Introduction
The autoimmune regulator, Aire, was first cloned by
German scientists in 1997. Aire is composed of four domains (LXXLL,
PHD, SAND and HSR) that are characteristic of transcription factors
(1,2), suggesting that Aire may also act as a
transcription factor. The mutation or deletion of Aire in humans
results in a severe autoimmune disease, autoimmune
polyendocrinopathy candidiasis ectodermal dystrophy (APECED); Aire
is thus considered to play a critical role in self-tolerance. Aire
is highly expressed in medullary thymic epithelial cells (mTECs),
where it leads to the elimination of autoreactive T cells by
regulating the expression of thousands of tissue-restricted
antigens (TRAs), which are presented to thymic T lymphocytes by
mTECs or cross-presented by dendritic cells (DCs).
Aire is also expressed in the peripheral immune
organs and tissues, especially in peripheral blood and the lymph
nodes. The two cell types that express Aire in the periphery are
myeloid, including DCs and macrophages (3,4), and
stromal, including epithelial cells (5,6).
Although the cell types that express Aire are known, the function
of Aire at these sites remains undefined. Ramsey et al
proposed that Aire may affect the maturation and antigen
presentation of DCs (7). Fletcher
et al reported that Aire expressed in the stromal cells of
the lymph nodes may be involved in peripheral immune tolerance by
regulating TRAs in a manner complementary to its role in mTECs
(6). However, whether Aire also
affects peripheral tolerance by other mechanisms remains
ambiguous.
CD4+ regulatory T (Treg) cells were first
identified by Sakaguchi et al in 1995 with the observation
that depletion of these cells in mice leads to autoimmunity in
multiple organs (8). Treg cells
represent an essential cell population in the maintenance of
peripheral immune tolerance. Miyara et al classified
CD4+Foxp3+ Treg cells into three subsets,
rTreg (CD4+CD45RA+Foxp3lo, resting
Treg), aTreg (CD4+CD45RA-Foxp3hi,
activating Treg) and non-Treg
(CD4+CD45RA-Foxp3lo) (9). rTreg and aTreg cells have a
suppressive function, while non-Treg cells do not suppress effector
T cells, but rather secrete a certain level of IL-17.
In the current study, in order to determine whether
the expression of Aire in the peripheral immune system influenced
Treg cells, the effects of macrophages overexpressing Aire on
CD4+Foxp3+ Treg cells and Treg subsets were
detected by co-culturing Aire-overexpressing RAW264.7 cells or
their supernatant with splenocytes.
Materials and methods
Cell culture and mice
RAW264.7 cells were obtained from the Shanghai Cell
Research Institute. The RAW264.7 cells were transfected with
pEGFPC1/Aire or pEGFPC1 plasmids and stable cell lines were
obtained following G418 selection (10). The cells were cultured in 10%
NCS-RPMI-1640 (Gibco-BRL, Carlsbad, CA, USA). Balb/c mice were
purchased from the Experimental Animal Center of Jilin University
and kept under pathogen-free conditions. All animal procedures were
approved by the Ethics Committee of Jilin University.
Co-culture of the RAW264.7 cell lines
with mouse splenocytes
The spleens were removed from the Balb/c mice and
gently dissociated into single-cell suspensions followed by
ammonium chloride lysis to remove red blood cells. RAW264.7 cells
overexpressing Aire (Aire cells) or empty vector (control cells)
were seeded onto 96-well plates at 1×105 per well in 100
μl. When the cells had attached to the dishes, they were
co-cultured with 1×106 freshly isolated splenocytes for
48 and 72 h. In addition, 100 μl supernatants from the Aire or
control cells were co-cultured with an equal number of splenocytes
for 48 and 72 h. Afterwards, the suspended splenocytes were
harvested for analysis by flow cytometry and real-time PCR.
RNA isolation and quantitative real-time
PCR
Total RNA was extracted from the harvested
splenocytes (as described above) or from the RAW264.7 cells using
TRIzol (Invitrogen, Carlsbad, CA, USA) and dissolved in
DEPC-treated water. The amount of total RNA was measured with an
ultraviolet spectrophotometer. A 1-μg sample of RNA was used to
synthesize cDNA with random primers and AMV reverse transcriptase
(Takara, Shiga, Japan) using the following reaction parameters:
30°C for 10 min, 45°C for 30 min and 5°C for 5 min for one cycle.
cDNA was used for real-time PCR with the following primers: GAPDH
sense, 5′-GAC TTC AAC AGC AAC TCC CAC TC-3′ and antisense, 5′-TAG
CCG TAT TCA TTG TCA TAC CAG-3′; and Foxp3 sense, 5′-TAC TCG CAT GTT
CGC CTA CTT CA-3′ and antisense, 5′-ATT CAT CTA CGG TCC ACA CTG
CT-3′. The reaction parameters used were as follows: 95°C for 30
sec, 95°C for 5 sec, and 60°C for 30 sec for 40 cycles. The samples
were detected with the ABI7300 real-time PCR instrument and the
results were analyzed using the formula 2−ΔΔCt.
The primers and reaction parameters used in RT-PCR
are shown in Table I. ImageMaster
VDS (Pharmacia Biotech, Tokyo, Japan) image analysis software was
employed to analyze the optical density value, and the mRNA
expression levels of various genes were expressed as the
gene/β-actin optical density value.
 | Table IPrimers and reaction parameters for
RT-PCR. |
Table I
Primers and reaction parameters for
RT-PCR.
| Gene | Sequences
(5′-3′) | Size (bp) | Reaction
parameters | Cycles |
|---|
| TGF-β | S:
GCCCTGGATACCAACTATTGC
AS: GCAGGAGCGCACAATCATGTT | 327 | 94°C for 2 min,
94°C for 30 sec, 58°C for 30 sec, 72°C for 30 sec, 72°C 10 min | 28 |
| β-actin | S:
TGGAATCCTGTGGCATCCATGAAAC
AS: TAAAACGCAGCACAGTAACAGTCCG | 360 | 94°C for 2 min,
94°C for 30 sec, 52°C for 30 sec, 72°C for 1 min, 72°C for 10
min | 25 |
Western blot analysis
The cells were lysed on ice in a buffer containing
50 mM Tris, pH 8.0, 150 mM NaCl, 1% Triton X-100, 1% sodium
desoxycholate, 0.1% SDS and a protease inhibitor cocktail (Roche,
Mannheim, Germany). Cellular lysates were centrifuged at 10,000 × g
for 10 min and the supernatants were subjected to western blot
analysis with anti-TGF-β (R&D Systems, Minneapolis, MN, USA) at
1:250 dilution. Finally, the signal was detected with a GeneGnome
Imaging System (Syngene, Cambridge, UK) using BeyoECL Plus
(Beyotime Biotech., Jiangsu, China).
