Introduction
Ventricular fibrillation (VF) induced by acute
myocardial infarction (AMI) frequently occurs without warning,
often leading to death within minutes in patients who do not
receive prompt medical attention. As is widely known, the cardiac
arrhythmia suppression trial (CAST) confirmed that traditional
antiarrhythmic drug therapy following myocardial infarction (MI)
effectively reduced ventricular premature beats and non-sustained
tachycardia, but these drugs were found to increase sudden death
and the total mortality rate compared to a placebo (1). Thus, it is critical to develop an
effective and safe approach to attenuate ischemia-induced VF in the
early phase of AMI in order to reduce sudden cardiac death (SCD).
Traditional Chinese medicine (TCM) has documented the use of
natural products, primarily plants (the source for over 80% of the
natural products), for over 2,000 years. The substances used
medicinally by different ethnic or cultural groups are viewed by
researchers as increasingly relevant and important sources of new
medicinal products.
Wenxin Keli is the first state-sanctioned TCM-based
antiarrhythmic drug and was developed by the Chinese Academy of
TCM. Baicalin, the major component of Wenxin Keli, is a flavone, a
type of flavonoid, and is found in several species in the genus
Scutellaria. This compound has protective effects against
heart injury in rats (2,3). Clinical studies have documented the
effects of Wenxin Keli in the clinical treatment of arrhythmias,
and no significant adverse reactions were observed. Recently,
Burashnikov et al(4) found
that Wenxin Keli possesses potent anti-atrial fibrillation (AF)
properties due to its ability to depress sodium channel-dependent
parameters in the atria. However, the effects of Wenxin Keli on
ischemia-induced ventricular arrhythmias in vivo remain to
be elucidated. In the present study, we demonstrated that long-term
oral treatment with Wenxin Keli is capable of attenuating
ischemia-induced ventricular arrhythmias in rats, and
ICa,L and Ito may be involved.
Materials and methods
Animal preparation and experimental
design
All experiments were performed in accordance with
the local Institutional Committee on Animal Research of Renmin
Hospital of Wuhan University (Wuhan, China) (permit no. 00015816).
Rats (250–300 g) were purchased from the Experiment Animal Center
of Tongji Medical College, Huazhong University of Science and
Technology (Wuhan, China). The investigation complied with the
Guide for the Care and Use of Laboratory Animals published by the
US National Institutes of Health (NIH publication no. 85–23,
revised 1996). Room temperature was maintained at 23°C with
constant humidity (55%), and the lights were maintained on a 12-h
light/dark cycle (8:00 am–8:00 pm light/8:00 pm–8:00 am dark). A
total of 34 rats were randomly divided into three groups: Group 1,
sham-operated group (n=8), rats underwent surgical procedures
without coronary artery ligation; Group 2, control group (n=13),
saline was administered for 3 weeks by gavage prior to coronary
artery occlusion; Group 3, Wenxin Keli group (n=13), Wenxin Keli (8
g/kg, qd, gavage) was administered for 3 weeks prior to coronary
artery occlusion.
After being anesthetized with sodium pentobarbital
[40 mg/kg, intraperitoneally (IP)], the rats were ventilated
artificially via a tracheal cannula using a constant volume rodent
ventilator (tidal volume, 3.0 ml; respiratory rate, 70
strokes/min). The right common carotid artery was cannulated to
measure the mean arterial blood pressure (MBP). Lead II of the
electrocardiogram was monitored with subcutaneous stainless steel
electrodes. A computer-based EP system (LEAD2000B; Jinjiang Ltd.,
Chengdu, China) was used to record the heart rate and the
electrocardiogram. Under sterile conditions, a left thoracotomy was
performed in the fourth intercostal space. After pericardiotomy, a
5–0 prolene suture was tied around the left anterior descending
coronary artery at 2–3 mm from its origin. A successful myocardial
ischemia model was confirmed by ST segment elevation in Lead II and
by regional cyanosis of the myocardial surface.
