Introduction
Antiphospholipid syndrome (APS) is characterized
clinically by recurrent fetal loss and/or thrombosis, and
serologically by the persistent presence of antiphospholipid
antibodies (aPLs). The aPLs mainly include anti-cardiolipin
antibodies (aCLs), lupus anticoagulant (LA) and anti-β2GPI
antibodies (anti-β2GPI). The poor obstetric outcomes in pregnant
women with APS are also characterized by the occurrence of growth
retardation and pre-eclampsia (1).
For decades, it has been widely adopted by
researchers that higher titers of aPLs induce clinical
manifestations in APS (2–5), although the approaches varied
regarding the division of low/high titers. The latest revised
approaches suggest that due to medium or high titers of IgG or
IgM-class aCL antibodies, medium and/or high titers of IgG or
IgM-class anti-β2GPI antibodies and positivity for LA, testing for
APS should be performed twice, at least 12 weeks apart (1).
Accumulating evidence demonstrates that APS is an
autoantibody-mediated systemic autoimmune disease (6–7).
CD4+ T helper cells (Th cells) play central roles in
immuno-regulation and immuno-stimulation. Th cells are divided into
4 subtypes: IFN-γ-secreting Th1, IL-4-secreting Th2,
IL-17-producing Th17 cells and
CD4+CD25+Foxp3+ regulatory T cells
(Tregs). Although there are various studies available on the role
of Th1 and Th2 cells in autoimmune diseases, their mechanisms in
APS have not been fully elucidated yet. Moreover, the published
data concerning the Th1/Th2 type of cellular immune response in APS
have been inaccurate both in human and mouse models. Certain
studies report a Th2-predominant state (8–10),
however, there are studies reporting a shift to a Th1 response
(11–13). The roles and mechanisms of Treg in
APS are both unclear. We found a sole study in a mouse model of APS
reporting the downregulation of the number and function of
CD4+CD25+Foxp3+ Treg cells
(14). Although Th17 cells have
been thoroughly studied in autoimmune diseases, they have never
been reported in APS. Thus, the present study aimed to closely
examine Th differentiation and to identify the Th1/Th2 paradigm in
APS, to delineate the way Th17 and Treg subtypes change in APS, to
determine their roles and to examine whether they are correlated
with the aPL titers.
Materials and methods
Patients and aPL antibody
preparation
Serum samples were obtained from 4 outpatients of
the Obstetrics and Gynecology Department of the Second Xiangya
Hospital, Hunan, China. The study was approved by the local
Research Ethics Committee and all patients provided informed
consent. The outpatients were diagnosed with APS characterized by
at least two fetal losses and positive aPL antibodies (aCL- and/or
anti-β2GPI-positive and LA-negative). The diagnoses were made on
two occasions, at least 12 weeks apart. Serum samples from patients
were collected after the clinical event, without immunosuppressive
therapy. The serum samples were boiled (30 min at 56°C) and IgG was
purified by ammonium sulfate precipitation. The titers of aCL-IgG
and anti-β2GPI-IgG were measured with an enzyme-linked
immunosorbent assay (ELISA) kit (Euroimmun, Lübeck, Germany), and
were found to be 56 and 170 U/ml, respectively. Control human serum
samples from 4 healthy non-autoimmune individuals were obtained in
the same way. Purified samples were stored at −80°C for later
use.
Peripheral mononuclear cell (PBMC)
isolation and sample preparation
Heparinized venous blood was obtained from healthy
adult volunteers negative for aPLs. PBMCs were isolated by
centrifugation (900 × g for 30 min, at room temperature) with
Ficoll-Hypaque (EZ-Sep™ Human Lymphocyte Separation Medium). Cells
were then collected, washed 3 times with PBS and suspended in
RPMI-1640 (Gibco, Carlsbad, CA, USA), supplemented with 100 M/ml
penicillin (Gibco), 100 μg/ml streptomycin (Gibco) and 10% fetal
calf serum.
