Introduction
Cerebrovascular injury is one of the three major
causes of death and is the leading cause of adult disability. The
annual incidence rate in China is 130–300 million, with 60–100
million deaths and 75% of survivors suffering disabilities of
various degrees. Despite the increasing progress in emergency
treatment and early rehabilitation in patients with cerebrovascular
injury, treatment options for neurological dysfunction that
presents at a later stage are lacking.
Regenerative medicine and stem cell research have
progressed significantly in the 21st century, offering novel routes
for the treatment of neurological disorders. Mesenchymal stem cells
(MSCs), unlike hematopoietic stem cells, are present in bone
marrow. Bone mesenchymal stem cells (BMSCs) have become a
progressive research field in modern biology and medicine. MSCs are
derived from the mesoderm early in development and may be exploited
as an ideal source of seed cells, which exhibit the potential to be
induced into osteogenic, chondrogenic and adipogenic cells, or even
tendon and adipose tissues (1–4).
MSCs are easy to obtain, culture and expand in vitro, and
are readily induced into designated tissues. Currently, BMSCs are
widely used. However, MSCs are present in extremely low amounts in
bone marrow, accounting for 0.01–0.001% of the bone marrow-derived
cells (5). Increasing evidence
indicates that MSCs with osteogenic potential may be isolated from
a diverse range of tissues, including adipose (6) and perinatal tissues, such as
umbilical cord (7), placenta
(8,9), umbilical cord blood (10,11)
and amniotic fluid (12,13), or even fetal blood, bone marrow and
liver (14–17).
Placenta, a temporary organ, is important for
maintaining maternal and fetal oxygen and nutrients during
embryonic development. The full-term placenta comprises amnion and
chorion, and our previous findings (18) indicate that MSCs may be obtained
and expanded from the amnion (amniotic MSCs; AMSCs) and chorion
(chorionic MSCs; CMSCs) of placenta (placental MSCs; PMSCs) in
vitro; their biological characteristics remain well maintained,
similar to those of BMSCs. In addition, a cell bank of PMSCs may be
set up in advance for clinical trials, suggesting that PMSCs have a
wide application prospect (18).
In this study, we aimed to establish a stable and
reliable method for the isolation and amplification of human
amniotic mesenchymal stem cells (hAMSCs) in vitro. Following
induction into neural cells, the hAMSCs were transplanted into the
ischemic tissue of rats subjected to focal cerebral ischemia by
middle cerebral artery occlusion (MCAO). The survival, migration
and differentiation of the implanted cells and the recovery of
neurological function were assessed in rats 1–8 weeks later to
examine the potential therapeutic benefit of the hAMSC-derived
neuron-like cell transplantation in the treatment of focal cerebral
ischemia.
Materials and methods
Cell culture
hAMSCs were obtained from normal post-partum
placenta. The amnion and villus layer were bluntly separated and
repeatedly washed with D-Hank’s solution, including double
resistant (100 U/ml penicillin and 100 μg/ml streptomycin). After
rinsing, the amnion was cut into several 1×1-mm sections with
ophthalmic scissors and digested at 37°C in a water bath for ~30
min with the action of 2.5 g/l trypsin (Gibco-BRL, Carlsbad, CA,
USA). The digestion of the amnion was terminated with Dulbecco’s
modified Eagle’s medium (DMEM) containing 5% calf serum and
filtered through a 200 mesh cell sieve. The filtered amnion
products were digested again in a 37°C water bath for ~0.5 h with
the addition of 1.0 g/l collagenase II (Sigma-Aldrich, St. Louis,
MO, USA). Subsequent termination and filtrations were performed as
described above. Finally, the harvested cell suspensions were
centrifuged at 1,000 rpm for 5 min and the cell pellet was
resuspended in low-glucose DMEM (L-DMEM; Hyclone Laboratories,
Inc., South Logan, UT, USA) supplemented with 10% fetal bovine
serum (FBS; Hyclone) and 1% penicillin-streptomycin (Invitrogen
Life Technologies, Carlsbad, CA, USA). The cells were then plated
in 25-cm2 culture flasks at a density of
1×106 cells/ml and incubated at 37°C with 5% carbon
dioxide. The medium was changed every 2 days. When the established
adherent cell colonies reached 70% confluence, they were detached
with 2.5 g/l trypsin and replated at a ratio of 1:2 in
25-cm2 flasks.
Differentiation of hAMSCs
Second or third generation hAMSCs were plated onto
6-well plates. When 60% confluence was achieved, the harvested
cells were washed with phosphate-buffered saline (PBS). To induce
neural differentiation, the hAMSCs were incubated with serum-free
medium containing DMSO (2%) and butylated hydroxyanisole (BHA) (100
μM). The media were changed every 3 days and culturing was
continued for 14–21 days. The neural induced cells were then
confirmed by neuron-specific enolase (NSE) and glial fibrillary
acidic protein (GFAP) immunofluorescence staining.
