Introduction
Liver fibrosis is the common sequel of diverse
chronic liver disease, characterized by increased deposition and
altered composition of the extracellular matrix (ECM). Its
pathogenesis is a multi-cellular event, involving Kupffer cells,
hepatocytes, intrahepatic lymphocytes and hepatic stellate cells
(HSCs), among others. It is generally accepted that HSCs are
central to the process of fibrosis as they are the major source of
ECM and their activation to form myofibroblasts is the key event in
fibrosis, characterized by increasing proliferation, chemotaxis,
contractility and fibrogenesis (1). A number of studies in animal models
and data in human liver disease emphasize that fibrosis is
reversible (2). To date, no
effective pharmacologic treatment able to prevent the progression
of liver fibrosis is available. Interleukin-10 (IL-10) is a
cytokine that downregulates pro-inflammatory responses and has a
potential modulatory effect on liver fibrosis (3,4).
Recombinant human IL-10 has been produced and tested in clinical
trials. It was shown in a study by Nelson et al that the
administration of IL-10 to patients with hepatitis C virus (HCV)
infection attenuated liver inflammation and subsequent fibrosis
(5). Furthermore, the inhibitory
effect of IL-10 on hepatic fibrogenesis was confirmed by our
previous study in experimental rat models of liver cirrhosis
induced by carbon tetrachloride (6).
However, the elimination half-life of recombinant
IL-10 in vivo is relatively short, and its non-targeted
administration may lead to systemic side-effects (7). Therefore, increasing numbers of
studies have aimed to use IL-10 gene transfer to maintain the level
of IL-10, especially in the target organs, with as low a frequency
of administration as possible. A previous study revealed that
electro-transfer of the IL-10 gene into the anterior tibialis
muscle was followed by attenuation of CCl4-induced liver
fibrosis in mice (8). However,
liver-targeting gene transfer is undoubtedly more acceptable in the
management of liver diseases, due to its conceivably fewer systemic
side-effects. We hypothesize that hepatocytes are the ideal target
cells of IL-10 gene transfer due to their quantitative dominance in
the liver and their expression of several specific molecules,
including the asialoglycoprotein receptor, which should facilitate
the targeting gene transfer.
To confirm this hypothesis, BRL cells, an
immortalized rat hepatocyte line, were transfected with the IL-10
gene by asialoglycoprotein receptor-mediated liposomes and
co-cultured with primary HSCs. We then investigated whether the
IL-10 expressed by the BRL cells affected the activation of HSCs
in vitro.
Materials and methods
Vector construction
Construction of the IL-10 eukaryotic expression
vector was performed as described previously (9). Briefly, the total RNA of peripheral
blood mononuclear cells from Sprague-Dawley rats was isolated using
a Purescript RNA Isolation kit (Gentra Systems, Inc., Minneapolis,
MN, USA) and cDNA was obtained by reverse transcription (RT;
Promega, Madison, WI, USA). The full-length coding region of IL-10
containing restriction sites was amplified by nested polymerase
chain reaction (PCR; Promega). In the first PCR step, 2 μl cDNA
product was used as the template to amplify specific fragments
without primer. Then, 2 μl product of the first PCR was used as the
template to amplify the full-length coding region of IL-10 with
nested primers, including the restriction sites of HindIII
and BamHI. PCR was performed with an initial denaturation at
94°C for 5 min, followed by 30 cycles at 94°C for 45 sec, annealing
at 61°C (nested primer at 58°C) for 45 sec and at 72°C for 60 sec,
with a final extension at 72°C for 7 min. The sequences of the
primers were as follows: outer primer: sense,
5′-cgcagccttgcagaaaacagagc-3′; antisense,
5′-gctctatttatgtcctgcagtccag-3′; nested primer: sense,
5′-cgaagcttgccaccatgcttggctcagcac-3′; and antisense, 5′-cgtcta
gatcaatttttcattttgagtg-3′. The PCR product was digested with
HindIII and BamHI (Promega), then cloned into the
eukaryotic expression vector pcDNA3.0 (Invitrogen, Carlsbad, CA,
USA) to establish the recombinant plasmid pcDNA3.0-IL-10, which was
verified by restriction endonuclease digestion and direct DNA
sequencing.
Cell transfection and RT-PCR
BRL cells, an immortalized normal rat hepatocyte
line obtained from cell bank of Academia Sinica, Shanghai, China,
were routinely seeded into 50-ml plastic flasks and cultivated in
DMEM supplemented with 10% fetal bovine serum (FBS) and 1%
antibiotics (penicillin/streptomycin) in a humidified incubator
containing 5% CO2 at 37°C. Cells in the logarithmic
growth phase were transfected with plasmid pcDNA3.0-IL-10 using
TransFast™ liposomes (Promega) and JetPEI™-Gal liposomes (Polyplus
Transfection, New York, NY, USA), separately. The transfection
system contained 3 mg DNA and 9.6 ml liposome JetPEI-Gal or 9 ml
liposome TransFast per flask. Transfection was performed according
to the manufacturer’s instructions and continued for 24 h. The
cells were then trypsinized and replated onto 6-well plates at
5×105 cells/well in 2 ml DMEM containing 10% FBS. The
total RNA was isolated from the cells of each group at 0, 1, 2, 4,
8, 12 and 16 days post-transfection and then reverse transcribed to
cDNA as a template. IL-10 and β-actin were amplified together by
PCR. The primer for IL-10 was the nested primer described above.
The sequences of the β-actin primers were sense,
5′-ccaaccgtgaaaagatgacc-3′ and antisense,
5′-caggaggagcaatgatcttg-3′. The PCR was carried out as follows:
pre-denaturation at 95°C for 5 min, 22 amplification cycles
(denaturation at 94°C for 45 sec, annealing at 57°C for 45 sec and
extension at 72°C for 1 min) and a final extension at 72°C for 7
min. The product of PCR was detected on 1.8% agarose gel.
ELISA
The BRL cells were seeded into plastic cell culture
flasks and cultured in DMEM containing 10% FBS. The cells in the
logarithmic growth phase were separately transfected with plasmids
pcDNA3.0-IL-10 and pcDNA3.0 using JetPEI-Gal liposomes and the
transfection was continued for 24 h. The supernatants of the two
groups (BRL/pcDNA3.0-IL-10, BRL/pcDNA3.0) were harvested and
refreshed every day until 16 days post-transfection. The IL-10 in
the supernatant 1, 2, 4, 8, 12, 16, 20 and 24 days
post-transfection was detected by ELISA (Biosource, Camarillo, CA,
USA) according to the manufacturer’s instructions.
MTT assay
BRL cells were seeded in 96-well microplates at
5×103 cells/well in 200 μl DMEM containing 10% FBS.