Assessment of ventricular
arrhythmias
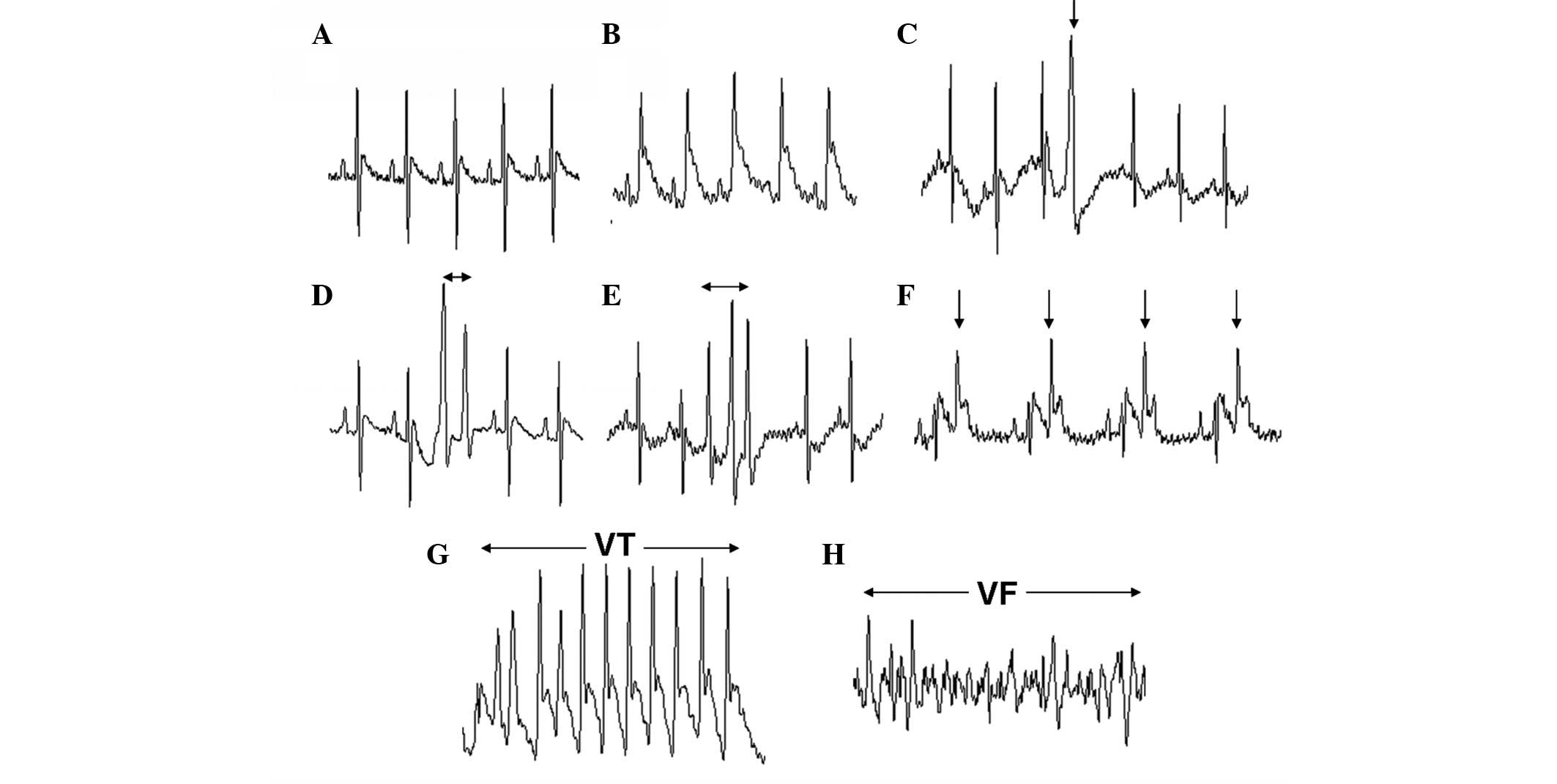
Ischemia-induced ventricular arrhythmias were
identified in accordance with the Lambeth Conventions as in our
previous study (5) (Fig. 1). Ventricular ectopic beats (VEBs)
were defined as identifiable premature QRS complexes. Ventricular
tachycardia (VT) was defined as the occurrence of ≥4 consecutive
VEBs at a rate faster than the resting sinus rate. VF was defined
as unidentifiable and low voltage QRS complexes. Other multipart
forms of VEBs, such as bigeminy, couplets (two consecutive VEBs)
and triplets (three consecutive VEBs), were evaluated as separate
episodes (Fig. 1). VF may be
sustained or may spontaneously revert to a normal sinus rhythm. VF
lasting for >5 min was considered irreversible.
The severity of the arrhythmias was quantified by
the following scoring system (6,7): a
total of 0–50 VEBs with no other arrhythmias during the 30-min
ischemia period resulted in a score of 0; a total of 50–500 VEBs in
a score of 1; a total of >500 VEBs or one episode of
spontaneously reversible VT or VF in a score of 2; a total of 2–30
episodes of spontaneously reversible VT and/or VF in a score of 3;
a total of >30 episodes of spontaneously reversible VT and/or VF
in a score of 4; and irreversible VF in a score of 5.
Whole-cell patch clamp recording
Isolation of cardiac ventricular
myocytes and patch clamp recordings
Ventricular myocytes were isolated by collagenase
type 2 (Type II; Sigma, St. Louis, MO, USA) perfusion from normal
adult rats as previously described (8). All steps were performed at 37°C in
solutions gassed with 95% O2 + 5% CO2. The
ventricles were cut off, cut into small pieces and gently stirred
in Tyrode's solution plus 1 mg/ml bovine serum albumin to collect
ventricular myocytes.