PBMCs at the concentration of 1×107
cells/ml were added onto 12-well microtiter plates, and incubated
with 5% PBS (control group), 5% normal human IgG (negative group)
or different titers of aPLs (5,10,15 or 25%), for 48 h at 37°C with
5% CO2.
Flow cytometry
Flow cytometric analysis of the Th cell subtypes was
performed using FITC anti-human CD4 (clone OKT4; Biolegend, San
Diego, CA, USA), PE anti-human IFN-r (clone 4S.B3; Biolegend), APC
anti-human IL-4 (clone 8D4–8; Biolegend), PE/Cy7 anti-human IL-17A
(clone BL168; Biolegend), PE anti-human CD25 (clone BC96;
Biolegend) and APC anti-human Poxp3 (clone 236A/E7; eBioscience,
San Diego, CA, USA).
For intracellular cytokine staining, PBMCs at the
concentration of 2×106 cells/ml were stimulated with
phorbol myristate acetate (PMA; 20 ng/ml; Sigma, St. Louis, MO,
USA) and ionomycin (1,000 ng/ml; Sigma) for 1 h, then incubated
with GolgiPlug (1 μl/ml; BD, USA) for 4 h. Then the stimulated
cells were collected and washed twice with PBS, and then stained
with FITC anti-human CD4 for 30 min on ice in the dark. The cells
were then washed twice with PBS, and fixed in fixation buffer
(Biolegend) for 30 min at room temperature in the dark. Subsequent
to this, cells were washed twice with permeabilizing solution
buffer (Biolegend) for 6 min at room temperature. They were then
stained with PE anti-human IFN-γ, APC anti-human IL-4, PE/Cy7
anti-human IL-17A for 30 min on ice in the dark. After washing, the
cells were fixed in 1% paraformaldehyde and stored at 4°C in the
dark for subsequent detection.
For Treg staining, PBMCs at the concentration of
2×106 cells/ml were collected and washed twice with PBS,
and stained for 30 min on ice in the dark with FITC anti-human CD4
and PE anti-human CD25. The cells were then washed twice with PBS
and fixed in fixation buffer (Biolegend) for 30 min at room
temperature in the dark. Subsequent to this, cells were washed
twice with permeabilizing solution buffer (Biolegend) for 6 min at
room temperature. They were then stained with APC anti-human Poxp3
for 30 min on ice in the dark. After washing, the cells were fixed
in 1% paraformaldehyde and stored at 4°C in the dark for subsequent
detection.
The cells were analyzed by Beckman Coulter FC500
(Beckman, Miami, FL, USA). A total of 10,000 cells were counted in
each sample. A gate was set on the lymphocytes using characteristic
forward scatter (FSC) and side scatter (SSC) parameters.
Isotype-matched FITC mouse IgG1 antibody, PE mouse IgG1 antibody,
APC mouse IgG1 antibody and PE/Cy7 mouse IgG1 antibody (Biolegend)
were used as controls.
Statistical analysis
Each experiment was repeated 3 times with similar
results. Statistical analysis was performed using SPSS 17.0.
Statistically significant differences in the 6 experimental groups
were analyzed by one-way ANOVA. Differences between 2 groups were
analyzed for homogeneity of variance, by the least significant
difference (LSD) or by Dunnet T3. Correlations in the 2 indices
were analyzed by the Pearson test. P<0.05 was considered to
indicate a statistically significant difference.
Results
aPL titers and groups
The aPLs were detected and recorded as follows: aCL
IgGs were 56 U/ml, anti-β2GPI IgGs were 170 U/ml. In accordance
with the literature, we produced concentrations of 5, 10, 15 and
25% aPLs and these formed the aPL groups. There were 2.8, 5.6, 8.4
and 14 U/ml aCL IgG and 8.5, 17, 25.5 and 42.5 U/ml anti-β2GPI IgG
in the groups, respectively. The group with 5% aPLs was the
negative concentration group (aPL titers <10 U/ml), the 10%
group had aPL titers <20 U/ml, the 15% group had aPL titers
>20 U/ml but <40 U/ml, whereas the 25% group with a
medium/high concentration, had aPL titers >40 U/ml.
