246 and
NCT04995523; 106-108).
B- and T-lymphocyte attenuator (BTLA), also known as
CD272, belongs to the CD28 Ig superfamily. The protein structure of
BTLA is comparable with that of PD-1 and CTLA-4. BTLA is expressed
on B cells, T cells, NK cells, DCs and macrophages (109,110). The ligand of BTLA is herpes virus
entry mediator (HVEM), which belongs to the tumor necrosis factor
receptor family. HVEM is widely expressed in B cells, T cells, NK
cells, monocytes and DCs (109).
Engagement of BTLA leads to the recruitment of SHP-1 and SHP-2 in T
cells, thereby downregulating TCR signaling and the transmission of
inhibitory signals (111).
Results of previous studies have demonstrated that BTLA is highly
expressed in melanoma, lung cancer, RCC, lymphoma, B-cell small
lymphocytic lymphoma and chronic lymphocytic leukemia (109-112). At present, preclinical studies of
BTLA or HVEM inhibitors are ongoing, and subsequent clinical
studies will be developed (109-111).
V-domain Ig suppressor of T-cell activation (VISTA),
also known as C10 or f54, is a member of the CD28 family. VISTA is
a novel immune checkpoint expressed on myeloid cells and lymphoid
cells, which is upregulated in various tumors (113). VISTA has two proven ligands,
P-selectin glycoprotein ligand-1 (PSGL-1) and Ig superfamily member
11 (IGSF-11); PSGL-1 only functions at neutral pH and the affinity
declines fourfold at pH 6.0 (113). VISTA can serve as both a ligand
and receptor to suppress T cell-associated immune responses;
however, the mechanism remains to be elucidated (113). Several clinical trials of VISTA
inhibitors are ongoing for the treatment of multiple types of
cancer (NCT02812875, NCT02671955 and NCT04475523; 114-116).
Although ICIs have demonstrated high levels of
success in improving therapeutic efficacy in some patients,
previous studies have demonstrated that only ≤20-30% of patients
with NSCLC, melanoma or RCC benefit from ICIs (30,117-120). Non-responders include patients
who do not respond to treatment at all and patients who relapse
after remission to ICIs (121).
These non-responders endure high treatment costs and associated
levels of toxicity with little benefit from the treatment.
Moreover, inappropriate application may cause disease progression
(122). Therefore, the
development of predictive biomarkers is required for prescribing
ICIs in a personalized manner.
Results of a previous study suggested that PD-L1
expression in tumor cells and the tumor environment is positively
associated with the response to PD-1/PD-L1-blocking antibodies
(123). Immunohistochemistry
(IHC) analyses performed on patients with metastatic melanoma,
colon cancer, NSCLC, prostate cancer and RCC who received
PD-1/PD-L1 targeted therapy demonstrated that PD-L1 upregulation
acted as a potential biomarker (63,119,124-126). Different PD-L1 expression cutoffs
and scoring systems have been used in different trials of
FDA-approved drugs directed by PD-1/PD-L1 (123). However, PD-L1 may not be optimal
as a potential biomarker, as the overall response rate of
PD-1/PD-L1-blocking antibodies in patients with negative PD-L1
expression can also reach 0-20% (127,128). There are some limitations that
must be considered when selecting PD-L1 as an immunotherapy
biomarker. Notably, the expression of PD-L1 is induced and dynamic;
thus, different treatment methods may impact the expression of
PD-L1 in different stages of treatment (129,130). Besides, the expression between
primary and metastatic tumors may be heterogeneous. As a result,
the expression of PD-L1 at a certain time or location cannot
accurately reflect the expression of PD-L1 in tumors (131,132). In addition, different
commercially available PD-L1 IHC tests were used in different
trials, and different cutoff scores were set to detect or quantify
tumor PD-L1 expression, resulting in clinical failure to select
patients according to a unified standard (133).
Following the development of gene sequencing and
bioinformatics, genomics technology is more frequently used for
discovering biomarkers associated with ICIs. Previous clinical
studies revealed that mismatch repair deficiency (dMMR) or MSI-high
(MSI-H) are associated with response to ICIs (81,134). MMR is an important DNA repair
mechanism that makes alterations in DNA mismatches, and dMMR may
lead to MSI, which can be used for the clinical detection of dMMR
(135). dMMR or MSI-H are often
present in various types of cancer (136,137). The results of previous studies
suggested that dMMR tumors exhibit high neoantigen load, tumor
mutational burden (TMB), T-cell infiltration and upregulation of
multiple immune checkpoints, including PD-1, PD-L1, CTLA-4 and
LAG-3 (138-141), which may lead to high response
rates to ICIs. Notably, MSI has been recognized by the FDA as a
predictive biomarker for ICI responsiveness (142). Moreover, pembrolizumab was
specifically approved in the treatment of multiple solid tumors
with MSI-H or dMMR. This was the first FDA-approved tumor
immunotherapy that was not based on tumor tissue type and instead
based solely on genetic characteristics. Therefore, further
investigations should focus on identifying MMR and MMR-like tumors.
Further analysis of specific DNA repair gene sets, or
bioinformatics analysis of specific DNA damage characteristics
associated with specific DNA repair defects in the cancer genome,
are required to assess the potential sensitivity to ICIs (143).
TMB refers to the total number of base substitution,
insertion or deletion mutations in the coding region of exons of
evaluated genes in tumor tissue samples. Notably, a higher TMB may
affect the probability of immunogenic peptide generation; thus,
affecting the response of patients to ICIs (144,145). As a result, the association
between TMB and the efficacy of ICIs has been the focus of multiple
studies. Notably, TMB may be associated with clinical benefits of
ICIs in patients with melanoma, NSCLC, UC, squamous cell carcinoma
of head and neck, and small cell lung cancer, while those with
lower TMB, such as pancreatic cancer and prostate cancer, may
exhibit poor responses to ICIs (71,146-152). In 2020, TMB became the second
FDA-approved predictive biomarker for the efficacy of ICIs.
However, there are notable limitations. Firstly, different testing
platforms are used in the clinical studies of TMB at present, and
there is no standard definition of high TMB. Thus, the mutation
load of different tumors is varied and different cutoffs must be
established (146,147). Moreover, TMB alone may not
distinguish all responders from non-responders. For example, the
immunogenicity of tumor neoantigens may be improved by epigenetic
modification in tumors with low TMB, leading to improved
therapeutic responses to ICIs (62,153). However, for those tumors with
high TMB, there may be other immunosuppressive molecules in the
tumor immune microenvironment, such as IL-10 and metabolism-related
enzyme IDO, which may affect the efficacy of ICIs (9,154).
Consequently, TMB alone may be inadequate in predicting the
efficacy of ICIs. Moreover, further investigations into cost
control, application of dynamic biomarkers and blood-based TMB
detection are required.
TILs are the effector cells of antitumor immunity
and the target cells of ICIs. TILs act as a representative of
tumor-immune system interaction; therefore, assessing the presence
of TILs may aid in identifying patients who benefit from
immunotherapy. In a study of pembrolizumab in the treatment of
advanced melanoma, CD8+ T-cell infiltration in the tumor
tissue or invasion margin was revealed to be higher in responders
than in patients who did not respond (155). Results of previous studies have
also demonstrated that TILs may be used to predict the
immunotherapeutic response and prognosis of numerous types of
cancer, including breast cancer and CRC (156-160). Adding immunoscore based on TILs
to the existing tumor, lymph node and metastasis classification
system may improve the development of effective treatment plans and
allow clinicians to provide more accurate prognoses (161). However, the widespread
application of TILs as a predictive tool for immunotherapy response
requires further validation and standardization.
Specific gene mutations may exert effects on tumor
cells that impact immune surveillance. Notably, several single gene
biomarkers may impact treatment decisions with ICIs. In patients
with melanoma, several gene mutations, including BRAF, JAK1/2,
β2-microglobulin (β2M) and mutations in the interferon γ (IFN-γ)
pathway, are associated with the efficacy of immunotherapies
(162-167). SERPINB3 and SERPINB4 mutations
have also been shown to be associated with the response to
anti-CTLA4 immunotherapy in patients with melanoma, independent of
tumor stage, TMB and patient age (168). In addition, inactivation of PTEN
may be associated with resistance to ICIs in melanoma and uterine
leiomyosarcoma (169,170). Patients with NSCLC and STK11/LKB1
or EGFR mutations, or ALK rearrangements, exhibited decreased
efficacy and low response rates to ICIs. By contrast, KRAS/TP53
mutations were associated with improved clinical outcomes (171-173). In patients with RCC, PBRM1
mutations may be associated with clinical benefits of ICIs
(174-178). Moreover, high-throughput
clustered regularly interspaced short palindromic repeats screening
has identified numerous genes associated with improved clinical
benefits of ICIs, such as PTPN2, APLNR and SWI/SNF complex genes
(179-181).
A collection of peptides presented to the cell
surface by class I and class II human leukocyte antigen (HLA)
molecules are referred to as the immunopeptidome. Cancer cells may
have defective HLA-I functions, leading to abnormal tumor antigen
presentation and the destruction of antigen-MHC binding; thus,
evading immune surveillance and impacting the efficacy of
immunotherapy (182,183). Results of a previous study
demonstrated that loss of HLA expression impacted the response to
ICI therapy (184). Moreover, a
further study analyzed the HLA-I genotype of 1,535 patients with
advanced tumors treated with ICIs. The results demonstrated that in
patients with maximal heterozygosity at the HLA-I loci ('A', 'B',
and 'C'), OS was improved following treatment with ICIs, compared
with that of patients who were homozygous for at least one HLA
locus. This may improve the ability of providing a wider range of
tumor antigens to T cells (185).
Therefore, these studies indicated that the recognition of
neoantigens by peripheral T lymphocytes is the main mechanism of
antitumor immune response. The widespread application of these
technologies still requires further validation.
Considering the advantages of non-invasive surgery,
peripheral blood detection technology has remained the focus of
research surrounding hematological markers associated with the
efficacy of ICIs. In patients with metastatic melanoma treated with
ipilimumab, previous studies demonstrated that survival was
significantly associated with low serum lactate dehydrogenase,
absolute monocyte counts, myeloid-derived suppressor cells (MDSCs),
high CD8 effector-memory type 1 T cells, absolute eosinophil counts
and absolute lymphocyte counts (186-189). Moreover, baseline absolute
neutrophil counts and derived neutrophil-to-lymphocyte ratios have
been reported to be significantly associated with the survival of
patients with melanoma treated with ipilimumab (187). The neutrophil-to-lymphocyte ratio
was also shown to be significantly associated with survival in
patients with metastatic RCC (190). In patients with melanoma treated
with pembrolizumab, results of a previous study demonstrated that
increased relative lymphocyte count at baseline was associated with
improved clinical outcomes (191). In patients with NSCLC treated
with anti-PD-1/PD-L1 agents as second- or third-line therapies,
PD-L1 expression in circulating tumor cells exhibited potential as
a prognostic biomarker (192). In
addition, numerous features of peripheral blood components are
associated with the response to ICIs, including classical monocyte
(CD14+CD16−
CD33+HLA-DR+) frequency (193), serum vascular endothelial growth
factor level (194,195), soluble CD25 levels (188), and serum cytokine levels of
IFN-γ, IL-18, IL-6 and IL-8 (124,196,197). Moreover, circulating exosomes
containing PD-L1, PD-1 or CD28 may be associated with responses to
ICIs (198-200). These blood-based biomarkers may
be obtained in a clinical setting and do not incur any additional
costs to the patient. However, there is still much to learn from
more retrospective and prospective studies evaluating the value of
both approved and developing peripheral blood biomarkers, meanwhile
care should be taken to avoiding redundancy between biomarkers.
Previous studies have demonstrated that intestinal
microbiota may affect the occurrence and development of cancer,
through modulating immunity and regulating cell metabolism
(201,202). Within antitumor immunotherapy,
intestinal microbiota may impact the therapeutic effects of ICIs.
Results of a previous study demonstrated that tumors in
antibiotic-treated or germ-free mice did not respond to a CTLA
inhibitor; however, treatment response occurred following gavage
with Bacteroides fragilis (203). Moreover, results of a further
study demonstrated that treatment with an oral microorganism
combined with a PD-L1 antibody reduced tumor outgrowth in mice
(204). These studies
demonstrated that controlling the microbiota may aid in regulating
cancer immunotherapy. Notably, comparable results were observed in
the clinic. Results of a clinical retrospective study demonstrated
that clinical benefits were reduced in patients who used
antibiotics before and after treatment with ICIs, compared with
patients who did not use antibiotics. Notably, the antibiotics may
have impacted the homeostasis of the intestinal microflora
(205). Patients with NSCLC, RCC,
metastatic melanoma or UC with a high microbial diversity,
including specific species such as Ruminococcus,
Bifidobacterium or Enterococcus, exhibited favorable
responses to PD-1 inhibitors (205-207). Moreover, results of previous
studies demonstrated that germ-free mice that received fecal
microbiota transplantation from patients with cancer who responded
to ICIs exhibited improved responses to ICIs, compared with those
that received fecal microbiota transplantation from non-responders
(203,205,206). Collectively, these results
demonstrated that intestinal microbiota are influential in
antitumor immunity and responses to ICI. At present, the immune
regulation mechanism of intestinal microbiota is not fully
understood. With the development of the Human Microbiome Program
and the advancement of sequencing analysis technology, research on
intestinal microbiota is increasing, and metabolomics analysis
technology further enhances the exploration of the relationship
between intestinal microbiota and host immunity. Two caveats should
be mentioned regarding research on intestinal microbiota. First,
the differences in the application of microbial sequencing analysis
techniques among different studies have limited comparability
between research results. Second, the intestinal microbiota is
influenced by various factors, such as diet, medication, age and
environment, and there is also serious interference between
different microorganisms. Regardless, reasonable antibiotic
selection provides new possibilities for improving the antitumor
efficacy and reducing adverse reactions of ICIs (204).