Membrane currents were obtained and analyzed with an
EPC-9 patch clamp amplifier (HEKA Electronik, Lambrecht, Germany)
in the whole-cell mode by the Pulse/Pulsefit software program (HEKA
Elektronik). Single cardiac ventricular myocytes were placed in the
experimental chamber (1.5 ml) mounted on the stage of an inverted
microscope (IX70; Olympus, Tokyo, Japan) and perfused with external
solution including different concentrations of Wenxin Keli (1 and
10 g/l) for 5 min at a rate of 2–3 ml/min. The measurements were
performed at room temperature (20–25°C). Glass microelectrodes were
made using two-stage pulling with a resistance of 3.0–5.0 MΩ on
microelectrodes (PB-7; Narishige, Tokyo, Japan) filled with
internal solution. The mean capacitance of the cells was
92.92±35.52 pF, and the series resistances were <25 MΩ. All
currents were digitally sampled at 10 kHz, low-pass filtered at 1
kHz, and saved on a hard drive for post hoc analysis.
Measurement of ICa,L and
Ito
ICa,L was recorded using a whole-cell
patch clamp configuration. The pipette solution contained 120 mM
CsCl, 1.0 mM CaCl2, 5.0 mM MgCl2, 5.0 mM
Na2ATP, 11 mM EGTA, 10 mM HEPES and 11 mM glucose,
adjusted to pH 7.2 with CsOH. The external solution was Tyrode's
solution (135 mM NaCl, 5.4 mM KCl, 1.8 mM CaCl2, 1.0 mM
MgCl2, 0.33 mM NaH2PO4, 10 mM
HEPES and 10 mM glucose, adjusted to pH 7.35 with NaOH), including
1 or 10 g/l Wenxin Keli. In order to estimate the spontaneous
decline of ICa,L with time (run-down) during the first 5
min of recording, we added 5 mmol/l MgATP to the pipette solution
and commenced data acquisition after 5–15 min of equilibration
between the pipette solution and the intracellular contents.
The external solution used to record Ito
contained 30 mM NaCl, 110 mM choline chloride, 5.4 mM KCl, 1.0 mM
MgCl2, 0.33 mM NaH2PO4, 10 mM
HEPES, 10 mM glucose and 0.3 mM CdCl, adjusted to pH 7.35 with
NaOH. The pipette solution used to record Ito contained
45 mM KCl, 85 mM K-aspartate, 5 mM Na-pyruvate, 5.0 mM MgATP, 10 mM
EGTA, 10 mM HEPES and 11 mM glucose, adjusted to pH 7.2 with KOH.
HEPES, Na2ATP, CsCl, EGTA and CsOH were purchased from
