Introduction
Coronary artery disease (CAD), including its most
severe complication, myocardial infarction, is the leading cause of
mortality worldwide (1,2). There are several traditional risk
factors of CAD, including hypertension, diabetes mellitus,
dyslipidemia and smoking, however, these factors explain only
two-thirds of the observed clinical events (3,4). In
addition, not all individuals exposed to those risk factors develop
CAD, which is indicative of other causes, including genetic
susceptibility, which may contribute to variations in host
susceptibility to CAD. Identification of CAD susceptibility genes
highlights the link between CAD and inflammation and immunity, as
well as the biological insights that may be gained from a genetic
understanding of CAD (5,6).
Environmental pollutants, including polycyclic
aromatic hydrocarbons (PAHs), aldehydes and metals, contribute to
the incidence, severity and risk of CAD by affecting atherogenesis,
thrombosis and blood pressure. Cytochrome P450 1A1 (CYP1A1) is a
member of the super family of cytochrome P450 enzymes important for
detoxification of PAHs (7,8). The CYP1A1 gene codes for a phase I
enzyme associated with detoxification pathways that protect against
damage caused by reactive metabolites of a number of chemicals,
including steroids. Numerous mutations in CYP1A1 have been
described and a T→C mutation in the non-coding 3′-flanking region
of the gene (MspI, T6235C polymorphism, rs4646903) is the most
commonly studied polymorphism (9).
CYP1A1 T6235C in the 3′-flanking region, is associated with
increased transcript half-life and therefore increased enzyme
activity leading to elevated levels of activated metabolites
(9,10). The CYP1A1 T6235C polymorphism is
one of the most extensively studied genes for susceptibility to
various diseases over the last two decades. A number of studies
have investigated the correlation between the CYP1A1 T6235C
polymorphism and CAD risk, however, the results remain inconclusive
(11–16). Primarily, single studies have been
performed to investigate the correlation between the CYP1A1 T6235C
polymorphism and CAD risk. In the present study, a meta-analysis
was performed to increase the statistical power to examine the
correlation between the CYP1A1 T6235C polymorphism and CAD risk.
The meta-analysis of observational studies in epidemiology (MOOSE)
consensus statement was followed during stages of design,
implementation and reporting of this meta-analysis (17).
Materials and methods
Search strategy
A comprehensive search of PubMed, Embase and the
Chinese Biomedical Database (CBM) databases prior to June 6, 2012
was performed. Search terms for the CYP1A1 T6235C polymorphism and
CAD risk were combined and included ‘cytochrome P4501A1’, ‘CYP1A1’,
‘T6235C’, ‘rs4646903’ or ‘MspI’; and ‘coronary artery disease’,
‘coronary heart disease’ or ‘myocardial infarction’. There was no
language limitation. Review articles and bibliographies of relevant
literature were manually scanned to identify additional eligible
studies.
Study eligibility
The criteria used for the study selection were: i)
Evaluation of the correlation between the CYP1A1 T6235C
polymorphism and CAD risk; ii) studies with case-control design;
iii) studies with full text articles; and iv) sufficient data for
estimating an odds ratio (OR) with its corresponding 95% confidence
interval (CI). Studies investigating progression, severity,
phenotype modification, response to treatment or survival were
excluded from the present meta-analysis. Family-based association
studies were also excluded due to utilization of various study
designs. In cases where multiple articles publishing data on the
same population were identified, the publication with the most
complete data set was included.
Data extraction
Two investigators independently extracted data using
a standardized data extraction form. Discrepancies were resolved by
discussion and if consensus was not achieved the decision was made
by a third investigator. The information sought from each
publication included author, year of publication, source of
controls, country of origin, ethnicity of participants, adjustment
for known confounding variables and genotype information. In
studies of multiple ethnic groups, data were extracted separately
for each ethnic group. Ethnicity of the participants was
categorized as Caucasian, Asian and Africans.
Quality assessment
Quality assessment for case-control studies was
assessed using the Newcastle Ottawa scale (NOS) as recommended by
the Cochrane Non-Randomized Studies Methods Working Group (18–20).
