Ovarian cancer (OC) is considered to be the most
lethal of the three major types of gynecological cancer known,
which also include cervical and endometrial cancer (1). Different histological subtypes of OC
can be distinguished by their unique combination of risk factors,
cellular origins, molecular profile, clinical characteristics and
response to treatment. Epithelial ovarian cancer (EOC) accounts for
90% of all ovarian tumors, which can then be further subdivided
into the following four subtypes: Plasmacytoma, endometrioid
carcinoma, clear cell carcinoma and mucinous carcinoma. In total,
~10% ovarian malignancies are classified as non-epithelial, which
includes germ cell tumors, gonadal mesenchymal tumors and
metastatic tumors (2,3). As the principal female reproductive
organ, the ovaries are in charge of oogenesis, female sex hormone
production and secretion. Previous research indicates that OC
originates in the fallopian tubes rather than the ovary, as
previously thought (4,5). Ovarian malignancies are difficult to
detect in the early stages due to their position in the peritoneal
cavity and being shielded and they are frequently discovered in the
late stages. However, detecting early atypical OC remains difficult
due to molecular similarities between cells in the fallopian tubes,
ovaries and peritoneum (4).
Ovarian cancer is exceedingly common, second only to breast cancer
in terms of incidence and it has the greatest mortality rate among
the three primary gynecologic malignancies, posing a substantial
threat to women. OC is a very aggressive cancer that is resistant
to treatment, has a high recurrence rate and has a low 5-year
survival rate (6-9). In comparison to other gynecological
cancers, research in this field is wanting and needs more attention
and exploration. As a result, identifying early diagnostic markers
and developing further focused therapy options for this cancer is
critical.
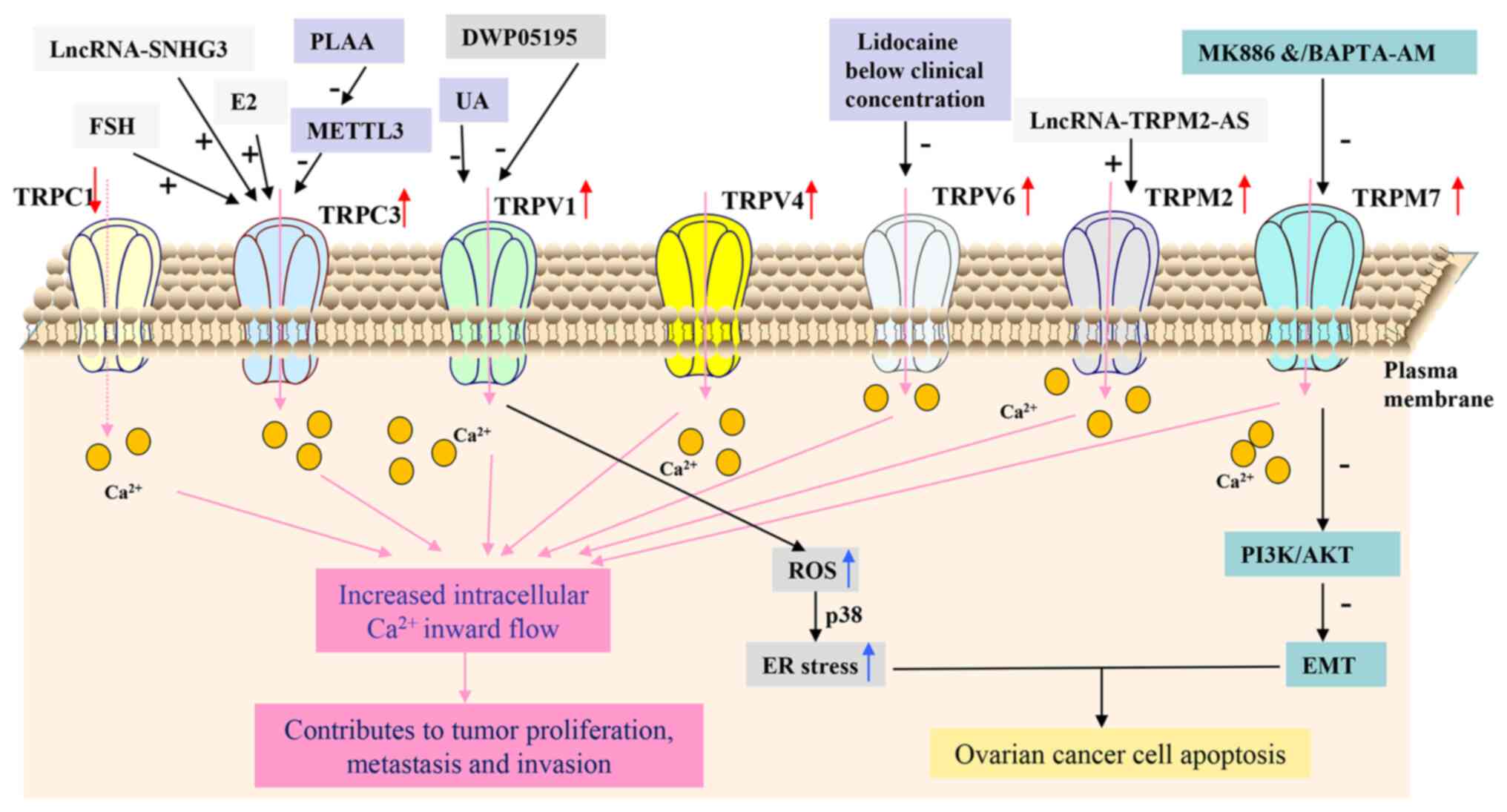
TRPC is a subfamily of TRP channels that can be
activated by hormones and growth factors, which can mediate
Ca2+ transport (19).
TRPC1, which is widely expressed, is involved in various
physiological processes, including cancer development (20), cell proliferation,
differentiation, migration, membrane permeability, fluid secretion
and apoptosis (21). In OC, the
mRNA expression levels of TRPC1 have been reported to be
significantly decreased, especially in drug-resistant cases. In
addition, this decrease may be associated with higher histological
tumor grades and drug resistance (22).
TRPC3 is an important member of the TRPC family and
has been shown to be involved in tumor proliferation, metastasis
and invasion in OC (23). The
protein levels of TRPC3 are considerably higher in human OC samples
compared with those in normal ovarian tissue (23-26). Relapse, metastasis and a poor
prognosis in human OC have all been associated with high TRPC3
expression (24-26). Downregulating TRPC3 expression in
human OC cells leads to a reduction in cell proliferation through
the suppression of epidermal growth factor-induced Ca2+
influx, dephosphorylation of cell division cycle 2 and
Ca2+/calmodulin (CaM)-dependent protein kinase IIα, in
addition to prolonged M phase progression (23). By contrast, follicle-stimulating
hormone (FSH), estrogen and long chain noncoding RNA (lncRNA) small
nucleolar RNA host gene (SNHG)3 can upregulate TRPC3 expression,
which contributes to the progression of human OC (24-26). Additionally, phospholipase
A2-activated protein has been found to inhibit OC cell invasion and
tumor metastasis via decreasing the levels of m6A-modified TRPC3
mRNA by inhibiting methyltransferase-like 3 expressions (27). Therefore, TRPC3 probably serves a
significant role in the progression of human OC, rendering it a
potential diagnostic and therapeutic target for this malignancy. In
particular, TRPC3 downregulation in aging fibroblasts has been
documented to increase endoplasmic-reticulum (ER)-mitochondrial
Ca2+ transfer, which enhances oxidative phosphorylation
in mitochondria and promotes the release of tumor-promoting
molecules such as interleukin-8 and matrix metalloproteinase 1
(28). However, it remains
unclear whether this mechanism would have a counteracting effect on
the downregulation of TRPC3 in the treatment pathway for OC, for
which further research is required.
TRPV1 is a non-selective cation channel that belongs
to the TRP channels family. It is particularly sensitive to
capsaicin, heat, protons, lipids, phorbols and phosphorylation
(29,30). Aberrant expression of TRPV1 has
been associated with malignant tumors in the female reproductive
system, including breast, ovarian and cervical cancer (31-33). During the development and
progression of OC, Han et al (33) previously found that high TRPV1
expression was present in the tissues of ovarian malignancies,
particularly in the plasma-type EOC. Therefore, it was proposed
that high TRPV1 expression can be applied as an independent
prognostic factor for the overall survival of patients with OC. In
addition, Han et al (33)
found that the expression of PTEN, a dual-lipoprotein phosphatase,
was negatively correlated with that of TRPV1 expression in
late-stage OC, whereby high TRPV1/low PTEN was confirmed by Cox
regression analysis to be a significant predictor of prognosis in
patients with OC. Subsequent in vitro functional studies
revealed that inhibiting TRPV1 can prevent the development of OC
cells (33). In another previous
study, Wang et al (34)
found that the TRPV1 antagonist DWP05195 significantly suppressed
the proliferation of five human OC cell lines A2780, SKOV3, OVCAR3,
TOV-21G and Hey8A by inducing C/EBP homologous protein expression,
ER stress and apoptosis through the accumulation of reactive oxygen
species (ROS). Cisplatin, which is used to treat OC, is known to
increase the risk of cytotoxicity. Ursolic acid treatment has been
reported to effectively prevent the development of cytotoxicity by
inhibiting the TRPV1/-Ca2+/calpain signaling pathway in
the cochlea (35). At present,
TRPV1 is one of the most extensively researched TRP channels.
However, the mechanism underlying its involvement in the
development of OC requires further study. These promising findings
provide a path for the future investigation of TRPV1 as a possible
therapeutic target for OC.
