Introduction
Gastric cancer is one of the most common
malignancies worldwide and ranks as the second leading cause of
cancer-related mortality (1).
During the past few decades, the incidence of distal gastric
carcinomas has decreased markedly, while adenocarcinoma of the
esophagogastric junction (AEG), including gastric cardiac
adenocarcinoma (GCA) and adenocarcinoma of the distal esophagus,
have shown a marked increase in western countries (2). Similar changes in the incidence of
gastric carcinomas at varying subsites have also been identified in
China (3), but the reason for
these changes is not clear. As previous studies have identified
differences in histological findings, phenotypic marker expression
and genetic alterations between adenocarcinomas of the gastric
cardia and distal stomach (4), the
carcinogenesis and development of GCA may be different from that of
adenocarcinomas of the distal stomach. These possible differences
therefore require further study.
One of the most clinically significant molecular
signaling networks that has been frequently studied over the past
decade is the mammalian target of rapamycin (mTOR) pathway. The
mTOR pathway is a critical nutritional and cellular energy
checkpoint sensor and regulator of cell growth in mammalian cells
(5–7). mTOR was originally identified as the
target of the macrolide antibiotic rapamycin. It is a Ser/Thr
protein kinase that mediates nutrient-dependent intracellular
signaling associated with cell growth, proliferation and
differentiation (8). Eukaryotic
translation initiation factor 4E binding protein 1 (4E-BP1) is the
first downstream substrate of mTOR, which is a small molecular
protein (9,10). Eukaryotic translation initiation
factor 4E (eIF4E) is an oncogene encoding a cap-binding protein,
and is also the main translation initiator. eIF4E is able to
specifically identify the mRNA 5′-end cap structure (m7 GPPPN,
where N is any nucleotide and m is a methyl group) and initiate
translation, so it is an extremely significant regulatory site of
translation in eukaryotes. 4E-BP1 suppresses eIF4E activity. The
protein exerts an inhibitory effect by binding to eIF4E and
preventing the assembly of the translation preinitiation complex.
Once 4E-BP1 is phosphorylated, it dissociates from eIF4E,
permitting cap-dependent translation to take place. Under the
stimulation of hormones, mitogens and other related factors, mTOR
modulates the activity of eIF4E by regulating the phosphorylation
of 4E-BP1. Activation of mTOR leads to the phosphorylation of
4E-BP1, resulting in the dissociation of 4E-BP1 from the mRNA
cap-binding protein eIF4E and promotion of protein synthesis. By
contrast, hypophosphorylated 4E-BP1 inhibits cap-dependent
translation (11).
As activation of the mTOR growth pathway has been
observed in numerous malignant tumors, mTOR has been suggested to
be an attractive molecular target for cancer therapy. Several
studies have shown that activation of the mTOR signaling pathway
and overexpression of mTOR is common in gastric cancers (12). Treatment strategies using
everolimus, the specific inhibitor of mTOR, have had a high
efficacy and safety in gastric cancer in phase II studies, and
therefore a global phase III study is now being prepared (13). The mTOR signaling pathway has
become a new target for gastric cancer therapy, but the role of the
mTOR signaling pathway in the varying subsites of gastric cancer
has not yet been reported.
The aim of this study was to evaluate the activation
of the mTOR/4E-BP1 signal transduction pathway in GCA, as it had
not often been studied in the past, and to explore the putative
role of the mTOR/4E-BP1 signal transduction pathway in the
carcinogenesis and progression of cardiac adenocarcinomas.
Patients and methods
Patients
A total of 33 patients with GCA who underwent
curative surgery at The Fouth Hospital of Hebei Medical University
and Cixian County Hospital between 2008 and 2009 were included in
this study. Fresh samples from pathologically representative tumor
regions and paired adjacent normal gastric mucosal tissues were
obtained. These tissues were stored at −80°C until the proteins and
RNA were extracted. The pathological nature of each specimen was
confirmed by examination with hematoxylin and eosin staining. Prior
treatments, including radiotherapy or chemotherapy, were not used
before surgery in any of the cases. The tumors were selected
carefully according to the definition of the Siewert II AEG, the
epicenters of which were at the gastroesophageal junction, i.e.,
from 1 cm above to 2 cm below the junction between the end of the
tubular esophagus and the beginning of the saccular stomach
(14). This study was approved by
the ethics committee of Hebei Medical University, Shijiazhuang,
China. Informed consent was obtained from all patients.
Western blot analysis
Whole-cell lysates were prepared from human GCA and
corresponding adjacent normal gastric mucosal tissues. Balanced
amounts of protein from each sample (100 μg) were fractionated by
SDS-PAGE. Subsequent to transferring the proteins onto
Immobilon-PVDF, the membranes were blocked with blocking buffer
containing 5% skimmed, dry milk and then incubated with the primary
antibody overnight at 4°C. The monoclonal antibody mTOR,
phosphorylated mTOR (p-mTOR; Ser2448), 4E-BP1, phosphorylated
4E-BP1 (p-4E-BP1; Ser65) and phosphorylated eIF4E (p-eIF4E) were
purchased from Cell Signaling Technology Inc. (Danvers, MA, USA)
and the monoclonal antibodies eIF4E and β-actin were purchased from
Santa Cruz Biotechnology Inc. (Santa Cruz, CA, USA). Primary
antibodies included mTOR (1:2,000), p-mTOR Ser2448 (1:1,000),
4E-BP1 (1:1,000), p-4E-BP1 Ser65 (1:1,000), eIF4E (1:200), p-eIF4E
(1:1,000) and β-actin (1:200) which were diluted in blocking
buffer. Depending on the primary antibodies used, either anti-mouse
or anti-rabbit horseradish peroxidase was used as a secondary
antibody. β-actin expression was used as a loading control and to
assess the protein extract quality.
