Introduction
Gap junctions (GJs) are formed in the cardiovascular
system by connexins 40, 37, 43 and 45 (Cx40, Cx37, Cx43 and Cx45,
respectively). These low resistance channels allow the transfer of
small molecules and ions between cells. The longitudinal coupling
of endothelial and vascular smooth muscle cells (VSMCs) via GJs
allows the spread of signals of membrane potential along the
vascular wall which elicits a coordinated dilation of the arteriole
over a considerable distance (1).
The significance of GJs in the pathogenesis of a vascular response
to injury and disease has been demonstrated in vitro and
in vivo. A previous study reported that a reduction in the
expression of the GJ protein Cx43 in mice causes the dysfunction of
the proliferation and migration of smooth muscle cells (SMCs) and
an inflammatory response resulting in the restriction of intimal
thickening in severe vascular injury (2). During the migration and proliferation
of VSMCs, Cx43 plays a significant role in intercellular signal
transduction. Although Cx40 is homologous to the major vascular
Cx43, the proteins have significantly different functions under
certain conditions. These functions include ascorbic acid
inhibiting the ability of a connexin-mimetic peptide targeted
against Cx40 to attenuate the transmission of endothelial
hyperpolarization to subintimal smooth muscle and a peptide
targeted against Cx43 to attenuate the spread of subintimal
hyperpolarization to subadventitial smooth muscle and the
associated mechanical relaxation (3).
In pregnant women, the endothelium-derived
hyperpolarizing factor-mediated vasorelaxation of subcutaneous
resistance arteries is associated with Cx43 but not Cx40 (4). Lipoprotein-derived phospholipid
oxidation products promote the upregulation of Cx43 in VSMCs and
endothelial cells (ECs), which causes a decrease of Cx40 in ECs and
an elevation of Cx40 in VSMCs (5).
Downregulation of the Cx40 protein expression and the resulting
inhibition of GJ intercellular communication contribute to coronary
vascular dysfunction in diabetes, an effect not observed with Cx43
(6). Extracellular calcium may
upregulate the expression of the cardiac GJ protein Cx40 but not
Cx43 (7). α-adrenoceptor
stimulation may affect Cx43 expression, but not Cx40 (8). These functional differences between
Cx40 and Cx43 support the conclusion that not all connexin isoforms
are regulated by the same mechanisms, even if they involve the same
molecular partners (9). Since
electron microscopy has confirmed the predominant role of Cx40 at
the myoendothelial junction in VSMCs (10), an increasing number of studies
attribute significance to the role of Cx40 in EC and VSMC GJs with
regard to the pathological change of vascular remodeling.
Angiotensin II (Ang II), a powerful vasoconstrictor,
may activate the mitosis of VSMCs, leading to the multiplication of
VSMCs, proliferation of fibroblasts, deposition of collagen and
arterial sclerosis (11). A
previous study showed that Cx40 hemichannels and extracellular ATP
are key molecular elements of the glomerular endothelial calcium
wave (12). Cx40, and probably
intercellular communication via Cx40-dependent GJs, not only
mediates the calcium-dependent inhibitory effects of Ang II and
intrarenal pressure of renin secretion and synthesis (1), but also affects renal autoregulation
(13) and the regulation of blood
pressure (14). Although these new
findings enable a better understanding of the correlations between
alteration in Cx40 expression and the renin-angiotensin system
(RAS), the role of connexins, particularly Cx40, in the migration
and proliferation of VSMCs has not been extensively studied. The
aim of this study was to prove that the alteration of Cx40 and Cx43
proteins in arteries submitted to balloon injury is associated with
RAS and to reveal the roles of Cx40 and Cx43 in the migration and
proliferation of VSMCs. The effects of ramipril, the Ang
II-converting enzyme inhibitor (ACEI), on Cx40 and Cx43 expression,
the structural changes of the GJ and the mechanism involving a
change in the Cx40 and Cx43 of ACEIs in VSMCs are also reported in
this study.