TRPV2 is typically found inside the cell membrane
and has been shown to regulate a number of pathological processes,
including cancer, through a signaling route that occurs outside the
membrane (36). TRPV2 activation
has been found to promote cell migration and cell invasiveness,
while the absence or modification of TRPV2-mediated signaling can
lead to uncontrolled proliferation and apoptotic (37). Cannabidiol (CBD) has been shown to
bind to the TRPV2 channel and has been associated with the
dysregulation of proliferation, cell differentiation and invasion
in a variety of cancer cell lines and animal models (36). CBD treatment of endometrial cancer
has been reported to reverse the cytotoxic effects of
chemotherapeutic agents, which is also enhanced by TRPV2
overexpression. Antitumor effects of CBD on OC have been previously
observed, both as a potential monotherapy and in combination with
conventional chemotherapeutic agents (36). Using PLGA-microparticles as
carriers of CBD in combination with paclitaxel, the therapeutic
efficacy for OC was increased without any worsening of
paclitaxel-related side effects (38). However, whether CBD can improve
chemotherapy prognosis for patients with OC by targeting TRPV2
remains to be elucidated. Additionally, TRPV2 also been proposed to
be a novel marker for type II EOC, especially for the plasmacytic
subtypes and high-grade tumors (39). Further research is required to
fully establish the role of TRPV2 in OC.
The present section discussed the TRP channels that
are most likely associated with OC, including TRPC1, TRPC3, TRPV1,
TRPV2, TRPV4, TRPV6, TRPM2 and TRPM7. While these channels have
been associated with various physiological processes, such as cell
proliferation, apoptosis, migration and invasion, in addition to
[Ca2+]i regulation, the precise mechanisms underlying
their roles in OC development remain to be elucidated.
Additionally, there are other TRP channels associated with cancer,
but their links to OC have not been confirmed. Therefore, further
research is required to fully understand the role and mechanism of
TRP channels in the development and therapeutic intervention of OC.
Fig. 1 and Table I summarize the TRP channels
discussed and their possible associations with OC.
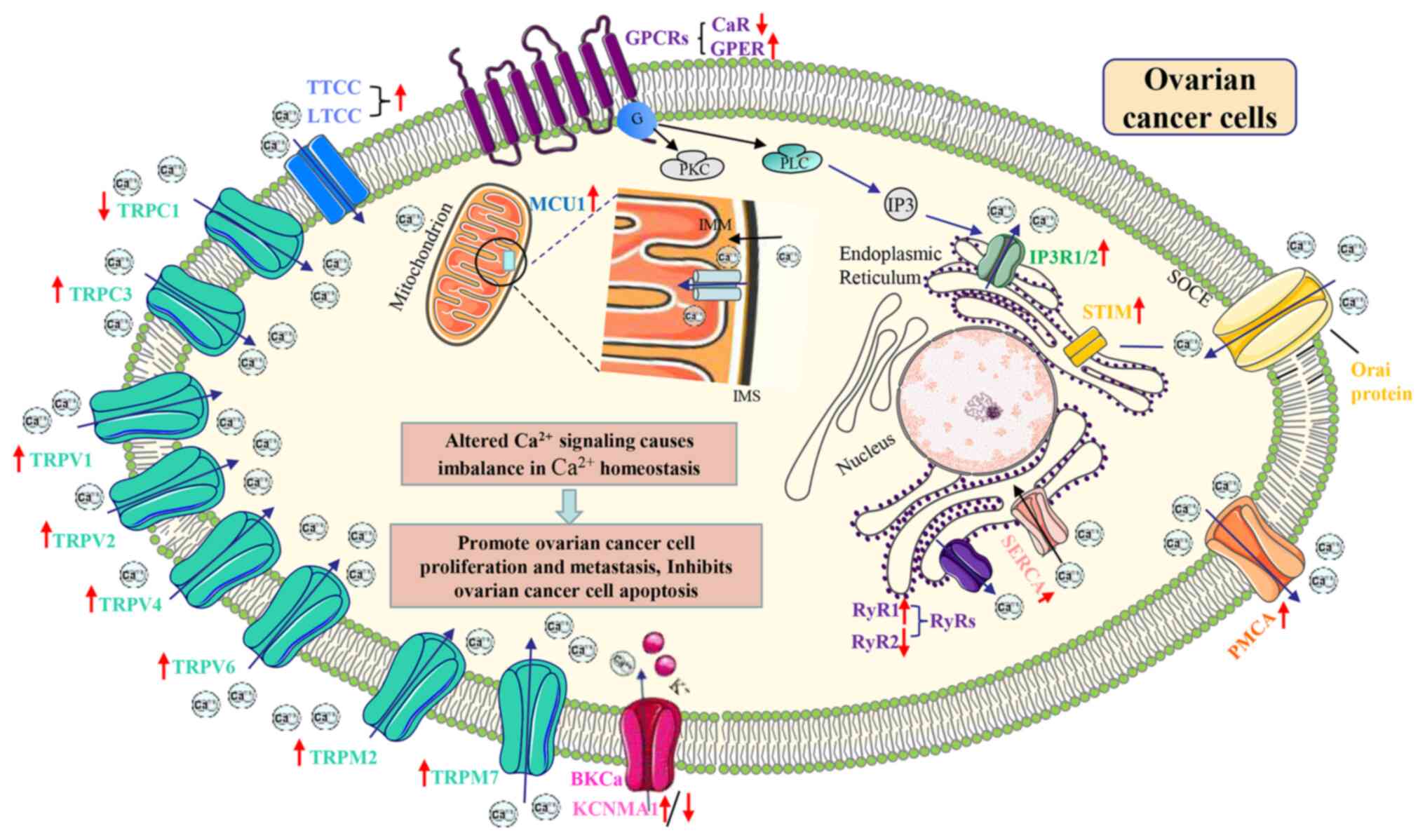
In addition, when the LTCC is affected by adverse
factors, such as serum gonadotropins and lysophosphatidic acid
(LPA), resulting in its abnormal activation of Ca2+
inward flow, it is strongly associated with the proliferation and
metastasis of OC cells (66,67). Nifedipine (a LTCC blocker) could
inhibit LPA-induced OC cell migration and adhesion (67). OC stem cells are a major
contributor to drug resistance in OC patients. LTCC blockers
(manidipine, lacidipine, benidipine and lomepizine) and trimebutine
maleate could inhibit the viability and proliferation of OC stem
cells by downregulating the expression of the LTCC gene, thereby
inducing apoptosis (68,69). CACNA1C, as an important type of
LTCC ion transmembrane channel, plays regulatory roles in the
development and progress of multiple tumors. Chang and Dong
(70) revealed that CACNA1C could
be a prognostic predictor of overall survival in OC and it was
closely related to immunity. In conclusion, the current research
indicates that TTCC and LTCC play an important role in OC cell
proliferation, cell cycle progression and metastasis and is also
linked to OC prognosis (Table
II).
The BKCa channel has been implicated in human cancer
development, including OC, by contributing to cell cycle
disruption, proliferation and migration (77,78). The BKCa channel opener NS1619 has
been found to reduce proliferation while inducing apoptosis in OC
cells by upregulating death-inducing proteins (such as P53, P21 and
Bax) (79). The α-subunit of BKCa
channel is encoded by the Ca2+-activated potassium
channel subunit α-1 (KCNMA1) gene, which has been shown to serve a
role in the formation of macromolecular signaling complexes through
the action of local Ca2+ introductory channels (80). The BKCa channel subunit KCNMA1
contributes to macromolecular signaling complexes, whereby KCNMA1
amplification is associated with higher proliferation rates and
higher degrees of malignancy in ovarian, endometrial and breast
cancers (81,82). However, a study reported that
knocking out KCNMA1 expression increases cisplatin resistance in OC
cells (68). A recent study found
that trimebutine maleate inhibits the viability of OC stem cells by
targeting the BKCa channel and can prevent drug resistance and
recurrence in OC (69). Further
investigation into the precise mechanism of BKCa channel-regulated
proliferation, apoptosis and resistance in OC cells is necessary to
determine their potential as biomarkers or therapeutic targets for
OC (Table II).
GPCRs form a class of receptor proteins that can
activate G proteins to elicit cascade reactions affecting a wide
range of biological functions including cancer progression
(83,84). GPCRs can exert different types of
effects on OC (Table III). The
Ca2+-sensing receptor (CaR) is a GPCR that mediates
Ca2+ signaling and disrupts normal epidermal
differentiation by sensing extracellular Ca2+ (85). The CaR rs17251221 G allele has
reported protective effects, reducing the risk of OC development
(86,87). By contrast,
lysophosphatidylglycerol-induced proliferation and migration of
human OC cells are mediated by pertussis toxin-sensitive GPCRs
(88). Previous studies have
highlighted the role of G protein-coupled estrogen receptor (GPER)
in OC pathogenesis, where its high expression is associated with
malignant OC, tumor cell invasion and poorer patient survival
(89,90). GPER activation may be involved in
OC initiation and progression, although GPER has also been reported
as possessing anti-cancer properties (91,92). Notably, OC cells treated with
GPER-specific agonist G1 exhibited increased levels of
apoptosis and impeded cancer progression (93). GPCRs are primarily stimulated by
most neurotransmitters and inflammation-related ligands, which can
in turn promote OC proliferation, metastasis and invasion, as
reviewed by Predescu et al (94). Despite the scarcity of animal
models and clinically relevant data, GPCRs have emerged as
promising therapeutic targets for OC (94). It is reported that
2-thioureidothiophene-3-carboxylates (TUTPs), a novel class of
antagonists for the GPCR C-X-C chemokine receptor type 2,
effectively inhibits C-X-C motif ligand 8-mediated cell migration
while exhibiting a synergistic effect with doxorubicin on OC cells
(95). These findings suggest
that TUTPs hold promise as potential anticancer agent for OC
treatment, highlighting the potential of GPCR-based approaches for
OC therapy.
Compared with IP3R1, IP3R3 has been shown to exert
both pro-proliferative and anti-apoptotic effects on cancer cells.
Elevated IP3R3 expression levels have been observed to enhance the
migratory and invasive properties of cancer cells by increasing
mitochondrial metabolism and driving anabolic pathways (107,108). By contrast, a recent study found
that OC cells become more resistant to chemotherapy-induced
apoptosis after IP3R3-mediated Ca2+ flux to mitochondria
was blocked (109). Therefore,
further research is necessary to elucidate the function of IP3R3 in
OC cells. By comparison, IP3R2 has received less attention.
Nonetheless, increased expression of the IP3R2 gene was observed in
iron-treated epithelial OC cells and cisplatin-resistant cells of
the same cell line, indicating its probable role in the development
and progression of OC (110).