After 24 h incubation, the supernatant was refreshed. The BRL cells
were divided into three groups (groups C, P and I) randomly and
group P were transfected with plasmid pcDNA3.0 and group I with
plasmid pcDNA3.0-IL-10 using JetPEI-Gal liposomes. At 24 and 48 h
post-transfection, 20 μl
3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide
(MTT; 5 mg/ml) was added to each well, and the plates were
incubated for an additional 4 h. The supernatant was then
discarded, 150 μl dimethyl sulfoxide (DMSO) was added to each well
and the optical density at 490 nm was measured with a microplate
reader.
Flow cytometric analysis
BRL cells were planted into 6-well plastic plates,
then grouped and transfected as described above. At 48 h
post-transfection, the cells were harvested and incubated with
Annexin V-FITC and PI (Bender MedSystems, Vienna, Austria) and the
cell apoptosis rate was analyzed by flow cytometry.
Hepatic stellate cell isolation and
culture
HSCs were isolated from male Sprague-Dawley rats
weighing 450–500 g by means of sequential perfusion with
collagenase and pronase E, and subsequent Nycodenz gradient
centrifugation as previously described elsewhere (10). Desmin immunocytochemistry
demonstrated an isolated HSC purity of >95%. The HSCs were
seeded into 6-well plastic tissue culture plates at a density of
2×105 cells/well in DMEM containing 20% FBS and
incubated in a humidified incubator containing 5% CO2 at
37°C. The culture medium was replaced with DMEM containing 10% FBS
24 h after plating.
Co-culture of BRL cells and primary
HSCs
BRL cells seeded at equal density in plastic cell
culture flasks were divided into three groups and separately
transfected with plasmid pcDNA3.0-IL-10, pcDNA3.0 and normal saline
as described previously. Post-transfection, the cells were digested
and seeded onto 30-mm filter inserts (Millipore, Billerica, MA,
USA) placed in 6-well culture plates at 2×105
cells/insert in 3 ml DMEM containing 10% FBS. After 24 h, the
inserts planted with BRL were placed into the 6-well plastic tissue
culture plates which had been planted with primary HSCs for 3 days
and the culture medium was refreshed. The co-culture cells were
divided into 4 groups: group H, HSCs; group C, HSCs/BRL transfected
with normal saline; group P, HSCs/BRL transfected with pcDNA3.0;
and group I, HSCs/BRL transfected with pcDNA3.0-IL-10. The
co-culturing was terminated after 48 h.
Western blotting
After the indicated number of days, the inserts with
BRL cells and supernatant were discarded. The HSCs were washed with
PBS twice and lysed with cell lytic buffer containing 50 mM Tris pH
8.0, 150 mM NaCl, 0.2 mg/ml NaN3, 1 mg/ml SDS, 0.1 mg/ml
aprotinin, 10 mg/ml NP-40, 5 mg/ml sodium deoxycholate and 0.1
mg/ml phenylmethylsulfonyl fluoride, and the supernatants were
obtained after centrifuging at 1,500 × g for 10 min. The protein
concentrations of the cytosolic extracts were determined by
Bradford protein assay. Equal amounts of protein were separated on
a 12% SDS-polyacrylamide gel and transferred onto nitrocellulose
membranes. Monoclonal antibodies of procollagen type I, α-SMA and
β-tubulin (Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA)
were used at dilution of 1:200, followed by incubation with
homologous secondary antibody labeled with HRP. The signals were
visualized using an ECL kit.
Terminal dUTP nick-end labeling (TUNEL)
assay for apoptosis of HSCs
After the indicated number of days, the inserts with
BRL cells and supernatant were discarded. The HSCs were washed with
PBS twice and fixed for 1 h at 37°C. A TUNEL assay was performed
following the manufacturer’s instructions (Beijing Zhongshan Co.,
Beijing, China). The cells were then analyzed under a microscope.
The apoptotic cells which had brown nuclei were counted among 3,000
cells/well.
MTT assay of HSCs
BRL cells seeded at equal density in plastic cell
culture flasks were divided into three groups (groups I, P and C)
and separately transfected with plasmid pcDNA3.0-IL-10, pcDNA3.0
and normal saline as described earlier. Post-transfection, the
cells were digested and seeded onto 6-well plastic cell culture
plates at 2×105 cells/well in 3 ml DMEM containing 10%
FBS. To the other three wells were added 3 ml DMEM containing 10%
FBS without BRL cells as group H. After 48 h, the supernatant of
each group was harvested.
The primary HSCs were isolated and planted into
96-well plastic cell culture plates at a density of
5×103 per well and incubated for 72 h. The supernatant
was refreshed with 100 μl DMEM containing 10% FBS. The HSCs were
divided into four groups (groups H, C, P and I) randomly, and the
harvested supernatants were added (100 μl per well). After 48 h,
the MTT assay was performed as previously described.
Statistical analysis
The data from the TUNEL assay were assessed using
the χ2 test. All other data are expressed as the mean ±
SE and were from at least three independent experiments assessed by
the method of analysis of variance (ANOVA). Data from three or more
groups were assessed using the Kruskall-Wallis test. When
significant, post hoc multiple comparisons were archived using the
Student-Newman-Keuls method. The analyses were performed using
SigmaStat software (SPSS, Chicago, IL, USA). P<0.05 was
considered to indicate a statistically significant result.
Results
Construction of IL-10 expression
vector
The IL-10 gene (559 bp) was prepared by nested PCR
and successfully cloned into plasmid pcDNA3.0. It was confirmed by
restriction endonuclease digestion and DNA sequencing.