In addition, epigenetic signatures, liquid
biomarkers and the tumor metabolism microenvironment may be
associated with responses to ICIs (206,208). In conclusion, there are numerous
potential biomarkers for predicting the effectiveness of ICIs;
however, these efficacy prediction biomarkers often do not work
alone, and different biomarkers interact in tumor specimens or
blood specimens. The combined application of multiple predictive
biomarkers can better screen out the population that will respond
best to ICIs, and maximize the clinical benefits of patients. Thus,
further large-scale prospective studies for comparison and
validation are required prior to use in clinical practice. Further
detection of multiple biomarkers, establishing standard biomarker
test procedures, and maintaining high repeatability and low costs
are all considerations that must be addressed.
ICIs exhibit potential in the treatment of some
advanced tumors; however, the majority of patients do not benefit
from ICIs as a single therapy due to the complexity of drug
resistance mechanisms. Improving the current understanding of
mechanisms limiting cancer immunotherapy may aid in the discovery
of novel therapeutic targets and provide potential combination
treatment strategies. Multiple tumor-intrinsic and -extrinsic
factors may contribute to both primary resistance, in which tumors
do not respond to initial therapy, and acquired resistance, such as
relapse after an initial response.
Tumor cells may escape from immune surveillance
through abnormal antigen processing and presentation. Genetic
mutations in β2M, a component of the MHC-I molecule required for
antigen presentation, are present in various cancer types and
associated with evading the T-cell immune response (166,183,209-212). Therefore, alterations in
antigen-presenting machinery must be taken into consideration prior
to ICI treatment.
Gene alterations in specific signaling pathways
also serve significant and complex roles in mediating immunotherapy
resistance. Alterations in oncogenic signaling pathways include: i)
Loss of IFN-γ signaling pathways; ii) upregulation of the
Wnt-β-catenin signaling pathway; and iii) loss of PTEN expression,
which enhances PI3K signaling. Loss-of-function mutations in JAK1
and/or JAK2 involved in the IFN-γ signaling pathway have been
observed in patients who exhibited resistance to PD-1 inhibitors
(167). Other genetic mutations
in the IFN-γ pathway, including IFN-γ receptor 1 and 2, and
interferon regulatory factor 1, have also been observed in patients
who exhibited resistance to CTLA-4 inhibitors (165). These mutations may inhibit IFN-γ
signal transduction and allow tumor cells to escape from T cells.
In addition, mutations in this pathway may lead to the
downregulation of PD-L1 expression following IFN-γ exposure, thus
reducing the therapeutic effect of PD-1/PD-L1 antibodies (213). Upregulation of the Wnt-β-catenin
signaling pathway has been detected in a subset of patients with
melanoma, and this was revealed to be associated with resistance to
ICIs (214). Notably, β-catenin
suppresses CCL4 secretion, a chemokine that attracts DCs,
subsequently leading to failure of T-cell activation and function
(215). Loss of PTEN expression
has also been associated with ICI resistance, resulting from
enhanced PI3K signaling (169,170,216). Moreover, mutations in the MAPK
signaling pathway may lead to cancer immune evasion, through
enhancing the expression of the immunoregulatory cytokines IL-6 and
IL-10 (217).
An additional tumor intrinsic mechanism is the
compensatory upregulation of other immune checkpoints, such as
VISTA, Tim-3 and TIGIT (218-220). Moreover, the transition of
epithelial cells to mesenchymal cells, characterized by increased
migration and invasion, and resistance to apoptosis, may also have
a role in ICI resistance (221-223). Numerous genes have been reported
to be associated with a lack of response to PD-1 blockade, known as
innate anti-PD-1 resistance signatures (80). Further understanding of these
resistance mechanisms is required for informing clinical management
and developing personalized therapies.
The highly immunosuppressive TME is considered the
tumor extrinsic mechanism of ICI resistance. The TME includes
immunosuppressive cells, cytokines, chemokines and metabolites that
restrain antitumor immunity (121). The immunosuppressive cells
include Tregs, MDSCs, tumor-associated macrophages (TAMs) and MSCs,
and these suppress immune responses through numerous mechanisms
(224-228). The immunosuppressive cytokines,
chemokines and metabolites, such as transforming growth factor-β
(TGF-β), CCL5, CXCL8 and IDO are secreted by cancer cells and
immunosuppressive cells in the TME (229-232). Collectively, these factors create
a functionally inhibitory microenvironment that causes resistance
to immunotherapy.
Acquired resistance refers to cancer that
progresses and relapses following initial responses to
immunotherapy. The potential mechanisms underlying acquired
resistance include the inability of T cells to recognize tumor
cells, due to lack of antigen expression or antigen presentation
function defects, upregulation of alternative immune checkpoints,
T-cell depletion, and immune escape. The mechanisms of abnormal
antigen recognition or upregulation of other immune checkpoints
have been aforementioned. The factors that lead to T-cell depletion
are multifaceted. For example, epigenetic dysfunction makes T cells
resistant to remodeling and activation (233). In addition, elevated IDO or
lactate dehydrogenase in the TME may diminish T-cell responses.
Furthermore, impaired production of memory T cells may lead to the
weakening of the effects of ICIs over time, leading to acquired
drug resistance (233).
Although resistance to immunotherapy may manifest
at different times, from initial therapy to relapse after an
initial response, similar or overlapping mechanisms enable tumor
cells to evade antitumor immune responses. For example, IFN-γ
signaling pathways are key factors for both primary and acquired
drug resistance (165,167,213,233). Moreover, resistance mechanisms
are dynamic (233). Therefore,
targeting a single drug resistance mechanism is unlikely to be
sufficient to eradicate immunotherapeutically refractory tumors.
Thus, the precise selection of sensitive populations, dynamic
monitoring of drug resistance, an increased search for synergistic
combination therapies, and the development of novel targets and
drugs are required to overcome resistance to immunotherapy.
The effectiveness of ICI therapy is limited by
various factors, and further investigations into reducing
resistance are required. To determine an optimal antitumor immune
response, combination treatments that combine ICIs with other
therapeutic strategies, such as surgery, radiotherapy, chemotherapy
and other forms of immunotherapy are required.
Immunotherapy may be combined with surgery in a
neoadjuvant setting (before surgery) or an adjuvant setting (after
surgery). Neoadjuvant treatment using chemotherapy or radiotherapy
before surgery exhibits specific advantages over adjuvant
treatment; however, current literature surrounding neoadjuvant
immunotherapy is lacking. From a biological standpoint, neoadjuvant
immunotherapy may reinvigorate exhausted cytotoxic T cells when
antigens are encountered, and the exposure to antigens during the
presence of major tumor mass may increase the breadth and
persistence of tumor-specific T-cell responses. Compared with
adjuvant immunotherapy, neoadjuvant immunotherapy may effectively
reduce tumor mass and improve the probability of complete surgical
resection (234). Besides,
neoadjuvant immunotherapy has been reported to be superior to
adjuvant immunotherapy in eradicating micrometastases, thereby
reducing the probability of recurrence (234). Moreover, fewer infusions of
neoadjuvant immunotherapy provides reduced exposure to
immunotherapy, limiting the development of resistant clones in
relapsed patients. The results of previous preclinical and clinical
studies have demonstrated that neoadjuvant immunotherapy can
improve response and survival rates, compared with the same therapy
administered in the adjuvant setting (234-236). In the first clinical trial that
performed a head-to-head comparison of neoadjuvant and adjuvant
ICIs for the treatment of stage III resectable melanoma, the
patients were treated either post-surgery for 12 weeks with a
combination of ipilimumab + nivolumab, or in a split design for 6
weeks before surgery and for 6 weeks post-surgery (NCT02437279).
The result showed that OS was 90% for patients treated with
neoadjuvant ICI therapy and 67% for patients treated with adjuvant
ICI therapy at a median follow-up time of 32 months (234). At the European Society of
Internal Oncology Immunooncology Conference in 2022, a phase II
CA209-8D8 study of neoadjuvant therapy for NSCLC based on
nivolumab, led by Professor Wu Yilong (Guangdong Provincial
People's Hospital, Guangzhou, China), also announced the advantages
of neoadjuvant immunotherapy (237). Specifically, patients can benefit
from nivolumab + chemotherapy regardless of PD-L1 expression, and
the neoadjuvant therapy does not affect the timing and feasibility
of surgery, nor does it increase the difficulty of surgery
(237). However, further
investigations into the optimal duration of neoadjuvant
immunotherapy and surgery, the optimal type of immunotherapy, and
the efficacy and safety of neoadjuvant immunotherapy are
required.
Radiotherapy and chemotherapy may induce apoptosis
of tumor cells, also known as immunogenic cell death, resulting in
greater antigen presentation and enhanced antitumor immune
responses (238). The results of
previous studies demonstrated improved efficacy when radiotherapy
or chemotherapy was used in combination with ICIs (239-243). Combined targeting of multiple
immune checkpoints, including CTLA-4, PD-1, LAG-3, Tim-3, OX40 and
glucocorticoid-induced tumor necrosis factor receptor exerts
significant survival benefits, compared with single targeting
(164,244-246). However, some immune checkpoints
are expressed only after initial T-cell priming; thus, ICIs may be
limited to tumors that require reverse exhaustion and restoration
of T-cell function (247). In
addition to antibody-based immunotherapy, the combination of ICIs
with other forms of immunotherapy, such as cancer vaccines,
oncolytic viruses or T-cell adoptive therapies are being explored
in clinical trials at present (248-252). Moreover, the combination of ICIs
with small molecule inhibitors targeting i) immunosuppressive
cells, such as MDSCs and TAMs; ii) cytokines, such as TGF-β; or
iii) metabolites, such as IDO, are being developed to enhance
responses to ICIs (229,253). Notably, these studies provide
guidance on delivering combination therapies. There are still a
number of issues that need to be addressed before these combination
therapies become clinical standards, including indications,
applicable population, combination medication sequence, medication
time, dosage, efficacy evaluation standards and adverse reaction
prediction. Therefore, further preclinical investigations and
clinical trial designs are required.
The application of ICIs, either alone or in
combination with other therapeutic strategies, has increased in
patients with refractory metastatic cancer, and also as adjuvant or
neoadjuvant therapy in the early stages of cancer. Although these
treatments are often well tolerated, immune-related adverse events
(irAEs) may also occur, resulting from activation of the immune
system and off-target immune attack on healthy tissues of the host,
which may affect almost any organ system with varying severities
(254). Notably, irAEs are often
graded using the National Cancer Institute Common Terminology
Criteria for Adverse events (254).
As a systemic adverse reaction, fatigue is the most
commonly reported, followed by infusion reactions (255). Moreover, adverse reactions of the
skin and gastrointestinal tract are the most common following
treatment with any approved ICI. Skin rash and pruritus are the
most widely reported symptoms of skin toxicity. Notably,
anti-CTLA-4 treatment causes the highest rate of adverse reactions,
occurring in 40-50% of cases, followed by anti-PD-1 treatment
(30-40%). In addition, anti-PD-L1 treatment causes the lowest rate
of adverse reactions, occurring in 1-7% of cases (255,256). Other skin toxicities include
vitiligo, photosensitive reaction and xerosis. Rare cases of
Stevens-Johnson syndrome and toxic epidermal necrolysis have been
reported, and these may be fatal (255). Often, the majority of skin
toxicities are low-grade and easily managed with emollients, oral
antihistamines and topical corticosteroids, while high-grade
adverse events require permanent cessation of ICIs.