It is known that the electron transport chain (ETC)
drives physiological mitochondrial Ca2+ uptake. However,
ETC overload and partial ETC inhibition can cause ROS production,
leading to oxidative damage to the mitochondrial membrane. This in
turn results in cell death and ROS-dependent tumor cell metastasis
and invasion (131-133). Several studies have shown that
modifying the Ca2+ concentration in mitochondria can be
a potential treatment method for OC (134-136). Gentisyl alcohol, which has
antibacterial, antifungal, antiviral and anticancer properties, is
observed to inhibit cell proliferation while inducing apoptosis in
human OC cells through DNA fragmentation (134). In addition, β-Sitosterol
(135), Campesterol (136), Stigmasterol (137), Osthole (138), Fucosterol (139), Laminarin (140), Chrysophanol (141), Chrysin (142) and Epothilone B (143) have all been shown to increase
ROS production by dose-dependently elevating Ca2+
concentrations in the cytoplasm and mitochondria of OC cells,
leading to oxidative stress through the endogenous pathway and
initiate apoptotic signaling (Table
IV). Mitochondrial Ca2+ overload activates the
unfolded protein response and the ER/mitochondrial axis, which then
disrupt [Ca2+]i homeostasis, initiate apoptosis and
inhibit cell proliferation (144). Treatment of cells with
β-Sitosterol or Campesterol impairs mitochondrial membrane
function, leading to the loss of membrane potential and disruption
of Ca2+ homeostasis (135,136). Furthermore, laminarin suppresses
the expression of the ER mitochondrial coupling protein
glucose-regulated protein 75 (GRP75) in OC cells (140), where the lack of GRP75
expression has been associated with Ca2+ overload
(145).
The high mortality rate of OC is largely attributed
to its resistance to currently available chemotherapeutic drugs
(7). Cisplatin is commonly used
for the treatment of malignant OC, but acquired resistance limits
its application. The inability to upregulate [Ca2+]i in
OC cells results in cisplatin resistance by reducing oxidative
stress (146). Bcl-2, a key
regulator of survival and apoptosis, is known to block
cisplatin-induced apoptosis by regulating Ca2+ signaling
in various cancer cell lines. Bcl-2 overexpression inhibits ER
mitochondrial Ca2+ signaling and increases cisplatin
resistance in OC cells (147).
ABT-737, a small-molecule Bcl-2 inhibitor, has been shown to
increase free Ca2+ levels in the mitochondria in
combination with cisplatin treatment of cisplatin-resistant OC
cells, thereby enhancing mitochondria-mediated cell apoptosis
(148). Increased mitochondrial
Ca2+ may induce apoptosis in cisplatin-resistant OC
cells, where the enrichment of GRP75 in the mitochondria-associated
ER membranes may be responsible for this effect (149). In paclitaxel-resistant OC cells,
lncRNA-RNA component of mitochondrial RNA processing
endoribonuclease (RMRP) has been shown to increase MICU1 expression
through miR-580-3p aggregation. By contrast, targeting lncRNA-RMRP
was found to inhibit the miR-580-3p/MICU1 axis to increase
paclitaxel sensitivity (150).
Overall, mitochondrial Ca2+ alterations probably serve a
significant role in the treatment of OC. Further in-depth studies
into MCU channels can aid in understanding their roles in the
occurrence, development and prognosis of OC. These are expected to
facilitate the development of novel therapeutic targets and search
for new therapeutic methods.
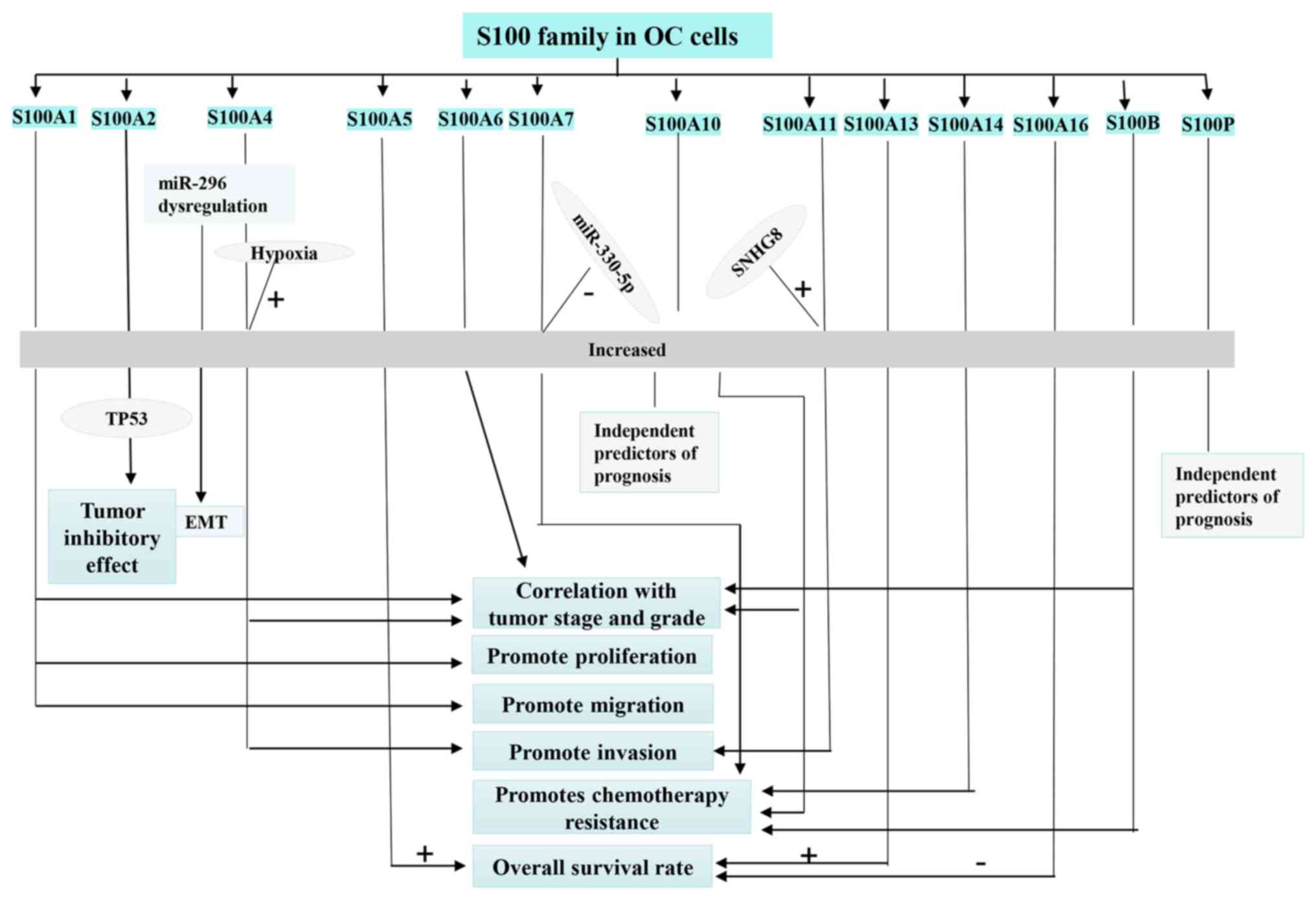
There are 21 members in the S100 family known to
date, all of which are found in human tissues and are acidic
Ca2+-binding proteins. These proteins are highly
homologous both in terms of sequence and structure, can switch
roles within a given biological process and are involved in a wide
variety of cellular events, such as proliferation, apoptosis,
migration, inflammation and differentiation (151). The proteins that make up the
S100 family can serve as both Ca2+ sensors on the inside
of cells and as extracellular factors promoting proliferation from
the outside. Therefore, aberrant expression of S100 proteins has
been proposed to be another factor in tumor development and
progression (152,153). In a previous review, Bresnick
et al (151) discussed
the importance of S100 family members in diagnosing and treating
cancer, how S100 signaling can affects the growth of tumors and how
S100 inhibitors were found to treat cancer. With the progression of
the disease, multi-drug resistance to tumor therapy remains to be a
problem. Hua et al (154)
found that the dysregulation of different S100 proteins can
contribute to the development of tumor drug resistance, which
worsens the prognosis of patients with cancer. A summary was also
provided of how S100 family members can affect tumor resistance to
therapy, pointing out that inhibition of S100 proteins can mediate
the response of tumors to therapy. Accumulating evidence suggests
multiple members of the S100 family are involved in OC development
and progression (Fig. 2 and
Table III) (153).
Compared with fallopian tube and normal ovarian
epithelial tissues, S100A1 expression tends to be significantly
higher in OC tissues, which is also associated with lymph node
metastasis, International Federation of Gynecology and Obstetrics
(FIGO) staging and tumor grade (155). S100A2 has also been hypothesized
to be a tumor suppressor that aids in the stabilization and
response to the transcription of mutant p53, thereby controlling
cell proliferation (156).
Higher expression levels of S100A2 have been shown to predict
superior overall survival in patients with OC expressing wild-type
TP53, but had no prognostic value in patients with mutant p53 OC.
This suggests that the interaction between S100A2 and TP53 may
mediate the tumor suppressive effects of S100A2 (153). The function of S100A3 in OC
remains to be elucidated. Kikuchi et al (157) found that S100A4 is highly
expressed in the nucleus in OC tissues; OC patients with stronger
nuclear S100A4 expression showed a significantly shorter survival
time compared those without. Subsequent treatment with the
recombinant S100A4 resulted in the translocation of S100A4 into the
nucleus, the enhancement of which enhanced OC cell invasiveness.
These findings suggest that the nuclear expression of S100A4 is
involved in the aggressive behavior of OC. Furthermore, nuclear
expression of S100A4 in combination with the nuclear HIF-1α protein
under hypoxic conditions has been demonstrated to induce hypoxia
response element-free methylation of the S100A4 gene and promote OC
aggressiveness (158). In
addition, miR-296 is an important upstream regulator of S100A4 and
aberrant regulation of the miR-296/S100A4 axis has been reported to
promote the EMT process and hasten OC progression (159). It was first proposed by Link
et al (160) that high
levels of circulating metastasis-associated in colon cancer 1 and
S100A4 transcripts could predict the prognosis of patients with OC,
because they were associated with advanced FIGO staging. Another
previous study has shown that the insulin-like growth factor 1
receptor 6-/integrin-/S100A4 molecular network can regulate the
organ-specific metastasis of chemoresistant epithelial OC cells.