Immunofluorescence
Immunofluorescence was performed on hAMSCs cultured
for 24 h. The cells were grown to 60% confluence on 6-well plates,
washed with PBS three times, fixed in 4% paraformaldehyde for 30
min, washed as previously described, permeabilized in 0.3% Triton
X-100 for 20 min and then rinsed with PBS three times. The cells
were then blocked with goat serum for 20 min, incubated with the
appropriate primary antibody in PBS for 2 h at 37°C, washed with
PBS three times, incubated with secondary antibodies in PBS for 30
min at 37°C (in the dark) and then viewed under a fluorescence
microscope. The following primary antibodies were used: rabbit
anti-human NSE (1:500) and rabbit anti-human GFAP (1:500), both
from Boster Biological Technology, Ltd). The secondary antibody for
immunofluorescence was goat anti-rabbit IgG (1:500; Sigma).
BrdU labeling and preparation of cell
suspension for transplantation
Third generation cells from the AMSCs were collected
and plated in 25-cm2 culture flasks and 6-well plates at
a density of 1×105 cells/ml. The cell pellet was
resuspended in L-DMEM supplemented with 10% FBS,
penicillin-streptomycin (100 μg/ml), 5 ng/ml bFGF and 10 μg/ml
BrdU, and then incubated at 37°C with 5% carbon dioxide for 48 h.
BrdU-labeled AMSCs were centrifuged at 1,000 rpm for 10 min and the
cell pellet was resuspended in PBS at 1×106 cells/μl.
Finally, 5-μl cell suspensions were used for cell
transplantation.
Animal model
Healthy male Wistar rats, aged 3–4 months and
weighing 250–300 g, were obtained from the Schistosomiasis
Prevention and Control Center of Jiangsu Province, China. Briefly,
the rats were placed in a supine position on an operating table
following the intraperitoneal injection of 10% chloral hydrate (4
m1/kg) anesthetic. A blunt dissection of the sternocleidomastoid
was made through the middle line neck incision, and the carotid
artery (CCA) was isolated and then separated into the right
external carotid artery (ECA), internal carotid artery (ICA) and
the wing jaw artery. A slipknot was left under the ECA and the wing
jaw artery, after threading deeply into all the arteries. The CCA
was clamped, a small incision was made in the proximal sidewall of
the ECA and a nylon suture filament (0.24 mm) was inserted and
advanced to a depth of ~18.5±0.5 mm away from the CCA bifurcation.
The suture was removed following a 2-h right MCAO, the ECA was
ligated and the skin was sutured.
Animal grouping
Out of 45 rats subjected to focal cerebral ischemia,
13 died and 8 did not exhibit paralysis of the limbs. The remaining
24 rats were randomly divided into two groups (n=12 per group).
hAMSC transplantation
Two weeks after MCAO, the rats were placed in a
stereotactic apparatus and the bregma was exposed through a median
head scalp incision. Coordinates were marked to enable targeting of
the striatum (1 mm anterior to the skull, left margin 2.5 mm; depth
4.5–5.5 mm), and a 5 μl BrdU-labeled AMSC suspension or PBS control
was then injected into the striatum with a Hamilton syringe for 10
min.
Neurological behavior evaluation
Neurological deficit evaluations were carried out
prior to the transplantation and 1, 3, 6 and 8 weeks after MCAO
using the neurological severity score (NSS; Table I), beam balance test (BBT; Table II) and elevated body swing test
(EBST). For the EBST, observers were instructed to record data only
when the rat head moved >10° from the vertical axis within 30
sec of the rat being raised by the tail. This procedure was
followed by 1 min of rest, and the test was then repeated 20 times.
For the three tests, recordings were taken on every last day of
weeks 1, 3, 6 and 8, and all rats were tested three times at
different time-points for each test, and the average result was
determined.
 | Table INeurological severity scores. |
Table I
Neurological severity scores.
| Grading | Score (normal, 0;
maximum, 5) |
|---|
| Normal walk | 0 |
| Flexion of forelimb
(raising the rat by the tail) | 1 |
| Circling toward the
paretic side (walking) | 2 |
| Falling down to the
paretic side (walking) | 3 |
| No spontaneous
walking, decreased consciousness | 4 |
| Ischemia-related
deaths | 5 |
 | Table IIBeam balance test. |
Table II
Beam balance test.
| Grading | Score (normal, 0;
maximum, 6) |
|---|
| Balances with steady
posture | 0 |
| Grasps side of
beam | 1 |
| Hugs the beam and one
limb falls down from the beam | 2 |
| Hugs the beam and two
limbs fall down from the beam, or spins on beam (>60 sec) | 3 |
| Attempts to balance
on the beam, but falls off (>40 sec) | 4 |
| Attempts to balance
on the beam, but falls off (>20 sec) | 5 |
| Falls off: no attempt
to balance or hang on to the beam (>20 sec) | 6 |
Triphenyltetrazolium chloride (TTC)
staining
TTC staining was used to show the ischemic area of
the brain tissue following 2 h of perfusion. A coronal cut was then
made in the brain tissue, 2 mm behind the optic chiasm, the latter
part of the brain was immersed in 1% TTC (Sigma-Aldrich) in PBS at
37°C for 30 min and then into 10% neutralized formalin
overnight.
Preparation of paraffin and frozen
sections
Eight weeks after MCAO, the rats were anesthetized
intraperitoneally with 400 mg/kg chloral hydrate, perfused
transcardially with 4% paraformaldehyde in PBS, and their brains
were quickly extracted. An ~2 cm ischemic area of brain tissue,
including the lateral ventricles and basal ganglia, striatum and
hippocampus, was excised and post-fixed in 4% paraformaldehyde.
