Introduction
Lung cancer is the leading cause of cancer-related
deaths, and 80% of lung cancer cases are non-small cell lung cancer
(NSCLC). Distant hematogenous metastasis is one of the main reasons
for poor prognosis of lung cancer patients (1,2). In
the blood circulation, lung cancer cells interact with a variety of
cellular components and cytokines, which affect the hematogenous
metastasis of cancer cells. In fact, platelet (PLT)activation may
play an important role in this event (3).
In the present study we first investigated the blood
PLT count in peripheral blood and the expression of platelet
P-selectin in patients with NSCLC and then compared these values
with a healthy control population. Purified PLTs were then
co-cultured with lung adenocarcinoma cell line A549 and human
vascular endothelial cells (HuvECs). A flow chamber simulation
assay was subsequently performed to investigate the formation of
PLT-lung cancer cell complexes and the effects on rolling and
adhesion of A549 calls on the surface of vascular endothelium.
Finally, the possible mechanism was further studied. Based on this,
we expected to elucidate the correlation between PLT activation and
hematogenous metastasis of lung cancer.
Materials and methods
Materials
Clinical data
One hundred and sixty-eight cases of primary lung
cancer excluding patients with hematological systemic diseases,
severe malnutrition, infection, cardiovascular diseases and
diabetes were enrolled in the present study after preliminary
diagnosis at the Department of Respiration of The Southwest
Hospital from June 2008 to August 2009 (Chongqing, China). The mean
patient age was 53±19 years. All of the cases were confirmed by
pathological examination, including 53 cases of squamous cell
carcinoma, 44 cases of NSCLC and 7 cases of other pathological
types such as large-cell carcinoma and carcinoid tumors.
TNM staging for lung cancer was performed in
accordance with the standards of AJCC and UICC. All of the patients
did not receive any drugs which might affect the function of the
platelets 2 week prior to the blood sample collection. Thirty
healthy volunteers including 19 females and 11 males were selected
as the control and their mean age was 44±9 years. In the present
study, all of the subjects read and signed informed consent forms.
This study was approved by the Ethics Committee of The Southwest
Hospital.
Cell line culture
A549 cells and HuvECs were purchased from the
Shanghai Cell Repository (Shanghai, China). A549 cells were
routinely cultured in Dulbecco’s modified Eagle’s medium (DMEM)
(Hyclone, USA) containing 10% fetal calf serum (Gibco, USA). HuvECs
were cultured in M200 medium (Hyclone) containing 20% fetal calf
serum and 2 mmol/l L-glutaminate (Sigma, USA). The cells were
cultured in an atmosphere of 5% CO2 at 37˚C and passaged
every 2–3 days.
Main reagents
Mouse anti-human integrin α3, integrin α5, integrin
β1, ICAM-1 and VCAM-1 antibodies were provided by Santa Cruz
Biotechnology, Ltd. (Santa Cruz, CA, USA). RNA Plus isolation and
RT-PCR kits were products of Takara Bio., Ltd. (Osaka, Japan).
Lipofectamine™ 2000 was obtained from Invitrogen Co. (Shanghai,
China). pSilencer 2.0-U6 plasmid carrying GFP was purchased from
the GenScript Corp., as well as the RNAi-negative control, the
pSilencer-PSGL1-NON RNAi plasmid.
Expression of P-selection in lung
cancer tissues of different pathological types
Laser scanning confocal microscopy (LSCM)
manipulation was performed as follows. i) Lung cancer tissue,
paracancerous tissue and distant tissue were removed and washed
with PBS three times and then cut into a size of ~0.3×0.5 cm. The
samples were consecutively cut into 5-μm sections. Sections
were fixed with cold acetone for 10 min and washed with 0.1 M PBS
three times each for 4 min. ii) Respectively, mouse anti-human
integrin α3, integrin α5, integrin β1, ICAM-1 or VCAM-1 antibody
(1:200) was added to the sections overnight at 4˚C in a humidity
chamber. iii) The sections were washed with 0.1 M PBS three times
each for 4 min. iv) Fluorescence-labeled rabbit anti-mouse IgG
(1:3000) was added and co-cultured for 30 min at 37˚C and then
washed with 0.1 M PBS three times each for 4 min. v)
4,6-Diamidino-2-phenylindole (DAPI) was used to stain the nuclei
for 10–20 min at 37˚C and then rinsed with 0.1 M PBS for three
times each for 4 min. vi) The sections were mounted with 70%
glycerine (v/v) followed by LSCM assay.
PLT isolation
PLT isolation was performed as follows. i) Blood
samples were collected from the subjects via vein in the morning.
Anticoagulated whole blood (7 ml) was collected and centrifuged at
2500 rpm for 12 min at room temperature. ii) The supernatants were
harvest and then centrifuged at 3200 rpm for 30 min at 4˚C. The
supernatants were extracted and PLTs were collected. iii) The
collected PLTs were washed with 0.01 M PBS and then centrifuged at
3200 rpm for 30 min at 4˚C.
Blood PLT count (BPC) assay
Based on the BPC in the peripheral blood prior to
chemotherapy, radiotherapy or surgery, BPC was classified into
decreased (<100×109/l), normal
(100–300×109/l) and increased (>300×109/l)
states. Meanwhile, the correlations between the change in
peripheral PLTs and tumor pathological type and metastasis stage in
the groups were compared and analyzed.
FACS assay of the expression of
P-selectin in peripheral blood
After isolation and purification, the PLTs were
re-suspended in 50 μl of PBS (0.1 M) followed by the
addition of 5 μl of P-selectin antibody (1:50). Then the
PLTs were incubated at 37˚C for 1 h. The PLTs were washed with PBS
(0.1 M) and centrifuged at 3000 rpm for 3.5 min. The process was
repeated. Subsequently, 5 μl of FITC-labeled secondary
antibody (1:50) was added and incubation was carried out at 37˚C
for 1 h. The PLTs were washed with PBS (0.1 M) twice and measured
using a Cell Lab Quanta SC Coulter flow cytometer(Beckman Coulter,
USA).
Small interference RNA (siRNA)
targeting PSGL1 and transfection
According to the PSGL-1 gene sequence in GenBank,
the specific siRNA sequence for PSGL-1 (GI: 68160948) available at
http://www.ncbi.nlm.nih.gov/nuccore/NM_003006.3 was
designed by using an siRNA design tool ‘siRNA sequence selector’.
Then three sequences including 5′-CCACGGATTCA GCAGCTAT-3′,
5′-GGAGATACAGACCACTCAA-3′, 5′-GGA AGCACAGACCACTCAA-3′ were
selected. After the primary experiment, the sequence
5′-GGAGATACAGACCACT CAA-3′ with best blockage was used for the
following transfection.
