Introduction
Depression is one of the most common types of mood
disorder and is associated with significant disability and
mortality. It is projected to become the second leading cause of
burden of disease in 2030 (1,2).
Although a number of theories have been put forward to explain the
pathology of depression, including the monoamine hypothesis, the
neurotransmitter receptor hypothesis and the neurotrophic factor
hypothesis, the precise mechanisms underlying the depression remain
unclear (3).
Considerable evidence suggests that dysregulation of
the hypothalamic-pituitary-adrenal (HPA) axis is associated with
depression (4,5). Hyperactivity of the HPA axis results
in increased levels of the glucocorticoid hormone cortisol in
depressed patients (6). Studies on
the effects of excessive glucocorticoid on the brain in depression
have to date focused on neuronal apoptosis. However, the majority
of studies have only examined the hippocampal neurons in response
to perturbed HPA-axis function, and little research has been
conducted on alterations to hypothalamic neurons and the cellular
and molecular basis for these changes. Previous studies have
revealed that the hypothalami of mice subcutaneously implanted with
corticosterone (CORT) pellets exhibited significant changes in the
expression of proteins involved in cell death (7). A recent study identified that the
antidepressant, sertraline, reduced depression-related behavior and
decreased the number of apoptotic cells in the hypothalamus in a
rat model of depression following myocardial infarction (8). Therefore, CORT-induced hypothalamic
cell damage may correlate with behavioral manifestations of
depression. Current antidepressants, which target monoaminergic
systems, are widely available on the pharmaceutical market.
However, approximately 30% of patients fail to respond to this
therapy (9). Therefore, the search
for novel drug targets for the treatment of major depression
continues.
Recently, we reported that icariin, a biologically
active purified compound from the Chinese herbal plant Epimedium
sagittatum Maxim was able to reverse social defeat-induced
depression-related behavior in mice, which is potentially mediated
through inhibition of CORT secretion and glucocorticoid receptor
(GR) downregulation (10).
Furthermore, we identified that icariin markedly suppresses
CORT-induced apoptosis in primary cultured rat hippocampal neurons,
presumably through blockade of p38MAPK activation (11) In the present study, we aim to
determine whether CORT is responsible for apoptosis in primary
cultured hypothalamic neurons, and to investigate the protective
effects of icariin by examining the activation of the
phosphoinositide 3-kinase/protein kinase B (PI3-K/Akt) signaling
pathway.
Materials and methods
Primary cultures of hypothalamic
cells
The experiments were performed on Sprague-Dawley
(SD) rats less than 24 h old purchased from the Shanghai Institute
of the Chinese Academy of Science. This study was approved by the
Fudan University experimental standards and followed the
international guidelines on the ethical treatment of experimental
animals. Primary cultures of dissociated hypothalamic cells were
prepared according to a previously described method (12) with specific modifications. The
hypothalamic tissue was dissected from neonatal SD rats, stripped
of the meninges and blood vessels, and minced in Hanks’ balanced
salt solution (HBSS) without Ca2+ and Mg2+.
The tissue was dissociated using 0.125% trypsin (Beijing Solarbio
Science and Technology Co., Ltd., Beijing, China) digestion for 15
min at 37°C and gentle titration through a series of glass pasteur
pipettes. The cells were then plated on poly-L-lysine (molecular
weight, 30,000–70,000; 0.1mg/ml; Sigma, St. Louis, MO, USA)
coated-glass coverslips, 96-well plates, or 100 mm dishes at a
density of 1×106 cell/ml and maintained at 37°C in a
humidified 5% CO2 incubator. Neurons were cultured in
neurobasal-A medium (Gibco BRL, Grand Island, NY, USA) and
supplemented with 2% B27 supplement (Gibco BRL), 10 μl/ml
penicillin-streptomycin, 2 mM glutamine (Solarbio), 5 ng/ml bovine
fibroblast growth factor 2 (FGF2; R&D Systems, Minneapolis, MN,
USA) and 1% fetal bovine serum (FBS). After a 24-h culture period,
cultures grown in serum-free neurobasal medium yielded greater than
80% neurons, as estimated by immunocytochemical staining with
antibodies against neurofilament proteins. The cultured neurons
were used for in vitro studies on day 8 (DIV 8).
Drug treatment
CORT (Sigma) was initially dissolved in ethanol as a
stock solution and then in culture media (final concentration of
ethanol, 0.1%). Icariin (purity >99%) was purchased from the
Shanghai Winherb Medical S&T Development Co., Ltd. (Shanghai,
China). Eight-day primary hypothalamic neurons were washed twice
with Mg2+-free, HEPES-buffered saline (HBS; 146 mM NaCl,
10 mM HEPES, 2 mM CaCl2, 5 mM KCl, 10 mM D-glucose, pH
7.4) and pretreated with different concentrations of icariin with
or without 50 μM LY294002 (Sigma) for 2 h at 37°C, and then exposed
to CORT for 24 h.
