Introduction
Cardiovascular disease (CVD) refers to any disorder
that affects the heart and/or circulatory system. This includes
coronary heart disease, cardiomyopathy, ischemic heart disease,
atherosclerosis and congestive heart failure. CVD has been the most
common cause of mortality worldwide over the last two decades
(1) and has increased the economic
burden due to the high costs of medical treatment (2,3). The
causative factors of CVD include obesity, diabetes, high blood
pressure, high blood cholesterol, smoking and family history. Among
these factors, hypercholesterolemia and unhealthy ratios of the two
smallest lipoprotein cholesterols, low-density lipoprotein (LDL)
cholesterol and high-density lipoprotein (HDL) cholesterol,
resulting from a high-fat diet and impaired lipid metabolism have
been closely linked to CVD incidence and mortality (4,5). LDL
cholesterol is often referred to as bad cholesterol since, while
circulating through the bloodstream, it tends to form deposits in
the walls of the arteries, thereby decreasing artery diameter and
resulting in atherosclerosis. Conversely, HDL cholesterol is
referred to as good cholesterol since it is able to maintain the
healthy state of the inner blood vessel walls by scavenging and
recycling cholesterol by transporting it to the liver where it is
reprocessed (6,7). Accordingly, the blood levels of LDL
and HDL cholesterol are closely associated with the development of
CVD and the LDL cholesterol/HDL cholesterol ratio is used to
calculate the CVD risk factor (4,5). To
reduce the incidence of CVD by achieving the desirable balance
between HDL and LDL cholesterol levels, dietary therapy may be
considered as a first line treatment.
In the present study, we evaluated dietary
supplementation with protamine and chitooligosaccharide (COS) as a
potential anti-CVD therapy. Protamine is a protein from salmon
sperm which contains high levels of arginine, a basic amino acid,
and helps to prevent DNA damage (8). In addition, protamine restricts fat
absorption in the intestine (9) by
markedly inhibiting triglyceride (TG) hydrolysis (10), a process required for the
absorption of lipids in the intestine (11). COS is an oligosaccharide made from
chitin or chitosan, a long-chain polymer of N-acetylglucosamine, by
chemical or enzymatic hydrolysis (12). Chitin and chitosan have limited use
as nutrient sources due to their low solubility and high viscosity
resulting from their polymeric structures (12,13).
By contrast, COS is widely used in food, pharmaceutical and
medicinal formulations as a biomaterial due to its high solubility,
low molecular weight, low viscosity and non-toxicity (14–16).
According to a recent study, COS restores healthy blood pressure,
reduces cholesterol levels and prevents alcoholic liver disease
(17). In addition, COS exerts
protective effects against infections and enhances antitumor
activity (18). COS has also been
shown to reduce plasma lipid levels in healthy men and TG levels in
obese diabetic mice (19–21).
The aim of this study was to evaluate the abilities
of protamine, COS and a mixture of the two compounds to reduce CVD
risk in vivo. We measured the TG, total cholesterol (T-CHO),
LDL cholesterol and HDL cholesterol levels in serum and feces
following the dietary administration of protamine, COS and
combinations of the two to male Sprague Dawley (SD) rats fed a
high-fat diet. We also examined their effects on lipid accumulation
in the rat liver tissues by histochemical analysis. Alterations in
lipid metabolism resulting from the experimental diets including
protamine and COS demonstrated that these two compounds help to
regulate hyperlipidemia and hypercholesterolemia, thereby
decreasing CVD risk in vivo.
Materials and methods
Animal adaptation
Healthy male SD rats were purchased from Central
Laboratory Animal, Inc. (Seoul, Korea). The 7-week-old male SD rats
were housed in a conventional animal facility at the Laboratory
Animal Research Center of Chungbuk National University (Cheongju,
Korea). The animals were allowed to acclimate for 1 week after
arrival. The rats were used for in vivo experiments in
accordance with the approved institutional guidelines of Chungbuk
National University.
High-fat and experimental diet
preparation
Salmon protamine (98% purity; hydrochloride salt;
Maruha Nichiro Foods, Tokyo, Japan) and COS (88% purity; CNA
Biotech, Cheongwon, Korea) were provided by LG Household and Health
Care Ltd. (Daejeon, Korea). A high-fat diet was prepared as a corn
oil suspension by mixing 6 ml corn oil (Sigma-Aldrich, St. Louis,
MO, USA), 80 mg cholic acid (Sigma-Aldrich), 2 mg cholesteryl
oleate (Sigma-Aldrich) and 1 mg margarine (Seoul Milk Ltd., Seoul,
Korea) in 6 ml distilled water (22). The experimental diets were made by
adding protamine, COS and a mixture of the two to the corn oil
suspension in high and low doses.
Administration of experimental diets to
rats
Male SD rats weighing 323.78±4.80 g were divided
into seven groups; 3 ml of each experimental diet was orally
administered once to each rat in the relevant group via a Zonde
needle. The vehicle group (n=5) was treated with the corn oil
suspension alone. The experimental groups (each group, n=5) were
administered the experimental diets of protamine, COS or a mixture
of the two with the corn oil suspension. The treatment groups were:
i) P100, 100 mg protamine/kg body weight (bw); ii) O300, 300 mg
COS/kg bw; iii) PO100/300, 100 mg protamine/kg bw and 300 mg COS/kg
bw; iv) P8.3, 8.3 mg protamine/kg bw; v) O25, 25 mg COS/kg bw; and
vi) PO8.3/25, 8.3 mg protamine/kg bw and 25 mg COS/kg bw. Prior to
the oral administration of the experimental diets, the rats were
starved for 18 h and blood samples were collected from the tail
vein (0 h). Following the oral administration of the diet, blood
samples were collected at 3, 9 and 24 h. Feces were collected from
each group at the same time points and stored at −20°C until
analysis.