Transfection efficiency
To select a vector for more efficient gene delivery
to hepatocytes, a frequently used liposome, TransFast, and an
asialoglycoprotein receptor-mediated liposome, JetPEI-Gal, were
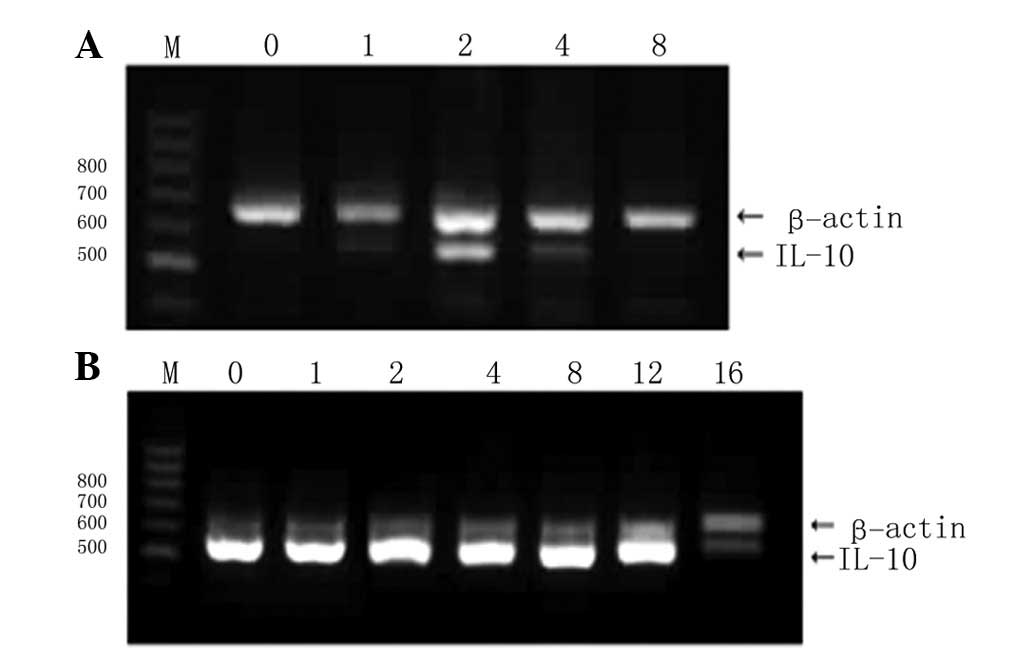
employed. As shown in Fig. 1A, the
TransFast liposomes displayed a lower transfection efficiency,
characterized by a low peak of IL-10 mRNA expression in the BRL
cells, followed by a rapid drop and disappearance at 8 days
post-transfection. The transfection efficiency of JetPEI-Gal
liposomes for hepatocytes was significantly higher. The expression
levels of IL-10 mRNA in the BRL cells transfected using the
JetPEI-Gal liposomes were much higher than those of β-actin. The
levels peaked quickly following transfection, were stable until 12
days post-transfection and then dropped at 16 days
post-transfection (Fig. 1B).
Expression of IL-10 protein by BRL
cells
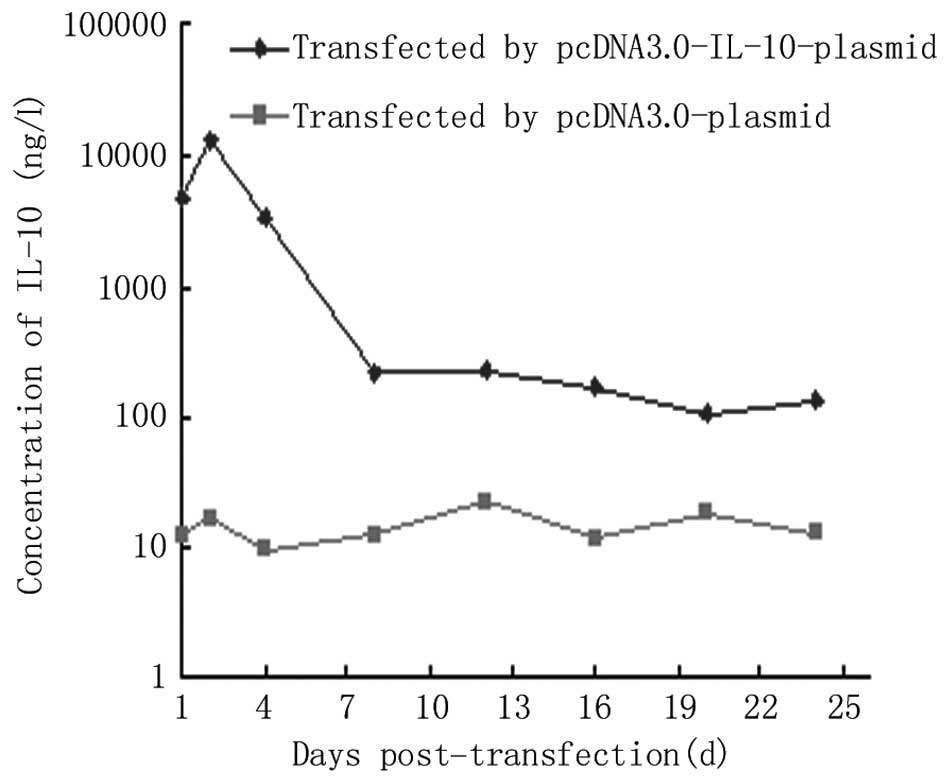
It would clearly be valuable if high and enduring
expression levels of IL-10 protein were able to be induced. While
almost no IL-10 was secreted by the BRL cells transfected with the
pcDNA3.0 plasmid, there was a high level of secretory IL-10 in the
supernatant of the BRL cells transfected with the pcDNA3.0-IL-10
plasmid which reached a peak of 12.78 μg/l at 2 days
post-transfection, then dropped rapidly and remained at a low level
from 8 days post-transfection for at least 16 days (Fig. 2). A divergence phenomenon between
the mRNA and protein expression levels of IL-10 in the late phase
was observed, implying the presence of translational suppression.
The results presented so far have been reported previously
(9).