Sigma. All the other chemicals were of analytical grade. Wenxin
Keli was provided by the Shandong Buchang Pharmaceutical Company
Co., Ltd. (Beijing, China).
Data analysis
All values were presented as the means ± SD. The
incidence of VT and VF was compared using the Fisher's exact test,
and the arrhythmia scores were analyzed with the Kruskal-Wallis
test. Patch clamp data were analyzed using one-way analysis of
variance (ANOVA). Statistical significance was defined as
P<0.05.
Results
Ventricular arrhythmias during
ischemia
The MBP and heart rate (HR) were continuously
recorded during the experiments, and the average MBP and heart rate
during the 30-min baseline and 30-min ischemia periods were
calculated. No significant differences were found in the HR and MBP
between the groups (P>0.05) at baseline. The MBP and heart rate
after 30-min ligation were lower than those before 30-min ligation,
but the differences were not statistically significant (P>0.05).
In this model of ischemia, severe ventricular arrhythmias peaked at
0–30 min following coronary artery ligation. Fig. 1 shows the different ventricular
arrhythmias during the 30-min ischemia.
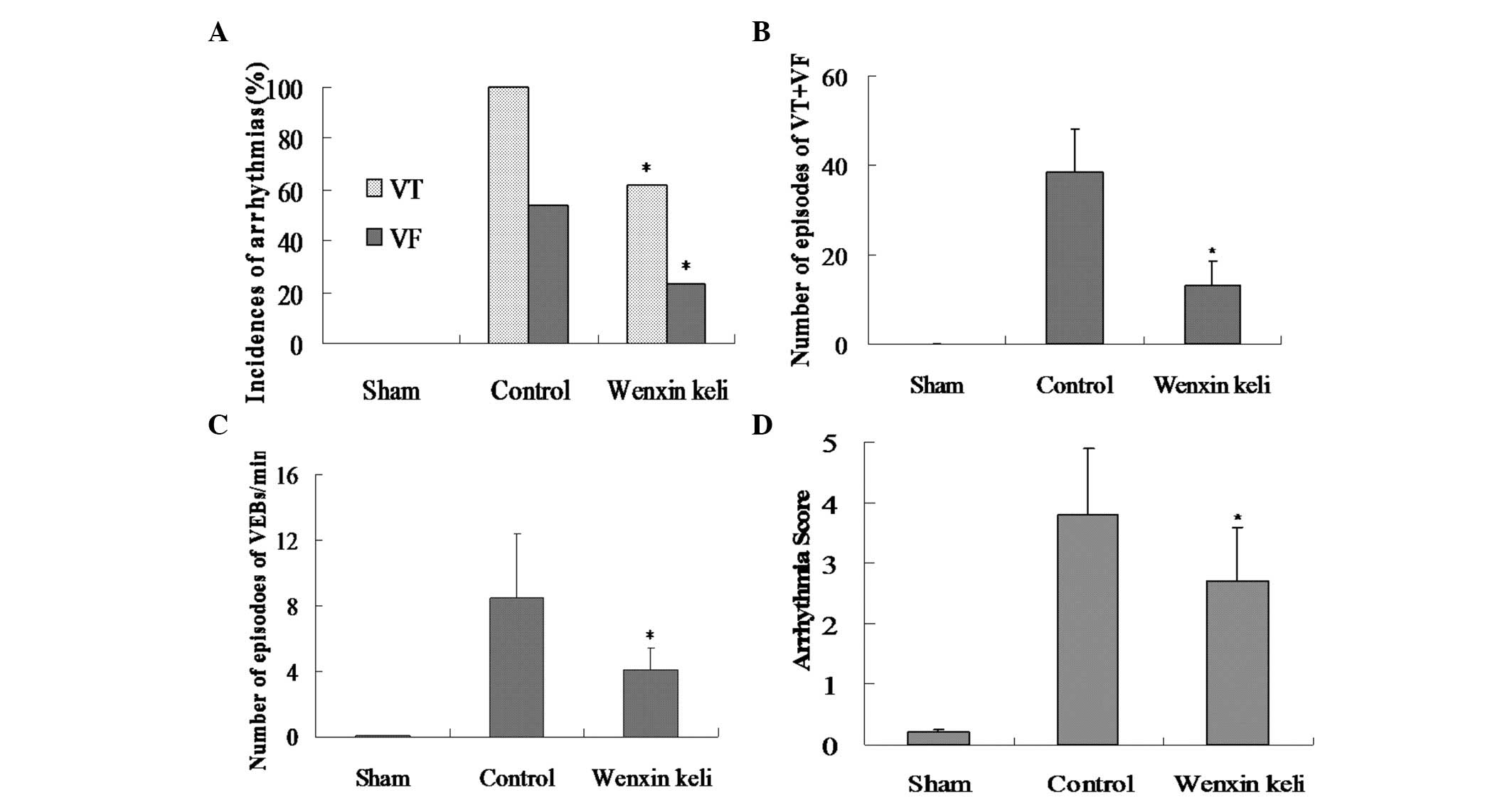
Incidence of VT and VF and number of
episodes of VT+VF
In the myocardial ischemia group, VT was observed in
100% (13/13) of the rat hearts, and 53.84% (7/13) of the hearts
exhibited VF. The administration of Wenxin Keli attenuated the
incidence of VT to 61.54% (8/13) and that of VF to 23.08% (3/13)
compared with the control group (Fig.
2A). The number of episodes of VT+VF in the Wenxin Keli group
(13.2±5.3) was significantly lower compared to that in the control
group (38.4±9.8) (P<0.05) (Fig.
2B).
Number of episodes of VEBs/min and
severity of arrhythmias
The number of episodes of VEBs/min in the Wenxin
Keli group (4.1±1.3) was significantly decreased compared to that
in the control group (8.5±3.9) (P<0.05) (Fig. 2C). The severity of ventricular
arrhythmias was significantly attenuated by Wenxin Keli (2.7±0.9)
compared to the severity in the myocardial ischemia group (3.8±1.1)
(P<0.05) (Fig. 2D).
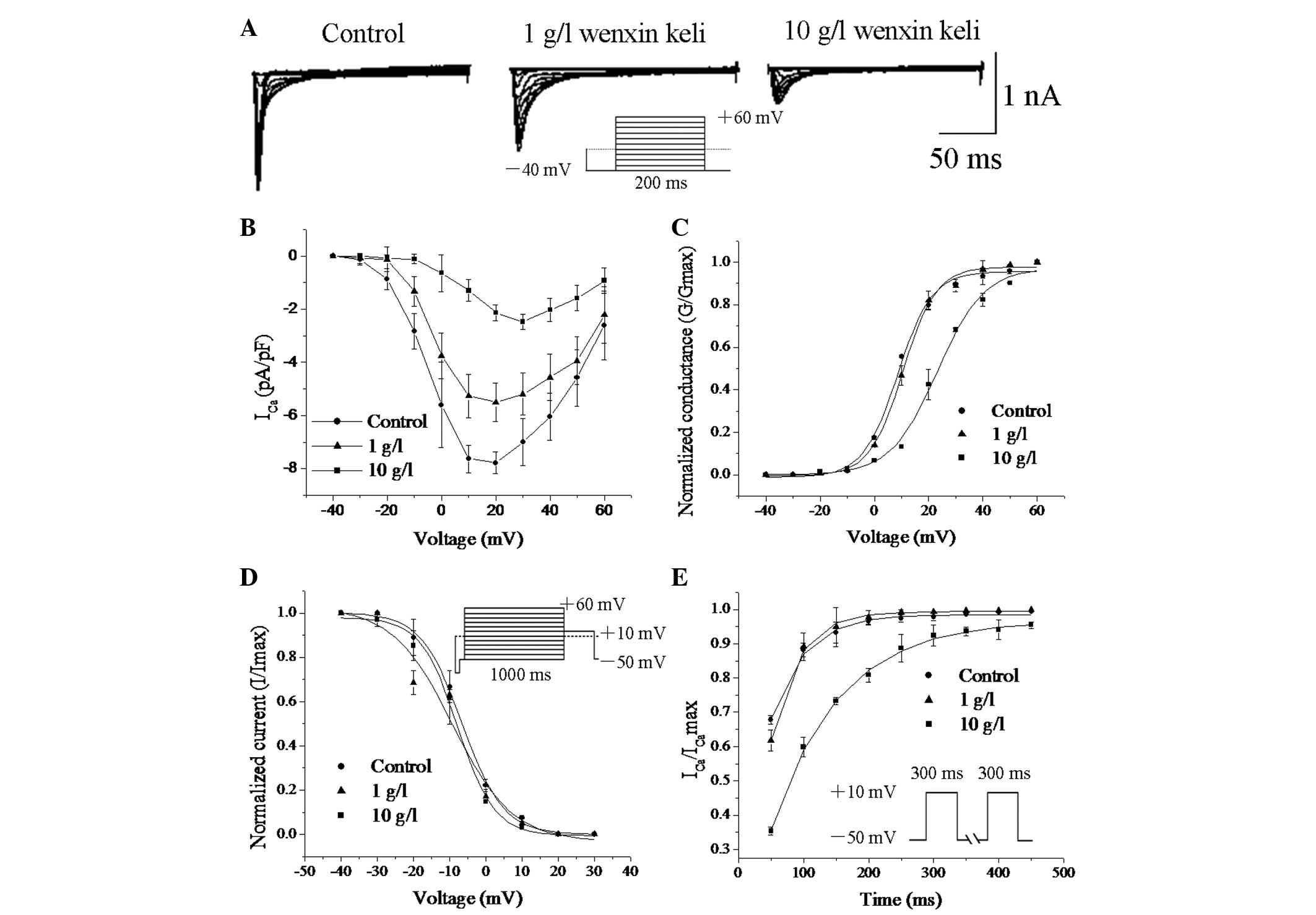