This instrument was developed to assess the quality of
non-randomized studies, specifically cohort and case-control. Based
on the NOS, case-control studies were judged based on three broad
perspectives: selection of study groups (4 criteria), comparability
of study groups (1 criteria) and ascertainment of outcome of
interest (3 criteria). Considering the variability in quality of
observational studies identified on our initial literature search,
we considered studies that met ≥5 of the NOS criteria as high
quality (18–20). Hardy-Weinberg Equilibrium (HWE)
served as a surrogate to assess study quality. The effect of HWE
was associated with problems in the design and conduct of genetic
association studies and therefore studies with departures from HWE
were judged as low quality (21).
Statistical analysis
For assessment of deviation from HWE in the reported
genotype frequencies among controls, the appropriate
goodness-of-fit χ2 test was carried out (21). Pooled ORs and 95% CIs were
performed to assess the strength of the correlation between the
CYP1A1 T6235C polymorphism and CAD risk. Pooled ORs with
corresponding 95% CIs for all studies were calculated and subgroup
analyses were then performed in the ethnic groups (Caucasians,
Asians and Africans). Pooled ORs were performed for the allele (C
vs. T), homozygous (CC vs. TT), dominant (CC+CT vs. TT) and
recessive models (CC vs. CT+TT). Statistical heterogeneity among
studies was estimated with Q and I2 statistics (22,23).
A P-value for the Q statistic >0.10 or I2 value
<50% indicated a lack of marked heterogeneity among the studies.
On the basis of heterogeneity test results, the fixed-effects
(Mantel-Haenszel) or random-effects model (DerSimonian and Laird)
was selected to calculate pooled OR (24,25).
Potential publication bias was investigated using the Begg’s funnel
plot and an asymmetric plot indicated possible publication bias.
