Introduction
Adjuvant FOLFOX-4 chemotherapy following surgery is
recommended as an effective therapy for patients with stage II and
III colon cancer (1).
Nevertheless, a high percentage of patients encounter recurrence as
a primary cause of mortality, which is mainly associated with
chemotherapeutic response (2–4).
Over the past 10 years, combination chemotherapies have improved
response rates and prolonged overall survival in colorectal cancer
(CRC) patients (5). To date,
oxaliplatin (a platinum drug) combined with fluorouracil
(5FU)/leucovorin (FL) in the FOLFOX-4 regimen is frequently
prescribed to treat CRC.
The prediction of sensitivity or resistance to
chemotherapy by analysis of genetic variations is of major interest
in choosing the first-line chemotherapy most likely to be
efficient. A single nucleotide polymorphism (SNP) is a point
mutation that is observed in 1% of a general population. Genetic
polymorphisms in the sequences of drug transporter genes have been
found to affect the therapeutic response, toxicity and survival of
cancer patients. Furthermore, SNPs may affect CRC susceptibility
when exposed to exogenous and endogenous carcinogenesis.
The formation of glutathione conjugates is a
well-known mechanism by which platinating agents inhibit tumor
growth. It is also known to be one of the mechanisms involved in
the resistance to oxaliplatin in CRC (6). Glutathione conjugates may be
substrates of ATP-binding cassette (ABC) multidrug transporters.
For example, glutathione detoxifies oxaliplatin by conjugation
(7,8); the conjugate is eliminated by
MRPs/ABCCs, either alone, as a co-substrate, or in its conjugated
form (9). Intracellular
glutathione content may be reduced by the high expression of
MRPs/ABCCs (10).
The human multidrug resistance protein 2
(MRP2/ABCC2/cMOAT) gene is a member of the ABC transporter family
that is located on chromosomal locus 10q24 and consists of 32 exons
(11). MRP2 is presented at the
apical membrane of polarized cells, including hepatocytes, renal
proximal tubule epithelia and intestinal epithelia (12). It is the optimal pump to eliminate
relatively hydrophilic compounds, including glucuronide,
glutathione and sulfate conjugates (12). Among several MRP2 SNPs, the G1249A
SNP is known to be associated with alterations in mRNA levels
(13,14). This MRP2 polymorphism results in an
amino acid alteration from Val to Ile at position 417, located in
membrane spanning domain 2 of the protein. According to previous
reports (15,16), mutations in this domain of MRP2 may
alter the specificity of its substrates but not its transporter
activity. In the present study, we investigated the association of
G1249A MRP2 polymorphism with the poor response to FOLFOX-4
chemotherapy, a platinum-base regimen, and the short-term survival
of CRC patients.
Materials and methods
Patients and treatment
The subjects included 50 primary CRC patients (30
male, 20 female; median age 57 years), who received radical
resection in Chamran Hospital and Hazrat Rasuol Akram Hospital,
Tehran, Iran. Patients were eligible if they had undergone adjuvant
FOLFOX-4 chemotherapy following the radical resection of
histologically confirmed stage II (T2 and T3, N0, M0) or stage III
(any T, N1 and 2, M0) CRC. Patients who had received preoperative
chemotherapy and radiotherapy were excluded. A total of 50
unrelated healthy individuals who did not have a family history of
cancer and were gender/age-matched with the patients were also
enrolled in this study. The protocol of the study was approved by
the Faculty of Medicine and Health Sciences Ethics Committee,
University Putra Malaysia.
The patients received 12 cycles of a FOLFOX-4
regimen which consisted a 2-week cycle of oxaliplatin (85
mg/m2) combined with leucovorin (200 mg/m2)
on day 1, bolus 5FU (400 mg/m2) and continuous infusion
of 5FU (600 mg/m2). Follow-up consisted of a
carcinoembryonic antigen (CEA) test at 3-month intervals for 2
years and at 6-month intervals thereafter. Colonoscopy and CT scans
were usually performed at 6-month intervals in the first 2 years
and annually thereafter; however, these tests were mandatory
following an elevated CEA level. Development of new recurrent or
metastatic lesions following surgery was considered as a relapse.
Local relapse was histopathologically/cytologically confirmed by
the examination of specimens. Written informed patient consent was
obtained from the patients.
