Introduction
In 2020, ~770,000 patients succumbed due to gastric
cancer (GC), rendering GC the fourth most common cause of
cancer-related mortality worldwide, surpassed only by lung,
colorectal and liver cancers (1). As
GC exhibits histological and genetic heterogeneity, the development
of biomarker-based molecularly targeted therapeutics has lagged
behind that of other cancer types (2). However, The Cancer Genome Atlas
research program has succeeded in characterizing GC at the genomic
level, yielding a system for classifying GC into four molecular
subtypes according to the Epstein-Barr virus-positive status,
microsatellite instability, genomic stability and chromosomal
instability (CIN), with the aim of simplifying the treatment and
diagnosis of GC (2,3).
The CIN subtype accounts for ~50% of GC cases and is
characterized by the amplification of receptor tyrosine kinase
(RTK) genes, a high frequency of TP53 mutations (in 70% of
cases) and CIN, which is indicated by a high frequency of
aneuploidy (4,5). In addition, the CIN subtype is known to
exhibit intratumoral heterogeneity, which is involved in tumor
relapse owing to the acquisition of cellular insensitivity to
targeted drugs (6-8).
Therefore, elucidating the molecular mechanisms of CIN in GC is of
utmost therapeutic importance. However, these mechanisms are not
yet fully understood.
The kinetochore-associated 1 (KNTC1) gene
encodes kinetochore-associated protein 1 (KNTC1), a protein
component of the outer kinetochore that is essential for the
association of chromosomes and spindle microtubules. KNTC1 forms a
complex with ZW10 and ZWILCH during mitosis. This complex, which is
known as the RZZ complex, is involved in the activation of the
spindle assembly checkpoint (SAC), the kinetochore-dependent
recruitment of the Mad1/Mad3 and dynein/dynactin complex and the
formation of the kinetochore corona in the outermost layer of the
kinetochore (9-14).
In particular, the activation of the SAC delays anaphase when there
is a lack of proper connection between kinetochores and spindle
microtubules, allowing for homogenous chromosome segregation
(15,16). The depletion or loss of function of
various kinetochore proteins, including KNTC1, has been reported to
cause lagging chromosomes, resulting in abnormal chromosome
segregation and subsequent aneuploidy and CIN in Drosophila
and Caenorhabditis elegans (16-18). However, the role of the
KNTC1 gene in GC is poorly understood.
It was hypothesized that the abnormal function of
KNTC1 may be associated with the mechanism of CIN in GC.
Therefore, the present study investigated the role of KNTC1
in GC CIN.
Materials and methods
Cell lines and culture conditions
The human GC cell lines, NCI-N87 (exhibiting human
epidermal growth factor receptor type 2 gene amplification; cat.
no. CRL-5822, American Type Culture Collection), KATOIII
[exhibiting fibroblast growth factor receptor type 2 gene
amplification; cat. no. JCRB0611, Japan Collection of Research
Bioresources (JCRB) Cell Bank] and MKN74 (without amplification of
RTK genes; cat. no. JCRB0255, JCRB), and the human normal
fibroblast cell line, TIG-1-20 (cat. no. JCRB0501, JCRB), were used