Semi-quantitative reverse transcription
polymerase chain reaction (RT-PCR)
Total RNA was extracted by a single-step method from
the tumors and corresponding benign tissues using guanidinium
isothiocyanate. The integrity of the total RNA was identified by 1%
agarose gel electrophoresis. Quantitation was achieved using an
ultraviolet spectrophotometer. The expression of mTOR, 4E-BP1 and
eIF4E in GCA and normal gastric mucosa at the mRNA level was
determined using the RT-PCR method. Relative expression was
calculated as the ratio between the density of the target gene and
that of GAPDH by a BIO-LD densitometric image analyzer. The primers
were as follows: mTOR, forward 5′-AGAGAGGAC ACAAGCAC-3′ and reverse
5′-CACAGATAATGGCAA TG-3′; eIF4E, forward 5′-TAATCAGGAGGTTGCT-3′ and
reverse 5′-TTCTCACTTCCCACA-3′; 4E-BP1, forward
5′-GGGGACTACAGCACGAC-3′ and reverse 5′-CGCCCG CTTATCTTCT-3′; GAPDH,
forward 5′-GGAAGGTGA AGGTCGGAGT-3′ and reverse 5′-CCTGGAAGATGGTGA
TGGG-3′. PCR products were visualized by ethidium bromide staining
of 1.5% agarose gel.
Statistical analysis
SPSS 13.0 software was employed to analyze all data.
The significance of differences between two groups was determined
using the paired-samples t-test. The Fisher’s exact test was used
to test possible associations between the expression levels of
members of the mTOR/4E-BP1 pathway and clinicopathological factors.
P<0.05 was considered to indicate a statistically significant
difference.
Results
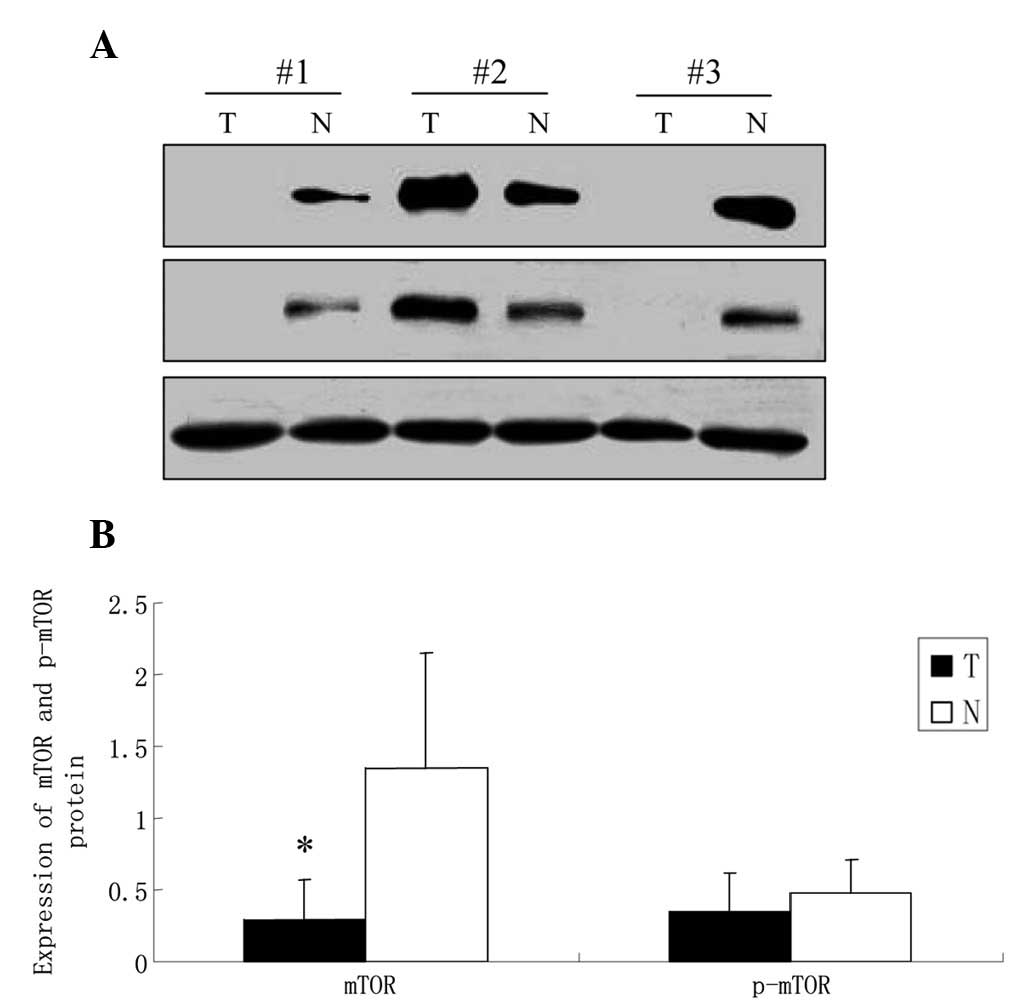
Western blot analysis
Western blot analysis results showed that the
expression of mTOR was significantly decreased in GCA compared with
that in the corresponding normal gastric mucosa (0.296±0.27 vs.