Analysis of serum and fecal lipids
Blood (0.5–1 ml) was collected using a Vacuum Serum
Separation Tube (SST; Green Cross Corp., Yongin, Gyeonggi, Korea)
and left at room temperature for 1 h. Serum was isolated from the
blood samples by centrifuging at 3,000 rpm at 4°C for 20 min and
then stored at −20°C. Serum analysis was conducted using a Hitachi
Clinical Analyzer 7080 (Hitachi Korea, Ltd., Seoul, Korea) to
measure the serum concentrations of various lipid components,
including TG, T-CHO, HDL cholesterol and LDL cholesterol. The
atherogenic index (AI), cardiac risk factor (CRF) and CVD risk were
determined based on serum LDL cholesterol, HDL cholesterol and
T-CHO concentrations. The AI was calculated as (T-CHO - HDL
cholesterol)/HDL cholesterol, the CRF was calculated as (T-CHO/HDL
cholesterol) and the CVD risk was calculated as (LDL
cholesterol/HDL cholesterol).
Rat feces from each group was collected for fecal
lipid analysis, and then fecal crude fat was extracted according to
the Rose-Gottlieb method (23).
For this procedure, 5 g feces were placed into a Mojonnier fat
extractor, 6 ml NH4OH (OCI Co., Ltd., Ulsan, Korea) was
added and the mixture was left for 3 min. This mixture was combined
with 12 ml 95% alcohol (OCI Co., Ltd.), 25 ml diethyl ether (OCI
Co., Ltd.) and 25 ml petroleum ether (OCI Co., Ltd.) and then left
at room temperature for 1–2 h. Finally, fecal crude fat was
collected by withdrawing the ether phase at 75°C and analyzed to
determine the TG and T-CHO levels. Fecal TG was measured with a
triglyceride assay kit (Cayman Chemical Co., Ann Arbor, MI, USA)
and fecal T-CHO was analyzed with an Enzychrome cholesterol assay
kit (BioAssay Systems, Hayward, CA, USA) according to the
manufacturer’s instructions.
Histological analysis by Oil Red O
staining
Twenty-four hours after the oral administration of
the experimental diets, liver tissues were harvested from the
sacrificed rats and immediately frozen in a deep freezer at −80°C.
The frozen liver tissues were cryo-sectioned (6-μm thick), fixed in
a 10% formalin solution (OCI Co., Ltd.) at 4°C for 5 min and then