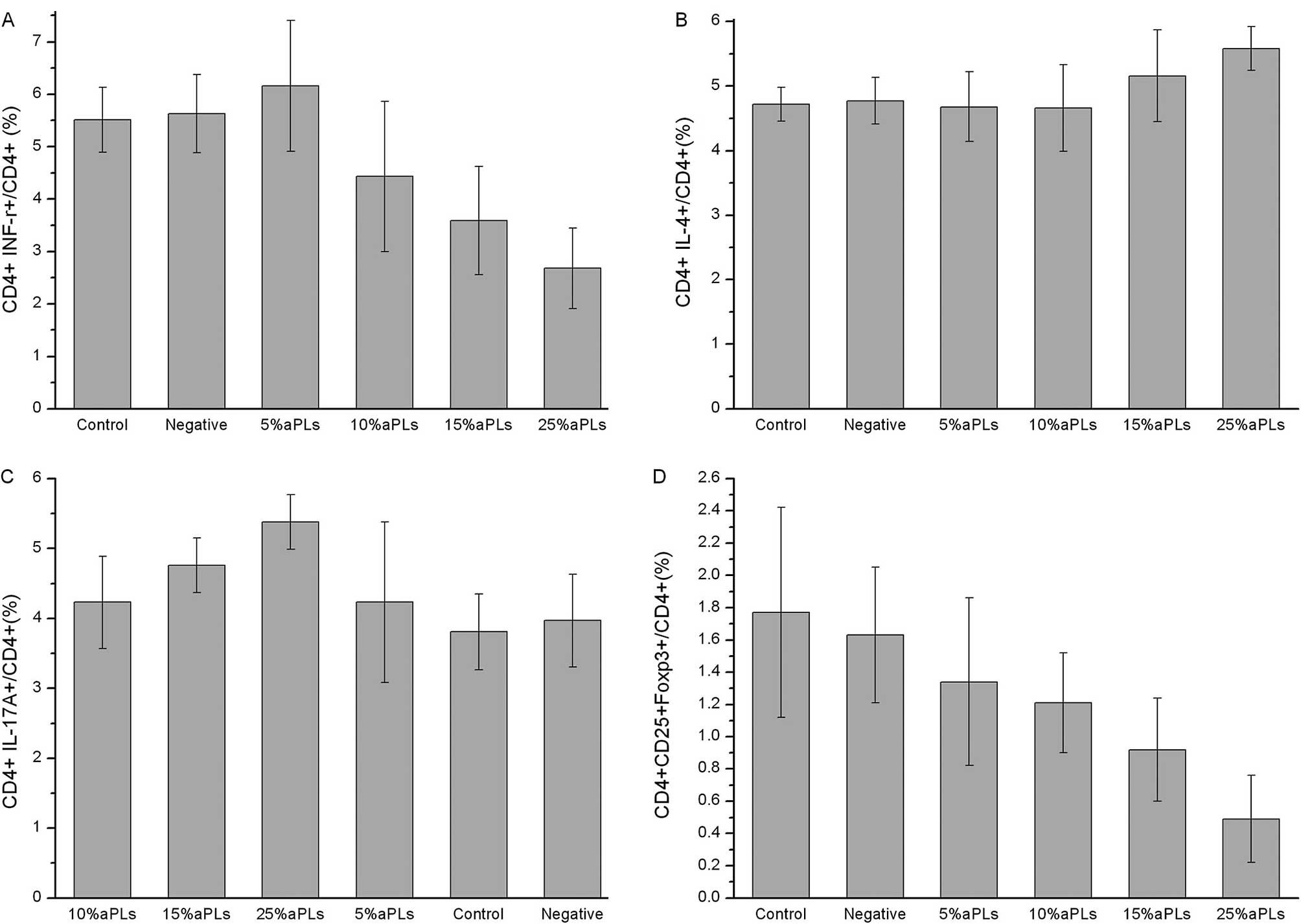
Th1/Th2/Th17/Treg subtype changes
detected by flow cytometry
The data in Table I
demonstrate that the Th1, Th2, Th17 and Treg cell expression and
the Th1/Th2 ratio changed in PBMCs cultured with different titers
of aPLs. We found that the subtypes of the Th cells were changed