Assessement of cell viability and
death
After exposure to various concentrations of CORT,
icariin and LY294002, cell viability was determined using the
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl (MTT) assay system
(Beyotime Institute of Biotechnology, Shanghai, China) (11). Cell cytotoxicity following various
treatments was evaluated by lactate dehydrogenase (LDH) release.
This was acheived using a Quantitative Detection kit according to
the manufacturer’s instructions (GenMed Scientifics Inc.,
Arlington, MA, USA) (11).
Cell morphology evaluation
To evaluate the morphological changes in primary
cultures of hypothalamic cells, cultures were observed under a
Leica DFIL inverted microscope with a phase-contrast optic lens.
Images were captured using Leica QWin plus 3 Image Processing
Software (Media Cybernetics, Silver Spring, MD, USA) through a
Leica DFC300 FX camera device. We analyzed neurite preference from
five images per condition.
Measurement of caspase-3 activity
Caspase-3 activity in primary cultures of
hypothalamic lysate was determined using the Chemicon caspase
colorimetric activity assay kit according to the manufacturer’s
instructions (11). In brief,
treated cells were resuspended in 50 μl of cell lysis buffer and
incubated on ice for 30 min. After centrifugation for 5 min at
10,000 × g, the supernatant was transferred to a fresh tube
followed by the addition of reaction buffer and caspase-3 substrate
for 4 h at 37°C in the dark. The free chromophore
p-nitroaniline (pNA) was quantified using a
microtiter plate reader at 405 nm. The fold increase in caspase-3
activity was determined by comparing the absorbance from each
sample with untreated neurons.
Determination of mitochondrial membrane
potential (MMP)
Following treatment, hypothalamic cell cultures
grown on 96-well plates were loaded with Rhodamine 123 (Rho 123)
(10 μM) (Beyotime Institute of Biotechnology, Shanghai, China) at
37°C in the dark for 15 min and then washed 3 times with
phosphate-buffered saline (PBS). The cell fluorescence intensity of
Rho 123 was quantified using a fluorescence microplate reader
(TECAN Infinite 200 microplate reader; Tecan Trading AG, Männedorf,
Switzerland) with excitation at 485 nm and emission at 530 nm. The
background fluorescence signal of Rho 123 was determined without
cells and subtracted from those obtained in hypothalamic
neurons.
Analysis of intracellular reactive oxygen
species (ROS)
Intracellular ROS levels were analyzed by H2DCF-DA
assay according to the method previously described (13). Cells (1×106 cells/well)
were rinsed with Kreb’s ringer solution and 10 mM H2DCF-DA was
added. After 15 min incubation at 37°C, cells were washed with PBS,
harvested and pelleted by centrifugation and then resuspended in
0.5 ml PBS. Fluorescence intensity was then monitored using a
fluorescence microplate reader. The data was analyzed and expressed
as a percentage of the control. DCF labeled cells were observed
under fluorescence microscopy.
Measurement of antioxidant enzyme
activities
Following treatment, the cultures were washed twice
with PBS, then placed into ice-cold PBS (0.1 M, containing 0.05 mM
EDTA) and homogenized. The homogenate was then centrifuged at 4°C
at 10,000 × g for 30 min, after which the protein concentration was
determined using the Bradford method, using bovine serum albumin
(BSA) as a reference standard. Measurement of superoxide dismutase
(SOD) activity was performed according to the reagent kit
manufacturer’s instructions.
Western blot analysis
Following treatment, cells in each of the 6-well
plates were rinsed twice with cold PBS, followed by the addition of
cell lysis buffer containing 150 mM NaCl, 50 mM Tris-HCl, 5 mM
EDTA, 1% Nonidet P-40, 0.5% deoxycholate, 1% SDS with proteinase
inhibitor cocktail (Sigma) on ice for 15 min, and then centrifuged
for 20 min at 12,000 × g. The supernatant was collected and the
protein concentration was measured using the Bradford method. Fifty
milligrams of total protein was dissolved in sample buffer and
heated for 5 min prior to loading onto polyacrylamide gels.
Proteins were then transferred to poly(vinylidine difluoride)
filter membranes, and blocked with 5% non-fat dry milk in
Tris-buffered saline/0.05% Tween-20. The membrane was incubated
with a monoclonal antibody against phospho-Akt (Ser473), Akt (Santa
Cruz Biotechnology Inc., Santa Cruz, CA, USA), followed by
incubation with horseradish peroxidase-conjugated (HRP) secondary
antibodies and visualized using an enhanced chemiluminescence
kit.
Statistical analysis
All data are expressed as the mean ± SD. Differences
between groups without particular comments were generally examined
for statistical significance using one-way ANOVA analysis with a
post-hoc Dunnett’s test. P≤0.05 indicated a statistically
significant difference.