rinsed three times with distilled water. A 5% Oil Red O working
solution was prepared by dissolving Oil Red O powder
(Sigma-Aldrich) in propylene glycol (OCI Co., Ltd.) and used to
stain the sectioned tissues according to the manufacturer’s
instructions. Counter-staining was conducted with hematoxylin
(Sigma-Aldrich) and the sections were then mounted in glycerine
(OCI Co., Ltd.). Lipid-containing cells were detected as those
containing red inclusions using a light microscope (BX51
U-LH100HGWIG, Olympus, Tokyo, Japan; magnifications, ×40 and
×400).
Statistical analysis
Data were analyzed with GraphPad Prism software (San
Diego, CA, USA). In vitro data were presented as the mean ±
SEM. One-way ANOVA was performed followed by Dunnett’s multiple
comparison test. P<0.05 was considered to indicate a
statistically significant result (24–26).
Results
Serum lipid concentrations
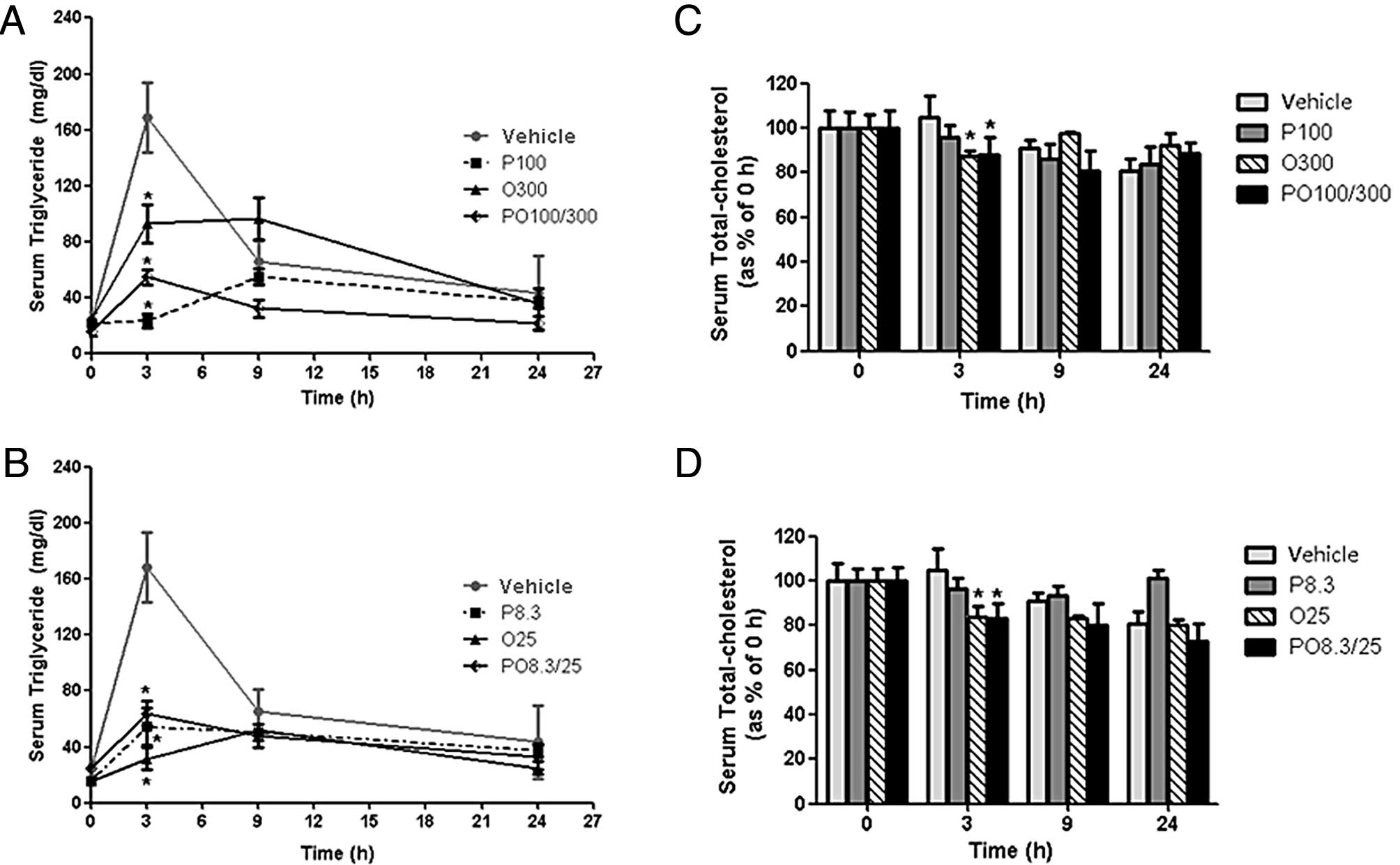
Serum TG concentrations in the vehicle control group
rapidly increased following the intake of a high-fat diet within 3
h. By contrast, treatment with high and low doses of protamine, COS
and their mixtures (P100, O300, PO100/300, P8.3, O25 and PO8.3/25
groups) significantly inhibited serum TG concentrations by 84.3,
47.1, 51.0, 50.7, 71.0 and 63.0% at 3 h, respectively, compared
with those of the vehicle group (Fig.
1A and B). Serum T-CHO concentrations were less effectively
reduced compared with the serum TG concentrations. However, the
serum T-CHO levels of the O25 and PO8.3/25 groups were
significantly decreased by 19.7 and 20.6%, respectively, at 3 h
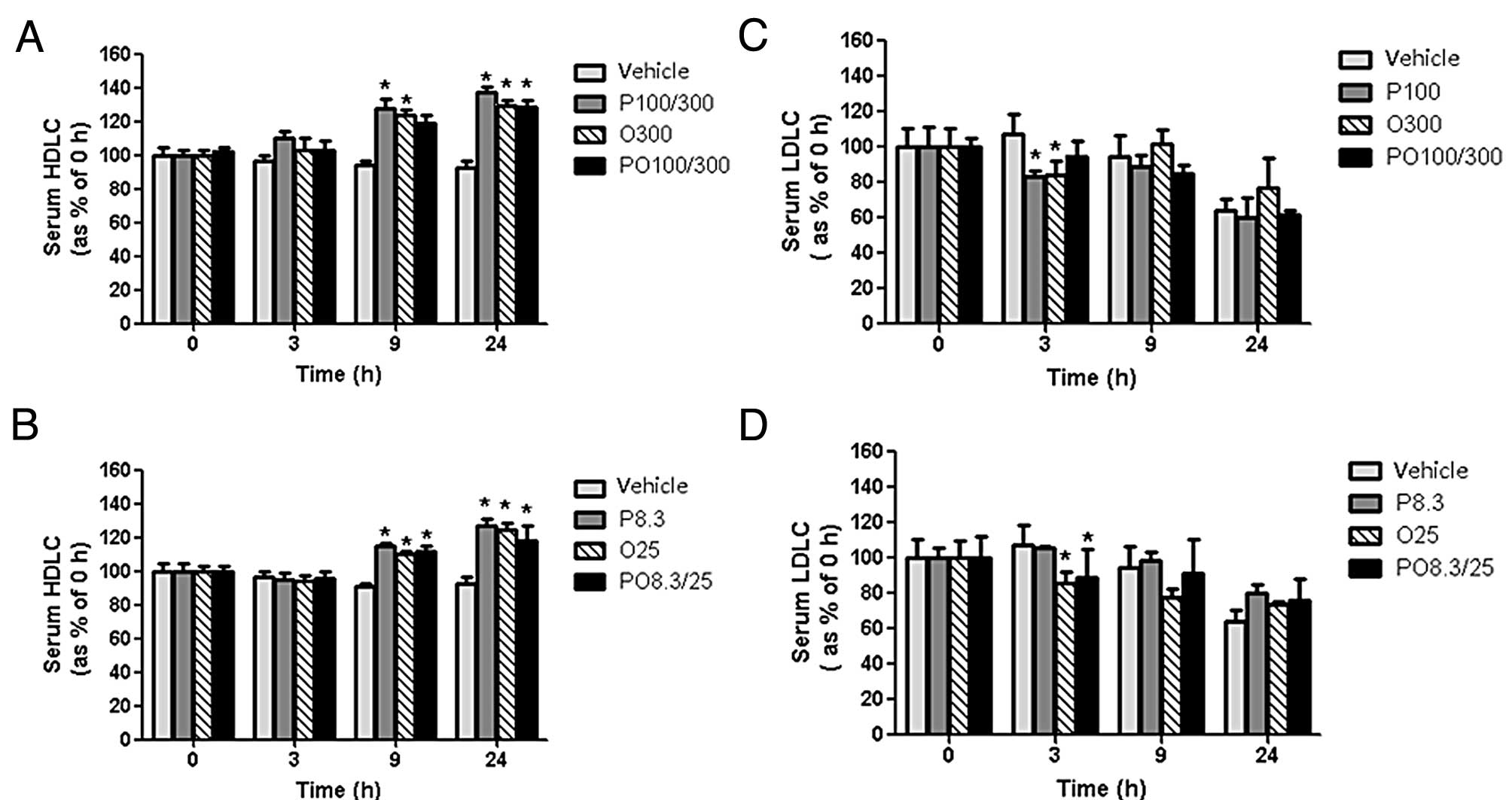
compared with those of the vehicle group (Fig. 1C and D). Serum HDL cholesterol
concentrations of the P100, O300, PO100/300, P8.3, O25 and PO8.3/25
groups were significantly increased by 32.8, 27.3, 26.5, 25.1, 23.5
and 19.1%, respectively, at 24 h compared with those of the vehicle
group (Fig. 2A and B). Conversely,
the P100, O300, O25 and PO100/300 treatments decreased serum LDL
cholesterol levels by 30.5, 37.7, 35.6 and 44.4%, respectively,
after 3 h compared with those of the vehicle control (Fig. 2C and D).