in the present study. The cells were cultured in RPMI-1640 medium
(cat. no. 30264-56; Nacalai Tesque, Inc.) containing a 10% fetal
bovine serum and 0.5% penicillin and streptomycin mixture (cat. no.
09367-34; Nacalai Tesque, Inc.) at 37˚C in an atmosphere containing
5% CO2. The cell cultures were grown in a CO2
incubator (cat. no. MHE-S1301A2-PJ; PHC Holdings Corporation).
Measurement of the frequency of
lagging chromosomes
The NCI-N87, KATOIII, MKN74 and TIG-1-20 cells were
seeded into 4-well culture slides (cat. no. 192-004; Watson Bio
Lab) and cultured for 24 h at 37˚C. After 24 h, the cells were
transfected with small interfering RNA (siRNA) targeting
KNTC1, as described below. Following transfection, the cells
are incubated for an additional 3 days and fixed for 20 min at room
temperature with 4% paraformaldehyde (cat. no. 006775-1L; Bioenno
Tech, LLC). The slides were washed twice for 5 min each with
phosphate-buffered saline (cat. no. 73111; Kanto Chemical Co.) and
sealed using coverslips and VECTASHIELD Vibrance Antifade Mounting
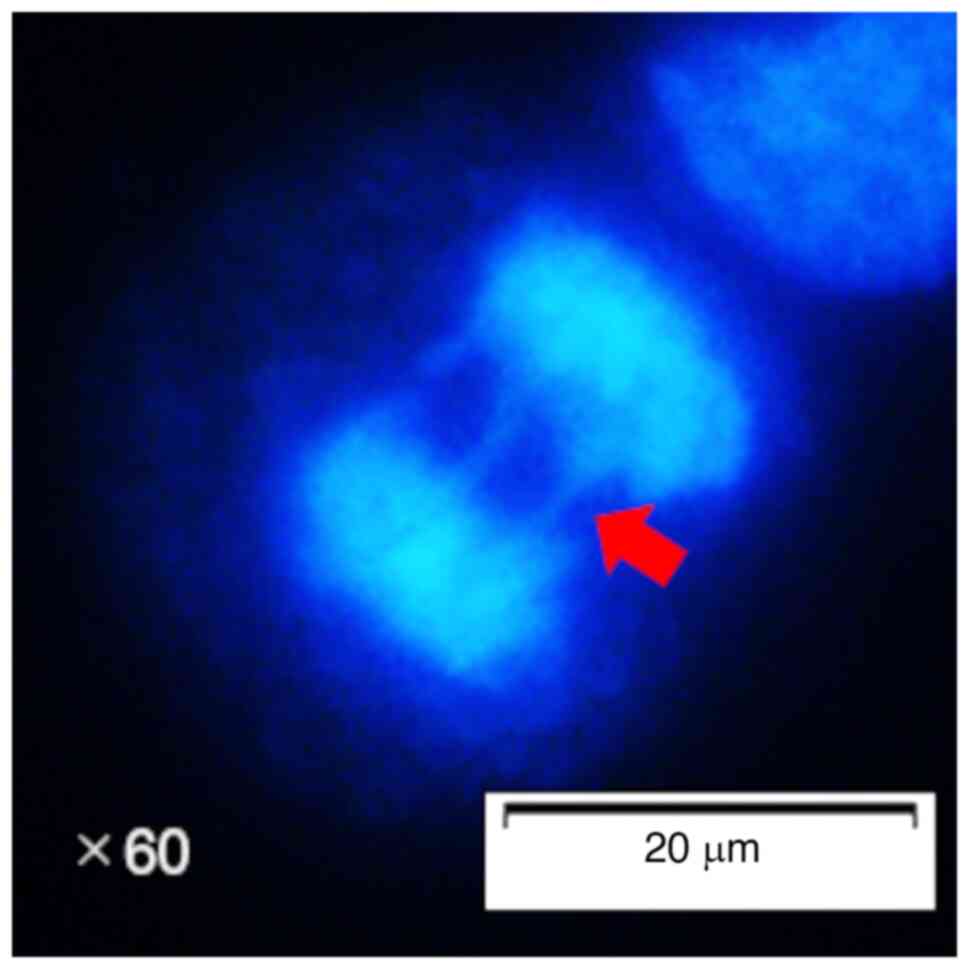
Medium with DAPI (cat. no. H-1800; Vector Laboratories, Inc.). A
total of 50 cells in anaphase, defined according to visible sister
chromatid separation, were then observed using a fluorescence
microscope (BX53F; Olympus Corporation). Among these 50 cells, the
number of lagging chromosomes was counted (Figs. 1 and S1), and the percentage was calculated.
Reverse transcriptionquantitative
polymerase chain reaction (RTqPCR)
Total RNA was extracted from the NCI-N87, KATOIII,
MKN74 and TIG-1-20 cells using an RNeasy mini kit (cat. no. 74104;
Qiagen, Inc.). cDNA synthesis was performed using reverse
transcription with Superscript Ⅳ VILO Master Mix with ezDNase (cat.
no. 11766050; Invitrogen; Thermo Fisher Scientific, Inc.). cDNA
synthesis reaction was performed at 25˚C for 10 min, 50˚C for 10
min, and 85˚C for 5 min. KNTC1 (Hs00938554_m1) and
GAPDH (No. 1902206) primers were obtained from Thermo Fisher
Scientific, Inc. (primer sequence information not available). qPCR
was performed using TaqMan Fast Advanced Master Mix (cat. no.
4444556; Applied Biosystems; Thermo Fisher Scientific, Inc.) using
the following reaction conditions: An initial denaturation at 95˚C
for 20 sec, then 40 cycles at 95˚C for 3 sec and 60˚C for 30 sec.
mRNA expression was analyzed using the 2-ΔΔCq
calculation (19).
Western blot analysis
The NCI-N87, KATOIII, MKN74 and TIG-1-20 cells were
lysed in RIPA buffer (cat. no. 08714-04; Nacalai Tesque, Inc.). The
cell lysates were then treated using ultrasound and centrifuged at
20,630 x g for 10 min at room temperature. The lysates were mixed
with sample buffer (cat. no. 30566-22; Nacalai Tesque, Inc.) with
2-mercapto ethanol and incubated at 100˚C for 3 min. Lysates
containing 5-7 µg protein were separated by 10% sodium dodecyl
sulfate-polyacrylamide gel electrophoresis using Mini
Trans-Blot® Cell (cat. no. 153BR78145; Bio-Rad
Laboratories, Inc.). The separated proteins were then transferred
from the gel to polyvinylidene fluoride membranes (cat. no.
1704272; Bio-Rad Laboratories, Inc.) using the
Trans-Blot®TurboTM System (cat. no.