Genetic and pharmacological inhibition of S100A4 was found to
significantly reduce distant metastasis and completely eliminated
lung invasion by advanced chemoresistant epithelial OC cells
(161). S100A5 is a novel member
of the S100 protein family that can interact with Ca2+,
Zn2+ and Cu2+ (162). High S100A5 expression was
previously reported to predict overall survival in all patients
with EOC (153).
High expression of S100A11 in the serum of patients
with OC and increased proliferation, migration and invasion of OC
cells are attributed to the lncRNA SNHG8, which regulates OC
progression by targeting miR-1270 and S100A11 (168,169). Patients with grade II, stage
I+II and p53 mutant OC had a longer overall survival if S100A13
levels were elevated (153).
Serum S100A14 levels was found to be consistently higher in
patients with OC, where a link was also found between elevated
S100A14 and resistance to platinum-based chemotherapy (170). Higher levels of S100A16, a
member of the S100 family isolated from astrocytomas (171), have also been associated with
worse prognosis in patients with OC, particularly those with grade
II, III and stage III EOC (153). S100B protein is overexpressed in
OC tissues compared with that in normal ovaries and is in turn
associated with advanced tumor stage, decreased differentiation and
shorter overall survival (172).
In addition, S100B has been documented to mediate chemotherapy
resistance in OC cells through p53 (173) and controls the stemness of OC
stem cell-like cells (172).
Although high S100P expression is associated with a worse prognosis
in OC patients in terms of overall survival and progression free
survival, S100P has been shown to increase chemosensitivity of OC
cells to carboplatin and paclitaxel in vitro (174,175). Overall, a comprehensive
understanding of the function of S100 family members is clinically
instructive for the diagnosis and prognosis of OC patients.
According to results from a previous survey, S100 protein mRNA
expression is strongly associated with overall survival in patients
with OC, with high levels of S100 family members S100A10, S100A11,
S100A16, S100B and S100P predicting worse overall survival, while
S100A1, S100A2, S100A5, S100A6 and S100A13 were associated with
longer overall survival, depending in part on OC subtype and
clinicopathological features (153,176).
Several promising approaches are currently proposed
and make use of current knowledge to assess S100 proteins as
potential therapeutic targets of cancer therapy, as evidenced by
the aforementioned studies. However, additional research is
required to firmly establish S100 proteins as reliable biomarkers
for OC therapy and to further characterize their roles in OC
pathophysiology. Although these initial findings show promise, the
true extent of the function of S100 proteins in OC remains unknown,
which requires unravelling it can be fully exploited in the
clinic.
Data sharing is not applicable to this article, as
no data sets were generated or analyzed during the current
study.
Not applicable.
QG and FD wrote the original draft of the
manuscript. QG, YZ, JL, MF, CZ and BJ reviewed and edited the
manuscript. QG, FD, YZ, HD and TX supervised the present study. QG,
JQ, JC, FD, JL, MF and JQ performed project administration: Data
authentication is not applicable. All authors have read and agreed
to the published version of the manuscript.
Not applicable.
The authors declare that they have no competing
interests.
Not applicable.
The present study was supported partly by the National Nature
and Science Foundation of China (grant nos. 82271724, 81873841,
81741024 and 81401244), Ministry of Science and Technology (grant
no. 2019YFA0802600), Suzhou City Wei Sheng Ren Cai program (grant
no. GSWS2019029), General Programs of Jiangsu Commission of Health
(grant no. M2021087) and Nature and Science Foundation of Jiangsu
(grant no. BK20221243).
|
1
|
Matz M, Coleman MP, Sant M, Chirlaque MD,
Visser O, Gore M and Allemani C; the CONCORD Working Group: The
histology of ovarian cancer: Worldwide distribution and
implications for international survival comparisons (CONCORD-2).
Gynecol Oncol. 144:405–413. 2017. View Article : Google Scholar
|
|
2
|
Lisio MA, Fu L, Goyeneche A, Gao ZH and
Telleria C: High-grade serous ovarian cancer: Basic sciences,
clinical and therapeutic standpoints. Int J Mol Sci. 20:9522019.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Matulonis UA, Sood AK, Fallowfield L,
Howitt BE, Sehouli J and Karlan BY: Ovarian cancer. Nat Rev Dis
Primers. 2:160612016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mallen A, Soong TR, Townsend MK, Wenham
RM, Crum CP and Tworoger SS: Surgical prevention strategies in
ovarian cancer. Gynecol Oncol. 151:166–175. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Menon U, Karpinskyj C and Gentry-Maharaj
A: Ovarian cancer prevention and screening. Obstet Gynecol.
131:909–927. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2019. CA Cancer J Clin. 69:7–34. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Torre LA, Trabert B, DeSantis CE, Miller
KD, Samimi G, Runowicz CD, Gaudet MM, Jemal A and Siegel RL:
Ovarian cancer statistics, 2018. CA Cancer J Clin. 68:284–296.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
O'Malley DM: New therapies for ovarian
cancer. J Natl Compr Canc Netw. 17:619–621. 2019.PubMed/NCBI
|
|
9
|
Zhang M, Cheng S, Jin Y, Zhao Y and Wang
Y: Roles of CA125 in diagnosis, prediction and oncogenesis of
ovarian cancer. Biochim Biophys Acta Rev Cancer. 1875:1885032021.
View Article : Google Scholar
|
|
10
|
Berridge MJ, Lipp P and Bootman MD: The
versatility and universality of calcium signalling. Nat Rev Mol
Cell Biol. 1:11–21. 2000. View Article : Google Scholar
|
|
11
|
Altamura C, Greco MR, Carratù MR, Cardone
RA and Desaphy JF: Emerging roles for ion channels in ovarian
cancer: Pathomechanisms and pharmacological treatment. Cancers
(Basel). 13. pp. 6682021, View Article : Google Scholar
|
|
12
|
Caravia L, Staicu CE, Radu BM, Condrat CE,
Crețoiu D, Bacalbașa N, Suciu N, Crețoiu SM and Voinea SC: Altered
organelle calcium transport in ovarian physiology and cancer.
Cancers (Basel). 12:22322020. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Monteith GR, McAndrew D, Faddy HM and
Roberts-Thomson SJ: Calcium and cancer: Targeting Ca2+ transport.
Nat Rev Cancer. 7:519–530. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
McConkey DJ and Orrenius S: The role of
calcium in the regulation of apoptosis. Biochem Biophys Res Commun.
239:357–366. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Prevarskaya N, Skryma R and Shuba Y:
Calcium in tumour metastasis: New roles for known actors. Nat Rev
Cancer. 11:609–618. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Pulliam TL, Goli P, Awad D, Lin C,
Wilkenfeld SR and Frigo DE: Regulation and role of CAMKK2 in
prostate cancer. Nat Rev Urol. 19:367–380. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Venkatachalam K, Luo J and Montell C:
Evolutionarily conserved, multitasking TRP channels: Lessons from
worms and flies. Handb Exp Pharmacol. 223:937–962. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chen JP, Wang J, Luan Y, Wang CX, Li WH,
Zhang JB, Sha D, Shen R, Cui YG, Zhang Z, et al: TRPM7 promotes the
metastatic process in human nasopharyngeal carcinoma. Cancer Lett.
356:483–490. 2015. View Article : Google Scholar
|
|
19
|
Chen X, Sooch G, Demaree IS, White FA and
Obukhov AG: Transient receptor potential canonical (TRPC) channels:
Then and now. Cells. 9:19832020. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
He B, Liu F, Ruan J, Li A, Chen J, Li R,
Shen J, Zheng D and Luo R: Silencing TRPC1 expression inhibits
invasion of CNE2 nasopharyngeal tumor cells. Oncol Rep.
27:1548–1554. 2012.PubMed/NCBI
|
|
21
|
Ong HL and Ambudkar IS: The dynamic
complexity of the TRPC1 channelosome. Channels (Austin). 5:424–431.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liu X, Zou J, Su J, Lu Y, Zhang J, Li L
and Yin F: Downregulation of transient receptor potential cation
channel, subfamily C, member 1 contributes to drug resistance and
high histological grade in ovarian cancer. Int J Oncol. 48:243–252.
2016. View Article : Google Scholar
|
|
23
|
Yang SL, Cao Q, Zhou KC, Feng YJ and Wang
YZ: Transient receptor potential channel C3 contributes to the
progression of human ovarian cancer. Oncogene. 28:1320–1328. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tao X, Zhao N, Jin H, Zhang Z, Liu Y, Wu
J, Bast RC Jr, Yu Y and Feng Y: FSH enhances the proliferation of
ovarian cancer cells by activating transient receptor potential
channel C3. Endocr Relat Cancer. 20:415–429. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Li S, Jiang K, Li J, Hao X, Chu W, Luo C,
Zhu Y, Xie R and Chen B: Estrogen enhances the proliferation and
migration of ovarian cancer cells by activating transient receptor
potential channel C3. J Ovarian Res. 13:202020. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Liu EL, Zhou YX, Li J, Zhang DH and Liang
F: Long-chain non-coding RNA SNHG3 promotes the growth of ovarian
cancer cells by targeting miR-339-5p/TRPC3 axis. Onco Targets Ther.
13:10959–10971. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Shen Z, Gu L, Liu Y, Wang L, Zhu J, Tang
S, Wei X, Wang J, Zhang S, Wang X, et al: PLAA suppresses ovarian
cancer metastasis via METTL3-mediated m6A modification
of TRPC3 mRNA. Oncogene. 41:4145–4158. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Farfariello V, Gordienko DV, Mesilmany L,
Touil Y, Germain E, Fliniaux I, Desruelles E, Gkika D, Roudbaraki
M, Shapovalov G, et al: TRPC3 shapes the ER-mitochondria
Ca2+ transfer characterizing tumour-promoting
senescence. Nat Commun. 13:9562022. View Article : Google Scholar
|
|
29
|
Caterina MJ, Schumacher MA, Tominaga M,
Rosen TA, Levine JD and Julius D: The capsaicin receptor: A
heat-activated ion channel in the pain pathway. Nature.
