1. Introduction
Breast cancer, a malignancy arising from the
epithelial cells of the breast, has witnessed a steady increase in
incidence over the years (1). As
per the 2020 Global Cancer Statistics Report published in CA: A
Cancer Journal for Clinicians, breast cancer surpassed lung cancer
to become the world's most prevalent type of cancer in 2020
(2). In that year, an estimated
2.3 million new breast cancer cases were diagnosed, representing
11.7% of all global cancer cases, and 685,000 individuals succumbed
to the disease, accounting for 6.9% of global cancer-related deaths
(2). Among the types of cancer
affecting females, breast cancer stands out with a quarter of the
incidence rate and a sixth of the mortality rate, ranking first in
terms of incidence in 159 countries and mortality in 110 countries
(2). The mainstay of treatment
for breast cancer is surgical excision, radiotherapy and adjuvant
targeted therapies; however, clinical studies have found that the
long-term use of this treatment does not improve patient survival
(3). In light of these findings,
regulated cell death (RCD) has emerged as a promising avenue for
both breast cancer prevention and treatment strategies.
In 2018, the Cell Death Committee refined the
definition of RCD by emphasizing its process mechanisms and
updating the classification system (4). RCD encompasses a diverse array of
developmental and immunological pathways that culminate in distinct
modes of cell demise, resulting in varied morphological
transformations and immunological consequences (5). This type of cell death can occur
intrinsically, without the interference of external factors,
serving as an inherent component of physiological programs, such as
development or tissue renewal (6,7).
This entirely physiological form of RCD is often referred to as
programmed cell death. However, RCD can also arise from disruptions
in the intracellular or extracellular microenvironment when these
disturbances are too severe or prolonged, exceeding the capacity of
adaptive responses to maintain cellular homeostasis (8). Oxidative stress, characterized by
the generation of reactive oxygen species (ROS), has been
implicated as a potential trigger for various forms of RCD. The
production of ROS and the effectiveness of antioxidant defenses are
reportedly influenced by the surrounding environment (5). In recent years, research on RCD in
cancer has increasingly focused on modes, such as cuproptosis,
ferroptosis, pyroptosis, immunogenic cell death and autosis.
Therefore, the present review primarily discusses and summarizes
the regulatory mechanisms, relevant genes and potential drugs
associated with cuproptosis, ferroptosis, pyroptosis and other such
modes of RCD in the context of breast cancer.
2. Cuproptosis and breast cancer
Copper (symbol, Cu) serves as an essential cofactor
for all living organisms, with intracellular levels meticulously
maintained within a narrow range. It plays a pivotal role in
various physiological processes, including mitochondrial
respiration, antioxidant activity and macromolecular biosynthesis.
However, exceeding the threshold of homeostatic mechanisms can
induce detrimental effects, regardless of whether copper levels are
deficient or excessive (9-11).
Cuproptosis, a recently discovered cell death pathway, arises
specifically from copper overload and operates independently of
other known death mechanisms. Copper ions directly bind to
lipoproteins within the tricarboxylic acid cycle (TCA) metabolic
pathway, causing anomalous aggregation and interfering with the
iron-sulfur cluster scaffold protein in the respiratory complex.
This disruption culminates in a proteotoxic stress response,
ultimately leading to cell death (12-15).
Cuproptosis is distinguished from other
types of RCD
Cuproptosis, a unique form of RCD, is specifically
induced by copper ion carriers. Notably, inhibitors targeting known
cell death pathways, including ferroptosis, necrosis, apoptosis and
oxidative stress, exhibit a limited ability to prevent copper ion
carrier-induced cell death. This distinct mechanism significantly
differentiates cuproptosis from traditional modes of cell death,
such as apoptosis and necroptosis. Mitochondrial respiration plays
a crucial role in the regulation of cuproptosis. Treatment with
mitochondrial antioxidants, fatty acids and inhibitors of
mitochondrial function significantly influences copper ion carrier
sensitivity, suggesting dependence on mitochondrial activity rather
than adenosine triphosphate (ATP) production (16). Key genes contributing to
copper-induced death include FDX1 and six genes involved in
protein S-acylation, all essential for mitochondrial aerobic
metabolism. The knockdown of FDX1 (encoding a protein
converting divalent copper ions to toxic monovalent forms) and six
lipoylated protein genes (LIPT1, DLD, LIAS, DLAT, PDHA1 and
PDHB) has been shown to successfully rescue cells from
copper ion-mediated death (16).
FDX1 acts as an upstream regulator of protein lipoylation, a
conserved lysine post-translational modification limited to four
enzymes within metabolic complexes regulating carbon entry into the
TCA cycle. It has been established that copper directly binds and
induces the oligomerization of lipoylated DLAT. Notably,
FDX1 deletion eliminates protein lipoylation, preventing
copper binding by DLAT and DLST, indicating the critical role of
the lipoyl moiety in copper binding. This unique death mechanism
observed in cuproptosis aligns with observations in a genetic model
of copper homeostasis dysregulation. Studies using a mouse model of
Wilson's disease further suggest that copper overload induces
cellular effects identical to those triggered by copper ion
carriers, confirming the shared mechanism between copper
homeostasis dysregulation and copper ion carrier-induced cell death
(17).
Mechanisms involved in cuproptosis
While cuproptosis holds promise, several aspects
remain enigmatic. The specific roles of key factors, such as
FDX1 require further investigation. Additionally, the
mechanisms underlying cuproptosis inhibition in healthy cells are
unclear. Furthermore, previous studies suggest that hte various
possible cuproptosis-related mechanisms require better integration.
Finally, characteristic morphological and molecular changes in
cuproptosis-affected cells have not been fully described (18-20).
Copper, crucial for enzymes, necessitates meticulous
control at low levels for normal physiological function. Studies
highlight its role in cancer progression (21-25). Notably, patients with breast
cancer exhibit significantly higher serum levels of copper compared
to the controls, suggesting its potential in early detection and
monitoring. In triple-negative breast cancer (TNBC), inhibiting
mitochondrial copper forces tumor cells to switch from respiration
to glycolysis, reducing energy production and ultimately hindering
tumor growth and improving prognosis (26-30). The novel concept of cuproptosis
sheds light on the copper-cancer link. Elesclomol, a copper ion
carrier, binds to environmental copper and delivers it into cells,
triggering cell death. This approach may be most effective in
cancers highly expressing mitochondrial lipoylated proteins and
relying heavily on respiration. Moreover, it could be particularly
useful in apoptosis-resistant cancers, providing a novel strategy
with which to eliminate cancer cells by leveraging the unique
properties of copper (31,32).
Building upon the anticancer properties of copper, chelators and
carriers are currently undergoing preclinical and clinical
evaluations in various tumor types. Copper chelation therapies,
such as tetrathiomolybdate and ATN-224 have reached phase II
clinical trials in breast cancer. Disulfiram, a copper ion carrier,
is under phase II investigation for malignant glioma, while
elesclomol holds promise for the treatment of melanoma in phase II
trials (33-36).
Relevant targets for cuproptosis in
breast cancer
Intracellular copper overload has recently been
linked to a novel form of cell death known as cuproptosis.
Independent of traditional pathways, cuproptosis does not activate
caspase-3 and remains unaffected by apoptosis inhibitors (15). Genes associated with this process
include FDX1, LIPT1, LIAS, DLD, DBT, GCSH, DLST, DLAT, PDHA1,
PDHB, SLC31A1, ATP7A and ATP7B. These genes primarily
regulate processes, such as glycolysis, the TCA cycle, and steroid
and vitamin D metabolism. Notably, SLC31A1 facilitates copper
uptake, while ATP7A and ATP7B are responsible for copper efflux,
maintaining intracellular copper levels (37-41). The overexpression of SLC31A1 and
the deletion of ATP7B can increase susceptibility to cuproptosis,
whereas the knockdown of nine specific genes (FDX1, LIAS, LIPT1,
DLD, DLAT, PDHA1, PDHB, GCSH and DBT) confers resistance
(15). While the role of copper
in breast cancer, particularly its impact on the immune
microenvironment and immunotherapy, has been well-established
(42), the association between
seven key cuproptosis-associated genes and breast cancer remains
unexplored. These genes include PGK1 (mitochondrial
metabolism and tumorigenesis), SLC family members SLC52A2
and SLC16A6 (metabolic transport), SEC14L2 (vitamin E
uptake), RAD23B (nucleotide excision repair and apoptosis),
CCL5 (inflammatory cell migration) and MAL2
(transcytosis in hepatocellular carcinoma) (43-58).
Song et al (59) analyzed the protein expression of
the cuproptosis-related genes, FDX1, LIPT1, MTF1, DLD,
DLAT, PDHA1 and PDHB, in breast cancer tissues and their
mRNA expression in cuproposis-inducible breast cancer cell models.
Notably, RAD23B expression was found to be positively associated
with breast cancer progression, drug resistance and a poor
prognosis of patients with breast cancer. Notably, both PD1 and
PDL1 expression exhibited a positive correlation with RAD23B
expression, suggesting that patients with higher RAD23B levels may
be more responsive to immune checkpoint blockade therapy targeting
the programmed cell death 1 (PD-1)/PD-L1 axis (59).
Recent advances in cuproptosis-related
drugs
In addition, the crucial role of cuproptosis in
tumor cells presents an opportunity to develop novel anticancer
drugs. One such example is the platelet vesicle (PV)-coated cuprous
oxide nanoparticle (Cu2O)/TBP-2 cuproptosis
sensitization system (PTC). Modified by AIE photosensitizer
(TBP-2), Cu2O and PV mimicry, PTC can enhance its
long-term blood circulation and tumor targeting ability.
Subsequently, PTC is rapidly degraded to release copper ions under
acidic conditions and hydrogen peroxide in tumor cells. Under light
irradiation, TBP-2 rapidly enters the cell membrane and generates
hydroxyl radicals to consume glutathione and inhibit copper efflux.
Accumulated copper can cause lipoylated protein aggregation and
iron-sulfur protein loss, which result in proteotoxic stress and
ultimately, in cuproptosis. PTC inhibits tumor cell proliferation
and invasion through cuproptosis. Notably, PTC research, primarily
in patients with lung metastases from breast cancer, have shown the
significant inhibition of metastatic tumor cell growth and
multiplication in the lungs (60). Furthermore, the hyaluronic
acid-dopamine (HD)/berberine hydrochloride (BER)/glucose oxidase
(GOx)/Cu hydrogel reactor system provides a promising avenue for
multiple breast cancer treatments. This system effectively inhibits
tumor growth through a combination of approaches: GOx and copper
sulfate convert accumulated glucose into hydroxyl radicals within
tumor cells, enacting starvation/chemokinetic therapy.
Additionally, Cu induces cuproptosis, further hindering tumor cell
growth. BER, included as a chemotherapeutic agent, synergizes with
the starvation/chemokinetic/cuproptosis modalities. This 'hydrogel
multiplicity effect' allows the system to potentially reduce the
size of breast cancer pre-operatively, facilitating surgical
resection (61). While these
novel drugs harness cuproptosis for therapeutic purposes, their
clinical efficacy remains to be determined as they are currently
limited to biological experiments. However, their potential offers
hope for future advancements in breast cancer treatment.
3. Ferroptosis and breast cancer
Ferroptosis proposed by Dixon et al (62) in 2012, is a unique form of cell
death distinct from apoptosis and necrosis, triggered by a compound
known as RSL3 (62). It is
characterized by the iron-dependent accumulation of lipid
peroxidation to lethal levels, affecting cellular structures and
metabolism. This includes mitochondrial atrophy, increased membrane
density, disrupted membrane integrity and the depletion of
intracellular NADH (63). Three
key mechanisms drive ferroptosis: i) Transferrin and L-glutaminase
regulation in cancer cells (64);
ii) the depletion of glutathione, leading to glutathione peroxidase
4 (GPX4) inactivation (a core antioxidant enzyme) and the
subsequent disruption of the antioxidant system in cancer cells
(65); and iii) the peroxidation
of unsaturated fatty acids in cell membranes by divalent iron or
esterases in cancer cells (66).
Ferroptosis targets in breast cancer
The mammary gland specifically regulates
ferroptosis, a type of RCD. Adipocytes, fat cells in the mammary
gland, significantly influence breast cancer cell growth, and
promote migration and invasion. In breast cancers expressing the
ACSL3 gene, adipocytes protect cancer cells from ferroptosis
by providing oleic acid, creating a unique tumor microenvironment
(67-69). Hypercholesterolemia, high blood
cholesterol, promotes breast cancer development and metastasis by
resisting ferroptosis directly or through its metabolite,
27-hydroxycholesterol (70,71). When treated with statins, cancer
cells may increase their uptake of exogenous cholesterol or boost
their cholesterol production, highlighting the need for real-time
tumor monitoring in patients with breast cancer taking statins
(72). Ferroptosis holds
significant relevance to breast cancer, offering potential for
clinical screening and prognosis. Sha et al (73) identified the expression of ACSL4,
a positive regulator of ferroptosis, as an independent predictor of
the pathological complete response to neoadjuvant chemotherapy,
with a higher expression suggesting a greater sensitivity. Studies
have also shown that GPX4, an antioxidant enzyme, regulates
mitochondria-mediated apoptosis in cancer cells through the
modulation of EGR1, functioning as a tumor suppressor in
well-differentiated breast cancers and potentially serving as a
therapeutic target (74). Zhang
et al (75) identified
long non-coding RNAs (lncRNAs) closely related to ferroptosis
through Cox regression analysis, which can accurately predict the
prognosis of patients with breast cancer. These lncRNAs, as
characteristic molecules of pyroptosis (another form of RCD), may
play a role in antitumor immune processes and hold potential as
therapeutic targets (76).