Flow cytometry analysis
The cells were collected and counted, and
1×106 cells were suspended in PBS (total volume 100 μl).
PE-Cy7-anti-CD4 (eBioscience, San Diego, CA, USA) and
PE-anti-CD45RA (Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA)
antibodies were added to the cells which were then incubated at 4°C
for 40 min. The cells were then fixed with the Foxp3
fixation/permeabilization concentrate and diluent (eBioscience) for
1 h and then treated with 0.1% saponin (Sigma, St. Louis, MO, USA)
and AF647-anti-Foxp3 antibody (eBioscience) at 4°C for 1 h. The
cells were then washed with PBS and resuspended in 2%
paraformaldehyde for analysis using a BD FACSCalibur flow
cytometer.
ELISA
A TGF-β1 ELISA (R&D Systems) kit was employed
and the assay was performed according to the manufacturer’s
instructions.
TGF-β blocking
The supernatants secreted from the Aire or control
cells were neutralized with TGF-β antibody (R&D Systems) for 1
h. Freshly isolated splenocytes were then added to the supernatants
and incubated for 48 and 72 h. The splenocytes were harvested for
FACS analysis.
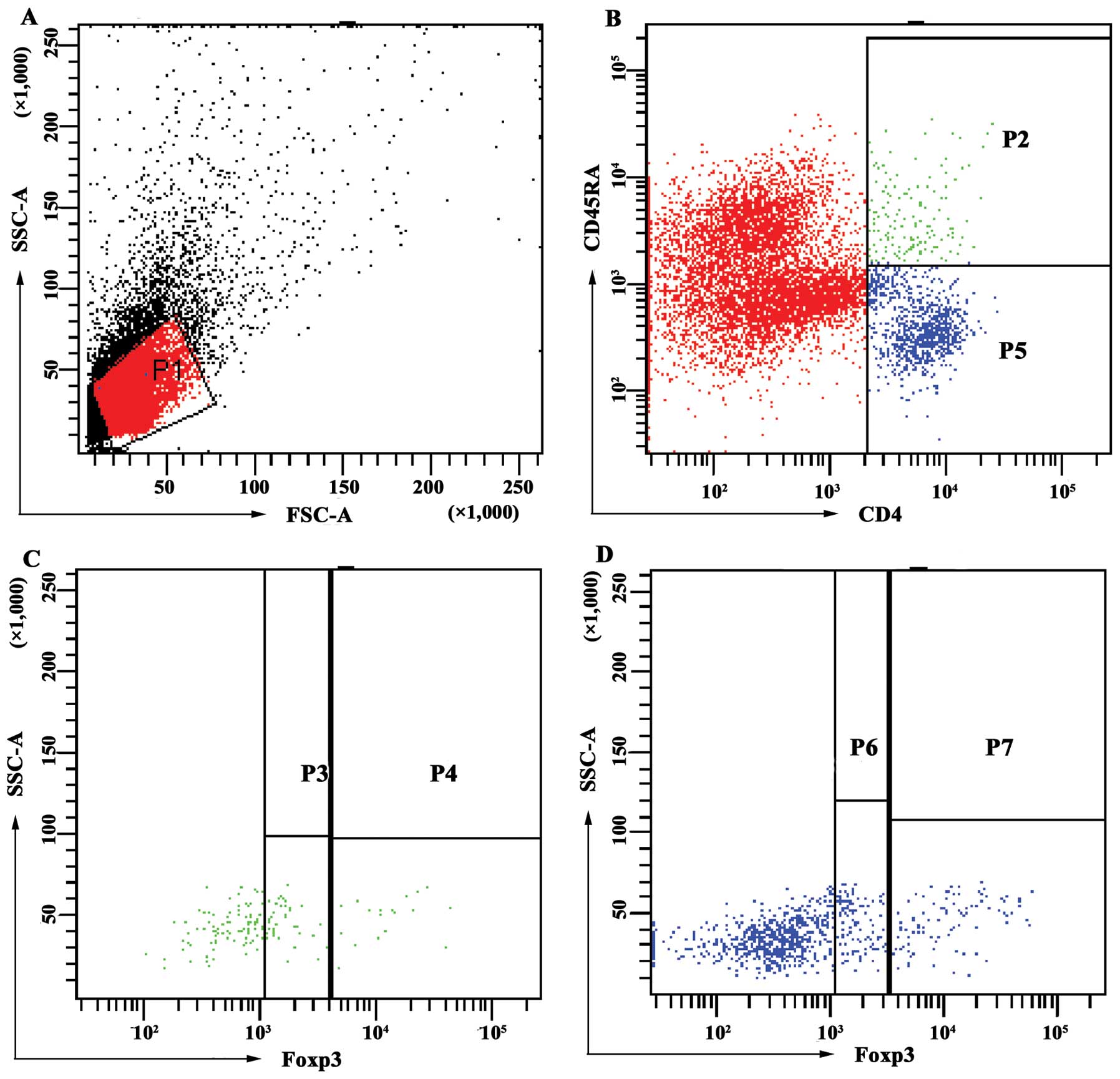
Statistical analysis
The percentages of each subset of
CD4+Foxp3+ T cells among the CD4+
T cells were calculated using the following formulae (Fig. 1): i)
CD4+Foxp3+ T = (P3 + P4 + P6 + P7)/(P2 + P5);
ii) rTreg (CD4+CD45RA+Foxp3lo T) =
P3/(P2 + P5); iii) aTreg
(CD4+CD45RA-Foxp3hi T) = P7/(P2 +
P5); iv) non-Treg
(CD4+CD45RA-Foxp3lo T) = P6/(P2 +
P5); v) CD4+CD45RA+Foxp3hi T =
P4/(P2 + P5).
All data are expressed as the means ± SD.
Differences were compared between the two groups using Student’s
t-tests. A value of p<0.05 was considered to indicate a
statistically significant result.