Coronal brain slices (5-μm) were then consecutively sampled using
paraffin sections or frozen sections.
Perl’s Prussian Blue stain for
hemosiderin
Sections were transferred to distilled water with
xylene and ethanol, placed into the working solution (an equal
parts mixture of ferrocyanide and hydrochloric acid) for 15 min,
rinsed with distilled water and then with tap water. Sections were
then stained with neutral red for 1 min, rinsed well with tap
water, dehydrated with ethanol and finally cleared with xylene. Out
of 400 slices, every 20th slice was stained using this method to
confirm the needle placement and injected sites.
Statistical analysis
Data were presented as the mean ± standard
deviation. Comparisons of neurological scores were carried out by
ANOVA (F test, q test), using SPSS 10.0. The paired t-test was used
for the cell count. In the analysis, a value of P<0.05 indicated
a statistically significant result.
Results
AMSC culture and cell phenotype
Adherent cells were observed 4 h after the cells
were plated, and clone-like growth was observed 48 h later. The
morphology of these cells was similar to that of BMSCs:
spindle-shaped with fibroblast-like colonies adhering to the
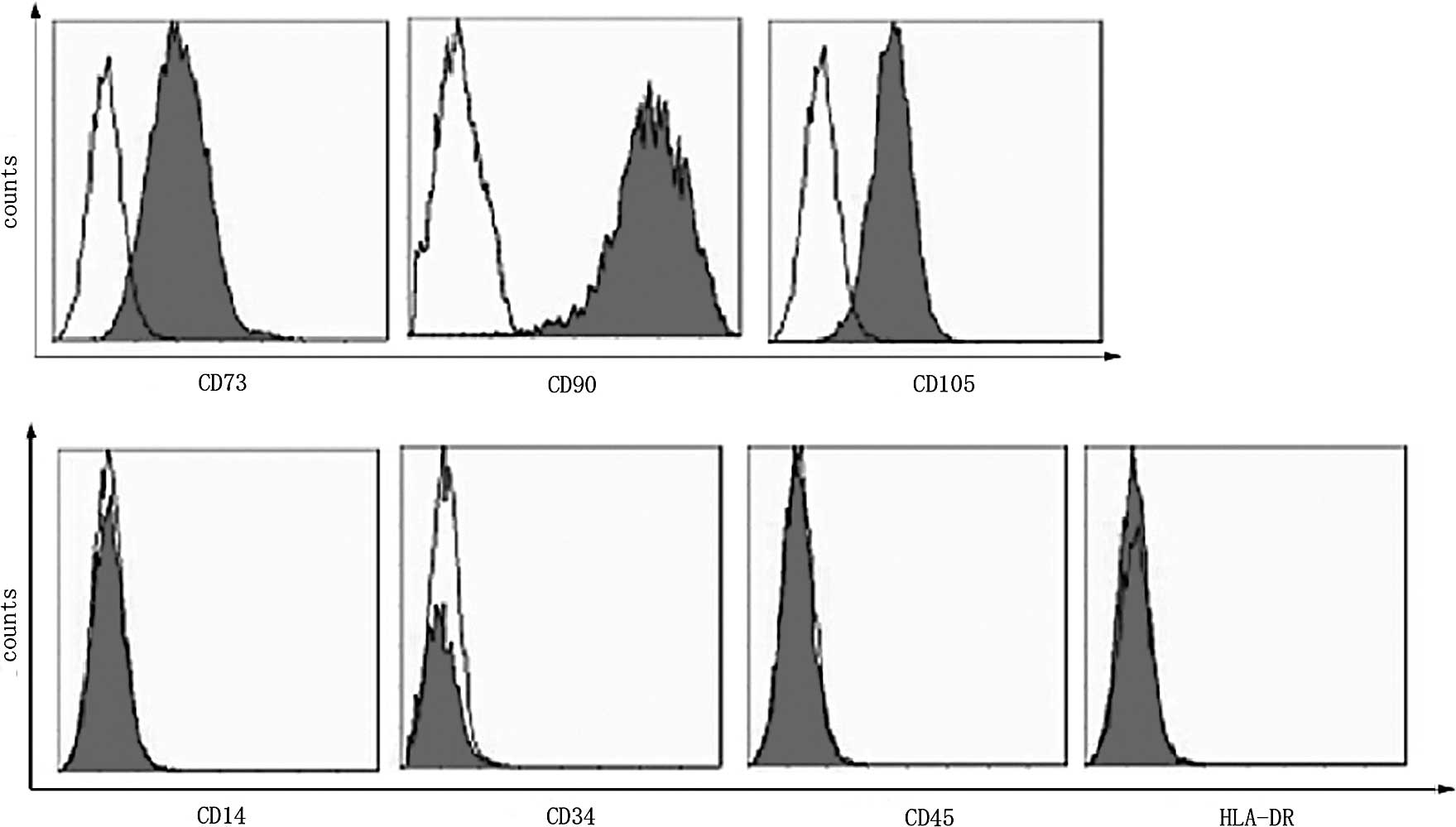
plastic surface. Flow analysis (Fig.