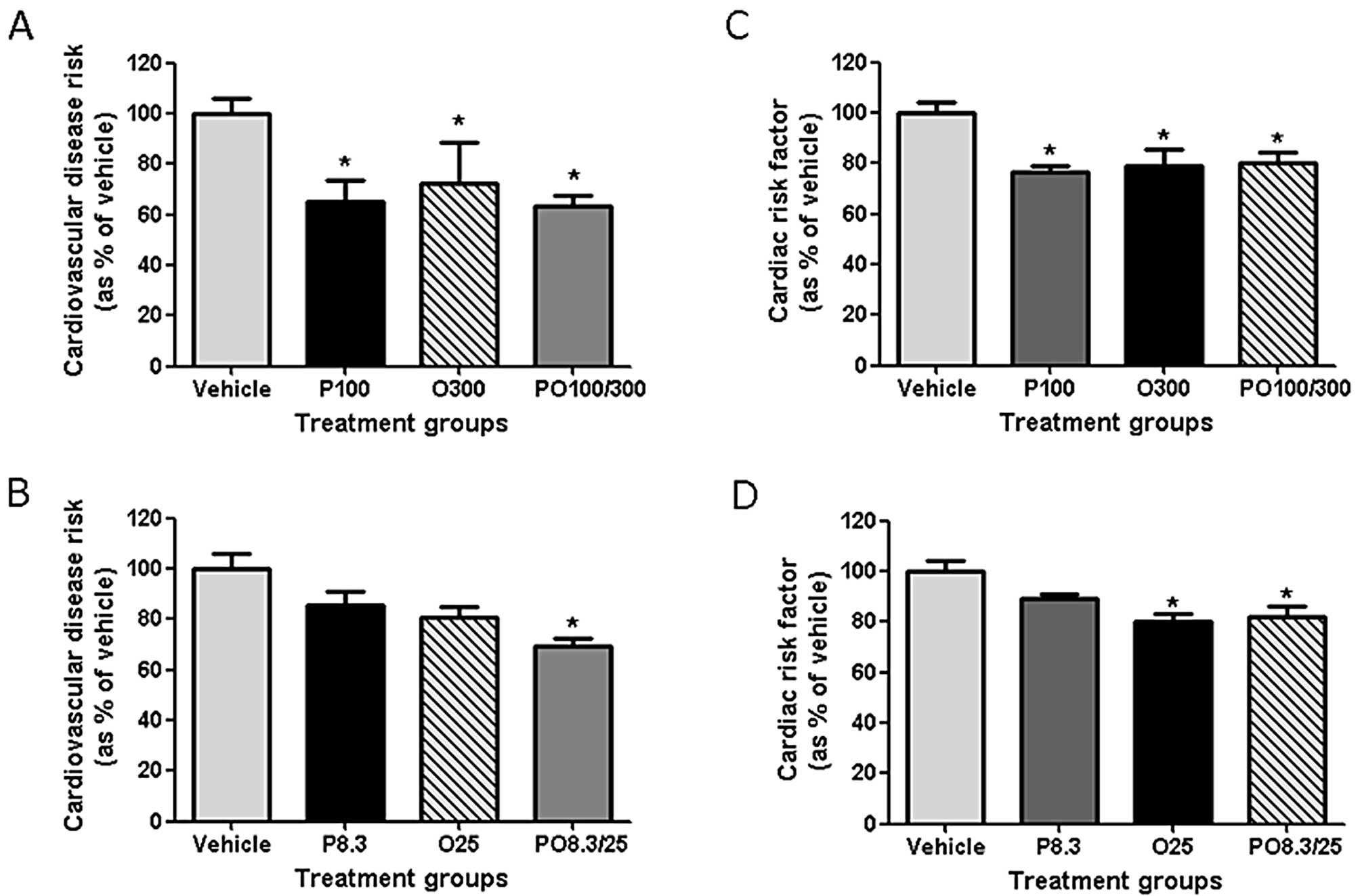
CVD risk factors
The CVD risk, CRF and AI of rats fed protamine, COS
or their mixtures were significantly decreased compared with those
of the vehicle group (Fig. 3 and
Table I). The CVD risk values of
the high-dose treatment groups (P100, O300 and PO100/300) were
decreased by 34.8, 27.5 and 36.6%, respectively, at 24 h. Among the
rats treated with low doses, only the PO8.3/25 group revealed a
significantly decreased CVD risk (30.8%; Fig. 3A and B). The CRF values of the
P100, O300 and PO100/300 groups were decreased by 23.4, 21.2 and
19.5%, respectively, at 24 h. The CRF values of the O25 and
PO8.3/25 groups were decreased by 19.7 and 17.8%, respectively
(Fig. 3C and D). The AI values of
all treatment groups were significantly decreased (Table I).
