Introduction
The management of ovarian cancer is a major
challenge for gynecological oncologists. Since the symptoms of
ovarian cancer are non-specific, more than 2/3 of the cases are
diagnosed at advanced stages (1,2).
Debulking surgery followed by chemotherapy continues to be the
standard treatment for advanced ovarian cancer (3). Despite a high response to the initial
therapy, many of the patients relapse eventually and succumb to
chemotherapy-resistant diseases. As a result, the overall 5-year
survival rate of advanced ovarian cancer remains low, at
approximately 30% (1,4). The poor outcome of ovarian cancer
necessitates our efforts towards better understanding its
biological behavior and identifying new prognostic and therapeutic
targets.
The mediator complex subunit 19 (MED19) was
originally recognized in a search for mutants with increased
aerobic expression of the CYC7 gene (5,6). The
mediator complex is a multiprotein transcriptional co-activator
that is expressed ubiquitously in eukaryotes and is required for
the induction of RNA polymerase II transcription (7–9). A
series of mediator complexes has been identified in mammals by
multidimensional protein identification technology. These complexes
include the thyroid hormone receptor-associated protein/
SRB-Med-containing co-factor (TRAP/SMCC), the activator-recruited
factor-large (ARC-L), vitamin D receptor-interacting protein
(DRIP), mouse mediator, positive co-factor 2 (PC2) and the
co-factor required for Sp1 transcriptional activation (CRSP)
complexes (10).
The aberrant transcription of genes is believed to
be one of the causes of human cancer. The silencing of MED19 may
interfere with the transcriptional process and has been
demonstrated to inhibit the growth of pancreatic cancer (11). The role of MED19 in ovarian cancer,
however, has never been reported. The goal of this study was to
investigate the effect of MED19 gene silencing on cell viability
and tumor growth in ovarian cancer. Our results demonstrated that
MED19 expression is correlated with malignancy and histological
grading of human ovarian tumors. The knockdown of MED19 with
lentivirus-mediated RNAi reduced the viability of ovarian cancer
cells in vitro and inhibited tumor growth in xenografted
nude mice. These data suggest that MED19 may serve as a potential
target in the treatment of ovarian cancer.
Materials and methods
Cell lines
The SKOV-3 and HEY human ovarian cancer cell lines
were purchased from ATCC (Manassas, VA, USA). The cells were
maintained in RPMI-1640 medium (Gibco, Grand Island, NY, USA)
containing 10% fetal bovine serum (FBS), 100 U/ml penicillin and
100 μg/ml streptomycin and were cultured in a humidified atmosphere
of 5% CO2 at 37°C.
Tissue microarray and
immunohistochemistry
Tissue microarrays of ovarian disease (OV1005) were
obtained from Alenabio (Xi'an, China), and contained 18 ovarian
cystadenoma, 7 varian borderline cystadenoma and 52 ovarian
carcinoma tissue cores with 24 stage I, 9 stage II, 18 stage III
and 1 stage IV ovarian cancer samples.
Tissue microarray sections were stained with rabbit
anti-MED19 antibody (1:250, Abcam Inc., Cambridge, MA, USA) using a
goat anti-rabbit ABC immunohistochemistry kit (Mingrui Inc.,
Shanghai, China). The immunostaining patterns for MED19 were
assessed by Image-Pro Plus software (Media Cybernetics, Silver
Spring, MD, USA).
Construction of lentiviral vectors
To generate lentivirus expressing RNAi specific for
the MED19 gene, the RNA interference sequence for human MED19
(aaGGTGAAGGAGA AGCTAAGT) was designed with the manufacturer's RNAi
Designer program, and the negative control construct was created
with a scrambled sequence (TTCTCCGAACGTGTC ACGT). The segments of
nucleotides were cloned into the HpaI and XhoI sites
of the pGCSIL-GFP vector (GeneChem, Shanghai, China) to generate
pGCSIL-GFP-MED19 (siMed) and pGCSIL-GFP-nonsense (siNon).
Lentivirus transduction
Cells were plated at 40–50% confluence and incubated
at 37°C. After 24 h of incubation, cells were infected with
recombinant lentiviral vectors at a multiplicity of infection of 40
and then incubated at 37°C for 10 h. The viral supernatant was then
replaced with fresh medium. At day 5 post-transduction, the
knockdown efficiency of RNAi was determined by quantitative
real-time RT-PCR and western blot analysis.
