Cancer stem cells (CSCs) constitute a distinct set
of cells found within a tumor that share similar properties to
normal stem cells (1). These
cells are important for cancer development and progression, as well
as in making tumors resistant to chemotherapy and radiation therapy
(2). CSCs exhibit self-renewal
potential and can differentiate into various cell types found
within the tumor, allowing them to continually regenerate the tumor
and form more aggressive tumors, even after initial treatment has
been administered. They have been identified in various types of
cancers, including glioma, breast cancer, lung carcinoma, and
leukemia (3-5). Due to their resistance to
conventional cancer treatments, they are considered to be one of
the contributing factors for tumor recurrence following treatment
(3-5).
The CSC niche, similar to the adult stem cell niche,
is a component of the tumor microenvironment (TME) that regulates
stem cell activities through interactions between cells and
secreted molecules (6). The TME
consists of a variety of components, such as cytokine networks,
immune cells, perivascular cells, fibroblasts, extracellular matrix
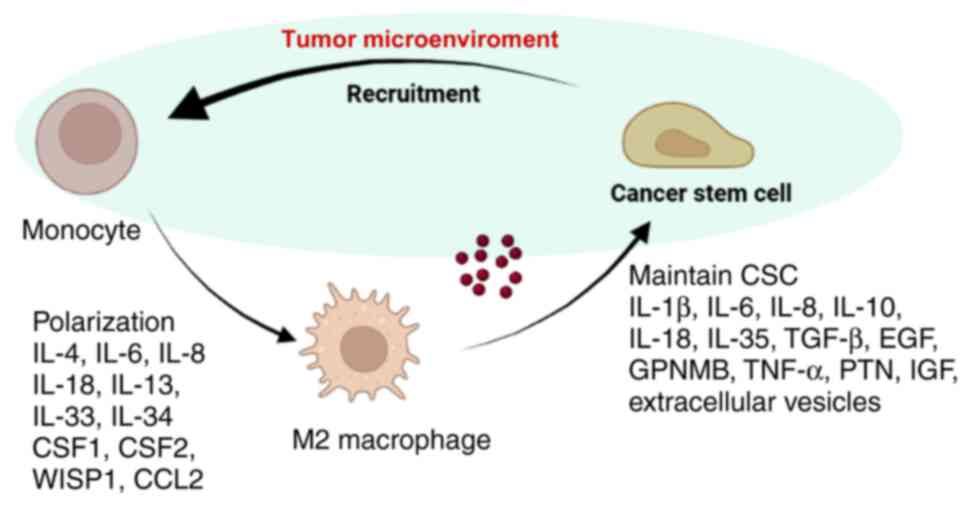
(ECM) components, and endothelial cells (7). Tumor-associated macrophages (TAMs)
are the most prevalent immune cells in the TME and can be recruited
by CSCs to participate in TME formation, which can aid in CSC
survival. M2 macrophages, which are alternatively activated
macrophages, have a significant influence on cancer development and
progression (8), unlike M1
macrophages, which are responsible for the immune response against
infections and inflammation (8).
Exosomes are small extracellular vesicles that range
from 40 to 160 nm in diameter and originate from endosomes
(9). It has been shown that
nearly all cells secrete exosomes, which can be found in various
biofluids and cell culture media (10). Exosomes represent a novel
mechanism for intercellular communication between donor and
recipient cells, and individuals with cancer have been found to
possess increased quantities of exosomes compared with healthy
individuals, highlighting the potential role of exosome-mediated
cellular and communication in cancer. This communication promotes
tumor formation, angiogenesis, metastasis, progression, immune
evasion, and drug resistance (11). Due to their favorable
biocompatibility properties and capacity for customization to
target specific cells, exosomes hold promise as carriers for
therapeutic payloads like microRNAs (miRNAs/miRNAs), small
interfering RNA (siRNAs), and small-molecule drugs. This potential
creates possibilities for transforming conventional cancer
treatment approaches (12).
Nevertheless, the quest for suitable exosomal molecules and donor
cells to establish an effective exosomal drug delivery system
remains a substantial challenge.
Extensive research has been performed to understand
the complex system of communication facilitated by exosomes within
the TME (9). Within the TME,
macrophages play a pivotal role in intercellular communication
through the release of exosomes. Macrophages are broadly
categorized into two types based on their activation status:
Classically activated M1 macrophages and alternatively activated M2
macrophages, which are influenced by a range of stimuli (13). TAMs exhibit a mixed M1/M2
phenotype, and macrophage-derived exosomes may vary based on their
parental cell properties. For example, exosomes derived from M2
macrophages may contain higher levels of specific miRNAs compared
to those derived from M1 macrophages, thereby impacting cancer
progression and drug resistance (14). Hence, understanding the specific
exosomes secreted by distinct macrophage phenotypes may offer
therapeutic opportunities. Macrophage-derived exosomes constitute a
substantial proportion of blood-borne exosomes and these may be
used as potential biomarkers for diagnosing cancer through
minimally invasive liquid biopsies (15). Moreover, exosomes released by
macrophages can trigger immune responses that restrain cancer
progression, highlighting their possible application in anti-tumor
treatments (16,17). Currently, the role of M2-derived
exosomes in maintaining cancer cell stemness is an active area of
investigation for developing novel therapeutic approaches to target
this process and improve outcomes in cancer patients.
CSCs are a subpopulation of tumor cells that exhibit
self-renewal and differentiation abilities, similar to those of
normal stem cells (5,18). CSCs are hypothesized to be
responsible for initiating and maintaining tumor growth, as well as
conferring resistance to chemotherapy and radiation therapy. These
versatile cancer cells have the capacity to differentiate into
various cell types found in tumors, which enables them to drive
primary tumor growth and contribute to the development of new
tumors (19). Various surface
markers, such as CD34+/CD38−, are used to identify CSCs in a wide
range of cancers (20). By way of
their pluripotency, CSCs play a pivotal role in tumorigenesis,
cellular proliferation, and metastasis. Furthermore, CSCs can
self-renew, making them functionally immortal. Although only a
small percentage of cancer cells exhibit stemness properties, they
can differentiate into a range of cancer cell types that constitute
the majority of tumor cells (18). CSCs are often more tumorigenic
than non-stem cancer cells. Although chemotherapy and radiotherapy
can effectively kill a significant portion of the tumor mass, CSCs
are typically resistant to these treatments, making it difficult to
achieve significant clinical improvement (2,4,21).
CSCs also have the capacity to generate a wide range of cell types
within a tumor, resulting in heterogeneous progeny (22). This diversity of phenotypes stems
from the inherent plasticity of CSCs, allowing them to transition
between different cell states or phenotypic states. These
transitions can occur spontaneously or in response to signals from
the tumor microenvironment, such as changes in oxygen levels,
nutrient availability, or interactions with other cells and
signaling molecules (22). The
phenotypic variations exhibited by CSCs are extensive and can vary
depending on the tumor type and context (22). There are several common phenotypic
states observed in CSCs: i) Stem-like state: CSCs maintain stem
cell-like properties, characterized by their ability to self-renew
and differentiate into multiple cell lineages. These cells often
show increased expression of stem cell markers and signaling
pathways associated with stemness (23). ii) Differentiated state: CSCs can
undergo partial or complete differentiation into various cell types
present in the tumor, resembling the non-CSC population. This
differentiation can lead to the formation of bulk tumor cells with
limited self-renewal potential (24). iii) Hybrid state: CSCs can exhibit
a hybrid phenotype that combines both stem-like and differentiated
characteristics. These cells possess certain stem cell properties
while also displaying markers or features associated with more
differentiated cells. The hybrid state may confer increased
resistance to therapies and enhanced metastatic potential (24). iv) Epithelial-to-mesenchymal
transition (EMT): CSCs can undergo a process known as EMT, which is
associated with increased invasiveness and metastatic potential.
During EMT, CSCs lose epithelial characteristics and acquire
mesenchymal traits, including enhanced motility, resistance to
apoptosis, and ECM remodeling abilities (25). v) Metabolic plasticity: CSCs can
adapt their metabolic profile to utilize different energy sources
and survive in a range of different microenvironments. They can
switch between glycolysis and oxidative phosphorylation (a process
known as the Warburg effect), which provides them with a survival
advantage under nutrient-deprived conditions (26). Understanding and targeting the
cellular plasticity of CSCs is crucial for developing effective
therapeutic strategies against cancer. The ability of CSCs to
transition between different phenotypic states enables them to
evade therapies, contribute to tumor heterogeneity, and drive tumor
relapse and metastasis (25).
Overall, the characteristics of CSCs offer a valuable avenue for
comprehending cancer development and the potential treatment of
various cancer types.
Within the scientific community, an ongoing debate
persists regarding the specific mechanism underlying the origin of
macrophages. However, it is widely recognized that macrophages can
be classified into two distinct lineages: Bone marrow-derived
macrophages and tissue-resident macrophages (27,28). Tissue-resident macrophages develop
during embryonic development and are self-sustaining within their
specific area, while bone marrow-derived macrophages arise from
monocytes differentiated by bone marrow progenitors (29). These monocytes migrate from the
bloodstream to tissues during normal and inflammatory conditions
and are activated by various factors. These macrophage populations
have distinct distributions within the TME, with tissue-resident
macrophages spreading to neighboring tumor cells early on,
promoting EMT and increasing invasion (30). Furthermore, tissue-resident
macrophages raise regulatory T-cell numbers to help tumor cells
escape from the immune system (31). Hence, tissue-resident macrophages
may present a promising target for treating tumors. There are
various macrophage subtypes that can be characterized with specific
markers.