Results
Icariin protects hypothalamic neurons
against CORT-induced cytotoxicity
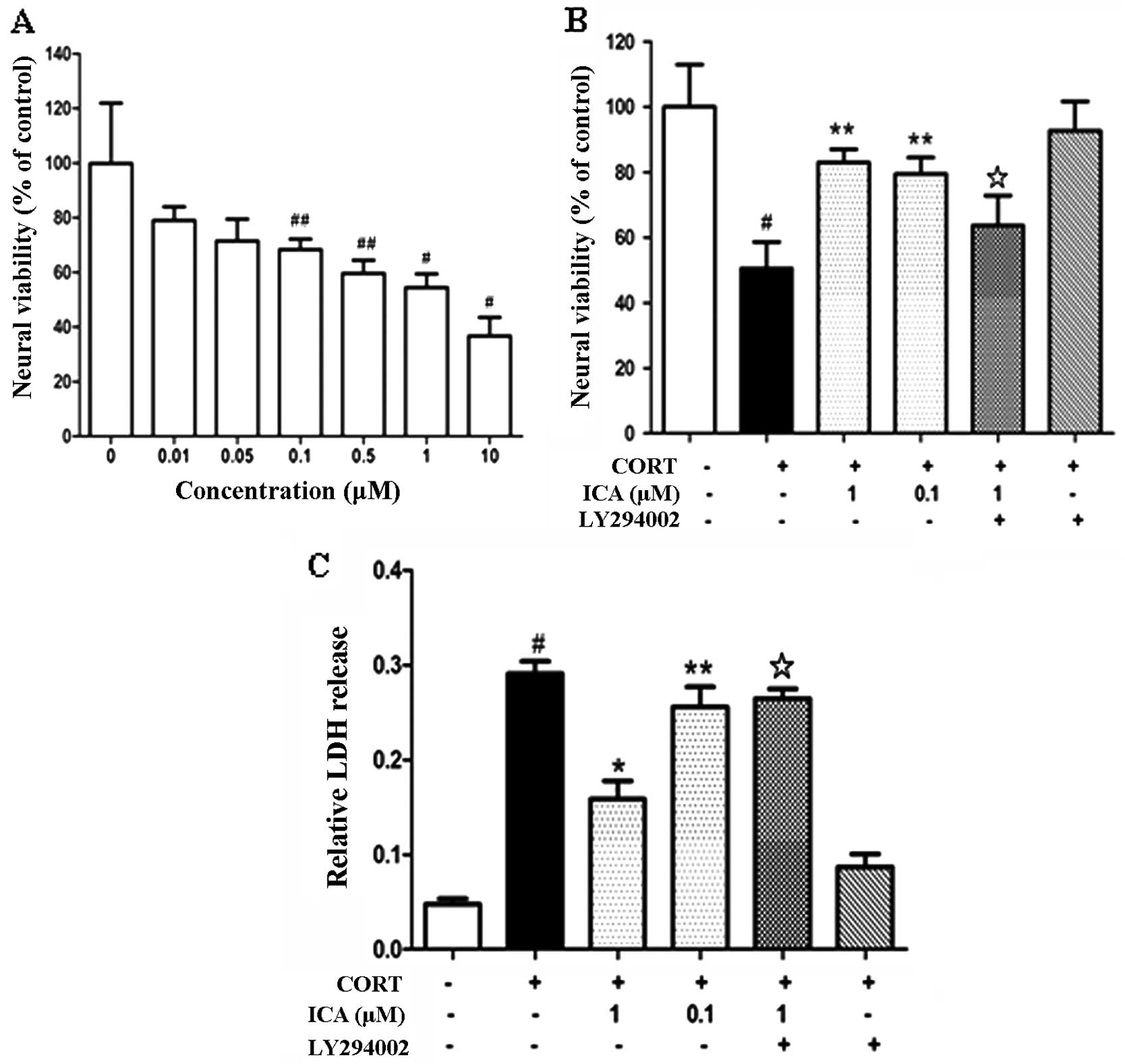
Cultured hypothalamic neurons were exposed to an
increasing concentration of CORT (0.01–5 μM) for 24 h and cell
viability was assessed using an MTT reduction assay. CORT induced
cell death in a concentration-dependent manner (Fig. 1A). Exposure to CORT (1 μM) for 24 h
was used for inducing excitotoxic neuronal injury in the subsequent
experiments.
To investigate the neuroprotective effects of
icariin on CORT-induced neuronal damage, hypothalamic neurons were
pretreated with icariin at 0.1 μM and 1 μM for 2 h, followed by
challenge with CORT (1 μM) for 24 h. Icariin pretreatment at 0.1 μM
and 1 μM enhanced cell viability compared with the CORT-treated
control (Fig. 1B). The protective
effect of icariin on CORT-induced cytotoxicity was also analyzed
using the LDH test. Pretreatment with 0.1 μM and 1 μM icariin
caused a marked decrease in LDH release (Fig. 1C).
