Introduction
Ultraviolet (UV) irradiation (200–400 nm) causes a
number of acute and chronic skin effects, which can result in
inflammation, immunosuppression, premature skin aging and the
development of skin malignancies (1). UVA irradiation (320–400 nm), which is
not absorbed in the ozone layer, comprises more than 95% of the UV
light that reaches the earth. UVA penetrates the epidermis and
affects the epidermal and dermal layers of the skin. At the
cellular level, UVA exposure causes significant oxidative stress
via generation of reactive oxygen species (ROS), such as singlet
oxygen, hydroxyl radical, superoxide anion and hydrogen peroxide
(2). ROS are rapidly removed by
non-enzymatic, particularly glutathione (GSH), and enzymatic
antioxidants (catalase, superoxide dismutase, glutathione
peroxidase and glutathione reductase), and that maintains the
pro-oxidant/antioxidant balance, resulting in cell and tissue
stabilization. However, a surplus of ROS may overwhelm the skin
anti-oxidant defense mechanisms causing pro-oxidant/antioxidant
disequilibrium. Overproduction of ROS induces oxidation of nucleic
acids, proteins and membrane lipids, which also lead to
intracellular GSH and NADH/NADPH depletion, and therefore energy
loss from the cell. UV-generated ROS also affect the regulation of
the gene expression of signaling molecules/cascades such as
mitogen-activated protein kinases and interrelated inflammatory
cytokines, as well as NF-κB and activator protein-1 (3).
Magnesium ascorbyl phosphate (MAP) is a vitamin C
derivative and is more stable than vitamin C (4,5). MAP
has been used in cosmetic and dermatological products as it has a
number of favorable effects on the skin (6). As an antioxidant, MAP can scavenge
and destroy aggressive oxidizing agents and radicals. Due to the
ability of MAP to suppress the pigmentation of the skin and
increase the decomposition of melanin (7), it can be used to whiten the skin.
Coenzyme Q10 (CoQ10) is a
bioactive, vitamin-like molecule present in all eukaryotic cells
containing mitochondria. CoQ10 is located in the
hydrophobic middle region of the phospholipid bilayer of the
mitochondrial membrane and plays a role in the electron transport
chain process, where it accepts electrons from reducing equivalents
produced from fatty acid and glucose breakdown and delivers them to
electron acceptors (8).
CoQ10 in its reduced form (ubiquinol) acts as a
principal fat-soluble cellular antioxidant that plays an important
role in neutralizing free radicals, inhibiting lipid peroxidation
of membranes and in protecting mitochondrial membrane proteins and
DNA (9).
Among the cutaneous antioxidants, the tripeptide,
GSH (γ-glutamylcysteinylglycine), plays a pivotal role in
protecting skin cells from oxidative damage by directly scavenging
ROS or acting as a co-enzyme in GSH-peroxidase or GSH-S-transferase
catalyzed reactions (10,11). Previous studies have shown that GSH
is also involved in DNA repair and apoptosis (12,13).
Moreover, GSH plays a role in many important biological processes,
such as mitochondrial respiration, inflammatory response, signal
transduction, regulation of gene expression and cell proliferation
(14).
In this study, we investigated the UVA protective
activity of MAP or CoQ10 on human keratinocytes, using
human keratinocyte-derived HaCaT cells as an experimental model. We
focused on the effects of MAP or CoQ10 on ROS-induced
cellular oxidative stress, particularly on intracellular GSH
levels.
Materials and methods
Materials
Human keratinocytes (HaCaT cells) were obtained from
the Food Industry Research and Development Institute (Taiwan).
Dulbecco's modified Eagle's medium (DMEM), heated-inactivated fetal
calf serum (FCS), penicillin-streptomycin solution and trypsin-EDTA
solution were from Gibco™ Invitrogen Corp. (Carlsbad, CA, USA).
Sterile dimethylsulfoxide (DMSO)
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT),
MAP and CoQ10 were purchased from Sigma-Aldrich (St.
Louis, MO, USA).