1.348±0.80, P<0.05). There was no difference identified in the
level of p-mTOR between the tumor and the corresponding normal
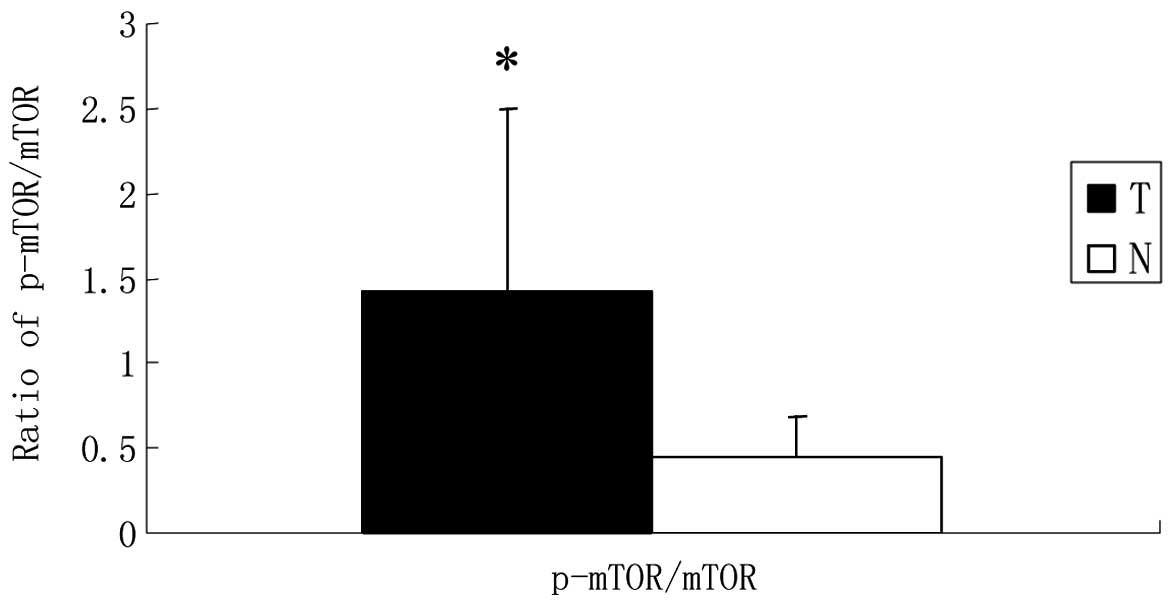
gastric tissues (0.348±0.27 vs. 0.475±0.23, P>0.05; Fig. 1). Further analysis showed that the
ratio of p-mTOR to mTOR in GCA (1.425±1.07) was significantly
higher than that in the corresponding normal gastric mucosa
(0.450±0.24, P<0.05; Fig. 2),
suggesting that mTOR was activated to a greater extent in GCA. In
comparison with expression in the corresponding normal gastric
mucosa, the expression of 4E-BP1 in the tumor tissues was
significantly decreased (2.210±0.87 vs. 4.498±1.78, P<0.05),
while western blot analysis detected an increased level of p-4E-BP1
in tumor tissues compared with the normal tissues (2.165±0.86 vs.
1.184±0.45, P<0.05; Fig. 3).
There was no significant difference in eIF4E expression between GCA
and the corresponding normal gastric mucosa (2.194±0.80 vs.
2.033±0.73, P>0.05), while the level of p-eIF4E was
significantly higher in GCA in comparison to the corresponding
normal gastric mucosa (1.822±0.63 vs. 0.997±0.38, P<0.05;
Fig. 3).