1) showed that the AMSCs expressed the typical MSC markers
(CD73, CD105 and CD90), but were negative for hematopoietic markers
(CD34 and CD45), the monocytic marker (CD14) and HLA-DR. A large
number of BrdU-positive cells were observed using fluorescence
microscopy, suggesting successful transplantation of the AMSCs.
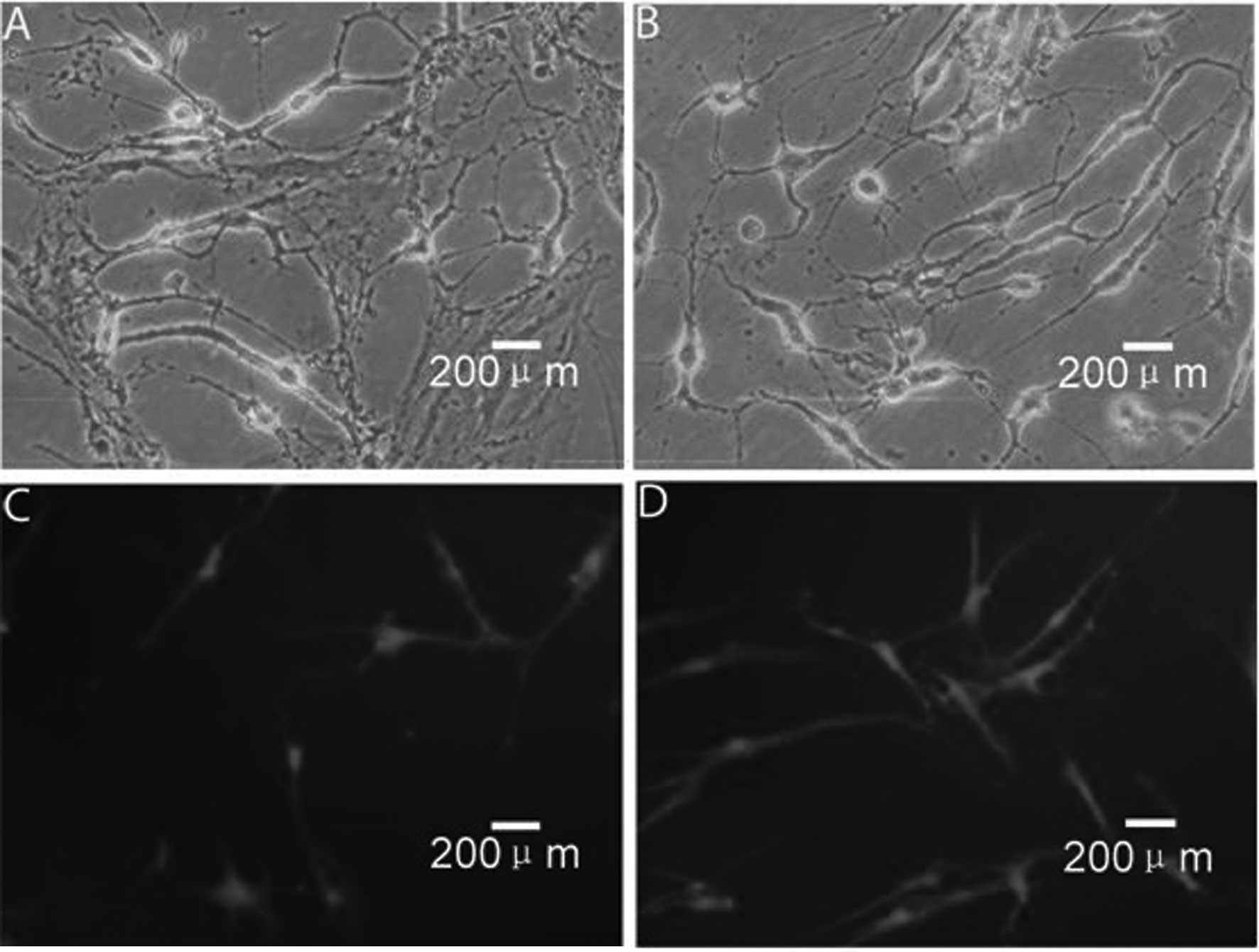
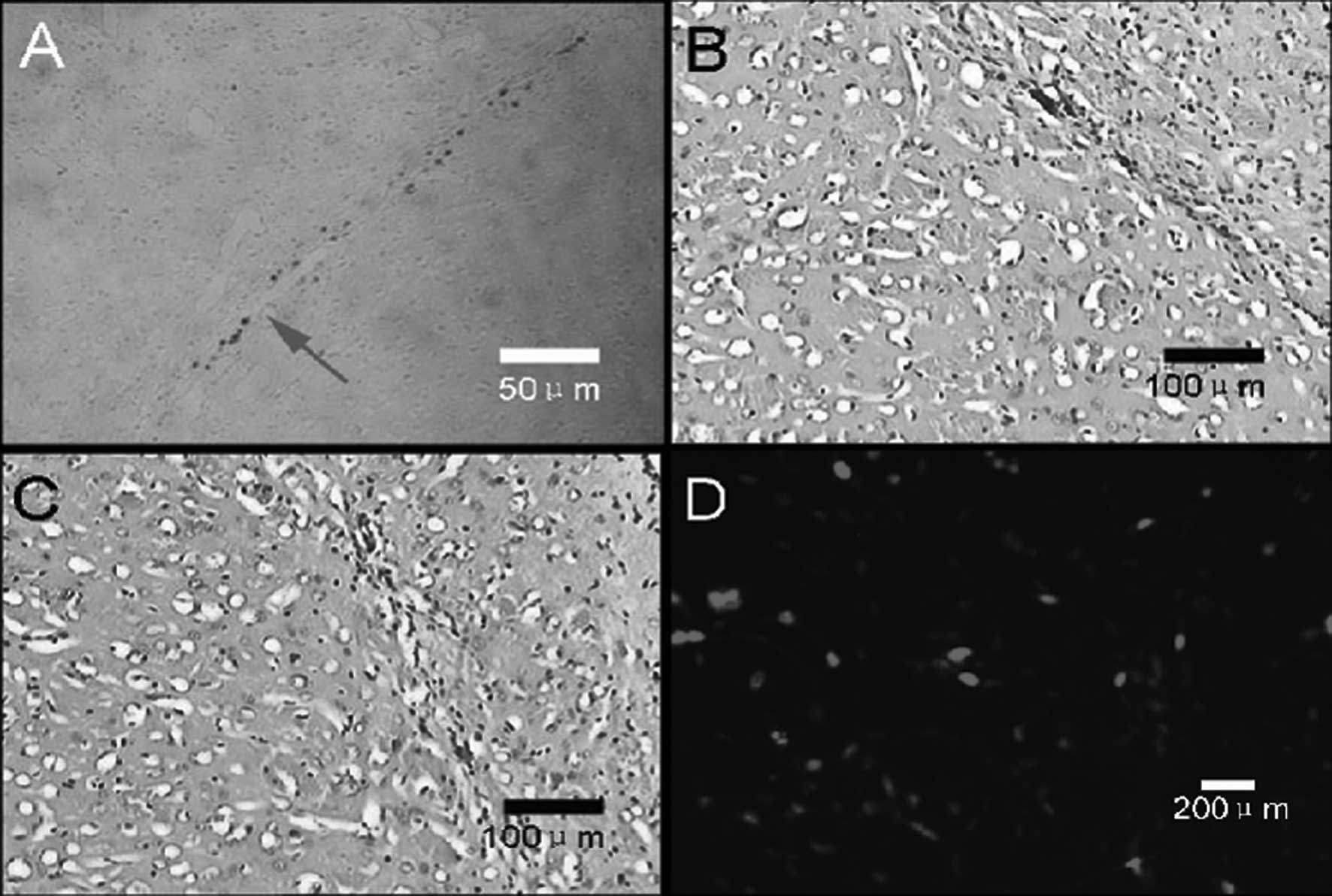
Neural induction of AMSCs
Morphological changes, including condensed cell
bodies with outgrowth in a few sites, were detected in some of the
cells 2 h after incubation (Fig.