Funnel-plot asymmetry was further determined by the Egger’s linear
regression test method (26).
Stata Version 12 (Stata Corp, College Station, TX, USA) was used
for statistical analyses. Two-sided P<0.05 was considered to
indicate a statistically significant difference for all
analyses.
Results
Characteristics of included studies
The search criteria identified 38 abstracts.
Following elimination of studies that did not meet the criteria and
exclusion of 19 records, 9 full-text publications were
preliminarily identified for further evaluation (11–16,27–29).
Each original manuscript was reviewed and data were extracted. Two
publications were excluded, including a case-only study(27) and a study with limited data
(28). Therefore, 7 studies with a
total of 2,903 CAD cases and 2,304 controls were included in the
meta-analysis (11–16,29).
All 7 studies were hospital-based case-control studies. There were
4 studies on Caucasian populations (11,12,14,15),
2 on Asians (16,29) and 1 on Africans (13). The number of cases varied between
114 and 873 and controls varied between 53 and 932. The CYP1A1
T6235C genotype distribution in the control groups were all
consistent with HWE, with the exception of 1 study (29). According to the quality criteria,
there were 5 studies with high quality (12–16).
The remaining studies were considered low quality (11,29).
Quantitative synthesis
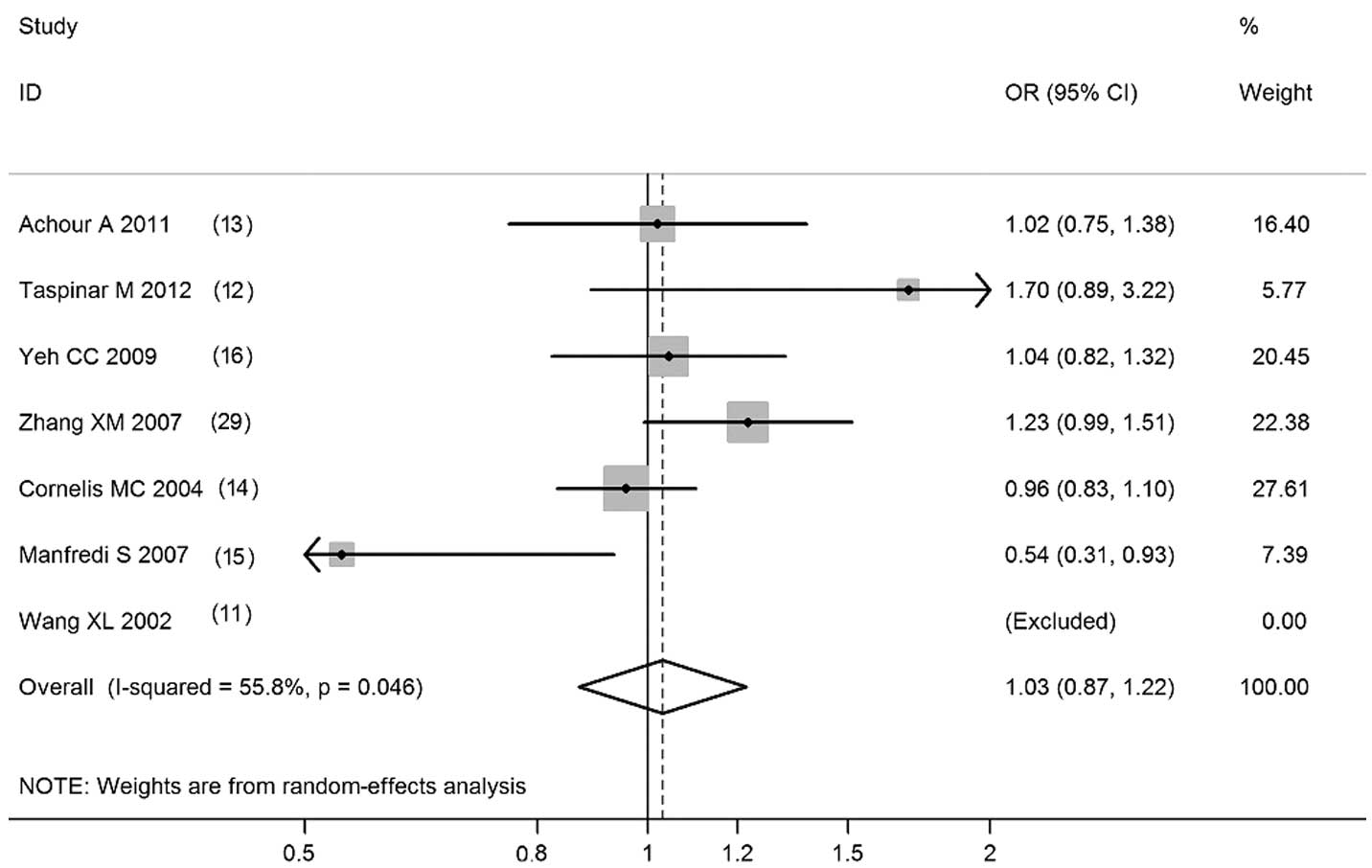
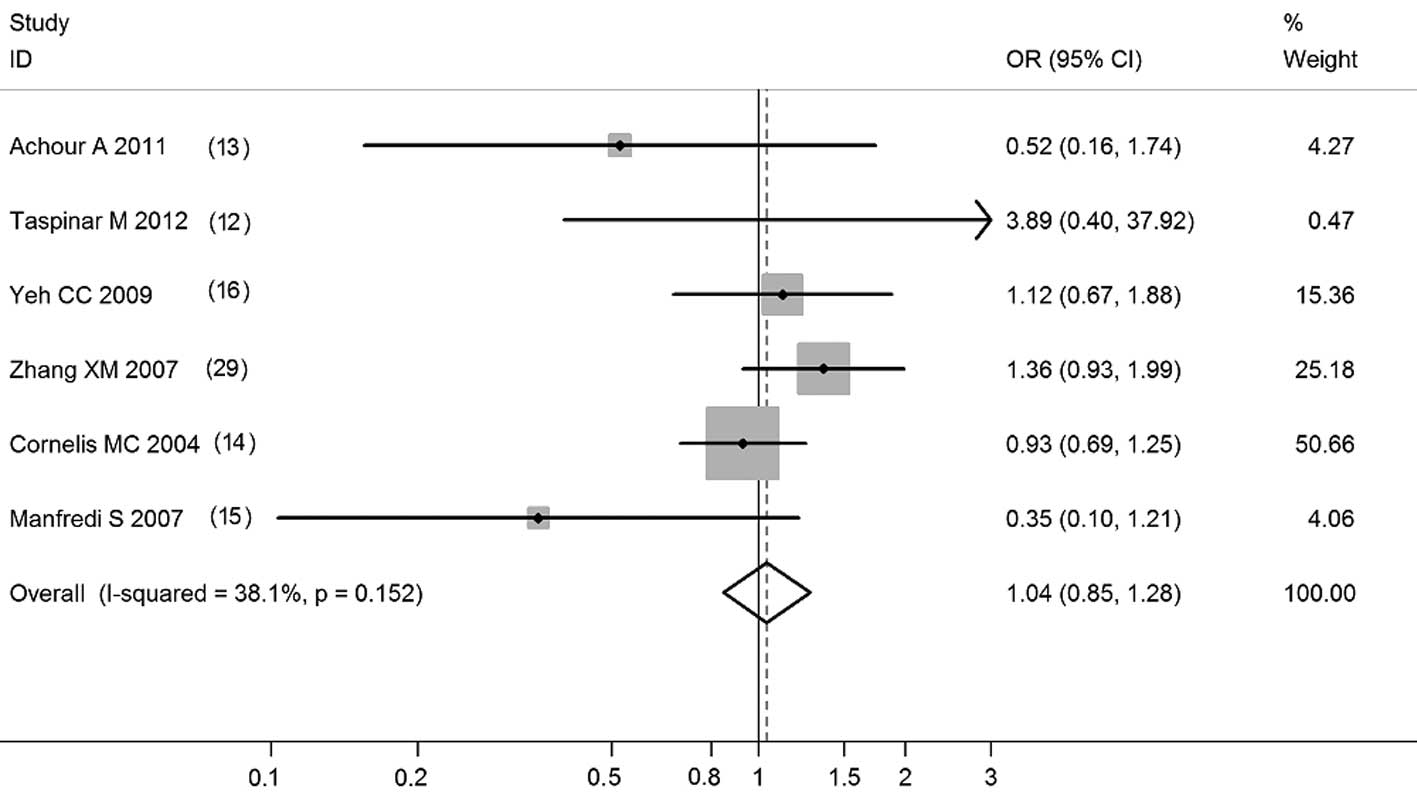
Pooled ORs and the corresponding 95% CIs are shown
in Table I. Overall, the CYP1A1
T6235C polymorphism was not correlated with CAD risk (C vs. T:
OR=1.03; 95% CI, 0.87–1.22; P=0.728; CC vs. TT: OR=1.04; 95% CI,
0.84–1.29; P=0.699; CC+TC vs. TT: OR=1.04, 95% CI, 0.93–1.18;
P=0.478; CC vs. TC+TT: OR=1.04, 95% CI, 0.85–1.28; P=0.704;
Figs. 1–4). Meta-analyses of the 5 high-quality
studies demonstrated that the CYP1A1 T6235C polymorphism was not