Materials and methods
Animals and balloon injury
A total of 28 male New Zealand white rabbits from
the animal center of Zhejiang University (Hangzhou, China),
weighing 2.5–3.0 kg, were randomly divided into four groups (n=7 in
each): control, sham injury, injury and injury plus ramipril
(Novartis Pharmaceuticals, Basel, Switzerland). The rabbits in the
injury and drug intervention groups were subjected to the surgical
procedures for balloon injury and the sham-operated rabbits were
subjected only to the separation of the femoral artery.
Experimental procedures complied with the ethics and
regulations of Zhejiang University to minimize the number of
animals used, as well as pain experienced. Ethyl urethane (20%; 5
ml/kg, i.v.) was introduced for anesthesia. The right femoral
arteries were separated and exposed and a 2.5 F coronary artery
balloon catheter was inserted from a small incision under the
guidance of fluoroscopic viewing. An aerocyst of ~3 mm in diameter
and 20 mm in length was aerated and inflated at 8 times the
atmospheric pressure and pulled along the whole iliac artery, until
the end of the aortic bifurcation, and was then deflated and
retracted 20 mm. The same procedure was repeated three times to
ensure an overall endothelial denudation within the same arterial
segments. Following the withdrawal of catheters, the femoral artery
was ligated and a layered suture incision was performed. Heparin
sulfate (1000 units) was administered intramuscularly (i.m.) to
prevent thrombosis.
Following surgery, the rabbits were placed in the
animal care unit for three days and benzylpenicillin sodium
(400,000 U/day, i.m.) was administered consecutively. If one rabbit
died, another was recruited and underwent the same surgical
process. Over the three-day care period, the rabbits were submitted
to the same experimental conditions for two weeks. Concomitantly,
ramipril (0.5 mg/kg/day) was added to the daily diet in the injury
plus ramipril group. When these procedures were finished, the
animals were sacrificed with an overdosed i.v. injection of
pentobarbital sodium (180 mg/kg) and the ballooned iliac arteries
were isolated and cut into four segments for analyses using
transmission electron microscopy, immunohistochemistry, western
blotting and reverse transcription-polymerase chain reaction
(RT-PCR).
Pathological analysis and
immunohistochemistry
The pathological segments and connexin expression
were visualised through hematoxylin and eosin (H&E) staining
and immunohistochemistry as described previously (15). Balloon-injured tissues were
pretreated overnight with 4% paraformaldehyde in phosphate-buffered
saline (PBS, pH 7.4) and sliced to 5 μm for streptavidin peroxidase
staining examination. The sections were incubated overnight at 4°C
with anti-Cx40 monoclonal antibody (1:100; Alpha Diagnostics, San
Antonio, TX, USA) or anti-Cx43 monoclonal antibody (1:100;
Chemicon, Temecula, CA, USA). Following three more rinses of PBS,
the sections were developed with peroxidase-labeled reagent
(Histofine simple-stain kit, Nichivei, Japan) for 20 min. Following
dehydration with graded alcohols and cleaning in xylene, the
location of Cx40 and Cx43 was visualized using
3,3′-diaminobenzidine.
Transmission electron microscopy
Ballooned segments were fixed with 2% glutaraldehyde
in a 0.1 M sodium cacodylate buffer (pH 7.4) for 3 h and washed
three times with the buffer. The specimens were then treated with
cacodylate-buffered 2% osmium tetroxide, dehydrated in a graded
ethanol series and embedded in an epoxy resin. Thin sections were
then collected and stained with uranyl acetate, contrasted with
lead citrate and imaged under a Philips TECNA10 electron microscope
(FEI Co., Hillsboro, OR, USA). The PC image analysis software
(Foster Findlay Associates, Newcastle upon Tyne, UK) was used to
measure the size of the GJs.
Western blot analysis
Western blotting was carried out in accordance with
the relevant literature (16).