Results
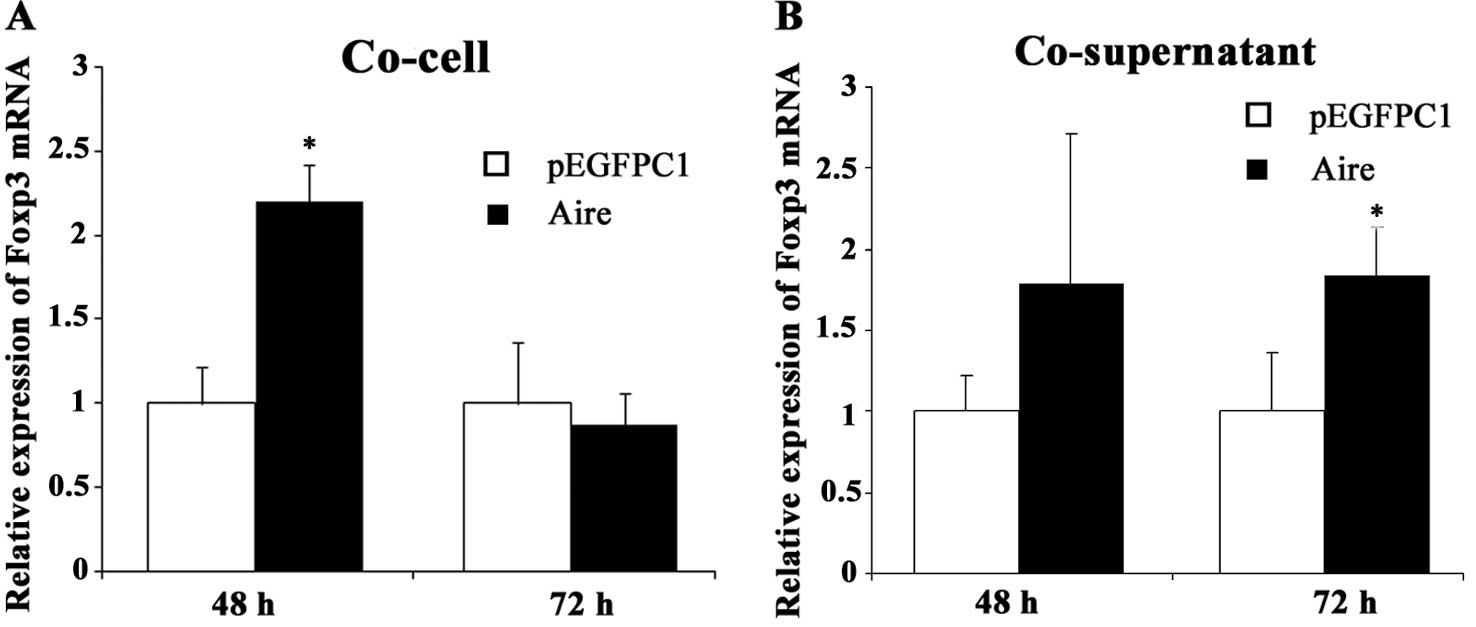
Aire upregulates Foxp3 mRNA expression in
splenocytes
To observe the effects of Aire on Treg cells, we
measured the expression of Foxp3 mRNA, a specific marker of
CD4+ Treg cells that is critical in the development and
suppressive function of CD4+ Treg cells. The Foxp3 mRNA
levels were upregulated in the splenocytes co-cultured for 48 h
with Aire cells compared with those in the control cells (Fig. 2A). There was a similar change when
the splenocytes were co-cultured with the supernatant of the Aire
cells for 72 h (Fig. 2B). These
results indicate that Treg cells are upregulated by the
Aire-overexpressing RAW264.7 cells. It appears that the effect of
the Aire cells on Foxp3 expression occurs earlier by cell-cell
contact than by contact with the supernatant.
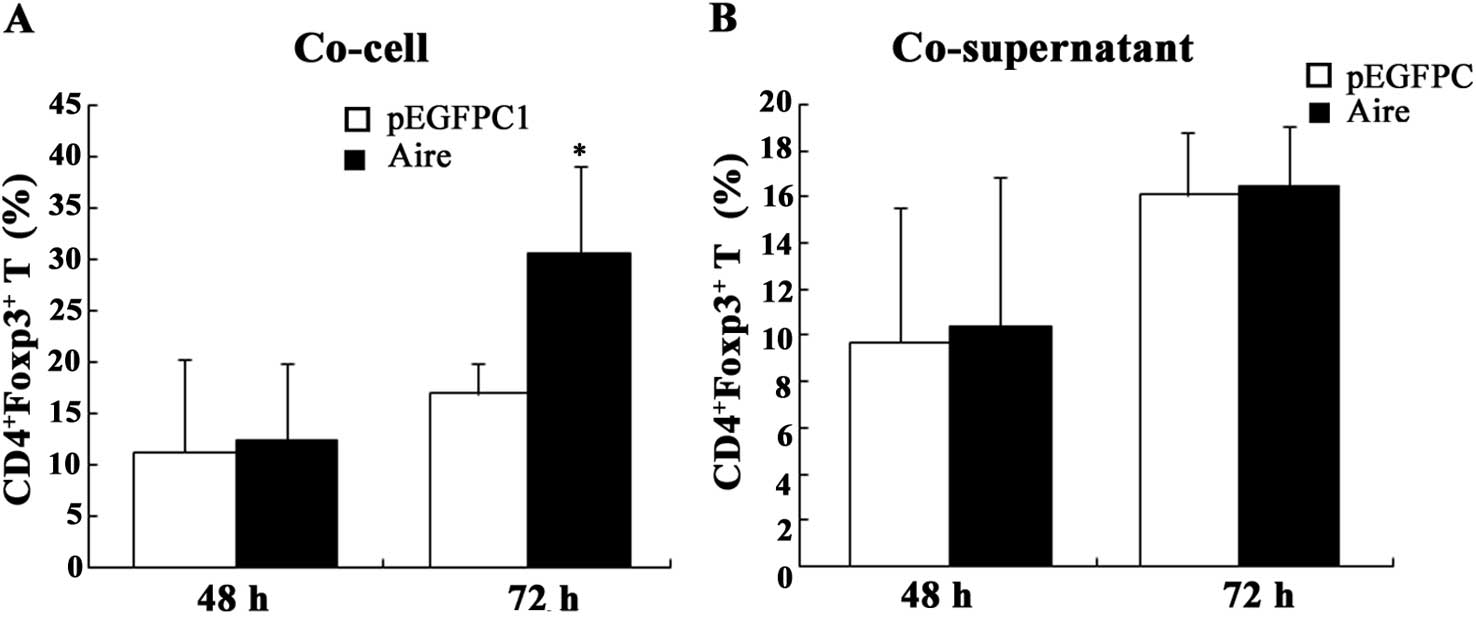
Aire increases
CD4+Foxp3+ T cell production
The data presented in the previous section show an
increase in the mRNA levels of a specific marker of CD4+
Treg cells. We then detected the number of Treg cells by FACS. The
percentage of CD4+Foxp3+ T cells was higher
when the spleen cells were co-cultured with Aire cells at 72 h, but
not at 48 h. We observed no differences in the percentage of
CD4+Foxp3+ T cells when the splenocytes were
co-cultured with the supernatants of Aire or control cells at
either 48 or 72 h (Fig. 3). These
data reveal that the percentage of CD4+Foxp3+
Treg cells was increased by the Aire cells. Moreover, the Aire
cells were able to affect the percentage of
CD4+Foxp3+ T cells through cell-cell contact,
but not through a product present in the supernatant.