Current evidence suggests that MTHFD2, a mitochondrial enzyme
involved in folate metabolism, is highly expressed in embryos and
various tumors. As a potential regulator of ferroptosis in breast
cancer, it may serve as a crucial molecular biomarker and a novel
therapeutic target for predicting the prognosis of patients with
TNBC (77,78). Furthermore, Yadav et al
(79) found that breast cancer
cells can evade cell death by overexpressing SLC7A11, which resists
ferroptosis by influencing the tumor microenvironment. Notably,
miR-5096 can target and downregulate SLC7A11, inducing ferroptosis
in breast cancer cells and inhibiting tumor growth (79-81). Zhang et al (82) constructed a nomogram based on nine
ferroptosis-related genes. These ferroptosis-related genes were
significantly associated with the level of immune cell infiltration
in patients with breast cancer, suggesting their potential use as
therapeutic targets or biomarkers (82-90). Additionally, studies have shown
that the deletion of CircRHOT1 inhibits breast cancer cell
proliferation and induces apoptosis, while the knockdown of CLCA2,
REEP6, SPDEF and CRAT can predict breast cancer prognosis based on
metabolic gene classification (91,92). Research on ferroptosis-related
regulatory mechanisms and genes is ongoing, holding promise for
improved breast cancer screening and treatment. It can be expected
that these research findings may translate into clinical
applications in the near future.
Related drugs that can induce
ferroptosis
Currently, promising drug studies are investigating
the potential of targeting ferroptosis, a form of cell death, in
the treatment of breast cancer. Several novel drugs have been
proposed that act on this pathway to enhance existing therapies.
Zhang et al (93)
constructed heparanase (HPSE)-driven sequential release
nanoparticles, which consisted of β-cyclodextrin-grafted heparin
[NLC/H(D + F + S) NPs] co-modified with doxorubicin (DOX),
di-ferric iron (Fe2+) and a TGF-β receptor inhibitor
(SB431542); co-loading modification effectively enhanced
intracellular ROS levels and activated the ferroptosis pathway. The
increased production of ROS also triggered apoptosis, reduced an
enzyme linked to tumor invasion (MMP-9) and synergized with
ferroptosis for the treatment of breast cancer (93). Similarly, polydopamine
nanoparticles loaded with iron and DOX exhibit a wide range of
anticancer effects (94).
Cinnamaldehyde dimers formulated into lipid-like materials deplete
glutathione, a key antioxidant, and when combined with the
anticancer drug, sorafenib, significantly enhance ferroptosis and
trigger a potent immune response in mice, leading to complete tumor
eradication (95,96). Erastin@FA-exo, a folic
acid-labeled exosome carrying the ferroptosis inducer, erastin,
inhibits the expression of GPX4, depleting intracellular
glutathione and upregulating cysteine dioxygenase, leading to
excessive ROS production, both hallmarks of ferroptosis. This
approach effectively reduces the survival of TNBC cells in
vivo and exhibits high biocompatibility compared to
conventional erastin, potentially reducing side-effects and paving
the way for improved clinical applications (97). These novel drugs offer exciting
new possibilities for the clinical treatment of breast cancer by
harnessing the power of ferroptosis.
Several common clinical drugs have significant
inhibitory effects on breast cancer cell growth. For example,
metformin reduces the protein stability of SLC7A11 by inhibiting
its UFMyation process, and SLC7A11 opposes ferroptosis by affecting
the tumor microenvironment to resist the ferroptosis of tumor
cells, thereby inhibiting the growth of breast cancer cells
(81,82,98). Siramesine and lapatinib initially
induce ferroptosis during the death process in breast cancer cells,
but this transforms into autophagy after 24 h (99). In this process, ROS production
plays a key role, and cystine transport inhibition, ferroportin-1
and transferrin are involved in the induction of ferroptosis
(99,100). Everolimus, a targeted
therapeutic agent for breast cancer, can also undergo ferroptosis
by inducing the activation of the FKBP1A/SLC3A2 axis. The specific
mechanism is that its related protein, FK506-binding protein 1A
(FKBP1A), binds to SLC3A2 and negatively regulates SLC3A2
expression during the everolimus-induced ferroptosis of breast
cancer cells and the promotion of antiproliferative Th9 lymphocytes
(101). This finding suggests
that everolimus may be more effective in breast cancer patients who
are more sensitive to it, potentially increasing the efficacy of
chemotherapy and reducing the dose of chemotherapeutic agents
needed (101). Ketamine inhibits
breast cancer cell proliferation by targeting the KAT5/GPX4 axis to
induce ferroptosis (73).
Additionally, targeting GPX4 cann enhance the anticancer effects of
gefitinib, suggesting that the study of the two drugs together may
provide a new direction for clinical treatment (102,103). Simvastatin has been reported to
inhibit HMGCR expression and downregulate the mevalonic acid
pathway and GPX4, thereby inducing ferroptosis in TNBC cells
(104). Holo lactoferrin induces
ferroptosis in cancer cells and sensitizes TNBC cells to
radiotherapy (105). Holo
lidocaine promotes ferroptosis in ovarian and breast cancer cells
via the miR-382-5p/SLC7A11 axis (106).
Some common plant extracts have also been found to
exert a promoting effect on ferroptosis. For example, curcumin has
been shown to significantly downregulate GPX4 and upregulate HO-1,
and both HO-1 and GPX4 enhance ferroptosis in breast cancer
(71,72,107). Red ginseng polysaccharides, an
effective extracted component of ginseng, also promote ferroptosis,
inhibit GPX4 expression and exert antitumor effects (108). These findings suggest that
ferroptosis may be a novel therapeutic target for breast cancer.
DMOCPTL, a derivative of the natural product, chamomile lactone,
can directly bind to GPX4 protein to induce GPX4 ubiquitination and
induce ferroptosis. This substance effectively inhibits the growth
of breast tumors without significant cytotoxicity, rendering it a
potential treatment option for patients with TNBC (74). On the whole, the latest research
findings on ferroptosis provide new hope for the development of
more effective treatments for breast cancer. However, further
research is required in order to develop a complete treatment plan
that utilizes ferroptosis.
4. Pyroptosis and breast cancer
Pyroptosis, a novel type of RCD distinct from
apoptosis, was first proposed by Cookson and Brennan (109) in 2001, as a rapid death
mechanism observed in Salmonella-infected macrophages
dependent on caspase-1 activation. Gasdermin (GSDM) proteins, the
key molecules in pyroptosis, induce cell membrane lysis (110). There are two main categories of
pyroptosis mechanisms: Classical and non-classical pathways. The
classical pyroptosis pathway is triggered by exogenous or
endogenous microbial infections that stimulate the production of
inflammasomes. These inflammasomes activate caspase-1 proteins,
which in turn disrupt the integrity of the cell membrane.
Additionally, this pathway promotes the activation and release of
the inflammatory cytokines, interleukin (IL)-1β and IL-18. The
combined action of caspase-1 proteins and inflammation leads to
pyroptosis (111-113). The non-classical pyroptosis
pathway, on the other hand, is mediated by
lipopolysaccharide-activated caspase-4/5/11 (114).
Mechanisms and genes involved in
pyroptosis in breast cancer
Pyroptosis and its association with tumors have
become a prominent research area in recent years, and the present
review specifically summarizes the association between pyroptosis
and breast cancer. Breast cancer exhibits a unique regulatory
mechanism associated with pyroptosis. Mitochondrial uncoupling
protein 1, linked to an production of body heat, has a high
expression in breast cancer cells. This leads to mitochondrial
swelling and autophagy, activating GSDME, stimulating antitumor
immunity, and ultimately resulting in pyroptosis. This process
inhibits breast cancer cell proliferation and holds potential as a
prognostic marker (115-117). PD-L1, exhibiting nuclear
transcriptional activity, participates in the pyroptosis pathway
and modulates the tumor microenvironment. In breast cancer cells,
this manifests as TNF-α activating caspase-8, which, in the
presence of GSDMC and hypoxia-activated nPD-L1, converts apoptosis
into pyroptosis, leading to tumor necrosis in hypoxic areas
(118). As identified in the
literature, the dysregulation of numerous pyroptosis genes is
associated with breast cancer prognosis. The high expression of
CASP6, CASP5, TIRAP, SCAF11, NLRP7, PLCG1, GSDMC, GSDMD and
NLRC4 is associated with a poor prognosis, while the high
expression of ELANE, CASP9, CASP8, GSDMB, CASP4, CASP1, TNF,
NOD1, PYCARD, NLRP6, NLRP3, NLRP2, IL6, NLRP1, IL18 and
IL1B is associated with improved outcomes (27,119-130). Furthermore, several genes
emerged as potential therapeutic targets for breast cancer.
DRD2, for instance, inhibits NF-κB signaling activation by
binding to β-arrestin2, downregulating DDX5 and eEF1A2. This
combined action suppresses the NF-κB signaling pathway and p65
phosphorylation. Additionally, DRD2 modulates the tumor
microenvironment and promotes macrophage M1 polarization,
ultimately triggering pyroptosis in breast cancer cells (131). In a previous study,
SCAF11 expression was found to be elevated in breast tumor
cell lines, and its high levels were shown to be associated with a
poor prognosis (120). Silencing
SCAF11 using siRNA significantly reduced the proliferation and
colony growth of BT549 and T47D breast cancer cell lines. GSEA
analysis revealed that SCAF11 co-expressed genes were primarily
involved inflammatory and immune-related pathways (131). Moreover, SCAF11
expression exhibited a positive correlation with immune
checkpoints, such as PD-L1, B7H3 and PDCD1LG2. Based on these
findings, SCAF11, as a pyroptosis regulatory gene, warrants
exploration as a potential therapeutic target for breast cancer
patients (131).
Anti-breast cancer drugs that can
leverage pyroptosis
Research on pyroptosis in breast cancer has led to
investigations into the potential of existing drugs to induce this
cell death process. DOX exhibits a three-pronged approach: It
dose-dependently reduces the viability of MDA-MB-231 and T47D
cells, activates caspase-3 through GSDME, induces the accumulation
of intracellular ROS, and subsequently stimulates the
phosphorylation of JNK and the activation of caspase-3, and
culminates in pyroptosis, exerting its anticancer effects (132). Tetraarsenic arsenic hexaoxide
exerts its anticancer effects by targeting a crucial factor in
breast cancer cells, mitochondrial STAT3. Inhibiting its activation
leads to mitochondrial ROS-mediated pyroptosis (133,134). Nigericin, derived from
Streptomyces hydrophobicus, triggers pyroptosis in TNBC
cells by inducing potassium efflux and subsequent mitochondrial ROS
production. This process activates the caspase-1/GSDMD pathway.
Moreover, combining nigericin with anti-PD-1 antibodies exhibits
synergy in treating advanced triple-negative breast cancer
(135). Notably, trimethylamine
N-oxide (TMAO), a metabolite produced by the Clostridium
genus, induces pyroptosis in tumor cells by activating the
endoplasmic reticulum kinase, PERK. This, in turn, enhances CD8
T-cell-mediated antitumor immunity in TNBC models in vivo.
As immunotherapy is a crucial option for these patients, the
ability of TMAO to boost its efficacy suggests its potential
application in the treatment of TNBC (136). Azurocidin-1, a protein
originating from neutrophils and predominantly stored in
azurophilic granules, exerts its effects on pyroptosis in TNBC
cells through the regulation of the pNF-κB/NLRP3/caspase-1/GSDMD
axis. The identification of Azurocidin-1 holds promise in the
development of novel immunotherapeutic approaches for the treatment
of TNBC (137). The treatment of
breast cancer cells with docosahexaenoic acid has been found to
increase the activation of caspase-1 and GSDMD, enhance the
secretion of IL-1β, promote the translocaton of high-mobility group
protein B1 (HMGB1) to the cytoplasm and to lead to the formation of
membrane pores. These findings suggest that docosahexaenoic acid
induces the pyroptosis-programmed death of breast cancer cells and
exerts an anti-breast cancer effect (138). Xihuangwan, a traditional Chinese
medicine, has been found to induce pyroptosis via the cyclic
AMP-activated protein kinase (cAMP)/protein kinase A signaling
pathway, and inhibit the proliferation, migration and invasion of
breast cancer cells (139).
Dihydroartemisinin, a plant extract, promotes the
AIM2/caspase-3/DFNA5 axis in breast cancer cells and induces
pyroptosis, inhibiting breast cancer growth (140). In addition, Ganoderma
lucidum extract (GLE) activates cysteine 3 and further cleaves
GSDME proteins to form membrane pores in cell membranes, thereby
releasing large amounts of inflammatory factors in breast cancer
cells, leading to pyroptosis and inhibiting the growth and
multiplication of breast cancer cells. GLE also disrupts multiple
steps of tumor metastasis, including adhesion, migration, invasion,
colonization and angiogenesis. Overall, GLE offers a potential
approach for the treatment of breast cancer that could complement
chemotherapy or immunotherapy for cancer metastasis (141).