Protein extracts were lysed in a lysis buffer (0.05% Igepal, 50 mM
Tris-HCl, 5 mM EDTA, 150 mM NaCl, 1% deoxycholic acid, 0.1% SDS and
1% Triton X-100). The whole-cell lysates were centrifuged (10,000
rpm) at 4°C for 30 min and the supernatants were preserved for
protein quantification. The aliquots of total proteins containing
Cx40 (2 mg) and Cx43 (2.8 mg) were separated on 12.5%
SDS-polyacrylamide gels and transferred to a nitrocellulose
membrane. The membranes were then soaked in PBS containing 0.1%
Tween and 5% non-fat dried milk for 30 min. The membranes were
developed overnight with anti-Cx43 (BD Transduction Laboratories™,
BD Biosciences, San Jose, CA, USA, 1:1,000), anti-Cx40 monoclonal
antibody (Alpha Diagnostic International, San Antonio, TX, USA
1:1,000) or anti-actin antibody (PharMingen, San Jose, CA, USA,
1:500). After serial washes, the membranes were incubated with
horseradish peroxidase-conjugated goat anti-rabbit IgG (1:1,000) or
anti-mouse IgG (1:500, Chemicon) for 1 h. Specific signals were
visualized with enhanced chemiluminescence and exposed to Hyperfilm
(Amersham-Pharmacia, Buckinghamshire, UK). The relative expression
level of the protein was represented as the optical density ratio
of connexins to actin using the ChemiDoc XRS system (Bio-Rad
Laboratories, Hercules, CA, USA).
RT-PCR
Samples of 2.5 μg total RNA were extracted using
TRIzol reagent (Gibco-BRL, Rockville, MA, USA) and
reverse-transcribed to produce cDNA using the typical protocol
(17). Briefly, the levels of Cx40
and Cx43 mRNA expression were determined through a
semi-quantitative PCR using glyceraldehyde 3′-phosphate
dehydrogenase (GAPDH) as a reference. RT-PCR primers were designed
(Gene Runner, Hastings Software) as follows: GAPDH-1, 5′-GCG CCT
GGT CAC CAG GGC TGC TT-3′ and GAPDH-2, 5′-TGC CGA AGT GGT CGT GGA
TGA CCT-3′; Cx40-1, 5′-ATG CAC ACT GTG CGC ATG CAG GA-3′ and
Cx40-2, 5′-CAG GTG GTA GAG TTA GCC AG-3′; and Cx43-1, 5′-CAT CTT
CAT GCT GGT GGT GT-3′ and Cx43-2, 5′-TAG TTC GCC CAG TTT TGC TC-3′.
The size of the products, including Cx40 (399 bp), Cx43 (283 bp)
and GAPDH (465 bp) was scaled to match and incorporate each target
gene. The PCR mixture (specific primers, reaction buffer, reverse
transcriptase and dNTP) was submitted to 28 (Cx40) or 30 (Cx43)
amplified cycles. PCR cycling was performed as follows:
preincubation for 2 min at 37°C, denaturation for 5 min at 94°C,
annealing for 45 sec at 57°C, elongation for 10 min at 72°C and
45°C for 15 sec. Gel electrophoresis using 2% agarose was performed
to confirm the mRNA expression of the RT-PCR products (10 ml). The
amplicon specimens were quantified by measuring the optical density
(OD) at 260 nm (Bio-Rad Laboratories).
Statistical analysis
Data were shown as the mean ± SD. Multi-group
comparisons of data sets were analyzed through one-way ANOVA and
Student-Newman-Keuls t-tests with SPSS 15.0 software (Chicago, IL,
USA). 2α=0.05 was considered to indicate a statistically
significant result.