2A), with more cells showing these neural cell-like changes 1 h
later (Fig. 2B). In addition to
the morphological changes, differentiated cells expressed NSE, a
marker for neural progenitor cells, and GFAP, a marker for
astrocytes (Fig. 2C and D,
respectively).
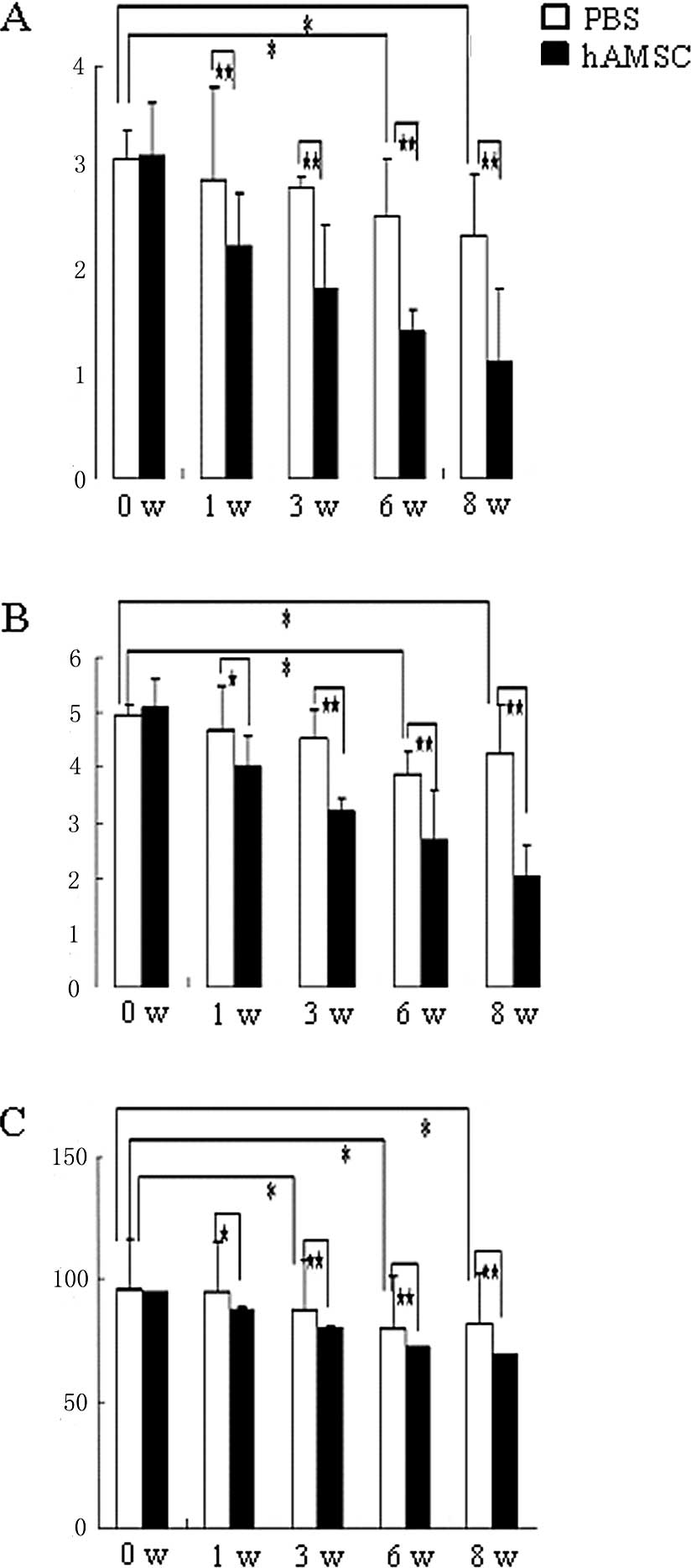
Neurological function score
The rats were tested for neurological function at
different time-points using the NSS, BBT and EBST tests (Fig. 3A-C). In each test, the neurological
behaviors were markedly improved, and there was a significant