 | Table IAtherogenic index (AI) of
Sprague-Dawley (SD) rats fed a high-fat diet containing protamine,
chitooligosaccharide (COS) and a mixture of these two
compounds. |
Table I
Atherogenic index (AI) of
Sprague-Dawley (SD) rats fed a high-fat diet containing protamine,
chitooligosaccharide (COS) and a mixture of these two
compounds.
| Group | Vehiclea | P100 | O300 | PO100 | P8.3 | O25 | PO8.3 |
|---|
| AIb | 1.79±0.11 | 1.14±0.07c | 1.20±0.19c | 1.25±0.11c | 1.49±0.05c | 1.24±0.08c | 1.29±0.11c |
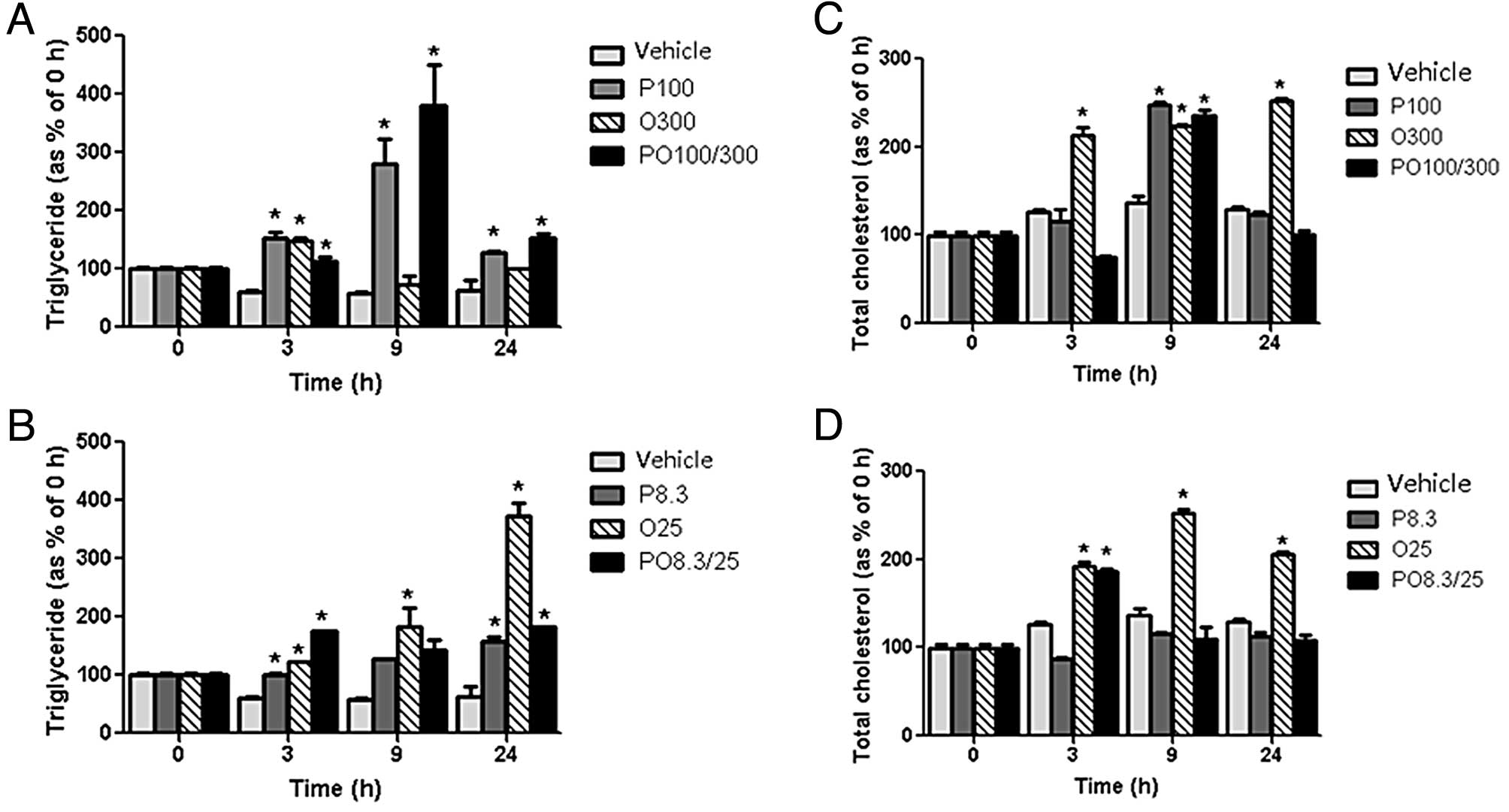
Fecal TG and cholesterol
concentrations
Protamine, COS, and mixtures of the two
significantly increased the TG and T-CHO concentrations in the
feces compared with those of the vehicle group in rats fed a
high-fat diet. The fecal TG concentrations generally increased over
time within 24 h. In particular, the P100 and PO100/300 groups
revealed highly increased levels of fecal TG at 9 h. The fecal TG
concentrations of the O25 group also increased significantly at 24
h (494.9%) compared with those of the vehicle group (Fig. 4A and B). The fecal T-CHO
concentrations of the rats treated with the experimental diets were
increased to a lesser degree. The fecal T-CHO concentrations of the
P100, O300 and O25 groups were increased by 82.2, 64.4 and 84.5%,
respectively, at 9 h (Fig. 4C and
D).