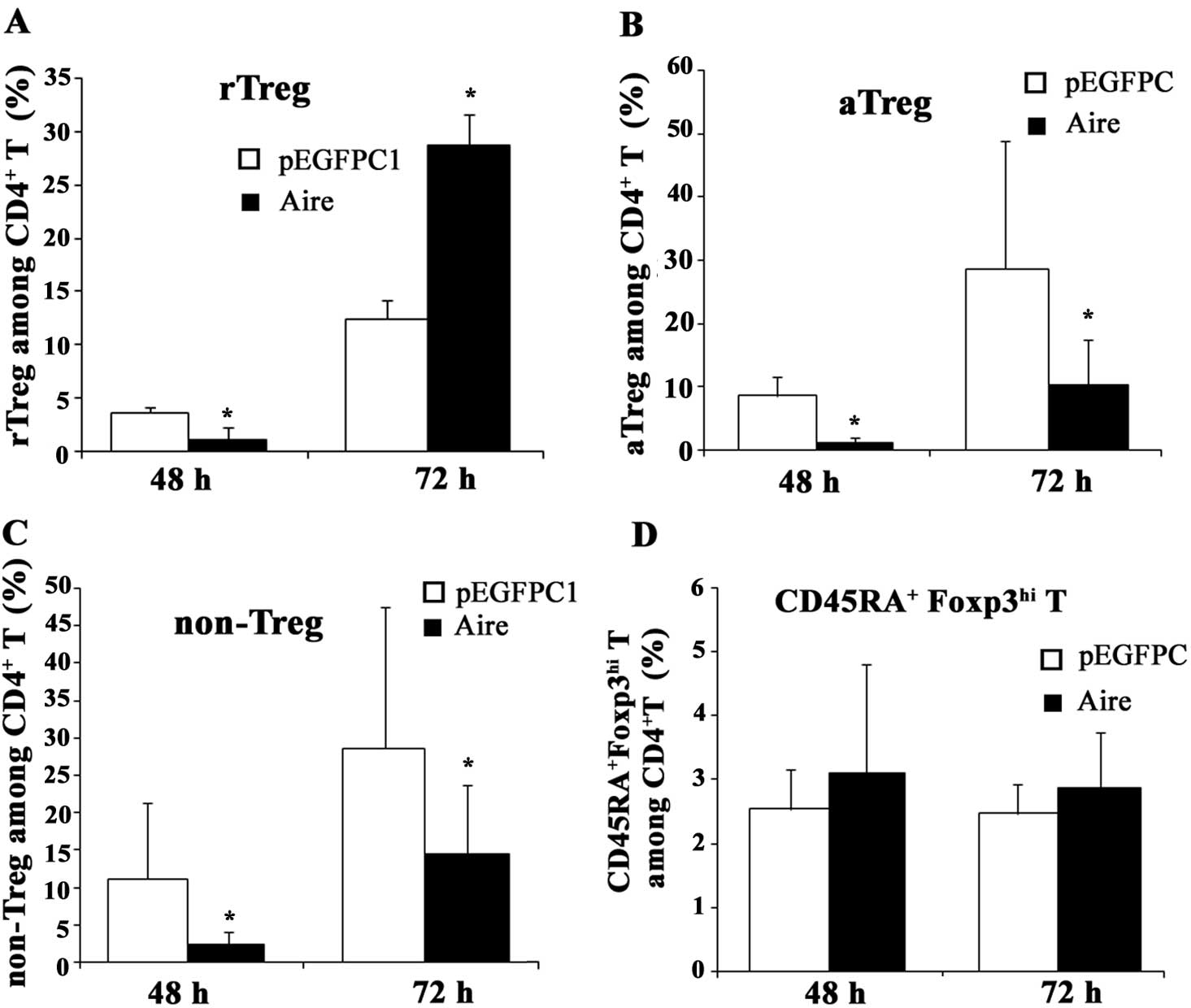
Aire affects the subsets of
CD4+Foxp3+ T cells
The population of CD4+Foxp3+
Treg cells is composed of three different subsets: rTreg
(CD4+CD45RA+Foxp3lo T cells),
aTreg (CD4+CD45RA-Foxp3hi T cells)
and non-Treg (CD4+CD45RA-Foxp3lo T
cells) which differ in expression of Foxp3 and CD45RA. To
investigate the influence of Aire on these subsets of
CD4+Foxp3+ Treg cells, we measured the change
in the percentage of each subset after co-culturing with Aire or
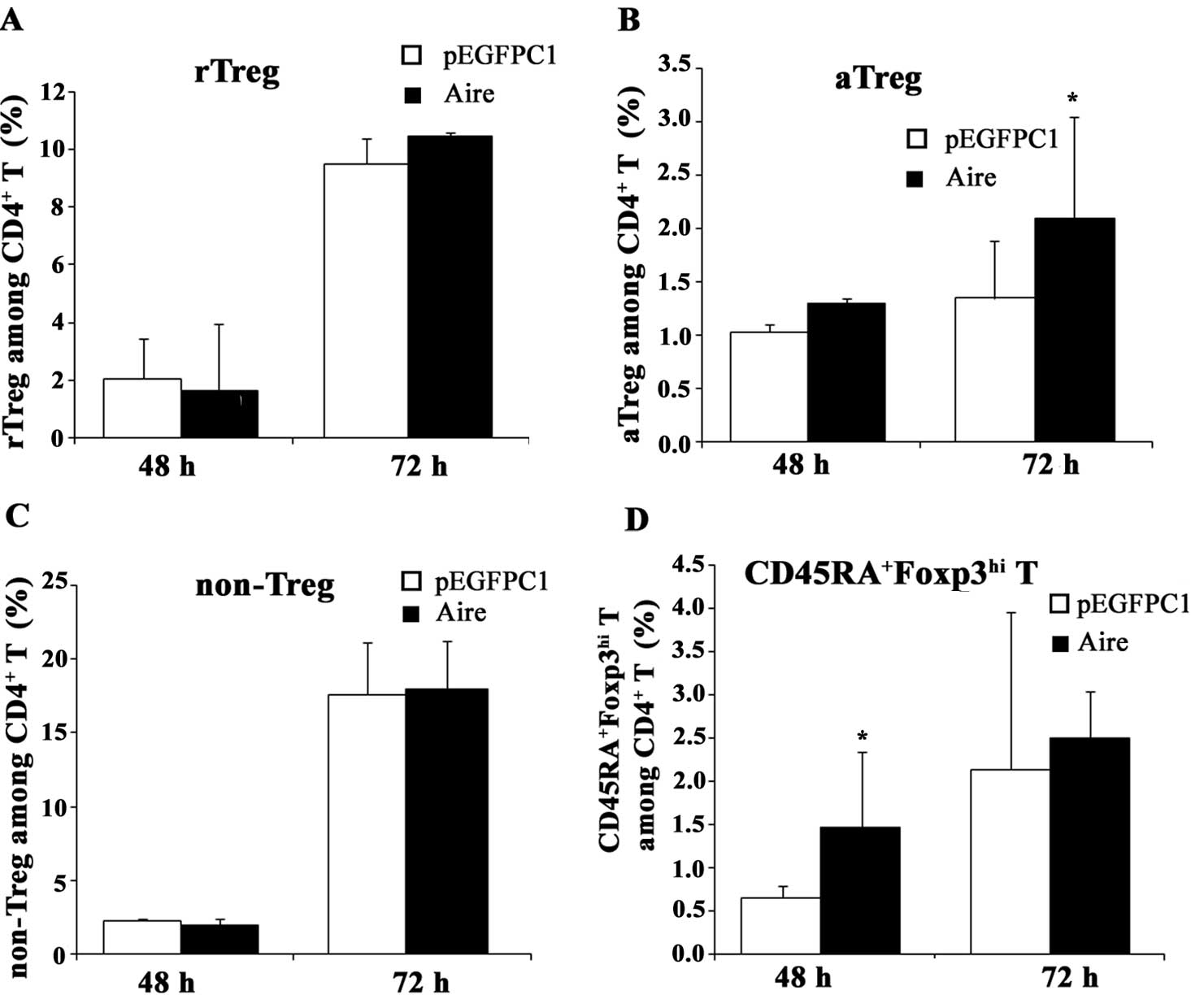
control cells or their supernatants. When the splenocytes and Aire
cells were co-cultured for 48 and 72 h, the percentage of rTreg
cells among the CD4+ T cells decreased at 48 h and
significantly increased at 72 h, but the percentages of aTreg and
non-Treg cells were lower than in the control cells (Fig. 4). When the spleen cells were
cultured with the supernatants secreted from the Aire or control