Wenxin Keli inhibits
ICa,L
Fig. 3A shows the
voltage-dependent ICa,L traces recorded in the absence
and presence of Wenxin Keli (protocol, HP=−40, 200 ms pulses of
voltages between −40 and +60 mV in 10 mV steps preceded by a 50 ms
prepulse of −40 mV). Wenxin Keli markedly reduced the amplitude of
ICa,L. The current-voltage (I-V) correlations for the
ICa,L density shown in Fig.
3B indicate that Wenxin Keli significantly inhibited
ICa,L at −10 to +60 mV in a concentration-dependent
manner.
The activation conductance variable (G/Gmax) of
ICa,L was determined from the I-V relationship for each
cell (Fig. 3B) and was fitted to
the Boltzmann distribution to obtain the half activation (V0.5) and
slope values. The V0.5 of ICa,L activation positively
shifted by 9.6 mV in the cells treated with 10 g/l of Wenxin Keli
(12.78±8.7 mV in the control group to 22.38±5.1 mV in the Wenxin
Keli group; n=5; P<0.05) (Fig.
3C), whereas no change was observed when using 1 g/l of Wenxin
Keli. The values of the variables (I/Imax) for the
voltage-dependent inactivation of ICa,L were determined
with the double-pulse protocol (a 1,000 ms prepulse of potentials
between −50 and +60 mV in 10 mV steps, followed by a fixed 400 ms
test pulse of 10 mV) (Fig. 3D),
and these data were also fitted to the Boltzmann distribution. The
V0.5 of ICa,L inactivation was not significantly changed
by the administration of Wenxin Keli.
The time-dependent recovery of ICa,L
following inactivation was studied with the double-pulse protocol
consisting of two identical pulses (holding potential from −50 to
+10 mV for 300 ms) in variable intervals from 50 to 500 ms in 50 ms
increments (Fig. 3E). The recovery
curves were fitted to a mono-exponential function. The recovery
time constant of ICa,L was slowed by 10 g/l Wenxin Keli
(55.76±5.98 ms in control, 104.13±4.71 ms in 10 g/l; n=6;
P<0.05), whereas no change was observed for 1 g/l Wenxin Keli
(59.82±7.24 ms). These results demonstrate that Wenxin Keli
inhibits ICa,L by decelerating the activation process
and delaying recovery from inactivation without changing the
inactivation process.