The construction of the pSilencer-PSGL1-shRNA vector
was completed by Shanghai GeneChem Co., Ltd. and then the
manipulation was performed as follows. i) The oligonucleotide
fragments were annealed to form double-strand structure. ii)
pSilencer-PSGL1-shRNA was digested with restriction enzymes to
retrieve the line empty vector. iii) The target fragments were
connected with the linearized empty vectors. iv) The plasmid was
transfected into fresh E.coli DH5α and screened on an LB
plate containing ampicillin (100 μg/ml) and the positive
colonies were picked for sequence identification (Takara Corp.,
Dalian, China).
A day before transfection, 0.5–2×105
tumor cells were inoculated into 6-well plates. The
pSilencer-PSGL1-shRNA plasmids were transfected into the A549 cells
according to the methods described in the instructions for
Lipofectamine™ 2000. The culture plates were incubated at 37˚C in
an incubator with 5% CO2. After a 48-h transfection, the
cells were collected to perform the subsequent FACS operations. The
cells after a 48-h transfection were digested and removed from the
plates and were washed twice with pre-cooled PBS and then
centrifuged at 1500 rpm for 5 min. The cells were re-suspended and
adjusted to a concentration of 20×106/l. They were
selected by FACS to obtain GFP-positive cells; the screening
process was sterile and double-antibiotics (penicillin and
streptomycin) were allowed to be added if necessary.
Assay of PLT adhesion under a scanning
electron microscope (SEM)
The cells were co-cultured as follows: i) A549, ii)
A549 + inactivated PLTs, iii) A549 + activated PLTs, iv) A549
(siRNA targeting PSGL-1) + activated PLTs, v)A549 + activated PLTs
+ P-selectin antibody.
After the co-culture, the cells were fixed overnight
at 4˚C and washed with 0.9% NS twice each for 10 min. Then the
specimens were dehydrated using 30, 50, 70, 80, 90 and 100%
alcohol, respectively, 4 min for each step. Then the specimens were
administered 50, 70, 90, 95 and 100% tert butyl alcohol,
respectively 4 min for each step. Finally, the specimens were
observed using an S-3400N SEM (Hitachi, Japan).
Assay of tumor cell adhesion
The temperature in the flow chamber was maintained
at 37˚C, and the cells were placed in an atmosphere of mixed gases
containing 95% O2 and 5% CO2. This system
produces stable laminar shear stress ranging from 0 to 48
dyn/cm2. It simulates different blood flow conditions
after the regulation. Previously, flow rates (Q, 0.5 ml/min; τ,
9.75 dyn/cm2), (Q, 0.3 ml/min; τ, 5.85
dyn/cm2) and (Q, 0.1 ml/min; τ, 1.95 dyn/cm2)
were used to monitor the rolling and adherence of lung cancer cells
on the surface of the endothelial cells.
Based on the previous study, we selected 0.1 ml/min
as an appropriate flow rate. After the co-culture of PLTs and lung
cancer cells, the cells were co-cultured again as following: i)
HuvEC+A549; ii) HuvEC+A549 + inactivated PLTs; iii) HuvEC+A549
(siRNA targeting PSGL-1) + activated PLTs; iv) HuvEC+A549 +
activated PLTs + P-selectin antibody.
HuvECs (1×106/ml) were inoculated on
coverslips in 6-well plates. HuvECs were co-cultured with PLTs and
A549 for 24 h. After that, the coverslips were placed in the flow
chamber. The perfusate was prepared and the flow rate was adjusted.
The treated PLTs and A549 cells were injected into the flow
chamber. The rolling time and adhesion of A549 on vascular
endothelium were observed and recorded along with the perfusate
flow. The rolling and adhesion of A549 cells on the surface of the
vascular endothelium and PLT aggregation were observed and recorded
using an inverted fluorescence microscope in the absence or
presence of a variety of flow rates. Meanwhile, a video was used to
record the time required for the lung cancer cells to pass through
per visual field unit.
RT-PCR assay of mRNA expression of
integrin α3, integrin α5, integrin β1, ICAM-1 and VCAM-1
The cells were collected using centrifugation of
1000 rpm for 10 min. Then total RNA was prepared in accordance with
the manufacturer’s instructions. After DNase I treatment, 2
μg of RNA was reverse transcribed with AMV reverse
transcriptase. A master mix containing the reaction buffer, dNTPs,
Taq polymerase and 2 μl cDNA in a 25-μl reaction
mixture was transferred to different PCR tubes. Forward and reverse
primers corresponding to different individual genes were added to
the PCR tubes and subjected to PCR amplification using primers
against various genes.
These reactions were performed for 30 cycles. The
annealing temperature was maintained at 56˚C; the resting
conditions included denaturation at 94˚C for 30 sec followed by
extension at 72˚C for 45 sec. The PCR products were determined
using 1.5% agarose gel electrophoresis and ethidium bromide
staining. Images of the gels were analyzed using the Quantity One
software (Bio-Rad), which compares the relative density between
objective straps and β-actin. The primers pairs are listed in
Table I.
 | Table IPrimer pairs for RT-PCR. |
Table I
Primer pairs for RT-PCR.
| Gene | Primer pairs | Product size
(bp) |
|---|
| Integrin α3 | PF:
5′-CACTCTGCTGGTGGACTATACACT-3′
PR: 5′-TACTTGAGGGGGCTTCCTACAT-3′ | 135 |
| Integrin α5 | PF:
5′-CTGTGACTACTTTGCCGTGAAC-3′
PR: 5′-GATGAGGGACTGTAAACCGAAG-3′ | 110 |
| Integrin β1 | PF:
5′-CGTAGCAAAGGAACAGCAGAG-3′
PR: 5′-GGTCAATGGGATAGTCTTCAGC-3′ | 142 |
| ICAM-1 | PF:
5′-GTCACCTATGGCAACGACTCCT-3′
PR: 5′-AGTGTCTCCTGGCTCTGGTTC-3′ | 119 |
| VCAM-1 | PF:
5′-CCGATCACAGTCAAGTGTTCAG-3′
PR: 5′-CTCTTGGTTTCCAGGGACTTC-3′ | 137 |
| β-actin | PF:
5′-TTCTACAATGAGCTGCGTGTG-3′˚
PR: 5′-AGTAACAGTCCGCCTAGAAGCAT-3′ | 874 |
Western blot assay of protein
expression of integrin α3, integrin α5, integrin β1, ICAM-1 and
VCAM-1 proteins
The tissue samples were dissolved in lysis buffer
containing 7 mol/l urea, 2 mol/l thiourea and 4% CHAPS (w/v). They
were centrifuged at 40,000 × g for 1 h and then the supernatants
were harvested. The total protein concentration was determined
using the Bradford method.