Cell culture
HaCaT cells were grown in DMEM supplemented with
heated-inactivated FCS (10%; v/v), streptomycin (100 U/ml) and
penicillin (0.1 mg/ml) in a humidified atmosphere with 5%
CO2 at 37°C. The culture medium was changed three times
a week. The cells were subcultured following trypsinization. For
the experiment, HaCaT cells were seeded in a 6-well plate at a
density of 1×105 cells per cm2.
UVA irradiation and treatment with MAP or
CoQ10
The keratinocytes were pre-treated with MAP (125
μM-1 mM) or CoQ10 (2.5–10 μM) at 37°C for 1 h, and
irradiated and incubated in serum-free medium at 37°C for an
additional 24 h. The irradiated and non-irradiated control cells
were treated with serum-free medium. Prior to UV irradiation, the
cells were washed with phosphate-buffered saline (PBS) and covered
with a thin layer of PBS. The dishes with keratinocytes were
irradiated (UVA; 4–32 J/cm2) on ice-cold plates to
eliminate UVA thermal stimulation. In parallel, non-irradiated
cells were treated similarly and were kept in the dark in an
incubator. For irradiation, a solar simulator Bio-Sun (Vilber
Lourmat, Marne-la-Vallée, France) with a fixed wavelength (365 nm)
was used.
MTT assay
The cell viability was monitored following UVA
irradiation and pre-treatment with MAP or CoQ10. MTT was
used to quantify the metabolically active living cells.
Mitochondrial dehydrogenases metabolize MTT to a purple formazan
dye, which is measured photometrically at 570 nm using a
spectrophotometer (15).
Intracellular GSH level
Intracellular GSH was estimated using a reaction
with 5,5′-dithiobis-(2-nitrobenzoic acid) (DTNB) (16). The keratinocytes rinsed with PBS
were scraped into cooled perchloric acid (1%; v/v) and sonicated.
The aliquots were frozen for protein determination by Bradford
assay. The suspension was centrifuged (10 min; 13,000 rpm; 4°C) and
the supernatant was used for estimation of GSH in reaction with the
reaction mixture (800 mmol/l Tris/HCl, 20 mmol/l EDTA, pH 8.2; 20
mg/ml DTNB). The absorbance was read on a microplate reader at 412
nm.
Statistical analysis
The mean ± standard error (SE) was calculated from
at least three repeated groups in all experiments. A statistical
significance between groups was determined by the Student's t-test.
P<0.05 was considered to indicate a statistically significant
difference between the two groups.
Results
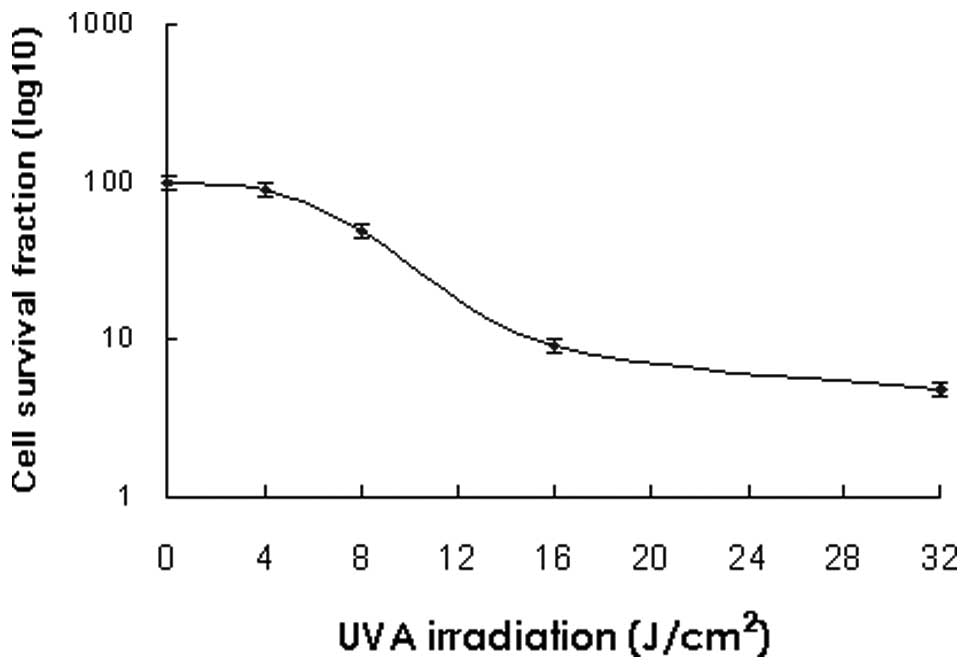
Cell survival fractions following UVA
irradiation at various doses
A comparison of cell survival fractions in HaCaT
cells following irradiation with UVA at various doses from 4–32
J/cm2 is shown in Fig.