Results
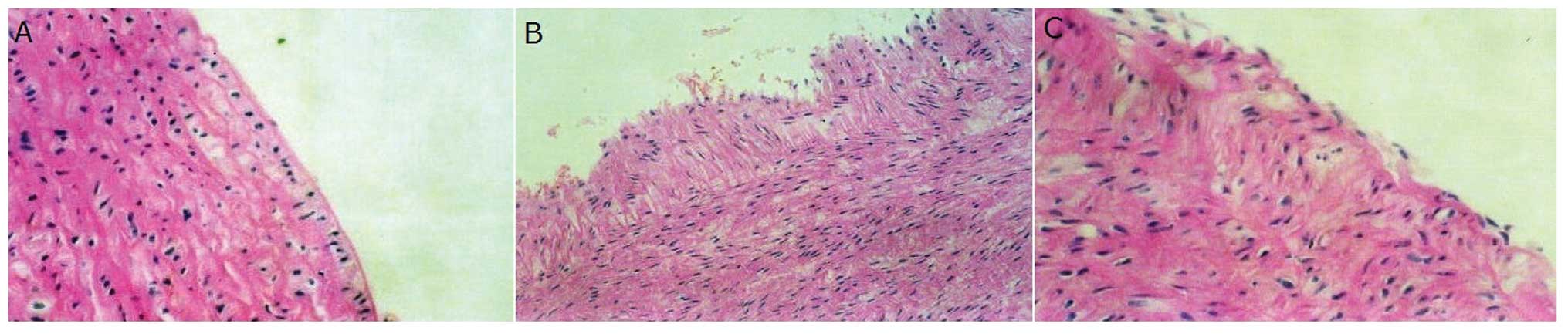
Pathological alterations in the arterial
wall
Following balloon injury, the arterial wall became
uneven and the neointima began to develop in comparison to the
control condition. Following treatment with ramipril, however, the
neointima area was markedly reduced (Fig. 1).
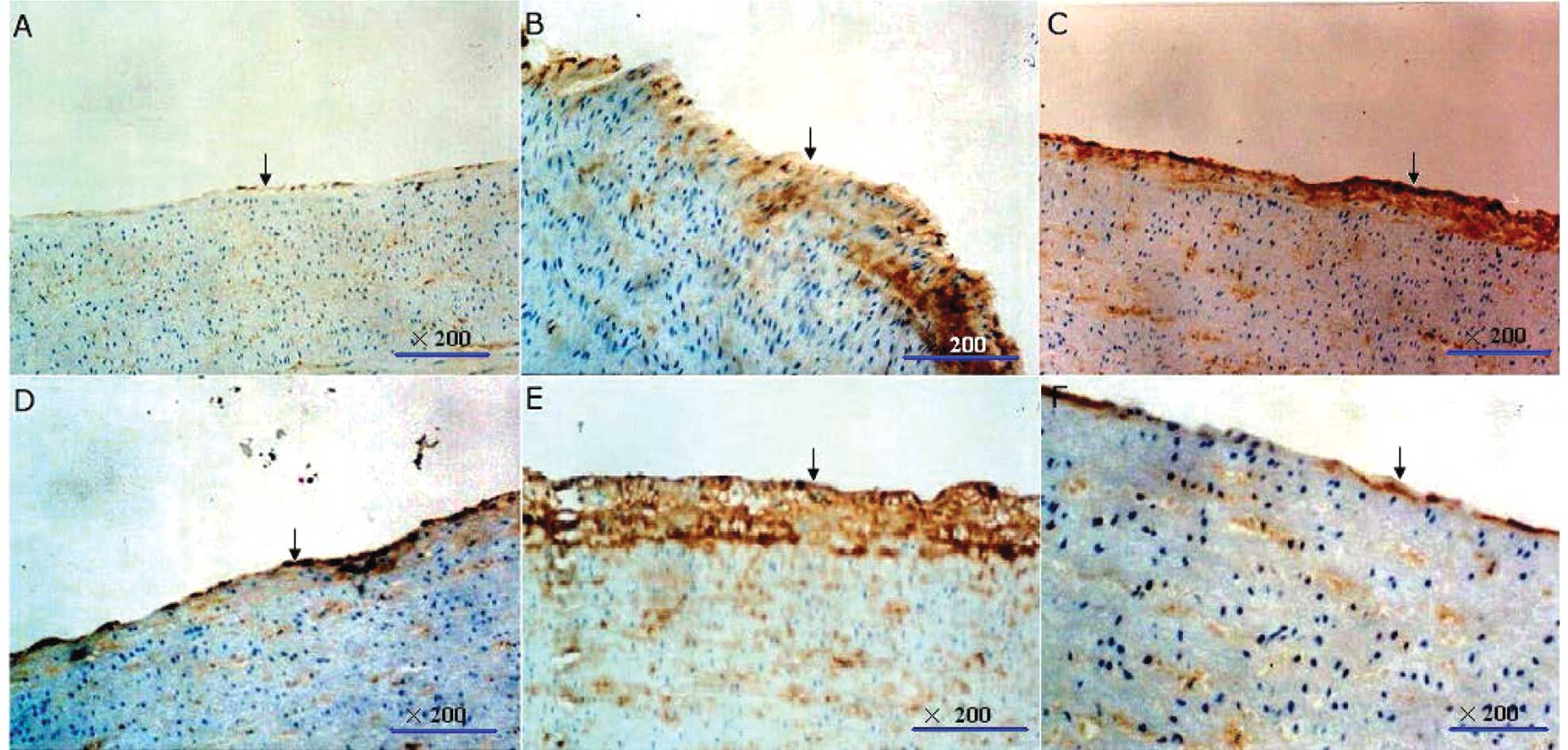
Distribution of Cx40 and Cx43 in the
arterial wall
Balloon injury led to copious immunolabeled Cx40 and
Cx43 staining in arteries, located mainly in the neointima and
media. Ramipril markedly reduced the immunostaining of Cx43 but had
a lesser effect on the immunolabeled Cx40 in the neointima area
(Fig. 2).