RNA extraction and quantitative real-time
RT-PCR
Total RNA was extracted from SKOV-3 cells with
TRIzol reagent (Invitrogen, Carlsbad, CA, USA). The RNA expression
levels of MED19 were detected by quantitative real-time RT-PCR
using the SYBR-Green RT-PCR kit (Takara) according to the
manufacturer's instructions. Briefly, the cDNA was synthesized
using the RevertAid First-Strand cDNA Synthesis kit (Fermentas,
Lithuania). The cDNA was used as the template and was subjected in
triplicate to denaturation at 94°C for 1 min and 40 cycles at 94°C
for 5 sec and at 60°C for 31 sec in an ABI PRISM 7000 Sequence
Detection System (Applied Biosystems, Foster City, CA, USA). The
specific primer pairs were as follows: MED19 (107 bp) sense,
5′-TGACAGGCAGCACGAATC-3′ and antisense, 5′-CAGGTCAGGCAGGAAGTTAC-3′;
β-actin (202 bp) sense, 5′-GGCGGCACCACCATGTACCCT-3′ and antisense,
5′-AGGGGCCGGACTCGTCATACT-3′. The fold change in the expression of
MED19 was calculated using the −ΔΔCt method, with β-actin as the
internal control.
Western blot analysis
The cultured cells were washed with
phosphate-buffered saline and then lysed with RIPA solution (Bocai,
Shanghai, China). The lysate was cleared by centrifugation at
12,000 rpm for 30 min and the protein was then quantified using the
bicinchoninic acid (BCA) method (Bocai) according to the
manufacturer's instructions. A total of 30 μg/lane proteins were
resolved by SDS-PAGE and transferred onto a PVDF membrane
(Millipore, Billerica, MA, USA). After blocking with 5% BSA-TBST
for 1 h at room temperature, the membrane was incubated with
anti-MED19 (1:250, Abcam Inc.) and anti-GAPDH antibody (1:5,000;
Kangchen, Shanghai, China) overnight at 4°C. The membrane was then
incubated with HRP-conjugated secondary antibodies (Kangchen)
(1:5,000) for 1 h at 37°C. Chemiluminescent detection was performed
using the ECL kit (Perkin-Elmer, Waltham, MA, USA).
MTT assay
Cell viability and proliferation were evaluated
using a modified 3-(4,5-dimethyldiazol-2-yl)-2,5-diphenylte
trazolium bromide (MTT) assay. Cells in exponential growth were
plated at a final concentration of 4×103 cells/well in
96-well culture plates and were harvested at 1, 2, 3, 4 and 5 days
later accordingly. A total of 20 μl of MTT (5 mg/ml)
(Sigma-Aldrich, St. Louis, MO, USA) was added to the cells. After
incubation for an additional 4 h, the reaction was stopped by
removal of the supernatant and 100 μl DMSO was added to dissolve
the formazan product. After 30 min, the optical density (OD) of
each well was measured at 570 nm using an ELISA reader.
BrdU incorporation assay
DNA synthesis in the proliferating cells was
determined by measuring the BrdU incorporation. SKOV-3 and HEY
cells transduced with siMed or siNon were seeded in 96-well culture
plates at a density of 4×103 cells/well and cultured for
24 or 48 h. The cells were then incubated with BrdU (BD Pharmingen,
San Diego, CA, USA) at a final concentration of 10 μM for 2–24 h.
The medium was removed at the end of incubation, and the cells were
fixed with 70% alcohol and labeled with a peroxidase-conjugated
anti-BrdU antibody (Sigma-Aldrich). After incubation for 60 min,
the cells were washed 3 times with phosphate-buffered saline.
Tetramethylbenzidine was added thereafter as the peroxidase
substrate for 30 min and the absorbance values were measured at 490
nm using an ELISA reader.
Colony formation assay
The effect of MED19 gene silencing on colony
formation in SKOV-3 cells was analyzed by colony formation assays.
Cells (3×102) were plated in 10-cm culture dishes and
cultured in 10% FBS RPMI-1640 and 5% CO2 at 37°C for 2
weeks. The cell colonies were washed twice with phosphate-buffered
saline and fixed by 4% PFA for 15 min. Cells were then stained with
Giemsa for 20 min and washed twice with ddH2O. Visible
colonies were manually counted.
Cell cycle analysis by flow
cytometry
Cellular DNA content was detected with a flow
cytometer (BD, Franklin Lakes, NJ, USA), in which 5×104
events were collected. The list mode data were regrouped into DNA
histograms, and individual cell cycle phase fractions were
quantified using ModFit 3.0 analysis software (Verity Software
Inc., Topsham, ME, USA).
Tumor xenografts in nude mice
Female BALB/c mice were treated according to the
protocol approved by the Ethics Committee of Fudan University.
Six-week-old mice (n=4 per group) were injected subcutaneously
either with parent SKOV-3 cells, siNon-transduced SKOV-3 cells or
siMed-transduced SKOV-3 cells (1×107 cells in 200 μl
RPMI-1640) in the right forelimbs. The mice were sacrificed at day
42 after injection. Tumor size was measured with calipers weekly
and tumor volume was calculated using the following formula: tumor
volume (mm3) = tumor length (mm) × tumor width (mm)/2.