Current research has identified a novel therapeutic
molecule, a bionic nanoparticle of indocyanine green and
decitabine, which synergistically upregulates GSDME expression
through DNA methylation inhibition and enhances caspase-3-mediated
cleavage of GSDME, leading to cancer cell pyroptosis and inhibiting
primary breast cancer and distant metastasis (142). Co-assembled carrier-free
chemo-photodynamic nanoplatforms (A-C/NPs) of cytarabine (Ara-C)
and chlorine e6 (Ce6) can induce tumor cell pyroptosis and enhance
the body's immune response to breast cancer. Their specific
mechanisms are the following: A-C/NPs trigger GSDME-mediated
pyroptosis in a controlled manner via ROS accumulation, and Ara-C
stimulates the maturation of cytotoxic T-lymphocytes, synergizing
with Ce6-mediated immunogenic cell death to jointly enhance the
anticancer effects of A-C/NPs. In a previous study using a mouse
model of breast cancer, A-C/NPs were found to markedly inhibit
in situ, metastatic and recurrent tumor growth (143). Current research on cellular
focalization-related drugs focuses on GSDME cleavage and the
regulation of the caspase-1/caspase-3 pathway. Relevant experiments
have demonstrated the effectiveness of this approach for breast
cancer (74,109,112). However, further studies are
warranted to investigate potential adverse effects on the organism
and to establish clinical application guidelines, including dosing
information. Therefore, advancing the use of pyroptosis-related
drugs in breast cancer should prioritize research on potential
drawbacks associated with GSDME cleavage and caspase-1/caspase-3
pathway regulation.
5. Other forms of cell death and breast
cancer
Current academic research on RCD in breast cancer
extends beyond previously mentioned modes to include immunogenic
cell death, autophagic cell death and others, all of which can both
promote and inhibit the growth and metastasis of breast cancer
cells. As regards immunogenic cell death, researchers have found
that Trametes robiniophila Murr. (Huaier) increases the
release of ATP and HMGB1 by promoting cell surface calreticulin
exposure. Its therapeutic effects are linked to endoplasmic
reticulum stress through the cAMP/PKR/eIF2α axis, both of which
trigger immunogenic cell death in TNBC cells (144). Breast cancer cells produce
angiopoietin-like 7 (Angptl7), a tumor-specific factor localized in
the perineal regions, which contributes to the formation of
necrotic apoptosis and the metastatic dissemination of the tumor
core. Functional studies have shown that Angptl7 deficiency allows
central necrosis and autophagy, ultimately protecting the growth of
breast cancer cells and promoting their metastasis.
Mechanistically, Angptl7 promotes vascular permeability and
supports perineal positioning vascular remodeling (145). Current research suggests that
autophagy can serve as a form of nutritional support for cellular
self-repair. However, it may also contribute to tumor dormancy in
breast cancer, potentially promoting chemoresistance and relapse
(146,147).
6. Co-modulation of conventional
chemotherapeutic agents by multiple types of RCD
Role of DOX in various types of RCD in
breast cancer
In the present review, the summary of RCD in breast
cancer reveals that DOX, a classic antitumor drug, interacts with
various cell death pathways. In ferroptosis, DOX downregulates GPX4
and triggers excessive lipid peroxidation through the
DOX-Fe2+ complex in the mitochondria, ultimately leading
to ferroptosis-mediated cell death. Of note, the combination of
ferrostatin-1 and zVAD-FMK effectively regulates ferroptosis and
prevents DOX-induced cardiomyocyte death, offering potential
therapeutic avenues (148). For
pyroptosis, DOX accumulation leads to a cascade of events,
beginning with ROS generation. ROS then stimulate the
phosphorylation of JNK (specifically p-JNK), which in turn
activates caspase-3, a key pyroptosis executioner. Additionally,
DOX-induced ROS production also affects the cleavage of caspase-8,
further promoting caspase-3 activation. Ultimately, activated
caspase-3 cleaves GSDME, triggering the characteristic membrane
rupture and pyroptotic cell death in breast cancer cells (132). Furthermore, DOX exhibits a
direct interaction with the pyroptosis-associated protein, GSDMD,
mitigating the cardiotoxic effects associated with the drug, a
finding with potential clinical implications (149,150). Based on these underlying
mechanisms, novel technologies have been employed to develop
targeted breast cancer drugs that focus on RCD. Several promising
therapeutic modalities have emerged.
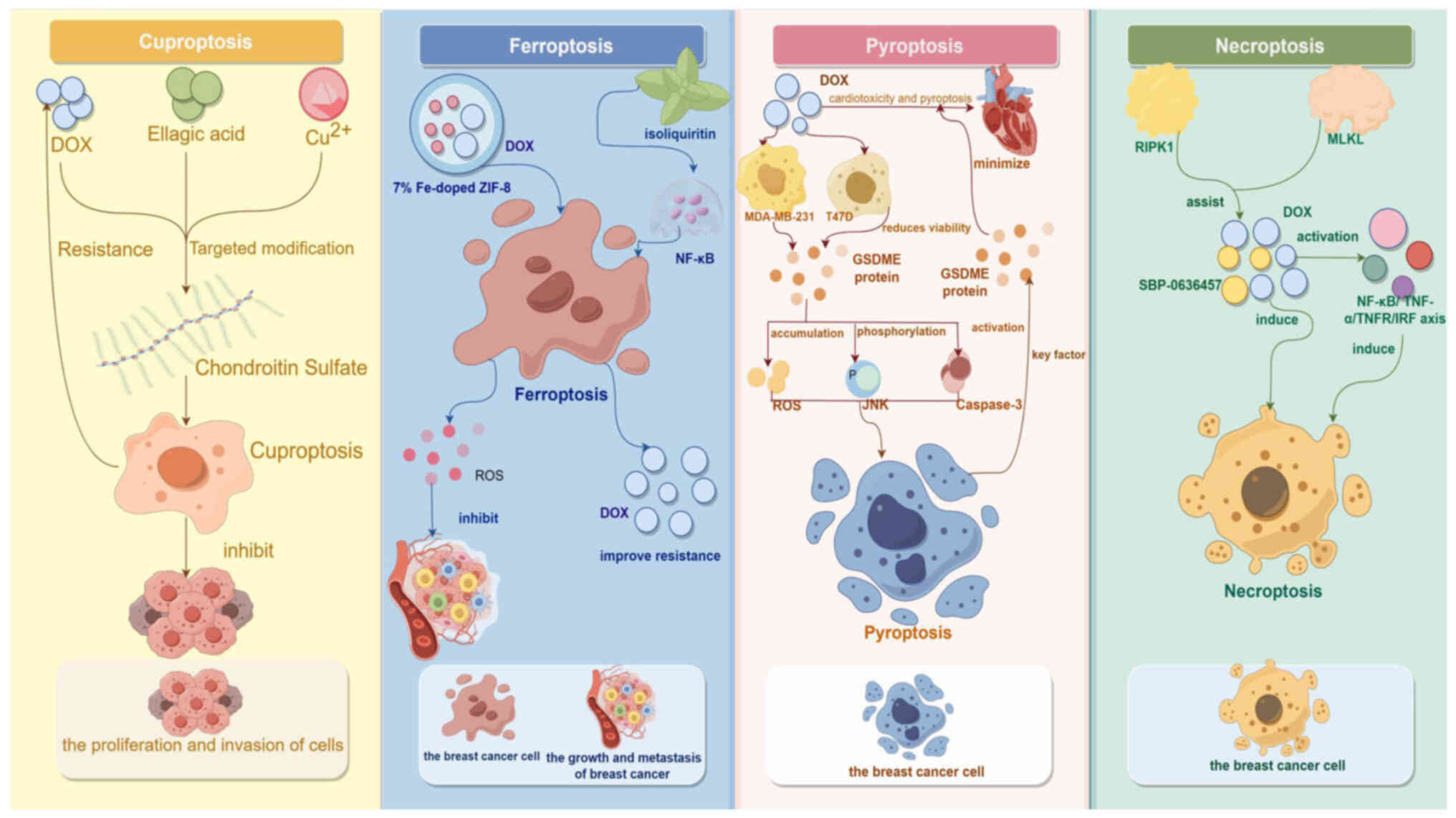
As previously demonstrated, DOX-loaded hedgehog
pathway inhibitor ellagic acid (EA) was combined with
Cu2+ to develop nanoscale metal-organic frameworks
(EA-Cu) modified by targeted chondroitin sulfate (151). This approach was shown to
achieve the inhibition of stemness maintenance by inhibiting the
hedgehog pathway through EA, while Cu2+ disrupted
mitochondrial metabolism. This combination reduces the stemness
characteristics of tumor cells and enhanced the effectiveness of
DOX-mediated chemotherapy. The co-action of EA and Cu induces
cuproptosis, thereby enhancing anticancer effects and preventing
the development of DOX resistance (151). In summary, CS/NPs demonstrate
notable antitumor effects by inducing cuproptosis and significantly
inhibiting cancer cell stemness, suggesting their potential to
overcome resistance to cancer chemotherapy (151).
Emerging therapeutic modalities targeting
ferroptosis utilize this interaction. One such modality utilizes
degraded bimetallic nanoparticles-7% Fe-doped ZIF-8 encapsulated
with DOX, a classical drug used in the treatment of breast cancer.
This approach inhibits the growth and metastasis of breast cancer
cells by utilizing ferroptosis to induce the production of ROS in
cancer cells (152). Several
studies have explored strategies with which to mitigate the
side-effects of DOX, while harnessing its antitumor effects. For
example, isoliquiritin, a natural compound, inhibits the NF-κB
signaling pathway, which regulates ferroptosis in breast cancer and
improves resistance to DOX (153). Additionally, decreasing the
levels of GSDME protein, a key factor in pyroptosis, can minimize
DOX-induced cardiotoxicity and pyroptosis in breast cancer cells
(132). While DOX itself can
induce necroptosis, activating the NF-κB/TNF-α/TNFR/IRF axis
further enhances this process, resulting in the
SBP-0636457/DOX-induced necrosis of breast cancer cells (154) (Fig. 1).
Adjuvant drugs can also be utilized to modulate the
anticancer effects of DOX through the regulation of RCD. It has
been shown that plant extracts containing magnoflorine (Mag)
significantly enhance the effects of DOX on the induction of
autophagy by increasing the expression of light chain 3 (LC3)-II.
Notably, combined treatment with DOX and Mag significantly inhibits
the activation of PI3K/AKT/mTOR signaling pathway (155). Conversely, it promotes the p38
MAPK pathway, leading to the induction of both autophagy and
apoptosis. These findings suggest that Mag may potentiate the
anticancer effects of DOX and enhance the sensitivity of breast
cancer cells to this chemotherapeutic agent (155). As a cornerstone of breast cancer
chemotherapy, the diverse roles of DOX in regulating cell death
remain under active investigation. Future research within the
academic community is expected to uncover additional functions of
doxorubicin and targeted drugs in this context.
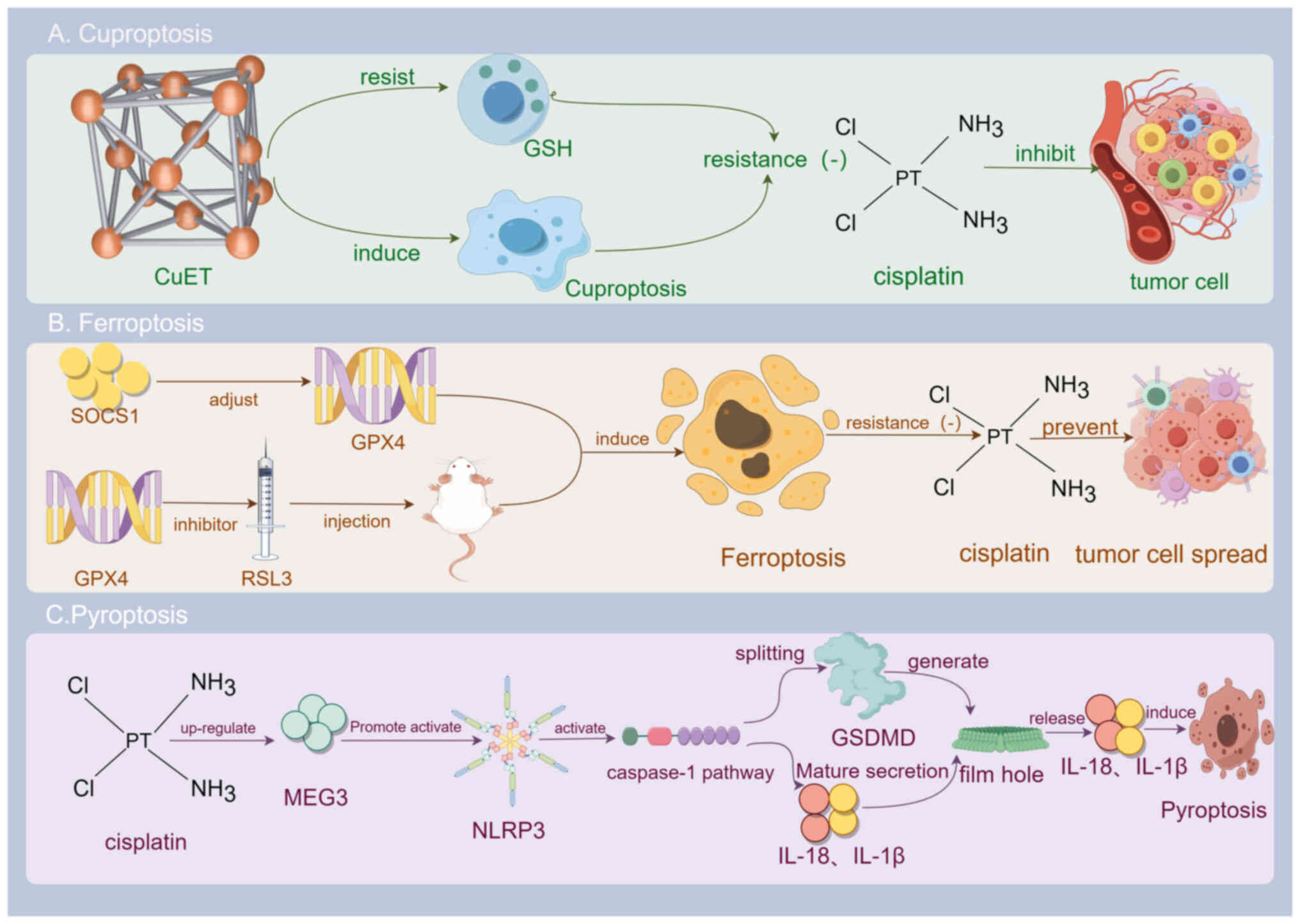
RCD and cisplatin
In addition to DOX, cisplatin also exhibits diverse
interactions with RCD pathways in breast cancer. As a potent
metallochemotherapeutic agent, cisplatin can overcome resistance
through various mechanisms. One approach involves the induction of
cuproptosis by constructing copper(II) bis(diethyldithiocarbamate)
(CuET). This increases CuET distribution in the cytoplasm and
cytoskeleton, effectively bypassing cisplatin resistance (156). Furthermore, cisplatin has been
shown to induce ferroptosis, another form of RCD, to overcome
resistance (157). The
overexpression of the ferroptosis driver, SOCS1, inhibits
proliferation and promotes ferroptosis in TNBC cells, modulating
cisplatin resistance (158).