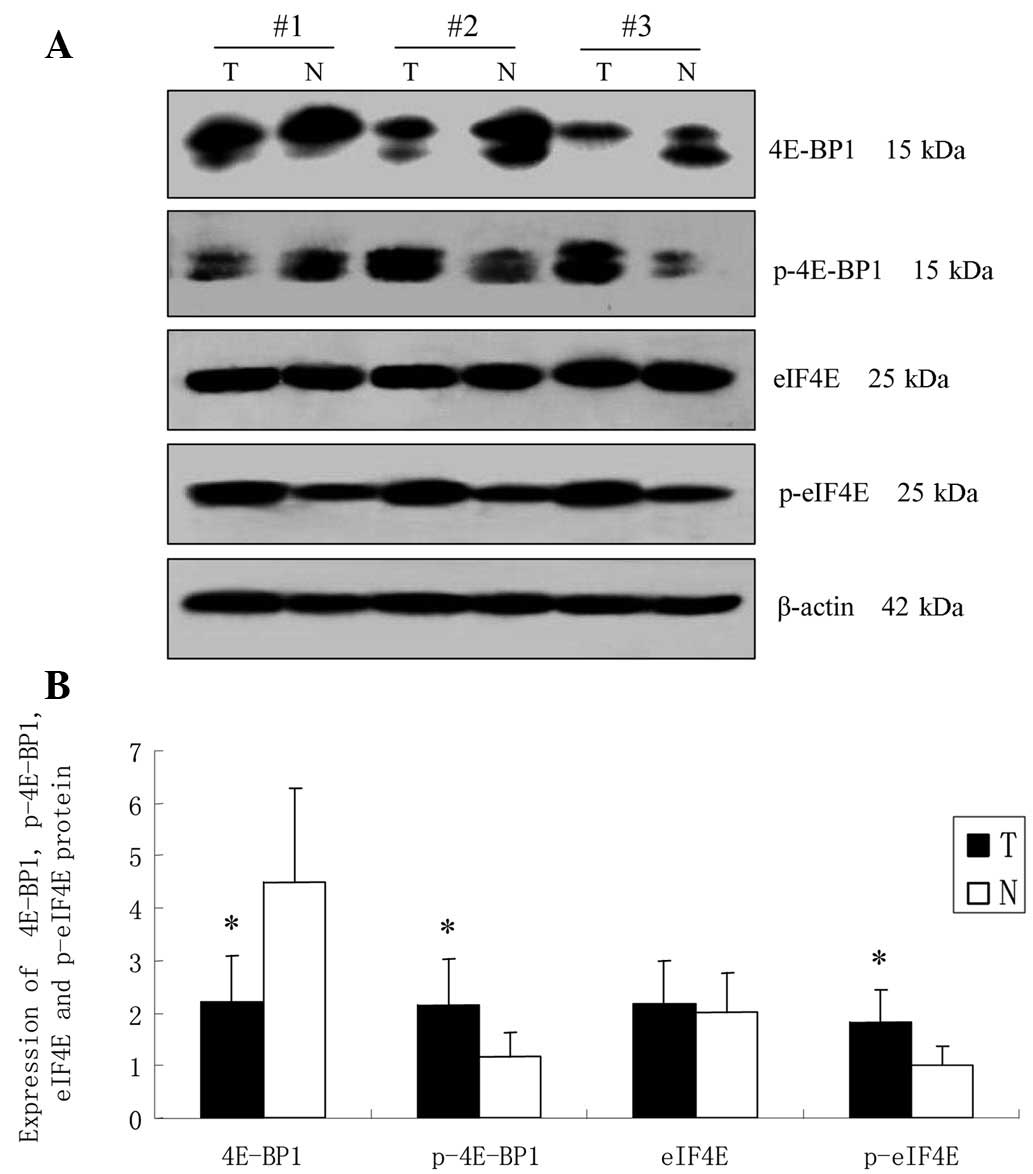 | Figure 3Expression of 4E-BP1, p-4E-BP1, eIF4E
and p-eIF4E at the protein level in GCA and paired normal gastric
mucosa investigated by western blot analysis. (A) Representative
immunoblots showing the expression of 4E-BP1, p-4E-BP1, eIF4E and
p-eIF4E in cases 1, 2 and 3. β-actin served as a loading control.
(B) Intensities of the immunoreactive bands were quantified by
densitometric scanning. T, GCA tumor tissues; N, normal gastric
mucosa. Data are the mean ± SD. *P<0.05 compared with
normal. 4E-BP1, eukaryotic translation initiation factor 4E binding
protein 1; p-4E-BP1, phosphorylated 4E-BP1; eIF4E, eukaryotic
translation initiation factor 4E; p-eIF4E, phosphorylated eIF4E;
GCA, gastric cardiac adenocarcinoma. |
The expression of mTOR, p-mTOR, mTOR/p-mTOR, 4E-BP1,
p-4E-BP1 and eIF4E in GCA was not corelated with age,
differentiation, tumor size, infiltration depth or lymph node
metastasis (P>0.05), while the level of p-eIF4E was closely
correlated with lymph node metastasis (P<0.05; Table I). The level of p-eIF4E in the
lymph node metastasis group was markedly higher compared with that
of the non-lymph node metastasis group. There was no correlation
identified between the level of p-eIF4E in GCA and the age, gender,
differentiation, tumor size or infiltration depth (P>0.05).
 | Table ICorrelations between p-mTOR/mTOR,
4E-BP1, p-4E-BP1, eIF4E and p-eIF4E and clinical pathological
characteristics in GCA. |
Table I
Correlations between p-mTOR/mTOR,
4E-BP1, p-4E-BP1, eIF4E and p-eIF4E and clinical pathological
characteristics in GCA.