Bodyweight was also measured to assess any side-effects.
Statistical analysis
Immunohistochemistry data were analyzed by one-way
ANOVA using SPSS software (Release 15.0, SPSS Inc.). Data from the
in vitro studies were expressed as the means ± SE. The
difference between the 2 groups was analyzed by one-way ANOVA
followed by Dunnett's test. P<0.05 was considered to indicate a
statistically significant difference. Representative images from
microscopy and western blot analysis are shown.
Results
Expression levels of MED19 in benign,
borderline and malignant ovarian tumor tissue
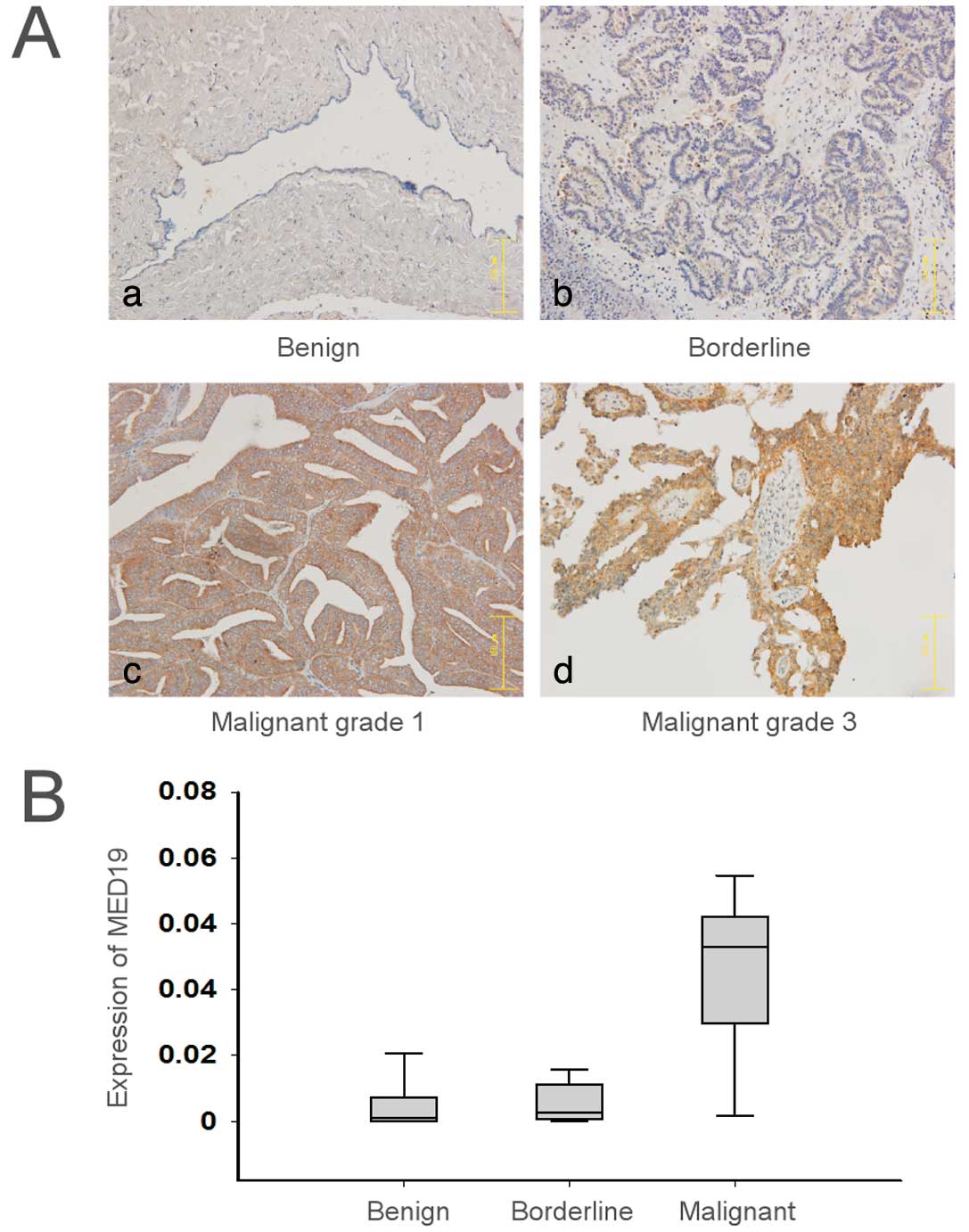
MED19 expression in ovarian tumor tissue was
examined by immunohistochemistry in a tissue microarray that
contained ovarian cystadenoma, borderline cystadenoma and carcinoma
samples. Following the incubation of the tissue microarrays with
anti-MED19 antibody, the staining was mainly confined to the tumor
tissue, whereas the majority of the mesenchymal tissue was
unstained (Fig. 1A). The mean
MED19 expression was significantly lower in the benign
(0.008±0.009) or borderline ovarian cystadenomas (0.006±0.006)
compared to the ovarian carcinomas (0.054±0.030) (P<0.01)
(Fig. 1B). Among the ovarian
carcinoma tissues, MED19 expression was higher in stage II and III
than in stage I tumors (P<0.05). MED19 expression in the ovarian
carcinoma tissues, however, did not correlate with age, tumor stage
or histological type (P>0.05) (Table I).