1. The cell survival fraction was 89.9% when the keratinocytes
was irradiated with UVA at a dose of 4 J/cm2. The cell
survival fractions were 48.4, 9.1 and 4.8%, at doses of 8, 16 and
32 J/cm2, respectively. At each dose investigated, a
characteristic dose-response curve was observed with decreased
survival at increased doses of UVA irradiation.
Modulation of cell viability by MAP in
UVA-irradiated cells
Keratinocytes were pre-treated with MAP (125 μM to 1
mM) prior to UVA irradiation (8 J/cm2). The cell
survival fractions were 51.6, 55.5, 64.8 and 76.7%, following the
addition of MAP at concentrations of 125, 250, 500 μM and 1 mM,
respectively (Fig. 2). MAP
pre-treatment suppressed the UVA-induced decrease in cell viability
in a concentration-dependent manner. The results showed that MAP is
capable of protecting the keratinocytes against UVA
irradiation.
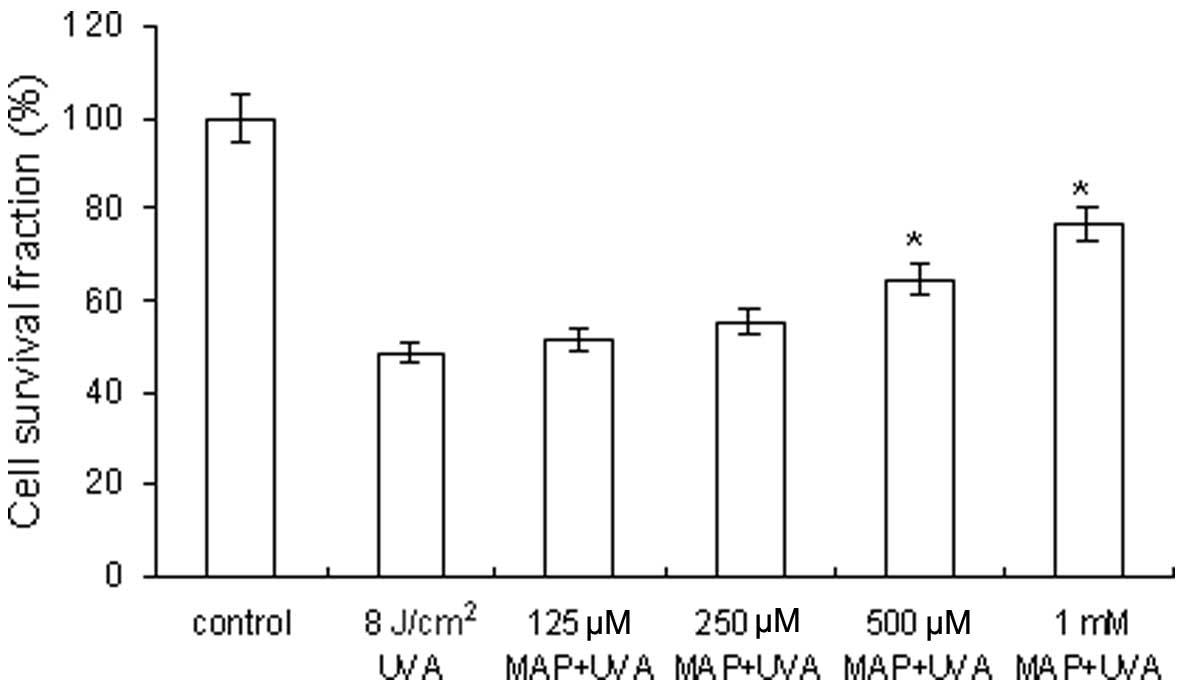 | Figure 2Modulation of cell viability by MAP in