DNA extraction and genotyping
Sections (5–10 μm) of paraffin-embedded tissues
(n=50) were cut with a microtome and placed in a 1.5-ml
microcentrifuge tube. To avoid intersample contamination, the blade
was cleaned with xylene after cutting each paraffin block. DNA was
isolated from the paraffin-embedded tissue samples using a QiAamp
DNA FFPE tissue kit (Qiagen, Hilden, Germany) according to the
manufacturer's instructions. DNA was also extracted from 50 blood
samples of the unrelated healthy individuals using a kit
(Qiagen).
Single nucleotide polymorphism was analyzed using a
polymerase chain reaction-restriction fragment length polymorphism
(PCR-RFLP) technique. The following PCR primers were used for
genotyping: forward, 5′-GGGCAAAGAAGTGTGTGGAT-3′; and reverse,
5′-ACATCAGGTTCACTGTTTCTCCCA-3′ (17). The PCR amplification conditions
consisted of an initial denaturation step at 94°C for 3 min,
followed by 35 cycles of a denaturation step at 94°C for 20 sec, an
annealing step at 56.4°C for 15 sec and an extension step at 72°C
for 20 sec with a final extension step at 72°C for 5 min. The PCR
products were digested with 5 units NcoI at 37°C for 15 h.
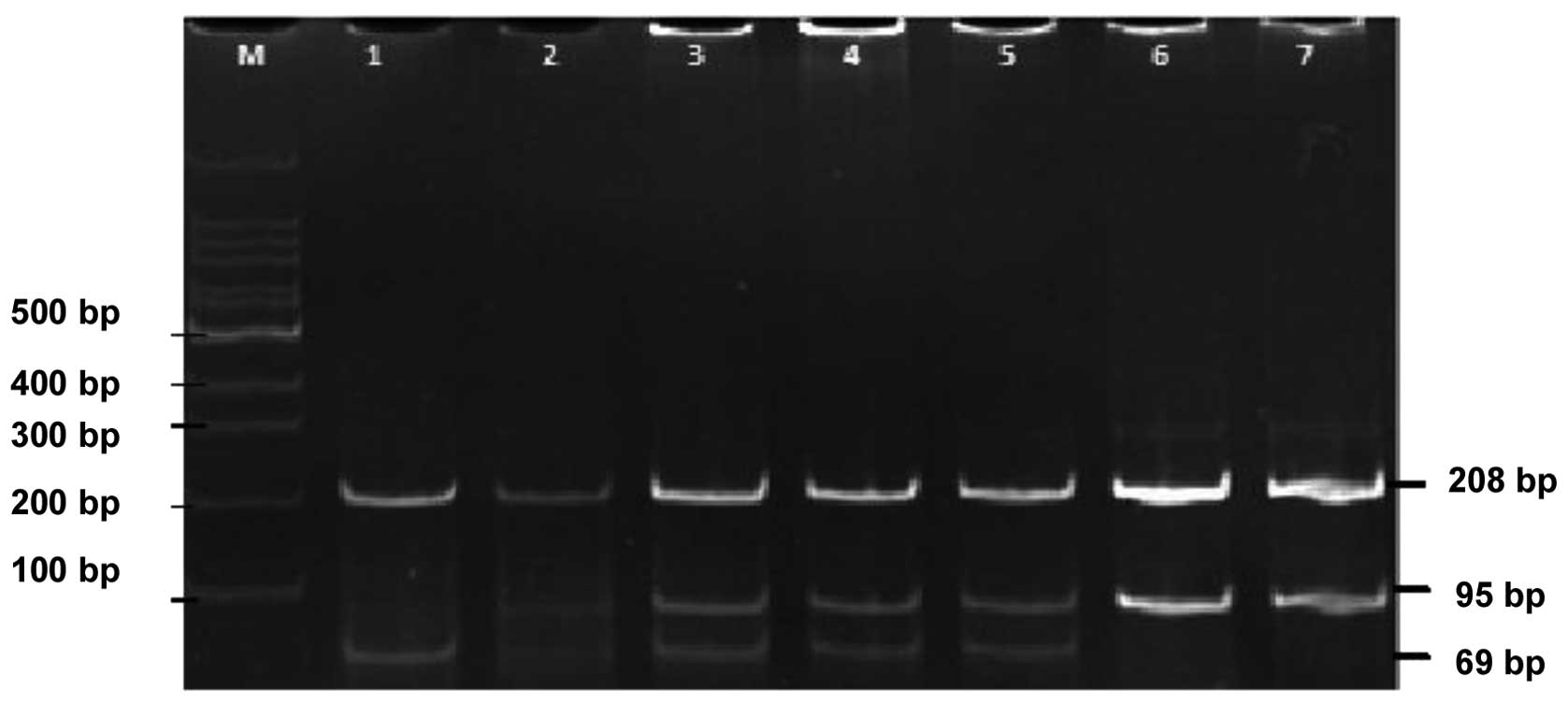
The digested products were thereafter electrophoresed on a 10%
acrylamide gel followed by ethidium bromide staining for genotype
determination. The fragments obtained were 208 and 95 bp for the
wild-type genotype GG; 208, 69 and 26 bp for the mutant genotype
AA; and 208, 95, 69 and 26 bp for the GA genotype (Fig. 1). The accuracy of PCR-RFLP was
confirmed by direct sequencing using a Big-Dye Terminator Cycle
Sequencing kit (Applied Biosystems, Foster City, CA, USA).