Macrophages are a heterogeneous population of immune
cells with a range of phenotypes and functions that actively
regulate tumor progression. Among these, the M1 and M2 macrophage
subtypes hold significant roles in tumor regulation. M1 macrophages
exhibit a pro-inflammatory phenotype when exposed to Type 1 T
helper cytokines, such as IFN-γ, and TNF-α. They secrete anti-tumor
pro-inflammatory cytokines such as IL-8, TNF-α, IL-1β, and IFN-γ
(32,33). Conversely, M2 macrophages are
primarily activated by Type 1 T helper (Th2) cytokines, including
IL-13 and IL-4, resulting in anti-inflammatory properties and
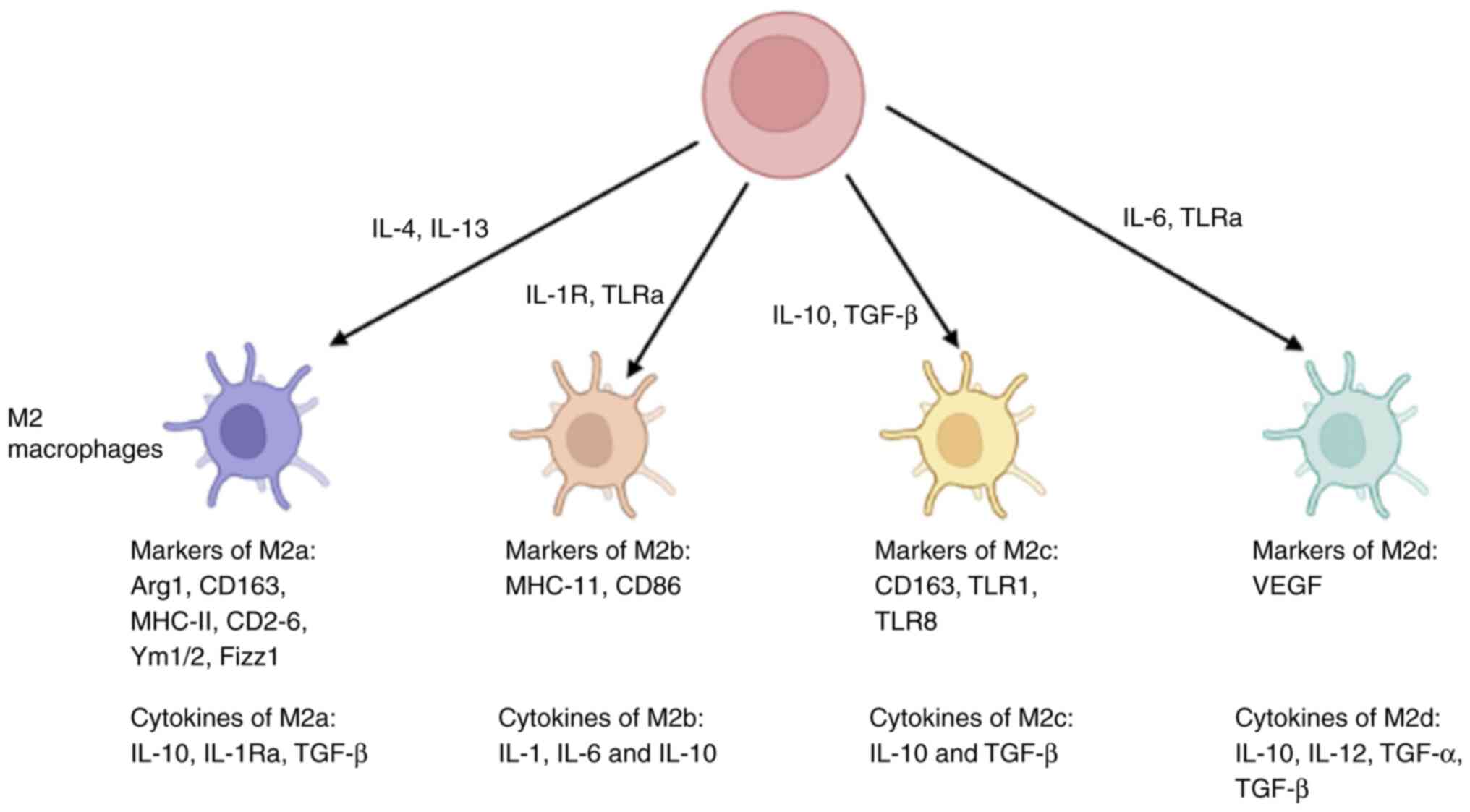
tumorigenesis. M2 macrophages can be further classified into
distinct subsets based on specific stimuli and markers. The M2a
subset, characterized by CD206 and CD68, contributes to fibrosis,
allergic responses, and parasite elimination. The M2b subset,
identified by CD86 receptors, plays a vital role in immune
responses (34,35). The M2c subset, distinguished by
the expression of CD163 receptors, is induced by IL-10, TGF-β, or
glucocorticoids and serves a critical function in anti-inflammatory
processes (36,37). Finally, the M2d subset, associated
with tumor progression, exhibits increased secretion of vascular
endothelial growth factor (VEGF) and IL-10, along with reduced
expression of TNF-α and IL-12 (38,39). Nonetheless, the precise mechanism
underlying the programming of M2d macrophages remains a subject of
controversy. Fig. 1 provides a
depiction of the expression of markers associated with different
subtypes of M2 macrophages.
Macrophages in the TME may have divergent effects on
cancer progression based on their polarization status. Initially,
macrophages may exhibit a pro-inflammatory response and inhibit
tumor growth; however, the evidence supporting this remains limited
(40). As a tumor expands, Th2
cells guide macrophages toward a pro-tumor phenotype, which
promotes tumor development (41).
M2 macrophages have been shown to regulate multiple aspects of
tumorigenesis, such as angiogenesis, metastasis, and
chemo-resistance. M2 macrophages can promote tumor cell
intravasation and extravasation by secreting VEGF and epidermal
growth factor (42,43). They also modulate tumor metastasis
by regulating EMT and promoting ECM degradation. The majority of
TME-associated macrophages tend to be M2, which creates an
immunosuppressive microenvironment (44). An overview of the interactions
between CSCs and M2 macrophages is shown in Fig. 2. Therefore, gaining insights into
the characteristics of M2 macrophages can enhance our understanding
of cancer states and enable the development of new approaches for
inhibiting or eradicating cancer.
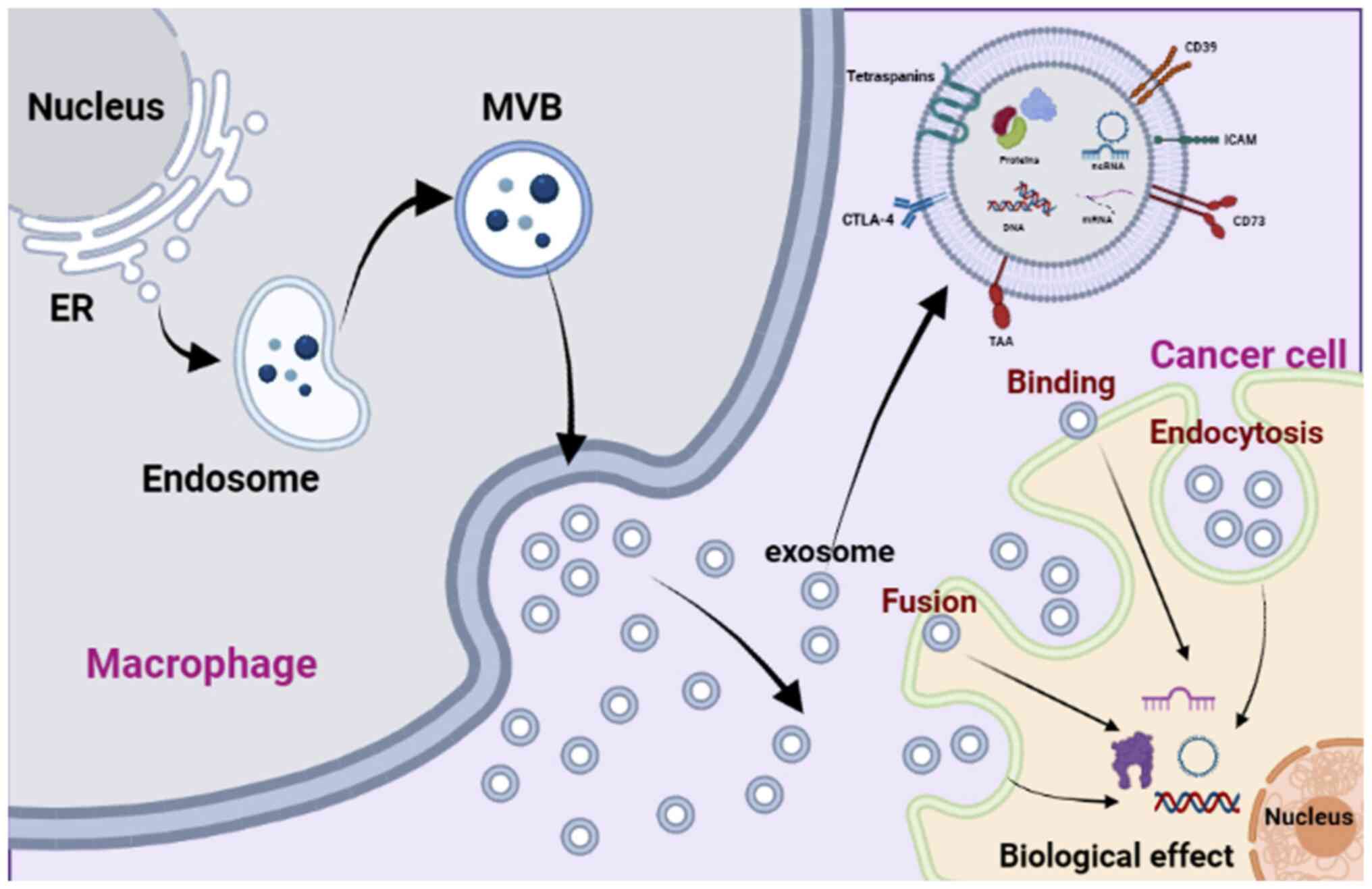
The process by which exosomes are formed from
macrophages follows a similar pattern to that observed in other
cells. It is initiated by the inward budding of the cellular
membrane, leading to the formation of endosomes (9). These endosomes then generate
intraluminal vesicles (ILVs) within the cytoplasm, gradually
transforming into multivesicular bodies (MVBs) (9). Throughout this process, the sorting
of exosomal cargo can be influenced by various external factors.
For example, in response to IL-4 stimuli, macrophage-derived
exosomes selectively incorporate miRNAs (45). Moreover, macrophages activate the
peroxisome proliferator-activated receptor γ pathway and transfer
phosphatase and tensin homolog (PTEN) into exosomes when exposed to
the microenvironment of apoptotic lung cancer cells undergoing
irradiation (46). Additionally,
the activation of the P2X7 signaling pathway triggered by
extracellular ATP enables macrophages to transfer IL-1β and other
proteins into exosomes, leading to an elevation in intracellular
calcium levels (Fig. 3) (47,48).
Typically, exosomes from the inward budding of the
cellular membrane can create endosomes, which develop ILVs that
eventually mature into MVBs through cargo sorting and external
factors. In the typical scenario, MVBs are predominantly degraded
by lysosomes, and only a small fraction of them are released as
exosomes through exocytosis facilitated by Rab proteins and small
GTPases (49). However, when
lysosomes in macrophages are defective, increased secretion of
exosomes is observed (50),
indicating the substantial reliance on macrophage-derived exosomes
on lysosomal function. Conversely, autophagy has a diminishing
effect on exosomal secretion since autophagosomes can merge with
MVBs to form amphisomes, which can then be degraded by lysosomes
(51). Conversely, reducing the
expression of lysosome-associated membrane protein-2 and
lysosome-associated membrane protein-1 hinders the fusion of
amphisomes with lysosomes, resulting in an increased release of
exosomes. Notably, not only endogenous factors but also exogenous
stimuli, such as cellular stress impact the secretion of
macrophage-derived exosomes (52). For example, macrophages release a
higher number of exosomes upon stimulation by lipopolysaccharide
(LPS), which upregulates the expression of Rab27b and Rab27a.