UVA-irradiated cells. The cell survival fractions were 51.6, 55.5,
64.8 and 76.7%, when MAP was added at concentrations of 125, 250,
500 μM and 1 mM, respectively, prior to UVA irradiation at a dose
of 8 J/cm2. MAP, magnesium ascorbyl phosphate; UVA,
ultraviolet A. *P<0.05, comparison with the group 8
J/cm2 UVA. |
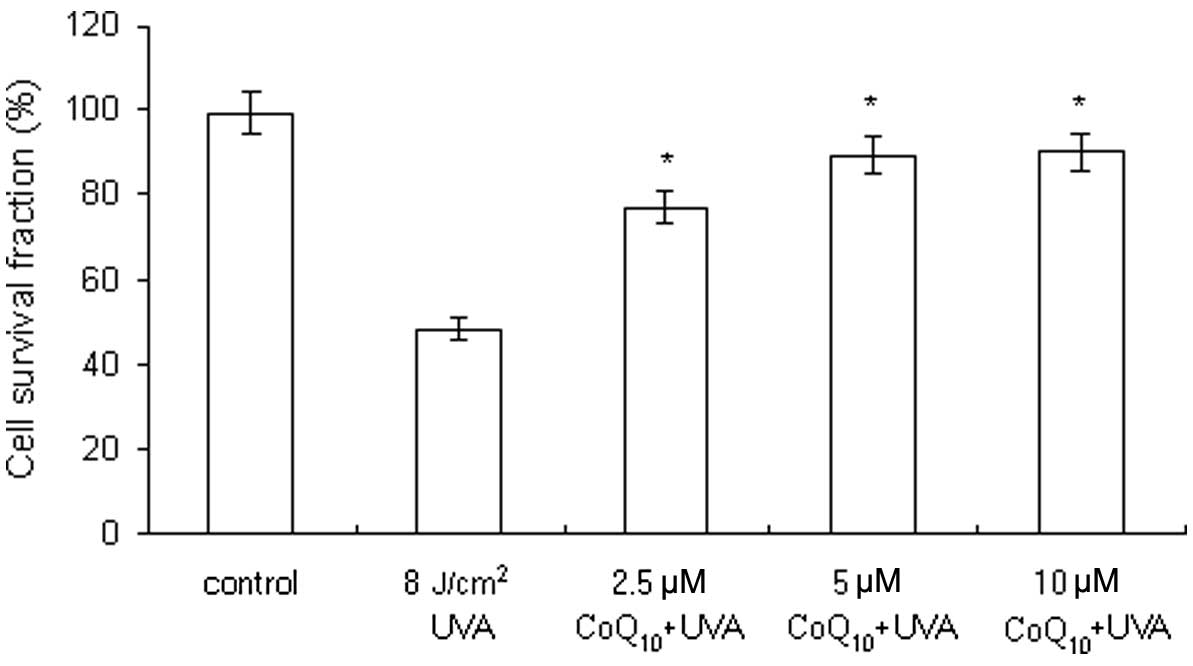
Modulation of cell viability by
CoQ10 in UVA-irradiated cells
Keratinocytes were pre-treated with CoQ10
(2.5–10 μM) prior to UVA irradiation (8 J/cm2). The cell
survival fractions were 77.2, 89.4 and 90.1%, following the
addition of CoQ10 at the concentrations of 2.5, 5 and 10
μM, respectively (Fig. 3).
CoQ10 pre-treatment suppressed the UVA-induced decrease
in cell viability in a concentration-dependent manner. The results
revealed that CoQ10 is capable of protecting the
keratinocytes against UVA irradiation.