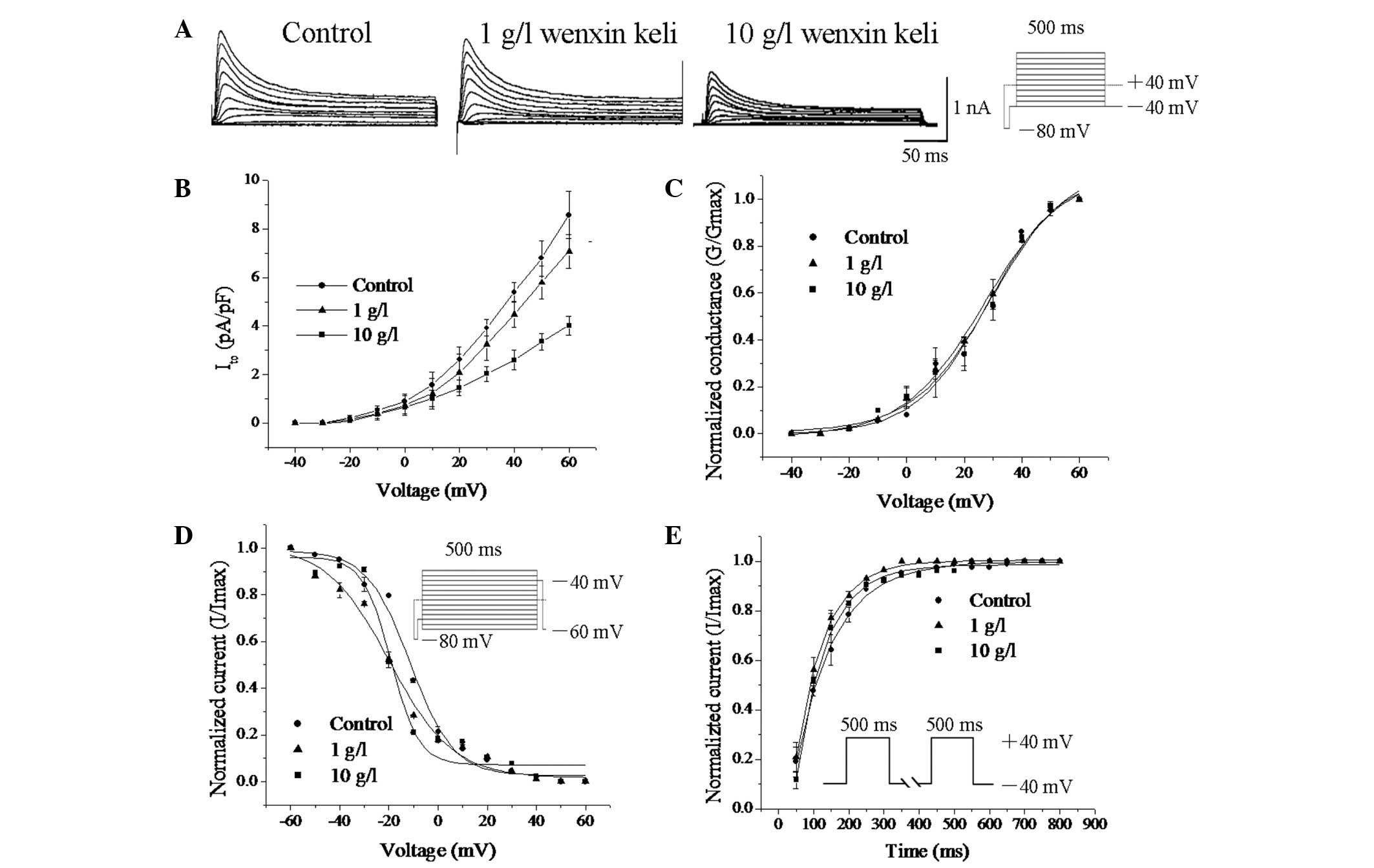
Wenxin Keli inhibits Ito
Fig. 4A illustrates
the voltage-dependent Ito trace in the absence and
presence of Wenxin Keli (500 ms depolarization step pulses from −40
to +60 mV with a step size of 10 mV). Wenxin Keli at 10 g/l
markedly reduced the amplitude of Ito. The I-V
relationship for the Ito density, shown in Fig. 4B, indicated that Wenxin Keli
inhibited Ito in a concentration-dependent manner. The
peak amplitude decreased to 15.31±7.21% at 1 g/l and to 53.25±4.74%
at 10 g/l (n=6, P<0.05).
By fitting the activation process of Ito
to the Boltzmann distribution, we found that Wenxin Keli (1 and 10
g/l) had no significant effect on voltage-dependent activation
(Fig. 4C). Steady-state
inactivation was analyzed using a double-pulse protocol: a 1,000 ms
prepulse of potentials between −60 and +60 mV in 10 mV steps,
followed by a fixed 400 ms test pulse of +40 mV. The V0.5 of
voltage-dependent inactivation was negatively shifted by treatment
with Wenxin Keli (−11.30±2.6 mV for control, −19.75±3.02 mV for 1
g/l, and −19.21±4.15 mV for 10 g/l, n=6, P<0.05) (Fig. 4D).
Recovery of Ito after inactivation was
investigated with a paired-pulse protocol (HP=−80 mV, a 500-ms
conditioning pulse of +40 mV was separated from a 50-ms test pulse
of −40 mV by a gradually prolonged recovery interval between 50 and
800 ms) (Fig. 4E), and the
recovery curves were fitted with a mono-exponential function. No
significant changes in the recovery time constants were observed.