 | Table IExpression levels of MED19 in ovarian
cancer and clinical factors. |
Table I
Expression levels of MED19 in ovarian
cancer and clinical factors.
| Clinical factors | N | MED19 expression | P-value |
|---|
| Age (years) |
| <55 | 31 | 0.057±0.033 | |
| ≥55 | 23 | 0.051±0.025 | 0.472 |
| Tumor stage |
| I, II | 35 | 0.054±0.031 | |
| III, IV | 19 | 0.057±0.030 | 0.821 |
| Pathological
grade |
| Grade 1 | 10 | 0.041±0.025 | |
| Grades 2 and 3 | 40 | 0.059±0.031 | 0.047 |
| Histological
type |
| Serous | 36 | 0.057±0.028 | |
| Mucinous | 4 | 0.049±0.032 | |
| Endometroid | 14 | 0.050±0.038 | |
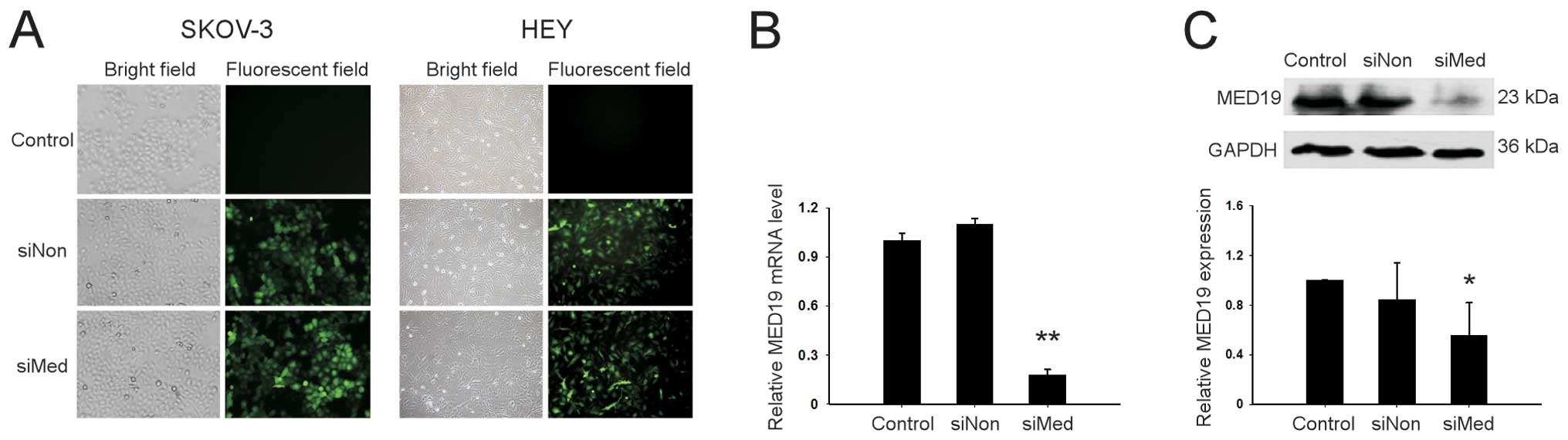
Knockdown of MED19 with lentiviral
transduction in ovarian cancer cells
The SKOV-3 and HEY human ovarian cancer cells were
infected with lentivirus that contained either siMed or siNon.
Efficient transduction was confirmed by GFP expression in cells
observed under a fluorescent microscope (Fig. 2A). Twenty-four hours after
transduction, the MED19 mRNA expression was downregulated by 84% in
the cells transduced with siMed compared to those transduced with
siNon (P<0.01) (Fig. 2B). A
similar result was noted in western blot analysis which showed that
MED19 protein expression was downregulated in the SKOV-3 cells
following siMed transduction (Fig.
2C).