Structural changes of the GJs in the
arterial wall
Balloon injury precipitated the remodeling of GJs in
the neointima consisting of SMCs by increasing the size and volume
of GJs. This increase contributes to the proliferation of VSMCs,
which may be inhibited by ramipril.
Expression of Cx40 and Cx43 protein and
mRNA
The levels of protein and mRNA expression of Cx40
and Cx43 were significantly increased in the rabbit iliac arterial
wall subjected to balloon injury. Ramipril markedly inhibited the
elevated Cx43 protein and mRNA expression in balloon-injured
arteries, but did not cause significant changes in the Cx40 protein
and mRNA expression.
Discussion
GJs are one of the most ubiquitous and ancient forms
of intercellular connection involved in signal exchange for the
maintenance of homeostasis and cell growth regulation in
multicellular organisms (18).
Over the past two decades, a number of studies have described a
role for GJ intercellular communication in the proliferation and
differentiation of numerous types of cells, including SMCs
(19). We investigated the effect
of ramipril, the ACEI, on the expression of Cx40 and Cx43 in a
rabbit model of balloon injury. Immunohistochemical analysis and
electron microscopy revealed that ramipril inhibits balloon
injury-induced neointimal formation, Cx43 expression in neointima
and structural changes of GJs between VSMCs. Further studies using
RT-PCR and western blot analysis indicated that ramipril inhibits
the increase of Cx43 protein and mRNA expression in balloon-injured
arteries but not Cx40. These results provide a better understanding
of the mechanism of GJs involved in the regulation of VSMC
proliferation.
In normal conditions, complete ECs are crucial for
the maintenance of the physiology of the blood vessel. The ligation
of ECs and completeness of the endothelium are regulated by Cx40
and Cx43. Following balloon injury, various cytokines and growth
factors are synthesized and secreted by local platelets, SMCs,
inflammatory cells and ECs to promote the migration and
proliferation of SMCs in arterial media. During the migration and
proliferation of arterial SMCs, the cells transform from a
contractile to a synthetic phenotype. Previously, it was shown that
Cx43 expression is enhanced in the cultured synthetic phenotype
VSMCs compared with its contractile counterparts (11). Moreover, Cx43 expression was
maintained at a relatively high level during the proliferation and
migration of VSMCs in the neointima following balloon injury. The
present study, in agreement with previous findings, demonstrates a
significant upregulation of Cx40 and Cx43 protein and mRNA
expression in proliferated VSMCs following balloon injury in rabbit
iliac artery. Thus far the exact mechanisms of upregulation of Cx40
and Cx43 under this pathological condition have not been
elucidated, but it is evident that growth factors in the blood
circulation in the synthetic state contribute to the regulation of
connexin expression in cultured VSMCs.