Data analysis
Data analysis was performed using the SPSS software
V11.0 (SPSS, Inc., Chicago, IL, USA). The χ2 and
Fisher's exact tests were used to analyze the data. Any possible
association between the development of the disease and the studied
polymorphism was evaluated by calculating odds ratios (ORs) and 95%
confidence intervals (CIs) from contingency tables and using a
two-sided Fisher's exact test. Survival analysis was performed
using the Kaplan-Meier method and differences between the survival
curves were evaluated with a log-rank test. Disease-free survival
(DFS) was defined in months from the day of surgery to the first
event of documented relapse or death. For those patients who did
not relapse, data were recorded during the last follow-up. Overall
survival (OS) was defined as the time from the date of coloctomy to
death; data on survivors were censored at the last follow-up.
Results
A total of 50 patients diagnosed with stage II/III
CRC (median age 60 years and range 17–77 years) were enrolled in
the study. Of these, 60 % were males and 52% were in UICC stage
III. Other clinicopathological characteristics of the patients are
listed in Table I. All patients
were treated with 12 cycles of the FOLFOX-4 regimen for 6 months.
The follow-up duration was 24–48 months. Fifteen cases (30%)
experienced an early relapse, either a local recurrence or distant
metastasis, within 1 year of follow-up.
 | Table IPatients and tumor
characteristics. |
Table I
Patients and tumor
characteristics.
| Characteristics | Total cases | (%) |
|---|
| Gender |
| Male | 30 | 60 |
| Female | 20 | 40 |
| Age (years) |
| >50 | 38 | 76 |
| <50 | 12 | 24 |
| Tumor size (cm) |
| <5 | 28 | 56 |
| >5 | 22 | 44 |
| Location |
| Colon | 35 | 70 |
| Rectum | 15 | 30 |
| Depth of tumor
invasion |
| T4 | 3 | 6 |
| T3 | 47 | 94 |
| Lymph node
metastasis |
| Negative | 22 | 44 |
| Positive | 28 | 56 |
| Stage |
| II | 22 | 44 |
| III | 28 | 56 |
| Histologya |
| WD + MD | 48 | 96 |
| PD | 2 | 4 |
G1249A polymorphism and CRC risk
Table II presents
OR estimates of CRC risk for each genotype of the MRP2 gene SNP. Of
the 50 patients, the MDR2 wild-type genotype (1249GG) was observed
in 32% of patients, whereas 56% were heterozygous (1249GA) and 12%
were homozygous (1249AA) for the mutation. The 1249GG, 1249GA and
1249AA genotypes were found in 44, 46 and 10% of the controls,
respectively. For the GG, AA and GA genotypes, no statistically
significant association was identified between the SNP and CRC
risk. In addition, no significant trend was identified in the
number of alleles with respect to CRC occurrence.
 | Table IIGenotypes and allele frequencies of
the G1249A polymorphism in 50 colorectal cancer (CRC) patients and
healthy controls. |
Table II
Genotypes and allele frequencies of
the G1249A polymorphism in 50 colorectal cancer (CRC) patients and
healthy controls.