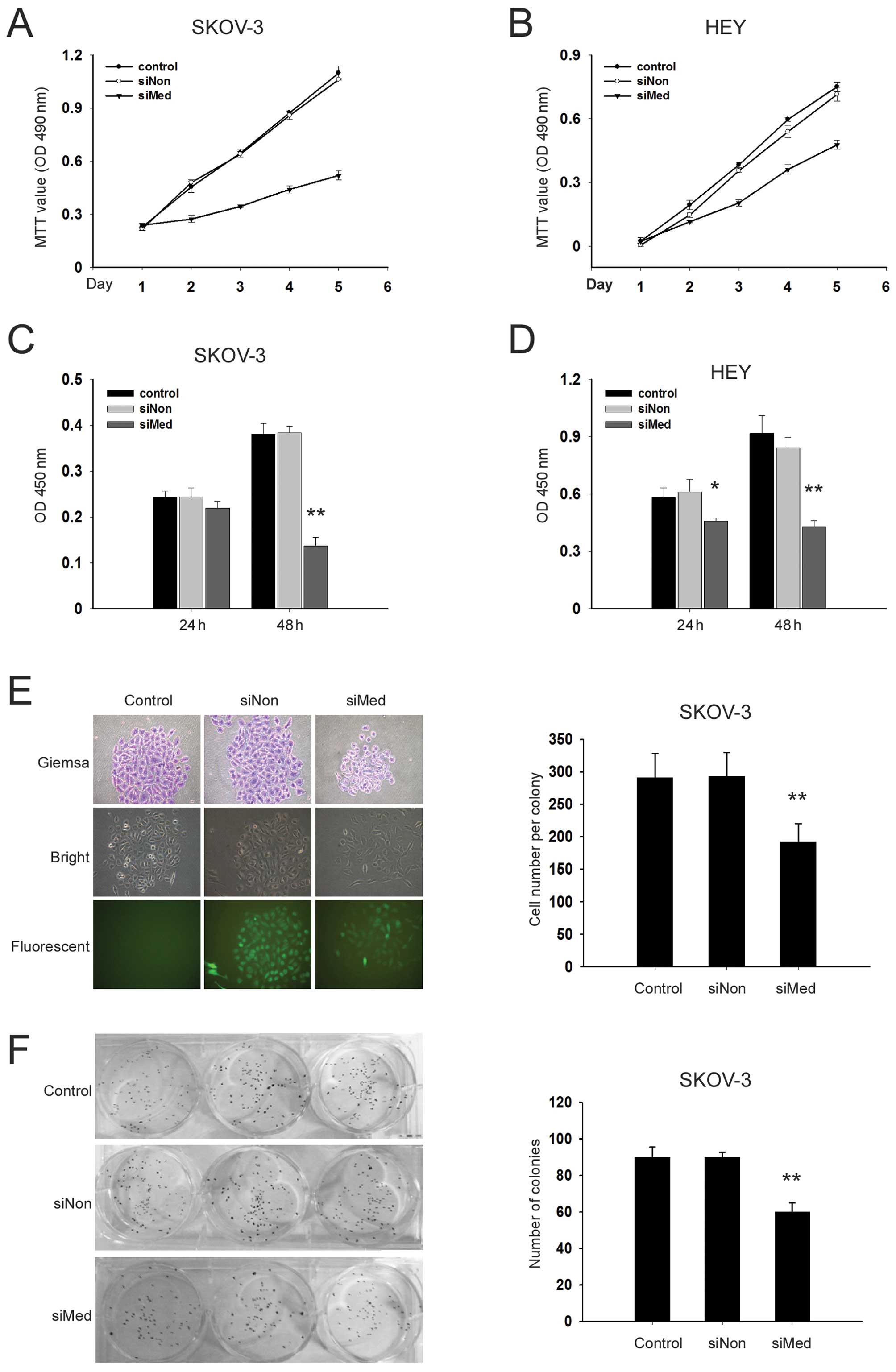
Knockdown of MED19 inhibits the
proliferation of ovarian cancer cells
The proliferation dynamics of the parental,
siNon-transduced and siMed-transduced cells were determined by MTT
assays. Cell proliferation was monitored for 5 consecutive days
following lentiviral infection. The results showed that the growth
of SKOV-3 and HEY cells transduced with siMed was significantly
inhibited compared with the siNon-transduced and parental cells
(Fig. 3A and B). A 70% reduction
in viability was noted in the SKOV-3 cells with MED19 gene
silencing compared with the siNon group on day 5.
To further investigate the effects of MED19 gene
silencing on DNA synthesis in ovarian cancer cells, a BrdU
incorporation assay was performed. Following treatment with siMed
for 24 h, the rate of DNA synthesis was not significantly affected
in the SKOV-3 and HEY cells. After 48 h of transduction with siMed,
however, the DNA synthesis was suppressed by 39% in the SKOV-3
cells and 41% in the HEY cells compared to the cells that were
transduced with siNon (Fig. 3C and
D).