difference between the AMSC-transplanted and the PBS-injected
groups.
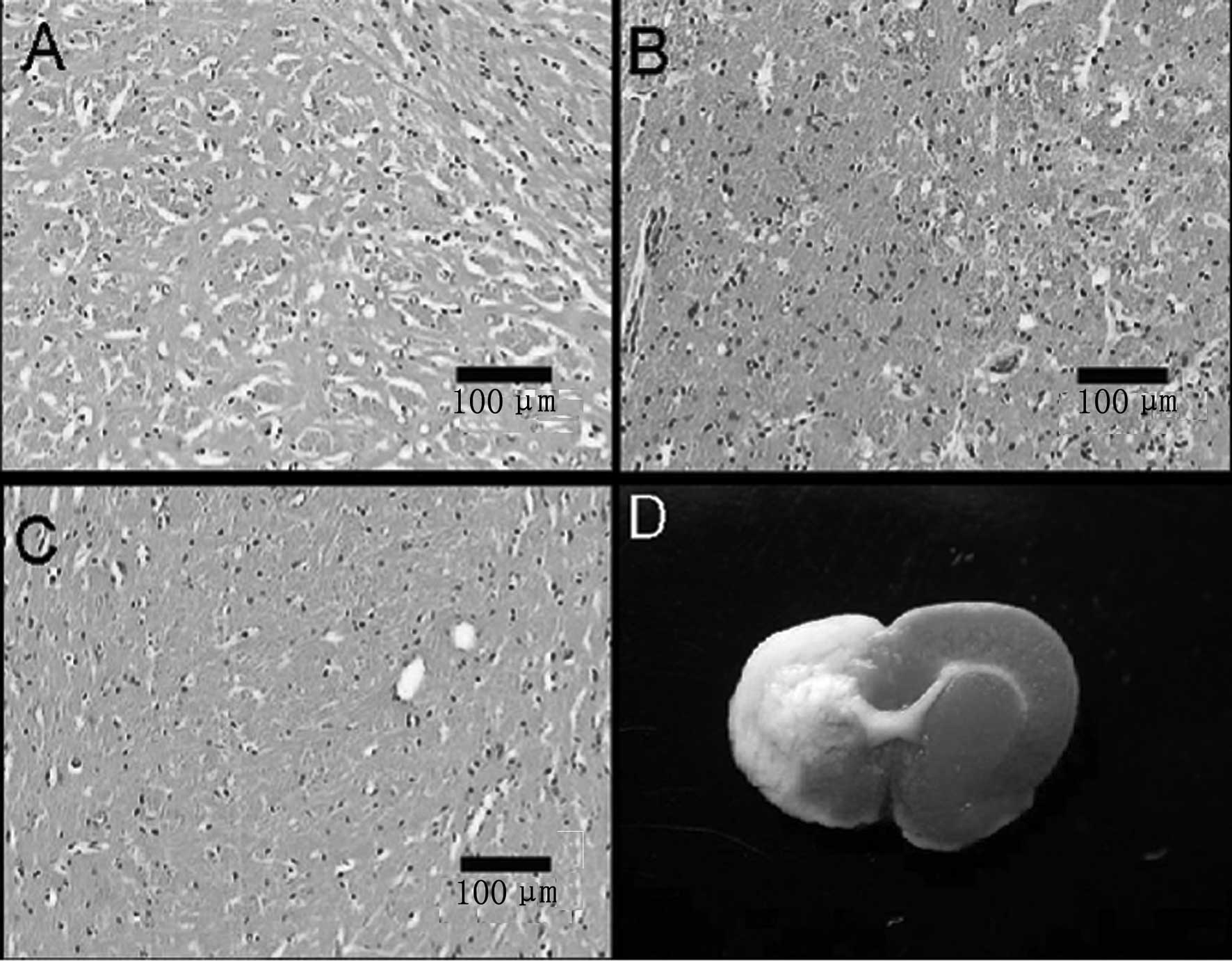
TTC and H&E staining
TTC staining is a standard for the measurement of
infarct size and has previously been used for assessment of infarct
size resulting from apoptosis and necrosis (6). Normal brain tissue was shown in gray,
while the ischemic area was white in TTC staining (Fig. 4D). The ischemic region was observed
in the dorsolateral striatum and was lateral to the ischemic
hemisphere, which is consistent with the blood supply area of the
middle cerebral artery (Fig. 4A).