significantly in the 15% aPL group and even moreso in the 25% aPL
group, compared to the normal group. In the 5% and 10% aPL groups
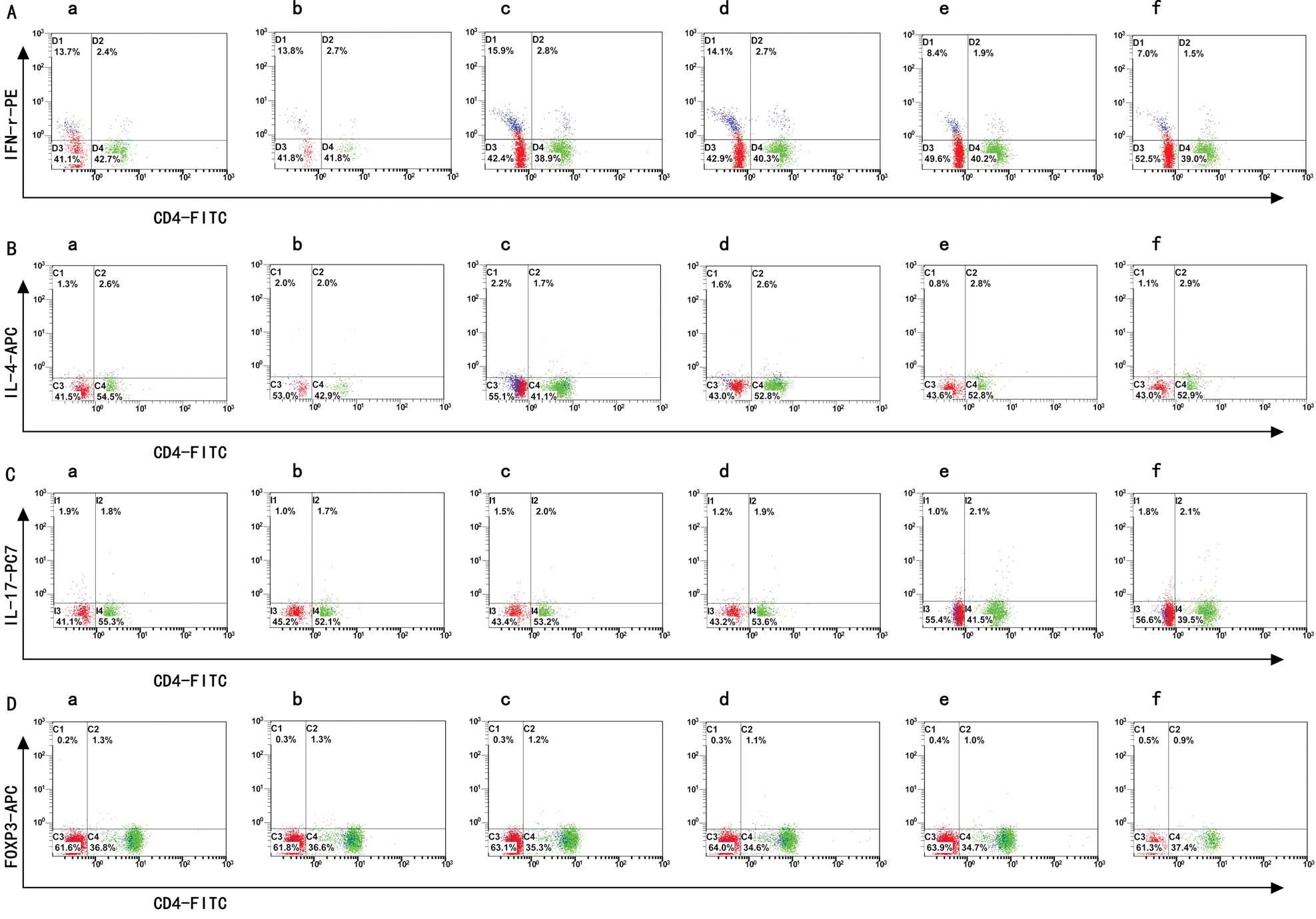
Th expression showed no difference. The flow cytometry images are
shown in Fig. 1, while the bar
graphs of Th1, Th2, Th17 and Treg cell expression are shown in
Fig. 2.