| Genotype N (%) | Allele (%) |
|---|
|
|
|
|---|
| GG | GA | AA | G | A |
|---|
| Patient | 16 (32) | 28 (56) | 6 (12) | 60 | 40 |
| Control | 22 (44) | 23 (46) | 5 (10) | 67 | 33 |
| P-value | 0.151 | 0.121 | 0.500 | 0.189 | - |
| Odds ratio | 2.597 | 0.667 | 0.814 | - | 0.739 |
Correlation between G1249A polymorphism
and clinicopathological markers
No significant association was observed between the
G1249A genotypes (GG, GA and AA) and the patient's characteristics,
including age, gender, tumor location, tumor size, deep tumor
invasion, disease stage and the presence or absence of lymph node
metastasis (P>0.05; Table
III).
 | Table IIICharacteristics of patients according
to G1249A genotypes. |
Table III
Characteristics of patients according
to G1249A genotypes.
| Characteristics | GG | GA or AA | P-value |
|---|
| Gender | | | 0.247 |
| Male (n=30) | 8 | 22 | |
| Female (n=20) | 8 | 12 | |
| Age (years) | | | 0.120 |
| >50 (n=38) | 10 | 28 | |
| <50 (n=12) | 6 | 6 | |
| Tumor size (cm) | | | 0.120 |
| <5 (n=28) | 6 | 6 | |
| >5 (n=22) | 10 | 28 | |
| Location | | | 0.572 |
| Colon (n=35) | 11 | 24 | |
| Rectum (n=15) | 5 | 10 | |
| Depth of tumor
invasion | | | 0.237 |
| T3 (n=47) | 14 | 33 | |
| T4 (n=3) | 2 | 1 | |
| Lymph node
metastasis | | | 0.587 |
| Negative
(n=22) | 8 | 14 | |
| Positive
(n=28) | 8 | 20 | |
| Stage | | | 0.587 |
| II (n=22) | 8 | 14 | |
| III (n=28) | 8 | 20 | |
| Histologya | | | - |
| WD + MD
(n=48) | 14 | 34 | |
| PD (n=2) | 2 | 0 | |
Correlation between prognostic and
clinicopathological markers