cells, no significant changes were observed in the rTreg and
non-Treg cell populations, but the percentage of aTreg cells was
higher in the Aire group at 72 h (Fig.
5). These results suggest that Aire stimulates the production
and/or induction of rTreg cells and inhibits the formation of aTreg
and non-Treg cells through cell-cell contacts. Aire may also
promote macrophages to secrete a specific substance that is able to
induce the production of aTreg cells. In addition, we also
identified a previously unreported subset of
CD4+Foxp3+ T cells,
CD4+CD45RA+Foxp3hi T, which was
also affected by Aire (Figs. 4 and
5), although its function remains
unknown. We propose that this population is a transition state from
rTreg to aTreg cells and may have a similar function to them.
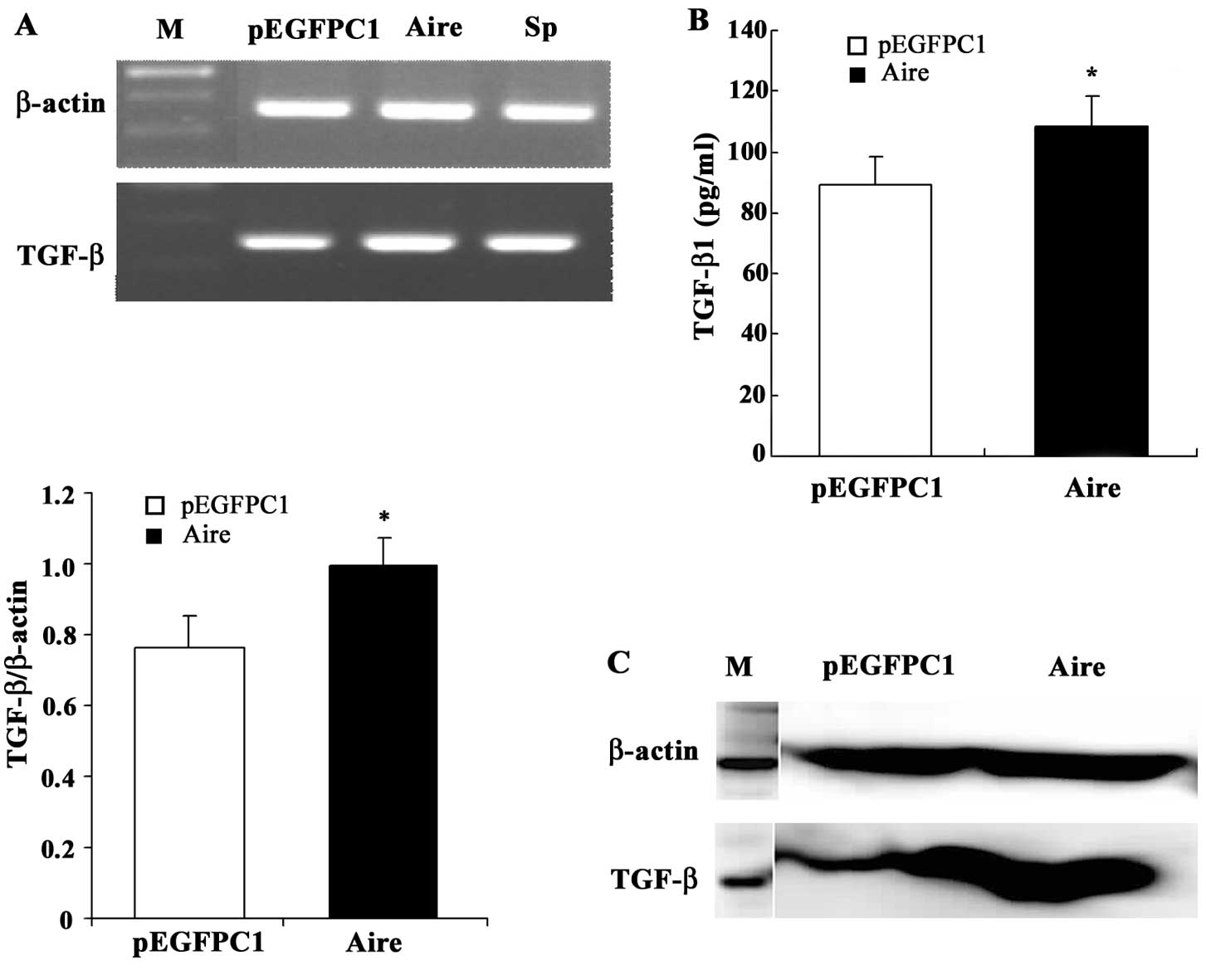
TGF-β levels are higher in RAW264.7 cells
expressing Aire
As shown in Fig. 5,
the supernatants of Aire cells may affect the composition of Treg