690BR009070; Bio-Rad Laboratories, Inc.). The membranes were
blocked for 5 min at room temperature with Every Blot blocking
buffer (cat. no. 12010020; Bio-Rad Laboratories, Inc.) and
incubated with anti-ZW10 (1:1,000; cat. no. 24561-1-AP,
Proteintech, Inc.) and anti-α-tubulin (1:5,000; cat. no. 3873,
clone DM1A, Cell Signaling Technology, Inc.) antibodies overnight
at 4˚C. Following incubation, the membranes were washed in
Tris-buffered saline containing 0.1% Tween-20 (TBS-T). The
membranes were then incubated with appropriate secondary antibodies
(1:5,000; anti-mouse IgG, cat. no. 7076, Cell Signaling Technology,
Inc. and 1:5,000; anti-rabbit IgG, cat. no. 7074, Cell Signaling
Technology, Inc.) for 1 h at room temperature and washed with
TBS-T. Protein bands were detected using a ChemiDoc MP Imaging
System (Bio-Rad Laboratories, Inc.). Quantitative analysis was
performed using ImageLab software version 4.1 (Bio-Rad
Laboratories, Inc.).
siRNA-mediated knockdown of KNTC1
The sequence (GGAAUGAUAUUGAGCUGCUAACAAA) of human
KNTC1 siRNA was designed from Thermo Fisher Scientific, Inc.
(cat. no. HSS114610). KNTC1 siRNA or negative control (cat.
no. 12935-300; Invitrogen; Thermo Fisher Scientific, Inc.) was
combined with Lipofectamine RNAiMAX (cat. no. 13778-030;
Invitrogen; Thermo Fisher Scientific, Inc.) and incubated for 15
min at room temperature. The NCI-N87, KATOIII, MKN74 and TIG-1-20
cells were transfected with the KNTC1 siRNA or negative
control-Lipofectamine RNAiMAX complexes and incubated at 37˚C for 3
days. At 3 days following the addition of the KNTC1 siRNA or
negative control-Lipofectamine RNAiMAX complexes, the cells were
harvested and processed for RT-qPCR and western blot analysis, and
for the analysis of the frequency of lagging chromosomes.
Statistical analysis
All the statistical analyses were performed using
Statcel 4 (https://oms-publ.main.jp/main/4steps4-hyo1/). The
one-way ANOVA and Tukey-Kramer test were performed to assess
differences in frequency of lagging chromosomes and KNTC1
mRNA expression. The Student's t test was used to assess the
efficiency of KNTC1 knockdown and the frequency of lagging
chromosomes and ZW10 protein expression following KNTC1
knockdown. Data are presented as the mean ± standard deviation.
P-values #x003C;0.05 were considered to indicate statistically
significant differences.
Results
Frequency of lagging chromosomes and
expression levels of KNTC1 mRNA
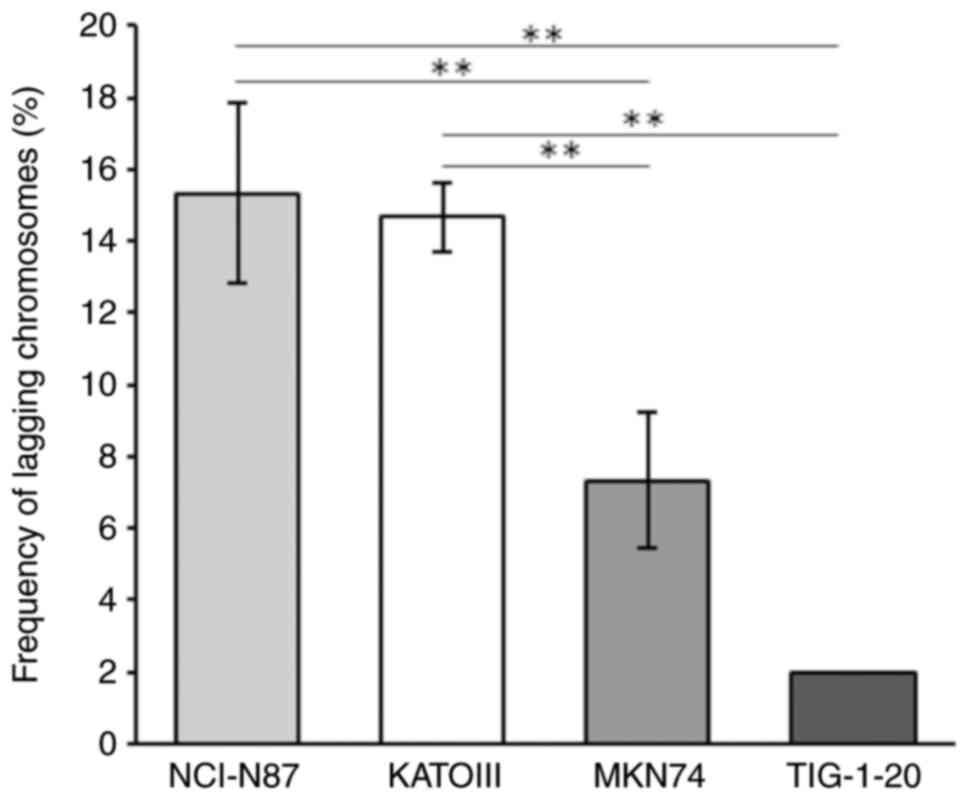
To investigate the CIN status in GC cells, the
frequency of lagging chromosomes was analyzed. The frequency of
lagging chromosomes was significantly higher in the NCI-N87 and
KATOIII cells, which exhibited the amplification of RTK genes, than
in the MKN74 cells, which did not exhibit the amplification of RTK
genes (Fig. 2). Only a small number
of lagging chromosomes were observed in the TIG-1-20 cells.