389:816–824. 1997. View
Article : Google Scholar : PubMed/NCBI
|
|
30
|
Gunthorpe MJ, Benham CD, Randall A and
Davis JB: The diversity in the vanilloid (TRPV) receptor family of
ion channels. Trends Pharmacol Sci. 23:183–191. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wang Z, Dong J, Tian W, Qiao S and Wang H:
Role of TRPV1 ion channel in cervical squamous cell carcinoma
genesis. Front Mol Biosci. 9:9802622022. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lucido CT, Wynja E, Madeo M, Williamson
CS, Schwartz LE, Imblum BA, Drapkin R and Vermeer PD: Innervation
of cervical carcinoma is mediated by cancer-derived exosomes.
Gynecol Oncol. 154:228–235. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Han GH, Chay DB, Nam S, Cho H, Chung JY
and Kim JH: Prognostic significance of transient receptor potential
vanilloid type 1 (TRPV1) and phosphatase and tension homolog (PTEN)
in epithelial ovarian cancer. Cancer Genomics Proteomics.
17:309–319. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wang YY, Lee KT, Lim MC and Choi JH: TRPV1
antagonist DWP05195 induces ER stress-dependent apoptosis through
the ROS-p38-CHOP pathway in human ovarian cancer cells. Cancers
(Basel). 12:17022020. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Di Y, Xu T, Tian Y, Ma T, Qu D, Wang Y,
Lin Y, Bao D, Yu L, Liu S and Wang A: Ursolic acid protects against
cisplatin-induced ototoxicity by inhibiting oxidative stress and
TRPV1-mediated Ca2+-signaling. Int J Mol Med. 46:806–816. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Santoni G, Amantini C, Maggi F, Marinelli
O, Santoni M, Nabissi M and Morelli MB: The TRPV2 cation channels:
From urothelial cancer invasiveness to glioblastoma multiforme
interactome signature. Lab Invest. 100:186–198. 2020. View Article : Google Scholar
|
|
37
|
Liberati S, Morelli MB, Amantini C,
Farfariello V, Santoni M, Conti A, Nabissi M, Cascinu S and Santoni
G: Loss of TRPV2 homeostatic control of cell proliferation drives
tumor progression. Cells. 3:112–128. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Fraguas-Sánchez AI, Fernández-Carballido
A, Delie F, Cohen M, Martin-Sabroso C, Mezzanzanica D, Figini M,
Satta A and Torres-Suárez AI: Enhancing ovarian cancer conventional
chemotherapy through the combination with cannabidiol loaded
microparticles. Eur J Pharm Biopharm. 154:246–258. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Griffiths C, Aikins J, Warshal D and
Ostrovsky O: Can cannabidiol affect the efficacy of chemotherapy
and epigenetic treatments in cancer? Biomolecules. 11:7662021.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Dutta B, Arya RK, Goswami R, Alharbi MO,
Sharma S and Rahaman SO: Role of macrophage TRPV4 in inflammation.
Lab Invest. 100:178–185. 2020. View Article : Google Scholar :
|
|
41
|
Wang K, Feng X, Zheng L, Chai Z, Yu J, You
X, Li X and Cheng X: TRPV4 is a prognostic biomarker that
correlates with the immunosuppressive microenvironment and
chemoresistance of anti-cancer drugs. Front Mol Biosci.
8:6905002021. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Zhang C, Xu C, Ma C, Zhang Q, Bu S, Zhang
DL, Yu L and Wang H: TRPs in ovarian serous cystadenocarcinoma: The
expression patterns, prognostic roles, and potential therapeutic
targets. Front Mol Biosci. 9:9154092022. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Yu S, Huang S, Ding Y, Wang W, Wang A and
Lu Y: Transient receptor potential ion-channel subfamily V member
4: A potential target for cancer treatment. Cell Death Dis.
10:4972019. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Bödding M and Flockerzi V: Ca2+ dependence
of the Ca2+-selective TRPV6 channel. J Biol Chem. 279:36546–36552.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Gees M, Colsoul B and Nilius B: The role
of transient receptor potential cation channels in Ca2+ signaling.
Cold Spring Harb Perspect Biol. 2:a0039622010. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Lehen'kyi V, Flourakis M, Skryma R and
Prevarskaya N: TRPV6 channel controls prostate cancer cell
proliferation via Ca(2+)/NFAT-dependent pathways. Oncogene.
26:7380–7385. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Xu X, Li N, Wang Y, Yu J and Mi J: Calcium
channel TRPV6 promotes breast cancer metastasis by NFATC2IP. Cancer
Lett. 519:150–160. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Xue H, Wang Y, MacCormack TJ, Lutes T,
Rice C, Davey M, Dugourd D, Ilenchuk TT and Stewart JM: Inhibition
of transient receptor potential vanilloid 6 channel, elevated in
human ovarian cancers, reduces tumour growth in a xenograft model.
J Cancer. 9:3196–3207. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Jiang Y, Gou H, Zhu J, Tian S and Yu L:
Lidocaine inhibits the invasion and migration of TRPV6-expressing
cancer cells by TRPV6 downregulation. Oncol Lett. 12:1164–1170.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Wang X, Li G, Zhang Y, Li L, Qiu L, Qian
Z, Zhou S, Wang X, Li Q and Zhang H: Pan-cancer analysis reveals
genomic and clinical characteristics of TRPV channel-related genes.
Front Oncol. 12:8131002022. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Tong Q, Zhang W, Conrad K, Mostoller K,
Cheung JY, Peterson BZ and Miller BA: Regulation of the transient
receptor potential channel TRPM2 by the Ca2+ sensor calmodulin. J
Biol Chem. 281:9076–9085. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Orfanelli U, Wenke AK, Doglioni C, Russo
V, Bosserhoff AK and Lavorgna G: Identification of novel sense and
antisense transcription at the TRPM2 locus in cancer. Cell Res.
18:1128–1140. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Ding Y, Tan X, Abasi A, Dai Y, Wu R, Zhang
T, Li K, Yan M and Huang X: LncRNA TRPM2-AS promotes ovarian cancer
progression and cisplatin resistance by sponging miR-138-5pto
release SDC3 mRNA. Aging (Albany NY). 13:6832–6848. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Dai W, Bai Y, Hebda L, Zhong X, Liu J, Kao
J and Duan C: Calcium deficiency-induced and TRP channel-regulated
IGF1R-PI3K-Akt signaling regulates abnormal epithelial cell
proliferation. Cell Death Differ. 21:568–581. 2014. View Article : Google Scholar :
|
|
55
|
Abed E, Martineau C and Moreau R: Role of
melastatin transient receptor potential 7 channels in the
osteoblastic differentiation of murine MC3T3 cells. Calcif Tissue
Int. 88:246–253. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Yee NS, Kazi AA and Yee RK: Cellular and
developmental biology of TRPM7 channel-kinase: Implicated roles in
cancer. Cells. 3:751–777. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Wang J, Xiao L, Luo CH, Zhou H, Hu J, Tang
YX, Fang KN and Zhang Y: Overexpression of TRPM7 is associated with
poor prognosis in human ovarian carcinoma. Asian Pac J Cancer Prev.
15:3955–3958. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Wang J, Liao QJ, Zhang Y, Zhou H, Luo CH,
Tang J, Wang Y, Tang Y, Zhao M, Zhao XH, et al: TRPM7 is required
for ovarian cancer cell growth, migration and invasion. Biochem
Biophys Res Commun. 454:547–553. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Liu L, Wu N, Wang Y, Zhang X, Xia B, Tang
J, Cai J, Zhao Z, Liao Q and Wang J: TRPM7 promotes the
epithelial-mesenchymal transition in ovarian cancer through the
calcium-related PI3K/AKT oncogenic signaling. J Exp Clin Cancer
Res. 38:1062019. View Article : Google Scholar
|
|
60
|
Catterall WA: Voltage-gated calcium
channels. Cold Spring Harb Perspect Biol. 3:a0039472011. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Li W, Zhang SL, Wang N, Zhang BB and Li M:
Blockade of T-type Ca(2+) channels inhibits human ovarian cancer
cell proliferation. Cancer Invest. 29:339–346. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Jang SJ, Choi HW, Choi DL, Cho S, Rim HK,
Choi HE, Kim KS, Huang M, Rhim H, Lee KT and Lee JY: In vitro
cytotoxicity on human ovarian cancer cells by T-type calcium
channel blockers. Bioorg Med Chem Lett. 23:6656–6662. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Dziegielewska B, Casarez EV, Yang WZ, Gray
LS, Dziegielewski J and Slack-Davis JK: T-type Ca2+ channel
inhibition sensitizes ovarian cancer to carboplatin. Mol Cancer
Ther. 15:460–470. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Mir R, Stanzani E, Martinez-Soler F,
Villanueva A, Vidal A, Condom E, Ponce J, Gil J, Tortosa A and
Giménez-Bonafé P: YM155 sensitizes ovarian cancer cells to
cisplatin inducing apoptosis and tumor regression. Gynecol Oncol.
132:211–220. 2014. View Article : Google Scholar
|
|
65
|
Fornaro L, Vivaldi C, Lin D, Xue H,
Falcone A, Wang Y, Crea F and Bootman MD: Prognostic relevance of a
T-type calcium channels gene signature in solid tumours: A
correlation ready for clinical validation. PLoS One.
12:e01828182017. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Mertens-Walker I, Bolitho C, Baxter RC and
Marsh DJ: Gonadotropin-induced ovarian cancer cell migration and
proliferation require extracellular signal-regulated kinase 1/2
activation regulated by calcium and protein kinase C{delta}. Endocr
Relat Cancer. 17:335–349. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Kim EK, Ha JM, Kim YW, Jin SY, Ha HK and
Bae SS: Inhibitory role of polyunsaturated fatty acids on
lysophosphatidic acid-induced cancer cell migration and adhesion.
FEBS Lett. 588:2971–2977. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Lee H, Kim JW, Kim DK, Choi DK, Lee S, Yu
JH, Kwon OB, Lee J, Lee DS, Kim JH and Min SH: Calcium channels as
novel therapeutic targets for ovarian cancer stem cells. Int J Mol
Sci. 21:23272020. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Lee H, Kwon OB, Lee JE, Jeon YH, Lee DS,
Min SH and Kim JW: Repositioning trimebutine maleate as a cancer
treatment targeting ovarian cancer stem cells. Cells. 10:9182021.