Icariin suppresses hypothalamic neuronal
apoptosis induced by CORT
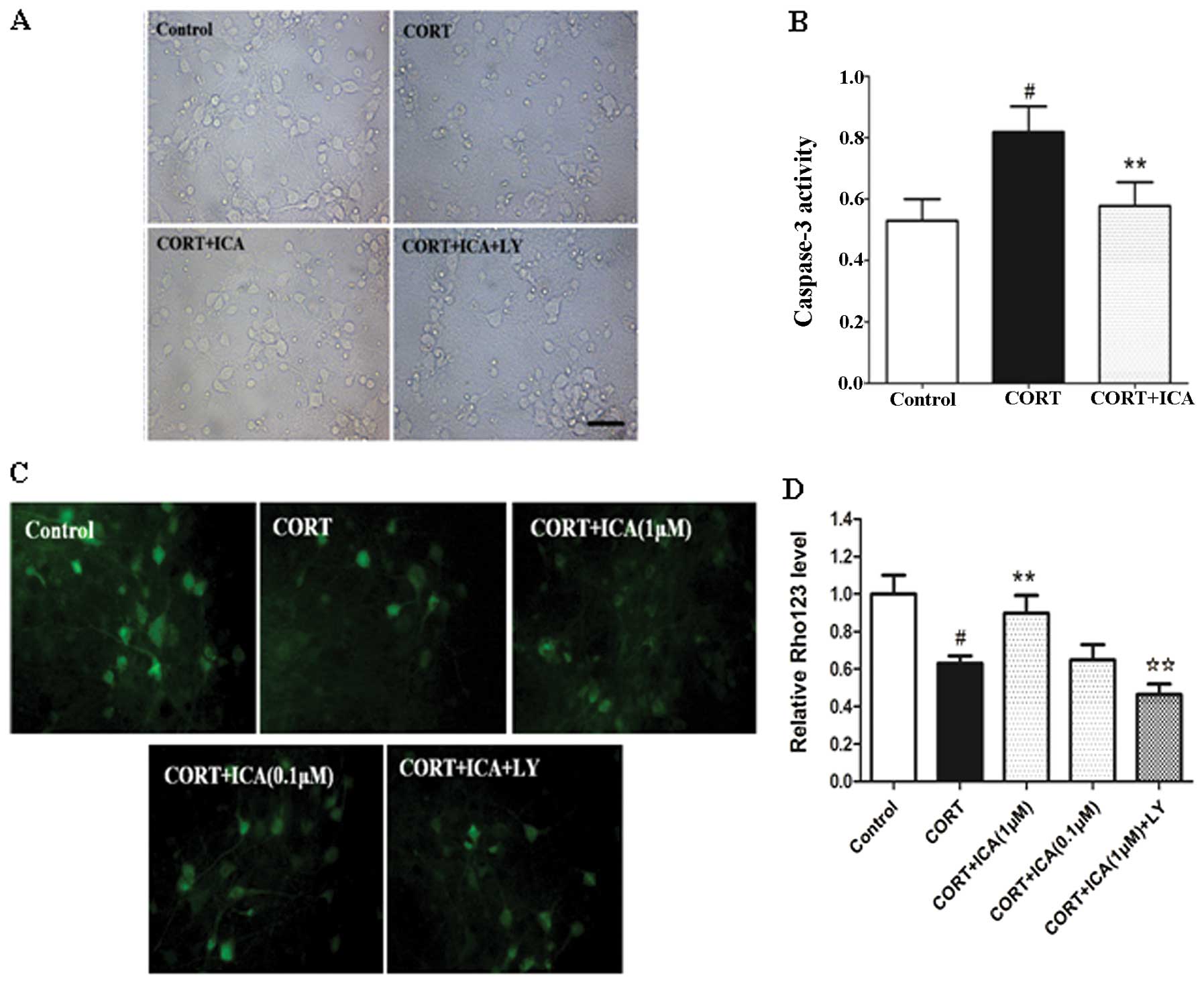
Morphological analysis at the subcellular level
remains the most conclusive method for distinguishing apoptosis
from necrosis. The protective effect of icariin on CORT-induced
cytotoxicity was also analyzed by microscopic examination. Cultured
hypothalamic neurons were pretreated with or without 1 μM icariin
for 2 h, followed by challenge with CORT (1 μM) for 24 h. Cells
treated with vehicle were healthy with networks of neurites and
vacuole-free cell bodies. The synapse connections between neurons
were clearly observed. Neurite fragmentation, shrinkage of cell
bodies and evident cell loss were observed when the neuronal
culture was exposed to 1 μM CORT for 24 h. Pretreatment of icariin
with 1 μM was able to protect cortical neurons against CORT
toxicity as demonstrated by the fine morphology (Fig. 2A).