However, this effect can be counteracted by IL-25 (53). In the tumor microenvironment,
hypoxia can also enhance the release of macrophage-derived exosomes
compared to physiological conditions (54). These exosomes become independent
components of the tumor microenvironment and can affect other cells
through various mechanisms. For instance, For example,
LPS/IFN-γ-induced macrophage-derived exosomes can bind to
extracellular endoplasmic reticulum aminopeptidase 1, which
promotes macrophage phagocytosis and nitric oxide synthesis
(55). Macrophage-derived exosome
surface markers are specific to cell and/or tissue types and can
determine the types of recipient cells (56). Different proteins, such as RAB27A
and syntaxin 3, are used by macrophages from various tissues to
regulate exosomal biogenesis and docking with recipient cells
(57). Overall, these findings
suggest that macrophage-derived exosomes play a role in
cell-to-cell communication and have a wide range of effects
depending on their contents and surface markers.
M2 macrophage-derived exosomes transfer various
biomolecules, including growth factors, cytokines, miRNAs, long
non-coding RNAs (lncRNAs), and circular RNAs (circRNAs) (Table I) have been shown to promote
self-renewal and survival of CSCs (58). They can also modulate the
signaling pathways and molecular processes that regulate CSCs,
thereby maintaining and expanding the cancer stem cell population
(58).
Additionally, M2 macrophage-derived exosomes have
been shown to modify the behavior of immune cells, thereby
suppressing the anti-tumor immune response (59). This may further promote the
survival and expansion of the cancer stem cell populations and
contribute to tumor progression.
Exosomes, which mediate cell-to-cell interactions,
possess the ability to exchange genetic molecules, such as miRNAs
(60). miRNAs are key to the
development, polarization, and metabolic control of macrophages
(61-63). To maintain a balanced mRNA
environment, exosomes can rapidly eliminate excessive miRNAs in an
activation-dependent manner (45). Consequently, exosomes derived from
macrophages stimulated with LPS or IL-4 exhibit noticeable
enrichment of specific miRNAs (64). Numerous studies have demonstrated
that alterations in exosomal miRNAs derived from macrophages can
significantly impact important post-transcriptional control
functions in neighboring cells, including cancer cells.
To date, a wide range of miRNAs sourced from
exosomes of M2 macrophages have been implicated in the regulation
of cancer stemness. In human colon cancer, exosomal miR-155-5p
derived from M2 macrophages has been shown to augment the
proliferation and anti-apoptotic capabilities of SW48 and HT29
cells. It also promotes immune escape by downregulating zinc finger
CCCH-type containing 12B and upregulating IL-6, thereby increasing
CD3+ T cell proliferation and the proportion of IFN-γ+ T cells
(65). Another study revealed
that the collaborative action of exosomal miR-155-5p and miR-21-5p
from M2 macrophages promotes cell migration and invasion in colon
cancer by suppressing BRG1 expression. (66); BRG1 is a crucial regulator in
maintaining colorectal cancer stem cells (67). In pancreatic cancer, exosomal
miR-155-5p and miR-221-5p derived from M2 macrophages were found to
stimulate angiogenesis and pancreatic cancer growth by inhibiting
E2F2 expression (68). E2F2
transcriptionally regulates multiple targets involved in various
characteristics of CSCs, including proliferation, self-renewal,
metastasis, and drug resistance (69). Furthermore, exosomal miR-193b-3p
from M2 macrophages was observed to enhance the proliferation,
migration, invasion, and glutamine uptake of pancreatic cancer
cells by downregulating tripartite motif containing 62 (70). Similarly, exosomal miR-365 from M2
macrophages suppressed the expression of BTG2, activating the
FAK/AKT pathway and promoting pancreatic cancer development
(71). Inhibiting the FAK/AKT
signaling pathway was found to reduce the viability of human breast
cancer stem cells (72).
Additionally, exosomal miR-501-3p derived from M2 macrophages was
identified to hinder the tumor suppressor TGFβ receptor 3 gene,
thereby facilitating the progression of pancreatic cancer through
the activation of the TGF-β signaling pathway (73). It is important to note that the
TGF-β signaling pathway has been demonstrated to enhance pancreatic
cancer stemness (74,75). Interestingly, miR-21-5p from
extracellular vesicles derived from M2 macrophages was found to
promote the differentiation and activity of pancreatic cancer stem
cells by suppressing Krüppel-like factor 3 (KLF3) expression
(76). In lung cancer, exosomal
miR-1911-5p from M2 macrophages facilitated cell migration and
invasion in lung adenocarcinoma by downregulating zinc finger and
BTB domain containing 4 expression, which is mediated by CUGBP
Elav-like family member 2 (77).
Additionally, exosomal miR-3917 from M2 macrophages promoted lung
cancer progression by inhibiting G protein-coupled receptor kinase
6 (78), a factor involved in
maintaining self-renewal of hematopoietic stem cells (79). Furthermore, exosomal miR-501-3p
from M2 macrophages represses WD repeat domain 82, contributing to
the progression of lung cancer (80). Notably, M2 macrophage-derived
exosomal miR-942 suppresses forkhead box protein O1 (FOXO1)
expression, promoting the progression of lung adenocarcinoma
(81), FOXO1 acts as a key
inhibitor of cancer cell stemness in various cancer types (82,83). In gastric cancer, exosomal
miR-487a from M2 macrophages advances disease progression by
suppressing TIA1 expression (84). Moreover, exosomal miR-588 from M2
macrophages contributes to cisplatin resistance in gastric cancer
cells by partially inhibiting cylindromatosis expression (85), which regulates the proliferation
of esophageal cancer stem-like cells through the cylindromatosis
pathway (86).
In glioblastoma, M2 macrophage-derived exosomal
miR-27b-3p represses mixed-lineage leukemia 4/positive regulatory
domain 1 signaling, resulting in the activation of IL-33 and
maintenance of stem-like properties in glioblastoma stem cells
(87). Studies on hepatocellular
carcinoma indicated that exosomal miR-27b-3p from M2 macrophages
enhanced tumor development by suppressing KLF3 (88), whereas inhibition of KLF3 was
found to stimulate the differentiation and activity of pancreatic
cancer stem cells (76). In
epithelial ovarian cancer, exosomal miR-221-3p from M2 macrophages
promotes disease progression by suppressing cyclin-dependent kinase
inhibitor 1B. Similarly, exosomal miR-221-3p derived from M2
macrophages enhance the growth and metastasis of osteosarcoma by
repressing suppressor of cytokine signaling 3, activating
JAK2/STAT3 signaling (89), which
plays a critical role in maintaining cancer cell stemness (90). In oral squamous cell carcinoma,
exosomal miR-31-5p from M2 macrophages hinders the tumor suppressor
large tumor suppressor kinase 2 gene, facilitating cancer
progression by inhibiting the Hippo signaling pathway (91), which promotes the transition EMT
and the maintenance of cancer stem cells (92). Conversely, in glioma, M2
macrophage-derived exosomal miR-15a and miR-92a suppress cyclin D1
and RAP1B, respectively, resulting in the inhibition of cell
migration and invasion via the PI3K/AKT/mTOR pathway (93). The PI3K/AKT/mTOR signaling pathway
has been shown to regulate cancer stemness (94). Notably, exosomal miR-223 derived
from macrophages exhibits divergent effects in different cancer
types, despite its involvement in regulating the biological
function of cancer stem cells. The expression levels of miR-223
vary across different cancer types, with increased expression in
metastatic gastric and ovarian cancers, but decreased expression in
hepatocellular and esophageal cancer (95,96). This indicates a contradictory role
for miR-223 in cancer. Furthermore, exosomal miR-223 from
macrophages can promote drug resistance in epithelial ovarian
carcinoma cells by inhibiting PTEN expression (97), while in breast cancer cells, it
can induce invasion and metastasis by suppressing Mef2c expression
(98). However, conflicting
findings suggest that exosomal miR-223 from macrophages inhibits
cancer cell proliferation in hepatocellular cancer cells by
downregulating stathmin 1 and insulin-like growth factor 1 receptor
expression (99). It is possible
that miR-223 induces macrophages to adopt either an anti-tumor or
pro-tumor phenotype in different pathological conditions, leading
to pleiotropic effects in cancer cells, exhibiting both suppressive
and promotive roles.
lncRNAs are RNA molecules that are >200
nucleotides in length and lack protein-coding capacity. Despite
their non-coding nature, they play crucial roles in various
cellular processes, including regulation of gene expression,
epigenetic modifications, chromatin remodeling, and mRNA
processing. In recent years, extensive research has highlighted the
significance of lncRNAs in cancer development and progression
(100,101). Dysregulation of lncRNAs can
disrupt normal cellular processes, leading to uncontrolled cell
growth, survival, and metastasis, which are all characteristic
features of cancer. Previous research has primarily focused on
studying the functions and mechanisms of lncRNAs within individual
cells, but there has been limited investigation into the role of
lncRNAs carried by exosomes secreted by macrophages in facilitating
communication between cells. However, recent studies have shown
that exosomes released by macrophages can deliver a specific lncRNA
called HIF-1α-stabilizing lncRNA to breast cancer cells. This
delivery process then influences the glycolysis of cancer cells by
interacting with a protein called prolyl hydroxylase domain 2,
leading to the stabilization of HIF-1α (102). HIF-1α is known to play a role in
the development of cancer stemness under conditions of hypoxia
(103). These findings suggest
that exosomal lncRNAs derived from macrophages have a positive
impact on cancer stemness. Additionally, Yin et al (104) discovered that exosomal lncRNA
SET-binding factor 2 antisense RNA 1 derived from M2 macrophages
can be transferred to pancreatic cancer cells, promoting cancer
progression by suppressing miR-122-5p and increasing the expression
of a protein called X-linked inhibitor of apoptosis protein.
Notably, miR-122-5p has been associated with cervical cancer stem
cell self-renewal and differentiation (105). In hepatocellular carcinoma,
exosomes derived from M2 macrophages play a role in promoting
malignancy by transferring lncMMPA to tumor cells. This transfer
inhibits miR-548 and leads to the upregulation of aldehyde
dehydrogenase 1 family member A3 (ALDH1A3) expression (106), whereas inhibition of ALDH1A3 can
impede cancer cell stemness (107). In bladder cancer, M2
macrophage-derived lncRNA H19 promotes autophagy in bladder cells
by stabilizing Unc-51-like kinase 1 (108). Notably, lncRNA H19 has been
extensively studied for its involvement in promoting cancer
stemness across various cancer types (109-111). In lung cancer, exosomal lncRNA
AGAP2 antisense RNA 1 derived from M2 macrophages enhances
radiotherapy immunity by reducing miRNA-296 and increasing NOTCH2
expression. Activation of the Notch2 signaling pathway has been
associated with promoting lung cancer stemness (112). In esophageal cancer, exosomal
lncRNA AFAP1 antisense RNA 1 from M2 macrophages affects cell
migration and metastasis by repressing miR-26a expression, thereby
promoting activating transcription factor 2 activity (113). It is worth noting that miR-26a
can regulate the activating enhancer binding Protein 2 α/Nanog
signaling axis related to glioma cancer stemness (114). In gastric cancer, M2
macrophage-derived lncRNA colorectal neoplasia differentially
expressed (CRNDE) contributes to cisplatin resistance (115). Furthermore, lncRNA CRNDE has
been implicated in the regulation of biological characteristics of
glioma stem cells (116). In
summary, these findings shed light on the role of exosomal lncRNAs
in mediating interactions between cells within TME.