Proliferation and apoptosis of BRL
cells
Having demonstrated the high transfection efficiency
of JetPEI-Gal liposomes to hepatocytes, it was important to
ascertain the effects of plasmid transfection and IL-10 gene
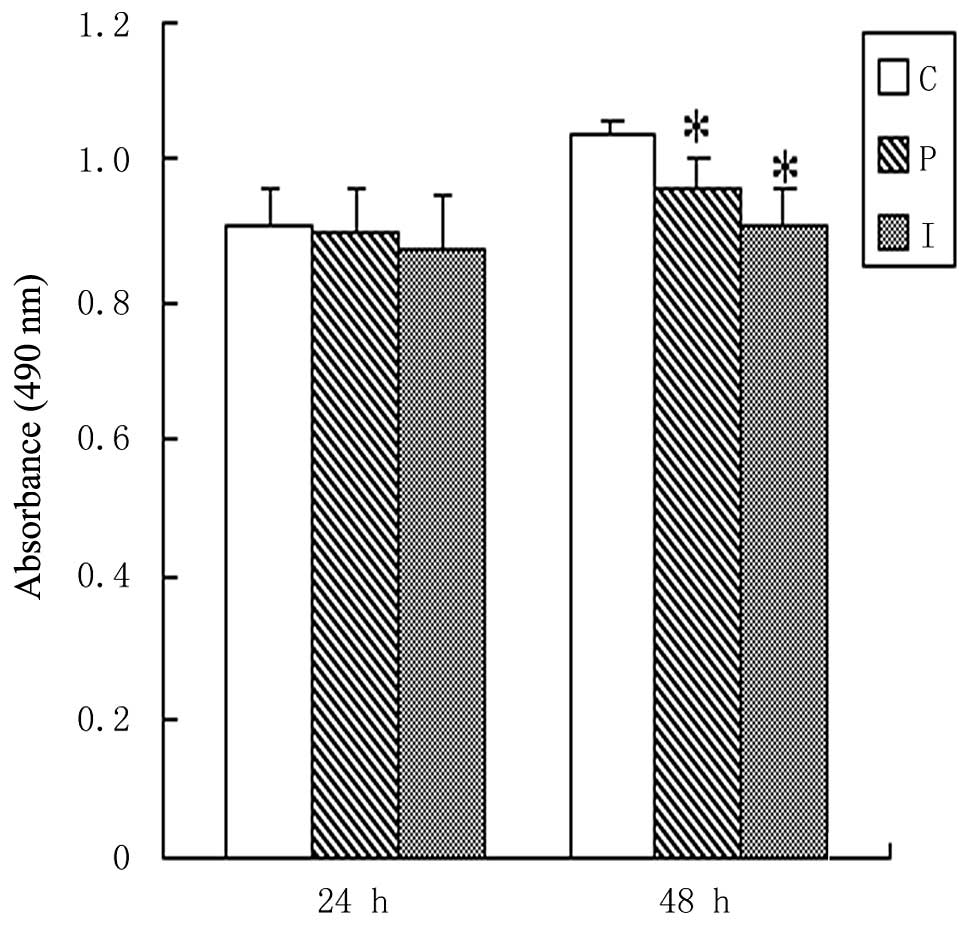
expression on the proliferation and apoptosis of BRL cells. No
significant deviations among the viable counts of the control and
transfected cells were observed during the first 24 h
post-transfection (P>0.05). At 48 h post-transfection, the
viable count of the BRL cells transfected with the pcDNA3.0 plasmid
was visibly but slightly decreased compared with that of the
control BRL cells (P<0.01), but there was no significant
deviation between the BRL cells transfected with the pcDNA3.0
plasmid and those transfected with the pcDNA3.0-IL-10 plasmid
(P>0.05; Fig. 3). The apoptosis
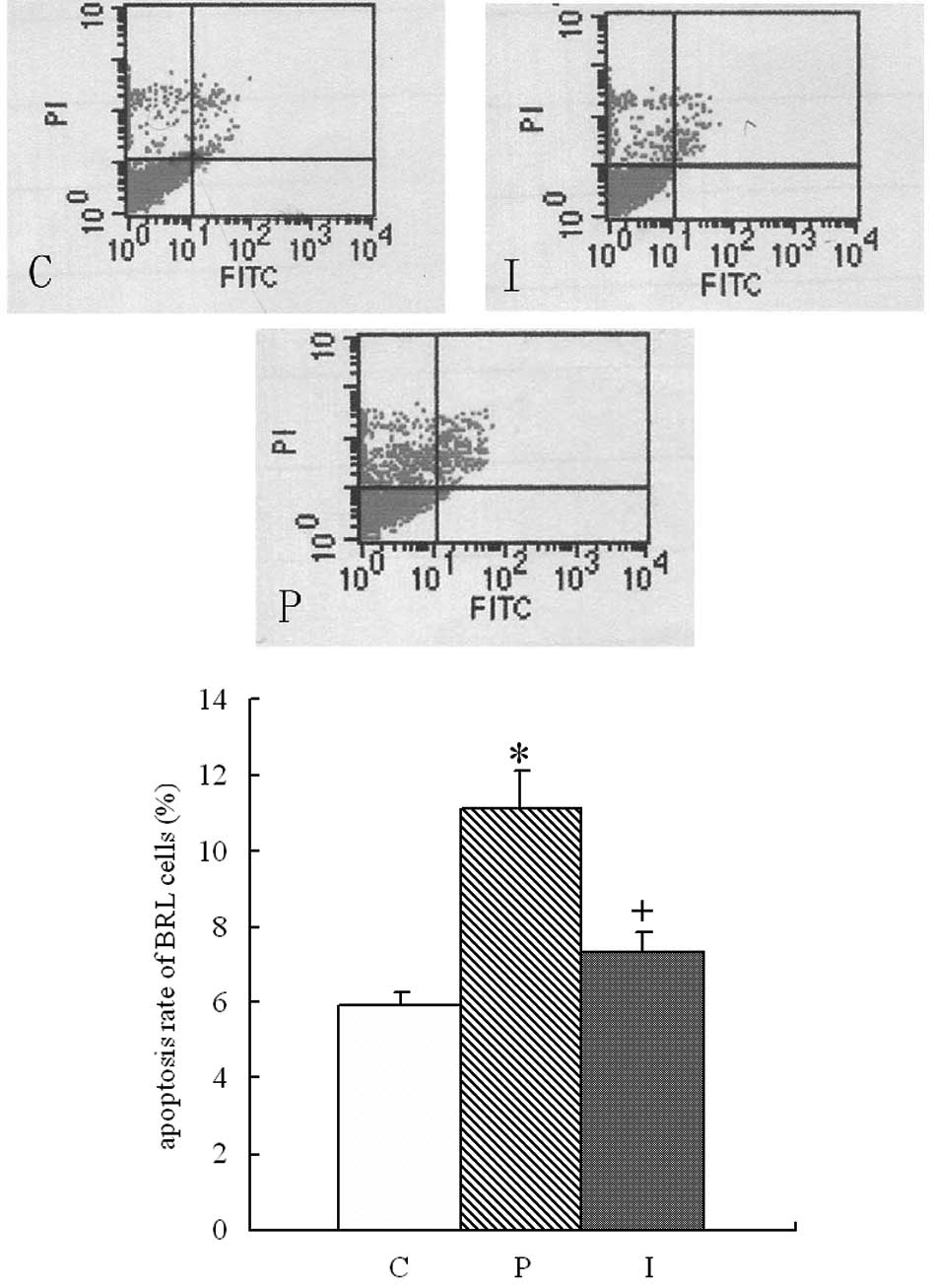
of each group was detected by flow cytometry. As shown in Fig. 4, the BRL cells transfected with the
pcDNA3.0 plasmid had a significantly higher apoptosis rate
(11.13±0.97%) than the control BRL cells (5.91±0.37%; P<0.01).
This increased apoptosis was markedly attenuated in the BRL cells
transfected with pcDNA3.0-IL-10 plasmid, which had an apoptosis
rate of 7.36±0.51% (P<0.01).