Gastrointestinal toxicities often present as diarrhea and/or
colitis. In total, in a previous study, ~30% of patients who
received anti-CTLA-4 treatment, 20% of patients who received
anti-PD-1 treatment and 45% of patients who received combination
treatment developed diarrhea (257,258). Prompt recognition and
intervention are crucial in preventing additional complications,
such as colonic perforation. It is generally recommended that all
patients receiving ICIs who present with diarrhea should undergo
stool analyses for enteric pathogens and Clostridium
difficile toxins. Patients with grade ≥2 diarrhea may require
steroid treatment, whereas patients with grade 4 diarrhea/colitis
or recurrent diarrhea should stop ICI treatment permanently
(259). Endocrine toxicity
associated with ICI therapy may involve the thyroid, pituitary or
adrenal gland. The most common adverse effect is hypophysitis;
however, others include hypothyroidism, hyperthyroidism,
thyroiditis, primary adrenal insufficiency, type 1 diabetes
mellitus and hypoparathyroidism. Therefore, examination of thyroid
function pre-treatment and monitoring during treatment are
essential (260). Hepatic adverse
events that occur following ICI therapy often present as increases
in asymptomatic transaminase, with or without increases in
bilirubin; however, autoimmune-like hepatitis with increased
severity and acute liver failure may also occur. Patients with
grade ≥2 toxicities should be treated with systemic steroid
treatment (253). Pulmonary
irAEs, such as pneumonitis, are uncommon; however, these may be
fatal. The incidence rate of pulmonary irAEs is higher following
treatment with anti-PD-1 and/or combined treatment, compared with
anti-CTLA-4 treatment. Timing of systemic steroid treatment is
crucial and potential infection should be excluded (261). Other irAEs, such as neurologic,
ocular, renal, hematological, rheumatologic and cardiovascular
toxicities are rare. Following single drug treatment, the incidence
rate of these events is <2%; however, following the development
of grade 3-4 adverse reactions, patients should stop ICI treatment
permanently (259).
Although treatment options are available for irAEs,
these can progress, and in some cases, be life threatening.
Management of irAEs is often complex and requires close
collaboration with clinical experts. Further identification of
predictive biomarkers of irAEs, such as T-cell or B-cell
biomarkers, microbiome biomarkers and genomic biomarkers, will aid
in guiding treatment decisions (262). Furthermore, it is necessary to
encourage the establishment of a large-scale pharmacovigilance
registration system and collect the records of irAEs in real-world
patients following treatment with ICIs. This can not only verify
the existing conclusions obtained through real-world large sample
data, but also use these records for new research, such as
determining the clinical characteristics of various irAEs,
exploring their important risk factors, and providing an important
basis for the diagnosis and treatment of irAEs.
Immunotherapy has emerged as a novel cancer
treatment. The present review summarized the history and novel
developments of ICIs. However, the number of patients benefiting
from ICIs remains low, and further studies should focus on
understanding the specific interactions between tumors and the
immune system, and resistance mechanisms relevant to immunotherapy.
Notably, the immune system of each patient is dynamic and
constantly evolving, highlighting that personalized treatment
options are required. Future research should focus on developing
ICIs for use in an increased number of patients with cancer. In
addition, ICIs should also be developed for use in all fields of
oncology, to expand the options available for combination
strategies. ICIs may be combined with surgery, chemotherapy,
radiotherapy, targeted therapy and other forms of immunotherapy;
however, efficient toxicity management strategies are required.
Moreover, identification of novel biomarkers that
predict response or resistance is essential for accurately
selecting specific ICIs. Existing biomarkers, such as PD-L1, dMMR,
MSI-H and TMB, are widely used in clinical practice; however,
factors such as tumor type, tumor heterogeneity, tumor dynamics and
testing procedures may impact the accuracy of these biomarkers.
Therefore, optimizing existing biomarkers, and developing new
biomarkers or new biomarker systems that integrate immune
profiling, tumor biology and treatment history are key in future
investigations.
The field of immunotherapy is challenging, but also
exhibits potential. In the future, immunotherapy will require
developments at a multi-directional level. Further investigations
should explore novel inhibitory checkpoints and pathways, and also
integrate other fields, such as cancer biology, genetics and
epigenetics. Moreover, further high-quality clinical trials of ICIs
are required, to advance evidence-based medicine and develop new
cancer treatment options.
Not applicable.
HC and JHW contributed to the conception and design
of the review. YJW and SY wrote the first draft of the manuscript.
LW and WL wrote sections of the manuscript. Data authentication is
not applicable. All authors contributed to manuscript revision, and
read and approved the final manuscript.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
Not applicable.
This work was supported by the National Natural Science
Foundation of China (grant no. 82100238), the Science and
Technology Program of Guangzhou (grant no. 202201011046), the
High-level Hospital Construction Project (grant no. DFJH201923) and
the Medical Scientific Research Foundation of Guangdong Province
(grant no. A2019063).
|
1
|
Li K and Tian H: Development of
small-molecule immune checkpoint inhibitors of PD-1/PD-L1 as a new
therapeutic strategy for tumour immunotherapy. J Drug Target.
27:244–256. 2019. View Article : Google Scholar
|
|
2
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Dunn GP, Bruce AT, Ikeda H, Old LJ and
Schreiber RD: Cancer immunoediting: From immunosurveillance to
tumor escape. Nat Immunol. 3:991–998. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Dillman RO: Cancer immunotherapy. Cancer
Biother Radiopharm. 26:1–64. 2011.PubMed/NCBI
|
|
5
|
Stewart TJ and Smyth MJ: Improving cancer
immunotherapy by targeting tumor-induced immune suppression. Cancer
Metastasis Rev. 30:125–140. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sharma P, Wagner K, Wolchok JD and Allison
JP: Novel cancer immunotherapy agents with survival benefit: Recent
successes and next steps. Nat Rev Cancer. 11:805–812. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Pardoll DM: The blockade of immune
checkpoints in cancer immunotherapy. Nat Rev Cancer. 12:252–264.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sharma P and Allison JP: The future of
immune checkpoint therapy. Science. 348:56–61. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Topalian SL, Drake CG and Pardoll DM:
Immune checkpoint blockade: A common denominator approach to cancer
therapy. Cancer Cell. 27:450–461. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hoos A: Development of immuno-oncology
drugs-from CTLA4 to PD1 to the next generations. Nat Rev Drug
Discov. 15:235–247. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wei SC, Duffy CR and Allison JP:
Fundamental mechanisms of immune checkpoint blockade therapy.
Cancer Discov. 8:1069–1086. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sharma P and Allison JP: Immune checkpoint
targeting in cancer therapy: Toward combination strategies with
curative potential. Cell. 161:205–214. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sharpe AH: Mechanisms of costimulation.
Immunol Rev. 229:5–11. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Rudd CE, Taylor A and Schneider H: CD28
and CTLA-4 coreceptor expression and signal transduction. Immunol
Rev. 229:12–26. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen L and Flies DB: Molecular mechanisms
of T cell co-stimulation and co-inhibition. Nat Rev Immunol.
13:227–242. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Brunet JF, Denizot F, Luciani MF,
Roux-Dosseto M, Suzan M, Mattei MG and Golstein P: A new member of
the immunoglobulin superfamily-CTLA-4. Nature. 328:267–270. 1987.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Krummel MF and Allison JP: CD28 and CTLA-4
have opposing effects on the response of T cells to stimulation. J
Exp Med. 182:459–465. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Linsley PS, Brady W, Urnes M, Grosmaire
LS, Damle NK and Ledbetter JA: CTLA-4 is a second receptor for the
B cell activation antigen B7. J Exp Med. 174:561–569. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Walunas TL, Lenschow DJ, Bakker CY,
Linsley PS, Freeman GJ, Green JM, Thompson CB and Bluestone JA:
Pillars article: CTLA-4 can function as a negative regulator of T
cell activation. Immunity. 1994. 1: 405-413. J Immunol.
187:3466–3474. 2011.PubMed/NCBI
|
|
20
|
Linsley PS, Greene JL, Brady W, Bajorath
J, Ledbetter JA and Peach R: Human B7-1 (CD80) and B7-2 (CD86) bind
with similar avidities but distinct kinetics to CD28 and CTLA-4
receptors. Immunity. 1:793–801. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gibson HM, Hedgcock CJ, Aufiero BM, Wilson
AJ, Hafner MS, Tsokos GC and Wong HK: Induction of the CTLA-4 gene
in human lymphocytes is dependent on NFAT binding the proximal
promoter. J Immunol. 179:3831–3840. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Waterhouse P, Penninger JM, Timms E,
Wakeham A, Shahinian A, Lee KP, Thompson CB, Griesser H and Mak TW:
Lymphoproliferative disorders with early lethality in mice
deficient in Ctla-4. Science. 270:985–988. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tivol EA, Borriello F, Schweitzer AN,
Lynch WP, Bluestone JA and Sharpe AH: Loss of CTLA-4 leads to
massive lymphoproliferation and fatal multiorgan tissue
destruction, revealing a critical negative regulatory role of
CTLA-4. Immunity. 3:541–547. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Read S, Greenwald R, Izcue A, Robinson N,
Mandelbrot D, Francisco L, Sharpe AH and Powrie F: Blockade of
CTLA-4 on CD4+CD25+ regulatory T cells abrogates their function in
vivo. J Immunol. 177:4376–4383. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wing K, Onishi Y, Prieto-Martin P,
Yamaguchi T, Miyara M, Fehervari Z, Nomura T and Sakaguchi S:
CTLA-4 control over Foxp3+ regulatory T cell function. Science.
322:271–275. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Schneider H, Smith X, Liu H, Bismuth G and
Rudd CE: CTLA-4 disrupts ZAP70 microcluster formation with reduced
T cell/APC dwell times and calcium mobilization. Eur J Immunol.
38:40–47. 2008. View Article : Google Scholar
|
|
27
|
Wang XB, Fan ZZ, Anton D, Vollenhoven AV,
Ni ZH, Chen XF and Lefvert AK: CTLA4 is expressed on mature
dendritic cells derived from human monocytes and influences their
maturation and antigen presentation. BMC Immunol. 12:212011.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Boasso A, Herbeuval JP, Hardy AW, Winkler
C and Shearer GM: Regulation of indoleamine 2,3-dioxygenase and
tryptophanyl-tRNA-synthetase by CTLA-4-Fc in human CD4+ T cells.
Blood. 105:1574–1581. 2005. View Article : Google Scholar
|
|
29
|
Leach DR, Krummel MF and Allison JP:
Enhancement of antitumor immunity by CTLA-4 blockade. Science.
271:1734–1736. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hodi FS, O'Day SJ, McDermott DF, Weber RW,
Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel
JC, et al: Improved survival with ipilimumab in patients with
metastatic melanoma. N Engl J Med. 363:711–723. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hammers HJ, Plimack ER, Infante JR, Rini
BI, McDermott DF, Lewis LD, Voss MH, Sharma P, Pal SK, Razak ARA,
et al: Safety and efficacy of nivolumab in combination with
ipilimumab in metastatic renal cell carcinoma: The CheckMate 016
study. J Clin Oncol. 35:3851–3858. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kwon ED, Drake CG, Scher HI, Fizazi K,
Bossi A, van den Eertwegh AJ, Krainer M, Houede N, Santos R,
Mahammedi H, et al: Ipilimumab versus placebo after radiotherapy in
patients with metastatic castration-resistant prostate cancer that
had progressed after docetaxel chemotherapy (CA184-043): A
multicentre, randomised, double-blind, phase 3 trial. Lancet Oncol.
15:700–712. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Le DT, Lutz E, Uram JN, Sugar EA, Onners
B, Solt S, Zheng L, Diaz LA Jr, Donehower RC, Jaffee EM and Laheru
DA: Evaluation of ipilimumab in combination with allogeneic
pancreatic tumor cells transfected with a GM-CSF gene in previously
treated pancreatic cancer. J Immunother. 36:382–389. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhao X and Subramanian S: Intrinsic
resistance of solid tumors to immune checkpoint blockade therapy.
Cancer Res. 77:817–822. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Duffy AG, Ulahannan SV, Makorova-Rusher O,
Rahma O, Wedemeyer H, Pratt D, Davis JL, Hughes MS, Heller T,
ElGindi M, et al: Tremelimumab in combination with ablation in
patients with advanced hepatocellular carcinoma. J Hepatol.
66:545–551. 2017. View Article : Google Scholar :
|
|
36
|
Selby MJ, Engelhardt JJ, Quigley M,
Henning KA, Chen T, Srinivasan M and Korman AJ: Anti-CTLA-4
antibodies of IgG2a isotype enhance antitumor activity through
reduction of intratumoral regulatory T cells. Cancer Immunol Res.
1:32–42. 2013. View Article : Google Scholar
|
|
37
|
Marangoni F, Zhakyp A, Corsini M, Geels
SN, Carrizosa E, Thelen M, Mani V, Prüßmann JN, Warner RD, Ozga AJ,
et al: Expansion of tumor-associated Treg cells upon disruption of
a CTLA-4-dependent feedback loop. Cell. 184:3998–4015.e19. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ishida Y, Agata Y, Shibahara K and Honjo
T: Induced expression of PD-1, a novel member of the immunoglobulin
gene superfamily, upon programmed cell death. EMBO J. 11:3887–3895.