As the HEY cells were not capable of forming
colonies in this study, SKOV-3 cells were used to examine the
effects of MED19 gene silencing on colony forming potential. The
results showed that the siMed-transduced SKOV-3 cells formed fewer
cells in each colony and 33% fewer colonies compared to the
siNon-transduced cells (P<0.05) (Fig. 3E and F).
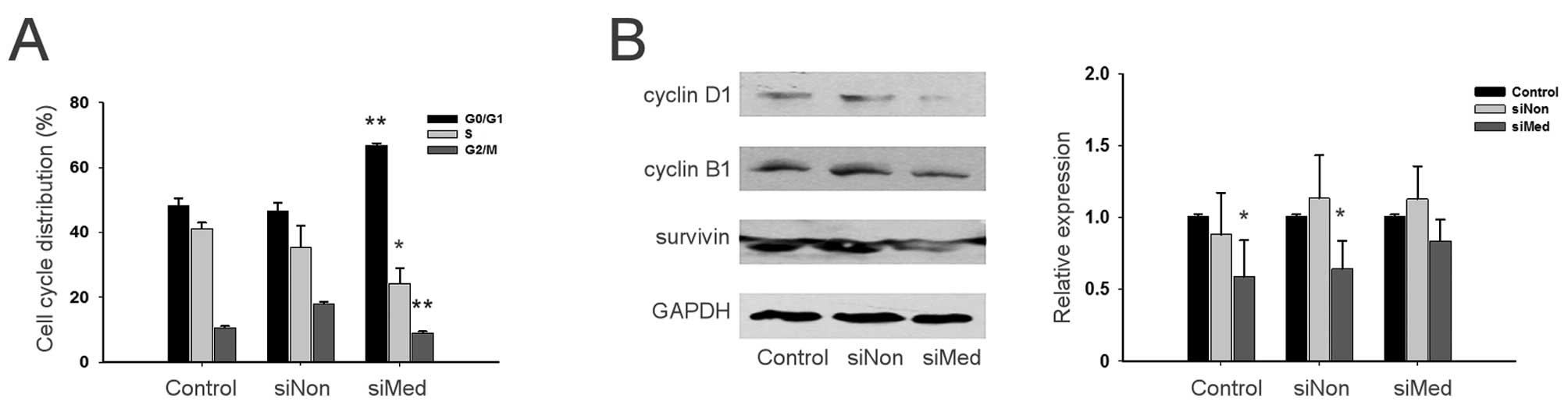
Knockdown of MED19 induces cell cycle
arrest in ovarian cancer cells
Cellular DNA contents of parental, siNon-transduced
and siMed-transduced cells were analyzed by fluorescence-activated
cell sorting to determine their cell cycle status. The results
demonstrated that the siMed-transduced SKOV-3 and HEY cells had a
higher percentage of cells in the G0/G1 phase (66.8 vs. 46.6% in
SKOV-3 cells and 73.9 vs. 68.8% in HEY cells) and a lower
percentage of cells progressing into the S phase (24.4 vs. 35.4% in
SKOV-3 cells and 17.3 vs. 22.8% in HEY cells) compared to the
siNon-transduced cells (Fig.
4A).
Expression of cyclin D1, cyclin B1 and
survivin detected by western blot analysis to verify cell cycle
status
The transduction of SKOV-3 and HEY cells with siMed
decreased the expression levels of cyclin D1 and cyclin B1. The
expression level of survivin was also downregulated by MED19 gene
silencing (Fig. 4B), which
indicated that the knockdown of MED19 arrested the cell cycle
progression of ovarian cancer cells.