| | p-mTOR/ mTOR | 4E-BP1 | p-4E-BP1 | eIF4E | p-eIF4E |
|---|
| |
|
|
|
|
|
|---|
| Clinicopathological
factors | All N (%) | H | L | P-value | H | L | P-value | H | L | P-value | H | L | P-value | H | L | P-value |
|---|
| Age (years) |
| <60 | 17 | 13 | 4 | 0.465 | 12 | 5 | 0.721 | 10 | 7 | 1.000 | 10 | 7 | 1.000 | 13 | 4 | 1.000 |
| ≥60 | 16 | 10 | 6 | | 10 | 6 | | 9 | 7 | | 10 | 6 | | 12 | 4 | |
| Differentiation |
| Poor | 15 | 8 | 7 | 0.126 | 11 | 4 | 0.712 | 9 | 6 | 1.000 | 8 | 7 | 0.493 | 13 | 2 | 0.242 |
| Well/moderate | 18 | 15 | 3 | | 11 | 7 | | 10 | 8 | | 12 | 6 | | 12 | 6 | |
| Lengh of tumor
(cm) |
| <5 | 17 | 14 | 3 | 0.141 | 12 | 5 | 0.721 | 9 | 8 | 0.728 | 12 | 5 | 0.296 | 12 | 5 | 0.688 |
| ≥5 | 16 | 9 | 7 | | 10 | 6 | | 10 | 6 | | 8 | 8 | | 13 | 3 | |
| Depth of tumor |
| T1 | 7 | 6 | 1 | 0.397 | 5 | 2 | 1.000 | 5 | 2 | 0.670 | 6 | 1 | 0.202 | 6 | 1 | 0.652 |
| T2/3/4 | 26 | 17 | 9 | | 17 | 9 | | 14 | 12 | | 14 | 12 | | 19 | 7 | |
| Lymph node
metastasis |
| Positive
(N1/2/3) | 19 | 13 | 6 | 1.000 | 14 | 5 | 0.459 | 10 | 9 | 0.723 | 11 | 8 | 1.000 | 17 | 2 | 0.047a |
| Negative (N0) | 14 | 10 | 4 | | 8 | 6 | | 9 | 5 | | 9 | 5 | | 8 | 6 | |
RT-PCR analysis
The relative expression of mTOR, 4E-BP1 and eIF4E in
GCA and corresponding normal tissues at the mRNA level were
detected by semi-quantitative RT-PCR (Fig. 4). The optical density of the mTOR
mRNA was 3.305±0.78 in tumor tissues and 7.564±1.60 in normal
tissues. The statistical results revealed that the mTOR mRNA
expression in tumor tissues was markedly lower than that in the
normal tissues (P<0.05). mRNA expression of 4E-BP1 in GCA tumor
tissues was reduced significantly in comparison to the
corresponding normal tissues (4.388±1.34 compared with 7.117±1.77,
P<0.05). The optical density of the eIF4E mRNA was 4.912±1.54 in
tumor tissues, which was markedly higher than that of the normal
control group (1.095±0.32, P<0.05).
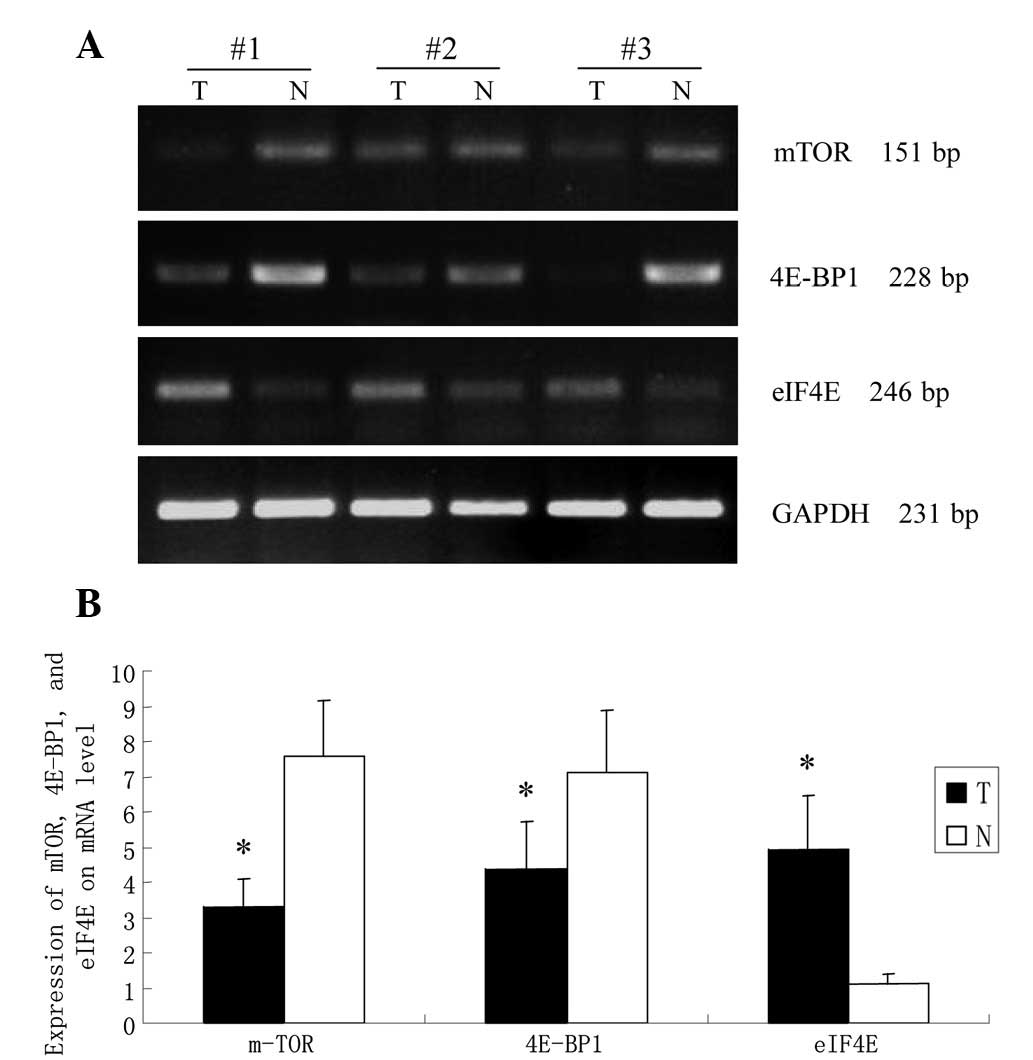 | Figure 4RT-PCR analysis of mTOR, 4E-BP1 and
eIF4E mRNA expression in GCA and paired normal gastric mucosa. (A)
Representative expression of 4E-BP1, p-4E-BP1, eIF4E and p-eIF4E at
the mRNA level. GAPDH was used to normalize any differences in mRNA
loading between lanes. (B) Intensities of the electrophoresis bands
were quantified by densitometric scanning. T, GCA tumor tissues; N,
normal gastric mucosa. Values are mean ± SD. *P<0.05
vs. normal. mTOR, mammalian target of rapamycin; 4E-BP1, eukaryotic
translation initiation factor 4E binding protein 1; eIF4E,
eukaryotic translation initiation factor 4E; GCA, gastric cardiac
adenocarcinoma; p-4E-BP1, phosphorylated 4E-BP1; p-eIF4E,
phosphorylated eIF4E. |
Discussion
The mTOR/4E-BP1 signaling pathway, which is
activated in a variety of malignances, is closely corelated with
tumor occurrence and progression. Aberrant activation of the mTOR
signaling pathway has been identified in numerous cancers,
including colorectal cancer (15),
lung cancer, renal cell carcinoma (16), breast cancer (17) and cervical carcinoma (18). Several previous studies using other
cancer models have identified mTOR signaling as a potential target
for anticancer therapy (19). mTOR
is a Ser/Thr protein kinase that mediates nutrient-dependent
intracellular signaling corelated with cell growth, proliferation
and differentiation. Osaki et al(20) revealed that mTOR plays a
significant role in the resistance to Fas-mediated apoptosis in the
human gastric carcinoma cell line MKN-45. p-mTOR is the activated
form of mTOR, and the level of this form may better reflect the
activation status of the pathway. mTOR phosphorylation is
frequently detected in ovarian cancer and may be targeted to
disrupt ovarian tumor cell growth (21). Overexpression of p-mTOR predicted
the angiogenic phenotype of human gastric cancer (22) and was significantly corelated with
tumor progression and outcome (23).