1992. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Nishimura H, Nose M, Hiai H, Minato N and
Honjo T: Development of lupus-like autoimmune diseases by
disruption of the PD-1 gene encoding an ITIM motif-carrying
immunoreceptor. Immunity. 11:141–151. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Dong H, Zhu G, Tamada K and Chen L: B7-H1,
a third member of the B7 family, co-stimulates T-cell proliferation
and interleukin-10 secretion. Nat Med. 5:1365–1369. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Freeman GJ, Long AJ, Iwai Y, Bourque K,
Chernova T, Nishimura H, Fitz LJ, Malenkovich N, Okazaki T, Byrne
MC, et al: Engagement of the PD-1 immunoinhibitory receptor by a
novel B7 family member leads to negative regulation of lymphocyte
activation. J Exp Med. 192:1027–1034. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Agata Y, Kawasaki A, Nishimura H, Ishida
Y, Tsubata T, Yagita H and Honjo T: Expression of the PD-1 antigen
on the surface of stimulated mouse T and B lymphocytes. Int
Immunol. 8:765–772. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Keir ME, Butte MJ, Freeman GJ and Sharpe
AH: PD-1 and its ligands in tolerance and immunity. Annu Rev
Immunol. 26:677–704. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Latchman Y, Wood CR, Chernova T, Chaudhary
D, Borde M, Chernova I, Iwai Y, Long AJ, Brown JA, Nunes R, et al:
PD-L2 is a second ligand for PD-1 and inhibits T cell activation.
Nat Immunol. 2:261–268. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
45
|
Okazaki T and Honjo T: The PD-1-PD-L
pathway in immunological tolerance. Trends Immunol. 27:195–201.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Zamani MR, Aslani S, Salmaninejad A, Javan
MR and Rezaei N: PD-1/PD-L and autoimmunity: A growing
relationship. Cell Immunol. 310:27–41. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Barber DL, Wherry EJ, Masopust D, Zhu B,
Allison JP, Sharpe AH, Freeman GJ and Ahmed R: Restoring function
in exhausted CD8 T cells during chronic viral infection. Nature.
439:682–687. 2006. View Article : Google Scholar
|
|
48
|
Pauken KE and Wherry EJ: Overcoming T cell
exhaustion in infection and cancer. Trends Immunol. 36:265–276.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Ahmadzadeh M, Johnson LA, Heemskerk B,
Wunderlich JR, Dudley ME, White DE and Rosenberg SA: Tumor
antigen-specific CD8 T cells infiltrating the tumor express high
levels of PD-1 and are functionally impaired. Blood. 114:1537–1544.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Yaghoubi N, Soltani A, Ghazvini K,
Hassanian SM and Hashemy SI: PD-1/PD-L1 blockade as a novel
treatment for colorectal cancer. Biomed Pharmacother. 110:312–318.
2019. View Article : Google Scholar
|
|
51
|
Patsoukis N, Wang Q, Strauss L and
Boussiotis VA: Revisiting the PD-1 pathway. Sci Adv.
6:eabd27122020. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Sharpe AH, Wherry EJ, Ahmed R and Freeman
GJ: The function of programmed cell death 1 and its ligands in
regulating autoimmunity and infection. Nat Immunol. 8:239–245.
2007. View
Article : Google Scholar : PubMed/NCBI
|
|
53
|
Zhong X, Tumang JR, Gao W, Bai C and
Rothstein TL: PD-L2 expression extends beyond dendritic
cells/macrophages to B1 cells enriched for V(H)11/V(H)12 and
phosphatidylcholine binding. Eur J Immunol. 37:2405–2410. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Sharpe AH and Pauken KE: The diverse
functions of the PD1 inhibitory pathway. Nat Rev Immunol.
18:153–167. 2018. View Article : Google Scholar
|
|
55
|
Ribas A and Hu-Lieskovan S: What does
PD-L1 positive or negative mean? J Exp Med. 213:2835–2840. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Dong H, Strome SE, Salomao DR, Tamura H,
Hirano F, Flies DB, Roche PC, Lu J, Zhu G, Tamada K, et al:
Tumor-associated B7-H1 promotes T-cell apoptosis: A potential
mechanism of immune evasion. Nat Med. 8:793–800. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Brown JA, Dorfman DM, Ma FR, Sullivan EL,
Munoz O, Wood CR, Greenfield EA and Freeman GJ: Blockade of
programmed death-1 ligands on dendritic cells enhances T cell
activation and cytokine production. J Immunol. 170:1257–1266. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Konishi J, Yamazaki K, Azuma M, Kinoshita
I, Dosaka-Akita H and Nishimura M: B7-H1 expression on non-small
cell lung cancer cells and its relationship with tumor-infiltrating
lymphocytes and their PD-1 expression. Clin Cancer Res.
10:5094–5100. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Brahmer JR, Drake CG, Wollner I, Powderly
JD, Picus J, Sharfman WH, Stankevich E, Pons A, Salay TM, McMiller
TL, et al: Phase I study of single-agent anti-programmed death-1
(MDX-1106) in refractory solid tumors: Safety, clinical activity,
pharmacodynamics, and immunologic correlates. J Clin Oncol.
28:3167–3175. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Gettinger S, Rizvi NA, Chow LQ, Borghaei
H, Brahmer J, Ready N, Gerber DE, Shepherd FA, Antonia S, Goldman
JW, et al: Nivolumab monotherapy for first-line treatment of
advanced non-small-cell lung cancer. J Clin Oncol. 34:2980–2987.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Rizvi NA, Mazières J, Planchard D,
Stinchcombe TE, Dy GK, Antonia SJ, Horn L, Lena H, Minenza E,
Mennecier B, et al: Activity and safety of nivolumab, an anti-PD-1
immune checkpoint inhibitor, for patients with advanced, refractory
squamous non-small-cell lung cancer (CheckMate 063): A phase 2,
single-arm trial. Lancet Oncol. 16:257–265. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Motzer RJ, Escudier B, McDermott DF,
George S, Hammers HJ, Srinivas S, Tykodi SS, Sosman JA, Procopio G,
Plimack ER, et al: Nivolumab versus everolimus in advanced
renal-cell carcinoma. N Engl J Med. 373:1803–1813. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Topalian SL, Hodi FS, Brahmer JR,
Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD,
Sosman JA, Atkins MB, et al: Safety, activity, and immune
correlates of anti-PD-1 antibody in cancer. N Engl J Med.
366:2443–2454. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Robert C, Ribas A, Wolchok JD, Hodi FS,
Hamid O, Kefford R, Weber JS, Joshua AM, Hwu WJ, Gangadhar TC, et
al: Anti-p rogrammed-death-receptor-1 treatment with pembrolizumab
in ipilimumab-refractory advanced melanoma: A randomised
dose-comparison cohort of a phase 1 trial. Lancet. 384:1109–1117.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Ribas A, Puzanov I, Dummer R, Schadendorf
D, Hamid O, Robert C, Hodi FS, Schachter J, Pavlick AC, Lewis KD,
et al: Pembrolizumab versus investigator-choice chemotherapy for
ipilimumab-refractory melanoma (KEYNOTE-002): A randomised,
controlled, phase 2 trial. Lancet Oncol. 16:908–918. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Garon EB, Rizvi NA, Hui R, Leighl N,
Balmanoukian AS, Eder JP, Patnaik A, Aggarwal C, Gubens M, Horn L,
et al: Pembrolizumab for the treatment of non-small-cell lung
cancer. N Engl J Med. 372:2018–2028. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Patnaik A, Kang SP, Rasco D, Papadopoulos
KP, Elassaiss-Schaap J, Beeram M, Drengler R, Chen C, Smith L,
Espino G, et al: Phase I study of pembrolizumab (MK-3475; anti-PD-1
monoclonal antibody) in patients with advanced solid tumors. Clin
Cancer Res. 21:4286–4293. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Rihawi K, Gelsomino F, Sperandi F, Melotti
B, Fiorentino M, Casolari L and Ardizzoni A: Pembrolizumab in the
treatment of metastatic non-small cell lung cancer: A review of
current evidence. Ther Adv Respir Dis. 11:353–373. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Suzman DL, Agrawal S, Ning YM, Maher VE,
Fernandes LL, Karuri S, Tang S, Sridhara R, Schroeder J, Goldberg
KB, et al: FDA approval summary: Atezolizumab or pembrolizumab for
the treatment of patients with advanced urothelial carcinoma
ineligible for cisplatin-containing chemotherapy. Oncologist.
24:563–569. 2019. View Article : Google Scholar
|
|
70
|
U.S. Food and Drug: FDA approves first
treatment for advanced form of the second most common skin cancer.
https://www.fda.gov/news-events/press-announcements/fda-approves-first-treatment-advanced-form-second-most-common-skin-cancer-0.
Accessed January 20, 2023
|
|
71
|
Rosenberg JE, Hoffman-Censits J, Powles T,
van der Heijden MS, Balar AV, Necchi A, Dawson N, O'Donnell PH,
Balmanoukian A, Loriot Y, et al: Atezolizumab in patients with
locally advanced and metastatic urothelial carcinoma who have
progressed following treatment with platinum-based chemotherapy: A
single-arm, multicentre, phase 2 trial. Lancet. 387:1909–1920.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Peters S, Gettinger S, Johnson ML, Jänne
PA, Garassino MC, Christoph D, Toh CK, Rizvi NA, Chaft JE,
Carcereny Costa E, et al: Phase II trial of atezolizumab as
first-line or subsequent therapy for patients with programmed
death-ligand 1-selected advanced non-small-cell lung cancer
(BIRCH). J Clin Oncol. 35:2781–2789. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Balar AV, Galsky MD, Rosenberg JE, Powles
T, Petrylak DP, Bellmunt J, Loriot Y, Necchi A, Hoffman-Censits J,
Perez-Gracia JL, et al: Atezolizumab as first-line treatment in
cisplatin-ineligible patients with locally advanced and metastatic
urothelial carcinoma: A single-arm, multicentre, phase 2 trial.
Lancet. 389:67–76. 2017. View Article : Google Scholar
|
|
74
|
Petrylak DP, Powles T, Bellmunt J, Braiteh
F, Loriot Y, Morales-Barrera R, Burris HA, Kim JW, Ding B, Kaiser
C, et al: Atezolizumab (MPDL3280A) monotherapy for patients with
metastatic urothelial cancer: Long-term outcomes from a phase 1
study. JAMA Oncol. 4:537–544. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Socinski MA, Jotte RM, Cappuzzo F, Orlandi
F, Stroyakovskiy D, Nogami N, Rodriguez-Abreu D, Moro-Sibilot D,
Thomas CA, Barlesi F, et al: Atezolizumab for first-line treatment
of metastatic nonsquamous NSCLC. N Engl J Med. 378:2288–2301. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Hamilton G and Rath B: Avelumab: Combining
immune checkpoint inhibition and antibody-dependent cytotoxicity.
Expert Opin Biol Ther. 17:515–523. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Antonia SJ, Villegas A, Daniel D, Vicente
D, Murakami S, Hui R, Yokoi T, Chiappori A, Lee KH, de Wit M, et
al: Durvalumab after chemoradiotherapy in stage III non-small-cell
lung cancer. N Engl J Med. 377:1919–1929. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Sunshine J and Taube JM: PD-1/PD-L1
inhibitors. Curr Opin Pharmacol. 23:32–38. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Gay CL, Bosch RJ, Ritz J, Hataye JM, Aga
E, Tressler RL, Mason SW, Hwang CK, Grasela DM, Ray N, et al:
Clinical trial of the anti-PD-L1 antibody BMS-936559 in HIV-1
infected participants on suppressive antiretroviral therapy. J
Infect Dis. 215:1725–1733. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Hugo W, Zaretsky JM, Sun L, Song C, Moreno
BH, Hu-Lieskovan S, Berent-Maoz B, Pang J, Chmielowski B, Cherry G,
et al: Genomic and transcriptomic features of response to anti-PD-1
therapy in metastatic melanoma. Cell. 165:35–44. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Le DT, Uram JN, Wang H, Bartlett BR,
Kemberling H, Eyring AD, Skora AD, Luber BS, Azad NS, Laheru D, et
al: PD-1 blockade in tumors with mismatch-repair deficiency. N Engl
J Med. 372:2509–2520. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Workman CJ and Vignali DAA: Negative
regulation of T cell homeostasis by lymphocyte activation gene-3
(CD223). J Immunol. 174:688–695. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Goldberg MV and Drake CG: LAG-3 in cancer
immunotherapy. Curr Top Microbiol Immunol. 344:269–278. 2011.
|
|
84
|
Anderson AC, Joller N and Kuchroo VK:
Lag-3, Tim-3, and TIGIT: Co-inhibitory receptors with specialized
functions in immune regulation. Immunity. 44:989–1004. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Demeure CE, Wolfers J, Martin-Garcia N,
Gaulard P and Triebel F: T Lymphocytes infiltrating various tumour
types express the MHC class II ligand lymphocyte activation gene-3
(LAG-3): Role of LAG-3/MHC class II interactions in cell-cell
contacts. Eur J Cancer. 37:1709–1718. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Gandhi MK, Lambley E, Duraiswamy J, Dua U,
Smith C, Elliott S, Gill D, Marlton P, Seymour J and Khanna R:
Expression of LAG-3 by tumor-infiltrating lymphocytes is coincident
with the suppression of latent membrane antigen-specific CD8+
T-cell function in Hodgkin lymphoma patients. Blood. 108:2280–2289.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Matsuzaki J, Gnjatic S, Mhawech-Fauceglia
P, Beck A, Miller A, Tsuji T, Eppolito C, Qian F, Lele S, Shrikant
P, et al: Tumor-infiltrating NY-ESO-1-specific CD8+ T cells are
negatively regulated by LAG-3 and PD-1 in human ovarian cancer.