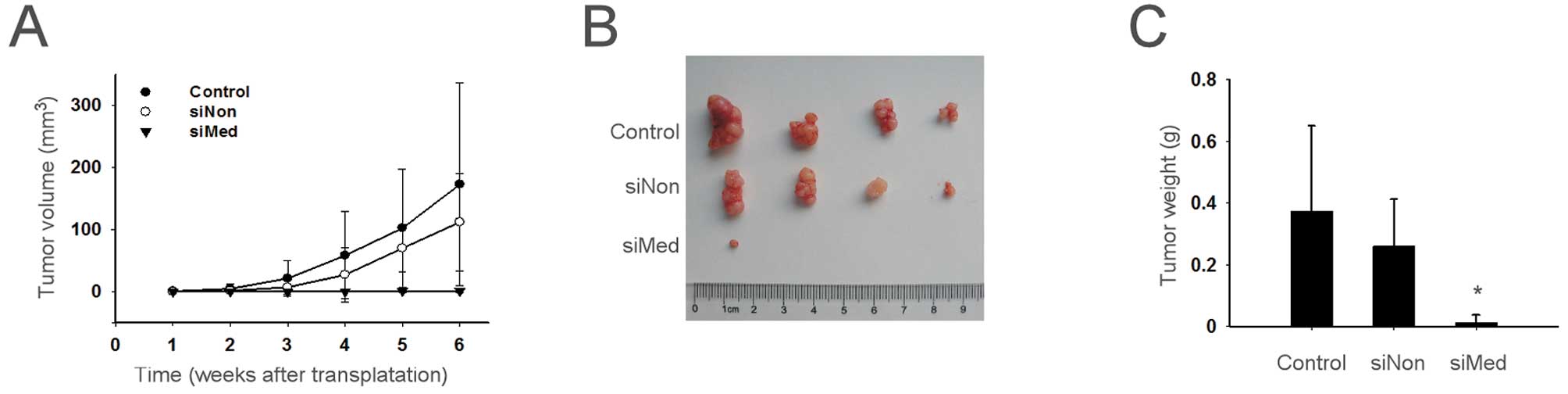
Knockdown of MED19 inhibits the growth of
ovarian cancer in vivo
The effect of MED19 gene silencing on the growth of
ovarian cancer was analyzed in nude mice that were injected with
siMed-transduced cells. Compared with the mice that were injected
with parental and siNon-transduced cells, tumor growth was
significantly delayed in the siMed group, which was evident from 2
weeks after transplantation until the day when the mice were
sacrificed (Fig. 5). At day 42,
the xenograft tumor weights were 372.5±277.6 mg in the mice
injected with parental cells, 260.0±126.6 mg in the siNon group and
12.5±2.5 mg in the siMed group. No marked change in behavior,
appearance or body weight was observed in the studied mice.
Discussion
Ovarian cancer is one of the most lethal
gynecological malignancies. Although new chemotherapeutic agents
have improved the survival rate for ovarian cancer patients over
the past few decades, overall mortality from the disease still
remains high. For patients in stage III and IV, the 5-year survival
is approximately 34 and 18%, respectively (13–16).
The reduced 5-year survival rates necessitate the need to develop
an improved understanding of cell growth and death in ovarian
cancer to identify new etiologic, prognostic or therapeutic
targets.
MED19 is a component of the mediator complex, which
is a co-activator for DNA binding factors that activate
transcription via RNA polymerase II. Mediator is recruited to
promoters by direct interactions with regulatory proteins and
serves as a scaffold for the assembly of a functional
pre-initiation complex with RNA polymerase II and general
transcription factors. In this capacity, MED19 plays an essential
role in regulating the assembly and/or activity of RNA polymerase
II transcription complexes on core promoters (12). Though MED19 gene silencing has been
reported to inhibit the growth of pancreatic cancer, the role of
MED19 in ovarian cancer remains unknown. Therefore, we genetically
downregulated the expression of MED19 in the SKOV-3 and HEY human
ovarian cancer cell lines by infecting them with MED19-specific
RNAi-expressing lentivirus and determined the role of MED19 in the
proliferation and growth of tumor cells. We found that the
downregulation of endogenous MED19 expression inhibited the growth,
decreased the proliferation and led to cell cycle arrest in ovarian
cancer cells. To the best of our knowledge, this is the first study
to show that the mediator complex is important in ovarian cancer
cell growth and proliferation.
The expression of MED19 correlated with the
progression of ovarian carcinoma, suggesting the relevance of MED19
to the tumorigenesis of ovarian cancer. The higher MED19 expression
was associated with a poorer differentiation, indicating that MED19
is also involved in the regulation of differentiation. Due to the
lack of follow-up data, we were unable to determine whether a
correlation exists between the survival rates and MED19 expression
in ovarian cancer.
Gene therapy, an applied form of biotechnology, is a
new, innovative approach to the treatment of human cancer (17–20).
The therapeutic efficiency of cancer gene therapy strategies
strongly depends on the efficiency of gene transfer. Lentivirus, a
member of slow viruses of the Retroviridae family, has been
characterized as having a long incubation period. Lentivirus is
capable of delivering a large amount of genetic information into
the host cell and is one of the most efficient methods of gene
delivery. Several studies have focused on evaluating the
therapeutic effects of gene silencing in a variety of diseases,
particularly since antisense technology allows for the highly
specific and effective downregulation of target gene expression
(17–20). RNAi is a useful tool for the
functional analysis of genes and may be a potential therapeutic
strategy for various diseases, including cancers. Thus, the
combination of lentivirus and RNAi may provide a novel therapeutic
strategy for cancer gene therapy.
Our study using the 2 human ovarian cancer cell
lines, SKOV-3 and HEY, showed that the lentivirus-mediated
downregulation of MED19 decreased cell proliferation and growth.
