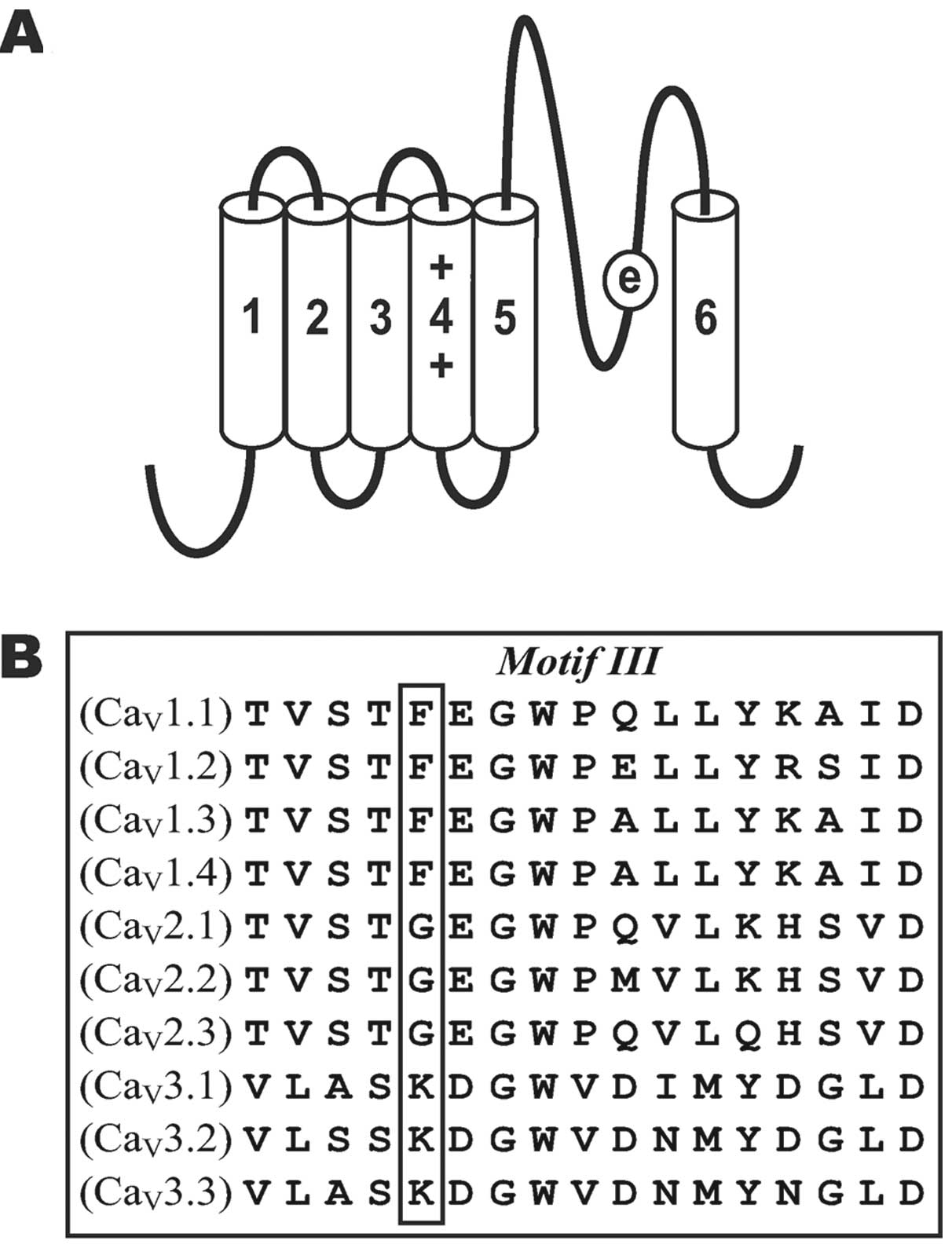
Introduction
L-type calcium channels transport Ca2+
into myocytes and are important in the regulation of heart rhythm,
excitation-contraction coupling and gene expression (1). Voltage-gated Ca2+ channels
(VGCCs) are heteromultimeric complexes composed of α1, β, α2/δ and
occasionally γ-subunits. The pore-forming α1 subunit contains all
the structural determinants required for ion permeation,
voltage-dependent gating and drug binding. The membrane topology of
the α1 subunit consists of four homologous domains (I, II, III and
IV), with six transmembrane segments (S1–S6) in each (Fig. 1A). Segments connecting with S5 and
S6 in each domain contain four negatively charged glutamate
residues that shape the pore of the channel and form binding sites
for ions, known as the selectivity filter (EEEE locus) (2).
The nature of multi-ion permeation through the pore
of voltage-gated Ca2+ channels has previously been
investigated. Hess and Tsien (3)
and Almers and McCleskey (4)
suggested that Ca2+ binds the channel more strongly than
other ions and that there is an electrostatic interaction between
ions within the channel. Such channel selectivity and ion
interactions demonstrate a phenomenon known as the anomalous mole
fraction effect (AMFE). The AMFE arises when a mixture of two
permeant ions produce less current than either permeant ion alone
at the same total ion concentration. The AMFE is a complex
phenomenon that depends on the holding voltage, total ion
concentration and the intrinsic binding properties of the channel.
Thus, AMFE is an important indicator of ion-ion interactions in the
pore of voltage-gated Ca2+ channels.