To investigate whether various clinicopathological
data of the patients correlate with chemotherapy response, disease
recurrence was evaluated according to the clinicopathological
factors. None of those factors were identified to be associated
with clinical response in the CRC patients (P>0.05). In
addition, all factors were examined using the Kaplan-Meier method.
These parameters did not correlate with the OS or DFS of the
patients (all P>0.05), with the exception of deep tumor
invasion. The correlation between deep tumor invasion and survival
rates, although significant, was not suitable for evaluation, since
the number of patients with T4 type tumors was very small.
Correlation between G1249A polymorphism
and prognosis
To identify any possible correlation between the
genotypes of the G1249A polymorphism and the survival rates and
early recurrence, the patients were divided into the normal group
(GG) and the mutation group (GA and AA). The polymorphic genotype
of MRP2 was not different between the patients who responded and
those who did not respond to the adjuvant FOLFOX-4 chemotherapy
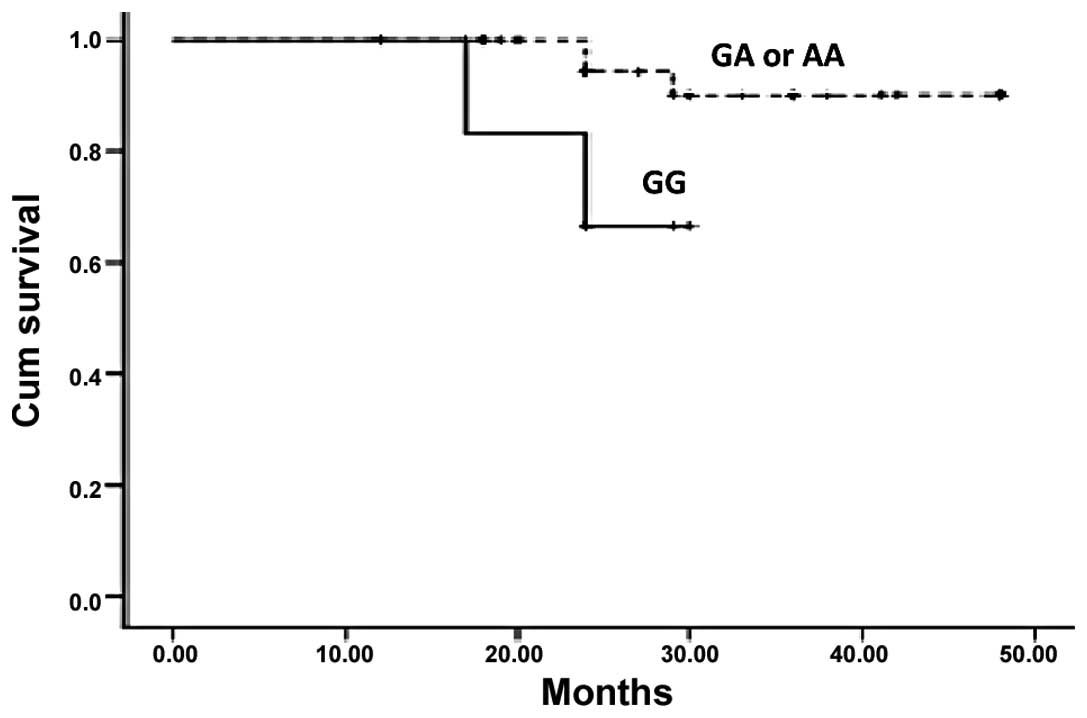
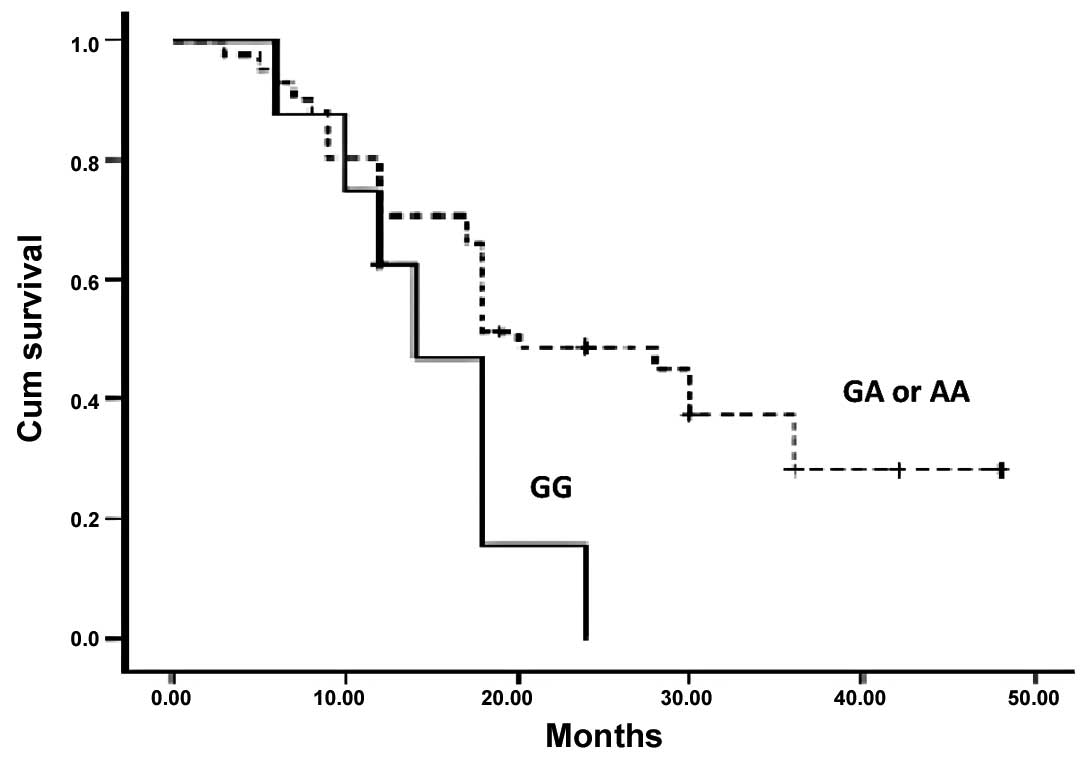
(P=0.468, P>0.05). However, a significant association between
G1249A genotypes and survival rates was observed when Kaplan-Meier
survival curves were plotted (Figs.
2 and 3). Thus patients with a
mutant allele A, either homozygous or heterozygous, of G1249A had
higher DFS and OS than patients with wild-type allele G (P=0.045
and 0.043).