 | Table IFrequencies of Th1/Th2/Th17/Treg
expression in different groups detected by flow cytometry (mean ±
SD). |
Table I
Frequencies of Th1/Th2/Th17/Treg
expression in different groups detected by flow cytometry (mean ±
SD).
| Th1 (%) | Th2 (%) | Th1/Th2 ratio
(%) | Th17 (%) | Treg (%) |
|---|
| Control | 5.51±0.62 | 4.72±0.26 | 1.24±0.12 | 3.81±0.54 | 1.77±0.65 |
| Negative | 5.63±0.75 | 4.77±0.36 | 1.18±0.15 | 3.97±0.66 | 1.63±0.42 |
| 5% aPLs | 6.16±1.25 | 4.68±0.54 | 1.32±0.29 | 4.23±1.15 | 1.34±0.52 |
| 10% aPLs |
.4.43±1.43b | 4.66±0.67 | 0.97±0.35 | 4.23±0.66 | 1.21±0.31 |
| 15% aPLs |
..3.59±1.03a,b | 5.16±0.71 |
.0.70±0.18a | 4.76±0.39 |
0.92±0.32a |
| 25% aPLs |
...2.68±0.77a,b,c |
....5.58±0.34a,b,c |
..0.49±0.16a,b |
.5.38±0.39a |
0.49±0.27a,b,c |
Th subtype changes with aPL titers
As shown in Table
II, changes of Th cells were found to be dose-dependent with
aPLs titers. Th1 expression, Th1/Th2 ratio and Treg expression were
decreased along with a higher aPL concentration, although the Th2
and Th17 expression was increased.
 | Table IICorrelation between Th subtypes and
aPL titers. |
Table II
Correlation between Th subtypes and
aPL titers.
| R | P-value |
|---|
|
CD4+INF-γ+/CD4+(Th1) | −0.702 | 0.000 |
|
CD4+IL-4+/CD4+(Th2) | 0.468 | 0.009 |
| Th1/Th2 ratio | −0.752 | 0.000 |
|
CD4+IL-17A+/CD4+(Th17) | 0.617 | 0.000 |
|
CD4+CD25+Foxp3+/CD4+(Treg) | −0.727 | 0.000 |
Discussion
Th1/Th2 imbalance in APS
In this study we found that the Th1 frequencies were
lower after a 48-h culturing with aPLs, while the Th2 frequencies
showed a rising tendency, and the Th1/Th2 ratio was expressly
decreased. In conclusion, aPL antibodies at higher concentrations
induce significant Th2 dominance.
Certain studies demonstrate that a shift from Th1-
to Th2-driven humoral immunity had been found in normal pregnancies
and considered to be beneficial for an immunologically successful
continuation of the pregnancy (15,16).
Regarding the Th1/Th2 imbalance in patients with recurrent
abortion, most scientists reported an increased Th1 expression
(17,18), while immunotherapy may induce the
dominance of Th2 cells (18),
which may reduce the abortion rate. The participants with recurrent
abortion discussed in the aforementioned articles, however, had no
positive aPL antibodies, thus it remains uncertain whether the
Th1/Th2 paradigm shift in recurrent abortion is caused by aPLs. We
only found a few relevant studies and their conclusions were
contradictory (8–13), possibly resulting from differences
in the experimental approaches. Th1 and Th2 responses have both
been reported to play a prominent role in the pathogenesis of
aPL-associated tissue injuries. For a normal pregnancy, the aPL
level must be maintained within an appropriate range, as an
overshift to either side may induce a miscarriage.