Ba2+ currents through L-type
Ca2+ channels, as well as most other
high-voltage-activated Ca2+ channels, are approximately
twice the size of Ca2+ currents (2,5). The
differences between Ba2+ and Ca2+ conductance
depend on the binding affinities of single cations to the
selectivity filter of the channel (3,4,6,7). The
binding affinity of a single Ba2+ ion to the selectivity
filter is reported to be 70-fold lower than that of
Ca2+(8). Therefore, the
repulsive forces exerted by the entry of a second ion promote the
exit of a Ba2+ ion more readily than Ca2+,
which increases the exit rate and ionic flux. However, how ions
permeate through the pore of voltage-gated Ca2+ channels
and interact with a second ion remains a controversial topic.
There are several numeric simulation models
dependent on the binding-repulsion events in the pore that predict
the ion-ion interactions in the pores of Ca2+ channels.
The Ca2+ channel model of a pair of high-affinity
binding sites and ion interactions with electrostatic repulsion is
widely known (3,4). Findings of previous studies indicated
that the Ca2+ channel pore contains only a single
high-affinity binding site and that ions compete for binding
moieties (9,10). Dang and McCleskey (7) revealed the stepwise model, which has
a single high-affinity Ca2+ binding site flanked by two
low-affinity divalent cation binding sites, each composed of two
carboxyl groups from the EEEE locus. Newer structure-based models
have aimed to define the volume of the Ca2+ channel pore
using structure theory. The ionic diameter of Ba2+ is
much larger than that of Ca2+ and the resulting
Ba2+ ions show a higher degree of crowding in the pore,
causing a faster exit rate and larger conductance (11–13).
Mutagenesis studies indicate that the glutamate
residue in the pore of domain III is the important determinant for
ion permeation (9,10,14–16).
The domain III pore segments of all 10 families of voltage-gated
Ca2+ channels (Fig. 1B)
are highly conserved. All L-type Ca2+ channels
(CaV1.1–4) possess a phenylalanine (F) at position 1144,
located next to Glu-1145. However, all non-L-type
high-voltage-activated Ca2+ channels
(CaV2.1–3) possess a glycine (G) and all
low-voltage-activated Ca2+ channels
(CaV3.1–3) possess a lysine (K). The physicochemical
properties of the amino acid residue at position 1144 are important
to the permeation of multiple Ba2+ and Ca2+
ions through the pore (17). Amino
acid substitutions at position 1144 of the CaV1.2
channel reduce barium currents without affecting calcium currents.
Therefore, we substituted Phe-1144 (F) with G (F/G) and K (F/K) and
observed the effects of mutation on the voltage and concentration
dependency of AMFE.
Materials and methods
Amino acid residue substitution
Site-directed mutagenesis was used to substitute
amino acid residues. Mutant fragments of the channel were generated
by polymerase chain reaction (PCR) using mutagenic primers. The
mutant PCR products were gel-purified, digested using restriction
endonucleases and subcloned into a digested CaV1.2
vector. The presence of the mutation and the integrity of each
mutant were confirmed by qualitative restriction map analysis and
directional DNA sequence analysis of the entire subcloned region.
Functional expression of the mutant cDNAs was confirmed by western
blot analysis and whole-cell patch-clamp electrophysiology.
Cell culture and transfections
HEK293 cells were grown at 37°C in 6% CO2
and DMEM-F12 medium supplemented with 10% fetal bovine serum and 1%
penn-strep antibiotics. cDNAs encoding wild-type and mutant
CaV1.2 channels were co-transfected with α2δ
(18), β2a (19) and CaM1234(20) into HEK293 cells by calcium
phosphate precipitation, as previously described (17,20,21).
CaM1234 was used to eliminate complications that could
arise from Ca2+/CaM-dependent changes in channel gating,
as it encodes an inactive form of calmodulin that has been shown to
eliminate Ca2+-dependent inactivation and facilitation
(17,20–24).
All cDNAs were expressed using pcDNA3 mammalian expression plasmids
(Invitrogen, Carlsbad, CA, USA).
Patch-clamp electrophysiology
Whole-cell patch-clamp recordings were obtained as
previously described (17,21). Briefly, whole-cell currents were
recorded at room temperature 2–3 days following transfection.
Pipettes were pulled from borosilicate glass using a P-97
Flaming/Brown micropipette puller (Sutter Instruments, Novato, CA,
USA) and fire-polished on an MF200 microforge (World Precision
Instruments, Sarasota, FL, USA). External solutions contained:
N-methyl-D-glutamine (NMG)-aspartate, 130 mM; HEPES, 10 mM;
4-aminopyridine, 10 mM. BaCl2 and CaCl2 were
used to give the desired molar ratio of the two ions and the total
divalent cation concentration was maintained at 2, 10 or 20 mM. The
osmolarity was adjusted to 300 mmol/kg with glucose and the pH was
adjusted to 7.4 using 1 mM NMG base solution. Pipettes had
resistances of 2.5–3.0 MΩ when filled with internal solution. The
peak tail currents were evoked at potentials from −90 to +50 mV of
all channels at each ion concentration.
Data acquisition and analysis
Data were acquired using a HEKA EPC-9/2 amplifier
and PULSE/PULSEFIT software (HEKA Electronik, Lambrecht, Germany).