Proc Natl Acad Sci USA. 107:7875–7880. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Li FJ, Zhang Y, Jin GX, Yao L and Wu DQ:
Expression of LAG-3 is coincident with the impaired effector
function of HBV-specific CD8(+) T cell in HCC patients. Immunol
Lett. 150:116–122. 2013. View Article : Google Scholar
|
|
89
|
Andrews LP, Marciscano AE, Drake CG and
Vignali DA: LAG3 (CD223) as a cancer immunotherapy target. Immunol
Rev. 276:80–96. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Ruffo E, Wu RC, Bruno TC, Workman CJ and
Vignali DAA: Lymphocyte-activation gene 3 (LAG3): The next immune
checkpoint receptor. Semin Immunol. 42:1013052019. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Gleason MK, Lenvik TR, McCullar V, Felices
M, O'Brien MS, Cooley SA, Verneris MR, Cichocki F, Holman CJ,
Panoskaltsis-Mortari A, et al: Tim-3 is an inducible human natural
killer cell receptor that enhances interferon gamma production in
response to galectin-9. Blood. 119:3064–3072. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Anderson AC: Tim-3, a negative regulator
of anti-tumor immunity. Curr Opin Immunol. 24:213–216. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Nakayama M, Akiba H, Takeda K, Kojima Y,
Hashiguchi M, Azuma M, Yagita H and Okumura K: Tim-3 mediates
phagocytosis of apoptotic cells and cross-presentation. Blood.
113:3821–3830. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Tang D and Lotze MT: Tumor immunity times
out: TIM-3 and HMGB1. Nat Immunol. 13:808–810. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Huang YH, Zhu C, Kondo Y, Anderson AC,
Gandhi A, Russell A, Dougan SK, Petersen BS, Melum E, Pertel T, et
al: CEACAM1 regulates TIM-3-mediated tolerance and exhaustion.
Nature. 517:386–390. 2015. View Article : Google Scholar
|
|
96
|
Fourcade J, Sun Z, Benallaoua M, Guillaume
P, Luescher IF, Sander C, Kirkwood JM, Kuchroo V and Zarour HM:
Upregulation of Tim-3 and PD-1 expression is associated with tumor
antigen-specific CD8+ T cell dysfunction in melanoma patients. J
Exp Med. 207:2175–2186. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Li H, Wu K, Tao K, Chen L, Zheng Q, Lu X,
Liu J, Shi L, Liu C, Wang G and Zou W: Tim-3/galectin-9 signaling
pathway mediates T-cell dysfunction and predicts poor prognosis in
patients with hepatitis B virus-associated hepatocellular
carcinoma. Hepatology. 56:1342–1351. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Yang ZZ, Grote DM, Ziesmer SC, Niki T,
Hirashima M, Novak AJ, Witzig TE and Ansell SM: IL-12 upregulates
TIM-3 expression and induces T cell exhaustion in patients with
follicular B cell non-Hodgkin lymphoma. J Clin Invest.
122:1271–1282. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Gao X, Zhu Y, Li G, Huang H, Zhang G, Wang
F, Sun J, Yang Q, Zhang X and Lu B: TIM-3 expression characterizes
regulatory T cells in tumor tissues and is associated with lung
cancer progression. PLoS One. 7:e306762012. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Markwick LJ, Riva A, Ryan JM, Cooksley H,
Palma E, Tranah TH, Manakkat Vijay GK, Vergis N, Thursz M, Evans A,
et al: Blockade of PD1 and TIM3 restores innate and adaptive
immunity in patients with acute alcoholic hepatitis.
Gastroenterology. 148:590–602 e10. 2015. View Article : Google Scholar
|
|
101
|
Stanietsky N, Simic H, Arapovic J, Toporik
A, Levy O, Novik A, Levine Z, Beiman M, Dassa L, Achdout H, et al:
The interaction of TIGIT with PVR and PVRL2 inhibits human NK cell
cytotoxicity. Proc Natl Acad Sci USA. 106:17858–17863. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Levin SD, Taft DW, Brandt CS, Bucher C,
Howard ED, Chadwick EM, Johnston J, Hammond A, Bontadelli K,
Ardourel D, et al: Vstm3 is a member of the CD28 family and an
important modulator of T-cell function. Eur J Immunol. 41:902–915.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Boles KS, Vermi W, Facchetti F, Fuchs A,
Wilson TJ, Diacovo TG, Cella M and Colonna M: A novel molecular
interaction for the adhesion of follicular CD4 T cells to
follicular DC. Eur J Immunol. 39:695–703. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Kurtulus S, Sakuishi K, Ngiow SF, Joller
N, Tan DJ, Teng MW, Smyth MJ, Kuchroo VK and Anderson AC: TIGIT
predominantly regulates the immune response via regulatory T cells.
J Clin Invest. 125:4053–4062. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Manieri NA, Chiang EY and Grogan JL:
TIGIT: A Key inhibitor of the cancer immunity cycle. Trends
Immunol. 38:20–28. 2017. View Article : Google Scholar
|
|
106
|
U.S. National Library of Medicine:
Zimberelimab (AB122) With TIGIT Inhibitor Domvanalimab (AB154) in
PD-1 Relapsed/Refractory Melanoma. https://clinicaltrials.gov/ct2/show/NCT05130177?term=NCT05130177&draw=2&rank=1.
Accessed January 20, 2023
|
|
107
|
U.S. National Library of Medicine: COM902
(A TIGIT Inhibitor) in Subjects With Advanced Malignancies.
https://clinicaltrials.gov/ct2/show/NCT04354246?term=NCT04354246&draw=2&rank=1.
Accessed January 20, 2023
|
|
108
|
U.S. National Library of Medicine: Study
to Assess the Safety and Efficacy of AZD2936 in Participants With
Advanced or Metastatic Non-small Cell Lung Cancer (ARTEMIDE-01).
https://clinicaltrials.gov/ct2/show/NCT04995523?term=NCT04995523&draw=2&rank=1.
Accessed January 20, 2023
|
|
109
|
Watanabe N, Gavrieli M, Sedy JR, Yang J,
Fallarino F, Loftin SK, Hurchla MA, Zimmerman N, Sim J, Zang X, et
al: BTLA is a lymphocyte inhibitory receptor with similarities to
CTLA-4 and PD-1. Nat Immunol. 4:670–679. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Karakatsanis S, Bertsias G, Roussou P and
Boumpas D: Programmed death 1 and B and T lymphocyte attenuator
immunoreceptors and their association with malignant
T-lymphoproliferative disorders: Brief review. Hematol Oncol.
32:113–119. 2014. View Article : Google Scholar
|
|
111
|
Pasero C and Olive D: Interfering with
coinhibitory molecules: BTLA/HVEM as new targets to enhance
anti-tumor immunity. Immunol Lett. 151:71–75. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
M'Hidi H, Thibult ML, Chetaille B, Rey F,
Bouadallah R, Nicollas R, Olive D and Xerri L: High expression of
the inhibitory receptor BTLA in T-follicular helper cells and in
B-cell small lymphocytic lymphoma/chronic lymphocytic leukemia. Am
J Clin Pathol. 132:589–596. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Hosseinkhani N, Derakhshani A, Shadbad MA,
Argentiero A, Racanelli V, Kazemi T, Mokhtarzadeh A, Brunetti O,
Silvestris N and Baradaran B: The role of V-domain Ig suppressor of
T cell activation (VISTA) in cancer therapy: Lessons learned and
the road ahead. Front Immunol. 12:6761812021. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
U.S. National Library of Medicine: A Study
of CA-170 (Oral PD-L1 PD-L2 and VISTA Checkpoint Antagonist) in
Patients With Advanced Tumors and Lymphomas. https://clinicaltrials.gov/ct2/show/NCT02812875?term=NCT02812875&draw=2&rank=1.
Accessed January 20, 2023
|
|
115
|
U.S. National Library of Medicine: A Study
of Safety Pharmacokinetics, Pharmacodynamics of JNJ-61610588 in
Participants With Advanced Cancer. https://clinicaltrials.gov/ct2/show/NCT02671955?term=NCT02671955&draw=2&rank=1.
Accessed January 20, 2023
|
|
116
|
U.S. National Library of Medicine: Phase 1
Study of CI-8993 Anti-VISTA Antibody in Patients With Advanced
Solid Tumor Malignancies. https://clinicaltrials.gov/ct2/show/NCT04475523?term=NCT04475523&draw=2&rank=1.
Accessed January 20, 2023
|
|
117
|
Motzer RJ, Rini BI, McDermott DF, Redman
BG, Kuzel TM, Harrison MR, Vaishampayan UN, Drabkin HA, George S,
Logan TF, et al: Nivolumab for metastatic renal cell carcinoma:
Results of a randomized phase II trial. J Clin Oncol. 33:1430–1437.
2015. View Article : Google Scholar
|
|
118
|
Gettinger SN, Horn L, Gandhi L, Spigel DR,
Antonia SJ, Rizvi NA, Powderly JD, Heist RS, Carvajal RD, Jackman
DM, et al: Overall survival and long-term safety of nivolumab
(anti-programmed death 1 antibody, BMS-936558, ONO-4538) in
patients with previously treated advanced non-small-cell lung
cancer. J Clin Oncol. 33:2004–2012. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Hamid O, Robert C, Daud A, Hodi FS, Hwu
WJ, Kefford R, Wolchok JD, Hersey P, Joseph RW, Weber JS, et al:
Safety and tumor responses with lambrolizumab (anti-PD-1) in
melanoma. N Engl J Med. 369:134–144. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
McDermott DF, Sosman JA, Sznol M, Massard
C, Gordon MS, Hamid O, Powderly JD, Infante JR, Fassò M, Wang YV,
et al: Atezolizumab, an anti-programmed death-ligand 1 antibody, in
metastatic renal cell carcinoma: Long-term safety, clinical
activity, and immune correlates from a phase Ia study. J Clin
Oncol. 34:833–842. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Augustin RC, Delgoffe GM and Najjar YG:
Characteristics of the tumor microenvironment that influence immune
cell functions: Hypoxia, oxidative stress, metabolic alterations.
Cancers (Basel). 12:38022020. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Wang DR, Wu XL and Sun YL: Therapeutic
targets and biomarkers of tumor immunotherapy: Response versus
non-response. Signal Transduct Target Ther. 7:3312022. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Doroshow DB, Bhalla S, Beasley MB, Sholl
LM, Kerr KM, Gnjatic S, Wistuba II, Rimm DL, Tsao MS and Hirsch FR:
PD-L1 as a biomarker of response to immune-checkpoint inhibitors.
Nat Rev Clin Oncol. 18:345–362. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Herbst RS, Soria JC, Kowanetz M, Fine GD,
Hamid O, Gordon MS, Sosman JA, McDermott DF, Powderly JD, Gettinger
SN, et al: Predictive correlates of response to the anti-PD-L1
antibody MPDL3280A in cancer patients. Nature. 515:563–567. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Herbst RS, Baas P, Kim DW, Felip E,
Pérez-Gracia JL, Han JY, Molina J, Kim JH, Arvis CD, Ahn MJ, et al:
Pembrolizumab versus docetaxel for previously treated,
PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010):
A randomised controlled trial. Lancet. 387:1540–1550. 2016.
View Article : Google Scholar
|
|
126
|
Zou W, Wolchok JD and Chen L: PD-L1
(B7-H1) and PD-1 pathway blockade for cancer therapy: Mechanisms,
response biomarkers, and combinations. Sci Transl Med.
8:328rv42016. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Patel SP and Kurzrock R: PD-L1 expression
as a predictive biomarker in cancer immunotherapy. Mol Cancer Ther.
14:847–856. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Mahoney KM and Atkins MB: Prognostic and
predictive markers for the new immunotherapies. Oncology (Williston
Park). 28(Suppl 3): S39–S48. 2014.
|
|
129
|
Kim J, Myers AC, Chen L, Pardoll DM,
Truong-Tran QA, Lane AP, McDyer JF, Fortuno L and Schleimer RP:
Constitutive and inducible expression of b7 family of ligands by
human airway epithelial cells. Am J Respir Cell Mol Biol.