Biochemical markers for caspase-3 activation
following treatment with CORT were consistent with apoptotic cell
death. Following 24-h CORT treatment, caspase-3 activity increased
in the hypothalamic neurons. In the cultured hypothalamic neurons
exposed to 1 μM CORT plus 1 μM icariin, caspase-3 activity was
similar to that the of the control (Fig. 2B)
Mitochondria are recognised to be involved in
apoptosis. Permeability changes can lead to caspase-dependent
cytotoxicity and downstream apoptotic signaling, while a loss of
mitochondrial transmembrane potential, denoted as mitochondrial
dysfunction, leads to cytochrome c release from the mitochondria
and triggers other apoptotic factors. In the present study, we
evaluated mitochondrial transmembrane potential using Rho 123 as
fluorescent dye. Dye distribution was examined under inverted
microscope (Leica DFIL). In the control cultures, Rho 123 was
markedly aggregated in hypothalamic neurons, reflecting the
baseline for healthy mitochondria in cells. CORT-treated neurons
demonstrated a decrease in Rho 123 of 37%. Pretreatment of 1 μM,
but not 0.1 μM icariin, significantly inhibited these changes
(Fig. 2C and D).
Icariin inhibits intracellular
accumulation of ROS and prevents loss of antioxidant enzyme
activities in CORT-treated cells
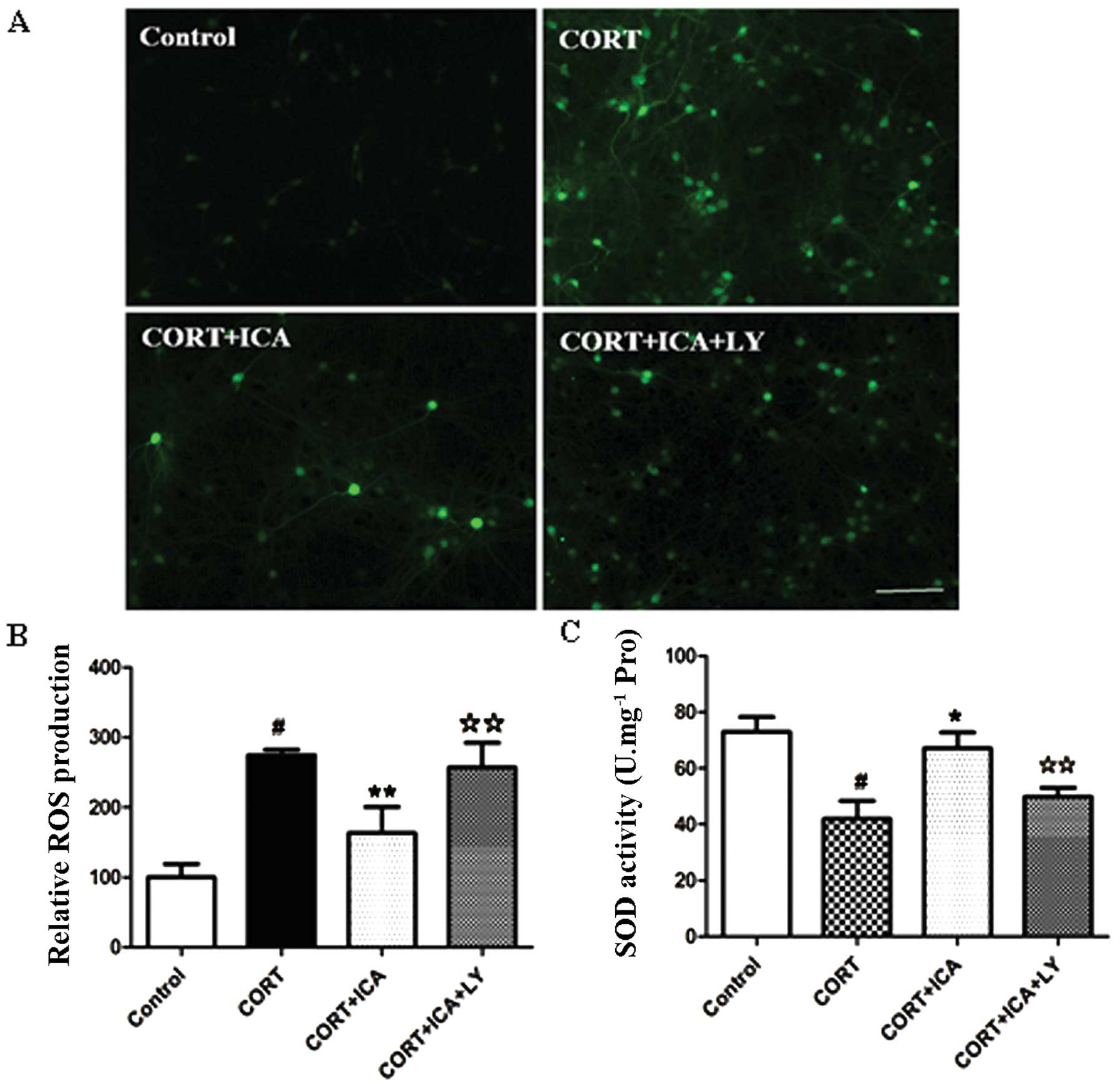
To determine whether icariin attenuates cell death
by blocking ROS generation, we detected the intracellular level of
ROS using H2DCF-DA fluorescent dye. Treatment of hypothalamic
neurons with 1 μM CORT for 24 h resulted in a significantly
increased DCF signal compared with the control group, but this was
significantly reduced by icariin pretreatment (Fig. 3A and B).