CircRNAs are a type of non-coding RNA characterized
by a closed-loop structure and play crucial roles in gene
regulation, development, and disease progression. Emerging studies
suggest that circRNAs may participate in modulating CSCs across
various cancer types (117,118). Specifically, certain circRNAs
have been shown to influence the differentiation and self-renewal
of CSCs in breast, glioblastoma, and colorectal cancer (119). Additionally, certain circRNAs
have been found to modulate the expression of genes that are
important for CSC maintenance and survival (120). One mechanism through which
circRNAs exert their influence on CSCs is by interacting with
miRNAs. By acting as miRNA sponges, circRNAs can bind to and
sequester miRNAs, thereby regulating the expression of miRNA target
genes crucial for CSC function. For example, Yu et al
(121), revealed that
macrophage-derived exosomes regulate gastric cancer cell resistance
to oxaliplatin by encapsulating circ_0008253. However, the role of
circ_0008253 in CSCs has not yet been investigated. Another study
by Chen et al (122)
revealed that M2 macrophage-derived exosomal circ_0020256 enhances
cholangiocarcinoma progression by targeting miR-432-5p/E2F3 axis.
Gu et al (123)
identified that M2 macrophage-derived exosomal circ_0001610 reduces
endometrial cancer radiosensitivity. Moreover, M2
macrophage-derived exosomal circ_TNFRSF21 facilitates angiogenesis
in cutaneous squamous cell carcinoma through the regulation of
miR-3619-5p/Rho-associated coiled-coil containing protein
serine/threonine kinase signaling (124). Nevertheless, the specific roles
of these circRNAs in CSCs remain to be investigated.
Furthermore, macrophage-derived exosomes can
influence the adaptability of cancer cells and contribute to
metastasis. Kim et al (46) demonstrated that macrophages can
deliver an increased amount of PTEN protein to recipient cells via
exosomes when exposed to irradiated apoptotic lung cancer cells,
thereby impeding EMT. Conversely, exosomal ADAM domain 15, a
protein secreted by M2 macrophages, inhibits cancer cell migration
and growth (129). In addition
to macromolecular proteins, cytokines also play a critical role in
cancer and can be found in M2 macrophage-derived exosomes. For
instance, mouse macrophages release various cytokines in exosomes
upon LPS stimulation (64). In
one study, M2 macrophages cultured with apoptotic breast cancer
cells after chemotherapy increased the production of IL-6 in their
exosomes, which were then transferred to cancer cells (130). This process promoted cancer cell
metastasis and proliferation by enhancing STAT phosphorylation.
While cytokines have been extensively investigated in the context
of non-exosomal secretion pathways, exploring whether these
cytokines exhibit enhanced efficacy through exosomal pathways would
be valuable.
Several studies have provided compelling evidence
that macrophage-derived exosomes contain a diverse array of
components, including miRNAs, lncRNAs, proteins, mRNA, tRNA and
ribosomes (131). Recent
research has highlighted the significant role of exosomal
mitochondrial/nuclear DNA derived from cancer cells in tumor
immunity (132). However, the
presence of functional endogenous DNA in macrophage-derived
exosomes remains uncertain. Nonetheless, artificial dsDNA has been
detected in macrophage-derived exosomes in pancreatic cancer
(14). Importantly, M2
macrophage-derived exosomes enriched with arginase-1 can stimulate
the migration and proliferation of glioblastoma cells (133). Moreover, once macrophage-derived
exosomes are released into the extracellular microenvironment, they
may serve as primitive particles within the ECM (9). This is supported by the observation
that components in macrophage-derived exosomes are capable of
synthesizing thromboxane B2, thromboxane, and specific proteins
(126). Further investigation is
warranted to explore the potential independent functions of
macrophage-derived exosomes separate from their parent cells.
Both preclinical and clinical investigations have
underscored the therapeutic potential of targeting two signaling
pathways, namely the C-C chemokine receptor type 2 (CCR2)-C-C Motif
chemokine ligand 2 (CCL2) axis and the C-C Motif chemokine ligand
12 (CXCL12)-C-X-C Motif chemokine receptor 4 (CXCR4) pathway, to
hinder the recruitment and infiltration of TAMs into the TME. These
approaches hold promise for patients with solid tumors (134). For example, an anti-CCL2
antibody (carlumab) inhibited macrophage infiltration in mice and
is presently being tested in clinical trials (NCT 00992186) for the
treatment of solid tumors and metastatic castrate-resistant
prostate cancer (135). However,
carlumab alone only produces a temporary reduction in serum CCL2
levels without significant antitumor effects. Nevertheless, when
combined with conventional chemotherapeutic regimens such as
paclitaxel and carboplatin, it enhances the antitumor response.
Similarly, clinical trials have revealed that inhibiting the
CXCL12-CXCR4 signaling pathway can result in TAM exclusion and
effective treatment of solid tumors (136). For example, a CXCR4 antagonist
called (Plerixafor) impedes tumor angiogenesis by inhibiting the
release of VEGF-A from TAMs and has been employed in the treatment
of solid tumors and pediatric cancers (137-139). LY2510924, a CXCR4 antagonist,
has also undergone clinical trials (NCT02737072) for the treatment
of solid tumors (134).
TAM recruitment and polarization are heavily
influenced by colony-stimulating factor-1 receptor
(CSF-1R)/colony-stimulating factor-1 (CSF-1) pathway, thus, there
have been endeavors to block this signal in TAMs for the treatment
of solid tumors (140). Clinical
trials have been conducted with Emactuzumab (RG7155) (NCT01494688).
This treatment has demonstrated a reduction in CD163+/CSF-1R+
macrophages in diffuse-type giant cell tumors and an increase in
CD8+/CD4+ ratio (134). Clinical
trials are also underway to explore the combination of Emactuzumab
with chemotherapy (NCT02760797) and immunotherapy (NCT02323191) for
solid tumor treatment. Clinical trials have utilized
CSF-1R-specific inhibitors for the treatment of solid tumors. Both
CSF-1R inhibitors and antibodies have exhibited therapeutic
improvements in clinical trials. An example of this is the
utilization of the CSF-1R inhibitor BLZ945, either alone or in
conjunction with anti-programmed cell death protein 1 (PD1)
antibody immunotherapy (NCT02829723), which has demonstrated the
ability to impede macrophage recruitment and promote a change in
macrophage polarization towards phenotypes that are beneficial in
combating tumors (134). The
efficacy of this combination is presently being assessed in
clinical trials as a potential treatment for advanced-stage solid
tumors.
Although strategies that aim to eliminate or hinder
the recruitment of TAMs can delay tumor progression, they often
have systemic toxicities as they target all macrophages and can be
quickly compensated by TAMs. Moreover, discontinuation of CCR2/CCL2
inhibitors can lead to accelerated metastasis in breast cancer due
to the sudden release of monocytes that were previously trapped in
the bone marrow (141). To
overcome these limitations, alternative approaches have garnered
interest, such as re-educating macrophages to adopt an anti-tumor
phenotype. One such approach involves using inhibitors that block
receptor signals on macrophages responsible for modulating
phagocytosis. Tumor cells frequently overexpress a signaling
molecule called CD47, which acts as a 'do not eat me' signal and
suppresses macrophage phagocytic capacity by interacting with
signal regulatory protein α (SIRPα). Anti-CD47 antibodies can
disrupt the CD47-SIRPα axis, restoring the ability of macrophages
to engulf tumors (126). Several
conventional anti-CD47 antibodies have shown success in preclinical
and clinical trials. For example, the anti-CD47 antibody Hu5F9-G4
inhibits the interaction between CD47 and SIRPα, promoting
macrophage-mediated phagocytosis and the elimination of cancer
cells; this antibody has been tested in clinical trials
(NCT02953509 and NCT02216409) for the treatment of solid tumors and
various hematological malignancies (142). Additionally, polypeptides or
recombinant proteins derived from SIRPα, such as engineered
high-affinity SIRPα proteins, can act as decoys by binding to CD47
and disrupting CD47-SIRPα signaling. Studies have demonstrated that
the recombinant protein TTI-621, consisting of the N-terminal
domain of SIRPα fused to human IgG1, suppresses tumor growth by
enhancing macrophage-mediated phagocytosis of solid tumor cells
(143). Currently, TTI-621 is
undergoing clinical investigation for the treatment of solid tumors
(NCT02663518 and NCT02890368). However, CD47 is expressed on both
healthy and tumor cells. Therefore, targeting CD47 will inevitably
lead to the elimination of healthy cells that express CD47,
including red blood cells and thrombocytes. The unintended
consequences of this approach, such as thrombocythemia and anemia,
result in a reduced maximum tolerated dose during clinical trials.
Consequently, these off-target effects limit the impact on the
tumor (144).
CD40, a member of the TNF receptor superfamily, is
expressed on various antigen-presenting cells and certain tumor
cells. Activation of TAMs by agonistic anti-CD40 antibodies has
been shown to stimulate the secretion of pro-inflammatory cytokines
such as nitric oxide and TNF-α, activating effector T cells and
restoring tumor immune surveillance. Selicrelumab in combination
with immunotherapy, such as atezolizumab, has been tested in
clinical trials (NCT02304393) for the treatment of solid tumors and
has been demonstrated to significantly enhance macrophage
phagocytic activity (134).
Toll-like receptor (TLR) activation is essential for
stimulating the innate immune response of macrophages, driving them
toward the M1 phenotype associated with anti-tumor activity. The
activation of multiple TLR signals enhances macrophage phagocytic
activity and promotes anti-tumor responses. For example, TLR4 and
TLR5 agonists have been observed to polarize a greater number of
CD206+ M2 TAMs towards the CD86+ M1 phenotype, effectively
suppressing tumor growth without significant toxicity (145). Clinical trials have evaluated
the TLR9 agonist, IMO-2125, for the treatment of refractory solid
tumors and metastatic melanoma (NCT04126876 and NCT03052205),
resulting in macrophage polarization towards an anti-tumor
phenotype and subsequent tumor regression (134). However, TLR agonists can
upregulate the expression of programmed death-ligand 1 (PD-L1) in
macrophages, limiting the anti-tumor response. To overcome this
limitation, the combination of IMO-2125 with immunotherapy, such as
ipilimumab, has been explored to enhance the effectiveness of
cancer treatment. Additionally, SD101, another TLR9 agonist, is
currently undergoing clinical trials (NCT03007732) in combination
with PD-1 blockade to augment therapeutic efficacy (134). Table II provides a summary of ongoing
clinical trials investigating therapeutic agents targeting M2
macrophages for cancer treatment.