Discussion
Among platinum drugs, oxaliplatin is the favored
drug for the therapy of CRC. Oxaliplatin combined with 5FU/LV (the
FOLFOX-4 regimen) is currently used for the treatment of stage
II/III CRC patients who have undergone complete resection of the
primary tumor. Expression of ABC-transporter proteins, particularly
MRP2, has been demonstrated to be associated with resistance to
platinum-based anticancer drugs, including cisplatin (18,19).
Hence, variations in the MRP2 gene may be important for evaluating
and/or predicting the response to platinum drugs. The role of MRP2
genetic polymorphisms on the response of CRC to platinum-based
chemotherapy has not previously been reported. Among the
polymorphisms of the MRP2 gene, G1249A has been found to be a
common SNP that affects mRNA levels (13,14).
Owing to its possible effect on gene expression, we anticipated
that this polymorphism of the MRP2 gene may affect the tumor
response to adjuvant FOLFOX-4 chemotherapy in CRC. To examine our
hypothesis, we investigated whether functional polymorphism of
G1249A in the MRP2 gene affected CRC occurrence and/or correlated
with early recurrence in patients treated with the FOLFOX-4
regimen. We selected a homogeneous population in stage II/III who
had not received any preoperative treatment. The genotype
frequencies of the MRP2 G1249A polymorphism did not change during
malignancy and were identical in the tumor and the matched normal
tissues in our selected population. Therefore, de novo
mutation of the MRP2 gene, which may affect cancer development and
drug response, does not appear to occur in CRC. In addition, we
analyzed the genetic polymorphism in the DNA isolated from
paraffin-embedded samples obtained from the patients' matched
normal tissues and in the peripheral blood of the controls. A
previous study has verified that the genotyping results of DNA
isolated from tissue are equivalent to those of DNA isolated from
blood (20). The frequency of the
A allele for the G1249A polymorphism in the controls was 33%, which
is inconsistent with that reported in the literature (11). No association was observed in the
present study between the SNP of the MRP2 gene in exon 10 and the
development of CRC. By contrast, an association between this
polymorphic genotype and the risk of primary colorectal
adenocarcinoma has been previously reported in Japanese patients
(21).
We have also analyzed this SNP to investigate its
significance in relation to platinum-based chemotherapy. The
genotype frequencies of G1249A were not different between the
patients who relapsed within one year and those who did not. Our
results indicate that the mutant allele in exon 10 was not related
to the response to adjuvant FOLFOX-4 chemotherapy. Similar results
were obtained by Sun et al(11) in Chinese advanced non-small cell
lung cancer (NSCLC) patients who were treated with a platinum-based
drug. By contrast, a significant correlation between G1249A and the
response to chemotherapy was observed in advanced ovarian cancer by
Xingsheng et al(22). In
addition, in Kaplan-Meier curves, the adenocarcinoma patients with
a GG genotype of G1249A demonstrated a significantly shorter
overall survival and disease-free survival than patients with AA or
GA genotypes. Thus, we demonstrated that G1249A MRP2 is a molecular
predictive marker for the survival of patients with stage II/III
CRC treated with adjuvant FOLFOX-4 therapy following curative
resection.
In conclusion, the MRP2 G1249A polymorphism was not
associated with the incidence of CRC in the Iranian population. In
addition, this polymorphism did not affect the prognosis of the
disease in our population. Although, the considered patient
population was small in size, it was very homogeneous, and hence
was suitable for prognostic evaluation.
References
|
1
|
Kuebler JP, Wieand HS, O'Connell MJ, et
al: Oxaliplatin combined with weekly bolus fluorouracil and
leucovorin as surgical adjuvant chemotherapy for stage II and III
colon cancer: results from NSABP C-07. J Clin Oncol. 25:2198–2204.
2007. View Article : Google Scholar
|
|
2
|
Chen LT and Whang-Peng J: Current status
of clinical studies for colorectal cancer in Taiwan. Clin
Colorectal Cancer. 4:196–203. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
de Gramont AD, Tournigand C, André T,
Larsen AK and Louvet C: Adjuvant therapy for stage II and III
colorectal cancer. Semin Oncol. 34(Suppl 1): S37–S40. 2007.
|
|
4
|
Schippinger W, Samonigg H, Schaberl-Moser
R, et al: A prospective randomised phase III trial of adjuvant
chemotherapy with 5-fluorouracil and leucovorin in patients with
stage II colon cancer. Br J Cancer. 97:1021–1027. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gonzalez-Haba E, García MI, Cortejoso L,
et al: ABCB1 gene polymorphisms are associated with adverse
reactions in fluoropyrimidine-treated colorectal cancer patients.