These results demonstrate that Wenxin Keli inhibits Ito
by accelerating its inactivation without changing its activation
process or recovery from inactivation.
Discussion
Ventricular arrhythmias, particularly spontaneous or
induced ventricular tachyarrhythmias and fibrillation, are
frequently observed post-infarction in various animal models of MI
(9,10). The majority of sudden cardiac
deaths are thought to be due to ventricular arrhythmias. Thus, the
treatment of ventricular arrhythmias, particularly VF, is important
in order to reduce the risk of sudden cardiac death
post-infarction. In the present study, Wenxin Keli was shown to
prevent ventricular arrhythmias in vivo following long-term
administration in a rat model of MI. Our data also demonstrated
that the antiarrhythmic effect of Wenxin Keli is associated with
the inhibition of ICa,L and Ito.
Traditional antiarrhythmic drugs may paradoxically
precipitate lethal arrhythmias; these drugs occasionally intensify
rather than inhibit arrhythmias (11,12).
Basic research into the clinical application of traditional Chinese
medicine has been conducted. It has been recognized worldwide that
traditional Chinese medicine has broad clinical prospects due to
its advantages with respect to multiple targets, significant
efficacy and safety. Wenxin Keli is useful for treating functional
arrhythmia and arrhythmia as a complication of infective
cardiomyopathy in the elderly and children. It has been shown that
the combined use of Wenxin Keli and amiodarone has a better effect
on the conversion rate of AF, shortening the conversion time and
decreasing the required dosage of amiodarone in treating AF,
compared with treatment with amiodarone alone. The use of Wenxin
Keli also protects against the adverse effects of the long-term use
of amiodarone (13,14). In addition, Wenxin Keli is capable
of greatly improving isoproterenol-induced cardiac dysfunction and
protecting against aconitine-induced arrhythmia in rats (15). Moreover, Wenxin Keli produces
atrial-selective depression of INa-dependent parameters
in isolated canine coronary perfused preparations and effectively
suppresses AF and prevents its induction (4,16).
Therefore, it is implied that Wenxin Keli has good clinical
prospects.
It is generally accepted that cardiac repolarization
and refractoriness are determined by the balance of inward
Ca2+ currents and outward K+ currents. The
L-type Ca2+ channel is considered to be the primary
route for calcium influx into cardiac myocytes and an important
determinant of calcium homeostasis. The increased ICa,L
may contribute to the prolongation of the action potential duration
and increase the frequency of early afterdepolarizations (EADs), as
demonstrated for L-type Ca2+ channel agonists (17–21).
Pathological remodeling of the myocardium depends on the persistent
activation of L-type calcium channels, which alters calcium
homeostasis and is responsible for the induction of hypertrophic
growth (22,23). In addition, more calcium entered
the cell through the L-type Ca2+ channel during
depolarization, leading to calcium overload and triggering cell
death signals (24).
Ito is a key regulator of phase one action potential
repolarization and is the primary cause of spike-and-dome
morphology (25). In addition,
Ito is important in human ventricle repolarization. Its
voltage-dependent activation and inactivation kinetics are much
faster than those of other cardiac K currents. Increased
Ito density may eliminate the plateau, which is the
primary mechanism responsible for the occurrence and maintenance of
VF. The data from our study demonstrate that Wenxin Keli
significantly inhibits ICa,L and Ito in adult
rat ventricular myocytes. This substance reduced the amplitude of
ICa,L, decelerated the activation process and slowed
down its recovery from inactivation, whereas the inactivation
process remained unaffected. In addition, Wenxin Keli inhibited
Ito and accelerated its inactivation without changing
the activation process or the recovery of its inactivation. These
effects of Wenxin Keli on ICa,L and Ito may
be protective against cardiac fibrillation.
In conclusion, the present study demonstrates that
Wenxin Keli attenuated ischemia-induced ventricular arrhythmias and
inhibited ICa,L and Ito. The regulation of
ICa,L and Ito contributed, at least in part,
to the antiarrhythmic action of Wenxin Keli.
Acknowledgements
This study was financially supported by the
Fundamental Research Funds for the Central Universities (no.
201030201 01000195 and 201130202020003). The authors are grateful
to Shandong Buchang Pharmaceutical Co., Ltd. for generously
providing Wenxin Keli. The authors would also like to thank
Professor Na Luo (School of Foreign Language, Wuhan University of
Science and Technology, Wuhan, China) for providing assistance in
writing the manuscript.
References
|
1
|
The cardiac arrhythmia suppression trial.
N Engl J Med. 321:1754–1756. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Xiping Z, Hua T, Hanqing C, et al: The
protecting effects and mechanisms of Baicalin and Octreotide on
heart injury in rats with SAP. Mediators Inflamm. 2007:194692007.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Woo AY, Cheng CH and Waye MM: Baicalein
protects rat cardiomyocytes from hypoxia/reoxygenation damage via a
prooxidant mechanism. Cardiovasc Res. 65:244–253. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Burashnikov A, Petroski A, Hu D, et al:
Atrial-selective inhibition of sodium channel current by Wenxin
Keli is effective in suppressing atrial fibrillation. Heart Rhythm.