Leak and capacitive transients were corrected by -P/4 compensation.
Series resistance was <6 MΩ and compensated to 70%. Tail
currents were sampled at 50 kHz and filtered at 5.0 kHz. Pulse
protocols are described in each figure legend. Data analysis was
performed using FitMaster (HEKA Electronik) and Origin 7
(OriginLab, Northampton, MA, USA). The significance of differences
for the smallest normalized current and
IBa/ICa among wild-type, F/G and F/K channels
were analyzed using one-way ANOVA with Tukey’s test. The
significance of differences between wild-type and F/K was analyzed
using two-sample independent t-tests. Data were reported as the
means ± SE. Statistically significant results (P<0.05) are
indicated in the figures by an asterisk or a pound sign.
Results
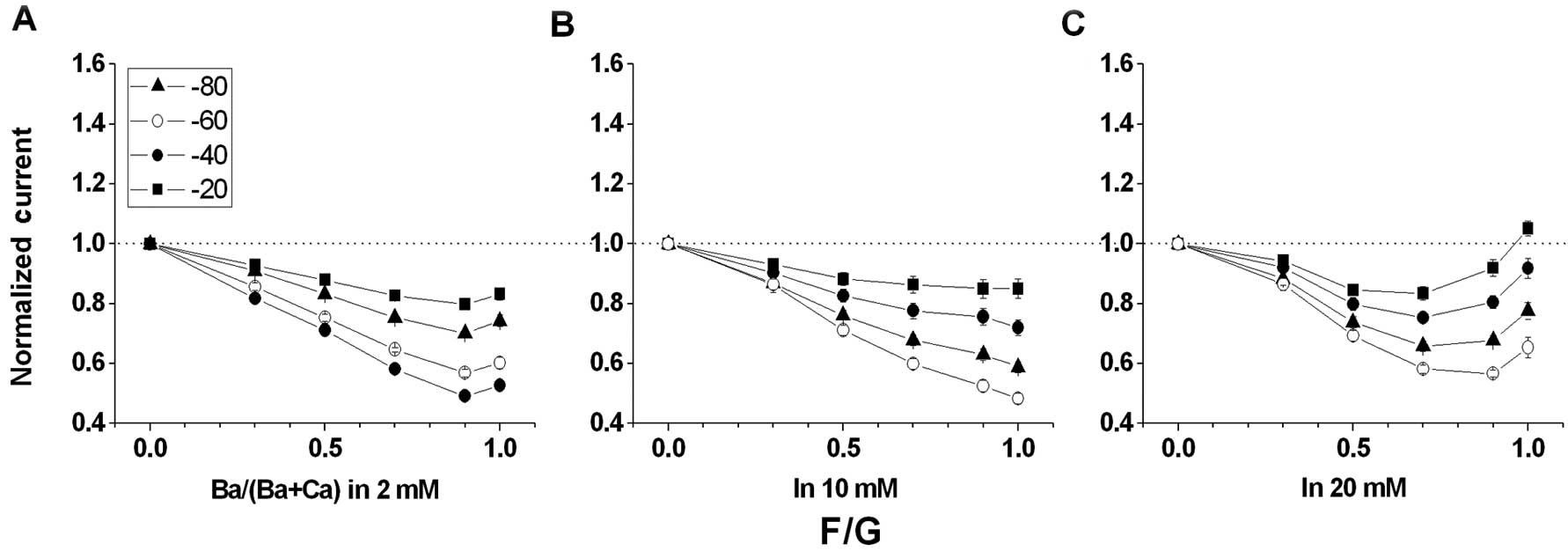
F/G does not promote AMFE
Unlike wild-type channels (Fig. 2), the tail current amplitudes for
F/G were reduced almost monotonically as
Ba2+/(Ba2+ + Ca2+) progressed to
0.7, but failed to increase as Ba2+/(Ba2+ +
Ca2+) approached 1.0 in 2 and 10 mM external solutions
(Fig. 3A and B). The relative
current amplitudes for F/G were indistinguishable when
Ba2+/(Ba2+ + Ca2+) = 1.0. However,
as shown in Fig. 3C, the tail
current amplitudes for F/G increased as
Ba2+/(Ba2+ + Ca2+) approached 1.0
in 20 mM external solution. Compared with a previous report
(25), we found that the
conditions at 20 mM for F/G did not detect AMFE, as the maximum
AMFE appeared when the total divalent cation concentrations were
kept low (i.e., 2 mM).