Total protein (25 μg) in each sample was
separated by 12% SDS-PAGE. The proteins were transferred onto a
sheet of polyvinylidene fluoride (PVDF) membrane with wet transfer
100 V for 1.5 h. The membrane was probed with primary mouse
anti-human integrin α3, integrin α5, integrin β1, ICAM-1 and VCAM-1
antibodies (1:1000) overnight at 4˚C. The membrane was washed three
times with Tris-buffered saline (TBS) for 5 min each and secondary
rabbit anti-mouse IgG (1:2500) (Zhongshan Golden Bridge, Beijing,
China) was added. The membrane was washed once with TBS for another
5 min. Then it was stained by an enhanced chemiluminescence (ECL)
reagent (Pierce, USA) and imaged on X-ray film by autoradiography.
β-actin was used as a control. The band intensity was measured by
Quantity One software (Bio-Rad) and the relative proteins levels
were expressed as the ratios between the densities of objective
proteins and β-actin.
Statistics and presentation of
data
All experiments were performed at least three times,
and representative data were presented. SPSS 12.0 statistical
software was used to analyze the differences. The differences among
groups were analyzed using one-way ANOVA or χ2 test. A
P-value of <0.05 was considered to denote statistical
significance.
Results
Expression of P-selectin in the different
lung tissues
Compared with lung squamous carcinoma, the
expression of P-selectin in lung adenocarcinoma tissue was
significantly enhanced (Fig. 1);
the expression of P-selectin in the cancer tissue was stronger than
that in the paracancerous and distant normal tissues, respectively
(Fig. 1).
Correlation between BPC in the peripheral
blood and pathological types
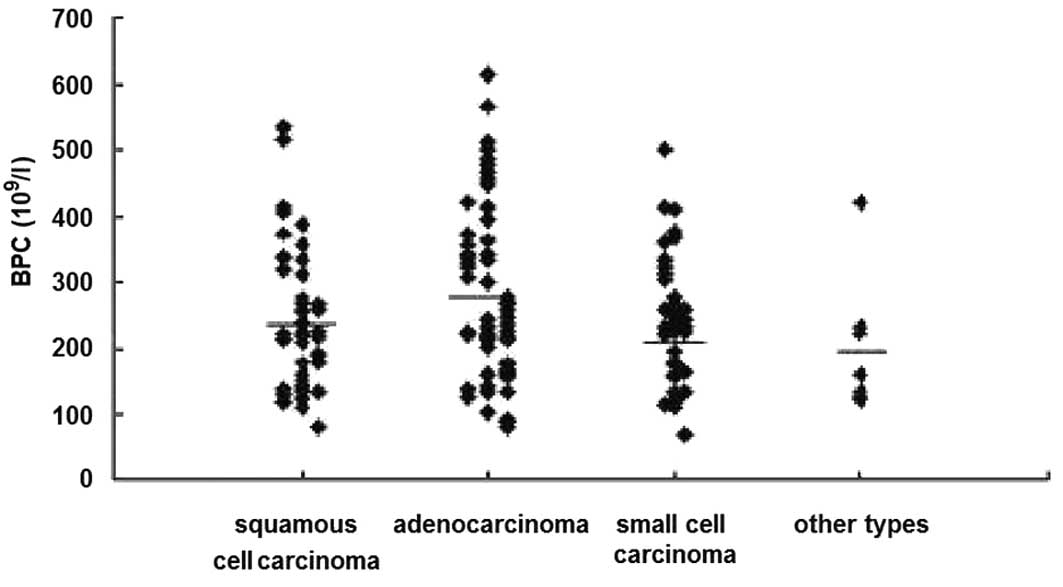
The PLT count was decreased, was normal and was
increased in 3.57% (6/168), 69.05% (116/168) and 27.38% (46/168) of
the 168 lung cancer patients, respectively. The PLT was increased
in 22.64% (12/53) of squamous cell carcinoma, 37.09% (23/62) of
adenocarcinoma, 22.70% (10/44) of small cell carcinoma cases and
14.20% (1/7) of other pathological types, respectively (Fig. 2).
The mean BPC was (233.85±102.23)x109/l,
(269.89±128.20)x109/l, (238.41±92.04)x109/l
in lung squamous cell carcinoma, adenocarcinoma and small-cell
carcinoma cases, respectively. Meanwhile, the BPC in the healthy
control group was sustained at a normal level. Although no
significant differences among groups were noted, there was an
obvious increased tendency in BPC in the lung adenocarcinoma.
Correlations between BPC and hematogenous
metastasis and clinical stage
Generally, hematogenous metastasis suggests poor
prognosis and a clinical TNM stage of IV. Here, we analyzed the
correlations between the change in BPC and TNM stage and
hematogenous metastasis.
The results showed that the BPC was increased in
27.38% (46/168) of the lung cancer patients. No increased BPC was
found in stage I and II in the 46 patients. However, the BPC was
increased in 19.35% of the patients in stage III. Furthermore, the
BPC was significantly increased in 36.96% of the patients in stage
IV (metastasis). Compared with stage I+II and stage III, there were
significant differences (P<0.01) (Table II).
 | Table IICorrelations between BPC and clinical
stages. |
Table II
Correlations between BPC and clinical
stages.
| Clinical stages
(TNM) |
|---|
|
|
|---|
| BPC | I+II | III | IV | Total |
|---|
|
<100×109/l | 1 | 3 | 2 | 6 |
|
100–300×109/l | 13 | 47 | 56 | 116 |
|
>300×109/l | 0 | 12 | 34a | 46 |
| Total | 14 | 62 | 92 | 168 |
FACS assay of P-selectin in peripheral
blood
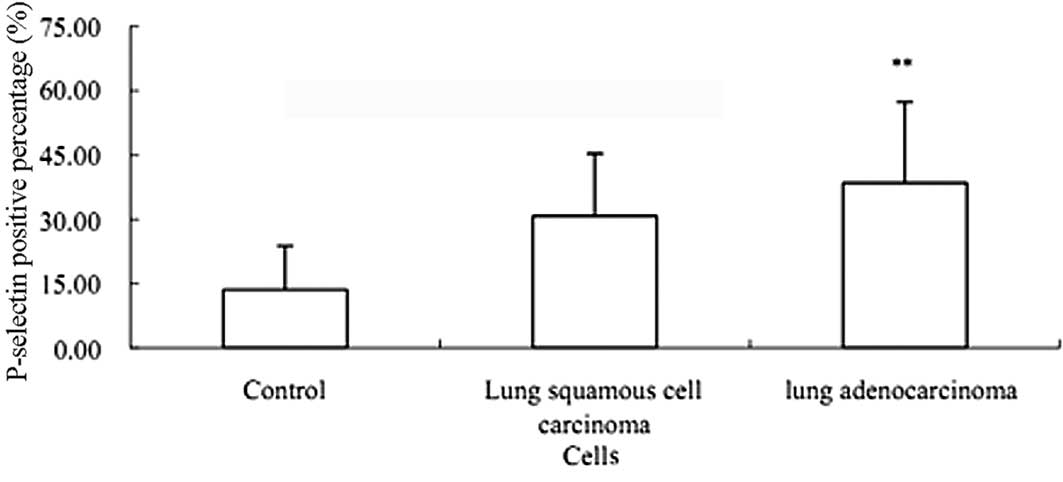
The FACS results showed that the expression of PLT
activation marker P-selectin in lung adenocarcinoma was
significantly enhanced compared with that in the healthy control
(P<0.01) (Fig. 3). However,
there was no significant difference between adenocarcinoma and
squamous cell carcinoma (P>0.05) (Fig. 3).