In the ischemic hemisphere, H&E staining indicated a large
necrotic area in the 8th week after transplantation. Within this
region, there was a significant loss of neurons, with only a few
remaining astrocytes, and a marked interstitial edema. The
surviving neurons had varying degrees of morphological changes,
with the most significant changes occurring in pyramidal cells,
which showed a shrunken cell body, retracted processes and loss of
Nissl bodies. Furthermore, the chromatin became cloudy and the
nuclear membrane decreased in size (Fig. 4B). Fewer degenerated cells were
observed in the AMSC-transplanted area (Fig. 4C).
Determination of injection sites, needle
passage and the BrdU-labeled transplanted AMSCs
The injection sites and needle tracks were
identified in each of the 24 rats using specific hemosiderin
staining (Fig. 5A) and showed that
17 rats were injected into the striatum and the other 7 rats in the
cerebral cortex. Small amounts of lymphocytic infiltration and
glial cell proliferation were observed around the needle tracks
(Fig. 5B and C). In the
experimental group, BrdU-positive AMSCs near the ischemic lesion
were found to be distributed around the needle passages (Fig. 5D), with some cells residing at a
distance of 2 mm away. No BrdU-positive cells were observed around
the needle passages in the control group.
Discussion
Cell replacement therapy has become a developing and
promising approach for the treatment of central nervous system
injury and disease. In this study, we used the focal cerebral
ischemia model in rats and implanted hAMSCs in the ischemic
hemisphere using stereotaxic targeting to the striatum or cortex.
We observed that cell survival and differentiation of the hPMSCs in
the cerebral ischemic rat brain was associated with recovery of
neurological function. We found that PMSCs implanted into ischemic
tissue in rats resulted in improved neurological function and
balance beam test performances relative to the control group.
Similarly, histological staining showed PMSC survival within the
ischemic region.
Silva et al analyzed the gene expression of
MSCs and found that MSCs, not only code the genes of mesenchymal
tissue, but also the genes of endothelial and epithelial tissues
(19). These results provide a
theoretical basis for the potential differentiation of MSCs. MSCs
may be used to replace a variety of cells due to their inherent
plasticity of cross-system and even cross-germ layer
differentiation. Deng et al showed that the bone marrow MSCs
of rats spontaneously express neural-specific proteins (20), such as NSE, β-III tubulin, NFM and
S100-β. In this study, the expression of NSE and GFAP was detected
following the induction of the hAMSCs by BHA. Furthermore,
placental amnion develop from embryonic ectoderm, thus we
speculated that the amniotic MSCs are more readily induced to
differentiate into astrocytes and neuronal cells than are MSCs
derived from other sources. Therefore, hAMSCs have broad
application prospects in the treatment of nervous system damage and
repair cell research.
When determining the best time-points for the
transplantation of hAMSCs following ischemic injury, it is
important to consider the release of toxic neurotransmitters and
oxygen-free radicals at the early stage of transplantation, and the
effect of scar formation on the growth and differentiation of the
transplanted cells at chronic infarction. For example, Li et
al found that when cells were transplanted 1 or 7 days after
acute stroke, nerve toxins, free radicals and pro-inflammatory
mediators led to the further development of ischemic injury and
affected the transplanted cells which underwent apoptotic cell
death in the ischemic penumbra (21). In addition, inflammation activates
microglia and inhibits the growth and survival rate of endogenous
neural cells. Fukunaga et al considered the best treatment
window for BMSC transplantation to be at least 1 month after the
patient experienced a stroke (22). In the present study, we
transplanted cells 2 weeks after stroke and found that hAMSCs are
dense within the ischemic lesion, suggesting that they migrate
and/or proliferate within the injured tissue. Furthermore, we found
that 8 weeks after cell transplantation, neurological function was
improved compared with the control group.
In this study, we showed that the transplantation of
hAMSCs markedly improves neurological recovery following MCAO
through stereotaxic injection. Additionally, that the recovery was
likely to be associated with the secretion function of the
implanted MSCs, as it has been reported that the ratio of cell
survival and differentiation reaches approximately 80% in
vitro (23) and only 3–10%
in vivo (24). However, the
mechanism underlying recovery is unclear and should be
investigated. However, the approach we have described in this study
offers a promising new route for the treatment of neurological
disorders, including ischemic stroke.