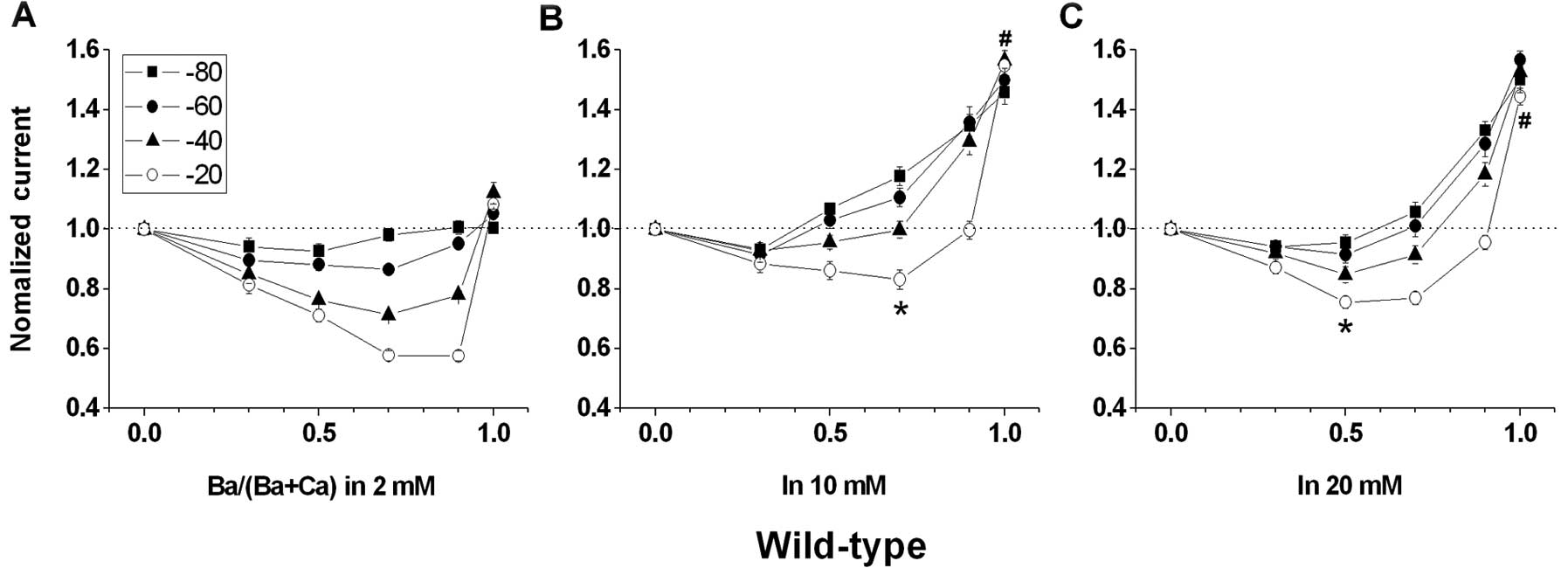 | Figure 2Voltage and concentration dependency
of IBa/ICa and the anomalous mole fraction
effect (AMFE) for wild-type channels. Cells were stepped from a
holding potential of −90 to +50 mV for 100 msec, and peak tail
currents were measured at molar ratios of external Ba2+
to total divalent cation concentrations
[Ba2+/(Ba2+ + Ca2+)] of 0.0, 0.3,
0.5, 0.7, 0.9 and 1.0. The combined divalent concentration
(Ba2+ + Ca2+) at each data point was kept at
(A) 2, (B) 10 and (C) 20 mM, respectively. AMFE is observed when
peak tail currents, normalized to 1.0 when
Ba2+/(Ba2+ + Ca2+) = 0, are
plotted against Ba2+/(Ba2+ + Ca2+)
at holding potentials of −80, −60, −40 and −20 mV. The dashed line
corresponds to peak tail currents measured in an external solution
containing 0.0 mM Ba2+. (A) AMFE is evident at 2 mM
combined divalent concentration. The most pronounced AMFE is at a
holding potential of −20 mV. The smallest normalized current is
0.576±0.018 [Ba2+/(Ba2+ + Ca2+) =
0.9] and IBa/ICa is 1.082±0.035, n=6. (B)
AMFE is evident at 10 mM combined divalent concentration. The most
pronounced AMFE is at a holding potential of −20 mV. The smallest
normalized current is 0.831±0.033 [Ba2+/(Ba2+
+ Ca2+) = 0.7] and IBa/ICa is
1.547±0.052, n=9. Each is different from that at 2 mM combined
divalent concentration and shown as ‘*’ and
‘#’, respectively. (C) AMFE is evident at 20 mM combined
divalent concentration. The most pronounced AMFE is at a holding
potential of −20 mV. The smallest normalized current is 0.769±0.022
[Ba2+/(Ba2+ + Ca2+) = 0.5] and
IBa/ICa is 1.445±0.027, n=9. Each is
different from that at 2 mM combined divalent concentration and
shown as ‘*’ and ‘#’, respectively. |
Voltage and concentration dependency of
IBa/ICa
The experimental conditions were established to
detect changes in AMFE at a varied series of recording holding
voltages and combined divalent concentrations. The AMFE was
determined by measuring peak tail currents in the presence of
various molar ratios of Ca2+ and Ba2+. Under
conditions that promote AMFE, a difference was detected for
wild-type and F/K channels, IBa/ICa
determined at the concentration of 2 mM compared with
IBa/ICa determined at higher concentrations
(i.e., 10 and 20 mM) (Figs. 2 and
4). Furthermore, we plotted the
voltage range of −80 to −20 mV at which we could record the clear
peak tail current of all three types of channels at each ion
concentration. For each category of wild-type and F/K channels,
IBa/ICa values were similar at each ion
concentration, while tail currents were evoked at potentials from
−80 to −20 mV. These observations suggest that the extracellular
divalent ion concentration is a critical parameter in determining
the selectivity properties of the pore.