correlated with risk of CAD in 4 genetic models (Table I). Ethnic subgroup analyses
revealed no significant correlation was detected in Caucasians,
Asians and Africans (Table I).
 | Table IMeta-analysis of the correlation
between the CYP1A1 T6235C polymorphism and CAD risk. |
Table I
Meta-analysis of the correlation
between the CYP1A1 T6235C polymorphism and CAD risk.
| Studies | Contrast model | Participantsa | OR (95% CI) | POR | I2
(%) |
|---|
| Total | C vs. T | 6 (4511) | 1.03 (0.87–1.22) | 0.728 | 55.8 |
| CC vs. TT | 6 (4511) | 1.04 (0.84–1.29) | 0.699 | 46.6 |
| CC/CT vs. TT | 7 (5207) | 1.04 (0.93–1.18) | 0.478 | 23.4 |
| CC vs. CT/TT | 6 (4511) | 1.04 (0.85–1.28) | 0.704 | 38.1 |
| High quality | C vs. T | 5 (3758) | 0.98 (0.88–1.09) | 0.698 | 48.6 |
| CC vs. TT | 5 (3758) | 0.92 (0.71–1.18) | 0.513 | 30.8 |
| CC/CT vs. TT | 5 (3758) | 0.99 (0.86–1.13) | 0.861 | 23.5 |
| CC vs. CT/TT | 5 (3758) | 0.93
(0.73–1.19) | 0.573 | 24.4 |
| Caucasian | C vs. T | 3 (2286) | 0.94
(0.58–1.52) | 0.792 | 72.3 |
| CC vs. TT | 3 (2286) | 0.82
(0.31–2.13) | 0.680 | 54.6 |
| CC/CT vs. TT | 4 (2982) | 1.02
(0.73–1.41) | 0.919 | 50.5 |
| CC vs. CT/TT | 3 (2286) | 0.91
(0.69–1.21) | 0.526 | 48.2 |
| Asian | C vs. T | 2 (1442) | 1.14
(0.98–1.34) | 0.099 | 0.0 |
| CC vs. TT | 2 (1442) | 1.33
(0.95–1.84) | 0.093 | 0.0 |
| CC/CT vs. TT | 2 (1442) | 1.14
(0.92–1.42) | 0.234 | 0.0 |
| CC vs. CT/TT | 2 (1442) | 1.27
(0.93–1.73) | 0.127 | 0.0 |
| African | C vs. T | 1 (783) | 1.02
(0.75–1.38) | 0.897 | NA |
| CC vs. TT | 1 (783) | 0.53
(0.16–1.79) | 0.310 | NA |
| CC/CT vs. TT | 1 (783) | 1.08
(0.78–1.51) | 0.648 | NA |
| CC vs. CT/TT | 1 (783) | 0.52
(0.16–1.74) | 0.289 | NA |
Publication bias
Begg’s funnel plot and Egger’s test were performed
to assess publication bias. The shapes of the funnel plots did not
reveal any evidence of marked asymmetry in all comparison models.