cells. This suggests that substances in the supernatant may induce
the production of Treg cells. Several studies have reported that
TGF-β induces the production of Treg cells. Therefore, we examined
the TGF-β levels in the Aire and control cells. The TGF-β mRNA and
protein levels were higher in the Aire cells than in the control
cells (Fig. 6). These data suggest
that TGF-β is the factor in the supernatant responsible for the
induction of Treg cells.
Anti-TGF-β antibody blocks the effects of
RAW264.7 cells overexpressing Aire on
CD4+Foxp3+ T cells
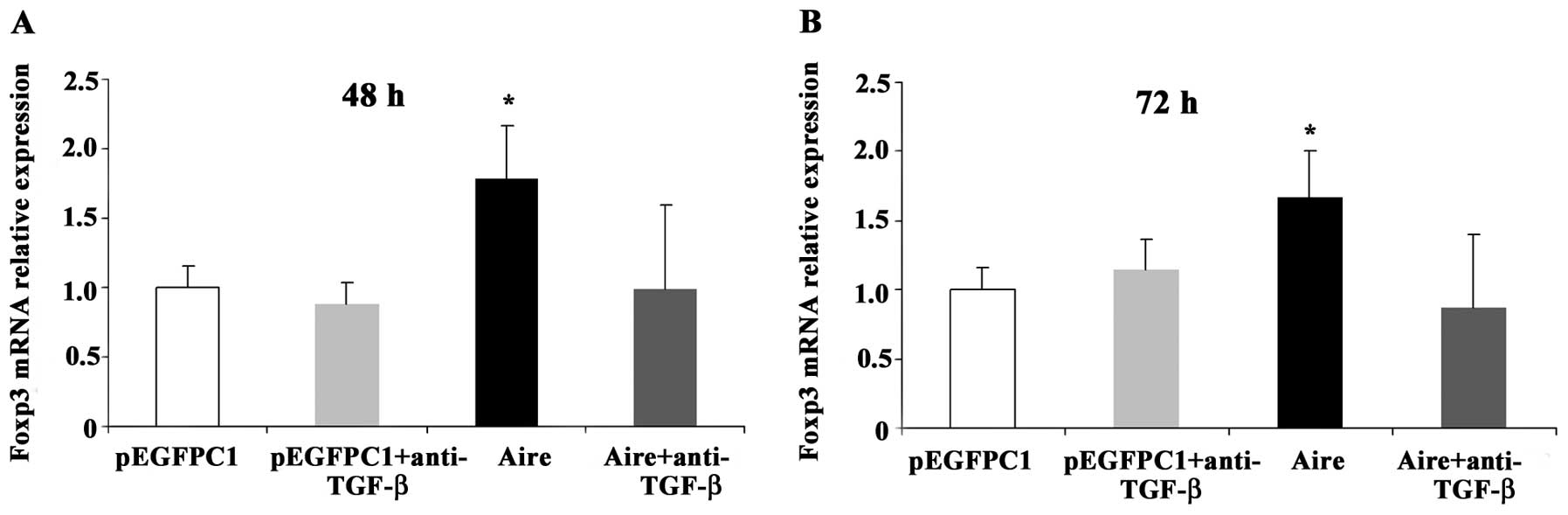
To confirm whether TGF-β mediates the effects of
Aire on CD4+Foxp3+ T cells, an anti-TGF-β
antibody was used to block the supernatants of the Aire cells prior
to the addition of the supernatants to the splenocytes. The
expression levels of Foxp3 mRNA were elevated in the splenocytes
cultured with the supernatant from the Aire cells at 48 and 72 h.