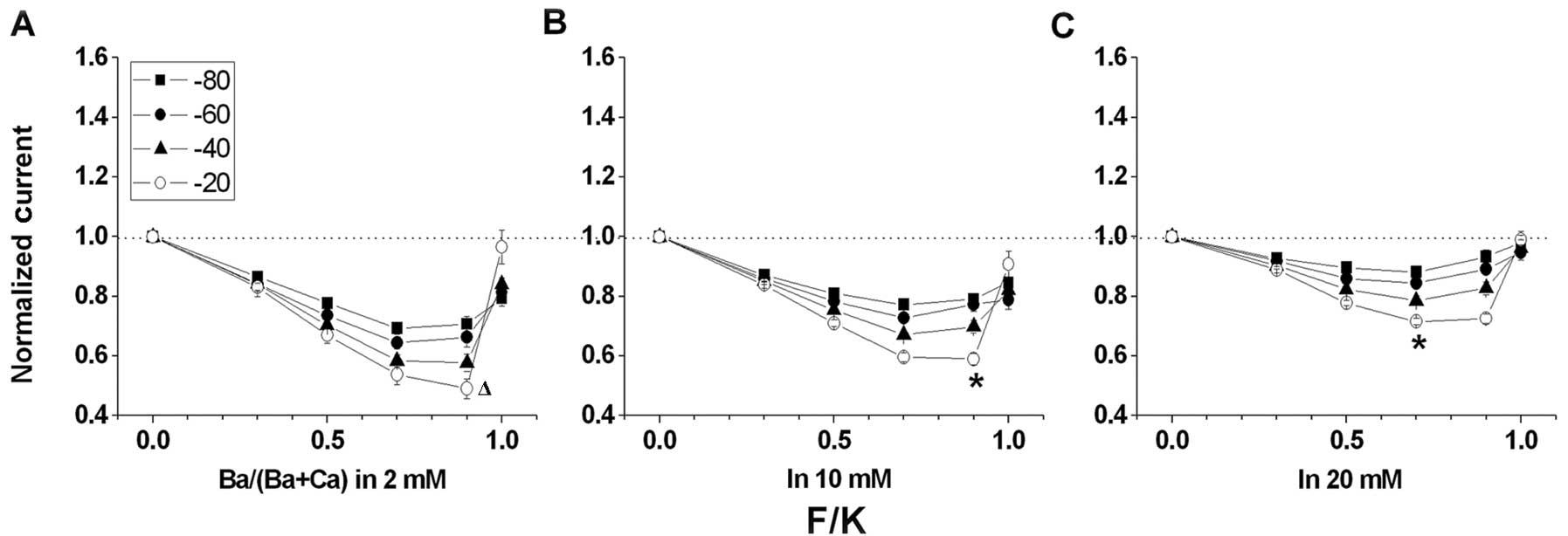 | Figure 4Voltage and concentration dependency
of IBa/ICa and the anomalous mole fraction
effect (AMFE) for F/K mutant channels. Cells were stepped from a
holding potential of −90 to +50 mV for 100 msec, and peak tail
currents were measured at molar ratios of external Ba2+
to total divalent cation concentrations
[Ba2+/(Ba2+ + Ca2+)] of 0.0, 0.3,
0.5, 0.7, 0.9 and 1.0. The combined divalent concentration
(Ba2+ + Ca2+) at each data point was kept at
(A) 2, (B) 10 and (C) 20 mM, respectively. AMFE is observed when
peak tail currents, normalized to 1.0 when
Ba2+/(Ba2+ + Ca2+) = 0, are
plotted against Ba2+/(Ba2+ + Ca2+)
at holding potentials of −80, −60, −40 and −20 mV. The dashed line
corresponds to peak tail currents measured in an external solution
containing 0.0 mM Ba2+. (A) AMFE is evident at 2 mM
combined divalent concentration. The most pronounced AMFE is at a
holding potential of −20 mV. The smallest normalized current is
0.491±0.021 [Ba2+/(Ba2+ + Ca2+) =
0.9] and IBa/ICa is 0.966±0.440, n=5. The
smallest normalized current of F/K is distinguished from that of
wild-type (Δ). (B) AMFE is evident at 10 mM combined divalent
concentration. The most pronounced AMFE is at a holding potential
of −20 mV. The smallest normalized current is 0.590±0.019
[Ba2+/(Ba2+ + Ca2+) = 0.9], which
is different from that at 2 mM combined divalent concentration and
shown as an asterisk and IBa/ICa is
0.908±0.044, n=8. (C) AMFE is evident at 20 mM of the combined
divalent concentration. The most pronounced AMFE is at a holding
potential of −20 mV. The smallest normalized current is 0.716±0.010
[Ba2+/(Ba2+ + Ca2+) = 0.7], which
is different from that at 2 mM combined divalent concentration and
shown as ‘*’ and IBa/ICa is
0.990±0.027, n=9. |
Voltage and concentration dependence of
AMFE
The AMFE of the indicated holding voltages and
external solution concentrations for wild-type and mutant channels
were recorded, respectively. While the holding potential declined
from −80 to −20 mV, AMFEs for wild-type and F/K channels became
more pronounced (Figs. 2 and
4). The smallest normalized
currents exhibited differently when the total divalent cation
concentrations remained at 2 mM compared to 10 or 20 mM (Fig. 2). Consistent with a previous report
(25), AMFE was greatest when tail
currents were evoked at relatively positive potentials (−20 mV) and
when the total divalent cation concentrations were kept low (i.e.,
2 mM).