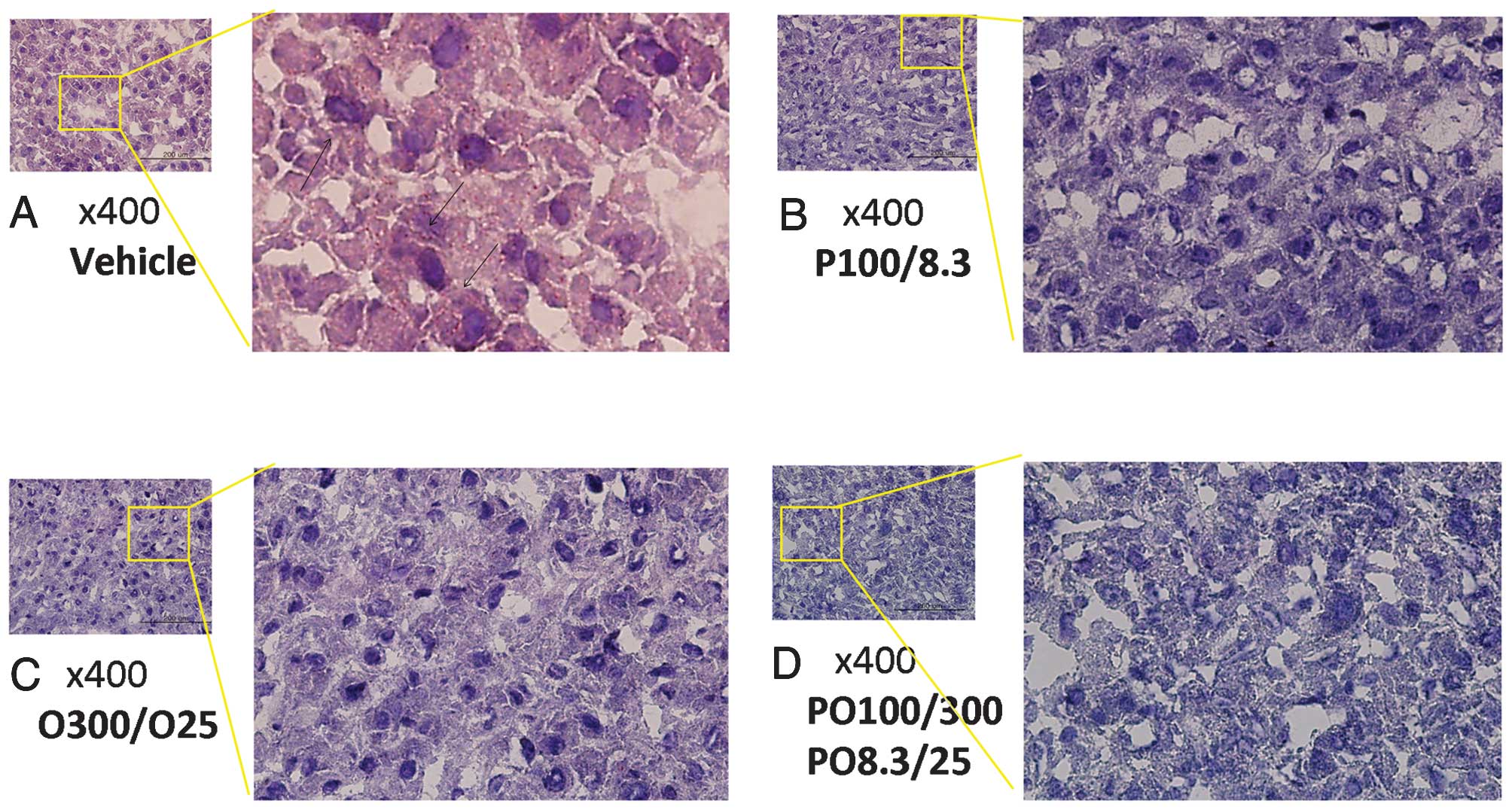
Lipid accumulation in liver tissues
Lipid accumulation in the rat liver tissues was
detected by Oil Red O staining and histological analysis. The red
inclusions corresponded to lipids derived from TG and T-CHO from
the high-fat diet. Significant levels of lipid accumulation were
observed in the vehicle group, but increases in lipid accumulation
were not observed in all experimental diet groups treated with
protamine, COS or a mixture of the two compounds (Fig. 5).
Discussion
A high-fat diet and dysfunctional lipid metabolism
are closely associated with the increasing prevalence of being
overweight and of obesity, and are influential causes of CVD
(27,28). In particular, dyslipidemia,
characterized by high levels of very low-density lipoproteins
(VLDL) and TG and decreased HDL and LDL levels in the serum, is
generally known to be a strong predictor for the development of CVD
(29). In the present study, we
evaluated the effects of a dietary therapy including protamine and
COS, two prominent candidates for positively affecting lipid
metabolism, on CVD risk in rats. We measured the improvements of
lipid metabolism following the administration of protamine, COS and
their mixtures in vivo by analyzing serum and fecal lipid
levels as well as hepatic lipid accumulation within 24 h of the
oral administration of a high-fat diet along with protamine and COS
to SD rats. The dose-dependent effects of protamine (30,31)
and COS were also examined. The factors CVD risk, CRF and AI were
then calculated based on serum T-CHO, HDL cholesterol and LDL
cholesterol concentrations.
Protamine, COS and mixtures of the two compounds
effectively reduced the serum levels of TG, T-CHO and LDL
cholesterol compared with the vehicle. By contrast, these compounds
significantly increased the serum levels of HDL cholesterol. When
serum cholesterol levels were translated into risk factor values,
protamine, COS and their mixtures were shown to decrease AI, CVD
risk and CRF. The AI was significantly reduced in all experimental
diet groups compared with the vehicle control group. CVD risk and
CRF were also significantly reduced in all rats treated with high
doses as well as in the low-dose PO8.3/25 group, indicating that
high doses of protamine and COS were more effective than low doses
for reducing these risk factors. Additionally, a combination of
protamine and COS had greater effects than treatment with protamine
or COS alone at low doses. However, the additive effect of
protamine and COS was not evident at high doses. We therefore
conclude that protamine and COS have effects that are beneficial
for the prevention of diseases associated with arteriosclerosis and
cardiac failure by improving lipid metabolism.