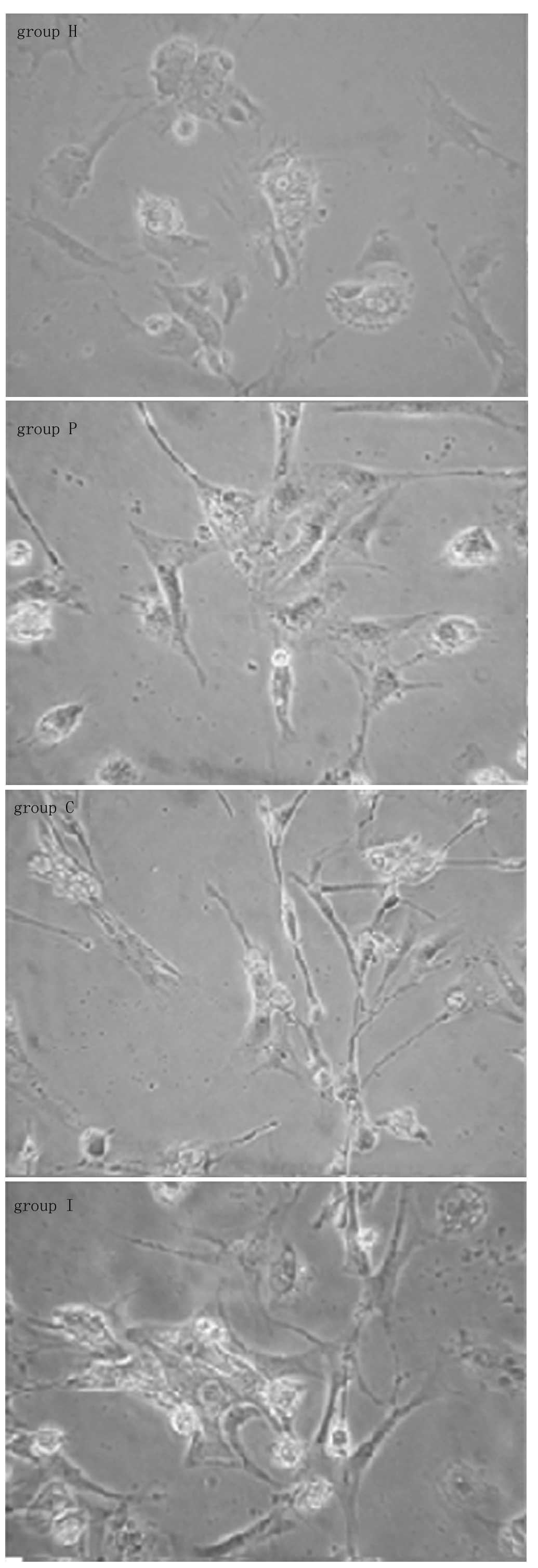
Phenotype diversity of HSCs
Following isolation and primary culturing for 5
days, the HSCs of group H fully stretched to a stellate shape. The
HSCs cocultured with BRL cells (groups C, P and I) clearly
contracted into fusiform or dendritic shapes, as shown in Fig. 5.
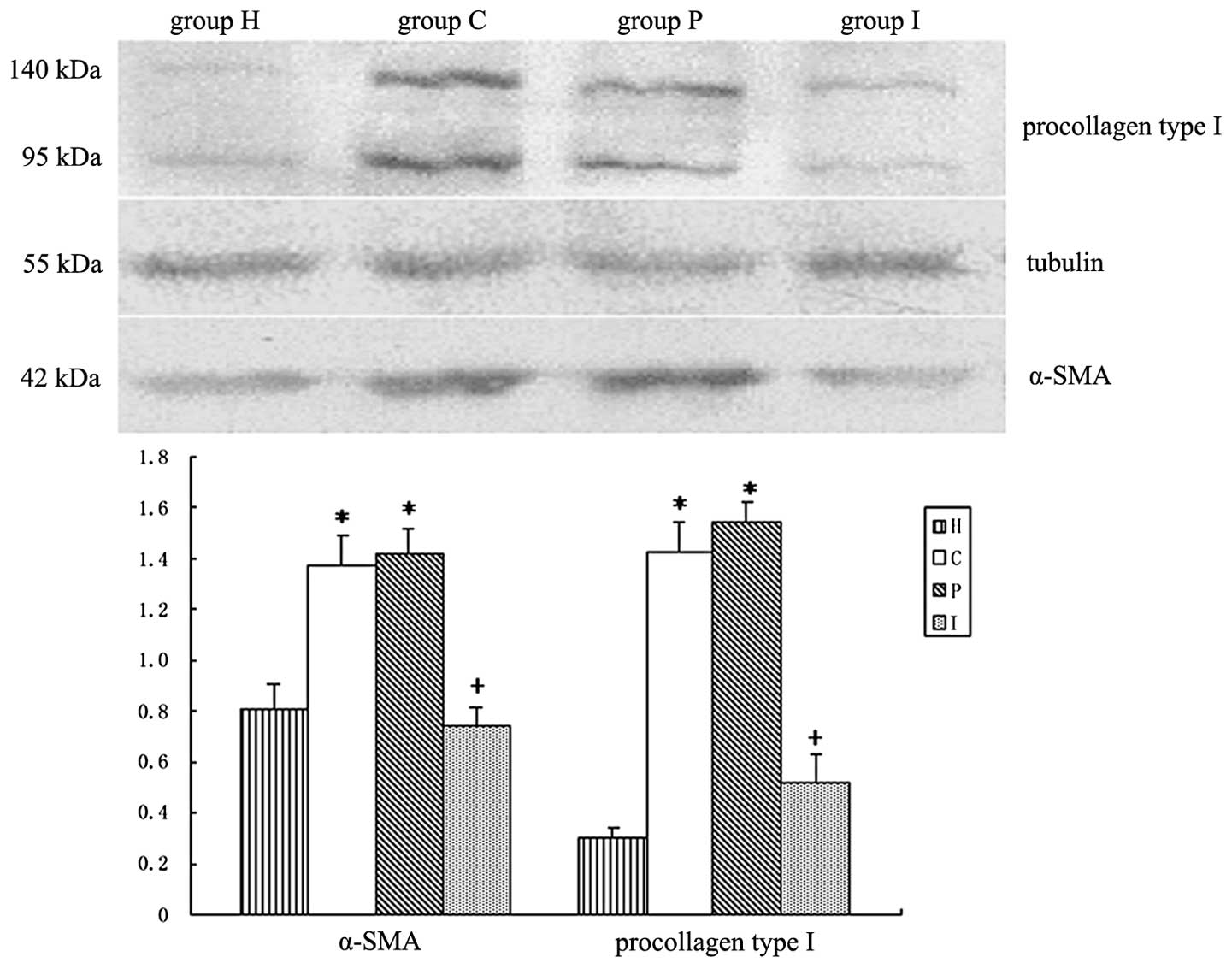
Expression of α-SMA and procollagen type
I in HSCs
HSC activation is accompanied by increased
expression of α-SMA and the production of collagen types I and III.