Since Cx40 and Cx43 are capable of being homomeric
(made from six identical connexins) or heteromeric, and the full GJ
channel may be homotypic (consisting of two identical connexons) or
heterotypic, great diversity is possible. The permeability and
regulatory properties may be different between heteromeric and
homomeric channels and the regulation of certain signals for
passing through its GJ channels may be provided by the heteromeric
channels. It has been reported that Cx40 and Cx43 exist in
heterotypic channels, but the functional channels may be formed
only via particular heterotypic pairings. For instance, the Cx40
protein is highly restricted in its ability to form heterotypic
channels and is only functional when interacting with Cx37, but not
Cx43. Additionally, functional Cx40/Cx43 heterotypic junctions have
been largely disputed and a number of studies have clearly shown
that Cx40 and Cx43 are not compatible to form heterotypic junctions
(20). In our study, transmission
electron microscopy revealed that larger and more abundant GJs
appeared among VSMCs of the neointima in balloon-injured arteries,
while smaller and fewer GJs presented between the medial VSMCs of
the arteries from healthy or ramipril-treated rabbits, indicating
that intercellular communication via GJs plays a crucial role in
the migration and proliferation of VSMCs in the balloon-injured
condition. These results show that an increasing Cx40 and Cx43
expression in the neointima of injured arteries may result in the
enhancement of direct intercellular electromechanical and
biochemical signaling, which is associated closely with VSMC
proliferation. The ACEI ramipril is able to prevent the
proliferation of VSMCs by inhibiting the expression of Cx43 mRNA
and protein.
Our study revealed that ramipril was not able to
alter the expression of Cx40 mRNA and protein in contrast to Cx43,
indicating that the expression of these connexins are regulated
differently. Moreover, Cx43 is the major connexin expressed in the
working myocardium of the heart and SMCs of the blood vessels,
whereas Cx40 is predominantly expressed in the atrium,
atrioventricular node, AV bundle and ECs. In contrast to the
distribution combining the incompatibility of Cx40 and Cx43,
hemichannels may partially determine that direct signal
communications are performed mainly through homotypic Cx40 and Cx43
GJs, which promotes the increase of VSMC growth and differentiation
in pathological conditions. These findings suggest that
intercellular communication is spatially regulated by the selective
expression of different connexins. Thus, the upregulation in the
expression of Cx40 mRNA and protein in proliferated VSMCs following
balloon injury may not be due to Ang II. More experimental studies
are required to clarify why it is not Cx40 but Cx43 GJs that are
responsible for the suppression of ACEI on the proliferation of
VSMCs following balloon injury.
In conclusion, this study has shown that different
roles were performed by Cx40 and Cx43 GJs involved in the
suppression of ACEI on the proliferation of VSMCs under
pathological conditions.
References
|
1
|
Schmidt VJ, Wölfle SE, Boettcher M and de
Wit C: Gap junctions synchronize vascular tone within the
microcirculation. Pharmacol Rep. 60:68–74. 2008.
|
|
2
|
Chadjichristos CE, Matter CM, Roth I,
Sutter E, Pelli G, Lüscher TF, Chanson M and Kwak BR: Reduced
connexin43 expression limits neointima formation after balloon
distension injury in hypercholesterolemic mice. Circulation.
113:2835–2843. 2006.
|
|
3
|
Edwards DH, Chaytor AT, Bakker LM and
Griffith TM: Modulation of gap-junction-dependent arterial
relaxation by ascorbic acid. J Vasc Res. 44:410–422. 2007.
|
|
4
|
Lang NN, Luksha L, Newby DE and
Kublickiene K: Connexin 43 mediates endothelium-derived
hyperpolarizing factor-induced vasodilatation in subcutaneous
resistance arteries from healthy pregnant women. Am J Physiol Heart
Circ Physiol. 292:H1026–H1032. 2007.
|
|
5
|
Isakson BE, Kronke G, Kadl A, Leitinger N
and Duling BR: Oxidized phospholipids alter vascular connexin
expression, phosphorylation, and heterocellular communication.