Egger’s test was further used to provide statistical evidence of
funnel plot asymmetry and the results were consistent with no
evidence of publication bias (Egger’s test values: C vs. T:
P=0.690; CC vs. TT: P=0.661; CC/TC vs. TT: P=0.450; CC vs. TC/TT:
P=0.693). Therefore, a low risk of publication bias was detected in
the present meta-analysis.
Discussion
CYP1A1 is a phase I extrahepatic metabolic enzyme
involved in the bioactivation of carcinogenic PAHs, including
benzopyrene. PAHs present in smoked foods, tobacco smoke and
ubiquitous in urban environments in large cities are considered to
be responsible for an elevated risk of specific types of cancer and
cardiovascular diseases. Considering the importance of CYP1A1, it
is biologically plausible that CYP1A1 polymorphisms may modulate
the risk of cancer and it is well documented that CYP1A1 is
important in arachidonic acid metabolism. The metabolites generated
by the process are associated with cardiovascular physiology
(16,30,31).
In addition, CYP1A1 is involved in the metabolic activation of
tobacco-derived PAHs, molecules associated with carcinogenesis and
atherosclerosis. A number of CYP1A1 polymorphisms increase
transcript half-life, leading to increased enzyme activity and a
subsequent elevation in levels of activated metabolites (9,10).
Therefore, a correlation between CYP1A1 polymorphisms and CAD risk
is possible.
A number of previous studies have evaluated the
correlation between the CYP1A1 T6235C polymorphism and CAD risk,
however, the effect of this polymorphism on CAD remains
inconclusive. Previously, small sample genetic association studies
were performed. These studies had various designs and methodologies
and insufficient power, qualities associated with an increased risk
of false results. Combining data from all eligible studies by
meta-analysis reduces random error and increases the accuracy of
genetic association data. To provide a more robust estimate of the
hypothesized correlation, a meta-analysis was performed. In the
present study, a comprehensive meta-analysis of literature on the
correlation between the CYP1A1 T6235C polymorphism and CAD risk was
performed. On the basis of our inclusion criteria, 7 studies with a
total of 2,903 cases and 2,304 controls were included in the
meta-analysis. Overall, the CYP1A1 T6235C polymorphism was not
correlated with CAD risk (C vs. T: OR=1.03; 95% CI, 0.87–1.22;
P=0.728; CC vs. TT: OR=1.04; 95% CI, 0.84–1.29; P=0.699; CC+TC vs.
TT: OR=1.04; 95% CI, 0.93–1.18; P=0.478; CC vs. TC+TT: OR=1.04; 95%
CI, 0.85–1.28; P=0.704). Meta-analyses of five high-quality studies
demonstrated that the CYP1A1 T6235C polymorphism was not correlated
with risk of CAD in four genetic models. Ethnic subgroup analyses
revealed that no significant correlation was identified in
Caucasian, Asian and African populations. Therefore, this
meta-analysis indicates that the CYP1A1 T6235C polymorphism is not
correlated with CAD risk.
Four polymorphisms, including T6235C (a substitution
in the 3′ non-coding region), A2455G (isoleucine to valine
transition at codon 462), T3205C (a transition mutation in the 3′
non-coding region) and C2453A (threonine to asparagine transition
at codon 461) were previously identified in the CYP1A1 gene
(32). C2453A is extremely rare
and T3205C exists only in African and African-American individuals.
Therefore, the majority of studies have focused on the CYP1A1
T6235C and CYP1A1 A2455G polymorphisms (32). The CYP1A1 A2455G polymorphism was
previously found to be correlated with risk of several common types
of cancer, however, the CYP1A1 T6235C polymorphism is not
correlated with this risk (33–35).