Treg downregulation in APS model
Recently,
CD4+CD25+Foxp3+ Treg cells were
recognized to play a crucial role in the maintenance of normal
immune tolerance. The functions of effector T cells, such as Th1,
Th2 and Th17 were regulated by Treg cells. In certain autoimmune
diseases, such as systemic lupus erythematosus (SLE), rheumatoid
arthritis (RA), primary Sjogren’s syndrome (pSs) and multiple
sclerosis (MS), Treg cells have been reported to decrease in number
(19–23). Decreased Treg cells also
contributed to recurrent abortion in the absence of
antiphospholipid syndrome (24–26).
In the present study, we demonstrated that the
frequencies of Treg cells were significantly lower subsequent to
aPL culturing, both in the 15% and the 25% aPL group, compared to
the control group. The aPLs induced significant Treg downregulation
even at lower concentrations, which was partly consistent with the
study conducted by Fu et al on APS in a mouse model
(14). Treg downregulation may be
another reason for recurrent abortion in APS.
Th17 upregulation
Th17, a novel subtype of T helper cells, actively
participates in inflammation and autoimmunity and is distinct from
the well-described Th1 and Th2 cells (27–28).
Th17 cells have been reported to accumulate in individuals with
recurrent abortions without APS (29–31);
their role in APS, however, has never been reported. In the present
study, we have initially found that the frequencies of Th17 cells
were higher subsequent to aPL-culturing, and were also
dose-dependent.
In addition, a Th17/Treg imbalance was observed in
our study, which may be another cause of APS, as naïve
CD4+ T cells (nTh) differentiate into Treg cells under
the influence of TGF-β. However, when exposed to TGF-β and IL-6,
nTh cells develop into Th17 cells (32). The Th17/Treg cells have a complex
relationship, and Th17 cells may affect Treg cell-induced
transplant tolerance.
The possible pathogenic titers of
aPLs
The latest revised approach to diagnosis is that APS
should be detected twice, at least 12 weeks apart, due to medium
and high titers antibodies. After decades of clinical research on
APS, scholars have found that aPL >40 GPL or MPL unit (1 μg/ml
affinity purification of IgG or IgM anti-cardiolipin antibody) may
lead to clinical manifestations, while others found aPL even >20
GPL or MPL unit may affect prognosis. In in vitro studies
Ferrara et al found that aPL antibodies upregulate tissue
factor expression in epithelial cells in a dose-dependent manner,
which is associated with thrombogenic effects (33), while Mulla et al found a
decreased viability in trophoblast cells in response to the
elevated anti-β2GPI antibody titers (34). Since aPL concentrations are
associated with the disease, it is necessary to determine the
appropriate concentration that would provide sufficient but not
excessive treatment.
In the present study, we distinguished 4 groups with
different aPL concentrations (5, 10, 15 and 25% groups) and found
the expression of the four Th subsets and Th1/Th2 ratios have
dose-dependent changes. At higher concentrations the differences in
the Th subtypes were statistically significant. In the 25% aPL
group, all 4 Th subtypes and the Th1/Th2 ratio showed significant
changes. In the 15% aPL group, only the Th1 and Treg expression and
Th1/Th2 ratio showed significant changes. In other words, the Th
subtypes changed even if the concentration was less than 40 GPL
units. The results of the present study revealed that clinical
treatment was required not only for patients with an aPL titer
>40 GPL or MPL unit, but also for patients with lower titers. In
order to determine the specific aPL concentration required for
treatment, further research is needed.
In conclusion, the data presented in this study
demonstrated that the aPL titers play a crucial role in the
pathogenesis of APS. These results also demonstrated that there is
a Th1/Th2 imbalance, a Th17 upregulation and a Treg downregulation
in APS, and that these factors are positively correlated with the
antibody titers, suggesting a potential role of Th cells in the
pathogenesis of APS. The Th cell changes provide a novel method for
the treatment of patients with APS.