The utilization of macrophage-derived exosomes in
cancer therapy is potentially significant. These exosomes have the
ability to deliver targeted drugs and nanomaterials to specific
recipient cells through cargo transfer. They exhibit
biocompatibility and can facilitate drug transport across natural
barriers, including the blood-brain barrier, enabling the delivery
of brain-derived neurotrophic factors for the treatment of central
nervous system diseases and brain tumor therapy while minimizing
toxicity (146). Furthermore,
macrophage-derived exosomes have the potential to mitigate adverse
reactions in drug therapy. Studies have demonstrated that
cisplatin-loaded exosomes derived from M1 macrophages enhance the
anti-cancer effects of a drug by suppressing cancer cell
proliferation, inducing apoptosis, and improving drug sensitivity
(147). Additionally,
macrophages, particularly M1 macrophages, can transport paclitaxel
and overcome drug resistance mechanisms, resulting in potent
anti-tumor effects (148).
Exploring the biogenesis and composition of
macrophage-derived exosomes holds promise for advancing anti-tumor
therapies. Inhibiting exosome formation and secretion may offer the
potential for anti-tumor treatment. Exosomes released by
macrophages can induce cancer cells to express PD-L1 proteins,
which play a crucial role in tumor immune escape. However, the use
of GW4869, an inhibitor of exosomal secretion, can counteract this
induction (149,150). Inhibiting exosome biogenesis
in vivo may have implications for normal cellular functions,
potentially leading to adverse effects on other intracellular
transport processes. Therefore, it is crucial to exercise caution
and conduct thorough investigations when developing cancer
therapeutic strategies targeting exosome biogenesis. In terms of
composition, ovatodiolide, a macrocyclic bioactive compound, has
demonstrated its ability to reduce the abundance of M2
macrophage-derived exosomal miR-21, consequently suppressing
bladder carcinogenesis (151).
This discovery suggests that macrophage-derived exosomal miRNAs
hold promising clinical potential for cancer treatment. In
conclusion, the development of therapeutic approaches for the
complex roles of macrophage-derived exosomes should prioritize
optimizing treatment efficacy while minimizing adverse reactions
during the design of clinical trials.
M2 macrophages and the exosomes they release play a
vital role in supporting CSCs and facilitating cancer progression.
Through secretion of growth factors, cytokines, and
immunosuppressive substances, M2 macrophages contribute to tumor
growth and progression. These M2-derived exosomes further promote
tumor development by transferring genetic material and influencing
the immune response against cancer. The exploration of M2
cell-derived exosomes holds significant implications for the
development of novel approaches in cancer treatment. Two potential
avenues for therapeutic intervention involve targeting M2
macrophages and their exosomes, as well as utilizing exosome-based
therapies. By directing efforts towards M2 macrophages and their
exosomes, it becomes possible to limit their support to the tumor
and stimulate an immune response against cancer. Additionally,
exosomes can serve as a therapeutic tool by delivering therapeutic
agents to cancer cells and modulating the immune response. Further
research is required to fully comprehend the role of M2-derived
exosomes in cancer and to develop effective therapeutic strategies.
Specifically, there is a need to obtain deeper insights into the
mechanisms by which M2-derived exosomes contribute to the
maintenance of cancer stem cells and to develop targeted
therapeutic strategies focusing on M2-derived exosomes in cancer
due to their critical involvement in disease progression. In
conclusion, studying M2 cell-derived exosomes in cancer has the
potential to offer a better understanding of the mechanisms driving
cancer progression and to guide the development of innovative
therapeutic approaches. However, extensive research is essential to
gain a comprehensive understanding of the role of M2 cell-derived
exosomes in cancer.
Not applicable.
YL, WZ and RZ were involved in the conceptualization
of the study. XL, LY, CC, JL and XY were involved in the
preparation of the figures and tables, and edited the manuscript.
WZ, JL and RZ were involved in the curation of the data for
inclusion in the review. WZ and YL were involved in the writing and
preparation of the original draft of the manuscript. YL was
involved in funding acquisition. All authors have read and agreed
to the published version of the manuscript. Data authentication is
not applicable.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
Not applicable.
The present study was supported by the Open Research Fund
Program of Hubei-MOST KLOS & KLOBME (grant no. 202203) and the
Science and Technology Project of Yantian District in Shenzhen
City, Guangdong Province, China (grant no. YTWS20220206).
|
1
|
Clevers H: The cancer stem cell: Premises,
promises and challenges. Nat Med. 17:313–319. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Nassar D and Blanpain C: Cancer stem
cells: Basic concepts and therapeutic Implications. Annu Rev
Pathol. 11:47–76. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Dawood S, Austin L and Cristofanilli M:
Cancer stem cells: Implications for cancer therapy. Oncology
(Williston Park). 28:1101–1107. 11102014.PubMed/NCBI
|
|
4
|
Vlashi E and Pajonk F: Cancer stem cells,
cancer cell plasticity and radiation therapy. Semin Cancer Biol.
31:28–35. 2015. View Article : Google Scholar
|
|
5
|
Walcher L, Kistenmacher AK, Suo H, Kitte
R, Dluczek S, Strauß A, Blaudszun AR, Yevsa T, Fricke S and
Kossatz-Boehlert U: Cancer stem cells-origins and biomarkers:
Perspectives for targeted personalized therapies. Front Immunol.
11:12802020. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Plaks V, Kong N and Werb Z: The cancer
stem cell niche: How essential is the niche in regulating stemness
of tumor cells? Cell Stem Cell. 16:225–238. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Neophytou CM, Panagi M, Stylianopoulos T
and Papageorgis P: The role of tumor microenvironment in cancer
metastasis: Molecular mechanisms and therapeutic opportunities.
Cancers (Basel). 13:20532021. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liu J, Geng X, Hou J and Wu G: New
insights into M1/M2 macrophages: Key modulators in cancer
progression. Cancer Cell Int. 21:3892021. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kalluri R and LeBleu VS: The biology,
function, and biomedical applications of exosomes. Science.
367:eaau69772020. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhou B, Xu K, Zheng X, Chen T, Wang J,
Song Y, Shao Y and Zheng S: Application of exosomes as liquid
biopsy in clinical diagnosis. Signal Transduct Target Ther.
5:1442020. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Nicolini A, Ferrari P and Biava PM:
Exosomes and cell communication: From tumour-derived exosomes and
their role in tumour progression to the use of exosomal cargo for
cancer treatment. Cancers (Basel). 13:8222021. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang Y, Liu Q, Zhang X, Huang H, Tang S,
Chai Y, Xu Z, Li M, Chen X, Liu J and Yang C: Recent advances in
exosome-mediated nucleic acid delivery for cancer therapy. J
Nanobiotechnology. 20:2792022. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Long KB, Collier AI and Beatty GL:
Macrophages: Key orchestrators of a tumor microenvironment defined
by therapeutic resistance. Mol Immunol. 110:3–12. 2019. View Article : Google Scholar
|
|
14
|
Binenbaum Y, Fridman E, Yaari Z, Milman N,
Schroeder A, Ben David G, Shlomi T and Gil Z: Transfer of miRNA in
macrophage-derived exosomes induces drug resistance in pancreatic
adenocarcinoma. Cancer Res. 78:5287–5299. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ismail N, Wang Y, Dakhlallah D, Moldovan
L, Agarwal K, Batte K, Shah P, Wisler J, Eubank TD, Tridandapani S,
et al: Macrophage microvesicles induce macrophage differentiation
and miR-223 transfer. Blood. 121:984–995. 2013. View Article : Google Scholar :
|
|
16
|
Behzadi E, Hosseini HM, Halabian R and
Fooladi AAI: Macrophage cell-derived exosomes/staphylococcal
enterotoxin B against fibrosarcoma tumor. Microb Pathog.
111:132–138. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cheng L, Wang Y and Huang L: Exosomes from
M1-polarized macrophages potentiate the cancer vaccine by creating
a pro-inflammatory microenvironment in the lymph node. Mol Ther.
25:1665–1675. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Batlle E and Clevers H: Cancer stem cells
revisited. Nat Med. 23:1124–1134. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Akbar Samadani A, Keymoradzdeh A, Shams S,
Soleymanpour A, Elham Norollahi S, Vahidi S, Rashidy-Pour A, Ashraf
A, Mirzajani E, Khanaki K, et al: Mechanisms of cancer stem cell
therapy. Clin Chim Acta. 510:581–592. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhao W, Li Y and Zhang X: Stemness-related
markers in cancer. Cancer Transl Med. 3:87–95. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Babaei G, Aziz SG and Jaghi NZZ: EMT,
cancer stem cells and autophagy; the three main axes of metastasis.
Biomed Pharmacother. 133:1109092021. View Article : Google Scholar
|
|
22
|
Das PK, Pillai S, Rakib MA, Khanam JA,
Gopalan V, Lam AKY and Islam F: Plasticity of cancer stem cell:
Origin and role in disease progression and therapy resistance. Stem
Cell Rev Rep. 16:397–412. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Huang T, Song X, Xu D, Tiek D, Goenka A,
Wu B, Sastry N, Hu B and Cheng SY: Stem cell programs in cancer
initiation, progression, and therapy resistance. Theranostics.
10:8721–8743. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhu P and Fan Z: Cancer stem cells and
tumorigenesis. Biophys Rep. 4:178–188. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chen W, Dong J, Haiech J, Kilhoffer MC and
Zeniou M: Cancer stem cell quiescence and plasticity as major
challenges in cancer therapy. Stem Cells Int. 2016:17409362016.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yang L, Shi P, Zhao G, Xu J, Peng W, Zhang
J, Zhang G, Wang X, Dong Z, Chen F and Cui H: Targeting cancer stem
cell pathways for cancer therapy. Signal Transduct Target Ther.
5:82020. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Shi C and Pamer EG: Monocyte recruitment
during infection and inflammation. Nat Rev Immunol. 11:762–774.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
van Furth R and Cohn ZA: The origin and
kinetics of mononuclear phagocytes. J Exp Med. 128:415–435. 1968.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Epelman S, Lavine KJ and Randolph GJ:
Origin and functions of tissue macrophages. Immunity. 41:21–35.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Casanova-Acebes M, Dalla E, Leader AM,
LeBerichel J, Nikolic J, Morales BM, Brown M, Chang C, Troncoso L,
Chen ST, et al: Tissue-resident macrophages provide a
pro-tumorigenic niche to early NSCLC cells. Nature. 595:578–584.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Gratchev A, Schledzewski K, Guillot P and
Goerdt S: Alternatively activated antigen-presenting cells:
Molecular repertoire, immune regulation, and healing. Skin
Pharmacol Appl Skin Physiol. 14:272–279. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Orecchioni M, Ghosheh Y, Pramod AB and Ley
K: Macrophage polarization: Different gene signatures in M1(LPS+)
vs classically and M2(LPS-) vs alternatively activated macrophages.