Setting β-tubulin as an internal reference, the expression levels
of procollagen type I and α-SMA by HSCs were detected by western
blot analysis. As shown in Fig. 6,
BRL cells significantly stimulated the expression of procollagen
type I and α-SMA by HSCs. In the BRL cells transfected with the
IL-10 gene, this stimulation was suppressed (Table I).
 | Table IRelative gray scale of α-SMA and
procollagen type I for HSCs (mean ± SD, n=4). |
Table I
Relative gray scale of α-SMA and
procollagen type I for HSCs (mean ± SD, n=4).
| Group | α-SMA/tubulin | Procollagen type
I/tubulin |
|---|
| Group H | 0.812±0.095 | 0.298±0.047 |
| Group C | 1.376±0.108a | 1.428±0.110a |
| Group P | 1.416±0.097a | 1.538±0.083a |
| Group I | 0.741±0.068b | 0.515±0.114b |
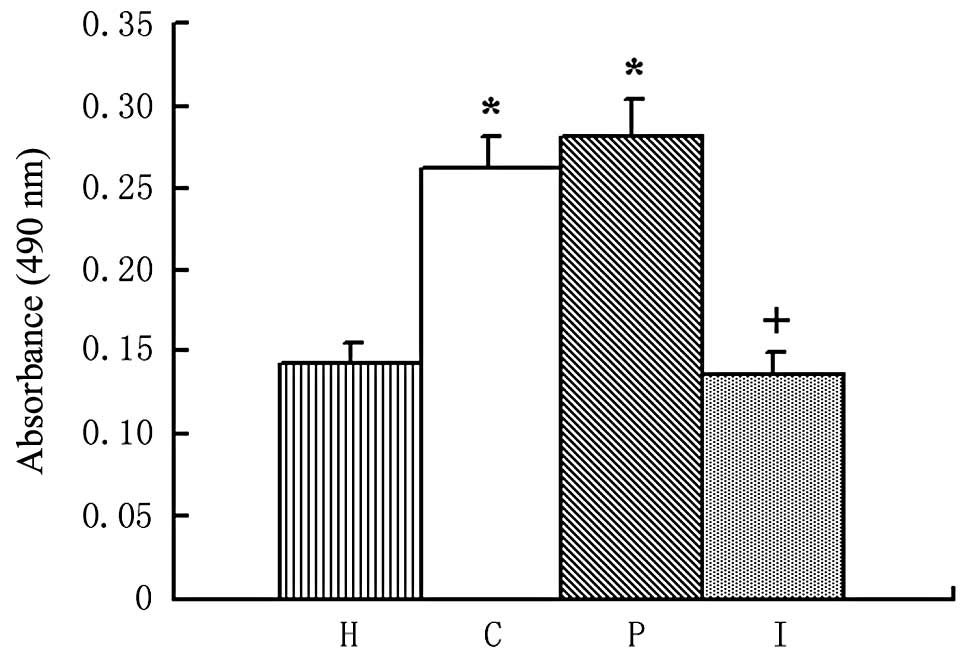
Proliferation of HSCs
Increased proliferation is an important feature of
HSC activation. In MTT assays, HSCs displayed a marked increase in
proliferation when cultured in the supernatant of BRL cells
(P<0.01). By contrast, when cultured in the supernatant of BRL
cells transfected with the IL-10 gene, the increased proliferation
of the HSCs was significantly attenuated (P<0.01), as shown in
Fig. 7 and Table II.
 | Table IIAbsorbance values of HSCs (mean ± SD,
n=8). |
Table II
Absorbance values of HSCs (mean ± SD,
n=8).
| Group | Absorbance |
|---|
| Group H | 0.143±0.013 |
| Group C | 0.261±0.021a |
| Group P | 0.282±0.020a |
| Group I | 0.135±0.014b |
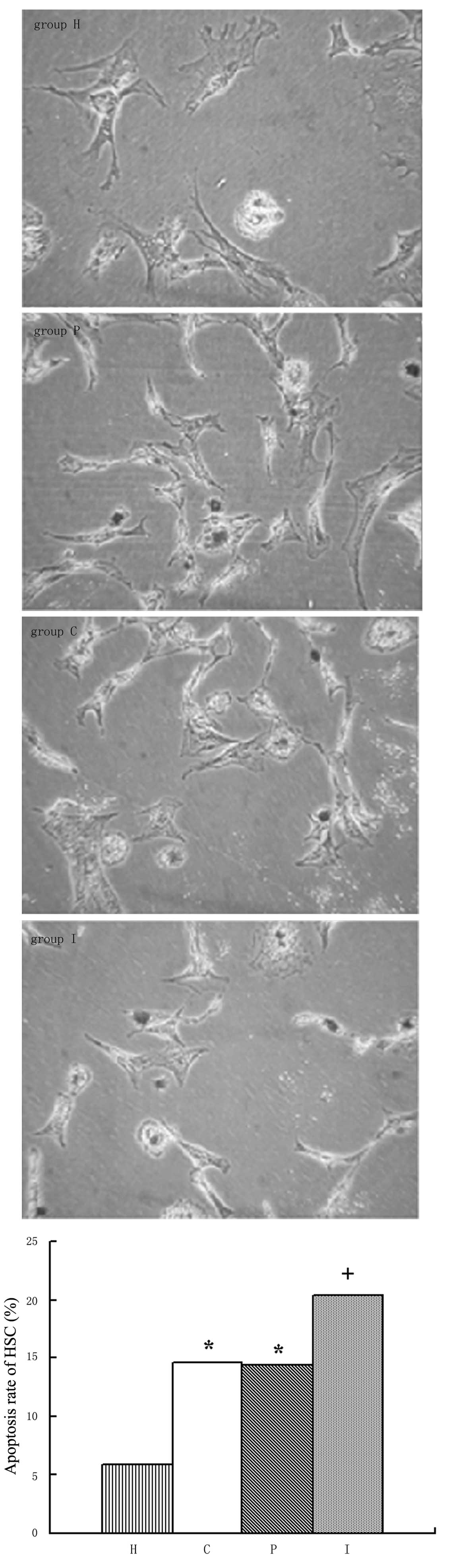
Apoptosis of HSCs
The apoptosis of activated HSCs may be involved in
the reversion of liver fibrosis. We aimed to establish whether BRL
cells with or without IL-10 gene modification affected the
apoptosis of HSCs. Following isolation and primary culturing for
several days, the HSCs displayed such strong adhesion that it was
difficult to digest them from the culture plates and separate them
from each other. Therefore, TUNEL assays but not flow cytometry,
were employed to detect the apoptosis rate of the HSCs. As shown in
Fig. 8, there were few apoptotic
HSCs in group H; the apoptosis rate was 5.9%. However, the
apoptosis rate was clearly increased in the HSCs cocultured with
BRL cells (14.65% in group C and 14.45% in group P), which is in
parallel with the increased proliferation described earlier. It is
a notable finding that further increased apoptosis (20.35%) was
detected in the HSCs co-cultured with the BRL cells transfected
with the IL-10 gene (P<0.01; Table III).