View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Chang X and Dong Y: CACNA1C is a
prognostic predictor for patients with ovarian cancer. J Ovarian
Res. 14:882021. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Niemeyer BA: Changing calcium: CRAC
channel (STIM and Orai) expression, splicing, and posttranslational
modifiers. Am J Physiol Cell Physiol. 310:C701–C709. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Khan HY, Mazahir I, Reddy S, Fazili F and
Azmi A: Roles of CRAC channel in cancer: Implications for
therapeutic development. Expert Rev Precis Med Drug Dev. 5:371–382.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Abdelazeem KNM, Droppova B, Sukkar B,
Al-Maghout T, Pelzl L, Zacharopoulou N, Ali Hassan NH, Abdel-Fattah
KI, Stournaras C and Lang F: Upregulation of Orai1 and STIM1
expression as well as store-operated Ca2+ entry in ovary
carcinoma cells by placental growth factor. Biochem Biophys Res
Commun. 512:467–472. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Schmidt S, Liu G, Liu G, Yang W, Honisch
S, Pantelakos S, Stournaras C, Hönig A and Lang F: Enhanced Orai1
and STIM1 expression as well as store operated Ca2+ entry in
therapy resistant ovary carcinoma cells. Oncotarget. 5:4799–4810.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Zahid M, Beseler CL, Hall JB, LeVan T,
Cavalieri EL and Rogan EG: Unbalanced estrogen metabolism in
ovarian cancer. Int J Cancer. 134:2414–2423. 2014. View Article : Google Scholar :
|
|
76
|
Lv X, Miao C, Liu M, Wang X, Wang L and
Wang D: 17β-Estradiol via Orai1 activates calcium mobilization to
induce cell proliferation in epithelial ovarian cancer. J Biochem
Mol Toxicol. 34:e226032020. View Article : Google Scholar
|
|
77
|
Ouadid-Ahidouch H, Roudbaraki M, Delcourt
P, Ahidouch A, Joury N and Prevarskaya N: Functional and molecular
identification of intermediate-conductance Ca(2+)-activated K(+)
channels in breast cancer cells: Association with cell cycle
progression. Am J Physiol Cell Physiol. 287:C125–C134. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Kunzelmann K: Ion channels and cancer. J
Membr Biol. 205:159–173. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Han X, Xi L, Wang H, Huang X, Ma X, Han Z,
Wu P, Ma X, Lu Y, Wang G, et al: The potassium ion channel opener
NS1619 inhibits proliferation and induces apoptosis in A2780
ovarian cancer cells. Biochem Biophys Res Commun. 375:205–209.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Berkefeld H, Sailer CA, Bildl W, Rohde V,
Thumfart JO, Eble S, Klugbauer N, Reisinger E, Bischofberger J,
Oliver D, et al: BKCa-Cav channel complexes mediate rapid and
localized Ca2+-activated K+ signaling. Science. 314:615–620. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Oeggerli M, Tian Y, Ruiz C, Wijker B,
Sauter G, Obermann E, Güth U, Zlobec I, Sausbier M, Kunzelmann K
and Bubendorf L: Role of KCNMA1 in breast cancer. PLoS One.
7:e416642012. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Samuel P, Pink RC, Caley DP, Currie JM,
Brooks SA and Carter DR: Over-expression of miR-31 or loss of
KCNMA1 leads to increased cisplatin resistance in ovarian cancer
cells. Tumour Biol. 37:2565–2573. 2016. View Article : Google Scholar
|
|
83
|
Lundstrom K: Structural genomics of GPCRs.
Trends Biotechnol. 23:103–108. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Oldham WM and Hamm HE: Heterotrimeric G
protein activation by G-protein-coupled receptors. Nat Rev Mol Cell
Biol. 9:60–71. 2008. View Article : Google Scholar
|
|
85
|
Tu CL, Oda Y, Komuves L and Bikle DD: The
role of the calcium-sensing receptor in epidermal differentiation.
Cell Calcium. 35:265–273. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Rodland KD: The role of the
calcium-sensing receptor in cancer. Cell Calcium. 35:291–295. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Yan S, Yuan C, Yang Q, Li X, Yang N, Liu
X, Dong R, Zhang X, Yuan Z, Zhang N and Kong B: A genetic
polymorphism (rs17251221) in the calcium-sensing receptor is
associated with ovarian cancer susceptibility. Oncol Rep.
34:2151–2155. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Park KS, Kim MK, Im DS and Bae YS: Effect
of lysophosphatidylglycerol on several signaling molecules in
OVCAR-3 human ovarian cancer cells: Involvement of pertussis
toxin-sensitive G-protein coupled receptor. Biochem Pharmacol.
73:675–681. 2007. View Article : Google Scholar
|
|
89
|
Smith HO, Arias-Pulido H, Kuo DY, Howard
T, Qualls CR, Lee SJ, Verschraegen CF, Hathaway HJ, Joste NE and
Prossnitz ER: GPR30 predicts poor survival for ovarian cancer.
Gynecol Oncol. 114:465–471. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Yan Y, Liu H, Wen H, Jiang X, Cao X, Zhang
G and Liu G: The novel estrogen receptor GPER regulates the
migration and invasion of ovarian cancer cells. Mol Cell Biochem.
378:1–7. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Heublein S, Mayr D, Friese K,
Jarrin-Franco MC, Lenhard M, Mayerhofer A and Jeschke U: The
G-protein-coupled estrogen receptor (GPER/GPR30) in ovarian
granulosa cell tumors. Int J Mol Sci. 15:15161–15172. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Yan Y, Jiang X, Zhao Y, Wen H and Liu G:
Role of GPER on proliferation, migration and invasion in
ligand-independent manner in human ovarian cancer cell line SKOV3.
Cell Biochem Funct. 33:552–559. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Ignatov T, Modl S, Thulig M, Weißenborn C,
Treeck O, Ortmann O, Zenclussen A, Costa SD, Kalinski T and Ignatov
A: GPER-1 acts as a tumor suppressor in ovarian cancer. J Ovarian
Res. 6:512013. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Predescu DV, Crețoiu SM, Crețoiu D,
Pavelescu LA, Suciu N, Radu BM and Voinea SC: G protein-coupled
receptors (GPCRs)-mediated calcium signaling in ovarian cancer:
Focus on GPCRs activated by neurotransmitters and
inflammation-associated molecules. Int J Mol Sci. 20:55682019.
View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Xue D, Chen W and Neamati N: Discovery,
structure-activity relationship study and biological evaluation of
2-thioureidothiophene-3-carboxylates as a novel class of C-X-C
chemokine receptor 2 (CXCR2) antagonists. Eur J Med Chem.
204:1123872020. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Seo MD, Velamakanni S, Ishiyama N,
Stathopulos PB, Rossi AM, Khan SA, Dale P, Li C, Ames JB, Ikura M
and Taylor CW: Structural and functional conservation of key
domains in InsP3 and ryanodine receptors. Nature. 483:108–112.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Vermassen E, Parys JB and Mauger JP:
Subcellular distribution of the inositol 1,4,5-trisphosphate
receptors: Functional relevance and molecular determinants. Biol
Cell. 96:3–17. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Giannini G, Clementi E, Ceci R, Marziali G
and Sorrentino V: Expression of a ryanodine receptor-Ca2+ channel
that is regulated by TGF-beta. Science. 257:91–94. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Santulli G, Nakashima R, Yuan Q and Marks
AR: Intracellular calcium release channels: An update. J Physiol.
595:3041–3051. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Ando H, Hirose M and Mikoshiba K: Aberrant
IP3 receptor activities revealed by comprehensive
analysis of pathological mutations causing spinocerebellar ataxia
29. Proc Natl Acad Sci USA. 115:12259–12264. 2018. View Article : Google Scholar
|
|
101
|
Díaz-Muñoz M, de la Rosa Santander P,
Juárez-Espinosa AB, Arellano RO and Morales-Tlalpan V: Granulosa
cells express three inositol 1,4,5-trisphosphate receptor isoforms:
Cytoplasmic and nuclear Ca2+ mobilization. Reprod Biol Endocrinol.
6:602008. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Hanson CJ, Bootman MD and Roderick HL:
Cell signalling: IP3 receptors channel calcium into cell death.
Curr Biol. 14:R933–R935. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Lahiri S, Roy A, Li J, Mokashi A and Baby
SM: Ca2+ responses to hypoxia are mediated by IP3-R on Ca2+ store
depletion. Adv Exp Med Biol. 536:25–32. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Lencesova L, Vlcek M, Krizanova O and
Hudecova S: Hypoxic conditions increases H2S-induced ER stress in
A2870 cells. Mol Cell Biochem. 414:67–76. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Yu Y, Xie Q, Liu W, Guo Y, Xu N, Xu L, Liu
S, Li S, Xu Y and Sun L: Increased intracellular Ca2+
decreases cisplatin resistance by regulating iNOS expression in
human ovarian cancer cells. Biomed Pharmacother. 86:8–15. 2017.
View Article : Google Scholar
|
|
106
|
Xie Q, Xu Y, Gao W, Zhang Y, Su J, Liu Y,
Guo Y, Dou M, Hu K and Sun L: TAT-fused IP3R-derived peptide
enhances cisplatin sensitivity of ovarian cancer cells by
increasing ER Ca2+ release. Int J Mol Med. 41:809–817. 2018.
|
|
107
|
Rezuchova I, Hudecova S, Soltysova A,
Matuskova M, Durinikova E, Chovancova B, Zuzcak M, Cihova M,
Burikova M, Penesova A, et al: Type 3 inositol 1,4,5-trisphosphate
receptor has antiapoptotic and proliferative role in cancer cells.
Cell Death Dis. 10:1862019. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Sneyers F, Rosa N and Bultynck G: Type 3
IP3 receptors driving oncogenesis. Cell Calcium.
86:1021412020. View Article : Google Scholar
|
|
109
|
Xue Y, Morris JL, Yang K, Fu Z, Zhu X,
Johnson F, Meehan B, Witkowski L, Yasmeen A, Golenar T, et al:
SMARCA4/2 loss inhibits chemotherapy-induced apoptosis by
restricting IP3R3-mediated Ca2+ flux to mitochondria.