33:280–289. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Chen J, Jiang CC, Jin L and Zhang XD:
Regulation of PD-L1: A novel role of pro-survival signalling in
cancer. Ann Oncol. 27:409–416. 2016. View Article : Google Scholar
|
|
131
|
Meng X, Huang Z, Teng F, Xing L and Yu J:
Predictive biomarkers in PD-1/PD-L1 checkpoint blockade
immunotherapy. Cancer Treat Rev. 41:868–876. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Mansfield AS, Murphy SJ, Peikert T, Yi ES,
Vasmatzis G, Wigle DA and Aubry MC: Heterogeneity of programmed
cell death ligand 1 expression in multifocal lung cancer. Clin
Cancer Res. 22:2177–2182. 2016. View Article : Google Scholar
|
|
133
|
Topalian SL, Taube JM, Anders RA and
Pardoll DM: Mechanism-driven biomarkers to guide immune checkpoint
blockade in cancer therapy. Nat Rev Cancer. 16:275–287. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Lee V, Murphy A, Le DT and Diaz LA Jr:
Mismatch repair deficiency and response to immune checkpoint
blockade. Oncologist. 21:1200–1211. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Lynch HT, Jascur T, Lanspa S and Boland
CR: Making sense of missense in Lynch syndrome: The clinical
perspective. Cancer Prev Res (Phila). 3:1371–1374. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Boland CR and Goel A: Microsatellite
instability in colorectal cancer. Gastroenterology.
138:2073–2087.e3. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Ratner D and Lennerz JK: Implementing
keytruda/pembrolizumab testing in clinical practice. Oncologist.
23:647–649. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Schwitalle Y, Kloor M, Eiermann S,
Linnebacher M, Kienle P, Knaebel HP, Tariverdian M, Benner A and
von Knebel Doeberitz M: Immune response against frameshift-induced
neopeptides in HNPCC patients and healthy HNPCC mutation carriers.
Gastroenterology. 134:988–997. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Drescher KM, Sharma P, Watson P, Gatalica
Z, Thibodeau SN and Lynch HT: Lymphocyte recruitment into the tumor
site is altered in patients with MSI-H colon cancer. Fam Cancer.
8:231–239. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Llosa NJ, Cruise M, Tam A, Wicks EC,
Hechenbleikner EM, Taube JM, Blosser RL, Fan H, Wang H, Luber BS,
et al: The vigorous immune microenvironment of microsatellite
instable colon cancer is balanced by multiple counter-inhibitory
checkpoints. Cancer Discov. 5:43–51. 2015. View Article : Google Scholar :
|
|
141
|
Goel G and Sun W: Advances in the
management of gastrointestinal cancers-an upcoming role of immune
checkpoint blockade. J Hematol Oncol. 8:862015. View Article : Google Scholar
|
|
142
|
Mouw KW, Goldberg MS, Konstantinopoulos PA
and D'Andrea AD: DNA damage and repair biomarkers of immunotherapy
response. Cancer Discov. 7:675–693. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Alexandrov LB, Nik-Zainal S, Wedge DC,
Aparicio SA, Behjati S, Biankin AV, Bignell GR, Bolli N, Borg A,
Børresen-Dale AL, et al: Signatures of mutational processes in
human cancer. Nature. 500:415–421. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Schumacher TN and Schreiber RD:
Neoantigens in cancer immunotherapy. Science. 348:69–74. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
145
|
Gubin MM, Zhang X, Schuster H, Caron E,
Ward JP, Noguchi T, Ivanova Y, Hundal J, Arthur CD, Krebber WJ, et
al: Checkpoint blockade cancer immunotherapy targets
tumour-specific mutant antigens. Nature. 515:577–581. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Snyder A, Makarov V, Merghoub T, Yuan J,
Zaretsky JM, Desrichard A, Walsh LA, Postow MA, Wong P, Ho TS, et
al: Genetic basis for clinical response to CTLA-4 blockade in
melanoma. N Engl J Med. 371:2189–2199. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
147
|
Van Allen EM, Miao D, Schilling B, Shukla
SA, Blank C, Zimmer L, Sucker A, Hillen U, Foppen MHG, Goldinger
SM, et al: Genomic correlates of response to CTLA-4 blockade in
metastatic melanoma. Science. 350:207–211. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
148
|
Rizvi NA, Hellmann MD, Snyder A, Kvistborg
P, Makarov V, Havel JJ, Lee W, Yuan J, Wong P, Ho TS, et al: Cancer
immunology. Mutational landscape determines sensitivity to PD-1
blockade in non-small cell lung cancer. Science. 348:124–128. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
149
|
Hellmann MD, Callahan MK, Awad MM, Calvo
E, Ascierto PA, Atmaca A, Rizvi NA, Hirsch FR, Selvaggi G,
Szustakowski JD, et al: Tumor mutational burden and efficacy of
nivolumab monotherapy and in combination with ipilimumab in
small-cell lung cancer. Cancer Cell. 33:853–861.e4. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
150
|
Hellmann MD, Ciuleanu TE, Pluzanski A, Lee
JS, Otterson GA, Audigier-Valette C, Minenza E, Linardou H, Burgers
S, Salman P, et al: Nivolumab plus ipilimumab in lung cancer with a
high tumor mutational burden. N Engl J Med. 378:2093–2104. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
151
|
Chalmers ZR, Connelly CF, Fabrizio D, Gay
L, Ali SM, Ennis R, Schrock A, Campbell B, Shlien A, Chmielecki J,
et al: Analysis of 100,000 human c'ancer genomes reveals the
landscape of tumor mutational burden. Genome Med. 9:342017.
View Article : Google Scholar
|
|
152
|
Yarchoan M, Hopkins A and Jaffee EM: Tumor
mutational burden and response rate to PD-1 inhibition. N Engl J
Med. 377:2500–2501. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
153
|
Lawrence MS, Stojanov P, Polak P, Kryukov
GV, Cibulskis K, Sivachenko A, Carter SL, Stewart C, Mermel CH,
Roberts SA, et al: Mutational heterogeneity in cancer and the
search for new cancer-associated genes. Nature. 499:214–218. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
154
|
Pardoll D: Cancer and the immune system:
Basic concepts and targets for intervention. Semin Oncol.
42:523–538. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
155
|
Tumeh PC, Harview CL, Yearley JH, Shintaku
IP, Taylor EJ, Robert L, Chmielowski B, Spasic M, Henry G, Ciobanu
V, et al: PD-1 blockade induces responses by inhibiting adaptive
immune resistance. Nature. 515:568–571. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
156
|
Adams S, Gray RJ, Demaria S, Goldstein L,
Perez EA, Shulman LN, Martino S, Wang M, Jones VE, Saphner TJ, et
al: Prognostic value of tumor-infiltrating lymphocytes in
triple-negative breast cancers from two phase III randomized
adjuvant breast cancer trials: ECOG 2197 and ECOG 1199. J Clin
Oncol. 32:2959–2966. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
157
|
Salgado R, Denkert C, Campbell C, Savas P,
Nuciforo P, Aura C, de Azambuja E, Eidtmann H, Ellis CE, Baselga J,
et al: Tumor-infiltrating lymphocytes and associations with
pathological complete response and event-free survival in
HER2-positive early-stage breast cancer treated with lapatinib and
trastuzumab: a secondary analysis of the NeoALTTO trial. JAMA
Oncol. 1:448–454. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
158
|
Basile D, Pelizzari G, Vitale MG, Lisanti
C, Cinausero M, Iacono D and Puglisi F: Atezolizumab for the
treatment of breast cancer. Expert Opin Biol Ther. 18:595–603.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
159
|
Denkert C, von Minckwitz G, Darb-Esfahani
S, Lederer B, Heppner BI, Weber KE, Budczies J, Huober J, Klauschen
F, Furlanetto J, et al: Tumour-infiltrating lymphocytes and
prognosis in different subtypes of breast cancer: A pooled analysis
of 3771 patients treated with neoadjuvant therapy. Lancet Oncol.
19:40–50. 2018. View Article : Google Scholar
|
|
160
|
Galon J, Costes A, Sanchez-Cabo F,
Kirilovsky A, Mlecnik B, Lagorce-Pagès C, Tosolini M, Camus M,
Berger A, Wind P, et al: Type, density, and location of immune
cells within human colorectal tumors predict clinical outcome.
Science. 313:1960–1964. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
161
|
Pagès F, Mlecnik B, Marliot F, Bindea G,
Ou FS, Bifulco C, Lugli A, Zlobec I, Rau TT, Berger MD, et al:
International validation of the consensus immunoscore for the
classification of colon cancer: A prognostic and accuracy study.
Lancet. 391:2128–2139. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
162
|
Khalili JS, Liu S, Rodríguez-Cruz TG,
Whittington M, Wardell S, Liu C, Zhang M, Cooper ZA, Frederick DT,
Li Y, et al: Oncogenic BRAF(V600E) promotes stromal cell-mediated
immunosuppression via induction of interleukin-1 in melanoma. Clin
Cancer Res. 18:5329–5340. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
163
|
Frederick DT, Piris A, Cogdill AP, Cooper
ZA, Lezcano C, Ferrone CR, Mitra D, Boni A, Newton LP, Liu C, et
al: BRAF inhibition is associated with enhanced melanoma antigen
expression and a more favorable tumor microenvironment in patients
with metastatic melanoma. Clin Cancer Res. 19:1225–1231. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
164
|
Wolchok JD, Chiarion-Sileni V, Gonzalez R,
Rutkowski P, Grob JJ, Cowey CL, Lao CD, Wagstaff J, Schadendorf D,
Ferrucci PF, et al: Overall survival with combined nivolumab and
ipilimumab in advanced melanoma. N Engl J Med. 377:1345–1356. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
165
|
Gao J, Shi LZ, Zhao H, Chen J, Xiong L, He
Q, Chen T, Roszik J, Bernatchez C, Woodman SE, et al: Loss of IFN-γ
pathway genes in tumor cells as a mechanism of resistance to
anti-CTLA-4 therapy. Cell. 167:397–404.e9. 2016. View Article : Google Scholar
|
|
166
|
Zaretsky JM, Garcia-Diaz A, Shin DS,
Escuin-Ordinas H, Hugo W, Hu-Lieskovan S, Torrejon DY,
Abril-Rodriguez G, Sandoval S, Barthly L, et al: Mutations
associated with acquired resistance to PD-1 blockade in melanoma. N
Engl J Med. 375:819–829. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
167
|
Shin DS, Zaretsky JM, Escuin-Ordinas H,
Garcia-Diaz A, Hu-Lieskovan S, Kalbasi A, Grasso CS, Hugo W,
Sandoval S, Torrejon DY, et al: Primary resistance to PD-1 blockade
mediated by JAK1/2 mutations. Cancer Discov. 7:188–201. 2017.
View Article : Google Scholar :
|
|
168
|
Riaz N, Havel JJ, Kendall SM, Makarov V,
Walsh LA, Desrichard A, Weinhold N and Chan TA: Recurrent SERPINB3
and SERPINB4 mutations in patients who respond to anti-CTLA4
immunotherapy. Nat Genet. 48:1327–1329. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
169
|
Peng W, Chen JQ, Liu C, Malu S, Creasy C,
Tetzlaff MT, Xu C, McKenzie JA, Zhang C, Liang X, et al: Loss of
PTEN promotes resistance to T cell-mediated immunotherapy. Cancer
Discov. 6:202–216. 2016. View Article : Google Scholar :
|
|
170
|
George S, Miao D, Demetri GD, Adeegbe D,
Rodig SJ, Shukla S, Lipschitz M, Amin-Mansour A, Raut CP, Carter
SL, et al: Loss of PTEN is associated with resistance to Anti-PD-1
checkpoint blockade therapy in metastatic uterine leiomyosarcoma.
Immunity. 46:197–204. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
171
|
Dong ZY, Zhong WZ, Zhang XC, Su J, Xie Z,
Liu SY, Tu HY, Chen HJ, Sun YL, Zhou Q, et al: Potential predictive
value of TP53 and KRAS mutation status for response to PD-1
blockade immunotherapy in lung adenocarcinoma. Clin Cancer Res.
23:3012–3024. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
172
|
Skoulidis F, Goldberg ME, Greenawalt DM,
Hellmann MD, Awad MM, Gainor JF, Schrock AB, Hartmaier RJ, Trabucco
SE, Gay L, et al: STK11/LKB1 mutations and PD-1 inhibitor
resistance in KRAS-mutant lung adenocarcinoma. Cancer Discov.
8:822–835. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
173
|
Lisberg A, Cummings A, Goldman JW,
Bornazyan K, Reese N, Wang T, Coluzzi P, Ledezma B, Mendenhall M,
Hunt J, et al: A phase II study of pembrolizumab in EGFR-mutant,
PD-L1+, tyrosine kinase inhibitor Naïve patients with advanced
NSCLC. J Thorac Oncol. 13:1138–1145. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
174
|
Cancer Genome Atlas Research Network:
Comprehensive molecular characterization of clear cell renal cell
carcinoma. Nature. 499:43–49. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
175
|
Kapur P, Peña-Llopis S, Christie A,
Zhrebker L, Pavía-Jiménez A, Rathmell WK, Xie XJ and Brugarolas J:
Effects on survival of BAP1 and PBRM1 mutations in sporadic
clear-cell renal-cell carcinoma: A retrospective analysis with
independent validation. Lancet Oncol. 14:159–167. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
176
|
Pawłowski R, Mühl SM, Sulser T, Krek W,
Moch H and Schraml P: Loss of PBRM1 expression is associated with
renal cell carcinoma progression. Int J Cancer. 132:E11–E17. 2013.