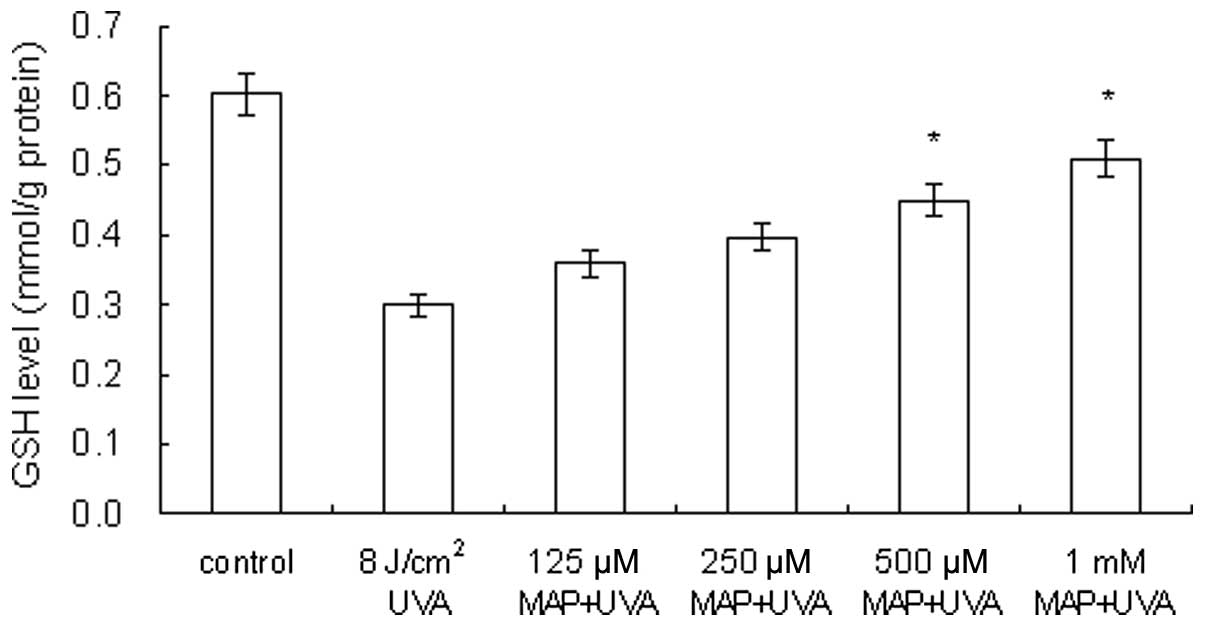
Prevention of UVA-induced GSH depletion
by MAP
As demonstrated in Fig.
4, in UVA-irradiated HaCaT cells (8 J/cm2) the GSH
level was decreased to 50% of the level of the control cells
(0.6→0.3 mmol/g protein). When MAP was added prior to UVA
irradiation, the GSH levels within the cells were 0.344, 0.388,
0.456 and 0.50 mmol/g protein, at MAP concentrations of 125, 250,
500 μM and 1 mM, respectively. The application of MAP to
UVA-irradiated keratinocytes led to dose-dependent prevention of
GSH depletion.
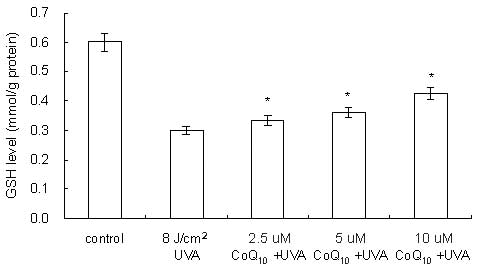
Prevention of UVA-induced GSH depletion
by CoQ10
As demonstrated in Fig.
5, in UVA-irradiated HaCaT cells (8 J/cm2) the GSH
level was decreased to 50% of the level of the control cells
(0.6→0.3 mmol/g protein). When CoQ10 was added prior to
UVA irradiation, the GSH levels within the cells were 0.328, 0.350
and 0.394 mmol/g protein, at CoQ10 concentrations of
2.5, 5 and 10 μM, respectively. CoQ10 application to
UVA-irradiated keratinocytes led to a dose-dependent prevention of
GSH depletion.