The AMFE can be attenuated or accentuated
by pore substitution
The normalized current for wild-type in 2 mM
external solution decreased to ~0.58 when
Ba2+/(Ba2+ + Ca2+) = 0.9 before
rapidly climbing to ~1.08 as Ba2+/(Ba2+ +
Ca2+), approaching 1.0 (Fig. 2A). In contrast to F/G (Fig. 3), F/K (Fig. 4) exhibited robust AMFE, similar to
wild-type. The relative current amplitudes for F/K are
indistinguishable when Ba2+/(Ba2+ +
Ca2+) = 1, yet F/K presents an AMFE, indicating that the
IBa/ICa and AMFE may be altered
independently. In 2 mM external solution the AMFE for F/K (Fig. 4A) was greater than that for
wild-type (Fig. 2A) and the
normalized current for F/K reached a minimum value of 0.49 compared
with 0.58 for wild-type.
Discussion
General
Mutagenesis was applied in the pore segment to
assess the effects on the voltage and concentration dependency of
IBa/ICa and the AMFE of CaV1.2
channels. Of the substitutions investigated in this study, F/G does
not promote AMFE. However, F/K exhibits the voltage and
concentration dependence of AMFE, which is more pronounced than
that of wild-type channels.
Glycine and lysine substitutions of
Phe-1144 affect AMFE through different mechanisms
The multi-ion interaction within the pore of the
Ca2+ channel has long been a topic of research.
Traditional theories (3,4) have indicated that an electrostatic
interaction occurs between one ion bound to the pore and a second
entering the pore. The electrostatic interaction causes an
intricate adjustment of the conductance and activity relationship
between the ions. The phenomenon of AMFE is an important
demonstration of this interaction. AMFE appears when a mixture of
two classes of permeant ions generates less current than either
class of permeant ion alone. AMFE is an intricate phenomenon that
is determined by the holding voltage and total ion concentration,
as well as the inherent binding characteristics of the channel.
Thus, AMFE is a significant biophysical probe of ion-ion
interactions in the open pore of VGCCs.
Furthermore, Williamson and Sather (26) suggested that the unitary channel
conductance is proportional to the volume of the side chain
introduced by the amino acid residue at position 1144 adjacent to
the residue of the selectivity filter. As the van der Waals volume
of the positively charged lysine is similar to that of
phenylalanine, F/K would be expected to demonstrate conductance
resembling that of wild-type. However, a previous finding (17) that revealed the Ba2+
conductance of F/K is similar to that of F/G but not wild-type, is
not compatible with this hypothesis. We suggest that Glu-1145 may
produce a more restricted interaction with its neighboring residue
as a positively charged lysine but a less restricted interaction
with its neighboring residue as a neutral glycine. Moreover, one
would predict that there are extra subtle differences between the
conductance properties of the two mutants as both F/G and F/K
reduce Ba2+ currents (17). Such differences may be further
revealed by observing their respective AMFEs.
We studied AMFE at a variety of voltages and
concentrations to examine how the mutations would affect ion-ion
interactions in the open pore of Ca2+ channels. The
results indicate that AMFE for F/G is lower than that for
wild-type, while for F/K it is more pronounced than that for
wild-type, although the voltage and concentration dependency remain
the same. These results suggest that the characteristics of the
amino acid residue at position 1144 are capable of accentuating or
attenuating the AMFE. Thus, the finding that glycine and lysine
substitutions at position 1144 are important determinants in
modifying the AMFE suggests that F/G and F/K reduce Ba2+
conductance through different mechanisms.
Phe-1144 substitutions confer to
structure-based models for Ca2+ channel permeation
The pore selectivity filter, the EEEE locus, is the
critical structure for high Ca2+ selectivity while
simultaneously promoting the high rates of Ca2+ flux of
the voltage-gated calcium channel, as it forms the Ca2+
binding site (27). However, our
findings are consistent with an increasing number of studies
indicating that residues near or even far from the EEEE locus also
participate in channel permeation (21,26,28,29).
The traditional two-site, three-barrier models for the fundamental
properties of ion selectivity and permeation through
Ca2+ channels are based on measurable forces and binding
energies. Therefore, these models cannot be used for channel
structural studies, as the forces and binding energies cannot be
measured for realistic channel structures. There are several later
models based on structures to simulate many of the biophysical
characteristics of ion permeation for the Ca2+ channel
pore (11–13,30–32).
Lipkind and Fozzard (12) suggested a structural model that may
be considered as a structural correlate to the stepwise model of
Dang and McCleskey (7). The former
model concludes that there are three binding sites formed by the
eight carboxyl groups from the EEEE locus: a central, single
high-affinity divalent cation binding site composed of four of the
carboxyl groups flanked by two low-affinity divalent cation binding
sites on either side, each composed of two carboxyl groups. On the
basis of this model, we conclude that substitutions at position
1144 change the interactions between one of the two low-affinity
binding sites and Ba2+.
The early barrier models (3,4)
proposed that the selectivity filter has a defined volume.