View Article : Google Scholar
|
|
177
|
Nam SJ, Lee C, Park JH and Moon KC:
Decreased PBRM1 expression predicts unfavorable prognosis in
patients with clear cell renal cell carcinoma. Urol Oncol.
33:340.e9–e16. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
178
|
Miao D, Margolis CA, Gao W, Voss MH, Li W,
Martini DJ, Norton C, Bossé D, Wankowicz SM, Cullen D, et al:
Genomic correlates of response to immune checkpoint therapies in
clear cell renal cell carcinoma. Science. 359:801–806. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
179
|
Manguso RT, Pope HW, Zimmer MD, Brown FD,
Yates KB, Miller BC, Collins NB, Bi K, LaFleur MW, Juneja VR, et
al: In vivo CRISPR screening identifies Ptpn2 as a cancer
immunotherapy target. Nature. 547:413–418. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
180
|
Patel SJ, Sanjana NE, Kishton RJ,
Eidizadeh A, Vodnala SK, Cam M, Gartner JJ, Jia L, Steinberg SM,
Yamamoto TN, et al: Identification of essential genes for cancer
immunotherapy. Nature. 548:537–542. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
181
|
Pan D, Kobayashi A, Jiang P, Ferrari de
Andrade L, Tay RE, Luoma AM, Tsoucas D, Qiu X, Lim K, Rao P, et al:
A major chromatin regulator determines resistance of tumor cells to
T cell-mediated killing. Science. 359:770–775. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
182
|
Shukla SA, Rooney MS, Rajasagi M, Tiao G,
Dixon PM, Lawrence MS, Stevens J, Lane WJ, Dellagatta JL, Steelman
S, et al: Comprehensive analysis of cancer-associated somatic
mutations in class I HLA genes. Nat Biotechnol. 33:1152–1158. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
183
|
McGranahan N, Rosenthal R, Hiley CT, Rowan
AJ, Watkins TBK, Wilson GA, Birkbak NJ, Veeriah S, Van Loo P,
Herrero J, et al: Allele-specific HLA loss and immune escape in
lung cancer evolution. Cell. 171:1259–1271.e11. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
184
|
Rodig SJ, Gusenleitner D, Jackson DG,
Gjini E, Giobbie-Hurder A, Jin C, Chang H, Lovitch SB, Horak C,
Weber JS, et al: MHC proteins confer differential sensitivity to
CTLA-4 and PD-1 blockade in untreated metastatic melanoma. Sci
Transl Med. 10:eaar33422018. View Article : Google Scholar : PubMed/NCBI
|
|
185
|
Chowell D, Morris LGT, Grigg CM, Weber JK,
Samstein RM, Makarov V, Kuo F, Kendall SM, Requena D, Riaz N, et
al: Patient HLA class I genotype influences cancer response to
checkpoint blockade immunotherapy. Science. 359:582–587. 2018.
View Article : Google Scholar :
|
|
186
|
Simeone E, Gentilcore G, Giannarelli D,
Grimaldi AM, Caracò C, Curvietto M, Esposito A, Paone M, Palla M,
Cavalcanti E, et al: Immunological and biological changes during
ipilimumab treatment and their potential correlation with clinical
response and survival in patients with advanced melanoma. Cancer
Immunol Immunother. 63:675–683. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
187
|
Martens A, Wistuba-Hamprecht K, Geukes
Foppen M, Yuan J, Postow MA, Wong P, Romano E, Khammari A, Dreno B,
Capone M, et al: Baseline peripheral blood biomarkers associated
with clinical outcome of advanced melanoma patients treated with
ipilimumab. Clin Cancer Res. 22:2908–2918. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
188
|
Martens A, Wistuba-Hamprecht K, Yuan J,
Postow MA, Wong P, Capone M, Madonna G, Khammari A, Schilling B,
Sucker A, et al: Increases in absolute lymphocytes and circulating
CD4+ and CD8+ T cells are associated with positive clinical outcome
of melanoma patients treated with ipilimumab. Clin Cancer Res.
22:4848–4858. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
189
|
Wistuba-Hamprecht K, Martens A, Heubach F,
Romano E, Geukes Foppen M, Yuan J, Postow M, Wong P, Mallardo D,
Schilling B, et al: Peripheral CD8 effector-memory type 1 T-cells
correlate with outcome in ipilimumab-treated stage IV melanoma
patients. Eur J Cancer. 73:61–70. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
190
|
Kuzman JA, Stenehjem DD, Merriman J,
Agarwal AM, Patel SB, Hahn AW, Alex A, Albertson D, Gill DM and
Agarwal N: Neutrophil-lymphocyte ratio as a predictive biomarker
for response to high dose interleukin-2 in patients with renal cell
carcinoma. BMC Urol. 17:12017. View Article : Google Scholar : PubMed/NCBI
|
|
191
|
Weide B, Martens A, Hassel JC, Berking C,
Postow MA, Bisschop K, Simeone E, Mangana J, Schilling B, Di
Giacomo AM, et al: Baseline biomarkers for outcome of melanoma
patients treated with pembrolizumab. Clin Cancer Res. 22:5487–5496.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
192
|
Dall'Olio FG, Gelsomino F, Conci N,
Marcolin L, De Giglio A, Grilli G, Sperandi F, Fontana F,
Terracciano M, Fragomeno B, et al: PD-L1 expression in circulating
tumor cells as a promising prognostic biomarker in advanced
non-small-cell lung cancer treated with immune checkpoint
inhibitors. Clin Lung Cancer. 22:423–431. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
193
|
Krieg C, Nowicka M, Guglietta S, Schindler
S, Hartmann FJ, Weber LM, Dummer R, Robinson MD, Levesque MP and
Becher B: High-dimensional single-cell analysis predicts response
to anti-PD-1 immunotherapy. Nat Med. 24:144–153. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
194
|
Yuan J, Zhou J, Dong Z, Tandon S, Kuk D,
Panageas KS, Wong P, Wu X, Naidoo J, Page DB, et al: Pretreatment
serum VEGF is associated with clinical response and overall
survival in advanced melanoma patients treated with ipilimumab.
Cancer Immunol Res. 2:127–132. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
195
|
Zang J, Hu Y, Xu X, Ni J, Yan D, Liu S, He
J, Xue J, Wu J and Feng J: Elevated serum levels of vascular
endothelial growth factor predict a poor prognosis of
platinum-based chemotherapy in non-small cell lung cancer. Onco
Targets Ther. 10:409–415. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
196
|
Powles T, Eder JP, Fine GD, Braiteh FS,
Loriot Y, Cruz C, Bellmunt J, Burris HA, Petrylak DP, Teng SL, et
al: MPDL3280A (anti-PD-L1) treatment leads to clinical activity in
metastatic bladder cancer. Nature. 515:558–562. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
197
|
Schalper KA, Carleton M, Zhou M, Chen T,
Feng Y, Huang SP, Walsh AM, Baxi V, Pandya D, Baradet T, et al:
Elevated serum interleukin-8 is associated with enhanced intratumor
neutrophils and reduced clinical benefit of immune-checkpoint
inhibitors. Nat Med. 26:688–692. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
198
|
Chen G, Huang AC, Zhang W, Zhang G, Wu M,
Xu W, Yu Z, Yang J, Wang B, Sun H, et al: Exosomal PD-L1
contributes to immunosuppression and is associated with anti-PD-1
response. Nature. 560:382–386. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
199
|
Yang Y, Li CW, Chan LC, Wei Y, Hsu JM, Xia
W, Cha JH, Hou J, Hsu JL, Sun L and Hung MC: Exosomal PD-L1 harbors
active defense function to suppress T cell killing of breast cancer
cells and promote tumor growth. Cell Res. 28:862–864. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
200
|
Tucci M, Passarelli A, Mannavola F, Stucci
LS, Ascierto PA, Capone M, Madonna G, Lopalco P and Silvestris F:
Serum exosomes as predictors of clinical response to ipilimumab in
metastatic melanoma. Oncoimmunology. 7:e13877062017. View Article : Google Scholar
|
|
201
|
Garrett WS: Cancer and the microbiota.
Science. 348:80–86. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
202
|
Zitvogel L, Ayyoub M, Routy B and Kroemer
G: Microbiome and anticancer immunosurveillance. Cell. 165:276–287.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
203
|
Vétizou M, Pitt JM, Daillère R, Lepage P,
Waldschmitt N, Flament C, Rusakiewicz S, Routy B, Roberti MP, Duong
CP, et al: Anticancer immunotherapy by CTLA-4 blockade relies on
the gut microbiota. Science. 350:1079–1084. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
204
|
Sivan A, Corrales L, Hubert N, Williams
JB, Aquino-Michaels K, Earley ZM, Benyamin FW, Lei YM, Jabri B,
Alegre ML, et al: Commensal Bifidobacterium promotes antitumor
immunity and facilitates anti-PD-L1 efficacy. Science.
350:1084–1089. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
205
|
Routy B, Le Chatelier E, Derosa L, Duong
CPM, Alou MT, Daillère R, Fluckiger A, Messaoudene M, Rauber C,
Roberti MP, et al: Gut microbiome influences efficacy of PD-1-based
immunotherapy against epithelial tumors. Science. 359:91–97. 2018.
View Article : Google Scholar
|
|
206
|
Gopalakrishnan V, Spencer CN, Nezi L,
Reuben A, Andrews MC, Karpinets TV, Prieto PA, Vicente D, Hoffman
K, Wei SC, et al: Gut microbiome modulates response to anti-PD-1
immunotherapy in melanoma patients. Science. 359:97–103. 2018.
View Article : Google Scholar
|
|
207
|
Matson V, Fessler J, Bao R, Chongsuwat T,
Zha Y, Alegre ML, Luke JJ and Gajewski TF: The commensal microbiome
is associated with anti-PD-1 efficacy in metastatic melanoma
patients. Science. 359:104–108. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
208
|
Arora S, Velichinskii R, Lesh RW, Ali U,
Kubiak M, Bansal P, Borghaei H, Edelman MJ and Boumber Y: Existing
and emerging biomarkers for immune checkpoint immunotherapy in
solid tumors. Adv Ther. 36:2638–2678. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
209
|
Sucker A, Zhao F, Real B, Heeke C,
Bielefeld N, Maβen S, Horn S, Moll I, Maltaner R, Horn PA, et al:
Genetic evolution of T-cell resistance in the course of melanoma
progression. Clin Cancer Res. 20:6593–6604. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
210
|
Rooney MS, Shukla SA, Wu CJ, Getz G and
Hacohen N: Molecular and genetic properties of tumors associated
with local immune cytolytic activity. Cell. 160:48–61. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
211
|
Sade-Feldman M, Jiao YJ, Chen JH, Rooney
MS, Barzily-Rokni M, Eliane JP, Bjorgaard SL, Hammond MR, Vitzthum
H, Blackmon SM, et al: Resistance to checkpoint blockade therapy
through inactivation of antigen presentation. Nat Commun.
8:11362017. View Article : Google Scholar : PubMed/NCBI
|
|
212
|
Gettinger S, Choi J, Hastings K, Truini A,
Datar I, Sowell R, Wurtz A, Dong W, Cai G, Melnick MA, et al:
Impaired HLA class I antigen processing and presentation as a
mechanism of acquired resistance to immune checkpoint inhibitors in
lung cancer. Cancer Discov. 7:1420–1435. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
213
|
Benci JL, Xu B, Qiu Y, Wu TJ, Dada H,
Twyman-Saint Victor C, Cucolo L, Lee DSM, Pauken KE, Huang AC, et
al: Tumor interferon signaling regulates a multigenic resistance
program to immune checkpoint blockade. Cell. 167:1540–1554.e12.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
214
|
Spranger S, Bao R and Gajewski TF:
Melanoma-intrinsic β-catenin signalling prevents anti-tumour
immunity. Nature. 523:231–235. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
215
|
Wang B, Tian T, Kalland KH, Ke X and Qu Y:
Targeting Wnt/β-catenin signaling for cancer immunotherapy. Trends
Pharmacol Sci. 39:648–658. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
216
|
Cancer Genome Atlas Network: Genomic
classification of cutaneous melanoma. Cell. 161:1681–1696. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
217
|
Sumimoto H, Imabayashi F, Iwata T and
Kawakami Y: The BRAF-MAPK signaling pathway is essential for
cancer-immune evasion in human melanoma cells. J Exp Med.
203:1651–1656. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
218
|
Koyama S, Akbay EA, Li YY, Herter-Sprie
GS, Buczkowski KA, Richards WG, Gandhi L, Redig AJ, Rodig SJ,
Asahina H, et al: Adaptive resistance to therapeutic PD-1 blockade
is associated with upregulation of alternative immune checkpoints.