 | Table IIIApoptosis rates of HSCs. |
Table III
Apoptosis rates of HSCs.
| Group | Positive cell
population | Apoptosis rate
(%) |
|---|
| Group H | 118 | 5.9 |
| Group C | 293 | 14.65a |
| Group P | 289 | 14.45a |
| Group I | 407 | 20.35ab |
Discussion
Considerable evidence suggests that IL-10 is a
promising antifibrotic agent. Nevertheless, the unfavorable
pharmacokinetic profile of IL-10 may limit the clinical application
of this cytokine. In healthy human volunteers, intravenous
administration of recombinant human IL-10 resulted in a rapid
disappearance from the circulation (11). Another study in rats with liver
fibrosis induced by bile duct ligation revealed that renal
clearance contributed to an even shorter half-life of IL-10 and an
increased hepatic uptake (2-fold), possibly mediated by markedly
elevated IL-10 receptor expression in the fibrotic livers (12). Moreover, the distribution in
non-target tissues and its multiple effects in normal physiological
processes also strongly hamper the clinical application of
IL-10.
It is conceivable that liver-targeting IL-10 gene
amplification may maintain a more enduring drug concentration in
hepatic tissue with minimal side-effects in non-target tissues. The
selection of suitable target cells and gene delivery system is
essential. HSCs appear to be the optimal target cells due to their
key role in liver fibrosis. The successful modification of IL-10
with mannose 6-phosphate (M6P), which targets the mannose
6-phosphate/insulin-like growth factor II (M6P/IGFII) receptor
expressed on activated HSCs, has been reported (7). However, in addition to inhibiting the
activation of HSCs by direct interaction with the IL-10 receptor
(13), IL-10 has anti-fibrotic
activity, mediated mainly by the inhibition of the production of
superoxide and TNF by Kupffer cells, reduction of the production of
proinflammatory cytokines (TNF-α, IL-12, and IFN-γ), reduction of
neutrophil infiltration and attenuation of the increase of
CD8+ T cells in the liver (3,14,15).
Therefore, a liver-targeting IL-10 gene delivery system may be more
efficient than a HSC-targeting one. We hypothesize that hepatocytes
would be the ideal target cells of IL-10 gene transfer for the
following reasons: i) hepatocytes constitute the majority cells in
the liver, so a high expression level of the target gene may be
anticipated; ii) hepatocytes are adjacent to all other hepatic
cells, including HSCs, and may have a paracrine action; iii)
several hepatocyte-specific expression molecules, including the
asialoglycoprotein receptor, have been well studied. Delivery
systems focused on these molecules are considerably mature and even
commercialized, and so may be utilized directly in experimental and
clinical treatment (16,17).
In certain IL-10 gene-targeting studies, viral
delivery, especially using an adenoviral vector, has displayed a
high transfection efficiency followed by significant attenuation of
the fibrotic process or acute rejection following organ
transplantation (18,19). However, apart from the biological
safety issues, adenoviral vectors may provoke an intensive immune
response leading to a marked reduction of transfection efficiency
upon further administration, which is inappropriate when treating a
chronic disease such as liver fibrosis. Common liposomes have high
safety but low transfection efficiency. The liposome JetPEI-Gal, a
liposome linked to galactose which is able to specifically bind to
the asialoglycoprotein receptors on liver cell membranes, should
significantly increase the targeting and transfection efficiency
for hepatocytes. In the present study, an eukaryotic expression
vector for the rat IL-10 gene was successfully constructed and
transfected into BRL cells. When delivered by the
asialoglycoprotein receptor-mediated JetPEI-Gal liposomes, high
expression levels of IL-10 were detected in BRL cells and their
supernatant. It was confirmed that JetPEI-Gal liposomes had
markedly higher transfection efficiency for hepatocytes than common
liposomes. Furthermore, JetPEI-Gal liposomes are able to prolong
the expression of IL-10 by hepatocytes. There was a divergence
phenomenon between the mRNA and protein expression levels of IL-10
from 4 days post gene transfer. This supression of translation was
possibly due to the super-high cell density at the late phase of
cell culture. Whether these divergence phenomena appear in
vivo requires further study.
BRL cells transfected with the pcDNA3.0 plasmid
present a decelerating proliferation and an accelerating apoptosis.
This may be due to the damage induced by a high quantity of
plasmids entering the cells and the following heavy plasmid load.
In the present study, we identified that the apoptosis of BRL cells
induced by plasmid transfection was attenuated by IL-10 gene
expression, which supports the efficacy of hepatocyte-targeting
IL-10 gene therapy.
It has been reported that heptocytes are able to
accelerate the proliferation of HSCs (20,21).
Gressner et al found that when cultured in the serum-reduced
supernatant of hepatocytes, HSCs appeared to undergo increased
proliferation, but no diversion of phenotypes (22). In the present study, it was shown
that hepatocytes not only accelerated the proliferation of HSCs but
also promoted the activation of HSCs, which was characterized by
enhanced contraction and increased expression of procollagen type I
and α-SMA. Saile et al reported that apoptosis was rare in
quiescent HSCs, but markedly increased in activated HSCs, which
suggests that apoptosis is an important mechanism in terminating
the proliferation of activated HSCs (23). Our findings demonstrate that
apoptosis becomes detectable in parallel with the HSC activation
induced by BRL cells.
It has been verified that endogenous and exogenous
IL-10 are able to inhibit liver fibrosis (3–6). In
the present study, BRL cells transfected with the IL-10 gene
displayed the ability to attenuate the proliferation of HSCs and
their expression of procollagen type I and α-SMA. As liver injury
resolves, apoptosis of activated HSCs may be involved in the
reversion of liver fibrosis (24).
When HSCs were cocultured with IL-10 gene-modified BRL cells, a
further acceleration of HSC apoptosis was identified in our study.