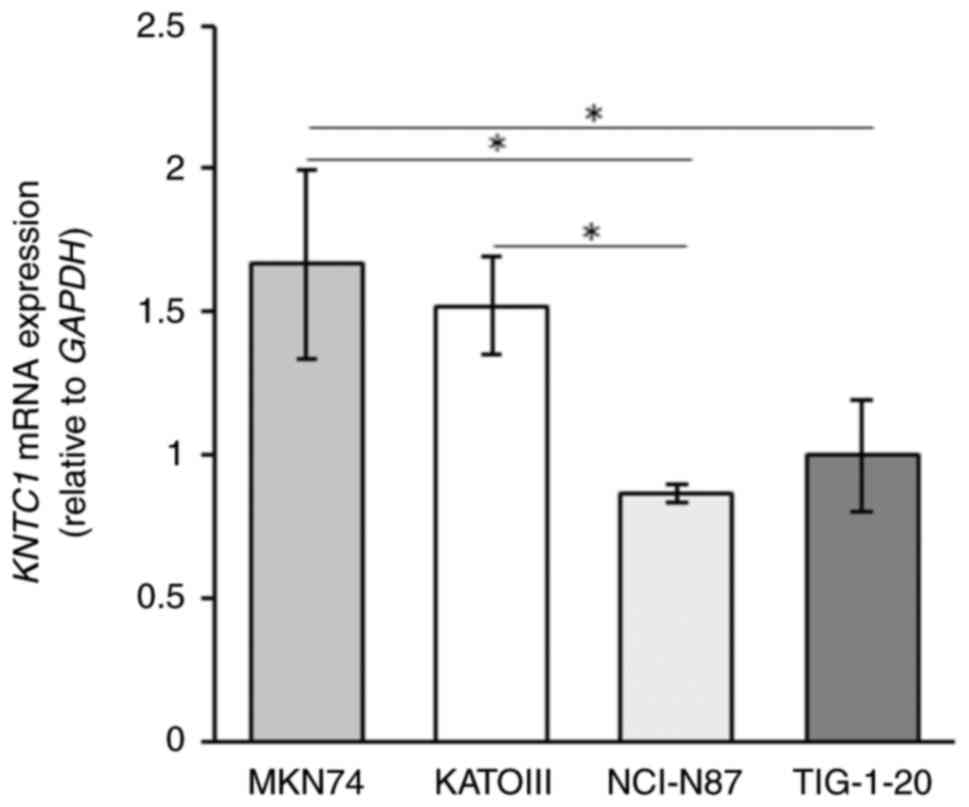
The expression levels of KNTC1 mRNA were
higher in the MKN74 cells than in the NCI-N87 and KATOIII cells,
and were inversely associated with the frequency of lagging
chromosomes (Fig. 3). In addition,
the mRNA expression level of KNTC1 in the NCI-N87 cells was
significantly lower than that in TIG-1-20 cells.
Frequency of lagging chromosomes and
expression of ZW10 following KNTC1 knockdown
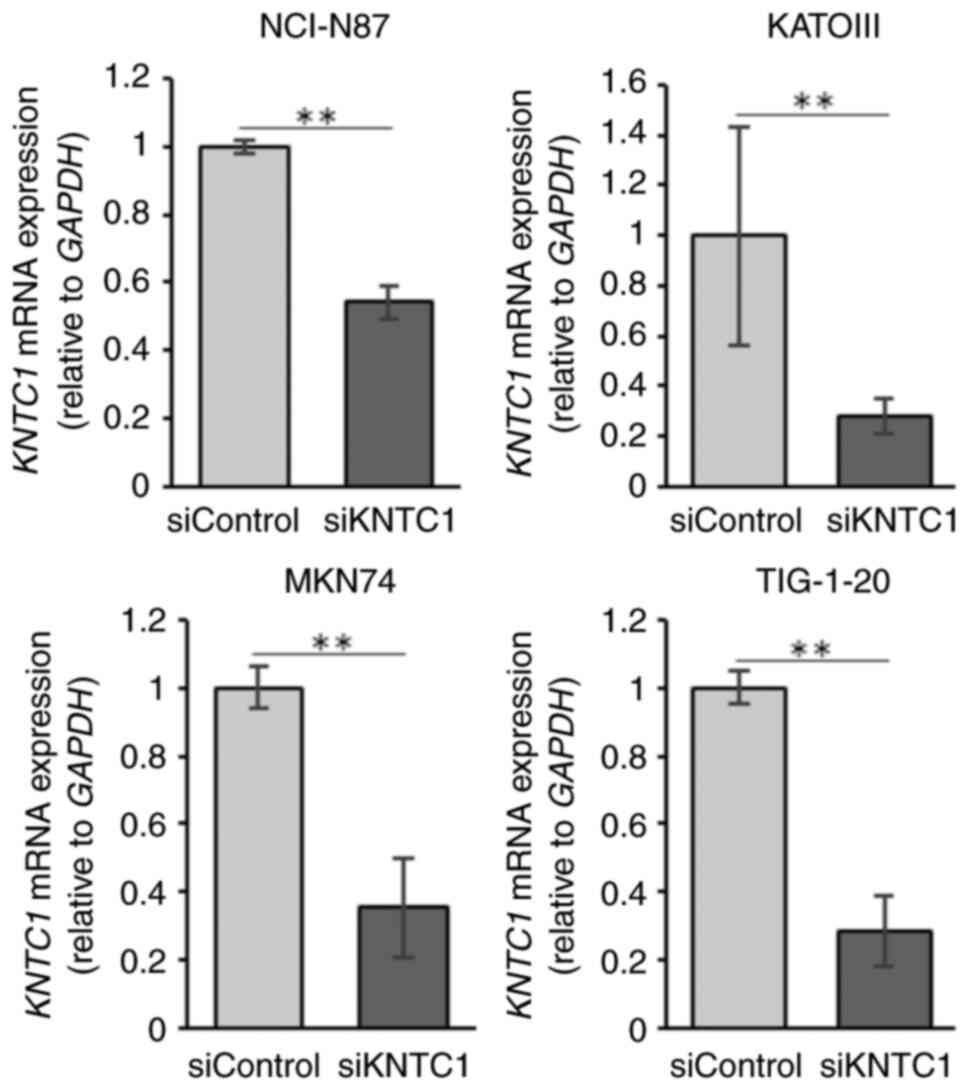
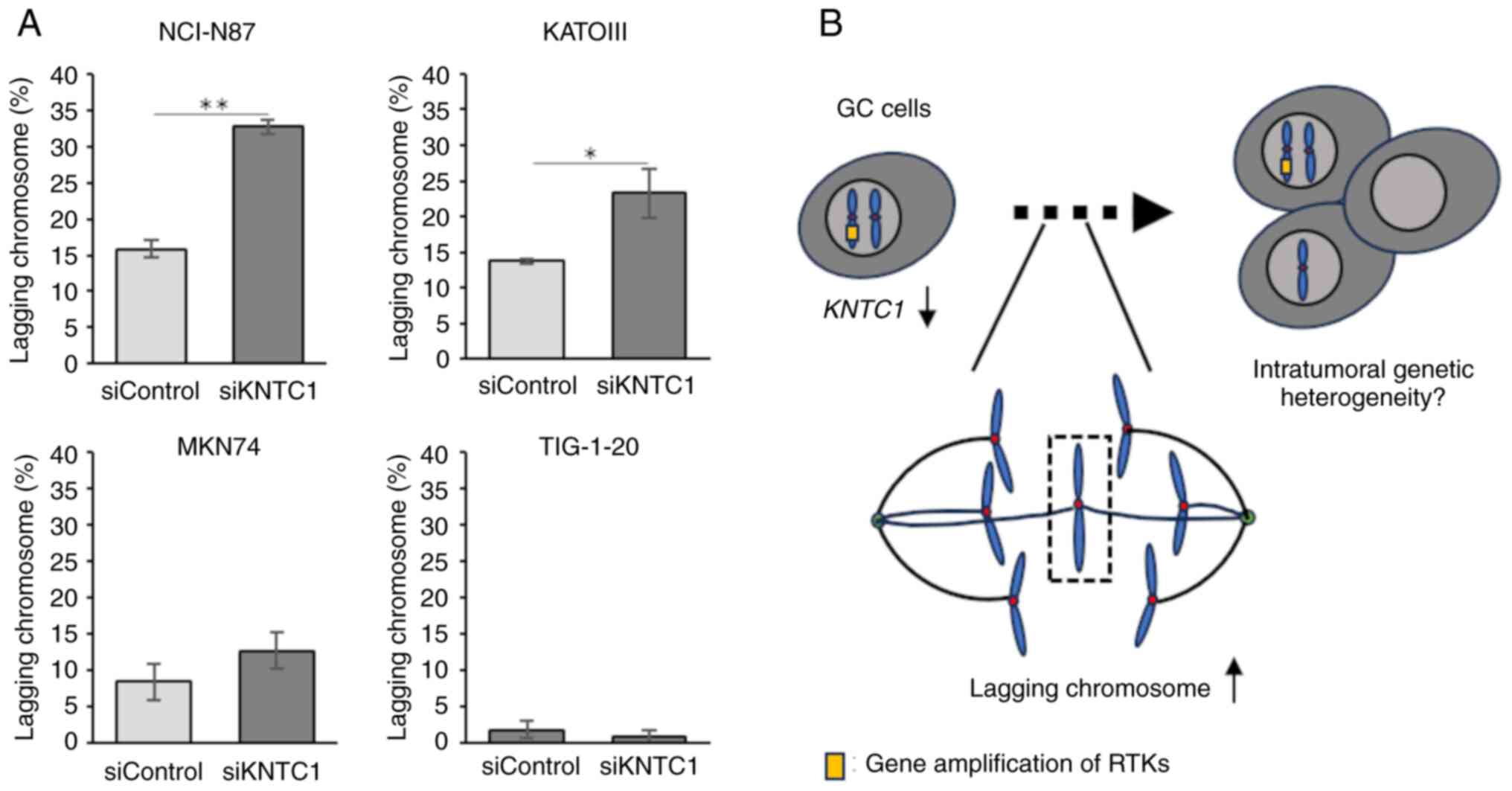
The present study then investigated whether the
knockdown of KNTC1 increased the frequency of lagging
chromosomes. The confirmation of the knockdown efficiency
KNTC1 siRNA was performed using RT-qPCR (Fig. 4). The NCI-N87, KATOIII and MKN74
cells exhibited increased frequencies of lagging chromosomes
following the knockdown of KNTC1 (Fig. 5A). These differences were
statistically significant in the NCI-N87 and KATOIII cells. By
contrast, no increases in lagging chromosomes were observed in the
TIG-1-20 cells following the knockdown of KNTC1.