Front Immunol. 10:10842019. View Article : Google Scholar
|
|
33
|
Tong Y, Guo YJ, Zhang Q, Bi HX, Kai K and
Zhou RY: Combined treatment with dihydrotestosterone and
lipopolysaccharide modulates prostate homeostasis by upregulating
TNF-α from M1 macrophages and promotes proliferation of prostate
stromal cells. Asian J Androl. 24:513–520. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hu W, Lin J, Lian X, Yu F, Liu W, Wu Y,
Fang X, Liang X and Hao W: M2a and M2b macrophages predominate in
kidney tissues and M2 subpopulations were associated with the
severity of disease of IgAN patients. Clin Immunol. 205:8–15. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wen Y, Lu X, Ren J, Privratsky JR, Yang B,
Rudemiller NP, Zhang J, Griffiths R, Jain MK, Nedospasov SA, et al:
KLF4 in macrophages attenuates TNFα-mediated kidney injury and
fibrosis. J Am Soc Nephrol. 30:1925–1938. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Loegl J, Hiden U, Nussbaumer E,
Schliefsteiner C, Cvitic S, Lang I, Wadsack C, Huppertz B and
Desoye G: Hofbauer cells of M2a, M2b and M2c polarization may
regulate feto-placental angiogenesis. Reproduction. 152:447–455.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lurier EB, Dalton D, Dampier W, Raman P,
Nassiri S, Ferraro NM, Rajagopalan R, Sarmady M and Spiller KL:
Transcriptome analysis of IL-10-stimulated (M2c) macrophages by
next-generation sequencing. Immunobiology. 222:847–856. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wang Q, Ni H, Lan L, Wei X, Xiang R and
Wang Y: Fra-1 protooncogene regulates IL-6 expression in
macrophages and promotes the generation of M2d macrophages. Cell
Res. 20:701–712. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ferrante CJ, Pinhal-Enfield G, Elson G,
Cronstein BN, Hasko G, Outram S and Leibovich SJ: The
adenosine-dependent angiogenic switch of macrophages to an M2-like
phenotype is independent of interleukin-4 receptor alpha (IL-4Rα)
signaling. Inflammation. 36:921–931. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Cassetta L and Pollard JW: Targeting
macrophages: Therapeutic approaches in cancer. Nat Rev Drug Discov.
17:887–904. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
DeNardo DG, Barreto JB, Andreu P, Vasquez
L, Tawfik D, Kolhatkar N and Coussens LM: CD4(+) T cells regulate
pulmonary metastasis of mammary carcinomas by enhancing protumor
properties of macrophages. Cancer Cell. 16:91–102. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Wyckoff J, Wang W, Lin EY, Wang Y, Pixley
F, Stanley ER, Graf T, Pollard JW, Segall J and Condeelis J: A
paracrine loop between tumor cells and macrophages is required for
tumor cell migration in mammary tumors. Cancer Res. 64:7022–7029.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Esser S, Lampugnani MG, Corada M, Dejana E
and Risau W: Vascular endothelial growth factor induces VE-cadherin
tyrosine phosphorylation in endothelial cells. J Cell Sci.
111:1853–1865. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Su S, Liu Q, Chen J, Chen J, Chen F, He C,
Huang D, Wu W, Lin L, Huang W, et al: A positive feedback loop
between mesenchymal-like cancer cells and macrophages is essential
to breast cancer metastasis. Cancer Cell. 25:605–620. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Squadrito ML, Baer C, Burdet F, Maderna C,
Gilfillan GD, Lyle R, Ibberson M and De Palma M: Endogenous RNAs
modulate microRNA sorting to exosomes and transfer to acceptor
cells. Cell Rep. 8:1432–1446. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Kim YB, Ahn YH, Jung JH, Lee YJ, Lee JH
and Kang JL: Programming of macrophages by UV-irradiated apoptotic
cancer cells inhibits cancer progression and lung metastasis. Cell
Mol Immunol. 16:851–867. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Välimäki E, Cypryk W, Virkanen J, Nurmi K,
Turunen PM, Eklund KK, Åkerman KE, Nyman TA and Matikainen S:
Calpain activity is essential for ATP-driven unconventional
vesicle-mediated protein secretion and inflammasome activation in
human macrophages. J Immunol. 197:3315–3325. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Qu Y, Franchi L, Nunez G and Dubyak GR:
Nonclassical IL-1 beta secretion stimulated by P2X7 receptors is
dependent on inflammasome activation and correlated with exosome
release in murine macrophages. J Immunol. 179:1913–1925. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Pegtel DM and Gould SJ: Exosomes. Annu Rev
Biochem. 88:487–514. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Yang Q, Nanayakkara GK, Drummer C, Sun Y,
Johnson C, Cueto R, Fu H, Shao Y, Wang L, Yang WY, et al:
Low-intensity ultrasound-induced anti-inflammatory effects are
mediated by several new mechanisms including gene induction,
immunosuppressor cell promotion, and enhancement of exosome
biogenesis and docking. Front Physiol. 8:8182017. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Xu J, Camfield R and Gorski SM: The
interplay between exosomes and autophagy-partners in crime. J Cell
Sci. 131:jcs2152102018. View Article : Google Scholar
|
|
52
|
Babuta M, Furi I, Bala S, Bukong TN, Lowe
P, Catalano D, Calenda C, Kodys K and Szabo G: Dysregulated
autophagy and lysosome function are linked to exosome production by
Micro-RNA 155 in alcoholic liver disease. Hepatology. 70:2123–2141.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Li ZG, Scott MJ, Brzóska T, Sundd P, Li
YH, Billiar TR, Wilson MA, Wang P and Fan J: Lung epithelial
cell-derived IL-25 negatively regulates LPS-induced exosome release
from macrophages. Mil Med Res. 5:242018.PubMed/NCBI
|
|
54
|
Brahimi-Horn MC, Chiche J and Pouysségur
J: Hypoxia and cancer. J Mol Med (Berl). 85:1301–1307. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Goto Y, Ogawa Y, Tsumoto H, Miura Y,
Nakamura TJ, Ogawa K, Akimoto Y, Kawakami H, Endo T, Yanoshita R
and Tsujimoto M: Contribution of the exosome-associated form of
secreted endoplasmic reticulum aminopeptidase 1 to exosome-mediated
macrophage activation. Biochim Biophys Acta Mol Cell Res.
1865:874–888. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Sancho-Albero M, Navascués N, Mendoza G,
Sebastián V, Arruebo M, Martín-Duque P and Santamaría J: Exosome
origin determines cell targeting and the transfer of therapeutic
nanoparticles towards target cells. J Nanobiotechnology. 17:162019.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Lai B, Wang J, Fagenson A, Sun Y, Saredy
J, Lu Y, Nanayakkara G, Yang WY, Yu D, Shao Y, et al: Twenty novel
disease group-specific and 12 new shared macrophage pathways in
eight groups of 34 diseases including 24 inflammatory organ
diseases and 10 types of tumors. Front Immunol. 10:26122019.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Bryl R, Piwocka O, Kawka E, Mozdziak P,
Kempisty B and Knopik-Skrocka A: Cancer stem cells-the insight into
non-coding RNAs. Cells. 11:36992022. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Zheng N, Wang T, Luo Q, Liu Y, Yang J,
Zhou Y, Xie G, Ma Y, Yuan X and Shen L: M2 macrophage-derived
exosomes suppress tumor intrinsic immunogenicity to confer
immunotherapy resistance. Oncoimmunology. 12:22109592023.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Valadi H, Ekström K, Bossios A, Sjöstrand
M, Lee JJ and Lötvall JO: Exosome-mediated transfer of mRNAs and
microRNAs is a novel mechanism of genetic exchange between cells.
Nat Cell Biol. 9:654–659. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Roy S: miRNA in macrophage development and
function. Antioxid Redox Signal. 25:795–804. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Li H, Jiang T, Li MQ, Zheng XL and Zhao
GJ: Transcriptional regulation of macrophages polarization by
MicroRNAs. Front Immunol. 9:11752018. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Yao Q, Song Z, Wang B and Zhang JA:
Emerging roles of microRNAs in the metabolic control of immune
cells. Cancer Lett. 433:10–17. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
McDonald MK, Tian Y, Qureshi RA, Gormley
M, Ertel A, Gao R, Aradillas Lopez E, Alexander GM, Sacan A,
Fortina P and Ajit SK: Functional significance of
macrophage-derived exosomes in inflammation and pain. Pain.
155:1527–1539. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Ma YS, Wu TM, Ling CC, Yu F, Zhang J, Cao
PS, Gu LP, Wang HM, Xu H, Li L, et al: M2 macrophage-derived
exosomal microRNA-155-5p promotes the immune escape of colon cancer
by downregulating ZC3H12B. Mol Ther Oncolytics. 20:484–498. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Lan J, Sun L, Xu F, Liu L, Hu F, Song D,
Hou Z, Wu W, Luo X, Wang J, et al: M2 macrophage-derived exosomes
promote cell migration and invasion in colon cancer. Cancer Res.
79:146–158. 2019. View Article : Google Scholar
|
|
67
|
Yoshikawa T, Fukuda A, Omatsu M, Namikawa
M, Sono M, Fukunaga Y, Masuda T, Araki O, Nagao M, Ogawa S, et al:
Brg1 is required to maintain colorectal cancer stem cells. J
Pathol. 255:257–269. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Yang Y, Guo Z, Chen W, Wang X, Cao M, Han
X, Zhang K, Teng B, Cao J, Wu W, et al: M2 macrophage-derived
exosomes promote angiogenesis and growth of pancreatic ductal
adenocarcinoma by targeting E2F2. Mol Ther. 29:1226–1238. 2021.
View Article : Google Scholar :
|
|
69
|
Xie D, Pei Q, Li J, Wan X and Ye T:
Emerging role of E2F family in cancer stem cells. Front Oncol.
11:7231372021. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Zhang K, Li YJ, Peng LJ, Gao HF, Liu LM
and Chen H: M2 macrophage-derived exosomal miR-193b-3p promotes
progression and glutamine uptake of pancreatic cancer by targeting
TRIM62. Biol Direct. 18:12023. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Li X, Xu H, Yi J, Dong C, Zhang H, Wang Z,
Miao L and Zhou W: miR-365 secreted from M2 Macrophage-derived
extracellular vesicles promotes pancreatic ductal adenocarcinoma
progression through the BTG2/FAK/AKT axis. J Cell Mol Med.
25:4671–4683. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Thakur R, Trivedi R, Rastogi N, Singh M
and Mishra DP: Inhibition of STAT3, FAK and Src mediated signaling
reduces cancer stem cell load, tumorigenic potential and metastasis
in breast cancer. Sci Rep. 5:101942015. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Yin Z, Ma T, Huang B, Lin L, Zhou Y, Yan
J, Zou Y and Chen S: Macrophage-derived exosomal microRNA-501-3p
promotes progression of pancreatic ductal adenocarcinoma through
the TGFBR3-mediated TGF-β signaling pathway. J Exp Clin Cancer Res.
38:3102019. View Article : Google Scholar
|
|
74
|
Katoh M and Katoh M: WNT signaling and
cancer stemness. Essays Biochem. 66:319–331. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Tang Q, Chen J, Di Z, Yuan W, Zhou Z, Liu
Z, Han S, Liu Y, Ying G, Shu X and Di M: TM4SF1 promotes EMT and
cancer stemness via the Wnt/β-catenin/SOX2 pathway in colorectal
cancer. J Exp Clin Cancer Res. 39:2322020. View Article : Google Scholar
|
|
76
|
Chang J, Li H, Zhu Z, Mei P, Hu W, Xiong X
and Tao J: microRNA-21-5p from M2 macrophage-derived extracellular
vesicles promotes the differentiation and activity of pancreatic
cancer stem cells by mediating KLF3. Cell Biol Toxicol. 38:577–590.