References
|
1
|
Miyakis S, Lockshin MD, Atsumi T, et al:
International consensus statement on an update of the
classification criteria for definite antiphospholipid syndrome
(APS). J Thromb Haemost. 4:295–306. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Silver RM, Porter TF, van Leeuween I, Jeng
G, Scott JR and Branch DW: Anticardiolipin antibodies: clinical
consequences of ‘low titers’. Obstet Gynecol. 87:494–500. 1996.
|
|
3
|
Levine SR, Salowich-Palm L, Sawaya KL, et
al: IgG anticardiolipin antibody titer > 40 GPL and the risk of
subsequent thrombo-occlusive events and death. A prospective cohort
study. Stroke. 28:1660–1665. 1997.
|
|
4
|
Erkan D, Barbhaiya M, George D,
Sammaritano L and Lockshin M: Moderate versus high-titer
persistently anticardiolipin antibody positive patients: are they
clinically different and does high-titer anti-beta 2-glycoprotein-I
antibody positivity offer additional predictive information? Lupus.
19:613–619. 2010. View Article : Google Scholar
|
|
5
|
Tuhrim S, Rand JH, Wu XX, et al: Elevated
anticardiolipin antibody titer is a stroke risk factor in a
multiethnic population independent of isotype or degree of
positivity. Stroke. 30:1561–1565. 1999. View Article : Google Scholar
|
|
6
|
Bakimer R, Fishman P, Blank M, Sredni B,
Djaldetti M and Shoenfeld Y: Induction of primary antiphospholipid
syndrome in mice by immunization with a human monoclonal
anticardiolipin antibody (H-3). J Clin Invest. 89:1558–1563. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Pierangeli SS and Harris EN: Induction of
phospholipid-binding antibodies in mice and rabbits by immunization
with human beta 2 glycoprotein 1 or anticardiolipin antibodies
alone. Clin Exp Immunol. 93:269–272. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Krause I, Blank M, Levi Y, Koike T, Barak
V and Shoenfeld Y: Anti-idiotype immunomodulation of experimental
anti-phospholipid syndrome via effect on Th1/Th2 expression. Clin
Exp Immunol. 117:190–197. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fischer K, Collins H, Taniguchi M,
Kaufmann SH and Schaible UE: IL-4 and T cells are required for the
generation of IgG1 isotype antibodies against cardiolipin. J
Immunol. 168:2689–2694. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Soltesz P, Der H, Veres K, et al:
Immunological features of primary anti-phospholipid syndrome in
connection with endothelial dysfunction. Rheumatology (Oxford).
47:1628–1634. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Karakantza M, Theodorou GL, Meimaris N, et
al: Type 1 and type 2 cytokine-producing CD4+ and CD8+ T cells in
primary antiphospholipid syndrome. Ann Hematol. 83:704–711.
2004.
|
|
12
|
Amital H, Gilburd B and Shoenfeld Y:
Probiotic supplementation with Lactobacillus casei (Actimel)
induces a Th1 response in an animal model of antiphospholipid
syndrome. Ann N Y Acad Sci. 1110:661–669. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Visvanathan S and McNeil HP: Cellular
immunity to beta 2-glycoprotein-1 in patients with the
antiphospholipid syndrome. J Immunol. 162:6919–6925.
1999.PubMed/NCBI
|
|
14
|
Fu J, Fy Q and Si CP: Changes of CD4+CD25+
regulatory T cells and foxp3 expression in rats with experimental
anti-phospholipid antibody syndrome. Matern Child Healthcare Chin.
25:821–824. 2010.
|
|
15
|
Lin H, Mosmann TR, Guilbert L,
Tuntipopipat S and Wegmann TG: Synthesis of T helper 2-type
cytokines at the maternal-fetal interface. J Immunol.
151:4562–4573. 1993.PubMed/NCBI
|
|
16
|
Marzi M, Vigano A, Trabattoni D, et al:
Characterization of type 1 and type 2 cytokine production profile
in physiologic and pathologic human pregnancy. Clin Exp Immunol.
106:127–133. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sugiura-Ogasawara M, Furukawa TA, Nakano
Y, Hori S, Aoki K and Kitamura T: Depression as a potential causal
factor in subsequent miscarriage in recurrent spontaneous aborters.