Nat Commun. 7:105012016. View Article : Google Scholar : PubMed/NCBI
|
|
219
|
Gao J, Ward JF, Pettaway CA, Shi LZ,
Subudhi SK, Vence LM, Zhao H, Chen J, Chen H, Efstathiou E, et al:
VISTA is an inhibitory immune checkpoint that is increased after
ipilimumab therapy in patients with prostate cancer. Nat Med.
23:551–555. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
220
|
Saleh R and Elkord E: Acquired resistance
to cancer immunotherapy: Role of tumor-mediated immunosuppression.
Semin Cancer Biol. 65:13–27. 2020. View Article : Google Scholar
|
|
221
|
Brabletz T, Kalluri R, Nieto MA and
Weinberg RA: EMT in cancer. Nat Rev Cancer. 18:128–134. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
222
|
Wang L, Saci A, Szabo PM, Chasalow SD,
Castillo-Martin M, Domingo-Domenech J, Siefker-Radtke A, Sharma P,
Sfakianos JP, Gong Y, et al: EMT- and stroma-related gene
expression and resistance to PD-1 blockade in urothelial cancer.
Nat Commun. 9:35032018. View Article : Google Scholar : PubMed/NCBI
|
|
223
|
Terry S, Savagner P, Ortiz-Cuaran S,
Mahjoubi L, Saintigny P, Thiery JP and Chouaib S: New insights into
the role of EMT in tumor immune escape. Mol Oncol. 11:824–846.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
224
|
Arce Vargas F, Furness AJS, Solomon I,
Joshi K, Mekkaoui L, Lesko MH, Miranda Rota E, Dahan R, Georgiou A,
Sledzinska A, et al: Fc-optimized anti-CD25 depletes
tumor-infiltrating regulatory T cells and synergizes with PD-1
blockade to eradicate established tumors. Immunity. 46:577–586.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
225
|
Shitara K and Nishikawa H: Regulatory T
cells: A potential target in cancer immunotherapy. Ann N Y Acad
Sci. 1417:104–115. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
226
|
Gebhardt C, Sevko A, Jiang H,
Lichtenberger R, Reith M, Tarnanidis K, Holland-Letz T, Umansky L,
Beckhove P, Sucker A, et al: Myeloid cells and related chronic
inflammatory factors as novel predictive markers in melanoma
treatment with ipilimumab. Clin Cancer Res. 21:5453–5459. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
227
|
Ruffell B and Coussens LM: Macrophages and
therapeutic resistance in cancer. Cancer Cell. 27:462–472. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
228
|
Arlauckas SP, Garris CS, Kohler RH,
Kitaoka M, Cuccarese MF, Yang KS, Miller MA, Carlson JC, Freeman
GJ, Anthony RM, et al: In vivo imaging reveals a tumor-associated
macrophage-mediated resistance pathway in anti-PD-1 therapy. Sci
Transl Med. 9:eaal36042017. View Article : Google Scholar : PubMed/NCBI
|
|
229
|
Mariathasan S, Turley SJ, Nickles D,
Castiglioni A, Yuen K, Wang Y, Kadel EE III, Koeppen H, Astarita
JL, Cubas R, et al: TGFβ attenuates tumour response to PD-L1
blockade by contributing to exclusion of T cells. Nature.
554:544–548. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
230
|
Highfill SL, Cui Y, Giles AJ, Smith JP,
Zhang H, Morse E, Kaplan RN and Mackall CL: Disruption of
CXCR2-mediated MDSC tumor trafficking enhances anti-PD1 efficacy.
Sci Transl Med. 6:237ra672014. View Article : Google Scholar : PubMed/NCBI
|
|
231
|
Holmgaard RB, Zamarin D, Munn DH, Wolchok
JD and Allison JP: Indoleamine 2,3-dioxygenase is a critical
resistance mechanism in antitumor T cell immunotherapy targeting
CTLA-4. J Exp Med. 210:1389–1402. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
232
|
Holmgaard RB, Zamarin D, Li Y, Gasmi B,
Munn DH, Allison JP, Merghoub T and Wolchok JD: Tumor-expressed IDO
recruits and activates MDSCs in a treg-dependent manner. Cell Rep.
13:412–424. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
233
|
Sharma P, Hu-Lieskovan S, Wargo JA and
Ribas A: Primary, adaptive, and acquired resistance to cancer
immunotherapy. Cell. 168:707–723. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
234
|
O'Donnell JS, Hoefsmit EP, Smyth MJ, Blank
CU and Teng MWL: The promise of neoadjuvant immunotherapy and
surgery for cancer treatment. Clin Cancer Res. 25:5743–5751. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
235
|
Liu J, Blake SJ, Yong MC, Harjunpää H,
Ngiow SF, Takeda K, Young A, O'Donnell JS, Allen S, Smyth MJ and
Teng MW: Improved efficacy of neoadjuvant compared to adjuvant
immunotherapy to eradicate metastatic disease. Cancer Discov.
6:1382–1399. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
236
|
Amaria RN, Reddy SM, Tawbi HA, Davies MA,
Ross MI, Glitza IC, Cormier JN, Lewis C, Hwu WJ, Hanna E, et al:
Neoadjuvant immune checkpoint blockade in high-risk resectable
melanoma. Nat Med. 24:1649–1654. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
237
|
Liu SY, Dong S, Liao RQ, Jiang B, Zhang
JT, Lin JT, Zhang S, Yang J, Nie Q, Yang X, et al: LBA2 phase II
study of PD-L1 expression guidance on neoadjuvant (NA) Nivolumab
(Nivo) monotherapy with or without platinum-doublet chemotherapy in
resectable NSCLC. ESMO. 16(Suppl 1): S103632022.
|
|
238
|
Hannani D, Sistigu A, Kepp O, Galluzzi L,
Kroemer G and Zitvogel L: Prerequisites for the antitumor
vaccine-like effect of chemotherapy and radiotherapy. Cancer J.
17:351–358. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
239
|
Teng F, Kong L, Meng X, Yang J and Yu J:
Radiotherapy combined with immune checkpoint blockade
immunotherapy: Achievements and challenges. Cancer Lett. 365:23–29.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
240
|
Ngiow SF, McArthur GA and Smyth MJ:
Radiotherapy complements immune checkpoint blockade. Cancer Cell.
27:437–438. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
241
|
Twyman-Saint Victor C, Rech AJ, Maity A,
Rengan R, Pauken KE, Stelekati E, Benci JL, Xu B, Dada H, Odorizzi
PM, et al: Radiation and dual checkpoint blockade activate
non-redundant immune mechanisms in cancer. Nature. 520:373–377.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
242
|
Langer CJ, Gadgeel SM, Borghaei H,
Papadimitrakopoulou VA, Patnaik A, Powell SF, Gentzler RD, Martins
RG, Stevenson JP, Jalal SI, et al: Carboplatin and pemetrexed with
or without pembrolizumab for advanced, non-squamous non-small-cell
lung cancer: A randomised, phase 2 cohort of the open-label
KEYNOTE-021 study. Lancet Oncol. 17:1497–1508. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
243
|
Wang C, Wang J, Zhang X, Yu S, Wen D, Hu
Q, Ye Y, Bomba H, Hu X, Liu Z, et al: In situ formed reactive
oxygen species-responsive scaffold with gemcitabine and checkpoint
inhibitor for combination therapy. Sci Transl Med. 10:eaan36822018.
View Article : Google Scholar : PubMed/NCBI
|
|
244
|
Larkin J, Chiarion-Sileni V, Gonzalez R,
Grob JJ, Cowey CL, Lao CD, Schadendorf D, Dummer R, Smylie M,
Rutkowski P, et al: Combined nivolumab and ipilimumab or
monotherapy in untreated melanoma. N Engl J Med. 373:23–34. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
245
|
Redmond WL, Linch SN and Kasiewicz MJ:
Combined targeting of costimulatory (OX40) and coinhibitory
(CTLA-4) pathways elicits potent effector T cells capable of
driving robust antitumor immunity. Cancer Immunol Res. 2:142–153.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
246
|
Mayes PA, Hance KW and Hoos A: The promise
and challenges of immune agonist antibody development in cancer.
Nat Rev Drug Discov. 17:509–527. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
247
|
Popovic A, Jaffee EM and Zaidi N: Emerging
strategies for combination checkpoint modulators in cancer
immunotherapy. J Clin Invest. 128:3209–3218. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
248
|
Kuai R, Ochyl LJ, Bahjat KS, Schwendeman A
and Moon JJ: Designer vaccine nanodiscs for personalized cancer
immunotherapy. Nat Mater. 16:489–496. 2017. View Article : Google Scholar :
|
|
249
|
Madan RA, Mohebtash M, Arlen PM, Vergati
M, Rauckhorst M, Steinberg SM, Tsang KY, Poole DJ, Parnes HL,
Wright JJ, et al: Ipilimumab and a poxviral vaccine targeting
prostate-specific antigen in metastatic castration-resistant
prostate cancer: A phase 1 dose-escalation trial. Lancet Oncol.
13:501–508. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
250
|
Chesney J, Puzanov I, Collichio F, Singh
P, Milhem MM, Glaspy J, Hamid O, Ross M, Friedlander P, Garbe C, et
al: Randomized, open-label phase II study evaluating the efficacy
and safety of talimogene laherparepvec in combination with
ipilimumab versus ipilimumab alone in patients with advanced,
unresectable melanoma. J Clin Oncol. 36:1658–1667. 2018. View Article : Google Scholar :
|
|
251
|
Liu X, Ranganathan R, Jiang S, Fang C, Sun
J, Kim S, Newick K, Lo A, June CH, Zhao Y and Moon EK: A chimeric
switch-receptor targeting PD1 augments the efficacy of
second-generation CAR T cells in advanced solid tumors. Cancer Res.
76:1578–1590. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
252
|
Gay F, D'Agostino M, Giaccone L, Genuardi
M, Festuccia M, Boccadoro M and Bruno B: Immuno-oncologic
approaches: CAR-T cells and checkpoint inhibitors. Clin Lymphoma
Myeloma Leuk. 17:471–478. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
253
|
De Henau O, Rausch M, Winkler D, Campesato
LF, Liu C, Cymerman DH, Budhu S, Ghosh A, Pink M, Tchaicha J, et
al: Overcoming resistance to checkpoint blockade therapy by
targeting PI3Kγ in myeloid cells. Nature. 539:443–447. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
254
|
Abdel-Wahab N, Shah M and Suarez-Almazor
ME: Adverse events associated with immune checkpoint blockade in
patients with cancer: A systematic review of case reports. PLoS
One. 11:e01602212016. View Article : Google Scholar : PubMed/NCBI
|
|
255
|
Palmieri DJ and Carlino MS: Immune
checkpoint inhibitor toxicity. Curr Oncol Rep. 20:722018.
View Article : Google Scholar : PubMed/NCBI
|
|
256
|
Boutros C, Tarhini A, Routier E, Lambotte
O, Ladurie FL, Carbonnel F, Izzeddine H, Marabelle A, Champiat S,
Berdelou A, et al: Safety profiles of anti-CTLA-4 and anti-PD-1
antibodies alone and in combination. Nat Rev Clin Oncol.
13:473–486. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
257
|
Cousin S, Seneschal J and Italiano A:
Toxicity profiles of immunotherapy. Pharmacol Ther. 181:91–100.
2018. View Article : Google Scholar
|
|
258
|
Sznol M, Ferrucci PF, Hogg D, Atkins MB,
Wolter P, Guidoboni M, Lebbé C, Kirkwood JM, Schachter J, Daniels
GA, et al: Pooled analysis safety profile of nivolumab and
ipilimumab combination therapy in patients with advanced melanoma.
J Clin Oncol. 35:3815–3822. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
259
|
Kottschade LA: Incidence and management of
immune-related adverse events in patients undergoing treatment with
immune checkpoint inhibitors. Curr Oncol Rep. 20:242018. View Article : Google Scholar : PubMed/NCBI
|
|
260
|
Martins F, Sofiya L, Sykiotis GP, Lamine
F, Maillard M, Fraga M, Shabafrouz K, Ribi C, Cairoli A,
Guex-Crosier Y, et al: Adverse effects of immune-checkpoint
inhibitors: Epidemiology, management and surveillance. Nat Rev Clin
Oncol. 16:563–580. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
261
|
Haanen JBAG, Carbonnel F, Robert C, Kerr
KM, Peters S, Larkin J and Jordan K; ESMO Guidelines Committee:
Management of toxicities from immunotherapy: ESMO clinical practice
guidelines for diagnosis, treatment and follow-up. Ann Oncol.
28(Suppl 4): iv119–iv142. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
262
|
Sullivan RJ and Weber JS: Immune-related
toxicities of checkpoint inhibitors: Mechanisms and mitigation
strategies. Nat Rev Drug Discov. 21:495–508. 2022. View Article : Google Scholar
|