Additionally, inhibiting the ferroptosis-related gene, GPX4, which
eliminates ROS crucial for ferroptosis, sensitizes tumor cells to
cisplatin. Research using nude mouse models has demonstrated that
combining cisplatin with the GPX4 inhibitor, RSL3, significantly
reduces tumor growth compared to either treatment alone (159). These findings suggest that GPX4
inhibition suppresses ferroptosis and enhances the anticancer
effects of cisplatin. Cisplatin can also promote pyroptosis,
another RCD pathway. By upregulating MEG3, it activates the NLRP3
inflammasome, leading to caspase-1-dependent pyroptosis. This
activation cleaves GSDMD, releasing fragments that form membrane
pores. Moreover, caspase-1 promotes the maturation and secretion of
IL-18 and IL-1β, ultimately inducing the focal death of breast
cancer cells and exerting antitumor effects (160) (Fig. 2). Notably, cisplatin can also
induce autophagy, a cellular self-degradation process. It
upregulates several autophagy-related genes, including AMBRA1,
ATG3, ATG4C, ATG4D, ATG5, ATG7, ATG13, ATG14, ATG16L2, Beclin1,
DRAM1, GABARAP, GABARAPL1, GABARAPL2, HDAC6, IRGM, MAP1LC3B and
ULK1 involved in the induction, vesicle nucleation and
elongation phases of autophagy, suggesting that it inhibits cell
proliferation and growth in breast cancer through multiple RCD
mechanisms (161). Overall,
these findings suggest that inducing various forms of RCD can
effectively reduce cisplatin resistance and enhance its anticancer
effects in breast cancer.
7. Comparison and co-action of multiple
types of RCD
Specific features of RCD
RCD stands apart from accidental cell death, which
results from uncontrolled damage exceeding the survival threshold
of a cell. Unlike its chaotic counterpart, RCD is a genetically
controlled and orderly process that maintains internal stability
(162). The present review
showcases the ability of RCD to selectively target tumor cells,
hindering their growth and spread. As such, RCD emerges as a
promising avenue for cancer therapy.
Comparison of different types of
mechanisms for RCD
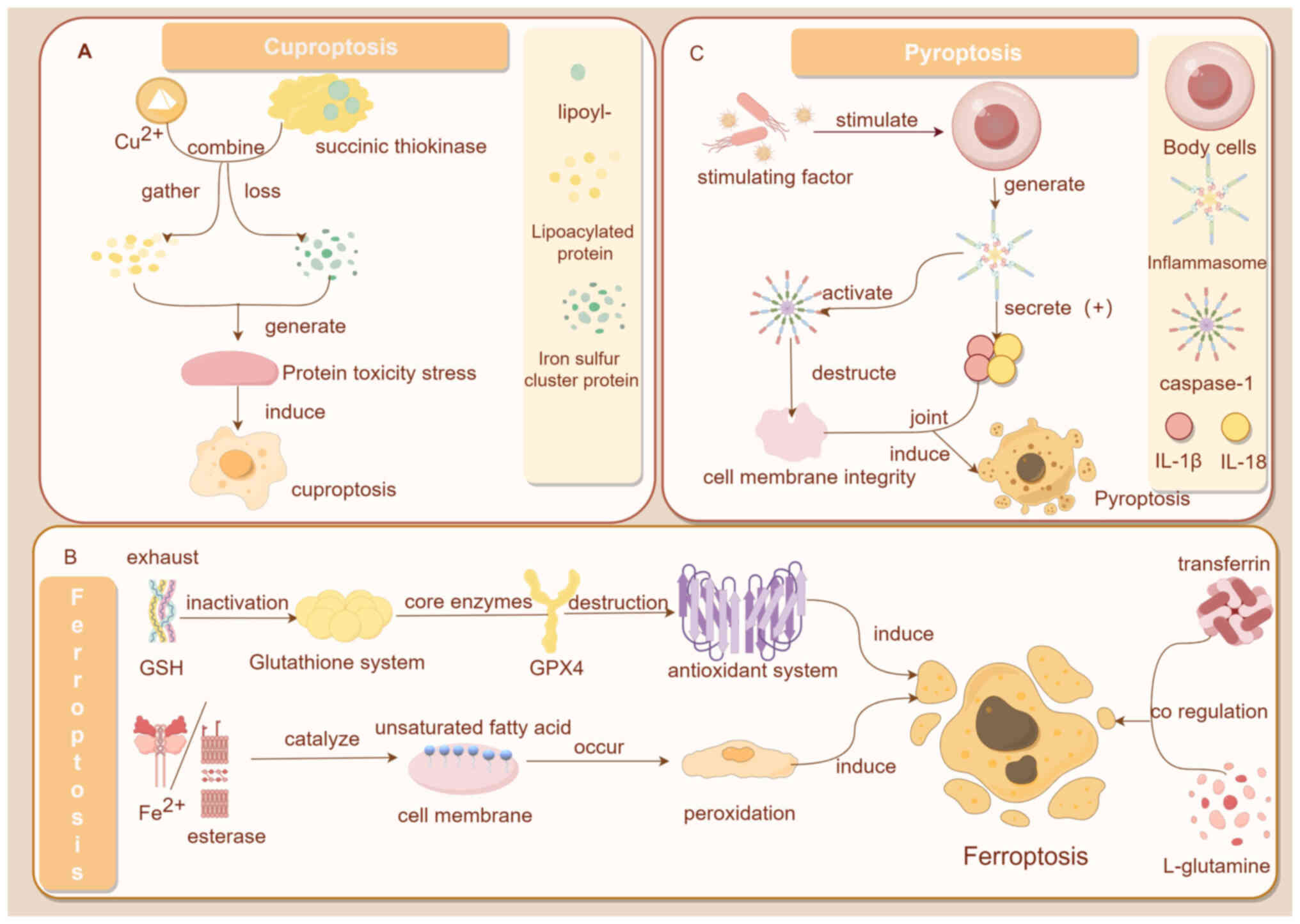
Various forms of RCD exist, each with unique
regulatory mechanisms. Cuproptosis is triggered by the direct
interaction of copper with thiooctylated components of the TCA
cycle. This interaction leads to the aggregation of these proteins
and the depletion of iron-sulfur cluster proteins, inducing
proteotoxic stress and, ultimately, cell death (16). By contrast, ferroptosis is
characterized by iron-dependent lipid peroxidation damage within
the mitochondria, primarily caused by the reduced activity of the
GPX4 enzyme (163). Pyroptosis,
on the other hand, is activated by external stimuli that induce the
formation of inflammatory vesicles. These vesicles activate
caspase-1, which disrupts the cell membrane and leads to the
release of IL-1β and IL-18 cytokines. The combined action of these
cytokines then induces pyroptosis in the affected cell (164) (Fig. 3). Although the detailed mechanisms
of each RCD type differ, they all share the characteristic of being
initiated by the intrinsic regulatory processes of the cell. Cells
that have not initiated these processes remain alive. This
understanding highlights the potential of RCD as a novel approach
to cancer therapy. Research in this area has already shown promise
in improving the accuracy of treatment and patient outcomes.
Oxidative stress: A common mode of
causing multiple types of RCD
Oxidative stress, characterized by the accumulation
of ROS, plays a critical role in triggering various forms of RCD in
cancer cells. Recent studies have shed light on the crucial role of
MTF1, a gene associated with cuproptosis. Notably, its
expression differs in tumor cells compared to normal cells, and
reducing MTF1 levels can elevate ROS production and initiate
cuproptosis (165). Similarly,
the buildup of ROS is crucial for ferroptosis, another form of RCD.
Polyunsaturated fatty acids, a hallmark of ferroptosis, are
produced when ROS attack the double bonds in lipids (166). For pyroptosis, ROS trigger the
activation of the NLRP3 inflammasome, which initiates and activates
NLRP3 synthesis. This inflammasome acts as the key player in
initiating pyroptosis (167). In
summary, oxidative stress plays a pivotal role in triggering
various forms of RCD in cancer cells. This understanding opens new
avenues for developing synergistic cancer therapies that leverage
multiple RCD modalities.
RCD and conventional chemotherapeutic
agents
As aforementioned, the induction of cuproptosis,
ferroptosis and pyroptosis can enhance the anticancer properties of
drugs, such as DOX and cisplatin, while reducing their resistance
and side-effects. This research also suggests the potential to
develop predictive models based on lncRNAs associated with
cuproptosis. These models could help determine the sensitivity of
patient with breast cancer to various chemotherapeutic agents (such
as lapatinib, phenelzine, vincristine and etanercept), aiding in
the selection of personalized treatment regimens (168). Furthermore, the protein, RelB,
provides evidence for the influence of ferroptosis on
chemotherapeutic response. RelB promotes resistance to tamoxifen by
upregulating GPX4, an enzyme that inhibits ferroptosis (169). Similarly, miR-155-5p supports
the role of pyroptosis in response to therapy. In vivo
research has demonstrated that decreasing miR-155-5p levels
triggers pyroptosis and enhances the effectiveness of the drug,
cetuximab, against TNBC cells (170). These findings suggest that RCD
research can not only lead to the discovery of new drugs, but may
also shed light on the anticancer potential and resistance
mechanisms of existing drugs.
Interactions of forms of RCD
There is a potential association between various
RCD modes. Cuproptosis and ferroptosis regulators exhibit the same
mutation frequency in breast cancer. As previously demonstrated,
the knockdown of the ferroptosis regulator, ATF2, in breast cancer
cells (MCF7) resulted in marked changes in cuproptosis regulators
(DLST, GCSH, PDHA1, LIPT1 and DLD) (171). Furthermore, the unsupervised
clustering of cuproptosis and ferroptosis regulators identified
three distinct copper/ferroptosis regulator clusters named
CuFecluster A, B and C. These three regulator clusters have
different biological functions. The three regulatory factor
clusters have distinct biological functions, which strengthen the
experimental basis for using RCD for tumor therapy. CuFecluster B
is associated with the full activation of immunity, including the
B-cell receptor signaling pathway, natural killer cell-mediated
cytotoxicity, antigen processing and presentation,
cytokine-cytokine receptor interactions and chemokine signaling
pathway. Additionally, CuFecluster B is enriched in various
activated immune cells and is classified as an immunoinflammatory
phenotype. CuFecluster A is associated with various cellular
proliferative processes, particularly mismatch repair, DNA
replication and the cell cycle. Notably, CuFecluster A is more
strongly associated with innate immune cells (including
myeloid-derived suppressor cells, eosinophils, natural killer
cells, monocytes, mast cells and macrophages). Finally, CuFecluster
C exhibits a limited association with immune cells and suppresses
immune responses, consistent with the main features of the immune
desert phenotype (171).
Different clusters of regulatory factors can be harnessed,
therefore, to open new avenues for future breast cancer therapy. In
conclusion, while this study has summarized the interactions and
derived functions of cuproptosis and ferroptosis in breast cancer,
associations with other RCDs are still under investigation.
Nevertheless, this work provides valuable insights for exploring
the relationships between other regulatory death pathways in the
future, potentially leading to new benefits for breast cancer
patients.
Summarizing the role of various forms of RCD in
tumor cells in breast cancer reveals that various RCD pathways can
inhibit cancer cell proliferation and invasion. Additionally, they
can reduce resistance to conventional chemotherapeutic drugs.
Combining multiple RCD modalities in breast cancer therapy holds
promise for synergistic effects and offers a promising new avenue
for treatment.