Construction and identification of siRNA
targeting PSGL-1
The complementary double-strand PSGL-1 siRNA was
directionally sub-cloned into pSilencer-GFP2.0 U6 eukaryotic
expression vector by using DNA recombination technology. The
pSilencer-PSGL1-shRNA eukaryotic expression plasmid was obtained.
The strain for plasmid cloning was sent to the Takara Corp. for
sequencing identification and the sequence results were
identified.
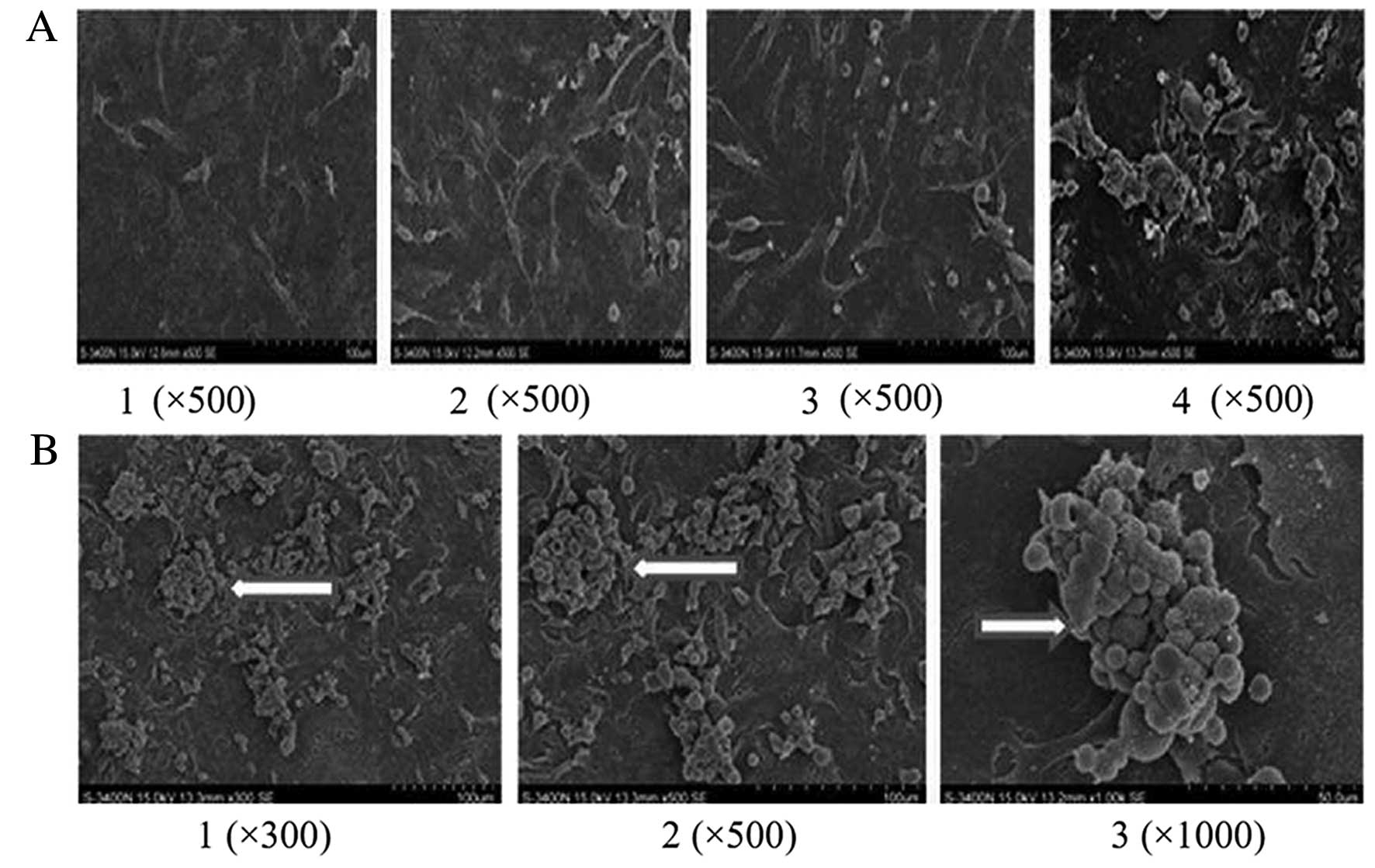
SEM assay of PLT aggregation after
co-culture
A small number of lung cancer cells assembled with
PLTs in the control group. After RNAi targeting PSGL-1 in A549
cells, the interactions between activated PLTs and lung cancer
cells were halted and little PLT aggregation was noted (Fig. 4A). Activated PLTs increased the
interactions between platelets and cancer cells (Fig. 4B).