In the present study, we investigated the expression
of the mTOR pathway in GCA and normal gastric tissues. The results
showed that mTOR and p-mTOR were expressed in GCA and normal
gastric mucosa. The expression of mTOR in cancer tissues was
significantly lower than that in the normal gastric mucosa, while
the level of p-mTOR in cancer and normal gastric mucosa
demonstrated no significant difference. Therefore, simple analysis
of the protein expression status of mTOR and p-mTOR may be
insufficient to reveal the correlation between mTOR and GCA. When
comparing the ratio of p-mTOR to mTOR between GCA and corresponding
normal gastric mucosa, the data showed a considerably higher ratio
of p-mTOR to mTOR in the tumor tissue in comparison to the benign
gastric tissue. The present study suggested that the
phosphorylation of mTOR may be a significant step in the
progression of GCA. The activation of mTOR is mainly through
increased mTOR phosphorylation rather than protein
overexpression.
4E-BP1 is a translation initiation inhibitory factor
and the first downstream substrate of mTOR (24). Hypophosphorylated 4E-BP1 binds to
and thereby inactivates the cap-binding protein eukaryotic
translation initiation factor 4E (eIF4E), then following
phosphorylation by mTOR 4E-BP1 releases eIF4E and allows its
binding to the cap structure of the mRNA and the subsequent
beginning of protein translation (25). Study results, particularly PTEN and
mTOR expression data, suggest that 4E-BP1 overexpression is
strongly associated with prostate cancer (26). Certain investigators have suggested
that p4E-BP1 may play a central role in determining the growth
self-sufficiency capacity of tumors (27). The p-4E-BP1 level was identified to
be significantly increased in poorly differentiated groups of
endometrial carcinoma, and is closely correlated with the tumor
stage (28). High levels of
p-4E-BP1 indicated a poor prognosis in human melanomas (29). Overexpression of p-4E-BP1 in
cervical carcinoma was strongly correlated with shortened
disease-free and overall survival (30).
In the present study, RT-PCR and western blot
analysis results indicated that compared with normal gastric
mucosa, the expression of 4E-BP1 was markedly decreased in GCA,
while the level of p-4E-BP1 was significantly elevated. These
results indicated that GCA cells grow faster than normal, so they
are required to synthesize more protein to support active cell
proliferation and division. 4E-BP1 directly blocks translation by
binding to eIF4E and preventing translation. Hyperphosphorylation
of 4E-BP1 releases it from eIF4E enabling assembly of the eIF4F
complex, which permits translation to proceed. Translation
initiation is the rate-limiting step of protein synthesis.
eIF4E does not only play a central role in the
regulation of protein translation (31,32)
but is also a proto-oncogene (33). As eIF4E is the least abundant among
the initiation factors and is considered to be the rate-limiting
factor for cap-dependent translation initiation, changes in the
levels of eIF4E profoundly affect the translation rates. High
levels of eIF4E may lead to overexpresssion of a number of
proto-oncogenes, growth factors and cell cycle-related proteins
(34). eIF4E is dysregulated in a
wide variety of human cancers. Numerous studies have shown that
eIF4E is elevated in a number of solid tumors, including breast,
bladder, colon, head and neck, prostate, cervical and lung cancers
and lymphomas (35). High eIF4E
expression was correlated with a decreased overall survival rate in
lung adenocarcinoma patients and may be a better clinical marker
for predicting the prognosis in these cases (36). The activity of eIF4E is not only
regulated by the expression of protein, but also by the
phosphorylation level. eIF4E phosphorylation enhances its mRNA
transport functions and its transformation activity in cell culture
(37).