2022. View Article : Google Scholar :
|
|
77
|
Guan B, Dai X, Zhu Y and Geng Q: M2
macrophage-derived exosomal miR-1911-5p promotes cell migration and
invasion in lung adenocarcinoma by down-regulating CELF2-activated
ZBTB4 expression. Anticancer Drugs. 34:238–247. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Song S, Zhao Y, Wang X, Tong X, Chen X and
Xiong Q: M2 macrophages-derived exosomal miR-3917 promotes the
progression of lung cancer via targeting GRK6. Biol Chem.
404:41–57. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Le Q, Yao W, Chen Y, Yan B, Liu C, Yuan M,
Zhou Y and Ma L: GRK6 regulates ROS response and maintains
hematopoietic stem cell self-renewal. Cell Death Dis. 7:e24782016.
View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Lei J, Chen P, Zhang F, Zhang N, Zhu J,
Wang X and Jiang T: M2 macrophages-derived exosomal microRNA-501-3p
promotes the progression of lung cancer via targeting WD repeat
domain 82. Cancer Cell Int. 21:912021. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Wei K, Ma Z, Yang F, Zhao X, Jiang W, Pan
C, Li Z, Pan X, He Z, Xu J, et al: M2 macrophage-derived exosomes
promote lung adenocarcinoma progression by delivering miR-942.
Cancer Lett. 526:205–216. 2022. View Article : Google Scholar
|
|
82
|
Yu JM, Sun W, Wang ZH, Liang X, Hua F, Li
K, Lv XX, Zhang XW, Liu YY, Yu JJ, et al: TRIB3 supports breast
cancer stemness by suppressing FOXO1 degradation and enhancing SOX2
transcription. Nat Commun. 10:57202019. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Firat E and Niedermann G: FoxO proteins or
loss of functional p53 maintain stemness of glioblastoma stem cells
and survival after ionizing radiation plus PI3K/mTOR inhibition.
Oncotarget. 7:54883–54896. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Yang X, Cai S, Shu Y, Deng X, Zhang Y, He
N, Wan L, Chen X, Qu Y and Yu S: Exosomal miR-487a derived from m2
macrophage promotes the progression of gastric cancer. Cell Cycle.
20:434–444. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Cui HY, Rong JS, Chen J, Guo J, Zhu JQ,
Ruan M, Zuo RR, Zhang SS, Qi JM and Zhang BH: Exosomal microRNA-588
from M2 polarized macrophages contributes to cisplatin resistance
of gastric cancer cells. World J Gastroenterol. 27:6079–6092. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Xu DD, Zhou PJ, Wang Y, Zhang L, Fu WY,
Ruan BB, Xu HP, Hu CZ, Tian L, Qin JH, et al: Reciprocal activation
between STAT3 and miR-181b regulates the proliferation of
esophageal cancer stem-like cells via the CYLD pathway. Mol Cancer.
15:402016. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Zhao G, Ding L, Yu H, Wang W, Wang H, Hu
Y, Qin L, Deng G, Xie B, Li G and Qi L: M2-like tumor-associated
macrophages transmit exosomal miR-27b-3p and maintain glioblastoma
stem-like cell properties. Cell Death Discov. 8:3502022. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Tian B, Zhou L, Wang J and Yang P:
miR-660-5p-loaded M2 macrophages-derived exosomes augment
hepatocellular carcinoma development through regulating KLF3. Int
Immunopharmacol. 101:1081572021. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Li X and Tang M: Exosomes released from M2
macrophages transfer miR-221-3p contributed to EOC progression
through targeting CDKN1B. Cancer Med. 9:5976–5988. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Liu W, Long Q, Zhang W, Zeng D, Hu B, Liu
S and Chen L: miRNA-221-3p derived from M2-polarized
tumor-associated macrophage exosomes aggravates the growth and
metastasis of osteosarcoma through SOCS3/JAK2/STAT3 axis. Aging
(Albany NY). 13:19760–19775. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Yuan Y, Wang Z, Chen M, Jing Y, Shu W, Xie
Z, Li Z, Xu J, He F, Jiao P, et al: Macrophage-derived exosomal
miR-31-5p promotes oral squamous cell carcinoma tumourigenesis
through the large tumor suppressor 2-mediated hippo signalling
pathway. J Biomed Nanotechnol. 17:822–837. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Li Z, Wang Y, Zhu Y, Yuan C, Wang D, Zhang
W, Qi B, Qiu J, Song X, Ye J, et al: The Hippo transducer TAZ
promotes epithelial to mesenchymal transition and cancer stem cell
maintenance in oral cancer. Mol Oncol. 9:1091–1105. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Yao J, Wang Z, Cheng Y, Ma C, Zhong Y,
Xiao Y, Gao X and Li Z: M2 macrophage-derived exosomal microRNAs
inhibit cell migration and invasion in gliomas through
PI3K/AKT/mTOR signaling pathway. J Transl Med. 19:992021.
View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Gao XF, He HQ, Zhu XB, Xie SL and Cao Y:
LncRNA SNHG20 promotes tumorigenesis and cancer stemness in
glioblastoma via activating PI3K/Akt/mTOR signaling pathway.
Neoplasma. 66:532–542. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Gao Y, Lin L, Li T, Yang J and Wei Y: The
role of miRNA-223 in cancer: Function, diagnosis and therapy. Gene.
616:1–7. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Haneklaus M, Gerlic M, O'Neill LA and
Masters SL: miR-223: Infection, inflammation and cancer. J Intern
Med. 274:215–226. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Zhu X, Shen H, Yin X, Yang M, Wei H, Chen
Q, Feng F, Liu Y, Xu W and Li Y: Macrophages derived exosomes
deliver miR-223 to epithelial ovarian cancer cells to elicit a
chemoresistant phenotype. J Exp Clin Cancer Res. 38:812019.
View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Yang M, Chen J, Su F, Yu B, Su F, Lin L,
Liu Y, Huang JD and Song E: Microvesicles secreted by macrophages
shuttle invasion-potentiating microRNAs into breast cancer cells.
Mol Cancer. 10:1172011. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Aucher A, Rudnicka D and Davis DM:
MicroRNAs transfer from human macrophages to hepato-carcinoma cells
and inhibit proliferation. J Immunol. 191:6250–6260. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Bhan A, Soleimani M and Mandal SS: Long
noncoding RNA and cancer: A new paradigm. Cancer Res. 77:3965–3981.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Peng WX, Koirala P and Mo YY:
LncRNA-mediated regulation of cell signaling in cancer. Oncogene.
36:5661–5667. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Chen F, Chen J, Yang L, Liu J, Zhang X,
Zhang Y, Tu Q, Yin D, Lin D, Wong PP, et al: Extracellular
vesicle-packaged HIF-1α-stabilizing lncRNA from tumour-associated
macrophages regulates aerobic glycolysis of breast cancer cells.
Nat Cell Biol. 21:498–510. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Zhang Q, Han Z, Zhu Y, Chen J and Li W:
Role of hypoxia inducible factor-1 in cancer stem cells (Review).
Mol Med Rep. 23:172021.
|
|
104
|
Yin Z, Zhou Y, Ma T, Chen S, Shi N, Zou Y,
Hou B and Zhang C: Down-regulated lncRNA SBF2-AS1 in M2
macrophage-derived exosomes elevates miR-122-5p to restrict XIAP,
thereby limiting pancreatic cancer development. J Cell Mol Med.
24:5028–5038. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Gao Z, Wang Q, Ji M, Guo X, Li L and Su X:
Exosomal lncRNA UCA1 modulates cervical cancer stem cell
self-renewal and differentiation through microRNA-122-5p/SOX2 axis.
J Transl Med. 19:2292021. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Xu M, Zhou C, Weng J, Chen Z, Zhou Q, Gao
J, Shi G, Ke A, Ren N, Sun H and Shen Y: Tumor associated
macrophages-derived exosomes facilitate hepatocellular carcinoma
malignance by transferring lncMMPA to tumor cells and activating
glycolysis pathway. J Exp Clin Cancer Res. 41:2532022. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Gelardi ELM, Colombo G, Picarazzi F,
Ferraris DM, Mangione A, Petrarolo G, Aronica E, Rizzi M, Mori M,
La Motta C and Garavaglia S: A selective competitive inhibitor of
aldehyde dehydrogenase 1A3 hinders cancer cell growth, invasiveness
and stemness in vitro. Cancers (Basel). 13:3562021. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Guo Y, Sun W, Gao W, Li L, Liang Y, Mei Z,
Liu B and Wang R: Long noncoding RNA H19 derived from M2
tumor-associated macrophages promotes bladder cell autophagy via
stabilizing ULK1. J Oncol. 2022:34654592022. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Ren J, Ding L, Zhang D, Shi G, Xu Q, Shen
S, Wang Y, Wang T and Hou Y: Carcinoma-associated fibroblasts
promote the stemness and chemoresistance of colorectal cancer by
transferring exosomal lncRNA H19. Theranostics. 8:3932–3948. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Shima H, Kida K, Adachi S, Yamada A, Sugae
S, Narui K, Miyagi Y, Nishi M, Ryo A, Murata S, et al: Lnc RNA H19
is associated with poor prognosis in breast cancer patients and
promotes cancer stemness. Breast Cancer Res Treat. 170:507–516.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Wang F, Rong L, Zhang Z, Li M, Ma L, Ma Y,
Xie X, Tian X and Yang Y: LncRNA H19-derived miR-675-3p promotes
epithelial-mesenchymal transition and stemness in human pancreatic
cancer cells by targeting the STAT3 pathway. J Cancer.
11:4771–4782. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Zhang F, Sang Y, Chen D, Wu X, Wang X,
Yang W and Chen Y: M2 macrophage-derived exosomal long non-coding
RNA AGAP2-AS1 enhances radiotherapy immunity in lung cancer by
reducing microRNA-296 and elevating NOTCH2. Cell Death Dis.
12:4672021. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Mi X, Xu R, Hong S, Xu T, Zhang W and Liu
M: M2 macrophage-derived exosomal lncRNA AFAP1-AS1 and MicroRNA-26a
affect cell migration and metastasis in esophageal cancer. Mol Ther
Nucleic Acids. 22:779–790. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Huang W, Zhong Z, Luo C, Xiao Y, Li L,
Zhang X, Yang L, Xiao K, Ning Y, Chen L, et al: The
miR-26a/AP-2α/Nanog signaling axis mediates stem cell self-renewal
and temozolomide resistance in glioma. Theranostics. 9:5497–5516.
2019. View Article : Google Scholar :
|
|
115
|
Xin L, Zhou LQ, Liu C, Zeng F, Yuan YW,
Zhou Q, Li SH, Wu Y, Wang JL, Wu DZ and Lu H: Transfer of LncRNA
CRNDE in TAM-derived exosomes is linked with cisplatin resistance
in gastric cancer. EMBO Rep. 22:e521242021. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Zheng J, Li XD, Wang P, Liu XB, Xue YX, Hu
Y, Li Z, Li ZQ, Wang ZH and Liu YH: CRNDE affects the malignant
biological characteristics of human glioma stem cells by negatively
regulating miR-186. Oncotarget. 6:25339–25355. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Feng Z, Meng S, Zhou H, Xu Z, Tang Y, Li
P, Liu C, Huang Y and Wu M: Functions and potential applications of
circular RNAs in cancer stem cells. Front Oncol. 9:5002019.