8. Importance of multiple types of RCD for
breast cancer
Importance of cuproptosis for breast
cancer cells
It has been established that cuproptosis-associated
genes can predict the prognosis of patients with breast cancer and
provide information about the immune microenvironment. Cox
regression analyses identified high expression of the
cuproptosis-associated gene, SLC31A1, as an independent prognostic
factor for a shorter overall survival. Additionally, a high SLC31A1
expression has been shown to be associated with dysregulated immune
responses; specifically, it has been shown to be negatively
associated with the level of infiltration of CD8 T-cells and
activated natural killer cells (53). Furthermore, targeting cuproptosis
with existing drugs may provide new avenues for the treatment of
breast cancer. Zinc pyrithione (ZnPT), typically used for fungal
treatment, promotes the aggregation of DLAT both in
vitro and in vivo. DLAT is a biomarker of
cuproptosis and ZnPT disrupts copper homeostasis, eventually
leading to cuproptosis in TNBC cells. This, in turn, inhibits their
viability and proliferation (172). Overall, cuproptosis plays a
crucial role in predicting and treating breast cancer, holding
significant value for exploring genetic detection methods and
repurposing existing drugs for anticancer effects.
Importance of ferroptosis for breast
cancer cells
In ferroptosis, the activation status is of the
pathway is significantly associated with clinical outcomes and
intra-tumor heterogeneity in breast cancer. The detection of
NDUFA13 expression levels provides a means with which to infer this
activation status (173).
Notably, ferroptosis-related genes extend beyond predicting patient
prognosis, also playing an immunologically active role in
immunotherapy.
For example, compared with normal samples, tumor
samples exhibit a significantly lower expression of the
ferroptosis-related gene, HIC1. Notably, HIC1
expression varies across different clinical stages of breast
cancer. Furthermore, HIC1 significantly participates in
immune-related biological functions and signaling pathways, with
its expression being directly associated with the response to
PD-1/PD-L1 inhibitors in cancer therapy (174). Beyond enhancing the
chemotherapeutic efficacy (as aforementioned), ferroptosis can also
potentiate radiotherapy in breast cancer. Constructing tumor
microenvironment-degradable nanohybrids that incorporate
ferroptosis in a dual radiosensitization mode markedly improves
therapeutic efficacy and anti-metastatic efficiency (175). Overall, ferroptosis is
significantly associated with early breast cancer invasion and
recurrence, highlighting its importance in treatment
comprehensiveness and predictive accuracy. Not only are
ferroptosis-related genes used for patient prognosis, but also
channel proteins are being explored to further enhance prediction
accuracy. Consequently, ferroptosis provides a multifaceted
approach for the treatment of breast cancer, capable of augmenting
the efficacy of both chemotherapy and radiotherapy.
Importance of pyroptosis for breast
cancer cells
In cellular pyroptosis, certain lncRNAs associated
with the pathway can predict the prognosis of patients with breast
cancer. For instance, a higher expression of RP11-459E5.1 has been
shown to be associated with a poorer overall survival, while high
levels of RP11-1070N10.3 and RP11-817J15.3 are associated with an
improved survival (176).
Additionally, pyroptosis-related genes can even predict the
potential target organs for breast cancer metastasis. The analysis
of patients with TNBC and brain metastases has revealed significant
differences in AIM2 and ZBP1 expression between primary tumors and
metastases. Notably, a high AIM2 expression predictsa worse
prognosis, while a high ZBP1 expression suggests improved outcomes,
suggesting their potential as biomarkers for TNBC brain metastasis
(177). Furthermore,
chemotherapeutic agents capable of inducing pyroptosis have
promising potential for use in the treatment of breast cancer.
Derivatives, such as 3-acyl isoquinoline-1 (2H)-ones can trigger
GSDME-mediated pyroptosis, leading to apoptosis and inhibiting the
proliferation of breast cancer cells without harming normal breast
cells (178). The unique
advantage of pyroptosis lies in the ability of related genes to
predict metastatic organs and the relative lack of toxicity of its
associated drugs towards normal cells, both contributing to
improved patient prognosis.
Across cuproptosis, ferroptosis and pyroptosis,
various molecules can be used to predict the prognosis of patients
with breast cancer. Additionally, targeting these pathways or their
mechanisms through drug development presents opportunities to
enhance treatment efficacy. All three types of RCD hold immense
potential for future research and breast cancer treatment. While
ferroptosis research currently boasts more applied and
comprehensive studies, including prognostic prediction encompassing
developmental stages, it is important to acknowledge the ongoing
investigation and promise of cuproptosis and pyroptosis as well.
Moreover, ferroptosis-related drugs may enhance not only
chemotherapy, but also radiotherapy, potentially rendering it the
first form of RCD to reach clinical application.
9. Conclusion and future perspectives
Breast cancer poses a significant threat to human
life and health. Its growth, development and metastasis are
intricately linked to the body's own gene regulation and immune
defense mechanisms. RCD, an intrinsic component of these
physiological programs, plays a crucial role in tumor regulation
and defense. The academic community is steadily uncovering and
proposing the regulatory mechanisms of cuproptosis, ferroptosis,
pyroptosis and other forms of RCD in breast cancer. The development
of novel drugs and ongoing clinical trials (presented in Table I) highlight the strong association
between these pathways and breast cancer, offering a promising new
direction for research. Several emerging drugs and clinical agents
have demonstrated the ability to induce RCD in breast cancer cells.
However, ongoing research is necessary to fully understand the
potential mechanisms of RCD and further explore and test related
drugs in clinical trials. By harnessing the power of RCD, it is
hoped that future advancements in treatment can improve treatment
efficacy, enhance the quality of life, and increase the survival
rate of patients with breast cancer.
 | Table IUtilization of scientifically and
technologically constructed drugs related to regulated cell
death. |
Table I
Utilization of scientifically and
technologically constructed drugs related to regulated cell
death.
| Drug | Target of
action/mechanism | Regulatory cell
death involved | Potency | (Refs.) |
|---|
| Type-I AIE
photosensitizer loaded biomimetic system | Lipoylated protein
aggregation and iron-sulfur protein loss | Cuproptosis | Inhibits lung
metastasis of breast cancer and prevents tumor rechallenge | (61) |
| HD/BER/GOx/Cu
hydrogel system | Produces
starvation/chemodynamic therapy and induces copper death | Cuproptosis | Preoperative
reduction of breast cancer size to facilitate surgical
excision | (62) |
| Heparanase-driven
sequential released nanoparticles | Effectively
enhances intracellular ROS levels and activates the iron death
pathway. Enhanced ROS also induces the apoptotic pathway and
reduces the expression of MMP-9 | Ferroptosis | New dosage regimens
for the treatment of breast cancer by intracellular and
extracellular mechanisms | (93) |
| Polydopamine
nanoparticles | Combination with
DOX induces ferroptosis in breast cancer cells | Ferroptosis | Possesses
anti-tumor activity and selectivity, increasing the accuracy and
effectiveness of targeted therapies | (94) |
| Cinnamaldehyde
dimers | Depletion of
glutathione, in combination with the anti-breast cancer drug
sorafenib (sorafenib.SRF), resulted in a significant enhancement of
iron death, and by promoting dendritic cell maturation and
CD8+ T-cell initiation | Ferroptosis | Increasing the
anticancer effects of sorafenib | (95) |
| erastin@FA-exo | Inhibits the
expression of GPX4 and upregulates the expression of CDO1 | Ferroptosis | May reduce side
effects in tumor therapy and may replace traditional erastin in
clinical practice | (96) |
| Biomimetic
nanoparticle (BNP) loaded with indocyanine green (ICG) and
decitabine (DCT) | Induced cleavage of
GSDME | Pyroptosis | Controlled tumor
growth and stimulated anticancer immune responses | (139) |
| Carrier-free
chemophotodynamic nanoplatform | Triggering
GSDME-Mediated ScorchDeath in a controlled manner via ROS
accumulation | Pyroptosis | Complement
chemotherapy or immunotherapy for cancer metastasis | (140) |
Availability of data and materials
Not applicable.
Authors' contributions
LA, CQ, WH, KZ, QH, LJ and HL searched the
literature for related studies for the review and prepared the
manuscript and figures. LL and NY provided constructive guidance
and made critical revisions. LA participated in the main editing of
the manuscript. LA, CQ, LL and NY participated in the design of the
review. All authors have read and approved the final manuscript.
Data authentication is not applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
Funding
The present study was supported by the Xinjiang Uygur Autonomous
Region Natural Science Foundation (Program no. 2023D01C40) and the
National Natural Science Foundation of China (Program no.
81660459).
References
|
1
|
Xia C, Dong X, Li H, Cao M, Sun D, He S,
Yang F, Yan X, Zhang S, Li N and Chen W: Cancer statistics in China
and United States, 2022: Profiles, trends, and determinants. Chin
Med J (Engl). 135:584–590. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cameron D, Piccart-Gebhart MJ, Gelber RD,
Procter M, Goldhirsch A, de Azambuja E, Castro G Jr, Untch M, Smith
I, Gianni L, et al: 11 years' follow-up of trastuzumab after
adjuvant chemotherapy in HER2-positive early breast cancer: Final
analysis of the HERceptin Adjuvant (HERA) trial. Lancet.
389:1195–1205. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Galluzzi L, Vitale I, Aaronson SA, Abrams
JM, Adam D, Agostinis P, Alnemri ES, Altucci L, Amelio I and
Andrews DW: Molecular mechanisms of cell death: Recommendations of
the Nomenclature Committee on Cell Death 2018. Cell Death Differ.
25:486–541. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tang D, Kang R, Berghe TV, Vandenabeele P
and Kroemer G: The molecular machinery of regulated cell death.
Cell Res. 29:347–364. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Conradt B: Genetic control of programmed
cell death during animal development. Annu Rev Genet. 43:493–523.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fuchs Y and Steller H: Programmed cell
death in animal development and disease. Cell. 147:742–758. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Galluzzi L, Bravo-San Pedro JM, Kepp O and
Kroemer G: Regulated cell death and adaptive stress responses. Cell
Mol Life Sci. 73:2405–2410. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lutsenko S: Human copper homeostasis: A
network of interconnected pathways. Curr Opin Chem Biol.
14:211–217. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gaggelli E, Kozlowski H, Valensin D and
Valensin G: Copper homeostasis and neurodegenerative disorders
(Alzheimer's, prion, and Parkinson's diseases and amyotrophic
lateral sclerosis). Chem Rev. 106:1995–2044. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Festa RA and Thiele DJ: Copper: An
essential metal in biology. Curr Biol. 21:R877–R883. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chillappagari S, Seubert A, Trip H,
Kuipers OP, Marahiel MA and Miethke M: Copper stress affects iron
homeostasis by destabilizing iron-sulfur cluster formation in
Bacillus subtilis. J Bacteriol. 192:2512–2524. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Macomber L and Imlay JA: The iron-sulfur
clusters of dehydratases are primary intracellular targets of
copper toxicity. Proc Natl Acad Sci USA. 106:8344–8349. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang Y, Zhang L and Zhou F: Cuproptosis: A
new form of programmed cell death. Cell Mol Immunol. 19:867–868.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tang D, Chen X and Kroemer G: Cuproptosis:
A coppertriggered modality of mitochondrial cell death. Cell Res.
32:417–418. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tsvetkov P, Coy S, Petrova B, Dreishpoon
M, Verma A, Abdusamad M, Rossen J, Joesch-Cohen L, Humeidi R,
Spangler RD, et al: Copper induces cell death by targeting
lipoylated TCA cycle proteins. Science. 375:1254–1261. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Elmore S: Apoptosis: A review of
programmed cell death. Toxicol Pathol. 35:495–516. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tsvetkov P, Detappe A, Cai K, Keys HR,
Brune Z, Ying W, Thiru P, Reidy M, Kugener G, Rossen J, et al:
Mitochondrial metabolism promotes adaptation to proteotoxic stress.
Nat Chem Biol. 15:681–689. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Skrott Z, Majera D, Gursky J, Buchtova T,
Hajduch M, Mistrik M and Bartek J: Disulfiram's anti-cancer
activity reflects targeting NPL4, not inhibition of aldehyde
dehydrogenase. Oncogene. 38:6711–6722. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Pan M, Zheng Q, Yu Y, Ai H, Xie Y, Zeng X,
Wang C, Liu L and Zhao M: Seesaw conformations of Npl4 in the human
p97 complex and the inhibitory mechanism of a disulfiram
derivative. Nat Commun. 12:1212021. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tsang T, Posimo JM, Gudiel AA, Cicchini M,
Feldser DM and Brady DC: Copper is an essential regulator of the
autophagic kinases ULK1/2 to drive lung adenocarcinoma. Nat Cell
Biol. 22:412–424. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Davis CI, Gu X, Kiefer RM, Ralle M, Gade
TP and Brady DC: Altered copper homeostasis underlies sensitivity
of hepatocellular carcinoma to copper chelation. Metallomics.
12:1995–2008. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ge EJ, Bush AI, Casini A, Cobine PA, Cross
JR, DeNicola GM, Dou QP, Franz KJ, Gohil VM, Gupta S, et al:
Connecting copper and cancer: From transition metal signalling to
metalloplasia. Nat Rev Cancer. 22:102–113. 2022. View Article : Google Scholar :
|
|
24
|
Hasinoff BB, Yadav AA, Patel D and Wu X:
The cytotoxicity of the anticancer drug elesclomol is due to
oxidative stress indirectly mediated through its complex with
Cu(II). J Inorg Biochem. 137:22–30. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tardito S, Bassanetti I, Bignardi C,
Elviri L, Tegoni M, Mucchino C, Bussolati O, Franchi-Gazzola R and
Marchiò L: Copper binding agents acting as copper ionophores lead
to caspase inhibition and paraptotic cell death in human cancer
cells. J Am Chem Soc. 133:6235–6242. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Pavithra V, Sathisha TG, Kasturi K,
Mallika DS, Amos SJ and Ragunatha S: Serum levels of metal ions in
female patients with breast cancer. J Clin Diagn Res. 9:BC25–BC27.