Arterioscler Thromb Vasc Biol. 26:2216–2221. 2006.
|
|
6
|
Makino A, Platoshyn O, Suarez J, Yuan JX
and Dillmann WH: Downregulation of connexin40 is associated with
coronary endothelial cell dysfunction in streptozotocin-induced
diabetic mice. Am J Physiol Cell Physiol. 295:C221–C230. 2008.
|
|
7
|
Dhein S, Duerrschmidt N, Scholl A, Boldt
A, Schulte JS, Pfannmüller B, Rojas-Gomez D, Scheffler A, Haefliger
JA, Doll N and Mohr FW: A new role for extracellular
Ca2+ in gap-junction remodeling: studies in humans and
rats. Naunyn Schmiedebergs Arch Pharmacol. 377:125–138. 2008.
|
|
8
|
Rojas, Gomez DM, Schulte JS, Mohr FW and
Dhein S: Alpha-1-adrenoceptor subtype selective regulation of
connexin 43 expression in rat cardiomyocytes. Naunyn Schmiedebergs
Arch Pharmacol. 377:77–85. 2008.
|
|
9
|
Bouvier D, Kieken F, Kellezi A and Sorgen
PL: Structural changes in the carboxyl terminus of the gap junction
protein connexin 40 caused by the interaction with c-Src and zonula
occludens-1. Cell Commun Adhes. 15:107–118. 2008.
|
|
10
|
Isakson BE, Best AK and Duling BR:
Incidence of protein on actin bridges between endothelium and
smooth muscle in arterioles demonstrates heterogeneous connexin
expression and phosphorylation. Am J Physiol Heart Circ Physiol.
294:H2898–H2904. 2008.
|
|
11
|
Cai W, Ruan LM, Wang YN and Chen JZ:
Effects of angiotensin II on connexin 43 of VSMCs in
arteriosclerosis. J Zhejiang Univ Sci B. 7:648–653. 2006.
|
|
12
|
Toma I, Bansal E, Meer EJ, Kang JJ, Vargas
SL and Peti-Peterdi J: Connexin 40 and ATP-dependent intercellular
calcium wave in renal glomerular endothelial cells. Am J Physiol
Regul Integr Comp Physiol. 294:R1769–R1776. 2008.
|
|
13
|
Takenaka T, Inoue T, Kanno Y, Okada H,
Hill CE and Suzuki H: Connexins 37 and 40 transduce purinergic
signals mediating renal autoregulation. Am J Physiol Regul Integr
Comp Physiol. 294:R1–R11. 2008.
|
|
14
|
Spray DC: Hypertension in connexin40-null
mice: a renin disorder. Kidney Int. 72:814–822. 2007.
|
|
15
|
Wang LH, Chen JZ, Sun YL, Zhang FR, Zhu
JH, Hu SJ and Wang DH: Regulation of connexin expression after
balloon injury: possible mechanisms for antiproliferative effect of
statins. Am J Hypertens. 18:1146–1153. 2005.
|
|
16
|
Duval N, Gomès D, Calaora V, Calabrese A,
Meda P and Bruzzone R: Cell coupling and Cx43 expression in
embryonic mouse neural progenitor cells. J Cell Science.
115:3241–3251. 2002.
|
|
17
|
Fruebis J, Gonzalez V, Silvestre M and
Palinski W: Effect of probucol treatment on gene expression of
VCAM-1, MCP-1, and M-CSF in the aortic wall of LDL
receptor-deficient rabbits during early atherogenesis. Arterioscler
Thromb Vasc Biol. 17:1289–1302. 1997.
|
|
18
|
Mennecier G, Derangeon M, Coronas V, Hervé
JC and Mesnil M: Aberrant expression and localization of connexin43
and connexin30 in a rat glioma cell line. Mol Carcinog. 47:391–401.
2008.
|
|
19
|
Ciovacco WA, Goldberg CG, Taylor AF,
Lemieux JM, Horowitz MC, Donahue HJ and Kacena MA: The role of gap
junctions in megakaryocyte-mediated osteoblast proliferation and
differentiation. Bone. 44:80–86. 2009.
|
|
20
|
Louault C, Benamer N, Faivre JF, Daniel
Potreau D and Bescond J: Implication of connexins 40 and 43 in
functional coupling between mouse cardiac fibroblasts in primary
culture. Biochim Biophys Acta. 1778:2097–2104. 2008.
|