Adhesion of lung cancer cells on vascular
endothelial cells under flow
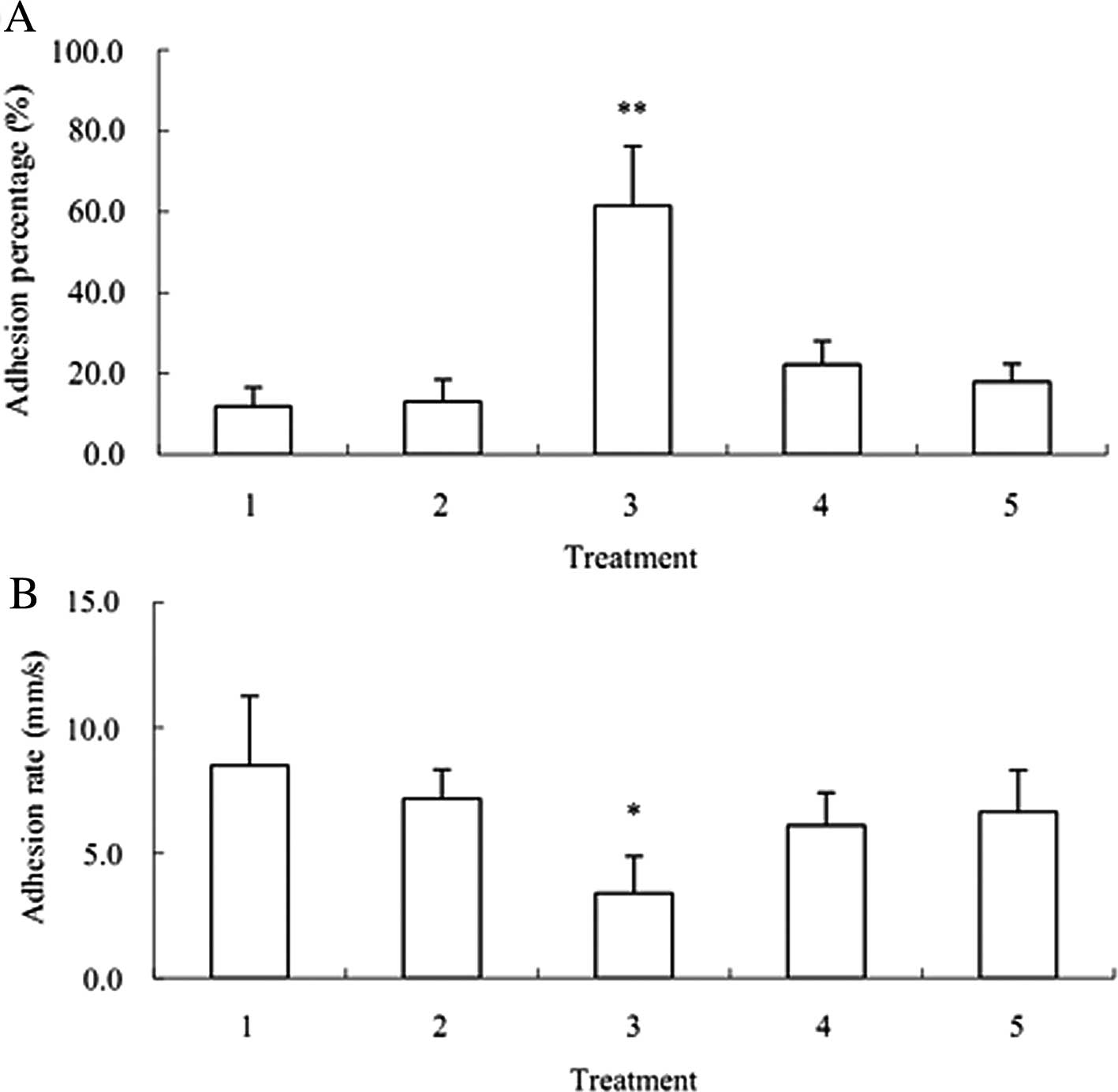
Blood flow simulation assay showed that the
co-culture of activated PLTs and A549 significantly attenuated the
rolling rate of A549 on HuvECs, which promoted the interaction
between A549 cells and HuvECs.
Compared with the control, the adhesion efficiency
reached 65% and the rolling rate of A549 was decreased by 60% after
the co-culture. However, the adhesion of A549 on HuvECs was 13% and
the rolling rate of A549 was decreased by only 11% after the
co-culture of HuvECs and A549 cells (Fig. 5).
After the RNAi targeting P-selectin specific ligand
PSGL-1, adhesion efficiency was 27% and the rolling rate of A549
was 76% after the co-culture of HuvECs, A549 and activated PLTs.
After the co-culture of HuvECs, A549 after RNAi targeting PSGL-1
and activated PLTs, the specific P-selectin antibody was employed
to block the binding site of PSGL-1. Then the adhesion efficiency
was 22% and the rolling rate of A549 was reduced by 20% (Fig. 5).
mRNA expression of integrin α3, integrin
α5, integrin β1, ICAM-1 and VCAM-1 are up-regulated after the
co-culture of HuvECs, A549 and activated PLTs
The RT-PCR results suggested that the mRNA levels of
integrin α3, integrin α5, integrin β1, ICAM-1 and VCAM-1 after the
co-culture of HuvECs, A549 and activated PLTs were significantly
increased compared with that after the co-culture of HuvECs and
A549 cells (P<0.05) (Fig.
6).
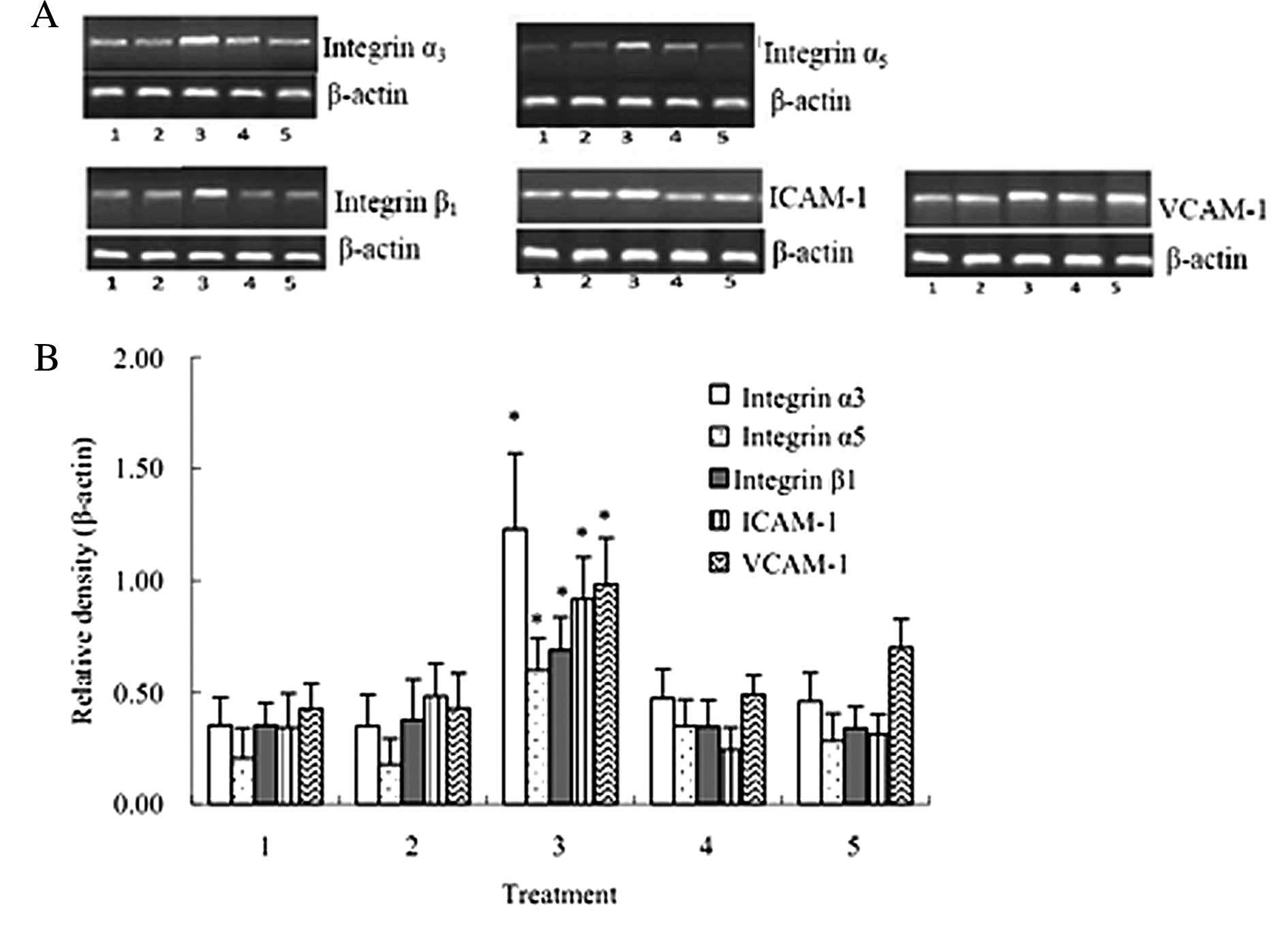 | Figure 6mRNA expression of integrin α3,
integrin α5, integrin β1, ICAM-1 and VCAM-1 (±SD, n=3). mRNA levels
of integrin α3, integrin α5, integrin β1, ICAM-1 and VCAM-1 after
the co-culture of HuvECs, A549 and activated PLTs were
significantly increased compared with that after the co-culture of
HuvECs and A549. 1, HuvECs+A549; 2, HuvECs+A549 + inactivated PLTs;
3, HuvECs+A549 + activated PLTs; 4, HuvECs+A549 (siRNA targeting
PSGL-1) + activated PLTs; 5, HuvECs+A549 + activated PLTs +