2015.PubMed/NCBI
|
|
27
|
Wu J, Zhu Y, Luo M and Li L: Comprehensive
analysis of pyroptosis-related genes and tumor microenvironment
infiltration characterization in breast cancer. Front Immunol.
12:7482212021. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Brady DC, Crowe MS, Turski ML, Hobbs GA,
Yao X, Chaikuad A, Knapp S, Xiao K, Campbell SL, Thiele DJ and
Counter CM: Copper is required for oncogenic BRAF signalling and
tumorigenesis. Nature. 509:492–496. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Cui L, Gouw AM, LaGory EL, Guo S,
Attarwala N, Tang Y, Qi J, Chen YS, Gao Z, Casey KM, et al:
Mitochondrial copper depletion suppresses triple-negative breast
cancer in mice. Nat Biotechnol. 39:357–367. 2021. View Article : Google Scholar
|
|
30
|
Blockhuys S, Zhang X and Wittung-Stafshede
P: Single-cell tracking demonstrates copper chaperone Atox1 to be
required for breast cancer cell migration. Proc Natl Acad Sci USA.
117:2014–2019. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kirshner JR, He S, Balasubramanyam V,
Kepros J, Yang CY, Zhang M, Du Z, Barsoum J and Bertin J:
Elesclomol induces cancer cell apoptosis through oxidative stress.
Mol Cancer Ther. 7:2319–2327. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Nagai M, Vo NH, Shin Ogawa L, Chimmanamada
D, Inoue T, Chu J, Beaudette-Zlatanova BC, Lu R, Blackman RK,
Barsoum J, et al: The oncology drug elesclomol selectively
transports copper to the mitochondria to induce oxidative stress in
cancer cells. Free Radic Biol Med. 52:2142–2150. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yadav AA, Patel D, Wu X and Hasinoff BB:
Molecular mechanisms of the biological activity of the anticancer
drug elesclomol and its complexes with Cu(II), Ni(II) and Pt(II). J
Inorg Biochem. 126:1–6. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Renier N, Reinaud O, Jabin I and Valkenier
H: Transmembrane transport of copper(i) by imidazole-functionalised
calix[4] arenes. Chem Commun (Camb). 56:8206–8209. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Chen L, Min J and Wang F: Copper
homeostasis and cuproptosis in health and disease. Signal Transduct
Target Ther. 7:3782022. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Smirnova J, Kabin E, Järving I, Bragina O,
Tõugu V, Plitz T and Palumaa P: Copper(I)-binding properties of
de-coppering drugs for the treatment of Wilson disease. α-Lipoic
acid as a potential anti-copper agent. Sci Rep. 8:14632018.
View Article : Google Scholar
|
|
37
|
He K, Chen Z, Ma Y and Pan Y:
Identification of high-copper-responsive target pathways in Atp7b
knockout mouse liver by GSEA on microarray data sets. Mamm Genome.
22:703–713. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Sheftel AD, Stehling O, Pierik AJ,
Elsässer HP, Mühlenhoff U, Webert H, Hobler A, Hannemann F,
Bernhardt R and Lill R: Humans possess two mitochondrial
ferredoxins, Fdx1 and Fdx2, with distinct roles in steroidogenesis,
heme, and Fe/S cluster biosynthesis. Proc Natl Acad Sci USA.
107:11775–11780. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Strushkevich N, MacKenzie F, Cherkesova T,
Grabovec I, Usanov S and Park HW: Structural basis for pregnenolone
biosynthesis by the mitochondrial monooxygenase system. Proc Natl
Acad Sci USA. 108:10139–10143. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zalewski A, Ma NS, Legeza B, Renthal N,
Flück CE and Pandey AV: Vitamin D-Dependent rickets type 1 caused
by mutations in CYP27B1 affecting protein interactions with
adrenodoxin. J Clin Endocrinol Metab. 101:3409–3418. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Moriya M, Ho YH, Grana A, Nguyen L,
Alvarez A, Jamil R, Ackland ML, Michalczyk A, Hamer P, Ramos D, et
al: Copper is taken up efficiently from albumin and
alpha2-macroglobulin by cultured human cells by more than one
mechanism. Am J Physiol Cell Physiol. 295:C708–C721. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Xie J, Yang Y, Gao Y and He J:
Cuproptosis: Mechanisms and links with cancers. Mol Cancer.
22:462023. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Lu Z and Hunter T: Metabolic kinases
moonlighting as protein kinases. Trends Biochem Sci. 43:301–310.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Zhang L, Li M, Cui Z, Chai D, Guan Y, Chen
C and Wang W: Systematic analysis of the role of SLC52A2 in
multiple human cancers. Cancer Cell Int. 22:82022. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Halestrap AP: The SLC16 gene
family-structure, role and regulation in health and disease. Mol
Aspects Med. 34:337–349. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Higuchi K, Sugiyama K, Tomabechi R,
Kishimoto H and Inoue K: Mammalian monocarboxylate transporter 7
(MCT7/Slc16a6) is a novel facilitative taurine transporter. J Biol
Chem. 298:1018002022. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Wright ME, Peters U, Gunter MJ, Moore SC,
Lawson KA, Yeager M, Weinstein SJ, Snyder K, Virtamo J and Albanes
D: Association of variants in two vitamin e transport genes with
circulating vitamin e concentrations and prostate cancer risk.
Cancer Res. 69:1429–1438. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Tanaka T, Bai Z, Srinoulprasert Y, Yang
BG, Hayasaka H and Miyasaka M: Chemokines in tumor progression and
metastasis. Cancer Sci. 96:317–322. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
de Marco MC, Martín-Belmonte F, Kremer L,
Albar JP, Correas I, Vaerman JP, Marazuela M, Byrne JA and Alonso
MA: MAL2, a novel raft protein of the MAL family, is an essential
component of the machinery for transcytosis in hepatoma HepG2
cells. J Cell Biol. 159:37–44. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Li DD, Yagüe E, Wang LY, Dai LL, Yang ZB,
Zhi S, Zhang N, Zhao XM and Hu YH: Novel copper complexes that
inhibit the proteasome and trigger apoptosis in triple-negative
breast cancer cells. ACS Med Chem Lett. 10:1328–1335. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Lee ZY, Leong CH, Lim KUL, Wong CCS,
Pongtheerawan P, Arikrishnan SA, Tan KL, Loh JS, Low ML, How CW, et
al: Induction of apoptosis and autophagy by ternary copper complex
towards breast cancer cells. Anticancer Agents Med Chem.
22:1159–1170. 2022. View Article : Google Scholar
|
|
52
|
Li X, Ma Z and Mei L: Cuproptosis-related
gene SLC31A1 is a potential predictor for diagnosis, prognosis and
therapeutic response of breast cancer. Am J Cancer Res.
12:3561–3580. 2022.PubMed/NCBI
|
|
53
|
Li L, Li L and Sun Q: High expression of
cuproptosis-related SLC31A1 gene in relation to unfavorable outcome
and deregulated immune cell infiltration in breast cancer: An
analysis based on public databases. BMC Bioinformatics. 23:3502022.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Li Z, Zhang H, Wang X, Wang Q, Xue J, Shi
Y, Wang M, Wang G and Zhang J: Identification of
cuproptosis-related subtypes, characterization of tumor
microenvironment infiltration, and development of a prognosis model
in breast cancer. Front Immunol. 13:9968362022. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Sha S, Si L, Wu X, Chen Y, Xiong H, Xu Y,
Liu W, Mei H, Wang T and Li M: Prognostic analysis of
cuproptosis-related gene in triple-negative breast cancer. Front
Immunol. 13:9227802022. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Guan X, Lu N and Zhang J: Construction of
a prognostic model related to copper dependence in breast cancer by
single-cell sequencing analysis. Front Genet. 13:9498522022.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Jiang ZR, Yang LH, Jin LZ, Yi LM, Bing PP,
Zhou J and Yang JS: Identification of novel cuproptosis-related
lncRNA signatures to predict the prognosis and immune
microenvironment of breast cancer patients. Front Oncol.
12:9886802022. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Zhao Q and Qi T: The implications and
prospect of cuproptosis-related genes and copper transporters in
cancer progression. Front Oncol. 13:11171642023. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Song S, Zhang M, Xie P, Wang S and Wang Y:
Comprehensive analysis of cuproptosis-related genes and tumor
microenvironment infiltration characterization in breast cancer.
Front Immunol. 13:9789092022. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Ning S, Lyu M, Zhu D, Lam JWY, Huang Q,
Zhang T and Tang BZ: Type-I AIE photosensitizer loaded biomimetic
system boosting cuproptosis to inhibit breast cancer metastasis and
rechallenge. ACS Nano. 17:10206–10217. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Lee SY, Seo JH, Kim S, Hwang C, Jeong DI,
Park J, Yang M, Huh JW and Cho HJ: Cuproptosis-Inducible
chemotherapeutic/cascade catalytic reactor system for combating
with breast cancer. Small. 19:e23014022023. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Dixon SJ, Lemberg KM, Lamprecht MR, Skouta
R, Zaitsev EM, Gleason CE, Patel DN, Bauer AJ, Cantley AM, Yang WS,
et al: Ferroptosis: An iron-dependent form of nonapoptotic cell
death. Cell. 149:1060–1072. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Xu T, Ding W, Ji X, Ao X, Liu Y, Yu W and
Wang J: Molecular mechanisms of ferroptosis and its role in cancer
therapy. J Cell Mol Med. 23:4900–4912. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Gao M, Monian P, Quadri N, Ramasamy R and
Jiang X: Glutaminolysis and transferrin regulate ferroptosis. Mol
Cell. 59:298–308. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Lu B, Chen XB, Ying MD, He QJ, Cao J and
Yang B: The role of ferroptosis in cancer development and treatment
response. Front Pharmacol. 8:9922018. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Stockwell BR, Friedmann Angeli JP, Bayir
H, Bush AI, Conrad M, Dixon SJ, Fulda S, Gascón S, Hatzios SK,
Kagan VE, et al: Ferroptosis: A regulated cell death nexus linking
metabolism, redox biology, and disease. Cell. 171:273–285. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Xie Y, Wang B, Zhao Y, Tao Z, Wang Y, Chen
G and Hu X: Mammary adipocytes protect triple-negative breast
cancer cells from ferroptosis. J Hematol Oncol. 15:722022.
View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Wang YY, Attané C, Milhas D, Dirat B,
Dauvillier S, Guerard A, Gilhodes J, Lazar I, Alet N, Laurent V, et
al: Mammary adipocytes stimulate breast cancer invasion through
metabolic remodeling of tumor cells. JCI Insight. 2:e874892017.
View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Yang D, Li Y, Xing L, Tan Y, Sun J, Zeng
B, Xiang T, Tan J, Ren G and Wang Y: Utilization of
adipocyte-derived lipids and enhanced intracellular trafficking of
fatty acids contribute to breast cancer progression. Cell Commun
Signal. 16:322018. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Liu W, Chakraborty B, Safi R, Kazmin D,
Chang CY and McDonnell DP: Dysregulated cholesterol homeostasis
results in resistance to ferroptosis increasing tumorigenicity and
metastasis in cancer. Nat Commun. 12:51032021. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Baek AE, Yu YA, He S, Wardell SE, Chang
CY, Kwon S, Pillai RV, McDowell HB, Thompson JW, Dubois LG, et al:
The cholesterol metabolite 27 hydroxycholesterol facilitates breast
cancer metastasis through its actions on immune cells. Nat Commun.
8:8642017. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Vallianou NG, Kostantinou A, Kougias M and
Kazazis C: Statins and cancer. Anticancer Agents Med Chem.
14:706–712. 2014. View Article : Google Scholar
|
|
73
|
Sha R, Xu Y, Yuan C, Sheng X, Wu Z, Peng
J, Wang Y, Lin Y, Zhou L, Xu S, et al: Predictive and prognostic
impact of ferroptosis-related genes ACSL4 and GPX4 on breast cancer
treated with neoadjuvant chemotherapy. EBioMedicine. 71:1035602021.
View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Ding Y, Chen X, Liu C, Ge W, Wang Q, Hao
X, Wang M, Chen Y and Zhang Q: Identification of a small molecule
as inducer of ferroptosis and apoptosis through ubiquitination of
GPX4 in triple negative breast cancer cells. J Hematol Oncol.
14:192021. View Article : Google Scholar
|
|
75
|
Zhang K, Ping L, Du T, Liang G, Huang Y,
Li Z, Deng R and Tang J: A Ferroptosis-Related lncRNAs signature
predicts prognosis and immune microenvironment for breast cancer.
Front Mol Biosci. 8:6788772021. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Ping L, Zhang K, Ou X, Qiu X and Xiao X: A
novel pyroptosis-associated long Non-coding RNA signature predicts
prognosis and tumor immune microenvironment of patients with breast
cancer. Front Cell Dev Biol. 9:7271832021. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Zhu Z and Leung GKK: More than a metabolic
enzyme: MTHFD2 as a novel target for anticancer therapy? Front
Oncol. 10:6582020. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Zhang H, Zhu S, Zhou H, Li R, Xia X and
Xiong H: Identification of MTHFD2 as a prognostic biomarker and
ferroptosis regulator in triple-negative breast cancer. Front
Oncol. 13:10983572023. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Yadav P, Sharma P, Sundaram S, Venkatraman
G, Bera AK and Karunagaran D: SLC7A11/xCT is a target of miR-5096
and its restoration partially rescues miR-5096-mediated ferroptosis
and anti-tumor effects in human breast cancer cells. Cancer Lett.