The present study observed that compared with the
normal gastric mucosa, the expression of eIF4E demonstrated no
significant difference in the GCA, while the level of eIF4E
phosphorylation was significantly increased. In addition, the level
of p-eIF4E in GCA was closely corelated with lymph node metastasis.
The phosphorylation of eIF4E in the lymph node metastasis group was
markedly increased compared with that of the non-lymph node
metastasis group. Thus, the level of eIF4E phosphorylation may be
correlated with carcinogenesis and progression in GCA and therefore
may serve as a molecular marker for the invasion and metastasis of
GCA.
Based on our results, we suggest that the
mTOR/4E-BP1 signaling pathway is activated in GCA and that
sustained activation of the mTOR signaling is able to induce gene
expression and lead to excessive cell proliferation and tumor
formation. mTOR/4E-BP1 signaling may play a significant role in the
carcinogenesis and progression of GCA. The signals are not only a
molecular marker for malignant changes, but they also provide the
rationale for targeting this pathway therapeutically in GCA
patients.
Acknowledgements
This work was supported by Key R&D Project of
Hebei Province (No. 07276102D), the Natural Science Foundation of
Hebei Province (No. H2012206134), the project of Educational
Commission of Hebei Province (No. 2011105) and the Department of
Health founded project of Hebei Province (No. 20120269).
References
|
1
|
Shah MA and Schwartz GK: Treatment of
metastatic esophagus and gastric cancer. Semin Oncol. 31:574–587.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Crane SJ, Richard Locke G III, Harmsen WS,
Diehl NN, Zinsmeister AR, Joseph Melton L III, et al: The changing
incidence of oesophageal and gastric adenocarcinoma by anatomic
sub-site. Aliment Pharmacol Ther. 25:447–453. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhao CY, Zhang XH, Xue LY, Xing LX, Wang
JL, Li XM, et al: Analysis of the changing trends of frequency and
localization of gastric cancers arising from different sites of the
stomach in population of the high incidence area of esophageal and
gastric cancers in Hebei province. Zhonghua Zhong Liu Za Zhi.
30:817–820. 2008.(In Chinese).
|
|
4
|
Tajima Y, Yamazaki K, Makino R, Nishino N,
Masuda Y, Aoki S, et al: Differences in the histological findings,
phenotypic marker expressions and genetic alterations between
adenocarcinoma of the gastric cardia and distal stomach. Br J
Cancer. 96:631–638. 2007. View Article : Google Scholar
|
|
5
|
Faried LS, Faried A, Kanuma T, Aoki H,
Sano T, Nakazato T, et al: Expression of an activated mammalian
target of rapamycin in adenocarcinoma of the cervix: A potential
biomarker and molecular target therapy. Mol Carcinog. 47:446–457.
2008. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Albert JM, Kim KW, Cao C and Lu B:
Targeting the Akt/mammalian target of rapamycin pathway
for radiosensitization of breast cancer. Mol Cancer Ther.
5:1183–1189. 2006.
|
|
7
|
Mori H, Inoki K, Masutani K, Wakabayashi
Y, Komai K, Nakagawa R, et al: The mTOR pathway is highly activated
in diabetic nephropathy and rapamycin has a strong therapeutic
potential. Biochem Biophys Res Commun. 384:471–475. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Fingar DC and Blenis J: Target of
rapamycin (TOR): an integrator of nutrient and growth factor
signals and coordinator of cell growth and cell cycle progression.
Oncogene. 23:3151–3171. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fingar DC, Richardson CJ, Tee AR, Cheatham
L, Tsou C and Blenis J: mTOR controls cell cycle progression
through its cell growth effectors S6K1 and 4E-BP1/eukaryotic
translation initiation factor 4E. Mol Cell Biol. 24:200–216. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Azar R, Alard A, Susini C, Bousquet C and
Pyronnet S: 4E-BP1 is a target of Smad4 essential for
TGFbeta-mediated inhibition of cell proliferation. EMBO J.
28:3514–3522. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Oulhen N, Mulner-Lorillon O and Cormier P:
eIF4E-binding proteins are differentially modified after ammonia
versus intracellular calcium activation of sea urchin unfertilized
eggs. Mol Reprod Dev. 77:83–91. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lang SA, Gaumann A, Koehl GE, Seidel U,
Bataille F, Klein D, et al: Mammalian target of rapamycin is
activated in human gastric cancer and serves as a target for
therapy in an experimental model. Int J Cancer. 120:1803–1810.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yoon DH, Ryu MH, Park YS, Lee HJ, Lee C,
Ryoo BY, et al: Phase II study of everolimus with biomarker
exploration in patients with advanced gastric cancer refractory to
chemotherapy including fluoropyrimidine and platinum. Br J Cancer.
106:1039–1044. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Siewert JR and Stein HJ: Classification of
adenocarcinoma of the oesophagogastric junction. Br J Surg.