The level of SOD provided further evidence of the
protective effects of icariin. Incubation with 1 μM CORT for 24 h
resulted in a significantly decreased activity of SOD (42.5%) in
the hypothalamic neurons compared to the controls. However,
pretreatment with 1 μM icariin resulted in a significant increase
in the activity of SOD (37.5%) when compared to cells treated with
CORT. Together, these results suggest that pretreatment with
icariin prevents ROS generation and attenuates changes in SOD
activity induced by treatment with CORT (Fig. 3C).
Phosphorylation of Akt in CORT-treated
neurons following exposure to icariin
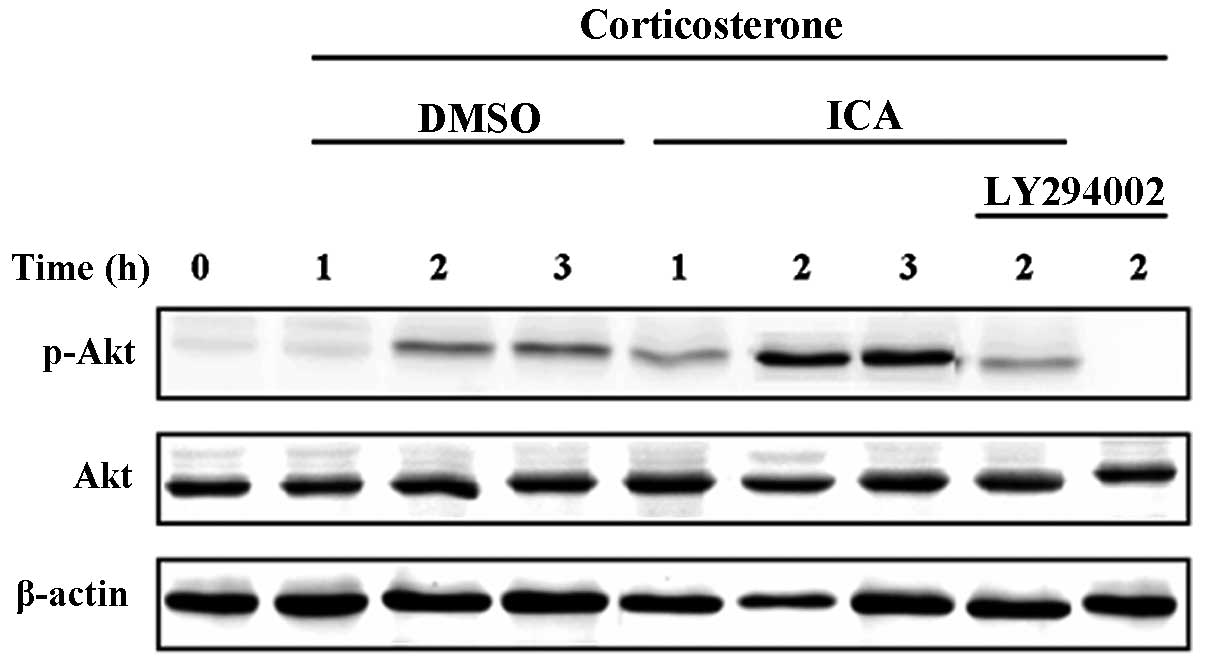
Activation of the PI3-K/Akt signal pathway by CORT
and icariin in cultured hypothalamic neurons was determined by
measuring phospho-Akt levels. Neurons were pretreated with icariin
or control media for 30 min followed by stimulation with CORT for
1, 2 and 3 h. Western blot analysis revealed a significant increase
in phospho-Akt at 2 h following treatment with CORT in cultured
hypothalamic neurons, similar levels were also recorded in the
cells treated for 3 h. In the icariin-treated groups, Akt
phosphorylation was increased compared with the CORT-treated
control group. As expected, the cells treated with LY294002
demonstrated a significant decrease in the amount of phosphorylated
Akt at 2 h (Fig. 4).