522:211–224. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Koppula P, Zhuang L and Gan B: Cystine
transporter SLC7A11/xCT in cancer: Ferroptosis, nutrient
dependency, and cancer therapy. Protein Cell. 12:599–620. 2021.
View Article : Google Scholar :
|
|
81
|
He J, Wang X, Chen K, Zhang M and Wang J:
The amino acid transporter SLC7A11-mediated crosstalk implicated in
cancer therapy and the tumor microenvironment. Biochem Pharmacol.
205:1152412022. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Zhang Y, Liang Y, Wang Y, Ye F, Kong X and
Yang Q: A novel ferroptosis-related gene signature for overall
survival prediction and immune infiltration in patients with breast
cancer. Int J Oncol. 61:1482022. View Article : Google Scholar :
|
|
83
|
Liu Q, Ma JY and Wu G: Identification and
validation of a ferroptosis-related gene signature predictive of
prognosis in breast cancer. Aging (Albany NY). 13:21385–21399.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Xu Y, Du Y, Zheng Q, Zhou T, Ye B, Wu Y,
Xu Q and Meng X: Identification of ferroptosis-related prognostic
signature and subtypes related to the immune microenvironment for
breast cancer patients receiving neoadjuvant chemotherapy. Front
Immunol. 13:8951102022. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Yang YF, Lee YC, Wang YY, Wang CH, Hou MF
and Yuan SF: YWHAE promotes proliferation, metastasis, and
chemoresistance in breast cancer cells. Kaohsiung J Med Sci.
35:408–416. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Qiao X, Zhang Y, Sun L, Ma Q, Yang J, Ai
L, Xue J, Chen G, Zhang H, Ji C, et al: Association of human breast
cancer CD44-/CD24-cells with delayed distant metastasis. Elife.
10:e654182021. View Article : Google Scholar
|
|
87
|
Gong Z, Li Q, Shi J, Liu ET, Shultz LD and
Ren G: Lipid-laden lung mesenchymal cells foster breast cancer
metastasis via metabolic reprogramming of tumor cells and natural
killer cells. Cell Metab. 34:1960–1976.e9. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Li Y, Jin K, van Pelt GW, van Dam H, Yu X,
Mesker WE, Ten Dijke P, Zhou F and Zhang L: c-Myb enhances breast
cancer invasion and metastasis through the Wnt/β-Catenin/Axin2
pathway. Cancer Res. 76:3364–3375. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Li Y, Zhang Y, Liu X, Wang M, Wang P, Yang
J and Zhang S: Lutein inhibits proliferation, invasion and
migration of hypoxic breast cancer cells via downregulation of
HES1. Int J Oncol. 52:2119–2129. 2018.PubMed/NCBI
|
|
90
|
Huo Q, Wang J and Xie N: High HSPB1
expression predicts poor clinical outcomes and correlates with
breast cancer metastasis. BMC Cancer. 23:5012023. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Zhang H, Ge Z, Wang Z, Gao Y, Wang Y and
Qu X: Circular RNA RHOT1 promotes progression and inhibits
ferroptosis via mir-106a-5p/STAT3 axis in breast cancer. Aging
(Albany NY). 13:8115–8126. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Zhou Y, Che Y, Fu Z, Zhang H and Wu H:
Triple-Negative breast cancer analysis based on metabolic gene
classification and immunotherapy. Front Public Health.
10:9023782022. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Zhang J, Yang J, Zuo T, Ma S, Xokrat N, Hu
Z, Wang Z, Xu R, Wei Y and Shen Q: Heparanase-driven sequential
released nanoparticles for ferroptosis and tumor microenvironment
modulations synergism in breast cancer therapy. Biomaterials.
266:1204292021. View Article : Google Scholar
|
|
94
|
Nieto C, Vega MA and Martín Del Valle EM:
Tailored-Made polydopamine nanoparticles to induce ferroptosis in
breast cancer cells in combination with chemotherapy. Int J Mol
Sci. 22:31612021. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Zhou Z, Liang H, Yang R, Yang Y, Dong J,
Di Y and Sun M: Glutathione depletion-induced activation of
dimersomes for potentiating the ferroptosis and immunotherapy of
'cold' tumor. Angew Chem Int Ed Engl. 61:e2022028432022. View Article : Google Scholar
|
|
96
|
Dattachoudhury S, Sharma R, Kumar A and
Jaganathan BG: Sorafenib inhibits proliferation, migration and
invasion of breast cancer cells. Oncology. 98:478–486. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Yu M, Gai C, Li Z, Ding D, Zheng J, Zhang
W, Lv S and Li W: Targeted exosome-encapsulated erastin induced
ferroptosis in triple negative breast cancer cells. Cancer Sci.
110:3173–3182. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Yang J, Zhou Y, Xie S, Wang J, Li Z, Chen
L, Mao M, Chen C, Huang A, Chen Y, et al: Metformin induces
Ferroptosis by inhibiting UFMylation of SLC7A11 in breast cancer. J
Exp Clin Cancer Res. 40:2062021. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Ma S, Henson ES, Chen Y and Gibson SB:
Ferroptosis is induced following siramesine and lapatinib treatment
of breast cancer cells. Cell Death Dis. 7:e23072016. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Ma S, Dielschneider RF, Henson ES, Xiao W,
Choquette TR, Blankstein AR, Chen Y and Gibson SB: Ferroptosis and
autophagy induced cell death occur independently after siramesine
and lapatinib treatment in breast cancer cells. PLoS One.
12:e01829212017. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Chen Z, Li R, Fang M, Wang Y, Bi A, Yang
L, Song T, Li Y, Li Q, Lin B, et al: Integrated analysis of
FKBP1A/SLC3A2 axis in everolimus inducing ferroptosis of breast
cancer and anti-proliferation of T lymphocyte. Int J Med Sci.
20:1060–1078. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Li H, Liu W, Zhang X, Wu F, Sun D and Wang
Z: Ketamine suppresses proliferation and induces ferroptosis and
apoptosis of breast cancer cells by targeting KAT5/GPX4 axis.
Biochem Biophys Res Commun. 585:111–116. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Song X, Wang X, Liu Z and Yu Z: Role of
GPX4-Mediated ferroptosis in the sensitivity of triple negative
breast cancer cells to gefitinib. Front Oncol. 10:5974342020.
View Article : Google Scholar
|
|
104
|
Yao X, Xie R, Cao Y, Tang J, Men Y, Peng H
and Yang W: Simvastatin induced ferroptosis for triple-negative
breast cancer therapy. J Nanobiotechnology. 19:3112021. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Zhang Z, Lu M, Chen C, Tong X, Li Y, Yang
K, Lv H, Xu J and Qin L: Holo-lactoferrin: the link between
ferroptosis and radiotherapy in triple-negative breast cancer.
Theranostics. 11:3167–3182. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Sun D, Li YC and Zhang XY: Lidocaine
promoted ferroptosis by targeting miR-382-5p/SLC7A11 axis in
ovarian and breast cancer. Front Pharmacol. 12:6812232021.
View Article : Google Scholar
|
|
107
|
Li R, Zhang J, Zhou Y, Gao Q, Wang R, Fu
Y, Zheng L and Yu H: Transcriptome Investigation and in vitro
verification of curcumin-induced HO-1 as a feature of ferroptosis
in breast cancer cells. Oxid Med Cell Longev. 2020:34698402020.
View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Zhai FG, Liang QC, Wu YY, Liu JQ and Liu
JW: Red ginseng polysaccharide exhibits anticancer activity through
GPX4 downregulation-induced ferroptosis. Pharm Biol. 60:909–914.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Cookson BT and Brennan MA:
Pro-inflammatory programmed cell death. Trends Microbiol.
9:113–114. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Shi J, Zhao Y, Wang K, Shi X, Wang Y,
Huang H, Zhuang Y, Cai T, Wang F and Shao F: Cleavage of GSDMD by
inflammatory caspases determines pyroptotic cell death. Nature.
526:660–665. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Järveläinen HA, Galmiche A and Zychlinsky
A: Caspase-1 activation by Salmonella. Trends Cell Biol.
13:204–209. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Martinon F, Burns K and Tschopp J: The
inflammasome: a molecular platform triggering activation of
inflammatory caspases and processing of proIL-beta. Mol Cell.
10:417–426. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Feng S, Fox D and Man SM: Mechanisms of
gasdermin family members in inflammasome signaling and cell death.
J Mol Biol. 430(18 Pt B): 3068–3080. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Shi J, Zhao Y, Wang Y, Gao W, Ding J, Li
P, Hu L and Shao F: Inflammatory caspases are innate immune
receptors for intracellular LPS. Nature. 514:187–192. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Xia J, Chu C, Li W, Chen H, Xie W, Cheng
R, Hu K and Li X: Mitochondrial Protein UCP1 inhibits the malignant
behaviors of triple-negative breast cancer through activation of
mitophagy and pyroptosis. Int J Biol Sci. 18:2949–2961. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Yu X, Shi M, Wu Q, Wei W, Sun S and Zhu S:
Identification of UCP1 and UCP2 as potential prognostic markers in
breast cancer: A study based on immunohistochemical analysis and
bioinformatics. Front Cell Dev Biol. 10:8917312022. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Zhang Z, Zhang Y, Xia S, Kong Q, Li S, Liu
X, Junqueira C, Meza-Sosa KF, Mok TMY, Ansara J, et al: Gasdermin E
suppresses tumour growth by activating anti-tumour immunity.
Nature. 579:415–420. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Yi M, Niu M, Xu L, Luo S and Wu K:
Regulation of PD-L1 expression in the tumor microenvironment. J
Hematol Oncol. 14:102021. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Zhang M, Wu K, Wang M, Bai F and Chen H:
CASP9 as a prognostic biomarker and promising drug target plays a
pivotal role in inflammatory breast cancer. Int J Anal Chem.
2022:10434452022. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Chu L, Yi Q, Yan Y, Peng J, Li Z, Jiang F,
He Q, Ouyang L, Wu S, Fu C, et al: A prognostic signature
consisting of pyroptosis-related genes and SCAF11 for predicting
immune response in breast cancer. Front Med (Lausanne).
9:8827632022. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Xu D, Ji Z and Qiang L: Molecular
characteristics, clinical implication, and cancer immunity
interactions of pyroptosis-related genes in breast cancer. Front
Med (Lausanne). 8:7026382021. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Zhou Y, Zheng J, Bai M, Gao Y and Lin N:
Effect of pyroptosis-related genes on the prognosis of breast
cancer. Front Oncol. 12:9481692022. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Jin H and Kim HJ: NLRC4, ASC and Caspase-1
are inflammasome components that are mediated by P2Y2R activation
in breast cancer cells. Int J Mol Sci. 21:33372020. View Article : Google Scholar :
|
|
124
|
Hergueta-Redondo M, Sarrió D,
Molina-Crespo Á, Megias D, Mota A, Rojo-Sebastian A, García-Sanz P,
Morales S, Abril S, Cano A, et al: Gasdermin-B promotes invasion
and metastasis in breast cancer cells. PLoS One. 9:e900992014.
View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Song C, Kendi AT, Lowe VJ and Lee S: The
A20/TNFAIP3-CDC20-CASP1 axis promotes inflammation-mediated
metastatic disease in triple-negative breast cancer. Anticancer
Res. 42:681–695. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Velloso FJ, Campos AR, Sogayar MC and
Correa RG: Proteome profiling of triple negative breast cancer
cells overexpressing NOD1 and NOD2 receptors unveils molecular
signatures of malignant cell proliferation. BMC Genomics.
20:1522019. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Miao H, Wang L, Zhan H, Dai J, Chang Y, Wu
F, Liu T, Liu Z, Gao C, Li L and Song X: A long noncoding RNA
distributed in both nucleus and cytoplasm operates in the
PYCARD-regulated apoptosis by coordinating the epigenetic and
translational regulation. PLoS Genet. 15:e10081442019. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Faria SS, Costantini S, de Lima VCC, de
Andrade VP, Rialland M, Cedric R, Budillon A and Magalhães KG:
NLRP3 inflammasome-mediated cytokine production and pyroptosis cell
death in breast cancer. J Biomed Sci. 28:262021. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Siersbæk R, Scabia V, Nagarajan S,
Chernukhin I, Papachristou EK, Broome R, Johnston SJ, Joosten SEP,
Green AR, Kumar S, et al: IL6/STAT3 signaling hijacks estrogen
receptor α enhancers to drive breast cancer metastasis. Cancer
Cell. 38:412–423.e9. 2020. View Article : Google Scholar
|
|
130
|
Wei Y, Huang H, Qiu Z, Li H, Tan J, Ren G
and Wang X: NLRP1 overexpression is correlated with the
tumorigenesis and proliferation of human breast tumor. Biomed Res
Int. 2017:49384732017. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Tan Y, Sun R, Liu L, Yang D, Xiang Q, Li
L, Tang J, Qiu Z, Peng W, Wang Y, et al: Tumor suppressor DRD2
facilitates M1 macrophages and restricts NF-κB signaling to trigger
pyroptosis in breast cancer. Theranostics. 11:5214–5231. 2021.
View Article : Google Scholar :
|
|
132
|
Zhang Z, Zhang H, Li D, Zhou X, Qin Q and
Zhang Q: Caspase-3-mediated GSDME induced Pyroptosis in breast
cancer cells through the ROS/JNK signalling pathway. J Cell Mol
Med. 25:8159–8168. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
An H, Heo JS, Kim P, Lian Z, Lee S, Park
J, Hong E, Pang K, Park Y, Ooshima A, et al: Tetraarsenic hexoxide
enhances generation of mitochondrial ROS to promote pyroptosis by
inducing the activation of caspase-3/GSDME in triple-negative
breast cancer cells. Cell Death Dis. 12:1592021. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Ma JH, Qin L and Li X: Role of STAT3
signaling pathway in breast cancer. Cell Commun Signal. 18:332020.