Furthermore, newer models (10,11,13,31)
proposed that the volume is formed by the eight carbonyl oxygen
atoms from the EEEE locus. Wang et al(17) used the ‘volume exclusion/charge
neutralization’ model to explain the reduced conductance of
Ba2+ but not Ca2+ in the pores of the F/G
mutant channels. The crystal diameters of Ca2+ and
Na+ ions are almost equal (2.00 vs. 2.04 Å,
respectively), but each Ca2+ ion carries twice as much
charge as an Na+ ion. Thus, Ca2+ is capable
of neutralizing the highly negatively charged EEEE to bind tightly
to the selectivity filter without the overcrowding suspected for
monovalent cations such as Na+. Ba2+ and
Ca2+ ions carry the same charge, but the ionic diameter
of Ba2+ is ~36% larger than that of Ca2+
(2.72 vs. 2.00 Å, respectively). Thus, for wild-type channels,
Ba2+ ions are expected to exhibit a higher degree of
crowding than Ca2+ ions, as multiple ions attempt to
bind and neutralize the negatively charged EEEE, resulting in an
increasing exit rate, lower binding affinity and higher conductance
relative to Ca2+. Our results resemble this ‘volume
exclusion/charge neutralization’ model. The substitutions at
position 1144 are assumed to alter the geometry and/or
electrostatic context of the selectivity filter, in order to lessen
the capability of overcrowding by Ba2+ ions, allowing it
to contain multiple larger Ba2+ ions; however, its
ability to contain multiple Ca2+ ions changes
little.
Residues at position 1144 participate in
the permeation of several classes of voltage-gated Ca2+
channels
Results of the present study have shown that
non-glutamate residues in the pore of L-type Ca2+
channels change the characteristics of ion permeation. The
CaV3-like mutant channel tested, F/K, promoted the AMFE
phenomenon that was more comparable with CaV1.2 channels
than CaV2 channels. Thus, the non-glutamate residues are
critical for determining the permeability characteristics of
voltage-gated Ca2+ channels. There are an increasing
number of other studies also supporting this conclusion (17,21,26,28,29,33).
Our previous study substituted an outer vestibule amino acid,
Glu-1126, of CaV1.2 channels and revealed that the
preference of the channel is approximately halved for passing
Ba2+, but remains the same for passing
Ca2+(21). Even
substitutions as far as 100 residues upstream of EEEE in
CaV2.2 channels reduce the preference of the channel for
passing Ba2+ over Ca2+ currents (33). Regardless of the precise mechanism
and simulation, our results provide further support for how the
nonglutamate residues participate in an electrostatic and chemical
environment contributing to ion permeation. More specifically, we
have investigated the voltage and concentration dependence of the
AMFE phenomenon of substituting mutant channels. The consequences
of this study are likely to expand the understanding of the
molecular mechanisms contributing to ion permeation of VGCCs.
Clinical implications
VGCCs are important in physiological functions of
the cardiovascular system. They are critical factors of
pathological changes in common or frequently encountered diseases
of the cardiovascular system, such as arrhythmia, hypertension and
coronary heart disease. Furthermore, they are functional targets of
multiple commonly used drugs, such as calcium channel blockers. Ion
permeation, as an essential biophysical property of VGCCs, is under
close attention from investigators worldwide; however, its
functional mechanisms have not been clearly elucidated.
Explorations of the functional mechanisms of calcium channels to
determine the essential qualities at the ion channel level of
physiological and pathological phenomenon as well as investigating
the theoretical basis at a molecular level of the pathogenesis and
therapeutics of diseases are underway.
Acknowledgements
We are grateful for the financial support from the
Superior Group Grant of Hubei Province Funding no. 2007ABC011 to
C.-X.H. and the Special Grant for Basic Scientific Research
Expenses of the Central Universities no. 302274994 to Z.L.
References
|
1
|
Catterall WA, Perez-Reyes E, Snutch TP and
Striessnig J: International Union of Pharmacology. XLVIII
Nomenclature and structure-function relationships of voltage-gated
calcium channels. Pharmacol Rev. 57:411–425. 2005. View Article : Google Scholar
|
|
2
|
Hille B: Ion Channels of Excitable
Membranes. 3rd edition. Sinaur Associates; Sunderland, MA: pp.
649–662. 2001
|
|
3
|
Hess P and Tsien RW: Mechanism of ion
permeation through calcium channels. Nature. 309:453–456. 1984.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Almers W and McCleskey EW: Non-selective
conductance in calcium channels of frog muscle: calcium selectivity
in single-file pore. J Physiol. 353:585–608. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bourinet E, Zamponi GW, Stea A, et al: The
alpha 1E calcium channel exhibits permeation properties similar to
low-voltage-activated calcium channels. J Neurosci. 16:4983–4993.
1996.
|
|
6
|
Yue DT and Marban E: Permeation in the
dihydropyridine-sensitive calcium channel. Multi-ion occupancy but
no anomalous mole-fraction effect between Ba2+ and
Ca2+. J Gen Physiol. 95:911–939. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Dang TX and McCleskey EW: Ion channel
selectivity through stepwise changes in binding affinity. J Gen
Physiol. 111:185–193. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kostyuk PG, Mironov SL and Shuba YM: Two
ion-selecting filters in the calcium channel of the somatic
membrane of mollusc neurons. J Membr Biol. 76:83–93. 1983.
View Article : Google Scholar
|
|
9
|
Yang J, Ellinor PT, Sather WA, Zhang JF
and Tsien RW: Molecular determinants of Ca2+ selectivity
and ion permeation in L-type Ca2+ channels. Nature.