P-selectin antibody. *P<0.05 as compared with
HuvECs+A549. |
Protein expression of integrin α3,
integrin α5, integrin β1, ICAM-1 and VCAM-1 are up-regulated after
the co-culture of HuvECs, A549 and activated PLTs
The results of western blot analysis showed that the
protein levels of integrin α3, integrin α5, integrin β1, ICAM-1 and
VCAM-1 the co-culture of HuvECs, A549 and activated PLTs were
significantly increased compared with that after the co-culture of
HuvECs and A549 cells (P<0.05) (Fig. 7).
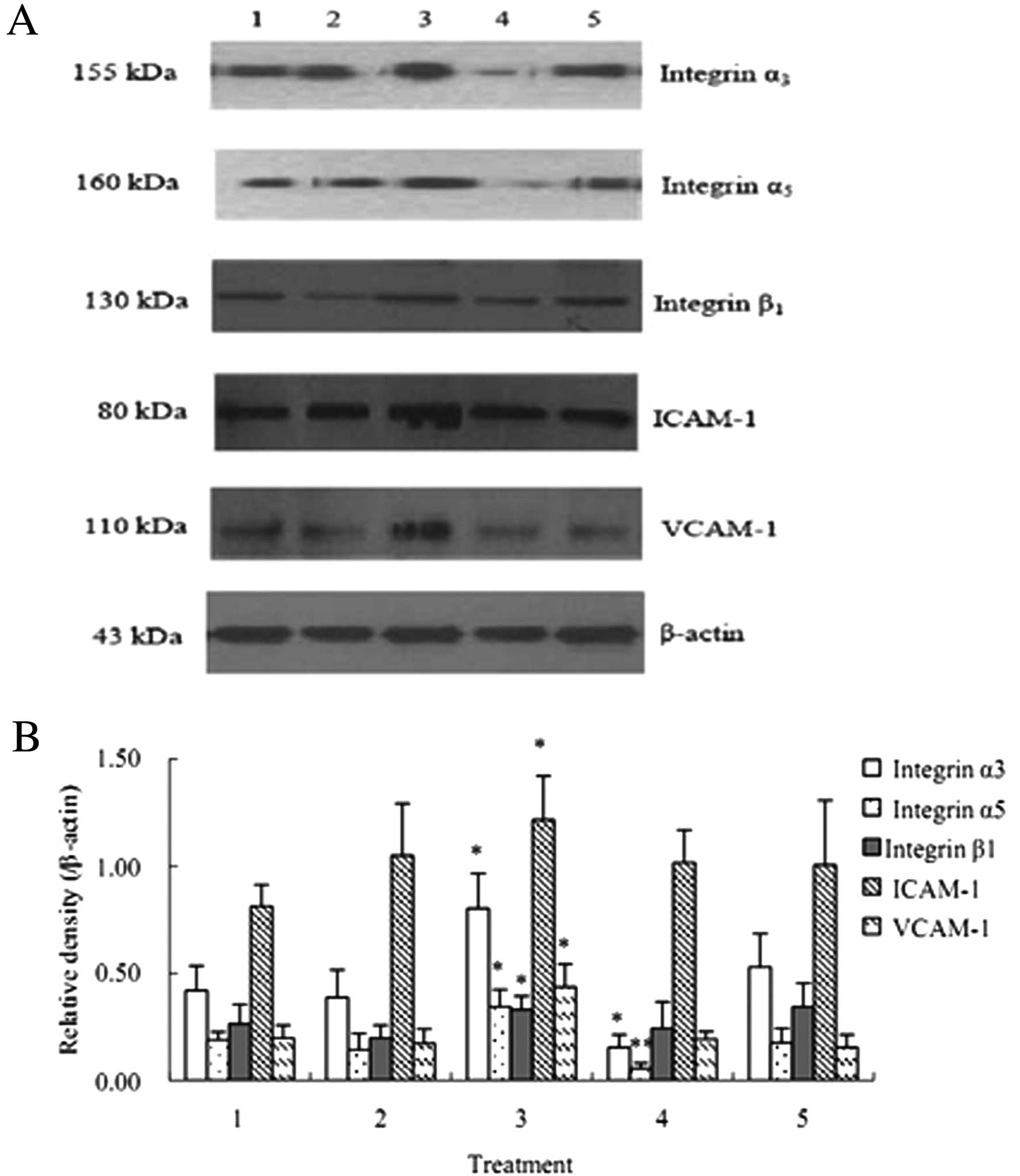 | Figure 7Protein expression of integrin α3,
integrin α5, integrin β1, ICAM-1 and VCAM-1 (mean ± SD, n=3). The
protein levels of integrin α3, integrin α5, integrin β1, ICAM-1 and
VCAM-1 after the co-culture of HuvECs, A549 and activated PLTs were
significantly up-regulated compared with that after the co-culture
of HuvECs and A549. 1, HuvECs+A549; 2, HuvECs+A549 + inactivated
PLTs; 3, HuvECs+A549 + activated PLTs; 4, HuvECs+A549 (siRNA
targeting PSGL-1) + activated PLTs; 5, HuvECs+A549 + activated PLTs
+ P-selectin antibody. *P<0.05,
**P<0.01 as compared with HuvECs+A549. |
Discussion
Lung cancer is a severe disease and its morbidity
and death rates are gradually increasing. Lung cancer is becoming
the leading cause of cancer-related deaths. Distant hematogenous
metastasis results in poor prognosis and the deaths of lung cancer
patients (1,2). Tumor hematogenous metastasis is a
complex multistep process including separation and leakage from the
primary tumor, promotion of angiogenesis and entry into the
circulatory system, escape from attack by circulatory blood and
immune factors, adhesion with endothelial cells in target organs,
and eventual passage through the vascular endothelium and formation
of metastasis via distant planting (3,6–8). At
present, more and more studies have confirmed that PLT activation
in the circulation is relevant to hematogenous metastasis of a
variety of malignant tumors (4,5,9),
while anti-platelet factor may inhibit tumor metastasis (10,11).