View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Yu T, Wang Y, Fan Y, Fang N, Wang T, Xu T
and Shu Y: CircRNAs in cancer metabolism: A review. J Hematol
Oncol. 12:902019. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Zhou P, Chen X, Shi K, Qu H and Xia J: The
characteristics, tumorigenicities and therapeutics of cancer stem
cells based on circRNAs. Pathol Res Pract. 233:1538222022.
View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Zhuang Z, Jia L, Li W and Zheng Y: The
emerging roles of circular RNAs in regulating the fate of stem
cells. Mol Cell Biochem. 476:231–246. 2021. View Article : Google Scholar
|
|
121
|
Yu D, Chang Z, Liu X, Chen P, Zhang H and
Qin Y: Macrophage-derived exosomes regulate gastric cancer cell
oxaliplatin resistance by wrapping circ 0008253. Cell Cycle.
22:705–717. 2023. View Article : Google Scholar
|
|
122
|
Chen S, Chen Z, Li Z, Li S, Wen Z, Cao L,
Chen Y, Xue P, Li H and Zhang D: Tumor-associated macrophages
promote cholangiocarcinoma progression via exosomal Circ_0020256.
Cell Death Dis. 13:942022. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Gu X, Shi Y, Dong M, Jiang L, Yang J and
Liu Z: Exosomal transfer of tumor-associated macrophage-derived
hsa_ circ_0001610 reduces radiosensitivity in endometrial cancer.
Cell Death Dis. 12:8182021. View Article : Google Scholar
|
|
124
|
Ma J, Huang L, Gao YB, Li MX, Chen LL and
Yang L: M2 macrophage facilitated angiogenesis in cutaneous
squamous cell carcinoma via circ_TNFRSF21/miR-3619-5p/ROCK axis.
Kaohsiung J Med Sci. 38:761–771. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Panis C, Pizzatti L, Souza GF and Abdelhay
E: Clinical proteomics in cancer: Where we are. Cancer Let.
382:231–239. 2016. View Article : Google Scholar
|
|
126
|
Zhu Y, Chen X, Pan Q, Wang Y, Su S, Jiang
C, Li Y, Xu N, Wu L, Lou X and Liu S: A comprehensive proteomics
analysis reveals a secretory path- and status-dependent signature
of exosomes released from tumor-associated macrophages. J Proteome
Res. 14:4319–4331. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
El-Arabey AA, Denizli M, Kanlikilicer P,
Bayraktar R, Ivan C, Rashed M, Kabil N, Ozpolat B, Calin GA, Salama
SA, et al: GATA3 as a master regulator for interactions of
tumor-associated macrophages with high-grade serous ovarian
carcinoma. Cell Signal. 68:1095392020. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Zheng P, Luo Q, Wang W, Li J, Wang T, Wang
P, Chen L, Zhang P, Chen H, Liu Y, et al: Tumor-associated
macrophages-derived exosomes promote the migration of gastric
cancer cells by transfer of functional apolipoprotein E. Cell Death
Dis. 9:4342018. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Lee HD, Koo BH, Kim YH, Jeon OH and Kim
DS: Exosome release of ADAM15 and the functional implications of
human macrophage-derived ADAM15 exosomes. FASEB J. 26:3084–3095.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Yu X, Zhang Q, Zhang X, Han Q, Li H, Mao
Y, Wang X, Guo H, Irwin DM, Niu G and Tan H: Exosomes from
macrophages exposed to apoptotic breast cancer cells promote breast
cancer proliferation and metastasis. J Cancer. 10:2892–2906. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Wang M, Zhou L, Yu F, Zhang Y, Li P and
Wang K: The functional roles of exosomal long non-coding RNAs in
cancer. Cell Mol Life Sci. 76:2059–2076. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Sharma A and Johnson A: Exosome DNA:
Critical regulator of tumor immunity and a diagnostic biomarker. J
Cell Physiol. 235:1921–1932. 2020. View Article : Google Scholar
|
|
133
|
Azambuja JH, Ludwig N, Yerneni SS,
Braganhol E and Whiteside TL: Arginase-1+ exosomes from
reprogrammed macrophages promote glioblastoma progression. Int J
Mol Sci. 21:39902020. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Chen Y, Jin H, Song Y, Huang T, Cao J,
Tang Q and Zou Z: Targeting tumor-associated macrophages: A
potential treatment for solid tumors. J Cell Physiol.
236:3445–3465. 2021. View Article : Google Scholar
|
|
135
|
Pienta KJ, Machiels JP, Schrijvers D,
Alekseev B, Shkolnik M, Crabb SJ, Li S, Seetharam S, Puchalski TA,
Takimoto C, et al: Phase 2 study of carlumab (CNTO 888), a human
monoclonal antibody against CC-chemokine ligand 2 (CCL2), in
metastatic castration-resistant prostate cancer. Invest New Drugs.
31:760–768. 2013. View Article : Google Scholar
|
|
136
|
Moisan F, Francisco EB, Brozovic A, Duran
GE, Wang YC, Chaturvedi S, Seetharam S, Snyder LA, Doshi P and
Sikic BI: Enhancement of paclitaxel and carboplatin therapies by
CCL2 blockade in ovarian cancers. Mol Oncol. 8:1231–1239. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Xu F, Wei Y, Tang Z, Liu B and Dong J:
Tumor-associated macrophages in lung cancer: Friend or foe?
(Review). Mol Med Rep. 22:4107–4115. 2020.PubMed/NCBI
|
|
138
|
DiPersio JF, Uy GL, Yasothan U and
Kirkpatrick P: Plerixafor. Nat Rev Drug Discov. 8:105–106. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Wang J, Tannous BA, Poznansky MC and Chen
H: CXCR4 antagonist AMD3100 (plerixafor): From an impurity to a
therapeutic agent. Pharmacol Res. 159:1050102020. View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Hume DA and MacDonald KP: Therapeutic
applications of macrophage colony-stimulating factor-1 (CSF-1) and
antagonists of CSF-1 receptor (CSF-1R) signaling. Blood.
119:1810–1820. 2012. View Article : Google Scholar
|
|
141
|
Bonapace L, Coissieux MM, Wyckoff J, Mertz
KD, Varga Z, Junt T and Bentires-Alj M: Cessation of CCL2
inhibition accelerates breast cancer metastasis by promoting
angiogenesis. Nature. 515:130–133. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
142
|
Advani R, Flinn I, Popplewell L, Forero A,
Bartlett NL, Ghosh N, Kline J, Roschewski M, LaCasce A, Collins GP,
et al: CD47 blockade by Hu5F9-G4 and rituximab in non-Hodgkin's
lymphoma. N Engl J Med. 379:1711–1721. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Petrova PS, Viller NN, Wong M, Pang X, Lin
GH, Dodge K, Chai V, Chen H, Lee V, House V, et al: TTI-621
(SIRPαFc): A CD47-blocking innate immune checkpoint inhibitor with
broad antitumor activity and minimal erythrocyte binding. Clin
Cancer Res. 23:1068–1079. 2017. View Article : Google Scholar
|
|
144
|
Bouwstra R, van Meerten T and Bremer E:
CD47-SIRPα blocking-based immunotherapy: Current and prospective
therapeutic strategies. Clin Transl Med. 12:e9432022. View Article : Google Scholar
|
|
145
|
Zheng JH, Nguyen VH, Jiang SN, Park SH,
Tan W, Hong SH, Shin MG, Chung IJ, Hong Y, Bom HS, et al: Two-step
enhanced cancer immunotherapy with engineered Salmonella
typhimurium secreting heterologous flagellin. Sci Transl Med.
9:eaak95372017. View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Yuan D, Zhao Y, Banks WA, Bullock KM,
Haney M, Batrakova E and Kabanov AV: Macrophage exosomes as natural
nanocarriers for protein delivery to inflamed brain. Biomaterials.
142:1–12. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
147
|
Li J, Li N and Wang J: M1
macrophage-derived exosome-encapsulated cisplatin can enhance its
anti-lung cancer effect. Minerva Med. Apr 8–2020.Epub ahead of
print. View Article : Google Scholar
|
|
148
|
Kim MS, Haney MJ, Zhao Y, Mahajan V,
Deygen I, Klyachko NL, Inskoe E, Piroyan A, Sokolsky M, Okolie O,
et al: Development of exosome-encapsulated paclitaxel to overcome
MDR in cancer cells. Nanomedicine. 12:655–664. 2016. View Article : Google Scholar
|
|
149
|
Bellmunt À M, López-Puerto L, Lorente J
and Closa D: Involvement of extracellular vesicles in the
macrophage-tumor cell communication in head and neck squamous cell
carcinoma. PLoS One. 14:e02247102019. View Article : Google Scholar : PubMed/NCBI
|
|
150
|
Trajkovic K, Hsu C, Chiantia S, Rajendran
L, Wenzel D, Wieland F, Schwille P, Brügger B and Simons M:
Ceramide triggers budding of exosome vesicles into multivesicular
endosomes. Science. 319:1244–1247. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
151
|
Wu ATH, Srivastava P, Yadav VK, Tzeng DTW,
Iamsaard S, Su EC, Hsiao M and Liu MC: Ovatodiolide, isolated from
Anisomeles indica, suppresses bladder carcinogenesis through
suppression of mTOR/β-catenin/CDK6 and exosomal miR-21 derived from
M2 tumor-associated macrophages. Toxicol Appl Pharmacol.
401:1151092020. View Article : Google Scholar
|
|
152
|
Guo J, Wang X, Guo Q, Zhu S, Li P, Zhang S
and Min L: M2 macrophage derived extracellular vesicle-mediated
transfer of MiR-186-5p promotes colon cancer progression by
targeting DLC1. Int J Biol Sci. 18:1663–1676. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
153
|
Wang P, Li GY, Zhou L, Jiang HL, Yang Y
and Wu HT: Exosomes from M2 macrophages promoted glycolysis in FaDu
cells by inhibiting PDLIM2 expression to stabilize PFKL. Neoplasma.
69:1041–1053. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
154
|
Song L, Luan B, Xu Q, Shi R and Wang X:
microRNA-155-3p delivered by M2 macrophages-derived exosomes
enhances the progression of medulloblastoma through regulation of
WDR82. J Transl Med. 20:132022. View Article : Google Scholar : PubMed/NCBI
|
|
155
|
Zhang Z, Hu J, Ishihara M, Sharrow AC,
Flora K, He Y and Wu L: The miRNA-21-5p payload in exosomes from M2
macrophages drives tumor cell aggression via PTEN/Akt signaling in
renal cell carcinoma. Int J Mol Sci. 23:30052022. View Article : Google Scholar : PubMed/NCBI
|