An unhealthy serum cholesterol profile (higher
levels of LDL cholesterol and lower levels of HDL cholesterol) is
known to promote atheroma development in atherosclerosis, which is
strongly associated with CVD (32,33).
Improvement of an unfavorable serum cholesterol profile by dietary
supplementation with protamine and COS was achieved by reversing
the unhealthy ratio of LDL cholesterol to HDL cholesterol. Serum
HDL cholesterol concentrations were significantly increased by
protamine and COS. However, the two compounds effectively reduced
the serum levels of LDL cholesterol. HDL cholesterol is considered
to have a variety of useful actions that tend to reduce the risk
for CVD. HDL cholesterol removes and recycles LDL cholesterol by
transporting it to the liver, an action which is generally thought
to produce a central anti-atherogenic effect (34). A previous study has shown that
there is an inverse correlation between HDL levels and the risk of
CVD in which the risk of CVD is increased by 14% with 5 mg/dl
decrements in HDL levels (35).
Unlike in serum, TG and T-CHO concentrations in
feces were markedly increased by protamine, COS and their mixtures
over the same period of time, indicating that TG and T-CHO were
effectively excreted from the body by rats fed the experimental
diets. When treated with high doses, the fecal TG concentrations
were maximized at 9 h. However, low-dose treatments resulted in the
maximum amount of TG being excreted in the feces at 24 h. Fecal
T-CHO concentrations were also considerably increased by protamine
and COS. Specifically, high-dose COS treatment increased fecal
T-CHO levels by the greatest amount. These results demonstrated
that protamine, COS and mixtures of the two interrupted lipid
accumulation in the liver and blood vessels. This was further shown
by histological analysis using Oil Red O staining. Liver tissues
obtained from rats fed high-fat diets revealed a large number of
lipid inclusions stained with the Oil Red O working solution, but
little lipid accumulation was observed in rats treated with
protamine, COS and their mixtures.
In summary, our results demonstrated that protamine,
COS and mixtures of the two compounds effectively reduced CVD risk,
CRF and AI by decreasing serum levels of TG, T-CHO and LDL
cholesterol and enhancing serum HDL cholesterol levels. The
reduction of serum TG and T-CHO levels may be explained by the
finding that protamine and COS promoted the excretion of TG and
T-CHO in feces, thereby preventing accumulation in the body.
However, future studies are required to elucidate the mechanism(s)
underlying the reduction of CVD risk through beneficial changes in
HDL cholesterol/LDL cholesterol ratios due to treatment with
protamine, COS and their mixtures. In conclusion, our results
suggest that protamine and COS are promising dietary supplements
for preventing CVD by alleviating hyperlipidemia and
hypercholesterolemia.
Acknowledgements
This study was supported by a National Research
Foundation of Korea (NRF) grant funded by the Ministry of
Education, Science and Technology (MEST) of the Republic of Korea
government (no. 2011-0015385). In addition, this study was also
supported by the Priority Research Centers Program through the NRF
funded by the MEST (2011-0031403).
References
|
1
|
Yokokawa H, Yasumura S, Tanno K, et al:
Serum low-density lipoprotein to high-density lipoprotein ratio as
a predictor of future acute myocardial infarction among men in a
2.7-year cohort study of a Japanese northern rural population. J
Atheroscler Thromb. 18:89–98. 2011.
|
|
2
|
Bae JM, Yang YJ, Li ZM and Ahn YO: Low
cholesterol is associated with mortality from cardiovascular
diseases: a dynamic cohort study in Korean adults. J Korean Med
Sci. 27:58–63. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kim JE, Jeon SM, Park KH, et al: Does
Glycine max leaves or Garcinia Cambogia promote
weight-loss or lower plasma cholesterol in overweight individuals:
a randomized control trial. Nutr J. 10:942011.
|
|
4
|
Ingelsson E, Schaefer EJ, Contois JH, et
al: Clinical utility of different lipid measures for prediction of
coronary heart disease in men and women. JAMA. 298:776–785. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Manninen V, Tenkanen L, Koskinen P, et al:
Joint effects of serum triglyceride and LDL cholesterol and HDL
cholesterol concentrations on coronary heart disease risk in the
Helsinki Heart Study. Implications for treatment. Circulation.
85:37–45. 1992. View Article : Google Scholar
|
|
6
|
Lowe ME: Molecular mechanisms of rat and
human pancreatic triglyceride lipases. J Nutr. 127:549–557.
1997.PubMed/NCBI
|
|
7
|
Lowe ME: The triglyceride lipases of the
pancreas. J Lipid Res. 43:2007–2016. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Aspedon A and Groisman EA: The
antibacterial action of protamine: evidence for disruption of
cytoplasmic membrane energization in Salmonella typhimurium.
Microbiology. 142:3389–3397. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hosomi R, Fukunaga K, Arai H, Kanda S,
Nishiyama T and Yoshida M: Effect of dietary protamine on lipid
metabolism in rats. Nutr Res Pract. 4:462–469. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang J, Kang MJ, Kim MJ, et al:
Pancreatic lipase inhibitory activity of Taraxacum
officinale in vitro and in vivo. Nutr Res Pract. 2:200–203.
2008. View Article : Google Scholar
|
|
11
|
Duarte-Vázquez MA, García-Padilla S,
Olvera-Ochoa L, et al: Effect of protamine in obesity induced by
high-fat diets in rats. Int J Obes (London). 33:687–692.