Pharmacogenomics. 11:1715–1723. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Casado E, De Castro J, Belda-Iniesta C, et
al: Molecular markers in colorectal cancer: genetic bases for a
customised treatment. Clin Transl Oncol. 9:549–554. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Perez RP, Hamilton TC and Ozols RF:
Resistance to alkylating agents and cisplatin: insights from
ovarian carcinoma model systems. Pharmacol Ther. 48:19–27. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
El-akawi Z, Abu-hadid M, Perez R, et al:
Altered glutathione metabolism in oxaliplatin resistant ovarian
carcinoma cells. Cancer Lett. 105:5–14. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Theile D, Grebhardt S, Haefeli WE and
Weiss J: Involvement of drug transporters in the synergistic action
of FOLFOX combination chemotherapy. Biochem Pharmacol.
78:1366–1373. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ballatori N, Hammond CL, Cunningham JB,
Krance SM and Marchan R: Molecular mechanisms of reduced
glutathione transport: role of the MRP/CFTR/ABCC and OATP/SLC21A
families of membrane proteins. Toxicol Appl Pharmacol. 204:238–255.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sun N, Sun X, Chen B, et al: MRP2 and
GSTP1 polymorphisms and chemotherapy response in advanced non-small
cell lung cancer. Cancer Chemother Pharmacol. 65:437–446. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Nies AT and Keppler D: The apical
conjugate efflux pump ABCC2 (MRP2). Pflugers Arch. 453:643–659.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Meyer zu Schwabedissen HE, Jedlitschky G,
Gratz M, et al: Variable expression of MRP2 (ABCC2) in human
placenta: influence of gestational age and cellular
differentiation. Drug Metab Dispos. 33:896–904. 2005.PubMed/NCBI
|
|
14
|
Kroetz DL, Liu W, Nguyen TD, et al:
1249G>A polymorphism of ABCC2 (MRP2) is associated with
altered gene expression in human liver. J Clin Oncol. 24(Suppl):
130722006.
|
|
15
|
Toh S, Wada M, Uchiumi T, et al: Genomic
structure of the canalicular multispecific organic
anion-transporter gene (MRP2/cMOAT) and mutations in the
ATP-binding-cassette region in Dubin-Johnson syndrome. Am J Hum
Genet. 64:739–746. 1999. View
Article : Google Scholar
|
|
16
|
Choi JH, Ahn BM, Yi J, et al: MRP2
haplotypes confer differential susceptibility to toxic liver
injury. Pharmacogenet Genomics. 17:403–415. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Naesens M, Kuypers DR, Verbeke K and
Vanrenterghem Y: Multidrug resistance protein 2 genetic
polymorphisms influence mycophenolic acid exposure in renal
allograft recipients. Transplantation. 82:1074–1084. 2006.
View Article : Google Scholar
|
|
18
|
Liedert B, Materna V, Schadendorf D,
Thomale J and Lage H: Overexpression of cMOAT (MRP2/ABCC2) is
associated with decreased formation of platinum-DNA adducts and
decreased G2-arrest in melanoma cells resistant to cisplatin. J
Invest Dermatol. 121:172–176. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Shen DW, Goldenberg S, Pastan I and
Gottesman MM: Decreased accumulation of [14C]carboplatin in human
cisplatin-resistant cells results from reduced energy-dependent
uptake. J Cell Physiol. 183:108–116. 2000.
|
|
20
|
Petrova DT, Nedeva P, Maslyankov S, et al:
No association between MDR1 (ABCB1) 2677G>T and 3435C>T
polymorphism and sporadic colorectal cancer among Bulgarian
patients. J Cancer Res Clin Oncol. 134:317–322. 2008. View Article : Google Scholar
|
|
21
|
Nishioka C, Sakaeda T, Nakamura T, et al:
MDR1, MRP1 and MRP2 genotypes and in vitro chemosensitivity in
Japanese patients with colorectal adenocarcinomas. Kobe J Med Sci.
50:181–188. 2004.PubMed/NCBI
|
|
22
|
Xingsheng Y, Sekine M, Kurata H, et al:
Single nucleotide polymorphisms in MRP2 gene and their significance
on chemosensitivity of advanced ovarian cancer. J Pract Obstet
Gynecol. 20:153–155. 2004.
|