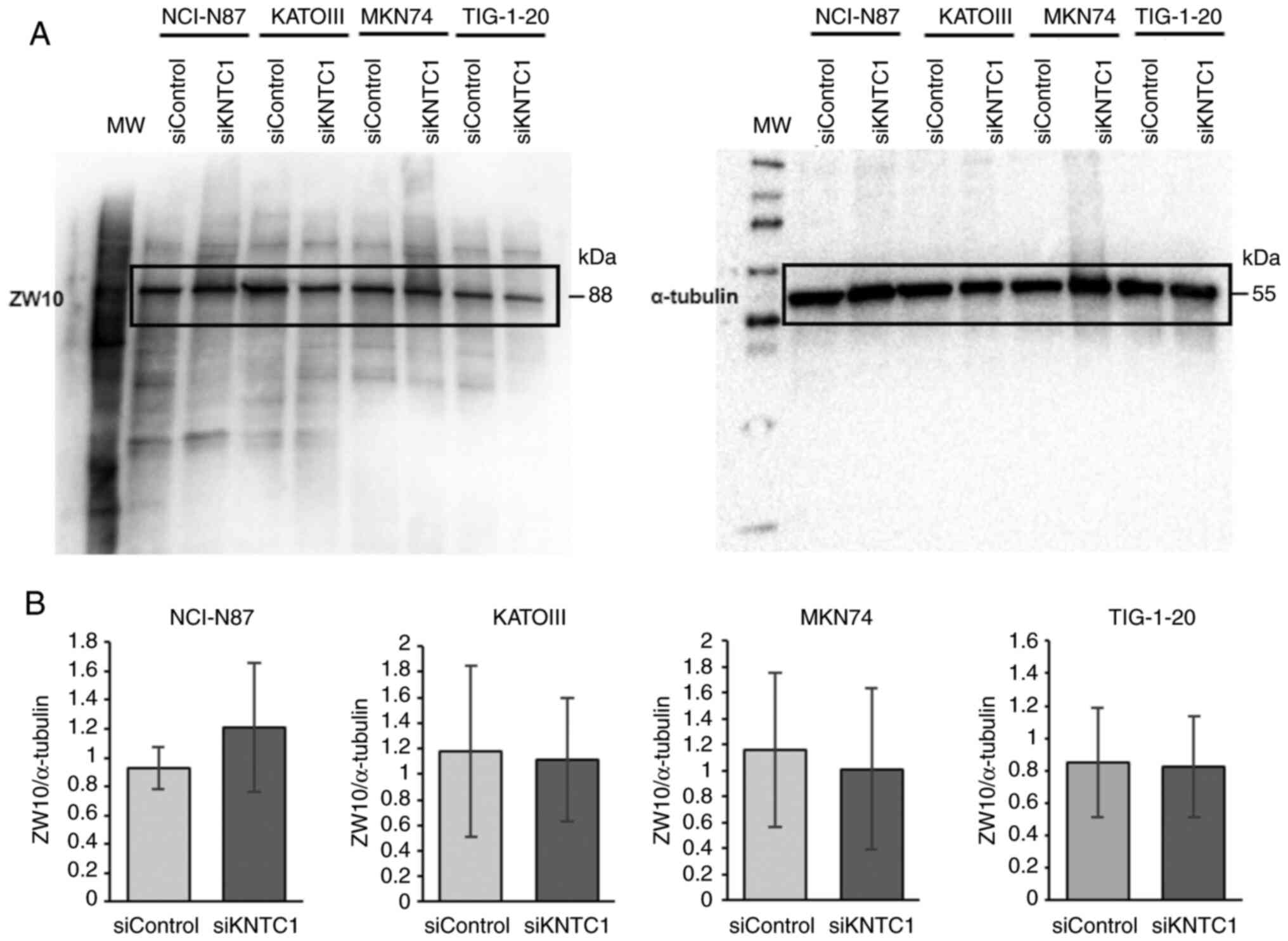
In addition, to determine whether ZW10 compensated
for KNTC1 function, ZW10 protein expression was investigated
following KNTC1 knockdown. However, no changes in ZW10
protein expression were observed in any of the cell lines tested
(Fig. 6).
Discussion
Deletion or loss of function of various kinetochore
proteins, including KNTC1, has been reported to cause chromosome
segregation abnormalities and induce aneuploidy and CIN in
Drosophila and C. elegans (16-18). However, the role
of KNTC1 in GC CIN is poorly understood. In the present
study, it was found that GC cells with the amplification of RTK
genes, including NCI-N87 and KATOIII cells, exhibited lower mRNA
expression levels of KNTC1 and KNTC1 expression
exhibited an inverse association with the frequency of lagging
chromosomes (Figs. 2 and 3). Moreover, the frequency of lagging
chromosomes in the NCI-N87, KATOIII and MKN74 cells increased
following the knockdown of KNTC1 (Fig. 5A). These findings suggest that the
suppression of the expression of the KNTC1 gene may
contribute to CIN in GC. In particular, suppression of the
expression of KNTC1 in GC cells exhibiting the amplification
of RTK genes may enhance CIN and could lead to intratumoral genetic
heterogeneity (Fig. 5B). Therefore,
the restoration of normal KNTC1 expression levels may
improve patient prognosis by alleviating intratumoral genetic
heterogeneity through appropriate kinetochore-microtubule
attachments.