9:125–131. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hu X, Wu B, Wang X, et al: Minocycline
attenuates ischemia-induced ventricular arrhythmias in rats. Eur J
Pharmacol. 654:274–279. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Demiryurek AT, Yildiz G, Esiyok S and
Altug S: Protective effects of poly (ADP-ribose) synthase
inhibitors on digoxin-induced cardiotoxicity in guinea-pig isolated
hearts. Pharmacol Res. 45:189–194. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Imani A, Faghihi M, Sadr SS, et al:
Noradrenaline reduces ischemia-induced arrhythmia in anesthetized
rats: involvement of alpha1-adrenoceptors and mitochondrial K ATP
channels. J Cardiovasc Electrophysiol. 19:309–315. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Nishimura N, Reien Y, Matsumoto A, et al:
Effects of nicorandil on the cAMP-dependent Cl-current in
guinea-pig ventricular cells. J Pharmacol Sci. 112:415–423. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tsuburaya R, Yasuda S, Ito Y, et al:
Shimokawa H, Eicosapentaenoic acid reduces ischemic ventricular
fibrillation via altering monophasic action potential in pigs. J
Mol Cell Cardiol. 51:329–336. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ravingerova T, Matejikova J, Pancza D and
Kolar F: Reduced susceptibility to ischemia-induced arrhythmias in
the preconditioned rat heart is independent of PI3-kinase/Akt.
Physiol Res. 58:443–447. 2009.PubMed/NCBI
|
|
11
|
Rosen MR and Hoffman BF: Mechanisms of
action of antiarrhythmic drugs. Circ Res. 32:1–8. 1973. View Article : Google Scholar
|
|
12
|
Gettes LS: The electrophysiologic effects
of antiarrhythmic drugs. Am J Cardiol. 28:526–535. 1971. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang M, Yu YB and Huang SE: Clinical
observation on effect and safety of combined use of wenxin granule
and amiodarone for conversion of auricular fibrillation. Zhongguo
Zhong Xi Yi Jie He Za Zhi. 26:445–448. 2006.(In Chinese).
|
|
14
|
Xie PY and Shen SH: Effect of combination
of Chinese and Western medicines on sinus rhythm maintenance in
patients with auricular fibrillation after conversion. Zhongguo
Zhong Xi Yi Jie He Za Zhi. 26:644–646. 2006.(In Chinese).
|
|
15
|
Zhou F, Hu SJ and Mu Y: Protection effect
of Wenxin Keli on isoproterenol induced heart failure in rats.
Zhongguo Zhong Yao Za Zhi. 32:1676–1679. 2007.(In Chinese).
|
|
16
|
Kalifa J and Avula UM: The Chinese herb
extract Wenxin Keli: atrial selectivity from the Far East. Heart
Rhythm. 9:132–133. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
January CT, Riddle JM and Salata JJ: A
model for early afterdepolarizations: induction with the
Ca2+ channel agonist Bay K 8644. Circ Res. 62:563–571.
1988.PubMed/NCBI
|
|
18
|
January CT and Riddle JM: Early
afterdepolarizations: mechanism of induction and block. A role for
L-type Ca2+ current Circ Res. 64:977–990.
1989.PubMed/NCBI
|
|
19
|
Marban E, Robinson SW and Wier WG:
Mechanisms of arrhythmogenic delayed and early afterdepolarizations
in ferret ventricular muscle. J Clin Invest. 78:1185–1192. 1986.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
De Ferrari GM, Viola MC, D'Amato E, et al:
Distinct patterns of calcium transients during early and delayed
afterdepolarizations induced by isoproterenol in ventricular
myocytes. Circulation. 91:2510–2515. 1995.PubMed/NCBI
|
|
21
|
Liu QN, Zhang L, Gong PL, et al:
Daurisoline suppressed early afterdepolarizations and inhibited
L-type calcium current. Am J Chin Med. 38:37–49. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zobel C, Rana OR, Saygili E, et al:
Mechanisms of Ca2+-dependent calcineurin activation in
mechanical stretch-induced hypertrophy. Cardiology. 10:7281–7290.
2007.
|
|
23
|
Sucharov CC, Mariner PD, Nunley KR, et al:
A beta1-adrenergic receptor CaM kinase II-dependent pathway
mediates cardiac myocyte fetal gene induction. Am J Physiol Heart
Circ Physiol. 291:H1299–H1308. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cano-Abad MF, Villarroya M, Garcia AG, et
al: Calcium entry through L-type calcium channels causes
mitochondrial disruption and chromaffin cell death. J Biol Chem.
276:39695–39704. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Guo W, Xu H, London B and Nerbonne JM:
Molecular basis of transient outward K+ current
diversity in mouse ventricular myocytes. J Physiol. 521:3587–3599.
1999.
|