However, when the splenocytes were incubated with the blocked
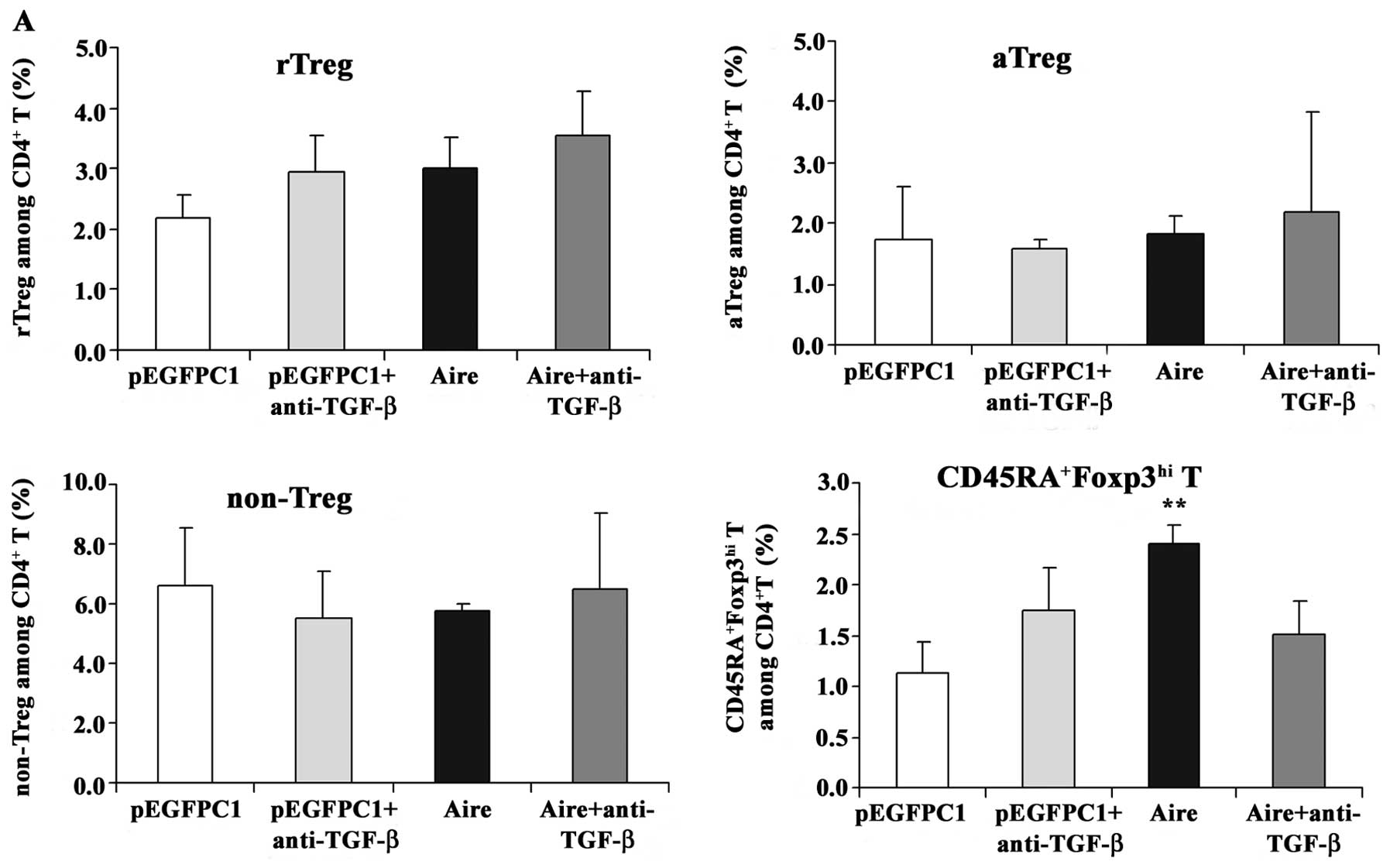
supernatants, the Foxp3 mRNA expression levels decreased (Fig. 7). We also evaluated the effects in
each subset of the CD4+Foxp3 T cells cultured with
supernatants neutralized with the anti-TGF-β antibody. The
supernatant from the Aire cells increased the percentage of
CD45RA+Foxp3hi T cells at 48 and 72 h, but
this increase did not occur when the supernatant was blocked with
anti-TGF-β (Fig. 8). The
percentage of aTreg cells increased at 72 h after culturing with
the supernatant from the Aire cells, but did not decrease to the
same level as that of the control group after treatment with the
neutralizing anti-TGF-β antibody. From these results, we can
conclude that Aire promotes TGF-β production and induces an
increase in the number of
CD4+CD45RA+Foxp3hi T cells. The
induction of aTreg cells following co-culture with Aire cells may
be mediated by other molecules besides TGF-β.
Discussion
The role of Aire in the central immune organs,
particularly in the thymus, is well defined. Aire functions to
eliminate autoreactive lymphocytes through negative selection by
regulating the expression of tissue restricted antigens (TRAs).
Aire is also thought to affect the positive selection of Treg cells
that mediate peripheral immune tolerance. This viewpoint is based
on the following observations: the number of Treg cells is lower in
APECED patients (11); and T cell
receptor (TCR)-influenza hemagglutinin (HA) mice crossed with
Aire-HA mice produce more
CD4+TCR-HA+Foxp3+ Treg cells
(12). Aire is also involved in
chemokine receptor XCR1 ligand (XCL1)-mediated medullary thymic DC
recruitment and contributes to Treg cell production (13). Furthermore, studies of mice with
deficiencies in Foxp3 and/or Aire show that mice deficient in the
two genes do not have a more severe manifestation than mice
deficient in a single gene (14).
Although the functions of Aire in the thymus have
been extensively studied, the functions of Aire in the periphery
remain unclear and debated. The aim of this study was to examine
the extrathymic functions of Aire using Aire-overexpressing
RAW264.7 cells.
We found that Aire overexpressed in RAW264.7 cells
affected the different subsets of CD4+Foxp3+
Treg cells. Through cell-cell contacts, Aire cells were able to
promote the production of rTreg cells and inhibit the production of
aTreg and non-Treg cells. However, the exact mechanisms by which
Aire affects Treg cells are not clear. A recent study reported that
some fungi induced the production of Treg cells through TLR3
(15), and we previously reported
that Aire promotes the expression of TLR3 (10). A pathway mediated through TLR3 is
likely to be one of the mechanisms by which Treg cells are induced.
A deficiency in Aire may affect the expression of TLR3 and
therefore result in autoimmune disease.
TGF-β is generally considered to be a potent inducer
of Treg cells (16). Given that
Aire overexpression upregulates TGF-β expression in macrophages and
induces aTreg production, Aire may also enhance the secretion of
TGF-β to induce the production of aTreg cells to suppress immune
responses and prevent autoimmune disease. In addition, based on
previous reports, IL-6 potently prevents the TGF-β-mediated
development of Treg cell induction and instead acts with TGF-β to
induce Th17 cell differentiation (17). Stimulation of Treg cells in the
presence of IL-6 results in loss of Foxp3 expression and
acquisition of a Th17 cell phenotype and function (18,19).