Discussion
The present study demonstrates that icariin prevents
CORT-induced apoptosis in primary cultured hypothalamic neurons.
Exposure of hypothalamic neurons to CORT resulted in a significant
loss of viability and apoptosis of the cells. In parallel, CORT
significantly increased the intracellular ROS elevation and
decreased SOD activity. However, pretreatment of the cells with
icariin prior to CORT exposure noticeably suppressed these
CORT-induced cellular events. Furthermore, icariin is able to
prevent CORT-induced hypothalamic cell death via activation of the
PI3-K/Akt pathway.
Patients with major depression and other
neurological afflictions frequently display hyperactivity of the
HPA axis (14). Patients with
major depression are known to have a higher level of cortisol than
patients without major depression (15). In rodents, studies have revealed
that repeated CORT injections induce behavioral and neurochemical
aspects of depression (16). For
example, chronic CORT injections increased the immobility time on
the forced swimming test (17).
Several studies have demonstrated that glucocorticoids induce
atrophy of dendritic processes, inhibition of neurogenesis and
cause overt loss of neurons by necrosis or apoptosis of the
hippocampus (18–20). Our recent study also revealed that
CORT may induce hippocampal neuronal damage (11). In the present study, we observed
morphological changes, including displayed neurite fragmentation,
shrinkage of cell bodies and evident cell loss in hypothalamic
neurons following CORT treatment. Furthermore, our results
demonstrated that exposure to CORT significantly decreased MMP and
increased caspase-3 activity. These results are consistent with
previous studies that the hypothalami of mice implanted with CORT
pellets exhibited significant changes in proteins related to cell
death pathways (7).
Several studies have demonstrated that ROS may lead
to neuronal apoptosis in neurodegenerative disorders (21). ROS is a natural by-product of the
normal metabolism of oxygen in the mitochondria, and accumulation
of ROS may be related to mitochondrial dysfunction and the
induction of apoptosis (22).
However, the antioxidant enzymes of SOD play protective roles in
all aerobic organisms by scavenging the generation of free radical
molecules. An imbalance between the generation of free radicals and
antioxidants may be involved in the pathogenesis of most
neurodegenerative diseases. In agreement with previously reported
results, our study demonstrated that CORT induces ROS generation
and suppresses SOD activity. The results suggest ROS are involved
in CORT-induced cytotoxicity.
The involvement of the PI3-K/Akt signal pathway in
neuronal survival involves different mechanisms. To identify the
intracellular signaling pathways that mediate the neuroprotective
actions of icariin, changes in the phosphorylation of key signaling
proteins were analyzed by immunoblots with phospho-specific
antibodies. In the present study, neurons were pretreated with
icariin or control media for 30 min followed by stimulation with
CORT for 1, 2 and 3 h. Western blot analysis revealed an increase
in phospho-Akt at 2 h following treatment with CORT in cultured
hypothalamic neurons and these levels of phospho-Akt were sustained
for at least 3 h. This is consistent with previous studies that
revealed CORT increases the phosphorylation of PI3-K/Akt in neurons
grown in neurobasal medium supplemented with B27 and 500 μm
L-glutamine (NBM+) (23). In the
icariin-treated groups, Akt phosphorylation was increased compared
with the CORT-treated control group. As expected, the cells treated
with LY294002 demonstrated a significant decrease in the amount of
phosphorylated Akt at 2 h. In addition, pretreatment with LY294002
significantly blocked the neuroprotective effects of icariin
against CORT-induced apoptosis. These results suggest that icariin
is able to prevent neuronal cell death induced by CORT in
hypothalamic neurons by modulating the activity of the PI3-K/Akt
pathway.
In conclusion, icariin was identified to be able to
prevent CORT-induced hypothalamic cell death via activation of the
PI3-K/Akt pathway. This study not only demonstrates the potential
pharmacological uses of icariin, but also reveals the key
neuroprotective role of the PI3-K/Akt pathway.
Acknowledgements
This study was supported by a grant from the
National Basic Science Program of China (No. 2009CB523000) and the
National Natural Science Foundation of China (No. 81102562).
Abbreviations:
|
CORT
|
corticosterone
|
|
PI3-K/Akt
|
phosphoinositide 3-kinase/protein
kinase B
|
|
MTT
|
3-(4,5)-dimethylthiahiazo(-z-y1)-3,5-di-phenytetrazoliumromide
|
|
LDH
|
lactate dehydrogenase
|
|
ROS
|
reactive oxygen species
|
|
HPA
|
hypothalamic-pituitary-adrenal
|
|
ICA
|
icarrin
|
|
GR
|
glucocorticoid receptor
|
|
MAPK
|
mitogen-activated protein kinases
|
|
Rho 123
|
rhodamine 123
|
|
MMP
|
mitochondrial membrane potential
|
|
SOD
|
superoxide dismutase
|
References
|
1
|
Mathers CD and Loncar D: Projections of
global mortality and burden of disease from 2002 to 2030. PLoS Med.