366:158–161. 1993.
|
|
10
|
Ellinor PT, Yang J, Sather WA, Zhang JF
and Tsien RW: Ca2+ channel selectivity at a single-locus
for high-affinity Ca2+ interactions. Neuron.
15:1121–1132. 1995.
|
|
11
|
Corry B, Allen TW, Kuyucak S and Chung SH:
Mechanisms of permeation and selectivity in calcium channels.
Biophys J. 80:195–214. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lipkind GM and Fozzard HA: Modeling of the
outer vestibule and selectivity filter of the L-type
Ca2+ channel. Biochemistry. 40:6786–6794. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Nonner W, Catacuzzeno L and Eisenberg B:
Binding and selectivity in L-type calcium channels: a mean
spherical approximation. Biophys J. 79:1976–1992. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kim MS, Morii T, Sun LX, Imoto K and Mori
Y: Structural determinants of ion selectivity in brain calcium
channel. FEBS Lett. 318:145–148. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mikala G, Bahinski A, Yatani A, Tang SQ
and Schwartz A: Differential contribution by conserved glutamate
residues to an ion-selectivity site in the L-type Ca2+
channel pore. FEBS Lett. 335:265–269. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tang SQ, Mikala G, Bahinski A, Yatani A,
Varadi G and Schwartz A: Molecular localization of ion selectivity
sites within the pore of a human L-type cardiac calcium-channel. J
Biol Chem. 268:13026–13029. 1993.PubMed/NCBI
|
|
17
|
Wang X, Ponoran TA, Rasmusson RL, Ragsdale
DS and Peterson BZ: Amino acid substitutions in the pore of the
Ca(V)1.2 calcium channel reduce barium currents without affecting
calcium currents. Biophys J. 89:1731–1743. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tomlinson WJ, Stea A, Bourinet E, Charnet
P, Nargeot J and Snutch TP: Functional properties of a neuronal
class C L-type calcium channel. Neuropharmacology. 32:1117–1126.
1993. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Perez-Reyes E, Castellano A, Kim HS, et
al: Cloning and expression of a cardiac/brain beta subunit of the
L-type calcium channel. J Biol Chem. 267:1792–1797. 1992.PubMed/NCBI
|
|
20
|
Peterson BZ, DeMaria CD, Adelman JP and
Yue DT: Calmodulin is the Ca2+ sensor for
Ca2+ -dependent inactivation of L-type calcium channels.
Neuron. 22:549–558. 1999.
|
|
21
|
Li Z, Wang XM, Gao GF, et al: A single
amino acid change in Ca(V)1.2 channels eliminates the permeation
and gating differences between Ca(2+) and Ba (2+). J Membr Biol.
233:23–33. 2010.PubMed/NCBI
|
|
22
|
Peterson BZ, Lee JS, Mulle JG, Wang Y, de
Leon M and Yue DT: Critical determinants of
Ca2+-dependent inactivation within an EF-hand motif of
L-type Ca2+ channels. Biophys J. 78:1906–1920. 2000.
|
|
23
|
Qin N, Olcese R, Bransby M, Lin T and
Birnbaumer L: Ca2+-induced inhibition of the cardiac
Ca2+ channel depends on calmodulin. Proc Natl Acad Sci
USA. 96:2435–2438. 1999.
|
|
24
|
Zuhlke RD, Pitt GS, Deisseroth K, Tsien RW
and Reuter H: Calmodulin supports both inactivation and
facilitation of L-type calcium channels. Nature. 399:159–162. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wakamori M, Strobeck M, Niidome T,
Teramoto T, Imoto K and Mori Y: Functional characterization of ion
permeation pathway in the N-type Ca2+ channel. J
Neurophysiol. 79:622–634. 1998.PubMed/NCBI
|
|
26
|
Williamson AV and Sather WA: Nonglutamate
pore residues in ion selection and conduction in voltage-gated
Ca2+ channels. Biophys J. 77:2575–2589. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sather WA and McCleskey EW: Permeation and
selectivity in calcium channels. Annu Rev Physiol. 65:133–159.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Dilmac N, Hilliard N and Hockerman GH:
Molecular determinants of Ca2+ potentiation of diltiazem
block and Ca2+-dependent inactivation in the pore region
of Ca(v)1.2. Mol Pharmacol. 64:491–501. 2003.
|
|
29
|
Dilmac N, Hilliard N and Hockerman GH:
Molecular determinants of frequency dependence and Ca2+
potentiation of verapamil block in the pore region of Ca(v)1.2. Mol
Pharmacol. 66:1236–1247. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Nonner W and Eisenberg B: Ion permeation
and glutamate residues linked by Poisson-Nernst-Planck theory in
L-type calcium channels. Biophys J. 75:1287–1305. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Boda D, Henderson D and Busath DD: Monte
Carlo simulations of the mechanism for channel selectivity: the
competition between volume exclusion and charge neutrality. J Phys
Chem. 104:11574–11577. 2001.
|
|
32
|
Gillespie D and Boda D: The anomalous mole
fraction effect in calcium channels: a measure of preferential
selectivity. Biophysi J. 95:2658–2672. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Feng ZP, Hamid J, Doering C, et al: Amino
acid residues outside of the pore region contribute to N-type
calcium channel permeation. J Biol Chem. 276:5726–5730. 2001.
View Article : Google Scholar : PubMed/NCBI
|