However, the mechanism has not been clarified thoroughly.
Platelet aggregation and increased expression of
active molecules are main characteristics of platelet activation.
P-selectin is an important cell adhesion molecule that normally
exits in platelet α particle. An absence and persistently low
expression were found in the resting state. P-selectin can be
rapidly expressed at the surface of PLTs by membrane fusion effect
when PLTs are activated. Therefore, P-selectin is commonly selected
as a marker for PLT activation (12,13).
Simanek et al confirmed that a high blood platelet count is
an important and independent factor for venous thrombosis in cancer
patients in the clinic (14). The
blood platelet count was reported to be a valuable prediction index
for the prognosis of a variety of cancers including malignant
melanoma, renal carcinoma, gastric carcinoma, and breast carcinoma
(4,15,16).
Iwasaki et al found that a high blood platelet count was a
significant independent factor for lung cancer postoperative
metastasis (17).
Dymicka-Piekarska et al suggested that soluble P-selectin is
associated with malignant invasion and metastasis of colon
carcinoma (18).
In the present study, we found that the expression
of P-selectin in lung adenocarcinoma was higher than that in
paracancerous tissue, distant tissue and lung squamous cell
carcinoma. Meanwhile, significant increased blood platelet count
and P-selectin were also found in the peripheral blood of NSCLC
patients. Compared with patients in stage I+II and III, the
increase in PLTs in the patients in stage IV was significant
(P<0.05). Furthermore, the FACS results showed that P-selectin
activation in lung adenocarcinoma was significantly enhanced
compared with that in the healthy control and lung squamous cell
carcinoma, suggesting a correlation between PLT activation,
P-selectin expression and hematogenous metastasis of NSCLC
particularly lung adenocarcinoma.
The cultured human lung adenocarcinoma cell line
A549 interacted with PLTs to form activated PLT-lung adenocarcinoma
cell complexes. It has been confirmed that adhesion between cancer
cells and PLTs is associated with the metastatic potential. The
adhesion molecules that may interact with tumor cells mainly
include GPIb-IX-V, GPIIb/IIIa and P-selectin (19–21).
However, the detail of their interactions particularly in the
presence of blood flow in vivo is not fully understood
(22,23). In the present study we found that
the rolling rate of A549 cells on the surface of vascular
endothelial cells was significantly decreased after co-culture of
activated PLTs and A549 in a flow state. Alternatively, the effect
of inactivated PLTs, P-selectin antibody or siRNA targeting
P-selectin ligand PSGL-1 on lung adenocarcinoma cells was
significantly attenuated, which was consistent with the study of
Borsig et al (24). They
found that heparin and its derivatives efficiently suppressed the
metastasis of various tumors via reducing the combination of tumor
cells and P-selectin in transplanted tumors in vivo
(25). These results suggest that
the activated PLT-lung adenocarcinoma cell complexes affect the
capture of lung adenocarcinoma cells on the surface of vascular
endothelium via P-selectin mediation.
Tumor cell capture and adhesion is a key process for
hematogenous metastasis (26),
which is regulated by a series of cellular adhesion molecules
including cadherins, selectin, integrin, immunoglobulin family and
CD44 (27). Generally, adhesion
capacity of individual tumor cells to vascular endothelial cells is
comparatively lower. Combined with the erosion blood flow, the
adhesion capacity is further attenuated. McCarty et al
observed that PLTs support the capture, rolling and stable adhesion
of colon carcinoma in a blood flow condition (28), which was similar to our study.
Integrin was found to play a crucial role in regulating stable
cellular adhesion (29,30). It was reported that in inflammatory
reactions P-selectin promotes leucocytic adhesion and migration via
initiating the activation of leucocyte integrin (31). In lung cancer, major histological
types express integrin β1. Integrin α2, α3, α6, αV and β1 were
mainly expressed in lung squamous cell carcinoma and small-cell
carcinoma, and integrin α3β1 and α5β1 were strongly expressed in
lung adenocarcinoma (32,33). Our results showed that mRNA and
protein levels of lung cancer cell membrane P-selectin ligand
glycoprotein PSGL-1, integrin α3, α and β1, endothelial cell
adhesion molecule ICAM-1, VCAM-1 gene were increased in different
degree after the co-culture of A549 cells, activated PLTs and
HuvECs.
In summary, the activation of circulatory PLTs
affected the hematogenous metastasis of NSCLC particularly lung
adenocarcinoma. The activated PLT-lung cancer cell complexes
protected the cancer cells from mechanical injury by blood flow and
escaped attack by the immune system. In addition, the complexes
up-regulated the expression of integrin α3, α5, β1 and endothelial
cell adhesion molecules ICAM-1 and VCAM-1, thereby promoting the
capture of NSCLC cells on the surface of vascular endothelial
cells, which may be one of the mechanisms for hematogenous
metastasis of lung cancer due to PLT activation. Importantly,
anti-PLT activation was found to play a potential role in
suppressing tumor metastasis (34–36).
Further study on the mechanism concerning the PLT promotion of
hematogenous metastasis of tumors will aid in the search for
specific target drugs to suppress tumor metastasis and to provide a
new direction for individualized treatment.