View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Wu L, Bai S, Huang J, Cui G, Li Q, Wang J,
Du X, Fu W, Li C, Wei W, et al: Nigericin boosts anti-tumor immune
response via inducing pyroptosis in triple-negative breast cancer.
Cancers (Basel). 15:32212023. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Wang H, Rong X, Zhao G, Zhou Y, Xiao Y, Ma
D, Jin X, Wu Y, Yan Y, Yang H, et al: The microbial metabolite
trimethylamine N-oxide promotes antitumor immunity in
triple-negative breast cancer. Cell Metab. 34:581–594.e8. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Lei S, Li S, Xiao W, Jiang Q, Yan S, Xiao
W, Cai J, Wang J, Zou L, Chen F, et al: Azurocidin 1 inhibits the
aberrant proliferation of triple-negative breast cancer through the
regulation of pyroptosis. Oncol Rep. 50:1882023. View Article : Google Scholar
|
|
138
|
Pizato N, Luzete BC, Kiffer LFMV, Corrêa
LH, de Oliveira Santos I, Assumpção JAF, Ito MK and Magalhães KG:
Omega-3 docosahexaenoic acid induces pyroptosis cell death in
triple-negative breast cancer cells. Sci Rep. 8:19522018.
View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Chen C, Yuan S, Chen X, Xie J and Wei Z:
Xihuang pill induces pyroptosis and inhibits progression of breast
cancer cells via activating the cAMP/PKA signalling pathway. Am J
Cancer Res. 13:1347–1362. 2023.PubMed/NCBI
|
|
140
|
Li Y, Wang W, Li A, Huang W, Chen S, Han F
and Wang L: Dihydroartemisinin induces pyroptosis by promoting the
AIM2/caspase-3/DFNA5 axis in breast cancer cells. Chem Biol
Interact. 340:1094342021. View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Zhong C, Li Y, Li W, Lian S, Li Y, Wu C,
Zhang K, Zhou G, Wang W, Xu H, et al: Ganoderma lucidum extract
promotes tumor cell pyroptosis and inhibits metastasis in breast
cancer. Food Chem Toxicol. 174:1136542023. View Article : Google Scholar : PubMed/NCBI
|
|
142
|
Zhao P, Wang M, Chen M, Chen Z, Peng X,
Zhou F, Song J and Qu J: Programming cell pyroptosis with
biomimetic nanoparticles for solid tumor immunotherapy.
Biomaterials. 254:1201422020. View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Li L, Tian H, Zhang Z, Ding N, He K, Lu S,
Liu R, Wu P, Wang Y, He B, et al: Carrier-Free nanoplatform via
evoking pyroptosis and immune response against breast cancer. ACS
Appl Mater Interfaces. 15:452–468. 2023. View Article : Google Scholar
|
|
144
|
Li C, Wang X, Chen T, Li W, Zhou X, Wang L
and Yang Q: Huaier induces immunogenic cell death via
CircCLASP1/PKR/eIF2α signaling pathway in triple negative breast
cancer. Front Cell Dev Biol. 10:9138242022. View Article : Google Scholar
|
|
145
|
Yamamoto A, Huang Y, Krajina BA, McBirney
M, Doak AE, Qu S, Wang CL, Haffner MC and Cheung KJ: Metastasis
from the tumor interior and necrotic core formation are regulated
by breast cancer-derived angiopoietin-like 7. Proc Natl Acad Sci
USA. 120:e22148881202023. View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Wen N, Lv Q and Du ZG: MicroRNAs involved
in drug resistance of breast cancer by regulating autophagy. J
Zhejiang Univ Sci B. 21:690–702. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
147
|
Wu Q and Sharma D: Autophagy and breast
cancer: connected in growth, progression, and therapy. Cells.
12:11562023. View Article : Google Scholar : PubMed/NCBI
|
|
148
|
Tadokoro T, Ikeda M, Ide T, Deguchi H,
Ikeda S, Okabe K, Ishikita A, Matsushima S, Koumura T, Yamada KI,
et al: Mitochondria-dependent ferroptosis plays a pivotal role in
doxorubicin cardiotoxicity. JCI Insight. 8:e1697562023. View Article : Google Scholar : PubMed/NCBI
|
|
149
|
Wang Y, Shi P, Chen Q, Huang Z, Zou D,
Zhang J, Gao X and Lin Z: Mitochondrial ROS promote macrophage
pyroptosis by inducing GSDMD oxidation. J Mol Cell Biol.
11:1069–1082. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
150
|
Dai S, Chen Y, Fan X, Han J, Zhong L,
Zhang Y, Liu Q, Lin J, Huang W, Su L, et al: Emodin attenuates
cardiomyocyte pyroptosis in doxorubicin-induced cardiotoxicity by
directly binding to GSDMD. Phytomedicine. 121:1551052023.
View Article : Google Scholar : PubMed/NCBI
|
|
151
|
Lu S, Tian H, Li B, Li L, Jiang H, Gao Y,
Zheng L, Huang C, Zhou Y, Du Z and Xu J: An ellagic acid
coordinated copper-based nanoplatform for efficiently overcoming
cancer chemoresistance by cuproptosis and synergistic inhibition of
cancer cell stemness. Small. Dec 3–2023.Epub ahead of print.
|
|
152
|
Zhong Y, Peng Z, Peng Y, Li B, Pan Y,
Ouyang Q, Sakiyama H, Muddassir M and Liu J: Construction of
Fe-doped ZIF-8/DOX nanocomposites for ferroptosis strategy in the
treatment of breast cancer. J Mater Chem B. 11:6335–6345. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
153
|
Wang J, Li Y, Zhang J and Luo C:
Isoliquiritin modulates ferroptosis via NF-κB signaling inhibition
and alleviates doxorubicin resistance in breast cancer.
Immunopharmacol Immunotoxicol. 45:443–454. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
154
|
Yu R, Wang L, Ji X and Mao C: SBP-0636457,
a novel smac mimetic, cooperates with doxorubicin to induce
necroptosis in breast cancer cells during apoptosis blockage. J
Oncol. 2022:23900782022. View Article : Google Scholar : PubMed/NCBI
|
|
155
|
Wei T, Xiaojun X and Peilong C:
Magnoflorine improves sensitivity to doxorubicin (DOX) of breast
cancer cells via inducing apoptosis and autophagy through AKT/mTOR
and p38 signaling pathways. Biomed Pharmacother. 121:1091392020.
View Article : Google Scholar
|
|
156
|
Lu Y, Pan Q, Gao W, Pu Y and He B:
Reversal of cisplatin chemotherapy resistance by
glutathione-resistant copper-based nanomedicine via cuproptosis. J
Mater Chem B. 10:6296–6306. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
157
|
Roh JL, Kim EH, Jang HJ, Park JY and Shin
D: Induction of ferroptotic cell death for overcoming cisplatin
resistance of head and neck cancer. Cancer Lett. 381:96–103. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
158
|
Wang Y, Pang X, Liu Y, Mu G and Wang Q:
SOCS1 acts as a ferroptosis driver to inhibit the progression and
chemotherapy resistance of triple-negative breast cancer.
Carcinogenesis. 44:708–715. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
159
|
Zhang X, Sui S, Wang L, Li H, Zhang L, Xu
S and Zheng X: Inhibition of tumor propellant glutathione
peroxidase 4 induces ferroptosis in cancer cells and enhances
anticancer effect of cisplatin. J Cell Physiol. 235:3425–3437.
2020. View Article : Google Scholar
|
|
160
|
Yan H, Luo B, Wu X, Guan F, Yu X, Zhao L,
Ke X, Wu J and Yuan J: Cisplatin induces pyroptosis via activation
of MEG3/NLRP3/caspase-1/GSDMD pathway in triple-negative breast
cancer. Int J Biol Sci. 17:2606–2621. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
161
|
Shen M, Duan WM, Wu MY, Wang WJ, Liu L, Xu
MD, Zhu J, Li DM, Gui Q, Lian L, et al: Participation of autophagy
in the cytotoxicity against breast cancer cells by cisplatin. Oncol
Rep. 34:359–367. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
162
|
Peng F, Liao M, Qin R, Zhu S, Peng C, Fu
L, Chen Y and Han B: Regulated cell death (RCD) in cancer: Key
pathways and targeted therapies. Signal Transduct Target Ther.
7:2862022. View Article : Google Scholar : PubMed/NCBI
|
|
163
|
Li J, Cao F, Yin HL, Huang ZJ, Lin ZT, Mao
N, Sun B and Wang G: Ferroptosis: Past, present and future. Cell
Death Dis. 11:882020. View Article : Google Scholar : PubMed/NCBI
|
|
164
|
Shi J, Gao W and Shao F: Pyroptosis:
Gasdermin-Mediated programmed necrotic cell death. Trends Biochem
Sci. 42:245–254. 2017. View Article : Google Scholar
|
|
165
|
Song L, Zeng R, Yang K, Liu W, Xu Z and
Kang F: The biological significance of cuproptosis-key gene MTF1 in
pan-cancer and its inhibitory effects on ROS-mediated cell death of
liver hepatocellular carcinoma. Discov Oncol. 14:1132023.
View Article : Google Scholar : PubMed/NCBI
|
|
166
|
Zheng D, Liu J, Piao H, Zhu Z, Wei R and
Liu K: ROS-triggered endothelial cell death mechanisms: Focus on
pyroptosis, parthanatos, and ferroptosis. Front Immunol.
13:10392412022. View Article : Google Scholar : PubMed/NCBI
|
|
167
|
Abais JM, Xia M, Zhang Y, Boini KM and Li
PL: Redox regulation of NLRP3 inflammasomes: ROS as trigger or
effector? Antioxid Redox Signal. 22:1111–1129. 2015. View Article : Google Scholar :
|
|
168
|
Li C and Zhang Y: Construction and
validation of a cuproptosis-related five-lncRNA signature for
predicting prognosis, immune response and drug sensitivity in
breast cancer. BMC Med Genomics. 16:1582023. View Article : Google Scholar : PubMed/NCBI
|
|
169
|
Xu Z, Wang X, Sun W, Xu F, Kou H, Hu W,
Zhang Y, Jiang Q, Tang J and Xu Y: RelB-activated GPX4 inhibits
ferroptosis and confers tamoxifen resistance in breast cancer.
Redox Biol. 68:1029522023. View Article : Google Scholar : PubMed/NCBI
|
|
170
|
Xu W, Song C, Wang X, Li Y, Bai X, Liang
X, Wu J and Liu J: Downregulation of miR-155-5p enhances the
anti-tumor effect of cetuximab on triple-negative breast cancer
cells via inducing cell apoptosis and pyroptosis. Aging (Albany
NY). 13:228–240. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
171
|
Shen Y, Li D, Liang Q, Yang M, Pan Y and
Li H: Cross-talk between cuproptosis and ferroptosis regulators
defines the tumor microenvironment for the prediction of prognosis
and therapies in lung adenocarcinoma. Front Immunol.
13:10290922022. View Article : Google Scholar
|
|
172
|
Yang X, Deng L, Diao X, Yang S, Zou L,
Yang Q, Li J, Nie J, Zhao L and Jiao B: Targeting cuproptosis by
zinc pyrithione in triple-negative breast cancer. iScience.
26:1082182023. View Article : Google Scholar : PubMed/NCBI
|
|
173
|
Li Y, Li T, Zhai D, Xie C, Kuang X, Lin Y
and Shao N: Quantification of ferroptosis pathway status revealed
heterogeneity in breast cancer patients with distinct immune
microenvironment. Front Oncol. 12:9569992022. View Article : Google Scholar : PubMed/NCBI
|
|
174
|
Wu Y, Lin Z, Tang X, Tong Z, Ji Y, Xu Y,
Zhou Z, Yang J, Li Z and Liu T: Ferroptosis-related gene HIC1 in
the prediction of the prognosis and immunotherapeutic efficacy with
immunological activity. Front Immunol. 14:11820302023. View Article : Google Scholar : PubMed/NCBI
|
|
175
|
Zeng L, Ding S, Cao Y, Li C, Zhao B, Ma Z,
Zhou J, Hu Y, Zhang X, Yang Y, et al: A MOF-Based potent
ferroptosis inducer for enhanced radiotherapy of triple negative
breast cancer. ACS Nano. 17:13195–13210. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
176
|
Yang X, Weng X, Yang Y and Jiang Z:
Pyroptosis-Related lncRNAs predict the prognosis and immune
response in patients with breast cancer. Front Genet.
12:7921062021. View Article : Google Scholar
|
|
177
|
Huang QF, Fang DL, Nong BB and Zeng J:
Focal pyroptosis-related genes AIM2 and ZBP1 are prognostic markers
for triple-negative breast cancer with brain metastases. Transl
Cancer Res. 10:4845–4858. 2021. View Article : Google Scholar
|
|
178
|
Ma L, Bian M, Gao H, Zhou Z and Yi W: A
novel 3-acyl isoquinolin-1(2H)-one induces G2 phase arrest,
apoptosis and GSDME-dependent pyroptosis in breast cancer. PLoS
One. 17:e02680602022. View Article : Google Scholar : PubMed/NCBI
|