Hum Reprod. 17:2580–2584. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yokoo T, Takakuwa K, Ooki I, Kikuchi A,
Tamura M and Tanaka K: Alteration of TH1 and TH2 cells by
intracellular cytokine detection in patients with unexplained
recurrent abortion before and after immunotherapy with the
husband’s mononuclear cells. Fertil Steril. 85:1452–1458.
2006.PubMed/NCBI
|
|
19
|
Bonelli M, Savitskaya A, von Dalwigk K, et
al: Quantitative and qualitative deficiencies of regulatory T cells
in patients with systemic lupus erythematosus (SLE). Int Immunol.
20:861–868. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Behrens F, Himsel A, Rehart S, et al:
Imbalance in distribution of functional autologous regulatory T
cells in rheumatoid arthritis. Ann Rheum Dis. 66:1151–1156. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li X, Qian L, Wang G, et al: T regulatory
cells are markedly diminished in diseased salivary glands of
patients with primary Sjogren’s syndrome. J Rheumatol.
34:2438–2445. 2007.PubMed/NCBI
|
|
22
|
Dalla Libera D, Di Mitri D, Bergami A, et
al: T regulatory cells are markers of disease activity in multiple
sclerosis patients. PLoS One. 6:e213862011.PubMed/NCBI
|
|
23
|
Miyara M, Gorochov G, Ehrenstein M, Musset
L, Sakaguchi S and Amoura Z: Human FoxP3+ regulatory T cells in
systemic autoimmune diseases. Autoimmun Rev. 10:744–755. 2011.
|
|
24
|
Sasaki Y, Sakai M, Miyazaki S, Higuma S,
Shiozaki A and Saito S: Decidual and peripheral blood CD4+CD25+
regulatory T cells in early pregnancy subjects and spontaneous
abortion cases. Mol Hum Reprod. 10:347–353. 2004.
|
|
25
|
Fraccaroli L, Alfieri J, Larocca L, et al:
A potential tolerogenic immune mechanism in a trophoblast cell line
through the activation of chemokine-induced T cell death and
regulatory T cell modulation. Hum Reprod. 24:166–175. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Arruvito L, Sotelo AI, Billordo A and
Fainboim L: A physiological role for inducible FOXP3(+) Treg cells.
Lessons from women with reproductive failure. Clin Immunol.
136:432–441. 2010.
|
|
27
|
McKenzie BS, Kastelein RA and Cua DJ:
Understanding the IL-23-IL-17 immune pathway. Trends Immunol.
27:17–23. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Steinman L: A brief history of T(H)17, the
first major revision in the T(H)1/T(H)2 hypothesis of T
cell-mediated tissue damage. Nat Med. 13:139–145. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang WJ, Hao CF, Qu QL, Wang X, Qiu LH and
Lin QD: The deregulation of regulatory T cells on
interleukin-17-producing T helper cells in patients with
unexplained early recurrent miscarriage. Hum Reprod. 25:2591–2596.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Liu YS, Wu L, Tong XH, et al: Study on the
relationship between Th17 cells and unexplained recurrent
spontaneous abortion. Am J Reprod Immunol. 65:503–511. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Nakashima A, Ito M, Shima T, Bac ND,
Hidaka T and Saito S: Accumulation of IL-17-positive cells in
decidua of inevitable abortion cases. Am J Reprod Immunol. 64:4–11.
2010.PubMed/NCBI
|
|
32
|
Ogura H, Murakami M, Okuyama Y, et al:
Interleukin-17 promotes autoimmunity by triggering a
positive-feedback loop via interleukin-6 induction. Immunity.
29:628–636. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ferrara DE, Swerlick R, Casper K, et al:
Fluvastatin inhibits up-regulation of tissue factor expression by
antiphospholipid antibodies on endothelial cells. J Thromb Haemost.
2:1558–1563. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Mulla MJ, Brosens JJ, Chamley LW, et al:
Antiphospholipid antibodies induce a pro-inflammatory response in
first trimester trophoblast via the TLR4/MyD88 pathway. Am J Reprod
Immunol. 62:96–111. 2009. View Article : Google Scholar : PubMed/NCBI
|