Nat Commun. 12:54042021. View Article : Google Scholar
|
|
110
|
Kucukkaya B, Erdag D, Akbas F and
Yalcintepe L: The effect of iron on the expression levels of
calcium related gene in cisplatin resistant epithelial ovarian
cancer cells. Explor Target Antitumor Ther. 2:309–322.
2021.PubMed/NCBI
|
|
111
|
Meissner G: The structural basis of
ryanodine receptor ion channel function. J Gen Physiol.
149:1065–1089. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Mariot P, Prevarskaya N, Roudbaraki MM, Le
Bourhis X, Van Coppenolle F, Vanoverberghe K and Skryma R: Evidence
of functional ryanodine receptor involved in apoptosis of prostate
cancer (LNCaP) cells. Prostate. 43:205–214. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Zhang L, Liu Y, Song F, Zheng H, Hu L, Lu
H, Liu P, Hao X, Zhang W and Chen K: Functional SNP in the
microRNA-367 binding site in the 3'UTR of the calcium channel
ryanodine receptor gene 3 (RYR3) affects breast cancer risk and
calcification. Proc Natl Acad Sci USA. 108:13653–13658. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Schmitt K, Molfenter B, Laureano NK, Tawk
B, Bieg M, Hostench XP, Weichenhan D, Ullrich ND, Shang V, Richter
D, et al: Somatic mutations and promotor methylation of the
ryanodine receptor 2 is a common event in the pathogenesis of head
and neck cancer. Int J Cancer. 145:3299–3310. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Andruska ND, Zheng X, Yang X, Mao C,
Cherian MM, Mahapatra L, Helferich WG and Shapiro DJ: Estrogen
receptor α inhibitor activates the unfolded protein response,
blocks protein synthesis, and induces tumor regression. Proc Natl
Acad Sci USA. 112:4737–4742. 2015. View Article : Google Scholar
|
|
116
|
Zheng X, Andruska N, Lambrecht MJ, He S,
Parissenti A, Hergenrother PJ, Nelson ER and Shapiro DJ: Targeting
multidrug-resistant ovarian cancer through estrogen receptor α
dependent ATP depletion caused by hyperactivation of the unfolded
protein response. Oncotarget. 9:14741–14753. 2018. View Article : Google Scholar
|
|
117
|
Williams CJ and Erickson GF: Morphology
and Physiology of the Ovary. Endotext. Feingold KR, Anawalt B,
Blackman MR, Boyce A, Chrousos G, Corpas E, de Herder WW, Dhatariya
K, Dungan K, Hofland J, et al: South Dartmouth, MA: MDText.com,
Inc.; 2000
|
|
118
|
Cui C, Merritt R, Fu L and Pan Z:
Targeting calcium signaling in cancer therapy. Acta Pharm Sin B.
7:3–17. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Bowen NJ, Walker LD, Matyunina LV, Logani
S, Totten KA, Benigno BB and McDonald JF: Gene expression profiling
supports the hypothesis that human ovarian surface epithelia are
multipotent and capable of serving as ovarian cancer initiating
cells. BMC Med Genomics. 2:712009. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Seo JA, Kim B, Dhanasekaran DN, Tsang BK
and Song YS: Curcumin induces apoptosis by inhibiting
sarco/endoplasmic reticulum Ca2+ ATPase activity in ovarian cancer
cells. Cancer Lett. 371:30–37. 2016. View Article : Google Scholar
|
|
121
|
Sun Z, Zhang H, Wang X, Wang QC, Zhang C,
Wang JQ, Wang YH, An CQ, Yang KY, Wang Y, et al: TMCO1 is essential
for ovarian follicle development by regulating ER Ca2+
store of granulosa cells. Cell Death Differ. 25:1686–1701. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Huang N, Yu Y and Qiao J: Dual role for
the unfolded protein response in the ovary: Adaption and apoptosis.
Protein Cell. 8:14–24. 2017. View Article : Google Scholar :
|
|
123
|
Peluso JJ: Basic fibroblast growth factor
(bFGF) regulation of the plasma membrane calcium ATPase (PMCA) as
part of an anti-apoptotic mechanism of action. Biochem Pharmacol.
66:1363–1369. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Solár P and Sytkowski AJ: Differentially
expressed genes associated with cisplatin resistance in human
ovarian adenocarcinoma cell line A2780. Cancer Lett. 309:11–18.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Kucukkaya B, Basoglu H, Erdag D, Akbas F,
Susgun S and Yalcintepe L: Calcium homeostasis in cisplatin
resistant epithelial ovarian cancer. Gen Physiol Biophys.
38:353–363. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Baughman JM, Perocchi F, Girgis HS,
Plovanich M, Belcher-Timme CA, Sancak Y, Bao XR, Strittmatter L,
Goldberger O, Bogorad RL, et al: Integrative genomics identifies
MCU as an essential component of the mitochondrial calcium
uniporter. Nature. 476:341–345. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Marchi S and Pinton P: The mitochondrial
calcium uniporter complex: Molecular components, structure and
physiopathological implications. J Physiol. 592:829–839. 2014.
View Article : Google Scholar :
|
|
128
|
Patron M, Checchetto V, Raffaello A,
Teardo E, Vecellio Reane D, Mantoan M, Granatiero V, Szabò I, De
Stefani D and Rizzuto R: MICU1 and MICU2 finely tune the
mitochondrial Ca2+ uniporter by exerting opposite effects on MCU
activity. Mol Cell. 53:726–737. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Denton RM: Regulation of mitochondrial
dehydrogenases by calcium ions. Biochim Biophys Acta.
1787:1309–1316. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Chakraborty PK, Mustafi SB, Xiong X,
Dwivedi SKD, Nesin V, Saha S, Zhang M, Dhanasekaran D, Jayaraman M,
Mannel R, et al: MICU1 drives glycolysis and chemoresistance in
ovarian cancer. Nat Commun. 8:146342017. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Hempel N and Trebak M: Crosstalk between
calcium and reactive oxygen species signaling in cancer. Cell
Calcium. 63:70–96. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Porporato PE, Payen VL, Pérez-Escuredo J,
De Saedeleer CJ, Danhier P, Copetti T, Dhup S, Tardy M, Vazeille T,
Bouzin C, et al: A mitochondrial switch promotes tumor metastasis.
Cell Rep. 8:754–766. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Moloney JN and Cotter TG: ROS signalling
in the biology of cancer. Semin Cell Dev Biol. 80:50–64. 2018.
View Article : Google Scholar
|
|
134
|
Ham J, Lim W, Kim K, Heo YM, Ryu SM, Lee
D, Kim JJ and Song G: Gentisyl alcohol inhibits proliferation and
induces apoptosis via mitochondrial dysfunction and regulation of
MAPK and PI3K/AKT pathways in epithelial ovarian cancer cells. Mar
Drugs. 17:3312019. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Bae H, Park S, Ham J, Song J, Hong T, Choi
JH, Song G and Lim W: ER-mitochondria calcium flux by β-sitosterol
promotes cell death in ovarian cancer. Antioxidants (Basel).
10:15832021. View Article : Google Scholar
|
|
136
|
Bae H, Park S, Yang C, Song G and Lim W:
Disruption of endoplasmic reticulum and ROS production in human
ovarian cancer by campesterol. Antioxidants (Basel). 10:3792021.
View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Bae H, Song G and Lim W: Stigmasterol
causes ovarian cancer cell apoptosis by inducing endoplasmic
reticulum and mitochondrial dysfunction. Pharmaceutics. 12:4882020.
View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Bae H, Lee JY, Song J, Song G and Lim W:
Osthole interacts with an ER-mitochondria axis and facilitates
tumor suppression in ovarian cancer. J Cell Physiol. 236:1025–1042.
2021. View Article : Google Scholar
|
|
139
|
Bae H, Lee JY, Song G and Lim W:
Fucosterol suppresses the progression of human ovarian cancer by
inducing mitochondrial dysfunction and endoplasmic reticulum
stress. Mar Drugs. 18:2612020. View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Bae H, Song G, Lee JY, Hong T, Chang MJ
and Lim W: Laminarin-derived from brown algae suppresses the growth
of ovarian cancer cells via mitochondrial dysfunction and ER
stress. Mar Drugs. 18:1522020. View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Lim W, An Y, Yang C, Bazer FW and Song G:
Chrysophanol induces cell death and inhibits invasiveness via
mitochondrial calcium overload in ovarian cancer cells. J Cell
Biochem. 119:10216–10227. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
142
|
Lim W, Ryu S, Bazer FW, Kim SM and Song G:
Chrysin attenuates progression of ovarian cancer cells by
regulating signaling cascades and mitochondrial dysfunction. J Cell
Physiol. 233:3129–3140. 2018. View Article : Google Scholar
|
|
143
|
Rogalska A, Szula E, Gajek A, Marczak A
and Jóźwiak Z: Activation of apoptotic pathway in normal, cancer
ovarian cells by epothilone B. Environ Toxicol Pharmacol.
36:600–610. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Giorgi C, Baldassari F, Bononi A, Bonora
M, De Marchi E, Marchi S, Missiroli S, Patergnani S, Rimessi A,
Suski JM, et al: Mitochondrial Ca(2+) and apoptosis. Cell Calcium.
52:36–43. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
145
|
Honrath B, Metz I, Bendridi N, Rieusset J,
Culmsee C and Dolga AM: Glucose-regulated protein 75 determines
ER-mitochondrial coupling and sensitivity to oxidative stress in
neuronal cells. Cell Death Discov. 3:170762017. View Article : Google Scholar
|
|
146
|
Ma L, Wang H, Wang C, Su J, Xie Q, Xu L,
Yu Y, Liu S, Li S, Xu Y and Li Z: Failure of elevating calcium
induces oxidative stress tolerance and imparts cisplatin resistance
in ovarian cancer cells. Aging Dis. 7:254–266. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
147
|
Xu L, Xie Q, Qi L, Wang C, Xu N, Liu W, Yu
Y, Li S and Xu Y: Bcl-2 overexpression reduces cisplatin
cytotoxicity by decreasing ER-mitochondrial Ca2+ signaling in SKOV3
cells. Oncol Rep. 39:985–992. 2018.
|
|
148
|
Xie Q, Su J, Jiao B, Shen L, Ma L, Qu X,
Yu C, Jiang X, Xu Y and Sun L: ABT737 reverses cisplatin resistance
by regulating ER-mitochondria Ca2+ signal transduction in human
ovarian cancer cells. Int J Oncol. 49:2507–2519. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
149
|
Li J, Qi F, Su H, Zhang C, Zhang Q, Chen
Y, Chen P, Su L, Chen Y, Yang Y, et al: GRP75-faciliated
mitochondria-associated ER membrane (MAM) integrity controls
cisplatin-resistance in ovarian cancer patients. Int J Biol Sci.