Discussion
UV irradiation is the principal factor in skin
cancers in humans. Several studies have shown that supplementation
with antioxidants can decrease UV-induced skin damage in
vitro and in vivo (17). In this study, we demonstrate the
ability of MAP and CoQ10 to prevent and reduce
UVA-related damage at a cellular level in human keratinocytes. In
particular, it was shown that treatment of HaCaT cells with MAP or
CoQ10 prior to UVA exposure increased cell viability and
suppressed intracellular GSH depletion. The cell viability assay
showed that MAP and CoQ10 protect against UVA-induced
cell death in a human keratinocyte cell line. It is well known that
during and after UVA irradiation, generation of ROS dramatically
increases in exposed cells (18,19).
As UVA-induced biological effects are primarily mediated by ROS,
their elimination is essential for UVA damage protection.
Application of MAP or CoQ10 led to a significant
increase in cell survival in irradiated HaCaT cells. MAP and
CoQ10 pre-treatment demonstrated maximal protection at
the highest concentration tested.
Pre-treatment of cells with MAP or CoQ10
resulted in concentration-dependent reduced GSH depletion. The
importance of GSH in protecting the skin from oxidative damage
caused by various chemicals and UV exposure is also well
documented. Among non-enzymatic antioxidants, GSH is the most
important as it also serves as the substrate for two major
antioxidant enzymes, GSH peroxidase and GSH transferase, and is
involved in vitamin C and vitamin E regeneration (20). The GSH level is directly associated
with the degree of lipid peroxidation in the cell membrane
(21), since GSH plays a role in
eliminating lipid peroxidation products, including
4-hydroxynonenal, by forming a GSH conjugate (22).
The cutaneous antioxidant system is complex and and
is not yet completely understood. Our results revealed that MAP and
CoQ10 can increase intracellular GSH levels. Previously,
Kagan et al showed that vitamin C can regenerate vitamin E
from the α-tocopheroxyl radical (23). α-lipoic acid has been shown to
elevate intracellular GSH levels in vitro by increasing
de novo synthesis (24).
The effect depends on the metabolic reduction of lipoic acid to
dihydrolipoic acid. Dihydrolipoic acid is released into the culture
medium where it reduces cystine. Cysteine thus formed is readily
taken up by the neutral amino acid transport system and utilized
from glutathione synthesis. By this mechanism, lipoic acid enables
cysteine to bypass the Xc- transport system, which is
weakly expressed in lymphocytes and inhibited by glutamate. Thereby
lipoic acid enables the key enzyme of glutathione synthesis,
γ-glutamylcysteine synthetase, which is regulated by uptake-limited
cysteine supply, to function at optimum conditions. The mechanisms
for the MAP and CoQ10 increased intracellular GSH levels
are not yet clear. Further studies are required to investigate this
mechanism.
However, from the results of the present study, we
can conclude that MAP and CoQ10 can protect
keratinocytes against UVA irradiation by suppressing GSH depletion.
Therefore, the protection mechanism is perhaps via increasing the
levels of GSH.
Acknowledgements
This study was supported by grant NSC
98-2314-B-238-001 from the National Science Council and grant
VIT-98-CM-01 from Vanung University, Taiwan.
References
|
1
|
Melnikova VO and Ananthaswamy HN: Cellular
and molecular events leading to the development of skin cancer.
Mutat Res. 571:91–106. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Svobodová A, Psotová J and Walterová D:
Natural phenolics in the prevention of UV-induced skin damage.
Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 147:137–145.
2003.PubMed/NCBI
|
|
3
|
Svobodova A, Walterova D and Vostalova J:
Ultraviolet light induced alteration to the skin. Biomed Pap Med
Fac Univ Palacky Olomouc Czech Repub. 150:25–38. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Segall AI and Moyano MA: Stability of
vitamin C derivatives in topical formulations containing lipoic
acid, vitamins A and E. Int J Cosmet Sci. 30:453–458. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Morisaki K and Ozaki S: Synthesis of novel
vitamin C phosphodiesters: stability and antioxidant activity.