These outcomes indicate that the CYP1A1 A2455G polymorphism may
have additional effects on the CYP1A1 enzyme compared with the
CYP1A1 T6235C polymorphism. Previous studies have concentrated on
the correlation between the CYP1A1 T6235C polymorphism and CAD
risk, however, limited studies have analyzed this correlation with
respect to the CYP1A1 A2455G polymorphism. Therefore, additional
studies are required to assess this correlation.
The present study is associated with several
limitations that must be considered when interpreting the results.
Firstly, inclusion of only seven studies with relatively small
sample sizes and poor validation was the main limitation of the
meta-analysis. In addition, only two studies on Asian and four on
Caucasian populations were included in the analysis. Two methods
were utilized to assess publication bias, however, these methods
may be associated with low power for the detection of publication
bias risk in cases of limited study numbers. Therefore, additional
studies of larger sample size and consistent design are required
for comprehensive analysis of this correlation. Secondly, the main
analysis was based on unadjusted estimates due to lack of adjusted
estimates. A more precise analysis is achieved when adjusted
estimates are available for all studies (36). Considerable variability in study
design and control selection was revealed in the present
meta-analysis. To obtain results from meta-analysis of homogeneous
studies, additional studies with adjusted estimates are required.
Thirdly, gene-environmental factor interactions were not fully
addressed due to insufficient data. Future studies must further
assess the possible gene-environmental interactions, including
gene-smoking interactions, in the correlation between the CYP1A1
T6235C polymorphism and CAD risk.
In conclusion, the present study has demonstrated
that the CYP1A1 T6235C polymorphism is not correlated with CAD
risk. Additional studies with larger sample size and consistent
design must be performed to confirm this finding.
References
|
1
|
White HD and Chew DP: Acute myocardial
infarction. Lancet. 372:570–584. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pfisterer ME, Zellweger MJ and Gersh BJ:
Management of stable coronary artery disease. Lancet. 375:763–772.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hansson GK: Inflammation, atherosclerosis
and coronary artery disease. N Engl J Med. 352:1685–1695. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Libby P and Theroux P: Pathophysiology of
coronary artery disease. Circulation. 111:3481–3488. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Samani NJ, Erdmann J, Hall AS, et al:
Genomewide association analysis of coronary artery disease. N Engl
J Med. 357:443–453. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Topol EJ, Smith J, Plow EF and Wang QK:
Genetic susceptibility to myocardial infarction and coronary artery
disease. Hum Mol Genet. 15:R117–R123. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nebert DW and Gonzalez FJ: P450 genes:
structure, evolution and regulation. Annu Rev Biochem. 56:945–993.
1987. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Whitlock JP Jr: Induction of cytochrome
P4501A1. Annu Rev Pharmacol Toxicol. 39:103–125. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sivaraman L, Leatham MP, Yee J, Wilkens
LR, Lau AF and Le Marchand L: CYP1A1 genetic polymorphisms and in
situ colorectal cancer. Cancer Res. 54:3692–3695. 1994.PubMed/NCBI
|
|
10
|
Landi MT, Bertazzi PA, Shields PG, et al:
Association between CYP1A1 genotype, mRNA expression and enzymatic
activity in humans. Pharmacogenetics. 4:242–246. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang XL, Greco M, Sim AS, Duarte N, Wang J
and Wilcken DE: Effect of CYP1A1 MspI polymorphism on cigarette
smoking related coronary artery disease and diabetes.
Atherosclerosis. 162:391–397. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Taspinar M, Aydos S, Sakiragaoglu O, et
al: Impact of genetic variations of the CYP1A1, GSTT1 and GSTM1
genes on the risk of coronary artery disease. DNA Cell Biol.