Acknowledgements
This study was supported by grants from the Major
State Basic Research Development Program of China (973 Program:
2007CB512402), the National Natural Science Foundation of China
(nos. 30930085 and 31000654). The authors especially thank Dr Shan
Jiang and Qun Xue for the valuable suggestions and critical review
of this manuscript.
References
|
1
|
Jiang Y, Jahagirdar BN, Reinhardt RL, et
al: Pluripotency of mesenchymal stem cells derived from adult
marrow. Nature. 418:41–49. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kadiyala S, Young RG, Thiede MA, et al:
Culture expanded canine mesenchymal stem cells possess
osteochondrogenic potential in vivo and in vitro. Cell Transplant.
6:125–134. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Reyes M, Lund T, Lenvik T, et al:
Purification and ex vivo expansion of postnatal human marrow
mesodermal progenitor cells. Blood. 98:2615–2625. 2001. View Article : Google Scholar
|
|
4
|
Väänänen HK: Mesenchymal stem cells. Ann
Med. 37:469–479. 2005.
|
|
5
|
Rao MS and Mattson MP: Stem cells and
aging: expanding the possibilities. Mech Ageing Dev. 122:713–734.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zuk PA, Zhu M, Mizuno H, et al:
Multilineage cells from human adipose tissue: implications for
cell-based therapies. Tissue Eng. 7:211–228. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sarugaser R, Lickorish D, Baksh D, et al:
Human umbilical cord perivascular (HUCPV) cells: a source of
mesenchymal progenitors. Stem Cells. 23:220–229. 2005.PubMed/NCBI
|
|
8
|
Parolini O, Alviano F, Bagnara GP, et al:
Isolation and characterization of cells from human term placenta:
outcome of the first international workshop on placenta derived
stem cells. Stem Cells. 26:300–311. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Soncini M, Vertua E, Gibelli L, et al:
Isolation and characterization of mesenchymal cells from human
fetal membranes. J Tissue Eng Regen Med. 1:296–305. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bieback K, Kern S, Klüter H, et al:
Critical parameters for the isolation of mesenchymal stem cells
from umbilical cord blood. Stem Cells. 22:625–634. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lee OK, Kuo TK, Chen WM, et al: Isolation
of multipotent mesenchymal stem cells from umbilical cord blood.
Blood. 103:1669–1675. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
De CP, Bartsch GJ, Siddiqui MM, et al:
Isolation of amniotic stem cell lines with potential for therapy.
Nat Biotechnol. 25:100–106. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lee OK, Kuo TK, Chen WM, et al: Isolation
of multipotent mesenchymal stem cells from umbilical cord blood.
Blood. 103:1669–1675. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chan J, O’Donoghue K, Gavina M, et al:
Galectin-1 induces skeletal muscle differentiation in human fetal
mesenchymal stem cells and increases muscle regeneration. Stem
Cells. 24:1879–1891. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Campagnoli C, Roberts IA, Kumar S, et al:
Identification of mesenchymal stem/progenitor cells in human
first-trimester fetal blood, liver, and bone marrow. Blood.
98:2396–2402. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chan J, O’Donoghue K, de la Fuente J, et
al: Human fetal mesenchymal stem cells as vehicles for gene
delivery. Stem Cells. 23:93–102. 2005. View Article : Google Scholar
|
|
17
|
Chan J, Waddington SN, O’Donoghue K, et
al: Widespread distribution and muscle differentiation of human
fetal mesenchymal stem cells after intrauterine transplantation in
dystrophic mdx mouse. Stem Cells. 25:875–884. 2007. View Article : Google Scholar
|
|
18
|
Miao ZN, Jin J, Chen L, et al: Isolation
of mesenchymal stem cells from human placenta: comparison with
human bone marrow mesenchymal stem cells. Cell Biol Int.
30:681–687. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Silva GV, Litovsky S, Assad JA, et al:
Mesenchymal stem cells differentiate into an endothelial phenotype,
enhance vascular density, and improve heart function in a canine
chronic ischemia model. Circulation. 111:150–156. 2005. View Article : Google Scholar
|
|
20
|
Deng J, Petersen EB, Steindler DA, et al:
Mesenchymal stem cells spontaneously express neural proteins in
culture and are neurogenic after transplantation. Stem Cells.
24:1054–1064. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li Y, Chopp M, Chen J, et al:
Intrastriatal transplantation of bone marrow nonhematopoietic cells
improves functional recovery after stroke in adult mice. J Cereb
Blood Flow Metab. 20:1311–1319. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Fukunaga A, Uchida K, Hara K, et al:
Differentiation and angiogenesis of central nervous system stem
cells implanted with mesenchyme into ischemic rat brain. Cell
Transplant. 8:435–441. 1999.PubMed/NCBI
|
|
23
|
Jori FP, Napolitano MA, Melone MA, et al:
Molecular pathways involved in neural in vitro differentiation of
marrow stromal stem cells. J Cellular Biochem. 94:645–655. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
McCully JD, Wakiyama H, Hsieh YJ, et al:
Differential contribution of necrosis and apoptosis in myocardial
ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol.
286:1923–1935. 2004. View Article : Google Scholar : PubMed/NCBI
|