18:2914–2931. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
150
|
Li L, Zeng S, Guo L, Huang P, Xi J, Feng
J, Li Q, Li Y, Xiao X, Yan R and Zhang J: Long noncoding RNA RMRP
contributes to paclitaxel sensitivity of ovarian cancer by
regulating miR-580-3p/MICU1 signaling. J Oncol.
2022:83019412022.PubMed/NCBI
|
|
151
|
Bresnick AR, Weber DJ and Zimmer DB: S100
proteins in cancer. Nat Rev Cancer. 15:96–109. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
152
|
Zimmer DB, Eubanks JO, Ramakrishnan D and
Criscitiello MF: Evolution of the S100 family of calcium sensor
proteins. Cell Calcium. 53:170–179. 2013. View Article : Google Scholar
|
|
153
|
Bai Y, Li LD, Li J and Lu X: Prognostic
values of S100 family members in ovarian cancer patients. BMC
Cancer. 18:12562018. View Article : Google Scholar : PubMed/NCBI
|
|
154
|
Hua X, Zhang H, Jia J, Chen S, Sun Y and
Zhu X: Roles of S100 family members in drug resistance in tumors:
Status and prospects. Biomed Pharmacother. 127:1101562020.
View Article : Google Scholar : PubMed/NCBI
|
|
155
|
Tian T, Li X, Hua Z, Ma J, Liu Z, Chen H
and Cui Z: S100A1 promotes cell proliferation and migration and is
associated with lymph node metastasis in ovarian cancer. Discov
Med. 23:235–245. 2017.PubMed/NCBI
|
|
156
|
Buckley NE, D'Costa Z, Kaminska M and
Mullan PB: S100A2 is a BRCA1/p63 coregulated tumour suppressor gene
with roles in the regulation of mutant p53 stability. Cell Death
Dis. 5:e10702014. View Article : Google Scholar : PubMed/NCBI
|
|
157
|
Kikuchi N, Horiuchi A, Osada R, Imai T,
Wang C, Chen X and Konishi I: Nuclear expression of S100A4 is
associated with aggressive behavior of epithelial ovarian
carcinoma: An important autocrine/paracrine factor in tumor
progression. Cancer Sci. 97:1061–1069. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
158
|
Horiuchi A, Hayashi T, Kikuchi N, Hayashi
A, Fuseya C, Shiozawa T and Konishi I: Hypoxia upregulates ovarian
cancer invasiveness via the binding of HIF-1α to a hypoxia-induced,
methylation-free hypoxia response element of S100A4 gene. Int J
Cancer. 131:1755–1767. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
159
|
Yan W, Chen J, Chen Z and Chen H:
Deregulated miR-296/S100A4 axis promotes tumor invasion by inducing
epithelial-mesenchymal transition in human ovarian cancer. Am J
Cancer Res. 6:260–269. 2016.PubMed/NCBI
|
|
160
|
Link T, Kuhlmann JD, Kobelt D, Herrmann P,
Vassileva YD, Kramer M, Frank K, Göckenjan M, Wimberger P and Stein
U: Clinical relevance of circulating MACC1 and S100A4 transcripts
for ovarian cancer. Mol Oncol. 13:1268–1279. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
161
|
Deo AN, Thorat R, Dhadve AC, De A, Rekhi B
and Ray P: IGF1R-α6 integrin-S100A4 network governs the
organ-specific metastasis of chemoresistant epithelial ovarian
cancer cells. Biochim Biophys Acta Mol Basis Dis. 1868:1662822022.
View Article : Google Scholar
|
|
162
|
Schäfer BW, Fritschy JM, Murmann P,
Troxler H, Durussel I, Heizmann CW and Cox JA: Brain S100A5 is a
novel calcium-, zinc-, and copper ion-binding protein of the
EF-hand superfamily. J Biol Chem. 275:30623–30630. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
163
|
Wei BR, Hoover SB, Ross MM, Zhou W, Meani
F, Edwards JB, Spehalski EI, Risinger JI, Alvord WG, Quiñones OA,
et al: Serum S100A6 concentration predicts peritoneal tumor burden
in mice with epithelial ovarian cancer and is associated with
advanced stage in patients. PLoS One. 4:e76702009. View Article : Google Scholar : PubMed/NCBI
|
|
164
|
Lin M, Xia B, Qin L, Chen H and Lou G:
S100A7 regulates ovarian cancer cell metastasis and chemoresistance
through MAPK signaling and is targeted by miR-330-5p. DNA Cell
Biol. 37:491–500. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
165
|
Nymoen DA, Hetland Falkenthal TE, Holth A,
Ow GS, Ivshina AV, Tropé CG, Kuznetsov VA, Staff AC and Davidson B:
Expression and clinical role of chemoresponse-associated genes in
ovarian serous carcinoma. Gynecol Oncol. 139:30–39. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
166
|
Lokman NA, Pyragius CE, Ruszkiewicz A,
Oehler MK and Ricciardelli C: Annexin A2 and S100A10 are
independent predictors of serous ovarian cancer outcome. Transl
Res. 171:83–95.e1-e2. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
167
|
Wang L, Yan W, Li X, Liu Z, Tian T, Chen
T, Zou L and Cui Z: S100A10 silencing suppresses proliferation,
migration and invasion of ovarian cancer cells and enhances
sensitivity to carboplatin. J Ovarian Res. 12:1132019. View Article : Google Scholar : PubMed/NCBI
|
|
168
|
Xuan L, Sun Z, Wang J and Gao S: lncRNA
SNHG8 promotes ovarian cancer progression through serving as sponge
for miR-1270 to regulate S100A11 expression. J Gene Med.
e33152021.
|
|
169
|
Li W, Cui Z, Kong Y, Liu X and Wang X:
Serum levels of S100A11 and MMP-9 in patients with epithelial
ovarian cancer and their clinical significance. Biomed Res Int.
2021:73412472021.PubMed/NCBI
|
|
170
|
Qian J, Ding F, Luo A, Liu Z and Cui Z:
Overexpression of S100A14 in human serous ovarian carcinoma. Oncol
Lett. 11:1113–1119. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
171
|
Sturchler E, Cox JA, Durussel I, Weibel M
and Heizmann CW: S100A16, a novel calcium-binding protein of the
EF-hand superfamily. J Biol Chem. 281:38905–38917. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
172
|
Yang T, Cheng J, Yang Y, Qi W, Zhao Y,
Long H, Xie R and Zhu B: S100B mediates stemness of ovarian cancer
stem-like cells through inhibiting p53. Stem Cells. 35:325–336.
2017. View Article : Google Scholar
|
|
173
|
Yang T, Cheng J, You J, Yan B, Liu H and
Li F: S100B promotes chemoresistance in ovarian cancer stem cells
by regulating p53. Oncol Rep. 40:1574–1582. 2018.PubMed/NCBI
|
|
174
|
Wang X, Tian T, Li X, Zhao M, Lou Y, Qian
J, Liu Z, Chen H and Cui Z: High expression of S100P is associated
with unfavorable prognosis and tumor progression in patients with
epithelial ovarian cancer. Am J Cancer Res. 5:2409–2421.
2015.PubMed/NCBI
|
|
175
|
Wang Q, He Z, Gao J, Hu S, Huang M, Liu M,
Zheng J and Tang H: S100P sensitizes ovarian cancer cells to
carboplatin and paclitaxel in vitro. Cancer Lett. 272:277–284.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
176
|
Ma N, Zhu L, Yang L, Cui Y and Zhan Y:
Prognostic values of S100 family mRNA expression in ovarian cancer.
Cancer Biomark. 25:67–78. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
177
|
Wu B, Yu C, Zhou B, Huang T, Gao L, Liu T
and Yang X: Overexpression of TROP2 promotes proliferation and
invasion of ovarian cancer cells. Exp Ther Med. 14:1947–1952. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
178
|
Dai S, Venturini E, Yadav S, Lin X, Clapp
D, Steckiewicz M, Gocher-Demske AM, Hardie DG and Edelman AM:
Calcium/calmodulin-dependent protein kinase kinase 2 mediates
pleiotropic effects of epidermal growth factor in cancer cells.
Biochim Biophys Acta Mol Cell Res. 1869:1192522022. View Article : Google Scholar : PubMed/NCBI
|
|
179
|
Chen LL, Xia LY, Zhang JP, Wang Y, Chen
JY, Guo C and Xu WH: Saikosaponin D alleviates cancer cachexia by
directly inhibiting STAT3. Phytother Res. 37:809–819. 2023.
View Article : Google Scholar
|
|
180
|
Laski J, Singha B, Wang X, Valdés YR,
Collins O and Shepherd TG: Activated CAMKKβ-AMPK signaling promotes
autophagy in a spheroid model of ovarian tumour metastasis. J
Ovarian Res. 13:582020. View Article : Google Scholar
|
|
181
|
Chen Z, Sun X, Xia Z, Wang J, Guo N and
Zhang Y: CaMKK2 promotes the progression of ovarian carcinoma
through the PI3K/PDK1/Akt axis. Comput Math Methods Med.
2022:71879402022.PubMed/NCBI
|
|
182
|
Tsuyoshi H, Wong VKW, Han Y, Orisaka M,
Yoshida Y and Tsang BK: Saikosaponin-d, a calcium mobilizing agent,
sensitizes chemoresistant ovarian cancer cells to cisplatin-induced
apoptosis by facilitating mitochondrial fission and G2/M arrest.
Oncotarget. 8:99825–99840. 2017. View Article : Google Scholar : PubMed/NCBI
|