31:211–218. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Achour A, Zaag I, Gueddah L, Trimeche B,
Slama FB and Zemni R: Role of CYP1A1 (T6235C) polymorphism and
cigarette smoking in the development of coronary heart disease in
Tunisian population. J Genet. 90:303–307. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cornelis MC, El-Sohemy A and Campos H:
Genetic polymorphism of CYP1A2 increases the risk of myocardial
infarction. J Med Genet. 41:758–762. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Manfredi S, Federici C, Picano E, Botto N,
Rizza A and Andreassi MG: GSTM1, GSTT1 and CYP1A1 detoxification
gene polymorphisms and susceptibility to smoking-related coronary
artery disease: a case-only study. Mutat Res. 621:106–112. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yeh CC, Sung FC, Kuo LT, Hsu WP and Chu
HY: Polymorphisms of cytochrome P450 1A1, cigarette smoking and
risk of coronary artery disease. Mutat Res. 667:77–81. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Stroup DF, Berlin JA, Morton SC, et al:
Meta-analysis of observational studies in epidemiology: a proposal
for reporting. Meta-analysis Of Observational Studies in
Epidemiology (MOOSE) group. JAMA. 283:2008–2012. 2000. View Article : Google Scholar
|
|
18
|
Wells G, Shea B, O’Connell D, et al: The
Newcastle-Ottawa Scale (NOS) for assessing the quality of
nonrandomised studies in meta-analyses. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
Accessed September 14, 2012
|
|
19
|
Boivin J, Griffiths E and Venetis CA:
Emotional distress in infertile women and failure of assisted
reproductive technologies: meta-analysis of prospective
psychosocial studies. BMJ. 342:d2232011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Millett GA, Flores SA, Marks G, Reed JB
and Herbst JH: Circumcision status and risk of HIV and sexually
transmitted infections among men who have sex with men: a
meta-analysis. JAMA. 300:1674–1684. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Rohlfs RV and Weir BS: Distributions of
Hardy-Weinberg equilibrium test statistics. Genetics.
180:1609–1616. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cochran WG: The combination of estimates
from different experiments. Biometrics. 10:101–129. 1954.
View Article : Google Scholar
|
|
23
|
Higgins JP, Thompson SG, Deeks JJ and
Altman DG: Measuring inconsistency in meta-analyses. BMJ.
327:557–560. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
DerSimonian R and Laird N: Meta-analysis
in clinical trials. Control Clin Trials. 7:177–188. 1986.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Mantel N and Haenszel W: Statistical
aspects of the analysis of data from retrospective studies of
disease. J Natl Cancer Inst. 22:719–748. 1959.PubMed/NCBI
|
|
26
|
Egger M, Davey Smith G, Schneider M and
Minder C: Bias in meta-analysis detected by a simple, graphical
test. BMJ. 315:629–634. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Jarvis MD, Palmer BR, Pilbrow AP, et al:
CYP1A1 MSPI (T6235C) gene polymorphism is associated with mortality
in acute coronary syndrome patients. Clin Exp Pharmacol Physiol.
37:193–198. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kim SJ, Kim MG, Kim KS, Song JS, Yim SV
and Chung JH: Impact of glutathione S-transferase M1 and T1 gene
polymorphisms on the smoking-related coronary artery disease. J
Korean Med Sci. 23:365–372. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhang XM, Xu G, Shan J, Jin GD, Huang CL
and Wu KS: Association of the MspI polymorphism of cytochrome
P4501A1 gene and smoking to the susceptibility to coronary artery
disease. Zhonghua Xin Xue Guan Bing Za Zhi. 35:536–539. 2007.(In
Chinese).
|
|
30
|
Nebert DW and Dalton TP: The role of
cytochrome P450 enzymes in endogenous signalling pathways and
environmental carcinogenesis. Nat Rev Cancer. 6:947–960. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Martucci CP and Fishman J: P450 enzymes of
estrogen metabolism. Pharmacol Ther. 57:237–257. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chen C, Huang Y, Li Y, Mao Y and Xie Y:
Cytochrome P450 1A1 (CYP1A1) T3801C and A2455G polymorphisms in
breast cancer risk: a meta-analysis. J Hum Genet. 52:423–435. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wang JJ, Zheng Y, Sun L, et al: CYP1A1
Ile462Val polymorphism and susceptibility to lung cancer: a
meta-analysis based on 32 studies. Eur J Cancer Prev. 20:445–452.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Huang M, Chen Q, Xiao J, Zhao X and Liu C:
CYP1A1 Ile462Val is a risk factor for ovarian cancer development.
Cytokine. 58:73–78. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Androutsopoulos VP, Tsatsakis AM and
Spandidos DA: Cytochrome P450 CYP1A1: wider roles in cancer
progression and prevention. BMC Cancer. 9:1872009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Peters J and Mengersen K: Selective
reporting of adjusted estimates in observational epidemiology
studies: reasons and implications for meta-analyses. Eval Health
Prof. 31:370–389. 2008. View Article : Google Scholar : PubMed/NCBI
|