85:1457–1459. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Johnson SM, Gulhati P, Rampy BA, Han Y,
Rychahou PG, Doan HQ, et al: Novel expression patterns of
PI3K/Akt/mTOR signaling pathway components in colorectal cancer. J
Am Coll Surg. 210:767–776. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kruck S, Bedke J, Hennenlotter J, Ohneseit
PA, Kuehs U, Senger E, et al: Activation of mTOR in renal cell
carcinoma is due to increased phosphorylation rather than protein
overexpression. Oncol Rep. 23:159–163. 2010.PubMed/NCBI
|
|
17
|
Zhou X, Tan M, Stone Hawthorne V, Klos KS,
Lan KH, Yang Y, et al: Activation of the Akt/mammalian target of
rapamycin/4E-BP1 pathway by ErbB2 overexpression predicts tumor
progression in breast cancers. Clin Cancer Res. 10:6779–6788. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ji J and Zheng PS: Activation of mTOR
signaling pathway contributes to survival of cervical cancer cells.
Gynecol Oncol. 117:103–108. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Faivre S, Kroemer G and Raymond E: Current
development of mTOR inhibitors as anticancer agents. Nat Rev Drug
Discov. 5:671–688. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Osaki M, Kase S, Adachi K, Takeda A,
Hashimoto K and Ito H: Inhibition of the PI3K-Akt signaling pathway
enhances the sensitivity of Fas-mediated apoptosis in human gastric
carcinoma cell line, MKN-45. J Cancer Res Clin Oncol. 130:8–14.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Altomare DA, Wang HQ, Skele KL, De Rienzo
A, Klein-Szanto AJ, Godwin AK and Testa JR: AKT and mTOR
phosphorylation is frequently detected in ovarian cancer and can be
targeted to disrupt ovarian tumor cell growth. Oncogene.
23:5853–5857. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yu G, Wang J, Chen Y, Wang X, Pan J, Li G,
et al: Overexpression of phosphorylated mammalian target of
rapamycin predicts lymph node metastasis and prognosis of chinese
patients with gastric cancer. Clin Cancer Res. 15:1821–1829. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Murayama T, Inokuchi M, Takagi Y, Yamada
H, Kojima K, Kumagai J, et al: Relation between outcomes and
localisation of p-mTOR expression in gastric cancer. Br J Cancer.
100:782–788. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Fingar DC, Salama S, Tsou C, Harlow E and
Blenis J: Mammalian cell size is controlled by mTOR and its
downstream targets S6K1 and 4EBP1/eIF4E. Genes Dev. 16:1472–1487.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sabatini DM: mTOR and cancer: insights
into a complex relationship. Nat Rev Cancer. 6:729–734. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kremer CL, Klein RR, Mendelson J, Browne
W, Samadzedeh LK, Vanpatten K, et al: Expression of mTOR signaling
pathway markers in prostate cancer progression. Prostate.
66:1203–1212. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Armengol G, Rojo F, Castellvi J, Iglesias
C, Cuatrecasas M, Pons B, et al: 4E-binding protein 1: a key
molecular ‘funnel factor’ in human cancer with clinical
implications. Cancer Res. 67:7551–7555. 2007.
|
|
28
|
Darb-Esfahani S, Faggad A, Noske A,
Weichert W, Buckendahl AC, Müller B, et al: Phospho-mTOR and
phospho-4EBP1 in endometrial adenocarcinoma: association with stage
and grade in vivo and link with response to rapamycin treatment in
vitro. J Cancer Res Clin Oncol. 135:933–941. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
O’Reilly KE, Warycha M, Davies MA, Rodrik
V, Zhou XK, Yee H, et al: Phosphorylated 4E-BP1 is associated with
poor survival in melanoma. Clin Cancer Res. 15:2872–2878.
2009.PubMed/NCBI
|
|
30
|
Benavente S, Vergés R, Hermosilla E,
Fumanal V, Casanova N, García A, et al: Overexpression of
phosphorylated 4E-BP1 predicts for tumor recurrence and reduced
survival in cervical carcinoma treated with postoperative
radiotherapy. Int J Radiat Oncol Biol Phys. 75:1316–1322. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Mamane Y, Petroulakis E, Rong L, Yoshida
K, Ler LW and Sonenberg N: eIF4E - from translation to
transformation. Oncogene. 23:3172–3179. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sonenberg N: Cap-binding proteins of
eukaryotic messenger RNA: functions in initiation and control of
translation. Prog Nucleic Acid Res Mol Biol. 35:173–207. 1988.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
De Benedetti A and Harris AL: eIF4E
expression in tumors: its possible role in progression of
malignancies. Int J Biochem Cell Biol. 31:59–72. 1999.PubMed/NCBI
|
|
34
|
Culjkovic B, Topisirovic I and Borden KL:
Controlling gene expression through RNA regulons: the role of the
eukaryotic translation initiation factor eIF4E. Cell Cycle.
6:65–69. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
De Benedetti A and Graff JR: eIF4E
expression and its role in malignancies and metastases. Oncogene.
23:3189–3199. 2004.PubMed/NCBI
|
|
36
|
Wang R, Geng J, Wang JH, Chu XY, Geng HC
and Chen LB: Overexpression of eukaryotic initiation factor 4E
(eIF4E) and its clinical significance in lung adenocarcinoma. Lung
Cancer. 66:237–244. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Topisirovic I, Ruiz-Gutierrez M and Borden
KL: Phosphorylation of the eukaryotic translation initiation factor
eIF4E contributes to its transformation and mRNA transport
activities. Cancer Res. 64:8639–8642. 2004. View Article : Google Scholar : PubMed/NCBI
|