References
|
1
|
DR BaldwinLung cancer: investigation and
stagingMedicine36155161200810.1016/j.mpmed.2007.12.004
|
|
2
|
A JemalR SiegelE WardCancer statistics,
2008CA Cancer J Clin587196200810.3322/CA.2007.0010
|
|
3
|
MF LeberT EfferthMolecular principles of
cancer invasion and metastasis (Review)Int J
Oncol34881895200919287945
|
|
4
|
L BorsigThe role of platelet activation in
tumor metastasisExpert Rev Anticancer
Ther812471255200810.1586/14737140.8.8.124718699763
|
|
5
|
T TsuruoN FujitaPlatelet aggregation in
the formation of tumor metastasisProc Jpn Acad Ser B Phys Biol
Sci84189198200810.2183/pjab.84.18918941298
|
|
6
|
AF ChambersGN NaumovHJ VargheseCritical
steps in hematogenous metastasis: an overviewSurg Oncol Clin N
Am10243255200111382585
|
|
7
|
TR GeigerDS PeeperMetastasis
mechanismsBiochim Biophys Acta1796293308200919683560
|
|
8
|
JA JoyceJW PollardMicroenvironmental
regulation of metastasisNat Rev
Cancer9239252200810.1038/nrc2618
|
|
9
|
S NobleJ PasiEpidemiology and
pathophysiology of cancer-associated thrombosisBr J
Cancer13S2S92008
|
|
10
|
A AmirkhosraviSA MousaM AmayaInhibition of
tumor cell-induced platelet aggregation and lung metastasis by the
oral GPIIb/IIIa antagonist XV454Thromb
Haemost90549554200312958625
|
|
11
|
E SierkoMZ WojtukiewiczInhibition of
platelet function: does it offer a chance of better cancer
progression control?Semin Thromb
Hemost33712721200710.1055/s-2007-99154018000800
|
|
12
|
RJ LudwigMP SchönWH BoehnckeP-selectin: a
common therapeutic target for cardiovascular disorders,
inflammation and tumour metastasisExpert Opin Ther
Targets1111031117200710.1517/14728222.11.8.110317665981
|
|
13
|
A MassaguerP EngelV TovarCharacterization
of platelet and soluble-porcine P-selectin (CD62P)Vet Immunol
Immunopathol96169181200310.1016/S0165-2427(03)00163-614592730
|
|
14
|
R SimanekR VormittagC AyHigh platelet
count associated with venous thromboembolism in cancer patients:
results from the Vienna Cancer and Thrombosis Study (CATS)J Thromb
Haemost8114120201010.1111/j.1538-7836.2009.03680.x19889150
|
|
15
|
HM VerheulHM PinedoThe importance of
platelet counts and their contents in cancerClin Cancer
Res932193221200312960106
|
|
16
|
M IkedaH FurukawaH ImamuraPoor prognosis
associated with thrombocytosis in patients with gastric cancerAnn
Surg Oncol9287291200210.1007/BF0257306711923136
|
|
17
|
A IwasakiW HamanakaT HarnadaSignificance
of platelet counts in patients who underwent surgical treatment for
lung metastasisInt Surg92103109200717518253
|
|
18
|
V Dymicka-PiekarskaJ Matowicka-KarnaM
GrykoRelationship between soluble P-selectin and inflammatory
factors (interleukin-6 and C-reactive protein) in colorectal
cancerThromb
Res120585590200710.1016/j.thromres.2006.11.00217169411
|
|
19
|
L ErpenbeckMP SchönDeadly allies: the
fatal interplay between platelets and metastasizing cancer
cellsBlood11534273436201010.1182/blood-2009-10-24729620194899
|
|
20
|
P JuraszD Alonso-EscolanoMW
RadomskiPlatelet-cancer interactions: mechanisms and pharmacology
of tumour cell-induced platelet aggregationBr J
Pharmacol143819826200410.1038/sj.bjp.070601315492016
|
|
21
|
M TrikhaZ ZhouJ TimarMultiple roles for
platelet GPIIb/IIIa and αvβ3 integrins in tumor growth,
angiogenesis, and metastasisCancer Res62282428332002
|
|
22
|
B FuchsU BuddeA SchulzFlow-based
measurements of von Willebrand factor (VWF) function: binding to
collagen and platelet adhesion under physiological shear rateThromb
Res125239245201010.1016/j.thromres.2009.08.02019853893
|
|
23
|
MR CominettiAC MartinJU RibeiroInhibition
of platelets and tumor cell adhesion by the disintegrin domain of
human ADAM9 to collagen I under dynamic flow
conditionsBiochimie9110451052200910.1016/j.biochi.2009.05.01219505527
|
|
24
|
L BorsigR WongRO HynesSynergistic effects
of L- and P-selectin in facilitating tumor metastasis can involve
non-mucin ligands and implicate leukocytes as enhancers of
metastasisProc Natl Acad Sci
USA9921932198200210.1073/pnas.26170409811854515
|
|
25
|
K BaumannD KowalczykT GutjahrSulfated and
non-sulfated glycopeptide recognition domains of P-selectin
glycoprotein ligand 1 and their binding to P- and E-selectinAngew
Chem Int Ed Engl4831743178200910.1002/anie.20080599919322854
|
|
26
|
M IiizumiS MohintaS BandyopadhyayK
WatabeTumor-endothelial cell interactions: therapeutic
potentialMicrovasc
Res74114120200710.1016/j.mvr.2007.04.00217498748
|
|
27
|
AP SkubitzAdhesion moleculesCancer Treat
Res1073053292002
|
|
28
|
OJ McCartySA MousaPF BrayK
KonstantopoulosImmobilized platelets support human colon carcinoma
cell tethering, rolling, and firm adhesion under dynamic flow
conditionsBlood9617891797200010961878
|
|
29
|
K Leycell adhesion under
flowMicrocirculation1612200910.1080/10739680802644415
|
|
30
|
MA ArnaoutSL GoodmanJP XiongStructure and
mechanics of integrin-based cell adhesionCell
Biology19495507200717928215
|
|
31
|
HB WangJT WangL ZhangP-selectin primes
leukocyte integrin activation during inflammationNat
Immunol8882892200710.1038/ni149117632516
|
|
32
|
A GogaliK CharalabopoulosS
ConstantopoulosIntegrin receptors in primary lung cancerExp
Oncol261061102004
|
|
33
|
KN SyrigosN KatirtzoglouE KotteasK
HarringtonAdhesion molecules in lung cancer: implications in the
pathogenesis and managementCurr Pharm
Des1421732183200810.2174/13816120878574016218781970
|
|
34
|
SR BarthelJD GavinoL DeschenyCJ
DimitroffTargeting selectins and selectin ligands in inflammation
and cancerExpert Opin Ther
Targets1114731491200710.1517/14728222.11.11.147318028011
|
|
35
|
L BorsigAntimetastatic activities of
modified heparins: selectin inhibition by heparin attenuates
metastasisSemin Thromb
Hemost33540546200710.1055/s-2007-98208617629852
|
|
36
|
DD MaratheA Buffone JrEV
ChandrasekaranFluorinated per-acetylated GalNAc metabolically
alters glycan structures on leukocyte PSGL-1 and reduces cell
binding to
selectinsBlood11513031312201010.1182/blood-2009-07-23148019996411
|