2009.PubMed/NCBI
|
|
12
|
Zhou TX, Chen YJ, Yoo JS, et al: Effects
of chitooligosaccharide supplementation on performance, blood
characteristics, relative organ weight, and meat quality in broiler
chickens. Poult Sci. 88:593–600. 2009. View Article : Google Scholar
|
|
13
|
Chae SY, Jang MK and Nah JW: Influence of
molecular weight on oral absorption of water soluble chitosans. J
Control Release. 102:383–394. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Choi BK, Kim KY, Yoo YJ, Oh SJ, Choi JH
and Kim CY: In vitro antimicrobial activity of a
chitooligosaccharide mixture against Actinobacillus
actinomycetemcomitans and Streptococcus mutans. Int J
Antimicrob Agents. 18:553–557. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ju C, Yue W, Yang Z, et al: Antidiabetic
effect and mechanism of chitooligosaccharides. Biol Pharm Bull.
33:1511–1516. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Shon YH and Nam KS: Inhibition of
polyamine biosynthesis in Acanthamoeba castellanii and
12-O-tetradecanoylphorbol-13-acetate-induced ornithine
decarboxylase activity by chitosanoligosaccharide. Biotechnol Lett.
25:701–704. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cho SY, Lee JH, Song MJ, et al: Effects of
chitooligosaccharide lactate salt on sleep deprivation-induced
fatigue in mice. Biol Pharm Bull. 33:1128–1132. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Suzuki K, Mikami T, Okawa Y, Tokoro A,
Suzuki S and Suzuki M: Antitumor effect of
hexa-N-acetylchitohexaose and chitohexaose. Carbohydr Res.
151:403–408. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Choi CR, Kim EK, Kim YS, et al:
Chitooligosaccharides decreases plasma lipid levels in healthy men.
Int J Food Sci Nutr. 63:103–106. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hayashi K and Ito M: Antidiabetic action
of low molecular weight chitosan in genetically obese diabetic
KK-Ay mice. Biol Pharm Bull. 25:188–192. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tang ZR, Yin YL, Nyachoti CM, et al:
Effect of dietary supplementation of chitosan and
galacto-mannan-oligosaccharide on serum parameters and the
insulin-like growth factor-I mRNA expression in early-weaned
piglets. Domest Anim Endocrinol. 28:430–441. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tsujita T, Matsuura Y and Okuda H: Studies
on the inhibition of pancreatic and carboxylester lipases by
protamine. J Lipid Res. 37:1481–1487. 1996.PubMed/NCBI
|
|
23
|
Gors S, Kucia M, Langhammer M, Junghans P
and Metges CC: Technical note: milk composition in mice -
methodological aspects and effects of mouse strain and lactation
day. J Dairy Sci. 92:632–637. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kang NH and Choi KC: 207 engineered human
amniotic fluid-derived stem cells by expressing cytosine deaminase
(cd) and thymidine kinase (hsv-tk) mediate targeting killing effect
in breast cancer cells. Reprod Fertil Dev. 24:2162012. View Article : Google Scholar
|
|
25
|
Kang NH, Yi BR, Lim SY, et al: Human
amniotic membrane-derived epithelial stem cells display anticancer
activity in BALB/c female nude mice bearing disseminated breast
cancer xenografts. Int J Oncol. 40:2022–2028. 2012.
|
|
26
|
Kim KY, Yi BR, Lee HR, et al: Stem cells
with fused gene expression of cytosine deaminase and interferon-β
migrate to human gastric cancer cells and result in synergistic
growth inhibition for potential therapeutic use. Int J Oncol.
40:1097–1104. 2012.PubMed/NCBI
|
|
27
|
Haslam DW and James WP: Obesity. Lancet.
366:1197–1209. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wang H and Peng DQ: New insights into the
mechanism of low high-density lipoprotein cholesterol in obesity.
Lipids Health Dis. 10:1762012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Poirier P, Giles TD, Bray GA, et al:
Obesity and cardiovascular disease: pathophysiology, evaluation,
and effect of weight loss: an update of the 1997 American Heart
Association Scientific Statement on Obesity and Heart Disease from
the Obesity Committee of the Council on Nutrition, Physical
Activity, and Metabolism. Circulation. 113:898–918. 2006.
|
|
30
|
Hosomi R, Fukunaga K, Arai H, Nishiyama T
and Yoshida M: Effects of dietary fish protein on serum and liver
lipid concentrations in rats and the expression of hepatic genes
involved in lipid metabolism. J Agric Food Chem. 57:9256–9262.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Moriyama T, Kishimoto K, Nagai K, et al:
Soybean beta-conglycinin diet suppresses serum triglyceride levels
in normal and genetically obese mice by induction of
beta-oxidation, downregulation of fatty acid synthase, and
inhibition of triglyceride absorption. Biosci Biotechnol Biochem.
68:352–359. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Artenie R, Ungureanu D, Artenie A,
Botnariu G and Anisie E: HDL-cholesterol - active or passive
participant in the pathogenesis of atherosclerosis. Rev Med Chir
Soc Med Nat Iasi. 107:282–287. 2003.(In Romanian).
|
|
33
|
Sancho-Rodriguez N, Avilés-Plaza FV,
Granero-Fernandez E, et al: Observational study of lipid profile
and LDL particle size in patients with metabolic syndrome. Lipids
Health Dis. 10:1622011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Shah PK, Kaul S, Nilsson J and Cercek B:
Exploiting the vascular protective effects of high-density
lipoprotein and its apolipoproteins: an idea whose time for testing
is coming, part I. Circulation. 104:2376–2383. 2001. View Article : Google Scholar
|
|
35
|
Gotto AM Jr, Whitney E, Stein EA, et al:
Relation between baseline and on-treatment lipid parameters and
first acute major coronary events in the Air Force/Texas Coronary
Atherosclerosis Prevention Study (AFCAPS/TexCAPS). Circulation.
101:477–484. 2000. View Article : Google Scholar
|