ZW10 is located in the cytoplasm and endoplasmic
reticulum (ER) during the interphase and is involved in transport
between the ER and Golgi apparatus (20). During mitosis, ZW10 and KNTC1 are
recruited to the kinetochore, where they form the RZZ complex with
ZWILCH (21). When KNTC1 is present,
the RZZ complex activates the SAC and recruits dynein/dynactin
during mitosis. Therefore, it was hypothesized that ZW10 may
compensate for the loss of function of KNTC1. However, ZW10 protein
expression levels were not altered when KNTC1 was knocked
down in all cell lines (Fig. 6).
This finding is consistent with the findings of a previous study
demonstrating that ZW10 protein levels were not altered by the
expression of a mutant KNTC1 gene, which severely affected
the localization of ZW10 in Drosophila (22); this suggests that ZW10 does not
compensate for the function of KNTC1. This finding also suggests
that there may be no association between ZW10 and CIN.
The overexpression of the KNTC1 gene has been
observed in several types of cancer, suggesting that KNTC1
promotes cell proliferation and viability (23-25).
However, in the present study, no inhibition of cell proliferation
or increase in apoptotic bodies were observed following
KNTC1 knockdown in KATOIII or MKN74 cells (Data S1 and Fig. S2), although increases in lagging
chromosomes were observed. In the NCI-N87 cells, differences in
cell proliferation were observed at 72 h following KNTC1
knockdown, but no apoptosis was observed. This difference suggests
that the role of the KNTC1 gene may vary by cancer type. In
fact, mRNA expression data from The Human Protein Atlas revealed
poor survival rates of patients with GC exhibiting a low expression
of KNTC1 (Data S1 and
Fig. S3). Previous findings that
the suppression of the expression of the KNTC1 gene
contributes to CIN may support these data (26-28).
In the future, additional large-scale studies using clinical
samples are warranted to clarify the association between the
KNTC1 gene and CIN and its causal association with patient
prognosis.
In conclusion, the present study demonstrated that
the knockdown of KNTC1 increased the frequency of lagging
chromosomes in GC cells. These finding suggest that the suppression
of the expression of KNTC1 may contribute to CIN in GC.
Supplementary Material
Giemsa-stained image of the lagging
chromosome in NCI-N87 cells. Chromosomes remained on the metaphase
plate and formed bridges in anaphase (red arrows).
Cell proliferation following
KNTC1 knockdown. (A) No decrease in cell proliferation was
observed in the MKN74, KATOIII and TIG-1-20 cells following
KNTC1 knockdown, whereas a decrease in cell proliferation
was observed in the NCI-N87 cells. All data were analyzed using the
Student's t-test. *P<0.05 vs. siControl. (B) No
increase in apoptosis was observed following KNTC1 knockdown
in all four cell lines. DAPI staining; 60X objective. KNTC1,
kinetochore-associated 1 gene.
Kaplan-Meier plot showing KNTC1
mRNA expression levels and outcomes of prognoses in patients with
gastric cancer. In gastric cancer, a low KNTC1 expression
was found to be associated with a poor prognosis. P=0.047, low
expression vs. high expression. Kaplan-Meier plots were obtained
from The Human Protein Atlas version 23.0) (https://www.proteinatlas.org/ENSG00000184445-KNTC1/cancer/stomach+cancer#STAD_TCGA).
KNTC1, kinetochore-associated 1 gene.
Supplementary materials and
methods
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
KK conceptualized the study. DO analyzed the data
and wrote the original draft of the manuscript. KK reviewed and
edited the original draft of the manuscript. KK and DO confirm the
authenticity of all the raw data. Both authors have read and
approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sung H, Ferlay J, Siegel RL, Sung H,
Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A and
Bray F: Global cancer statistics 2020: GLOBOCAN estimates of
incidence and mortality worldwide for 36 cancers in 185 countries.
CA Cancer J Clin. 71:209–249. 2021.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Sato Y, Okamoto K, Kawano Y, Kasai A,
Kawaguchi T, Sagawa T, Sogabe M, Miyamoto H and Takayama T: Novel
biomarkers of gastric cancer: Current research and future
perspectives. J Clin Med. 12(4646)2023.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Cancer Genome Atlas Research Network.
Comprehensive molecular characterization of gastric adenocarcinoma.
Nature. 513:202–209. 2014.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Ignatova EO, Kozlov E, Ivanov M, Mileyko
V, Menshikova S, Sun H, Fedyanin M, Tryakin A and Stilidi I:
Clinical significance of molecular subtypes of gastrointestinal
tract adenocarcinoma. World J Gastrointest Oncol. 14:628–645.
2022.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Sohn BH, Hwang JE, Jang HJ, Lee HS, Oh SC,
Shim JJ, Lee KW, Kim EH, Yim SY, Lee SH, et al: Clinical
significance of four molecular subtypes of gastric cancer
identified by the cancer genome atlas project. Clin Cancer Res.