Therefore, a new strategy via the reduction of MED19 expression
mediated by lentivirus may be useful in the treatment of human
ovarian cancer.
Many other mediator complexes exist in the mediator
gene family. Whether other members of the mediator complex are
abnormally regulated in human ovarian cancer or other human cancers
remains unknown. The effectiveness of MED19 RNAi therapy in human
ovarian cancer and other human cancer warrants further
investigation.
Acknowledgements
This research was sponsored by the Fundamental
Research Funds for the Central Universities of Fudan
University.
References
|
1
|
Colombo N, van Gorp T, Parma G, et al:
Ovarian cancer. Crit Rev Oncol Hemat. 60:159–179. 2006. View Article : Google Scholar
|
|
2
|
Jacobs IJ and Menon U: Progress and
challenges in screening for early detection of ovarian cancer. Mol
Cell Proteomics. 3:355–366. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Armstrong DK, Bundy B, Wenzel L, et al:
Intraperitoneal cisplatin and paclitaxel in ovarian cancer. N Engl
J Med. 354:34–43. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jemal A, Siegel R, Ward E, et al: Cancer
statistics 2007. CA Cancer J Clin. 57:43–66. 2007. View Article : Google Scholar
|
|
5
|
Rosenblum-Vos LS, Rhodes L, Evangelista
CC, et al: The ROX3 gene encodes an essential nuclear protein
involved in CYC7 gene expression in Saccharomyces
cerevisiae. Mol Cell Biol. 11:5639–5647. 1991.PubMed/NCBI
|
|
6
|
Gustafsson CM, Myers LC, Li Y, et al:
Identification of Rox3 as a component of mediator and RNA
polymerase II holoenzyme. J Biol Chem. 272:48–50. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jiang YW, Veschambre P, Erdjument-Bromage
H, et al: Mammalian mediator of transcriptional regulation and its
possible role as an end-point of signal transduction pathways. Proc
Natl Acad Sci USA. 95:8538–8543. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kornberg RD: Mediator and the mechanism of
transcriptional activation. Trends Biochem Sci. 30:235–239. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kim YJ, Bjorklund S, Li Y, et al: A
multiprotein mediator of transcriptional activation and its
interaction with the c-terminal repeat domain of RNA-polymerase II.
Cell. 77:599–608. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sato S, Tomomori-Sato C, Parmely TJ, et
al: A set of consensus mammalian mediator subunits identified by
multidimensional protein identification technology. Mol Cell.
14:685–691. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li XH, Fang DN and Zeng CM: Knockdown of
MED19 by short hairpin RNA-mediated gene silencing inhibits
pancreatic cancer cell proliferation. Cancer Biother Radiopharm.
26:495–501. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Shih IeM and Davidson B: Pathogenesis of
ovarian cancer: clues from selected overexpressed genes. Future
Oncol. 5:1641–1657. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Pisano C, Bruni GS, Facchini G, et al:
Treatment of recurrent epithelial ovarian cancer. Ther Clin Risk
Manag. 5:421–426. 2009.PubMed/NCBI
|
|
14
|
Nelson AE, Francis JE and Zorbas H:
Population screening and early detection of ovarian cancer in
asymptomatic women. Aust N Z J Obstet Gynaecol. 49:448–450. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Marth C, Hiebl S, Oberaigner W, et al:
Influence of department volume on survival for ovarian cancer:
results from a prospective quality assurance program of the
Austrian Association for Gynecologic Oncology. Int J Gynecol
Cancer. 19:94–102. 2009.PubMed/NCBI
|
|
16
|
Sato S, Tomomori-Sato C, Banks CA, et al:
Identification of mammalian Mediator subunits with similarities to
yeast Mediator subunits Srb5, Srb6, Med11, and Rox3. J Biol Chem.
278:15123–15127. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Martinez LA, Naguibneva I, Lehrmann H, et
al: Synthetic small inhibiting RNAs: efficient tools to inactivate
oncogenic mutations and restore p53 pathways. Proc Natl Acad Sci
USA. 99:14849–14854. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Rubinson DA, Dillon CP, Kwiatkowski AV, et
al: A lentivirus-based system to functionally silence genes in
primary mammalian cells, stem cells and transgenic mice by RNA
interference. Nat Genet. 33:401–406. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Brummelkamp TR, Bernards R and Agami R:
Stable suppression of tumorigenicity by virus-mediated RNA
interference. Cancer Cell. 2:243–247. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Crnkovic-Mertens I, Hoppe-Seyler F and
Butz K: Induction of apoptosis in tumor cells by siRNA-mediated
silencing of the livin/ML-IAP/KIAP gene. Oncogene. 22:8330–8336.
2003. View Article : Google Scholar : PubMed/NCBI
|