3:e4422006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ustun TB, Ayuso-Mateos JL, Chatterji S,
Mathers C and Murray CJ: Global burden of depressive disorders in
the year 2000. Br J Psychiatry. 184:386–392. 2004.PubMed/NCBI
|
|
3
|
Chopra K, Kumar B and Kuhad A:
Pathobiological targets of depression. Expert Opin Ther Targets.
15:379–400. 2011.
|
|
4
|
Pariante CM and Lightman SL: The HPA axis
in major depression: classical theories and new developments.
Trends Neurosci. 31:464–468. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Snyder JS, Soumier A, Brewer M, Pickel J
and Cameron HA: Adult hippocampal neurogenesis buffers stress
responses and depressive behaviour. Nature. 476:458–461. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Pariante CM: Risk factors for development
of depression and psychosis. Glucocorticoid receptors and pituitary
implications for treatment with antidepressant and glucocorticoids.
Ann NY Acad Sci. 1179:144–152. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Skynner HA, Amos DP, Murray F, et al:
Proteomic analysis identifies alterations in cellular morphology
and cell death pathways in mouse brain after chronic corticosterone
treatment. Brain Res. 1102:12–26. 2006. View Article : Google Scholar
|
|
8
|
Wann BP, Bah TM, Kaloustian S, et al:
Behavioural signs of depression and apoptosis in the limbic system
following myocardial infarction: effects of sertraline. J
Psychopharmacol. 23:451–459. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kulkarni SK and Dhir A: Current
investigational drugs for major depression. Expert Opin Investig
Drugs. 18:767–788. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wu J, Du J, Xu C, et al: Icariin
attenuates social defeat-induced down-regulation of glucocorticoid
receptor in mice. Pharmacol Biochem Behav. 98:273–278. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liu B, Zhang H, Xu C, et al:
Neuroprotective effects of icariin on corticosterone-induced
apoptosis in primary cultured rat hippocampal neurons. Brain Res.
1375:59–67. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yokosuka M, Ohtani-Kaneko R, Yamashita K,
Muraoka D, Kuroda Y and Watanabe C: Estrogen and environmental
estrogenic chemicals exert developmental effects on rat
hypothalamic neurons and glias. Toxicol In Vitro. 22:1–9. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang H, Xu Y, Yan J, et al: Acteoside
protects human neuroblastoma SH-SY5Y cells against
beta-amyloid-induced cell injury. Brain Res. 1283:139–147. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kunugi H, Hori H, Adachi N and Numakawa T:
Interface between hypothalamic-pituitary-adrenal axis and
brain-derived neurotrophic factor in depression. Psychiatry Clin
Neurosci. 64:447–459. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Keller J, Flores B, Gomez RG, et al:
Cortisol circadian rhythm alterations in psychotic major
depression. Biol Psychiatry. 60:275–281. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Iijima M, Ito A, Kurosu S and Chaki S:
Pharmacological characterization of repeated corticosterone
injection-induced depression model in rats. Brain Res. 1359:75–80.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ago Y, Arikawa S, Yata M, et al:
Antidepressant-like effects of the glucocorticoid receptor
antagonist RU-43044 are associated with changes in prefrontal
dopamine in mouse models of depression. Neuropharmacology.
55:1355–1363. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gerritsen L, Comijs HC, van der Graaf Y,
Knoops AJ, Penninx BW and Geerlings MI: Depression, hypothalamic
pituitary adrenal axis, and hippocampal and entorhinal cortex
volumes - The SMART Medea Study. Biol Psychiatry. 70:373–380.
2011.
|
|
19
|
de Quervain DJ, Aerni A, Schelling G and
Roozendaal B: Glucocorticoids and the regulation of memory in
health and disease. Front Neuroendocrinol. 30:358–370.
2009.PubMed/NCBI
|
|
20
|
Xu Y, Zhang C, Wang R, et al:
Corticosterone induced morphological changes of hippocampal and
amygdaloid cell lines are dependent on 5-HT7 receptor related
signal pathway. Neuroscience. 182:71–81. 2011. View Article : Google Scholar
|
|
21
|
Fatokun AA, Stone TW and Smith RA:
Oxidative stress in neurodegeneration and available means of
protection. Front Biosci. 13:3288–3311. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Huang SH, Lin CM and Chiang BH: Protective
effects of Angelica sinensis extract on amyloid
beta-peptide-induced neurotoxicity. Phytomedicine. 15:710–721.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhu ZH, Yang R, Fu X, Wang YQ and Wu GC:
Astrocyte-conditioned medium protecting hippocampal neurons in
primary cultures against corticosterone-induced damages via
PI3-K/Akt signal pathway. Brain Res. 1114:1–10. 2006. View Article : Google Scholar
|