This suggested that IL-10 plays its anti-fibrotic role not only by
inhibiting the proliferation and activation of HSCs but also by
enhancing the apoptosis of HSCs. These results are in agreement
with findings from our previous studies using recombinant rat IL-10
(25,26). They also demonstrate that IL-10
from hepatocytes with IL-10 gene amplification have an effect
equivalent to that of exogenous IL-10 on HSCs.
Acknowledgements
This study was supported by Natural Science
Foundation of Fujian Province, No. 2009J05065.
References
|
1
|
Friedman SL: Cytokines and fibrogenesis.
Semin Liver Dis. 19:129–140. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rockey DC: Current and future
anti-fibrotic therapies for chronic liver disease. Clin Liver Dis.
12:939–962. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Thompson K, Maltby J, Fallowfield J,
McAulay M, Millward-Sadler H and Sheron N: Interleukin-10
expression and function in experimental murine liver inflammation
and fibrosis. Hepatology. 28:1597–1606. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Louis H, Van Laethem JL, Wu W, et al:
Interleukin-10 controls neutrophilic infiltration, hepatocyte
proliferation, and liver fibrosis induced by carbon tetrachloride
in mice. Hepatology. 28:1607–1615. 1998. View Article : Google Scholar
|
|
5
|
Nelson DR, Lauwers GY, Lau JY and Davis
GL: Interleukin-10 treatment reduces fibrosis in patients with
chronic hepatitis C: a pilot trail of interferon nonresponders.
Gastroenterology. 118:655–660. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang XZ, Zhang LJ, Li D, Huang YH, Chen ZX
and Li B: Effects of transmitters and interleukin-10 on rat hepatic
fibrosis induced by CCl4. World J Gastroenterol.
9:539–543. 2003.PubMed/NCBI
|
|
7
|
Rachmawati H, Reker-Smit C, Lub-de Hooge
MN, van Loenen-Weemaes A, Poelstra K and Beljaars L: Chemical
modification of interleukin-10 with mannose 6-phosphate groups
yields a liver-selective cytokine. Drug Metab Dispos. 35:814–821.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hung KS, Lee TH, Chou WY, et al:
Interleukin-10 gene therapy reverses thioacetamide-induced liver
fibrosis in mice. Biochem Biophys Res Commun. 336:324–331. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chen YX, Huang YH, Chen ZX, Zheng WD and
Wang XZ: Construction of eukaryotic expression plasmid containing
rat interleukin-10 gene and its expression in BRL cells in
vitro. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 24:332–334.
2008.(In Chinese).
|
|
10
|
Ramm GA: Isolation and culture of rat
hepatic stellate cells. J Gastroenterol Hepatol. 13:846–851. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Huhn RD, Radwanski E, O’Connell SM, et al:
Pharmacokinetics and immunomodulatory properties of intravenously
administered recombinant human interleukin-10 in healthy
volunteers. Blood. 87:699–705. 1996.PubMed/NCBI
|
|
12
|
Rachmawati H, Beljaars L, Reker-Smit C, et
al: Pharmacokinetic and biodistribution profile of recombinant
human interleukin-10 following intravenous administration in rats
with extensive liver fibrosis. Pharm Res. 21:2072–2078. 2004.
View Article : Google Scholar
|
|
13
|
Mathurin P, Xiong S, Kharbanda KK, et al:
IL-10 receptor and coreceptor expression in quiescent and activated
hepatic stellate cells. Am J Physiol Gastrointest Liver Physiol.
282:G981–G990. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Safadi R, Ohta M, Alvarez CE, et al:
Immune stimulation of hepatic fibrogenesis by CD8 cells and
attenuation by transgenic interleukin-10 from hepatocytes.
Gastroenterology. 127:997–1000. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Louis H, Le Moine O, Peny MO, et al:
Production and role of interleukin-10 in concanavalin A-induced
hepatitis in mice. Hepatology. 25:1382–1389. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Di Stefano G, Derenzini M, Kratz F, Lanza
M and Fiume L: Liver-targeted doxorubicin: effects on rat
regenerating hepatocytes. Liver Int. 24:246–252. 2004.PubMed/NCBI
|
|
17
|
Wang S, Cheng L, Yu F, Pan W and Zhang J:
Delivery of different length poly(L-lysine)-conjugated ODN to HepG2
cells using N-stearyl lactobionamide-modified liposomes and their
enhanced cellular biological effects. Int J Pharm. 311:82–88. 2006.
View Article : Google Scholar
|
|
18
|
Lan L, Chen Y, Sun C, Sun Q, Hu J and Li
D: Transplantation of bone marrow-derived hepatocyte stem cells
transduced with adenovirus-mediated IL-10 gene reverses liver
fibrosis in rats. Transpl Int. 21:581–592. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tashiro H, Shinozaki K, Yahata H, et al:
Prolongation of liver allograft survival after interleukin-10 gene
transduction 24–48 hours before donation. Transplantation.
70:336–339. 2000.
|
|
20
|
Gressner AM, Lotfi S, Gressner G and Lahme
B: Identification and partial characterization of a
hepatocyte-derived factor promoting proliferation of cultured
fat-storing cells (parasinusoidal lipocytes). Hepatology.
16:1250–1266. 1992. View Article : Google Scholar
|
|
21
|
Faouzi S, Lepreux S, Bedin C, et al:
Activation of cultured rat hepatic stellate cells by tumoral
hepatocytes. Lab Invest. 79:485–493. 1999.PubMed/NCBI
|
|
22
|
Gressner AM, Lotfi S, Gressner G, Haltner
E and Kropf J: Synergism between hepatocytes and Kupffer cells in
the activation of fat storing cells (perisinusoidal lipocytes). J
Hepatol. 19:117–132. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Saile B, Knittel T, Matthes N, Schott P
and Ramadori G: CD95/CD95L-mediated apoptosis of the hepatic
stellate cell. A mechanism terminating uncontrolled hepatic
stellate cell proliferation during hepatic tissue repair. Am J
Pathol. 151:1265–1272. 1997.
|
|
24
|
Issa R, Williams E, Trim N, et al:
Apoptosis of hepatic stellate cells: involvement in resolution of
biliary fibrosis and regulation by soluble growth factors. Gut.
48:548–557. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang LJ, Zheng WD, Shi MN and Wang XZ:
Effects of interleukin-10 on activation and apoptosis of hepatic
stellate cells in fibrotic rat liver. World J Gastroenterol.
12:1918–1923. 2006.PubMed/NCBI
|
|
26
|
Chen YX, Wang XZ, Weng SG, Chen ZX, Huang
YH and Zhang LJ: Effects of interleukin-10 on the proliferation and
Fas/Fas ligand expression of hepatic stellate cells. Zhonghua Gan
Zang Bing Za Zhi. 11:637–640. 2003.(In Chinese).
|