23:4441–4449. 2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Nemtsova MV, Kuznetsova EB and Bure IV:
Chromosomal instability in gastric cancer: Role in tumor
development, progression, and therapy. Int J Mol Sci.
24(16961)2023.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Grillo F, Fassan M, Sarocchi F, Fiocca R
and Mastracci L: HER2 heterogeneity in gastric/gastroesophageal
cancers: From benchside to practice. World J Gastroenterol.
22:5879–5887. 2016.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Wakatsuki T, Yamamoto N, Sano T, Chin K,
Kawachi H, Takahari D, Ogura M, Ichimura T, Nakayama I, Osumi H, et
al: Clinical impact of intratumoral HER2 heterogeneity on
trastuzumab efficacy in patients with HER2-positive gastric cancer.
J Gastroenterol. 53:1186–1195. 2018.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Gassmann R, Essex A, Hu JS, Maddox PS,
Motegi F, Sugimoto A, O'Rourke SM, Bowerman B, McLeod I, Yates III
JR, et al: A new mechanism controlling kinetochore–microtubule
interactions revealed by comparison of two dynein-targeting
components: SPDL-1 and the Rod/Zwilch/Zw10 complex. Genes Dev.
22:2385–2399. 2008.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Lara-Gonzalez P, Westhorpe FG and Taylor
SS: The spindle assembly checkpoint. Curr Biol. 22:R966–R980.
2012.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Vallee RB, Varma D and Dujardin DL: ZW10
function in mitotic checkpoint control, dynein targeting, and
membrane trafficking: Is dynein the unifying theme? Cell Cycle.
5:2447–2451. 2006.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Cmentowski V, Ciossani G, d'Amico E,
Wohlgemuth S, Owa M, Dynlacht B and Musacchio A: RZZ-Spindly and
CENP-E form an integrated platform to recruit dynein to the
kinetochore corona. EMBO J. 42(e114838)2023.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Pereira C, Reis RM, Gama JB, Celestino R,
Cheerambathur DK, Carvalho AX and Gassmann R: Self-assembly of the
RZZ complex into filaments drives kinetochore expansion in the
absence of microtubule attachment. Curr Biol. 28:3408–3421.e8.
2018.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Barbosa J, Conde C and Sunkel C:
RZZ-SPINDLY-DYNEIN: You got to keep 'em separated. Cell Cycle.
19:1716–1726. 2020.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Zhang G, Lischetti T, Hayward DG and
Nilsson J: Distinct domains in Bub1 localize RZZ and BubR1 to
kinetochores to regulate the checkpoint. Nat Commun.
6(7162)2015.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Lara-Gonzalez P, Pines J and Desai A:
Spindle assembly checkpoint activation and silencing at
kinetochores. Semin Cell Dev Biol. 117:86–98. 2021.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Scaërou F, Aguilera I, Saunders R, Kane N,
Blottière L and Karess R: The rough deal protein is a new
kinetochore component required for accurate chromosome segregation
in Drosophila. J Cell Sci. 112:3757–3768. 1999.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Williams BC, Li Z, Liu S, Williams EV,
Leung G, Yen TJ and Goldberg ML: Zwilch, a new component of the
ZW10/ROD complex required for kinetochore functions. Mol Biol Cell.
14:1379–1391. 2003.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Hirose H, Arasaki K, Dohmae N, Takio K,
Hatsuzawa K, Nagahama M, Tani K, Yamamoto A, Tohyama M and Tagaya
M: Implication of ZW10 in membrane trafficking between the
endoplasmic reticulum and Golgi. EMBO J. 23:1267–1278.
2004.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Défachelles L, Raich N, Terracol R, Baudin
X, Williams B, Goldberg M and Karess RE: RZZ and Mad1 dynamics in
Drosophila mitosis. Chrom Res. 23:333–342. 2015.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Williams BC and Goldberg ML: Determinants
of drosophila zw10 protein localization and function. J Cell Sci.
107:785–798. 1994.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Zhengxiang Z, Yunxiang T, Zhiping L and
Zhimin Y: KNTC1 knockdown suppresses cell proliferation of colon
cancer. 3 Biotech. 11(262)2021.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Liu L, Chen H, Chen X, Yao C, Shen W and
Jia C: KNTC1 as a putative tumor oncogene in pancreatic cancer. J
Cancer Res Clin Oncol. 149:3023–3031. 2023.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Liu CT, Min L, Wang YJ, Li P, Wu YD and
Zhang ST: shRNA-mediated knockdown of KNTC1 suppresses cell
viability and induces apoptosis in esophageal squamous cell
carcinoma. Int J Oncol. 54:1053–1060. 2019.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Bakhoum SF and Cantley LC: The
multifaceted role of chromosomal instability in cancer and its
microenvironment. Cell. 174:1347–1360. 2018.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Bakhoum SF, Ngo B, Laughney AM, Cavallo
JA, Murphy CJ, Ly P, Shah P, Sriram RK, Watkins TBK, Taunk NK, et
al: Chromosomal instability drives metastasis through a cytosolic
DNA response. Nature. 553:467–472. 2018.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Sansregret L, Vanhaesebroeck B and Swanton
C: Determinants and clinical implications of chromosomal
instability in cancer. Nat Rev Clin Oncol. 15:139–150.
2018.PubMed/NCBI View Article : Google Scholar
|