In our previous study, although IL-6 expression in RAW264.7 cells
overexpressing Aire increased following treatment with LPS, it was
significantly lower than in the LPS-treated control cells
(unpublished data). This means that the expression of Aire may
inhibit IL-6 expression, thus leading to the induction of Treg cell
production and suppression of Th17 cell production.
Patients with a mutation in Aire (APECED) often
contract a cutaneous Candida albicans infection in addition
to autoimmune diseases. As previously mentioned, overexpression of
Aire in macrophages enhances TLR3 expression and promotes the
production of Treg cells. In addition, C. albicans is one of
the fungi that induces Treg cells via TLR3 (15). This result may provide an
explanation for the manifestation of C. albicans in
Aire-deficient patients. A recent study showed increased secretion
of IL-17A in response to C. albicans in patients with
autoimmune polyendocrine syndrome type 1 (20). Non-Treg cells have the ability to
produce IL-17 and overexpression of Aire in macrophages inhibits
the production of non-Treg. This suggests that APECED patients, who
are deficient in Aire, may not be able to suppress the
overproliferation and activation of non-Treg cells that then
produce a large amount of IL-17 and mediate the response to C.
albicans.
In conclusion, we found that the overexpression of
Aire in RAW264.7 cells may affect the induction of different
subsets of Treg cells. This finding yields a possible explanation
for the prevalence of C. albicans infections in patients
with autoimmune polyendocrine syndrome type 1 and may also lead to
new therapeutics for the treatment of autoimmune disease and
transplantation.
Acknowledgements
The current study was supported by grants from the
National Natural Science Foundation of China (nos. 30872307 and
81001304). We would like to thank Professor Xuejin Su in the
Department of Pathology at Jilin University for help with the FACS
experiments.
References
|
1
|
Nagamine K, Peterson P, Scott HS, et al:
Positional cloning of the APECED gene. Nat Genet. 17:393–398. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
The Finnish-German APECED Consortium. An
autoimmune disease, APECED, caused by mutations in a novel gene
featuring two PHD-type zinc-finger domains. Nat Genet. 17:399–403.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Suzuki E, Kobayashi Y, Kawano O, et al:
Expression of AIRE in thymocytes and peripheral lymphocytes.
Autoimmunity. 41:133–139. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pöntynen N, Strengell M, Sillanpää N,
Saharinen J, Ulmanen I, Julkunen I and Peltonen L: Critical
immunological pathways are downregulated in APECED patient
dendritic cells. J Mol Med (Berlin). 86:1139–1152. 2008.PubMed/NCBI
|
|
5
|
Cohen JN, Guidi CJ, Tewalt EF, et al:
Lymph node-resident lymphatic endothelial cells mediate peripheral
tolerance via Aire-independent direct antigen presentation. J Exp
Med. 207:681–688. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fletcher AL, Lukacs-Kornek V, Reynoso ED,
et al: Lymph node fibroblastic reticular cells directly present
peripheral tissue antigen under steady-state and inflammatory
conditions. J Exp Med. 207:689–697. 2010. View Article : Google Scholar
|
|
7
|
Ramsey C, Hässler S, Marits P, Kämpe O,
Surh CD, Peltonen L and Winqvist O: Increased antigen presenting
cell-mediated T cell activation in mice and patients without the
autoimmune regulator. Eur J Immunol. 36:305–317. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sakaguchi S, Sakaguchi N, Asano M, Itoh M
and Toda M: Immunologic self-tolerance maintained by activated T
cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a
single mechanism of self-tolerance causes various autoimmune
diseases. J Immunol. 155:1151–1164. 1995.
|
|
9
|
Miyara M, Yoshioka Y, Kitoh A, et al:
Functional delineation and differentiation dynamics of human
CD4+ T cells expressing the Foxp3 transcription factor.
Immunity. 30:899–911. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhu W, Yang W, He Z, et al: Overexpressing
autoimmune regulator regulates the expression of toll-like
receptors by interacting with their promoters in RAW264.7 cells.
Cell Immunol. 270:156–163. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kekäläinen E, Tuovinen H, Joensuu J, et
al: A defect of regulatory T cells in patients with autoimmune
polyendocrinopathy-candidiasis-ectodermal dystrophy. J Immunol.
178:1208–1215. 2007.PubMed/NCBI
|
|
12
|
Aschenbrenner K, D’Cruz LM, Vollmann EH,
et al: Selection of Foxp3+ regulatory T cells specific
for self antigen expressed and presented by Aire+ medullary thymic
epithelial cells. Nat Immunol. 8:351–358. 2007.
|
|
13
|
Lei Y, Ripen AM, Ishimaru N, et al:
Aire-dependent production of XCL1 mediates medullary accumulation
of thymic dendritic cells and contributes to regulatory T cell
development. J Exp Med. 208:383–394. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chen Z, Benoist C and Mathis D: How
defects in central tolerance impinge on a deficiency in regulatory
T cells. Proc Natl Acad Sci USA. 102:14735–14740. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Romani L: Immunity to fungal infections.
Nat Rev Immunol. 11:275–288. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chen W, Jin W, Hardegen N, et al:
Conversion of peripheral CD4+CD25- naive T
cells to CD4+CD25+ regulatory T cells by
TGF-beta induction of transcription factor Foxp3. J Exp Med.
198:1875–1886. 2003.
|
|
17
|
Bettelli E, Carrier Y, Gao W, et al:
Reciprocal developmental pathways for the generation of pathogenic
effector TH17 and regulatory T cells. Nature. 441:235–238. 2006.
View Article : Google Scholar
|
|
18
|
Zheng SG, Wang J and Horwitz DA: Cutting
edge: Foxp3+CD4+CD25+ regulatory T
cells induced by IL-2 and TGF-β are resistant to Th17 conversion by
IL-6. J Immunol. 180:7112–7116. 2008.PubMed/NCBI
|
|
19
|
Xu L, Kitani A, Fuss I and Strober W:
Cutting edge: regulatory T cells induce CD4+CD25-Foxp3-
T cells or are self-induced to become Th17 cells in the absence of
exogenous TGF-β. J Immunol. 178:6725–6729. 2007.PubMed/NCBI
|
|
20
|
Ahlgren KM, Moretti S, Lundgren BA, et al:
Increased IL-17A secretion in response to Candida albicans
in autoimmune polyendocrine syndrome type 1 and its animal model.
Eur J Immunol. 41:235–245. 2011.PubMed/NCBI
|