Carbohydr Res. 286:123–138. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Spiclin P, Gasperlin M and Kmetec V:
Stability of ascorbyl palmitate in topical microemulsions. Int J
Pharm. 222:271–279. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Austria R, Semenzato A and Bettero A:
Stability of vitamin C derivatives in solution and topical
formulations. J Pharm Biomed Anal. 15:795–801. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Pastore A, Giovamberardino GD, Bertini E,
Tozzi G, Gaeta LM, Federici G and Piemonte F: Simultaneous
determination of ubiquinol and ubiquinone in skeletal muscle of
pediatric patients. Anal Biochem. 342:352–355. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Frei B, Kim MC and Ames BN: Ubiquinol-10
is an effective lipid-soluble antioxidant at physiological
concentrations. Proc Natl Acad Sci USA. 87:4879–4883. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Afaq F and Mukhtar H: Effects of solar
radiation on cutaneous detoxification pathways. J Photochem
Photobiol. B63:61–69. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hayes JD and McLellan LI: Glutathione and
glutathione-dependent enzymes represent a co-ordinately regulated
defence against oxidative stress. Free Radic Res. 31:273–300. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fonnum F and Lock EA: The contributions of
excitotoxicity, glutathione depletion and DNA repair in chemically
induced injury to neurones: exemplified with toxic effects on
cerebellar granule cells. J Neurochem. 88:513–531. 2004. View Article : Google Scholar
|
|
13
|
Hall AG: The role of glutathione in the
regulation of apoptosis. Eur J Clin Invest. 29:238–245. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sies H: Glutathione and its role in
cellular functions. Free Radic Biol Med. 27:916–921. 1999.
View Article : Google Scholar
|
|
15
|
Green LM, Reade JL and Ware CF: Rapid
colorimetric assay for cell viability: application to the
quantitation of cytotoxic and growth inhibitory lymphokines. J
Immunol Methods. 70:257–268. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sedlak J and Lindsay RH: Estimation of
total, protein-bound, and nonprotein sulfhydryl groups in tissue
with Ellman's reagent. Anal Biochem. 25:192–205. 1968. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Swindells K and Rhodes LE: Influence of
oral antioxidants on ultraviolet radiation-induced skin damage in
humans. Photodermatol Photoimmunol Photomed. 20:297–304. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tyrrell RM: The molecular and cellular
pathology of solar ultraviolet radiation. Mol Aspects Med. 15:1–77.
1994.PubMed/NCBI
|
|
19
|
Morita A and Krutmann J: Ultraviolet A
radiation-induced apoptosis. Methods Enzymol. 319:302–309. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Svobodova A, Rambouskova J, Walterova D
and Vostalova J: Protective effects of phenolic fraction of blue
honeysuckle fruits against UVA-induced damage to human
keratinocytes. Arch Dermatol Res. 300:225–233. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Schneider LA, Dissemond J, Brenneisen P,
Hainzl A, Briviba K, Wlaschek M and Scharffetter-Kochanek K:
Adaptive cellular protection against UVA-1-induced lipid
peroxidation in human dermal fibroblasts shows donor-to-donor
variability and is glutathione dependent. Arch Dermatol Res.
297:324–328. 2006. View Article : Google Scholar
|
|
22
|
Tarozzi A, Marchesi A, Hrelia S, Angeloni
C, Andrisano V, Fiori J, Cantelli-Forti G and Hrelia P: Protective
effects of cyanidin-3-O-beta-glucopyranoside against UVA-induced
oxidative stress in human keratinocytes. Photochem Photobiol.
81:623–629. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kagan V, Witt E, Goldman R, Scita G and
Packer L: Ultraviolet light-induced generation of vitamin E
radicals and their recycling. A possible photosensitizing effect of
vitamin E in skin. Free Radic Res Commun. 16:51–64. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Han D, Handelman G, Marcocci L, Sen CK,
Roy S, Kobuchi H, Tritschler HJ, Flohé L and Packer L: Lipoic acid
increases de novo synthesis of cellular glutathione by improving
cystine utilization. Biofactors. 6:321–338. 1997. View Article : Google Scholar : PubMed/NCBI
|